Abstract
Global literature examining the association between mental health of women living with HIV (WLWH) and child development is scarce. In this study, we examined the relationship between mothers’ mental health and their children’s social development outcomes 6 months later. Data for these analyses come from several waves of interviews of 600 WLWH in the South Indian state of Andhra Pradesh, India. These women were enrolled in a 2×2 factorial clinical trial designed to assess the impact of food supplementation and nutrition education, both in addition to ASHA support, on adherence to ART and improved health outcomes for the women and one of their children. They were assessed on food security, stigma, social support, quality of life, depressive symptoms and child development outcomes. Results of longitudinal GEE regression analysis indicate that mother’s depressive symptoms were significantly negatively associated with child’s social quotient 6 months later. These findings have important implications for targeted health interventions, integrating mental health, both for WLWH and their children in India.
Keywords: Depression, Stigma, Women, Child development, HIV, India
Introduction
Caregiver physical and mental health have been found to impact children’s development in different populations(Black et al., 2007; Cluver, Boyes, Orkin, & Sherr, 2013; Sherr et al., 2014), including those living with HIV(Dorsey et al., 1999). Not only are there the physical health aspects of the disease, mothers living with HIV are more likely to have mental health issues than are non-infected caregivers (Chhagan et al., 2014; Nothling, Martin, Laughton, Cotton, & Seedat, 2013). This is partly due to the stigma surrounding HIV, which not only affects the woman living with HIV (WLWH) but often also the rest of the family, including her children (Murphy, Marelich, & Herbeck, 2012).
Children, either HIV-infected or HIV-exposed but uninfected, born to WLWH have worse developmental outcomes than unexposed children (McHenry et al., 2018). In infants, nutritional outcomes are affected (Kaaya et al., 2016); in young children, cognitive and motor development are affected (McHenry et al., 2018); and in older children, working memory, processing speed, and executive function are most affected(Phillips et al., 2016).Additionally, even though HIV-infected children on ART have improved neuro developmental outcomes (Gomez et al., 2018; Laughton et al., 2012), they still have worse developmental outcomes compared to uninfected children (Nozyce et al., 2006).
Previous interventions targeting HIV-infected or HIV-exposed children’s development have used multiple methods to try to improve their development (McHenry, McAteer, Oyungu, Deathe, & Vreeman, 2019; McHenry et al., 2018; Sherr et al., 2014). Several interventions targeting mental and cognitive development of HIV-infected or affected children have shown improvements in child cognitive development (Boivin et al., 2013; Boivin et al., 2010; Potterton, Stewart, Cooper, & Becker, 2010; Rotheram-Borus, Lee, Gwadz, & Draimin, 2001; Rotheram-Borus et al., 2006).Children living with HIV in Uganda who used cognitive rehabilitation computer games improved their maze learning and detection speed compared to control group (Boivin et al., 2010). Massage therapy with infants born to HIV-infected mothers was found to improve motor skills, stress behaviors, habituation, range of state, excitability, and scores on hearing and speech tests(Perez et al., 2015; Scafidi & Field, 1996).A United States-based study found that a family-based coping skills intervention improved conduct problems and emotional distress in adolescent children of parents with HIV (Rotheram-Borus et al., 2001). Another study that followed up the infants of these adolescents, indicated that they had improved development and fewer behavioral symptoms compared to the control group (Rotheram-Borus et al., 2006). HIV-positive children who received targeted, play-based interventions showed improved development over control group children (Potterton et al., 2010). These interventions all focused on the children of HIV-infected parents, rather than looking at the effect of a parental intervention on child development.
Providing nutritional supplements to HIV-infected prenatal and postpartum women in Tanzania was shown to be protective against motor delays in infants but did not affect cognitive ability(McGrath et al., 2006),and providing nutritional supplements to the children of HIV-infected mothers has also been shown to improve children’s growth and development in Uganda and South Africa (Boivin et al., 2017; Tomlinson, Rotheram-Borus, Scheffler, & le Roux, 2017). However, the Ugandan caregiver training program to increase children’s cognitive development did not produce better child outcomes than did health and nutrition training (Boivin et al., 2017), and the South African study found that health promotion programs need to address the mother’s mental health and the needs of HIV-positive women (Tomlinson et al., 2017). A 2019 systematic review found that while caregiver training for HIV-infected mothers had some benefits to children, additional research was needed before recommending any particular intervention(McHenry et al., 2019).
A search of PubMed failed to find any published interventions in India that targeted HIV-positive mothers to improve child outcomes. The data presented here were collected as part of a quasi-experimental trial of a community-based behavioral and nutritional intervention for women living with HIV in rural South India ( Nyamathi et al., 2012). The parent study built on and extended a previous, successful pilot that used India’s network of community health workers, ASHAs (Accredited Social Health Activists) in the state of Andhra Pradesh to address the needs of HIV-positive mothers to improve nutritional status, coping, and depressive symptoms ( Nyamathi et al., 2012).The present paper was designed to examine the effect of mother’s mental health on child’s future development 6 months later.
