Abstract
Purpose:
To validate the furosemide stress test (FST) for predicting the progression of acute kidney injury (AKI).
Materials and Methods:
We performed a multicenter, prospective, observational study in patients with stage I or II AKI. The FST (1mg/kg for loop diuretic naïve patients and 1.5mg/kg in patients previously exposed to loop diuretics) was administered. Subsequent urinary flow rate (UFR) recorded and predictive ability of urinary output was measured by the area under the curve receiver operatic characteristics AuROC). Primary outcome was progression to Stage III AKI. Secondary outcomes included in-hospital mortality and adverse events.
Results:
We studied 92 critically ill patients. 23 patients progressed to stage III AKI and had significantly lower UFR (p<0.0001). The UFR during the first 2 hours was most predictive of progression to stage III AKI (AuROC=0.87), with an ideal cut-off of less than 200mls, with a sensitivity of 73.9% and specificity of 90.0%.
Conclusion:
In ICU patients without severe CKD with mild AKI, a UFR of less than 200mls in the first 2 hours after an FST is predictive of progression to stage III AKI. Future studies should focus on incorporating a FST as part of a clinical decision tool for further management of critically ill patients with AKI.
Keywords: Furosemide Stress Test, Acute Kidney Injury, Intensive Care Unit
Introduction
Acute Kidney Injury (AKI) is a common clinical syndrome in hospitalized patients. AKI affects nearly 5% of all hospitalized patients and up to 60% of patients admitted to the intensive care unit (ICU).1–4 However, despite advances in supportive care, patients with AKI have a high mortality rate (50 – 70%).1 Currently, there are no proven therapeutic agents for the prevention or treatment of AKI.
The ability to predict the course of AKI with accuracy and reliability is limited. Conventionally markers of kidney function (i.e., serum creatinine [sCr] and urine output [u/o]) measure surrogate parameters and are poor at predicting worsening AKI.5 While novel biomarkers of AKI have shown some promise, results have been inconsistent for the prediction of progressive AKI.6,7,8 This prompted characterization of the furosemide stress test (FST).9–14 The FST is a standardized intravenous furosemide diuretic challenge, in the setting of early AKI, designed to predict the progression of AKI.15 Chawla and colleagues studied 77 critically ill patients with stage 1 or 2 AKI and evaluated their response to a FST. Non-responders (i.e., <200 mL of urine output in 2 hours following FST) were more likely to have AKI progression.9 However, this pilot study utilized two distinct cohorts of critically ill patients (retrospective [n=22] and prospective [n=54]), and there has been limited prospective validation of these findings.
Accordingly, we aimed to perform a multi-centre prospective study to further characterize and validate the FST for predicting worsening AKI among critically ill patients. We hypothesized that in patients with stage 1 or 2 AKI, a urinary response of less than 200 ml over the first 2 hours following administration of 1.0 or 1.5 mg/kg of intravenous furosemide (depending on a patient’s prior loop diuretic exposure status), will be predictive of worsening renal function, defined as progression to stage III AKI or receipt for RRT.
Materials and Methods
Patient population
Patients were recruited from 5 academic center ICUs in the United States and Canada (University of Chicago Medicine, Chicago, Il, USA; University of California San Francisco Medical Centre, San Francisco, CA, USA; Johns Hopkins Hospital, Baltimore, MA, USA; St. Michael’s Hospital, Toronto, ON, Canada; and University of Alberta Hospital, Edmonton, AB, Canada) between January 2014 to August 2017. All ICUs were in tertiary, urban academic medical centers, had greater than 10 beds and nursing was usually 1:1 but could be 1:2 or 1:3 depending on patient disease severity (see Appendix). Adult patients (age > 18 years of age) with AKI Network (AKIN) stage I or II (defined by either urine output or sCr criteria) with an indwelling bladder catheter deemed to be euvolemic or hypervolemic were screened for eligibility. Patients were screened for study eligibility throughout their ICU stay. We applied the following exclusions: a baseline eGFR <30 ml/min/1.73 m2, prior kidney transplant, evidence of obstructive uropathy, AKI secondary to glomerulonephritis, volume depletion (as determined by the clinical team), evidence of active bleeding, pregnancy, presence of stage III AKI or those who had received RRT in the preceding 3 months. For eGFR calculation, baseline sCr was defined as lowest recorded sCr within the preceding 6 months.
