Key Points
Question
Is endotracheal aspirate culture (EAC) use variable across the US, and is EAC use associated with antibiotic use in children receiving mechanical ventilation?
Findings
In this cross-sectional study of 31 pediatric hospitals and 152 132 patients in the US, EAC use varied widely between hospitals, and higher rates were correlated with increased antibiotic use. Patients who underwent an EAC culture were significantly more likely to receive antibiotics after adjustment for patient demographic characteristics, patient complexity, complex conditions, duration, and episodes of mechanical ventilation.
Meaning
In this study, EACs are associated with increased antibiotic prescribing in hospitalized mechanically ventilated children may represent an opportunity for diagnostic and antibiotic stewardship.
Abstract
Importance
Endotracheal aspirate cultures are commonly collected from patients with mechanical ventilation to evaluate for ventilator-associated pneumonia or tracheitis. However, the respiratory tract is not sterile, making differentiating between colonization from bacterial infection challenging, and results may be unreliable owing to variable specimen quality and sample processing across laboratories. Despite these limitations, clinicians routinely interpret bacterial growth in endotracheal aspirate cultures as evidence of infection, sometimes regardless of organism significance, prompting antibiotic treatment.
Objective
To assess the variability in endotracheal aspirate culture rates and the association between culture rates and antibiotic prescribing among patients with mechanical ventilation across children’s hospitals in the US.
Design, Setting, and Participants
Cross-sectional retrospective analysis of data obtained from the Children’s Hospital Association Pediatric Health Information System database between January 1, 2016, through December 31, 2019. Participants were all patients hospitalized with mechanical ventilation aged less than 18 years.
Exposures
A charge for an endotracheal aspirate culture on a ventilated day.
Main Outcomes and Measures
Endotracheal aspirate culture rate and antibiotic days of therapy per ventilated days. For mechanical ventilation, clinical transaction classification codes for mechanical ventilation other unspecified ventilator assistance were used. To identify respiratory cultures, the laboratory test code for aerobic culture was used and relevant keywords (ie, respiratory tract, sputum) were used to identify sources in the hospital charge description master.
Results
A total of 152 132 patients were identified among 31 hospitals. Among these patients, 79 691 endotracheal aspirate cultures were collected on a ventilator-day (patients aged less than 1 year, 44%; 1-4 years, 27%, 5-11 years. 16%, and 12-18 years, 13%; 3% were Asian; 17% Hispanic; 21% non-Hispanic Black; 45% Non-Hispanic White patients; 14% were other; 56% of patients were male, 44% were female). The overall median rate of culture use was 46 per 1000 ventilator-days (IQR, 32-73 cultures per 1000 ventilator-days). The endotracheal aspirate culture rate was positively correlated with the hospital’s antibiotic days of therapy rate (R = 0.46; P = .009). In a multivariable model adjusting for patient-level and hospital-level characteristics and among patients with mechanical ventilation, each additional endotracheal aspirate culture was associated with 2.87 (95% CI, 2.74-3.01) higher odds of receiving additional days of therapy compared with patients who did not receive and endotracheal aspirate culture.
Conclusions and Relevance
In this study, notable variability was found in endotracheal aspirate culture rates across US pediatric hospitals and pediatric intensive care units, and endotracheal aspirate culture use was associated with increased antibiotic use. These findings suggest an opportunity for diagnostic and antibiotic stewardship to standardize testing and treatment of suspected ventilator-associated infections in pediatric patients with mechanical ventilation pediatric patients.
This cross-sectional study characterizes the variability in the rate of endotracheal aspirate culture use and assesses the association between endotracheal aspirate culture use and antibiotic use across US pediatric hospitals.
Introduction
Ventilator-associated pneumonia (VAP) is the most frequently acquired infection in patients with mechanical ventilation and the second most common nosocomial infection in the intensive care unit (ICU).1 In addition to increased length of stay, VAP is associated with an extended duration of ventilation, increased risk of morbidity and mortality, and high costs.2,3 There is no standard definition for VAP, which makes the diagnosis challenging. Therefore, some clinicians may have a low threshold to obtain endotracheal aspirate cultures (EACs) to evaluate for suspected VAP or VAT.1,4,5
The results from EACs are relatively nonspecific for infection and may be unreliable.6,7,8,9,10,11,12,13,14,15,16 Sample collection and quality of specimens are variable, which may affect results.16,17 Endotracheal secretion samples are from a nonsterile site, making the differentiation between colonization and infection in the culture challenging. There is also a substantial amount of variability in the way EACs are processed and reported,18 which further complicates result interpretation and may be a factor in EAC unreliability. Clinicians may misinterpret reported growth of bacteria in EAC results as being an indicator of infection and then treat with antibiotics.1,19,20 Therefore, overuse of EACs may potentiate avoidable antibiotic treatment in patients with mechanical ventilation and contribute to avoidable harms such as Clostridioides difficile infections, antimicrobial resistance,21 and adverse drug reactions.22,23 Ultimately, EAC practices may vary widely within and across institutions. We aimed to characterize the variability in the rate of EAC use and assess the association between EAC use and antibiotic use across US pediatric hospitals.
