Abstract
Objective
Rhizoma Coptidis is an herb that has been frequently used in many traditional formulas for the treatment of diabetic mellitus (DM) over thousands of years. Berberine, the main active component of Rhizoma Coptidis, has been demonstrated to have the potential effect of hypoglycemia. To determine the potential advantages of berberine for diabetic care, we conducted this systematic review and meta-analysis to examine the efficacy and safety of berberine in the treatment of patients with type 2 DM.
Methods
Eight databases including PubMed, Embase, Web of Science, the Cochrane library, China National Knowledge Infrastructure (CNKI), Chinese Biomedical Database (SinoMed), Wanfang Database, and Chinese VIP Information was searched for randomized controlled trials (RCTs) reporting clinical data regarding the use of berberine for the treatment of DM. Publication qualities were also considered to augment the credibility of the evidence. Glycemic metabolisms were the main factors studied, including glycosylated hemoglobin (HbA1c), fasting plasm glucose (FPG), and 2-hour postprandial blood glucose (2hPG). Insulin resistance was estimated by fasting blood insulin (FINS), homeostasis model assessment-insulin resistance (HOMA-IR), and body mass index (BMI). Lipid profiles were also assessed, including triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL), along with inflammation factors such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). Serum creatinine (Scr), blood urea nitrogen (BUN), and adverse events were applied to evaluate the safety of berberine.
Results
Forty-six trials were assessed. Analysis of berberine applied alone or with standard diabetic therapies versus the control group revealed significant reductions in HbA1c (MD = −0.73; 95% CI (−0.97, −0.51)), FPG (MD = −0.86, 95% CI (−1.10, −0.62)), and 2hPG (MD = −1.26, 95% CI (−1.64, −0.89)). Improved insulin resistance was assessed by lowering FINS (MD = −2.05, 95% CI (−2.62, −1.48)), HOMA-IR (MD = −0.71, 95% CI (−1.03, −0.39)), and BMI (MD = −1.07, 95% CI (−1.76, −0.37)). Lipid metabolisms were also ameliorated via the reduction of TG (MD = −0.5, 95% CI (−0.61, −0.39)), TC (MD = 0.64, 95% CI (−0.78, −0.49)), and LDL (MD = 0.86, 95% CI (−1.06, −0.65)) and the upregulation of HDL (MD = 0.17, 95% CI (0.09, 0.25)). Additionally, berberine improved the inflammation factor.
Conclusion
There is strong evidence supporting the clinical efficacy and safety of berberine in the treatment of DM, especially as an adjunctive therapy. In the future, this may be used to guide targeted clinical use of berberine and the development of medications seeking to treat patients with T2DM and dyslipidemia.
1. Introduction
Diabetes mellitus (DM) is a chronic, noncommunicable disease that has become a worldwide threat to public health. The global prevalence of DM has been remarkably increased to 463 million among adults and has been predicted to surge to about 700 million by the year 2045. In 2019, approximately 4.2 million adults had an estimated cause of death related to diabetes and its complications. This comes out to about one death every eight seconds [1]. Though 10% of the global health expenditure is currently spent on DM, this disease remains in the top 4 causes of noncommunicable disease deaths [2]. Thus, it is essential to explore more effective and safe strategies for the prevention and treatment of DM. China currently has the largest number of type 2 diabetic patients, so this condition has become a leading public health challenge in China [3].
In addition, with the development of society and the change of diet structure, the characteristics of the diabetic population are changing. The classic symptoms, like polydipsia, polyphagia, polyuria, and weight loss, are less often recognized in those with type 2 diabetes mellitus (T2DM). It has been reported that 44% of type 2 diabetic respondents had no classic symptoms in the previous year [4]. Moreover, patients with T2DM are more likely to be overweight or obese, which indicates insulin resistance and dyslipidemia along with hyperglycemia [5, 6]. For T2DM patients, glycemic or lipid values and their variability can significantly predict all-cause mortality and the occurrence of complications, including micro- and macrovascular complications [7, 8]. Growing evidence has indicated that chronic inflammation, hyperglycemia, and dyslipidemia are all involved in insulin resistance, pathogenesis of T2DM, and systematic diabetic complications [9, 10]. In T2DM patients, IL-6 and TNF-α levels are strikingly increased. This is also associated with a downregulation of several drug metabolizing enzymes, which leads to poor drug effects [11].
Huanglian (Rhizoma Coptidis) is an herb that has been frequently used in many traditional formulas for the treatment of T2DM. This treatment has been used for thousands of years. Alkaloids from Huanglian have been widely used for the treatment of diabetes and hyperglycemia with inconspicuous toxicities and side effects [12]. Among the different alkaloids, berberine is an important lead compound with a wide spectrum of pharmacological activities, including antitumor, anti-inflammatory, hypoglycemic, hypolipidemic, and antiobesity effects [13]. Additionally, evidence has shown that berberine regulates the gut microbiota as well [14, 15], which is closely associated with systematic inflammation that adds to the progression of T2DM. Systematic reviews have previously reported the efficacy of berberine on the regulation of glycemic and lipid metabolisms [16, 18]. However, its comprehensive effects on glycemic metabolisms, lipid profiles, and inflammation factors of patients with T2DM have not been well evaluated. Since berberine is the main active component of Rhizoma Coptidis, we conducted this meta-analysis to comprehensively evaluate the efficacy and safety of berberine and Rhizoma Coptidis on T2DM.
2. Methods
2.1. Search Strategy and Study Selection
This study was performed according to the Cochrane Handbook for Systematic Reviews of Interventions and was reported according to Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [19]. The protocol has been registered on PROSPERO as CRD42020155086.
The literature research was conducted with the use of the following eight databases with no time restriction: PubMed (up to March 29, 2021), Embase (up to March 29, 2021), Web of Science (ended up to March 29, 2021), the Cochrane library (up to March 29, 2021), China National Knowledge Infrastructure (CNKI) (up to April 14, 2021), Chinese Biomedical Database (SinoMed) (up to April 14, 2021), Wanfang Database (up to April 14, 2021), and Chinese VIP Information (up to April 14, 2021). The MeSH and free-text terms were applied based on the characteristics of specific database as follows: berberine, huanglian, Rhizoma Coptidis, traditional Chinese medicine, diabetes mellitus, insulin resistance, and randomized controlled trials. No restrictions on publication language or date were set. The terms were searched as “diabetes mellitus” OR “insulin resistance” OR “metabolic syndrome” OR “Xiaoke syndrome” AND “TCM” OR” Traditional Chinese Medicine” OR “herbal medicines” OR “plant medicines” OR “berberine” OR “huanglian” OR “ Coptidis “ AND “randomized controlled trial” OR “controlled clinical trial” OR “random” OR “double-blind” OR “single-blind.” The full electronic search strategy for PubMed was provided in Supplementary Files 1 according to the search history.
The included clinical studies fulfilled the following criteria:
Types of studies: only RCTs were eligible for this review. Single-blinded and open label trials were also considered. The sample size was greater than or equal to 60 participants, and the intervention duration was no less than four weeks
Types of participants: adults aged 18 years or older with T2DM or prediabetes were included
Types of interventions: intervention with berberine or Rhizoma Coptidis was considered. The control intervention included placebo, life interventions such as changes in exercise or dietary habits, or any active antihyperglycemia intervention
Types of outcomes: primary outcomes included glycosylated hemoglobin (HbA1c), fasting plasma glucose (FPG), and 2-hour postprandial plasma glucose (2hPG)
Secondary outcomes included insulin resistance and the associated index of fasting plasma insulin, homeostasis model assessment-insulin resistance (HOMA-IR), and body mass index (BMI). Lipid profiles include triglyceride, total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) along with inflammation markers, such as C-reactive protein.
Safety outcomes included the incidence of adverse reactions and adverse reactions.
The excluded criteria were as follows:
Other TCM treatments applied in either the treatment or control group
If the publication was a review, an abstract, a protocol, or had no outcomes of interest, it would not be considered
Full texts were not available
2.2. Data Collection and Analysis
Titles and abstracts were screened independently by two investigators. The NoteExpress 3.4 literature management software was used to screen references. It was suggested to reviewers to contact the author for the complete information if this was indicated. If there was a disagreement, another reviewer would help determine a solution. Kappa statistics were employed to analyze the consistency of the results.
Data extraction was carried out independently by two authors using standard data extraction forms that included participant details, the interventions used in both treatment and control group, and the primary and secondary outcomes. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses.
2.3. Assessment of Risk of Bias in Included Studies
Two reviewers independently assessed the quality of each article, using the risk of bias assessment tool in the Cochrane. Another reviewer was asked to help decide if there was a disagreement.
2.4. Measures of Treatment Effect
Categorical outcomes were expressed as risk ratio (RR) with 95% confident intervals (CI), while continuous measurement was pooled by the standardized mean difference (SMD) or mean difference (MD). If data were missing or unclear, it was suggested that reviewers contact the authors of studies to request this information.
2.5. Assessment of Heterogeneity
Heterogeneity was analyzed using a Chi2 test with an alpha of 0.1. The I2 statistic was used for statistical significance. When I2 was less than or equal to 50% and P > 0.1, the heterogeneity was acceptable. In addition, when I2 was greater than 50% and P < 0.1, the heterogeneity among the trials was significant.
2.6. Data Synthesis and Analysis
Revman 5.4 software was used to conduct the data synthesis and analysis. In the analysis, pooling data was processed as overall RR and/or MD. A random effects model was applied if there was any heterogeneity observed. If pooling was not possible, the data were summarized descriptively.
Subgroup analysis was planned to explore the source of heterogeneity according to the differences of treatment methods among trials. Sensitivity analyses were conducted using a leave-one-out method to identify studies contributing to significant heterogeneity, which had an I2 value of greater than 50%. Funnel plots were planned to assess publication bias as well.
3. Results
A total of 46 clinical trials were considered and included in the quantitative meta-analysis (Figure 1). As to the literature screening and selection, the result of kappa statistics was k = 0.785, indicating a good consistency. Of these, 38 were related to berberine in the treatment of T2DM, and four [20–23] were for prediabetes. Two trials [24, 25] were aimed at evaluating the effectiveness and safety of Rhizoma Coptidis in the treatment of T2DM, and two [26, 27] were for root dry extracts including berberine. The publication time of the included studies was from 2004 to 2021.
Figure 1.
