Abstract
Chromone glycosides comprise an important group of secondary metabolites. They are widely distributed in plants and, to a lesser extent, in fungi and bacteria. Significant biological activities, including antiviral, anti-inflammatory, antitumor, antimicrobial, etc., have been discovered for chromone glycosides, suggesting their potential as drug leads. This review compiles 192 naturally occurring chromone glycosides along with their sources, classification, biological activities, and spectroscopic features. Detailed biosynthetic pathways and chemotaxonomic studies are also described. Extensive spectroscopic features for this class of compounds have been thoroughly discussed, and detailed 13C-NMR data of compounds 1–192, have been added, except for those that have no reported 13C-NMR data.
Keywords: chromone glycosides, chemical structure, activity, benzo-γ-pyrone, 13C-NMR data
1. Introduction
Chromone glycosides are a class of secondary metabolites with various medicinal properties. They are widely distributed in many plant genera and, to a lesser extent, in some fungal species and other sources [1]. Several biological activities have been reported for various chromone glycosides. For example, aloesin and its analogues, from Aloe, are used in cosmetic preparations to treat hyperpigmentation induced by UV radiation, owing to their role in inhibition of tyrosinase enzyme [2,3]. Additionally, 8-[C-β-d-[2-O-(E)-cinnamoyl]glucopyranosyl]-2-[(R)-2-hydroxypropyl]-7-methoxy-5-methylchromone), isolated from certain Aloe species, was reported to have potent topical anti-inflammatory activity comparable to the effect of hydrocortisone without affecting thymus weight [3]. Macrolobin, from Macrolobium latifolium, has a remarkable acetylcholinesterase inhibitory activity with an IC50 value of 0.8 µM. Uncinosides A and B, isolated from the Chinese herbal medicine Selaginella uncinata, showed potent anti-RSV (respiratory syncytial virus) activity with IC50 values of 6.9 and 1.3 µg/mL. Taking into consideration the broad biological activities of chromone glycosides, this review summarizes the naturally occurring chromone glycosides and categorizes these compounds on their structural basis, in addition to their sources, bioactivities and spectroscopic features. Importantly, this review will shed more light toward the NMR features of chromone glycosides to help natural product researchers in the identification of various chemical structures. Scientific databases as SciFinder, PubMed, and Google Scholar were used to collect the relevant literature data.
2. Biosynthesis
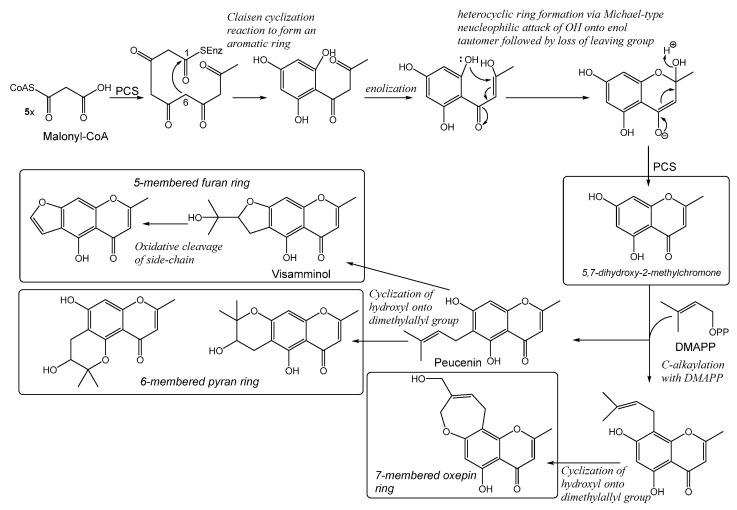
Chromones are biosynthesized through the acetic acid pathway by the condensation of five acetate molecules. These compounds, generally, have a methyl group at C-2 and are oxygenated at C-5 and C-7 [4]. Pentaketide Chromone Synthase (PCS) is a key enzyme in the biosynthesis process that catalyzes the formation of a pentaketide chromone (5,7-dihydroxy-2-methylchrome) from five-step decarboxylative condensations of malonyl-CoA, followed by the Claisen cyclization reaction to form an aromatic ring. However, it is unclear whether the heterocyclic ring closure of the pentaketide chromone is enzymatic or not, because the ring closure can take place due to spontaneous Michael-like ring closure, as in the case of flavanone formation from chalcone in vitro. PCS also accepts acetyl-CoA, resulting from decarboxylation of malonyl-CoA, as a starter substrate, but it is a poor substrate for PCS [5].The pentaketide chromone has been isolated from several plants and is known to be the biosynthetic precursor of the chromone derivatives with additional heterocyclic rings (e.g., furano-, pyrano- and oxepino-chromone glycosides). Scheme 1 ([6] with modifications) shows the sequence of steps utilized in the biosynthesis of these compounds, fully consistent with the biosynthetic rationale developed above. The key intermediate is 5,7-dihydroxy-2-methylchromone [5,6]. For many years, the cyclization had been postulated to involve an intermediate epoxide, such that nucleophilic attack of the phenol onto the epoxide group might lead to formation of either five-membered furan, six-membered pyran or the seven-membered oxepin heterocycles, as commonly encountered in natural products [6].
Scheme 1.
Proposed mechanisms for the enzymatic formation of 5,7-dihydroxy-2-methylchromone and its derivatives.
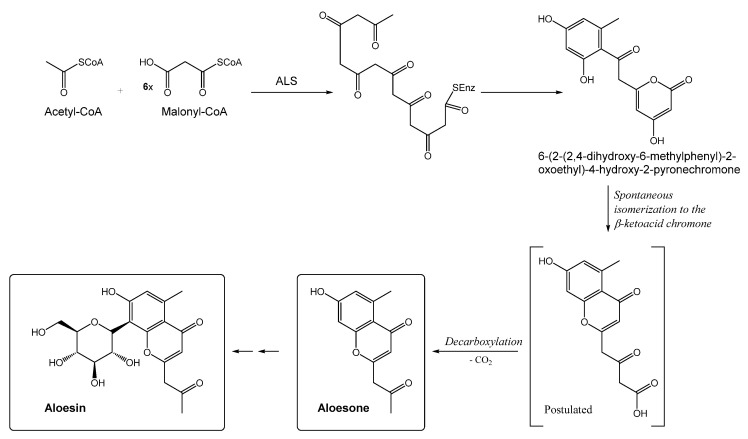
Aloesone Synthase (ALS) (Scheme 2, [5] with modifications) is a key enzyme in the biosynthesis of heptaketide chromone aloesone derivatives, such as aloesone 7-O-β-d-glucopyranoside (53) in rhubarb and anti-inflammatory aloesone 8-C-β-d-glucopyranoside (aloesin, 98) in Aloe (A. arborescens). ALS efficiently catalyzes the formation of a heptaketide aromatic pyrone 6-(2-(2,4-dihydroxy-6-methylphenyl)-2-oxoethyl)-4-hydroxy-2-pyronechromone from acetyl-CoA and six molecules of malonyl-CoA through an aldol cyclization. The unstable heptaketide pyrone (or acid form) would then undergo subsequent spontaneous isomerization to the β-ketoacid chromone, which is followed by decarboxylation to produce the heptaketide aloesone [5].
Scheme 2.
Proposed mechanisms for the enzymatic formation of aloesone and its derivatives.
3. Taxonomy
We have reviewed the literature concerning the occurrence of chromone glycosides, and we have found that 192 different chromone glycosides have been isolated from different natural sources, including angiosperms, ferns, lichens, fungi and actinobacteria (Table 1). The occurrence of chromone glycosides is mostly confined to botanical families: Apiaceae, Fabaceae, Myrtaceae, Asphodelaceae, Ranunculaceae, Rubiaceae, Hypericaceae, Ericaceae, Amaryllidaceae, Polygonaceae and Araceae. However, few chromone glycosides are also present in Asteraceae, Eucryphiaceae, Saxifragaceae, Smilacaceae, Pentaphylaceae, Salicaceae, Meliaceae, Euphorbiaceae, Staphyleaceae, Amaranthaceae, Aquifoliaceae, Rosaceae, Bignoniaceae, Olacaceae, Pinaceae, Selaginellaceae, Gentianaceae, Cannabaceae, Euphorbiaceae, Cucurbitaceae, Thymelaeaceae and Poaceae. Many of the naturally occurring chromone-8-C-glycosides such as the well-known chromone glycoside aloesin (98) were reported from genus Aloe. Until now, chromone glycosides with additional heterocyclic moieties such as pyrano-, oxepino- and pyrido-chromone glycosides were only isolated from Saposhnikovia divaricate, Eranthis species and Schumanniophyton magnificum, respectively. Another interesting category comprises hybrids of furano-chromones with cycloartane triterpenes, which were reported from Cimicifuga foetida. Actinobacteria also constitute an important source of the chromone alkaloid aminoglycosides, which are isolated from Streptomyces, Saccharothrix and Actinomycete species.
Table 1.
The distribution of chromone glycosides reported through this review.
| Family | Genus | Species | Compounds | ||
|---|---|---|---|---|---|
| Plants (Angiosperms) | 1 | Ericaceae | Rhododendron | ovatum | 2 |
| spinuliferum | 3 | ||||
| collettianum | 55 | ||||
| Calluna | vulgaris | 13 | |||
| 2 | Rubiaceae | Schumanniophyton | magnificum | 7, 25, 156 | |
| Knoxia | corymbosa | 20, 24, 35, 36 | |||
| Adina | rubescens | 20 | |||
| Neonauclea | sessilifolia | 26, 166 | |||
| 3 | Amaryllidaceae | Gethyllis | ciliaris | 10 | |
| Pancratium | biflorum | 20, 66 | |||
| maritimum | 20, 47 | ||||
| 4 | Polygonaceae | Polygonum | capitatum | 15 | |
| Rheum | austral | 50 | |||
| sp. | 52, 53 | ||||
| 5 | Apiaceae | Ammi | visnaga | 20, 140, 146 | |
| Peucedanum | austriacum | 20 | |||
| japonicum | 149 | ||||
| Cnidium | monnieri | 57, 58, 123–130 | |||
| juponicum | 123, 124 | ||||
| Bupleurum | chinense | 58 | |||
| Angelica | archangelica | 125 | |||
| genuflexa | 146, 149 | ||||
| japonica | 146, 149 | ||||
| Archangelica | litoralis | 125 | |||
| Saposhnikovia | divaricata | 143–146, 149, 150 | |||
| Ledebouriella | seseloides | 144 | |||
| Diplolophium | buchananii | 144, 146, 151 | |||
| Sphallerocarpus | gracilis | 144 | |||
| Glehnia | littoralis | 149 | |||
| 6 | Hypericaceae | Hypericum | henryi | 22, 23 | |
| erectum | 22, 38 | ||||
| sikokumontanum | 38, 39, 60, 61 | ||||
| japonicum | 82, 83 | ||||
| 7 | Ranunculaceae | Delphinium | hybridum | 28 | |
| Cimicifuga | heracleifolia | 141 | |||
| foetida | 146, 147, 157–165 | ||||
| Eranthis | hyemalis | 131, 132, 152, 153, 154 | |||
| cilicica | 133, 134, 153, 155 | ||||
| 8 | Myrtaceae | Myrtus | communis | 34 | |
| Syzygium | aromaticum | 66, 71, 80, 86 | |||
| Baeckea | frutescens | 66, 67, 70, 72, 85, 87 | |||
| Kunzea | ambigua | 67, 70, 72–74, 80, 85, 88, 89 | |||
| Eucalyptus | globulus | 80 | |||
| maidenii | 82, 83 | ||||
| grandis | 83 | ||||
| urograndi | 83 | ||||
| 9 | Fabaceae | Cassia | multijuga | 51, 77 | |
| siamea | 59 | ||||
| obtusifolia | 68, 69 | ||||
| spectablis | 78 | ||||
| obtusifolia | 84 | ||||
| Macrolobium | latifolium | 64 | |||
| Aspalathus | linearis | 65 | |||
| Abrus | mollis | 80 | |||
| Ononis | vaginalis | 135 | |||
| 10 | Asphodelaceae | Aloe | vera | 75, 76, 90–98, 103, 104–110, 112–117, 120–122 | |
| barbadensis | 97, 98, 103, 107, 108 | ||||
| rupestris | 99 | ||||
| cremnophila | 101 | ||||
| nobilis | 111, 118, 119 | ||||
| 11 | Araceae | Scindapsus | officinalis | 18–20, 29, 31, 32, 56, 57 | |
| 12 | Asteraceae | Mutisia | acuminate | 1 | |
| 13 | Eucryphiaceae | Eucryphia | cordifolia | 4 | |
| 14 | Saxifragaceae | Astilbe | thunbergii | 4 | |
| 15 | Smilacaceae | Smilax | glabra | 4 | |
| 16 | Pentaphylaceae | Eurya | japonica | 5 | |
| 17 | Salicaceae | Salix | matsudana | 6 | |
| 18 | Meliaceae | Dysoxylum | binectariferum | 7 | |
| 19 | Euphorbiaceae | Acalypha | fruticose | 7 | |
| 20 | Staphyleaceae | Staphylea | bumalda | 7 | |
| 21 | Amaranthaceae | Salicornia | europaea | 12 | |
| 22 | Aquifoliaceae | Ilex | hainanensis | 13 | |
| 23 | Rosaceae | Dasiphora | parvifolia | 16, 17 | |
| 24 | Bignoniaceae | Tecomella | undulata | 20, 27 | |
| 25 | Olacaceae | Scorodocarpus | borneensis | 26 | |
| 26 | Pinaceae | Pseudotsuga | sinensis | 37 | |
| 27 | Selaginellaceae | Selaginella | uncinata | 46, 48 | |
| 28 | Gentianaceae | Swertia | punicea | 54 | |
| 29 | Cannabaceae | Humulus | lupulus | 62 | |
| 30 | Euphorbiaceae | Chrozophora | prostrata | 79 | |
| 31 | Cucurbitaceae | Cucumis | melo | 136 | |
| 32 | Thymelaeaceae | Aquilaria | sinensis | 137, 139 | |
| 33 | Poaceae | Imperata | cylindrical | 138 | |
| Ferns | 1 | Polypodiaceae | Drynaria | fortunei | 7, 28, 30, 32, 33, 57 |
| 2 | Dryopteridaceae | Dryopteris | fragrans | 20, 21 | |
| 3 | Onocleaceae | Matteuccia | intermedia | 49 | |
| Lichens | Roccellaria | mollis | 41, 42, 45 | ||
| Schismatomma | accedens | 41, 42, 45 | |||
| Roccella | galapagoensis | 41, 42, 45 | |||
| Lobodirina | cerebriformis | 44 | |||
| Fungi | Armillaria | tabescens | 11 | ||
| Orbiocrella | sp. | 40, 43 | |||
| Stemphylium | botryosum | 63 | |||
| Actinobacteria | Streptomyces | phaeoverticillatus var. takatsukiensis | 168 | ||
| pluricolorescens | 169–171 | ||||
| sp. | 172–178, 180 | ||||
| griseoruber | 179 | ||||
| Saccharothrix | sp. | 181–183 | |||
| Actinomycete | 184–192 |
4. Chromone Glycosides
Chromone glycosides belong to a group of oxygen-containing heterocyclic compounds with a benzo-γ-pyrone skeleton. Naturally occurring chromone glycosides can be either O-glycosides or C-glycosides. For O-glycosides, the most frequently encountered group is the 7-O-glycosides; however, 2-, 3-, 5-, 8-, 11- and 13-O-glycosides also exist but to a lower extent. For an example, only one 6-O-glycoside 11 has been reported from nature, and from fungi, not higher plants [1]. Glycosylation can also be detected at side chains for chromones, at C-11 and C-12 as in compounds 56–59, at the hydroxyprenyl and hydroxyisoprenyl side chains as in 123 and 128, respectively, or at the phenyl ethyl moiety as 139. The most abundant among chromone glycosides is the glucoside from. However, other sugar moieties such as xylose, arabinose and rhamnose were also detected in 3-, 7- and 11-O-glycosides.
4.1. Chromone O-glycosides
4.1.1. 2-O-Glycosides
This category includes compound 1 (Figure 1), 2-hydroxy-5-methylchromone-β-d-glucopyranoside, isolated from the aerial parts of Mutisia acuminata var. hirsuta, a member of family Asteraceae [7]. The authors did not report biological activity for this compound.
Figure 1.
