Abstract
Urinary tract infection (UTI) develops after a pathogen adheres to the inner lining of the urinary tract. Cases of UTIs are predominantly caused by several Gram-negative bacteria and account for high morbidity in the clinical and community settings. Of greater concern are the strains carrying antimicrobial resistance (AMR)-conferring genes. The gravity of a UTI is also determined by a spectrum of other virulence factors. This study represents a pilot project to investigate the burden of AMR among uropathogens in East Africa. We examined bacterial samples isolated in 2017–2018 from in- and out-patients in Kenya (KY) and Uganda (UG) that presented with clinical symptoms of UTI. We reconstructed the evolutionary history of the strains, investigated their population structure, and performed comparative analysis their pangenome contents. We found 55 Escherichia coli and 19 Klebsiella pneumoniae strains confirmed uropathogenic following screening for the prevalence of UTI virulence genes including fimH, iutA, feoA/B/C, mrkD, and foc. We identified 18 different sequence types in E. coli population while all K. pneumoniae strains belong to ST11. The most prevalent E. coli sequence types were ST131 (26%), ST335/1193 (10%), and ST10 (6%). Diverse plasmid types were observed in both collections such as Incompatibility (IncF/IncH/IncQ1/IncX4) and Col groups. Pangenome analysis of each set revealed a total of 2862 and 3464 genes comprised the core genome of E. coli and K. pneumoniae population, respectively. Among these are acquired AMR determinants including fluoroquinolone resistance-conferring genes aac(3)-Ib-cr and other significant genes: aad, tet, sul1, sul2, and cat, which are associated with aminoglycoside, tetracycline, sulfonamide, and chloramphenicol resistance, respectively. Accessory genomes of both species collections were detected several β-lactamase genes, blaCTX-M, blaTEM and blaOXA, or blaNDM. Overall, 93% are multi-drug resistant in the E. coli collection while 100% of the K. pneumoniae strains contained genes that are associated with resistance to three or more antibiotic classes. Our findings illustrate the abundant acquired resistome and virulome repertoire in uropathogenic E. coli and K. pneumoniae, which are mainly disseminated via clonal and horizontal transfer, circulating in the East African region. We further demonstrate here that routine genomic surveillance is necessary for high-resolution bacterial epidemiology of these important AMR pathogens.
Keywords: antimicrobial resistance, pangenome, lmic, public health
1. Introduction
Antimicrobial resistance (AMR) has raised alarms as a global health threat. AMR is often fueled by misuse and abuse of antibiotics including self-medication [1,2] and unrestricted access to antimicrobial drugs [3,4,5], and is further accelerated by industrialization, poor waste disposal, and poor hygiene levels. AMR pathogens are frequently detected in food, clinical, and environmental settings in East Africa. Despite facing broad challenges, significant efforts have recently been put in place to curb AMR in East African countries. For instance, Kenya (KY) has adapted the National Action Plan that incorporates One Health measures to prevent AMR and is highly supported by multiple governmental policies (NAPCAR 2017) [6]. Similarly, an extensive evaluation of the AMR situation in Uganda (UG) was assessed by the Uganda National Academy of Sciences (UNAS) supported by the Global Antibiotic Resistance Partnership (GARP)-Uganda (UNAS 2015) [7]. High prevalence of multi-drug resistant bacteria particularly extended-spectrum beta-lactamase (ESBL)-producing strains is significantly recorded in both countries.
Urinary tract infection (UTI) develops after a pathogen’s adherence to the inner lining of the urinary tract. UTIs occur among patients of all age groups and account for high morbidity in the clinical and community settings [8]. Following binding within the urinary tract, uropathogens either cause asymptomatic or commensal connection or severe disease. About 1% of the population have asymptomatic bacteriuria (ABU), wherein a pathogen (≥105 cfu mL−1) inhabits the tract without eliciting mucosal host response [9,10]. Infections in the lower urinary tract region (e.g., cystitis) are recognized by symptoms such as dysuria. Successful virulent strains can induce pyelonephritis where rapid immune response is mobilized via cytokine secretion and influx of immune cells. UTIs are either uncomplicated or complicated. Uncomplicated UTI cases are usually observed in patients who are otherwise healthy, while complicated UTIs are diagnosed in compromised patients (e.g., if they have anatomical or functional anomalies in their urinary tract or are under long-term catheterization) [11] Treatment of these complicated UTI cases is often confounded by AMR uropathogens usually caused by Gram-negative bacteria [12]. Uncomplicated UTIs are frequently caused by uropathogenic Escherichia coli (E. coli (UPEC)) while complicated cases might be caused by several pathogens such as Proteus mirabilis, Providencia stuartii, Morganella morganii, Klebsiella pneumoniae (K. pneumoniae), and Pseudomonas aeruginosa [8]. Recurrent UTI cases are also common, particularly when urinary tract anomalies linger, or treatment failed to kill resistant bacteria [13], leading to more severe type of infections. Due to the lack of active investigation of UTI cases in East Africa, particularly in the community, access to accurate data can be challenging.
An increasing number of studies have employed whole genome sequencing (WGS) and analyses for disease surveillance in both hospital and community settings [14,15,16]. The high-resolution genotyping that WGS provides allows one to investigate and describe the population structure and evolutionary history of the isolates, as well as tracing their spread. Outbreaks have been robustly detected and described using high-throughput methodologies designed for bacterial pathogens [17,18,19,20]. Comprehensive AMR gene databases and prediction tools are also available that help assess AMR gene content in whole genomes with high accuracy [21].
Here, we used WGS to investigate the prevalence of acquired AMR-conferring genes in E. coli and K. pneumoniae isolated from urine samples taken from patients in rural areas of KY and UG that presented UTI-like symptoms. Our analysis of AMR determinants was limited to those associated with the pan genome and mutation in core genes responsible for antibiotic resistance were not investigated. We further explored their phylogenetic relationships of the isolates collected with other currently circulating African and global strains. This study represents a pilot project of the HATUA consortium. HATUA stands for Holistic Approach to Unravel Antibiotic Resistance in East Africa and the team is comprised of researchers from different disciplines that aim to tackle the main drivers of AMR among uropathogens in East Africa.
2. Methods
2.1. Study Design and Patient Recruitment
A total of N = 150 bacterial isolates were obtained from patients in KY (n = 91) and UG (n = 59) presenting UTI-like symptoms, as part of a larger study. Ethical Review Board of University of St Andrews ethical approval, Approval code MD14548 and KY (KEMRI/SERU/P00112/3865) approved verbal consent taken from all the patients. Important patient data such as name, age, gender, location was recorded, and unique identification number were assigned to each patient.
2.2. Library Preparation and Whole Genome Sequencing
Bacterial genomic DNA for the isolates were extracted using the QIAxtractor (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Library preparation was conducted according to the Illumina protocol and sequenced (96-plex) on an Illumina MiSeq platform (Illumina, San Diego, CA, USA) using 250 bp paired-end reads.
2.3. Read Library Quality Control, Mapping and De Novo Genome Assembly
Illumina MiSeq read libraries were rid of sequencing adapters and ambiguous bases using Fastp [22]. Sets that passed the quality filtering were de novo assembled using Unicycler v4.6 [23] pipeline in normal mode to merge contigs.
The read libraries were mapped to reference sequences using SMALT v7.6 (http://www.sanger.ac.uk/resources/software/SMALT/ (accessed on 18 December 2019) [24] and the resulting SAM files were converted to BAM format, sorted and PCR duplicates removed using SAMtools v1.19 [25]. Strain TOP52_1721_U1 [26] was used the reference genome for the K. pneumoniae samples while the strain EC958 [27] was employed as the reference sequence for the E. coli population.
2.4. Species Identification, Sero- and Sequence Typing, Genome Annotation and Screening for UTI Virulence/AMR Genes
Prediction of bacterial species was carried out by uploading the assemblies on PathogenWatch website (https://pathogen.watch (accessed on 31 October 2019)) [28], which runs Speciator (https://gitlab.com/cgps/mash-speciator (accessed on 31 October 2019)) for its species assignment. Speciator employs Mash [29] to identify the most identical strain (≥90% identity) in a reference collection of complete genomes found in the NCBI RefSeq database (https://www.ncbi.nlm.nih.gov/refseq/ (accessed on 31 October 2019)) [30]. The strains are then grouped according to their species designation and were screened for UTI pathogen determinants. Multi-locus sequence typing was performed by running SRST2 v.0.2.0 [31] based on the Achtman scheme [32] for E. coli and Pasteur [33] for K. pneumoniae isolates. Antigenic (O polysaccharide and H flagellin) profiles of E. coli samples were identified by employing Serotypefinder v.2.0 (https://cge.cbs.dtu.dk/services/SerotypeFinder/ (accessed on 13 December 2019)) [34] at 85% ID threshold and 60% minimum length.