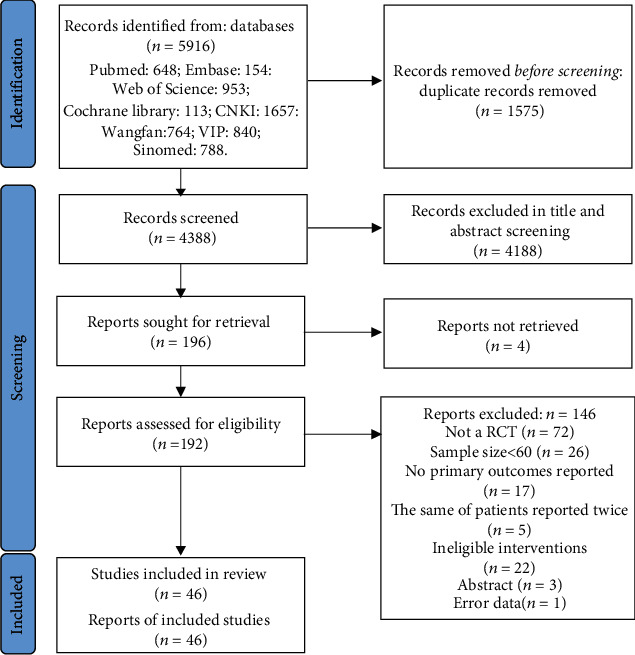
Flow diagram.
A total of 4,158 participants were enrolled, including 2,063 participants in the experimental group and 2,095 participants in the control group. The intervention duration ranged from 4 weeks to 6 months. Eleven trials compared berberine with placebo or none, while four trials compared berberine with metformin. Specific characteristics of the included trials are illustrated in Table 1.
Table 1.
Specific characteristics of included trials.
| Study | Participants | No. (T/C) | Age | Sex (M/F) | Course (year) | Intervention | Control | Duration (weeks) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Zhang et al. 2020 [15] | T2DM | 98/103 | T: 53 (42–61) C: 54 (46–61) |
T: 59/39 C: 61/42 |
Newly diagnosed | Berberine 0.5 g bid | Placebo | 12 | ①②③④⑤⑥⑦⑧⑨⑩ |
| Hu 2020 [28] | T2DM | 60/58 | T: 43.21 ± 8.14 C: 42.85 ± 6.23 |
T: 34/26 C: 30/28 |
T: 2.31 ± 1.06 C: 2.61 ± 0.84 |
Berberine 0.3 g tid Metformin 0.5 g tid |
Placebo Metformin 0.5 g tid |
24 | ①③④⑤⑥⑦⑧⑨⑩ |
| Sanjari et al. 2020 [26] | T2DM | 42/38 | T: 51.8 ± 9.3 C: 46.5 ± 10 |
T: 18/24 C: 7/31 |
T: 4.5 ± 0.25 C: 4.25 ± 0.42 |
Berberis integerrima root extract 480 mg | Metformin 1 g/d | 12 | ①②③⑥⑦⑧⑨ |
| Li et al. 2021 [20] | Prediabetes | 35/35 | T: 54.11 ± 8.14 C: 42.85 ± 6.23 |
T: 22/13 C: 17/18 |
— | Berberine 0.3 g tid | Life intervention | 24 | ①②③⑥⑦⑧⑨⑩ |
| Tahmasebi et al. 2019 [27] | T2DM | 40/40 | T: 54.05 ± 8.00 C: 53.07 ± 7.74 |
T: 17/23 C: 15/25 |
Less than 10 years | 1000 mg dry extract and 157.3 mg berberine | Placebo | 6 | ①②④⑥⑦⑧⑨⑩ |
| Ma 2019 [24] | T2DM | 50/50 | T: 50.8 ± 3.6 C: 50.2 ± 4.2 |
T: 31/19 C:28/22 |
T: 1.77 ± 0.57 C: 1.81 ± 0.53 |
C+Huanglian 3 g tid | Metformin 0.5 g tid | 8 | ①②③④⑥⑦⑧⑨ |
| Deng et al. 2019 [29] | T2DM | 35/35 | T: 54.6 ± 2.5 C: 55.3 ± 2.6 |
T:23/12 C:21/14 |
T: 6.04 ± 2.43 C: 6.61 ± 2.75 |
C+berberine 0.3 g tid | Glipizide 5 mg tid | 12 | ①②③④⑥⑦⑧⑨ |
| Chen et al. 2018 [30] | T2DM | 40/40 | T: 53.27 ± 8.15 C: 52.71 ± 7.89 |
T: 22/18 C: 24/16 |
T: 5.59 ± 3.74 C: 5.64 ± 3.58 |
C+berberine 0.5 g tid | Metformin 0.5 g tid | 12 | ①②③⑩ |
| Wu 2018 [31] | T2DM | 63/63 | T: 58.61 ± 8.37 C: 58.43 ± 8.26 |
T: 37/26 C: 38/25 |
NA | C+berberine 0.2 g tid | Metformin 0.5 g tid | 12 | ①②③⑤⑥⑦⑧⑨⑩ |
| Rashidi et al. 2018 [32] | T2DM | 40/41 | T: 50.18 ± 4.22 C: 45.12 ± 9.55 |
T: 14/28 C: 19/22 |
T: 4.5 ± 1.8 C: 4.3 ± 2.04 |
Berberine 0.5 g bid | Placebo | 4 | ①②④⑥⑦⑧⑨⑩ |
| Li et al. 2018 [33] | T2DM | 57/57 | T: 53 ± 15 C: 57 ± 12 |
T: 22/35 C: 24/33 |
T: 5.92 (1.92, 8) C:7.25 (2, 10.2) |
C+berberine 300 mg tid | Antidiabetic agents | 24 | ①②③⑤⑥⑦⑩ |
| Sha et al. 2018 [34] | T2DM | 30/30 | T: 58.79 ± 12.27 C: 60.46 ± 11.73 |
T: 15/13 C: 14/15 |
T: 7.08 ± 2.68 C: 8.68 ± 4.13 |
Berberine 500 mg tid | NA | 12 | ①③④⑩ |
| Zhao 2018 [22] | Prediabetes | 32/32 | T: 54.7 ± 0.8 C: 54.9 ± 0.16 |
T: 24/8 C: 26/6 |
— | Berberine 0.3-0.5 g tid | Life intervention | 12 | ①②③⑤⑥⑦ |
| Guan et al. 2017 [35] | T2DM | 53/53 | T: 65.74 ± 3.85 C: 65.28 ± 3.29 |
T: 29/24 C: 28/25 |
T: 6.14 ± 2.44 C: 6.25 ± 2.41 |
C+berberine 0.5 g tid | Metformin 0.5 g tid | 4 | ①③ |
| Zhang 2017 [36] | T2DM | 40/40 | T: 58.24 ± 6.15 C: 58.13 ± 6.24 |
T: 21/19 C: 23/17 |
T: 5.8 ± 1.9 C: 5.7 ± 1.8 |
Berberine 3 g tid | Metformin 750 mg/d | 12 | ①②③④ |
| 40/40 | T: 58.91 ± 6.58 C: 58.13 ± 6.24 |
T:22/18 C:23/17 |
T: 6.0 ± 1.7 C: 5.7 ± 1.8 |
C+berberine 3 g tid | Metformin 750 mg/d | 12 | ①②③④ | ||
| Xing 2017 [37] | T2DM | 35/35 | T: 53.3 ± 6.2 C: 54.1 ± 6.1 |
T: 18/17 C: 17/18 |
T: 5.9 ± 2.8 C: 5.8 ± 2.7 |
Berberine 500 mg tid | Metformin 250 mg tid | 12 | ①③④⑤⑥⑦⑧⑨⑩ |
| Sun 2017 [38] | T2DM | 91/91 | T: 58.95 ± 10.57 C: 58.34 ± 11.21 |
T: 53/38 C: 51/40 |
T: 3.98 ± 1.62 C: 3.64 ± 1.75 |
C+berberine 30 mg tid | Metformin 0.5 g tid | 8 | ①②③④⑥⑦⑧⑨⑩ |
| Chen et al. 2017 [39] | Prediabetes | 50/50 | T: 36.5 ± 14.4 C: 35.2 ± 15.1 |
T: 31/19 C: 30/20 |
— | Berberine 0.5 g tid | Metformin 0.5 g tid | 24 | ①②⑤ |
| Li et al. 2017 [40] | T2DM | 30/30 | T: 50.54 ± 3.78 C: 51.24 ± 3.91 |
T: 18/12 C: 17/13 |
T: 6.04 ± 2.43 C: 6.61 ± 2.75 |
C+berberine 0.3 g tid | Sitagliptin 100 mg tid | 12 | ①②③ |
| Li 2016 [41] | T2DM | 90/90 | T: 56.2 ± 2.9 C: 57.8 ± 2.7 |
T: 55/35 C: 55/35 |
T: 3.7 ± 1.9 C: 3.8 ± 1.7 |
C+berberine 0.3 g tid | Metformin 1.7 g/d | 12 | ①②③④ |
| Lang and Zhu 2016[42] | T2DM | 50/50 | 46.3 ± 4.7 | 63/37 | NA | Berberine 500 mg tid | Placebo | 12 | ①②③ |
| Du 2016 [43] | T2DM | 37/36 | T: 67.8 ± 4.6 C: 56.5 ± 7.1 |
T: 21/16 C: 18/18 |
T: 10.9 ± 6.3 C: 11.6 ± 6.9 |
C+berberine 4 pills tid | Metformin 0.5 g tid | 4 | ①②③ |
| Li 2016 [44] | T2DM | 60/60 | T: 65.85 ± 4.78 C: 67.92 ± 4.73 |
T: 26/24 C: 28/32 |
T: 5.32 ± 1.08 C: 5.28 ± 1.31 |
C+berberine 0.5 g tid | Glipizide 5 mg tid | 12 | ③④⑥⑦⑧⑨ |
| Cui 2016 [45] | T2DM | 40/40 | T: 50.85 ± 7.26 C: 51.59 ± 8.97 |
T: 28/12 C: 30/10 |
T: 8.65 ± 3.33 C: 9.07 ± 3.47 |
C+berberine 0.5 g tid | Metformin 0.5 g tid | 16 | ①②③⑥⑦ |
| Ren et al. 2016 [46] | T2DM | 31/30 | T: 47.74 ± 7.39 C: 46.87 ± 7.74 |
T: 21/10 C: 19/11 |
Newly diagnosed | C+berberine 0.5 g tid | Life interverntion | 12 | ①②③⑥⑦⑧⑨ |
| Wang 2015 [25] | T2DM | 39/36 | T: 51.6 ± 11.7 C: 53.4 ± 11.3 |
T: 22/17 C: 21/15 |
T: 1.7 ± 0.78 C: 1.9 ± 0.71 |
C+Huanglian 3 g tid | Metformin 0.5 g tid | 8 | ①②③④⑥⑦⑧⑨ |
| Yu 2015 [47] | T2DM | 49/48 | T: 51.7 ± 4.6 C: 50.9 ± 3.8 |
T: 23/26 C: 25/23 |
T: 4.1 ± 0.5 C: 4.3 ± 0.7 |
C+berberine 0.5 g tid | Metformin 0.5 g qd | 12 | ①③⑥⑦ |
| Zhu et al. 2015 [48] | T2DM | 59/59 | T: 66.4 ± 7.6 C: 65.6 ± 7.2 |
T: 35/24 C: 33/26 |
T: 4.5 ± 1.8 y C: 4.3 ± 2.04 |
