Abstract
Purpose
This study explored the performance of prenatal ultrasonography in the differential diagnosis of cystic biliary atresia (CBA) and choledochal cyst (CC).
Methods
Fetuses diagnosed with hepatic hilar cyst in the second trimester were included in this study. A series of prenatal ultrasound examinations were performed in the second and third trimesters. The diameter of the gallbladder (GB) and hepatic cyst were measured, as well as the wall thickness of the GB. The GB-cyst connection, visibility of the right hepatic artery (RHA), and other concomitant abnormalities were carefully evaluated. A neonatal transabdominal ultrasound examination was performed within 1 week after birth, and clinical data were followed up to 6 months after birth.
Results
Between January 1, 2016 and January 31, 2020, 53 fetuses diagnosed with hepatic hilar cyst were recruited. Eight were excluded because they were lost to follow-up. Among the 45 cases included in this study, 10 were diagnosed with CBA and 35 with CC after birth. Statistically significant differences were found in GB width, wall thickness, change in GB width, change in cyst length, GB-cyst connection, and RHA visibility between the CBA and CC groups. GB width showed the best diagnostic performance with an area under the curve (AUC) of 0.899. The combination of GB width, GB wall thickness, and GB-cyst connection yielded a comparable AUC of 0.971.
Conclusion
The GB should be carefully evaluated in fetuses with hepatic hilar cyst. Prenatal ultrasound findings could provide suggestive parameters for the differential diagnosis of CBA from CC.
Keywords: Ultrasonography, Cystic biliary atresia, Choledochal cyst, Fetus, Prenatal diagnosis
Introduction
Recent advances in ultrasonography have increased the rate of prenatal diagnoses of biliary malformations. Choledochal cyst (CC) is the most common congenital biliary abnormality in Asia, with an incidence of 1 in 1,000; however, it remains rare in western countries, where it has an incidence of 1 in 100,000-150,000 [1,2]. CC is usually asymptomatic, and sometimes does not require intervention. Although some complications may occur, most patients can recover through a choledochojejunostomy [3,4].
Cystic biliary atresia (CBA) is a subtype of biliary atresia (BA), which is complicated with an extrahepatic biliary cyst. CBA can be easily misdiagnosed as CC both prenatally and postnatally [5]. Although CBA has a relatively favorable prognosis compared with other subtypes of BA, if infants with CBA are left untreated, progressive liver cirrhosis may lead to death by 2 years of age [6]. Kasai portoenterostomy (KP) significantly improves the survival rate of children with BA, especially those with CBA [7]. However, the timing of KP is critical; if KP is performed within 45 days, the prognosis is significantly improved [8,9]. Conversely, if liver cirrhosis has occurred when a patient seeks medical attention, the patient might need liver transplantation.
CC and CBA may share the same ultrasonography pattern, with the detection of a hepatic hilar cyst during the second and third trimesters of pregnancy; no ultrasound differential parameter has been unequivocally accepted [10]. Previous studies reported that in CBA, the cysts were smaller and decreased with advanced gestational age, whereas they grew in CC [11-13]. However, because of the limited sample size, the differences were not statistically significant. Moreover, those studies were based on retrospective analysis of prenatal ultrasound images of infants with CBA, and thus the information available is limited. Therefore, the purpose of this study was to explore ultrasound parameters that can differentiate CBA from CC prenatally. An accurate diagnosis is desirable in order for patients to receive timely and effective treatment after birth.
Materials and Methods
Compliance with Ethical Standards
This study was approved by the Clinical Research Ethics Committee of Shengjing Hospital of China Medical UniversIty (No. 2016PS179J), and written informed consent was obtained.
Subjects and Prospective Sequential Prenatal Ultrasonography
This was a retrospective study of prospectively collected data from fetuses diagnosed with hepatic hilar cyst in second-trimester ultrasound examinations. Sequential prenatal ultrasonography was performed later at gestational ages of 22-26 weeks, 30-34 weeks, and 36-39 weeks. Those who did not receive sequential ultrasound examinations and/or were lost to follow-up after birth were excluded. All pregnant women underwent three detailed prenatal ultrasound examinations performed by one operator (L.C.) with more than 10 years of experience with prenatal ultrasound. The Voluson E8/E10 Ultrasound Diagnostic Instrument (GE, Boston, MA, USA) was used with 4.0- to 8.0-MHz transabdominal transducers. First, a two-dimensional ultrasound scan was used to detect fetal biometry and the relationship between the gallbladder (GB) and cyst, and to evaluate other structural abnormalities. The maximum lumen length and maximum width of the GB and cyst from inner wall to inner wall were measured, as well as the wall thickness of the GB. Color Doppler ultrasonography was used to evaluate visibility of the right hepatic artery (RHA). All parameters were measured three times, and the average values were calculated.
