Abstract
Phase separation is the driving force behind formation of various biomolecular condensates (BioMCs), which sub-compartmentalize certain cellular components in a membraneless manner to orchestrate numerous biological processes. Many BioMCs are composed of proteins and RNAs. While the features and functions of proteins are well studied, less attention was paid to the other essential component RNAs. Here, we describe how RNA contributes to the biogenesis, dissolution, and properties of BioMCs as a multivalence providing scaffold for proteins/RNA to undergo phase separation. Specifically, we focus on N6-methyladenosine (m6A), the most widely distributed dynamic post-transcriptional modification, which would change the charge, conformation, and RNA-binding protein (RBP) anchoring of modified RNA. m6A RNA-modulated phase separation is a new perspective to illustrate m6A-mediated various biological processes. We summarize m6A main functions as “beacon” to recruit reader proteins and “structural switcher” to alter RNA–protein and RNA–RNA interactions to modulate phase separation and regulate the related biological processes.
Keywords: phase separation, N6-methyladenosine (m 6 A), biomolecular condensate, multivalence, RNA modification, RNA–RNA interaction, RNA–protein interaction
Introduction
Compartmentalization is a common strategy of cells to ensure timely and spatial execution and coordination of various biochemical reactions. While many compartments called membrane-bound organelles are surrounded by phospholipid bilayers, membraneless organelles, biomolecular condensates (BioMCs) lacking lipid bilayers, also constitute another form of cellular compartments. Although BioMCs and membrane-bound organelles are both efficient to accomplish biochemical reactions within the organelles, they differ significantly in their biogenesis, component, sensitivity to the environment, and so on (Aguilera-Gomez and Rabouille, 2017). In 2009, P granules, a type of protein-rich BioMCs essential for zygogenesis in Caenorhabditis elegans, were found to exhibit gel-like behaviors (Brangwynne et al., 2009). Since then, phase separation, especially liquid–liquid phase separation (LLPS), has gained broad attention as a physicochemical mechanism for forming both nuclear and cytoplasmic membraneless structures. To date, many distinct BioMCs are reportedly driven by phase separation, including stress granules (SGs) (Molliex et al., 2015; Guillén-Boixet et al., 2020; Sanders et al., 2020; Yang et al., 2020), processing bodies (PBs) (Smith et al., 2016), spindle apparatus (Jiang et al., 2015), and centrosome (Woodruff et al., 2017) in the cytoplasm along with nucleolus (Brangwynne et al., 2011; Feric et al., 2016; Yao et al., 2019) and paraspeckles (Hennig et al., 2015; West et al., 2016; Yamazaki et al., 2018) in the nucleus. In addition to conventional condensates, burgeoning BioMCs participating in gene expression such as heterochromatin (Strom et al., 2017), super enhancer (Hnisz et al., 2017; Sabari et al., 2018), and mediator complex (Boija et al., 2018; Guo et al., 2019) are all formed and modulated by phase separation.
Based on the current literature, phase separation is elicited by multivalent low-affinity interactions, which usually happen among protein–protein, protein–RNA, and RNA–RNA (Banani et al., 2016). Through phase separation, protein and/or RNA components concentrate to form “droplets” distinct from surrounding dilute phase, which exhibit unique properties such as spherical shape and rapid dynamics (Banani et al., 2017; Alberti et al., 2019), thereby exerting various functions. Apart from well-known membraneless cellular compartments (e.g., SGs and PBs) functioning as important organelles via phase separation, it was reported recently that phase separation might associate with oncogenic fusion protein degradation by heat stress (Maimaitiyiming et al., 2021). The fluidity of BioMCs allows them to organize dynamically and function efficiently. Inversely, the arrest of BioMCs’ dynamics is correlated with some pathological processes (Mathieu et al., 2020). Take TDP-43 as an example, abnormal nuclear shuttle and decreased nuclear pore complex caused by persistent stress or cell aging could lead to the accumulation of TDP-43 in the cytoplasm; the abnormal TDP-43 accumulation results in decreased dynamics of phase-separated TDP-43 droplets and converts the droplets into gel or solid aggregations, which could induce neurotoxicity (Gasset-Rosa et al., 2019). Furthermore, the decreased RNA-binding capacity of TDP-43 induced by mutation in RNA-recognition motif also exhibits reduced dynamics and promotes similar pathological progression (Mann et al., 2019). Therefore, it is worth to further study the mechanism by which BioMCs assemble and function, so as to exploit ways to modulate this physiological process for developing novel treatment approaches for diseases caused by abnormal phase separation.
Many BioMCs are ribonucleoprotein (RNP) granules containing RNA and RNA-binding proteins (RBPs) (Banani et al., 2016; Banani et al., 2017), and the heterogeneity of composition may dictate the heterogeneity of function (Banani et al., 2016). The majority of the available literatures focus on the contribution of proteins to phase separation. By performing targeted mutagenesis, many studies have demonstrated that low-complexity domains (LCDs)/intrinsically disordered regions (IDRs) in RBPs contribute to multivalence and are essential in RNP granule formation (Boeynaems et al., 2018). However, in sharp contrast to the role of RBPs in phase separation, much less attention was paid to RNA. Notably, IDRs of some RBPs provide structural flexibility to make adequate contact with their partner RNAs (Molliex et al., 2015; Basu and Bahadur, 2016). In addition, RNA-binding domains (RBDs) in RBPs are required for BioMCs’ assembly, while IDRs are dispensable in certain cases (Guillén-Boixet et al., 2020; Sanders et al., 2020; Yang et al., 2020). Consistent with these findings, several studies showed that the addition of RNA lowers the concentration threshold for RBPs to trigger phase separation (Gao et al., 2019; Ries et al., 2019; Guillén-Boixet et al., 2020; Wang et al., 2020; Yang et al., 2020). These findings indicate that RNA plays a role in phase separation, at least by interacting with RBPs. Furthermore, protein-free total RNA extracted from yeast self-assembles into droplets (van Treeck et al., 2018), implying RNA–RNA interaction potentially contributes to phase separation as well.
Here, we mainly focus on the contribution of RNA in phase separation. Given post-transcriptional RNA modification is widely distributed and of critical importance to RNA processing and function (Zaccara et al., 2019), we summarize current studies on how RNA modification, especially N6-methyladenosine (m6A), contributes to phase separation and discuss its potential biological significance.
RNA Modulates the Formation and Properties of Biomolecular Condensates by Regulating Phase Separation
The interaction between biological macromolecules is the core event in phase separation, and the valence as well as affinity of the interaction are the key parameters for regulating phase separation (Tauber et al., 2020a). Previously, the role of proteins in phase separation has been widely reported. IDRs are rich in disorder-promoting amino acids (such as Q, S, N, Y, and G) and prone to form pi–pi, cation–pi, or bipolar interactions. Thus, proteins containing IDRs are generally involved in phase separation through providing multivalent interactions (Oldfield and Dunker, 2014). Accruing evidence indicated that RNA, as a flexible and variable molecule with the properties to interact with multiple partners including protein and RNA (Roden and Gladfelter, 2021), is a potentially powerful multivalency provider in phase separation. In this part, we discuss how RNA affects the assembly and properties of BioMCs through modulating phase separation.
RNA: An Innate Multivalence Provider
On one hand, RNA provides multivalency by introducing a non-specific negative charge to modulate phase separation (Figure 1). Most of the interactions that modulate phase separation are electrostatic in nature. To a certain extent, polymers with opposite charges can promote the condensation of biological macromolecules. For instance, during RNP granule formation, LCDs of protein components can drive phase separation through non-covalent charged interaction (Wang et al., 2018), and this type of interaction occurs either between LCDs or between LCDs and other domains of proteins (Monahan et al., 2017; Qamar et al., 2018). Similarly, adding cationic spermine to the solution promotes the condensation of negatively charged poly-U RNA into small droplets, which exhibit fluidity and temperature sensitivity. This phase-separated system reaches its highest turbidity at a specific ratio of positive and negative charges (Aumiller et al., 2016). It indicates that the negative charge of RNA favors phase separation once oppositely charged substances are added to the system by providing multivalency for the charge–charge interaction.
FIGURE 1.

RNA regulates biomolecular condensates’ (BioMCs) properties through providing multivalence. RNA acts as an important multivalence provider through interacting with other bio-macromolecules (such as proteins and RNA) via charge–charge, sequence-specific, and structure-dependent interactions.
Unlike DNA, most cellular RNAs are single-stranded, leading to complete exposure of phosphate backbones and bases to the surrounding environment. This is beneficial for establishing an interaction between RNA and positively charged molecules. In a model composed of poly-U RNAs and cationic peptides, dephosphorylation at a serine is sufficient to cause a charge–charge interaction between the negatively charged RNA and the positively charged peptides, thereby mediating phase separation. Conversely, phosphorylation at this serine reverses this process, causing the formed droplets to dissolve (Aumiller and Keating, 2016). In addition, some protein domains rich in positive charges, such as the arginine/glycine-rich (RGG) domain, are believed to bind to negatively charged RNA non-specifically to promote protein–RNA interaction (Elbaum-Garfinkle et al., 2015; Lin et al., 2015; Saha et al., 2016; Yoshizawa et al., 2018), which displays the potential of RNA to influence phase separation through charge–charge interaction. It is worth noting that although the addition of oppositely charged biomolecules initially promotes droplet assembly through mechanisms including charge–charge interactions, excess amount of spermine will cause dissolution of small droplets formed by poly-U RNA (Aumiller et al., 2016), and superabundant RNA in the phase-separated system will trigger a charge inversion and disassembly of RNP. This phenomenon is called reentrant phase transition (Banerjee et al., 2017). Collectively, RNA can provide negative charge to regulate the generation and depolymerization of BioMCs in a sequence-independent manner.
On the other hand, RNA also provides protein or RNA binding sites in a sequence- and structure-dependent manner to enhance RNA–protein or RNA–RNA interactions (Figure 1). RNA structure is relatively flexible, allowing it to either maintain an unstructured state and expose the combination motif for other molecules or fold into complicated structures such as hairpin, helical regions, tetra loops, G-quadruplexes, etc. (Miao and Westhof, 2017). The structural diversity allows RNA to form multiple conformations. Although the RNA structure is thought to play a role to limit access of RBPs to its target specific RNA motifs, certain non-specific RBPs bind to RNA via recognizing RNA structure (Jankowsky and Harris, 2015). For instance, the Whi3 protein preferentially binds to stem loops formed by mRNAs such as CLN3, BNI1, and SPA2 and regulates secondary structures of these mRNAs (Langdon et al., 2018). Collectively, RNA can interact with other molecules in various ways including non-specific charge–charge, sequence-dependent, and structure-dependent manners, which increase the probability to form RNA-dependent massive complexes, thereby promoting phase separation. Interestingly, numerous in vitro experiments have shown that adding RNA to the protein solution could result in the reduction of protein concentration through forming phase-separated droplets (van Treeck and Parker, 2018; Tauber et al., 2020a), but other studies showed that excessive or high-affinity RNAs prevent phase separation by competitively inhibiting protein–protein interaction (Maharana et al., 2018; Gasset-Rosa et al., 2019; Mann et al., 2019), suggesting the ratio of the amount between RNA and protein is a critical parameter for phase separation. Notably, longer RNAs are preferred to promote the formation of phase-separated structures as they might possess multiple sites to interact with other partners. Proving this notion, in some phase-separated intracellular granules, such as P-granule (Saha et al., 2016) and stress granule (Khong et al., 2017), there is a preference for enrichment of longer RNAs. Together, these findings suggest that RNA plays an important role in phase separation through modulating RNA–protein interaction.
