Abstract
Objectives
The Oxford–AstraZeneca COVID-19 vaccine (ChAdOx1 nCoV-19, Vaxzevira or Covishield) builds on two decades of research and development (R&D) into chimpanzee adenovirus-vectored vaccine (ChAdOx) technology at the University of Oxford. This study aimed to approximate the funding for the R&D of ChAdOx and the Oxford–AstraZeneca vaccine and to assess the transparency of funding reporting mechanisms.
Methods
We conducted a scoping review and publication history analysis of the principal investigators to reconstruct R&D funding the ChAdOx technology. We matched award numbers with publicly accessible grant databases. We filed freedom of information (FOI) requests to the University of Oxford for the disclosure of all grants for ChAdOx R&D.
Results
We identified 100 peer-reviewed articles relevant to ChAdOx technology published between January 2002 and October 2020, extracting 577 mentions of funding bodies from acknowledgements. Government funders from overseas (including the European Union) were mentioned 158 times (27.4%), the UK government 147 (25.5%) and charitable funders 138 (23.9%). Grant award numbers were identified for 215 (37.3%) mentions; amounts were publicly available for 121 (21.0%). Based on the FOIs, until December 2019, the biggest funders of ChAdOx R&D were the European Commission (34.0%), Wellcome Trust (20.4%) and Coalition for Epidemic Preparedness Innovations (17.5%). Since January 2020, the UK government contributed 95.5% of funding identified. The total identified R&D funding was £104 226 076 reported in the FOIs and £228 466 771 reconstructed from the literature search.
Conclusion
Our study approximates that public and charitable financing accounted for 97%–99% of identifiable funding for the ChAdOx vaccine technology research at the University of Oxford underlying the Oxford–AstraZeneca vaccine until autumn 2020. We encountered a lack of transparency in research funding reporting.
Keywords: vaccines, health policy, COVID-19
Key questions.
What is already known?
The Oxford–AstraZeneca vaccine relies on two decades of research and development (R&D) into the chimpanzee adenovirus-vectored vaccine (ChAdOx) technology at the University of Oxford.
The Oxford–AstraZeneca COVID-19 vaccine plays an important role in the global vaccine rollout especially in resource-limited settings as it provides a cheaper alternative to the Pfizer/BioNTech and Moderna mRNA vaccines and does not require the same cold-chain management.
What are the new findings?
Funders of ChAdOx platform research by grant mention in academic publications were 99% public and charitable bodies, of which 27.4% was overseas governments (including the European Union), 25.5% the UK government, 23.9% philanthropy, 19.6% research institution and 2.6% public–private partnership.
Freedom of information (FOI) requests to the University of Oxford showed 97% public and charitable funding for the ChAdOx platform; the European Commission (34.0%), Wellcome Trust (20.4%) and Coalition for Epidemic Preparedness Innovations (17.5%) were the biggest funders of ChAdOx research until the start of the COVID-19 pandemic, but since January 2020, the UK government contributed 95.5% of identifiable R&D funding until October 2020.
What do the new findings imply?
The scale of high-risk public funding for the R&D of the ChAdOx technology underlying the Oxford–AstraZeneca vaccine compels advocacy for global equitable access to the health technology beyond the favourable pricing currently implemented.
Difficulty in identifying funding amounts from the academic literature compared with FOIs shows a severe lack of transparency in research funding reporting.
Introduction
The ChAdOx1 nCoV-19 vaccine, commonly known as the Oxford–AstraZeneca vaccine, Covishield, or Vaxzevira, is one of four vaccines that received conditional approval for the prevention of COVID-19 in the UK (November 2021)1 2 The Oxford–AstraZeneca vaccine has been approved and licensed for use in over 170 countries, and approximately 1 billion doses have been administered globally as of late November 2021.3 4 The vaccine makes use of a novel technology that relies on a chimpanzee adenovirus-vector (ChAdOx) to encode the production of the SARS-CoV-2 spike protein, which induces an immune response.5 It is of particular importance in resource-limited settings as it does not require the same cold-chain management and is more affordable than the mRNA-based COVID-19 vaccines developed by Pfizer/BioNTech and Moderna.6
Although the Oxford–AstraZeneca vaccine itself was developed in response to the COVID-19 pandemic, the underlying ChAdOx vaccine platform relies on two decades of research and development (R&D) by the Oxford Vaccine Group at the Jenner Institute, University of Oxford, led by Professor Sarah Gilbert (SG) and Professor Adrian Hill (AH). Vaccines using the ChAdOx technology have previously undergone clinical trials in human participants for other infectious diseases, including hepatitis C virus and malaria, where it has been shown to induce a powerful immune response during phase I clinical trials.7 8 Before the emergence of SARS-CoV-2, the ChAdOx technology was being used to develop a vaccine for Middle East Respiratory Syndrome coronavirus (MERS-CoV), which is closely related to the novel coronavirus.9 When the pandemic emerged, this ChAdOx1 MERS-CoV vaccine had already undergone its first clinical trials in non-human primates and humans (phase I) and was rapidly adapted to induce an immune response to SARS-CoV-2.10 The resultant ChAdOx nCoV-19 vaccine was undergoing phase I/II clinical trials in NHS Trusts across the UK when a deal with biopharmaceutical company AstraZeneca was announced in late April 2020.11–14 Shortly after this, the UK government committed £65.5 million towards the commercialisation and manufacturing of the Oxford–AstraZeneca vaccine.15 However, it is not known who funded the early stages of R&D into the ChAdOx technology at the University of Oxford.
Previous studies have shown that public funding has played a significant role in the medical innovation system for many decades, particularly in early-phase R&D and notably in vaccine research.16–18 Between 2000 and 2019, the US National Institutes of Health (NIH) funded over $17.2 billion in published research on vaccine technologies, providing the foundation for the COVID-19 vaccines currently entering the market.19 Despite a number of public statements involving funding pledges for the development of the Oxford–AstraZeneca vaccine,6 it remains largely unknown which funding bodies have contributed to the ChAdOx technology. In this study, we aimed to identify the funding to the University of Oxford for the R&D of the ChAdOx technology with a specific focus on the research into the adenovirus-vectored vaccine technology conducted at the Jenner Institute and its subsequent application to the Oxford–AstraZeneca vaccine. This study has three objectives: (1) to approximate the funding for the R&D of the ChAdOx platform led by SG and AH and the subsequent application to SARS-CoV-2; (2) to identify the main funders based on disclosures in academic publications and freedom of information (FOI) requests to the University of Oxford; (3) to assess the transparency in R&D funding reporting mechanisms by comparing information available in the public realm with disclosures by the University of Oxford in response to FOI requests.
