Abstract
Male mice lacking both the Ink4c and Ink4d genes, which encode two inhibitors of D-type cyclin-dependent kinases (Cdks), are infertile, whereas female fecundity is unaffected. Both p18Ink4c and p19Ink4d are expressed in the seminiferous tubules of postnatal wild-type mice, being largely confined to postmitotic spermatocytes undergoing meiosis. Their combined loss is associated with the delayed exit of spermatogonia from the mitotic cell cycle, leading to the retarded appearance of meiotic cells that do not properly differentiate and instead undergo apoptosis at an increased frequency. As a result, mice lacking both Ink4c and Ink4d produce few mature sperm, and the residual spermatozoa have reduced motility and decreased viability. Whether or not Ink4d is present, animals lacking Ink4c develop hyperplasia of interstitial testicular Leydig cells, which produce reduced levels of testosterone. The anterior pituitary of fertile mice lacking Ink4c or infertile mice doubly deficient for Ink4c and Ink4d produces normal levels of luteinizing hormone (LH). Therefore, the failure of Leydig cells to produce testosterone is not secondary to defects in LH production, and reduced testosterone levels do not account for infertility in the doubly deficient strain. By contrast, Ink4d-null or double-null mice produce elevated levels of follicle-stimulating hormone (FSH). Because Ink4d-null mice are fertile, increased FSH production by the anterior pituitary is also unlikely to contribute to the sterility observed in Ink4c/Ink4d double-null males. Our data indicate that p18Ink4c and p19Ink4d are essential for male fertility. These two Cdk inhibitors collaborate in regulating spermatogenesis, helping to ensure mitotic exit and the normal meiotic maturation of spermatocytes.
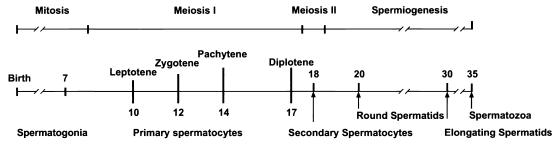
Spermatogenesis in mammals is characterized by a well-defined sequence of mitotic and meiotic divisions that lead to the production of mature spermatozoa (27). In newborn mice, male germ cell precursors undergo self-renewal in the testis between days 1 and 7 postpartum (pp) (Fig. 1). From day 7 pp onward, inception of spermatogenesis begins synchronously in a cohort of precursors, starting with at least two mitotic divisions followed by one round of meiosis. The early cell divisions lead to the development of type A and type B spermatogonia, the latter of which undergo premeiotic replication and enter meiosis as primary spermatocytes. Meiosis I is characterized by a prolonged prophase that allows chromatid exchange through crossing over. Segregation of homologous chromosomes occurs at the end of meiosis I, and resulting secondary spermatocytes then proceed through a second meiotic division in which haploid germ cells are generated. These differentiate to form round spermatids and, eventually, mature elongated spermatozoa (spermiogenesis). The first round of spermatogenesis is followed by additional waves, enabling continuous sperm production throughout the life of the animals.
FIG. 1.
Idealized timing of the first wave of spermatogenesis. The time line from birth onward indicates the temporal sequence of events in the first 35 days pp (27). Intervals in which mitotic cell division, meiosis I, meiosis II, and spermiogenesis occur are indicated above the time line, noting different stages during prophase of meiosis I. Spermatogonia populate the seminiferous tubules after birth, giving rise to spermatocytes, spermatids, and spermatozoa, as indicated below the time line.
Spermatogenesis is regulated hormonally through the pituitary-gonadal axis. The anterior lobe of the pituitary gland produces the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH). In males, FSH stimulates Sertoli cells, whose number determines the thickness of the seminiferous epithelium and, in turn, the size of the testis (36). LH induces interstitial Leydig cells to produce testosterone, a gonadal steroid necessary for spermatogenesis (19).
Cyclin-dependent kinases (Cdks) likely govern both the mitotic and meiotic divisions that characterize spermatogenesis, but it remains unclear which classes of enzymes are required for the various processes. Using immunohistochemical methods, cyclins D2 and D3 and their catalytic partner Cdk4 were seen to be expressed at the periphery of the seminiferous tubules between days 1 and 13 pp in spermatogonia undergoing mitosis (7, 22, 28, 33, 34, 46). By contrast, little cyclin D2 and Cdk4 expression was observed later in differentiated spermatocytes and spermatids (7, 28), although cyclin D3 expression was maintained (33, 46). cyclin D2-null male mice are fertile but have reduced testicular size and low sperm counts (39), whereas Cdk4-null mice are for the most part sterile at birth or become so at an early age (32, 42). Atrophic seminiferous tubules observed within the testes of Cdk4-null mice showed reduced numbers of spermatogonia and spermatocytes, suggesting that their proliferative capacity was reduced. Cdk6, the other known catalytic partner of D-type cyclins, was expressed at lower levels in testes of Cdk4-null animals (42). These findings together suggest that cyclins D2 and D3 in combination with Cdk4, and possibly Cdk6, may regulate G1 progression in spermatogonia in the postnatal testis and that cyclin D3 may play an additional role later in germ cell development.
The cyclin D-dependent kinases are negatively regulated by small polypeptide Cdk inhibitors encoded by four distinct Ink4 genes (38). Two of the Ink4 gene products, p16Ink4a and p15Ink4b, are not detectably expressed during mouse fetal development and are first observed in tissues of young adult animals (48). Disruption of either Ink4a or Ink4b leads to no developmental defects, and the young animals are healthy and fertile (25, 35). By contrast, the other Ink4 family members, p18Ink4c and p19Ink4d, are expressed during mouse embryogenesis and into adult life, particularly in the central nervous system and testis (48–50). Ink4c-null male mice are fertile and manifest no apparent testicular abnormalities, but they exhibit organomegaly and eventually develop midlobe pituitary tumors and hyperplasia of Leydig cells (16, 17, 25). Mice expressing a Cdk4 knock-in allele encoding a mutant form of the kinase (R24C) that cannot be inhibited by Ink4 proteins are fertile and also manifest Leydig cell hyperplasia (32). Ink4d-null mice are fertile and enjoy a normal life span, but they do not manifest Leydig cell abnormalities and instead display marked testicular atrophy associated with increased apoptosis in seminiferous tubules and reduced sperm counts (50). These findings suggest that expression of unrestrained cyclin D-dependent kinases can not only increase Leydig cell proliferation but can also have additional effects on male germ cell development, whether their expression is cell autonomous or not.
To address possible redundant roles for p18Ink4c and p19Ink4d in testicular development, we bred mice lacking Ink4c (25) with Ink4d-null mice (50) to derive animals lacking both genes. We show that deletion of both Ink4c and Ink4d (referred to here as Ink4cd double-null mice) results in complete infertility in males but has no effect on female reproductive function. Our data suggest that inappropriate regulation of cyclin D-dependent kinases in male germ cell progenitor cells inhibits them from undergoing meiosis.
MATERIALS AND METHODS
Generation of mouse strains and mouse embryo fibroblasts.
Mouse strains (C57BL/6 × 129Svj) deficient in Ink4c (25) or Ink4d (50) were intercrossed. Ink4d-deficient females were bred with Ink4c-null males to yield compound heterozygotes. Interbreeding generated wild-type and single- and double-null animals at the expected Mendelian frequencies. Genotyping was performed by Southern blotting with mouse tail DNA from weanling pups, as previously described (25, 50). Primary mouse embryonic fibroblasts (MEFs) were isolated from 13.5- to 14.5-day-old embryos as described previously and were propagated on a 3T9 schedule (48).
Sperm counts.
Two-cauda epididymides from 10- to 14-week-old male mice were harvested at the same time of day (between 12:00 and 2:00 p.m.). The sperm-containing fluid was squeezed out of the cauda, which was later cut into pieces. The sperm fluid and the pieces of cauda were suspended in 1 ml of Dulbecco's modified Eagle medium containing 25 mM HEPES buffer (pH 7.5) and 4 mg of bovine serum albumin per ml and were incubated at 37°C for 20 min. Suspensions of spermatozoa (20 μl) were fixed in 480 μl of 10% formalin. We used a hematocytometer to determine the number of spermatozoa.
Histologic and immunohistochemical methods.
