Abstract
Background:
Patient-level surveillance of antimicrobial use (AMU) in Canadian hospitals empowers the reduction of inappropriate AMU and was piloted in 2017 among 14 hospitals in Canada. We aimed to describe AMU on the basis of patient-level data in Canadian hospitals in 2018 in terms of antimicrobial prescribing prevalence and proportions, antimicrobial indications, and agent selection in medical, surgical and intensive care wards.
Methods:
Canadian adult, pediatric and neonatal hospitals were invited to participate in the standardized web-based cross-sectional Global Point Prevalence Survey of Antimicrobial Consumption and Resistance (Global-PPS) conducted in 2018. An identified site administrator assigned all wards admitting inpatients to specific surveyors. A physician, pharmacist or nurse with infectious disease training performed the survey. The primary outcomes were point prevalence rates for AMU over the study period regarding prescriptions, indications and agent selection in medical, surgical and intensive care wards. The secondary outcomes were AMU for resistant organisms and practice appropriateness evaluated on the basis of quality indicators. Antimicrobial consumption is presented in terms of prevalence and proportions.
Results:
Forty-seven of 118 (39.8%) hospitals participated in the survey; 9 hospitals were primary care centres, 15 were secondary care centres and 23 were tertiary or specialized care centres. Of 13 272 patients included, 33.5% (n = 4447) received a total of 6525 antimicrobials. Overall, 74.1% (4832/6525) of antimicrobials were for therapeutic use, 12.6% (n = 825) were for medical prophylaxis, 8.9% (n = 578) were for surgical prophylaxis, 2.2% (n = 143) were for other use and 2.3% (n = 147) were for unidentified reasons. A diagnosis or indication was documented in the patient’s file at the initiation for 87.3% (n = 5699) of antimicrobials; 62.9% (n = 4106) of antimicrobials had a stop or review date; and 72.0% (n = 4697) of prescriptions were guided by local guidelines.
Interpretation:
Overall, three-quarters of AMU was for therapeutic use across participating hospitals. Canadian hospitals should be further incentivized to create and adapt local guidelines on the basis of recent antimicrobial resistance data.
Antimicrobial resistance is a substantial threat to public health1 and increases mortality, morbidity and health care costs.2 Antimicrobial overuse and misuse accelerates the development of antimicrobial resistance. 1,3 A global response is warranted to ensure rational antimicrobial use (AMU), given that antimicrobial resistance is commutable between countries. In 2017, Canada released Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action to reinforce its strategy on antimicrobial resistance and to complement the World Health Organization’s Global Action Plan on Antimicrobial Resistance.4,5 Surveillance of AMU is a core component of Canada’s framework for action, as it allows trend monitoring and identification of areas of concerns.
The Canadian Nosocomial Infection Surveillance Program (CNISP) monitors AMU in participating hospitals using daily defined doses with monthly data points.6 Surveillance of AMU at the community and hospital level is important to identify areas of concern; however, the methods used in this surveillance do not involve the collection of patient-level and qualitative information, most notably information on the indication for AMU, the appropriate choice of antimicrobials, and the dosing and duration of antimicrobial therapy, which is required to interpret quantitative community-and hospital-level data and guide stewardship interventions.7
Patient-level surveillance of AMU is a key factor in reducing antimicrobial overuse and misuse. Recently, CNISP published the results of 3 national point prevalence surveys, limited to health care–acquired infections.8 In Canada, patient-level AMU surveillance has been performed on a national level through a pilot point prevalence survey in 2017, which included only 14 hospitals.9 A broader Canadian sample would provide better national representation and support the evaluation of hospital-based interventions and benchmarking in the future.
The Global Point Prevalence Survey of Antimicrobial Consumption and Resistance (Global-PPS) is an international collaborative created in 2014 to monitor AMU and antimicrobial resistance in hospitals worldwide. Global-PPS locally documents, on a single day, patient-level antimicrobial prescribing practices. The advantage of the Global-PPS’s standardized surveillance method is that it is adapted to all types of hospitals and allows data comparison locally, nationally and internationally. Global-PPS identifies areas for improvement and, through repeat surveys, measures the impact of interventions.
Our main objective was to describe AMU on the basis of patient-level data in Canadian hospitals collected as part of the Global-PPS in 2018 in terms of antimicrobial prescribing prevalence and proportions, antimicrobial indications and agent selection in medical, surgical and intensive care wards. In addition, we measured AMU for resistant organisms and evaluated practice appropriateness on the basis of quality indicators.
Methods
Design, setting and participants
A cross-sectional point prevalence survey on antimicrobial consumption and resistance was performed across Canada between June and December 2018; 1 hospital performed the survey beginning January 2019. Adult, pediatric and neonatal hospitals in Canada were invited to participate in the 2018 Global-PPS through email and live presentations at the annual conferences of the CNISP (n = 87), the Association of Medical Microbiology and Infectious Disease Canada and the Association des Médecins Microbiologistes Infectiologues du Québec (n = 31).
CNISP was created through a collaboration between the Canadian Hospital Epidemiology Committee, a subcommittee of the Association of Medical Microbiology and Infectious Disease Canada, and the Public Health Agency of Canada, which funds the program. Participation is on a voluntary basis and has expanded over time to include participants ranging from small hospitals to large urban academic centres. CNISP now has 87 participating sentinel hospitals across all 10 provinces and 1 territory.