The GB length used for comparison between the CBA and CC groups was defined as the maximum length of the GB among the three examinations at different gestational ages. Similarly, the GB width and cyst length were defined as the maximum width of the GB and maximum length of the cyst measured among the three examinations. The maximum changes in GB length, GB width, and cyst length among different gestational ages were calculated, and the wall thickness of the GB at the maximum GB width was recorded for comparison.
Tests of Interobserver Agreement
Interobserver agreement of measurements and identification of GB length, GB width, GB wall thickness, cyst length, GB-cyst connection, and visibility of RHA were compared between two operators (Z.Y., with 20 years of experience; L.C., with 10 years of experience) in 20 randomly selected fetuses (6 with CBA and 14 with CC). Each of these 20 fetuses was independently scanned by each operator. Three measurements of each parameter were obtained by each operator. To evaluate interobserver variability, the mean values of each parameter were compared between operators.
Follow-up
All neonates were examined by two pediatricians and followed up for at least 6 months after birth. A detailed transabdominal ultrasound examination was performed within 1 week of birth. The reference standard for the diagnosis of CBA and CC was based on postnatal surgery, liver biopsy, and cholangiography. In patients who did not undergo surgery, the postnatal diagnosis of CC was based on postnatal ultrasound, biochemical examinations, magnetic resonance cholangiopancreatography, and clinical follow-up.
Statistical Analysis
Quantitative parameters are presented as mean±standard deviation or median with interquartile range (IQR). Continuous variables were first tested for normality, and the Mann-Whitney U test was performed to compare variables that did not follow a normal distribution. Categorical variables were compared using the chi-square test and Fisher exact test. Binary logistic regression was used for the multivariate analysis, and receiver operating characteristic curves were used to determine the diagnostic effectiveness and threshold. To assess interobserver measurement errors, the Bland-Altman test and kappa consistency analysis were used. Statistical analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, NY, USA), MedCalc version 12.7.0 (MedCalc Software, Ostend, Belgium), and GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA). P<0.05 were considered to indicate statistical significance.
Results
Clinical Characteristics
Between January 1, 2016 and January 31, 2020, 50,297 women received standard second-trimester ultrasound examinations at the authors’ hospital, of whom 53 were diagnosed with fetal hepatic hilar cyst. Eight patients were excluded because they were lost to follow-up; therefore, 45 patients were included in this study (Fig. 1). All infants received postnatal ultrasonography within 1 week after birth, and hepatic hilar cysts were found in 44 cases. In one case, the cyst disappeared at 33 weeks prenatally.
Fig. 1. Flow diagram of the inclusion criteria for patients.
n, number of patients; CBA, cystic biliary atresia; CC, choledochal cyst.
CBA was confirmed in 10 infants who underwent cholangiography and KP at 10-59 days after birth. Jaundice cleared in nine infants (90%) by 6 months of age, and one received liver transplantation at 9 months. The 10 CBA infants were all female. Eight fetuses received a prenatal genetic test and abnormal copy number variations were identified in two cases (one case of 3p24.2 duplication and 12q13.12 duplication, one case of 2q22. 3-q23.1 duplication).
Thirty-five infants were confirmed to have CC after birth. Of these, 25 were female and 10 were male (ratio, 2.5:1). Twenty-five cases (71.4%) underwent surgery at the age of 1-10 months; in 17 cases (48.6%), surgery was chosen because of cholestasis, cholangitis, or a palpable abdominal mass, and the other eight underwent surgery without any symptoms. Ten patients received conservative treatment and remained asymptomatic until follow-up (follow-up period: 6 months to 4 years). Genetic testing was performed in 20 fetuses, and no abnormal chromosomal abnormalities were found. The clinical details of the 10 CBA cases and 35 CC cases are shown in Table 1.
Table 1.
Clinical characteristics of the cases included in this study
| Characteristic | CBA group (n=10) | CC group (n=35) | P-value |
|---|---|---|---|
| Maternal age (year) | 31.0±4.5 | 28.5±3.8 | 0.453 |
| Gestational age at diagnosis (week) | 23.0±3.1 | 22.8±2.2 | 0.845 |
| Sex | <0.001 | ||
| Female | 10 (100) | 25 (71.4) | |
| Male | 0 | 10 (28.6) | |
| Incidence (‰) | 0.2 | 0.7 | <0.001 |
| Body weight (g) | 3,131.0±229.8 | 3,304.0±364.0 | 0.164 |
| Operation | 10 (100) | 25 (71.4) | <0.001 |
Values are presented as mean±SD or number (%) unless otherwie indicated.
