Abstract
Objectives
To characterise the antibody response for 12 weeks following second dose of the Pfizer/BioNTech BNT162b2 mRNA vaccine in hospital workers of a Korean general hospital.
Methods
We measured the level of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) anti-receptor binding domain (anti-RBD) and neutralising antibodies every week in the first 4 weeks, and at weeks 8 and 12 following the second dose of vaccination in 71 hospital workers.
Results
The initial median level of anti-RBD and neutralising antibodies were 3898.0 U/mL (interquartile range [IQR], 2107.5–5478.5) and 97.54 % (IQR, 96.85–97.81), respectively. The levels declined the fastest and the most significantly between weeks 1 and 2 (p < 0.01, both), and continuously decreased thereafter, and were 1163.0 U/mL (683.4–1743.0) and 94.87% (89.24–96.99) at weeks 12. The antibodies levels showed a trend of rapid decrease in the older group over time. The slope of the decrease in the antibodies level was observed for each individual. Within 8 weeks, the anti-RBD antibody levels decreased to less than half of the initial levels in most of the participants (88.7%: 63/71). The SARS-CoV-2 anti-RBD and neutralising antibodies levels showed a strong positive correlation (Spearman’s coefficient = 0.7833).
Conclusions
Considerably high levels of SARS-CoV-2 anti-RBD and neutralising antibodies were produced following the second dose of vaccination. The levels decreased continuously, showing a tendency to decline over time; however, reasonable levels persisted up to weeks 12. Moreover, considering individual variations in antibody response following vaccination, a further inter-individual analysis is needed.
Keywords: Severe acute respiratory syndrome coronavirus 2, Antibody response, BNT162b2, Receptor binding domain
1. Introduction
In the pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which began in 2019 in China, several coronavirus disease 2019 (COVID-19) vaccines have been developed and approved for use worldwide [1], [2]. The BNT162b2 mRNA COVID-19 vaccine (Pfizer, New York, US and BioNTech, Mainz, Germany), which contains nucleoside-modified RNA (modRNA) encoding the SARS-CoV-2 full-length spike, was administered in many parts of the world and assessed for various aspects, including safety, immunogenicity, and efficacy [3], [4], [5], [6], [7]. Polack, et al. reported that the BNT162b2 was 95% effective in preventing Covid-19 (95% CI, 90.3 to 97.6) [7]. Meanwhile, several studies have focused on the antibody response following vaccination. Reports exist stating the role of antibodies in protective immunity against SARS-CoV 2 infection, similar to other viral infections [8], [9], [10]. The first vaccination drive using BNT162b2, which targeted healthcare workers, started on 27 February 2021 in Korea. However, to date, only a few studies have investigated the antibody response following vaccination in Korea. Herein, we attempted to characterise the antibody response for 12 weeks following the second dose of the BNT162b2 vaccine in healthcare workers of a Korean general hospital.
2. Materials and methods
2.1. Study design and participants
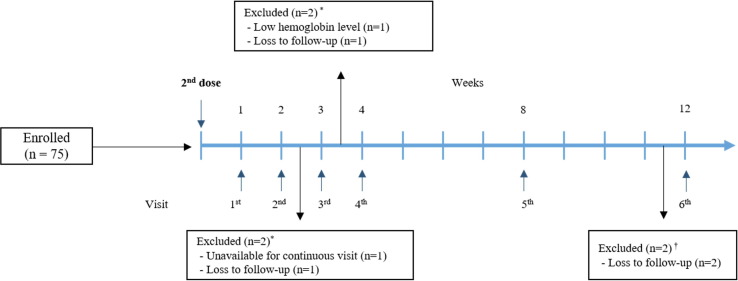
This prospective, single-centre, observational longitudinal study enrolled the Chungnam National University Sejong Hospital workers who had received two doses of the Pfizer/BioNTech BNT162b2 mRNA vaccine. Information regarding the recruitment of participants was posted on the groupware electronic bulletin board, and 71 workers who agreed to participate signed the informed consent form. We confirmed that all participants had never been previously infected with SARS-CoV-2 through medical records and questionnaires. After the second dose, the participants visited the blood collection centre every week for 4 weeks. They subsequently visited at weeks 8 and 12 (Fig. 1 ). We measured the levels of SARS-CoV-2 antibodies, including immunoglobulin M/immunoglobulin G (IgM/IgG), anti-receptor-binding domain (anti-RBD), and neutralising antibodies, at each visit. We measured anti-nucleocapsid(anti-N) antibody levels every month to denote prior SARS-CoV-2 exposure.