Method
Design
Data for these analyses come from 600 WLWH in the South Indian state of Andhra Pradesh, India. Participants were assessed at baseline and followed every 6 months for 18 months. These women were enrolled in a quasi-experimental 2×2 factorial clinical trial designed to assess the impact of food supplementation and nutrition education, both in addition to ASHA support, on adherence to ART and improved health outcomes for the women and one of their children. The length of the intervention was 6 months and outcomes were assessed at 6 months (immediately after the intervention and at 12 and 18 months. Specifically, the four groups included: 1) Asha-support (Asha; control); 2) Asha support + nutrition training (Asha+ NEd); 3) Asha support + nutrition supplements (Asha+ NSupl); and 4) Asha support + nutrition training + nutrition supplements (Asha+NEd+NSupl). Participants were recruited from 16 Primary Health Centers (PHCs) located in Nellore and Prakasam districts of Andhra Pradesh. The PHCs were grouped into four regional clusters to increase feasibility and promote intervention fidelity by reducing the risk of cross-contamination. Details of the trial design have been described elsewhere( Nyamathi et al., 2016)( Nyamathi et al., 2019). Human Subjects Protection Committee clearances were obtained both in the US and in India.
Participants
Women needed to fulfill the following inclusion criteria for study enrollment: 1) 18–50 years of age; 2) diagnosed with HIV and receiving ART for at least three months; 3) have CD4 levels above 100; and 4) reporting to have a child aged 3–8 living with them. Only one child per woman was included in the study.
Screening Procedures
Study flyers were developed in collaboration with our community partners and posted in selected Primary Health Clinics (PHCs), targeting local WLWH. Those who indicated interest were given a screening appointment with study staff in a private area at the PHC to assess age, HIV and ART status and having a child (3–8 years) to determine eligibility. Eligible and consented women completed the interviewer-administered baseline questionnaire in Telugu (the local language), using a tablet format.
Measures
Sociodemographic characteristics
Sociodemographic characteristics included age at baseline and at first HIV diagnosis, education, employment status, religion, marital status, monthly income, and number of children. For the children, we documented age, gender, and HIV status.
Food Insecurity.
The Household Food Insecurity Access Scale (Coates, Swindale, & Bilinsky, 2007) was used to measure food insecurity. This scale, used widely in India and elsewhere (Pasricha et al., 2010; Tsai et al., 2011; Vargas Puello, Alvarado Orellana, & AtalahSamur, 2013; Weiser et al., 2011) includes 9 items that assess the frequency, in the previous4 weeks, of worrying about not having enough food (1 item), and perceived insufficient quality (3 items) and quantity of food (5 items), due to a lack of resources. Response options range from 0 “Never” to 3 “Often” and were summed across all items.
Stigma Fears.
This scale was developed by our team, based on formative research with people living with HIV/AIDS (PLWH) participating in an earlier study (M. L. Ekstrand et al., 2020). Participants indicated on a 4-point Likert-type scale, how worried (from 0, “Not at all worried” to 3 “Very worried”) they were of stigmatizing or discriminatory reactions by family members (6 items), friends (6 items), health care workers (HCW, 5 items), people at work (4 items) and community members (12 items) if they were to disclose their HIV status to members of these social groups. A mean score was taken over all items. Cronbach’s alpha was 0.92 at baseline.
Internalized Stigma
Internalized Stigma was computed as the mean of 8 items from an Internalized Stigma Scale, which has been used in our previous research with Indian PLWH ( Ekstrand, Bharat, Ramakrishna, & Heylen, 2012; Ekstrand, Chandy, Heylen, Steward, & Singh, 2010; Ekstrand et al., 2011). Response options range from 0, “Not at all” to 3, “A great deal”. Alpha for the eight-item scale was 0.79 (baseline).
Vicarious Stigma
Vicarious Stigma was the mean of 10 items assessing how often (0 “Never” to 3 “Frequently”) the respondent had heard stories about other PLWH experiencing discrimination such as denial of care, or being ostracized by their family or village because of their HIV status. This measure was developed and used previously among Indian PLWH (Steward, Bharat, Ramakrishna, Heylen, & Ekstrand, 2013; Steward et al., 2011; Steward et al., 2008).Reliability in this sample at baseline was α=0.82.
Stigma-Avoidant Coping Strategies
Stigma-Avoidant Coping Strategies were assessed by asking how often the WLWH used strategies such as lying about the reason for medical visits to try to keep others from knowing that they had HIV/AIDS (0 “Never” to 3 “Often”) and was based on a scale used in our previous research in the region (Steward et al., 2008). A scale score was constructed by calculating the mean over all 9 items (α =0.81 at baseline).
Social Support
Social Support was assessed by the RAND Corporation’s Medical Outcomes Study (MOS) Social Support Scale (Sherbourne & Stewart, 1991), modified for India. The 18-item scale assesses frequency of support from friends and (extended) family or partners on a scale ranging from 1, “None of the time” to 5 “All of the time”. We computed the mean over all items to obtain a total social support score (α =0.94 at baseline).
Quality of Life
Quality of Life during the past week was assessed by asking the WLWH how satisfied they were with 10 aspects of life such as health, work, mood, family relationships, etc. (Endicott, Nee, Harrison, & Blumenthal, 1993). Responses were on a 4-point Likert-type scale ranging from 0 “Very unsatisfied” to 3 “Very satisfied”. We calculated the mean score over all items (α =0.81 at baseline).
Depressive Symptoms
Depressive Symptoms were assessed using the Center for Epidemiological Studies in Depression (CES-D) scale(Radloff, 1977). The CES-D scale is a widely used measure with well-established reliability and validity to assess depressive symptoms globally (Baron, Davies, & Lund, 2017; Meffert et al., 2019; Thai, Jones, Harris, & Heard, 2016)and in India(Chokkanathan & Mohanty, 2013; A. Nyamathi et al., 2011). It is a short (10 item) self-report scale designed to measure depressive symptomatology in the general population. Depressive symptoms in the past week are rated on a 4 point scale ranging from 0-“Rarely or none of the time” to 3“Most or all of the time.” Total scores range from 0–30.