CBA, cystic biliary atresia; CC, choledochal cyst; SD, standard deviation.
Imaging Morphology
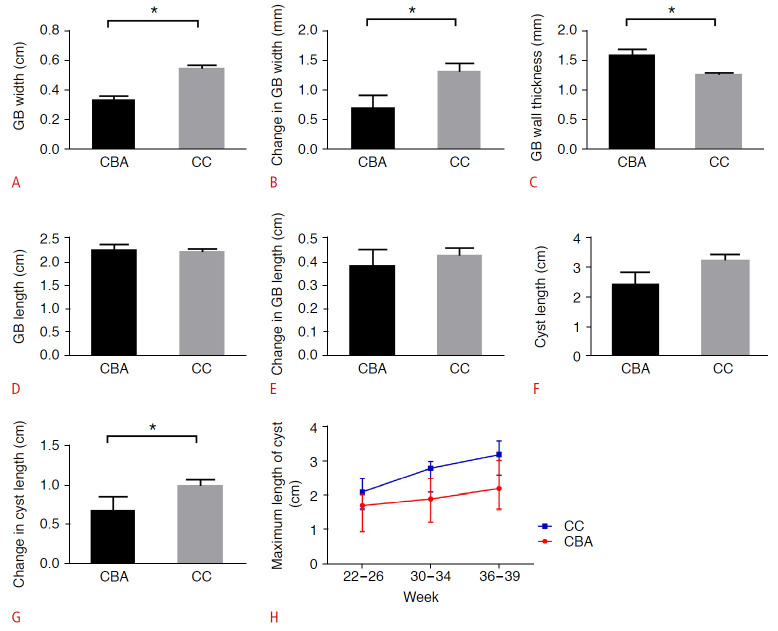
The GBs all presented with abnormal morphology in the CBA group, either with a thick and rigid GB wall or with a tortuous tubular shape. In contrast, the GBs in the CC group were well-filled, with smooth pear-shaped structures (Fig. 2). The GB width in the CBA group (median [IQR], 0.3 cm [0.3-0.4 cm]) was significantly smaller than that in the CC group (0.6 cm [0.4-0.6 cm], P<0.001) (Fig. 3A). The change in GB width was also significantly smaller in fetuses with CBA (1.0 mm [0-1.0 mm]) than in CC fetuses (2.0 mm [1.0-2.0 mm], P=0.038) (Fig. 3B). The wall thickness of the GB in fetuses with CBA (1.6 mm [1.3-1.8 mm]) was significantly higher than in fetuses with CC (1.3 mm [1.1-1.4 mm], P=0.003) (Fig. 3C). There were no significant differences in the GB length and change in GB length between the two groups (P=0.869 and P=0.989, respectively) (Fig. 3D, E).
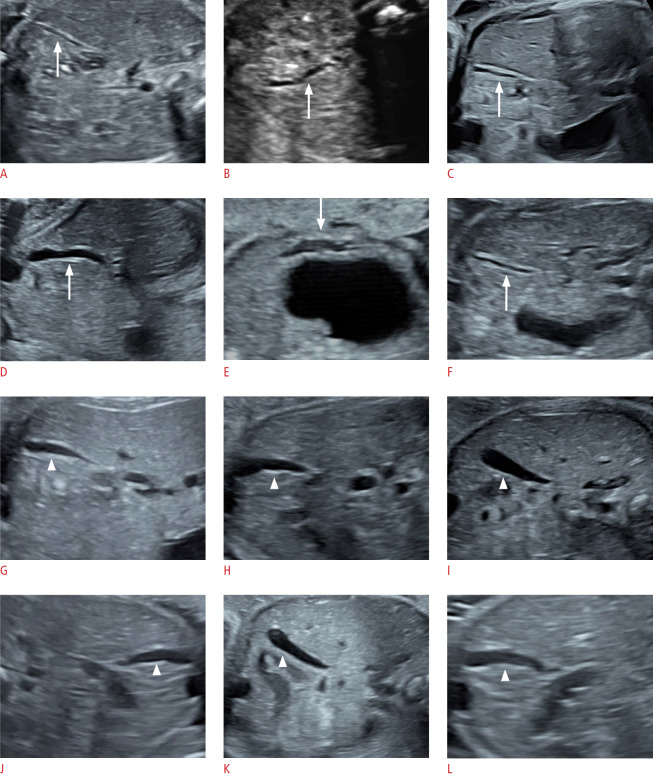
Fig. 2. Longitudinal ultrasound images of gallbladders (GBs) in cystic biliary atresia (CBA) and choledochal cyst (CC) fetuses.