Fig. 1.
Study design. *Four participants who lost within 4 weeks were excluded from the analysis. †Two participants who lost at week 12 were included in the analysis.
2.2. SARS-CoV-2 IgM/IgG antibody detection
The levels of SARS-CoV-2 IgM/IgG antibodies were measured using the COVID-19 IgM/IgG Combo FIA kit (SDBiosensor Inc., Suwon, Korea). This test was based on colloidal gold-labelled immunochromatography, and nucleocapsid and spike proteins of SARS-CoV-2 were used as antigens, according to the manufacturer’s instructions. The results were determined as positive or negative using a STANDARD™ F2400 Analyzer (SDBiosensor Inc., Suwon, Korea).
2.3. SARS-CoV-2 anti-N antibody detection
The levels of anti-N antibodies were measured using the semi-quantitative Elecsys anti-SARS-CoV-2 assay on a Cobas e411 analyser (Roche Diagnostics Rotkreuz, Switzerland). The clinical sensitivity ranged from 78.5 to 87.7%, and specificity ranged from 97.6 to 100% [11]. Results were reported as a cut-off index (signal sample/cut-off), with values > 1 considered positive.
2.4. SARS-CoV-2 anti-RBD antibody detection
The levels of anti-RBD antibodies were measured using the quantitative Elecsys anti-SARS-CoV-2 S assay on a Cobas e411 analyser (Roche Diagnostics Rotkreuz, Switzerland). It quantifies the total antibodies against the RBD of the viral spike (S) using electrochemiluminescence sandwich immunoassay [12]. The clinical sensitivity ranged from 67.1 to 82.5%, and specificity was 100% [13]. Results were reported as concentrations (U/mL), with a manufacturer’s cut-off > 15 U/mL considered positive.
2.5. SARS-CoV-2 neutralising antibody detection
Neutralising antibody tests were manually performed using a commercially available surrogate virus neutralisation assay (sVNT, Genscript, Netherlands) according to the manufacturer’s instructions. The assay based on antibody-mediated blockage of the interaction between virus (RBD) and host (angiotensin-converting enzyme 2, ACE2). The clinical sensitivity ranged from 95 to 100%, and specificity was 99.93% [14]. Optical density (OD) was measured at 450 nm using a microplate reader of the DS2 automation system (Dynex Technologies, USA).
2.6. Ethical considerations
This study was approved by the Institutional Review Board of Chungnam National University Sejong Hospital (IRB File No. 2021-04-004).
2.7. Statistical analysis
All the data were validated for normality using the Shapiro-Wilk normality test before statistical analyses. Statistical significance between the two periods or groups was determined using paired t-test, Wilcoxon signed-rank test, or unpaired Mann-Whitney U test. Multiple group comparisons were performed using one-way analysis of variance test followed by Tukey’s post-hoc test for normally distributed parameters or Kruskal-Wallis with Dunn’s post-hoc test for parameters that did not show a normal distribution. Spearman’s rank correlation coefficient was calculated to determine the correlation between SARS-CoV-2 anti-RBD and SARS-CoV-2 neutralising antibodies. A probability value of p < 0.05 was considered statistically significant for all the analyses. R software (version 4.1.0, R Foundation for Statistical Computing, Vienna, Austria) was used for all the statistical analyses, and graphs were created in Python using seaborn libraries.
3. Results
3.1. Participant demographics and anti-N antibody assay results
Overall, 71 hospital workers were included; the median (interquartile range, IQR) age was 35 (28–40) years, and 45.1% (32/71) were males. There were 25 people in their 20 s, 26 people in their 30 s, and 20 people in their 40 s and over. Two participants (N = 70, 71), both men in their 30 s, were missing at week 12. We performed anti-N antibody assay at week 1, 4, 8, and 12, respectively. All the participants were confirmed as SARS-CoV-2 naïve through the negative results.