Child Development
Child Development was assessed using the Vineland Social Maturity Scale (VSMS), a developmental checklist that assesses significant social, motor, cognitive and adaptive milestones from birth to 15 years of age. This was initially developed by Doll (1953) and adapted to the Indian culture (Malin, 1971). The scoring yields a social age and social quotient (100 * social age / calendar age).
Data Analysis
Descriptive statistics for the demographic and psychosocial characteristics of the WLWH and the target children consisted of frequency tabulations for categorical variables and mean and SD, or median and interquartile range (IQR) for continuous variables, depending on the normality of their distribution. The research question of interest was the effect of mother’s mental health on child’s future development. We examined this effect in longitudinal analyses of child social quotient predicted by mother’s depressive symptoms (CES-D score) 6 months prior, specifically a GEE model for a continuous variable (family=Gaussian, link=identity, robust standard errors), using child’s data from the 6, 12 and 18 mo, follow-up interviews and mother’s data from baseline, 6, and 12 months. Between baseline and 6-month follow-up, the mothers received the intervention, hence we originally planned to control for any intervention effects in the analyses. However, as shown in the Results section, mother’s psychosocial variables improved dramatically over time for all participants, regardless of intervention group, so there were no intervention effects on the mother variables that needed to be controlled for in our analyses. We examined if there was a direct intervention effect on the child outcome, but as there was not (see supplemental results), we did not retain it in the analyses. There was a difference in the outcome between the intervention groups at the start, however, which remained throughout the follow-up period, so we did include a main effect for intervention group.Other predictors included were mother’s marital status and child’s gender (time-invariant) and child’s age at each wave (time-varying). These predictor variables were selected based on their theoretical relationship to the outcome variable(Chhagan et al., 2014; Nothling, Martin, Laughton, Cotton, & Seedat, 2013).
Descriptive analyses were performed in SPSS v26, GEEanalyses in Stata v16.
Results
Sample characteristics at baseline: Findings indicate that the WLWH were, on average, in their mid-thirties and diagnosed with HIV at a mean age of 30.2. About half the sample were widowed (51.3%) and illiterate (48.7%). Nearly all WLWH worked (96.5%) as casual/day laborers, living with a median (IQR) household income of 2000INR (1500 – 2700), about 26 USD. (Table 1 near here).
Psychosocial vulnerability factors of WLWH pre- and post-intervention: Descriptive findings (Median and IQR) on stigma types (vicarious, internalized, stigma avoidant coping and fears), depressive symptoms, food insecurity and social support indicate a significant reduction in scores from baseline to 6 months follow up, which took place shortly after completion of the intervention. Quality of life on the other hand improved significantly from baseline to 6 months follow up (Table 2 near here). Results from the 12- and 18-months follow-up show these improvements were sustained over the following year.
Children of WLWH: Descriptive findings indicate that nearly half of the children of the WLWH in the study were male. About 18% of them were HIV+ at baseline (Table 1). Their average age at the 6 month follow up was 7 years 10 months. Their average social age was about 7 years 1 month, and the mean (SD) social quotient was 88.8 (11.0). Details for the 12 and 18 months follow-up are shown in Table 3. (Table 3 near here)
Predictors of child development: Results of the GEE longitudinal analysis indicate that mother’s depressive symptoms were significantly negatively associated with child’s social quotient 6 months later (b = −0.09, 95% CI: −0.16 – −0.02]). Boys’ social quotient was on average 2.43 (95% CI: 1.68 – 3.18)], p <0.001) higher than girls’. Child’s age was significantly associated with social quotient as well, as shown in Figure 1. (Table 4 and Figure 1 near here)
Table 1:
Sample Characteristics of WLWH and their child at BL (n=600)
| Variablesa | Frequency | Percent |
|---|---|---|
| Marital Status: | ||
| Married | 238 | 39.7 |
| Widowed | 308 | 51.3 |
| Divorced/Separated | 54 | 9.0 |
| Number of children | ||
| 1 | 207 | 34.5 |
| 2 | 296 | 49.3 |
| ≥3 | 97 | 16.2 |
| Occupation | ||
| Casual/day labor | 579 | 96.5 |
| Other | 20 | 3.3 |
| None | 1 | 0.2 |
| Religion | ||
| Hindu | 439 | 73.2 |
| Christian | 117 | 19.5 |
| Muslim | 44 | 7.3 |
| Education | ||
| None | 292 | 48.7 |
| < 5 yrs | 98 | 16.3 |
| 5–9 yrs | 123 | 20.5 |
| ≥10 yrs | 87 | 14.5 |
| Child male gender | 294 | 49.0 |
| Child HIV+ | 109 | 18.2 |
|
|
||
| Mean | (SD) | |
|
|
||
| Age at BL (years) | 34.3 | (7.0) |
| Age when first diagnosed HIV+ | 30.2 | (7.2) |
| Monthly HH income, INR, Median (IQR) | 2000 | (1500–2700) |
| Food insecurity (0–27) | 21.15 | (3.44) |
| Child age (years) | 7.4 | (1.7) |
Variables pertain to mother unless otherwise indicated.