Study procedures
The study was approved by the local research ethics board at each study site. Informed consent was obtained prior to study enrollment. Once enrolled, 7 ml of whole blood and 50 ml of urine was collected to serve as a baseline biochemical profile. Next, a single bolus of furosemide was administered. Prior furosemide exposure was determined if the patient had received furosemide within the last 7 days. If the patient was furosemide naïve (i.e., no prior loop diuretic exposure), then a dose of 1.0 mg/kg of furosemide was administered intravenously; if they had had previous exposure, a dose of 1.5 mg/kg of furosemide was administered. The patient’s urine output was then recorded hourly for the next 24 hours. The clinical team had the option to replace all or part of the furosemide-induced urine output as per a pre-determined protocol (see Appendix Figure).
Outcomes
The primary outcome was progression to AKIN stage III (receipt for RRT, increase in sCr to 3x baseline, u/o < 0.3 ml/kg/hour × 24hrs) within 30 days of FST. Secondary outcomes included in-hospital mortality and a composite of progression to stage III and in-hospital mortality. Additionally, we also captured adverse events related to the FST (i.e., hypokalemia, hypomagnesemia, allergic reactions to furosemide, clinically significant hypotension defined as either requiring additional fluid boluses or new or increased vasopressor doses).
Statistics
We assessed the distribution of demographic and clinical variables. Differences between proportions of patient with baseline demographics were assessed with the chi-square, Student t, and non-parametric Kruskal-Wallis tests, as appropriate. Hourly urine output amount response to the FST was compared descriptively between patient groups, either non-progress vs. progress to AKIN stage III AKI or alive vs. death within 30 days. The primary analysis was to assess predictive ability of the urinary output, on the primary endpoint of progression to AKIN stage III. We tested various combinations of hourly and cumulative urinary outputs to determine optimal operating characteristics of the FST. Predictive ability of urinary output was measured by the area under the curve receiver operatic characteristics (AuROC) in a multivariate predictive logistic regression model which was built on all possible candidate variables with stepwise selection strategy for significant predictors. Sensitivity and specificity were also calculated for determining the best among the predictive models. Multivariate logistic regression was also used to create a clinical model using the Acute Physiology and Chronic Health Evaluation (APACHE II) score, baseline urinary flow rate (UFR) and sCr at the time of the FST, which was used to explain roles of them on the primary outcome, using stepwise selection with entry and stay criterion. UFR was determined by calculating the average hourly urinary flow rate for the 6 hours preceding the FST. All means are reported with standard error (SE), unless otherwise specified. Statistical analysis was performed using Statistical Analysis System (SAS) Enterprise Guide 7.1 (Cary, NC, USA). Methodology used to calculate APACHE II score, SOFA and eGFR is as previously described.16–18
Results
We evaluated a total of 92 patients (see Appendix a study flow diagram). The mean age was 64.2 ± 1.5 years; 64% were male. Forty-two patients (46%) were African-American, 41 (45%) were Caucasian.. The proportion of patients with CKD, hypertension (HTN), congestive hearth failure (CHF) and diabetes mellitus (DM) was not significantly different between those who did and did not progress. (Table 1). There was no difference in the prevalence of significant risk factors (i.e., NSAID, aminoglycoside, amphotericin, IV contrast exposure; cardiac-surgery and sepsis) in those who did and did not progress. Baseline clinical data was similar between those who and did not progress, except for baseline urine flow rate (UFR). UFR was lower at baseline among progressors compared with non-progressors (51.0 [9.5] ml/hr vs. 80.1 [7.75] ml/hr, p=0.028). Progressors had higher APACHE II scores (22.1 [1.71] vs. 18.9 [1.03]), lower baseline eGFR (56.8 [4.79] vs. 67.9 [3.66]) and higher baseline sCr (1.27 [0.08] vs. 1.22 [0.06]) compared to non-progressors but none of these achieved statistical significance.
Table 1.