Methods
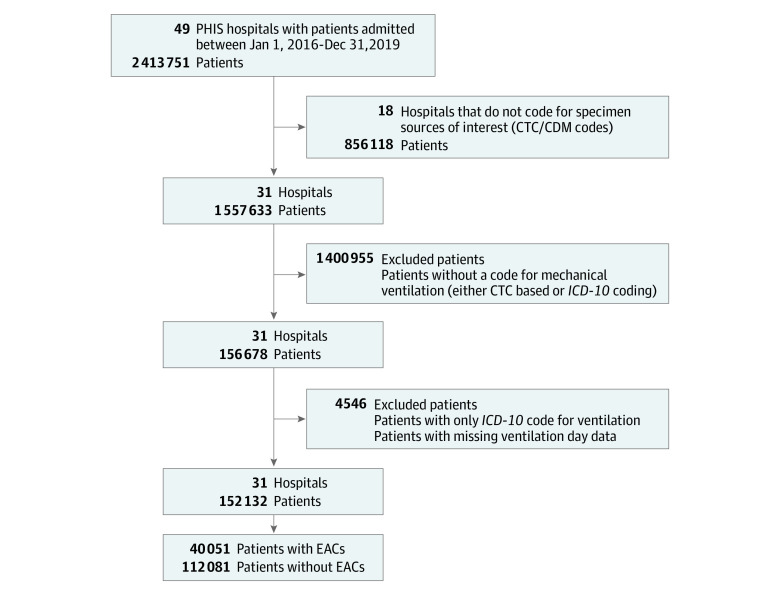
This study was a retrospective multicenter cross-sectional analysis of clinical and billing data from the Pediatric Health Information System (PHIS, Children’s Hospital Association, Overland Park, Kansas) from January 1, 2016, through December 31, 2019. PHIS is a national database that compiles administrative data including clinical and resource use from more than 49 children’s hospitals geographically dispersed across the US. Hospitals that did not continuously submit billing data to PHIS during the study period were excluded (n = 18). Data were obtained for all inpatient units and further stratified into ICU and neonatal ICU (NICU) groups for analysis. Of note, the ICU subgroup contained data from pediatric ICUs (PICUs) and pediatric cardiac ICUs because division of these units varies by hospital and is not easily distinguished in the PHIS database. This study was classified as exempt research (secondary research not requiring informed patient consent) per the University of Colorado institutional review board. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies.
Patient Population
This study included all patients less than age 18 years who were hospitalized at a participating PHIS hospital during the study period and had at least 1 day of mechanical ventilation. We identified patients with mechanical ventilation as those with daily Clinical Transaction Codes (CTCs) for mechanical ventilation (n = 152 132), which includes all types of invasive ventilation (ie, tracheostomy, endotracheal, and nasotracheal). The CTC code is associated with a day of service each time it is used and allows for the calculation of ventilator-days for each patient. Patients who only had an International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) procedure code for ventilation without mechanical ventilation CTC codes and patients with missing ventilation coding data were excluded (n = 4546) because the duration of ventilation could not be ascertained. To assess the accuracy of mechanical ventilation billing codes, we performed a validation using local Children’s Hospital Colorado data. This validation demonstrated that the use of ICD-10 codes for intubation and ventilation excluded data on patients with tracheostomy and made duration of ventilation undeterminable; they were therefore excluded.
Primary Outcomes
For this study, we defined the rate of EAC use as the total number of EACs billed for on a ventilated day per 1000 total ventilator-days. We identified EACs when the patient had a code for respiratory aerobic culture coinciding with a charge for ventilation on the same calendar day. Specimen source billing data were reviewed for consistency by a microbiologist (A.P.) and infectious disease physician (A.S.) and refereed by an additional infectious disease physician (S.K.P.). Because laboratory test descriptions and specimen source descriptions may be nonspecific (ie, aerobic culture, other specimen source), associated hospital charge description data were reviewed for details that specified the specimen source (eTable 1 in the Supplement). All respiratory tract, nasopharyngeal, sputum, other, and unspecified sources with charge descriptions that included descriptors such as bacterial culture and tracheal aspirate from a patient receiving mechanical ventilation were considered to be an EAC specimen. We excluded bronchoalveolar lavage specimens, nasopharyngeal specimens used for methicillin-resistant Staphylococcus aureus screening, viral respiratory testing, anaerobic culture, and any additional testing unrelated to standard aerobic bacterial culture such as Pneumocystis direct fluorescent antibody and fungal testing. Rates of use were calculated for all inpatient units, the ICU subgroup, and the NICU subgroup.
We defined the rate of antibiotic use as an antibiotic day of therapy (DOT) occurring on a ventilated day per 1000 ventilator-days. An antibiotic DOT was identified when the patient had a billing code for an antibiotic coinciding with a charge for ventilation on the same calendar day. We included a comprehensive list of antibiotics given via enteral or intravenous methods commonly prescribed for ventilator-associated infections (VAIs) (eTable 2 in the Supplement). We excluded rarely used antibiotics and inhaled antibiotics (eTable 3 in the Supplement). We further subanalyzed anti-Pseudomonal and anti-Staphylococcal antibiotics because Pseudomonal and Staphylococcal organisms are commonly recovered from EACs and are associated with chronic colonization.24,25 Additionally, we considered a subanalysis of antibiotics for highly drug-resistant organisms (eg, ceftazidime-avibactam); however, there was an insufficient volume of DOT. Rates of use were calculated for all inpatient units, the ICU subgroup, and the NICU subgroup.
Patient and Hospital-Level Characteristics
Characteristics associated with EAC use and antibiotic use were considered a priori and informed by previously reported associations conducted using the PHIS database.26 Variables collected for each patient for each hospital admission included age (<1 year, 1-4 years, 5-11 years, and 12-18 years), race and ethnicity (Asian, non-Hispanic Black, non-Hispanic White, other), sex, the presence of any chronic condition, the patient’s cumulative number of ventilator-days and their number of ventilator episodes during the admission. Variables characterizing the hospital included case-mix index27 and geographic location (Midwest, Northeast, South, West). Patient race and ethnicity data were collected at each individual hospital at the time of patient registration, and were not determined by the investigators.28
Statistical Analysis
Characteristics were summarized using frequencies and percentages for categorical variables, and their association with EAC or antibiotic use was first compared using χ2 tests. The Shapiro-Wilk test was used to evaluate the distribution of each variable, and Pearson (normal) and Spearman (nonnormal) correlation tests were used accordingly to assess the correlation between EAC use and antibiotic use. All correlation analyses were performed using R studio, version 1.4.1106 (R Studio). Covariates that were significantly associated with receiving an antibiotic on a ventilated day were entered into the final model for adjustment to accurately assess the association between EAC and antibiotic use in patients with mechanical ventilation patients.