Genome composition of the draft assemblies was assessed using Prokka v.1.10 [35] Acquired AMR genes were identified by aligning the genome sequences to the 2158 gene homolog subset of the Comprehensive Antibiotic Resistance Database (CARD) v. 3.0.8 (https://card.mcmaster.ca/ (accessed on 08 November 2019)) [36] and BacWGSTdb 2.0 [37] Clustering based on the distribution of AMR genes among isolates was drawn using Phandango v.1.3.0 [38]. Plasmid and replicon typing was carried out by comparing the genomes against the PlasmidFinder database v. 2.1.1 [39] at 99% identity threshold.
2.5. Bacterial Sample Collection and Antimicrobial Susceptibility Testing
To determine concordance between the AMR gene content and sample phenotype, antibiotic susceptibility testing and phenotypic detections of ESBL were performed by disc diffusion methods on a subset of n = 16 isolates from KY. The tests were carried out according to CLSI (2016) guidelines [40]. Isolates were examined for the insusceptibility to 9 different classes of antibiotics including Penicillin (ampicillin (AMP)), Penicillin + β-lactamase inhibitors (ampicillin-clavulanic acid (AMC)), Chloramphenicol (Chloramphenicol (CHL)), Sulfonamide (Trimethoprim-sulfamethoxazole (SXT)) and Quinolones (nalidixic acid (NA)), and Fluoroquinolone (Ciprofloxacin (CIP)). Resistance to ESBL Cephalosphorins was also assessed by testing the strains with Ceftriaxone (CRO), Ceftazidime (CAZ), cefotaxime (CTX), and Cefepime (FEP) (Supplementary Table S1).
2.6. Phylogenetic Reconstruction
Phylogenetic relationships and sequence variations between the samples were determined by constructing phylogenetic trees based on their chromosomal single-nucleotide polymorphism (SNP)s. Mobile genetic elements (MGEs) were further excluded using an internal script. Non-recombinant SNPs were determined using ClonalframeML v. 1.12 [41] and were used to create a maximum-likelihood midpoint-rooted phylogeny using RAxML v8.0.19 [42] using a General Time Reversible + gamma (GTR + G) substitution model with 100 bootstraps. Phylogenies were visualized using iToL (https://itol.embl.de/ (accessed on 20 January 2020)) [43] and FigTree v1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 20 January 2020)) [44].
2.7. Pangenome Analyses
The resulting annotation files from Prokka v.1.10 [35] were used as the basis for generating a pangenome for each species set. This step was completed by running Roary v3.11.2 [45] with a 100% BLAST v2.6.0 identity threshold using the MAFFT v7.3 setting [46]. Pangenome outputs were also used to assess the accessory genome composition of each bacterial population and as basis for reconstructing core genome phylogenies.
3. Results
3.1. Patient and Bacterial Strain Profiles
From the total of N = 150 strains, we collected from urine samples of patients, n = 81 were identified as E. coli and n = 19 were K. pneumoniae. The respondents were either to be admitted or visiting rural hospitals in KY and from clinics in the countryside of UG.
3.2. Genomic and Pangenomic Characterization Confirmed the Virulence Factors Present in Uropathogenic E. coli and K. pneumoniae
A subset (n = 55) from the total n = 81 E. coli and all n = 19 K. pneumoniae were confirmed uropathogenic following a thorough characterization of their pangenome contents. One thousand one hundred forty-four (1144) and 3464 core genes were found across the strains in E. coli and K. pneumoniae populations, respectively. These include known UTI virulence markers that are responsible for urinary tract (mucosal) surface binding (type 1 fimbrial adhesin-coding fimH) and colonization (mrkD; K. pneumoniae only), iron (Fe(2+)) transport (feoA/B/C), enterobactin synthase production (entB), formate transport (focA), cell division (zapA), succinate-acetate/proton symport (satP), anaerobic sulfatase-maturation (chuR; found in 100% and 95% of E. coli and K. pneumoniae, respectively). Other important virulence genes were also found, albeit not conserved among all the isolates: iutA (ferric aerobactin receptor: 44.6% in E. coli, 10% in K. pneumoniae), papA (fimbrial major pilin protein: E. coli only (41%)), papD (import of P pilus subunits into the periplasm: 44.6% in E. coli, 10% in K. pneumoniae), hlyE (hemolysin E: 80.4% in E. coli, 10% in K. pneumoniae), fyuA (pesticin receptor: 73.2% in E. coli, 15% in K. pneumoniae), kpsT (polysialic acid transport ATP-binding protein: E. coli only (26.8%)) and pic (serine protease pic autotransporter: E. coli only (5.4%); Table 1).
Table 1.
UTI virulence marker genes present in the pangenome of N = 55 E. coli and N = 19 K. pneumoniae isolates (including the reference genomes for each species collection). Proportion of the samples containing the gene are shown in count of strains with gene over the total strains and % values.
| Gene | Protein Product | Strain Count and % in E. coli Collection | Strain Count and % in K. pneumoniae Collection |
|---|---|---|---|
| fim H | Type 1 fimbrin D-mannose specific adhesin | 56/56 (100) | 20/20 (100) |
| feo A/B/C | Fe(2+) transport protein A/B/C | 56/56 (100) | 20/20 (100) |
| ent B | Enterobactin synthase component B | 56/56 (100) | 20/20 (100) |
| foc A | Formate transporter | 56/56 (100) | 20/20 (100) |
| zap A | Cell division protein | 56/56 (100) | 20/20 (100) |
| sat P | Succinate-acetate/proton symporter | 56/56 (100) | 20/20 (100) |
| chu R | Anaerobic sulfatase-maturating enzyme | 56/56 (100) | 19/20 (95) |
| mrk D | Type 3 fimbrial adhesin | 0 | 20/20 (100) |
| hly E | Hemolysin E | 45/56 (80.4) | 2/20 (10) |
| fyu A | Pesticin receptor | 41/56 (73.2) | 3/20 (15) |
| iut A | Ferric aerobactin receptor | 25/56 (44.6) | 2/20 (10) |
| pap D | Import of P pilus subunits into the periplasm | 26/56 (46.4) | 2/20 (10) |
| pap A | Fimbrial major pilin protein | 23/56 (41.1) | 0 |
| kps T | Polysialic acid transport ATP-binding protein | 15/56 (26.8) | 0 |
| pic | Serine protease pic autotransporter | 3/56 (5.4) | 0 |
3.3. Prevalence of AMR Genes in E. coli and K. pneumoniae Uropathogens from KY and UG
All n = 55 E. coli and n = 19 K. pneumoniae isolates harbored type 1 fimbrin. Among the UPEC, fimH30 was the most common allele, followed by fimH41; n = 4/55 samples had type fimH22 and n = 2/55 singleton were found with fimH22.
We further detected multiple acquired AMR-conferring elements in the genomes of the two species collections. Alignment of the sequences against CARD v.3.0.7 with 98–100% identity revealed that the n = 55 E. coli (n = 31 from KY, n = 24 from UG) were detected with the ciprofloxacin-conferring gene, marA. Majority (n = 47/55) were also aminoglycoside resistant and harbors either aadA or aac(6′)-Ib/(3′)-Ib alleles or both. Only n = 11/55 were not detected with resistant genes for ESBL cephalosphorins. Of the n = 44/55 that produce ESBLs, n = 10/44 had blaCTX-M (allele type 15 or 88), n = 24/44 had blaTEM (type 30/2/220) and n = 9/44 had blaOXA-1/140 and n = 2/44 (both from UG) had all 3 ESBL genes. Sulfonamide resistance genes were widely observed, n = 39 had either sul1 only, sul2 only or sul3 only, or both sul1 and sul2. Tetracycline resistance gene, tet(A) was present in n = 34 of 55. Twenty-four (N = 24/55) contained macrolide resistance-conferring mphA, n = 9/55 (n = 6 from KY, n = 3 from UG) harbored catB3 and were chloramphenicol resistant. Ninety-five percent (95%, n = 52/55) had at least one gene that codes for efflux pump proteins with n = 4/55 having yojI-pmrF-emrR-bacA-acrS/B/E-msbA-evgA-kdpE-mdtP-eptA cassette and n = 1/55 containing a mixture of yojI, pmrF, emrR, bacA, acrS/B/E, msbA, evgA, kdpE, mdtP, eptA, emtK, cpxA (Table 2 and Table 3). Two KY isolates (71 and 72) were found to have the fluoroquinolone resistance-conferring gene aac(3′)-Ib-cr while its variant aac(6′)-Ib-cr was present in UG isolates BN19, BN38 and BN44 (Supplementary Figure S1a).