Participation in the current survey was on a voluntary basis for all 118 hospitals contacted. Each participating institution has the advantage and opportunity of comparing their own uniformized data set to the collective aggregated data.
Details of the Global-PPS methods have been described elsewhere.10,11 The methodology we employed was similar to that used in the Canadian Global-PPS conducted in 2017,9 using the standardized protocol and forms developed by the Global-PPS partnership12 (Appendix 1, available at www.cmajopen.ca/content/9/4/E1242/suppl/DC1). Briefly, a point prevalence survey of antimicrobial prescribing was conducted among voluntarily participating hospitals. They reported the total numbers of inpatients admitted on the day of the point prevalence survey. Detailed information was collected for inpatients with an active antimicrobial prescription, including details on prescription, indication and antimicrobial quality indicators as described below.
Outcomes
The primary outcomes were point prevalence rates for AMU over the study period regarding prescriptions, indications and agent selection in medical, surgical and intensive care wards. The secondary outcomes were AMU for resistant organisms and practice appropriateness evaluated on the basis of quality indicators.
Data collection
All inpatient wards were surveyed once on a single day; however, different wards could be surveyed on separate days. All patients present at 8 am in the surveyed ward were to be included. Data collection was done using 2 forms: a ward form for collection of data for denominators (number of beds and admitted patients at 8 am on the day of the survey) and a patient form for collection of data on basic patient characteristics and AMU (type, dose, route, indication, diagnosis) and microbial resistance data. The patient form applied only to in-ward patients receiving at least 1 antimicrobial agent the day of the survey. The included agents were systemic antibiotics, antibiotics used as intestinal anti-infectives, systemic antimycotics and antifungals, antituberculosis agents, nitroimidazole derivatives and antiprotozoals used as antibiotic agents, antivirals and antimalarials. Topical antimicrobials were excluded.
Data for a set of quality indicators were also collected for patients receiving an antimicrobial. The indicators included the following: documentation of diagnosis in the chart at the start of the antimicrobial, local guideline compliance, and documentation of the stop or review date and whether therapy was empirical or targeted. If therapy was targeted, the targeted resistance type was recorded.
The treating physician’s diagnosis was recorded using standardized categories.10 The type of indication was categorized using standardized definitions. The categories were as follows: community-acquired infection, hospital-acquired infection, surgical prophylaxis as 1 dose, 1 day or more than 1 day, medical prophylaxis defined as prophylaxis not related to surgery (e.g., antifungals for chemotherapy), other and unknown.10 Finally, biomarker data were recorded (C-reactive protein, procalcitonin or other) if they supported prescribing decisions. Antibiotics were categorized using the World Health Organization’s AWaRe classification.13
Participating hospitals were required to report all of the data except for the patient’s weight. Notably, as agreed upon by the research ethics boards that approved the study, the age of neonates was not recorded because it was possible to retrieve the exact age using the variables “date of admission” and “age of neonate in days.” Missing details for certain variables had to be recorded as unknown (e.g., diagnosis, indication). Missing details for quality indicators were encoded as “No.” Additionally, lack of availability of data for the quality indicator “guideline compliance” had to be recorded as “not assessable” if there were no local guidelines for the specific indication, or as “no information” when the indication for the antimicrobial was unknown.
An identified site administrator, usually the antibiotic stewardship program director, assigned all wards admitting inpatients to specific surveyors. A physician, pharmacist or nurse with infectious disease training performed the survey. All surveyors participated in an initial 1-hour introductory webinar and a subsequent specific training webinar. The site administrator provided oversight to ensure survey completion and reviewed each case for validation and to avoid duplication. The necessary detailed information was retrieved from medical charts and was not discussed with the ward staff, nor was direct feedback provided, to enhance objective data collection.
Data were submitted by the participating hospital using the Global-PPS’s freely available web-based application. This allowed for anonymized data entry, validation and feedback reporting.10
Statistical analysis
Antimicrobial consumption data are presented as proportions. The prevalence of antimicrobial prescribing was calculated as the proportion of patients on at least 1 antimicrobial divided by the number of inpatients on the ward, with its 95% confidence interval (CI). Patients on single or multiple antimicrobials had the same weight in the numerator. Biomarker data and dose differences among patients receiving the same antimicrobial were not analyzed. Data were analyzed using SPSS version 25.
Ethics approval
The Global-PPS was considered exempt from ethics review as a quality assurance project or was approved by the research ethics boards at participating hospitals as required by institution-specific policies.
Results
Forty-seven of 118 Canadian hospitals (39.8%) participated in the 2018 Global-PPS. The median hospital size was 276 (interquartile range 157–465.5) beds. Overall, 802 units and 13 272 patients were included in the survey; about 1 in every 6 acute care beds in Canada were surveyed.14
Thirty (63.8%) of the participating hospitals were university-affiliated centres. Eleven hospitals (23.4%) were from western Canada (British Columbia and Saskatchewan), 22 (46.8%) were from central Canada (Ontario and Quebec) and 14 (28.8%) were from the Atlantic Provinces (New Brunswick, Newfoundland and Labrador, Nova Scotia and Prince Edward Island). Nine hospitals were primary care centres (10.0% of patients; 1325/13 272), 15 were secondary care centres (29.7% of patients; 3947/13 272) and 23 were tertiary or specialized care centres (60.3% of patients; 8000/13 272). Two tertiary care centres were exclusively pediatric centres. Table 1 presents baseline patient characteristics (the age of neonates was not recorded for privacy reasons).