C+berberine 100 mg tid | Gliclazide modified release tablets 30 mg qd | 12 | ①②③⑥⑦⑧⑨ |
| Shu 2014 [49] | T2DM | 32/32 | T: 62.80 ± 12.20 C: 61.21 ± 13.52 |
T: 19/13 C: 20/12 |
T: 6.53 ± 2.61 C: 6.79 ± 2.93 |
C+berberine 300 mg tid | Metformin 0.5 g tid | 24 | ①④ |
| Zhou 2014 [50] | T2DM | 33/33 | 65.3 ± 2.5 | 46/20 | 7.4 ± 1.4 | C+berberine 300 mg tid | Metformin 0.5 g bid | 12 | ①②③⑤⑥⑦⑧⑨ |
| Cao 2012 [51] | T2DM | 38/40 | T: 51.26 ± 14.71 C: 52.53 ± 6.37 |
T: 22/16 C: 24/16 |
Newly diagnosed | C+berberine 0.5 g tid | Metformin 0.5 g tid | 16 | ①②③④⑤⑥⑦⑧⑨⑩ |
| Zhou and Huang 2012 [52] | T2DM | 45/45 | 46.67 ± 8.52 | 48/44 | 4.81 ± 0.29 | C+berberine 0.5 g tid | Metformin 0.5 g tid | 12 | ①②④⑤⑥⑦⑧⑨ |
| Zhang and Yuan 2012 [53] | T2DM | 38/38 | No difference | T: 21/17 C: 20/18 |
No difference | C+berberine 0.5 g-0.8 tid | Metformin 0.5 g tid | 12 | ①②③ |
| Xue et al. 2012 [54] | T2DM | 44/45 | No difference | No difference | No difference | C+berberine 0.5 g tid | Metformin 1.5 g/d SU | 12 | ①②③④ |
| Lou et al. 2011 [55] | T2DM | 30/30 | 36-74 | 32/28 | Newly diagnosed | C+berberine 0.3 g tid | Metformin 1.5 g/d | 24 | ①②③④⑥⑦⑧⑨ |
| Qiu et al. 2011 [56] | T2DM | 49/51 | T: 42.2 ± 11.4 C: 54 ± 12.5 |
T: 26/23 C: 28/23 |
NA | C+berberine 0.3 g tid | Antidiabetic drugs | 8 | ①②③④⑩ |
| Meng et al. [57] 2011 |
T2DM | 30/30 | T: 51 ± 13.3 C: 53 ± 13.9 |
T: 17/13 C: 16/14 |
Newly diagnosed | C+berberine 0.3 g tid | Insulin injection | 12 | ①②③ |
| Ye 2010 [58] | T2DM | 40/40 | T: 43∙5 ± 9∙8 C: 42∙5 ± 8∙6 |
T: 24/16 C: 22/18 |
T: 0.84 ± 0.36 C: 0.8 ± 0.35 |
C+berberine 0.5 g tid | Glimepiride 0.1 mg bid Metformin 0.5 g tid |
12 | ①②③⑤⑥⑦⑧⑨ |
| Sheng and Xie 2010 [59] | T2DM | 30/30 | T: 52 ± 11 C: 51 ± 8 |
T: 18/12 C: 11/19 |
T: 5 ± 3 C: 6 ± 3 |
C+berberine 0.5 g tid | Glipizide 5 mg bid Metformin 0.5 g tid |
12 | ①④⑤⑩ |
| Xu et al. 2008 [60] | T2DM | 32/32 | 43.4 ± 2.1 | 34/30 | Newly diagnosed | C+berberine 0.3 g tid | Pioglitazone 30 mg qd | 12 | ①②⑦ |
| Zhang et al. 2008 [61] | T2DM | 59/57 | T: 51 ± 9 C: 51 ± 10 |
T: 30/28 C: 31/21 |
Newly diagnosed | Berberine 0.5 g bid | Placebo | 12 | ①②③④⑤⑥⑦⑧⑨⑩ |
| Li et al. 2008 [62] | T2DM | 33/32 | T: 47.5 C: 49.2 |
T: 17/16 C: 16/16 |
T: 9.01 ± 1.99 C: 8.11 ± 2.24 |
Berberine 500 mg tid | Metformin 250 mg tid | 12 | ①②③④⑤⑥⑦⑧⑨⑩ |
| Liu and Hu 2008 [63] | T2DM | 30/30 | T: 52 ± 9.81 C: 53.07 ± 8.51 |
T: 10/20 C: 14/16 |
T: 9.01 ± 1.99 C: 8.11 ± 2.24 |
C+berberine 0.3-0.5 g tid | Metformin 500 mg tid | 8 | ①②③④ |
| Ju et al. 2007 [23] | Prediabetes | 46/44 | T: 46.7 ± 4.2 C: 45.6 ± 4.4 |
T: 28/18 C: 26/18 |
— | Berberine 0.6 g/d | NA | 48 | ①②⑤⑥⑦⑧⑨⑩ |
| Zhang 2005 [64] | T2DM | 48/46 | 43 ± 11 | 50/44 | 6-10 | SU+berberine 0.3 g tid | Metformin 1.5 g/d + SU | 12 | ①②③④ |
| Cao 2004 [65] | T2DM | 30/30 | T: 55.3 ± 11.5 C: 55.4 ± 10.7 |
T: 13/17 C: 12/18 |
T: 0.8 ± 0.28 C: 0.825 ± 0.275 |
Berberine 0.5 g tid Life intervention |
Life intervention | 12 | ①②③④⑤⑥⑦⑧⑨⑩ |
Note: ① FBG: fasting blood glucose; ② 2hPBG: 2-hour postprandial blood glucose; ③ HbA1c: glycated hemoglobin; ④ FINS: fasting insulin; ⑤ BMI: body mass index: ⑥ TC: total cholesterol; ⑦ TG: triglyceride; ⑧ HDL-C: high-density lipoprotein cholesterol; ⑨ LDL-L: low-density lipoprotein cholesterol; ⑩HOMA-IR.
All the included studies were RCT designs and twenty-two of them provided information on random sequence generation. Five studies reported information on allocation concealment procedures and the blindness of outcome assessments. Five studies were conducted with blinding of both participants and personnel. No risks of incomplete outcome data or selective reporting were found for any of the recruited studies. The risks of bias assessment across the recruited studies are illustrated in Figure 2. To comprehensively illustrate the effect of berberine for diabetes, glucose metabolism-related index (HbA1c, FPG, and 2hPG), insulin resistance-related index (FINS, HOMA-IR, and BMI), lipid profiles (TC, TG, HDL, and LDL), and inflammation index (CRP, IL-6, and TNF-α) were reported, along with the safety including effect of berberine on serum creatinine (SCR), blood urea nitrogen (BUN), and any associated adverse events.
Figure 2.
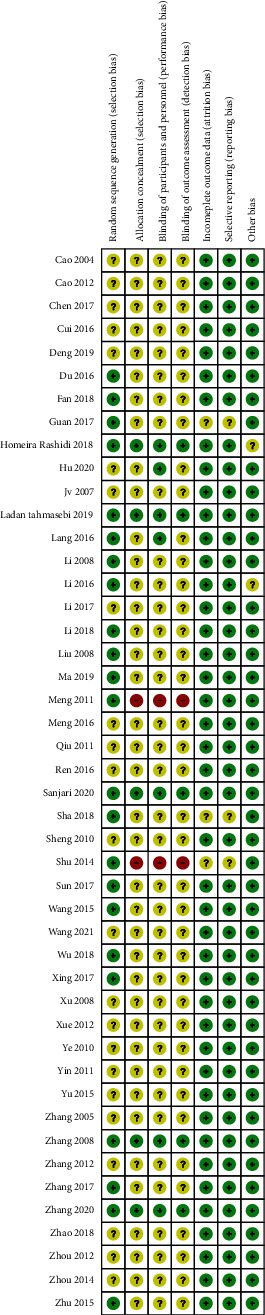
Risk of bias of assessment. The quality of each article independently using the risk of bias assessment tool in the Cochrane.
3.1. Berberine for Glucose Metabolism of T2DM
Forty trials, 45 trials, and 36 trials provided the data of HbA1c, FPG, and 2hPG, respectively. The meta-analyses showed that berberine remarkably decreased the HbA1c level (MD = −0.75, 95% (−1.00, −0.51); P < 0.05; I2 = 98%, 95% CI (0.97, 0.98)), the FPG level (MD = −0.89, 95% CI (−1.13, −0.64), P < 0.05, I2 = 97, 95% CI (0.96, 0.97)), and the 2hPG level (MD = −1.31, 95% CI (−1.69, −0.93), P < 0.05, I2 = 96%, 95% CI (0.96, 0.97)).
Subgroup analysis showed that berberine could slightly lower the HbA1c level (MD = −0.38, 95% CI (−0.49, −0.27), P < 0.05) and the FPG level (MD = −0.58, 95% CI (−0.81, −0.35), P < 0.05) but remarkably reduce the 2hPG level (MD = −1.48, 95% CI (−2.16, −0.79), P < 0.05) when it was used alone. When combined with antidiabetic agents, berberine strikingly reduce the HbA1c level (MD = −0.91, 95% CI (−1.25, −0.56), P < 0.05), the FPG level (MD = −1.06, 95% CI (−1.34, −0.79), P < 0.05), and the 2hPG level (MD = −1.34, 95% CI (−1.73, −0.96), P < 0.05). However, compared with Western medicine, there was no significant difference observed in the HbA1c level, the FPG level, or the 2hPG level when berberine was applied alone (Figures 3–5).