Table 2:
Stigma, quality of life and depressive symptoms and social support scores of WLWH at baseline, 6- and 12-month follow-up
| Baseline | 6-month follow-upa | 12-month follow-upa | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Median | IQR | Median | IQR | % with best possible scoreb | Median | IQR | % with best possible scoreb | |
| Vicarious stigma (0–3) | 2.60 | (2.30 – 2.80) | 0 | (0 – 0) | 88.8 | 0 | (0 – 0) | 95.3 |
| Internalized stigma (0–3) | 2.88 | (2.75 – 3) | 0 | (0 – 0) | 97.5 | 0 | (0 – 0) | 100 |
| Stigma-avoidant coping (0–3) | 2.56 | (2.33 – 2.67) | 0 | (0 – 0) | 92.3 | 0 | (0 – 0) | 98.0 |
| Stigma fears (0–3) | 2.91 | (2.73 – 3) | 0 | (0 – 0) | 95.5 | 0 | (0 – 0) | 89.3 |
| Quality of life (0–3) | 0.30 | (0 – 0.40) | 3 | (2.9 – 3) | 74.3 | 3 | (3 – 3) | 78.5 |
| Depression: CES-D score (0–30) | 8 | (7 – 9) | 0 | (0 – 0) | 97.3 | 0 | (0 – 0) | 93.5 |
| Food insecurity (0–27) | 21 | (20 – 23) | 0 | (0 – 0) | 98.5 | 0 | (0 – 0) | 100 |
| Social support (1–5) | 1 | (1 – 1.11) | 5 | (5 – 5) | 79.7 | 5 | (5 – 5) | 83.5 |
Change from Baseline to 6 months and BL to 12 months follow-up: p<0.001 for all variables based on Wilcoxon Signed Rank test.
Best possible score is highest possible score on QOL and Social Support and lowest possible score on all others
Table 3:
Descriptive data of children of WLWH at 6- to 18-mo follow up (n=600)
| 6-month FU | 12-month FU | 18-month FU | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |
|
|
||||||
| Age in months | 94.3 | (20.0) | 100.5 | (20.0) | 106.8 | (20.0) |
| Raw Vineland score | 65.2 | (10.1) | 67.7 | (9.1) | 70.3 | (8.7) |
| Social age based on Vineland, mo | 85.3 | (23.8) | 91.9 | (22.7) | 99.6 | (23.3) |
| Social quotienta | 88.8 | (11.0) | 90.3 | (8.9) | 92.3 | (8.2) |
Social quotient = (social age / calendar age) x 100.
Figure 1:
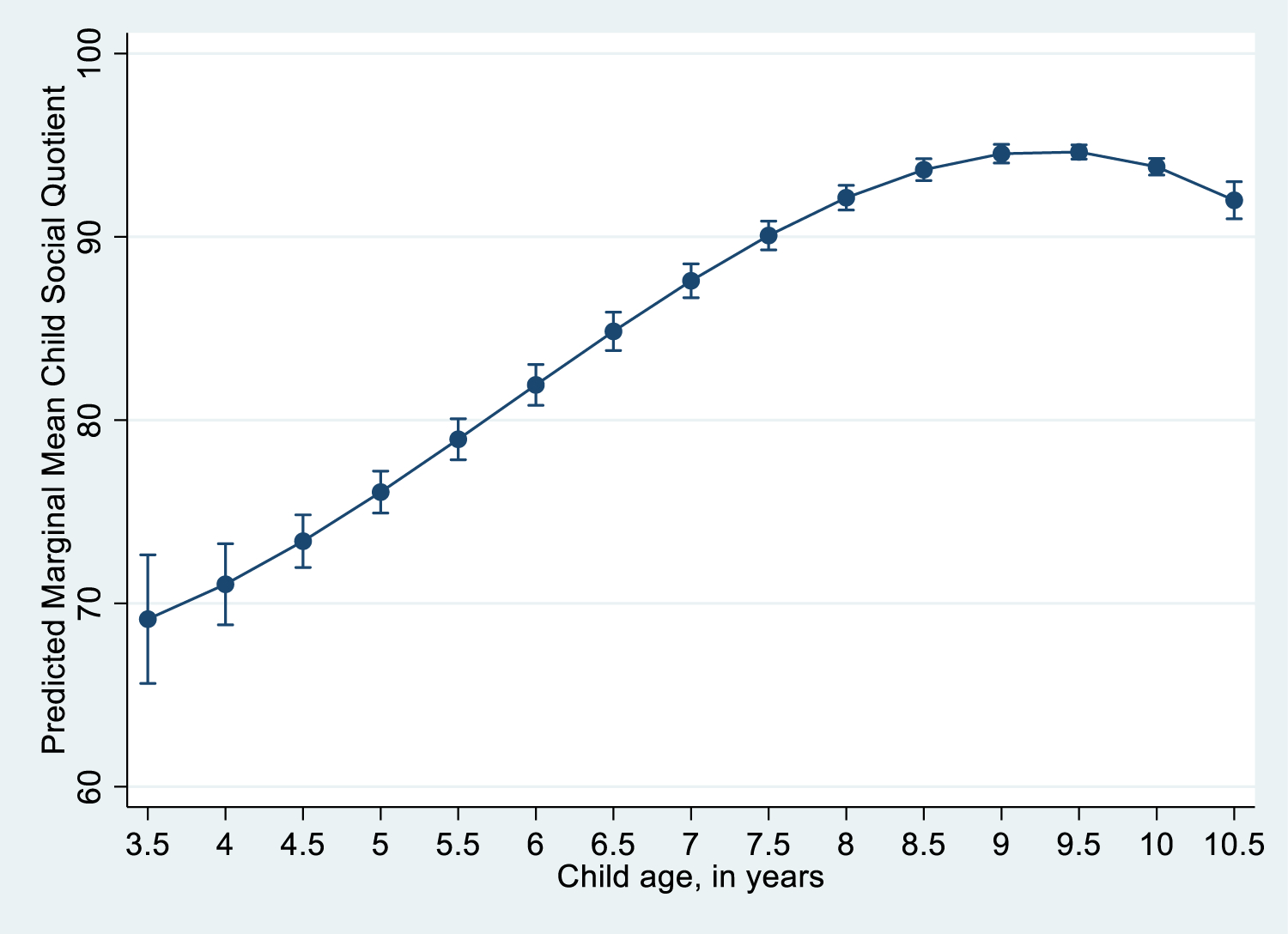
Social Quotient by child’s age
Table 4:
GEE model for repeated measures of child development (Social Quotient) predicted by mother’s depression 6mo prior, and demographics
| Predictors | Coefficient | 95% CI | p-value |
|---|---|---|---|
| Unmarried | 0.35 | (−0.41; 1.12) | 0.361 |
| Depressive symptoms (CES-D-10 score) 6 months prior | −0.09 | (−0.16; −0.02) | 0.011 |
| Intervention groupa | |||
| Asha only | Reference | - | - |
| Asha + NEd | −3.26 | (−4.35; −2.16) | <0.001 |
| Asha + NSupl | −2.09 | (−3.21; −0.97) | <0.001 |
| Asha + NEd + NSupl | −4.73 | (−5.88; −3.58) | <0.001 |
| Child male gender | 2.43 | (1.68; 3.18) | <0.001 |
| Child age (per 6-month increase)b | |||
| Linear | 0.50 | (0.29; 0.72) | <0.001 |
| Quadratic | −0.06 | (−0.08; −0.05) | <0.001 |
| Cubic | −0.0006 | (−0.0008; −0.0003) | <0.001 |
Asha, Accredited Social Health Activists; Ned, nutrition education; NSupl, nutrition supplements.
centered at overall median of 108 months= 9 years
Discussion
We examined the association between mother’s mental health in 600 WLWH and their children’s future developmental outcomes 6 months later, following mothers’ receiving one of four types of intervention. Data for these analyses come from a larger study examining the efficacy of a nurse-led and ASHA-supported nutrition intervention targeting the mother on child growth and development in the high HIV prevalence South Indian state of Andhra Pradesh, India (Nyamathi et al., 2016; Nyamathi et al., 2012; Nyamathi et al., 2019; Shin et al, 2020).