Adjusted EAC use and antibiotic DOT rates per 1000 total ventilator-days were calculated using generalized linear mixed-effects models with Poisson distributions, controlling for hospital-level clustering and allowing for the presence of correlated data (within hospital), nonconstant variability (across hospitals), and responses that are not normally distributed. We used the same approach to assess DOT per 1000 total ventilator-day values for all patients, patients in the ICU, patients in the NICU across institutions, and the antibiotic groups (all antibiotics, anti-Pseudomonal, and anti-Staphylococcal). Covariates that had a significant univariate association with antibiotic use and determined a priori to be clinically relevant were included in the final model. Of note, the association between number of chronic conditions as well as individual chronic conditions and EAC use was assessed (eTable 4 in the Supplement) and had significant collinearity. Due to this collinearity, a binary variable of any complex chronic condition was instead included in the final model. Statistical significance for covariate selection and the main model was based on a 2-sided analysis and a P value less than .01 because of the large sample size. Analyses were performed using SAS software, version.9.4 (SAS Institute).
Results
EAC Use
Of the 51 PHIS hospitals, 31 hospitals met inclusion criteria, and 152 132 patients were admitted and received mechanical ventilation on a ventilated day during the study period (Figure 1). Among these patients, 79 691 EAC were collected on a ventilator day. Most patients in the study cohort were less than 1 year of age (44%) followed by 1-4 years (27%), 5-11 years (16%) and 12-18 years (13%). For race and ethnicity, 3% were Asian; 17% Hispanic; 21% non-Hispanic Black; 45% Non-Hispanic White patients; 14% were other. With respect to sex, 56% of patients were male, 44% were female. The Table describes demographic characteristics, culture, and antibiotic use for all included hospitals.
Figure 1. Flowchart of Identification of Patients With Mechanical Ventilation and Hospitalization.
CTC/CDM indicates Clinical Transaction Codes/cut-down method; EACs, endotracheal aspirate cultures; ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; PHIS, Pediatric Health Information System.
Overall, the unadjusted median rate of EAC use for all units and all hospitals was 46 per 1000 total ventilator-days (IQR, 32-73 cultures per 1000 total ventilator-days) (Figure 2). EAC use for the ICU group was 100 per 1000 total ventilator-days (IQR, 63-137 per 1000 total ventilator-days) and for the NICU group was 24 per 1000 total ventilator-days (IQR, 11-34 per 1000 total ventilator-days). Before adjustment, numerous variables were significantly associated with receiving an EAC test, including case mix index (OR, 1.03 [95% CI, 1.02-1.03]; P < .001), non-Hispanic Black race (OR, 1.16 [95% CI, 1.13-1.20]; P < .001), and presence of any chronic condition (OR, 2.13 [95% CI, 2.06-2.20]; P < .001) (Table).
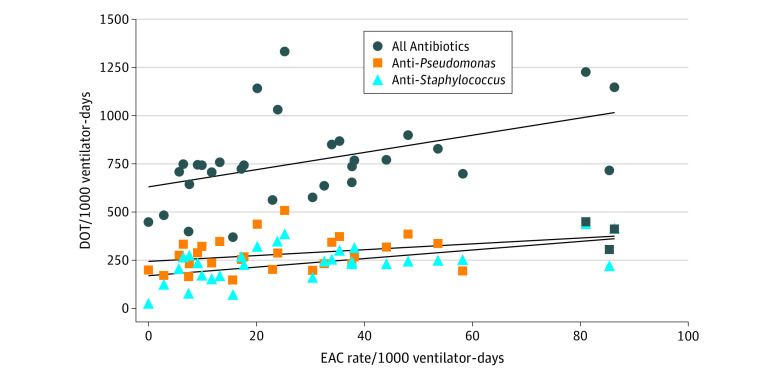
Figure 2. EAC Rate vs Days of Therapy, All Units (2016-2019).
DOT indicates day of therapy; EAC, endotracheal aspirate culture.
Table. Association of Patient and Hospital-Level Characteristics With Receiving an Endotracheal Aspirate Culture (EAC) and Antibiotic DOT.