3.2. 100% positivity of SARS-CoV-2 IgG in the qualitative assay
We performed the COVID-19 IgM/IgG Combo test for the anti-SARS-CoV-2 binding antibodies, which showed 100% positivity for IgG in all samples during the study period. IgM was positive in 12.7% (9/71) at week 1 and slightly increased to 15.5% (11/71) at week 2. Thereafter, it gradually decreased to 9.9% (7/71) at week 3 and 7.0% (5/71) at week 4. IgM positivity was not observed at weeks 8 and 12.
3.3. Changes in SARS-CoV-2 anti-RBD antibody levels after second dose of vaccine
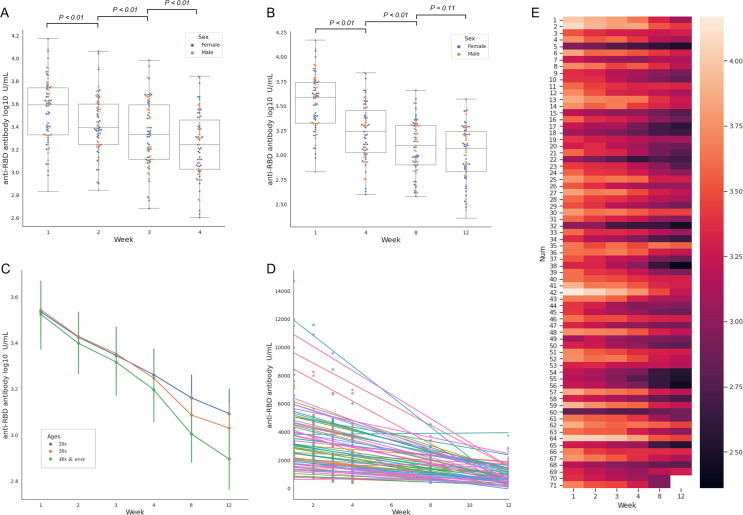
Anti-RBD antibody levels are summarised in Table 1 . The initial median antibody level was 3898.0 U/mL (IQR, 2107.5–5478.5) at week 1. The fastest and most significant decline was observed between weeks 1 and 2 (2432.0, 1742.5–3927.0) (p < 0.01) (Supplementary Material Fig. S1A). It continuously decreased to 2152.0 (1288.0–3894.5) and 1753.0 (1053.5–2834.5) at weeks 3 and 4, respectively. The differences in both periods were significant (p < 0.01); however, the decline between weeks 3 and 4 was steeper than between weeks 2 and 3. The median antibody level was 1245.0 (794.4–2036.0) at week 8, and the difference between weeks 4 and 8 was significant (p < 0.01). Notably, at week 12, the median antibody level was 1163.0 (683.4–1743.0), which was slightly lower than that at week 8; however, it was not significant (p = 0.11) (Supplementary Material Fig. S1B). Log-transformed data for normalisation demonstrated a similar pattern (Fig. 2 A,B). The initial median antibody level was 3.59 log10 U/mL (IQR, 3.33–3.74) at week 1. At week 2, it significantly decreased to 3.39 (3.24–3.60; p < 0.01). It significantly decreased continuously to 3.33 (3.11–3.59), 3.24 (3.03–3.46), and 3.10 (2.90–3.31) at weeks 3, 4, and 8, respectively (p < 0.01). At week 12, the median antibody level was 3.07 (2.83–3.24), slightly lower than that at week 8; however, the difference was not significant (p = 0.11).
Table 1.
The median levels of Anti–SARS-CoV-2 anti-RBD and neutralising antibodies in the first 12 weeks following the second dose of the BNT162b2 mRNA vaccine.