Testes were placed in Bouin's fixative (Electron Microscopy Sciences, Fort Washington, Pa.) for a period between 6 h and overnight, depending on the size of the testis. After fixation, the testes were stored in 70% ethanol at 4°C until processed. Tissues were dehydrated in increasing concentrations of ethanol, embedded in paraffin wax, and sectioned at a thickness of 5 μm. Sections were stained with hematoxylin and eosin or were processed for immunohistochemical analysis with antibodies to either germ cell nuclear antigen 1 (GCNA1; a kind gift of George Enders, University of Kansas Medical Center) or the steroidogenic enzyme P450 side chain cleavage (P450scc; Chemicon International Inc., Temecula, Calif.). The anti-P450scc antibody was used at a dilution of 1:100. Detection of the proliferating-cell nuclear antigen (PCNA) was performed with 12-μm frozen sections of testis fixed in 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.6. PCNA was detected as described previously (21).
In situ hybridization and apoptosis assay.
Male mice were anesthetized with intraperitoneal injections of ketamine and xylazine (ratio of 0.4 to 0.6 [vol/vol]) and perfused intracardially with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.6) for 20 min. Isolated testes were postfixed for 4 h at 4°C, transferred to 25% sucrose solution in 0.1 M sodium phosphate buffer (pH 7.6), and incubated at 4°C for an additional 24 h. A microtome cryostat (Microm, Walldorf, Germany) was used to section tissue into 12-μm cryosections, which were mounted on Fischerbrand Superfrost Plus slides (Fischer Scientific, Pittsburg, Pa.) and stored at −20°C. In situ hybridization was performed as previously described (49). Briefly, sections were hybridized with radiolabeled antisense and sense riboprobes generated from mouse Ink4c and Ink4d cDNAs (49), from a PstI fragment containing the 3′ end of the Cdk4 gene (nucleotides 588 to 867), and from a KpnI-SacI fragment containing the 3′ end of the Cdk6 gene (nucleotides 549 to 1000). Apoptotic cells were visualized by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick 3′-end labeling (TUNEL) assay (TdT FragEL DNA fragmentation detection kit; Calbiochem, La Jolla, Calif.).
Protein analysis.
Sequential immunoprecipitation and immunoblotting were performed as described previously (49). Analyses of mouse p18Ink4c and p19Ink4d were performed with commercially available polyclonal antisera: M-167 for immunoprecipitation and immunoblotting of p19Ink4d, M-20 for immunoprecipitation of p18Ink4c, and M-168 for immunoblotting of p18Ink4c (Santa Cruz Biotechnologies, Inc., Santa Cruz, Calif.). Mouse Cdk4 was precipitated from cell lysates with antiserum (Rz) to the C-terminal peptide of mouse Cdk4 (26) and was immunoblotted with antibody C-22 (Santa Cruz Biotechnologies, Inc.). Mouse Cdk6 was detected with antiserum to a C-terminal peptide (49).
Hormone measurements.
Blood samples were collected from 10- to 14-week-old male mice (between 12:00 and 2:00 p.m.) and were kept on ice. Serum was separated and stored at −80°C. The amounts of serum testosterone were measured by a standard coated-tube radioimmunoassay kit (Diagnostic Systems Laboratories, Webster, Tex.). Interassay and intra-assay controls were included. Serum FSH measurements were carried out by radioimmunoassays (8) performed with antibodies and standards generously provided by the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases (Washington, D.C.).
RESULTS
Phenotypic features of mice lacking Ink4c and Ink4d.
Mice containing disrupted Ink4c alleles were bred with Ink4d-null animals, and intercrosses between heterozygotes yielded mice lacking both genes at the expected Mendelian frequency. Ink4c-deficient mice exhibit organomegaly (16, 25), whereas Ink4d-null animals do not differ from their wild-type littermates in size (50). Female mice doubly deficient for both Ink4c and Ink4d exhibited a mean increase in body weight relative to wild-type and Ink4d-null female littermates but still remained 8% smaller than Ink4c-null animals (data not shown). In contrast, Ink4cd double-null males were similar in size to Ink4c-deficient males. Despite expression of high levels of Ink4c and Ink4d in the developing mouse brain (49), the doubly deficient mice displayed no overt neurological defects. However, we recently recognized that Ink4d-null mice are deaf (B. Chen, F. Zindy, M. F. Roussel, and N. Segil, unpublished observations). Other Cdk inhibitors may well compensate for the lack of these Ink4 proteins in the brain, as animals lacking p19Ink4d and p27Kip1, but neither alone, exhibit profound neurologic defects leading to the death of the animals in the first few weeks of life (47).
Ink4c-null animals develop progressively expanding adenomas of the intermediate lobe of the pituitary gland with nearly complete penetrance by 8 to 10 months of age, and by 14 months at least half of the animals die as a consequence (16, 25). Ink4c-null mice also develop pheochromocytomas at a low but significant frequency (ca. 10%) (17, 25). At least one independently derived strain exhibits hyperplasia of pancreatic β-islet cells (25), mirroring observations with mice expressing an Ink4-resistant mutant Cdk4 allele in place of the wild-type gene (32). Ink4cd double-null mice exhibited each of these features of the Ink4c-null strain with similar types, frequencies, and rates of tumor development. The fact that Ink4d loss did not synergize with Ink4c deficiency in this respect is consistent with the previous finding that p19Ink4d is not a tumor suppressor in mice (50).
To determine whether the combined loss of Ink4c and Ink4d affects cell proliferation, we explanted MEFs into culture from 13.5-day-old single- and double-null embryos that normally express both proteins (48). Primary MEFs from the various knockout strains could not be distinguished from those derived from wild-type littermates with respect to their proliferative rates, their ability to arrest in medium containing limiting concentrations of serum, or their rate of entry into S phase following serum withdrawal and restimulation. The cells underwent replicative senescence after 20 to 30 population doublings, associated with the induction during progressive cell culture of various stress response proteins, including p16Ink4a, p15Ink4b, p21Cip1, and p19Arf (reviewed in reference 37). Like nonimmortalized MEF strains, Ink4cd double-null cells were resistant to transformation by oncogenic Ras. In short, the combined loss of Ink4c and Ink4d had no overt effects on the fibroblast cell cycle or replicative senescence.
Sterility in Ink4cd double-null males.
Ink4cd double-null females exhibited no obvious fertility defects, but all doubly deficient males were sterile, and test matings with wild-type females failed to induce pregnancy. Atrophy of the testes in 6-month-old Ink4cd double-null males was more pronounced than that in males lacking Ink4d alone (P < 0.005) (Table 1). In contrast, the testes of organomegalic Ink4c-null mice were larger than those of their wild-type littermates (P < 0.0001). The number of spermatozoa in fluid extruded from the epididymides of Ink4d-null males was significantly lower than that of both wild-type (P = 0.03) and Ink4c-null (P < 0.001) males (Table 1). More strikingly, the sperm counts of double-null males were 85% lower than those of wild-type littermates (P < 0.001) (Table 1). In addition, the morphology of residual epididymal spermatozoa in the double-null mice was abnormal and more than 60% of spermatozoa were nonviable (data not shown).
TABLE 1.
Phenotypes of Ink4c-null, Ink4d-null, and Ink4cd double-null male mice
| Strain genotype | Fertility | Testis wt (mg)a | Sperm countb | Seminiferous tubule histochemistry | Testosterone (ng/ml)b | Leydig cells | FSH (ng/ml)b |
|---|---|---|---|---|---|---|---|
| Wild type | Fertile | 124 ± 10.3 | 3.0 × 107 ± 0.8 × 107 | Normal | 13.5 ± 2.8 | Normal | 18.4 ± 3.6 |
| Ink4c−/− | Fertile | 149 ± 12.7 | 4.5 × 107 ± 1.4 × 107 | Normal | 3.52 ± 1.3 | Hyperplasticc | 19.1 ± 5.1 |
| Ink4d−/− | Fertile | 101 ± 18.4 | 2.0 × 107 ± 0.7 × 107 | Meiotic delay, increased apoptosis | 9.89 ± 2.8 | Normal | 35.8 ± 7.5 |
| Ink4cd−/− | Infertile | 84.2 ± 9.9 | 0.44 × 107 ± 0.3 × 107 | Pronounced meiotic delay, greatly increased apoptosis | 2.84 ± 0.56 | Hyperplasticc | 45.0 ± 11 |
Testes from 6-month-old mice (wild type, n = 10; Ink4c null, n = 18; Ink4d null, n = 14; Ink4cd double null, n = 15). Data are averages ± standard deviations.