Methods
Scoping review of the academic literature to identify primary research on ChAdOx and the Oxford–AstraZeneca vaccine
We performed a scoping review of the literature using a systematic search of MEDLINE and Embase between 26 October and 30 November 2020 to identify all relevant academic publications which included primary research involving the ChAdOx technology. Our search strategies (online supplemental file 1) were developed in collaboration with an academic librarian from Imperial College London. To identify further articles, we conducted a PubMed search of the complete publication history of SG and AH, the primary investigators of the ChAdOx technology at the Jenner Institute. Abstracts were manually screened by two independent reviewers using Rayyan QCRI20 based on the following inclusion criteria: (1) peer-reviewed primary research articles; (2) mentioning of the relevant vaccine technology as identified in preliminary background research and described in the search strategy (i.e., using the terms ChAdOx1, ChAdOx2, chimpanzee adenovirus-vectored, etc); and (3) including at least one author affiliated to the University of Oxford (figure 1 and online supplemental file 1). Non-English studies and review articles, conference abstracts, clinical trial registry entries, and opinion pieces not containing any primary data were excluded.
Figure 1.
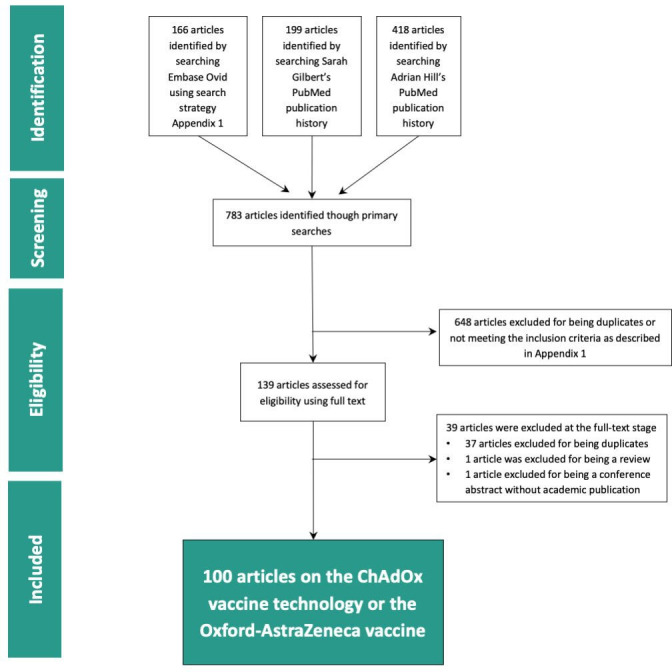
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the ChAdOx funding scoping review. ChAdOx, chimpanzee adenovirus-vectored vaccine.
bmjgh-2021-007321supp001.pdf (83.8KB, pdf)
Data extraction from funding acknowledgement statements in the academic literature
The full text of all selected articles were downloaded into EndNote V.7.8 and duplicates were removed. Two authors extracted information from all acknowledgement sections, funding statements and conflict of interest declarations from the academic publications on the ChAdOx technology and entered them into an Excel spreadsheet (online supplemental file 2). First, we ranked funding bodies and other actors by the absolute number of mentions extracted from the included articles. Next, we quantified the proportion of grants that listed an award number and conducted a separate analysis in which we removed any duplicate mentions of funder names if they were linked to the same award number. Meanwhile, using the award numbers, we searched the following publicly available databases to identify grants towards the development of the ChAdOx technology; UK Research and Innovation (UKRI), European Commission, Wellcome Trust, Bill & Melinda Gates Foundation, Coalition for Epidemic Preparedness Innovations (CEPI) and World Report, the latter of which includes all grants administered by the US NIH. Grants in currencies other than British pound sterling (GBP) were converted into GBP using the following conversion rates on 28 February 2021: US$1=0.72 GBP and €1=0.87 GBP.21 Funding declarations from the academic literature were matched to grant amounts where publicly available (online supplemental file 2). Additionally, we used previously collected open-access data (publicmeds4covid.com), which tracks government investment in COVID-19 research.22 Funders were categorised into the following funding types: overseas government (including the European Union (EU)), UK government, charity/philanthropy, public–private partnership (PPP), research institution (including the University of Oxford), and industry.
bmjgh-2021-007321supp002.pdf (402.2KB, pdf)
FOI requests
We filed several requests under the Freedom of Information Act (2000) to ask the University of Oxford for the disclosure of all funding (including all financial support, grants, donations, etc) for both the ChAdOx technology and the ChAdOx1 nCoV-19 vaccine. The FOIs and correspondence with the University of Oxford are publicly available on the online platform WhatDoTheyKnow.com.23 To remain within the limits of the maximum amount of time (18 hours) a public authority is legally required to spend on responding to a single FOI request, we had to limit the final disclosure request to grants received by the principal investigators, SG and AH, since 2000 to the most recent date available. We received a list of relevant grants on 27 January 2021. We filed further requests for disclosure of all grants received from public entities and AstraZeneca for the development of the ChAdOx1 nCoV-19 vaccine specifically since 1 January 2020 to the date of the request (25 October 2021).
Analysis of grant disclosures by the University of Oxford
Two authors independently classified the grants into the following categories based on the project names pertaining to each grant, provided by the University of Oxford: (1) funding towards the COVID-19 vaccine specifically, (2) funding towards the R&D of the ChAdOx technology, (3) funding for the fellowships/salary/research/equipment/infrastructure (later coded as ‘other vaccine research’) that may have contributed to the development of the ChAdOx technology but is not directly identifiable (not displayed) and (4) other research funding not relevant to the R&D of the ChAdOx technology (not displayed). Based on this categorisation, we found that all ‘prepandemic’ grants given for R&D up to 31 December 2019 funded the ChAdOx vaccine platform technology, and all grants from 1 January 2020 were ‘pandemic’ R&D funding specific to the Oxford–AstraZeneca vaccine. We will use these terms to pertain to this specific cut-off date for the remainder of the paper. Funders were additionally categorised into the following funding types: overseas government (including the EU), UK government, charity/philantropy, PPP, research institution (including the University of Oxford), industry and other, which included anonymous donors that could not be classified.
Results
Funding based on disclosure statements in academic publications on the ChAdOx technology
We identified 100 published peer-reviewed articles relevant to the Oxford–AstraZeneca vaccine or the ChAdOx technology (online supplemental files 1 and 2). Publication dates ranged from January 2002 to November 2020. The concordance between the two independent reviewers was 93.61%. Funding acknowledgement statements differed in completeness between articles, with some only noting funding bodies and others detailing specific grants using grant titles or award numbers. In total, we extracted 577 mentions of funding bodies, with or without reference to specific grants. Of these, we were able to identify award numbers for 215 mentions (37.3%). Grant amounts were available in the public realm for 121 mentions (21.0%) (figure 2). Of the 215 mentions for which we ascertained award numbers, 73 mentions (12.7% of total mentions) corresponded to a previously identified award number. These mentions were not excluded from the total number due to the low proportion of mentions for which we were able to identify award numbers. However, grants identified as being duplicates based on having the same award numbers were excluded when calculating the amount of funding provided by that funding body. The total amount of funding we were able to reconstruct based on the academic literature was £228 466 771.