Table 2.
Genomic characteristics of uropathogenic E. coli strains isolated in this study for acquired AMR-associated genes and plasmid replicon types. Asterisk (*) next to the O antigen type means undefined; NF means not found; ‘Yes’ means the strain is either ESBL-producing or MDR; and ‘No’ means the sample is either non-ESBL or non-MDR.
| Sample Name | Serotype | Sequenc Type Pasteur | AMR Phenotype | fimH | Aminoglycosides | Macrolide | Ciplrofloxacin | β-Lactamase Inhibitors/ESBL Cephalosporins | Phenicols | Fluoroquinolones | Quinolone | Sulfonamide | Tetracycline | Folate Pathway Inhibitors | Antibiotic Effulx/Regulation | ESBL | MDR | Plasmid Replicon |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | O25:H4 | 131 | AMP, CTX, CHL, SXT, CIP, NA | H30 | aac(3)-Ib, aadA5 | mphA | marA | bla CTX -M-27/99 | NF | NF | NF | sul1, sul2 | tet(A) | dfrA17 | gadW | Yes | Yes | IncFIA, IncFII |
| 6 | O25:H4 | 131 | AMP, CTX, CRO, CHL, SXT, CIP, NA | H30 | aac(3)-Ib, aadA5 | mphA | marA | bla CTX -M-27/99 | NF | NF | NF | sul1, sul2 | tet(A) | dfrA17 | gadW | Yes | Yes | IncFIA, IncFII |
| 7 | O*:H5 | 1193 | CRO, CHL, SXT, CIP, NA | H41 | aac(3)-Ib, aadA17 | mphA | marA | bla TEM-220 | NF | NF | NF | NF | NF | NF | bacA, tolC, evgA | Yes | Yes | IncQ1, IncFIA, Col156, Col(BS512) |
| 8 | O75:H5 | 1193 | AMP, FOX, SXT, CIP, NA | H41 | aac(3)-Ib, aadA17 | mphA | marA | bla TEM-220 | NF | NF | NF | NF | NF | NF | bacA, tolC, evgA | Yes | Yes | IncFIA, IncQ1, Col(BS512), Col156 |
| 9 | O6:H1 | 73 | AMP, FOX, SXT, CIP, NA | H30 | NF | NF | marA | NF | NF | NF | NF | NF | NF | NF | mexB | No | No | IncX1 |
| 10 | O6:H1 | 73 | AMP, CTX | H30 | NF | NF | marA | NF | NF | NF | NF | NF | NF | NF | acrB | No | No | NF |
| 11 | O25:H4 | 131 | AMP, CTX, CRO, CHL, SXT, CIP, NA | H30 | aac(3)-Ib, aadA5 | mphA | marA | bla CTX -M-27/99 | NF | NF | NF | sul1, sul2 | tet(A) | NF | acrB, gadW, pmrF | Yes | Yes | IncFIA, IncFII, Col156 |
| 12 | O25:H4 | 131 | AMP, CTX, CRO, SXT, CIP, NA | H30 | aac(3)-Ib, aadA5 | mphA | marA | bla CTX -M-27/99 | NF | NF | NF | sul1, sul2 | tet(A) | NF | acrB, gadW, pmrF | Yes | Yes | IncFIA, IncFII, Col156 |
| 13 | O55:H7 | 335 | AMP, SXT, NA | H30 | aac(3)-Ib | NF | marA | bla TEM-220 | NF | NF | NF | sul1 | tet(A) | dfrA7 | evgA, cpxA, gadW | Yes | Yes | IncQ1 |
| 14 | O55:H7 | 335 | AMP, SXT, NA | H30 | aac(3)-Ib | NF | marA | bla TEM-220 | NF | NF | NF | sul1 | tet(A) | dfrA7 | evgA, cpxA | Yes | Yes | IncQ1 |
| 15 | O55:H7 | 335 | AMP, CTX, SXT, NA | H30 | aac(3)-Ib | NF | marA | bla TEM-220 | NF | NF | NF | sul1 | tet(A) | dfrA7 | evgA, cpxA | Yes | Yes | IncQ1 |
| 16 | O55:H7 | 335 | AMP, SXT, NA | H30 | aac(3)-Ib | NF | marA | bla TEM-220 | NF | NF | NF | sul1 | tet(A) | dfrA7 | evgA, cpxA | Yes | Yes | IncQ1 |
| 20 | O25:H4 | 131 | AMP, CTX, FOX, SXT, CIP, NA | H30 | aac(3)-Ib, aadA5 | mphA | marA | bla CTX -M-27/99 | NF | NF | NF | sul1, sul2 | tet(A) | dfrA17 | acrB, gadW | Yes | Yes | IncFIA, IncFII, Col156 |
| 21 | O75:H5 | 1193 | AMP, FOX, SXT, CIP, NA | H41 | aac(3)-Ib | mphA | marA | bla TEM-220 | NF | NF | NF | NF | NF | dfrA17 | evgA, tolC, bacA | Yes | Yes | IncQ1, IncFIA, Col156, Col(BS512) |
| 23 | O25:H4 | 131 | AMP, CTX, CRO, CAZ, FEP, CHL, SXT, CIP, NA | H30 | aac(3)-Ib, aadA5 | mphA | marA | bla CTX -M-27/99 | NF | NF | NF | sul1, sul2 | tet(A) | dfrA17 | acrB, gadW | Yes | Yes | IncFIA, IncFII, Col156 |
| 24 | O25:H4 | 131 | AMP, CTX, CRO, SXT, CIP, NA | H30 | aac(3)-Ib, aadA5 | mphA | marA | bla CTX -M-27/99 | NF | NF | NF | sul1, sul2 | tet(A) | dfrA17 | acrB, gadW | Yes | Yes | IncFIA, IncFII, Col156 |
| 60 | O*:H5 | 1193 | ND | H41 | aac(3)-Ib, aadA5 | mphA | marA | bla TEM-220 | NF | NF | NF | NF | NF | dfrA17 | acrS, bacA, tolC | Yes | Yes | IncQ1, IncFIA, Col156, Col(BS512) |
| 63 | O25:H4 | 131 | ND | H30 | aac(3)-Ib, aadA5 | mphA | marA | bla CTX -M-27/99 | NF | NF | NF | sul1, sul2 | tet(A) | dfrA17 | gadW | Yes | Yes | IncFII, Col156, IncFIA |
| 64 | O25:H4 | 131 | ND | H30 | aac(3)-Ib, aadA5 | mphA | marA | bla CTX -M-27/99 | NF | NF | NF | sul1, sul2 | tet(A) | dfrA17 | gadW | Yes | Yes | IncFIA, IncFII, Col156 |
| 67 | O25:H4 | 131 | ND | H30 | aac(3)-Ib, aadA5 | mphA | marA | blaCTX-M-15/88, blaOXA-1/140 | catB3 | NF | NF | sul1 | tet(A) | dfrA17 | acrS, gadW | Yes | Yes | IncFIA |
| 68 | O89:H4 | 44 | ND | H54 | aac(3)-Ib, aadA5 | mphA | marA | blaCTX-M-15/88, blaOXA-1/140 | catB3 | NF | NF | sul1 | tet(A) | dfrA17 | acrS, gadW | Yes | Yes | IncFIA, IncFII |
| 71 | O25:H4 | 131 | ND | H30 | aadA5 | NF | marA | blaCTX-M-15/88, blaOXA-1/140 | catB3 | aac(3)-Ib-cr | QnrB2 | sul1 | tet(A) | dfrA17 | gadW | Yes | Yes | IncFIA, IncFII, IncY |
| 72 | O25:H4 | 131 | ND | H30 | aadA5 | NF | marA | blaCTX-M-15/88, blaOXA-1/140 | catB3 | aac(3)-Ib-cr | NF | sul2 | NF | dfrA14 | gadW | Yes | Yes | IncFIA, IncFII |
| 73 | O25:H4 | 131 | ND | H30 | aac(3)-Ib, aadA5 | mphA | marA | blaCTX-M-15/88, blaOXA-1/140 | catB3 | NF | NF | sul1 | tet(A) | dfrA17 | acrS, gadW | Yes | Yes | IncFIA, IncFII |