Total number recovered per mouse using 10- to 14-week-old males. Numbers represent averages ± standard deviations from five or more animals of each genotype.
Hyperplastic Leydig cells did not appear to differentiate based on the relatively low levels of histochemically detectable P450scc, the rate-limiting enzyme for steroid biogenesis.
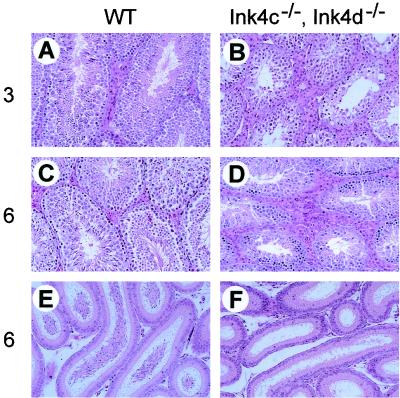
Histologic comparison of transverse and sagittal sections of testes from 3-month-old and 6-month-old Ink4cd double-null and wild-type mice revealed that steroidogenic Leydig cells were abnormal in doubly deficient males. Hyperplasia of the Leydig cells was already apparent at 3 months of age (Fig. 2B versus A) and was even more obvious at 6 months of age (Fig. 2D versus C). The excessive proliferation of Leydig cells in Ink4cd double-null males seemed somewhat more severe than that seen in Ink4c-null males (data not shown). Consistent with the sterility of Ink4cd double-null males, the number of mature sperm observed in the epididymal lumena of 6-month-old animals was much less than that seen in sections of wild-type testes (Fig. 2F versus E).
FIG. 2.
Leydig cell hyperplasia and reduced numbers of sperm in Ink4cd doubly deficient mice. Hematoxylin and eosin staining of sections of testes from 3-month-old (A) and 6-month-old (C) wild-type (WT) mice show normal seminiferous tubule architecture and interstitial Leydig cells. In the testes of 3-month-old (B) and 6-month-old (D) Ink4cd double-null males, Leydig cell hyperplasia is apparent. Although the 6-month-old wild-type epididymis (E) was replete with mature spermatozoa, the epididymis of the 6-month-old Ink4cd double-null mouse (F) had few mature spermatozoa. Magnification, ×200.
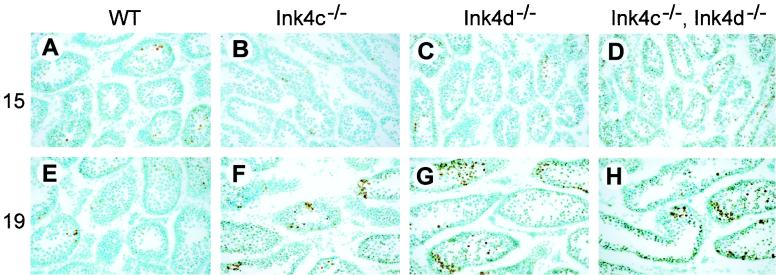
Apoptosis normally plays a role in regulating production of mature functional sperm. However, the decreased number of sperm in Ink4d-null mice is associated with an increased number of apoptotic germ cells; this is reflected by increases in the number of multinucleated giant cells, of cells with condensed nuclei, and of DNA free ends detected by TUNEL assay (50). In testes from wild-type mice, as the first cohort of germ cells reaches the pachytene stage at day 14 or 15 pp (Fig. 1), apoptosis of tubular cells increases (Fig. 3A) but then declines by day 19 pp as some cells complete meiosis I (Fig. 3E). Apoptosis was also observed at day 15 pp in testes from Ink4c-null mice (Fig. 3B), but unlike that of testes from wild-type mice, was even more evident at day 19 pp (Fig. 3F). The tubules in testes of Ink4d-null (Fig. 3C) and Ink4cd double-null mice (Fig. 3D) also revealed elevated apoptosis through day 19 pp (Fig. 3G and H) and even into adulthood (data not shown). Analysis of multiple sections from at least five animals of each genotype suggested that the apoptotic indices in testes from mice at day 19 pp lacking Ink4d were higher than those of animals lacking Ink4c (Fig. 3G and H versus F), although we did not quantify these effects by grain counts. Therefore, despite Leydig cell hyperplasia, the Ink4cd doubly deficient males had small testes with tubular atrophy, germ cell apoptosis, reduced sperm production, and loss of viability of the remaining spermatozoa, leading to male infertility.
FIG. 3.
Increased apoptotic index in germ cells from Ink4cd doubly deficient mice. Sections of testes of 15-day-old (A through D) and 19-day-old (E through H) mice of indicated genotypes were subjected to TUNEL assay to score apoptotic cells. As visualized by the brown precipitate, testes from Ink4d-null (G) and Ink4cd double-null (H) mice at day 19 pp showed increased numbers of apoptotic cells within the seminiferous tubules. WT, wild type.
Altered hormone levels in males lacking Ink4c, Ink4d, or both.
A complex network of hormonal signals orchestrates spermatogenesis. Gonadotrophs in the anterior lobe of the pituitary gland secrete LH and FSH, which stimulate Leydig cells to produce testosterone. To investigate the possible contribution of the pituitary-gonadal axis to male sterility, we measured the levels of testosterone, FSH, and LH in 10- to 14-week-old males of all genotypes (Table 1).
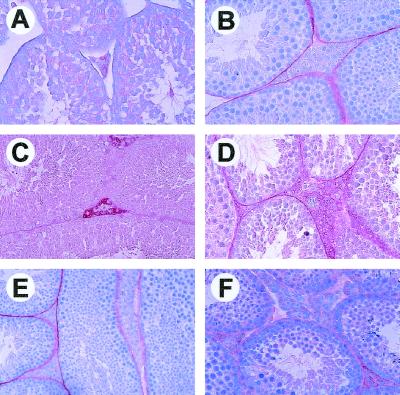
The onset of mature Leydig cell function at puberty is characterized by their ability to terminally differentiate and produce testosterone and other androgens at levels seen in adult males. As noted above, however, spermatogenesis begins earlier in life, arguing that adequate levels of testosterone to support this process are available soon after birth. One marker of Leydig cell differentiation is P450scc, the steroidogenic enzyme responsible for conversion of cholesterol to pregnenolone, the first and rate-limiting step in the steroidogenic pathway (29). As expected, in testis sections from 1-month-old wild-type males, Leydig cells failed to show P450scc immunoreactivity (Fig. 4A) above background levels (Fig. 4B). By 3 months of age, however, Leydig cells of wild-type males showed strong P450scc immunoreactivity, as demonstrated by the red precipitate within the interstitial space of the testis (Fig. 4C). Similarly, Leydig cells of testes from adult Ink4d-null males also showed up-regulated levels of P450scc (Fig. 4D) correlating with nearly normal levels of testosterone in their serum (Table 1). However, the levels of P450ssc immunoreactivity was severely reduced in Leydig cells of Ink4c-null (Fig. 4E) and Ink4cd double-null testes (Fig. 4F), suggesting a failure of Leydig cell differentiation. In turn, levels of serum testosterone were reduced by approximately 75% in Ink4c-null and Ink4cd doubly deficient males (Table 1). The levels of LH, released by the anterior lobe of the pituitary, were equivalent in males of all genotypes (data not shown), suggesting that the apparent failure of Leydig cell differentiation in mice lacking Ink4c was not secondary to pituitary dysfunction. We presume that the hyperplasia of Leydig cells observed somewhat later in life in the two mouse strains lacking Ink4c reflects a consequence of the same pathologic process; namely, their failure to differentiate and produce testosterone. However, despite the decline in serum testosterone levels, Ink4c-null mice remain fertile.
FIG. 4.
Impaired differentiation of Leydig cells from Ink4c-null and Ink4cd double-null mice. P450scc staining of Leydig cells is indicated by red precipitate. (A) Immature Leydig cells from 1-month-old wild-type mice lack P450scc staining. (B) Absence of staining of 3-month-old wild-type type testis with preimmune serum used as negative control. P450scc staining in Leydig cells from 3-month-old wild-type (C) and Ink4d-null (D) mice. Testes from 3-month-old Ink4c-null (E) and Ink4cd doubly deficient (F) mice failed to reveal significant P450scc staining.