| Week 1 | Week 2 | Week 3 | Week 4 | Week 8 | Week 12* | ||
|---|---|---|---|---|---|---|---|
| Anti-S RBD Ab (U/mL) | Total (n = 71) |
3898.0 (2107.5–5478.5) |
2432.0† (1742.5–3927.0) |
2152.0† (1288.0–3894.5) |
1753.0† (1053.5–2834.5) |
1245.0† (794.4–2036.0) |
1163.0 (683.4–1743.0) |
| Male (n = 32) |
3961.5 (2095.0–5706.0) |
2685.5 (1747.8–4221.5) |
2442.0 (1271.8–4268.8) |
1967.0 (1037.0–2756.0) |
1371.5 (814.5–1974.8) |
1304.5 (791.4–1709.8) |
|
| Female§ (n = 39) |
3817.0 (2342.0–5107.0) |
2378.0 (1866.5–3522.5) |
2135.0 (1351.5–3571.5) |
1619.0 (1128.0–2817.5) |
1192.0 (794.4–2118.5) |
919.4 (644.0–1720.5) |
|
| 20 s (n = 25) |
3968.0 (2099.0–5465.0) |
2760.0 (1818.0–3860.0) |
2152.0 (1262.0–3794.0) |
2017.0 (1040.0–3282.0) |
1503.0 (842.9–2362.0) |
1300.0 (734.7–1913.0) |
|
| 30 s (n = 26) |
3954.0 (2443.2–4822.5) |
2539.5 (1956.5–3959.0) |
2205.5 (1527.5–3618.5) |
1826.0 (1217.2–2434.8) |
1305.0 (905.1–1932.8) |
1194.0 (769.5–1625.8) |
|
| 40 s (n = 20) |
3610.5 (2038.2–5706.0) |
2277.5 (1693.5–3796.8) |
1892.5 (1286.8–4206.8) |
1570.5 (935.9–2609.5) |
1054.0 (539.2–1792.5) |
801.7 (465.6–1297.2) |
|
| Neutralising Ab (Inhibition %) | Total (n = 71) |
97.54 (96.85–97.81) |
96.93† (96.33–97.15) |
96.82† (96.06–97.15) |
96.87 (96.20–97.13) |
96.31† (93.89–97.33) |
94.87† (89.24–96.99) |
| Male (n = 32) |
97.46 (96.60–97.83) |
96.87 (96.34–97.15) |
96.74 (95.95–97.15) |
96.70 (96.08–97.11) |
96.41 (94.06–97.29) |
95.64 (90.42–97.00) |
|
| Female (n = 39) |
97.54 (97.08–97.79) |
96.97 (96.36–97.23) |
96.87 (96.25–97.17) |
96.92 (96.28–97.13) |
96.14 (93.72–97.33) |
94.62 (88.52–96.95) |
|
| 20 s (n = 25) |
97.54 (97.26–97.79) |
96.93 (96.59–97.09) |
96.82 (96.47–97.15) |
96.93 (96.30–97.18) |
96.77 (94.58–97.48) |
96.09 (91.07–97.60) |
|
| 30 s (n = 26) |
97.61 (96.90–97.90) |
96.95 (96.30–97.19) |
96.95 (96.19–97.19) |
96.90 (96.46–97.12) |
96.64 (94.19–97.30) |
95.64 (89.63–97.11) |
|
| 40 s (n = 20) |
97.46 (96.51–97.73) |
96.87 (96.21–97.11) |
96.69 (95.49–97.02) |
96.73 (95.27–97.00) |
95.78 (90.17–96.90) |
93.39 (84.49–96.02) |
|
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RBD, receptor binding domain of viral spike protein.
* Two participants were loss, so total number of participants were 69. †p < 0.01.
Fig. 2.
SARS-CoV-2 anti-RBD antibody log-scale responses for (A) weekly change for 4 weeks, (B) monthly. Change for 12 weeks. SARS-CoV-2 anti-RBD antibodies (C) change according to age groups, (D) regression model, and (E) heatmap for individuals. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RBD, receptor-binding domain of viral spike protein.
3.4. Tendency of rapid decrease in the SARS-CoV-2 anti-RBD antibody levels in the older group over time
We analysed whether the anti-S RBD antibody levels differed based on sex and age. There was no significant difference in antibody levels between men and women (Table 1). The Kruskal-Wallis analysis was performed to determine whether there were differences in the mean antibody levels among the different age groups (20 s, 30 s, and 40 s and over). Although the analysis results for each week showed no significant differences among the three age groups (Table 1), the mean antibody level showed a trend of rapid decrease in the older group over time (Fig. 2C).