RNA–RNA interaction can occur in a variety of ways including Watson–Crick base-pairing, non-Watson–Crick interaction, and base stacking. For two random long RNAs, these kinds of interactions are potentially widespread (van Treeck and Parker, 2018). Longer length, higher GC content, and binding with RBPs are favorable for this kind of interaction, while a structured and translated state inhibits it (van Treeck and Parker, 2018; Roden and Gladfelter, 2021). Multiple RNAs self-assemble in vitro independent of RBPs (Aumiller et al., 2016; Langdon et al., 2018; Boeynaems et al., 2019). For instance, the protein-free total RNA extracted from yeast undergoes self-assembly in an environment mimicking physiological state, implying that RNA–RNA interaction alone is sufficient to mediate phase separation (van Treeck et al., 2018). The formation of paraspeckles is also reportedly mediated by RNA–RNA interaction (Chujo and Hirose, 2017). Likewise, Barr body (Cerase et al., 2019), a well-studied BioMCs that potentially assembles through phase separation, exhibited intermolecular interactions among XISTs (Lu et al., 2016). Other studies also showed the importance of RNA–RNA interaction in BioMC formation via modulating phase separation. Ras-GAP SH3 domain-binding proteins (G3BPs) are important assembly factors of SGs, and knockout of G3BPs disrupts SGs’ formation (Guillén-Boixet et al., 2020; Yang et al., 2020; Hofmann et al., 2021); Tauber et al. (2020b) found that promoting RNA–RNA interaction by inhibiting eIF4A, a disruptive protein for RNA–RNA interaction, results in SG reformation in G3BPs knockout cells.
Notably, similar to protein aggregation diseases, short nucleotide repeats containing RNAs undergo sol-gel transition by multivalent base-pairing (Gasset-Rosa et al., 2019), and inhibition of aberrant RNA–RNA interaction by adding monomeric RBP leads to disassembly of RNA droplets (Sheth and Parker, 2003; Chujo and Hirose, 2017). As a whole, the complicated network structure formed by RNA–RNA interactions might directly trigger phase separation or provide platforms for proteins to condensate and undergo phase separation.
The Role of RNA in Phase Separation: Driver, Regulator, and Buster
As an important biomacromolecule with relatively large size, complicated sequence, and flexible structure, RNA serves as a scaffold for the interaction between biomacromolecules including RNA–protein and RNA–RNA, to achieve multivalence and modulate phase separation. Specifically, RNA regulates the valence and affinity of interaction with other biomacromolecules, playing different roles in phase separation. First, an increase of regional concentration of specific RNAs is the driver of local BioMC formation. In the nucleus, this is often mediated by transcription activities, which is in synergy with the establishment of some nuclear structures. For example, paraspeckles are formed at the local transcription site of lncRNA NEAT1 (Chujo and Hirose, 2017), and pre-rRNA transcription is involved in the formation as well as maintenance of nucleolus (Brangwynne et al., 2011; Feric et al., 2016; Yao et al., 2019). In the cytoplasm, the formation of SGs and PBs is dependent on the increase of the pool of untranslated naked RNAs. Inhibiting RNA degradation or blocking the initiation of translation at the overall level promotes the biogenesis of SGs and PBs (Sheth and Parker, 2003; Mazroui et al., 2006). On the contrary, degrading mRNA or trapping them on the ribosomes inhibits the biogenesis of SGs (Khong and Parker, 2018; Burke et al., 2019; Guillén-Boixet et al., 2020; Yang et al., 2020), suggesting the indispensable role of RNAs in the formation of BioMCs.
In addition to acting as seeds and platforms for formation of RNP granules, RNA also serves as a regulator for the properties, such as dynamics and subcompartmentalization, of RNP granules (Tauber et al., 2020a; Trcek et al., 2020). Generally, higher valency among biomacromolecules represents a slower exchange rate (Banani et al., 2016), and this provides an explanation for why RNA leads to slower dynamics in RNP granule both in vitro and in vivo (Banani et al., 2016; Tauber et al., 2020a). In physiological conditions, specific RNAs determine the liquid-like dynamics of specific RNP granules and create immiscible granules (Zhang et al., 2015; Langdon et al., 2018). In repeat expansion diseases, RNA foci exhibited less dynamics and gelation in a repeat length-dependent manner (Fay et al., 2017; Jain and Vale, 2017). For specific RBPs, higher affinity of RNAs induces less dynamics, indicating a lower off-rate from RNAs (Tauber et al., 2020a). This was proved by domain-swapping experiments of G3BP1 (Yang et al., 2020). Specifically, adding more RBDs (KH domain; ZnF domain; G3BP1 RBD) resulted in less dynamics of G3BP1. The internal substructures of RNP granules remain largely unknown (Feric et al., 2016). Recently, Trcek et al. (2020) showed that mRNAs could self-assemble into homotypic assemblies within granules, and the regulation of spatial organization is due to sequence but not general RNA–RNA interactions.
Contrary to acting as “seeds” or “glue,” when the concentration of RNA is excessively high, it exhibits a destructive effect on the already formed BioMCs. This effect may be caused by charge inversion due to the introduction of massive negatively charged RNA or hindering of protein–protein interaction by excess RNA (Banerjee et al., 2017; Mann et al., 2019). Given that the concentration of RNA in the nucleus is more than 10 times higher than that of the cytoplasm (Maharana et al., 2018), RNA in the nucleus may serve as buffer to limit the abnormal aggregation of FUS, TDP43, hnRNPA1, and other nuclear proteins (Maharana et al., 2018; Mann et al., 2019). On the contrary, under senescence or repeated external stimuli, TDP43 abnormally locate and accumulate in the cytoplasm to produce pathological aggregation (Gasset-Rosa et al., 2019). Notably, a dynamic RNA/protein ratio change may play a role in controlling RNP granule with tunable lifetimes through either promoting or preventing phase separation (Banerjee et al., 2017; Henninger et al., 2021). For instance, Henninger et al. (2021) reported that newborn RNAs contribute to feedback control of transcriptional condensates by reentrant phase transition. Specifically, small non-coding RNAs produced at the initiation of transcription promote the formation of transcription condensates mediated by phase separation, but the large amount of long-strand RNA produced by transcription elongation will in turn promote the dispersion of the transcription condensates, by which newborn RNA completes feedback regulation. Collectively, RNA plays various roles during phase separation depending on the context.
RNA m6A Modification Modulates Phase Separation
As mentioned above, the multivalent interaction is the key event for both formation and modulation of phase separation-mediated BioMCs. Recent studies showed that the interaction is widely affected by post-translational modification (PTM) of proteins participating in phase separation (Hofweber and Dormann, 2019). For instance, arginine methylation at RGG regions in LCDs reduces the phase separation potential of hnRNPA2 by disrupting arginine-mediated arginine–aromatic interactions (Ryan et al., 2018). Delocalized pi system can be provided by both aromatic amino acids and nucleobases, implying RNA–protein interaction is also a potential target modulated by arginine methylation. Proving this notion, arginine demethylation at the RGG region of G3BP1, which is considered important for RNA binding and initiation of SG establishment (3–6), is prerequisite for SG assembly (Tsai et al., 2016).
Similar to post-translational modifications of proteins, RNA is subject to numerous post-transcriptional modifications (over 160), which play critical roles in modulating the properties of RNA and regulating RNA metabolism (Machnicka et al., 2013). Unlike 5-methylcytosine (m5C) on the CpG island of DNA (Roels et al., 2020), m6A is the most abundant and well-studied modification on eukaryotic mRNA. m6A is a dynamic and reversible modification regulated by three groups of “m6A modifiers,” including “m6A writers” (m6A methyltransferases), “m6A readers” (m6A-binding proteins), and “m6A erasers” (m6A demethylases) (Zaccara et al., 2019; Liang et al., 2020). The m6A writers (METTL3/METTL14/WATP complex, METTL16, etc.) catalyze m6A in a site- and transcript-specific manner, and the m6A erasers (FTO and ALKBH5) specifically remove the methyl group. These enzymes make m6A modification reversible and adjustable. Notably, the function of m6A modification is extensively decided by its readers (YTH domain containing proteins, IGF2BPs, HNRNPs, etc.). Each of these readers exhibits distinct function in regulating the fate of m6A-modified RNA. For instance, YTHDC1 regulates alternative splicing (Xiao et al., 2016) and nuclear export (Roundtree et al., 2017), YTHDC2 promotes translation initiation (Hsu et al., 2017) and RNA degradation (Wojtas et al., 2017), YTHDF1 enhances the translation (Wang et al., 2015), YTHDF2 promotes RNA degradation (Wang et al., 2013), and YTHDF3 exhibits similar functions with both YTHDF1 and YTHDF2 depending on the context (Shi et al., 2017). Therefore, m6A modification is involved in the elaborate regulation of many bioprocesses including cellular stress responses (Zhou et al., 2015; Xiang et al., 2017; Ji et al., 2021; Ninomiya et al., 2021), tumorigenesis (Deng et al., 2018; Liang et al., 2020; Rosselló-Tortella et al., 2020), and differentiation (Edens et al., 2019; Song et al., 2019).
Recently, several groups have reported that multivalent m6A-modified RNAs act as scaffolds to gather YTHDF proteins and thus lead to phase separation both in vitro and in vivo (Gao et al., 2019; Ries et al., 2019; Fu and Zhuang, 2020; Wang et al., 2020). These m6A-modified mRNA–YTHDF protein complexes are subsequently partitioned into SGs and potentially influence the fate of m6A-containing mRNA stored in SGs (Gao et al., 2019; Ries et al., 2019; Fu and Zhuang, 2020). These studies reveal the strong potential of m6A-modified RNA over regulating phase separation. However, it remains elusive what molecular mechanisms are exploited by m6A modification other than multivalently recruiting YTHDF proteins to influence phase separation and regulate multiple bioprocesses.