| Variable | Patients with mechanical ventilation, No. (%) | Odds ratio (95% CI) of receiving EAC | Odds ratio (95% CI) of receiving antibiotics | |||
|---|---|---|---|---|---|---|
| Without EAC (n = 112 081) | With EAC (n = 40 051) | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Case-mix index, median (IQR) | 6.34 (2.91-14.36) | 8.05 (3.89-16.45) | 1.03 (1.02-1.03)a | 1.03 (1.02-1.03)a | 1.17 (1.17-1.17)a | 1.11 (1.11-1.12)a |
| Geographic location | ||||||
| Midwest | 37 039 (33) | 12 539 (31.3) | 0.67 (0.65-0.69)a | 0.83 (0.42-1.62) | 0.58 (0.56-0.60)a | 1.01 (0.73-1.38) |
| Northeast | 24 269 (21.7) | 5875 (14.7) | 0.48 (0.46-0.50)a | 0.42 (0.19-0.91) | 0.56 (0.54-0.59)a | 0.83 (0.57-1.20) |
| South | 33 492 (29.9) | 12 908 (32.2) | 0.76 (0.74-0.79)a | 0.81 (0.42-1.56) | 1.02 (0.98-1.07) | 1.11 (0.81-1.51) |
| West | 17 281 (15.4) | 8729 (21.8) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Age, y | ||||||
| <1 | 65 256 (58.2) | 17 450 (43.6) | 0.63 (0.61-0.65)a | 0.61 (0.59-0.64)a | 1.54 (1.48-1.60)a | 1.27 (1.21-1.33)a |
| 1-4 | 20 228 (18) | 10 761 (26.9) | 1.25 (1.20-1.30)a | 1.31 (1.25-1.36)a | 1.05 (1.01-1.10)a | 1.09 (1.04-1.14)a |
| 5-11 | 14 087 (12.6) | 6518 (16.3) | 1.09 (1.04-1.14)a | 1.05 (1.00-1.10) | 1.24 (1.18-1.30)a | 1.25 (1.18-1.32)a |
| 12-18 | 12 510 (11.2) | 5322 (13.3) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Sex | ||||||
| Male | 63 336 (56.5) | 22 414 (56) | 0.98 (0.96-1.00) | 1.01 (0.98-1.03) | 1.00 (0.97-1.02) | 1.01 (0.98-1.04) |
| Female | 48 745 (43.5) | 17 637 (44) | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| Race and ethnicity | ||||||
| Asian | 3789 (3.4) | 1167 (2.9) | 0.92 (0.86-0.99) | 1.04 (0.97-1.12) | 1.07 (0.99-1.15) | 0.95 (0.87-1.03) |
| Hispanic | 17 176 (15.3) | 6919 (17.3) | 1.21 (1.17-1.24)a | 1.08 (1.04-1.12)a | 1.34 (1.29-1.40)a | 0.98 (0.94-1.03) |
| Non-Hispanic Black | 21 688 (19.4) | 8446 (21.1) | 1.16 (1.13-1.20)a | 1.20 (1.16-1.24)a | 0.87 (0.84-0.90)a | 0.80 (0.77-0.83)a |
| Non-Hispanic White | 53 575 (47.8) | 17 910 (44.7) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Otherb | 15 853 (14.1) | 5609 (14) | 1.06 (1.02-1.10)a | 1.09 (1.05-1.14)a | 1.03 (0.99-1.07) | 0.98 (0.94-1.03) |
| Any CCC | 84 029 (75) | 34 626 (86.5) | 2.13 (2.06-2.20)a | 0.95 (0.91-0.99)a | 3.66 (3.56-3.76)a | 1.55 (1.50-1.60)a |
| Cumulative ventilator-days | ||||||
| 1-3 | 72 351 (64.6) | 7993 (20) | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| 4-14 | 31 176 (27.8) | 18 763 (46.8) | 5.45 (5.29-5.61)a | 2.60 (2.50-2.71)a | 3.80 (3.68-3.93)a | 1.88 (1.81-1.95)a |
| >14 | 8554 (7.6) | 13 295 (33.2) | 14.07 (13.58-14.58)a | 6.81 (6.40-7.24)a | 11.01 (10.25-11.83)a | 1.87 (1.72-2.03)a |
| No. of ventilation episodes | ||||||
| 1 | 95 594 (85.3) | 28 662 (71.6) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| ≥2 | 16 487 (14.7) | 11 389 (28.4) | 2.30 (2.24-2.37)a | 0.86 (0.83-0.90)a | 3.81 (3.63-3.99)a | 1.25 (1.18-1.32)a |
| Receiving EAC | ||||||
| No | NA | NA | NA | NA | 1 [Reference] | 1 [Reference] |
| Yes | NA | NA | NA | NA | 4.68 (4.49-4.88)a | 2.87 (2.74-3.01)a |
Abbreviations: CCC, complex chronic condition; EAC, endotracheal aspirate; NA, not applicable.
Statistical significance defined as P < .001.
Including American Indian, Alaskan Native, Native Hawaiian Pacific Islander, and other meaning all other race and ethnicity groups including those records that have multiple race groups indicated on their patient record.
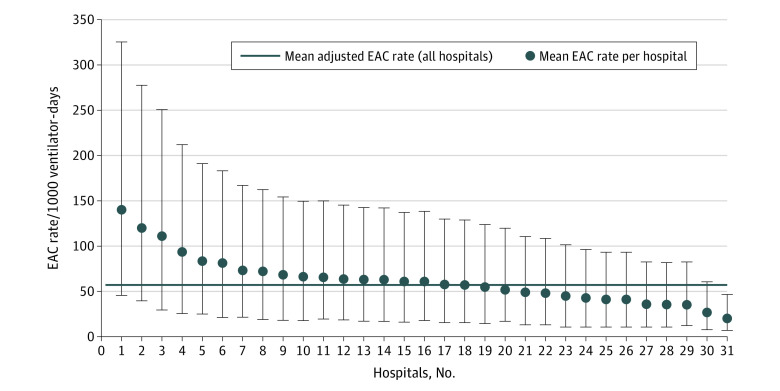
After adjustment, the median rate of EAC for all hospitals and units was 57 per 1000 total ventilator-days. The adjusted EAC rates for individual institutions ranged from 20 per 1000 total ventilator-days (95% CI, 13-26) to 119 per 1000 total ventilator-days (95% CI, 80-158) (Figure 3). Case-mix index was significantly associated with receiving an EAC (adjusted odds ratio [AOR], 1.03 [95% CI, 1.02-1.03]), and the odds of receiving EAC testing was highest in the 1–4-year age group (AOR, 1.31 [95% CI, 1.25-1.36]) (Table). The odds of EAC testing were significantly higher in non-Hispanic Black patients compared with non-Hispanic White patients (AOR, 1.08 [95% CI, 1.04-1.12]). The variables that were associated with EAC use in the final model included the presence of underlying chronic conditions (AOR, 0.95 [95% CI, 0.91-0.99]; P < .001), the number of cumulative ventilator-days a patient had (>14 days, AOR, 2.60 [95% CI, 2.50-2.71]; P < .001), and the total number of ventilation episodes (2 or more, AOR, 0.86 [95% CI, 0.83-0.90]; P < .001) (Table).
