Abstract
Sepsis is a significant cause for mortality among critically ill patients. Metabolic derangements that develop in sepsis are often considered to be pathologic, contributing to sepsis morbidity and mortality. However, alterations in metabolism during sepsis are multifaceted and are incompletely understood. Acute anorexia during infection is an evolutionarily conserved response, suggesting a potential protective role of anorexia in the host response to infection. In animal models of bacterial inflammation, fasting metabolic programs associated with acute anorexia, such as Fibroblast Growth Factor 21 and ketogenesis, are associated with improved survival. Other fasting metabolic pathways such as fatty acid oxidation and autophagy are also implicated in preventing acute kidney injury. Global metabolic changes during sepsis and current clinical interventions can potentially affect disease tolerance mechanisms and modify the risk of acute kidney injury.
Keywords: Bacterial Sepsis, Fasting metabolism, Acute Kidney Injury
Introduction
Effective host defense against pathogens requires both disease resistance, which is focused on pathogen recognition and clearance, as well as disease tolerance, which involves the activation of pathways that limit tissue damage resulting from the inflammatory response [1]. Disease tolerance pathways include metabolic and physiologic responses that can occur at the cellular, tissue and systemic levels. Metabolic derangements are common in sepsis, and include hyperlipidemia, dysglycemia, proteolysis, and those associated with poor appetite. Whether these metabolic changes are entirely reflective of dysregulated and pathologic responses is not clear. For example, while lipolysis and the resulting hyperlipidemia could be viewed as maladaptive, there is evidence that lipoproteins are capable of binding and neutralizing endotoxin, and the lipid substrates could be used as fuel for organs dependent on fatty acid oxidation (FAO). The purpose of dysglycemia, the most commonly observed disorder, could be used to redirect glucose utilization and perhaps fuel the immune system for the initial antimicrobial response. Proteolysis is a concerning phenomenon in sepsis as it leads to muscle atrophy, however, its purpose could be used to fuel the liver’s synthesis of acute phase proteins, many of which are important in host defense. Anorexia of acute illness has traditionally been considered a maladaptive response in the face of a presumed hyper-catabolic state. However, anorexia of infection can be protective or detrimental depending on the organism and pathogen, suggesting that adaptive metabolic programs activated in the setting of infectious stimuli are context specific [2].
Fasting metabolism as a potential mechanism of disease tolerance in bacterial inflammation
In bacterial infection, hypocaloric glucose supplementation during the period of infection-induced anorexia is detrimental and promotes mortality without impacting pathogen burden [2]. Moreover, glucose supplementation in the lipopolysaccharide (LPS) model of sterile bacterial inflammation also increases mortality, further supporting the role of infection-induced anorexia in mediating disease tolerance. The mechanism by which glucose provision during bacterial inflammation-induced anorexia promotes mortality is incompletely understood. Insights from normal fasting metabolism suggest that a shift towards lipid metabolism and associated downstream pathways may play a critical role in supporting disease tolerance. Moreover, aspects of the fasting metabolic response resulting from intermittent fasting and caloric restriction are proposed to promote longevity and to mitigate many disease conditions. During normal fasting, metabolism shifts towards lipid metabolism initiated by adipose lipolysis, liberating free fatty acids (FFA) that are delivered to the periphery. Fatty acids activate hepatic Peroxisome Proliferator Activated Receptor Alpha (PPARa), a nuclear receptor and master regulator of lipid metabolism, resulting in ketone production and the induction of fibroblast growth factor 21 (FGF21), a starvation adaptation hormone. Exogenous glucose supplementation after LPS challenge, will suppress circulating FFA, FGF21, and ketones.
FGF21 as a mediator of cardioprotection during bacterial inflammation
FGF21, a member of the endocrine growth factor family that also includes FGF15/19 and FGF23, requires the co-receptor beta-Klotho (KLB) for downstream signaling via FGF receptors. FGF21 is induced by a variety of metabolic stressors and mediates starvation adaptation [3]. Similar to fasting, FGF21 is produced by the liver in response to bacterial inflammation. Hepatic-derived FGF21 is required for survival during bacterial inflammation, as mice with liver-specific Fgf21 deletion are more susceptible to mortality in both cecal ligation and puncture and endotoxemia models [4]. Interestingly, the action of FGF21 in bacterial inflammation appears to be independent of well-described KLB-expressing target tissues, the hypothalamus and the adipose tissue. Instead, FGF21 supports cardiac function possibly through an indirect effect via the hindbrain, where KLB-expressing neurons are found in the area postrema and the nucleus of the solitary tract, regions that support autonomic function, including cardiovascular function. This protective FGF21 pathway in sepsis adds to a growing body of evidence that supports the concept that in response to specific challenges, certain physiologic responses, often considered maladaptive, such as anorexia of acute illness or metabolic derangements of sepsis, are in fact coordinated defense mechanisms to promote survival and tissue protection.