In this part, based on the current literature, we describe how m6A modification alters the properties of target RNA (including RNA conformation, the capacity for protein-binding, and the affinity to interact with other RNAs) and further discuss how these changes potentially contribute to phase separation.
m6A Acts as a “Beacon” to Recruit Reader Proteins
Interacting with diverse m6A readers is recognized as a major mechanism for regulating various fate for m6A-harboring RNA. By now, a large group of RBPs have been verified to directly bind to m6A-modified RNA (Zaccara et al., 2019). Among them, YTH domain-harboring proteins are the first group of readers to be discovered in an m6A pull-down assay (Dominissini et al., 2012). Structural studies revealed that m6A resides in a deep cleft formed by three hydrophobic residues in YTH domain–m6A-modified RNA complex, and the methyl–pi interaction between the methyl group of m6A and the rings of the two tryptophan residues constitutes the basis of the preference of YTH domain toward m6A modification (Liao et al., 2018). The members of cytosolic YTHDF family (YTHDF1/2/3) share high similarity in length, amino acid composition, and conformation; except for C-terminal YTH domain of around 15 kDa, YTHDF family proteins also contain an around 40-kDa LCD including prion-like domain (Patil et al., 2018). This structural feature implies potential for YTHDFs to be involved in phase separation. Indeed, the LCDs of all three YTHDFs are sufficient to trigger phase separation in a concentration-dependent manner without RNA in vitro (Gao et al., 2019); notably, glutamine (Q)-rich domain is important for the capacity to undergo phase separation, as changing all Q to alanine (A) in this region led to loss of phase separation potential (Wang et al., 2020). Consistently, the YTH domain alone failed to undergo phase separation even at a high concentration (Ries et al., 2019). Although the YTH domain is not required for phase separation in vitro, it plays an important role in phase separation of YTHDFs through binding to m6A-modified RNA. The addition of m6A-modified RNA can lower the concentration threshold needed to form YTHDF condensates in an m6A valency-dependent manner (Gao et al., 2019; Ries et al., 2019; Wang et al., 2020), but the enhancing effect of multivalence by m6A-modified RNA disappears when the m6A-binding capability of YTHDFs is compromised either by mutation or deletion of YTH domain (Gao et al., 2019; Wang et al., 2020). These studies suggest that multivalent m6A-modified RNA may act as scaffolds to concentrate YTHDFs in a small area leading to phase separation.
More importantly, the phenomena observed in vitro may have an important implication in vivo given the tight correlation between m6A-modified RNA–YTHDFs complex and biomolecular condensates including SGs, PBs, and neuronal RNA granules (Molliex et al., 2015; Gao et al., 2019; Ries et al., 2019; Fu and Zhuang, 2020; Patil et al., 2018). First, m6A-modified RNA–YTHDFs complex colocalized with SGs formed by overexpressing G3BP1 or various stresses including oxidative stress, heat shock, and ER stress (Gao et al., 2019; Ries et al., 2019; Fu and Zhuang, 2020). Second, excluding the interference of length, which is an important parameter for RNA targeting into biomolecular condensates (van Treeck et al., 2018), the SGs mediated by various stresses consistently show a preference for m6A-modified mRNA in a valency- and stoichiometry-dependent manner (Wang et al., 2020). In addition to SGs, PBs also exhibit the preference in a stoichiometry-dependent manner regardless of the length of mRNA (Wang et al., 2020). Third, the capability of m6A binding is essential for YTHDFs to partition into BioMCs in vivo. For instance, METTL14 knockout reduced targeting of YTHDF2 to PBs under normal conditions and markedly reduced the relocation of YTHDF2 into SGs under stress. Similarly, the compromised m6A-binding capacity resulting from introducing mutation to YTH domain lowered YTHDF2 content in SGs as well (Ries et al., 2019). For YTHDF1 and 3, knockdown of any or both of them disturbed SG formation, and YTHDF protein expression was able to restore SGs, while the overexpression of YTH domain-deficient truncation failed to do so. Consistently, by overexpressing a dominant-negative YTHDF1 to compete m6A binding with endogenous wild-type YTHDFs, SG formation was partially impaired under stress conditions (Fu and Zhuang, 2020). It is worth noting that when N-IDR was swapped with CRY2olig domain, which can undergo blue light-induced oligomerization (Taslimi et al., 2014), blue light succeeded to oligomerize recombinant YTHDF1 but failed to induce SG assembly in YTHDF1/3 KD cells, even under stress conditions (Fu and Zhuang, 2020). These findings suggest that neither oligomerization of YTHDFs nor YTHDF interaction with other proteins is sufficient for phase separation and SG assembly. On the other hand, it is evident that m6A acts as a “beacon” to recruit readers (Figure 2A) and mRNA harboring multivalent m6A modification and serves as scaffolds to gather multiple reader proteins, which may enhance phase separation and modulate SGs. However, although YTHDF1/3 knockdown largely reduces SG formation, SG assembly seems not fully dependent on m6A modification (Fu and Zhuang, 2020). A recent study showed that SGs assemble from the summation of a multitude of RNA–protein, RNA–RNA, and protein–protein interactions rather than only one of them (Matheny et al., 2021), and this explains why the length of mRNAs as well as the number of potential interactions would play a major role in the formation of condensates (Khong et al., 2017). Given the different conditions applied and heterogeneity of SG assembly mechanism (van Leeuwen and Rabouille, 2019), both m6A modification and YTHDF proteins contribute to phase separation of m6A RNA–YTHDF complex.
FIGURE 2.
RNA N6-methyladenosine (m6A) modification regulates RNA–RNA and RNA–protein interactions of modified RNA. (A) RNA m6A modification acts as a “beacon” to directly recruit various m6A readers in both the nucleus and cytoplasm. (B) RNA m6A modification modulates RNA–RNA and RNA–protein interaction through “structural switcher” function. m6A modification promotes the instability of RNA base complementary pairing and thus leads to deconstruction of the corresponding structure, reshaping the spectrum of RNA–protein and RNA–RNA interaction.
Another member of YTH domain-harboring protein with the potential to undergo phase separation is YTHDC1, which mainly localizes in the nucleus and also contains a large LCD similar to YTHDFs (Patil et al., 2016). YTHDC1 was first found to localize in dot-like subnuclear condensates named “YT bodies” (recognized as nuclear speckles now), a membraneless structure that exhibited dynamics and is subjected to regulation by transcription state (Nayler et al., 2000). The function of YTHDC1 is tightly correlated with nuclear bioprocesses that are deemed to be driven or modulated by phase separation. First, YTHDC1 may participate in remodeling chromatin structure and gene silencing mediated by heterochromatin through phase separation (Peng et al., 2020). To be specific, nascent RNA with m6A sites recruits KDM3B, one of the histone demethylases, through the scaffolding role of YTHDC1 and thus decreases the H3K9me2 levels to potentially participate in chromosome remodeling (Li et al., 2020a). In addition, YTHDC1 also binds to transcripts of retrotransposons in an m6A-dependent manner in mouse ESCs and further catalyzes H3K9me3 modification of target chromosome by recruiting SETDB1 and NCL–KAP1 complex (Chen et al., 2021a; Liu et al., 2021). Specifically, LINE1, a lncRNA with multiple m6A peaks and YTHDC1 binding sites, forms the LINE1–NCL–KAP1complex, which plays a role in H3K9me3 installation and thereby modulates the expression of two cell state-related retrotransposons. Another evidence to prove this notion is Barr body, a lncRNA XIST–protein complex inducing heterochromatinization of the X chromosome, which is speculated to be mediated by phase separation (Cerase et al., 2019). YTHDC1 preferentially recognizes m6A residues on XIST and further recruits repressive proteins to chromatin to achieve gene silencing. It is worth noting that XIST harbors more than 70 m6A sites, implying a strong potential for scaffolding m6A readers (Patil et al., 2016). Second, YTHDC1 is able to reshape nuclear speckles and modulate transcription. Another lncRNA MALAT1 with m6A modification acted as scaffold to recruit YTHDC1 to nuclear speckles, and the YTHDC1 anchoring played an important role in maintaining the composition and genomic binding sites of nuclear speckles. Similar to that of lncRNA XIST (Patil et al., 2016), as many as 31 high-confidence MALAT1–m6A motifs were identified (Wang et al., 2021). Third, YTHDC1 participates in alternative splicing, which is also potentially modulated by phase separation (Peng et al., 2020; Ninomiya et al., 2021). YTHDC1 may shortly bind to methylated nascent RNA and further stabilize SRSF3 (and displace SRSF10) to promote exon inclusion, and the LCD in the C-terminus is important for its interaction with SRSF3/10 (Xiao et al., 2016), suggesting that this is probably a phase separation-dependent phenomenon. Ninomiya et al. (2021) found that YTHDC1 as well as m6A–writer complex components could be sequestered inside nuclear stress bodies by binding to the m6A-modified lncRNA HSATIII, thereby repressing the m6A-dependent splicing of pre-mRNAs in the nucleoplasm. Recently, Cheng et al. (2021) have proven that m6A-modified mRNA and YTHDC1 can form m6A–YTHDC1 condensates in a phase separation-dependent manner, and this condensate in acute myeloid leukemia cells may protect some mRNA of malignance from degradation.
As a whole, several m6A readers themselves have a potential to trigger phase separation, and their anchoring on m6A-modified RNA strongly enhances this potential.
m6A Acts as a “Structural Switcher” to Modulate the Spectrum of RNA–Protein and RNA–RNA Interaction
Liu et al. (2015) demonstrated that m6A acts as a “structural switcher” to change the conformation of RNA harboring m6A modification. Although m6A modification could not preclude the Watson–Crick base pairing between A and U, it induces the methylamino group rotating from energetically favored syn geometry on the Watson–Crick face to higher-energy anti-conformation, thus destabilizing the RNA duplex. The notion of m6A destabilizing base pairing was further verified by the kinetic research that showed introducing m6A significantly lowers the rate of duplex annealing, providing support for how m6A reshapes the kinetics of conformational transition toward single-string preference (Shi et al., 2019). On the other hand, at the unstructured region, m6A modification stabilizes the conformation due to stronger base–stacking interaction (Roost et al., 2015). Likewise, an in vivo transcriptome-wide RNA structure mapping study presented direct structural evidence that m6A affects RNA structure, favoring the transition from paired to unpaired RNA (Spitale et al., 2015).
By altering the RNA structure, m6A modification would help to recruit the RBPs that prefer to bind linear, unfolded RNAs (Figure 2B). In fact, some RBPs tend to bind to m6A-modified RNA because of the “structural switcher” function of m6A. Among them, several members of the heterogeneous nuclear ribonucleoprotein (HNRNP) family are well-studied (Liu et al., 2015; Zhou et al., 2016; Liu et al., 2017; Wu et al., 2018). For instance, HNRNP C recognizes the U-tract which are often buried by A-tract at the stem structure. The m6A modification of an A on the A-tract is capable to destabilize the region where the U-tract is located and increases the accessibility of the U-tract to HNRNP C (Liu et al., 2015; Zhou et al., 2016). Except for unmasking the target complementary sequences, m6A modification is capable of increasing the accessibility of its located region as well. HNRNP G binds to a purine-rich motif that includes the m6A site, and the m6A modification helps altering the structure to increase motif accessibility (Liu et al., 2017). Of note, HNRNP G binds to m6A-modified RNA through its LCD, which is able to undergo self-assembly (Liu et al., 2017); this suggests that the m6A modification leads to the “partner switch” of HNRNP G from protein to RNA. Another member of HNRNPs regarded to bind to m6A-mediated structural switch RNA is HNRNPA2/B1, which was revealed by structural, biochemical, and bioinformatics studies (Liao et al., 2018; Wu et al., 2018). Apart from HNRNPs, another group of m6A readers that may also bind to different targets in a structural switch-dependent manner are IGF2BPs (Sun et al., 2019), which were reported to enhance the stability and translation of m6A-modified mRNA (Huang et al., 2018). Therefore, in addition to recruit m6A readers directly binding to m6A sites, m6A-mediated structural switch of RNA contributes to binding multivalence for m6A-modified RNAs as well, which participates in the regulation of phase separation.