Figure 3. Adjusted EAC Rate per 1000 Ventilator-Days.
The whiskers represent 95% CIs. EAC indicates endotracheal aspirate culture.
Antibiotic Use
For all antibiotics, the unadjusted median DOT rate was 783 DOT per 1000 total ventilator-days across all units (IQR, 686-875 per 1000 total ventilator-days), 1181 DOT per 1000 total ventilator-days in the ICU (IQR, 1062-1387 per 1000 total ventilator-days), and 740 per 1000 total ventilator-days in the NICU (IQR, 648-838 per 1000 total ventilator-days). Before adjustment, numerous variables were associated with antibiotic use including age (<1 year, OR, 1.54 [95% CI, 1.48-1.60]; P < .001), race (non-Hispanic Black, OR, 0.87 [95% CI, 0.84-0.90]; P < .001) and cumulative ventilator days (4-14 days, OR, 3.80 [95% CI, 3.68-3.93]; P < .001) (Table).
Except for hospital geographic location, all patient and hospital characteristics that were significantly associated with receiving an antibiotic on a ventilator day were also significantly associated after adjustment. Non-Hispanic Black patients had significantly less odds of receiving antibiotics on a ventilated day than non-Hispanic White patients (AOR, 0.80 [95% CI, 0.77-0.83]). Additionally, the odds of receiving an antibiotic was 1.55 (95% CI, 1.50-1.60) times higher in patients with at least 1 chronic condition compared with patients without and odds of antibiotic use were higher in patients with more cumulative ventilator days (4-14 days, AOR 1.88 [95% CI, 1.81-1.95]).
Across all units, there was a positive correlation between EAC rate and DOT (R = 0.46; P = .009). In the ICU subgroup, the EAC rate was positively correlated with the DOT for all antibiotics (R = 0.48; P = .001) and anti-Staphylococcal drugs (R = 0.43; P = .015). However, in this ICU subgroup, the EAC rate was not correlated with anti-Pseudomonal antibiotics (R = 0.28; P = .12). In the NICU subgroup, the EAC rate had a positive correlation with antibiotic DOT for all antibiotics (R = 0.5; P = .005), anti-Pseudomonal antibiotics (R = 0.39; P = .03), and anti-Staphylococcal antibiotics (R = 0.51, P = .004).
After accounting for significant patient and hospital characteristics in the final model, patients who received EAC testing on a ventilated day had 3 times the odds of receiving an antibiotic than patients with mechanical ventilation who did not receive EAC testing (AOR, 2.87 [95% CI, 2.74-3.01]). Patients with mechanical ventilation with EACs were also more likely to receive anti-Pseudomonal antibiotics (AOR, 2.01 [95% CI, 1.95-2.07]) and anti-Staphylococcal antibiotics (AOR, 2.04, [95% CI, 1.97-2.10]) compared with patients with mechanical ventilation without EACs.
Discussion
In this observational study of EAC use in 31 pediatric hospitals from 2016 to 2019, we found variability in the rate of EAC use. Some patient-level characteristics were associated with higher EAC use such as age, presence of a complex chronic condition, cumulative ventilator-days, and the number of ventilation episodes, which aligns with clinical expectations. Some of the associations were not anticipated, such as non-Hispanic Black patients having a higher rate of EAC use but lower antibiotic use than White patients. More investigation is warranted for a better understanding of these observed differences in testing and treatment by race, which has been seen in other diagnostic evaluations.29 After adjustment for patient and hospital characteristics, there was still a 10-fold difference in the highest and lowest hospital-level rate of EAC use, suggesting that differences in patient populations do not fully explain variability in testing frequency.
When examining the association between EAC and antibiotic usage, we found a moderate positive correlation. The multivariable modeling also showed increased odds of antimicrobial use in patients with mechanical ventilation who received more EAC testing. Overall, the antibiotic rates in this population with mechanical ventilation and associated variables align with prior studies26,30 in pediatric populations. We aimed to explore whether trends could be found when focusing on anti-Pseudomonal or anti-Staphylococcal antibiotics because these organisms can cause chronic colonization and biofilm formation of airway devices.31 A strong correlation was not found for anti-Pseudomonal agents except in the NICU subgroup. Although this study included antibiotics prescribed only on ventilated days and medications commonly used to treat VAIs, billing data cannot provide the level of granularity to determine specific treatment indications. Particularly in the PICU setting, anti-Pseudomonal agents such as cefepime are commonly used for other infectious processes, so a portion of the captured antibiotic treatment may possibly be unrelated to VAI management.
Overall, one may expect that culture use is associated with antibiotic prescribing rates because clinicians will treat for suspected infections. The observational ecologic (ie, population-level) design of this study cannot determine directionality or causality between EAC testing frequency and antibiotic usage. Specifically, we did not have the data to independently adjudicate appropriateness of individual EACs or specific antibiotic treatment indications. However, prior studies suggest that EAC testing may be associated with additional antibiotic treatment.6,21,32,33,34,35 For example, in the first of two ventilator-associated infection studies (VAIN),36 a multicenter study across 22 PICUs, antibiotic treatment was associated with the patient having had a positive EAC, and in the second VAIN study, implementing guidelines to de-escalate antibiotic treatment if the patient did not exhibit signs of respiratory infection was only successful in 27% of cases, largely owing to clinicians continuing treatment for positive EACs.37 Furthermore, recent reports from pediatric hospitals implementing clinical decision support for EAC testing were associated with significant reductions in EAC use and antibiotic prescribing for indication of VAIs.38,39 Therefore, we believe that in the present study, at least a portion of the EAC testing among hospitals with higher EAC use may be associated with higher antibiotic usage.