A-F. In the CBA group, the GBs (arrow) present with abnormal morphology, either with a thick and rigid GB wall or with a tortuous tubular shape. G-L. In the CC group, the GBs (arrowhead) are well-filled, with smooth pear-shaped structures.
Fig. 3. The comparison of different parameters between the cystic biliary atresia (CBA) and choledochal cyst (CC) group.
A. The gallbladder (GB) width in the CBA group was significantly smaller than in the CC group. B. The change in GB width was significantly smaller in CBA fetuses than in CC fetuses. C. The wall thickness of the GB in fetuses with CBA was significantly higher than in fetuses with CC. D, E. There were no significant differences in the GB length and change in GB length between the two groups. F. There were no significant differences in cyst length between the two groups. G, H. The change in cyst length was significantly smaller in CBA fetuses than in CC fetuses. *P<0.05
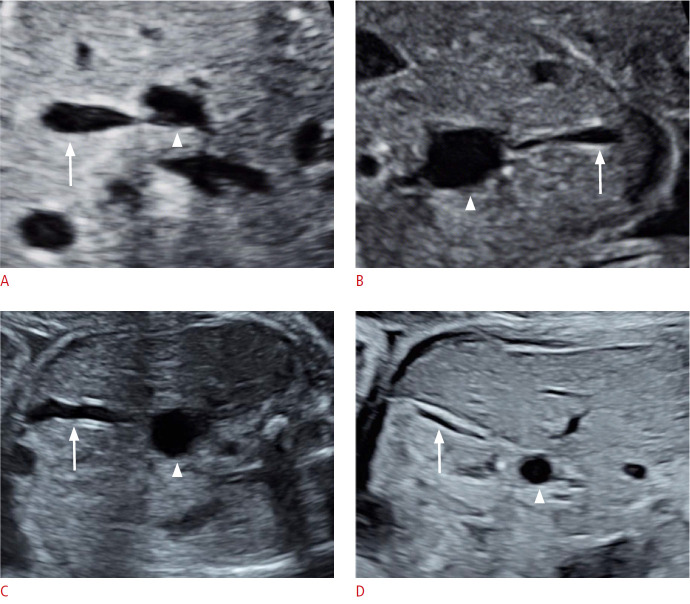
The cyst length in the CBA group (2.3 cm [1.6-3.2 cm]) was smaller than in the CC group (3.2 cm [2.6-3.6 cm]); however, the difference was not statistically significant (P=0.067) (Fig. 3F). Cyst size decreased in one case, remained unchanged in two cases, and increased in seven cases in the CBA group. In the CC group, cyst size remained unchanged in one case and increased in 34 cases. The change in cyst length was significantly smaller in CBA fetuses than in CC fetuses (P=0.021) (Fig. 3G, H). The cyst was found to be connected with the GB in six cases (60%) in the CBA group and in 32 cases (91.4%) in the CC group. There was a statistically significant difference in the detection rate of GB-cyst connection between the CBA group and the CC group (P=0.034) (Fig. 4).
Fig. 4. The relationship between the gallbladder (GB) and the cyst.
A, B. The cysts were connected with the GBs in two choledochal cyst cases. C, D. The cysts were not connected with GBs in two cystic biliary atresia cases. Arrow and arrowhead indicate GB and cyst, respectively.
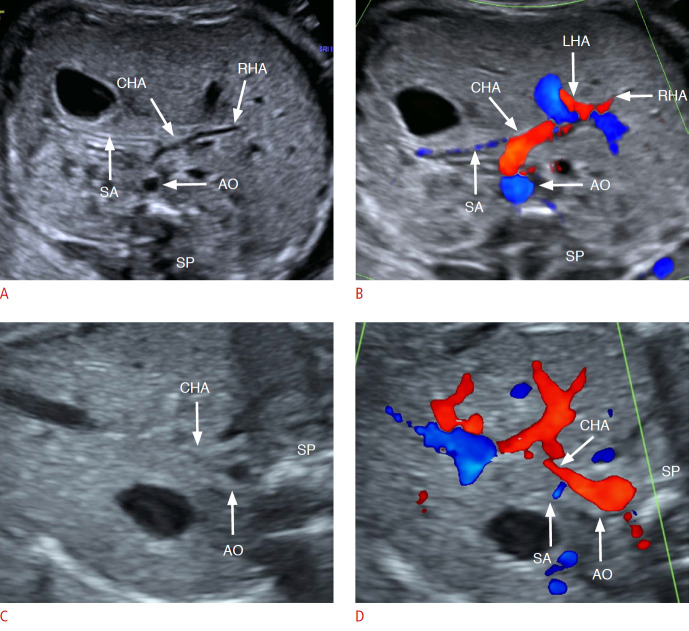
The RHA was visible on two-dimensional ultrasonography and color Doppler ultrasonography in five CBA fetuses in the third trimester and in no CC fetuses. There was a statistically significant difference in the detection rate of the RHA between the CBA group and the CC group (P<0.001) (Fig. 5).