Figure 2.
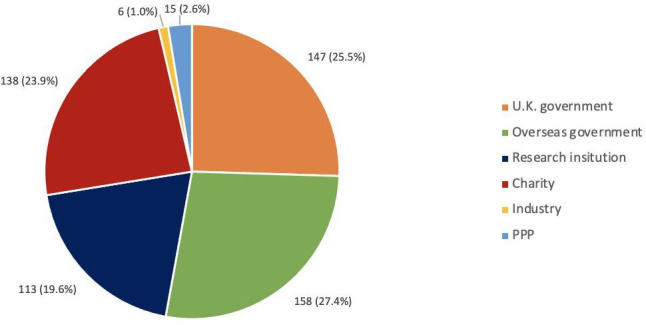
Number of mentions for each funder type from the academic literature identified in the scoping review. PPP, public–private partnership.
Overseas government bodies were mentioned in funding acknowledgement statements of peer-reviewed articles on ChAdOx 158 times (27.4%), followed by the UK government (147 mentions (25.5%)), and charities (138 mentions (23.9%)) (table 1 and figure 3). Funders from industry were mentioned 6 times (1.0%), and PPP funders (including CEPI, Program for Appropriate Technology in Health (PATH) malaria vaccine initiative, and Consultative Group for International Agricultural Research (CGIAR)) were mentioned 15 times (2.6%). Grant amounts could be matched with 27.9% of UK government mentions, 19.0% of overseas government (including EU) mentions, and 36% of charity mentions. Overseas government funders contributed the most funding for which grant amounts could be identified, namely, £105 715 805 (46.3%). This was followed by the UK government, which contributed £69 773 203 (30.5%), and charitable organisations, which contributed £52 977 763 (23.2%) based on traceable grants that could be linked to amounts in publicly available grant databases.
Table 1.
Number of mentions and amount of funding identified for each funder type from the academic literature identified in the scoping review
| Funder type | Mentions from the literature, n (%) | Percentage of mentions matched to a grant amount (%) | Total value of matched grants, £ (%) |
| Overseas government (including EU) | 158 (27.4) | 19.0 | 105 715 805 (46.3) |
| UK government | 147 (25.5) | 27.9 | 69 773 203 (30.5) |
| Charity | 138 (23.9) | 36.2 | 52 977 763 (23.2) |
| Research institution | 113 (19.6) | 0.0 | 0 (0.0) |
| PPP | 15 (2.6) | 0.0 | 0 (0.0) |
| Industry | 6 (1.0) | 0.0 | 0 (0.0) |
| Total | 577 | 21% of all mentions matched | 228 466 771 |
EU, European Union; PPP, public–private partnership; UK, United Kingdom.
Figure 3.
Number of mentions for which grant amounts were publicly available from the academic literature identified in the scoping review.
Table 2 provides an overview of individual funding bodies for whom grant amounts were identified from publicly available databases, ranked based on the total number of mentions. Here, we have only displayed funders mentioned across more than seven articles. The most frequently named funding body was the Wellcome Trust (107 (18.5%)), followed by the Jenner Institute (73 (12.7%)), the Medical Research Council (66 (11.4%)) and the United States’ NIH (64 (11.4%)). The top three funders for which we could retrieve most grant amounts from publicly available databases to match them with funder mentions in the acknowledgement section were UK Research and Innovation (UKRI) (72.2%), the European Commission (58.6%) and the Wellcome Trust (44.9%).
Table 2.
Number of mentions and amount of funding identified for the top 12 funders from the academic literature identified in the scoping review, ranked by number of mentions
| Rank in top funder list based on number of mentions | Funder name | Type of funder | Mentions from the literature, n (%) | Percentage of mentions matched to a grant amount (%) | Total value of matched grants, £ (%) |
| 1 | Wellcome Trust | Charity | 107 (18.5) | 44.90 | 41 075 570 (18.0) |
| 2 | Jenner Institute | Research institution | 73 (12.7) | 0.00 | 0 (0.0) |
| 3 | Medical Research Council (UK) | UK government | 66 (11.4) | 40.90 | 12 872 968 (5.6) |
| 4 | National Institute of Health (US) | Overseas government | 64 (11.1) | 20.30 | 61 217 268 (26.8) |
| 5 | National Institute of Health Research (UK) | UK government | 45 (7.8) | 0.00 | 0 (0.0) |
| 6 | European Commission | Overseas government | 29 (5.0) | 58.60 | 44 498 537 (19.5) |
| 7 | The Oxford Martin School | Research institution | 19 (3.3) | 0.00 | 0 (0.0) |
| 8 | UK Research and Innovation | UK government | 18 (3.1) | 72.20 | 56 416 780 (24.7) |
| 9 | European Malaria Vaccine Development Association | Public–private partnership | 14 (2.4) | 0.00 | 0 (0.0) |
| 10 | PATH | Charity | 11 (1.9) | 0.00 | 0 (0.0) |
| Malaria Vaccine Initiative | |||||
| 11 | Bill and Melinda Gates Foundation | Charity | 7 (1.2) | 28.60 | 11 902 193 (5.2) |
| 12 | European and Developing Countries Clinical Trial Partnership | Overseas government | 7 (1.2) | 0.00 | 0 (0.0) |
| 13–77 | Other | N/A | 117 (20.3) | 0.90 | 483 455 (0.2) |
| Total | 577 | 21 | 228 466 771 |
UK, United Kingdom; UKRI, UK Research and Innovation; US, United States.
Funding based on FOI requests to the University of Oxford
The University of Oxford disclosed two datasets in response to our FOI requests. The first dataset includes all grants received by SG and AH since 2000. We extracted the grants relevant to the R&D of the ChAdOx technology based on the project numbers and grant names with a cut-off of 31 December 2019. Grants received by the University of Oxford between January 2020 and October 2020 for the development of the Oxford–AstraZeneca vaccine were included in the second dataset. In total, the University of Oxford disclosed 189 grants, donations and payments between January 2004 and October 2020 (online supplemental file 3). We classified 133 as relevant to the R&D of the Oxford–AstraZeneca vaccine and underlying ChAdOx technology (table 3). The total disclosed R&D amount was £104 226 076, of which £69 313 380 was provided before 1 January 2020 and £34 912 696 on or after that date.
Table 3.