Figure 3.
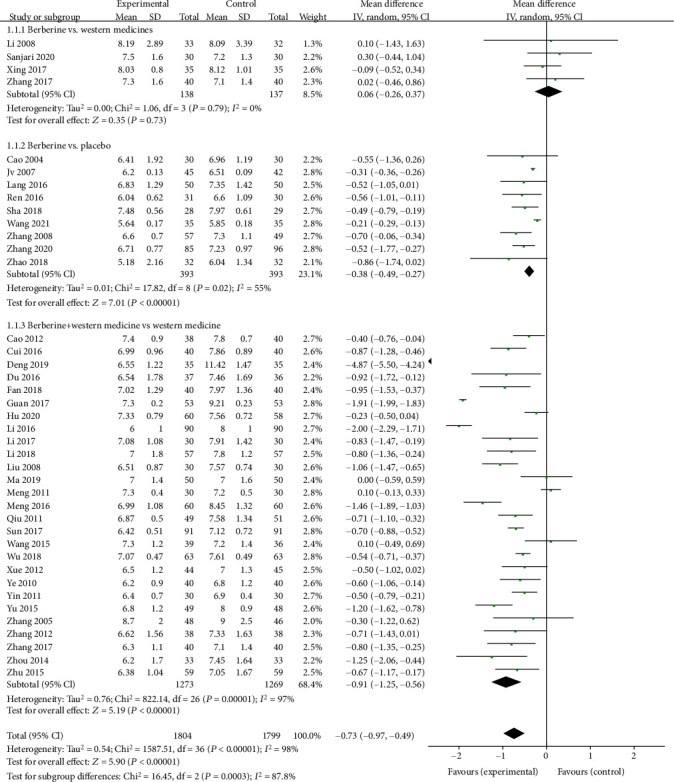
Meta-analysis of the effect of berberine on glycosylated hemoglobin (HbA1c).
Figure 4.
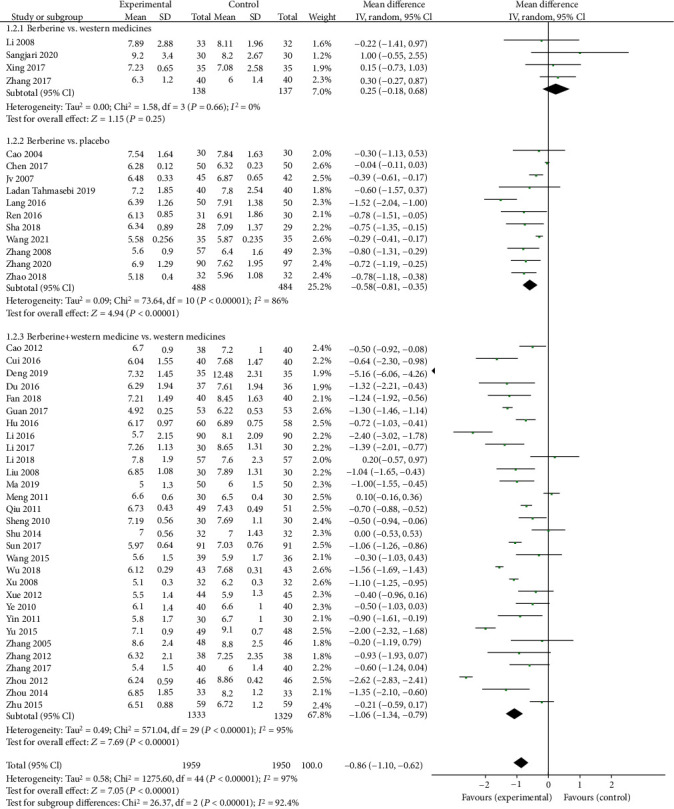
Meta-analysis of the effect of berberine on fasting plasma glucose (FPG).
Figure 5.
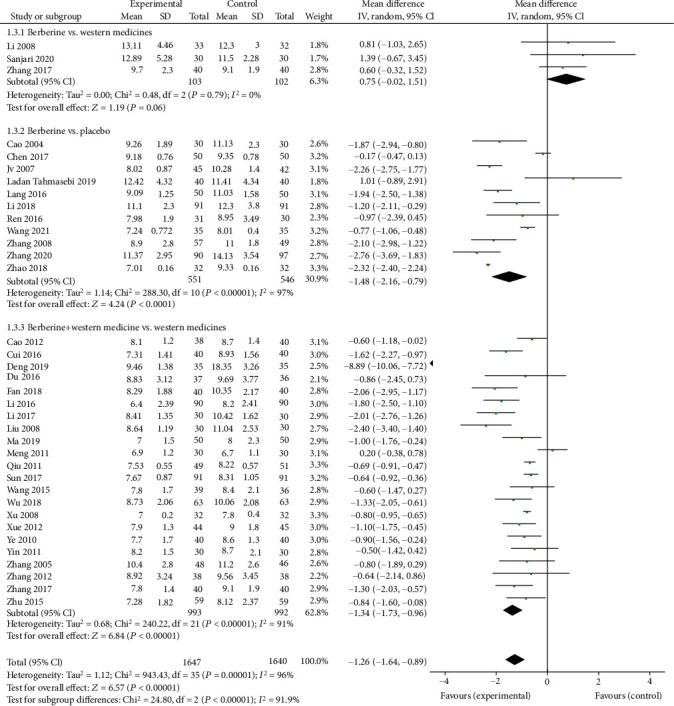
Meta-analysis of the effect of berberine on 2-hour postprandial plasma glucose (2hPG).
3.2. Berberine for Insulin Resistance-Associated Index of T2DM
Figure 6 shows the efficacy of berberine on the FINS level, the HOMA-IR level, and the BMI level of T2DM patients. The FINS concentration of the trial group decreased by 2.05 (95% CI (−2.62, −1.48), P < 0.05, I2 = 93%, 95% CI (0.91, 0.95)), the HOMA-IR level of the berberine group decreased by 0.71 (95% CI (−1.03, −0.39), P < 0.05, I2 = 96%, 95% CI (0.94, 0.97)), and the BMI level of the berberine group decreased by 1.07 (95% CI (−1.76, −0.37), P < 0.05, I2 = 91%, 95% CI (0.87, 0.94)).
Figure 6.
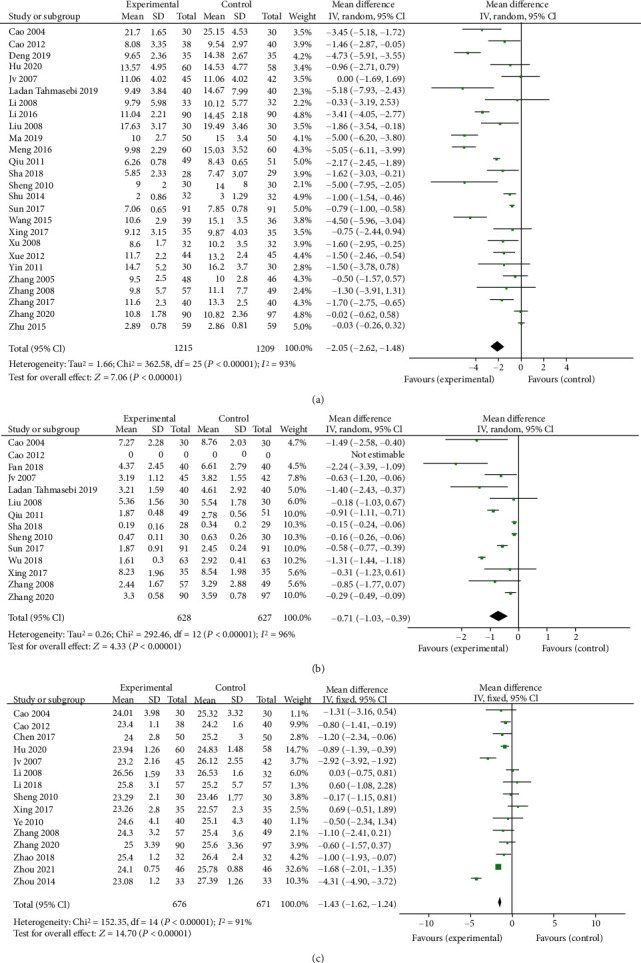
Meta-analysis of the effect of berberine on insulin resistance-associated index. (a) Fasting plasma insulin (FINS). (b) Homeostasis model assessment-insulin resistance (HOMA-IR). (c) Body mass index (BMI).
3.3. Berberine for Lipid Profiles of T2DM
The pooled results of berberine efficacy in the treatment of T2DM on lipid profiles are shown in Figures 7 and 8. Twenty-four trials were assessed on TG (Figure 7(a)), and the result showed that berberine significantly lowered the TG level in patients with T2DM (MD = −0.5, 95% CI (−0.61, −0.39), P < 0.05, I2 = 92%, 95% CI (0.89, 0.94)). Pooled results of 25 trials showed that the TC concentration of the berberine group decreased by 0.64 (95% CI (−0.78, −0.49), P < 0.05, I2 = 79%, 95% CI (0.69, 0.86)) (Figure 7(b)). There was a slightly upregulated tendency in HDL (MD = 0.17, 95% CI (0.09, 0.25), P < 0.05, I2 = 92%, 95% CI (0.89, 0.95)) (Figure 8(a)), as compared to the control group and the LDL concentration of the berberine group decreased by 0.86 (95% CI (−1.06, −0.66), P < 0.05, I2 = 92%, 95% CI (0.89, 0.94)) (Figure 8(b)).
Figure 7.