Characteristics and outcomes amongst those with and without Progressive AKI
| Variable | Total (n=92) | Non-progress (n=69) | Progress (n=23) | p value |
|---|---|---|---|---|
| Age (years), mean (SE) | 64.2 (1.53) | 63.7 (1.90) | 66.0(2.21) | 0.82 |
| Gender (male), n (%) | 59 (64.1) | 43 (62.3) | 16 (69.6) | 0.53 |
| Race, n (%) | 0.70 | |||
| African American | 42 (45.7) | 32 (46.4) | 10 (43.5) | |
| Caucasian | 41 (44.6) | 29 (42.0) | 12 (52.2) | |
| Native | 1 (1.1) | 1 (1.6) | 0 (0.00) | |
| Other | 8 (8.7) | 7 (10.1) | 1 (4.4) | |
| Type of ICU admission, n (%) | ||||
| Medical | 48 (52.2) | 33 (47.8) | 15 (65.2) | 0.92 |
| Surgical | 16 (17.4) | 12 (17.4) | 4 (17.4) | 1.0 |
| Cardiovascular Surgical | 15 16.3) | 11 (15.9) | 4 (17.4) | 0.99 |
| Other | 13 (14.1) | 13 (18.8) | 0 | <0.001 |
| Comorbidities, n (%) | ||||
| CKD | 21 (22.8) | 14 (20.3) | 7 (30.4) | 0.32 |
| HTN | 60 (65.2) | 47 (68.1) | 13 (56.5) | 0.31 |
| CHF | 32 (34.8) | 22 (31.9) | 10 (43.5) | 0.31 |
| DM | 39 (42.4) | 28 (40.6) | 11 (47.8) | 0.54 |
| Nephrotoxic exposure, n (%) | ||||
| NSAIDs | 10 (10.9) | 7 (10.1) | 3 (13.0) | 0.70 |
| Aminoglycosides | 2 (2.2) | 1 (1.5) | 1 (4.4) | 0.41 |
| Amphotericin | 1 (1.1) | 1 (1.5) | 0 (0.00) | 0.56 |
| Contrast | 19 (20.7) | 16 (23.2) | 3 (13.0) | 0.30 |
| Post-cardiac Surgery | 14 (15.2) | 10 (14.5) | 4 (17.4) | 0.74 |
| Sepsis | 22 (23.9) | 17 (24.6) | 5 (21.7) | 0.78 |
| Clinical Data, mean (SE) | ||||
| Baseline eGFR (mL/min) | 65.13 (3.03) | 67.9 (3.66) | 56.83 (4.79) | 0.14 |
| Serum Creatinine (mg/dL) | 1.23 (0.05) | 1.22 (0.06) | 1.27 (0.08) | 0.33 |
| Baseline UFR (ml/hr) | 72.78 (6.4) | 80.06 (7.75) | 50.95 (9.54) | 0.03 |
| Furosemide-naïve, n (%) | 41 (44.6) | 32 (46.4) | 9 (39.1) | 0.5 |
| SOFA | 1.52 (0.14) | 1.44 (0.16) | 1.75 (0.33) | 0.58 |
| APACHE II score | 19.68 (0.89) | 18.88 (1.03) | 22.09 (1.71) | 0.10 |
| AKI stage at enrollment, n (%) | 0.89 | |||
| AKIN I | 69 (75.0) | 52 (75.4) | 17 (73.9) | |
| AKIN II | 23 (25.0) | 17 (24.6) | 6 (26.1) | |
| Primary Outcomes, n (%) | ||||
| AKIN III | 23 (25.0) | 0 (0.00) | 23 (100.0) | <.0001 |
| RRT | 10 (10.9) | 0 (0.00) | 10 (43.5) | <.0001 |
| Death | 16 (17.4) | 9 (13.0) | 7 (30.4) | 0.06 |
| AKIN III/Death | 32 (34.8) | 9 (13.0) | 23 (100.0) | <.0001 |
| Secondary Outcomes, days (SE) | ||||
| Length of ICU stay | 9.15 (1.45) | 7.81 (1.6) | 13.17 (3.13) | 0.03 |
| Length of Hospital stay | 17.29 (2.4) | 16.65 (2.97) | 19.22 (3.59) | 0.09 |
Table 1. Patient characteristics for our cohort, non-progressors and progressors are depicted above. CKD – chronic kidney disease; HTN – hypertension; CHF – congestive heart failure; DM – diabetes mellitus; eGFR – estimated glomerular filtration rate; UFR – urine flow rate; SOFA – sequential organ failure assessment; APACHE – acute physiology. P-values for categorical (continuous) variables were from Chi-square (non-parametric Kruskal-Wallis) test.
Of the total cohort, 23 (25%) progressed to stage III AKI, and of those who progressed 10 (44%) received RRT while 7 (30%) died; these outcomes were not mutually exclusive. Of the 23 patients who progressed to stage III AKI, 20 (87%) met criteria as per sCr, and 3 (13%) met criteria as per initiation of RRT; no patients met criteria on urine output alone. Progressors had significantly longer ICU stays (p=0.03) compared to non-progressors and there was a trend towards higher inpatient mortality in progressors (p=0.06). (Table 1). In both progressors and non-progressors, the proportion of patients with AKIN stage I and II AKI was similar at baseline (73.9 vs. 75.4 and 26.1 vs. 24.6, p=0.89).