Funding given to support the research and development of the ChAdOx technology and the Oxford–AstraZeneca vaccine based on freedom of information to University of Oxford, sorted by funder type
| Funder type | ChAdOx technology (to SG and AH only), £ (%) | Oxford–AstraZeneca vaccine, £ (%) | Total, £ (%) |
| UK government | 5 511 316 (8.0) | 33 354 469 (95.5) | 38 865 785 (37.3) |
| Overseas government | 26 252 085 (37.9) | 0 (0.0) | 26 252 085 (25.2) |
| Charity | 21 468 904 (31.0) | 1 217 835 (3.5) | 22 686 739 (21.8) |
| PPP | 12 943 763 (18.7) | 272 286 (0.8) | 13 216 049 (12.7) |
| Research institution | 0 (0.0) | 68 106 (0.2) | 68 106 (0.1) |
| Industry | 1 970 370 (2.8) | 0 (0.0) | 1 970 370 (1.9) |
| Other | 1 166 941 (1.7) | 0 (0.0) | 1 166 941 (1.1) |
| Total | 69 313 380 | 34 912 696 | 104 226 076 |
An approximation of the total amount of funding received for the adenovirus vector technology and the Oxford–AstraZeneca vaccine, for each funder type, is given in the total column.
AH, Professor Adrian Hill; ChAdOx, chimpanzee adenovirus-vectored vaccine; PPP, public–private partnership; SG, Professor Sarah Gilbert; UK, United Kingdom.
bmjgh-2021-007321supp003.pdf (102.9KB, pdf)
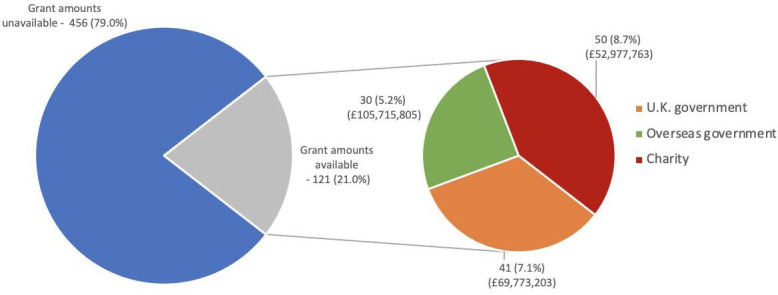
The largest funding source for the R&D investment into the prepandemic ChAdOx technology research by SG and AH was overseas governments, including the EU, which contributed £26 252 085 (37.9%) (figure 4). During the same period charitable funding accounted for £21 468 904 (31.0%), PPPs (including CEPI, CGIAR and PATH malaria vaccine initiative) contributed £12 943 763 (18.7%), and the UK government was the fourth largest funding source with £5 511 316 (8.0%). Industry funding accounted for £1 970 370 (2.8%).
Figure 4.
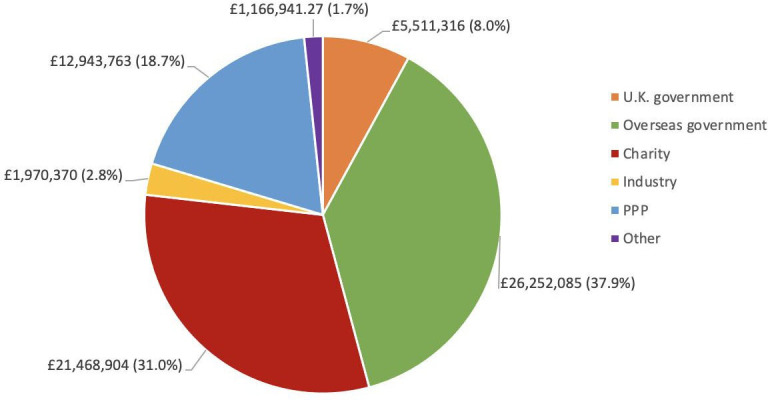
Funding given to support the research and development of the chimpanzee adenovirus-vectored vaccine technology until January 2020, based on freedom of information to the University of Oxford, sorted by funder type. PPP, public–private partnership.
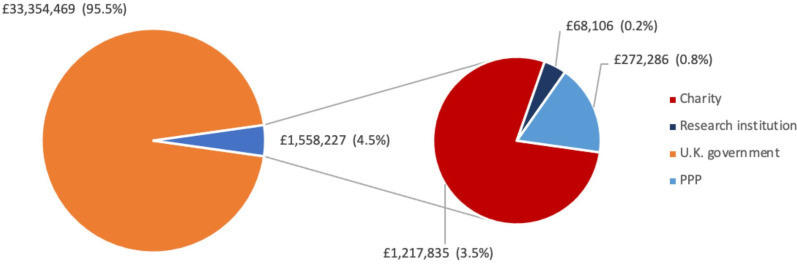
Since January 2020, the UK government was found to be the largest funder of Oxford–AstraZeneca vaccine R&D, contributing £33 354 469 (95.5%) (figure 5). Charitable funders accounted for £1 217 835 (3.5%), the majority of which came from the Wellcome Trust. PPP (specifically CEPI) accounted for £272 286 (0.8%) and research institutions accounted for £68 106 (0.2%).
Figure 5.
Funding given to support the R&D of the Oxford–AstraZeneca vaccine from January 2020 onwards, based on FOIs to the University of Oxford, sorted of funder type. PPP, public–private partnership.
Combining prepandemic and pandemic R&D funding, the UK government provided £38 865 785 (37.3%) of the R&D funding, making it the largest funder identified. Overseas government ranked the second highest funder, providing £26 252 085 (25.2%) of R&D funding while charitable funders contributed £22 686 739 (21.8%). Industry funders contributed £1 970 370 (1.9%).
Overall, based on FOI disclosure by the University of Oxford, public and charitable funding sources accounted for 97% of the R&D funding towards the ChAdOx technology and its application to SARS-CoV-2. Direct government funding added up to £65 117 870 (62.5%), while charitable sources accounted for £22 686 739 (21.8%). PPPs CEPI and PATH malaria vaccine initiative accounted for 12.7% of R&D funding. Private industry contributed 1.9% of R&D funding; 1.1% came from other sources.
Together, the top nine funders were responsible for 95.6% of the disclosed funding for the R&D of the ChAdOx technology and the Oxford–AstraZeneca vaccine (table 4). The remaining 10 funders contributed £4 574 803 (4.4%). Of the top funders identified, three were UK government funders, two EU funders and three charities. Before 1 January 2020, the biggest funders of the R&D into the ChAdOx technology were the European Commission (22.6%), Wellcome Trust (14.7%) and CEPI (11.9%). Since 1 January 2020, the Department of Health and Social Care was the largest funder as declared by the University of Oxford, contributing 89.3% of R&D funding. The University of Oxford on two occasions disclosed via FOI that they had not received any funding for the Oxford–AstraZeneca vaccine in the period from 1 January 2020 to 5 February 2021 (online supplemental file 3).