Table 1:
Baseline patient and hospital characteristics
| Characteristic | No. (%) of patients* n = 13 272 |
|---|---|
| Patients | |
| Adult | 12438 (93.7) |
| Age, yr, mean ± SD | 64.0 ± 17.9 |
| Sex, male | 6816 (54.8) |
| Pediatric | 427 (3.2) |
| Age, yr, mean ± SD | 7.6 ± 7.6 |
| Sex, male | 253 (59.0) |
| Neonate | 407 (3.1) |
| Age, yr | Not recorded |
| Sex, male | 294 (72.2) |
| Hospitals | |
| Type | |
| Primary† | 1325 (10.0) |
| Secondary‡ | 3947 (29.7) |
| Tertiary and specialized§ | 8000 (60.3) |
| Region | |
| Western¶ | 3862 (29.1) |
| Central** | 6440 (48.5) |
| Atlantic†† | 2970 (22.4) |
Note: SD = standard deviation.
Unless indicated otherwise.
Often referred to as a district hospital or first-level referral. It usually corresponds to a general hospital without teaching functions.
Often referred to as provincial hospital. It is a hospital highly differentiated by function with 5–10 clinical specialties, which takes some referrals from other (primary) hospitals. It often corresponds to a general hospital with teaching functions.
Often referred to as a central or regional hospital. It is a hospital with highly specialized staff and technical equipment (e.g., hematology, transplantation, cardiothoracic surgery, neurosurgery and specialized imaging units and an intensive care unit). Clinical services are highly differentiated by function.
British Columbia, Alberta, Saskatchewan and Manitoba.
Ontario and Quebec.
Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick.
Prevalence of antimicrobial use
Of the 13 272 admitted inpatients, 4447 (33.5%, 95% CI 30.7%–36.2%) received a total of 6525 antimicrobials. Missing data were identified only for indication, where 147 prescriptions had “unknown” indications (2.3%, n = 6525). Almost one-third (29.3%, n = 1325) of patients in primary care centres, 31.0% (n = 3947) of those in secondary care centres and 35.4% (n = 8000) of patients in tertiary or specialized care centres received antimicrobials (Table 2). Thirty-four percent (4230/12 438) of adults, 35.8% (153/427) of children and 15.7% (64/407) of neonates received at least 1 antimicrobial (Table 2).
Table 2:
Overall antimicrobial prevalence and use by hospital type, hospital location and ward type
| Characteristic | No. of patients | No. (%) of patients receiving antimicrobials | No. of antimicrobials received | No. (%) of antimicrobials received; indication | |||
|---|---|---|---|---|---|---|---|
| Therapeutic use | Medical prophylaxis | Surgical prophylaxis | Other or unknown | ||||
| Total | 13 272 | 4447 (33.5) | 6525 | 4832 (74.1) | 825 (12.6) | 578 (8.9) | 290 (4.4) |
| Hospital type | |||||||
| Primary | 1325 | 388 (29.3) | 518 | 415 (80.1) | 33 (6.4) | 38 (7.3) | 32 (6.2) |
| Secondary | 3947 | 1225 (31.0) | 1635 | 1316 (80.5) | 101 (6.2) | 156 (9.5) | 62 (3.8) |
| Tertiary and specialized | 8000 | 2834 (35.4) | 4372 | 3101(70.9) | 691 (15.8) | 384 (8.8) | 196 (4.5) |
| Region | |||||||
| West | 3862 | 1424 (36.9) | 2092 | 1572 (75.1) | 241 (11.5) | 198 (9.5) | 81 (3.9) |
| Central | 6440 | 2240 (34.8) | 3372 | 2462 (73.0) | 467 (13.8) | 293 (9.7) | 150 (4.4) |
| Atlantic | 2970 | 783 (26.4) | 1061 | 798 (75.2) | 117 (11.0) | 87 (8.2) | 59 (5.6) |
| Adult wards | |||||||
| All wards | 12 438 | 4230 (34.0) | 6171 | 4610 (74.7) | 749 (12.1) | 567 (9.2) | 245 (4.0) |
| Adult medical ward | 7686 | 2031 (26.4) | 2753 | 2319 (84.2) | 227 (8.2) | 86 (3.1) | 121 (4.4) |
| Hematology–oncology adult medical ward | 420 | 205 (48.8) | 387 | 199 (51.4) | 175 (45.2) | 4 (1.0) | 9 (2.3) |
| Transplant adult medical ward | 142 | 113 (79.6) | 305 | 123 (40.3) | 178 (58.4) | 0 | 4 (1.3) |
| Pneumology adult medical ward | 159 | 91 (57.2) | 196 | 156 (79.6) | 37 (18.9) | 1 (0.5) | 2 (1.0) |
| Adult surgical ward | 3083 | 1318 (42.8) | 1755 | 1205 (68.7) | 73 (4.2) | 403 (23.0) | 74 (4.2) |
| Adult intensive care unit | 948 | 472 (49.8) | 775 | 608 (78.5) | 59 (7.6) | 73 (9.4) | 35 (4.5) |
| Pediatric and neonatal wards | |||||||
| All wards | 834 | 217 (26.0) | 354 | 222 (62.7) | 76 (21.5) | 11 (3.1) | 45 (12.7) |
| Pediatric medical ward | 307 | 81 (26.4) | 115 | 90 (78.3) | 7 (6.1) | 3 (2.6) | 15 (13.0) |
| General neonatal medical ward | 151 | 7 (4.6) | 14 | 12 (87.5) | 2 (14.3) | 0 | 0 |
| Hematology–oncology pediatric medical ward | 25 | 21 (84.0) | 46 | 17 (37.0) | 24 (52.2) | 0 | 5 (10.9) |
| Pediatric surgical ward | 57 | 24 (42.1) | 32 | 20 (62.5) | 4 (12.5) | 5 (15.6) | 3 (9.4) |
| Pediatric intensive care unit | 38 | 21 (55.3) | 39 | 24 (61.5) | 9 (23.1) | 0 | 6 (15.4) |
| Neonatal intensive care unit | 256 | 63 (24.6) | 108 | 59 (54.6) | 30 (27.8) | 3 (2.8) | 16 (14.8) |
Therapeutic use was highest in primary and secondary care centres (80.1% [415/518] and 80.5% [1316/1635], respectively), while the rate of medical prophylaxis in tertiary or specialized centres was more than double that in other hospital types (Table 2).