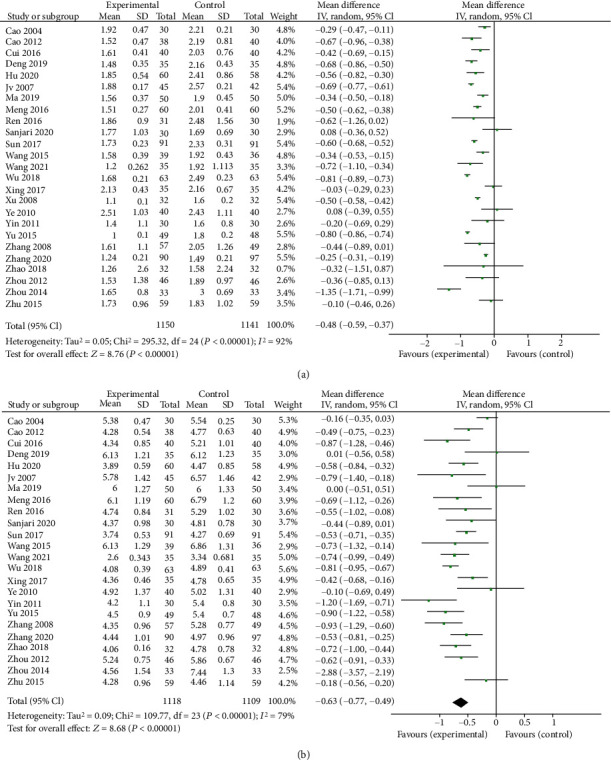
Meta-analysis of the effect of berberine on triglyceride (a) and total cholesterol (b).
Figure 8.
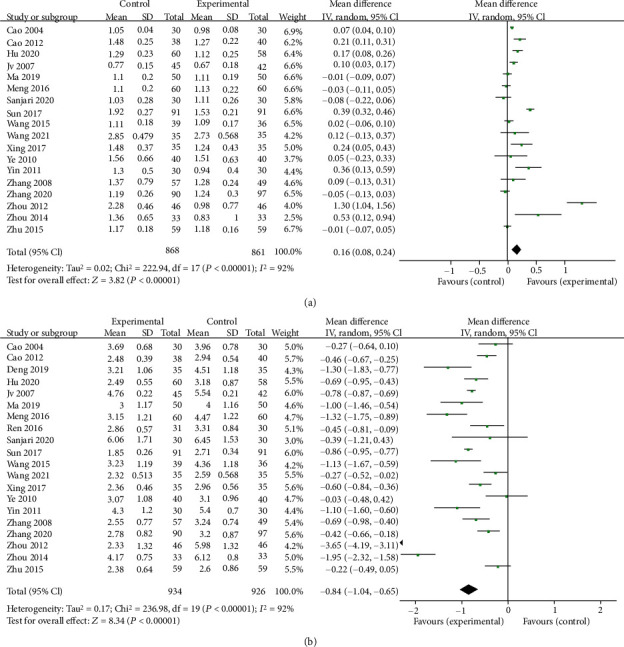
Meta-analysis of the effect of berberine on high-density lipoprotein (a) and low-density lipoprotein (b).
3.4. Berberine for Inflammation Factors of T2DM
Pooled results of six clinical trials proved that berberine markedly lowered the CRP levels in patients with T2DM (SMD = −2.13, 95% CI (−2.98, −1.28), P < 0.05, I2 = 96%, 95% CI (0.94, 0.97)).
Six trials were considered for the analysis of the efficacy of berberine on IL-6 levels. The IL-6 concentration in the trial group decreased by 1.83 (95% CI (−3.05, −0.61), P = 0.003; I2 = 97%, 95% CI (0.95, 0.98)).
Among them, five reported the efficacy of berberine on TNF-α, and the results found that berberine reduced the level of TNF-α in patients with T2DM to some extent (SMD = −1.44, 95% CI (−2.72, −0.16), P = 0.03, I2 = 97%, 95% CI (0.95, 0.98)) (Figure 9).
Figure 9.
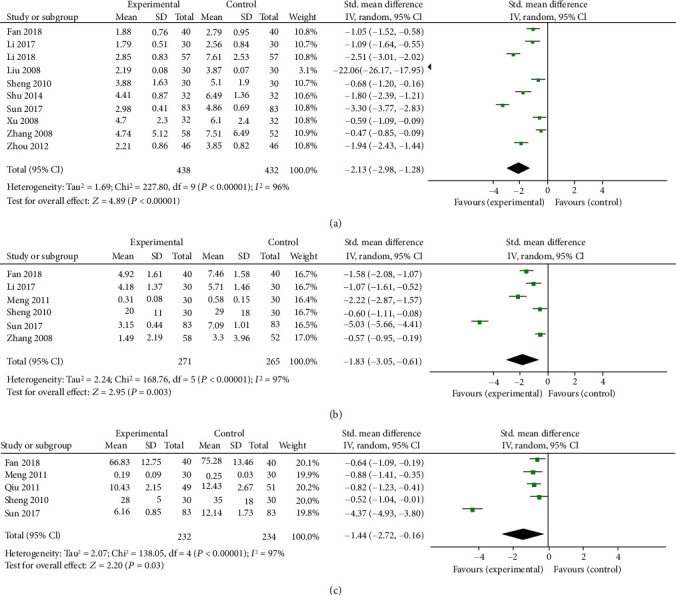
Meta-analysis of the effect of berberine on inflammation factors. (a) C-reaction protein (CRP). (b) Interleukin-6 (IL-6). (c) Tumor necrosis factor-α (TNF-α).
3.5. Safety of Berberine on Patients with T2DM
Figure 10(a) shows the effects of berberine on Scr in patients with T2DM. There were a total of 288 volunteers in the trial group and 294 in the control group. The Scr concentration of the berberine group decreased by 2.02 (95% CI (−3.63, −0.42), P = 0.01, I2 = 0%, 95% CI (0, 0.86)). Berberine was found to have a beneficial effect on Scr.
Figure 10.
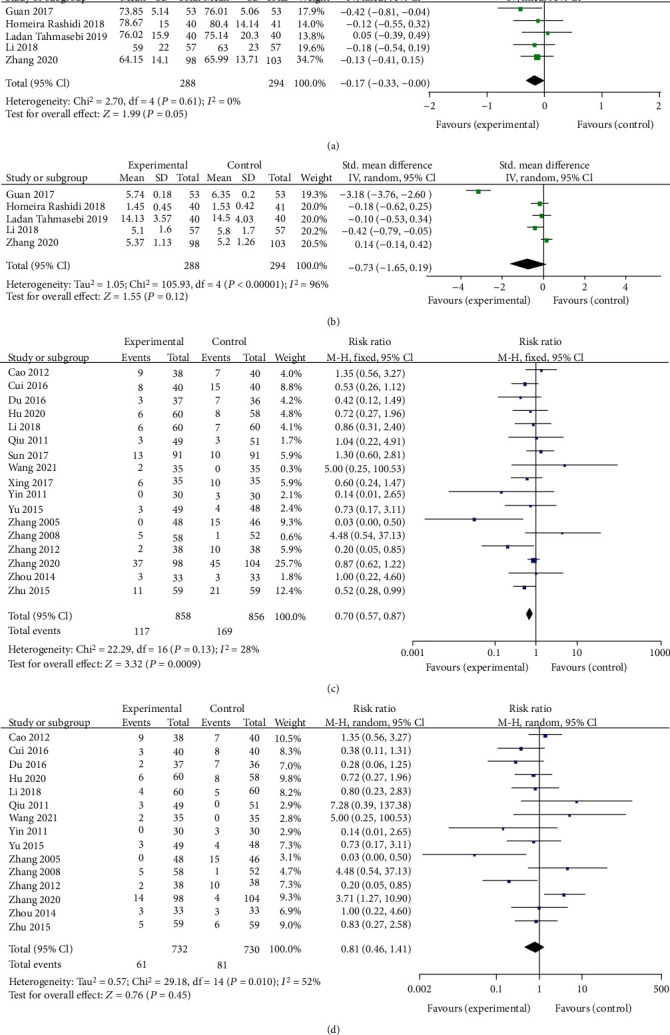
Meta-analysis of the safety of berberine in the treatment of diabetes mellitus. (a) Serum creatinine (Scr). (b) Blood urea nitrogen (BUN). (c) Total adverse events. (d) The gastrointestinal adverse events.
Figure 10(b) illustrates the effects of berberine on BUN. There were a total of 288 volunteers in the trial group and 294 in the control group. Compared to the control group, berberine had no significant effect on the BUN level (SMD = −0.29, 95% CI (−0.69, −0.11), P = 0.16, I2 = 97%, 95% CI (0.94, 0.98)).
The incidence of adverse events (AEs) was used to assess the safety of berberine in 17 studies, including a total of 858 patients in the berberine group and 856 in the control group. Pooled results of 17 trials showed that berberine applied for the treatment of T2DM appeared to have better safety compared to the control group in the incidence of AEs (RR = 0.70, 95% CI (0.57, 0.87), P = 0.0009, I2 = 28%, 95% CI (0, 0.6)) (Figure 10(c)). The main reported adverse events of berberine treatment were gastrointestinal responses like diarrhea, abdominal distention, or constipation. Among the 17 trials, 15 specifically reported the number of gastrointestinal AEs, which included 732 volunteers in the trial group and 730 in the control group. These results demonstrated that berberine did not have more gastrointestinal AEs as compared to the control group (RR = 0.81, 95% CI (0.46, 1.14), P = 0.45, I2 = 52%, 95% CI (0.13, 0.73)) (Figure 10(d)). The pooled results demonstrated that berberine was generally safe as a complementary or alternative therapy for the treatment of T2DM.
3.6. Publication Bias
A funnel plot was used to evaluate potential publication bias. Comparisons of HbA1c, FPG, and 2hPG were conducted using funnel plots. Approximately symmetrical dispersion points suggested rare publication bias. These results are shown in Figure 11.
Figure 11.