Fig. 5. Prenatal observation of the right hepatic artery (RHA) in fetuses with hepatic hilar cyst.
A, B. RHA was clearly visible in a fetus with cystic biliary atresia at 32 weeks by two-dimensional ultrasonography and color Doppler ultrasonography. C, D. RHA was invisible either by two-dimensional ultrasonography or color Doppler ultrasonography in a fetus with choledochal cyst (32 weeks), and even the common hepatic artery (CHA) was not clear enough by two-dimensional ultrasound. AO, aortaventralis; LHA, left hepatic artery; RHA, right hepatic artery; SA, splenic artery; SP, spine.
One CBA case presented with a persistent left superior vena cava. No other structural abnormalities were found in the other nine CBA patients and 35 CC patients.
Diagnostic Performance of Ultrasound Parameters to Differentiate CBA and CC
The area under the receiver operating characteristic curve (AUC), sensitivity, specificity, Youden index, and optimal cutoff values for the above parameters in the prenatal diagnosis of CBA are shown in Table 2. The GB width showed the best diagnostic performance as a single parameter (AUC, 0.899), and the combination of GB width, GB wall thickness, and GB-cyst connection provided the highest sensitivity and specificity of 90.0% and 91.4%, respectively (AUC, 0.971) (Fig. 6).
Table 2.
Diagnostic performance of ultrasound parameters in differentiating CBA and CC
| US parameter | Sensitivity (%) | Specificity (%) | AUC | Youden index | Cutoff value (mm) |
|---|---|---|---|---|---|
| GB width | 90.0 | 71.4 | 0.899 | 0.614 | 4.00 |
| Change in GB width | 90.0 | 42.9 | 0.704 | 0.329 | 1.00 |
| Wall thickness | 60.0 | 97.1 | 0.803 | 0.571 | 1.50 |
| Change in cyst length | 90.0 | 57.1 | 0.741 | 0.471 | 8.00 |
| GB-cyst connection | 91.4 | 40.0 | 0.657 | 0.314 | - |
| RHA | 50.0 | 100 | 0.750 | 0.500 | - |
| Combinationa) | 90.0 | 91.4 | 0.971 | 0.814 | - |
CBA, cystic biliary atresia; CC, choledochal cyst; US, ultrasonography; AUC, area under the curve; GB, gallbladder; RHA, right hepatic artery.
Combination: the combination of GB width, wall thickness, and GB-cyst connection.
Fig. 6. Comparison of receiver operating characteristic curves for different parameters alone or combined in the determination of cystic biliary atresia.
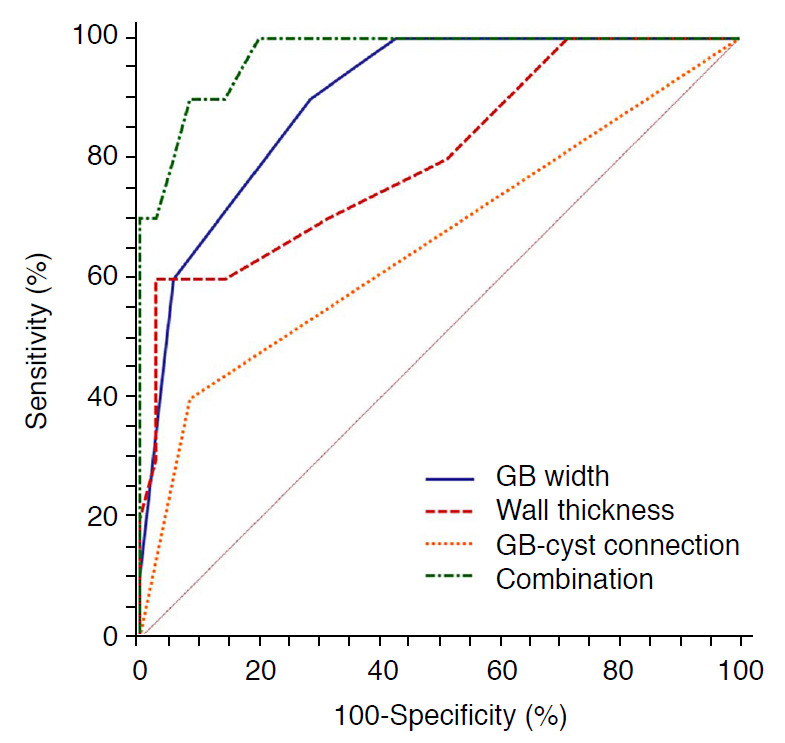
GB, gallbladder; Combination, the combination of GB width, wall thickness, and GB-cyst connection.