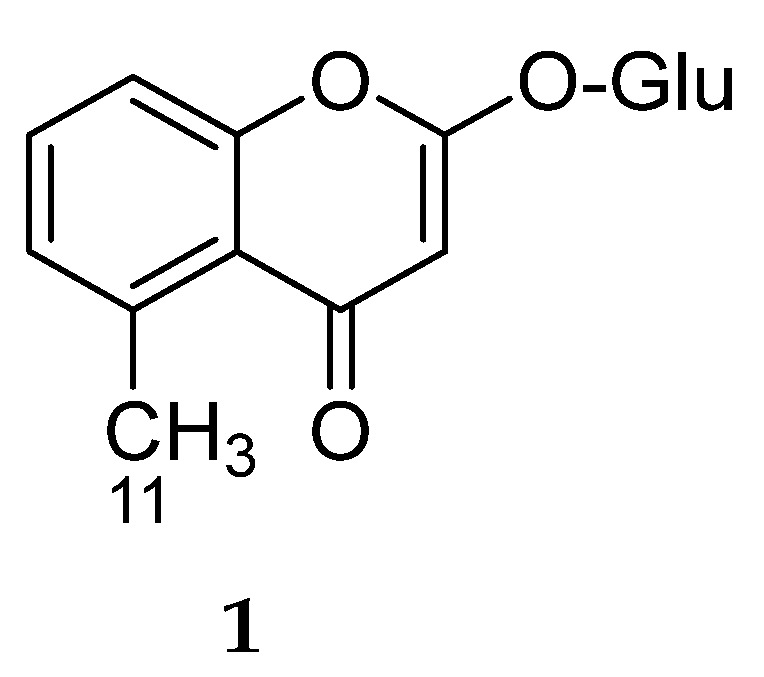
Structure of compound 1.
4.1.2. 3-O-Glycosides
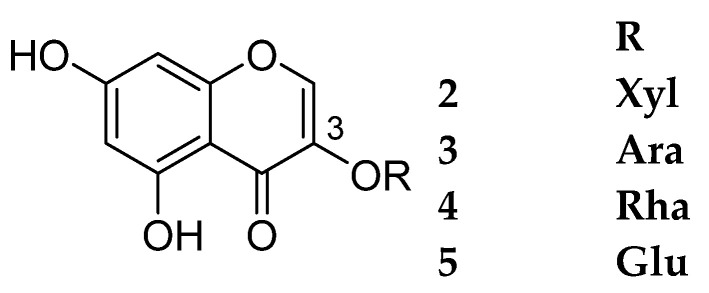
This category includes compounds 2–5. They share the same aglycone nucleus but with different sugar moieties at C-3. Eucryphin 4 was reported as a new compound in 1979 [8]; however, it was reported again in 1996 as a new compound under the name smiglanin [9]. In addition, 3,5,7-trihydroxychromone 3-O-β-d-xylopyranoside 2 was first reported in 2005 from Rhodadendron ovatum [10], but it was reported again as a new compound in 2013 [11]. Compounds 2–5 are shown in Figure 2. The sources and the reported biological activities are summarized in Table 2.
Figure 2.
Structures of compounds 2–5.
Table 2.
3-O-Chromone glycosides with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 2 | 3,5,7-trihydroxychromone-3-O- β-d-xylopyranoside |
Rhododendron ovatum roots [10] Eurya japonica stems [11] |
Inhibitory effects on LPS (Lipopolysaccharide)-induced NO (Nitric Oxide) production with inhibition rate 36.24 ± 1.29% at 20 μg/mL [11] |
| 3 | 3,5,7-trihydroxylchromone-3-O- α-l-arabinopyranoside |
Rhododendron spinuliferum aerial parts [12] |
Inhibition of NO production in LPS-stimulated RAW 264.7 cells with an IC50 value more than 100 mM [12] |
| 4 | Eucryphin (5,7-dihydroxy-3-(α-O-l-rhamnopyranosyl)- 4H-l-benzopyran-4-one) |
Eucryphia cordifolia bark [8] Astilbe thunbergii rhizomes [13] |
Norepinephrine-enhancing lipolytic effect 6.432 ± 0.014 FFA µmol/mL at 1000 µg [13] Enhancing effect on burn wound repair at 100 mg ointment per mouse [14] |
| Smiglanin (3,5,7-trihydroxychromone-3-O- α-l-rhamnopyranoside) |
Smilax glabra roots [9] | No reported biological activity | |
| 5 | 5,7-Dihydroxy-4H-chromen-4-one- 3-O-β-d-glucopyranoside |
Eurya japonica stems [11] | Inhibitory effects on LPS-induced NO production with inhibition rate 53.79 ± 1.78% at 20 μg/mL [11] |
4.1.3. 5-O-Glycosides
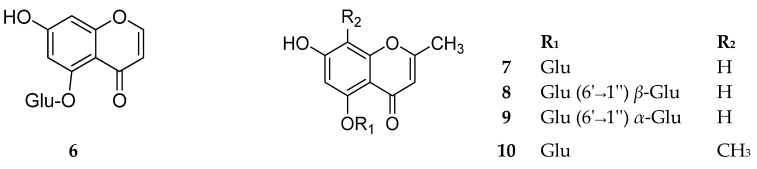
Among the naturally occurring 5-O-glycosides, Staphylosides A and B (8–9), isolated from Staphylea bumalda, are characterized by a presence of a disaccharide moiety attached to C-5. The disaccharide chain in 8 is β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside while in 9, is α-d-glucopyranosyl-(1→6)-β-d-glucopyranoside. Compounds 6–10 are shown in Figure 3. The sources and the reported biological activities (if any) are summarized in Table 3.
Figure 3.
Structures of compounds 6–10.
Table 3.
5-O-Chromone glycosides with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 6 | Matsudoside A (5-β-d-glucosyloxy-7- hydroxychromone) |
Salix matsudana leaves [15] | No reported biological activity |
| 7 | Schumaniofioside A (2-methyl-5,7-dihydroxychromone 5-O-β-d-glucopyranoside) |
Schumanniophyton magnificum root bark [16] Dysoxylum binectariferum fruits [17]. Acalypha fruticose aerial parts [18,19] Drynaria fortune rhizomes [13] |
Inhibition of proinflammatory cytokines TNF-α (39.51 ± 1.21%) and IL-6 (22.21 ± 0.58%) at 5 μM [17] Inhibition of NF-kB transcriptional activity and iNOS with IC50 value of 29.5 ± 6.5 µg/mL [19] |
| 8 | Staphyloside A | Staphylea bumalda leaves [20] | No reported biological activity |
| 9 | Staphyloside B | ||
| 10 | Isoeugenitol glucoside |
Gethyllis ciliaris underground parts [21] |
No reported biological activity |
4.1.4. 6-O-Glycosides
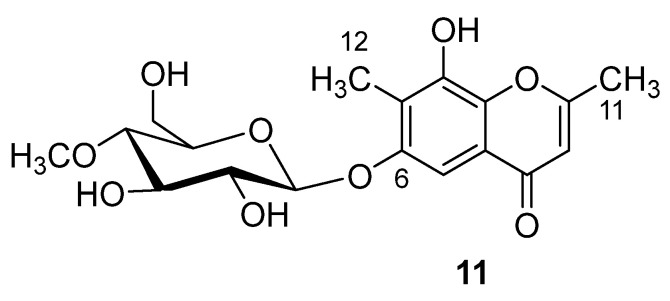
Compound 11 (Figure 4) has a unique structure for bearing 4-O-methylglucopyranosyl unit. Chemically, it is 6-O-(4-O-methyl-β-d-glucopyranosyl)-8-hydroxy-2,7-dimethyl-4H-benzopyran-4-one, isolated from the rice culture of the fungus Armillaria tabescens [1]. Although such compounds are not common in higher plants, several of them have previously been isolated from fungi [1].
Figure 4.
Structure of compound 11.
4.1.5. 7-O-Glycosides
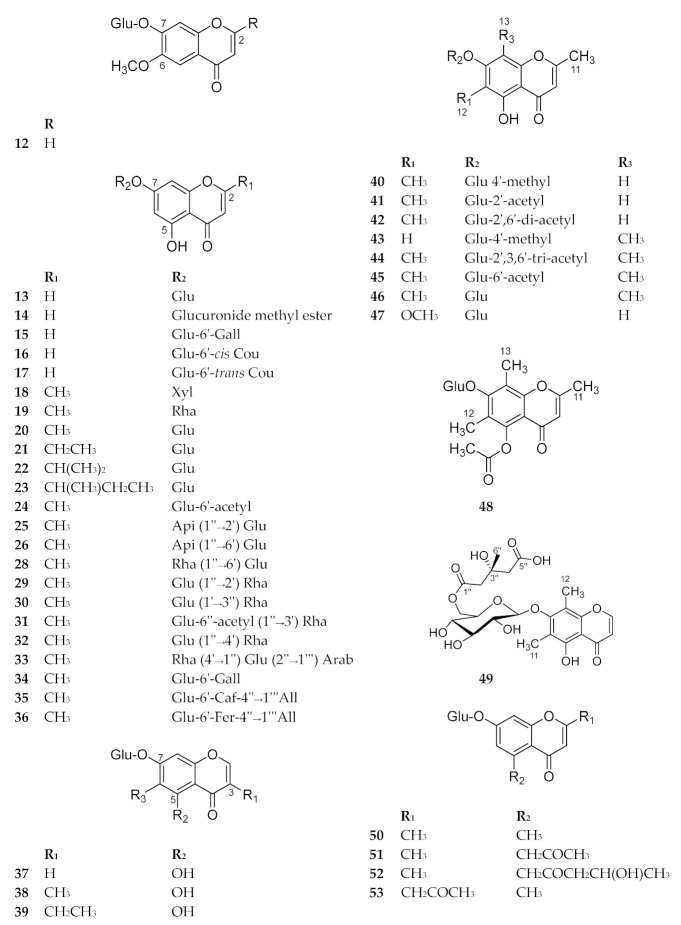
This subclass is characterized by the presence of sugar at C-7. Hyperimone A is the same as Urachromone A (22), reported at nearly the same time from different co-authors from the genus Hypericum. Takanechromone A (38) is the same as Hyperimone B, isolated from the same genus by different co-authors. They were reported each time as new compounds. We preferred to add only 13C-NMR data of one set of these compounds (Table 23). Several biological activities have been reported to some members of this subclass. Compounds 12–53 are shown in Figure 5. The sources and the reported biological activities (if any) are summarized in Table 4.
Figure 5.
Structures of compounds 12–53.
Table 4.
7-O-Chromone glycosides with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 12 | 7-O-β-d-glucopyranosyl-6-methoxychromone |
Salicornia europaea leaves and stems [25] |
No reported biological activity |
| 13 | 5,7-dihydroxychromone 7-O-β-d-glucopyranoside |
Ilex hainanensis leaves [26] Calluna vulgaris flowers [27] |
No reported biological activity |
| 14 | 5,7-dihydroxychromone 7-O-β-d-glucuronide methyl ester |
Davallia mariesii rhizomes [28] | No reported biological activity |
| 15 | 7-O-(6′-galloyl)-β-d-glucopyranosyl-5-hydroxychromone | Polygonum capitatum aerial parts [29] | No reported biological activity |
| 16 | 5-hydroxy-7-O-(6-O-p-cis-coumaroyl-β-d-glucopyranosyl)-chromone | Dasiphora parvifolia aerial parts [30] | No reported biological activity |
| 17 | 5-hydroxy-7-O-(6-O-p-trans-coumaroyl-β-d-glucopyranosyl)-chromone | Dasiphora parvifolia aerial parts [30] | No reported biological activity |
| 18 | Officinaliside A | Scindapsus officinalis stems [31] | No reported biological activity |
| 19 | 7-O-α-l-rhamnosyl-nereugenin | Scindapsus officinalis stems [31] | No reported biological activity |
| 20 | Undulatoside A (2-methyl-5,7-dihydroxychromone 7-O-β-d-glucopyranoside) |
Scindapsus officinalis stems [31] Ammi visnaga fruits [32] Knoxia corymbosa [33] Pancratium biflorum roots [34] Panacratium biflorum flowering bulbs [35] Pancratium maritimum L. fresh bulbs [36] Peucedanum austriacum [37] Tecomella undulata bark [38] Adina rubescens leaves [39] Dryopteris fragrans [40] Staphylea bumalda leaves [20] |
Immunomodulatory activity inhibited the proliferation of murine B lymphocytes in vitro at 10−5 M [33] Inhibition of nitric oxide production in lipopolysaccharide induced RAW 264.7 macrophages with an IC50 value of 49.8 μM [40] Weak antimigratory activity against human metastatic prostate cancer cells (PC-3M) at 50 µM [36] |
| 21 | Frachromone C (5-hydroxy-2-ethylchromone-7-O-β-d-glucopyranoside) |
Dryopteris fragrans whole plant [40] | Inhibition of nitric oxide production in lipopolysaccharide induced RAW 264.7 macrophages with an IC50 value of 45.8 μM [40] |
| 22 | Urachromone A (5-hydroxy-2-isopropylchromone-7-O-β-d-glucopyranoside) |
Hypericum henryi aerial parts [41] Hypericum erectum [42] |
No reported biological activity |
| Hyperimone A | |||
| 23 | Urachromone B | Hypericum henryi aerial parts [41] | No reported biological activity |
| 24 | Corymbosin K2
(7-O-β-d-6- acetylglucopyranosyl-5-hydroxy-2-methylchromone) |
Knoxia corymbosa [33] | Immunomodulatory activity inhibited the proliferation of murine B lymphocytes in vitro at 10−5 M [33] |
| 25 | Schumanniofioside B | Schumanniophyton magnificum root bark [16] | No reported biological activity |
| 26 | 5-hydroxy-2-methylchromone-7-O-β-d-apiofuranosyl-(1→6)- β-d-glucopyranoside |
Neonauclea sessilifolia roots [43] Scorodocarpus borneensis leaves [44] Staphylea bumalda leaves [20] |
No reported biological activity |
| 27 | Undulatoside B | Tecomella undulata [45] | No reported biological activity |
| 28 | 2-methyl-chromone-5,7-diol 7-O-α-l-rhamnopyranosyl-(1-6)-β-d-glucopyranoside |
Delphinium hybridum aerial parts [46] Drynaria fortunei rhizomes [22] |
No reported biological activity |
| 29 | Officinaliside C (7-O-[β-d-glucopyranosyl-(1-2)-α-l-rhamnopyranosyl]-5-hydroxy-2-methyl-4H-1-benzopyran-4-one) |
Scindapsus officinalis stems [31] | No reported biological activity |
| 30 | Drynachromoside C (5-hydroxy-2-methyl chromone-7-O-β-d-glucopyranosyl (1-3)-α-l-rhamnopyranoside) |
Drynaria fortunei rhizomes [22] | Inhibitory activity on triglyceride accumulation at 10 μM [22] |
| 31 | Officinaliside B (7-O-[6-acetyl-β-d-glucopyranosyl-(1-3)-α-l-rhamnopyranosyl]-5-hydroxy- 2-methyl-4H-1-benzopyran-4-one) |
Scindapsus officinalis stems [31] | Inhibition of NO production in LPS-stimulated RAW 264.7 cells with an IC50 value of 16.1 μM [31] |
| 32 | Drynachromoside A (5-hydroxy-2-methyl-4H-benzopyran-4-one-7-O-β-d-glucopyranosyl-(1-4)-α-l-rhamnopyranoside) |
Scindapsus officinalis stems [31] Drynaria fortunei rhizomes [47] |
Proliferative activity 10.1% on MC3T3-E1 (Mouse osteoblast) cells at 25 μg/mL [47] |
| 33 | Drynachromoside D (5-hydroxy-2-methyl chromone-7-O-α-l-arabinopyranosyl(1-2)-β-d-glucopyranosyl(1-4)-α-l-rhamnopyranoside) |
Drynaria fortunei rhizomes [22] | Inhibitory activity on triglyceride accumulation (inhibited PPARγ, C/EBPα and aP2 expression by 50%, 43% and 37% at 10 mM) [22] |
| 34 | Undulatoside A 6′-O-gallate | Myrtus communis leaves [48] | |
| 35 | Corymbosin K3 (7-O-[6-O-(4-O-trans-caffeoyl-β-d-allopyranosyl)]-β-d-glucopyranosyl-5-hydroxy-2- methylchromone) |
Knoxia corymbosa [33] | Immunomodulatory activity inhibited the proliferation of murine B lymphocytes in vitro at 10−5 M [33] |
| 36 | 7-O-[6-O-(4-O-trans-feruloyl-β-d-allopyranosyl)]- β-d-glucopyranosyl-5-hydroxy-2-methylchromone |
Knoxia corymbosa [33] | No reported biological activity |
| 37 | 5-hydroxy-6-methylchromone-7-O-β-d-glucopyranoside | Pseudotsuga sinensis [49] | No reported biological activity |
| 38 | Takanechromone A (5,7-dihydroxy-3-methylchromone- 7-O-β-d-glucopyranoside) |
Hypericum sikokumontanum aerial parts [50] Hypericum erectum [42] |
No reported biological activity |
| Hyperimone B | |||
| 39 | Takanechromone B (5,7-dihydroxy-3-ethylchromone- 7-O-β-d-glucopyranoside) |
Hypericum sikokumontanum aerial parts [50] |
No reported biological activity |
| 40 | 7-O-(4-O-Methyl-β-d-glucopyranosyl)eugenitol | The scale-insect pathogenic fungus Orbiocrella sp. [23] | No reported biological activity |
| 41 | Mollin | Lichens (Roccellaria mollis, Schismatomma accedens, Roccella galapagoensis) [51] | No reported biological activity |
| 42 | Roccellin | Lichens (Roccellaria mollis, Schismatomma accedens, Roccella galapagoensis) [51] | No reported biological activity |
| 43 | 7-O-(4-O-Methyl-β-d-glucopyranosyl)isoeugenitol | The scale-insect pathogenic fungus Orbiocrella sp. [23] | No reported biological activity |
| 44 | Lobodirin | Lobodirina cerebriformis lichen [51] | No reported biological activity |
| 45 | Galapagin | Lichens (Roccellaria mollis, Schismatomma accedens, Roccella galapagoensis) [51] | No reported biological activity |
| 46 | Uncinoside A (5-hydroxy-2,6,8- trimethylchromone 7-O-β-d-glucopyranoside) |
Selaginella uncinate Herb [24] | Antiviral activity against respiratory syncytial virus (RSV) with an IC50 value of 6.9 μg/mL, against parainfluenza type 3 virus (PIV 3) with an IC50 value of 13.8 μg/mL [24] |
| 47 | Pancrichromone | Pancratium maritimum L. fresh bulbs [36] | No reported biological activity |
| 48 | Uncinoside B (5-acetyoxyl-2,6,8-trimethylchromone 7-O-β -d-glucopyranoside) |
Selaginella uncinate herb [24] | Antiviral activity against respiratory syncytial virus (RSV) with an IC50 value of 1.3 μg/mL, against parainfluenza type 3 virus (PIV 3) with an IC50 value of 20.8 μg/mL [24] |
| 49 | Matteuinterin B | Matteuccia intermedia rhizomes [52] | |
| 50 | 2,5-dimethylchromone-7-O-β-d-glucopyranoside |
Rheum austral D. Don underground parts [53] Rumex gmelini Turcz. roots [54] |
Anti-oxidant activity (DPPH radical scavenging capacity with an IC50 value of 66.9 ± 1.3 μM) [53] |
| 51 | 5-acetonyl-7-β-d-glucopyranosyl-2-methylchromone | Cassia multijuga leaves [55,56] | No reported biological activity |
| 52 | 2-methyl-5-(2′-oxo-4′-hydroxyphenyl)-7-hydroxychromone 7-O-β-d-glucopyranoside | Chinese rhubarb (Rhei Rhizoma) [57] | No reported biological activity |
| 53 | Aloesone 7-O-β-d-glucopyranoside | Chinese rhubarb (Rhei Rhizoma) [57] | No reported biological activity |
Drynachromosides C (30) and D (33) exhibited inhibitory activity on triglyceride accumulation [22]. The effects of these compounds on mRNA expression of the three adipogenesis-related marker genes, PPARγ, C/EBPα and Ap2, in 3T3-L1 were investigated. The mRNA expression levels of PPARγ, C/EBPα and Ap2 were found to be dramatically downregulated. Compounds 40 and 43, having a unique sugar unit of 4-O-methyl-β-d-glucopyranose, were isolated from the scale-insect pathogenic fungus Orbiocrella sp. BCC 33248 [23]. Uncinosides A (46) and B (48) [24], isolated from the Chinese herbal medicine Selaginella uncinata, showed potent anti-RSV (respiratory syncytial virus) activity with IC50 values of 6.9 and 1.3 µg/mL, respectively. Uncinoside B (48) was found to have a TI value of 64.0, a large therapeutic index comparable to that of ribavirin with a TI value of 24.0, which is an approved drug for the treatment of RSV infection in humans. They also showed moderate antiviral activities against PIV 3 (parainfluenza type 3 virus) with IC50 values of 13.8 and 20.8 µg/mL and TI values of 6.0 and 4.0, respectively.