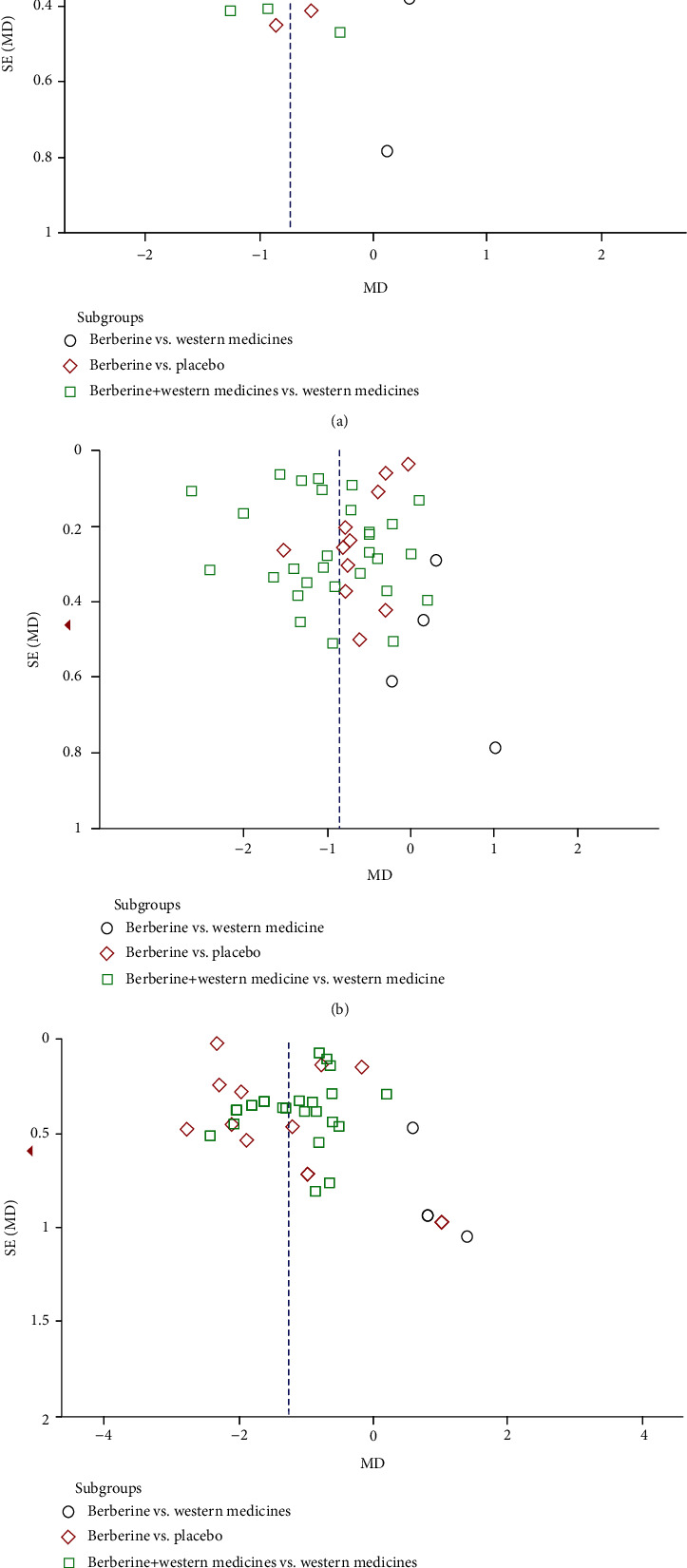
Funnel plots of comparison. A funnel plot was used to evaluate potential publication bias. Comparison in HbA1c, FPG, and 2hPG was conducted by funnel plots.
3.7. Sensitivity Analysis
According to the different interventions, daily dosage of berberine applied, the intervention duration, and disease courses, we conducted the subgroup analyses for main outcomes including HbA1c, FPG, and 2hPG (Supplementary Files 2). Results found that different interventions were the source of heterogeneity. Mutual conversion between a random-effects model and a fixed-effect model was conducted as a sensitivity analysis for testing the stability of the research. The results showed that the I2 value did not change in the mutual conversion. The results of meta-analyses were not changed either. This suggests that our findings were stable.
4. Discussion
In the current systematic review, we evaluated the efficacy and safety of berberine for the treatment of T2DM. Our findings suggested that berberine, used along or combined with antidiabetic agents, significantly improved glucose and lipid metabolisms along with inflammation markers.
Berberine showed effectiveness in lowering blood glucose comparable with metformin. As an adjunctive therapy, berberine presented better reduction of HbA1c, FPG, and 2hPG. In recent years, three meta-analyses [17, 66, 67] were conducted to explore the effects of berberine for the treatment of T2DM. The latest study was published in 2019, which first performed subgroup analyses to examine the source of heterogeneity and identify the potential factors which likely determine the effects of berberine. These results showed that berberine seemed to achieve better effects on glucose levels when patients aged less than 60 years were treated with a daily dosage of 1.5–2 g. After adding new research with limited sample size and intervention durations in the included criteria, our results suggested that low-dose berberine (< 1 g/d) achieve promising effects of FPG, especially for those patients with a disease duration of no more than five years. The efficacy of berberine seemed to decrease with an increased treatment course. Low and medium doses of (1–2 g) berberine showed the same effects on reducing HbA1c and 2hPG. Better efficacy on 2hPG was observed in patients with an intervention duration of no less than 12 weeks. For HbA1c, the highest level of efficacy was observed with an intervention duration of no more than eight weeks and less than twelve weeks, especially for those patients with T2DM that have had this condition for 5–10 years. It appeared that the early application of berberine mainly functioned to reduce FPG. With prolonged intervention duration and disease progression, this effect was mainly observed as the reduction of 2hPG.
The regulation of berberine on blood homeostasis is partly due to the improvement of insulin resistance, the hallmark of T2DM, which is given rise to obesity. Systematic reviews showed that berberine improved obesity parameters including BMI [68, 69]. Meanwhile, a case-control clinical trial reported that the HOMA-IR level of T2DM decreased by 73% with 500 mg (×3 daily) berberine for 3 months [70]. Our results showed that berberine remarkably lower fasting blood insulin, improve HOMA-IR, and decrease BMI, which demonstrated the advantages of berberine on improving insulin resistance.
Evidence suggests that clinically tested lipid-lowering nutraceuticals including berberine could be safely used to improve lipid levels in patients with mild-to-moderate dyslipidemia [71]. Our work also showed the plasma lipid profiles of diabetic patients were improved by berberine intake. The efficacy of berberine on dyslipidemia has been widely researched. Whether used alone or combined with other therapies, meta-analyses [16, 72] suggested that berberine improve obesity and hyperlipidemia by reducing TG, TC, and LDL and increasing HDL in the setting of several metabolic disorders along with improving glucose metabolism. This is consistent with the current meta-analysis specific to T2DM, which showed a remarkable lowering of TC, TG, and LDL, along with moderate upregulation of HDL.
In T2DM, obesity and dyslipidemia bring about low-grade inflammation and factor like IL-6 and TNF-α levels were found to be strikingly increased. This was associated with a downregulation of several drug metabolizing enzymes, which led to poor drug effect. Berberine has been demonstrated as a chronic inflammation regulator as well [73]. A meta-analysis proved that berberine supplementation ameliorates the state of chronic inflammation by lowering the serum level of CRP [74]. Our meta-analysis illustrated that berberine significantly downregulates CRP, IL-6, and TNF-α. This indicates that berberine as an additional therapy shows synergistic benefits with hypoglycemic agents. Above all, berberine is suggested to be applied to diabetic patients with insulin resistance and dyslipidemia, especially for those patients newly diagnosed with T2DM accompanied by obesity and dyslipidemia.
Regarding safety, our work demonstrated that berberine had no toxic effects on Scr and BUN. In addition, this treatment did not increase the risk of serious adverse events when the routine dosage ranged from 0.6 g to 1.5 g.
Berberine has been demonstrated to have comparable effects in the treatment of T2DM with antidiabetic drugs like metformin that display multiple targets and pathways. For patients with T2DM, the main function of berberine is as a SIRT1 or AMPK agonist to mimic energy restriction [75–77] and to target on NF-κB [78] to improve insulin resistance and inflammation as well as to alleviate the activation of ox-LDL-induced macrophages [79]. In addition, berberine stabilizes LDL receptor mRNA to increase the clearance rate of plasma LDL [80]. This corresponds to its effects of improving insulin resistance and the regulation of glycemic and lipid metabolisms. As we all know, dyslipidemia and inflammation are risk factors of micro- and macrovascular leisures, our meta-analysis suggest that berberine has potential benefits on diabetes with cardiovascular and chronic kidney disease, which has been reported in preclinical studies [81, 82].
Excessive statistical heterogeneity was induced by several factors in our work. First, different antidiabetic agents used as controls also had a distinct influence on the outcomes. Different dosage levels of berberine and the duration of treatment may have resulted in inconsistent efficacy and different disease courses. To account for these, subgroup analyses were used to assess primary outcomes, including HbA1c, FPG, and 2hPG. The results showed that the different interventions in the control groups seemed to be a potential impact factor of heterogeneity. Meanwhile, the literature qualities may have influenced the results. In the future, the association of each factor with the effect of berberine for the treatment of T2DM patients should be quantified by metaregression analysis.
5. Strengths and Limitations of the Current Study
Compared to previous meta-analyses, the current study included 46 trials and comprehensively showed the efficacy and safety of berberine for the treatment of T2DM. Subgroup analyses were also conducted to clarify how berberine is used. Additionally, GRADE criteria (Supplementary Files 3) were applied to determine the certainty in the estimate of effect for primary outcomes.
There are some limitations of this review. First, most of the trials were conducted among Chinese patients, which limited the widespread application of this data. Second, excessive statistical heterogeneity appeared in some comparisons; however, the primary source of heterogeneity could not be determined. Third, literature qualities were uneven, although the included trials were RCTs. Many of these studies did not report the methods of blinding and allocation concealment. Lastly, more information on the long-term intervention of berberine for the treatment of T2DM is needed to assess the occurrence risk of diabetic complications.
In conclusion, berberine positively regulated glucose metabolism and lipids, improving insulin resistance and inflammation in patients with T2DM. Thus, berberine was recommended as an adjunctive therapy for T2DM.
Acknowledgments
This study was supported by the Natural Science Foundation of China (Grant Nos. 81804083 and 82074361).
Abbreviations
- DM:
Diabetes mellitus
- RCT:
Randomized controlled trials
- HbA1c:
Glycosylated hemoglobin
- FPG:
Fasting plasm glucose
- 2hPG:
2-hour postprandial blood glucose
- FINS:
Fasting blood insulin
- HOMA-IR:
Homeostasis model assessment-insulin resistance
- BMI:
Body mass index
- TG:
Triglyceride
- TC:
Total cholesterol
- LDL:
Low-density lipoprotein
- HDL:
High-density lipoprotein
- CRP:
C-reactive protein
- IL-6:
Interleukin-6
- TNF-α:
Tumor necrosis factor-α
- Scr:
Serum creatinine
- BUN:
Blood urea nitrogen.
Contributor Information
Yaoxian Wang, Email: wyx3203@sina.com.
Wei Jing Liu, Email: liuweijing-1977@hotmail.com.
Data Availability
The original contributions presented in the study are included in the article material; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that they do not have any potential conflict of interest.