Normal levels of FSH were present in the serum of wild-type and Ink4c-null males, whereas FSH levels were increased in both Ink4d-null and Ink4cd double-null males (Table 1). The physiologic basis or consequence, if any, of the increased FSH levels remains unclear but occurs independently of production of the other gonadotroph product, LH. Normal levels of other pituitary hormones secreted by the anterior lobe, including corticotropin and thyroid-stimulating hormone, were produced in males of all genotypes (data not shown).
Expression of Ink4 proteins, Cdk4, and Cdk6 in testes.
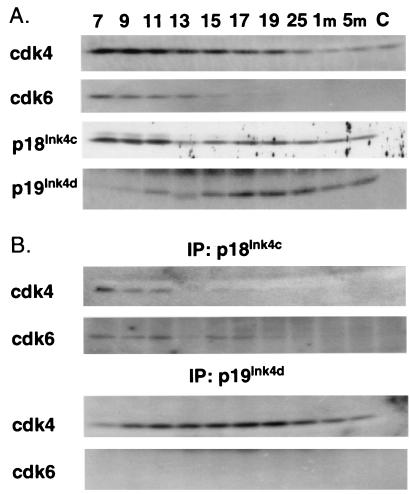
To understand the nature of sterility in Ink4cd double-null males, we first studied the expression of Ink4 proteins and cyclin D-dependent kinases during testicular development in wild-type mice. We isolated testes from animals between 7 days and 1 month pp, the period that marks the first wave of spermatogenesis (Fig. 1), and also from mice at 5 months of age. We then used sequential immunoprecipitation and immunoblotting to score for the presence of the various proteins in testicular tissue and to look for complexes between p18Ink4c or p19Ink4d with Cdk4 and Cdk6 (Fig. 5). As controls for antibody specificity, we used testes from the Ink4cd doubly deficient animals (Fig. 5, lanes C). Testes from at least five mice were pooled to make lysates, and analysis of the same lysates was repeated twice with similar results.
FIG. 5.
Expression of Ink4 proteins and Cdks during testis development in wild-type mice. (A) Lysates from whole testes isolated from wild-type males at 7, 9, 11, 13, 15, 17, 19, and 25 days pp and at 1 and 5 months (m) of age were normalized for protein content and precipitated with antibodies to Ink4 proteins or Cdks. Denatured precipitates were electrophoretically separated on denaturing gels and immunoblotted with antisera directed to the same proteins, as indicated at the left of the panels. (B) Lysates were precipitated with antibodies directed to either p18Ink4c or p19Ink4d, as indicated at the top of the panels. Immunoprecipitates (IP) were denatured, separated electrophoretically, and immunoblotted with antisera to the Cdks noted at the left of the panels. Controls performed with cells of Ink4cd double-null mice are in lanes C.
As previously described (34, 46), Cdk4 protein was most abundant in the immature testis (days 7 to 11 pp), after which its levels declined (Fig. 5A). This may reflect the fact that Cdk4 expression in Sertoli cells and spermatogonia is diluted by an increasing proportion of postmeiotic germ cells as the organ develops postnatally. Cdk6 expression was also maximal in the immature testis but, in contrast to that of Cdk4, was undetectable after day 17 pp (Fig. 5A). Since our antibodies detect recombinant Cdk4 and Cdk6 with nearly equal efficiency, we can conclude that the levels of Cdk4 eclipse those of Cdk6 at all times during maturation of the organ. The pattern of expression of p18Ink4c was biphasic (Fig. 5A): the first burst of expression occurred between days 7 and 11 pp, which marks the mitotic phase of spermatogenesis, and the second phase occurred between days 15 and 25 pp, peaking at about day 17 pp, which roughly corresponds to the end of meiosis I (Fig. 1). p18Ink4c was detected in complexes with both Cdk4 and Cdk6 at days 7 to 11 pp but was more difficult to visualize in complexes with Cdk4 at later times (Fig. 5B). In contrast, the level of p19Ink4d progressively increased from day 7 to day 17 pp and then slowly declined (Fig. 5A). p19Ink4d failed to bind to Cdk6 but formed complexes with Cdk4 from day 7 pp through adulthood (Fig. 5B).
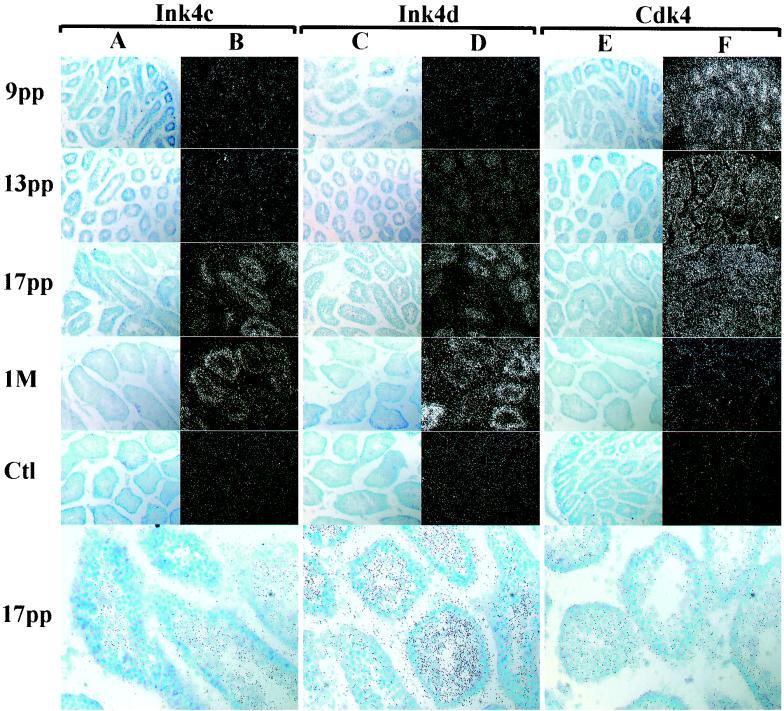
Because our antibodies proved inadequate for examining protein expression by immunohistochemistry in fixed tissue sections, we performed in situ hybridization using antisense probes (50) with sections of testes from wild-type mice in an attempt to localize cells expressing Ink4 and Cdk4 transcripts during the first wave of spermatogenesis (Fig. 6). At each stage, we never obtained specific hybridization with sense probes used as controls (Fig. 6, Ctl panels). At day 9 pp, Ink4c RNA detected with the antisense probe was expressed in a diffuse pattern throughout the testis (Fig. 6B), although for unknown reasons the relative intensity of RNA staining at this time was significantly weaker than what might have been predicted based on parallel protein expression studies (Fig. 5A). This underscores a potential shortcoming of this approach since disparities between mRNA expression and protein turnover could well reflect translational or posttranslational controls. Immunohistochemical staining of sections of human testes performed with a monoclonal antibody to p18Ink4c detected this protein in interstitial Leydig cells and tubular Sertoli cells as well as in germ cells (4).
FIG. 6.
Ink4c, Ink4d, and Cdk4 mRNAs expressed during normal testis development. In situ hybridizations were performed on sections of testes taken from wild-type animals at 9, 13, and 17 days and 1 month (M) pp as indicated at the left of the panels. Sections were hybridized with the antisense probes indicated at the top. Morphology of the tubules is revealed in bright-field images (A, C, and E), and the hybridization intensity is revealed by dark-field images (B, D, and F). Control hybridizations (Ct1) performed with sense strand probes at 1 month pp (Ink4c and Ink4d) and at day 9 pp (Cdk4) are shown. All magnifications are ×200. The three bottom bright-field panels indicate the localization of grains within tubules, as photographed at higher power (magnification, ×400).