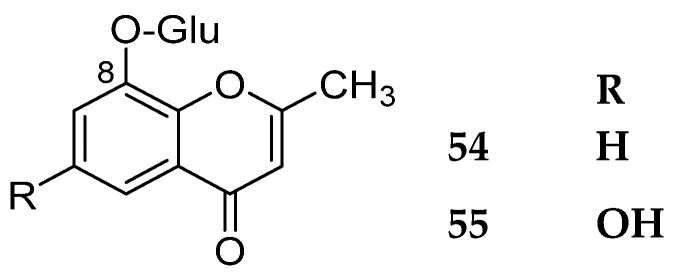
4.1.6. 8-O-Glycosides
Only two compounds 54–55 were reported in nature. They are shown in Figure 6. The sources and the reported biological activities (if any) are summarized in Table 5.
Figure 6.
Structures of compounds 54–55.
Table 5.
8-O-Chromone glycosides with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 54 | 8-O-β-d-Glucopyranosyl-2-methylchromone | Swertia punicea whole herb [58] | No reported biological activity |
| 55 | 8-O-β-d-Glucopyranosyl-6-hydroxy-2-methyl-4H-1-benzopyrane-4-one | Rhododendron collettianum aerial parts [59] | Inhibitory activity against tyrosinase enzyme with an IC50 value of 256.97 μM [59] |
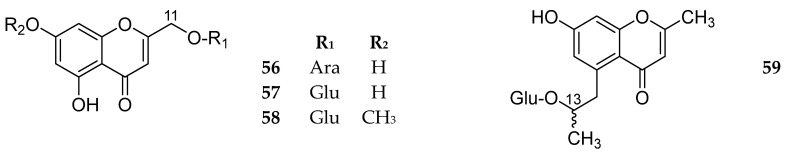
4.1.7. 11- and 13-O-Glycosides
Compound 57 was reported in 2012 as Monnieriside A [60] and was then reported as Drynachromoside B [22,31,47]. Compounds 56–59 are shown in Figure 7. The sources and the reported biological activities (if any) are summarized in Table 6.
Figure 7.
Structures of compounds 56–59.
Table 6.
11, 13-O-chromone glycosides with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 56 | Officinaliside D (2-hydroxymethyl-5,7-dihydroxy-4H-benzopyran-4- one-1′-O-α-l-arabinopyranoside) |
Scindapsus officinalis stems [31] | Inhibition of NO production in LPS-stimulated RAW 264.7 cells with an IC50 value of 19.1 µM [31] |
| 57 | Drynachromoside B |
Drynaria fortune rhizomes [22,47] Scindapsus officinalis stems [31] |
Mild inhibitory activity against MC3T3-E1 (mouse osteoblast) cells at 3.125 to 100 μg/ml [47] Triglyceride accumulation inhibitory effect at 0.1 to 10 μM [22] |
| Monnieriside A | Cnidium monnieri fruits [60] | No reported biological activity | |
| 58 | Saikochromoside A |
Bupleurum chinense [61] Cnidium monnieri fruits [60] |
No reported biological activity |
| 59 | 2-Methyl-5-propyl-7,12- dihydroxychromone-12-O-β-d-glucopyranoside |
Cassia siamea stem [62] | No reported biological activity |
4.1.8. Chromanone Glycosides
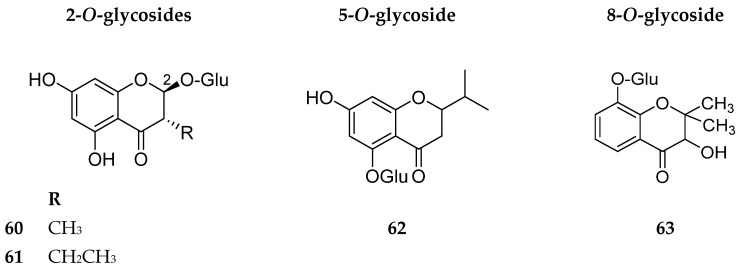
Chromanone glycosides or 2,3-dihydrochromone glycosides are not abundant in nature. Reviewing the literature, we encountered only four examples 60, 61, 62 and 63. Their structures are shown in Figure 8. The sources and biological activities (if any) of these compounds are summarized in Table 7.
Figure 8.
Structures of compounds 60–63.
Table 7.
Chromanone glycosides with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 60 | Takanechromanone A |
Hypericum sikokumontanum aerial parts [50] |
Anti-Helicobacter pylori at 100 µg/disc [50] |
| 61 | Takanechromanone B | ||
| 62 | 5-β-d-glucopyranosyloxy-7-hydroxy-2-isopropyl-chromanone | Humulus lupulus L. bracts [63] | No reported biological activity |
| 63 | Stemphylin (3-hydroxy-2, 2-dimethyl-5-α-d-glucopyranoside-2, 3-dihydrochromone) |
The liquid culture of the fungus Stemphylium botryosum [64] | Phytotoxic activity [64] |
4.2. Chromone C-Glycosides
In contrast to chromone O-glycosides, which are widely distributed and of common occurrence, C-glycoside derivatives are rarely found out.
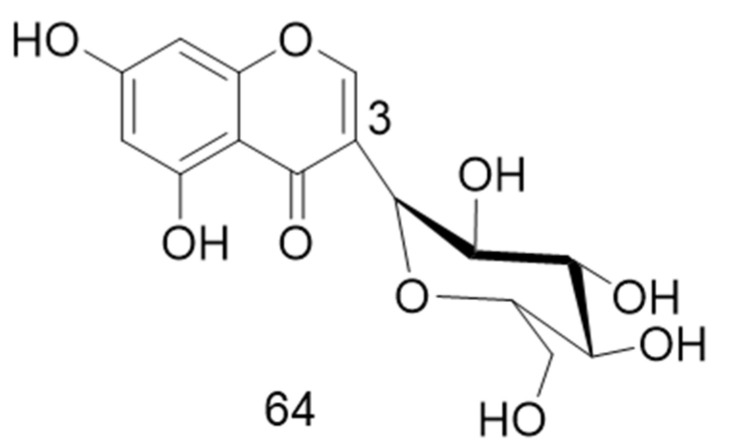
4.2.1. 3-C-Glycosides
This subclass includes the unusual 5,7-dihydroxychromone-3α-d-C-glucoside, named macrolobin, isolated from the aerial parts of Macrolobium latifolium [65]. Its structure is shown in Figure 9. Its source and biological activities are summarized in Table 8.
Figure 9.
Structure of compound 64.
Table 8.
3-C-Chromone glycoside with its source and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 64 | Macrolobin (5,7-dihydroxychromone- 3α-d-C-glucoside) |
Macrolobium latifolium aerial parts [65] | Inhibition of acetylcholinesterase enzyme with an IC50 value of 0.8 μM Antimicrobial activity against P. aeruginosa and Salmonella at 0.73 and 0.44 μM, respectively [65] |
4.2.2. 6-C-Glycosides
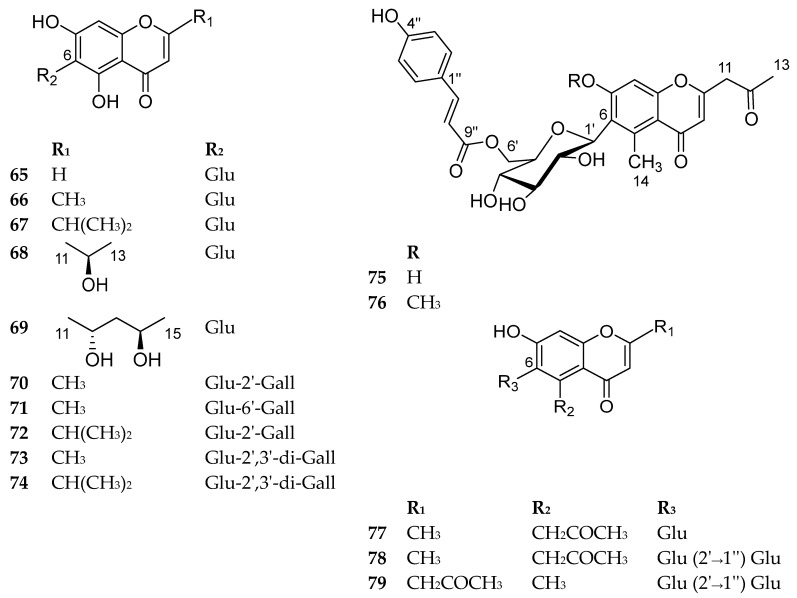
Compounds 65–79 are shown in Figure 10. The sources and the reported biological activities (if any) are summarized in Table 9.
Figure 10.
Structures of compounds 65–79.
Table 9.