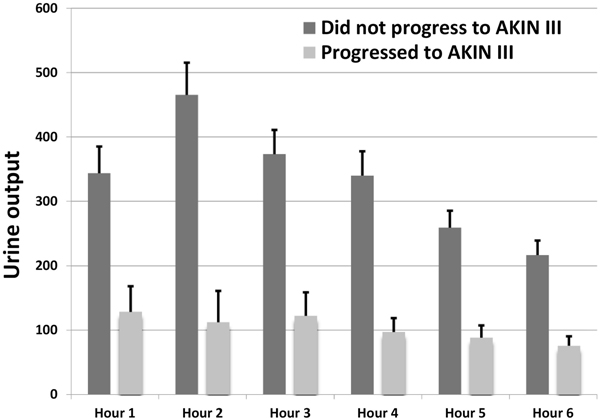
Urine output following FST
Table 2 provides the urine output for the first 6 hours following the FST (both individual hours and cumulative) for those patients who did and did not progress to stage III AKI. The highest UFR was within the first 3 hours following the FST (Figure 1, Table 2).9 For each hourly interval measured through the first 6 hours after furosemide, progressors had a lower UFR compared to non-progressors (p<0.001). Hourly urine output was not significantly predictive for patients who survived the index hospital stay vs. those who did not (Supplemental Table 1).
Table 2.
Urine output (Non-progress vs Progress to AKIN Stage III AKI) over the first 6 Post-FST hours
| Total (n=92) | Non-progress (n=69) | Progress (n=23) | p value | |
|---|---|---|---|---|
| Hour 1 | 289.73 (34.05) | 343.5 (41.57) | 128.43 (39.79) | 0.001 |
| Hour 2 | 377 (42.58) | 465.25 (50.2) | 112.26 (48.8) | <0.001 |
| Hour 3 | 310.44 (31.62) | 373.18 (37.53) | 122.22 (36.46) | <0.001 |
| Hour 4 | 279.1 (30.87) | 339.75 (37.85) | 97.17 (21.7) | <0.001 |
| Hour 5 | 216.62 (21.67) | 259.41 (26.29) | 88.26 (19.1) | <0.001 |
| Hour 6 | 181.48 (18.56) | 216.71 (22.76) | 75.78 (14.69) | <0.001 |
| 2 – hour total | 666.73 (71.87) | 808.74 (85.31) | 240.7 (83.5) | <0.001 |
| 3 – hour total | 977.17 (98.81) | 1181.93 (116.19) | 362.91 (116.13) | <0.001 |
| 4 – hour total | 1256.28 (123.56) | 1521.68 (145.42) | 460.09 (134.46) | <0.001 |
| 5 – hour total | 1472.9 (141.01) | 1781.08 (165.62) | 548.35 (151.09) | <0.001 |
| 6 – hour total | 1654.38 (156.06) | 1997.79 (183.39) | 624.13 (163.74) | <0.001 |
Table 2. The urine output in millilitres in response to the furosemide stress test is depicted above. Table 2 shows the response in patients who progressed or did not progress to AKIN stage III AKI. There was a significant difference at all time points between progressors and non-progressors to AKIN stage III AKI. Reported are the mean values with standard error in the parenthesis.
Figure 1. Urinary output in response to FST-.
For each hourly interval, there was a significantly higher urine output in patients who did not progress to AKIN stage III AKI in response to the furosemide stress test. This was most pronounced in hour 2 but was maintained for 6 hours following the furosemide stress test. AKIN – acute kidney injury network.
We found that the sum of the first 2 hours urine output after the FST had the highest AUC to predict the primary outcome (0.87) (Table 3). We also assessed the sensitivities and specificity of various 2-hour urine volumes to predict the progression to stage III AKI. Urine output of 200 ml or less in the first 2 hours had the best sensitivity and specificity (73.9% and 90.0%, respectively) to predict the primary outcome (Table 4).
Table 3.