Only during later periods of development was Ink4c RNA clearly localized to the mouse seminiferous tubules, appearing most evident by day 17 pp (Fig. 6B). Higher power resolution of grains scored at day 17 pp (Fig. 6A and B, bright field, bottom) demonstrated selective staining of intratubular cells corresponding to spermatocytes while sparing the more immature cells at the periphery. Ink4d RNA expression increased with the age of the testis (Fig. 6D), consistent with the graded expression of the protein observed by immunoblotting (Fig. 5A). Ink4d transcripts were detected in tubules at day 13 pp before the appearance of Ink4c RNA. By day 17 pp, intratubular spermatocytes were highly labeled (Fig. 6C and D, bright field, bottom). The temporal and physical localization of Ink4c and Ink4d RNAs within the tubules indicates that both Cdk inhibitors are primarily expressed in postmitotic spermatocytes, in agreement with immunohistochemical studies performed on human testes (4, 5). Cdk4 staining was detected in seminiferous tubules during the first meiotic wave (Fig. 6F, bright field, bottom), but the hybridization signal decreased in intensity as spermatogenesis progressed, in agreement with results of protein immunoblotting (Fig. 5A). These data suggest that, unlike the Ink4 proteins, Cdk4 is primarily expressed during earlier stages of spermatogenesis at the time when spermatogonia undergoing mitosis predominate in the tubules.
Impaired meiotic progression in Ink4cd doubly deficient mice.
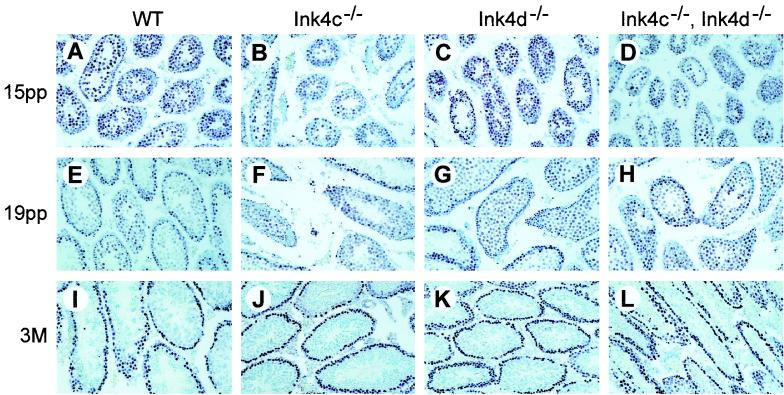
Because p18Ink4c and p19Ink4d, when expressed at high levels, were expected to inhibit Cdk4 activity and to block entry of cells into S phase, we anticipated that the loss of both inhibitors might increase premeiotic spermatogonial proliferation. To detect mitotically active spermatogonia, sections of testes from males of all genotypes were stained with an antibody to PCNA, a protein expressed in S phase (6). Up to day 15 pp, PCNA-positive cells were detected in testicular tubular cells of all genotypes (Fig. 7A through D). By day 19 pp, the number of PCNA-positive cells in tubules from wild-type and Ink4c-null mice was diminished, suggesting that many cells had exited the mitotic cycle (Fig. 7E and F versus A and B, respectively). In contrast, more PCNA-positive cells remained in some but not all tubules of Ink4d-deficient mice and were most evident in those of the doubly null strain (Fig. 7G and H). By adulthood, however, germ cell proliferation was restricted to a monolayer of cells along the basement membrane of the seminiferous tubules in males of all genotypes (Fig. 7I through L). The relative persistence of mitotic activity of the spermatogonial population in 19-day-old Ink4d-null and Ink4cd double-null males might therefore reflect a delay in the onset of the first wave of meiosis I.
FIG. 7.
PCNA-positive cells in seminiferous tubules of mice of different genotypes. Sections of testes from mice of the indicated genotypes (top) taken at day 15, day 19, and 3 months pp (indicated at the left) were immunostained with an antibody against PCNA. A deep purple precipitate indicates cellular proliferation that, in adult males, is eventually restricted to a single or double layer of premeiotic germ cells on the periphery of the seminiferous tubules (I through L). At day 19 pp, more widespread proliferation is observed in the tubules of single Ink4-null (F and G) and Ink4cd double-null males (H) as compared to the patterns in wild-type (WT) mice (E).
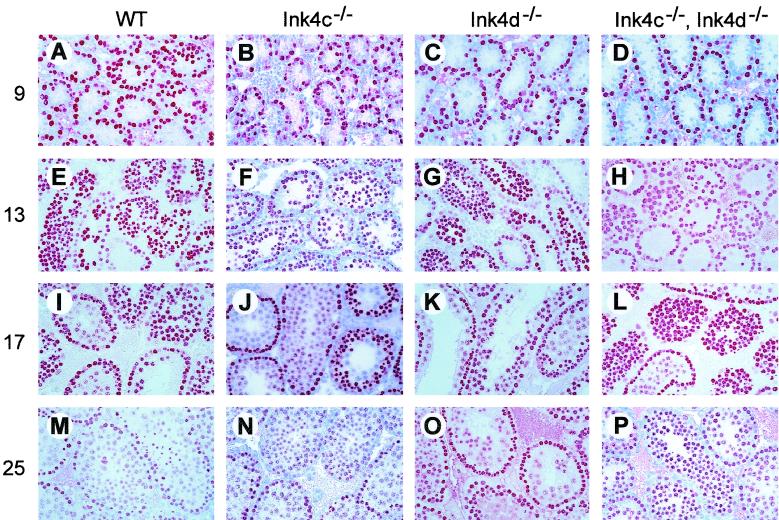
To determine if and when meiotic progression was impaired in Ink4d and Ink4cd double-null males, we compared the progress of germ cell differentiation through meiosis I using testis sections made from male mice of various genotypes sacrificed between days 9 and 25 pp. Sections were stained with an antibody against GCNA1, a germ cell nuclear antigen that is specifically expressed in premeiotic cells up to the pachytene stage of meiosis I (43). At early stages, densely stained premeiotic cells are localized close to the basement membranes of seminiferous tubules (Fig. 8A). Passage through meiosis I is marked by a progressive loss of intensity of GCNA1-positive red precipitate during early prophase I, resulting in a weaker punctate staining pattern in pachytene cells (Fig. 8E, I, and M).
FIG. 8.
Meiosis I is delayed in germ cells from Ink4cd doubly deficient mice. Testis sections from mice of the indicated genotypes (top) were immunostained using an antibody against GCNA1 (red precipitate). Testes were removed at the times (days pp) indicated at the left. GCNA1-positive germ cells are apparent within the testes as early as day 9 pp in all genotypes and are located at the periphery of the tubules (A through D). Their numbers increase through day 13 pp (E through H), although the intensity of staining of some cells starts to decrease. By day 17 pp, many cells within the lumena of the seminiferous tubules of wild-type (WT) (I) and Ink4c-null (J) males have lost their bright GCNA1 signal, indicative of progression of cells toward meiosis II. The proportion of GCNA1-positive cells continues to drop in these mice through day 25 pp (M and N). In Ink4d-null (K and O) and Ink4cd double-null (L and P) males, however, the number of GCNA1-positive cells remains higher from day 17 to day 25 pp, indicative of reduced progression through meiosis I.
At day 9 pp, the patterns of GCNA1 localization were similar in all genotypes (Fig. 8A through D), with at least one layer of stained spermatogonia observed on the tubular basement membrane, and by day 13 pp cells showed signs of entry into meiosis I (Fig. 8E through H). Whereas pachytene cells were detected at day 17 pp in Ink4c-null (Fig. 8J) and Ink4d-null testes (Fig. 8K), cells in many tubules of Ink4cd-deficient mice continued to stain brightly with GCNA1 (Fig. 8L), suggesting retarded progression through meiosis concomitant with increased apoptosis (see Fig. 3H above). By day 25 pp, many apoptotic cells were present within the tubular lumena of Ink4cd-deficient mice (Fig. 8P; confirmed by hematoxylin and eosin staining and TUNEL assays). In summary, these results show that p18Ink4c and p19Ink4d are required during the first wave of spermatogenesis to regulate the differentiation of mature viable sperm.
DISCUSSION
Impaired spermatogenesis in Ink4cd double null males.
Females lacking both Ink4c and Ink4d exhibit no reproductive abnormalities, whereas males lacking both Cdk inhibitors are infertile. Ink4cd double-null males produce low numbers of mature sperm, the majority of which exhibited abnormal morphology and reduced motility. Male sterility correlated with an ineffective exit of spermatogonia from the mitotic cycle and with a failure of residual postmitotic spermatocytes to correctly undergo the meiotic divisions required to generate haploid gametes. The temporally delayed entry into meiosis of Ink4cd-null germ cells was also associated with increased apoptosis of spermatocytes, leading ultimately to the production of mostly nonviable sperm (genotypes are summarized in Table 1).