Therapeutic use
Therapeutic use accounted for the majority of antimicrobial prescriptions: 74.7% (4610/6171) in adults, 65.1% (151/232) in children and 58.2% (71/122) in neonates (Table 2). Treatment was targeted in 39.4% (1906/4832) of these cases. A total of 29.6% (1428/4832) of antimicrobials were for respiratory tract infections, 11.5% (554/4832) were for urinary tract infections and 11.0% (531/4832) were for intraabdominal infections (Appendix 2, available at www.cmajopen.ca/content/9/4/E1242/suppl/DC1).
The overall prevalence of patients with hospital-acquired infections was 9.2% (1221/13 272). Community-acquired pneumonia accounted for 14.4% (696/4832) of antimicrobials while health care–acquired pneumonia accounted for 9.1% (440/4832) of antimicrobials.
Surgical prophylaxis
Cefazolin accounted for the majority of the surgical antimicrobial prophylaxis prescriptions (69.7%, 403/578). Overall, 37.9% (219/578) of patients received a single dose of medication for surgical prophylaxis, 31% (179/578) received prophylaxis for a duration of 1 day and 31.1% (180/578) received prophylaxis for more than 1 day. Appendix 3, available at www.cmajopen.ca/content/9/4/E1242/suppl/DC1, presents antimicrobial prevalence by surgical prophylaxis site in wards. Appendix 4, available at www.cmajopen.ca/content/9/4/E1242/suppl/DC1, presents antimicrobial prevalence by medical prophylaxis site in wards.
Antimicrobial class prevalence
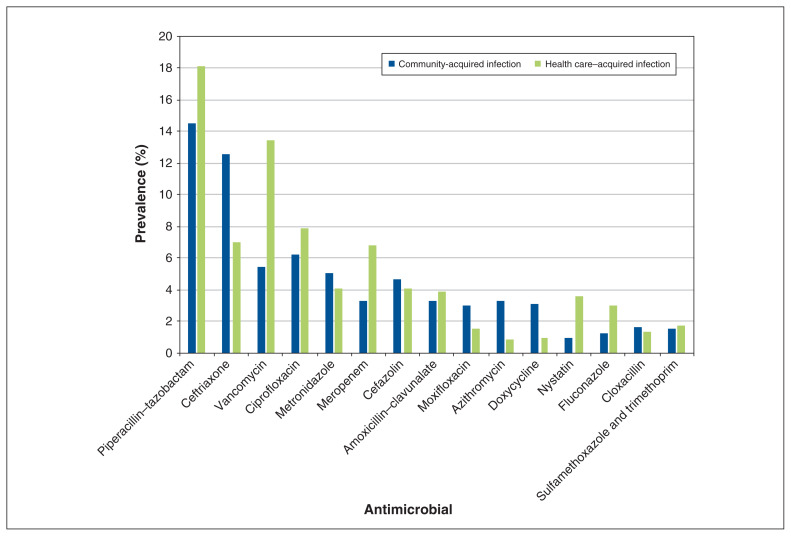
Antibiotics accounted for 83.6% (5454/6525) of antimicrobials prescribed (Appendices 5 and 6, available at www.cmajopen.ca/content/9/4/E1242/suppl/DC1). Penicillins with β-lactamase inhibitors (19.1%, 1042/5454), first-generation cephalosporins (13.4%, 730/5454), third-generation cephalosporins (11.1%, 606/5454) and fluoroquinolones (10.7%, 582/5454) were the most common classes of antibiotics prescribed. The most commonly prescribed antimicrobial agents were piperacillin-tazobactam (12.3%, 800/6525), cefazolin (9.7%, 631/6525), ceftriaxone (8.1%, 531/6525), vancomycin (6.9%, 453/6525) and ciprofloxacin (5.9%, 388/6525).
For the treatment of pneumonia, the combination of a penicillin with a β-lactamase inhibitor (27.2%, 308/1132) or third-generation cephalosporin monotherapy (19.3%, 218/1132) or fluoroquinolone monotherapy (12.5%, 142/1132) accounted for more than half of the antibiotics prescribed. Antivirals (7.3%, 477/6525), antifungals (7.2%, 473/6525), antimalarial agents (1.1%, 72/6525) and antituberculosis agents (0.7%, 47/6525) followed the antibiotics in prevalence.