3.5. Varied SARS-CoV-2 anti-RBD antibody response dynamics in the participants
Regression analysis showed that 95.8% (68/71) of the participants tended to decrease antibody levels over time (Fig. 2D, E). Although most participants showed a tendency of decreased antibodies, the slope of the decrease in the antibody levels was observed for each individual. A faster decrease was observed in participants with higher antibody levels at week 1 (Supplementary Material Fig. S2A, B). Three participants (Participant Number = 35, 60, 69) showed an increasing trend, and only one participant (Participant Number = 60) had a higher antibody level at week 12 (785.5 U/mL) than that at week 1 (675.9 U/mL).
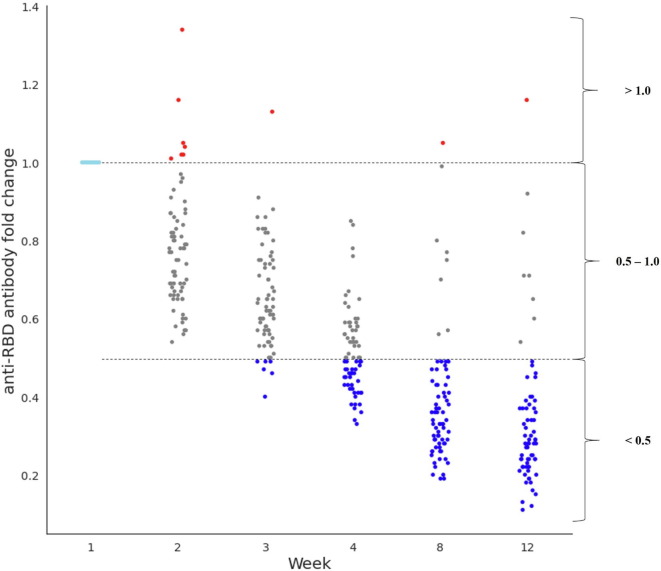
3.6. Time taken to drop by half or less of the initial antibody level
To determine when the antibody levels dropped by half or less, the change in pattern of each antibody level was converted into a fold change. At week 3, for the first time, 8.5% of the participants (6/71) showed antibody levels that were at least two-fold lower than that at week 1 (Fig. 3 ). Participants who had less than half of their initial antibody levels within a month (at week 4) showed an increase to 49.3% (35/71), and at week 8, 88.7% (63/71) of participants showed a decrease to less than half of the antibody levels compared to those at week 1. At week 12 (88.4%, 61/69), there was no difference compared to week 8, except for the loss of two participants.
Fig. 3.
SARS-CoV-2 anti-RBD antibody fold change according to weeks after 2nd dose vaccination. SARS-CoV. 2, severe acute respiratory syndrome coronavirus 2; RBD, receptor-binding domain of viral spike protein.
3.7. Similar but not identical SARS-CoV-2 neutralising antibody responses to the SARS-CoV-2 anti-RBD antibodies
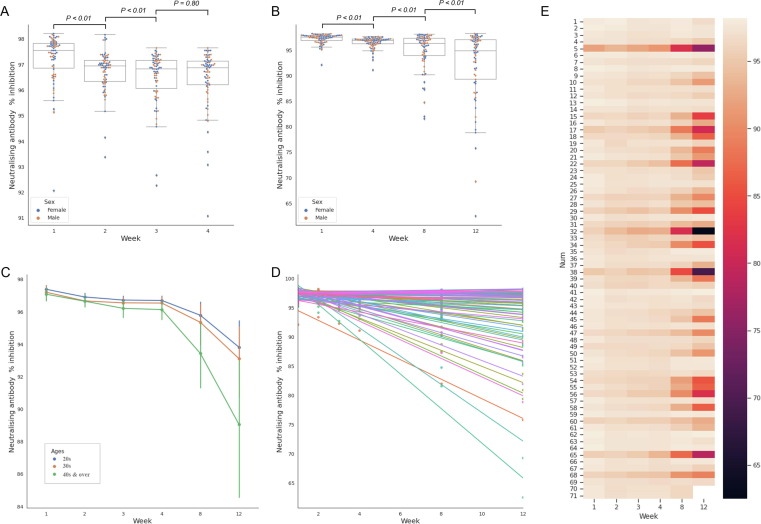
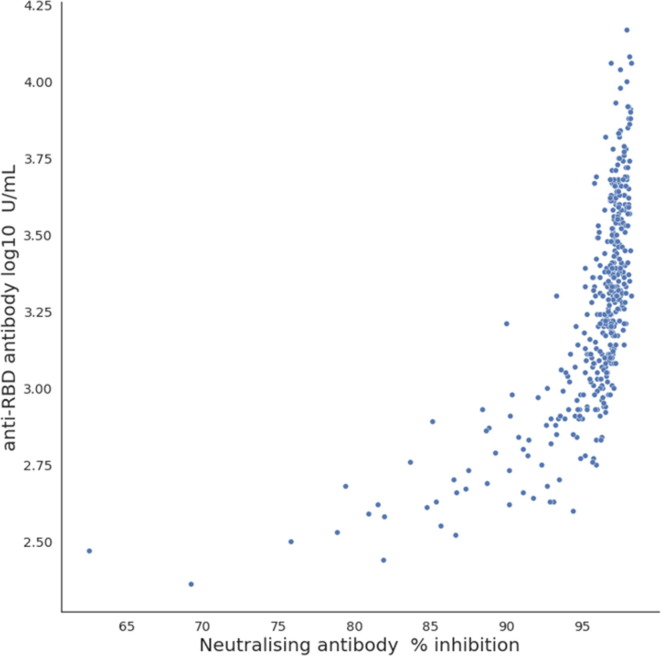