By day 19 pp during the first wave of spermatogenesis, mitotic cell divisions in the seminiferous tubules of wild-type mice, marked by PCNA-stained S-phase cells, become largely confined to spermatogonial cells that reside close to the basement membrane. In contrast, intralumenal spermatocytes at a further distance from the basement membrane have exited the cell cycle to undergo meiotic division and subsequent differentiation. Upon completion of meiosis, the latter cells differentiate to spermatids and mature spermatozoa that can be most easily visualized histologically in the epididymis. GCNA staining of cells in seminiferous tubules can distinguish the densely stained premeiotic cells at the tubular periphery from the more punctately stained cells in the pachytene stage of meiosis I within the lumen. These patterns of staining were distorted in the tubules of Ink4cd double-null mice (and, to a lesser extent, in Ink4d-null males), in which an expanded intralumenal population of mitotic S-phase cells was detected and fewer pachytene cells appeared, the latter only after a significant delay. Abnormalities in stages of meiotic progression were associated with an increased apoptotic index, and in turn, spermatids and spermatozoa were not readily seen in the seminiferous tubules of the Ink4cd doubly deficient mice.
Both p18Ink4c and p19Ink4d are expressed at relatively high levels in testes compared to those in other organs of neonatal mice (48). Ink4d RNA transcripts were first readily detected by in situ hybridization in the seminiferous tubules of wild-type mice by day 13 pp, consistent with the increase in overall testicular p19Ink4d protein expression observed by immunoblotting. This corresponds to the time of entry into meiosis by the first wave of developing germ cells. At later stages, Ink4d RNA expression remains restricted to tubular cells, while the overall p19Ink4d protein level in the testis is maintained as additional waves of spermatogenesis ensue. In agreement with these findings, immunohistochemical studies of human p19INK4d in testes revealed maximal levels of protein expression in primary and secondary spermatocytes (5). This argues that Ink4d is normally expressed in postmitotic spermatocytes, where it presumably functions to extinguish the activity of cyclin D-dependent kinases, thereby helping to prevent further mitotic divisions.
The overall pattern of p18Ink4c protein expression was more complex, with an earlier wave of protein synthesis (days 7 to 11 pp) followed by a nadir at day 13 pp and a return of expression from day 15 pp onward. By use of in situ hybridization, we were unable to clearly localize the earliest round of Ink4c expression to tubular germ cells and instead saw a more diffuse pattern of staining throughout the testis. However, Ink4c expression eventually became restricted to seminiferous tubular cells by day 17 pp onward, implying that the protein is expressed at high levels in spermatocytes. Similar results were obtained by others who applied immunohistochemical techniques to study p18INK4c in human testes (4). Loss of Ink4d leads to modest but still clearly defined inhibition of mature sperm cell production, but so far Ink4c has not been implicated in this process. Our data now suggest that p18Ink4c collaborates with p19Ink4d in helping to ensure the transition from mitotic to meiotic cell cycles during male germ cell maturation.
Evidence is accumulating that progression of meiosis I is monitored to eliminate germ cells that have sustained unrepaired DNA damage. For example, defects in genes, such as Atm (2, 12, 20, 44), ligase-4 (3, 15), and MSH 4 and MSH 5 (11, 23), that monitor or effect DNA repair routinely lead to male sterility. One possibility, then, is that Ink4 genes have effector functions in cell cycle checkpoint control, arresting cells with broken chromatids until DNA damage is repaired. TUNEL assays indicated that loss of Ink4d, or more dramatically, loss of Ink4d in collaboration with disabled Ink4c, led to increased apoptosis once spermatocytes entered prophase I. The increased number of apoptotic cells coincided with the progression of spermatocytes up to and through pachynema (31). In contrast, the germ cells of Ink4c-null mice, like those of wild-type littermates, did not undergo significantly increased apoptosis. Hence, lack of Ink4d had a greater impact than loss of Ink4c, although both genes can contribute.
Unlike the two Ink4 proteins, Cdk4 is expressed at maximal levels at earlier stages of spermatogenesis, where spermatogonia undergoing mitotic cell cycles predominate. Although its level declined as the animals aged, Cdk4 was still readily detected at reduced levels thereafter. Lower levels of Cdk6 relative to those of Cdk4 were also observed from day 7 to day 15 pp during the first wave of spermatogenesis but fell below the limit of detectability of our assays at later times. This indicates that Cdk4 is the major cyclin D-dependent catalytic subunit in the mouse testis but does not formally preclude the participation of Cdk6 in spermatogenesis in adult mice. Our results demonstrate that the effects of codeletion of Ink4c and Ink4d on spermatogenesis are not equivalent to those observed in the Ink4-resistant Cdk4 R24C knock-in strain, which is fertile (32). The mechanistic basis for these differences remains unclear. Under normal circumstances, p19Ink4d interacts preferentially with Cdk4 (data shown here), whereas p18Ink4d binds more selectively to Cdk6 (18). Possibly, the presence of the Cdk4 R24C mutant in place of wild-type Cdk4 might enhance the association of p19Ink4d with Cdk6, leading to a compensatory decrease in overall cyclin D-dependent kinase activity. A detailed analysis of spermatogenesis in the Cdk4 R24C knock-in strain has not been undertaken, and it is conceivable that the mice manifest subtle defects similar to those seen in males lacking Ink4d alone.
Disruption of the cyclin D2 gene, which is normally expressed in spermatogonia (7, 22, 28, 33, 34, 46), leads to testicular atrophy and reduced sperm counts (39), while loss of Cdk4 ultimately results in decreased numbers of spermatogonia and male infertility (32, 42). Together, these results indicate that cyclin D-dependent kinases are required for progression through the early mitotic divisions that characterize postnatal male germ cell development. However, their unopposed expression at later stages due to Ink4cd loss also prevents proper germ cell maturation, underscoring a requirement for precise temporal regulation of these enzymes.
In humans, testicular germ cell tumors are derived from primordial germ cells. Both seminomas and other germ cell tumor types appear to arise from cytogenetically identical carcinomas in situ that progress to invasive lesions (7). p19INK4d is undetectable in fetal germ cells that are thought to be the precursors of testicular tumors, and as might be expected, deletion of INK4d has not been seen in such tumors (5). Unlike p19INK4d, however, p18INK4c expression is abundant in carcinomas in situ, and loss of INK4c is frequently associated with progression from carcinomas in situ to invasive germ cell tumors in human testes (4). These findings point to the possibility for additional roles for p18INK4c in fetal germ cell development.
Effects of Ink4c and Ink4d loss on the pituitary-gonadal axis.
Although the noted defects in spermatogenesis in Ink4cd double-null mice are likely to be germ cell autonomous, the loss of Ink4c and Ink4d also affects the production of hormones that regulate germ cell development. The pituitary tumors observed in Ink4c-null or Ink4cd double-null mice arise gradually throughout life but with complete penetrance and primarily involve the intermediate lobe of the gland. Pituitary tumorigenesis is unlikely to be responsible for the observed early postnatal effects on germ cell production in Ink4cd double-null animals, because mice lacking Ink4c alone are fertile and produce normal numbers of sperm and their pituitary tumors arise much later in life and occur in a region of the gland that does not produce LH or FSH.
Whether Ink4d is present or not, Ink4c-null animals, like mice expressing the Ink4-resistant mutant form of Cdk4 (32), exhibit Leydig cell hyperplasia. However, these cells produced only low levels of P450scc, the rate-limiting enzyme for steroid biogenesis (29). In turn, serum testosterone levels in Ink4c-null and Ink4cd double-null mice at 10 to 14 weeks of age were equally low. Because Ink4c-null males had normal sperm counts and were fertile, decreased testosterone production in Ink4cd double-null males was not strictly associated with infertility. In fact, previous studies have demonstrated that the amount of testosterone synthesized by the rodent testis far exceeds that required for normal spermatogenesis (1, 51). Moreover, the initial failure of male gametogenesis in Ink4cd double-null mice was manifested before puberty during the very first wave of spermatogenesis, so the effects of Ink4c loss on Leydig cell function are unlikely to contribute to the reduced meiotic progression. In agreement, we were unable to rescue defective spermatogenesis in Ink4cd double-null males by enforced administration of testosterone (data not shown). Because Ink4c-null and Ink4cd double-null animals synthesized normal levels of LH, the failure to produce normal levels of testosterone does not reflect pituitary LH insufficiency. Instead, Ink4c expression appears to control the ability of pubertal Leydig cells to properly exit the cell cycle, up-regulate steroidogenic enzyme expression, and increase testosterone production. The fact that Ink4d-null mice produced near normal levels of testosterone connotes a distinct role for p18Ink4c, but not p19Ink4d, within Leydig cells.