Results of the GEE longitudinal analysis indicated that mother’s depressive symptoms were significantly negatively associated with child’s social quotient 6 months later. Several studies have examined the influence of antenatal and postnatal depression on infant’s nutritional status, growth and development in community samples. However, very few studies have been conducted on the impact of maternal depression in WLWH and their children. One such published study in Tanzania, indicated a negative relationship between postnatal depression in WLWH and infant nutritional and growth outcomes. These authors also found that chronicity of depression affected child outcomes (Kaaya et al., 2016)and hence underscored the need to target maternal mental health of WLWH (Kaaya et al., 2016; Tomlinson et al., 2017).
No known studies globally have examined the association between maternal depressive symptoms and child development following a nutritional intervention targeting WLWH. Much of the available literature has shown improved child development outcomes when nutritional intervention was given to WLWH in the perinatal period (McGrath et al., 2006) or when the intervention targeted HIV infected children of WLWH (Boivin et al., 2017; Tomlinson et al., 2017)as opposed to WLWH themselves (Boivin et al., 2013; Boivin et al., 2010; Perez et al., 2015; Potterton et al., 2010; Rotheram-Borus et al., 2001; Rotheram-Borus et al., 2006; Scafidi & Field, 1996).Studies on HIV infected or HIV exposed but uninfected children of WLWH have indicated poorer cognitive and motor development compared to unexposed children (McHenry et al., 2018). Although HIV infected children on ART showed better developmental outcomes, they were still lower than unexposed children (Nozyce et al., 2006). These studies (Boivin et al., 2013; Boivin et al., 2010; Boivin et al., 2017; McGrath et al., 2006; Perez et al., 2015; Potterton et al., 2010; Rotheram-Borus et al., 2001; Rotheram-Borus et al., 2006; Scafidi & Field, 1996) however, did not comment on the role of maternal depression. A recent systematic review found that while caregiver training for HIV-infected mothers had some benefits to children, additional research was needed before recommending any particular intervention (McHenry et al., 2019).
We also found that male children had a significantly higher social quotient than female children. In patriarchal cultures such as India, male children have a privileged position compared to their female counterparts. Male children have greater access to nutrition, education, employment, leisure, career/marriage choices compared to females and this gender disadvantage may explain this relationship (Satyanarayana, Chandra, Sharma, Sowmya, & Kandavel, 2016). We found that social quotient increased until about age 9, and after 9.5 began to trend downward again. Child development is known to steadily increase as their chronological age increases. However, there are certain critical periods where development may wax and wane before it finally plateaus.
Demographic data also suggests that this sample of WLWH have multiple psychosocial vulnerabilities in addition to being HIV+. A significant proportion of these women are widowed at a very young age with young children to care for, are illiterate, work as casual day laborers and hail from low income households. Other studies on WLWH have described similar risk factors (Chhagan et al., 2014; Dorsey et al., 1999; Laughton et al., 2012; Murphy et al., 2012; Nothling et al., 2013; Nozyce et al., 2006)including a recent Indian study(Reynolds et al., 2016). These risk factors can in turn affect both maternal mental health and infant/child developmental outcomes (Cluver et al., 2013; Sherr et al., 2014).