Of note, m6A does not consistently promote RNA–protein interactions. m6A also showed an ability to repress RBP binding; for instance, m6A modification may impede the formation of RNA structures needed for HUR binding (Wang et al., 2014). Several other “anti-readers” were verified by high-throughput screening (Edupuganti et al., 2017; Sun et al., 2019), such as LIN28A, EWSR1, G3BP1, and G3BP2, all of which were displaced when m6A appear in their binding sites. By recruiting and repelling RBPs, m6A potentially changes the spectrum of the RNA–protein interaction, which would contribute to the dynamic modulation of phase separation.
Another role for molecular switch mediated by m6A is to regulate the kinetics of RNA–RNA interactions (Figure 2B). As mentioned above, the addition of m6A may promote the dissolution of local duplex (e.g., steam, etc.) and tend to induce linear, unstructured conformation, which would accelerate the formation of trans-RNA–RNA interaction (van Treeck and Parker, 2018; Roden and Gladfelter, 2021). Therefore, m6A modification potentially serves as a kinetic regulator to reshape the RNA–RNA interaction spectrum, which influences phase separation. Taken together, m6A exhibits a huge potential to alter the conformation of m6A-harboring RNAs to affect their interaction with RBPs and RNAs, thereby modulating multivalence dynamics.
Phase Separation Provides Platforms for m6A-Regulating Bioprocesses
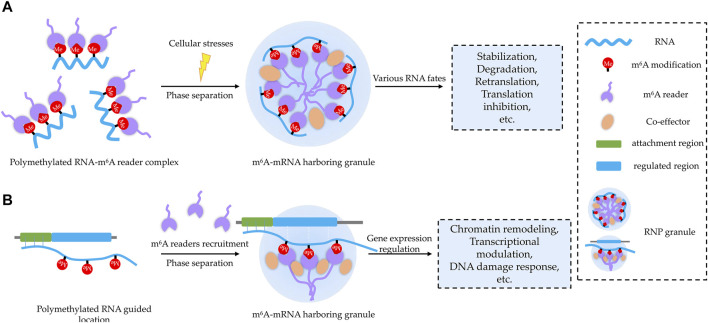
Although m6A exhibits a great potential to modulate phase separation, the biological processes regulated through this manner remain largely unknown. Based on current literatures, we propose that phase separation may provide platforms for m6A-regulating bioprocesses in two major working patterns with potential biological significance: cellular stress response and gene expression regulation (Figure 3).
FIGURE 3.
Two proposed working patterns for m6A-related BioMCs. (A) m6A modification acts as a sorting marker to decide RNA components and their associated molecular partners inside the condensates upon stress, and forming BioMCs will facilitate regulation of m6A RNA for stress response. (B) On nascent RNAs, m6A acts as a beacon to recruit m6A readers, and then m6A RNA–reader complexes stimulate formation of BioMCs, which play important roles for chromatin remodeling, transcriptional regulation, DNA damage response, etc.
For the former pattern, RNA m6A modification may work as a sorting marker to decide RNA-targeting BioMCs (Figure 3A), as m6A-modified RNA is enriched in BioMCs in a valency- and stoichiometry-dependent manner (Ries et al., 2019; Wang et al., 2020), at least in terms of SGs and PBs. Although the molecular mechanism of sorting needs to be further elucidated, it may correlate with the greater potential of higher levels of modified RNA to achieve multivalent interactions. When cells are exposed to environmental stresses, the translation is inhibited and mRNAs dissociate from ribosomes; the increased cytosol pool of free mRNAs, especially the non-translating ones, binds to RBPs and initiates SGs coalescing (van Leeuwen and Rabouille, 2019; Mateju et al., 2020). As m6A-modified RNA–m6A reader complex is preferentially recruited to SGs, the readers can tune the fate of target mRNA with help from other associated co-effectors (Ries et al., 2019; Wang et al., 2015; Fu and Zhuang, 2020; Huang et al., 2018; Stöhr et al., 2006). For instance, YTHDF1 was regarded to promote translation re-initiation for its moderately high co-localization with eIF3/RPS6 in the periphery of G3BP1 cores in SGs (Wang et al., 2015), and the interaction of YTHDF1 with the translation machinery (RPS10) at the periphery of SGs was also reportedly essential for translation initiation promotion (Fu and Zhuang, 2020). Whereas, Ries et al. (2019) showed that YTHDFs’ role in phase separation is independent of their role in translation or degradation. Thus, YTHDF proteins seem to exert dual functions in protein translation and in the formation of SGs. Given the different conditions employed (thermal instead of oxidative stress) and different targets studied (overall mRNA instead of YTHDF target ones), it needs further investigation to reveal how YTHDF proteins discriminate the mRNAs that will be regulated at the translation level and the mRNAs that will be relocated into condensates. Interestingly, both the levels of m6A modification and m6A readers were increased under certain cellular stresses (Meyer et al., 2015; Zhou et al., 2015; Xiang et al., 2017; Anders et al., 2018; Maimaitiyiming et al., 2020), supporting the notion that m6A participates in stress response through modulating phase separation.
For the latter pattern, m6A occurring on nascent RNAs could act as a “beacon” to recruit m6A readers, which could further interact with other co-effectors to reshape the chromatin, regulate transcription, participate in DNA damage response, etc. (Figure 3B). For instance, the YTHDC1 anchoring in nascent RNA recruits H3K9me2 demethylase KDM3B to change the histone methylation levels of target chromatin (Li et al., 2020a). Similarly, SETDB1 and NCL-KAP1 are directed to the transcripts of retrotransposons by binding to YTHDC1 and deposit H3K9me3 in two cell state-related retrotransposons (Chen et al., 2021a; Liu et al., 2021). It is also evident from XIST-mediated X-chromosome inactivation. Several gene-silencing proteins may precisely localize on XIST by binding to YTHDC1 in an m6A-dependent manner (Patil et al., 2016). In addition to reshaping the chromatin, m6A could regulate transcription as well. For instance, YTHDC1 recognizes m6A sites in lncRNA MALAT1, which harbors multiple m6A motifs, playing an important role in reshaping nuclear speckles and modulating the accessibility of nuclear speckles to diverse genes, thereby affecting gene expression (Wang et al., 2021). In addition to MALAT1, another lncRNA, NEAT1, which is required for paraspeckle assembly through phase separation (Yamazaki et al., 2018), was also reported to regulate gene expression in an m6A-dependent manner (Wen et al., 2020).
Apart from transcriptional regulation, m6A in DNA damage-associated RNAs may also play an important role in DNA damage repair (Xiang et al., 2017; Zhang et al., 2020), and YTHDC1 anchoring in m6A sites of damage-associated RNA would facilitate the stabilization of DNA–RNA hybrids at damage sites and mediate the recruitment of RAD51 and BRCA1 for homologous recombination-mediated repair (Zhang et al., 2020). Interestingly, several lncRNAs (XIST, MALAT1, NEAT1, LINE1, dilncRNA, etc.) seem essential for nuclear phase separation events, which may be due to their length, flexible structure, and potentially multiple m6A sites. Multiple m6A-bearing mRNAs are predominantly located in the nucleoplasm and probably associated with chromatin remodeling in terms of molecular function in gene ontology (Ries et al., 2019).
RNA modifications other than m6A might also take part in phase separation since they are capable of changing the pattern of RNA–RNA and RNA–protein interactions as well (Lewis et al., 2017; Drino and Schaefer, 2018). Take N1-methyladenosine (m1A) for example, it occurs at the Watson–Crick interface and endows a positive charge to the modified adenosine, thereby changing RNA structure and RNA–protein interactions (Safra et al., 2017). A recent study reported that m1A is significantly accumulated in SGs upon heat shock and oxidative stress, along with its writer TRMT6/61A, likely hyposensitizing cells to the stress (Alriquet et al., 2021). This finding supports the notion that m1A modification participates in phase separation. More studies are needed in the future to figure out if phase separation is a universal mechanism to mediate modified RNAs’ sorting and to regulate their fate as well as function.
Perspectives and Concluding Remarks
RNA m6A modification is an emerging layer of regulator over cellular BioMCs via phase separation (Liu et al., 2019a; Gao et al., 2019; Ries et al., 2019; Fu and Zhuang, 2020; Wang et al., 2020; Cheng et al., 2021; Lee et al., 2021). Under certain stress conditions, m6A modification acts as a sorting mark to enrich m6A-modified mRNAs in SGs and therefore potentially influence multiple cellular processes by modulating related mRNA re-translation after stress relief (Ries et al., 2019; Fu and Zhuang, 2020). These observations indicate the importance of m6A modification in stress-response mechanism and potentially in stress-related diseases. It has been reported that the arrest of BioMCs’ dynamics is correlated with some pathological processes (Mathieu et al., 2020). Therefore, an investigation of the details of m6A modification in phase separation would improve our understanding in stress response and related diseases.
Although specific RNAs harboring multivalent m6A modification have shown a strong potential to control cellular processes via phase separation, several technical problems limited the investigation of the biological function of m6A-mediated phase separation. First, a feasible approach to examine how an m6A-modified RNA regulates phase separation is condensate reconstitution experiment in vitro using artificially synthesized RNA and purified protein. However, it would be difficult to synthesize longer RNAs and add multiple m6A modifications in proper sites in vitro (Roden and Gladfelter, 2021). Second, the biomolecular condensates in vivo usually consist of numerous components including distinct RNA species and various RBPs, and it would be difficult to purify and include all components in an in vitro experiment. Third, the thermodynamic features of the heterotypic multicomponent interactions are different from in vivo condition and in vitro-purified components’ interaction in simplified model (Riback et al., 2020). Besides, unmodified RNA constitute a large portion of intracellular RNA and lots of m6A-modified mRNAs harbor only one m6A modification site (Dominissini et al., 2012; Zaccara et al., 2019); therefore, using multivalently modified RNA alone in reconstitute experiments may lead to false conclusions that deviate from physiological conditions.