Interobserver Agreement
Interobserver agreement among the two operators was assessed in 20 fetuses chosen at random (Table 3). The assessment of the ultrasound parameters (GB length, GB width, GB wall thickness, cyst length, GB-cyst connection, and RHA) showed excellent agreement.
Table 3.
Assessment of interobserver agreement
| US parameter | Test | Interobserver agreement |
|---|---|---|
| GB length | Bland-Altman | 0.98 |
| GB width | Bland-Altman | 0.99 |
| GB wall thickness | Bland-Altman | 0.96 |
| Cyst length | Bland-Altman | 0.98 |
| GB-cyst connection | Kappa | 100% |
| RHA | Kappa | 100% |
GB, gallbladder; RHA, right hepatic artery.
Discussion
CBA accounted for 22.2% of all hilar cysts in this study, suggesting that clinicians should be alert to the occurrence of BA when hilar cysts were observed on prenatal ultrasonography. Currently, the differential diagnosis of CBA and CC remains a challenge both pre- and post-birth, and the ultrasound features of fetal CBA have mostly been reported in individual retrospective cases [10,14]. To the authors’ knowledge, this is the first study based on prospectively collected data in fetuses with hepatic hilar cysts at different gestational timepoints, which enabled the identification of prenatal sonographic features that differentiate CBA from CC. As a final result, it was possible to obtain a panel of prenatal ultrasound parameters with good diagnostic performance in predicting CBA. For fetuses suspected to have CBA prenatally, a liver function test, transabdominal ultrasonography, and other necessary examinations will be recommended immediately after birth to help confirm the diagnosis in time. Early Kasai surgery can relieve the obstruction of the biliary tract before the child develops liver cirrhosis, which is critical for improving the prognosis.
Most previous studies focused on the size of the cyst in differentiating CBA from CC. An increase in the size of the cyst as gestational age advances may suggest CC based on a retrospective study of four CBA cases and six CC cases, although there was no statistical significance [11]. However, cyst size increased with gestational age in seven out of the 10 CBA cases in the present study. Other scholars suggested that CBA should be suspected if the cyst size decreases before and after birth [15]. In this study, the cyst size decreased only in one BA case. Casaccia et al. [10] proposed that small cyst size and an absent GB are sonographic findings suggestive of BA based on the sonographic features of two CBA and two CC fetuses. In this study, GB was visible in every CBA case, and cyst size in the CBA group was smaller than in the CC group, albeit to a statistically insignificant extent. Changes in cysts, rather than their size, were more meaningful for the prenatal diagnosis of CBA.
Regarding the differential diagnosis of CBA and CC, few scholars have studied the sonographic features of fetal GB. The present study found that the GB ultrasound findings were more valuable in the prenatal diagnosis of CBA, consistent with the ultrasound characteristics of infant BA [16,17]. Zhou et al. [16] developed a GB classification scheme that showed a good diagnostic performance for BA diagnosis in infants. The GB length-to-width ratio was identified as a suggestive ultrasound parameter for infant BA screening [17]. Morel et al. [14] reported one case of CBA with a crenelated and irregular GB wall associated with a biliary cyst on prenatal ultrasonography. To date, this is the first study to summarize the sonographic features of the fetal GB in the prenatal diagnosis of CBA. In the 10 CBA cases, all GBs presented with abnormal morphology. To avoid subjectivity in evaluating GB morphology, the length, width, wall thickness, and dynamic changes of the GB were analyzed. Finally, the GB was shown to be a critical factor for the differential diagnosis of CBA from CC. The GB width showed the best diagnostic performance as a single parameter with an AUC of 0.899.
Previous studies proposed that cyst communication with the GB may facilitate the diagnosis of CC [18]. In the 35 CC cases analyzed herein, a GB-cyst connection was observed in 32 cases (91.4%), except in three cases where the cysts were relatively long (≥4.3 cm). A GB-cyst connection was also observed in six cases (60%) in the CBA group. Therefore, a GB-cyst connection is not a diagnostic marker of CC. However, the observation of no connection between the GB and cyst with a cyst that is not very large warrants very careful observation of other sonographic features.