Area under receiver operation characteristics (AUC) for Urine output Post-FST for the progression to Stage III
| Urine output-Individual Hours | AUROC | Urine output-Cumulative | AUROC |
|---|---|---|---|
| Hour 1 | 0.86 | 1 – hour total | 0.86 |
| Hour 2 | 0.89 | 2 – hour total | 0.87 |
| Hour 3 | 0.84 | 3 – hour total | 0.86 |
| Hour 4 | 0.85 | 4 – hour total | 0.86 |
| Hour 5 | 0.83 | 5 – hour total | 0.86 |
| Hour 6 | 0.82 | 6 – hour total | 0.86 |
Table 3. The operating characteristics for progression to AKIN stage III AKI are depicted above. The column on the left shows AUROC per hourly urine output, while on the right shows AUROC for total urine output following the furosemide stress test. Urine output at the 2-hour period consistently had the best operating characteristics for progression to AKIN stage III AKI.
Table 4.
Sensitivity and specificity for test AKIN Stage III
| FST Index | Sensitivity | Specificity |
|---|---|---|
| Urine output in hour 2 (individual) | ||
| < 50 ml | 52.2% | 94.2% |
| < 100 ml | 65.2% | 87.0% |
| < 150 ml | 87.0% | 81.2% |
| < 200 ml | 87.0% | 73.9% |
| < 250 ml | 95.7% | 69.6% |
| Total urine output over 2 hours (cumulative) | ||
| < 100 ml | 43.5% | 94.2% |
| < 200 ml | 73.9% | 90.0% |
| < 300 ml | 78.3% | 84.1% |
| < 400 ml | 87.0% | 65.2% |
| < 500 ml | 87.0% | 55.1% |
Table 4. Specific urine output cut-offs for progression to AKIN stage III AKI were assessed. The 2-hour urine output of 200 mls offered the best combination of sensitivity and specificity.
In multivariable analysis, only post-FST urine output and sex remained significant predictors of AKI progression. APACHE II score and baseline urine output were not significant predictors of AKI progression (Supplemental Table 2a). In a second multivariable analysis including important clinical variables (i.e., sCr, baseline UFR and APACHE II), no baseline clinical parameters were found to the be significant for the prediction of progression to AKIN stage III AKI.
There were few occurrences of adverse events following the FST, with 9 (9.8%) episodes of clinically significant (i.e., requiring intervention by means of fluid administration or vasopressor support/titration) hypotension reported. With regard to electrolytes, there were 5 (5.4%) instances each of hypokalemia and hypomagnesemia reported, as defined by unit specific electrolyte replacement protocols. There were no critical life-threatening events recorded (Table 5). There was no difference in adverse events between those who did and did not progress to stage III AKI.
Table 5.
Total Number of adverse events following the FST
| Adverse Event | Total, n=19 (20.7%) | Non-progress, n=14 (20.3%) | Progress, n=5 (21.7%) | P-value |
|---|---|---|---|---|
| Hypotension | 9 (9.78) | 5 (7.25) | 4 (17.39) | 0.55 |
| Hypokalemia | 5 (5.43) | 5 (7.25) | 0 (0.00) | 0.54 |
| Hypomagnesemia | 5 (5.43) | 4 (5.80) | 1 (4.35) | 0.91 |
Table 5. The total number of adverse events relating to the furosemide stress test remained low. Importantly, no significant adverse events, as determined by the study team at each site, occurred.
Discussion
In this prospective, multicenter study, we evaluated the FST in 92 critically ill patients. These patients were from general medical, surgical and cardiovascular critical care units from 5 academic centers in the United States and Canada. The FST was found to predict the progression to AKIN stage III with an AUC of 0.87 and had incremental operating characteristics for the prediction of AKI when compared to usual clinical parameters (Supplemental Table 2). This is most likely secondary to our clinical parameters being not sufficiently specific for AKI, while the FST is focused solely on renal reserve. Additionally, the FST was safe in this group of critically ill patients.