6-C-Chromone glycosides with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 65 | 5,7-dihydroxy-6-C-glucosyl-chromone |
Aspalathus linearis fermented rooibos (red-brownish dry leaves) [66] |
No reported biological activity |
| 66 | Biflorin (6-β-C-glucopyranosyl-5,7-dihydroxy-2-methylchromone) |
Pancratium Biflorum roots [34] Syzygium aromaticum L. flower buds [67,68] Baeckea frutescens leaves [69,70] |
Inhibitory activity to phosphodiesterase and spared cyclic nucleotides at 10−9 M [34] Inhibition of LPS-induced production of nitric oxide (NO) and prostaglandin E2 (PGE2) in RAW 264.7 macrophages with IC50 values of 51.7 and 37.1 μM, respectively [67] |
| 67 | 6-β-C-glucopyranosyl- 5,7-dihydroxy-2-isopropylchromone |
Baeckea frutescens leaves [69,70] Kunzea ambigua leaves [71] |
Inhibitory activity 70.4% against EBV-EA (Epstein–Barr virus early antigen) activation induced by 12-O-tetradecanoylphorbol 13-acetate (TPA) at 500 mol ratio/TPA [71] |
| 68 | Obtusichromoneside C | Cassia obtusifolia seeds [72] | Weak inhibitory activity against human organic anion/cation transporters (OATs/OCTs) and organic anion transporting polypeptides (OATPs) at 50 μM [72] |
| 69 | Obtusichromoneside A | ||
| 70 | Kunzeachromone C |
Kunzea ambigua leaves [71] Baeckea frutescens leaves [70] |
Inhibition of copper-induced LDL oxidation with an IC50 value of 3.35 ± 0.36 μM [70] |
| 71 | 6-C-β-d-(6′-O-galloyl)glucosylnoreugenin |
Syzygium aromaticum flower buds [68,73] |
Cytotoxicity against human ovarian cancer cells (A2780) with an IC50 value of 66.78 ± 5.49 μM [68] Prolyl endopeptidase inhibitory effects with an IC50 value of 1.74 ± 0.03 μM [73] |
| 72 | 6-β-C-(2′-O-galloylglucopyranosyl)-5,7-dihydroxy- 2-isopropylchromone |
Baeckea frutescens leaves [69,70] Kunzea ambigua leaves [71] |
Inhibitory activity 68.4% against EBV-EA activation induced by TPA at 500 mol ratio/TPA [71] Inhibition of copper-induced LDL oxidation with an IC50 value of 3.90 ± 0.24 μM [70] |
| 73 | Kunzeachromone D | Kunzea ambigua leaves [71] | No reported biological activity |
| 74 | Kunzeachromone A | ||
| 75 | Aloeveraside B | Aloe vera resin [74,75,76] | Inhibition of urease enzyme (55% and 62%, respectively) at 1 mg/mL concentration, significant growth inhibition (70.5 and 76.4%) of the breast cancer cell line MDA-MB-231 at 100 μM, and antioxidant (80% and 60%) at 1 mg/mL [74] Anti-lipid peroxidation activity with IC50 values of 432.1 ± 0.6 and 469.5 ± 0.4 µmol/L, respectively [75] |
| 76 | Aloeveraside A | ||
| 77 | Acetonyl-6-glycosyl -7-hydroxy -2-methylchromone | Cassia multijuga leaves [55,56] | No reported biological activity |
| 78 | 5-acetonyl-7-hydroxy-6-C-glucopyranosyl-2-methyl chromone 2″-O-glucopyranoside |
Cassia spectablis seeds [77] | No reported biological activity |
| 79 | 2-acetonyl-5-methyl-7-hydroxy-6-C-glucopyranosyl chromone 2″-O-glucopyranoside |
Chrozophora prostrata roots [78] | No reported biological activity |
4.2.3. 8-C-Glycosides
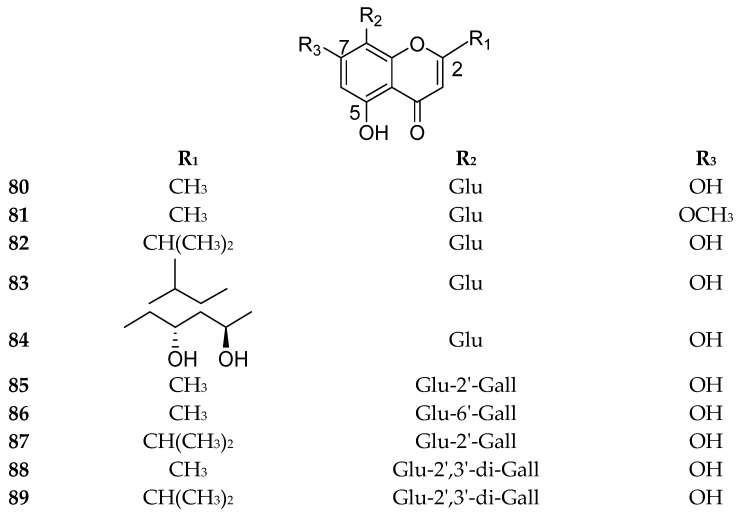
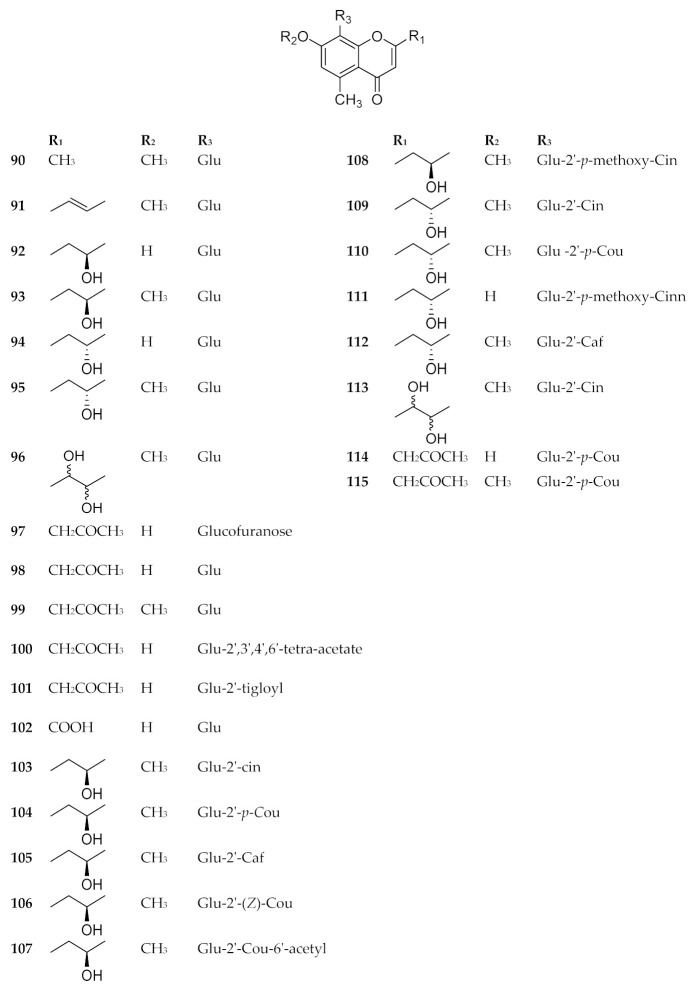
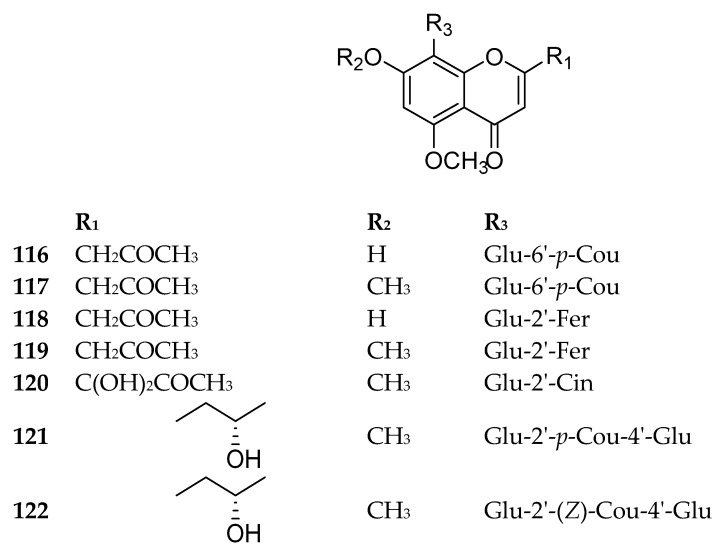
Many of the naturally occurring chromone-8-C-glycosieds can be found in genus Aloe. Approximately 26 chromone-8-C-glycosides were reported in the perennial plant Aloe vera, which is a well-known pharmaceutical herb used in traditional Chinese medicine [76]. Some significant bioactive chromone-8-C-glycosides were isolated and identified in Aloe vera, including Aloesin (98), aloeresin E (109), isoaloeresin D (110), aloeresin A (114) and other derivatives. For instance, aloeresin A (114) exhibited a promising therapeutic activity toward α-glucosidase enzyme [79], while the compound isobiflorin (80), isolated from the flower buds of Syzygium aromaticum, had the capacity to inhibit LPS-induced production of nitric oxide (NO) and prostaglandin E2 (PGE2) in RAW 264.7 macrophages [67]. A chromone-8-C-glycoside, 5,7-dihydroxy-2-isopropylchromone-8-β-d-glucoside, reported in Hypericum japonicum, showed an activity against Epstein–Barr virus [71]. Additionally, BACE1 (β-secretase), which is a possible potential target in the treatment of Alzheimer’s disease, was inhibited by some compounds as aloesin (98) [80], 7-O-methyl-aloeresin A (115) [81] and 2′-feruloyl-7-O-methylaloesin (119) [80]. Furthermore, tyrosinase, which is the key enzyme for controlling the production of melanin, was inhibited by aloeresin E (109) and isoaloeresin D (110) [82]. The compounds 80–122 are shown in Figure 11, Figure 12 and Figure 13. The sources and the reported biological activities (if any) are summarized in Table 10.
Figure 11.
Structures of compounds 80–89.
Figure 12.
Structures of compounds 90–115.
Figure 13.
Structures of compounds 116–122.
Table 10.
8-C-Chromone glycosides with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 80 | Isobiflorin |
Abrus mollis Hance. aerial parts [83] Syzygium aromaticum L. flower buds [67] Kunzea ambigua (SM.) Druce. leaves [71] Eucalyptus globulus leaves [84,85] Eugenia caryophyllata flower buds [86] |
Inhibition of LPS-induced production of nitric oxide (NO) with an IC50 > 60 μM and prostaglandin E2 (PGE2) ) with an IC50 value of 46.0 μM [67] |
| 81 | 7-methoxy-isobiflorin | Zhuyeqing Liquor; a famous traditional Chinese functional health liquor [87] |
No reported biological activity |
| 82 | 5,7-Dihydroxy-2-isopropylchromone- 8-β-d-glucoside |
Hypericum japonicum aerial parts [71,88,89] Eucalyptus maidenii bark [84] |
Inhibit Epstein–Barr virus early antigen induced by 12-O-tetradecanoylphorbol 13-acetate (TPA) in Raji cells (70.4%) at 500 mol ratio/TPA [71] |
| 83 | 5,7-Dihydroxy-2-(1-methylpropyl) chromone-8-β-d-glucoside |
Hypericum japonicum aerial parts [71,88] Eucalyptus grandis, Eucalyptus urograndi, and Eucalyptus maidenii bark [90] |
No reported biological activity |
| 84 | Obtusichromoneside B | Cassia obtusifolia seeds [72] | Inhibitory activity against human organic anion/cation transporters (OATs/OCTs) and organic anion transporting polypeptides (OATPs) at 50 μM [72] |
| 85 | Kunzeachromone E [8-β-C-(2′-galloylglucopyranosyl)- 5,7-dihydroxy-2-methylchromone] |
Kunzea ambigua leaves [71] Baeckea frutescens leaves [70] |
Inhibition activity toward copper-induced LDL oxidation with IC50 value of 3.98 ± 0.24 µM [70] |
| 86 | 8-C-β-d-(6′-O-galloyl)glucosylnoreugenin [2-Methyl-5,7-dihydroxy-chromone-8-β-d-(6′-O-galloyl)-glucopyranoside] |
Syzygium aromaticum L. leaves [91] Syzygium aromaticum L. flower buds [68,73] |
Cytotoxicity against human ovarian cancer cells (A2780) with an IC50 value of 87.50 ± 1.56 µM [68] Significant inhibition capacity against Prolyl Endopeptidase with IC50 value of 1.48 ± 0.02 µM [73] |
| 87 | 8-β-C-(2′-galloylglucopyranosyl)-5,7-dihydroxy-2-isopropylchromone | Baeckea frutescens leaves [70] | Active against copper-induced LDL oxidation with an IC50 value of 3.91 ± 0.18 µM [70] |
| 88 | Kunzeachromone F [2-Methyl-5,7-dihydroxy-chromone-8-β-d-(2’,3′-di-O-galloyl)-glucopyranoside] |
Kunzea ambigua leaves [71] | No reported biological activity |
| 89 | Kunzeachromone B [2-Isopropyl-5,7-dihydroxy-chromone-8-β-d-(2’,3′-di-O-galloyl)-glucopyranoside] |
Kunzea ambigua leaves [71] | No reported biological activity |
| 90 | 2,5-dimethyl-8-C-β-d-glucopyranosyl-7-hydroxy-chromone | Aloe vera [92] | No reported biological activity |
| 91 | 2-(E)-propenyl-7- methoxy-8-C-β-d-glucopyranosyl-5-methylchromone |
Aloe vera [76,80] | BACE1 (β-secretase) inhibitory activity with an IC50 value of 20.5 µM [80] |
| 92 | 8-C-β-d-glucosyl-(R)-aloesol | Aloe vera [76,80] | BACE1 (β-secretase) inhibitory activity (39.2%) at 100 μM [80] |
| 93 | 8-C-β-d-glucosyl-7-O-methyl-(R)-aloesol |
Aloe vera [76,80] and anerobic incubation of aloesin with bacterial mixture [93] |
BACE1 (β-secretase) inhibitory activity (26.8%) at 100 μM [80] |
| 94 | 8-C-β-d-glucosyl-(S)-aloesol |
Aloe vera [76] and anerobic incubation of aloesin with bacterial mixture [93] |
No reported biological activity |
| 95 | 8-C-β-d-glucosyl-7-O-methyl-(S)-aloesol |
Aloe vera [76] and anerobic incubation of aloesin with bacterial mixture [93] |
No reported biological activity |
| 96 | 8-C-β-d-glucosyl-7-O-methylaloediol | Aloe vera [76,80] | No reported biological activity |
| 97 | Neoaloesin A |
Aloe vera [76] Aloe barbadensis leaves [94] |
No reported biological activity |
| 98 | Aloesin |
Aloe vera [76,80] Aloe barbadensis leaves [95] |
Antioxidant activity (50 ± 1 μM trolox equivalent) at 100 mg of soluble solid/L solution [95] BACE1 inhibitory activity (37.5%) at 100 μM [80] Suppresses hyperpigmentation (40%) at 100 mg⁄g polyethylene glycol [2] |
| 99 | 7-O-methylaloesin | Aloe rupestris leaves exudate [96] | No reported biological activity |
| 100 | Aloesin-2″,3″,4″,6″-tetra-O-acetate | Anerobic incubation of aloesin with bacterial mixture [93] |
No reported biological activity |
| 101 | 2″-O-tigloylaloesin | Aloe cremnophila leaves exudate [97] | No reported biological activity |
| 102 | 8-C-β-d-glucopyranosyl-7-hydroxy-5-methylchromone-2-carboxylic acid | Herbal tea “muti” [98] | No reported biological activity |
| 103 | 8-[C-β-d-[2-O-(E)-cinnamoyl]glucopyranosyl]-2-[(R)-2-hydroxypropyl]-7-methoxy-5-methylchromone |
Aloe vera [76] Aloe barbadensis leaves [99] |
Topical anti-inflammatory activity at 200 µg/ear [99] |
| 104 | Aloeresin D | Aloe vera [76] | No reported biological activity |
| 105 | Rabaichromone | Aloe vera [76] | No reported biological activity |
| 106 | Allo-aloeresin D | Aloe vera [76] | No reported biological activity |
| 107 | Aloeresin K |
Aloe vera [76] Aloe barbadensis leaf skin [100] |
No reported biological activity |
| 108 | Aloeresin J |
Aloe vera [76] Aloe barbadensis leaf skin [100] |
No reported biological activity |
| 109 | Aloeresin E | Aloe vera leaves [76,82] | Inhibition of tyrosinase enzyme (40% and 80% at 50 and 100 ppm, respectively) [82] |
| 110 | Isoaloeresin D | Aloe vera leaves [76,82] | Inhibition of tyrosinase enzyme (20% and 40% at 50 and 100 ppm, respectively) [82] Antiviral activity against Pepper mild mottle virus; PMMoV (37.5 ± 6.5% at 1.5 mg/mL) [81] |
| 111 | 2′-O-[p-methoxy-(E)-cinnamoyl]-(S)-aloesinol | Aloe nobilis leaves [12] | BACE1 inhibitory activity (34.1%) at 100 μM [80] |
| 112 | Iso-rabaichromone | Aloe vera [76,82] | No reported biological activity |
| 113 | 8-C-glucosyl-(2′-O-cinnamoyl)-7-O-methylaloediol B | Aloe vera leaves [76] | No reported biological activity |
| 114 | Aloeresin A | Aloe vera [76] | Antioxidant activity [101] α-glucosidase inhibitory activities, with IC50 values of 11.94 and 2.16 mM against rat intestinal sucrase and maltase [79] |
| 115 | 7-O-methyl-aloeresin A | Aloe vera [76] | Tyrosinase inhibitory activity with an IC50 value of 9.8 μM [81] |
| 116 | 6′-O-coumaroyl-aloesin | Aloe vera [76] | Anti-lipid peroxidation activity with an IC50 value of 476.4 ± 0.9 µM [75] |
| 117 | 7-Methoxy-6′-O-coumaroyl-aloesin | Aloe vera [76] | Weak anticancer activity against breast cancer cell line, MDA-MB-231 (induce 30% decline in cell survival at 25 μM ) [102] |
| 118 | 2′-Feruloylaloesin | Aloe nobilis leaves [80] | Inhibition activity against β-secretase (36.4%) at 100 μM [80] Inhibition effect against mushroom tyrosinase (27 ± 0.57%) at 0.4 μM [103] |
| 119 | 2′-Feruloyl-7-O-methylaloesin | Aloe nobilis leaves [80] | Inhibition activity against BACE1 (β-secretase) (48.7%) at 100 μM [80] |
| 120 | 9-Dihydroxyl-2′-O-(Z)-cinnamoyl-7-methoxy-aloesin | Aloe vera [76] | Inhibition of tyrosinase enzyme (9.5 ± 9.0%) at 100 μM Antiviral against Pepper mild mottle virus; PMMoV (31.5 ± 4.2% inhibition at 1.5 mg/mL) [81] |
| 121 | 4′-O-β-d-glucosyl-isoaloeresin DI | Aloe vera [76] | No reported biological activity |
| 122 | 4′-O-β-d-glucosyl-isoaloeresin DII | Aloe vera [76] | No reported biological activity |
4.3. Phenyl and Isoprenyl Chromone Glycosides
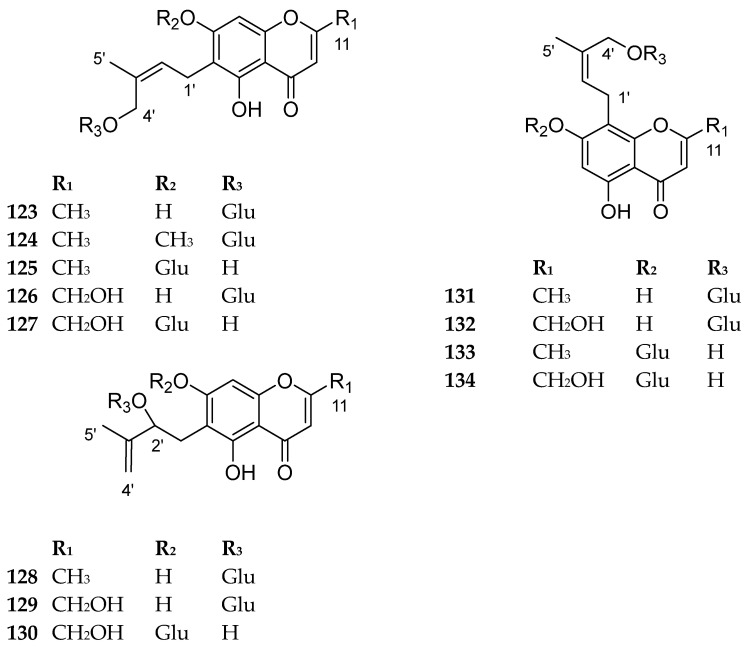
This category is characterized by a hydroxyl prenyl moiety at C-6 or C-8, or a hydroxyl isoprenyl moiety at C-6 only. The sugar moiety can be either situated at C-7 hydroxyl of the chromone nucleus or C-4’ of the hydroxyl prenyl or C-2’ of the hydroxyl isoprenyl moiety. Most of the compounds in this category were reported from the genus Cnidium, belonging to family Apiaceae. The reported biological activity associated with several compounds in this category is their significant inhibition of fat accumulation in differentiated adipocytes employing 3T3-L1 preadipocyte cells as an assay system [60]. The compounds 123–134 are shown in Figure 14. The sources and the reported biological activities (if any) are summarized in Table 11.