Apart from in vitro experiments, the in vivo experiment is another available system to study how an m6A-modified RNA regulates phase separation. However, cellular BioMCs are usually constituted by multitudinous components, resulting in difficulty to evaluate the specific effects from m6A-modified RNAs. Additionally, traditional routes of studying m6A modification of a particular RNA often require defining the m6A site, changing the stoichiometry of m6A modification, and observing the consequent phenomenon. For this end, m6A regulators (writers, readers, and erasers) are often intervened (increase, decrease, mutate, etc.) to modulate m6A modification levels on target RNA or change the interaction pattern between target RNA and certain m6A readers of interest. However, these measures would lead to uncontrollable off-target effects, because these regulators are shared by thousands of RNAs apart from the targeted one. An improved method is to reconstruct the target RNA to change its modifiable capacity and the spectrum of binding partners; in many cases, it contains the deletion or mutation applied to the target RNA. Though this improved method greatly eliminates the off-target possibility and makes the intervention more precise, some other risks occur. As the ideal research objects are long RNAs harboring multiple m6A sites, such intervention may result in deletion or sequence component changes of large RNA fragments, both of which are important parameters for interaction spectrum contributing to RNA-mediated phase separation. Therefore, most of the currently available methods are more or less defective.
The ideal strategy is to site specifically modulate m6A levels and interaction spectrum of target RNA without changing the primary sequence. Recently, several biological tools have been developed to achieve site-specific m6A editing (Table 1) (Rauch et al., 2018; Liu et al., 2019b; Rauch et al., 2019; Li et al., 2020b; Mo et al., 2020; Shinoda et al., 2020; Wilson et al., 2020; Zhao et al., 2020; Chen et al., 2021b). The approach for developing new editing biology tools that interfere with RNA modification is using gRNA as the locator for the target sequence and refined Cas protein as the adaptor for the functional effectors to anchor and modulate target RNA. m6A writers, erasers, and readers were integrated with Cas protein to regulate m6A modification at specific sites on target RNA (Liu et al., 2019b; Rau et al., 2019; Li et al., 2020b; Mo et al., 2020; Chen et al., 2021b). For instance, Wilson et al. (2020) created nucleus-localized and cytoplasm-localized dCas13 fusions with a truncated METTL3 methyltransferase domain (nucleus-localized) and modified METTL3:METTL14 complex (cytoplasm-localized), which were able to install m6A in specific sites of target RNAs. Similarly, dCas13b fusions with ALKBH5 succeeded to specifically remove m6A of targeted mRNA. It is worth noting that one of the engineered tools exhibited equal efficiency in eliminating multiple modifications on a single target as well (Li et al., 2020b).
TABLE 1.
Novel tools for site-specific m6A editing without primary sequence changed.
| Category | Reconstituted construct | Working pattern | Ref |
|---|---|---|---|
| CRISPR–CAS-based | The fusion of YTHDF1 and dCas13b | SgRNA guides editing system to targeted transcript, and fusioned m6A readers function to achieve translation/degradation modulation | Rauch et al. (2018) |
| The fusion of YTHDF2 and dCas13b | |||
| The fusion of M3M14 and dCas9 | PAMer and sgRNA guide editing system to targeted site and fusioned m6A writer/eraser function to install/erase m6A modification | Liu et al. (2019b) | |
| The fusion of ALKBH5/FTO and dCas9 | |||
| The fusion of ALKBH5 and dCas13b | SgRNA guides editing system to targeted transcript and fusioned ALKBH5 functions to erase m6A modification | Li et al. (2020b) | |
| The fusion of dCas13b and 10 copies of GCN4 peptides cooperates with scFv-fusion RNA demethylase | SgRNA guides dCas13b–GCN4 fusions to targeted transcript and further multiply recruit scFv fusion RNA demethylase to erase m6A modification | Mo et al. (2020) | |
| The fusion of METTL3/METTL3:METTL14 and dCas13 | SgRNA guides editing system to targeted transcript and fusioned METTL3/METTL3:METTL14 function to achieve transmethylation | Wilson et al. (2020) | |
| The RNA anchor probes containing dCas13b and CIBN (a truncated version of light-sensitive protein CIB1) cooperate with the effector probe containing CRY2PHR(the photolyase homology region of CRY2) and METTL3/METTL14 or FTO | The RNA anchor binds the targeted RNA via crRNA, and METTL3/METTL14 or FTO is recruited as the attraction of CRY2PHR and CIBN heterodimerization upon blue light irradiation to install/erase m6A modification | Zhao et al. (2020) | |
| The fusion of dCas13a and ALKBH5 | SgRNA guides editing system to targeted transcript, and fusioned ALKBH5 functions to erase m6A modification | Chen et al. (2021b) | |
| CRISPR–CAS-inspired | The fusion of an effector protein, a RNA hairpin-binding protein, and ss-RNA-binding protein. YTHDF1/YTHDF2 was employed as effector protein, TBP/SLBP as RNA hairpin-binding protein, and ORF5/HBEGF/β-defensin as ss-RNA-binding protein | gRNA guide editing system to targeted site, RNA hairpin-binding protein binds to the structure of gRNA, ss-RNA-binding protein stabilizes and protects the gRNA prior to target engagement, and the effector protein works in a proximity-dependent manner | Rauch et al. (2019) |
| Others | The fusion of programmable RNA-binding protein PUF and METTL14 | PUFs with specific mRNA-binding regions guide editing system to targeted transcript and fusioned METTL14/FTO function to install/erase m6A modification | Shinoda et al. (2020) |
| The fusion of programmable RNA-binding protein PUF and FTO |
Another interesting and practical engineered tool is “TRADES” (Mo et al., 2020). Distinct from regular engineering practice, in which Cas protein serves as an adaptor to make the distance between the functional element fused to it and targeted RNA complementary with gRNA closer, in the TRADES system, the dCas13b portion is fused to 10 repeated GCN4 peptides, which are able to efficiently recruit multiple scFv–m6A eraser (scFv-FTO/ALKBH5) fusions. This design provides a wider editing window for its flexible repeated GCN4 peptides, and it would help to intervene with m6A clusters and eliminate m6A modification when the m6A sites are only known vaguely. Apart from CRISPR–Cas13 system, the PUF RNA-binding protein and CRISPR–Cas-inspired RNA targeting system (CIRTS) were also applied to regulate site-specific m6A (Rauch et al., 2019, Shinoda et al., 2020).
Taken together, we have summarized that RNA, as an essential portion of most BioMCs, can serve as drivers, regulators, and busters of BioMCs through modulating phase separation by multivalently interacting with biomacromolecules (protein and RNA). More importantly, RNA m6A modification, as the most widespread modification of eukaryotic mRNA, shows a strong potential to regulate phase separation and thus exert various physiological functions. Phase separation has been recognized as an emerging explanation for a plethora of previously unknown phenomena. Thus, it merits to comprehensively investigate how m6A regulates phase separation and how phase separation participates in m6A-mediated biological processes.
Author Contributions
C-HH conceptualized the study. YM performed the methodology. LW contributed the software. C-HH and YM performed the validation. YS conducted the formal analysis. YS and LW conducted the investigation. YS and LW contributed resources. YS contributed in the writing—original draft preparation. YM and C-HH contributed in the writing—review and editing. YS performed visualization. XC, YM, and C-HH supervised the study. C-HH and XC were in charge of project administration. C-HH and XC acquired funding. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by grants from the National Natural Science Foundation of China, Grant No. 31972883 and 8200015; the Zhejiang Provincial Natural Science Foundation, Grant No. LY21H160032; and the Key research and development program of Zhejiang province, Grant No. 2019C03010.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aguilera-Gomez A., Rabouille C. (2017). Membrane-bound Organelles Versus Membrane-Less Compartments and Their Control of Anabolic Pathways in Drosophila. Developmental Biol. 428, 310–317. 10.1016/j.ydbio.2017.03.029 [DOI] [PubMed] [Google Scholar]
- Alberti S., Gladfelter A., Mittag T. (2019). Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 176, 419–434. 10.1016/j.cell.2018.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alriquet M., Calloni G., Martínez-Limón A., Delli Ponti R., Hanspach G., Hengesbach M., et al. (2021). The Protective Role of m1A During Stress-Induced Granulation. J. Mol. Cell Biol 12, 870–880. 10.1093/jmcb/mjaa023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders M., Chelysheva I., Goebel I., Trenkner T., Zhou J., Mao Y., et al. (2018). Dynamic m6A Methylation Facilitates mRNA Triaging to Stress Granules. Life Sci. Alliance 1, e201800113. 10.26508/lsa.201800113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumiller W. M., Keating C. D. (2016). Phosphorylation-mediated RNA/peptide Complex Coacervation as a Model for Intracellular Liquid Organelles. Nat. Chem 8, 129–137. 10.1038/nchem.2414 [DOI] [PubMed] [Google Scholar]
- Aumiller W. M., Pir Cakmak F., Davis B. W., Keating C. D. (2016). RNA-based Coacervates as a Model for Membraneless Organelles: Formation, Properties, and Interfacial Liposome Assembly. Langmuir 32, 10042–10053. 10.1021/acs.langmuir.6b02499 [DOI] [PubMed] [Google Scholar]