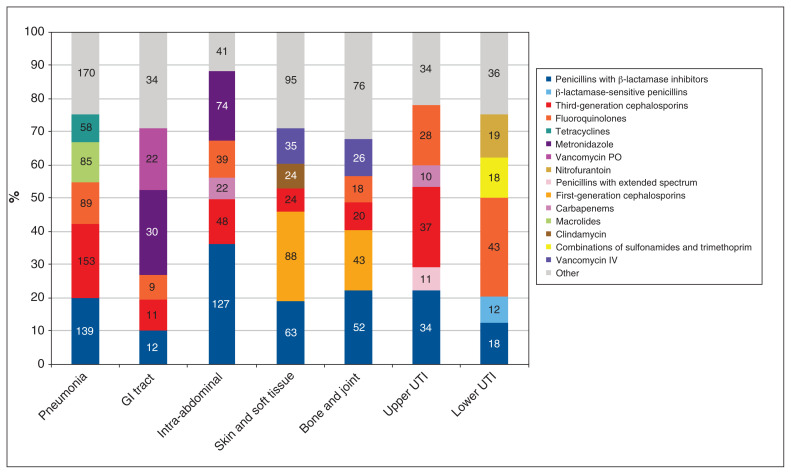
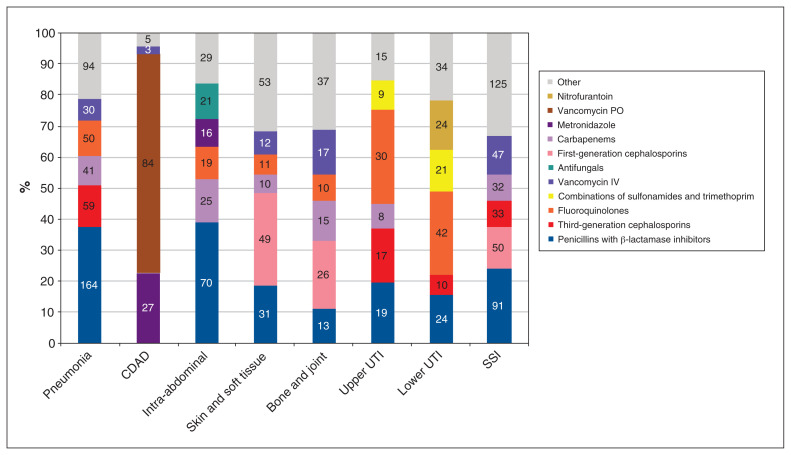
Antimicrobial indications for hospital- and community-acquired infections are presented in Figure 1 and Figure 2. The top 15 antimicrobials used for a therapeutic purpose are presented in Figure 3.
Figure 1:
Antimicrobial use by indication for community-acquired infections. The values in the columns indicate the number of each type of antimicrobial prescribed for patients. Note: GI = gastrointestinal, IV = intravenous, PO = by mouth, UTI = urinary tract infection.
Figure 2:
Antimicrobial use by indication for health care–acquired infection. The values in the columns indicate the number of each type of antimicrobial prescribed for patients. Note: CDAD = Clostridiodes difficile–associated diarrhea, IV = intravenous, PO = by mouth, SSI = surgical site infection, UTI = urinary tract infection.
Figure 3:
The top 15 antimicrobials used for a therapeutic purpose.
Antimicrobial stewardship
A diagnosis or indication was documented in the patient’s file at the initiation of 87.3% antimicrobials (5699/6525); 62.9% of antimicrobials had a stop or review date documented in the patient’s file (4106/6525). Local guidelines were available to guide 72.0% (4697/6525) of prescriptions and 83.6% (3928/4697) of those prescriptions were judged as complying with the recommended choice. Compliance with local guidelines was highest in central Canada (85.3%) (2125/2491) and lowest in the Atlantic Provinces (71.5%) (329/460).
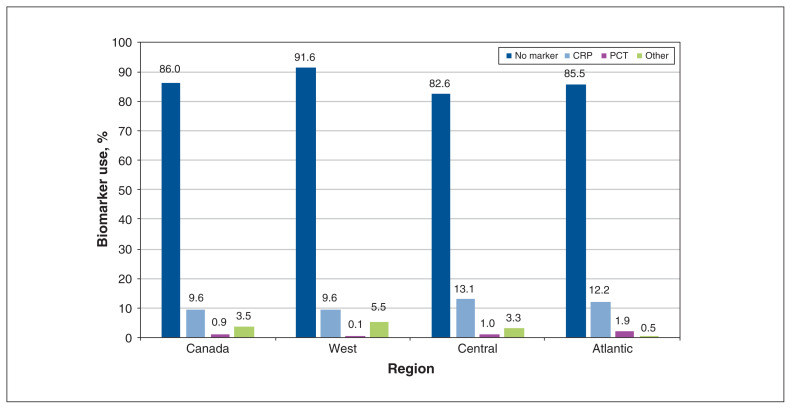
Stewardship data are presented in Table 3. Biomarker use to support prescribing decisions was 14.0% (915/6525) (Figure 4).