A furosemide challenge to assess renal tubular integrity and anticipate outcomes is a practice in patients with AKI. Loop diuretics, and furosemide in particular, reduce sodium reabsorption in the thick ascending limb of the loop of Henle, resulting in an increase in urinary sodium, water excretion and subsequent diuresis.19 For this to occur, both proximal, loop and distal renal tubular function must be intact. The standardization of a weight-based furosemide dose as a, ‘furosemide stress test,’ was first described by members of our group in 2013.9 However, this study involved two cohorts (one prospective and one retrospective), and has not been prospectively validated. This current study confirmed these findings. A urine output cut-off of 200 mL or less in the two hours following an FST predicted progressive AKI (sensitivity 73.9% and specificity 89.9%) and non-response to the FST was predictive of progression to AKIN III or 30-day mortality (p<0.001). A notable difference between their study and ours was that in our study the proportion of patients with AKIN stage I and II at the time of the FST was similar between responders and non-responders to the FST (p=0.89), while in Chawla et al. there were significantly more patients with stage II AKI who went on to progress to stage III (p=0.003). As our results were more balanced between patients with stage I and II AKI, this would suggest broader applicability of the FST. This may in turn have led to additional patients being recruited with stage I AKI, potentially having increased renal reserve and being more responsive to the FST, thus accounting for the increased incidence of hypotension found in our study vs. in Chawla et al. Additionally, Chawla et. al evaluated progression to AKIN Stage III AKI at 14 days, while we considered a longer time period and followed patients to 30 days which could potentially capture more patients progressing to severe AKI. However, we observed a lower rate of AKIN Stage III AKI (25% vs. 32.4%) which likely reflected our different patient cohorts. Finally, and most importantly, while Chawla et al. included a retrospective cohort, our data was a multicenter, prospective study involving a broad group of critically ill patients.
A furosemide challenge has also been evaluated when compared with kidney damage biomarkers to predict AKI progression.12 Matsuura et al. retrospectively analyzed 95 patients with AKIN stage I and II AKI, and their urinary response following a variable furosemide challenge, along with the predictive characteristics of plasma NGAL. The furosemide responsiveness (2-hour urine output divided by the diuretic dose) was determined to be the best predictor of progression to AKIN stage III AKI (AUC 0.87, 95% CI 0.73–0.94) and had better operating characteristics compared to plasma NGAL (AUC 0.80, 95% CI 0.67–0.88). This further highlights the clinical utility of the FST to test functional nephron reserve and predict progressive AKI.
The FST has also been studied in the evaluation and prediction of delayed graft function (DGF) and need of post-transplant RRT in patients undergoing kidney transplant.13 McMahon et al. defined the FST as 100 mg of furosemide (regardless of patient weight) intraoperatively after the anastomosis of the renal vessels. Patients with DGF had a significantly decreased urinary response to intraoperative furosemide (73 ml vs. 250 ml in 2 hours following the FST, p<0.001). Additionally, the urine output 6 hours after the FST provided an AUC of 0.85 with a cut-off of 600 ccs in the first 6 hours providing a sensitivity and specificity of 83% and 74%. While this data is limited by its retrospective nature, it again suggests that the FST may be used as a clinical prediction tool for the future need of RRT, not only in general medical/surgical patients, but also in those undergoing kidney transplants.
The FST provides a readily applicable mechanism to identify patients with AKI with an adverse prognosis who are optimally suited for trials testing strategies to improve AKI outcomes. This was illustrated in a study by Lumlertgul et al. who conducted a prospective, multicenter, open label trial of patients with AKI in any stage, in which FST non-responders were selected for randomization to either early or standard RRT initiation.20 Forty-four patients were FST responsive (made more than 200 ccs in the first 2 hours), while 118 were FST non-responsive and randomized to either early or standard RRT initiation. They concluded that the FST successfully excluded patients at low risk for the future need for RRT as only 6 of 44 (13.6%) of FST-responsive patients subsequently received RRT. Furthermore, a non-response to FST was highly predictive of receiving RRT with 45 of 60 patients randomized to standard RRT initiation, receiving RRT. However, these investigators included patients will all severity of AKI. Our study evaluated the FST in patients with mild to moderate (i.e., AKI stage I and II) and future work will be required to evaluate the FST for predicting progression to RRT in patients with severe (i.e., stage III) AKI.
A leading question for patients with AKI is the issue of when to initiate renal replacement therapy (RRT).21 Retrospective studies suggest that the early initiation of RRT improves outcome.22–25 While there exist several studies regarding outcomes and timing of initiation of RRT; results are conflicting.26–28 The recently published Artificial Kidney Initiation in Kidney Injury Trial (AKIKI), a multicenter randomized trial evaluating the timing of RRT in critically ill patients who have AKI but no potentially life threatening complications, assigned patients with stage III AKI who required mechanical ventilation, catecholamine infusion, or both to an early or delayed strategy of RRT.27 Patients were randomized to the early-strategy as soon as stage III AKI was documented, while in the delayed group, traditional criteria for RRT initiation were used (i.e., oliguria or anuria for greater than 72hrs after randomization; BUN greater than 112 md/dl, serum potassium greater than 6 mmol/l, serum potassium greater than 5.5. mmol/l despite medical treatment, pH below 7.15 and acute pulmonary edema responsible for severe hypoxemia). The investigators ultimately demonstrated that 49% of patients randomized to the delayed-treatment arm did not ultimately require RRT. These data would suggest that there is likely a subset of critically ill patients with severe AKI who may recover kidney function and avoid receipt of RRT. This could potentially minimize their risk of suffering RRT-related complications (i.e., dialysis line insertion, anticoagulation, etc.) and reduce unnecessary healthcare expenditures. An FST in these patients may assist clinicians in the decision on initiation of RRT.