- Banani S. F., Lee H. O., Hyman A. A., Rosen M. K. (2017). Biomolecular Condensates: Organizers of Cellular Biochemistry. Nat. Rev. Mol. Cell Biol 18, 285–298. 10.1038/nrm.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani S. F., Rice A. M., Peeples W. B., Lin Y., Jain S., Parker R., et al. (2016). Compositional Control of Phase-Separated Cellular Bodies. Cell 166, 651–663. 10.1016/j.cell.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P. R., Milin A. N., Moosa M. M., Onuchic P. L., Deniz A. A. (2017). Reentrant Phase Transition Drives Dynamic Substructure Formation in Ribonucleoprotein Droplets. Angew. Chem. Int. Ed. 56, 11354–11359. 10.1002/anie.201703191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Bahadur R. P. (2016). A Structural Perspective of RNA Recognition by Intrinsically Disordered Proteins. Cell. Mol. Life Sci. 73, 4075–4084. 10.1007/s00018-016-2283-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S., Alberti S., Fawzi N. L., Mittag T., Polymenidou M., Rousseau F., et al. (2018). Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol. 28, 420–435. 10.1016/j.tcb.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S., Holehouse A. S., Weinhardt V., Kovacs D., van Lindt J., Larabell C., et al. (2019). Spontaneous Driving Forces Give Rise to protein−RNA Condensates with Coexisting Phases and Complex Material Properties. Proc. Natl. Acad. Sci. USA 116, 7889–7898. 10.1073/pnas.1821038116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boija A., Klein I. A., Sabari B. R., Dall’Agnese A., Coffey E. L., Zamudio A. V., et al. (2018). Transcription Factors Activate Genes Through the Phase-Separation Capacity of Their Activation Domains. Cell 175, 1842–1855. 10.1016/j.cell.2018.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C. P., Eckmann C. R., Courson D. S., Rybarska A., Hoege C., Gharakhani J., et al. (2009). Germline P Granules Are Liquid Droplets that Localize by Controlled Dissolution/condensation. Science 324, 1729–1732. 10.1126/science.1172046 [DOI] [PubMed] [Google Scholar]
- Brangwynne C. P., Mitchison T. J., Hyman A. A. (2011). Active Liquid-like Behavior of Nucleoli Determines Their Size and Shape in Xenopus laevis Oocytes. Proc. Natl. Acad. Sci. 108, 4334–4339. 10.1073/pnas.1017150108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. M., Moon S. L., Matheny T., Parker R. (2019). RNase L Reprograms Translation by Widespread mRNA Turnover Escaped by Antiviral mRNAs. Mol. Cell 75, 1203–1217. 10.1016/j.molcel.2019.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerase A., Armaos A., Neumayer C., Avner P., Guttman M., Tartaglia G. G. (2019). Phase Separation Drives X-Chromosome Inactivation: A Hypothesis. Nat. Struct. Mol. Biol. 26, 331–334. 10.1038/s41594-019-0223-0 [DOI] [PubMed] [Google Scholar]
- Chen C., Liu W., Guo J., Liu Y., Liu X., Liu J., et al. (2021). Nuclear m6A Reader YTHDC1 Regulates the Scaffold Function of LINE1 RNA in Mouse ESCs and Early Embryos. Protein Cell 12, 455–474. 10.1007/s13238-021-00837-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhao Q., Zhao Y. L., Chai G. S., Cheng W., Zhao Z., et al. (2021). Targeted RNA N 6 ‐Methyladenosine Demethylation Controls Cell Fate Transition in Human Pluripotent Stem Cells. Adv. Sci. 8, 2003902. 10.1002/advs.202003902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Xie W., Pickering B. F., Chu K. L., Savino A. M., Yang X., et al. (2021). N6-Methyladenosine on mRNA Facilitates a Phase-Separated Nuclear Body that Suppresses Myeloid Leukemic Differentiation. Cancer Cell 39, 958–972. 10.1016/j.ccell.2021.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chujo T., Hirose T. (2017). Nuclear Bodies Built on Architectural Long Noncoding RNAs: Unifying Principles of Their Construction and Function. Mol. Cells 40, 889–896. 10.14348/molcells.2017.0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Su R., Weng H., Huang H., Li Z., Chen J. (2018). RNA N6-Methyladenosine Modification in Cancers: Current Status and Perspectives. Cell Res 28, 507–517. 10.1038/s41422-018-0034-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominissini D., Moshitch-Moshkovitz S., Schwartz S., Salmon-Divon M., Ungar L., Osenberg S., et al. (2012). Topology of the Human and Mouse m6A RNA Methylomes Revealed by m6A-Seq. Nature 485, 201–206. 10.1038/nature11112 [DOI] [PubMed] [Google Scholar]
- Drino A., Schaefer M. R. (2018). RNAs, Phase Separation, and Membrane-Less Organelles: Are Post-Transcriptional Modifications Modulating Organelle Dynamics? Bioessays 40, 1800085. 10.1002/bies.201800085 [DOI] [PubMed] [Google Scholar]
- Edens B. M., Vissers C., Su J., Arumugam S., Xu Z., Shi H., et al. (2019). FMRP Modulates Neural Differentiation Through m6A-dependent mRNA Nuclear Export. Cell Rep. 28, 845–854. 10.1016/j.celrep.2019.06.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edupuganti R. R., Geiger S., Lindeboom R. G. H., Shi H., Hsu P. J., Lu Z., et al. (2017). N6-methyladenosine (m6A) Recruits and Repels Proteins to Regulate mRNA Homeostasis. Nat. Struct. Mol. Biol. 24, 870–878. 10.1038/nsmb.3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum-Garfinkle S., Kim Y., Szczepaniak K., Chen C. C.-H., Eckmann C. R., Myong S., et al. (2015). The Disordered P Granule Protein LAF-1 Drives Phase Separation into Droplets with Tunable Viscosity and Dynamics. Proc. Natl. Acad. Sci. USA 112, 7189–7194. 10.1073/pnas.1504822112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay M. M., Anderson P. J., Ivanov P. (2017). ALS/FTD-Associated C9ORF72 Repeat RNA Promotes Phase Transitions In Vitro and in Cells. Cell Rep. 21, 3573–3584. 10.1016/j.celrep.2017.11.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feric M., Vaidya N., Harmon T. S., Mitrea D. M., Zhu L., Richardson T. M., et al. (2016). Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell 165, 1686–1697. 10.1016/j.cell.2016.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Zhuang X. (2020). m6A-binding YTHDF Proteins Promote Stress Granule Formation. Nat. Chem. Biol. 16, 955–963. 10.1038/s41589-020-0524-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Pei G., Li D., Li R., Shao Y., Zhang Q. C., et al. (2019). Multivalent m6A Motifs Promote Phase Separation of YTHDF Proteins. Cell Res 29, 767–769. 10.1038/s41422-019-0210-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasset-Rosa F., Lu S., Yu H., Chen C., Melamed Z. e., Guo L., et al. (2019). Cytoplasmic TDP-43 De-mixing Independent of Stress Granules Drives Inhibition of Nuclear Import, Loss of Nuclear TDP-43, and Cell Death. Neuron 102, 339–357. 10.1016/j.neuron.2019.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén-Boixet J., Kopach A., Holehouse A. S., Wittmann S., Jahnel M., Schlüßler R., et al. (2020). RNA-induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation. Cell 181, 346–361. 10.1016/j.cell.2020.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. E., Manteiga J. C., Henninger J. E., Sabari B. R., Dall’Agnese A., Hannett N. M., et al. (2019). Pol II Phosphorylation Regulates a Switch Between Transcriptional and Splicing Condensates. Nature 572, 543–548. 10.1038/s41586-019-1464-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig S., Kong G., Mannen T., Sadowska A., Kobelke S., Blythe A., et al. (2015). Prion-like Domains in RNA Binding Proteins Are Essential for Building Subnuclear Paraspeckles. J. Cell Biol 210, 529–539. 10.1083/jcb.201504117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger J. E., Oksuz O., Shrinivas K., Sagi I., LeRoy G., Zheng M. M., et al. (2021). RNA-mediated Feedback Control of Transcriptional Condensates. Cell 184, 207–225. 10.1016/j.cell.2020.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D., Shrinivas K., Young R. A., Chakraborty A. K., Sharp P. A. (2017). A Phase Separation Model for Transcriptional Control. Cell 169, 13–23. 10.1016/j.cell.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S., Kedersha N., Anderson P., Ivanov P. (2021). Molecular Mechanisms of Stress Granule Assembly and Disassembly. Biochim. Biophys. Acta (Bba) - Mol. Cell Res. 1868, 118876. 10.1016/j.bbamcr.2020.118876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofweber M., Dormann D. (2019). Friend or foe-Post-translational Modifications as Regulators of Phase Separation and RNP Granule Dynamics. J. Biol. Chem. 294, 7137–7150. 10.1074/jbc.TM118.001189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. J., Zhu Y., Ma H., Guo Y., Shi X., Liu Y., et al. (2017). Ythdc2 Is an N6-Methyladenosine Binding Protein that Regulates Mammalian Spermatogenesis. Cell Res 27, 1115–1127. 10.1038/cr.2017.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Weng H., Sun W., Qin X., Shi H., Wu H., et al. (2018). Recognition of RNA N6-Methyladenosine by IGF2BP Proteins Enhances mRNA Stability and Translation. Nat. Cell Biol 20, 285–295. 10.1038/s41556-018-0045-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Vale R. D. (2017). RNA Phase Transitions in Repeat Expansion Disorders. Nature 546, 243–247. 10.1038/nature22386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky E., Harris M. E. (2015). Specificity and Nonspecificity in RNA-Protein Interactions. Nat. Rev. Mol. Cell Biol 16, 533–544. 10.1038/nrm4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q., Zong X., Mao Y., Qian S.-B. (2021). A Heat Shock-Responsive lncRNA Heat Acts as a HSF1-Directed Transcriptional Brake via m6A Modification. Proc. Natl. Acad. Sci. USA 118, e2102175118. 10.1073/pnas.2102175118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Wang S., Huang Y., He X., Cui H., Zhu X., et al. (2015). Phase Transition of Spindle-Associated Protein Regulate Spindle Apparatus Assembly. Cell 163, 108–122. 10.1016/j.cell.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong A., Matheny T., Jain S., Mitchell S. F., Wheeler J. R., Parker R. (2017). The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules. Mol. Cell 68, 808–820. 10.1016/j.molcel.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong A., Parker R. (2018). mRNP Architecture in Translating and Stress Conditions Reveals an Ordered Pathway of mRNP Compaction. J. Cell Biol 217, 4124–4140. 10.1083/jcb.201806183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon E. M., Qiu Y., Ghanbari Niaki A., McLaughlin G. A., Weidmann C. A., Gerbich T. M., et al. (2018). mRNA Structure Determines Specificity of a polyQ-Driven Phase Separation. Science 360, 922–927. 10.1126/science.aar7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Wang R., Xiong F., Krakowiak J., Liao Z., Nguyen P. T., et al. (2021). Enhancer RNA m6A Methylation Facilitates Transcriptional Condensate Formation and Gene Activation. Mol. Cell 81, 3368–3385. 10.1016/j.molcel.2021.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. J. T., Pan T., Kalsotra A. (2017). RNA Modifications and Structures Cooperate to Guide RNA-Protein Interactions. Nat. Rev. Mol. Cell Biol 18, 202–210. 10.1038/nrm.2016.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chen Z., Chen F., Xie G., Ling Y., Peng Y., et al. (2020). Targeted mRNA Demethylation Using an Engineered dCas13b-ALKBH5 Fusion Protein. Nucleic Acids Res. 48, 5684–5694. 10.1093/nar/gkaa269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xia L., Tan K., Ye X., Zuo Z., Li M., et al. (2020). N6-Methyladenosine Co-transcriptionally Directs the Demethylation of Histone H3K9me2. Nat. Genet. 52, 870–877. 10.1038/s41588-020-0677-3 [DOI] [PubMed] [Google Scholar]