Figure 14.
Structures of compounds 123–134.
Table 11.
Prenyl and isoprenyl chromone glycosides with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 123 | Cnidimoside A |
Cnidium Juponicum whole plant [104,105] Cnidium monnieri fruits [60,106] |
Significant inhibition of fat accumulation at 300 μM in differentiated adipocytes [60] Antitumor and antimetastatic actions at 0.1–100 μM (in vitro) and 20, 50 mg/kg, twice daily (in vivo) [105] |
| 124 | Cnidimoside B |
Cnidium Juponicum whole plant [104] Cnidium monnieri fruits [60] |
Significant ihibition of fat accumulation at 300 μM in differentiated adipocytes [60] |
| 125 | 2-methyl-5-hydroxy-6-(2- butenyl-3-hydroxymethyl)-7-(β-d-glucopyranosyloxy)-4H- 1-benzopyran-4-one |
Cnidium monnieri fruits [60] Angelica archangelica [107] Archangelica litoralis [107] |
No reported biological activity |
| 126 | Hydroxycnidimoside A | Cnidium monnieri fruits [60,106] | Significant inhibition of fat accumulation at 300 μM in differentiated adipocytes [60] |
| 127 | Monnieriside B | Cnidium monnieri fruits [60,106] | |
| 128 | Monnieriside C | Cnidium monnieri fruits [60] | No reported biological activity |
| 129 | Monnieriside D | ||
| 130 | Monnieriside E | ||
| 131 | 7,8-Secoeranthin-β-d-glucopyranoside (8-{(2E)-4-[β-d-glucopyranosyl)oxy]-3-methylbut-2-enyl}-5,7- dihydroxy-2-methyl-4H-l-benzopyran-4-one) |
Eranthis hyemalis tubers [108] | No reported biological activity |
| 132 | 2-C-Hydroxy-7,8-seroeranthipn-β-d-glucopyranoside (8-{(2E)-4-[β-d-glucopyranosyl)oxy]-3-methylbut-2-enyl}-5,7- dihydroxy-2-(hydroxymethyl)-4H-1-benzopyran-4-on2) |
||
| 133 | 7-[(β-d-glucopyranosyl)oxy]-5-hydroxy-8-[(2E)-4-hydroxy-3-methylbut-2-enyl]-2-methyl-4H-1-benzopyran-4-one |
Eranthis cilicica tubers [109] |
No reported biological activity |
| 134 | 7-[(β-d-glucopyranosyl)oxy]-5-hydroxy-2-hydroxymethyl-8- [(2E)-4-hydroxy-3-methylbut-2-enyl]-4H-1-benzopyran-4-one |
4.4. Phenyl Ethyl Chromone Glycosides
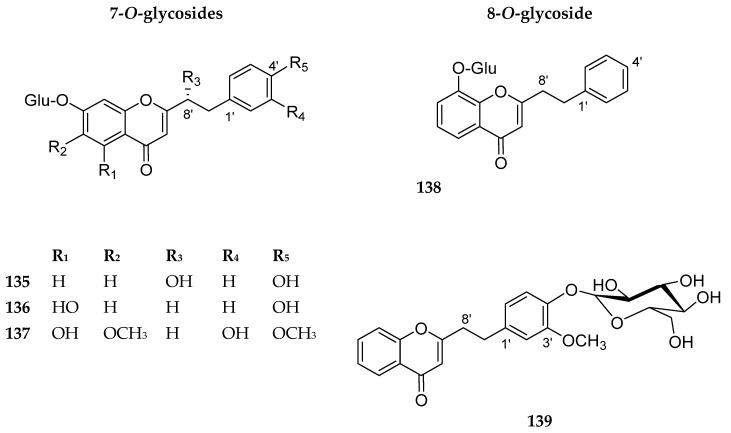
Reviewing the literature, we encountered five phenyl ethyl chromone glycosides. The phenyl ethyl moiety is usually located at C-2 of the chromone nucleus. The sugar moiety is attached to C-7 of the chromone skeleton in compounds 135–137, while in compound 138, the sugar is attached to C-8. In compound 139, the sugar is not attached directly to the basic chromone skeleton. Compounds 135–139 are shown in Figure 15. Their sources are summarized in Table 12. There are no reported biological activities of these compounds.
Figure 15.
Structures of compounds 135–139.
Table 12.
Phenyl ethyl chromone glycosides with their sources.
| No. | Compound | Source |
|---|---|---|
| 135 | Ononin glucoside | Ononis vaginalis whole plant [110] |
| 136 | 7-Glucosyloxy-5-hydroxy-2-[2-(4-hydroxyphenyl)ethyl]chromone | Cucumis melo seeds [111] |
| 137 | Aquilarinoside C (6,4′-dimethoxy-3′-hydroxy-2- (2-phenylethyl)chromone 7-O-β-d-glucopyranoside) |
Aquilaria sinensis stems [112] |
| 138 | 2-(2-phenylethyl) chromone-8-O-β-d-glucopyranoside | Imperata cylindrical rhizomes [113] |
| 139 | 2-[2-(4-glucosyloxy-3-methoxyphenyl)ethyl]chromone | Aquilaria sinensis resinous heartwood [114] |
4.5. Chromone Glycosides with Additional Heterocyclic Moieties
This category of chromone glycosides is further classified based on the additional heterocyclic moiety into furano-chromone glycosides, pyrano-chromone glycosides, oxepino-chromone glycosides and pyrido-chromone glycosides.
4.5.1. Furano-Chromone Glycosides
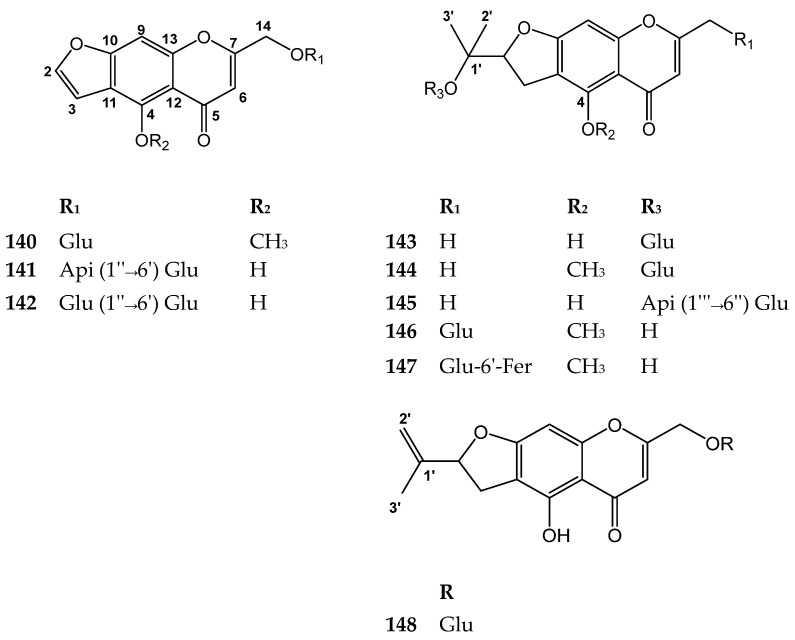
This subclass of compounds is characterized by presence of an additional furan, or a tetrahydrofuran ring fused with the benzo-δ-pyrone. Khellol glucoside (140), isolated from Ammi visnaga, is one of the important members in this subclass. It possess potent coronary vasodilator and bronchodilator activities [115]. It was reported to have a significant hypocholesterolemic effect. It lowered low-density lipoprotein cholesterol (LDL-C) by 73%, high-density lipoprotein cholesterol (HDL-C) by 23%, and total-C by 44%, after a single oral dose of 20 mg/kg per day after two weeks [116]. Compounds 140–148 are shown in Figure 16. The sources and the reported biological activities (if any) are summarized in Table 13.
Figure 16.
Structures of compounds 140–148.
Table 13.
Furano-chromone glycosides with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 140 | Khellol glucoside (Khellinin; Khelloside) |
Ammi visnaga fruits [117] Eranthhis hyernalis tubers [108] |
Potent coronary vasodilator and bronchodilator [115] Hypocholesterolemic effect at 20 mg/kg per day [116] |
| 141 | Norkhelloside |
Cimicifuga heracleifolia rhizomes [118] |
No reported biological activity |
| 142 | 7-[(O-β-d-glucopyranosyl-(1→6)- β-d-glucopyranosyl)oxy]methyl-4-hydroxy-5H-furo[3,2-g][1]benzopyran-5-one |
Eranthis cilicica tubers [109] | No reported biological activity |
| 143 | 4′-O-β-d-glucopyranosylvisamminol (Monnieriside G) |
Cnidium monnieri fruits [60] Saposhnikovia divaricata roots [119] |
Antitumor activity against SK-OV-3 with an IC50 value of 93.91 μM [120] |
| 144 | 4′-O-β-d-glucopyranosyl-5-O-methylvisamminol |
Ledebouriella seseloides roots and rhizomes [121] Saposhnikovia divaricata roots [119,122] Diplolophium buchananii aerial parts [123] Sphallerocarpus gracilis roots [124] |
Analgesic, antipyretic, anti-inflammatory, and anti-platelet aggregation activities [125,126] Antitumor activity with against H-460 cell line with an IC50 value of 86.91 μM [120] |
| 145 | (2’S)-4′-O-β-d-apiofuranosyl- (1→6)-β-d-glucopyranosylvisamminol |
Saposhnikovia divaricata roots [119] | Antitumor activities against PC-3 and SK-OV-3 cell lines with IC50 values of 48.5, 81.91 μM, respectively [120] |
| 146 | prim-O-glucosylcimifugin |
Ammi visnaga fruits [127] Angelica genuflexa roots [128] Eranthis hyernalis tubers [108] Angelica japonica roots [129] Cimicifuga foetida rhizomes [130] Diplolophium buchananii aerial parts [123] Saposhnikovia divaricata roots [131] |
Analgesic, antipyretic, anti-inflammatory, anti-platelet aggregation and antitumor activities [125,126,131] |
| 147 | Cimifugin-4′-O-[6″-feruloyl]-β-d-glucopyranoside | Cimicifuga foetida rhizomes [132] | No reported biological activity |
| 148 | Monnieriside F | Cnidium monnieri fruits [60] | No reported biological activity |
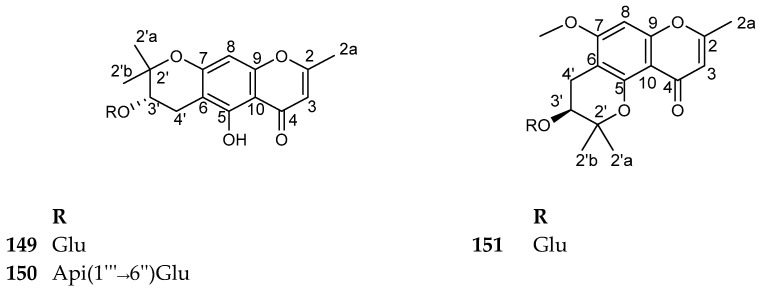
4.5.2. Pyrano-Chromone Glycosides
This subclass of compounds is characterized by the presence of an additional pyran ring fused with the benzo-δ-pyrone. Only three compounds were reported from nature until now. Of them, 3′-O-glucopyranosylhamaudol (Sec-O-glucopyranosylhamaudol) (149) and (3’S)-3′-O-β-d-apiofuranosyl-(1→6)-β-d-glucopyranosylhamaudol (150), isolated from the Saposhnikovia divaricata, showed weak anti-cancer activity. Both compounds were screened against three cancer cell lines, namely human prostatic cancer cell (PC-3), human ovarian carcinoma cell (SK-OV-3), and human lung cancer cell (H460) using the conventional MTT assay. Compound 149 showed a weak activity against H460, with an IC50 value of 94.25 ± 1.45 µM while compound 150, showed an activity against SK-OV-3 with an IC50 value of 86.21 ± 1.03 µM [119]. Compounds 149–151 are shown in Figure 17. The sources and the reported biological activities (if any) are summarized in Table 14.
Figure 17.
Structures of compounds 149–151.
Table 14.
Pyrano-chromone glycosides with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 149 | 3′-O-glucopyranosylhamaudol (Sec-O-glucopyranosylhamaudol) |
Angelica genuflexa roots [128] Angelica japonica roots [129] Glehnia littoralis roots [133] Peucedanum japonicum roots [134] Saposhnikovia divaricata roots [119,122] |
Antitumor activity against H-460 cell line with an IC50 value of 94.25 ± 1.45 μM [119] |
| 150 | (3’S)-3′-O-β-d-apiofuranosyl-(1→6)- β-d-glucopyranosylhamaudol |
Saposhnikovia divaricata roots [119] | Antitumor activity against SK-OV-3 with an IC50 value of 86.21 ± 1.03 μM [119] |
| 151 | (2’S)-2′-hydroxy-7-O-methylallopeucenin 2′-O-β-d-glucopyranoside |
Diplolophium buchananii aerial parts [123] |
No reported biological activity |
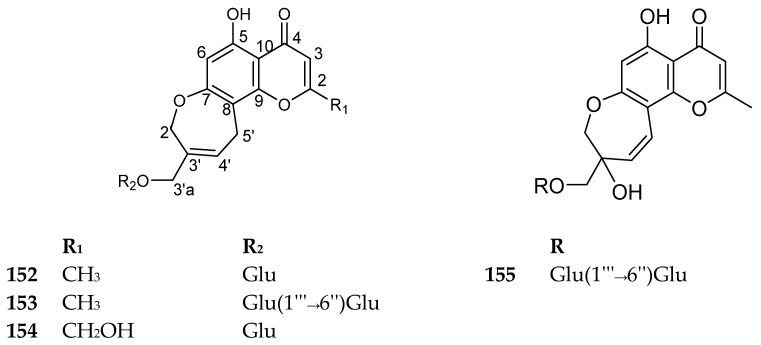
4.5.3. Oxepino-Chromone Glycosides
This subclass of compounds is characterized by the presence of an additional oxepin fused with the benzo-δ-pyrone. Only four compounds were reported from nature until now, and all of them were reported from Eranthis species. The compounds 152–155 are shown in Figure 18. The sources are summarized in Table 15. There are no reported biological activities for these compounds.
Figure 18.
Structures of compounds 152–155.
Table 15.
Oxepino-chromone glycosides with their sources.
| No. | Compound | Source |
|---|---|---|
| 152 | Eranthin β-d-glucopyranoside | Eranthis hyemalis tubers [108] |
| 153 | Eranthin β-d-gentiobioside |
Eranthis cilicica tubers [109] Eranthis hyemalis tubers [108] |
| 154 | 2-C-Hydroxyeranthin β-d-glucopyranoside | Eranthis hyemalis tubers [108] |
| 155 | 9-[(O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl)oxy]methyl-8,11-dihydro-5,9-dihydroxy-2-methyl-4Hpyrano[2,3-g][1]benzoxepin-4-one | Eranthis cilicica tubers [109] |
4.5.4. Pyrido-Chromone Glycosides
This subclass includes only the chromone alkaloidal glycoside; Schumanniofoside. This compound was found to reduce the lethal effect of black cobra (Naja melanoleuca) venom in mice [135]. The authors proved that this effect is greatest when the venom is mixed and incubated with the extract or schumanniofoside. They concluded that the mode of action is by oxidative inactivation of the venom. Schumanniophyton magnificum is used extensively in African ethno-medicine for the treatment of various diseases and, most commonly, the treatment of snake bites [135]. Its structure is shown in Figure 19. Its source and biological activity are summarized in Table 16.