A complicating feature of EACs is that the respiratory secretions are sampling a nonsterile body site; therefore EACs may be limited in their ability to differentiate between bacteria colonizing the airway from those acting as pathogens.16,19,40,41,42 Multiple studies have shown no association between bacterial growth or inflammation with clinical findings of respiratory infection across pediatric and adult populations.16,19,41,43 Various factors unrelated to the culture may affect the likelihood of infection such as inflammation, clinical status of the patient, and environmental exposures.25,44 Recognizing that VAI may have substantial morbidity if untreated, reporting of bacteria in EAC results presents a challenge for the clinician and may encourage antibiotic use regardless of the clinical relevance of the organism.24 The Infectious Disease Society of America recognized that EACs were less specific for infection and associated with more antibiotic use compared with bronchoalveolar lavage (BAL) specimens, but because of less patient risk and cost, the VAP guidelines recommended EACs over BAL cultures.45 Compounding these known limitations of EACs, variability occurs in sample quality, processing and reporting of EACs across US microbiology laboratories.18 Diagnostic stewardship interventions and formal guidelines for collecting, processing, and reporting for EACs are needed to support accurate interpretation and antibiotic treatment decisions for suspected VAI.
Clinician variability in pediatric VAI management practices has been described5,46 and the present study further confirms interhospital variability in EAC testing practices. These descriptions are not isolated to pediatric care, lack of standardization, potential harms of EAC overuse, and diagnostic stewardship interventions have been proposed for adult patients with mechanical ventilation.47,48,49 Additional study is warranted to examine the association between EAC testing patterns and antibiotic use across the ages spectrum, emergency and ambulatory care settings, while also considering clinician and institutional factors.
Limitations
This study has limitations. A primary limitation of this study is the use of billing data, which can lead to misclassification. The use of ventilator-days to identify patients did not capture patients or EACs from tracheostomies if the patient was not ventilated. If an EAC was charged under a different billing code, it could have been omitted or some EACs may have been mislabeled and in fact been a different specimen. To optimize data validity, we excluded hospitals with inconsistent data reporting. Additionally, to ensure patients with mechanical ventilation were correctly identified, we back-validated PHIS data with Children’s Hospital Colorado patient data. To reduce the risk of misclassification of specimen type, we reviewed details of hospital charge descriptions. Although we captured important hospital and patient-level characteristics, billing data cannot reflect all the potentially relevant patient clinical condition details or local environment such as illness severity, indications for testing and treatment, the role of antibiotic stewardship programs, or clinician experience that may be associated with EAC and antibiotic usage. In particular, we could not distinguish the type of artificial airway (tracheostomy vs endotracheal tube).
Conclusions
The results from this cross-sectional study found notable variability in EAC rates of use across pediatric hospitals, and EAC use was associated with higher odds of antibiotic use. Given the diagnostic limitations of EACs, these findings suggest that there may be opportunity for diagnostic and antibiotics stewardship to standardize EAC testing and treatment among children with mechanical ventilation.
eTable 1. Billing Codes
eTable 2. Included Antibiotics
eTable 3. Excluded Antibiotics
eTable 4. Association Between Complex Chronic Conditions and Receiving EAC Testing
References
- 1.Venkatachalam V, Hendley JO, Willson DF. The diagnostic dilemma of ventilator-associated pneumonia in critically ill children. Pediatr Crit Care Med. 2011;12(3):286-296. doi: 10.1097/PCC.0b013e3181fe2ffb [DOI] [PubMed] [Google Scholar]
- 2.Rello J, Ollendorf DA, Oster G, et al. ; VAP Outcomes Scientific Advisory Group . Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115-2121. doi: 10.1378/chest.122.6.2115 [DOI] [PubMed] [Google Scholar]
- 3.Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C; The Canadian Critical Trials Group . The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1249-1256. doi: 10.1164/ajrccm.159.4.9807050 [DOI] [PubMed] [Google Scholar]
- 4.Sick-Samuels AC, Fackler JC, Berenholtz SM, Milstone AM. Understanding reasons clinicians obtained endotracheal aspirate cultures and impact on patient management to inform diagnostic stewardship initiatives. Infect Control Hosp Epidemiol. 2020;41(2):240-242. doi: 10.1017/ice.2019.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willson DF, Kirby A, Kicker JS. Respiratory secretion analyses in the evaluation of ventilator-associated pneumonia: a survey of current practice in pediatric critical care. Pediatr Crit Care Med. 2014;15(8):715-719. doi: 10.1097/PCC.0000000000000213 [DOI] [PubMed] [Google Scholar]