| 74 | O*:H6 | 648 | ND | H30 | aac(3)-Ib, aadA5 | mphA | marA | blaCTX-M-15/88, blaOXA-1/140 | catB3 | NF | NF | sul1 | tet(A) | dfrA17 | acrS, gadW | Yes | Yes | IncFIA, IncFII |
| 87 | O*:H4 | 131 | ND | H30 | aac(3)-Ib, aadA5 | mphA | marA | bla CTX -M-27/99 | NF | NF | NF | sul1 | tet(A) | dfrA17 | gadW | Yes | Yes | IncFIA, IncFII |
| 97 | O25:H4 | 131 | ND | H30 | aac(3)-Ib, aadA5 | mphA | marA | bla CTX -M-27/99 | NF | NF | NF | sul1 | tet(A) | dfrA17 | gadW | Yes | Yes | IncFIA, IncFII, Col156 |
| 102 | O*:H7 | 335 | ND | H30 | aac(3)-Ib | NF | marA | bla TEM-220 | NF | NF | NF | sul1 | tet(A) | dfrA7 | cpXxA, evgA | Yes | Yes | IncQ1 |
| 103 | O25:H4 | 131 | ND | H30 | aac(3)-Ib, aadA5 | mphA | marA | bla CTX -M-27/99 | NF | NF | NF | sul2 | tet(A) | dfrA17 | NF | Yes | Yes | IncFIA, IncFII, Col156 |
| 105 | O55:H7 | 335 | ND | H30 | aac(3)-Ib | NF | marA | bla TEM-220 | NF | NF | NF | sul1 | tet(A) | dfrA7 | cpxA, evgA, acrB | Yes | Yes | IncQ1 |
| 106 | O*:H5 | 1193 | ND | H41 | aac(3)-Ib | mphA | marA | bla TEM-220 | NF | NF | NF | NF | NF | dfrA17 | evgA, tolC, mphA | Yes | Yes | IncQ1, IncFIA, Col156 |
| BN1 | O75:H5 | 1193 | ND | H41 | aac(3)-Ib | mphA | marA | bla TEM-220 | NF | NF | NF | NF | NF | dfrA17 | bacA, tolC, evgA | Yes | Yes | IncQ1, IncFIA |
| BN12 | O*:H9 | 410 | ND | H41 | aadA9, aac(3)-Ib | mphA | marA | bla TEM-220 | NF | NF | NF | NF | NF | dfrA17 | emrR | Yes | Yes | IncQ1, IncFIA |
| BN19 | O25:H4 | 131 | ND | H30 | aadA5, aac(3)-Ib, aac(3)-Iic/d/e | NF | marA | blaTEM-220, blaCTX-M-15, blaOXA-1, blaOXA-140 | catB3 | aac(6′)-Ib-cr | NF | sul1, sul2 | NF | NF | kdpE, gadW | Yes | Yes | IncFIA, IncFII |
| BN2 | O156:H7 | NF | ND | H22 | aac(3)-Ib | NF | marA | NF | NF | NF | NF | sul1 | tet(A) | dfrA17 | NF | No | Yes | Col |
| BN20 | O89:H9 | 10 | ND | H54 | aadA9, aac(3)-Ib | NF | marA | bla TEM-220 | NF | NF | NF | sul1 | tet(A) | dfrA1 | mdtP, msbA, acrB, baeS/R, yojI | Yes | Yes | IncQ1 |
| BN25 | O6:H11 | 48 | ND | H41 | aadA5, aac(3)-Ib | NF | marA | NF | NF | NF | NF | sul2 | NF | dfrA17 | yojI, pmrF, emrR, bacA, acrS/B/E, msbA, evgA, kdpE, mdtP, eptA | No | Yes | IncHI2A |
| BN26 | O9:H19 | 162* | ND | H30 | NF | NF | marA | NF | NF | NF | NF | NF | tet(A) | NF | emrR, mdtA | No | Yes | NF |
| BN27 | O*:H2 | 165 | ND | H54 | aac(6′)-Ib7, aac(3)-Ib, aadA9 | NF | marA | bla TEM-220 | NF | NF | qnrS1 | sul3 | tet(A) | NF | tolC, mdtO, msbA, acrB, baeS/R, acrD, gadX, evgA, pmrF | Yes | Yes | NF |
| BN3 | O18:H49 | 212 | ND | H30 | aac(6′)-Ib7, aac(3)-Ib, aadA9 | NF | marA | NF | NF | NF | NF | sul1 | tet(A) | dfrA15 | emrR | No | Yes | NF |
| BN37 | O8:H17 | 10 | ND | H41 | aac(3)-Ib, aadA5 | NF | marA | bla TEM-220 | NF | NF | NF | sul2 | tet(A) | dfrA15 | yojI, pmrF, emrR, bacA, acrS/B/E, msbA, evgA, kdpE, mdtP, eptA | Yes | Yes | IncFIA, IncFII |
| BN38 | O89:H10 | 617 | ND | H41 | NF | NF | marA | blaCTX-M-15/88, blaOXA-1/140 | catB3 | aac(6′)-Ib-cr | NF | sul1, sul2 | NF | NF | yojI, pmrF, emrR, bacA, acrS/B/E, msbA, evgA, kdpE, mdtP, eptA | Yes | Yes | IncFIA, IncFII |
| BN41 | O171:H21 | 155 | ND | H30 | aac(6′)-Ib7, aadA9 | NF | marA | bla TEM-220 | NF | NF | NF | sul2 | tet(A) | NF | emrR | Yes | Yes | IncHI1A, IncHI1B, IncFIA |
| BN42 | O29:H8 | 448 | ND | H30 | aadA13, aac(3)-Ib | NF | marA | bla CTX-M-5 | NF | NF | NF | sul2 | NF | dfrA14 | emrR, mphA | Yes | Yes | IncI, IncFII, IncFIA IncX4 |
| BN43 | O*:H4 | 6161 | ND | H30 | NF | NF | marA | NF | NF | NF | NF | sul2 | NF | dfrA14 | baeS | No | Yes | NF |
| BN44 | O8:H9 | 410 | ND | H41 | aac(6′)-Ib7, aadA9 | mphA | marA | blaCTX-M-5, blaOXA-1, blaTEM-30/220 | catB3 | aac(6′)-Ib-cr | NF | NF | NF | NF | emrR | Yes | Yes | IncFIA, IncQ1 |
| BN47 | O89:H9 | 10 | ND | H54 | acrD/E/F, aadA9, aac(3)-Ib, aac(6′)-Ib7 | NF | marA | bla TEM-220 | NF | NF | NF | sul1 | NF | dfrA1 | gadX, tolC, mdtF/N/O, emrK | Yes | Yes | Col440II, IncQ1 |
| BN48 | O17:H11 | NF | ND | H41 | aac(3)-Ib | NF | marA | bla TEM-7/75/177 | NF | NF | NF | sul1 | NF | dfrA7 | axyY | Yes | Yes | IncQ1 |
| BN49 | O45:H11 | 10 | ND | H41 | NF | NF | marA | NF | NF | NF | qnrB19 | NF | NF | NF | yojI, pmrF, emrR, bacA, acrS/B/E, msbA, evgA, kdpE, mdtP, eptA | No | No | Col |
| BN50 | O*:H4 | 167 | ND | H30 | NF | NF | marA | NF | NF | NF | NF | sul2 | NF | dfrA14 | baeS | No | Yes | NF |
| BN51 | O171:H21 | 6161 | ND | H30 | aac(6′)-Ib7, aadA9 | NF | marA | bla TEM-220 | NF | NF | NF | sul2 | tet(A) | NF | mdtA/B, emrR | Yes | Yes | IncHI1A, IncHI1B, IncFIA |
| BN55 | O185:H8 | 155 | ND | H30 | acrB, aadA1, aac(3)-Ib, aac(6′)-Ib7 | NF | marA | bla TEM-220 | NF | NF | NF | sul1 | tet(A) | dfrA1 | yojI, pmrF, emrR, bacA, acrS/B/E, msbA, evgA, kdpE, mdtP, eptA, emtK, cpxA | Yes | Yes | IncFIB, IncQ1 |
| BN56 | O*:H7 | 2163 | ND | H30 | NF | NF | marA | NF | NF | NF | NF | NF | NF | NF | emrR, baeS | No | No | NF |
| BN57 | O9:H19 | 162* | ND | H30 | NF | NF | marA | NF | NF | NF | NF | sul2 | tet(A) | NF | mdtA, emrR | No | Yes | NF |
| BN6 | O6:H1 | 73 | ND | H22 | aac(3)-Ib | NF | marA | bla TEM-220 | NF | NF | NF | sul1 | tet(A) | dfrA7 | NF | Yes | Yes | IncFIB |
Table 3.