Table 4.
Top nine funders ranked by total amount of funding given to support the research and development of the ChAdOx technology and Oxford–AstraZeneca vaccine, based on Freedom Of Information requests to the University of Oxford
| Rank based on total amount | Funder | ChAdOx technology (to SG and AH only), £ (%) | Oxford–AstraZeneca vaccine, £ (%) | Total, £ (%) |
| 1 | Department of Health and Social Care | 0 (0.0) | 31 179 621 (89.3) | 31 179 621 (29.9) |
| 2 | European Commission | 23 545 255 (34.0) | 0 (0.0) | 23 545 255 (22.6) |
| 3 | Wellcome Trust | 14 144 606 (20.4) | 1 217 835 (3.5) | 15 362 440 (14.7) |
| 4 | Coalition for Epidemic Preparedness and Innovations | 12 098 260 (17.5) | 272 286 (0.8) | 12 370 546 (11.9) |
| 5 | Medical Research Council | 3 080 837 (4.4) | 2 174 848 (6.2) | 5 255 685 (5.0) |
| 6 | Foundation for National Institute of Health (US) | 5 729 292 (8.3) | 0 (0.0) | 5 729 292 (5.5) |
| 7 | Innovate UK | 2 403 678 (3.5) | 0 (0.0) | 2 403 678 (2.3) |
| 8 | European & Developing Countries Clinical Trials Partnership | 2 209 747 (3.2) | 0 (0) | 2 209 747 (2.1) |
| 9 | Bill and Melinda Gates Foundation | 1 595 006 (2.3) | 0 (0.0) | 1 595 006 (1.5) |
| 10–20 | Other | £4 506 697 (6.5%) | 68 106 (0.2) | 4 574 803 (4.4) |
| Total | 69 313 379 | 34 912 696 | 104 226 076 |
Funders which contributed >£1 000 000 are shown.
AH, Professor Adrian Hill; ChAdOx, chimpanzee adenovirus-vectored vaccine; SG, Sarah Gilbert.
Discussion
Research conducted at the Jenner Institute of the University of Oxford led to the development of the ChAdOx vaccine platform on which the Oxford–AstraZeneca COVID-19 vaccine is built. Our study approximated that public and charitable funding accounted for 97%–99% of the identifiable funding towards the R&D of the ChAdOx technology and its application for SARS-CoV-2 at the University of Oxford until October 2020. Our study identified £104 226 076 of R&D funding reported in FOIs to the University of Oxford and £228 466 771 from the 21% of mentions with a matched grant amount in the scoping review for academic publications on the ChAdOx technology and the Oxford–AstraZeneca vaccine.
Due to insufficient identifiable information that could link the two datasets, we were not able to cross-match the funding between the academic literature and the FOIs, which is a major limitation of our study. Furthermore, only 21% of exact grant amounts for funder mentions in academic publications were retrievable from publicly available information. Receiving funding information through FOIs was largely successful, making it a useful novel method for reconstructing the cost of R&D for health technologies that are largely developed at public research institutions. However, UK institutions are legally required to spend a maximum of 18 hours collecting the requested data according to the Freedom of Information Act Regulation 4 (2004),24 limiting the scope of these FOI requests. Another limitation of this study is that due to its primary focus on prepandemic academic literature and grants received for SG and AH, funding for manufacturing scale-up and late-stage clinical trials of the Oxford–AstraZeneca vaccine was outside of our scope. For example, the University of Oxford received at least £65.5 million from the UK Department of Business, Energy and Industrial Strategy for the development of the COVID-19 vaccine and the relevant clinical trials.14 The UKRI database further listed two UKRI grants to the University of Oxford, worth £657 388.25 Additionally, the US government awarded US$125.6 million and over US$1.2 billion in funding to AstraZeneca for vaccine trials, manufacturing, and distribution of vaccine doses to the US government.26 27 A further nine donations totalling £1.8–2.9 million (included in online supplemental file 3) were reported by the University of Oxford in their response to our FOI, two of which came from charitable sources, totalling £50 000–100 000. The remaining seven donations were private or anonymous funders. All nine donations were not integrated into the FOI dataset as exact amounts were not provided and donor names or amounts were missing for 44.4% of donations. There is also circa £18 m worth of funding in the FOI regarding SG and AH that may be linked to the development of the vaccine, consisting of fellowship grants and general vaccine grants with descriptions too vague to attribute them to the development of ChAdOx specifically (listed in full in online supplemental file 3). Our approximation of the cost of the R&D of the ChAdOx technology is therefore conservative, as it most likely excludes important salary costs, some contributions towards the scale-up of manufacturing, and funding for clinical trials to the University of Oxford beyond October 2020.
By applying a methodology that included data collection through two different mechanisms, we are confident to have captured a good approximation of the R&D costs for the ChAdOx vaccine technology at the University of Oxford. However, our study was unable to identify any funding that was received for R&D conducted by Vaccitech, the spin-off company founded in 2016 by SG and AH to further develop the ChAdOx and Modified Vaccinia Ankara (MVA) viral vectors.28 This is because it is only possible to send FOIs to public institutions. The private contributions for the complete R&D of the ChAdOx technology might therefore have been higher than identified in our study, which focused on the research conducted at the University of Oxford. Finally, it was not possible to measure relevant non-monetary contributions to the ChAdOx R&D, such as the participation in clinical trials, for example, in South Africa and Brazil for the Oxford–AstraZeneca vaccine.29 30 Future research should focus on analysing the public and private contribution and risk-taking in the later stages of the R&D of ChAdOx nCoV-19, specifically the funding of clinical trials in humans conducted after the University of Oxford entered an agreement with AstraZeneca.