RHA dilatation is one of the most important signs in the ultrasonographic diagnosis of neonatal BA [19,20]. This study is the first to report the prenatal observation of RHA in CBA fetuses. Although the AUC of RHA in CBA prenatal diagnosis was only 50%, its specificity could reach 100%. Therefore, in fetuses with a hepatic cyst found on prenatal ultrasonography, the observation of a dilated RHA is highly indicative of CBA.
Some scholars have proposed that CBA is a clinically distinct variant of BA most commonly associated with type I BA [5,21]. Type I BA refers to atresia of the distal bile duct with a patent common hepatic duct, and has a relatively good prognosis. Type II BA has patency to the level of the common hepatic duct. In type III BA, the most proximal part of the extrahepatic biliary tract within the porta hepatis is entirely solid; the closer the atresia is to the hepatic hilum, the greater the probability of liver damage and a poor prognosis [6,22]. In the 10 CBA cases in the present study, nine were diagnosed with type I BA after birth, and jaundice cleared after KP. One fetus was diagnosed with type III BA and received a liver transplantation at 9 months. Therefore, the findings of this study indicate that fetuses with CBA who are diagnosed prenatally can be treated promptly after birth, achieving a good prognosis in most. Foo et al. [23] showed that prenatal diagnosis of CC resulted in earlier surgical treatment, which appeared to be safer than delayed surgery and can prevent potential long-term complications. Of the 35 CC fetuses in this study, 25 received surgery, and all children had a good prognosis during the follow-up period.
A limitation of this study is the relatively limited sample size due to the low incidence of CBA. Future studies are required to further validate the prenatal ultrasound features in a larger sample size through a multi-center collaboration.
In conclusion, the GB should be well-evaluated in fetuses diagnosed with hepatic hilar cyst, as doing so could provide suggestive parameters for the differential diagnosis of CBA from CC. Other diagnostic parameters are proposed, and their combination could improve the accuracy of prenatal diagnosis of CBA. An accurate diagnosis is critical for timely and effective treatment after birth.
Acknowledgments
We thank all the women who took part in this study at the Prenatal Diagnosis Center of Shengjing Hospital, China Medical University.
This study was supported by the National Natural Science Foundation of China (Grants 81600258, 81802540), and the 345 Talent Project of Shengjing Hospital.
Key point
In fetal sonography, observation of fetal gallbladder would provide critical factor in differential diagnosis between cystic biliary atresia and choledochal cyst. Prenatal visualization of right hepatic artery also can be important in diagnosis of cystic biliary atresia.
Footnotes
Author Contributions
Conceptualization: Chen L, Ren W. Data acquisition: Chen L, Wang B, Yang Z, Li J, Zhao D. Data analysis or interpretation: Chen L, He F, Zeng K. Drafting of the manuscript: Chen L, Yang Z. Critical revision of the manuscript: Ren W. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
References
- 1.Lipsett PA, Pitt HA. Surgical treatment of choledochal cysts. J Hepatobiliary Pancreat Surg. 2003;10:352–359. doi: 10.1007/s00534-002-0797-4. [DOI] [PubMed] [Google Scholar]
- 2.Jensen KK, Sohaey R. Antenatal sonographic diagnosis of choledochal cyst: case report and imaging review. J Clin Ultrasound. 2015;43:581–583. doi: 10.1002/jcu.22256. [DOI] [PubMed] [Google Scholar]
- 3.Jablonska B. Biliary cysts: etiology, diagnosis and management. World J Gastroenterol. 2012;18:4801–4810. doi: 10.3748/wjg.v18.i35.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weng R, Hu W, Cai S, Guo X, Luo Q. Prenatal diagnosis and prognosis assessment of congenital choledochal cyst in 21 cases. J Obstet Gynaecol. 2016;36:324–327. doi: 10.3109/01443615.2015.1050648. [DOI] [PubMed] [Google Scholar]