Genomic characteristics of uropathogenic K. pneumoniae strains isolated in this study for acquired AMR-associated genes and plasmid replicon types. NF means not found; ‘Yes’ means the strain is either ESBL-producing or MDR; and ‘No’ means the sample is either non-ESBL or non-MDR.
| Sample Name |
Sequence Type Pasteur | AMR Phenotype | Aminoglycosides | Ciplrofloxacin | Penicillins + β-lactamase Inhibitors |
ESBL Cephalosporins |
Phenicols | Fluoroquinolones | Quinolone | Sulfonamide | Tetracycline | Folate Pathway Inhibitors |
Antibiotic Effulx/Regulation | ESBL | MDR | Plasmid Replicon |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 26 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 27 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 28 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 29 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 30 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 31 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 32 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 33 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 34 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 35 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 36 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 55 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 56 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 89 | 11 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | bla LEN-4/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140, blaNDM-1 | catB3 | oqxB, aac(6′)-Ib-cr | qnrB9 | sul2 | NF | NF | NF | Yes | Yes | IncFII, IncFIB, IncR |
| 90 | NF | ND | aac(6′)-Ib7, aadA9 | NF | bla LEN-3/4/5/6 | blaSHV-28, blaCTX-M-15, blaOXA-1/140 | NF | NF | NF | sul1 | tet(A) | dfrA17 | yojI, pmrF, emrR, bacA, acrB, msbA, evgA, kdpE, mdtP, eptA, emtK, cpxA | Yes | Yes | IncN |
| BN14 | 0b8e | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | marA | bla LEN-3/4/5/6 | blaSHV-28, blaCTX-M-15, blaTEM-220 | catB3 | oqxA/B, aac(6′)-Ib-cr | qnrB6/17 | sul1 | tet(B) | dfrA27 | yojI, pmrF, emrR, bacA, acrB, msbA, evgA, kdpE, mdtP, eptA, emtK, cpxA | Yes | Yes | IncR, IncFII, IncFIA, Col, IncX4 |
| BN16 | 67b2 | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | marA | bla LEN-3/4/5/6 | bla CTX-M-15/88 | catB3 | oqxA/B, aac(6′)-Ib-cr | qnrB6/17 | sul1, sul2 | tet(B) | dfrA27 | yojI, pmrF, emrR, bacA, acrB, msbA, evgA, kdpE, mdtP, eptA, emtK, cpxA | Yes | Yes | IncR, IncFII, IncFIA, Col, IncX4 |
| BN7 | 6b6f | ND | aac(6′)-30/aac(6′)-Ib’/7/10, aadA9 | NF | NF | NF | NF | oqxA/B, aac(6′)-Ib-cr | qnrB6/17 | NF | NF | NF | NF | No | Yes | IncR |
All n = 19 K. pneumoniae isolates were resistant to aminoglycosides and had either aac(6′)-30/aac(6′)-Ib’/7/10-aadA9 (n = 10/19) or aac(6′)-Ib7-aadA9 (n = 1/19) combination. N = 18/19 are potentially sulfonamide insusceptible and contained either sul1 only (n = 2/19), sul2 only (n = 15/19) or both (n = 1). The β-lactamase blaLEN gene is present in all but the BN7 strain, with blaLEN-4/6 (n = 15 from KY) or blaLEN-3/4/5/6 alleles. BN7 was also the only susceptible isolate against ESBL cephalosphorins. The rest are ESBL producers: n = 15/19 had blaSHV-28, blaCTX-M-15, blaOXA-1/140 and blaNDM-1, n = 1/19 were observed with the blaSHV-28-CTX-M-15-OXA-1/140 combination and n = 1/19 had blaSHV-28, blaCTX-M-15/88 only. All n = 3 strains from UG had tet(B) and dfrA (17 or 27 allele type); these strains also contained efflux pump-expressing genes: yojI, pmrF, emrR, bacA, acrB, msbA, evgA, kdpE, mdtP, eptA, emtK, and cpxA. N = 2/19 (BN14 and BN16 from UG) had ciprofloxacin-resistance gene, marA. Only the strain 90 from KY was not resistant to phenicols, while the rest were detected with the cat gene, specifically, catB3 (Table 3; Supplementary Figure S2b). Overall, 80% of our E. coli uropathogens had ESBL genes (n = 15 strains from UG and n = 29 from KY) and 93% of these UPEC are MDR, while all the K. pneumoniae isolates are MDR and only n = 1 out of the total n = 19 (95%) are ESBL.
3.4. Population Structure of KY and UG Uropathogens
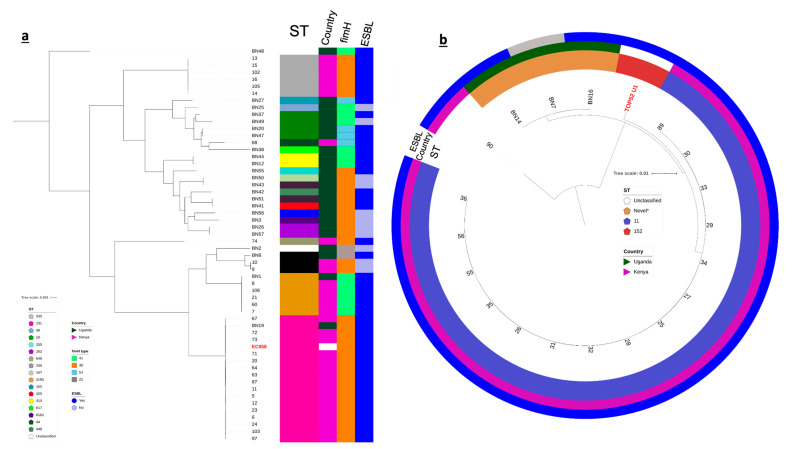
The UPEC collection was polyclonal. Eighteen (18) different sequence types were identified in the UPEC population (Achtman scheme). The most prevalent MLST sequence types were ST131 (n = 17/55, 31%), ST335 and ST1193 (n = 6/55, 11%) and ST10 (n = 4/55, 7%). These sequence types were usually associated with UTI cases (Nicolas-Chanoine et a. 2014; Afset et al. 2008; Yamaji et al. 2018); the globally disseminated ESBL-ST131 stood out to be the most dominant ST. Other clones were also observed: n = 3 ST73, n = 2 each from ST155, ST410, ST6161 and ST162, and singletons from ST44, ST48, ST165, ST167, ST212, ST448, ST617, ST648 and ST2163; n = 2 strains from UG (BN2 and BN48) were unclassified (Figure 1a, Table 2). E. coli isolates from UG belong to 15 STs and were thus more diverse compared to those collected from KY, which belong to only 6 STs (Figure 1a, Table 2). This difference in diversity is consistent with the number of serotypes found in UG relative to those from KY: Ugandan strains belong to 20 different O:H antigen combinations while the KYn ones were found to have 9 O:H types.
Figure 1.
Maximum likelihood phylogenies of core genomes of E. coli (a) and K. pneumoniae (b) uropathogens isolated from KY and UG; reference genomes are in red and font. The mid-point rooted phylograms was constructed using 2862 and 3464 core genes from E. coli and K. pneumoniae populations, respectively and visualized with iTol. The colored strips adjacent to the E. coli phylogeny represent (from left to right) the sequence type (ST), country of origin, type of fimH allele and the ESBL status of each strain. The colored rings around the K. pneumoniae phylogenetic tree indicate the ST, country of origin and the ESBL status of each isolate. “Novel*” means the sequence type of the sample/s did not match those in the database and may be novel. The scale bar indicates substitutions per site.
Fifteen out of nineteen (n = 15/19) K. pneumoniae isolates from KY belong to ST11 (Pasteur scheme); n = 3/19 UG had no defined sequence types (BN14: 0b8e, BN16; 67b2, BN7: 6b6f) and formed their own clade (Table 3; Figure 1b).
We compared our E. coli samples from the three most prevalent clones, ST131, ST335 and ST10, and our K. pneumoniae strains with previously published genomes listed in BacWGSTdb 2.0. Based on the metadata of the reference genomes, these strains were of different geographical origins (country/state) and were mostly isolated from human hosts and have caused disease (Supplementary Table S2). Computing for the pairwise SNP distances showed that strain CP023853 is the most closely related genome with our KY isolates with distances ranging from 910–1489; CP023853 was also sampled from a UTI patient in Sweden in 2009 (Supplementary Table S2; Supplementary Figure S2a). Our ST335 collection is solely composed of KY isolates, and all appeared to be genetically distant to the selected sequences in the database with a minimum of 4700 SNP differences between the two groups (Supplementary Figure S2a). In contrast, our E. coli ST10 strains were all from UG. The closest reference isolate was LSBS01 (isolated from a fecal sample; Supplementary Table S2), which was 2009 and 2070 SNPs apart from BN20 and BN70, respectively (Supplementary Figure S2a).