- Liang Y., Zhan G., Chang K.-J., Yang Y.-P., Wang L., Lin J., et al. (2020). The Roles of m6A RNA Modifiers in Human Cancer. J. Chin. Med. Assoc. 83, 221–226. 10.1097/JCMA.0000000000000251 [DOI] [PubMed] [Google Scholar]
- Liao S., Sun H., Xu C. (2018). YTH Domain: A Family of N 6 -methyladenosine (M 6 A) Readers. Genomics, Proteomics & Bioinformatics 16, 99–107. 10.1016/j.gpb.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Protter D. S. W., Rosen M. K., Parker R. (2015). Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol. Cell 60, 208–219. 10.1016/j.molcel.2015.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Gao M., He J., Wu K., Lin S., Jin L., et al. (2021). The RNA m6A Reader YTHDC1 Silences Retrotransposons and Guards ES Cell Identity. Nature 591, 322–326. 10.1038/s41586-021-03313-9 [DOI] [PubMed] [Google Scholar]
- Liu N., Dai Q., Zheng G., He C., Parisien M., Pan T. (2015). N6-methyladenosine-dependent RNA Structural Switches Regulate RNA-Protein Interactions. Nature 518, 560–564. 10.1038/nature14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Zhou K. I., Parisien M., Dai Q., Diatchenko L., Pan T. (2017). N 6-methyladenosine Alters RNA Structure to Regulate Binding of a Low-Complexity Protein. Nucleic Acids Res. 45, 6051–6063. 10.1093/nar/gkx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Y., Feng Y., Wu J.-J., Zou M.-L., Sun Z.-L., Li X., et al. (2019). m 6 A Facilitates YTHDF‐independent Phase Separation. J. Cell Mol Med 24, 2070–2072. 10.1111/jcmm.14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.-M., Zhou J., Mao Y., Ji Q., Qian S.-B. (2019). Programmable RNA N6-Methyladenosine Editing by CRISPR-Cas9 Conjugates. Nat. Chem. Biol. 15, 865–871. 10.1038/s41589-019-0327-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Zhang Q. C., Lee B., Flynn R. A., Smith M. A., Robinson J. T., et al. (2016). RNA Duplex Map in Living Cells Reveals Higher-Order Transcriptome Structure. Cell 165, 1267–1279. 10.1016/j.cell.2016.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnicka M. A., Milanowska K., Osman Oglou O., Purta E., Kurkowska M., Olchowik A., et al. (2013). MODOMICS: A Database of RNA Modification Pathways-2013 Update. Nucleic Acids Res. 41, D262–D267. 10.1093/nar/gks1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharana S., Wang J., Papadopoulos D. K., Richter D., Pozniakovsky A., Poser I., et al. (2018). RNA Buffers the Phase Separation Behavior of Prion-like RNA Binding Proteins. Science 360, 918–921. 10.1126/science.aar7366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimaitiyiming Y., Wang Q. Q., Hsu C.-H., Naranmandura H. (2020). Arsenic Induced Epigenetic Changes and Relevance to Treatment of Acute Promyelocytic Leukemia and beyond. Toxicol. Appl. Pharmacol. 406, 115212. 10.1016/j.taap.2020.115212 [DOI] [PubMed] [Google Scholar]
- Maimaitiyiming Y., Wang Q. Q., Yang C., Ogra Y., Lou Y., Smith C. A., et al. (2021). Hyperthermia Selectively Destabilizes Oncogenic Fusion Proteins. Blood Cancer Discov. 2, 388–401. 10.1158/2643-3230.BCD-20-0188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J. R., Gleixner A. M., Mauna J. C., Gomes E., DeChellis-Marks M. R., Needham P. G., et al. (2019). RNA Binding Antagonizes Neurotoxic Phase Transitions of TDP-43. Neuron 102, 321–338. 10.1016/j.neuron.2019.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateju D., Eichenberger B., Voigt F., Eglinger J., Roth G., Chao J. A. (2020). Single-Molecule Imaging Reveals Translation of mRNAs Localized to Stress Granules. Cell 183, 1801–1812. 10.1016/j.cell.2020.11.010 [DOI] [PubMed] [Google Scholar]
- Matheny T., van Treeck B., Huynh T. N., Parker R. (2021). RNA Partitioning into Stress Granules Is Based on the Summation of Multiple Interactions. RNA 27, 174–189. 10.1261/rna.078204.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C., Pappu R. V., Taylor J. P. (2020). Beyond Aggregation: Pathological Phase Transitions in Neurodegenerative Disease. Science 370, 56–60. 10.1126/science.abb8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazroui R., Sukarieh R., Bordeleau M.-E., Kaufman R. J., Northcote P., Tanaka J., et al. (2006). Inhibition of Ribosome Recruitment Induces Stress Granule Formation Independently of Eukaryotic Initiation Factor 2α Phosphorylation. MBoC 17, 4212–4219. 10.1091/mbc.e06-04-0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. D., Patil D. P., Zhou J., Zinoviev A., Skabkin M. A., Elemento O., et al. (2015). 5′ UTR m6A Promotes Cap-independent Translation. Cell 163, 999–1010. 10.1016/j.cell.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z., Westhof E. (2017). RNA Structure: Advances and Assessment of 3D Structure Prediction. Annu. Rev. Biophys. 46, 483–503. 10.1146/annurev-biophys-070816-034125 [DOI] [PubMed] [Google Scholar]
- Mo J., Chen Z., Qin S., Li S., Liu C., Zhang L., et al. (2020). TRADES: Targeted RNA Demethylation by SunTag System. Adv. Sci. 7, 2001402. 10.1002/advs.202001402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex A., Temirov J., Lee J., Coughlin M., Kanagaraj A. P., Kim H. J., et al. (2015). Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization. Cell 163, 123–133. 10.1016/j.cell.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan Z., Ryan V. H., Janke A. M., Burke K. A., Rhoads S. N., Zerze G. H., et al. (2017). Phosphorylation of the FUS Low‐complexity Domain Disrupts Phase Separation, Aggregation, and Toxicity. EMBO J. 36, 2951–2967. 10.15252/embj.201696394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler O., Hartmann A. M., Stamm S. (2000). The ER Repeat Protein YT521-B Localizes to a Novel Subnuclear Compartment. J. Cell Biol 150, 949–962. 10.1083/jcb.150.5.949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya K., Iwakiri J., Aly M. K., Sakaguchi Y., Adachi S., Natsume T., et al. (2021). m 6 A Modification of HSATIII lncRNAs Regulates Temperature‐dependent Splicing. EMBO J. 40, e107976. 10.15252/embj.2021107976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield C. J., Dunker A. K. (2014). Intrinsically Disordered Proteins and Intrinsically Disordered Protein Regions. Annu. Rev. Biochem. 83, 553–584. 10.1146/annurev-biochem-072711-164947 [DOI] [PubMed] [Google Scholar]
- Patil D. P., Chen C.-K., Pickering B. F., Chow A., Jackson C., Guttman M., et al. (2016). m6A RNA Methylation Promotes XIST-Mediated Transcriptional Repression. Nature 537, 369–373. 10.1038/nature19342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil D. P., Pickering B. F., Jaffrey S. R. (2018). Reading m6A in the Transcriptome: m6A-Binding Proteins. Trends Cell Biol. 28, 113–127. 10.1016/j.tcb.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Li E.-M., Xu L.-Y. (2020). From Start to End: Phase Separation and Transcriptional Regulation. Biochim. Biophys. Acta (Bba) - Gene Regul. Mech. 1863, 194641. 10.1016/j.bbagrm.2020.194641 [DOI] [PubMed] [Google Scholar]
- Qamar S., Wang G., Randle S. J., Ruggeri F. S., Varela J. A., Lin J. Q., et al. (2018). FUS Phase Separation Is Modulated by a Molecular Chaperone and Methylation of Arginine Cation-π Interactions. Cell 173, 720–734. 10.1016/j.cell.2018.03.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau K., Rösner L., Rentmeister A. (2019). Sequence-specific m6A Demethylation in RNA by FTO Fused to RCas9. RNA 25, 1311–1323. 10.1261/rna.070706.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S., He C., Dickinson B. C. (2018). Targeted m6A Reader Proteins to Study Epitranscriptomic Regulation of Single RNAs. J. Am. Chem. Soc. 140, 11974–11981. 10.1021/jacs.8b05012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S., He E., Srienc M., Zhou H., Zhang Z., Dickinson B. C. (2019). Programmable RNA-Guided RNA Effector Proteins Built from Human Parts. Cell 178, 122–134. 10.1016/j.cell.2019.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riback J. A., Zhu L., Ferrolino M. C., Tolbert M., Mitrea D. M., Sanders D. W., et al. (2020). Composition-dependent Thermodynamics of Intracellular Phase Separation. Nature 581, 209–214. 10.1038/s41586-020-2256-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries R. J., Zaccara S., Klein P., Olarerin-George A., Namkoong S., Pickering B. F., et al. (2019). m6A Enhances the Phase Separation Potential of mRNA. Nature 571, 424–428. 10.1038/s41586-019-1374-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden C., Gladfelter A. S. (2021). RNA Contributions to the Form and Function of Biomolecular Condensates. Nat. Rev. Mol. Cell Biol 22, 183–195. 10.1038/s41580-020-0264-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels J., Thénoz M., Szarzyńska B., Landfors M., De Coninck S., Demoen L., et al. (2020). Aging of Preleukemic Thymocytes Drives CpG Island Hypermethylation in T-Cell Acute Lymphoblastic Leukemia. Blood Cancer Discov. 1, 274–289. 10.1158/2643-3230.BCD-20-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roost C., Lynch S. R., Batista P. J., Qu K., Chang H. Y., Kool E. T. (2015). Structure and Thermodynamics of N6-Methyladenosine in RNA: A Spring-Loaded Base Modification. J. Am. Chem. Soc. 137, 2107–2115. 10.1021/ja513080v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosselló-Tortella M., Ferrer G., Esteller M. (2020). Epitranscriptomics in Hematopoiesis and Hematologic Malignancies. Blood Cancer Discov. 1 (1), 26–31. 10.1158/2643-3249.BCD-20-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree I. A., Luo G.-Z., Zhang Z., Wang X., Zhou T., Cui Y., et al. (2017). YTHDC1 Mediates Nuclear export of N6-Methyladenosine Methylated mRNAs. eLife 6, e31311. 10.7554/eLife.31311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan V. H., Dignon G. L., Zerze G. H., Chabata C. V., Silva R., Conicella A. E., et al. (2018). Mechanistic View of hnRNPA2 Low-Complexity Domain Structure, Interactions, and Phase Separation Altered by Mutation and Arginine Methylation. Mol. Cell 69, 465–479. 10.1016/j.molcel.2017.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabari B. R., Dall’Agnese A., Boija A., Klein I. A., Coffey E. L., Shrinivas K., et al. (2018). Coactivator Condensation at Super-enhancers Links Phase Separation and Gene Control. Science 361, 361. 10.1126/science.aar3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safra M., Sas-Chen A., Nir R., Winkler R., Nachshon A., Bar-Yaacov D., et al. (2017). The m1A Landscape on Cytosolic and Mitochondrial mRNA at Single-Base Resolution. Nature 551, 251–255. 10.1038/nature24456 [DOI] [PubMed] [Google Scholar]