Authors' Contributions
This study was designed by W.J. Liu and J.Guo. J. Guo and X.Q. Zhang searched literature. J. Guo and H.D. Chen screened and selected the eligible trials. W.J. Lou, Pingna Zhang, Y.H. Qiu, and C. Z performed the data collection. Analysis of the data was performed by J. Guo and H.D. Chen. J. Guo and X.Q. Zhang wrote the manuscript. Y. X Wang and W.J. Liu checked and revised the manuscript. J. Guo and H.D. Chen revised the manuscript according to reviewers' suggestions. Jing Guo and Hongdong Chen contributed equally to this study.
Supplementary Materials
Supplementary Files 1 shows the full electronic search strategy for PubMed according to the search history.
Supplementary Files 2 shows results of subgroup analyses for main outcomes including HbA1c, FPG, and 2hPG.
Supplementary Files 3 shows GRADE criteria to determine the certainty in the estimate of effect for primary outcomes including HbA1c, FPG, and 2hPG.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas . 9th. Brussels: [Google Scholar]
- 2.Balakumar P., Maung-U K., Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacological Research . 2016;113(Part A):600–609. doi: 10.1016/j.phrs.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 3.Hu C., Jia W. Diabetes in China: epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes . 2018;67(1):3–11. doi: 10.2337/dbi17-0013. [DOI] [PubMed] [Google Scholar]
- 4.Clark N. G., Fox K. M., Grandy S. Symptoms of diabetes and their association with the risk and presence of diabetes: findings from the study to help improve early evaluation and management of risk factors leading to diabetes (SHIELD) Diabetes Care . 2007;30(11):2868–2873. doi: 10.2337/dc07-0816. [DOI] [PubMed] [Google Scholar]
- 5.Daousi C. Prevalence of obesity in type 2 diabetes in secondary care: association with cardiovascular risk factors. Postgraduate Medical Journal . 2006;82(966):280–284. doi: 10.1136/pmj.2005.039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong X., Bian G., Zhen Z. TCM syndrome differentiation of 2,518 T2DM cases. WORLD JOURNAL OF INTEGRATED TRADITIONAL AND WESTERN MEDICINE. . 2008;3:26–28. [Google Scholar]
- 7.Wan E. Y. F., Yu E. Y. T., Chin W. Y., et al. Greater variability in lipid measurements associated with cardiovascular disease and mortality: a 10-year diabetes cohort study. Diabetes, Obesity and Metabolism. . 2020;22(10):1777–1788. doi: 10.1111/dom.14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S., Zhou J., Wong W. T., et al. Glycemic and lipid variability for predicting complications and mortality in diabetes mellitus using machine learning. BMC Endocrine Disorders . 2021;21(1):p. 94. doi: 10.1186/s12902-021-00751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray J. J. H., Foster‒Davies H., Salem A., et al. Glucagon-like peptide-1 receptor agonistsimprove biomarkers of inflammation and oxidative stress: a systematic review and meta-analysis of randomised controlled trials. Diabetes, obesity & metabolism . 2021;23(8):1806–1822. doi: 10.1111/dom.14399. [DOI] [PubMed] [Google Scholar]
- 10.Leite M. M., Dutra M. T., da Costa M. V. G., et al. Comparative evaluation of inflammatory parameters and substitute insulin resistance indices in elderly women with and without type 2 diabetes mellitus. Experimental Gerontology . 2021;150, article 111389 doi: 10.1016/j.exger.2021.111389. [DOI] [PubMed] [Google Scholar]
- 11.Darakjian L., Deodhar M., Turgeon J., Michaud V. Chronic inflammatory status observed in patients with type 2 diabetes induces modulation of cytochrome P450 expression and activity. International Journal of Molecular Sciences . 2021;22(9):p. 4967. doi: 10.3390/ijms22094967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma H., He K., Zhu J., Li X., Ye X. The anti-hyperglycemia effects of _Rhizoma Coptidis_ alkaloids: a systematic review of modern pharmacological studies of the traditional herbal medicine. Fitoterapia . 2019;134:210–220. doi: 10.1016/j.fitote.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Kong Y., Li L., Zhao L. G., Yu P., Li D. D. A patent review of berberine and its derivatives with various pharmacological activities (2016-2020) Expert Opinion on Therapeutic Patents . 2021:1–13. doi: 10.1080/13543776.2021.1974001. [DOI] [PubMed] [Google Scholar]
- 14.Di S., Han L., An X., et al. _In silico_ network pharmacology and _in vivo_ analysis of berberine-related mechanisms against type 2 diabetes mellitus and its complications. Journal of Ethnopharmacology . 2021;276, article 114180 doi: 10.1016/j.jep.2021.114180. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Gu Y., Ren H., et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study) Nature Communications . 2020;11(1):p. 5015. doi: 10.1038/s41467-020-18414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye Y., Liu X., Wu N., et al. Efficacy and safety of Berberine alone for several metabolic disorders: a systematic review and meta-analysis of randomized clinical trials. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.653887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang Y., Xu X., Yin M., et al. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: a systematic literature review and a meta-analysis. Endocrine Journal . 2019;66(1):51–63. doi: 10.1507/endocrj.EJ18-0109. [DOI] [PubMed] [Google Scholar]
- 18.Ju J., Li J., Lin Q., Xu H. Efficacy and safety of berberine for dyslipidaemias: a systematic review and meta-analysis of randomized clinical trials. Phytomedicine . 2018;50:25–34. doi: 10.1016/j.phymed.2018.09.212. [DOI] [PubMed] [Google Scholar]
- 19.Page M. J., McKenzie J. E., Bossuyt P. M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ . 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L., Ge H., Wei G. Clinical study of berberine and lifestyle intervention in the treatment of prediabetes. Chinese Journal of Gerontology. . 2021;41:1595–1598. [Google Scholar]
- 21.Chang H., Xu L., Cheng T., Chen X., Qi K., Xu L. Effect of lifestyle intervention and drug treatment on prediabetes lesions. Northwest Pharmaceutical Journal. . 2020;35:920–925. [Google Scholar]
- 22.Zhao J., Lv Q., Zhang C. Clinical study on effect of berberine on patients with prediabetes to postpone diabetes development. Journal of Practical Diabetology. . 2018;14:31–33. [Google Scholar]
- 23.Ju S., Tan L., Su W., Rong K. Clinical intervenion of berberine hydrochloride on impaired glucose tolerance combined with hyperlipidemia. Journal of Practical Traditional Chinese Medicine. . 2007:490–492. [Google Scholar]
- 24.Ma Y. Clinical study on treatment of type 2 diabetes with traditional Chinese medicine Rhizoma Coptidis. Guangming Journal of Chinese Medicine. . 2019;34:827–830. [Google Scholar]
- 25.Wang X. Clinical observation of traditional Chinese medicine Coptis chinensis treatment of type 2 diabetes mellitus. Journal of Liaoning University of Traditional Chinese Medicine. . 2015;17:168–170. [Google Scholar]
- 26.Sanjari M., Shamsinejad B., Khazaeli P., Safi Z., Mirrashidi F., Naghibzadeh-Tahami A. Safety and efficacy of Berberis integerrima root extract in patients with type 2 diabetes. A parallel intervention based triple blind clinical trial. Journal of Diabetes & Metabolic Disorders. . 2020;19(1):71–80. doi: 10.1007/s40200-019-00478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tahmasebi L., Zakerkish M., Golfakhrabadi F., Namjoyan F. Randomised clinical trial of _Berberis vulgaris_ root extract on glycemic and lipid parameters in type 2 diabetes mellitus patients. European Journal of Integrative Medicine . 2019;32, article 100998 doi: 10.1016/j.eujim.2019.100998. [DOI] [Google Scholar]
- 28.Hu T. Effect of berberine combined with metformin on body fat distribution and liver fat content in type 2 diabetes mellitus with NAFLD. Hangzhou Normal University . 2020;40 [Google Scholar]
- 29.Deng Y., Liao T., Li H. Hypoglycemic effect of berberine combined with glipizide on type 2 diabetes. China Medicine and Pharmacy. . 2019;9:50–52. [Google Scholar]
- 30.Fan C., Li Y., Song H., Wang Q. Effectiveness evaluation analysis on berberine tablets combination with metformin treating type 2 diabetes. Mordern Hospitals. . 2018;18:731–733. [Google Scholar]
- 31.Wu W. Effect of berberine hydrochloride on the efficacy and progress of diabetes mellitus of type 2 diabetes mellitus patients with obesity. Clinical Research and Practice. . 2018;3:46–47. [Google Scholar]
- 32.Rashidi H., Namjoyan F., Mehraban Z., Zakerkish M., Ghaderian S. B., Latifi S. M. The effects of active ingredients of barberry root (berberine) on glycemic control and insulin resistance in type 2 diabetic patients. Jundishapur Journal of Natural Pharmaceutical Products. . 2018;In Press(In Press, article e64180) doi: 10.5812/jjnpp.64180. [DOI] [Google Scholar]
- 33.Li Z., Liu B., Zhuang X., et al. Effects of berberine on the serum cystatin C levels and urine albumin/creatine ratio in patients with type 2 diabetes mellitus. National Medical Journal of China. . 2018;98:3756–3761. doi: 10.3760/cma.j.issn.0376-2491.2018.46.007. [DOI] [PubMed] [Google Scholar]
- 34.Sha W., Zhu Y., Lei T. Effects of berberine on glucagon-like peptide in patients with type 2 diabetes mellitus (syndrome of dampness-heat disturbing spleen) Journal of Shanghai University of Traditional Chinese Medicine. . 2018;32:7–10. [Google Scholar]
- 35.Guan C., Zhu Y., Ye Y., Chen R. Study on the evaluation of curative effect of berberine in the treatment of type 2 diabetes and its safety. China Modern Doctor. . 2017;55:82–84. [Google Scholar]
- 36.Zhang H. The study of sulfonylurea drugs combined with berberine and metformin in the treatment of diabetes mellitus. Diabetes New World. . 2017;20:82–83. [Google Scholar]
- 37.Xing W. Clinical study of Berberine combined with taurine in treatment of type 2 diabetes mellitus with insulin resistance. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease. . 2017;15:90–92. [Google Scholar]
- 38.Sun S. Effect of Berberine on the serum levels of IL-10, IL-6 and CRP in patients with type 2 diabetes mellitus. Journal of Changchun University of traditional Chinese Medicine. . 2017;33:431–433. [Google Scholar]
- 39.Chen Y., Sun G., Sun Y. Effect of berberine hydrochloride on weight and prognosis of patiens with prediabetes and obesity. Chinese Journal of Prevention and Control of Chronic Diseases. . 2017;25:54–56. [Google Scholar]