The lack of transparency around the costs of R&D of novel health technologies is a prevailing issue, with large disparities in estimates reported.31 Although there have been improvements in funding reporting in the past years, there are still major obstacles to investigating the funding of biomedical innovation based on disclosures made in the published scientific literature.32–34 Furthermore, the cumulative nature of scientific research makes it difficult to ascertain the R&D costs of previous innovation, which may have enabled the development of the ChAdOx technology and the Oxford–AstraZeneca vaccine.33 Of the grant mentions relevant to the R&D of ChAdOx identified through the scoping review, nearly four-fifths could not be matched to an amount using searchable online grant databases. This was because for many of these grants the award number was not given in the funding acknowledgement section of the article, or because the funder had no searchable database in which the exact grant amount was listed. Attempting to match grants without award numbers was unreliable and inconsistent. Another issue was a lack of publicly available grant information of particular types of funders, especially from the two main research institution funding bodies that contributed to the ChAdOx technology based on the funding acknowledgement statements, the Jenner Institute and The Oxford Martin School. Funding amounts from the private sector and PPPs were especially difficult to identify in this study as they usually do not disclose their grants in publicly accessible databases. As a result, the approximation of R&D costs of two decades of research into the ChAdOx technology on the basis of acknowledgements in academic articles is most likely a gross underestimation as only 21% of all mentions could be matched. Furthermore, due to a discrepancy in the titles of grants as disclosed by the University of Oxford in the FOI, which often excluded grant numbers, and the funder mentions in the academic literature, prevented the integration of the two datasets. Therefore, we here present two approximations of the funding of ChAdOx R&D at the University of Oxford. Initiatives to address the lack of transparency in R&D funding have been initiated, such as a 2019 World Health Assembly (WHA) resolution 72.8 which sought to improve ‘the transparency of markets for medicines, vaccines, and other health products’.35 However, the voluntary nature of such initiatives and opposition from the private sector as well as governments of high-income countries limit efforts to increase R&D transparency globally.36
In response to the pandemic, Oxford University Innovation (OUI), a subsidiary of the University of Oxford managing the university’s technology transfer, published a statement committing to non-exclusive, royalty-free licensing and affordable pricing for the duration of the pandemic.37 However, the University of Oxford shortly after releasing this statement entered an exclusive licensing agreement with the British-Swedish pharmaceutical company AstraZeneca for the COVID-19 vaccine.38 39 While AstraZeneca pledged to sell the vaccine globally at no profit during the pandemic, the price of the vaccine reportedly includes a profit margin of 20% on top of the production cost.40 41 The Oxford–AstraZeneca vaccine is offered at the lowest price of $5 per course, making it one of the most affordable vaccines available for COVID-19.6 Vaccine prices paid by countries are kept confidential, yet discrepancies in pricing have been reported with some lower-income countries seemingly paying more than higher-income countries.42 AstraZeneca has, in collaboration with the Serum Institute of India, committed a large number of vaccine doses to the COVAX facility.43 However, as of October 2021, AstraZeneca has only delivered 14% of the vaccine doses that were originally promised to COVAX.44Global equitable access is further hindered by bilateral purchasing agreements made between AstraZeneca and countries outside of COVAX.45 Given that the Oxford–AstraZeneca vaccine price is determined by the pandemic status and SARS-CoV-2 will likely become an endemic virus requiring repeated vaccinations, affordability of the vaccine postpandemic remains a concern.46
Despite a lack of research funding transparency, our findings show the dominance of government and charity funding throughout the R&D process of the ChAdOx technology, which accelerated during the pandemic. Public funding has been especially critical for vaccine research, where the failure rate is as high as 94%, and public risk-taking has enabled the rapid development of many COVID-19 vaccines.19 47 Prior to the pandemic, the ChAdOx technology has been studied in several diseases that the WHO identified as emerging infectious diseases requiring urgent R&D efforts in their Blueprint for Action to Prevent Epidemics48 including Nipah, MERS, and Ebola.49 In addition to government and charitable funders, PPPs are growing global health actors prominent in R&D efforts for diseases endemic to lower-income populations, for which a funding gap prevails.50 51 52 These public and charitable funding bodies include governments, charitable organisations, and the PPPs such as CEPI, PATH malaria vaccine initiative and CGIAR. Since the PPPs that contributed to ChAdOx were largely supported by public funding, we categorised them as public in our study.53 To recognise the public contributions and risk-taking in the R&D of the ChAdOx technology on which the Oxford–AstraZeneca vaccine relies, the benefits of this research should be shared fairly and equitably with the global population.39 54 55 As the ChAdOx vaccine platform is potentially applicable to many more global health challenges beyond the COVID-19, including emerging infectious diseases and pathogens of pandemic potential other than SARS-CoV-2, its mode of technology transfer is of global public health relevance with potential impact for equitable access and affordability of vaccines for other diseases.
Conclusion
Approximating the funding of ChAdOx to the University of Oxford offers a relevant and timely case study to understand wider trends in R&D taking place at universities and the importance of transparency in funding reporting. We found that public and charitable funders provided the majority of identifiable funding to the University of Oxford towards the R&D of the Oxford–AstraZeneca vaccine and the underlying ChAdOx technology until October 2020, which may have significant implications for the global discourse around vaccine nationalism and COVID-19 health technology access. Understanding who contributed to the development of ChAdOx is of importance to other global health challenges as well, considering that the vaccine platform may be used for multiple applications beyond SARS-CoV-2, offering an opportunity to rapidly and equitably develop affordable solutions to other existing and emerging infectious disease threats. However, a lack of transparency of funding reporting mechanisms hinders the discourse surrounding public and private contributions towards R&D and the cost of R&D. We therefore urge medical journal editors and research funders to further improve their funding reporting mechanisms by publishing funding and grant information more widely in a publicly accessible manner.
Acknowledgments
The authors thank Molly Pugh-Jones for filing one of the freedom of information (FOI) requests to the University of Oxford and Manuel Martin for his comments on an earlier version of the manuscript. We thank the Information Compliance Team at the University of Oxford for the careful composition of their responses to our FOI requests.
Footnotes
Handling editor: Seye Abimbola
SW and SK contributed equally.
Contributors: SK and SW conceived of the study and act as guarantors. FR and SK devised the methodology. FR made the search strategy. SK filed the freedom of information (FOI) requests and managed communication with the University of Oxford. FR and SK screened all articles. SC and HR extracted the data from the articles. YR searched grant databases using award numbers. SC and YR classified all the grants from the FOI. TP, SK and SW contributed to data management. SC and SK wrote the Methods and Results sections. SW, SC, SK, RO, AE-O, TP and RB wrote the first draft of the manuscript. SK, TP, and SW made the revisions to the manuscript. All authors contributed to and edited the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: The authors of this paper are all members of Universities Allied for Essential Medicines Europe. SW is a member of the Executive Committee of Universities Allied for Essential Medicines Global and FR is the National Coordinator of Universities Allied for Essential Medicines UK. SK and RO are members of the People’s Health Movement and the WHO Watch initiative. RO is currently policy director for Students for Global Health UK. However, views expressed in this paper are their own and are not necessarily shared with the organisations the authors are affiliated with.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. All datasets are available as supplementary to this article or on WhatDoTheyKnow.com under the title "Breakdown of funding for the ChAdOx1 nCoV-19 vaccine". Any quieries and requests for raw data should be addressed to the corresponding author Ms Sarai Keestra at s.m.keestra@amsterdamumc.nl.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study did not receive nor require ethics approval as it does not involve human and animal participants.