Implications of other fasting metabolic pathways in sepsis
During fasting adaptation, metabolism shifts from carbohydrate to lipid metabolism, favoring utilization of fat stores, FAO, and ketogenesis. Septic patients also exhibit this metabolic shift and a preference for lipid metabolism. Compared to other critically ill non-septic trauma patients, septic critically ill patients have a lower respiratory quotient, reflecting a shift towards lipid metabolism over carbohydrate metabolism [5]. In fact, septic patients have higher fat oxidation, while glucose oxidation is suppressed and cannot be induced with the provision of excess glucose [6].
While the pathogenesis of sepsis-associated acute kidney injury is multifactorial including changes in hemodynamics and microcirculation, inflammation and drug toxicity, growing evidence suggests that altered kidney metabolism is also a key contributor. FAO mediators Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1a) and PPARa have been shown to be critical in preventing septic kidney injury [7, 8]. Moreover, kidney proximal tubules increase mitochondrial respiratory capacity while glycolytic capacity is diminished during bacterial sepsis [9]. Although it was not directly tested whether FAO contributed to the increase in mitochondrial respiratory capacity, it is possible that proximal tubules may preferentially switch from glucose metabolism to FAO.
Ketogenesis is an important fasting adaptation pathway that fuels the brain during periods of nutrient deprivation. Ketones not only serve as an alternate fuel source, but also act as signaling molecules, drive post-translational modifications, and modify immune and oxidative stress responses [10]. While it was previously thought that ketogenesis is impaired in septic patients, ketogenesis is preserved in septic patients and is only impaired in the context of continuous glucose and amino acid infusions, even at hypocaloric levels. Both baseline ketone bodies and inducible ketone production after a lipid challenge are intact in septic patients not receiving these infusions [11]. These observations are similar to those in mice in which hypocaloric glucose supplementation during endotoxemia suppressed ketone production, which was associated with increased mortality, worse kidney function and suppressed ketogenic gene expression in the kidney ([2] and Huen et al unpublished observations). Further research is needed to determine whether ketone bodies directly mediate disease tolerance by limiting kidney injury and whether supporting ketogenesis in patients with bacterial sepsis could improve outcomes.
Another well-described fasting metabolic pathway is autophagy, a regulated cellular mechanism that removes and reuses unfolded or misfolded proteins and damaged organelles. Autophagy allows for recycling of nutrients, promotes cell survival, and is an important regulator of lipid metabolism. Autophagy is a critical component of disease tolerance in sepsis, by limiting mitochondrial dysfunction, cellular apoptosis and tissue damage via autophagic pathways such as mitophagy. The activation of autophagy during sepsis has been found to be protective in multiple vital organ systems including the kidney [12].
Taken together, these observations suggest that fasting metabolic changes in sepsis are likely regulated and a switch towards lipid metabolism could be the preferred metabolic state during septic conditions. As all of these fasting metabolic pathways are inhibited by glucose and insulin signaling pathways (Figure), the clinical implications of these fasting metabolic pathways suggest a need to reassess the nutritional and metabolic management of septic patients. A few recent clinical studies highlight the possibility that some of our attempts to normalize presumed metabolic changes could have detrimental effects on pathways that could be protective. Although there was no difference in mortality, permissive underfeeding in critically ill patients in which protein and volume intake were controlled resulted in lower insulin use and lower blood glucose, which was associated with lower incident need for renal replacement therapy [13]. Correction of hyperglycemia in critical illness was questioned by the NICE-SUGAR trial. While hypoglycemia is likely to contribute to increased mortality, the intensive glycemic control group also had significantly more exposure to both insulin and glucose [14]. Could it also be that exposure of excess insulin and glucose suppresses protective fasting metabolic pathways? In fact, insulin treatment in type 2 diabetics with confirmed COVID-19 infection was associated with increased mortality as well as organ damage, including acute kidney injury [15]. Although this was a retrospective cohort analysis, this relationship remained significant with propensity-score matching to control for baseline comorbidities and severity of disease, stratification based on glucose control at admission, and an analysis of patients without hypoglycemic episodes.