- 5.Caponcelli E, Knisely AS, Davenport M. Cystic biliary atresia: an etiologic and prognostic subgroup. J Pediatr Surg. 2008;43:1619–1624. doi: 10.1016/j.jpedsurg.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 6.Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704–1713. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 7.Lakshminarayanan B, Davenport M. Biliary atresia: a comprehensive review. J Autoimmun. 2016;73:1–9. doi: 10.1016/j.jaut.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Serinet MO, Wildhaber BE, Broue P, Lachaux A, Sarles J, Jacquemin E, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics. 2009;123:1280–1286. doi: 10.1542/peds.2008-1949. [DOI] [PubMed] [Google Scholar]
- 9.Bezerra JA, Wells RG, Mack CL, Karpen SJ, Hoofnagle JH, Doo E, et al. Biliary atresia: clinical and research challenges for the twenty-first century. Hepatology. 2018;68:1163–1173. doi: 10.1002/hep.29905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casaccia G, Bilancioni E, Nahom A, Trucchi A, Aite L, Marcellini M, et al. Cystic anomalies of biliary tree in the fetus: is it possible to make a more specific prenatal diagnosis? J Pediatr Surg. 2002;37:1191–1194. doi: 10.1053/jpsu.2002.34470. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka H, Sasaki H, Wada M, Sato T, Kazama T, Nishi K, et al. Postnatal management of prenatally diagnosed biliary cystic malformation. J Pediatr Surg. 2015;50:507–510. doi: 10.1016/j.jpedsurg.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Matsubara H, Oya N, Suzuki Y, Kajiura S, Suzumori K, Matsuo Y, et al. Is it possible to differentiate between choledochal cyst and congenital biliary atresia (type I cyst) by antenatal ultrasonography? Fetal Diagn Ther. 1997;12:306–308. doi: 10.1159/000264493. [DOI] [PubMed] [Google Scholar]
- 13.Shin HJ, Yoon H, Han SJ, Ihn K, Koh H, Kwon JY, et al. Key imaging features for differentiating cystic biliary atresia from choledochal cyst: prenatal ultrasonography and postnatal ultrasonography and MRI. Ultrasonography. 2021;40:301–311. doi: 10.14366/usg.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morel B, Kolanska K, Dhombres F, Jouannic JM, Franchi-Abella S, Ducou Le Pointe H, et al. Prenatal ultrasound diagnosis of cystic biliary atresia. Clin Case Rep. 2015;3:1050–1051. doi: 10.1002/ccr3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang PQ, Wu QQ, Wang L, Liang N, Han JJ, Zhang TJ, et al. The prenatal ultrasound diagnosis and evaluation of the prognosis of fetal congenital choledochal cysts. J China Med Imaging. 2019;30:36–38. [Google Scholar]
- 16.Zhou LY, Guan BY, Li L, Xu ZF, Dai CP, Wang W, et al. Objective differential characteristics of cystic biliary atresia and choledochal cysts in neonates and young infants: sonographic findings. J Ultrasound Med. 2012;31:833–841. doi: 10.7863/jum.2012.31.6.833. [DOI] [PubMed] [Google Scholar]
- 17.Choochuen P, Kritsaneepaiboon S, Charoonratana V, Sangkhathat S. Is "gallbladder length-to-width ratio" useful in diagnosing biliary atresia? J Pediatr Surg. 2019;54:1946–1952. doi: 10.1016/j.jpedsurg.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Koukoura O, Kelesidou V, Delianidou M, Athanasiadis A, Dagklis T. Prenatal sonographic diagnosis of biliary tract malformations. J Clin Ultrasound. 2019;47:292–297. doi: 10.1002/jcu.22705. [DOI] [PubMed] [Google Scholar]
- 19.Rozel C, Garel L, Rypens F, Viremouneix L, Lapierre C, Decarie JC, et al. Imaging of biliary disorders in children. Pediatr Radiol. 2011;41:208–220. doi: 10.1007/s00247-010-1829-x. [DOI] [PubMed] [Google Scholar]
- 20.Kim WS, Cheon JE, Youn BJ, Yoo SY, Kim WY, Kim IO, et al. Hepatic arterial diameter measured with US: adjunct for US diagnosis of biliary atresia. Radiology. 2007;245:549–555. doi: 10.1148/radiol.2452061093. [DOI] [PubMed] [Google Scholar]
- 21.Muise AM, Turner D, Wine E, Kim P, Marcon M, Ling SC. Biliary atresia with choledochal cyst: implications for classification. Clin Gastroenterol Hepatol. 2006;4:1411–1414. doi: 10.1016/j.cgh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Kasai M, Sawaguchi M, Akiyama T. A proposal of new classification of biliary atresia. J Jpn Soc Pediatr Surg. 1976;12:327–331. [Google Scholar]
- 23.Foo DC, Wong KK, Lan LC, Tam PK. Impact of prenatal diagnosis on choledochal cysts and the benefits of early excision. J Paediatr Child Health. 2009;45:28–30. doi: 10.1111/j.1440-1754.2008.01424.x. [DOI] [PubMed] [Google Scholar]