While our study only found that 43.4% of patients with decreased urine output following their FST required RRT, we did not have specific criteria to mandate the initiation of RRT. Additionally, our study was not powered with RRT initiation as a primary endpoint. Further study is required to determine the optimal operating characteristic of the FST to predict need for RRT and may integrate the FST into trial design for studies evaluating initiation of RRT.
This study had several important strengths. First, it is amongst the largest, prospective study to date evaluating the characteristics of the FST. Second, it was a multicentered, international study involving 5 distinct tertiary-care, urban, academic centers, which served to minimize any local biases, practice patterns and greatly increased the generalizability of our findings. Third, our study involved a strict, standardized, furosemide administration protocol that ensured that an adequate FST was administered. This ensured that if marginalized kidney’s had sufficient reserve in order to respond to furosemide, then a clinically significant urinary response would occur.
This study had several limitations. First, only patients with AKIN stage I and II were included in this study. We did not evaluate the FST in patients with already AKIN stage III AKI, but who had not yet progressed to requiring RRT. Recruitment for this study was lengthy, which may indicate that the FST, as per our protocol for patients with AKIN Stage I or II AKI may have limited generalizability; however, even though not studied, the FST may be useful in patients with more severe AKI. While this is an important patient cohort, the FST for prediction of need of RRT was not the primary purpose of this study and may be a focus of future work. We also did not record specific time until progression to AKIN Stage III AKI and reported only on progression within 30-days. This was done as the primary objective of this study was to use the FST as a function of renal reserve and not necessarily as a clinical decision tool. This would be feasible as future studies of the FST. Additionally, we do not have urinalysis, microscopy or biomarker data on this cohort to compare the performance of the FST to other novel markers of kidney function. As well, the treating clinicians were not blinded to the results of the FST. This may have influenced their decision on initiation of RRT and influenced our results. We also did not evaluate on how clinician altered their care in response to the FST (i.e., medication dosing, RRT initiation, nephrotoxin limitations) as our study sought to validate the existing FST. The clinical response to the FST would be an important aspect to evaluate in future studies. The FST is also limited to patients with a UFR of less than 100ml/h, and hence cannot be utilized in high-output non-oliguric AKI. Finally, we do not have post-discharge, long-term follow up on patient outcomes (long term need for RRT, development of CKD or major adverse kidney events) in this cohort.
The FST has been shown to be a safe and effective test in patients with mild to moderate AKI (AKIN stage I and II) to predict progression to AKIN stage III AKI in patients with mild to moderate AKI. This is consistent with previous published studies. This is an important finding as other markers of AKI have not been consistently shown to predict future kidney function /outcomes. The FST has been shown as a robust test of renal reserve. Our data supports the routine implementation of the FST into clinical practice, and future guidelines should consider the FST for prognosis and prediction of worsening AKI. The FST, along with clinical criteria and novel biomarkers, should be integrated in the design of clinical trials evaluating patients with AKI, and to determine need for RRT.
Conclusions
In summary, we have validated the safety and utility of the FST as a dynamic functional assessment of future renal function. The FST has very good predictive capacity to identify those patients who will progress to advanced stage AKI. Future studies should focus on incorporating a FST as part of a clinical decision tool for further management of critically ill patients with AKI and perhaps to guide the initiation of RRT.
Supplementary Material
Highlights.
AKI is common in critically ill patients
There is no previously validated test to predict progression of AKI
The Furosemide Stress Test is able to predict worsening AKI
Acknowledgements:
SMB is supported by a Canada Research Chair in Critical Care Nephrology.