Quantitative analysis was performed by converting the optical density observed in the assay into inhibition percentage (% inhibition) (Table 1). The initial median % inhibition was 97.54 % (IQR, 96.85–97.81) at week 1, and it significantly decreased to 96.93 (96.33–97.15) and 96.82 (96.06–97.15) at weeks 2 and 3, respectively (Fig. 4 A). Notably, the median % inhibition at week 4 (96.87; IQR, 96.20–97.13) was slightly higher than at week 3. Thereafter, the median % inhibition was significantly decreased at weeks 8 (96.31; IQR, 93.89–97.33) and 12 (94.87; IQR, 89.24–96.99) (Fig. 4B). There were no significant differences in the percentage of inhibition between men and women (Table 1). Similar to the SARS-CoV-2 anti-RBD antibody response, the SARS-CoV-2 neutralising antibodies showed a tendency to decrease more rapidly in the older group over time (Fig. 4C). Conversely, as opposed to the SARS-CoV-2 anti-RBD antibody response, the SARS-CoV-2 neutralising antibodies showed small differences among individuals at week 1, and as time passed, the inter-individual differences became larger (Fig. 4D,E). Overall, 31.9 % (22/69) of the participants showed a change in the percentage of inhibition of < 1% at week 12 compared with that at week 1, and three of them (Participant Number = 35, 41, 62) showed an elevated level. A decrease of > 10% was observed in 18.8 % (13/69) of the participants (Supplementary Material Fig. S3). The correlation analysis revealed a strong positive correlation (Spearman’s coefficient = 0.7833) between the SARS-CoV-2 neutralising antibodies and SARS-CoV-2 anti-RBD antibodies (p < 0.01; r2 = 0.4138) (Fig. 5 ).
Fig. 4.
SARS-CoV-2 neutralising antibody responses for (A) weekly change for 4 weeks, (B) monthly change for. 12 weeks, (C) change according to age groups, (D) regression model, and (E) heatmap for individual. SARS-CoV. 2, severe acute respiratory syndrome coronavirus 2; RBD, receptor-binding domain of viral spike protein.
Fig. 5.
Correlation between anti-RBD and neutralising antibodies following COVID-19 vaccination. SARS-CoV. 2, severe acute respiratory syndrome coronavirus 2; RBD, receptor binding domain of viral spike protein.