Women in India as per traditional gender norms of a patriarchal culture are primary caregivers for their spouse, children and extended family members. As a result, women experience a significant amount of stress which renders them vulnerable to common mental disorders such as depression(Reynolds et al., 2016). Rates of depression are disproportionately higher in women compared to men, and the effect is further compounded by social factors of poverty and food insecurity (Cornelius et al., 2017), which are highly prevalent in Andhra Pradesh (Athreya VB, 2008). WLWH have an increased vulnerability to depression (Chhagan et al., 2014; Murphy et al., 2012; Nothling et al., 2013) and are perhaps unable to provide adequate nutrition and stimulation necessary for child development. Studies have also indicated that caregivers physical and mental health impacts child development significantly(Black et al., 2007; Cluver et al., 2013; Sherr et al., 2014) including for those infected with HIV(Dorsey et al., 1999).
The strength of this manuscript is the finding that maternal depression is significantly negatively associated with child development. This has important program implications, such as identifying and treating maternal depression early as part of antenatal care and also track child development. Further, we were able to demonstrate this association using a longitudinal design in a vulnerable and under-served population. The present analysis also has limitations, in that, due to multicollinearity in maternal psychosocial variables, only maternal depression scores, which was our variable of interest, was retained in the model as a predictor. Further, children are known to have certain growth spurts and development is an ongoing process till the child reaches 16 years, after which it plateaus. While identifying deficits may provide an opportunity for early intervention, some of these mild deficits and a development lag may also improve spontaneously with time.
Supplementary Material
Funding Support:
Support for this research was provided by an Award R01MH098728 from the National Institute on Mental Health.
REFERENCES
- Athreya VB, B. R., Anuradha G, Gopinath R, Sakthi Velan A (2008). Report on the state of food insecurity in rural India. Retrieved from http://www.wfp.org/content/india-report-state-food-insecurity-rural-india-december-2008
- Baron EC, Davies T, & Lund C (2017). Validation of the 10-item Centre for Epidemiological Studies Depression Scale (CES-D-10) in Zulu, Xhosa and Afrikaans populations in South Africa. BMC Psychiatry, 17(1), 6. doi: 10.1186/s12888-016-1178-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Baqui AH, Zaman K, McNary SW, Le K, Arifeen SE, … Black RE (2007). Depressive symptoms among rural Bangladeshi mothers: implications for infant development. J Child Psychol Psychiatry, 48(8), 764–772. doi: 10.1111/j.1469-7610.2007.01752.x [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Bangirana P, Nakasujja N, Page CF, Shohet C, Givon D, … Klein PS (2013). A year-long caregiver training program to improve neurocognition in preschool Ugandan HIV-exposed children. J Dev Behav Pediatr, 34(4), 269–278. doi: 10.1097/DBP.0b013e318285fba9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Busman RA, Parikh SM, Bangirana P, Page CF, Opoka RO, & Giordani B (2010). A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology, 24(5), 667–673. doi: 10.1037/a0019312 [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Nakasujja N, Familiar-Lopez I, Murray SM, Sikorskii A, Awadu J, … Bass JK (2017). Effect of Caregiver Training on the Neurodevelopment of HIV-Exposed Uninfected Children and Caregiver Mental Health: A Ugandan Cluster-Randomized Controlled Trial. J Dev Behav Pediatr, 38(9), 753–764. doi: 10.1097/dbp.0000000000000510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhagan MK, Mellins CA, Kauchali S, Craib MH, Taylor M, Kvalsvig JD, & Davidson LL (2014). Mental health disorders among caregivers of preschool children in the Asenze study in KwaZulu-Natal, South Africa. Matern Child Health J, 18(1), 191–199. doi: 10.1007/s10995-013-1254-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokkanathan S, & Mohanty J (2013). Factor structure of the CES-D scale among older adults in Chennai, India. Aging Ment Health, 17(4), 517–525. doi: 10.1080/13607863.2012.751580 [DOI] [PubMed] [Google Scholar]
- Cluver L, Boyes M, Orkin M, & Sherr L (2013). Poverty, AIDS and child health: identifying highest-risk children in South Africa. S Afr Med J, 103(12), 910–915. doi: 10.7196/samj.7045 [DOI] [PubMed] [Google Scholar]
- Cornelius T, Jones M, Merly C, Welles B, Kalichman MO, & Kalichman SC (2017). Impact of food, housing, and transportation insecurity on ART adherence: a hierarchical resources approach. AIDS Care, 29(4), 449–457. doi: 10.1080/09540121.2016.1258451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey S, Forehand R, Armistead LP, Morse E, Morse P, & Stock M (1999). Mother Knows Best? Mother and Child Report of Behavioral Difficulties of Children of HIV-Infected Mothers. Journal of Psychopathology and Behavioral Assessment, 21(3), 191–206. doi: 10.1023/A:1022821314228 [DOI] [Google Scholar]