- Saha S., Weber C. A., Nousch M., Adame-Arana O., Hoege C., Hein M. Y., et al. (2016). Polar Positioning of Phase-Separated Liquid Compartments in Cells Regulated by an mRNA Competition Mechanism. Cell 166, 1572–1584. 10.1016/j.cell.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D. W., Kedersha N., Lee D. S. W., Strom A. R., Drake V., Riback J. A., et al. (2020). Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization. Cell 181, 306–324. 10.1016/j.cell.2020.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U., Parker R. (2003). Decapping and Decay of Messenger RNA Occur in Cytoplasmic Processing Bodies. Science 300, 805–808. 10.1126/science.1082320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Liu B., Nussbaumer F., Rangadurai A., Kreutz C., Al-Hashimi H. M. (2019). NMR Chemical Exchange Measurements Reveal that N6-Methyladenosine Slows RNA Annealing. J. Am. Chem. Soc. 141, 19988–19993. 10.1021/jacs.9b10939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Wang X., Lu Z., Zhao B. S., Ma H., Hsu P. J., et al. (2017). YTHDF3 Facilitates Translation and Decay of N6-Methyladenosine-Modified RNA. Cell Res 27, 315–328. 10.1038/cr.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda K., Suda A., Otonari K., Futaki S., Imanishi M. (2020). Programmable RNA Methylation and Demethylation Using PUF RNA Binding Proteins. Chem. Commun. 56, 1365–1368. 10.1039/c9cc09298f [DOI] [PubMed] [Google Scholar]
- Smith J., Calidas D., Schmidt H., Lu T., Rasoloson D., Seydoux G. (2016). Spatial Patterning of P Granules by RNA-Induced Phase Separation of the Intrinsically-Disordered Protein MEG-3. eLife 5, e21337. 10.7554/eLife.21337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song T., Yang Y., Wei H., Xie X., Lu J., Zeng Q., et al. (2019). Zfp217 Mediates m6A mRNA Methylation to Orchestrate Transcriptional and Post-transcriptional Regulation to Promote Adipogenic Differentiation. Nucleic Acids Res. 47, 6130–6144. 10.1093/nar/gkz312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitale R. C., Flynn R. A., Zhang Q. C., Crisalli P., Lee B., Jung J.-W., et al. (2015). Structural Imprints In Vivo Decode RNA Regulatory Mechanisms. Nature 519, 486–490. 10.1038/nature14263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr N., Lederer M., Reinke C., Meyer S., Hatzfeld M., Singer R. H., et al. (2006). ZBP1 Regulates mRNA Stability During Cellular Stress. J. Cell Biol 175, 527–534. 10.1083/jcb.200608071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom A. R., Emelyanov A. V., Mir M., Fyodorov D. V., Darzacq X., Karpen G. H. (2017). Phase Separation Drives Heterochromatin Domain Formation. Nature 547, 241–245. 10.1038/nature22989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Fazal F. M., Li P., Broughton J. P., Lee B., Tang L., et al. (2019). RNA Structure Maps Across Mammalian Cellular Compartments. Nat. Struct. Mol. Biol. 26, 322–330. 10.1038/s41594-019-0200-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taslimi A., Vrana J. D., Chen D., Borinskaya S., Mayer B. J., Kennedy M. J., et al. (2014). An Optimized Optogenetic Clustering Tool for Probing Protein Interaction and Function. Nat. Commun. 5, 4925. 10.1038/ncomms5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber D., Tauber G., Khong A., van Treeck B., Pelletier J., Parker R. (2020). Modulation of RNA Condensation by the DEAD-Box Protein eIF4A. Cell 180, 411–426. 10.1016/j.cell.2019.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber D., Tauber G., Parker R. (2020). Mechanisms and Regulation of RNA Condensation in RNP Granule Formation. Trends Biochem. Sci. 45, 764–778. 10.1016/j.tibs.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trcek T., Douglas T. E., Grosch M., Yin Y., Eagle W. V. I., Gavis E. R., et al. (2020). Sequence-Independent Self-Assembly of Germ Granule mRNAs into Homotypic Clusters. Mol. Cell 78, 941–950. 10.1016/j.molcel.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W.-C., Gayatri S., Reineke L. C., Sbardella G., Bedford M. T., Lloyd R. E. (2016). Arginine Demethylation of G3BP1 Promotes Stress Granule Assembly. J. Biol. Chem. 291, 22671–22685. 10.1074/jbc.M116.739573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen W., Rabouille C. (2019). Cellular Stress Leads to the Formation of Membraneless Stress Assemblies in Eukaryotic Cells. Traffic 20, 623–638. 10.1111/tra.12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Treeck B., Parker R. (2018). Emerging Roles for Intermolecular RNA-RNA Interactions in RNP Assemblies. Cell 174, 791–802. 10.1016/j.cell.2018.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Treeck B., Protter D. S. W., Matheny T., Khong A., Link C. D., Parker R. (2018). RNA Self-Assembly Contributes to Stress Granule Formation and Defining the Stress Granule Transcriptome. Proc. Natl. Acad. Sci. USA 115, 2734–2739. 10.1073/pnas.1800038115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Choi J.-M., Holehouse A. S., Lee H. O., Zhang X., Jahnel M., et al. (2018). A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 174, 688–699. 10.1016/j.cell.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang L., Diao J., Shi Y. G., Shi Y., Ma H., et al. (2020). Binding to m6A RNA Promotes YTHDF2-Mediated Phase Separation. Protein Cell 11, 304–307. 10.1007/s13238-019-00660-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Liu C., Zhang S., Yan H., Zhang L., Jiang A., et al. (2021). N6-methyladenosine Modification of MALAT1 Promotes Metastasis via Reshaping Nuclear Speckles. Developmental Cell 56, 702–715. 10.1016/j.devcel.2021.01.015 [DOI] [PubMed] [Google Scholar]
- Wang X., Lu Z., Gomez A., Hon G. C., Yue Y., Han D., et al. (2013). N6-methyladenosine-dependent Regulation of Messenger RNA Stability. Nature 505, 117–120. 10.1038/nature12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhao B. S., Roundtree I. A., Lu Z., Han D., Ma H., et al. (2015). N6-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 161, 1388–1399. 10.1016/j.cell.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li Y., Toth J. I., Petroski M. D., Zhang Z., Zhao J. C. (2014). N6-methyladenosine Modification Destabilizes Developmental Regulators in Embryonic Stem Cells. Nat. Cell Biol 16, 191–198. 10.1038/ncb2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S., Wei Y., Zen C., Xiong W., Niu Y., Zhao Y. (2020). Long Non-coding RNA NEAT1 Promotes Bone Metastasis of Prostate Cancer Through N6-Methyladenosine. Mol. Cancer 19, 171. 10.1186/s12943-020-01293-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. A., Mito M., Kurosaka S., Takumi T., Tanegashima C., Chujo T., et al. (2016). Structural, Super-resolution Microscopy Analysis of Paraspeckle Nuclear Body Organization. J. Cell Biol 214, 817–830. 10.1083/jcb.201601071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C., Chen P. J., Miao Z., Liu D. R. (2020). Programmable m6A Modification of Cellular RNAs with a Cas13-Directed Methyltransferase. Nat. Biotechnol. 38, 1431–1440. 10.1038/s41587-020-0572-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtas M. N., Pandey R. R., Mendel M., Homolka D., Sachidanandam R., Pillai R. S. (2017). Regulation of m6A Transcripts by the 3ʹ→5ʹ RNA Helicase YTHDC2 Is Essential for a Successful Meiotic Program in the Mammalian Germline. Mol. Cell 68, 374–387. 10.1016/j.molcel.2017.09.021 [DOI] [PubMed] [Google Scholar]
- Woodruff J. B., Ferreira Gomes B., Widlund P. O., Mahamid J., Honigmann A., Hyman A. A. (2017). The Centrosome Is a Selective Condensate that Nucleates Microtubules by Concentrating Tubulin. Cell 169, 1066–1077. 10.1016/j.cell.2017.05.028 [DOI] [PubMed] [Google Scholar]
- Wu B., Su S., Patil D. P., Liu H., Gan J., Jaffrey S. R., et al. (2018). Molecular Basis for the Specific and Multivariant Recognitions of RNA Substrates by Human hnRNP A2/B1. Nat. Commun. 9, 420. 10.1038/s41467-017-02770-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Laurent B., Hsu C.-H., Nachtergaele S., Lu Z., Sheng W., et al. (2017). RNA m6A Methylation Regulates the Ultraviolet-Induced DNA Damage Response. Nature 543, 573–576. 10.1038/nature21671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W., Adhikari S., Dahal U., Chen Y.-S., Hao Y.-J., Sun B.-F., et al. (2016). Nuclear M 6 A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 61, 507–519. 10.1016/j.molcel.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Yamazaki T., Souquere S., Chujo T., Kobelke S., Chong Y. S., Fox A. H., et al. (2018). Functional Domains of NEAT1 Architectural lncRNA Induce Paraspeckle Assembly Through Phase Separation. Mol. Cell 70, 1038–1053. 10.1016/j.molcel.2018.05.019 [DOI] [PubMed] [Google Scholar]
- Yang P., Mathieu C., Kolaitis R.-M., Zhang P., Messing J., Yurtsever U., et al. (2020). G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules. Cell 181, 325–345. 10.1016/j.cell.2020.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R.-W., Xu G., Wang Y., Shan L., Luan P.-F., Wang Y., et al. (2019). Nascent Pre-rRNA Sorting via Phase Separation Drives the Assembly of Dense Fibrillar Components in the Human Nucleolus. Mol. Cell 76, 767–783. 10.1016/j.molcel.2019.08.014 [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., Ali R., Jiou J., Fung H. Y. J., Burke K. A., Kim S. J., et al. (2018). Nuclear Import Receptor Inhibits Phase Separation of FUS Through Binding to Multiple Sites. Cell 173, 693–705. 10.1016/j.cell.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccara S., Ries R. J., Jaffrey S. R. (2019). Reading, Writing and Erasing mRNA Methylation. Nat. Rev. Mol. Cell Biol 20, 608–624. 10.1038/s41580-019-0168-5 [DOI] [PubMed] [Google Scholar]
- Zhang C., Chen L., Peng D., Jiang A., He Y., Zeng Y., et al. (2020). METTL3 and N6-Methyladenosine Promote Homologous Recombination-Mediated Repair of DSBs by Modulating DNA-RNA Hybrid Accumulation. Mol. Cell 79, 425–442. 10.1016/j.molcel.2020.06.017 [DOI] [PubMed] [Google Scholar]
- Zhang H., Elbaum-Garfinkle S., Langdon E. M., Taylor N., Occhipinti P., Bridges A. A., et al. (2015). RNA Controls PolyQ Protein Phase Transitions. Mol. Cell 60, 220–230. 10.1016/j.molcel.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Li B., Ma J., Jin W., Ma X. (2020). Photoactivatable RNA N 6 ‐Methyladenosine Editing with CRISPR‐Cas13. Small 16, 1907301. 10.1002/smll.201907301 [DOI] [PubMed] [Google Scholar]
- Zhou J., Wan J., Gao X., Zhang X., Jaffrey S. R., Qian S.-B. (2015). Dynamic m6A mRNA Methylation Directs Translational Control of Heat Shock Response. Nature 526, 591–594. 10.1038/nature15377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K. I., Parisien M., Dai Q., Liu N., Diatchenko L., Sachleben J. R., et al. (2016). N6-Methyladenosine Modification in a Long Noncoding RNA Hairpin Predisposes its Conformation to Protein Binding. J. Mol. Biol. 428, 822–833. 10.1016/j.jmb.2015.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]