- 40.Li P. Effect of berberine combined with Sitagliptin on blood glucose and inflammatory markers in obese patients with type 2 diabetes mellitus. Henan Medical Research. . 2017;26:903–904. [Google Scholar]
- 41.Li J. Clinical study of berberine combined with metformin in the treatment of type 2 diabetes mellitus with insulin resistance. China Health Care Nutrition. . 2016;26:167–168. [Google Scholar]
- 42.Lang C., Zhu K. Efficacy and safety of berberine in treatment of type 2 diabetes mellitus. Health Guide. . 2016:p. 212. [Google Scholar]
- 43.Du J. Evaluation on efficacy and safety of berberine on type 2 diabetes mellitus. Clinical Journal of Chinese Medicine. . 2016;8:120–121. [Google Scholar]
- 44.Li M. Effect of berberine combined with glipizide on type 2 diabetes mellitus. Diabetes New World. . 2016;19:19–21. [Google Scholar]
- 45.Cui J. Efficacy and safety of berberine combined with metformin in treatment of non-alcoholic fatty liver disease with type 2 diabetes mellitus. Nei Mongol Journal of Traditional Chinese Medicine. . 2016;35:67–68. [Google Scholar]
- 46.Ren Y., Wang Y., Yang J., Wang W., Gong Z., Zhao L. Effects of berberine on glycolipid metabolism and adiponectin in newly diagnosed type 2 diabetes mellitus. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease. . 2016;14:1921–1922. [Google Scholar]
- 47.Yu Y. Clinical efficacy studies of berberine adjuvant treatment of type II diabetes. Diabetes New World. . 2015:34–35. [Google Scholar]
- 48.Zhu J., Wang X., Lv W., Ji X. Gliclazide combined with berberine in the treatment of type 2 diabetes mellitus. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine. . 2015;25:915–917. [Google Scholar]
- 49.Shu S. Effects of berberine on serum adiponectin and high sensitivity C reactive protein levels in type 2 diabetic patients. China Pharmaceuticals. . 2014;23:33–34. [Google Scholar]
- 50.Zhou J. Application of berberine hydrochloride combined with metformin in treatment of diabetic hyperlipidemia. Xinxueguanbing Fangzhi Zhishi. . 2014:68–69. [Google Scholar]
- 51.Cao Y., Cai W., Zhang L., Fan Y. Clinical observation on the Berberine plus metformin in treatment of type 2 diabetes complicated by nonalcoholic fatty liver disease. Modern Preventive Medicine. . 2012;39:4885–4886. [Google Scholar]
- 52.Zhou Q., Huang S. Clinical study of berberine combined with metformin in treatment of obesity type 2 diabetes mellitus. Journal of Practical Diabetology. . 2012;8:33–35. [Google Scholar]
- 53.Zhang Y., Yuan X. Clinical observation of berberine in treatment of type 2 diabetes mellitus. Journal of Heze Medical College. . 2012;24:27–28. [Google Scholar]
- 54.Xue S., Xu J., Tie L. Efficacy of sulfonylurea antidiabetic drugs combined with berberine and metformin in treatment of diabetes mellitus. Shaanxi Journal of Traditional Chinese Medicine. . 2012;33:1335–1336. [Google Scholar]
- 55.Lou S., Yin H., Li Z., Liu C. Effect of berberine hydrochloride on blood glucose, insulin level and blood lipids in newly diagnosed type 2 diabetic patients. Acta Academiae Medicinae Xuzhou. . 2011;31:40–41. [Google Scholar]
- 56.Qiu L., Sheng H., Qu Y., Xu R., Wang S. Clinical efficiency of berberine bydrochloride in type 2 diabetes mellitus and its effect on serum adiponection and insulin resistance factor levels. Shanghai Medical Journal. . 2011;34:679–681. [Google Scholar]
- 57.Meng X., Zeng Y., Kong H., Shu X. Research on the effect of berberine on the function of endothelial vessels, IL-6 and TNF-a in newly diagnosed patients with type 2 diabetes mellitus. Chinese Journal of Prevention and Control of Chronic Diseases. . 2011;19:504–505. [Google Scholar]
- 58.Ye W. Clinical efficacy of berberine treatment of diabetes. Modern Hospitals. . 2010;10:9–10. [Google Scholar]
- 59.Sheng X., Xie D. The level of inflammatory factors in type 2 diabetes mellitus and the effect of berberine on them as an adjuvant therapy. Journal of New Medicine. . 2010;41:177–180. [Google Scholar]
- 60.Xu F., Yu F., Li Q., Tong A., Yu F. Effects of berberine and pioglitazone on type 2 diabetes acute insulin secretion and adiponectin. Hainan Medical Journal. . 2008;19:5–6. [Google Scholar]
- 61.Zhang Y., Li X., Zou D., et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. The Journal of Clinical Endocrinology & Metabolism. . 2008;93(7):2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 62.Li J., Ma X., Liang H. Treatment of diatebes and IR with berberine hydrochloride combined with intensified life inference. Liaoning Journal of Traditional Chinese Medicine. . 2008;35:1858–1859. [Google Scholar]
- 63.Liu H., Hu Q. Effect of berberine on islet β-cell function in type 2 diabetes mellitus (damp-heat type) Chinese Journal of Information on TCM. . 2008;15:12–14. [Google Scholar]
- 64.Zhang X. Application of berberine on diabetic patients with secondary failure of sulfonylureas. Clinical Journal of Traditional Chinese Medicine. . 2005;17:549–550. [Google Scholar]
- 65.Cao Y. Clinical research on multiple factor intervention of improving the insulin resistance. Shandong University of Traditional Chinese Medicine . 2004 [Google Scholar]
- 66.Dong H., Wang N., Zhao L., Lu F. Berberine in the treatment of type 2 diabetes mellitus: a systemic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine . 2012;2012:12. doi: 10.1155/2012/591654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lan J., Zhao Y., Dong F., et al. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. Journal of Ethnopharmacology . 2015;161:69–81. doi: 10.1016/j.jep.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 68.Asbaghi O., Ghanbari N., Shekari M., et al. The effect of berberine supplementation on obesity parameters, inflammation and liver function enzymes: a systematic review and meta-analysis of randomized controlled trials. Clinical Nutrition ESPEN . 2020;38:43–49. doi: 10.1016/j.clnesp.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Memon M. A., Khan R. N., Riaz S., Ain Q. U., Ahmed M., Kumar N. Methylglyoxal and insulin resistance in berberine-treated type 2 diabetic patients. Journal of Research in Medical Sciences : The Official Journal of Isfahan University of Medical Sciences . 2018;23 doi: 10.4103/jrms.JRMS_1078_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cicero A. F. G., Fogacci F., Stoian A. P., et al. Nutraceuticals in the management of dyslipidemia: which, when, and for whom? Could nutraceuticals help low-risk individuals with non-optimal lipid levels? Current Atherosclerosis Reports . 2021;23(10) doi: 10.1007/s11883-021-00955-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fogacci F., Grassi D., Rizzo M., Cicero A. F. G. Metabolic effect of berberine–silymarin association: a meta-analysis of randomized, double-blind, placebo-controlled clinical trials. Phytotherapy Research . 2019;33(4):862–870. doi: 10.1002/ptr.6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu Y. S., Li Z. M., Chen Y. T., et al. Berberine improves inflammatory responses of diabetes mellitus in Zucker diabetic fatty rats and insulin-resistant HepG2 cells through the PPM1B pathway. Journal of Immunology Research . 2020;2020:32. doi: 10.1155/2020/2141508.2141508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beba M., Djafarian K., Shab-Bidar S. Effect of berberine on C-reactive protein: a systematic review and meta- analysis of randomized controlled trials. Complementary Therapies in Medicine . 2019;46:81–86. doi: 10.1016/j.ctim.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Xu Y., Yu T., Ma G., et al. Berberine modulates deacetylation of PPARγ to promote adipose tissue remodeling and thermogenesis via AMPK/SIRT1 pathway. International Journal of Biological Sciences . 2021;17(12):3173–3187. doi: 10.7150/ijbs.62556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shan Y., Zhang S., Gao B., et al. Adipose tissue SIRT1 regulates insulin sensitizing and anti-inflammatory effects of berberine. Frontiers in Pharmacology . 2020;11, article 591227 doi: 10.3389/fphar.2020.591227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee Y. S., Kim W. S., Kim K. H., et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes (New York, N.Y.) . 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 77.Reddi K. K., Li H., Li W., Tetali S. D. Berberine, a phytoalkaloid, inhibits inflammatory response induced by LPS through NF-kappabeta pathway: possible involvement of the IKKalpha. MOLECULES . 2021;26(16) doi: 10.3390/molecules26164733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pei C., Zhang Y., Wang P., et al. Berberine alleviates oxidized low-density lipoprotein-induced macrophage activation by downregulating galectin-3 via the NF-κB and AMPK signaling pathways. Phytotherapy Research . 2019;33(2):294–308. doi: 10.1002/ptr.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou Y., Cao S., Wang Y., et al. Berberine metabolites could induce low density lipoprotein receptor up- regulation to exert lipid-lowering effects in human hepatoma cells. Fitoterapia . 2014;92:230–237. doi: 10.1016/j.fitote.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 80.Feng X., Sureda A., Jafari S., et al. Berberine in cardiovascular and metabolic diseases: from mechanisms to therapeutics. THERANOSTICS. . 2019;9(7):1923–1951. doi: 10.7150/thno.30787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu J., Zong G. N., Wu H., Zhang K. Q. Podoplanin mediates the renoprotective effect of berberine on diabetic kidney disease in mice. Acta Pharmacologica Sinica . 2019;40(12):1544–1554. doi: 10.1038/s41401-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ilyas Z., Perna S., Al-thawadi S., et al. The effect of berberine on weight loss in order to prevent obesity: a systematic review. Biomedicine & Pharmacotherapy . 2020;127, article 110137 doi: 10.1016/j.biopha.2020.110137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Files 1 shows the full electronic search strategy for PubMed according to the search history.
Supplementary Files 2 shows results of subgroup analyses for main outcomes including HbA1c, FPG, and 2hPG.
Supplementary Files 3 shows GRADE criteria to determine the certainty in the estimate of effect for primary outcomes including HbA1c, FPG, and 2hPG.
Data Availability Statement
The original contributions presented in the study are included in the article material; further inquiries can be directed to the corresponding authors.