4. Discussion
Herein, we characterised the SARS-CoV-2 antibody response for 12 weeks from the second dose of the BNT162b2 mRNA COVID-19 vaccine. In the qualitative test, all the participants showed 100% IgG positivity for 12 weeks. SARS-CoV-2 anti-RBD and SARS-CoV-2 neutralising antibodies were observed at much higher concentrations than the cut-off level in all participants, and both SARS-CoV-2 anti-RBD and SARS-CoV-2 neutralising antibodies showed a strong positive correlation. In most participants, the levels of both antibodies tended to decrease over time; however, they remained much higher than the cut-off level until the end of the study period. Notably, the higher the initial level, the faster the decrease rate in the SARS-CoV-2 anti-RBD antibodies, and the initial antibody level decreased to less than half within 8 weeks in most participants. Interestingly, some of the participants even demonstrated an increase in their antibody levels over time. Although not statistically significant, we confirmed the tendency of a more rapid decrease of antibodies in the older group over time.
In our study, IgM is observed in a small portion compared to IgG (IgM positivity rate: 12.7% at week 1 and 15.5% at week 2) and not observed over time. Lustig et al. reported that following the second vaccination, IgM positivity peaked at 27.7% on the 7th day and decreased rapidly thereafter [1]. Differences in portion and peak time may be due to different test method or the small number of participants in our study. However, the rapid decrease of IgM and robust IgG response, which are shown in both studies, are suitable for the common concept of the general process that occurred after vaccination [15]. Similar to other vaccination-induced humoral responses, the class switch occurs quickly due to the high immunogenicity of S-protein, and it leads to robust IgG response after BNT162b2 mRNA COVID-19 vaccination.
One of our major findings was that following the second vaccination, the anti-RBD antibody level reached a peak within 1 week and then decreased rapidly, and the rate of decrease slowed as time passed. Lustig et al. also reported that anti-RBD IgG reached its peak at 1 week [1]. In a study by Moss et al., although they did not measure the antibody levels at week 1, the antibody level measured for the first time (week 2) following the second inoculation was the highest and showed a decreasing pattern thereafter, similar to our results [16]. We think that this is related to the early transient burst of short-lived plasmablasts in the first week of the second vaccination by the memory B cells generated after the first vaccination [15]. Moreover, there is a report of a SARS-CoV-2 mRNA vaccine-induced persistent human germinal center response [17], which we think may be related to the slower rate of decrease over time.
The decline in the antibody levels varied individually; however, the participants who had initial high antibody levels showed a tendency to decrease more rapidly. There are some reports on inter-individual changes in antibody levels [1], [18]; however, few studies have reported inter-individual changes following vaccination. Interestingly, we observed a trend toward increasing antibodies level in some participants. An increase in anti-RBD antibody level was observed in participants 35, 60, 69. Participants 35, 41, 62 showed a trend of increasing neutralising antibody level. Participant number 35, a 27-year-old female, was the only participant who observed an increase in both antibodies. To our knowledge, there have been no reports on an increase in antibodies level over time following the second dose. Further studies are needed to determine the cause of increased antibody levels.
We analysed the antibody level changes following conversion to fold change; hence, we found that antibody levels in most participants decreased by less than half within 8 weeks. However, it was difficult to predict when the decrease was below the cut-off level since the rate of decrease slowed with time; thus, future research with a longer study period is warranted.
We observed a faster decline in the antibody levels in the older group over time. Some studies have reported significant differences in the antibody levels among different age groups [1], [19], and the criteria for the older age group was older (65 or 66 years) than that considered in our study (40 years). We believe that a significant result would be obtained if the number of participants was greater. Nevertheless, a decrease in antibody levels appears to be associated with age.
In our study, the anti-RBD and the neutralising antibodies showed a strong positive correlation; however, the adjusted R square value was low (0.4138). Some studies also analysed the correlation between the two antibodies, and most showed a positive correlation [1], [20], [21]. Chia et al. reported that the neutralising antibody response dynamics in patients who have recovered from COVID-19 vary greatly; hence, prediction of immune longevity can only be accurately determined at the individual level [10].
Bergwerk et al. reported breakthrough infections among health care workers who were fully vaccinated with BNT162b2 mRNA COVID-19 vaccine [22]. They suggested that neutralising antibody titres correlated with breakthrough infection. However, we did not observe breakthrough infection in our paticipants during the study period. Long-term follow-up of participants is required to establish an association between breakthrough infection and antibodies level.