- 6.Claassen CC, Keenan WJ. Challenging the “culture” of the tracheal aspirate. Neoreviews. 2019;20(3):e145-e151. doi: 10.1542/neo.20-3-e145 [DOI] [PubMed] [Google Scholar]
- 7.Booth GR, Al-Hosni M, Ali A, Keenan WJ. The utility of tracheal aspirate cultures in the immediate neonatal period. J Perinatol. 2009;29(7):493-496. doi: 10.1038/jp.2009.33 [DOI] [PubMed] [Google Scholar]
- 8.Brook I, Martin WJ, Finegold SM. Bacteriology of tracheal aspirates in intubated newborn. Chest. 1980;78(6):875-877. doi: 10.1378/chest.78.6.875 [DOI] [PubMed] [Google Scholar]
- 9.Campbell S, Forbes BA. The clinical microbiology laboratory in the diagnosis of lower respiratory tract infections. J Clin Microbiol. 2011;49(9 Supplement):S30-S33. doi: 10.1128/JCM.00789-11 [DOI] [Google Scholar]
- 10.Cline JM, Woods CR, Ervin SE, Rubin BK, Kirse DJ. Surveillance tracheal aspirate cultures do not reliably predict bacteria cultured at the time of an acute respiratory infection in children with tracheostomy tubes. Chest. 2012;141(3):625-631. doi: 10.1378/chest.10-2539 [DOI] [PubMed] [Google Scholar]
- 11.Dray S, Coiffard B, Persico N, Papazian L, Hraiech S. Are tracheal surveillance cultures useful in the intensive care unit? Ann Transl Med. 2018;6(21):421. doi: 10.21037/atm.2018.08.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau YL, Hey E. Sensitivity and specificity of daily tracheal aspirate cultures in predicting organisms causing bacteremia in ventilated neonates. Pediatr Infect Dis J. 1991;10(4):290-294. doi: 10.1097/00006454-199104000-00005 [DOI] [PubMed] [Google Scholar]
- 13.Seligman R, Seligman BG, Konkewicz L, Dos Santos RP. Accuracy of tracheal aspirate gram stain in predicting Staphylococcus aureus infection in ventilator-associated pneumonia. BMC Anesthesiol. 2015;15(1):19. doi: 10.1186/1471-2253-15-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tetenta S, Metersky ML. Tracheal aspirate Gram stain has limited sensitivity and specificity for detecting Staphylococcus aureus. Respirology. 2011;16(1):86-89. doi: 10.1111/j.1440-1843.2010.01855.x [DOI] [PubMed] [Google Scholar]
- 15.Thureen PJ, Moreland S, Rodden DJ, Merenstein GB, Levin M, Rosenberg AA. Failure of tracheal aspirate cultures to define the cause of respiratory deteriorations in neonates. Pediatr Infect Dis J. 1993;12(7):560-564. doi: 10.1097/00006454-199307000-00002 [DOI] [PubMed] [Google Scholar]
- 16.Willson DF, Conaway M, Kelly R, Hendley JO. The lack of specificity of tracheal aspirates in the diagnosis of pulmonary infection in intubated children. Pediatr Crit Care Med. 2014;15(4):299-305. doi: 10.1097/PCC.0000000000000106 [DOI] [PubMed] [Google Scholar]
- 17.Morris AJ, Tanner DC, Reller LB. Rejection criteria for endotracheal aspirates from adults. J Clin Microbiol. 1993;31(5):1027-1029. doi: 10.1128/jcm.31.5.1027-1029.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prinzi AM, Parker SK, Curtis DJ, Ziniel SI. The Pediatric Endotracheal Aspirate Culture Survey (PETACS): examining practice variation across pediatric microbiology laboratories in the United States. J Clin Microbiol. 2021;59(3):e02232-20. doi: 10.1128/JCM.02232-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yalamanchi S, Saiman L, Zachariah P. Decision-making around positive tracheal aspirate cultures: the role of neutrophil semiquantification in antibiotic prescribing. Pediatr Crit Care Med. 2019;20(8):e380-e385. doi: 10.1097/PCC.0000000000002014 [DOI] [PubMed] [Google Scholar]
- 20.Foglia E, Meier MD, Elward A. Ventilator-associated pneumonia in neonatal and pediatric intensive care unit patients. Clin Microbiol Rev. 2007;20(3):409-425. doi: 10.1128/CMR.00041-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit: a proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162(2 Pt 1):505-511. doi: 10.1164/ajrccm.162.2.9909095 [DOI] [PubMed] [Google Scholar]
- 22.Chiotos K, Gerber JS, Himebauch AS. How can we optimize antibiotic use in the PICU? Pediatr Crit Care Med. 2017;18(9):903-904. doi: 10.1097/PCC.0000000000001261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer JE, Ramser M, Fanconi S. Use of antibiotics in pediatric intensive care and potential savings. Intensive Care Med. 2000;26(7):959-966. doi: 10.1007/s001340051288 [DOI] [PubMed] [Google Scholar]
- 24.Harlid R, Andersson G, Frostell CG, Jörbeck HJ, Ortqvist AB. Respiratory tract colonization and infection in patients with chronic tracheostomy: a one-year study in patients living at home. Am J Respir Crit Care Med. 1996;154(1):124-129. doi: 10.1164/ajrccm.154.1.8680667 [DOI] [PubMed] [Google Scholar]
- 25.Siegel SJ, Weiser JN. Mechanisms of bacterial colonization of the respiratory tract. Annu Rev Microbiol. 2015;69:425-444. doi: 10.1146/annurev-micro-091014-104209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brogan TV, Thurm C, Hersh AL, et al. Variability in Antibiotic Use Across PICUs. Pediatr Crit Care Med. 2018;19(6):519-527. doi: 10.1097/PCC.0000000000001535 [DOI] [PubMed] [Google Scholar]
- 27.Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of Hospitalization Resource Intensity Scores for Kids (H-RISK) and Comparison across Pediatric Populations. J Hosp Med. 2018;13(9):602-608. doi: 10.12788/jhm.2948 [DOI] [PubMed] [Google Scholar]