Our K. pneumoniae collection, which was dominated by ST11 showed ~3500 SNP differences from strain 27 from KY while those that had no defined ST (e.g., BN14 and BN 16 from UG) appeared to be most closely related (minimum SNP distances of 3519 and 3255) to the human isolate references LXMM01 and VUBS01, respectively (Supplementary Table S2; Supplementary Figure S2b).
3.5. Plasmid Characterization
Genome assemblies of the KY and UG uropathogens were screened for the presence and type of plasmids using PlasmidFinder v.2.1.1. N = 47/55 in the E. coli collection were found with at least one plasmid. IncFIA was consistently found in n = 10 had both IncFIA and IncFII, n = 9 contained IncFIA, IncFII, Col156 types, n = 1 was detected with IncFIA, IncFII and IncY only or IncI only and IncFII-IncFIA-IncX4 plasmid combinations.
All the samples from the K. pneumoniae collection were found with at least one plasmid type. IncFII-IncFIB-IncR is the most common combination and is found among n = 15 isolates, while n = 2/19 was found with IncR, IncFII, IncFIA, Col, and IncX4. Notably, the strain 90 from KY had the blaCTX-M-1 gene-carrying plasmid IncN and BN7 from UG had the blaNDM-associated IncR.
4. Discussion
We assessed the prevalence of acquired AMR characteristics among uropathogenic E. coli and K. pneumoniae circulating in East African region using WGS. We recruited out-patients that presented UTI-like symptoms from rural areas in KY and UG, which represents a limitation of our sample collection. The lack of point-of-care diagnostic tool such as the use of dipstick test also contributed to some difficulties in our screening. This is evidenced by a high level of contaminants that comprised of strains that do not contain UTI determinants. Nevertheless, our in silico predictions using whole genome analysis revealed alarming rates of ESBL-producing and MDR strains in both our UPEC and K. pneumoniae collections, which reiterates the great necessity for effective interventions to curb their spread.
Our results firmly indicate a high diversity among E. coli uropathogens, which was more evident in samples taken from UG rather than KY. Strains that belong to the same clonal group had <200 core SNPs from each other. This rich genetic diversity is consistent with those observed in other isolates collected from rural or semi-rural communities of low/middle-income countries [47,48,49]. The widely disseminated UTI-causing clones ST131, ST335 and ST10 were common among our E. coli strains and dominated our Kenyan collection. This is unsurprising as these STs are reported to be circulating globally [50,51,52]. What is remarkable is the detection of emerging clones such as ST1193 and ST617 that were unusually associated with UTI [53,54] albeit observed in hospital settings. UPEC strains from UG are even more alarming as they represent higher number of unusual or novel UTI clones (i.e., ST155, ST448, and ST162) with potentially higher virulence levels [55,56,57] compared to those globally-known STs.
Several Klebsiella species were known to have broad-spectrum resistance to common antibiotics [58]. K. pneumoniae strains particularly those belonging to the hypervirulent ST11 have been extensively reported to cause severe infections [59,60] and have led to dire disease outcomes in intensive care units [61]. This K. pneumoniae clone has an alarming antibiotic resistance profile [62] making it difficult to treat. The dominance of ST11 strains in our samples that were mainly collected from outpatients suggests the strong presence of this clinically important bacterial pathogen in the community and pose an apparent threat to public health.
BlaCTX-M genes were present in 40% of our UPEC collection and in all but one K. pneumoniae strain (95%). Notably, the blaCTX-M-15 gene that confers resistance to last-resort antibiotics was found in high levels in both countries. This gene was detected with other ESBL determinants, blaTEM and blaOXA-1 in E. coli and with blaNDM in K. pneumoniae, concordant with those in uropathogens found from the Middle East [63] and Asia [64], among others. Consistent with previous findings in other African regions, tet genes in this study were also detected alongside ESBL genes blaCTX-M-15, blaOXA-1 and blaTEM in n = 30/55 E. coli and with blaLEN-3/4/5/6 among n = 3/19 K. pneumoniae [65,66] which stipulates their co-selection and co-transmission in KY and UG. The presence of these genes in the identified plasmid-associated contigs suggest that the mode of transfer may have been plasmid-mediated.
5. Conclusions
We underline in this pilot study the high frequency of AMR determinants associated with resistance to common antibiotic classes among E. coli and Klebsiella pneumoniae in East Africa, with specific focus on MDR and ESBL-producing strains from KY and UG. We further demonstrate that routine genomic surveillance is necessary for high-resolution investigation of bacterial epidemiology especially in less represented regions. Our findings have significant implications on improving interventions that aim to address the strong presence of AMR pathogens that cause UTI (particularly in low/middle-income countries).
Acknowledgments
We thank Alison Sandeman, the HATUA Program Manager and Andy Lynch from the Mathematical Institute at the University of St Andrews for their helpful feedback on the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10121547/s1. Table S1: Metadata of E. coli and K. pneumoniae strains isolated from urine samples including Antibiotic Sensitivity Test results of n = 16 isolates; Table S2: Comparison of host niche, disease implication, isolation source, collection year, antimicrobial and virulence gene contents between three most abundant E. coli clones, ST131, ST335 and ST10 (a) and K. pneumoniae ST11 (b) strains in our study and selected isolate genomes listed in BacWGSTdb 2.0. ND means not determined; Figure S1: Distribution of antimicrobial resistance genes (AMRGs; right panel) among E. coli (a) and K. pneumoniae (b) isolates from our HATUA Pilot collection. Left panel shows clustering of the strains in a phylogenetic tree according to the presence (green blocks) or absence (pink blocks) of AMRGs; Figure S2: Pairwise SNP distances in core genome multi-locus sequence type (cgMLST)-based alleles of the three most abundant E. coli clones, ST131, ST335, and ST10 (a) and K. pneumoniae ST11 (b) strains in our study and selected isolate genomes listed in BacWGSTdb. The assemblies of the reference sequences were downloaded from European Nucleotide Archive.
Author Contributions
Conceptualization, S.N.; methodology, A.G.D., K.P., W.S. and M.T.G.H.; software, and M.T.G.H.; validation, H.O., J.B. and K.P.; formal analysis, A.G.D. and M.T.G.H.; investigation, A.G.D.; resources, S.N.; data curation, A.G.D. and M.T.G.H.; writing—original draft preparation, A.G.D.; writing—review and editing, A.G.D., W.S., B.A., J.K., and M.T.G.H.; visualization, A.G.D.; supervision, M.T.G.H.; project administration, M.T.G.H.; funding acquisition, J.K., W.S., B.A., D.J.S. and M.T.G.H. All authors have read and agreed to the published version of the manuscript.
Funding
The Holistic Approach to Unravel Antibacterial Resistance in East Africa is a three-year Global Context Consortia Award (MR/S004785/1) funded by the National Institute for Health Research, Medical Research Council and the Department of Health and Social Care. The award is also part of the EDCTP2 program supported by the European Union.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, approved by the Institutional Ethics Committee of School of Biomedical Sciences, Makerere University (SBS-HDREC-514 on 13/10/2017) and the Kenya Medical Research Institute (KEMRI) with the approval of KEMRI Scientific and Ethics Review Unit (KEMRI/SERU/CMR/P00043/3329 on 04 October 2016).