Figure 19.
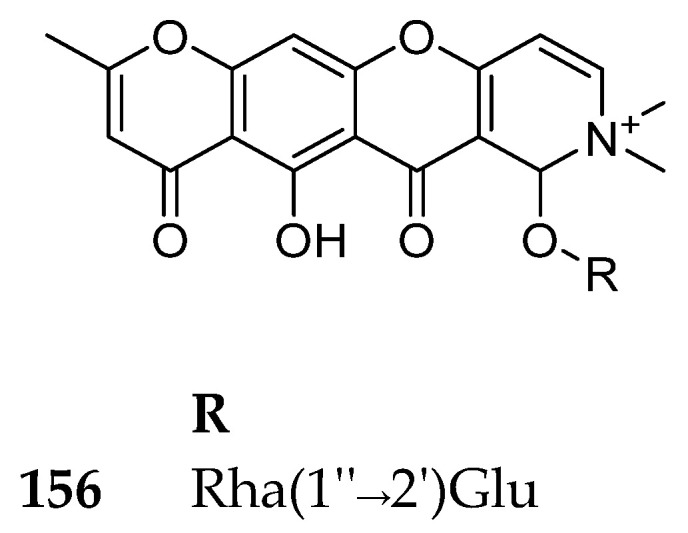
Structure of compound 156.
Table 16.
Pyrido-chromone glycosides with its source and biological activity.
4.6. Hybrids of Chromones with Other Classes of Secondary Metabolites
This is an interesting category, as the chromone skeleton is conjugated to another high molecular weight compound, as shown in the following subclasses.
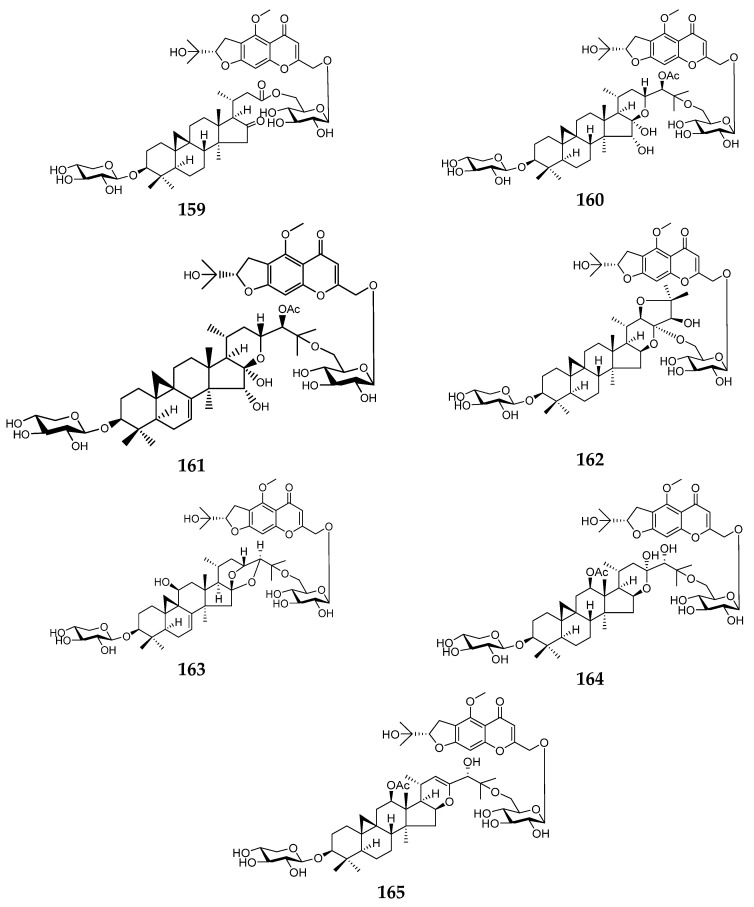
4.6.1. Hybrids of Furano-Chromones with Cycloartane Triterpenes
This subclass of compounds is a hybrid of cycloartane triterpene and chromone. The reported compounds were isolated from the rhizomes of Cimicifuga foetida. The compounds 157–165 are shown in Figure 20. The sources and the reported biological activities (if any) are summarized in Table 17.
Figure 20.
Structures of compounds 157–165.
Table 17.
Hybrids of furanochromones with cycloartane triterpenes with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 157 | Cimitriteromone A | Cimicifuga foetida rhizomes [130] | No reported biological activity |
| 158 | Cimitriteromone B | Cimicifuga foetida rhizomes [130] | Anti-proliferative activity with an IC50 value of 15.73 ± 0.59 μM [130] |
| 159 | Cimitriteromone C | Cimicifuga foetida rhizomes [130] | No reported biological activity |
| 160 | Cimitriteromone D | Cimicifuga foetida rhizomes [130] | Anti-proliferative activity with an IC50 value of 24.21 ± 0.61 μM [130] |
| 161 | Cimitriteromone E | Cimicifuga foetida rhizomes [130] | No reported biological activity |
| 162 | Cimitriteromone F | Cimicifuga foetida rhizomes [130] | No reported biological activity |
| 163 | Cimitriteromone G | Cimicifuga foetida rhizomes [130] | No reported biological activity |
| 164 | Cimitriteromone H | Cimicifuga foetida rhizomes [136] | No reported biological activity |
| 165 | Cimitriteromone I | Cimicifuga foetida rhizomes [136] | Anti-proliferative activity with an IC50 value of 27.14 ± 1.38 μM [136] |
4.6.2. Hybrids of Chromones with Secoiridoids
There are only two compounds (Figure 21) belonging to this class, sessilifoside (166) and 7″-O-β-d-glucopyranosylsessilifoside (167). Both compounds were isolated from the roots of Neonauclea sessilifolia roots [41]. The authors did not report biological activities for these compounds.
Figure 21.
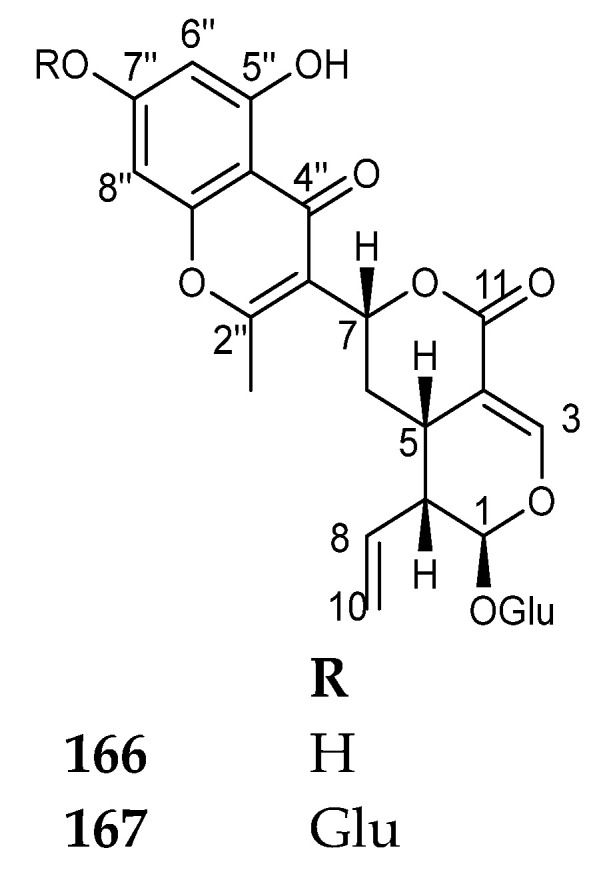
Structures of compounds 166–167.
4.6.3. Chromone Alkaloids Aminoglycosides
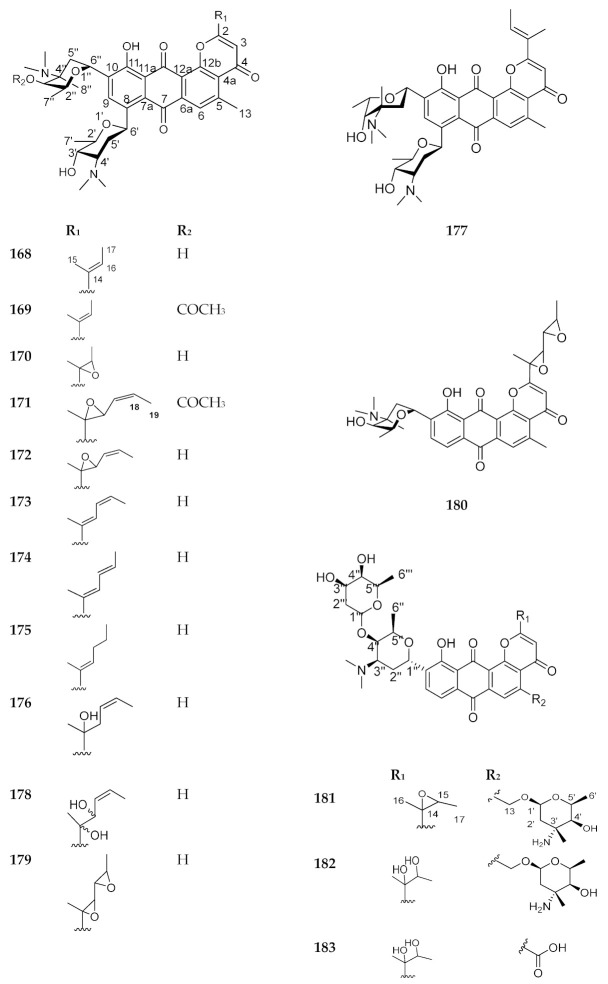
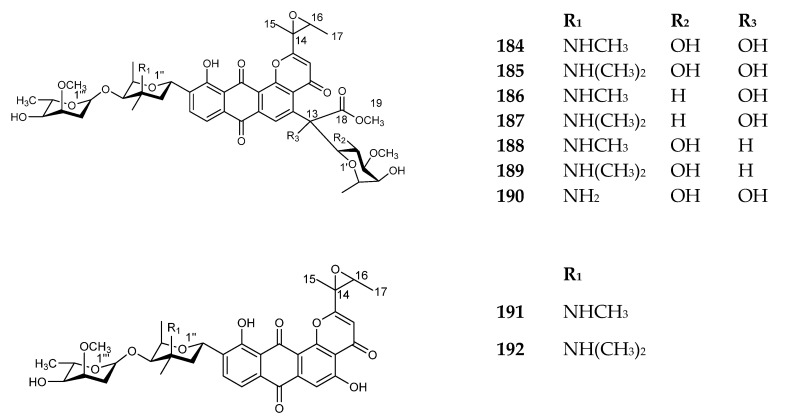
This category includes compounds 168–192. Compounds 168–180 were reported from a strain of Streptomyces, isolated from a soil sample. These compounds showed antimicrobial activity against Gram-positive bacteria, as well as a potent antitumor activity. Conversely, compounds 181–183 were isolated from Saccharothrix species, while compounds 184–192 were reported from Actinomycete and exhibited antitumor and antimicrobial activities [137]. Compounds 168–192 are shown in Figure 22 and Figure 23. The sources and the reported biological activities (if any) are summarized in Table 18.
Figure 22.
Structures of compounds 168–183.
Figure 23.
Structures of compounds 184–192.
Table 18.
Chromone alkaloids aminoglycosides with their sources and biological activities.
| No. | Compound | Source | Biological Activity |
|---|---|---|---|
| 168 | Kidamycin (rubiflavin B) |
Streptomyces phaeoverticillatus var. takatsukiensis [138] |
Antibiotic with MIC (minimum inhibitory concentration) ranges from 0.19–1.56 μg/mL and potent antitumor activity [138] |
| 169 | Neopluramycin | Streptomyces pluricolorescens [139,140] | Antibiotics and potent antitumor activity [140] |
| 170 | 14, 16-Epoxykidamycin | Streptomyces pluricolorescens [141] | Antibiotic against Gram-positive bacteria with MIC ranges from 0.5 to 10 μg/mL and antitumor activity [141] |
| 171 | Pluramycin A | Streptomyces pluricolorescens [139,140] | Antibiotic and antitumor activity [140] |
| 172 | Rubiflavin A | Streptomyces species [142] | Antibiotic and antitumor activity [142] |
| 173 | Rubiflavin C-1 | ||
| 174 | Rubiflavin C-2 | ||
| 175 | Rubiflavin D | ||
| 176 | Rubiflavin E | ||
| 177 | Rubiflavin F (isokidamycin) | ||
| 178 | PD 121,222 | Streptomyces species [143] | Antibiotic and potent antitumor activity [143] |
| 179 | Hedamycin | Streptomyces griseoruber [144,145] | Antibiotic and potent antitumor activity against HeLa cells [144] |
| 180 | Ankinomycin (deangolosaminylhedamycin) | Streptomyces species [146] | Antibiotic against Gram-positive bacteria with MICs ranges from 0.39–1.56 μg/mL and potent antitumor activity [146] |
| 181 | Pluraflavin A | Saccharothrix species [147] | Antibiotic and potent antitumor activity [147] |
| 182 | Pluraflavin B | ||
| 183 | Pluraflavin E | ||
| 184 | Altromycin A | Actinomycete, AB 1246E-26 [148,149] | Antibiotic against Gram-positive bacteria and potent antitumor activity [137] |
| 185 | Altromycin B | ||
| 186 | Altromycin C | ||
| 187 | Altromycin D | ||
| 188 | Altromycin E | Actinomycete, AB 1246E-26 [137] | |
| 189 | Altromycin F | ||
| 190 | Altromycin G | ||
| 191 | Altromycin H | ||
| 192 | Altromycin I |
5. Spectroscopic Features
5.1. UV Features
Most of the published work on chromones show several strong bands in the range of 200–320 nm [150,151]. In contrast to chromone, the pyrone ring of 4-chromanone contains no double bond. The ultraviolet absorption spectra of chromones and chromanones are summarized in Table 19 [150].
Table 19.
UV Band maxima of chromones and chromanone in 3-methylpentane at 25 °C.
| Chromones | Chromanones | |
|---|---|---|
| Band system | λmax | λmax |
| A | 360, 352, 345, 337, 324 | 363, 347 322(sh), 312 252, 246 219(sh), 213 |
| B | 301, 290, 283 | |
| C | 225, 246, 239, 227, 223, 216, 202 | |
The UV spectrum of chromones in alcohol shows two strong bands at λmax 245 and 299 nm [152,153,154]. Some data reported three bands at λmax 245, 303 and 297 nm [150]. 2-methyl-5,7-dihydroxy chromone shows bands at λmax 250, 255, 295 and 325 nm, meanwhile 2-methyl-5-hydroxy-7-O-glycosyl chromone shows bands at λmax 248, 255 and 290 nm [154]. The presence of an electron attracting group at C-2 resulted in a bathochromic shift in all bands [151]. The information gained from applying spectral shift reagents with flavonoids can be also applied to chromones. In the case of AlCl3, a bathochromic shift of 20–70 nm, which is non-reversible with acids, indicates a free hydroxyl group at position 5. Meanwhile, a bathochromic shift with NaOAc can be diagnostic for the presence of a free 7-hydroxyl group [154,155].
5.2. IR Features
Carbonyl region: The IR carbonyl stretching frequency for a chromone is observed at 1640~1660 cm−1, which is slightly higher than that of δ-pyrone (1650 cm−l) but is much lower than that of coumarins (1720–1740 cm−l) [25,153]. Despite that the OH group attached to C-5 of the chromone nucleus chelates strongly with the CO group, this intramolecular H-bonding has only a slight bathochromic effect on the CO stretching frequency [156]. All 5-hydroxychromones possess three significant maxima in the 1580–1700 cm−1 region. The two higher frequencies are intense at 1660 and 1630 cm−1, with a constant wavenumber separation of 34 ( ± 5) cm−1 in both carbon tetrachloride and chloroform.