- 28.Sills MR, Hall M, Colvin JD, et al. Association of Social Determinants With Children’s Hospitals’ Preventable Readmissions Performance. JAMA Pediatr. 2016;170(4):350-358. doi: 10.1001/jamapediatrics.2015.4440 [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care . Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. In: Smedley BD, Stith AY, Nelson AR, eds. National Academies Press; 2003. [PubMed] [Google Scholar]
- 30.Gerber JS, Newland JG, Coffin SE, et al. Variability in antibiotic use at children’s hospitals. Pediatrics. 2010;126(6):1067-1073. doi: 10.1542/peds.2010-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotterbeekx A, Xavier BB, Bielen K, et al. The endotracheal tube microbiome associated with Pseudomonas aeruginosa or Staphylococcus epidermidis. Sci Rep. 2016;6:36507-36507. doi: 10.1038/srep36507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilde AM, Nailor MD, Nicolau DP, Kuti JL. Inappropriate antibiotic use due to decreased compliance with a ventilator-associated pneumonia computerized clinical pathway: implications for continuing education and prospective feedback. Pharmacotherapy. 2012;32(8):755-763. doi: 10.1002/j.1875-9114.2012.01161.x [DOI] [PubMed] [Google Scholar]
- 33.Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi: 10.1007/s00134-007-0619-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamma PD, Turnbull AE, Milstone AM, Lehmann CU, Sydnor ER, Cosgrove SE. Ventilator-associated tracheitis in children: does antibiotic duration matter? Clin Infect Dis. 2011;52(11):1324-1331. doi: 10.1093/cid/cir203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Combes A, Luyt CE, Trouillet JL, Chastre J. Controversies in ventilator-associated pneumonia. Semin Respir Crit Care Med. 2010;31(1):47-54. doi: 10.1055/s-0029-1246288 [DOI] [PubMed] [Google Scholar]
- 36.Willson DF, Hoot M, Khemani R, et al. ; Ventilator-Associated INfection (VAIN) Investigators and the Pediatric Acute Lung Injury and Sepsis Investigator’s (PALISI) Network . Pediatric ventilator-associated infections: the Ventilator-Associated INfection Study. Pediatr Crit Care Med. 2017;18(1):e24-e34. doi: 10.1097/PCC.0000000000001001 [DOI] [PubMed] [Google Scholar]
- 37.Karsies T, Tarquinio K, Shein SL, et al. ; Pediatric Acute Lung Injury and Sepsis Investigator’s (PALISI) Network . Compliance with an antibiotic guideline for suspected ventilator-associated infection: the Ventilator-Associated INfection (VAIN2) Study. Pediatr Crit Care Med. 2021;22(10):859-869. doi: 10.1097/PCC.0000000000002761 [DOI] [PubMed] [Google Scholar]
- 38.Sick-Samuels AC, Linz M, Bergmann J, et al. Diagnostic stewardship of endotracheal aspirate cultures in a PICU. Pediatrics. 2021;147(5):e20201634. doi: 10.1542/peds.2020-1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ormsby J, Conrad P. Reducing unnecessary pediatric tracheal aspirate cultures using a quality improvement approach. Am J Infect Control. Published online May 27, 2019. doi: 10.1016/j.ajic.2019.04.095 [DOI] [Google Scholar]
- 40.Hill JD, Ratliff JL, Parrott JC, et al. Pulmonary pathology in acute respiratory insufficiency: lung biopsy as a diagnostic tool. J Thorac Cardiovasc Surg. 1976;71(1):64-71. doi: 10.1016/S0022-5223(19)40261-4 [DOI] [PubMed] [Google Scholar]
- 41.Durairaj L, Mohamad Z, Launspach JL, et al. Patterns and density of early tracheal colonization in intensive care unit patients. J Crit Care. 2009;24(1):114-121. doi: 10.1016/j.jcrc.2008.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klompas M. Does this patient have ventilator-associated pneumonia? JAMA. 2007;297(14):1583-1593. doi: 10.1001/jama.297.14.1583 [DOI] [PubMed] [Google Scholar]
- 43.Langston SJ, Pithia N, Sim MS, Garg M, de St Maurice A, Chu A. Lack of utility of tracheal aspirates in the management of suspected pneumonia in intubated neonates. Infect Control Hosp Epidemiol. 2020;41(6):660-665. doi: 10.1017/ice.2020.57 [DOI] [PubMed] [Google Scholar]
- 44.Casadevall A, Pirofski LA. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun. 1999;67(8):3703-3713. doi: 10.1128/IAI.67.8.3703-3713.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111. doi: 10.1093/cid/ciw353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Booth LD, Sick-Samuels AC, Milstone AM, Fackler JC, Gnazzo LK, Stockwell DC. Culture ordering for patients with new-onset fever: a survey of pediatric intensive care unit clinician practices. Pediatr Qual Saf. 2021;6(5):e463. doi: 10.1097/pq9.0000000000000463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kenaa B, O’Hara LM, Richert ME, et al. A qualitative assessment of the diagnosis and management of ventilator-associated pneumonia among critical care clinicians exploring opportunities for diagnostic stewardship. Infect Control Hosp Epidemiol. 2021;1-7. doi: 10.1017/ice.2021.130 [DOI] [PubMed] [Google Scholar]
- 48.Kenaa B, Richert ME, Claeys KC, et al. Ventilator-associated pneumonia: diagnostic test stewardship and relevance of culturing practices. Curr Infect Dis Rep. 2019;21(12):50. doi: 10.1007/s11908-019-0708-3 [DOI] [PubMed] [Google Scholar]
- 49.Albin OR, Pogue JM, Petty LA, Kaye KS. Asymptomatic bacterisputia: rethinking diagnostic stewardship in pneumonia. Infect Control Hosp Epidemiol. 2021;42(6):737-739. doi: 10.1017/ice.2021.109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Billing Codes
eTable 2. Included Antibiotics
eTable 3. Excluded Antibiotics
eTable 4. Association Between Complex Chronic Conditions and Receiving EAC Testing