Regardless of whether or not Ink4c was present, mice lacking Ink4d produced elevated levels of FSH. It has been suggested that FSH is required not for the initiation of spermatogenesis but rather for the maintenance of sperm viability and motility (24). Male mice lacking the FSH receptor (10) or lacking the FSH-β subunit (24) and men with a mutation in the FSH receptor (40) are fertile despite decreased testicular mass and reduced sperm motility and numbers. However, FSH treatment of oligospermic men results in increased spermatogenesis rather than improved spermiogenesis, arguing that FSH might increase the entry of spermatogonia into meiosis (14). Despite high levels of FSH, however, mice lacking Ink4d, or both Ink4d and Ink4c, have small testes and a reduction in sperm counts and viability, suggesting that the testis is unable to up-regulate spermatogenesis in the absence of the Ink4 proteins. We saw good correlation between testis size and sperm counts, a phenomenon also found in mice lacking other genes involved in testicular development, including cyclin D2 (39), Cdk4 (32, 42), E2F-1 (13, 45), and Egr4 (41).
In males, FSH enhances functions of Sertoli cells that, in turn, provide support for spermatogonial maturation (10, 36). In females, FSH plays a similar role in sustaining granulosa cell development, and disruption of cyclin D2, an FSH-responsive gene in these cells, leads to faulty granulosa cell function and female infertility (39). Disruption of Cdk4 also results in female infertility, but there is no apparent defect in granulosa cell development. Instead, female infertility appears to be due to defects in the hypothalamic-pituitary axis, where Cdk4 loss leads to a significant reduction in FSH production (32). Notably, this contrasts with the elevated FSH levels observed in both Ink4d-null and Ink4cd double-null animals. Although we have no clear explanation for the increased FSH production by the pituitary gland in these mice, this could well reflect a role of Cdk4 in regulating FSH levels in males. In this regard, Ink4d appears to play a more prominent role than Ink4c.
The rate of meiotic progression in the testis is governed by an inhibin-mediated feedback loop that is initiated in the Sertoli cells. Under normal conditions, the accumulation of pre- and perimeiotic germ cells stimulates Sertoli cells to produce inhibin, which selectively suppresses FSH production by gonadotrophs in the anterior pituitary (9, 10, 30). In light of the reduced rate of meiotic entry and progression, we might expect that inhibin levels would be reduced in Ink4cd double-null mice. This feedback mechanism would explain the selective increase in FSH seen in Ink4cd-null mice, with serum LH concentrations remaining unaffected. We attempted to analyze this possibility by staining for inhibin in the testes of these mice, but available commercial antibodies did not yield specific immunohistochemical signals in Sertoli cells. This formally leaves open the possibility that a surfeit of FSH produced by the anterior pituitary gland of Ink4cd-null mice acts through Sertoli cells to drive premeiotic hyperproliferation of Ink4cd-null spermatogonia. However, given that FSH levels in fertile Ink4d-null mice and infertile Ink4cd double-null mice are 2.0- and 2.5-fold greater, respectively, than those in wild-type animals, and that FSH is used to improve spermatogenesis in men with low sperm counts (14, 36), we believe that elevated FSH levels are unlikely to explain the abnormalities in meiotic progression observed in the Ink4cd double-null strain.
In summary, our results demonstrate that p18Ink4c and p19Ink4d are crucial for the progression of germ cells past the pachytene stage of spermatogenesis and are most consistent with the idea that this defect in progression is intrinsic rather than hormonally driven. Human INK4d and INK4c may be candidate markers for some forms of male-only infertility, including those caused by autosomal recessive disorders in which early meiotic arrest is a hallmark.
ACKNOWLEDGMENTS
We are indebted to Zhen Lu, Ming Wang, Esther van de Kamp, Rose Mathew, Myriam Chang, and Liyin Zhu for excellent technical assistance. We thank Jo-Anne Croxford and Patrick Sailor from the Animal Resource Center at St. Jude Children's Research Hospital for their help in handling the mice. We thank Beatriz Sosa-Pineda for her help with the analysis of the mouse pancreas, Shengjie Wu and Xiaoping Xiong for statistical analyses, Justine Cunningham and members of the Department of Biomedical Communications for assistance with photographic production, Julia-Cay Jones for scientific editing of the manuscript, and all members of the laboratory for helpful criticisms.
This work was supported in part by NIH grants CA-71907 (M.F.R.) and CA-89617 (J.W.P.), by Cancer Center grants CA-21765 (SJCRS) and P30-13330 (AECOM), and by the American Lebanese Syrian Associated Charities (ALSAC). C.J.S. is an investigator of the Howard Hughes Medical Institute.
F.Z. and W.D.B. contributed equally to this work.
REFERENCES
- 1.Awoniyi C A, Santulli R, Sprando R L, Ewing L L, Zirkin B R. Restoration of advanced spermatogenic cells in the experimentally regressed rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology. 1989;124:3043–3049. doi: 10.1210/endo-124-3-1217. [DOI] [PubMed] [Google Scholar]
- 2.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley J N, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia-telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 3.Barnes D E, Stamp G, Rosewell I, Denzel A, Lindahl T. Targeted disruption of the gene encoding DNA ligase IV leads to lethality in embryonic mice. Curr Biol. 1998;8:1395–1398. doi: 10.1016/s0960-9822(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 4.Bartkova J, Thullberg M, Rajpert-de Meyts E, Skakkebaek N E, Bartek J. Cell cycle regulators in testicular cancer: loss of p18INK4C marks progression from carcinoma in situ to invasive germ cell tumours. Int J Cancer. 2000;85:370–375. doi: 10.1002/(sici)1097-0215(20000201)85:3<370::aid-ijc13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Bartkova J, Thullberg M, Rajpert-de Meyts E, Skakkebaek N E, Bartek J. Lack of p19INK4d in human testicular germ-cell tumours contrasts with high expression during normal spermatogenesis. Oncogene. 2000;19:4146–4150. doi: 10.1038/sj.onc.1203769. [DOI] [PubMed] [Google Scholar]
- 6.Bravo R, MacDonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol. 1987;105:1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaganti R S K, Houldsworth J. Genetics and biology of adult human male germ cell tumors. Cancer Res. 2000;60:1475–1482. [PubMed] [Google Scholar]
- 8.Cohen P E, Hardy M P, Pollard J W. CSF-1 plays a major role in the development of reproductive function in male mice. Mol Endocrinol. 1997;11:1636–1650. doi: 10.1210/mend.11.11.0009. [DOI] [PubMed] [Google Scholar]
- 9.De Kretser D M, Meinhardt A, Meehan T, Phillips D J, O'Bryan M K, Loveland K A. The roles of inhibin and related peptides in gonadal function. Mol Cell Endocrinol. 2000;161:43–46. doi: 10.1016/s0303-7207(99)00222-1. [DOI] [PubMed] [Google Scholar]
- 10.Dierich A, Sairam M R, Monaco L, Fimia G M, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelmann W, Cohen P E, Kneitz B, Winand N, Heyer J, Kolodner R, Pollard J W, Kucherlapati R. Mammalian MutS homolog 5 is required for chromosome pairing in meiosis. Nat Genet. 1999;21:123–127. doi: 10.1038/5075. [DOI] [PubMed] [Google Scholar]
- 12.Elson A, Wang Y, Daugherty C J, Morton C C, Zhou F, Campos-Torres J, Leder P. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci USA. 1996;93:13084–13089. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field S J, Tsai F-Y, Kuo F, Zubiaga A M, Kaelin W G, Livingston D M, Orkin S H, Greenberg M E. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 14.Foresta C, Bettella A, Merico M, Garolla A, Plebani M, Ferlin A, Rossato M. FSH in the treatment of oligozoospermia. Mol Cell Endocrinol. 2000;161:73–88. doi: 10.1016/s0303-7207(99)00228-2. [DOI] [PubMed] [Google Scholar]