Figure. Protective Fasting Metabolic Programs.
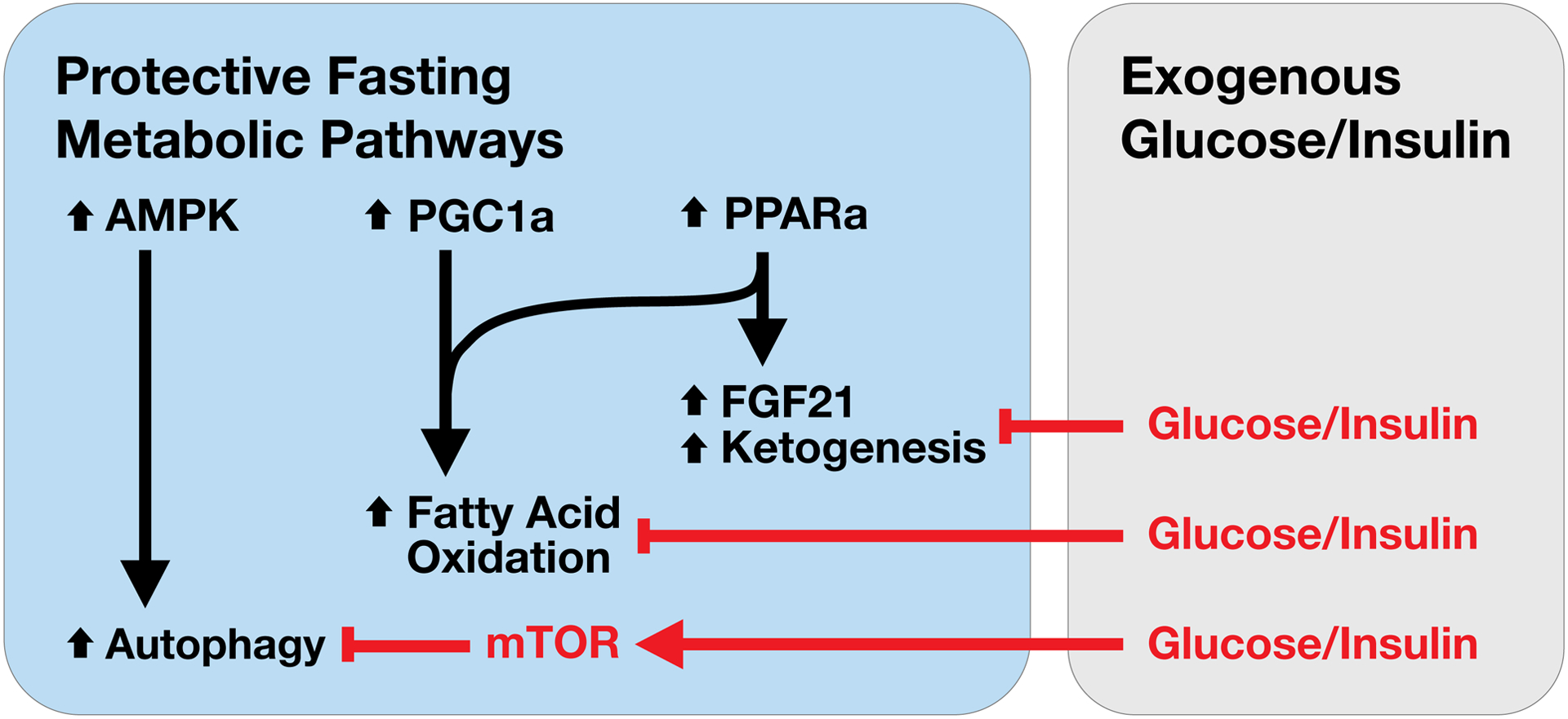
In response to fasting, Peroxisome Proliferator Activated Receptor Alpha (PPARa), co-activated by Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1a) mediates the upregulation of ketogenic and fatty acid oxidation gene programs. PPARa also regulates the induction of hepatic Fibroblast Growth Factor 21 (FGF21) expression during fasting. Adenosine monophosphate activated protein kinase (AMPK) activation will promote autophagy which is inhibited by mechanistic target of rapamycin (mTOR). PPARa, PGC1a and autophagy are all implicated as protective pathways that limit acute kidney injury (AKI). These protective fasting metabolic programs are suppressed by glucose and insulin activated pathways, suggesting a need to re-evaluate current nutrient and metabolic approaches that may lead to excessive glucose, and thus obligate insulin exposures in patients with sepsis and at risk for AKI.