- Ekstrand ML, Bharat S, Ramakrishna J, & Heylen E (2012). Blame, symbolic stigma and HIV misconceptions are associated with support for coercive measures in urban India. AIDS Behav, 16(3), 700–710. doi: 10.1007/s10461-011-9888-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand ML, Chandy S, Heylen E, Steward W, & Singh G (2010). Developing useful HAART adherence measures for India: the Prerana study. J Acquir Immune Defic Syndr, 53(3), 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand ML, Heylen E, Pereira M, D’Souza J, Nair S, Mazur A, … Chandy S (2020). A Behavioral Adherence Intervention Improves Rates of Viral Suppression Among Adherence-Challenged People Living with HIV in South India. AIDS Behav. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=31933020 [DOI] [PMC free article] [PubMed]
- Ekstrand ML, Shet A, Chandy S, Singh G, Shamsundar R, Madhavan V, … Kumarasamy N (2011). Suboptimal adherence associated with virological failure and resistance mutations to first-line highly active antiretroviral therapy (HAART) in Bangalore, India. Int Health, 3(1), 27–34. doi: 10.1016/j.inhe.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, & Blumenthal R (1993). Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol Bull, 29(2), 321–326. [PubMed] [Google Scholar]
- Gomez LA, Crowell CS, Njuguna I, Cranmer LM, Wamalwa D, Chebet D, … Benki-Nugent S (2018). Improved Neurodevelopment After Initiation of Antiretroviral Therapy in Human Immunodeficiency Virus-infected Children. Pediatr Infect Dis J, 37(9), 916–922. doi: 10.1097/inf.0000000000001942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaya S, Garcia ME, Li N, Lienert J, Twayigize W, Spiegelman D, & Smith Fawzi MC (2016). Association of maternal depression and infant nutritional status among women living with HIV in Tanzania. Matern Child Nutr, 12(3), 603–613. doi: 10.1111/mcn.12154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughton B, Cornell M, Grove D, Kidd M, Springer PE, Dobbels E, … Cotton MF (2012). Early antiretroviral therapy improves neurodevelopmental outcomes in infants. Aids, 26(13), 1685–1690. doi: 10.1097/QAD.0b013e328355d0ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin A (1971). Vineland Social Maturity Scale: Nagpur adaptation. Lucknow, India: Indian Psychological Corporation. [Google Scholar]
- McGrath N, Bellinger D, Robins J, Msamanga GI, Tronick E, & Fawzi WW (2006). Effect of maternal multivitamin supplementation on the mental and psychomotor development of children who are born to HIV-1-infected mothers in Tanzania. Pediatrics, 117(2), e216–225. doi: 10.1542/peds.2004-1668 [DOI] [PubMed] [Google Scholar]
- McHenry MS, McAteer CI, Oyungu E, Deathe AR, & Vreeman RC (2019). Interventions for developmental delays in children born to HIV-infected mothers: a systematic review. AIDS Care, 31(3), 275–282. doi: 10.1080/09540121.2018.1533629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry MS, McAteer CI, Oyungu E, McDonald BC, Bosma CB, Mpofu PB, … Vreeman RC (2018). Neurodevelopment in Young Children Born to HIV-Infected Mothers: A Meta-analysis. Pediatrics, 141(2). doi: 10.1542/peds.2017-2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert SM, Neylan TC, McCulloch CE, Maganga L, Adamu Y, Kiweewa F, … Valcour VG (2019). East African HIV care: depression and HIV outcomes. Glob Ment Health (Camb), 6, e9. doi: 10.1017/gmh.2019.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Marelich WD, & Herbeck DM (2012). Impact of maternal HIV health: a 12-year study of children in the Parents And Children Coping Together project. J Adolesc Health, 51(4), 313–318. doi: 10.1016/j.jadohealth.2011.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothling J, Martin CL, Laughton B, Cotton MF, & Seedat S (2013). Maternal post-traumatic stress disorder, depression and alcohol dependence and child behaviour outcomes in mother-child dyads infected with HIV: a longitudinal study. BMJ Open, 3(12), e003638. doi: 10.1136/bmjopen-2013-003638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozyce ML, Lee SS, Wiznia A, Nachman S, Mofenson LM, Smith ME, … Pelton S (2006). A behavioral and cognitive profile of clinically stable HIV-infected children. Pediatrics, 117(3), 763–770. doi: 10.1542/peds.2005-0451 [DOI] [PubMed] [Google Scholar]
- Nyamathi A, Ekstrand M, Heylen E, Ramakrishna P, Yadav K, Sinha S, … Arab L (2016). Relationships among adherence and physical and mental health among women living with HIV in rural India. AIDS Behav. doi: 10.1007/s10461-016-1631-3 [DOI] [PMC free article] [PubMed]
- Nyamathi A, Heravian A, Zolt-Gilburne J, Sinha S, Ganguly K, Liu E, … Leake B (2011). Correlates of depression among rural women living with AIDS in Southern India. Issues Ment Health Nurs, 32(6), 385–391. doi: 10.3109/01612840.2011.577269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyamathi A, Salem BE, Meyer V, Ganguly KK, Sinha S, & Ramakrishnan P (2012). Impact of an Asha intervention on depressive symptoms among rural women living with AIDS in India: comparison of the Asha-Life and Usual Care program. AIDS Educ Prev, 24(3), 280–293. doi: 10.1521/aeap.2012.24.3.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyamathi AN, Shin SS, Sinha S, Carpenter CL, Garfin DR, Rk P, … Ekstrand ML (2019). Sustained Effect of a Community-based Behavioral and Nutrition Intervention on HIV-related Outcomes among Women living with HIV in Rural India: A Quasi-experimental Trial. J Acquir Immune Defic Syndr. doi: 10.1097/qai.0000000000002044 [DOI] [PMC free article] [PubMed]