Hydroxyl region: The IR hydroxyl stretching vibration for a chromone was observed at 2500–3650 cm−1. A strong chelation in 5-hydroxychromones does not produce a considerable bathochromic shifts in both the OH and CO stretching bands [156]. Chelated 5-hydroxychromones produce no absorption maxima in the 3300–3600 cm−1 region, but a weak absorption envelope extends from 2400 to 3300 cm−1. The entire envelope is associated with various stretching modes of the chelated 5-OH group [156]. For 7-hydroxychromones, a steric buttressing effect is observed when the 7-OH group is flanked by a bulky substituent in the ortho-position (6 or 8). The free OH band appears as a doublet centered at 3615 cm−1, the separation of the components being ~26 cm−1. When a prenyl moiety is located in the ortho-position to the 7-OH group, an intramolecular OH interaction occurs, resulting in two OH stretching frequencies. When a 7-OH group is flanked by an OMe group, intense intramolecularly bonded OH stretching frequencies are found at ~3513 and 3517 cm−1, respectively [156]. The 2-hydroxymethyl group exhibits a free stretching frequency at ≈3615 cm−1. At concentrations higher than 0.15 M, a broad-bonded OH frequency at 3400 cm−1 occurs due to intermolecular H-bonding, and it consequently disappears on dilution [156].
5.3. 1H-NMR Features
In the following text, we try to give insight about the most characteristic 1H-NMR features of the benzo-δ-pyrone skeleton (Figure 24) and its glycosides. The δ-pyrone ring has two olefinic protons assigned as H-2 and H-3. In 2, 3 unsubstituted chromones, for example compound 12, the 1H-NMR spectrum shows two ortho-coupled doublets (J = 6.0 Hz), located downfield at δH 8.19 (H-2) and upfield at δH 6.26 (H-3) [25]. For 2-alkyl and 2-O-glycosyl chromones (compounds 21 and 1, respectively), they are characterized by an upfield singlet proton (H-3) at δH 6.11 and 5.98, respectively [7,40]. Meanwhile, 3-alkyl and 3-O-glycosyl chromones (compounds 39 and 2, respectively) are characterized by a downfield singlet proton (H-2) at δH 7.93 and 8.07, respectively [10,50].
Figure 24.
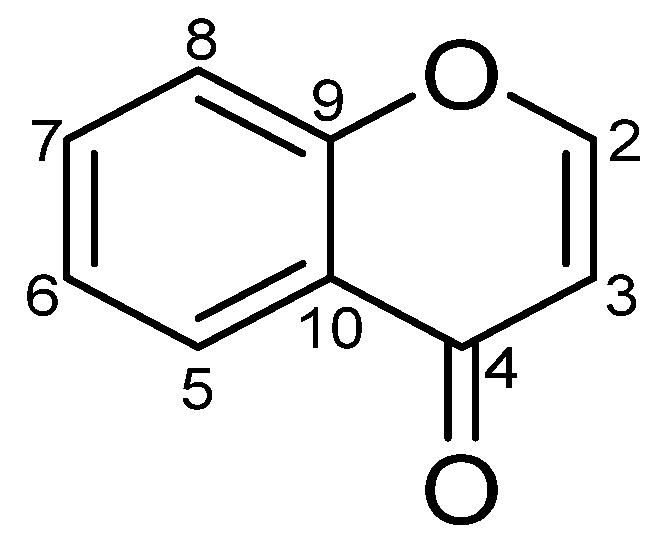
Basic skeleton of Chromone.
Chromanone glycosides or 2,3-dihydrochromone glycosides are characterized by an oxygenated proton (H-2) at δH 4.12 and 5.44 as in compounds 62 [63] and 60 [50], respectively. The splitting pattern of H-2 can be either d, dd or ddd, depending on the number of neighboring protons. A small coupling constant between H-2 and H-3 (J= 2.8 Hz) can determine that they are located in the equatorial–equatorial position [50]. Further information on the detailed configuration can be clarified from observing NOESY correlations. Unsubstituted chromanones at C-3, as in 62, show two geminal protons at δH 2.50 and 2.70. Their splitting pattern shows geminal (J3a-3b = 16.2 Hz) and vicinal (Jax-eq = 2.7 Hz or Jax-ax = 12.6 Hz ) couplings [63]. In 3-alkyl substituted chromanones, as in 60 and 61, H-3 is also detected at δH 2.79 [50].
Naturally occurring chromones often bear a hydroxyl or methoxy group at C-5 and/or C-7 and a methyl group at C-2 and/or C-5 [153]. The C-5 methyl is usually observed in 6-C and 8-C glycosides. In aprotic solvents such as DMSO-d6, the chelated 5-OH is detected as a singlet at δH 12.57; meanwhile, the 7-OH is detected at δH 10.00, as in compound 56 [31]. The C-2 methyl in Schumaniofioside A 7 can be detected at δH 2.33 (3H, s) [17]. Meanwhile, those located at C-5, can be detected more downfield at δH 2.64 (3H, s) as in 79 [78].
For the phenyl part of the benzo-δ-pyrone skeleton, the protons show chemical shift and coupling constant values similar to those observed for protons in substituted benzenes.
Sugar moiety: Xylosyl, arabinosyl and glucosyl chromones show an anomeric proton signal at δH ~4.73 (d, J = 7–7.6 Hz) [10,11,12]. The former moieties can be differentiated by the number of the oxygenated protons at the δH 3-5 region, in addition to the difference in 13C-NMR values. Rhamnosyl chromones show a distinct signal at δH ~1.25 (3H, d, J = 6.0 Hz) corresponding to CH3-6’ of α-l-rhamnose [8]. The most abundant chromone of C-glycosides is the 8-C-glycoside form, followed by 6-C-glycosides. However, we encountered a unique 3-C-glycoside named macrolobin 64 [65]. The anomeric proton in macrolobin is detected at δH 5.32 (d, J = 1.5 Hz), the small coupling constant being indicative of the α-anomer [65]. Biflorin 66 and isobiflorin 80, as representative for 6-C and 8-C-glycosides, respectively, show the anomeric proton signal at δH 4.55 (d, J = 9.8 Hz), and 4.63 (d, J = 9.8 Hz), respectively [69]. The former coupling constant value is higher than that observed in case of O-glycosides (J = 7–7.6 Hz) [11,12]. In 5-O, 7-O, 6-C, furano-, pyrano-, oxepino-chromone glycosides, the sugar moiety can be further substituted with another sugar, as in 8–9, 25–32, 78–79, 141–142, 150 and 153, respectively. In 6-C, 8-C and, to a lesser extent, 7-O-glycosides, the sugar moiety can be mono- or disubstituted with a phenolic acid moiety, commonly at C-2’ or C-6’ or C-2’ and C-3’. The most commonly occurring phenolic moiety is gallic acid, but other phenyl propanoids such as cinnamoyl, coumaroyl, feruloyl and coniferoyl moieties also exist. The galloyl moiety is characterized by a singlet aromatic signal integrated for two protons at δH 6.75 [27]. The cinnamoyl moiety is confirmed by two trans-coupled olefinic protons at δH 7.43 (d, J = 15.8 Hz) and 6.25 (d, J = 15.8 Hz), in addition to the aromatic signals of the benzene ring [30]. The presence of coumaroyl substitution is characterized by AA’ BB’ system for two pairs of ortho-coupled aromatic protons at δH 7.31 (2H, d, J = 8.6 Hz) and δH 6.74 (2H, d, J = 8.6 Hz), a trans-olefinic proton signals at δH 7.35 (1H, d, J = 16.1 Hz) and δH 6.03 (1H, d, J = 15.9 Hz) [29].
Prenyl and Isoprenyl chromone glycosides: In the case of 7-O-glycoside 127, hydroxyl prenyl moiety can be easily characterized by the allylic methylene protons at δH 3.53 (2H, m, H-1’), an olefinic proton at δH 5.39 (t, J = 6.4, H-2’), oxygenated methylene protons δH 4.20 and 4.46 (1H each, d, J = 12.0 Hz, H-4’), and an olefinic methyl at δH 1.75 (3H, d, J = 1.2, H-5″) [60]. Its isomeric 4′-O-glycoside 126 showed similar signals; however, the hydroxyl methylene protons (H-4’) were slightly downfield at δH 4.34 and 4.65 due to O-glycosidation [106]. Meanwhile, the hydroxyl isoprenyl group in the 7-O-glycoside 130 is characterized by the methylene protons at δH 2.96 (2H, m, H-1’), an oxygenated methine proton at δH 4.35 (H-2’, m), exomethylene protons at δH 4.74 and 4.91 (H-4’) and an olefinic methyl at δH 1.87 (3H, s, H-5’). Its isomeric 2′-O-glycoside 129 showed similar signals, but the oxygenated methine proton (H-2’) is shifted downfield at δH 4.74 due to O-glycosidation [60].
Phenyl ethyl chromone glycosides: The presence of the phenyl ethyl moiety in compound 136 can be detected by the methylene proton signals at δH 2.75 (2H each, dd, J = 14.8, 6.4 Hz, H-7’) and 3.19 (2H each, J = 14.8, 3.1 Hz, H-8’), in addition to the aromatic protons of the phenyl ring. The 8′-hydroxy phenyl ethyl moiety in 135 shows an oxygenated proton signal δH 5.85 (dd, J = 3.5, 5.9 Hz, H-8’) [110].
5.4. 13C-NMR Features
For better understanding of the differences in chemical shifts related to the substituents on the chromone moiety, we preferred to add the 13C-NMR data in Table 20, Table 21, Table 22, Table 23, Table 24, Table 25, Table 26, Table 27, Table 28, Table 29, Table 30, Table 31, Table 32, Table 33, Table 34, Table 35, Table 36, Table 37, Table 38, Table 39 and Table 40. For the numbering of the skeleton, the following figure (Figure 25) gives few examples for the numbering system of the skeleton with multiple substituents. Briefly, the basic chromone nucleus was assigned numbers 1–10. In the case of a substitution at C-2, numbers 11, 12…etc. were given to the substituents, followed by substitution at C-3 and so on. Sugar moiety, and substituents attached to it, were assigned numbers 1’, 2’, … and then 1″, 2″, … etc. For better understanding, the following figure shows representative examples for the numbering system. Some complicated structures have their own numbering system, shown on them within the review.
Table 20.
The 13C-NMR spectral data of compounds 1–14 except those which have no reported 13C-NMR data.
| C | 1 | 2 | 3 | 4 | 5 | 7 | 11 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 161.2 | 147.4 | 147.7 | 147.8 | 147.4 | 167.2 | 167.8 | 158.6 | 159.5 |
| 3 | 93.1 | 141.2 | 141.0 | 138.1 | 141.5 | 111.7 | 109.1 | 112.0 | 112.8 |
| 4 | 166.6 | 178.4 | 178.5 | 177.0 | 178.4 | 180.3 | 183.2 | 183.6 | 184.4 |
| 5 | 137.1 | 163.4 | 163.3 | 161.5 | 163.4 | 160.2 | 93.9 | 163.4 | 163.9 |
| 6 | 132.0 | 100.1 | 100.2 | 98.8 | 100.2 | 104.6 | 162.0 | 101.3 | 101.9 |
| 7 | 127.8 | 166.4 | 166.3 | 164.3 | 166.5 | 164.7 | 110.2 | 164.9 | 165.2 |
| 8 | 114.9 | 95.0 | 95.0 | 93.8 | 95.0 | 99.1 | 159.6 | 96.2 | 96.9 |
| 9 | 154.3 | 159.3 | 159.3 | 157.2 | 159.4 | 161.1 | 156.6 | 159.5 | 160.2 |
| 10 | 114.5 | 106.2 | 106.2 | 104.8 | 106.1 | 109.2 | 106.1 | 108.4 | 109.3 |
| 11 | 23.2 | - | - | - | - | 19.9 | 20.3 | - | - |
| 12 | - | - | - | - | - | - | 8.2 | - | - |
| 1′ | 100.1 | 104.6 | 104.4 | 100.6 | 104.2 | 105.0 | 101.7 | 101.6 | 102.1 |
| 2′ | 73.2 | 74.6 | 72.0 | 69.9 | 74.8 | 74.6 | 75.1 | 74.7 | 75.1 |
| 3′ | 77.5 | 77.1 | 73.6 | 70.2 | 77.4 | 77.3 | 78.4 | 77.9 | 77.8 |
| 4′ | 69.6 | 70.8 | 69.2 | 71.5 | 71.3 | 71.3 | 80.5 | 71.2 | 73.9 |
| 5′ | 76.7 | 67.1 | 67.1 | 69.7 | 78.5 | 78.7 | 78.1 | 78.4 | 77.5 |
| 6′ | 60.7 | - | - | 17.7 | 62.6 | 62.5 | 60.9 | 62.4 | 171.5 |
| OCH3 | - | - | - | - | - | - | - | - | 53.8 |
| Solvent | DMSO-d6 | CD3OD | CD3OD | DMSO-d6 | CD3OD | CD3OD | C5D5N | CD3OD | CD3OD |
| References | [7] | [10] | [12] | [9] | [11] | [17] | [1] | [27] | [28] |
Table 21.
The 13C-NMR spectral data of compounds 15–23 except which has no reported 13C-NMR data.
| C | 15 | 16 | 17 | 18 | 20 | 21 | 22 | 23 |
|---|---|---|---|---|---|---|---|---|
| 2 | 158.1 | 158.4 | 158.5 | 168.9 | 161.6 | 174.3 | 175.5 | 174.6 |
| 3 | 110.8 | 111.9 | 112.0 | 108.8 | 108.8 | 107.8 | 106.2 | 106.6 |
| 4 | 181.8 | 183.6 | 183.6 | 182.5 | 182.5 | 184.3 | 183.3 | 183.1 |
| 5 | 161.3 | 163.1 | 163.2 | 161.2 | 157.9 | 163.0 | 162.2 | 162.7 |
| 6 | 99.8 | 101.2 | 101.2 | 99.8 | 100.3 | 101.0 | 100.6 | 100.7 |
| 7 | 162.9 | 164.6 | 164.8 | 163.1 | 168.9 | 164.8 | 164.2 | 164.2 |
| 8 | 94.6 | 96.2 | 96.2 | 94.9 | 95.0 | 95.9 | 95.3 | 95.3 |
| 9 | 157.7 | 159.4 | 159.4 | 157.9 | 163.4 | 159.5 | 158.4 | 158.4 |
| 10 | 106.8 | 108.5 | 108.5 | 105.5 | 105.5 | 107.0 | 106.5 | 107.4 |
| 11 | - | - | - | 20.5 | 20.5 | 28.3 | 33.3 | 40.4 |
| 12 | - | - | - | - | - | 11.2 | 19.9 | 27.6 |
| 13 | - | - | - | - | - | - | 19.9 | 17.7 |
| 14 | - | - | - | - | - | - | - | 11.7 |
| 1′ | 99.7 | 101.5 | 101.5 | 100.6 | 99.9 | 101.6 | 101.7 | 101.7 |
| 2′ | 73.1 | 74.7 | 74.7 | 73.3 | 73.5 | 74.7 | 74.8 | 74.8 |
| 3′ | 76.2 | 77.9 | 77.9 | 76.6 | 77.6 | 77.8 | 78.5 | 78.5 |
| 4′ | 69.7 | 71.9 | 72.0 | 69.6 | 70.7 | 71.2 | 71.2 | 71.1 |
| 5′ | 74.1 | 75.8 | 76.0 | 66.2 | 76.8 | 78.4 | 79.3 | 79.2 |
| 6′ | 63.4 | 64.2 | 64.7 | - | 61.1 | 62.4 | 62.4 | 62.3 |
| 1″ | 119.5 | 127.4 | 127.3 | - | - | - | - | - |
| 2″ | 108.8 | 133.7 | 131.2 | - | - | - | - | - |
| 3″ | 145.6 | 115.8 | 117.0 | - | - | - | - | - |
| 4″ | 138.6 | 160.0 | 161.4 | - | - | - | - | - |
| 5″ | 145.6 | 115.8 | 117.0 | - | - | - | - | - |
| 6″ | 108.8 | 133.7 | 131.2 | - | - | - | - | - |
| 7″ | 165.9 | 145.1 | 146.9 | - | - | - | - | - |
| 8″ | - | 116.0 | 115.1 | - | - | - | - | - |
| 9″ | - | 168.1 | 168.9 | - | - | - | - | - |
| Solvent | DMSO-d6 | CD3OD | CD3OD | DMSO-d6 | DMSO-d6 | CD3OD | C5D5N | C5D5N |
| References | [29] | [30] | [30] | [31] | [157] | [40] | [41] | [41] |
Table 22.