Our study had several limitations. First, to confirm the correct antibody response, test results before and after the first vaccination are warranted; however, they were not included in our study. Second, the sample size was not large enough to be statistically significant among the three age groups. Third, older participants were excluded. The oldest participant was 60 years old. Fourth, there were inaccuracies due to the test method. The Roche kit used for the SARS-CoV-2 anti-RBD antibodies can measure up to 250 U/mL without dilution. According to the manufacturer’s information, accuracy could be reduced with dilution, and we used the result of dilution up to 50 times. The sVNT method for measuring neutralising antibodies was developed as a qualitative method; however, we converted the results into percentage of inhibition and used them for quantitative comparison. Furthermore, we used the WHO International Standard and Reference Panel [23] to convert the percentage of inhibition result into a U/mL unit during the experiment.; however, owing to the large difference between the predicted value and the measured value, it could not be used.
5. Conclusion
Following the second dose of the BNT162b2 mRNA vaccine, anti-SARS-CoV-2 antibodies decreased after 1 week, and the rate of decrease showed a tendency to slow down over time. However, since individual variation is very large, it is important to observe the antibody response at the individual level following vaccination.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.12.012.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Lustig Y., Sapir E., Regev-Yochay G., Cohen C., Fluss R., Olmer L., et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9(9):999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyriakidis N.C., López-Cortés A., González E.V., Grimaldos A.B., Prado E.O. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines. 2021;6(1) doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skowronski D.M., De Serres G. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021;384:1576–1577. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 5.Frenck R.W., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellerstein M. What are the roles of antibodies versus a durable, high quality T-cell response in protective immunity against SARS-CoV-2? Vaccine X. 2020;6:100076. doi: 10.1016/j.jvacx.2020.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addetia A., Crawford K.H.D., Dingens A., Zhu H., Roychoudhury P., Huang M.-L., et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58(11) doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chia W.N., Zhu F., Ong S.W.X., Young B.E., Fong S.-W., Le Bert N., et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2(6):e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padoan A., Bonfante F., Pagliari M., Bortolami A., Negrini D., Zuin S., et al. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBioMedicine. 2020;62:103101. doi: 10.1016/j.ebiom.2020.103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resman Rus K., Korva M., Knap N., Avšič Županc T., Poljak M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. JCV. 2021;139:104820. doi: 10.1016/j.jcv.2021.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins V., Fabros A., Kulasingam V., Loeffelholz M.J. Quantitative measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol. 2021;59(4) doi: 10.1128/JCM.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.-C., Tiu C., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 15.Siegrist C-A. Vaccine immunology. In 2018. p. 16-34.e7.
- 16.Parry H, Bruton R, Stephens C, Brown K, Amirthalingam G, Hallis B, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people. medRxiv. 2021 May 17.05.15.21257017. [DOI] [PMC free article] [PubMed]
- 17.Turner J.S., O’Halloran J.A., Kalaidina E., Kim W., Schmitz A.J., Zhou J.Q., et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596(7870):109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.L’Huillier A.G., Meyer B., Andrey D.O., Arm-Vernez I., Baggio S., Didierlaurent A., et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27:784–e1. doi: 10.1016/j.cmi.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grupper A., Sharon N., Finn T., Cohen R., Israel M., Agbaria A., et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16(7):1037–1042. doi: 10.2215/CJN.03500321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gozalbo-Rovira R., Gimenez E., Latorre V., Francés-Gómez C., Albert E., Buesa J., et al. SARS-CoV-2 antibodies, serum inflammatory biomarkers and clinical severity of hospitalized COVID-19 patients. J Clin Virol. 2020;131:104611. doi: 10.1016/j.jcv.2020.104611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham N.R., Whitaker A.N., Strother C.A., Miles A.K., Grier D., McElvany B.D., et al. Kinetics and isotype assessment of antibodies targeting the spike protein receptor-binding domain of severe acute respiratory syndrome-coronavirus-2 in COVID-19 patients as a function of age, biological sex and disease severity. Clin Transl Immunol. 2020;9(10) doi: 10.1002/cti2.v9.1010.1002/cti2.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO/BS.2020.2403 Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody [Internet]. [cited 2021 Oct 13]. Available from: https://www.who.int/publications/m/item/WHO-BS-2020.2403
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.