References
- 1.Medicines & Healthcare products Regulatory Agency . Conditions of authorisation for COVID-19 vaccine AstraZeneca, 2021. Available: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/conditions-of-authorisation-for-covid-19-vaccine-astrazeneca [Accessed 29 Mar 2021].
- 2.UK Government . Approved COVID-19 vaccines and countries and territories with approved proof of vaccination. Available: https://www.gov.uk/guidance/countries-with-approved-covid-19-vaccination-programmes-and-proof-of-vaccination#approved-vaccines [Accessed 1 Nov 2021].
- 3.University of Oxford . Oxford vaccine reaches one billion doses released, 2021. Available: https://www.ox.ac.uk/news/2021-07-29-oxford-vaccine-reaches-one-billion-doses-released [Accessed 1 Nov 2021].
- 4.Mallapaty S, Callaway E. What scientists do and don’t know about the Oxford-AstraZeneca COVID vaccine. Nature 2021;592:15–17. 10.1038/d41586-021-00785-7 [DOI] [PubMed] [Google Scholar]
- 5.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021;396:1979–93. 10.1016/S0140-6736(20)32466-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wouters OJ, Shadlen KC, Salcher-Konrad M, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet 2021;397:1023–34. 10.1016/S0140-6736(21)00306-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes E, Folgori A, Capone S, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med 2012;4:115ra1. 10.1126/scitranslmed.3003155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afolabi MO, Tiono AB, Adetifa UJ, et al. Safety and immunogenicity of ChAd63 and MVA ME-TRAP in West African Children and Infants. Mol Ther 2016;24:1470–7. 10.1038/mt.2016.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Doremalen N, Haddock E, Feldmann F, et al. A single dose of ChAdOx1 MERS provides protective immunity in rhesus macaques. Sci Adv 2020;6:eaba8399. 10.1126/sciadv.aba8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folegatti PM, Bittaye M, Flaxman A, et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis 2020;20:816–26. 10.1016/S1473-3099(20)30160-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ClinicalTrials.gov . A study of a candidate COVID-19 vaccine (COV001), 2020. Available: https://clinicaltrials.gov/ct2/show/NCT04324606?term=chadox&draw=3&rank=31 [Accessed 3 Nov 2021].
- 12.Oxford Vaccine Group . Oxford COVID-19 vaccine begins human trial stage, 2020. Available: https://www.ovg.ox.ac.uk/news/oxford-covid-19-vaccine-begins-human-trial-stage [Accessed 3 Nov 2021].
- 13.AstraZeneca . AstraZeneca and Oxford University announce landmark agreement for COVID-19 vaccine, 2020. Available: https://www.astrazeneca.com/media-centre/press-releases/2020/astrazeneca-and-oxford-university-announce-landmark-agreement-for-covid-19-vaccine.html [Accessed 3 Nov 2021].
- 14.University of Oxford . Funding and manufacturing boost for UK vaccine programme, 2020. Available: https://www.ox.ac.uk/news/2020-05-18-funding-and-manufacturing-boost-uk-vaccine-programme [Accessed 6 Apr 2021].
- 15.UK Government . Press release: funding and manufacturing boost for UK vaccine programme, 2020. Available: https://www.gov.uk/government/news/funding-and-manufacturing-boost-for-uk-vaccine-programme [Accessed 3 Nov 2021].
- 16.Galkina Cleary E, Beierlein JM, Khanuja NS, et al. Contribution of NIH funding to new drug approvals 2010-2016. Proc Natl Acad Sci U S A 2018;115:2329–34. 10.1073/pnas.1715368115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Vaccine Advisory Committee . United States vaccine research: a delicate fabric of public and private collaboration. National Vaccine Advisory Committee. Pediatrics 1997;100:1015–20. 10.1542/peds.100.6.1015 [DOI] [PubMed] [Google Scholar]
- 18.Mazzucato M. Putting the public back in public health. project syndicate, 2018. Available: https://www.project-syndicate.org/commentary/big-pharma-health-care-costs-by-mariana-mazzucato-2018-12 [Accessed 2 Nov 2021].
- 19.Kiszewski AE, Cleary EG, Jackson MJ, et al. NIH funding for vaccine readiness before the COVID-19 pandemic. Vaccine 2021;39:2458–66. 10.1016/j.vaccine.2021.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile APP for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Currency converter calculator. Available: https://www.xe.com/currencyconverter/ [Accessed 6 Apr 2021].
- 22.Universities Allied for Essential Medicines (UAEM), Student National Medical Association, American Medical Student Association . Tracking COVID-19 public investment in global COVID-19 research and development, 2020. Available: https://www.publicmeds4covid.org/ [Accessed 13 Mar 2021].
- 23.Keestra, Sarai & University of Oxford. WhatDoTheyKnow.com. Breakdown of funding for the ChAdOx1 nCoV-19 vaccine, 2020. Available: https://www.whatdotheyknow.com/request/breakdown_of_funding_for_the_cha#outgoing-1094220 [Accessed 10 Nov 2021].
- 24.UK Government . Freedom of information policy, 2020. Available: https://www.gov.uk/government/publications/slcs-freedom-of-information-policy/freedom-of-information-policy [Accessed 2 Nov 2021].
- 25.UK Research and Innovation . Find COVID-19 research and innovation supported by UKRI. Available: https://www.ukri.org/find-covid-19-research-and-innovation-supported-by-ukri/ [Accessed 6 Apr 2021].
- 26.GovTribe . Definitive contract 75A50120C00114. federal contract Award, 2020. Available: https://govtribe.com/award/federal-contract-award/definitive-contract-75a50120c00114 [Accessed 6 Apr 2021].
- 27.GovTribe . Other transaction IDV W15QKN2191003. federal contract IDV Award, 2020. Available: https://govtribe.com/award/federal-idv-award/other-transaction-idv-w15qkn2191003 [Accessed 6 Apr 2021].
- 28.Oxford Science Enterprises . The Backstory: Vaccitech and its role in co-inventing the Oxford COVID-19 vaccine., 2020. Available: https://oxfordscienceenterprises.com/news/the-backstory-vaccitech-and-its-role-in-co-inventing-the-oxford-covid-19-vaccine/ [Accessed 3 Nov 2021].
- 29.The University of the Witwatersrand, Johannesburg . Oxford Covid-19 vaccine trial. Available: https://www.wits.ac.za/covid19vaccine/oxford-covid-19-vaccine-trial/ [Accessed 6 Apr 2021].