Data Availability Statement
Raw and generated data for this Pilot study are available in the following links: https://doi.org/10.6084/m9.figshare.17105876 (supplementary files, accessed on 16 November 2021), https://doi.org/10.6084/m9.figshare.17091293 (draft genome assemblies, accessed on 16 November 2021) and https://doi.org/10.6084/m9.figshare.11965455 (Table 2 and Table 3, accessed on16 November 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Michael C.A., Dominey-Howes D., Labbate M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health. 2014;2:145. doi: 10.3389/fpubh.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rather I.A., Kim B.C., Bajpai V.K., Park Y.H. Self-medication and antibiotic resistance: Crisis, current challenges, and prevention. Saudi J. Biol. Sci. 2017;24:808–812. doi: 10.1016/j.sjbs.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan C., Cars O. Antibiotic resistance-problems, progress, and prospects. N. Engl. J. Med. 2014;371:1761–1763. doi: 10.1056/NEJMp1408040. [DOI] [PubMed] [Google Scholar]
- 4.French G.L. The continuing crisis in antibiotic resistance. Int. J. Antimicrob. Agents. 2010;36((Suppl. 3)):S3–S7. doi: 10.1016/S0924-8579(10)70003-0. [DOI] [PubMed] [Google Scholar]
- 5.Read A.F., Woods R.J. Antibiotic resistance management. Evol. Med. Public Health. 2014;2014:147. doi: 10.1093/emph/eou024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Government of Kenya . National Action Plan for the Prevention and Containment of Antimicrobial Resistance. Government of Kenya; Nairobi, Kenya: 2017. [Google Scholar]
- 7.UNAS. CDDEP. GARP-Uganda. Mpairwe Y., Wamala S. Antibiotic Resistance in Uganda: Situation Analysis and Recommendations. Uganda National Academy of Sciences; Kampala, Uganda: Center for Disease Dynamics, Economics & Policy; Washington, DC, USA: 2015. p. 107. [Google Scholar]
- 8.Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunin C.M., Zacha E., Paquin A.J. Urinary-tract infections in schoolchildren. I. Prevalence of bacteriuria and associated urologica. N. Engl. J. Med. 1962;266:1287–1296. doi: 10.1056/NEJM196206212662501. [DOI] [PubMed] [Google Scholar]
- 10.Lindberg U., Hanson L.A., Jodal U., Lidin-Janson G., Lincoln K.O., lling S. Asymptomatic bacteriuria in schoolgirls. II. Differences in Escherichia coli causing asymptomatic bacteriuria. Acta Paediatr. Scand. 1975;64:432–436. doi: 10.1111/j.1651-2227.1975.tb03860.x. [DOI] [PubMed] [Google Scholar]
- 11.Najar M.S., Saldanha C.L., Banday K.A. Approach to urinary tract infections. Indian J. Nephrol. 2009;19:129–139. doi: 10.4103/0971-4065.59333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levison M.E., Kaye D. Treatment of complicated urinary tract infections with an emphasis on drug-resistant gram-negative uropathogens. Curr. Infect. Dis. Rep. 2013;15:109–115. doi: 10.1007/s11908-013-0315-7. [DOI] [PubMed] [Google Scholar]
- 13.Nicolle L.E. Pivmecillinam in the treatment of urinary tract infections. J. Antimicrob. Chemother. 2000;46((Suppl. A)):35–39. doi: 10.1093/jac/46.suppl_1.35. [DOI] [PubMed] [Google Scholar]
- 14.Croucher N.J., Didelot X. The application of genomics to tracing bacterial pathogen transmission. Curr. Opin. Microbiol. 2015;23:62–67. doi: 10.1016/j.mib.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Goswami C., Fox S., Holden M., Connor M., Leanord A., Evans T.J. Genetic analysis of invasive Escherichia coli in Scotland reveals determinants of healthcare-associated versus community-acquired infections. Microb. Genom. 2018;4:e000190. doi: 10.1099/mgen.0.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Argimón S., Masim M., Gayeta J.M., Lagrada M.L., Macaranas P., Cohen V., Limas M.T., Espiritu H.O., Palarca J.C., Chilam J., et al. Integrating whole-genome sequencing within the National Antimicrobial Resistance Surveillance Program in the Philippines. Nat. Commun. 2020;11:2719. doi: 10.1038/s41467-020-16322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halachev M.R., Chan J.Z.-M., Constantinidou C.I., Cumley N., Bradley C., Smith-Banks M., Oppenheim B., Pallen M.J. Genomic epidemiology of a protracted hospital outbreak caused by multidrug-resistant Acinetobacter baumannii in Birmingham, England. Genome Med. 2014;6:61070. doi: 10.1186/s13073-014-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee R.S., Seemann T., Heffernan H., Kwong J.C., Gonҫalves da Silva A., Carter G.P., Woodhouse R., Dyet K.H., Bulach D.M., Stinear T.P., et al. Genomic epidemiology and antimicrobial resistance of Neisseria gonorrhoeae in New Zealand. J. Antimicrob. Chemother. 2018;73:353–364. doi: 10.1093/jac/dkx405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludden C., Decano A.G., Jamrozy D., Pickard D., Morris D., Parkhill J., Peacock S.J., Cormican M., Downing T. Genomic surveillance of Escherichia coli ST131 identifies local expansion and serial replacement of subclones. Microb. Genom. 2020;6:e000352. doi: 10.1099/mgen.0.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masim M.L., Argimón S., Espiritu H.O., Magbanua M.A., Lagrada M., Olorosa A.M., Cohen V., Gayeta J.M., Jeffrey B., Abudahab K., et al. Genomic surveillance of methicillin-resistant Staphylococcus aureus in the Philippines, 2013–2014. West. Pac. Surveill. Response J. 2021;12:6–16. doi: 10.5365/wpsar.2020.11.1.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldgarden M., Brover V., Haft D.H., Prasad A.B., Slotta D.J., Tolstoy I., Tyson G.H., Zhao S., Hsu C.H., McDermott P.F., et al. Validating the AMRFinder tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob. Agents Chemother. 2019;63:e00483-19. doi: 10.1128/AAC.00483-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S., Zhou Y., Chen Y., Gu J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wick R.R., Judd L.M., Gorrie C.L., Holt K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponstingl H., Ning Z. SMALT–A New Mapper for DNA Sequencing Reads. F1000 Research 2010. [(accessed on 18 December 2019)]. Available online: https://f1000research.com/posters/327.
- 25.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wylie K.M., Wylie T.N., Minx P.J., Rosen D.A. Whole-genome sequencing of Klebsiella pneumoniae isolates to track strain progression in a single patient with recurrent urinary tract infection. Front. Cell. Infect. Microbiol. 2019;9:14. doi: 10.3389/fcimb.2019.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forde B.M., Ben Zakour N.L., Stanton-Cook M., Phan M.-D., Totsika M., Peters K.M., Chan K.G., Schembri M.A., Upton M., Beatson S.A. The complete genome sequence of Escherichia coli EC958: A high quality reference sequence for the globally disseminated multidrug resistant E. coli O25b:H4-ST131 clone. PLoS ONE. 2014;9:e104400. doi: 10.1371/journal.pone.0104400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centre for Genomic Pathogen Surveillance (CGPS) Pathogenwatch Private Metadata. [(accessed on 31 October 2019)]. Available online: https://cgps.gitbook.io/pathogenwatch/how-to-use-pathogenwatch/private-metadata.