Conclusion
Activation of metabolic pathways in response to inflammatory conditions such as sepsis may represent defense mechanisms that promote disease tolerance. Identifying these pathways will be a critical step towards differentiating between pathologic versus inherently protective mechanisms. Understanding the regulation of protective metabolic pathways will be an important step towards the development of therapeutic targets and management approaches in nutrition and metabolism to limit organ damage such as acute kidney injury and to improve survival from sepsis.
Acknowledgements
S.C.H. was supported by NIH Grants K08DK110424, R35GM137984, and the American Society of Nephrology Carl W. Gottschalk Research Scholar Grant.
Footnotes
Conflict of Interest Statement
The author has no conflicts of interest to disclose.
References
- 1.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, Booth CJ, Medzhitov R. Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell. 2016;166:1512–1525.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kliewer SA, Mangelsdorf DJ. A Dozen Years of Discovery: Insights into the Physiology and Pharmacology of FGF21. Cell Metab. 2019;29:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huen SC, Wang A, Feola K, Desrouleaux R, Luan HH, Hogg R, Zhang C, Zhang Q-J, Liu Z-P, Medzhitov R. Hepatic FGF21 mediates tissue tolerance during bacterial inflammation by preserving cardiac function. bioRxiv. 20202020.10.05.310508. [Google Scholar]
- 5.Giovannini I, Boldrini G, Castagneto M, Sganga G, Nanni G, Pittiruti M, Castiglioni G. Respiratory quotient and patterns of substrate utilization in human sepsis and trauma. JPEN J Parenter Enteral Nutr. 1983;7:226–230. [DOI] [PubMed] [Google Scholar]
- 6.White RH, Frayn KN, Little RA, Threlfall CJ, Stoner HB, Irving MH. Hormonal and metabolic responses to glucose infusion in sepsis studied by the hyperglycemic glucose clamp technique. JPEN J Parenter Enteral Nutr. 1987;11:345–353. [DOI] [PubMed] [Google Scholar]
- 7.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121:4003–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwaki T, Bennion BG, Stenson EK, Lynn JC, Otinga C, Djukovic D, Raftery D, Fei L, Wong HR, Liles WC, Standage SW. PPARα contributes to protection against metabolic and inflammatory derangements associated with acute kidney injury in experimental sepsis. Physiol Rep. 2019;7:e14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Nourbakhsh N, Pham H, Tham R, Zuckerman JE, Singh P. Evolution of altered tubular metabolism and mitochondrial function in sepsis-associated acute kidney injury. Am J Physiol Renal Physiol. 2020;319:F229–F244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puchalska P, Crawford PA. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017;25:262–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beylot M, Guiraud M, Grau G, Bouletreau P. Regulation of ketone body flux in septic patients. Am J Physiol. 1989;257:E665–74. [DOI] [PubMed] [Google Scholar]
- 12.Yin X, Xin H, Mao S, Wu G, Guo L. The Role of Autophagy in Sepsis: Protection and Injury to Organs. Front Physiol. 2019;10:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, Mehta S, McIntyre L, Solaiman O, Sakkijha MH, Sadat M, Afesh L, PermiT TG. Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults. N Engl J Med. 2015;372:2398–2408. [DOI] [PubMed] [Google Scholar]
- 14.NICE-SUGAR SI, Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. [DOI] [PubMed] [Google Scholar]
- 15.Yu B, Li C, Sun Y, Wang DW. Insulin Treatment Is Associated with Increased Mortality in Patients with COVID-19 and Type 2 Diabetes. Cell Metab. 2021;33:65–77.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]