Table 3:
Documentation of indications, planned duration or review, and adherence to local guidelines, by region
| Indicator | Region; no. (%) of antimicrobial prescriptions* | |||
|---|---|---|---|---|
| Total | West | Central | Atlantic | |
| No. of hospitals | 47 | 11 | 22 | 14 |
| No. of antimicrobials received | 6525 | 2092 | 3372 | 1061 |
| No. of antimicrobials for therapeutic use | 4832 | 1572 | 2462 | 798 |
| Targeted treatment | 1906/4832 (39.4) | 716/1572 (45.5) | 936/2462 (38.0) | 254/798 (31.8) |
| Reasons in notes | 5699/6525 (87.3) | 1899/2092 (90.8) | 2922/3372 (86.7) | 878/1061 (82.8) |
| Stop or review date documented | 4106/6525 (62.9) | 1357/2092 (64.9) | 2095/3372 (62.1) | 564/1061 (53.2) |
| Guidelines available | 4697/6525 (72.0) | 1746/2092 (83.5) | 2491/3372 (73.9) | 460/1061 (43.4) |
| Compliant with guidelines | 3928/4697 (83.6) | 1474/1746 (84.4) | 2125/2491 (85.3) | 329/460 (71.5) |
| No guidelines available | 1604/6525 (24.6) | 281/2092 (13.4) | 763/3372 (22.6) | 560/1061 (52.8) |
| No information on guidelines because indication is unknown | 224/6525 (3.5) | 65/2092 (3.2) | 118/3372 (3.5) | 41/1061 (3.9) |
Unless indicated otherwise.
Figure 4:
Use of biomarkers to guide treatment. Note: CRP = C-reactive protein, PCT = procalcitonin.
Antimicrobial used to treat multidrug-resistant organisms
A total of 353 patients had targeted treatments against multidrug-resistant organisms out of the 1470 patients for whom AMU was for targeted use (24.0%). Of those, 34.8% (186/535), 18.2% (130/716) and 16.9% (37/219) were in the western, central and Atlantic regions of Canada, respectively. The most frequent multidrug-resistant organism was methicillin-resistant Staphylococcus aureus (MRSA) (23.2%, 82/353). The prevalence of patients with an MRSA infection was 9.5% (51/535), 4.2% (30/716) and 0.5% (1/219) in the western, central and Atlantic regions of Canada, respectively. AMU rates are presented on the basis of targeted treatment in Table 4.
Table 4:
Rate of antimicrobial use to treat multidrug-resistant organisms, by region
| Multidrug-resistant organism | Region; no. (%) of patients treated for multidrug-resistant organisms* | |||
|---|---|---|---|---|
| Total n = 1470 |
Region | |||
| West n = 535 |
Central n = 716 |
Atlantic n = 219 |
||
| MRSA | 82 (5.6) | 51 (9.5) | 30 (4.2) | 1 (0.5) |
| MRCoNS | 33 (2.2) | 12 (2.2) | 18 (2.5) | 3 (1.4) |
| VRE | 10 (0.7) | 4 (0.7) | 6 (0.8) | 0 |
| ESBL-producing Enterobacteriaceae | 41 (2.8) | 22 (4.1) | 11 (1.5) | 8 (3.7) |
| 3-ceph | 60 (4.1) | 26 (4.9) | 19 (2.7) | 15 (6.8) |
| CRE | 5 (0.3) | 5 (0.9) | 0 | 0 |
| ESBL-NF | 22 (1.5) | 12 (2.2) | 9 (1.3) | 1 (0.5) |
| CR-NF | 25 (1.7) | 9 (1.7) | 13 (1.8) | 3 (1.4) |
| Other multidrug-resistant organism | 75 (5.1) | 45 (8.4) | 24 (3.4) | 6 (2.7) |
Note: 3-ceph = third-generation cephalosporin-resistant Enterobacterales, CRE = carbapenem-resistant Enterobacterales, CR-NF = carbapenem-resistant nonfermenter Gram-negative bacilli, ESBL = extended-spectrum β-lactamase, ESBL-NF = ESBL-producing nonfermenter gram-negative bacilli, MRCoNS = methicillin-resistant coagulase-negative staphylococci, MRSA = methicillin-resistant Staphylococcus aureus, VRE = vancomycin-resistant enterococci.
The denominator for the percentages in this table is the number of patients receiving antimicrobials for targeted use.
Interpretation
Participation in this point prevalence study was voluntary; therefore, it was not designed to obtain representative results for Canadian hospitals. However, the study measured AMU in 18% of acute care beds in hospitals Canada and 8 of the 10 provinces.
Our findings on AMU on medical, surgical and intensive care wards are similar to data previously reported in Canada. As seen in the 2017 pilot study,9 respiratory tract infections accounted for the majority of the infections treated in all wards except for surgical wards, where intra-abdominal infections were most prevalent. Treatments for surgical site infections were more frequent on surgical wards, while treatments for pneumonias and urinary tract infections were more frequent on medical wards. The number of antimicrobials used for prophylaxis was similar for both types of wards, but antimicrobials were used predominantly for medical indications on medical wards and for surgical indications on surgical wards.
The proportion of AMU varied substantially between indications in the study. Third-generation cephalosporins were the most commonly used antimicrobials for community-acquired pneumonia, while penicillins with β-lactamase inhibitors were the most commonly used antimicrobials for the treatment of hospital-acquired pneumonia. Agent selection for community- and hospital-acquired pneumonia was similar to previously reported worldwide and European rates.10 However, lower use of penicillins with β-lactamase inhibitors was reported in the United States, where levofloxacin alone was the predominant choice for community- and hospital-acquired pneumonia.10
From 2002 to 2017, a substantial decrease in prescriptions for hospital-acquired infections was observed in Canada.8 When we compared our results with the 2017 survey,9 this decrease appears to have been maintained in 2018. At the patient level, similar rates of hospital-acquired infections were reported worldwide in 201510 and in Europe in 2016–2017.15
Our data indicate that piperacillin–tazobactam is being used more frequently than cefazolin than it was previously.6 We observed a general continuation in the order of most used antimicrobials between 2016 and 2018 in Canada. A major decrease in fluoroquinolone use was previously observed in Canadian hospitals that are part of the CNISP network.6 When we compared our results with point prevalence surveys performed in 2002, 2009 and 2017, our results were consistent with this trend.9,16
The proportion of patients receiving a single dose of surgical prophylaxis more than tripled between 2017 and 2018, whereas receipt of surgical prophylaxis for more than 1 day decreased by 20%.9 In Europe, surgical prophylaxis for more than 1 day represents 54% of prescriptions for surgical prophylaxis, 15 compared with 31% in this study. Our results indicate a trend toward surgical prophylaxis being administered for no more than 1 day in Canada.