Funding:
This study was partly funded from a grant from the University of Alberta Hospital Foundation (PRO052189) (SMB) as well as Coplon Grant Satellite Healthcare (JLK). The funding agencies had no role in the design or conduct of the study, in the collection, management, analysis or interpretation of the data, or in the preparation, review or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ. Acute renal failure in intensive care units--causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med. 1996;24(2):192–198. [DOI] [PubMed] [Google Scholar]
- 2.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74(2):243–248. [DOI] [PubMed] [Google Scholar]
- 3.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational. Int Care Med. 2015;4(8)1411–23. [DOI] [PubMed] [Google Scholar]
- 4.National Confidential Enquiry into Patient Outcome and Death. Adding Insult to Injury: A review of the care of patients who died in hospital with a primary diagnosis of acute kidney injury (acute renal failure). 2009. Available at https://www.ncepod.org.uk/2009report1/Downloads/AKI_report.pdf.
- 5.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193–207. [DOI] [PubMed] [Google Scholar]
- 6.Klein SJ, Brandtner AK, Lehner GF, et al. Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2018;44(3):323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephol. 2012;23(5):905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rewa O, Wald R, Adhikari NK, et al. Whole-blood neutrophil gelatinase-associated lipocalin to predict adverse events in acute kidney injury: A prospective observational cohort study. J Crit Care. 2015;30(6):1359–1364. [DOI] [PubMed] [Google Scholar]
- 9.Chawla LS, Davison DL, Brasha-Mitchell E, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17(5):R207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chawla LS, Ronco C. Renal Stress Testing in the Assessment of Kidney Disease. Kidney Int Rep. 2016;1(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyner JL, Chawla LS. Use of stress tests in evaluating kidney disease. Curr Opin Nephorl Hypertens. 2017;26(1): 31–35. [DOI] [PubMed] [Google Scholar]
- 12.Matsuura R, Komaru Y, Miyamoto Y, et al. Response to different furosemide doses predicts AKI progression in ICU patients with elevated plasma NGAL levels. Ann Intensive Care. 2018;8(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahon BA, Koyner JL, Novick T, et al. The prognostic value of the furosemide stress test in predicting delayed graft function following deceased donor kidney transplantation. Biomarkers. 2018;23(1):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penk J, Gist KM, Wald EL, et al. Furosemide response predicts acute kidney injury in children after cardiac surgery. LID - S0022–5223(19)30003–0 [pii] LID - 10.1016/j.jtcvs.2018.12.076 [doi]. (1097–685X (Electronic)). [DOI] [PubMed] [Google Scholar]
- 15.Powell TC, Warnock DG. The Furosemide Stress Test and Predicting AKI Outcomes. Journal of the American Society of Nephrology. 2015;26(8):1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knaus WA, Zimmerman JE, Wagner DP, et al. APACHE-acute physiology and chronic health evaluation: a physiologically based. Crit Care Med. 1981;9(8):591–597. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction.failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Int Care Med. 1996:22(7):707–710. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new predictin equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 19.Oh SW, Han SY. Loop Diuretics in Clinical Practice. Electrolyte Blood Press. 2015;13(1):17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lumlertgul N, Peerapornratana S, Trakarnvanich T, et al. Early versus standard initiation of renal replacement therapy in furosemide stress test non-responsive acute kidney injury patients (the FST trial). Crit Care. 2018;22(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu KD, Himmelfarb J, Paganini E, et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1(5):915–919. [DOI] [PubMed] [Google Scholar]
- 22.Conger JD. A controlled evaluation of prophylactic dialysis in post-traumatic acute renal failure. J Trauma. 1975;15(12):1056–1063. [DOI] [PubMed] [Google Scholar]
- 23.Kleinknecht D, Jungers P, Chanard J, Barbanel C, Ganeval D, Rondon-Nucete M. Factors influencing immediate prognosis in acute renal failure, with special reference to prophylactic hemodialysis. Adv Nephrol Necker Hosp. 1971;1:207–230. [PubMed] [Google Scholar]
- 24.Parsons FM, Hobson SM, Blagg CR, Mc CB. Optimum time for dialysis in acute reversible renal failure. Description and value of an improved dialyser with large surface area. Lancet. 1961;1(7169):129–134. [DOI] [PubMed] [Google Scholar]
- 25.Fischer RP, Griffin WO Jr., Clark DS. Postoperative renal failure. The Journal-lancet. 1968;88(2):42–46. [PubMed] [Google Scholar]
- 26.Wald R, Adhikari NK, Smith OM, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int. 2015;88(4):897–904. [DOI] [PubMed] [Google Scholar]
- 27.Gaudry S, Hajage D, Schortgen F, et al. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med. 2016;375(2):122–133. [DOI] [PubMed] [Google Scholar]
- 28.Zarbock A, Kellum JA, Schmidt C, et al. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality. JAMA. 2016;315(20):2190–2199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.