- Perez EM, Carrara H, Bourne L, Berg A, Swanevelder S, & Hendricks MK (2015). Massage therapy improves the development of HIV-exposed infants living in a low socio-economic, peri-urban community of South Africa. Infant Behav Dev, 38, 135–146. doi: 10.1016/j.infbeh.2014.12.011 [DOI] [PubMed] [Google Scholar]
- Phillips N, Amos T, Kuo C, Hoare J, Ipser J, Thomas KG, & Stein DJ (2016). HIV-Associated Cognitive Impairment in Perinatally Infected Children: A Meta-analysis. Pediatrics, 138(5). doi: 10.1542/peds.2016-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poda GG, Hsu CY, & Chao JC (2017). Malnutrition is associated with HIV infection in children less than 5 years in Bobo-Dioulasso City, Burkina Faso: A case-control study. Medicine (Baltimore), 96(21), e7019. doi: 10.1097/md.0000000000007019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potterton J, Stewart A, Cooper P, & Becker P (2010). The effect of a basic home stimulation programme on the development of young children infected with HIV. Dev Med Child Neurol, 52(6), 547–551. doi: 10.1111/j.1469-8749.2009.03534.x [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement, 1(3), 385–401. [Google Scholar]
- Reynolds NR, Satyanarayana V, Duggal M, Varghese M, Liberti L, Singh P, … Chandra PS (2016). MAHILA: a protocol for evaluating a nurse-delivered mHealth intervention for women with HIV and psychosocial risk factors in India. BMC health services research, 16(a), 352–352. doi: 10.1186/s12913-016-1605-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AM, Hall CS, & Martinez-Alier N (2014). Aetiology and management of malnutrition in HIV-positive children. Arch Dis Child, 99(6), 546–551. doi: 10.1136/archdischild-2012-303348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Lee MB, Gwadz M, & Draimin B (2001). An intervention for parents with AIDS and their adolescent children. Am J Public Health, 91(8), 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Lester P, Song J, Lin YY, Leonard NR, Beckwith L, … Lord L (2006). Intergenerational benefits of family-based HIV interventions. J Consult Clin Psychol, 74(3), 622–627. doi: 10.1037/0022-006x.74.3.622 [DOI] [PubMed] [Google Scholar]
- Satyanarayana VA, Chandra PS, Sharma MK, Sowmya HR, & Kandavel T (2016). Three sides of a triangle: gender disadvantage, resilience and psychological distress in a sample of adolescent girls from India. International Journal of Culture and Mental Health, 9(4), 364–372. doi: 10.1080/17542863.2016.1206949 [DOI] [Google Scholar]
- Scafidi F, & Field T (1996). Massage therapy improves behavior in neonates born to HIV-positive mothers. J Pediatr Psychol, 21(6), 889–897. [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, & Stewart AL (1991). The MOS social support survey. Soc Sci Med, 32(6), 705–714. [DOI] [PubMed] [Google Scholar]
- Sherr L, Cluver LD, Betancourt TS, Kellerman SE, Richter LM, & Desmond C (2014). Evidence of impact: health, psychological and social effects of adult HIV on children. Aids, 28 Suppl 3, S251–259. doi: 10.1097/qad.0000000000000327 [DOI] [PubMed] [Google Scholar]
- Shin SS, Satyanarayana VA, Ekstrand ML, Carpenter CL, Wang Q, Yadav K, Ramakrishnan P, Pamujula S, Sinha S, & Nyamathi AM (2020). The Effect of Community-Based Nutritional Interventions on Children of Women Living With Human Immunodeficiency Virus in Rural India: A 2 × 2 Factorial Intervention Trial. Clin Infect Dis, 71(6),1539–1546. doi: 10.1093/cid/ciz1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward WT, Bharat S, Ramakrishna J, Heylen E, & Ekstrand ML (2013). Stigma is associated with delays in seeking care among HIV-infected people in India. J Int Assoc Provid AIDS Care, 12(2), 103–109. doi: 10.1177/1545109711432315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward WT, Chandy S, Singh G, Panicker ST, Osmand TA, Heylen E, & Ekstrand ML (2011). Depression is not an inevitable outcome of disclosure avoidance: HIV stigma and mental health in a cohort of HIV-infected individuals from Southern India. Psychol Health Med, 16(1), 74–85. doi: 10.1080/13548506.2010.521568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward WT, Herek GM, Ramakrishna J, Bharat S, Chandy S, Wrubel J, & Ekstrand ML (2008). HIV-related stigma: adapting a theoretical framework for use in India. Soc Sci Med, 67(8), 1225–1235. doi: 10.1016/j.socscimed.2008.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai TT, Jones MK, Harris LM, & Heard RC (2016). Screening value of the Center for epidemiologic studies - depression scale among people living with HIV/AIDS in Ho Chi Minh City, Vietnam: a validation study. BMC Psychiatry, 16, 145. doi: 10.1186/s12888-016-0860-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstans S, Kerac M, Maleta K, Banda T, & Nesbitt A (2008). HIV prevalence in severely malnourished children admitted to nutrition rehabilitation units in Malawi: geographical & seasonal variations a cross-sectional study. BMC Pediatr, 8, 22. doi: 10.1186/1471-2431-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson M, Rotheram-Borus MJ, Scheffler A, & le Roux I (2017). Antenatal depressed mood and child cognitive and physical growth at 18-months in South Africa: a cluster randomised controlled trial of home visiting by community health workers. Epidemiol Psychiatr Sci, 1–10. doi: 10.1017/s2045796017000257 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