On any given day, the indication for a prescription was identified in most cases (87%). However, a substantial proportion of antimicrobial prescriptions in the Atlantic Provinces were not guided by local guidelines (53% v. 25% national average); implementation of local guidelines could help reduce misuse and overuse in this region. Moreover, higher guideline availability appeared to correlate with higher rates of targeted treatments in this study. Nevertheless, compliance to guidelines in 2018 was almost identical to previous Canadian9 and European findings.15 Despite similar rates of compliance, the decrease in AMU in children and neonates, combined with the evidence that a single dose of surgical prophylaxis seemed to be prioritized over surgical prophylaxis for more than 1 day, may indicate a more rational use of antimicrobials across Canada. Further evaluations should be performed to assess the impact of the point prevalence surveys on the participating hospitals and the rollout of enhanced antimicrobial stewardship interventions that may have been introduced in response to the survey results.
As seen in the 2017 survey, MRSA is still the most frequently treated multidrug-resistant organism.9 The rate of MRSA infections has been increasing since 2012 and this trend is believed to be driven by the increasing rate of community-acquired MRSA.17 A substantial proportion of multidrug-resistant organisms were identified in western Canada (34.8% v. 24.0% national average). Multidrug-resistant organisms were generally more uncommon in the Atlantic Provinces. The lower prevalence of MRSA in the Atlantic Provinces (0.5% v. 5.6% national average) appears to correlate with the lower empirical use of vancomycin in this region (2.6% v. 4.6% national average). Indeed, as vancomycin is usually recommended when treating an MRSA infection,18 it is plausible that the prevalence of vancomycin use is being driven by the prevalence of MRSA. This association is maintained for the rest of Canada.
Higher rates of AMU and lower rates of hospital-acquired infections have been reported in the United States;19,20 however, the difference in reporting methodology and period between surveys prevents rigorous comparison between countries. When similar methodology is used, the prevalence of AMU reported in this study is in line with previously reported global and European proportions.10,15
Future point prevalence surveys should be performed to evaluate the impact of existing stewardship interventions on trends in AMU across Canadian hospitals by region, by hospital type and by individual hospital, as well as to identify new tailored interventions that could have a substantial impact on the quality and quantity of antimicrobial prescribing practices. Future areas of consideration could include the use of more detailed diagnostic codes, graded appropriateness and collection of data regarding allergies to antimicrobials.
Limitations
The main limitation of point prevalence surveys is inherent to the method of a cross-sectional survey, namely the interpretation of data acquired at a single point in time. Although day-to-day variations occur, these surveys have moderate correlation with antimicrobial consumption measured in daily defined doses for the month and season of the survey.21 Surveys were performed between June and December (1 in January), which may partially correct for seasonal variation; however, a portion of the winter season was missed, where AMU may be different.
The point prevalence survey was carried out at centres where identification of microorganism and stewardship programs are mostly available, introducing selection and representiveness bias. Performing such a survey in hospitals where this expertise is not available is of future interest.
A total of 14 hospitals participated in the 2017 survey whereas 47 participated in 2018; comparison may be limited. Furthermore, the 40% (47/118) of hospitals that participated in the current survey did so on a voluntary basis, and this could be a source of selection bias.
Differences among surveyors regarding the interpretation of compliance with guidelines may also be a source of bias, as intersurveyor variance may be present; however, only a limited group of surveyors per site acquired the data, minimizing this variance. An important missing component of the survey was the validity of the infectious disease diagnosis. The surveyor recorded what the physician intended to treat as recorded in the medical files, which is not based on clinical case definitions provided with the Global-PPS protocol.
Conclusion
This study provided valid and reliable information on antimicrobial prescribing practices in participating Canadian hospitals. The results can inform national and local stewardship programs. The overall use of antimicrobials was similar to what was previously reported in Canada. However, the prevalence of AMU in pediatric and neonatal patients seems to be decreasing and surgical prophylaxis with 1 dose is being prioritized over surgical prophylaxis for more than a day; these 2 findings suggest that the use of antimicrobials is becoming more rational. Adherence to surgical prophylaxis guidelines was high throughout the surveyed hospitals, although it was lower in the Atlantic Provinces. New protocols have since been developed in this region.
Supplementary Material
Footnotes
Competing interests: Daniel Thirion has shares in Lumed. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Greg German and Charles Frenette were joint primary authors. Ines Pauwels, Herman Goossens and Ann Versporten oversee the Global Point Prevalence Survey of Antimicrobial Consumption and Resistance (Global-PPS). Greg German and Charles Frenette coordinated the Canadian 2018 Global-PPS. All authors coordinated or contributed to data collection, data processing, data analysis and drafting or critically revising the manuscript and agree to be accountable for all aspects of the work. All authors have approved the final version for publication.