- 29.Ondov B.D., Treangen T.J., Melsted P., Mallonee A.B., Bergman N.H., Koren S., Phillippy A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016;17:132. doi: 10.1186/s13059-016-0997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatusova T., DiCuccio M., Badretdin A., Chetvernin V., Nawrocki E.P., Zaslavsky L., Lomsadze A., Pruitt K.D., Borodovsky M., Ostell J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inouye M., Dashnow H., Raven L.A., Schultz M.B., Pope B.J., Tomita T., Zobel J., Holt K.E. SRST2: Rapid genomic surveillance for public health and hospital microbiology labs. Genome Med. 2014;6:90. doi: 10.1186/s13073-014-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Achtman M., Wain J., Weill F.X., Nair S., Zhou Z., Sangal V., Krauland M.G., Hale J.L., Harbottle H., Uesbeck A., et al. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog. 2012;8:e1002776. doi: 10.1371/journal.ppat.1002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diancourt L., Passet V., Nemec A., Dijkshoorn L., Brisse S. The population structure of Acinetobacter baumannii: Expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE. 2010;5:e10034. doi: 10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheutz F., Teel L.D., Beutin L., Pierard D., Buvens G., Karch H., Mellmann A., Caprioli A., Tozzoli R., Morabito S., et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J. Clin. Microbiol. 2012;50:2951–2963. doi: 10.1128/JCM.00860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 36.McArthur A.G., Waglechner N., Nizam F., Yan A., Azad M.A., Baylay A.J., Bhullar K., Canova M.J., De Pacale G., Ejim L., et al. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother. 2013;57:3348–3357. doi: 10.1128/AAC.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng Y., Zou S., Chen H., Yu Y., Ruan Z. BacWGSTdb 2.0: A one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res. 2021;49:D644–D650. doi: 10.1093/nar/gkaa821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadfield J., Croucher N.J., Goater R.J., Abudahab K., Aanensen D.M., Harris S.R. Phandango: An interactive viewer for bacterial population genomics. Bioinformatics. 2018;34:292–293. doi: 10.1093/bioinformatics/btx610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carattoli A., Zankari E., García-Fernández A., Larsen M.V., Lund O., Villa L., Aarestrup F.M., Hasman H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clinical and Laboratory Standards Institute . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. 30th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2016. Approved Standard; CLSI Document C24–Ed4. [Google Scholar]
- 41.Didelot X., Wilson D.J. ClonalFrameML: Efficient inference of recombination in whole bacterial genomes. PLoS Comput. Biol. 2015;11:e1004041. doi: 10.1371/journal.pcbi.1004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Letunic I., Bork P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019;47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rambaut A. FigTree v1.3.1. Institute of Evolutionary Biology, University of Edinburgh; Edinburgh, Scotland: 2010. [(accessed on 20 January 2020)]. Available online: http://tree.bio.ed.ac.uk/software/figtree/ [Google Scholar]
- 45.Page A.J., Cummins C.A., Hunt M., Wong V.K., Reuter S., Holden M.T., Fookes M., Falush D., Keane J.A., Parkhill J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salinas L., Cárdenas P., Johnson T.J., Vasco K., Graham J., Trueba G. Diverse commensal Escherichia coli clones and plasmids disseminate antimicrobial resistance genes in domestic animals and children in a semirural community in Ecuador. mSphere. 2019;4:e00316-19. doi: 10.1128/mSphere.00316-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimata K., Shima T., Shimizu M., Tanaka D., Isobe J., Gyobu Y., Watahiki M., Nagai Y. Rapid categorization of pathogenic Escherichia coli by multiplex PCR. Microbiol. Immunol. 2005;49:485–492. doi: 10.1111/j.1348-0421.2005.tb03752.x. [DOI] [PubMed] [Google Scholar]
- 49.Montealegre M.C., Roy S., Boni F., Hossain M.I., Navab-Daneshmand T., Caduff L., Faruque A.S.G., Islam M.A., Julian T.R. Risk factors for detection, survival, and growth of antibiotic-resistant and pathogenic Escherichia coli in household soils in rural Bangladesh. Appl. Environ. Microbiol. 2018;84:e01978-18. doi: 10.1128/AEM.01978-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben Zakour N.L., Alsheikh-Hussain A.S., Ashcroft M.M., Khanh N.T., Roberts L.W., Stanton-Cook M., Schembri M.A., Beatson S.A. Sequential acquisition of virulence and fluoroquinolone resistance has shaped the evolution of Escherichia coli ST131. mBio. 2016;7:e00347-16. doi: 10.1128/mBio.00347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanjar F., Rusconi B., Hazen T.H., Koenig S.S., Mammel M.K., Feng P.C., Rasko D.A., Eppinger M. Characterization of the pathogenome and phylogenomic classification of enteropathogenic Escherichia coli of the O157: Non-H7 serotypes. Pathog Dis. 2015;73:ftv033. doi: 10.1093/femspd/ftv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Day M.J., Hopkins K.L., Wareham D.W., Toleman M.A., Elviss N., Randall L., Teale C., Clearly P., Wiuff C., Doumith M., et al. Extended-spectrum β-lactamase-producing Escherichia coli in human-derived and foodchain-derived samples from England, Wales, and Scotland: An epidemiological surveillance and typing study. Lancet. Infect. Dis. 2019;19:1325–1335. doi: 10.1016/S1473-3099(19)30273-7. [DOI] [PubMed] [Google Scholar]
- 53.Valenza G., Werner M., Eisenberger D., Nickel S., Lehner-Reindl V., Holler C., Bogdan C. First report of the new emerging global clone ST1193 among clinical isolates of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli from Germany. J. Glob. Antimicrob. Resist. 2019;17:305–308. doi: 10.1016/j.jgar.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Mhaya A., Trabelsi R., Begu D., Aillerie S., M’zali F., Tounsi S., Gdoura R., Arpin C. Emergence of B2-ST131-C2 and A-ST617 Escherichia coli clones producing both CTX-M-15- and CTX-M-27 and ST147 NDM-1 positive Klebsiella pneumoniae in the Tunisian community. bioRxiv. 2019 doi: 10.1101/713461. [DOI] [Google Scholar]
- 55.Castellanos L.R., Donado-Godoy P., León M., Clavijo V., Arevalo A., Bernal J.F., Timmerman A.J., Mevius D.J., Wagenaar J.A., Hordijk J. High heterogeneity of Escherichia coli sequence types harbouring ESBL/AmpC genes on IncI1 plasmids in the colombian poultry chain. PLoS ONE. 2017;12:e0170777. doi: 10.1371/journal.pone.0170777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choudhury N.A., Paul D., Chakravarty A., Bhattacharjee A., Dhar Chanda D. IncX3 plasmid mediated occurrence of blaNDM-4 within Escherichia coli ST448 from India. J. Infect. Public Health. 2018;11:111–114. doi: 10.1016/j.jiph.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Zhang P., Wang J., Wang X., Bai X., Ma J., Dang R., Xiong Y., Fanning S., Bai L., Yang Z. Characterization of five Escherichia coli isolates co-expressing ESBL and MCR-1 resistance mechanisms from different origins in China. Front. Microbiol. 2019;10:1994. doi: 10.3389/fmicb.2019.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghenea A.E., Cioboată R., Drocaş A.I., Țieranu E.N., Vasile C.M., Moroşanu A., Țieranu C.G., Salan A.I., Popescu M., Turculeanu A., et al. Prevalence and antimicrobial resistance of Klebsiella strains isolated from a county hospital in Romania. Antibiotics. 2021;10:868. doi: 10.3390/antibiotics10070868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang J., Ye L., Guo L., Zhao Q., Chen R., Luo Y., Chen Y., Tian S., Zhao J., Shen D., et al. A nosocomial outbreak of KPC-2-producing Klebsiella pneumoniae in a Chinese hospital: Dissemination of ST11 and emergence of ST37, ST392 and ST395. Clin. Microbiol. Infect. 2013;19:E509–E515. doi: 10.1111/1469-0691.12275. [DOI] [PubMed] [Google Scholar]
- 60.Li J., Zou M.X., Wang H.C., Dou Q.Y., Hu Y.M., Yan Q., Liu W.E. An outbreak of infections caused by a Klebsiella pneumoniae ST11 clone coproducing Klebsiella pneumoniae carbapenemase-2 and RmtB in a Chinese Teaching Hospital. Chin. Med. J. 2016;129:2033–2039. doi: 10.4103/0366-6999.189049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu D., Dong N., Zheng Z., Lin D., Huang M., Wang L., Chan E.W., Shu L., Yu J., Zhang R., et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: A molecular epidemiological study. Lancet Infect. Dis. 2018;18:37–46. doi: 10.1016/S1473-3099(17)30489-9. [DOI] [PubMed] [Google Scholar]
- 62.Jayol A., Poirel L., Dortet L., Nordmann P. National survey of colistin resistance among carbapenemase-producing Enterobacteriaceae and outbreak caused by colistin-resistant OXA-48-producing Klebsiella pneumoniae, France, 2014. Euro surveillance. Eur. Commun. Dis. Bull. 2016;21:30339. doi: 10.2807/1560-7917.ES.2016.21.37.30339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alqasim A., Abu Jaffal A., Alyousef A.A. Prevalence of multidrug resistance and extended-spectrum β-lactamase carriage of clinical uropathogenic Escherichia coli isolates in Riyadh, Saudi Arabia. Int. J. Microbiol. 2018;2018:3026851. doi: 10.1155/2018/3026851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao R., Shi J., Shen Y., Li Y., Han Q., Zhang X., Gu G., Xu J. Phylogenetic distribution of virulence genes among ESBL-producing uropathogenic Escherichia coli isolated from long-term hospitalized patients. J. Clin. Diagn. Res. 2015;9:DC01–DC04. doi: 10.7860/JCDR/2015/13234.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mbelle N.M., Osei Sekyere J., Amoako D.G., Maningi N.E., Modipane L., Essack S.Y., Feldman C. Genomic analysis of a multidrug-resistant clinical Providencia rettgeri (PR002) strain with the novel integron ln1483 and an A/C plasmid replicon. Ann. N. Y. Acad. Sci. 2020;1462:92–103. doi: 10.1111/nyas.14237. [DOI] [PubMed] [Google Scholar]
- 66.Adefisoye M.A., Okoh A.I. Identification and antimicrobial resistance prevalence of pathogenic Escherichia coli strains from treated wastewater effluents in Eastern Cape, South Africa. Microbiol. Open. 2016;5:143–151. doi: 10.1002/mbo3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw and generated data for this Pilot study are available in the following links: https://doi.org/10.6084/m9.figshare.17105876 (supplementary files, accessed on 16 November 2021), https://doi.org/10.6084/m9.figshare.17091293 (draft genome assemblies, accessed on 16 November 2021) and https://doi.org/10.6084/m9.figshare.11965455 (Table 2 and Table 3, accessed on16 November 2021).