- 15.Frank K M, Sekiguchi J M, Seidl K J, Swat W, Rathbun G A, Cheng H L, Davidson L, Kangaloo L, Alt F W. Late embryonic lethality and impaired VDJ recombination in mice lacking DNA ligase IV. Nature. 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- 16.Franklin D S, Godfrey V L, Lee H, Kovalev G I, Schoonhoven R, Chen-Kiang S, Su L, Xiong Y. CDK inhibitors p18INK4c and p27Kip1 mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998;12:2899–2911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franklin D S, Godfrey V L, O'Brien D A, Deng C, Xiong Y. Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol Cell Biol. 2000;20:6147–6158. doi: 10.1128/mcb.20.16.6147-6158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan K L, Jenkins C W, Li Y, Nichols M A, Wu X, O'Keefe C L, Matera A G, Xiong Y. Growth suppression by p18, a p16INK4A/MTS1- and p15INK4B/MTS2-related CDK6 inhibitor, correlates with wild type Rb function. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- 19.Hedger M P, de Kretser D M. Leydig cell function and its regulation. Results Probl Cell Differ. 2000;28:69–110. doi: 10.1007/978-3-540-48461-5_4. [DOI] [PubMed] [Google Scholar]
- 20.Herzog K H, Chong M J, Kapsetaki M, Morgan J L, McKinnon P J. Requirement for Atm in ionizing radiation-induced cell death in the developing central nervous system. Science. 1998;280:1089–1091. doi: 10.1126/science.280.5366.1089. [DOI] [PubMed] [Google Scholar]
- 21.Kamijo T, Bodner S, van de Kamp E, Randle D H, Sherr C J. Tumor spectrum in ARF-deficient mice. Cancer Res. 1999;59:2217–2222. [PubMed] [Google Scholar]
- 22.Kang M J, Kim M K, Terhune A, Park J K, Kim Y H, Koh G Y. Cytoplasmic localization of cyclin D3 in seminiferous tubules during testicular development. Exp Cell Res. 1997;234:27–36. doi: 10.1006/excr.1997.3590. [DOI] [PubMed] [Google Scholar]
- 23.Kneitz B, Cohen P E, Avdievich E, Zhu L, Kane M F, Hou H, Kolodner R D, Pollard J W, Kucherlapati R, Edelmann W. MutS homolog 4 (MSH4) localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev. 2000;14:1085–1097. [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar T R, Wang Y, Lu N, Matzuk M M. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 25.Latres E, Malumbres M, Sotillo R, Martin J, Ortega S, Martin-Caballero J, Flores J M, Cordon-Cardo C, Barbacid M. Limited overlapping roles of P15(INK4b) and P18(INK4c) cell cycle inhibitors in proliferation and tumorigenesis. EMBO J. 2000;19:3496–3506. doi: 10.1093/emboj/19.13.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J-Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarrey J R. Development of the germ cell. In: Desjardins C, Ewing L L, editors. Cell and molecular biology of the testis. New York, N.Y: Oxford University Press; 1993. pp. 58–89. [Google Scholar]
- 28.Nakayama H, Nishiyama H, Higuchi T, Kaneko Y, Fukumoto M, Fujita J. Change of cyclin D2 mRNA expression during murine testis development detected by fragmented cDNA subtraction method. Dev Growth Differ. 1996;38:141–151. doi: 10.1046/j.1440-169X.1996.t01-1-00003.x. [DOI] [PubMed] [Google Scholar]
- 29.Payne A H, Youngblood G L. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol Reprod. 1995;52:217–225. doi: 10.1095/biolreprod52.2.217. [DOI] [PubMed] [Google Scholar]
- 30.Pierson T M, Wang Y, DeMayo F J, Matzuk M M, Tsai S Y, O'Malley B W. Regulable expression of inhibin A in wild-type and inhibin alpha null mice. Mol Endocrinol. 2000;14:1075–1085. doi: 10.1210/mend.14.7.0478. [DOI] [PubMed] [Google Scholar]
- 31.Print C G, Loveland K L. Germ cell suicide: new insights into apoptosis during spermatogenesis. Bioessays. 2000;22:423–430. doi: 10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Rane S G, Dubus P, Mettus R V, Galbreath E J, Boden G, Reddy E P, Barbacid M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat Genet. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- 33.Ravnik S E, Rhee K, Wolgemuth D J. Distinct patterns of expression of the D-type cyclins during testicular development in the mouse. Dev Genet. 1995;16:171–178. doi: 10.1002/dvg.1020160209. [DOI] [PubMed] [Google Scholar]
- 34.Rhee K, Wolgemuth D J. Cdk family genes are expressed not only in dividing but also in terminally differentiated mouse germ cells, suggesting their possible function during both cell division and differentiation. Dev Dyn. 1995;204:406–420. doi: 10.1002/aja.1002040407. [DOI] [PubMed] [Google Scholar]
- 35.Serrano M, Lee H-W, Chin L, Cordon-Cardo C, Beach D, DePinho R A. Role of the INK4a locus in tumor suppression and mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 36.Sharpe R M. Follicle stimulating hormone and spermatogenesis in the adult male. J Endocrinol. 1989;57:152–159. doi: 10.1677/joe.0.1210405. [DOI] [PubMed] [Google Scholar]
- 37.Sherr C J, DePinho R A. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 38.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 39.Sicinski P, Donaher J L, Geng Y, Parker S B, Gardner H, Park M Y, Robker R L, Richards J S, McGinnis L K, Biggers J D, Eppig J J, Bronson R T, Elledge S J, Weinberg R A. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–474. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- 40.Tapanainen J S, Aittomäki K, Min J, Vaskivuo T, Huhtaniemi I T. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet. 1997;15:205–206. doi: 10.1038/ng0297-205. [DOI] [PubMed] [Google Scholar]
- 41.Tourtellotte W G, Nagarajan R, Auyeung A, Mueller C, Milbrandt J. Infertility associated with incomplete spermatogenic arrest and oligozoospermia in Egr4-deficient mice. Development. 1999;126:5061–5071. doi: 10.1242/dev.126.22.5061. [DOI] [PubMed] [Google Scholar]
- 42.Tsutsui T, Hesabi B, Moons D S, Pandolfi P P, Hansel K S, Koff A, Kiyokawa H. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27Kip1 activity. Mol Cell Biol. 1999;19:7011–7019. doi: 10.1128/mcb.19.10.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D, Enders G C. Expression of a specific mouse germ cell nuclear antigen (GCNA1) by early embryonic testicular teratoma cells in 129/Sv-S1/+ mice. Cancer Lett. 1996;100:31–36. doi: 10.1016/0304-3835(95)04068-4. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y, Ashley T, Brainerd E E, Bronson R T, Meyn M S, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 45.Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson N J. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell. 1996;85:537–548. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q, Wang X, Wolgemuth D J. Developmentally regulated expression of cyclin D3 and its potential in vivo interacting proteins during murine gametogenesis. Endocrinology. 1999;140:2790–2800. doi: 10.1210/endo.140.6.6756. [DOI] [PubMed] [Google Scholar]
- 47.Zindy F, Cunningham J J, Sherr C J, Jogal S, Smeyne R J, Roussel M F. Postnatal neuronal proliferation in mice lacking Ink4d and Kip1 inhibitors of cyclin-dependent kinases. Proc Natl Acad Sci USA. 1999;96:13462–13467. doi: 10.1073/pnas.96.23.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zindy F, Quelle D E, Roussel M F, Sherr C J. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
- 49.Zindy F, Soares H, Herzog K-H, Morgan J, Sherr C J, Roussel M F. Expression of INK4 inhibitors in cyclin D-dependent kinases during mouse brain development. Cell Growth Differ. 1997;8:1139–1150. [PubMed] [Google Scholar]
- 50.Zindy F, van Deursen J, Grosveld G, Sherr C J, Roussel M F. INK4d-deficient mice are fertile despite testicular atrophy. Mol Cell Biol. 2000;20:372–378. doi: 10.1128/mcb.20.1.372-378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zirkin B R, Santulli R, Awoniyi C A, Ewing L L. Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology. 1989;124:3043–3049. doi: 10.1210/endo-124-6-3043. [DOI] [PubMed] [Google Scholar]