Funding: This research received no external funding. The Global Point Prevalence Survey of Antimicrobial Consumption and Resistance is coordinated at the University of Antwerp, Belgium, and sponsored through an unrestricted grant by bioMérieux.
Data sharing: Study data are strictly confidential and are stored anonymously at the coordinating center of the University of Antwerp. Requests for data should go through and will be evaluated by the group in Antwerp to ensure that data-sharing agreements for the respective participating countries are respected. Requests should be addressed to Dr. Ann Versporten (ann.versporten@uantwerpen.be) All requests for commercial reasons or use are systematically refused.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/9/4/E1242/suppl/DC1.
References
- 1.Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. PT. 2015;40:277–83. [PMC free article] [PubMed] [Google Scholar]
- 2.Abat C, Rolain JM, Dubourg G, et al. Evaluating the clinical burden and mortality attributable to antibiotic resistance: the disparity of empirical data and simple model estimations. Clin Infect Dis. 2017;65(Suppl 1):S58–63. doi: 10.1093/cid/cix346. [DOI] [PubMed] [Google Scholar]
- 3.Michael CA, Dominey-Howes D, Labbate M. The antimicrobial resistance crisis: causes, consequences, and management. Front Public Health. 2014;2:145. doi: 10.3389/fpubh.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global action plan on antimicrobial resistance. Geneva: World Health Organization; 2015. [accessed 2021 Apr. 16]. Available: www.who.int/antimicrobial-resistance/global-action-plan/en/ [Google Scholar]
- 5.Public Health Agency of Canada. Pan-Canadian framework for action on antimicrobial resistance and antimicrobial use. Can Commun Dis Rep. 2017;43:217–9. doi: 10.14745/ccdr.v43i11a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudnick W, Science M, Thirion DJG, et al. Antimicrobial use among adult inpatients at hospital sites within the Canadian Nosocomial Infection Surveillance Program: 2009 to 2016. Antimicrob Resist Infect Control. 2020;9:32. doi: 10.1186/s13756-020-0684-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNeil V, Cruickshank M, Duguid M. Safer use of antimicrobials in hospitals: the value of antimicrobial usage data. Med J Aust. 2010;193(S8):S114–7. doi: 10.5694/j.1326-5377.2010.tb04026.x. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell R, Taylor G, Rudnick W, et al. Trends in health care-associated infections in acute care hospitals in Canada: an analysis of repeated point-prevalence surveys. CMAJ. 2019;191:E981–8. doi: 10.1503/cmaj.190361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frenette C, Sperlea D, German GJ, et al. The 2017 global point prevalence survey of antimicrobial consumption and resistance in Canadian hospitals. Antimicrob Resist Infect Control. 2020;9:104. doi: 10.1186/s13756-020-00758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Versporten A, Zarb P, Caniaux I, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6:e619–29. doi: 10.1016/S2214-109X(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 11.Vandael E, Latour K, Goossens H, et al. Point prevalence survey of antimicrobial use and healthcare-associated infections in Belgian acute care hospitals: results of the Global-PPS and ECDC-PPS 2017. Antimicrob Resist Infect Control. 2020;9:13. doi: 10.1186/s13756-019-0663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Global Point Prevalence Survey of Antimicrobial Consumption and Resistance. The Global Point Prevalence Survey Group; [accessed 2021 Apr. 16]. Available: www.global-pps.com/ [Google Scholar]
- 13.The 2019 WHO AWaRe classification of antibiotics for evaluation and monitoring use. Geneva: The World Health Organization; 2019. [accessed 2021 Apr. 16]. Available: www.who.int/medicines/news/2019/WHO_releases2019AWaRe_classification_antibiotics/en/ [Google Scholar]
- 14.Sutherland JM, Crump RT. Alternative level of care: Canada’s hospital beds, the evidence and options. Healthc Policy. 2013;9:26–34. [PMC free article] [PubMed] [Google Scholar]
- 15.Plachouras D, Kärki T, Hansen S, et al. Antimicrobial use in European acute care hospitals: results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveill. 2018;23:1800393. doi: 10.2807/1560-7917.ES.23.46.1800393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor G, Gravel D, Saxinger L, et al. Prevalence of antimicrobial use in a network of Canadian hospitals in 2002 and 2009. Can J Infect Dis Med Microbiol. 2015;26:85–9. doi: 10.1155/2015/468987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canadian Antimicrobial Resistance Surveillance System — update 2018: executive summary. Ottawa: Public Health Agency of Canada; 2018. [accessed 2021 Apr. 16]. Available: https://www.canada.ca/en/public-health/services/publications/drugs-health-products/canadian-antimicrobial-resistance-surveillance-system-2018-report-executive-summary.html. [Google Scholar]
- 18.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 19.Magill SS, O’Leary E, Janelle SJ, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379:1732–44. doi: 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magill SS, O’Leary E, Ray SM, et al. Antimicrobial use in US hospitals: comparison of results from emerging infections program prevalence surveys, 2015 and 2011. Clin Infect Dis. 2021;72:1784–92. doi: 10.1093/cid/ciaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SB, Thirion DJG, Irfan N, et al. Antimicrobial utilization data: Does point prevalence data correlate with defined daily doses? Infect Control Hosp Epidemiol. 2019;40:920–1. doi: 10.1017/ice.2019.154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.