- 30.Pepperrell T, Rodgers F, Tandon P, et al. Making a COVID-19 vaccine that works for everyone: ensuring equity and inclusivity in clinical trials. Glob Health Action 2021;14:1892309. 10.1080/16549716.2021.1892309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The United Nations Secretary-General’s High-Level Panel on Access to Medicines . The United Nations Secretary-general’s high-level panel on access to medicines report - promoting innovation and access to health technologies, 2016. Available: https://static1.squarespace.com/static/562094dee4b0d00c1a3ef761/t/57d9c6ebf5e231b2f02cd3d4/1473890031320/UNSG+HLP+Report+FINAL+12+Sept+2016.pdf
- 32.Wallach JD, Boyack KW, Ioannidis JPA. Reproducible research practices, transparency, and open access data in the biomedical literature, 2015-2017. PLoS Biol 2018;16:e2006930. 10.1371/journal.pbio.2006930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wimmer S, Keestra SM. Public risk-taking and rewards during the COVID-19 pandemic - a case study of remdesivir in the context of global health equity. Int J Health Policy Manag 2020 10.34172/ijhpm.2020.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iqbal SA, Wallach JD, Khoury MJ, et al. Reproducible research practices and transparency across the biomedical literature. PLoS Biol 2016;14:e1002333. 10.1371/journal.pbio.1002333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Assembly . Improving the transparency of markets for medicines, vaccines, and other health products. World Health Organization, 2020. Available: https://apps.who.int/iris/handle/10665/329301 [Accessed 6 Apr 2021].
- 36.Silverman E, ed. Pharma pushes back against setting international standards for drug-pricing transparency. Stat, 2019. https://www.statnews.com/pharmalot/2019/05/08/pharma-transparency-resolution-drug-prices/ [Google Scholar]
- 37.Oxford University Innovation, University of Oxford . Expedited access for COVID-19 related IP. Available: https://innovation.ox.ac.uk/technologies-available/technology-licensing/expedited-access-covid-19-related-ip/ [Accessed 13 Mar 2021].
- 38.Statement of Sir Menelas Pangalos, Ph. D. Executive vice president biopharmaceutical research & development AstraZeneca before the subcommittee on oversight and investigations committee on energy and commerce U.S. house of representatives. Pathway to a vaccine: efforts to develop a safe, effective and accessible COVID-19 vaccine, 2020. Available: https://docs.house.gov/meetings/IF/IF02/20200721/110926/HHRG-116-IF02-Wstate-PangalosM-20200721.pdf
- 39.Keestra S. Structural violence and the biomedical innovation system: what responsibility do universities have in ensuring access to health technologies? BMJ Glob Health 2021;6. 10.1136/bmjgh-2020-004916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.AstraZeneca . AstraZeneca to supply Europe with up to 400 million doses of Oxford University’s vaccine at no profit, 2020. Available: https://www.astrazeneca.com/media-centre/press-releases/2020/astrazeneca-to-supply-europe-with-up-to-400-million-doses-of-oxford-universitys-vaccine-at-no-profit.html [Accessed 6 Apr 2021].
- 41.Mancini DP, Cookson C, eds. Vaccine deal allows AstraZeneca to take up to 20% on top of costs. Financial Times, 2020. https://www.ft.com/content/e359159b-105c-407e-b1be-0c7a1ddb654b [Google Scholar]
- 42.Paun C, Furlong A, eds. Poorer countries hit with higher price tag for Oxford/AstraZeneca vaccine. Politico, 2021. https://www.politico.eu/article/astrazeneca-vaccine-cost-higher-in-poorer-countries-coronavirus/ [Google Scholar]
- 43.AstraZeneca . AstraZeneca advances mass global rollout of COVID-19 vaccine through COVAX, 2021. Available: https://www.astrazeneca.com/media-centre/press-releases/2021/astrazeneca-advances-mass-global-rollout-of-covid-19-vaccine-through-covax.html [Accessed 6 Apr 2021].
- 44.Malpani RM, Maitland A. Dose of Reality: How rich countries and pharmaceutical corporations are breaking their vaccine promises. The People’s Vaccine Alliance 2021.
- 45.Callaway E. The unequal scramble for coronavirus vaccines - by the numbers. Nature 2020;584:506–7. 10.1038/d41586-020-02450-x [DOI] [PubMed] [Google Scholar]
- 46.Phillips N. The coronavirus is here to stay - here’s what that means. Nature 2021;590:382–4. 10.1038/d41586-021-00396-2 [DOI] [PubMed] [Google Scholar]
- 47.Pronker ES, Weenen TC, Commandeur H, et al. Risk in vaccine research and development quantified. PLoS One 2013;8:e57755. 10.1371/journal.pone.0057755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehand MS, Al-Shorbaji F, Millett P, et al. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antiviral Res 2018;159:63–7. 10.1016/j.antiviral.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Doremalen N, Lambe T, Sebastian S, et al. A single-dose ChAdOx1-vectored vaccine provides complete protection against Nipah Bangladesh and Malaysia in Syrian golden hamsters. PLoS Negl Trop Dis 2019;13:e0007462. 10.1371/journal.pntd.0007462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wheeler C, Berkley S. Initial lessons from public-private partnerships in drug and vaccine development. Bull World Health Organ 2001;79:728–34. [PMC free article] [PubMed] [Google Scholar]
- 51.Young R, Bekele T, Gunn A, et al. Developing new health technologies for neglected diseases: a pipeline portfolio review and cost model. Gates Open Res 2018;2:23. 10.12688/gatesopenres.12817.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coalition for Epidemic Preparedness Innovations . Our mission. Available: https://cepi.net/about/whyweexist/ [Accessed 6 Apr 2021].
- 53.Coalition for Epidemic Preparedness Innovations . Board of directors’ report and annual accounts 2020, 2020. Available: https://cepi.net/wp-content/uploads/2021/03/2020-Board-of-Directors-Report-and-Annual-Accounts-incl-Auditors-report.pdf
- 54.Nuffield Council on Bioethics . Fair and equitable access to COVID-19 treatments and vaccines, 2020. Available: https://www.nuffieldbioethics.org/publications/fair-and-equitable-access-to-covid-19-treatments-and-vaccines/read-the-briefing-note/factors-affecting-fair-and-equitable-access
- 55.Keestra S, Osborne R, Rodgers F. University patenting and licensing practices in the United Kingdom during the COVID-19 pandemic – implications for global equitable access to COVID-19 health technologies, 2021. Available: https://www.medrxiv.org/content/10.1101/2021.09.20.21263777v1 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-007321supp001.pdf (83.8KB, pdf)
bmjgh-2021-007321supp002.pdf (402.2KB, pdf)
bmjgh-2021-007321supp003.pdf (102.9KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. All datasets are available as supplementary to this article or on WhatDoTheyKnow.com under the title "Breakdown of funding for the ChAdOx1 nCoV-19 vaccine". Any quieries and requests for raw data should be addressed to the corresponding author Ms Sarai Keestra at s.m.keestra@amsterdamumc.nl.