The 13C-NMR spectral data of compounds 24–32 except which has no reported 13C-NMR data.
| C | 24 | 25 | 26 | 28 | 29 | 30 | 31 | 32 |
|---|---|---|---|---|---|---|---|---|
| 2 | 168.7 | 157.3 | 167.9 | 168.2 | 168.9 | 169.2 | 168.9 | 168.5 |
| 3 | 108.7 | 108.3 | 108.8 | 108.8 | 108.8 | 109.3 | 108.8 | 108.4 |
| 4 | 182.4 | 181.9 | 182.7 | 182.8 | 182.5 | 184.2 | 182.5 | 182.1 |
| 5 | 161.5 | 161.1 | 162.4 | 162.4 | 161.7 | 163.1 | 161.7 | 161.3 |
| 6 | 99.9 * | 108.7 | 100.6 | 100.7 | 100.2 | 100.8 | 100.0 | 99.6 |
| 7 | 162.9 | 162.6 | 163.9 | 163.9 | 161.9 | 163.4 | 161.9 | 161.6 |
| 8 | 94.9 | 108.2 | 95.3 | 95.1 | 95.2 | 95.7 | 95.1 | 94.7 |
| 9 | 157.7 | 168.2 | 158.3 | 158.3 | 157.9 | 159.5 | 157.9 | 157.5 |
| 10 | 105.5 | 105.0 | 106.3 | 106.3 | 105.7 | 106.8 | 105.6 | 105.2 |
| 11 | 20.3 | 19.9 | 19.9 | 20.1 | 20.5 | 20.4 | 20.5 | 20.1 |
| 1′ | 99.8 * | 99.3 | 101.9 | 102.0 | 98.8 | 99.6 | 98.5 | 98.1 |
| 2′ | 73.3 | 77.0 | 74.6 | 74.6 | 81.2 | 71.0 | 69.8 | 69.5 |
| 3′ | 76.5 | 75.8 | 78.5 | 78.5 | 70.4 | 82.5 | 82.1 | 70.3 |
| 4′ | 70.2 | 68.9 | 71.5 | 71.6 | 70.9 | 72.4 | 70.7 | 81.4 |
| 5′ | 74.1 | 76.0 | 77.4 | 77.5 | 69.4 | 70.9 | 68.7 | 68.5 |
| 6′ | 63.7 | 60.5 | 69.0 | 68.0 | 18.3 | 18.1 | 18.0 | 17.9 |
| 1″ | - | 108.7 | 111.2 | 102.7 | 105.0 | 105.9 | 104.8 | 104.4 |
| 2″ | - | 76.7 | 77.9 | 72.0 | 74.5 | 75.4 | 74.1 | 74.5 |
| 3″ | - | 79.2 | 80.3 | 72.8 | 77.2 | 77.8 | 76.8 | 77.1 |
| 4″ | - | 73.9 | 75.1 | 74.1 | 70.0 | 71.1 | 70.6 | 70.1 |
| 5″ | - | 64.1 | 65.8 | 69.8 | 76.7 | 77.7 | 74.8 | 76.7 |
| 6″ | - | - | - | 18.6 | 61.5 | 62.2 | 64.1 | 61.2 |
| CH3CO | 20.9, 170.5 | - | - | - | - | - | 21.1, 170.6 | - |
| Solvent | DMSO-d6 | CDCl3, DMSO-d6 | C5D5N | C5D5N | DMSO-d6 | DMSO-d6 | DMSO-d6 | DMSO-d6 |
| References | [33] | [16] | [44] | [46] | [31] | [22] | [31] | [47] |
* Data interchangeable.
Table 23.
The 13C-NMR spectral data of compounds 33–41.
| C | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 168.3 | 168.2 | 168.3 | 168.3 | 157.8 | 154.8 | 154.7 | 168.6 | 168.4 |
| 3 | 108.2 | 108.7 | 108.3 | 108.2 | 110.6 | 120.3 | 125.8 | 108.7 | 108.4 |
| 4 | 181.4 | 182.7 | 181.9 | 181.9 | 181.5 | 183.8 | 183.5 | 182.5 | 182.3 |
| 5 | 161.1 | 162.3 | 161.1 | 161.2 | 157.7 | 163.0 | 163.1 | 158.4 | 185.1 |
| 6 | 99.5 | 100.6 | 99.8 | 99.5 | 108.9 | 100.9 | 100.8 | 109.1 | 108.5 |
| 7 | 161.4 | 163.7 | 162.6 | 162.6 | 160.9 | 164.6 | 164.6 | 161.1 | 155.56 |
| 8 | 94.5 | 94.6 | 94.3 | 94.5 | 93.3 | 95.8 | 95.8 | 93.3 | 97.7 * |
| 9 | 157.3 | 158.3 | 157.4 | 157.4 | 155.4 | 159.4 | 159.5 | 155.9 | 160.3 |
| 10 | 105.0 | 106.3 | 105.1 | 105.1 | 106.0 | 107.4 | 107.6 | 105.1 | 105.0 |
| 11 | 19.9 | 19.9 | 19.8 | 19.8 | 7.4 | 10.2 | 19.3 | 20.5 | 19.9 |
| 12 | - | - | - | - | - | - | 13.4 | 7.9 | 6.9 |
| 1′ | 98.0 | 101.6 | 99.5 | 98.1 | 100.1 | 101.6 | 101.6 | 100.2 | 93.1 * |
| 2′ | 69.5 | 74.4 | 72.9 | 73.0 | 73.1 | 74.7 | 74.7 | 73.8 | 77.3 |
| 3′ | 69.8 | 78.1 | 76.1 | 76.2 | 76.4 | 77.8 | 77.8 | 76.5 | 74.5 |
| 4′ | 80.9 | 71.3 | 69.9 | 69.9 | 69.6 | 71.2 | 71.2 | 79.4 | 69.7 |
| 5′ | 68.4 | 75.8 | 73.9 | 73.9 | 77.1 | 78.4 | 78.4 | 76.1 | 73.3 |
| 6′ | 17.9 | 64.5 | 63.4 | 63.4 | 60.6 | 62.4 | 62.4 | 60.7 | 60.5 |
| 1″ | 102.3 | 121.0 | 128.5 | 127.9 | - | - | - | - | - |
| 2″ | 81.4 | 110.4 | 114.8 | 114.9 | - | - | - | - | - |
| 3″ | 76.6 | 147.4 | 146.9 | 148.9 | - | - | - | - | - |
| 4″ | 69.8 | 140.8 | 147.7 | 149.3 | - | - | - | - | - |
| 5″ | 77.6 | 147.4 | 120.7 | 122.5 | - | - | - | - | - |
| 6″ | 61.0 | 110.4 | 115.9 | 115.8 | - | - | - | - | - |
| 7″ | - | 167.1 | 144.6 | 144.7 | - | - | - | - | - |
| 8″ | - | - | 115.7 | 115.8 | - | - | - | - | - |
| 9″ | - | - | 166.1 | 166.2 | - | - | - | - | - |
| 1‴ | 103.5 | - | 99.5 | 99.5 | - | - | - | - | - |
| 2‴ | 71.2 | - | 70.3 | 71.2 | - | - | - | - | - |
| 3‴ | 71.7 | - | 71.0 | 71.6 | - | - | - | - | - |
| 4‴ | 66.4 | - | 67.1 | 67.1 | - | - | - | - | - |
| 5‴ | 64.2 | - | 75.0 | 74.8 | - | - | - | - | - |
| 6‴ | - | - | 61.0 | 61.0 | - | - | - | - | - |
| OCH3 | - | - | - | 55.8 | - | - | - | 60.1 | - |
| CH3CO | - | - | - | - | - | - | - | - | 20.7, 169.6 |
| Solvent | DMSO-d6 | C5D5N | DMSO-d6 | DMSO-d6 | DMSO-d6 | CD3OD | CD3OD | DMSO-d6 | DMSO-d6 |
| References | [47] | [48] | [33] | [33] | [49] | [50] | [50] | [23] | [51] |
* Data interchangeable.
Table 24.
The 13C-NMR spectral data of compounds 43–52 except those which have no reported 13C-NMR data.
| C | 43 | 45 | 46 | 47 | 48 | 49 | 50 | 52 |
|---|---|---|---|---|---|---|---|---|
| 2 | 168.7 | 168.4 | 168.4 | 169.5 | 170.0 | 160.0 | 164.9 | 164.9 |
| 3 | 108.4 | 108.0 | 108.0 | 108.4 | 109.1 | 112.5 | 101.9 | 118.6 |
| 4 | 182.9 | 182.7 | 182.7 | 182.4 | 185.0 | 185.0 | 178.8 | 177.6 |
| 5 | 159.5 | 152.6 | 155.9 | 152.5 | 157.8 | 158.8 | 141.8 | 137.9 |
| 6 | 98.3 | 109.7 | 114.3 | 135.2 | 116.3 | 117.3 | 116.6 | 110.5 |
| 7 | 160.9 | 158.5 | 158.8 | 157.8 | 160.2 | 161.2 | 160.4 | 159.8 |
| 8 | 104.6 | 114.3 | 109.8 | 94.7 | 111.6 | 112.9 | 111.5 | 99.6 |
| 9 | 155.0 | 155.9 | 152.6 | 155.6 | 154.6 | 155.5 | 159.3 | 158.5 |
| 10 | 105.2 | 106.5 | 106.5 | 106.2 | 108.2 | 110.6 | 117.1 | 115.7 |
| 11 | 20.5 | 20.0 | 20.1 | 20.2 | 20.5 | 10.3 | 22.8 | 19.4 |
| 12 | 8.1 | 8.8 | 9.0 | - | 8.9 | 10.5 | 19.9 | 48.7 |
| 13 | - | 8.9 | 9.1 | - | 9.5 | - | - | 205.3 |
| 14 | - | - | - | - | - | - | - | 52.0 |
| 15 | - | - | - | - | - | - | - | 62.7 |
| 16 | - | - | - | - | - | - | - | 23.6 |
| 1′ | 100.3 | 104.3 | 104.4 | 100.8 | 105.7 | 106.5 | 100.3 | 102.1 |
| 2′ | 73.9 | 76.0 | 74.1 | 74.1 | 75.3 | 76.5 | 77.6 | 73.0 |
| 3′ | 76.5 | 73.9 | 76.3 | 78.0 | 75.6 | 78.5 | 73.6 | 76.3 |
| 4′ | 79.4 | 70.0 | 69.9 | 69.9 | 71.7 | 72.5 | 70.1 | 69.4 |
| 5′ | 76.1 | 73.4 | 77.0 | 77.0 | 77.7 | 76.4 | 76.9 | 77.0 |
| 6′ | 60.7 | 63.0 | 61.0 | 61.2 | 64.3 | 65.3 | 61.0 | 60.5 |
| 1″ | - | - | - | - | - | 173.1 | - | - |
| 2″ | - | - | - | - | - | 47.3 | - | - |
| 3″ | - | - | - | - | - | 71.4 | - | - |
| 4″ | - | - | - | - | - | 46.9 | - | - |
| 5″ | - | - | - | - | - | 176.6 | - | - |
| 6″ | - | - | - | - | - | 28.4 | - | - |
| OCH3 | 60.1 | - | - | 56.7 | - | - | - | - |
| CH3CO | - | 20.4, 170.1 | - | - | 20.4, 172.5 | - | - | - |
| Solvent | DMSO-d6 | DMSO-d6 | * | DMSO-d6 | * | CD3OD | CDCl3 | DMSO-d6 |
| References | [23] | [51] | [24] | [36] | [24] | [52] | [53] | [57] |
* The authors did not report the NMR solvent.
Table 25.
The 13C-NMR spectral data of compounds 53–64 except those which have no reported 13C-NMR data.
| C | 53 | 54 | 55 | 56 | 57 | 60 | 61 | 62 | 64 |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 161.0 | 166.3 | 169.9 | 166.6 | 166.5 | 105.9 | 104.7 | 83.5 | 148.0 |
| 3 | 117.2 | 110.7 | 109.3 | 107.6 | 106.9 | 46.2 | 52.9 | 42.2 | 140.5 |
| 4 | 178.1 | 177.4 | 184.0 | 182.2 | 182.5 | 199.2 | 198.9 | 193.7 | 178.9 |
| 5 | 141.3 | 117.8 | 96.0 | 162.0 | 162.0 | 165.3 | 165.2 | 161.5 | 163.6 |
| 6 | 113.0 | 125.0 | 162.9 | 99.5 | 98.8 | 97.5 | 97.5 | 100.0 | 100.2 |
| 7 | 159.9 | 119.2 | 101.0 | 165.1 | 164.8 | 168.4 | 168.4 | 166.6 | 166.2 |
| 8 | 99.7 | 147.4 | 164.7 | 94.5 | 93.6 | 97.0 | 97.0 | 99.1 | 95.3 |
| 9 | 158.8 | 147.3 | 159.3 | 158.1 | 158.2 | 160.4 | 160.2 | 166.2 | 159.3 |
| 10 | 116.1 | 125.4 | 119.0 | 104.3 | 104.2 | 102.0 | 102.4 | 106.7 | 106.6 |
| 11 | 47.3 | 20.1 | 20.3 | 66.1 | 66.0 | 13.8 | 23.7 | 33.2 | - |
| 12 | 202.5 | - | - | - | - | - | 11.8 | 18.2 * | - |
| 13 | 29.8 | - | - | - | - | - | - | - | - |
| 14 | 22.3 | - | - | - | - | - | - | - | - |
| 1’ | 101.3 | 102.4 | 101.6 | 102.8 | 102.7 | 104.1 | 104.1 | 104.0 | 102.1 |
| 2’ | 73.1 | 74.8 | 74.7 | 73.9 | 73.6 | 75.0 | 75.0 | 74.6 | 72.0 |
| 3’ | 76.3 | 78.6 | 78.3 | 76.4 | 76.6 | 77.9 | 77.9 | 77.4 | 74.0 |
| 4’ | 69.5 | 71.2 | 71.4 | 67.7 | 70.1 | 71.0 | 71.0 | 71.2 | 71.5 |
| 5’ | 77.0 | 79.2 | 77.8 | 63.6 | 76.8 | 78.0 | 78.0 | 78.5 | 72.4 |
| 6’ | 60.9 | 62.4 | 62.4 | - | 61.3 | 62.5 | 62.5 | 62.5 | 62.5 |
| Solvent | DMSO-d6 | C5D5N | CD3OD | DMSO-d6 | CD3OD | CD3OD | CD3OD | CD3OD | CD3OD |
| References | [57] | [58] | [59] | [31] | [60] | [54] | [50] | [63] | [65] |
* Data interchangeable.
Table 26.
The 13C-NMR spectral data of compounds 66–72.
| C | 66 | 67 | 68 | 69 | 70 | 71 | 72 |
|---|---|---|---|---|---|---|---|
| 2 | 167.3 | 174.7 | 168.3 | 168.4 | 167.8 | 169.4 | 174.4 |
| 3 | 107.8 | 105.1 | 108.6 | 108.8 | 107.0 | 109.2 | 105.3 |
| 4 | 181.8 | 182.2 | 181.8 | 181.8 | 182.0 | 184.3 | 182.2 |
| 5 | 160.6 | 160.6 | 160.6 | 160.6 | 160.4 | 162.3 | 160.4 |
| 6 | 108.7 | 108.7 | 108.6 | 108.8 | 108.1 | 108.9 | 107.0 |
| 7 | 163.2 | 163.3 | 163.1 | 163.1 | 162.4 | 165.0 | 163.4 |
| 8 | 93.3 | 93.4 | 93.3 | 93.3 | 93.9 | 95.2 | 93.7 |
| 9 | 156.6 | 156.7 | 156.6 | 156.6 | 157.0 | 159.4 | 156.9 |
| 10 | 103.0 | 103.3 | 103.2 | 103.2 | 102.9 | 105.1 | 103.1 |
| 11 | 19.8 | 32.3 | 43.1 | 66.2 | 20.0 | 20.4 | 32.4 |
| 12 | - | 19.8 | 64.1 | 46.0 | - | - | 19.8 |
| 13 | - | 19.8 | 23.3 | 63.8 | - | - | 19.8 |
| 14 | - | - | - | 43.8 | - | - | - |
| 15 | - | - | - | 23.5 | - | - | - |
| 1′ | 73.0 | 73.0 | 72.9 | 72.9 | 70.8 | 75.6 | 70.7 |
| 2′ | 70.1 | 70.2 | 70.0 | 70.0 | 72.0 | 72.6 | 72.0 |
| 3′ | 78.9 | 78.9 | 78.8 | 78.8 | 76.7 | 80.1 | 76.6 |
| 4′ | 70.6 | 70.6 | 70.5 | 70.5 | 70.8 | 71.9 | 70.7 |
| 5′ | 81.4 | 81.6 | 81.4 | 81.4 | 82.0 | 80.2 | 81.8 |
| 6′ | 61.4 | 61.5 | 61.4 | 61.4 | 61.6 | 65.2 | 61.5 |
| 1″ | - | - | - | - | 119.9 | 121.6 | 119.9 |
| 2″ | - | - | - | - | 108.9 | 110.4 | 108.7 |
| 3″ | - | - | - | - | 145.4 | 146.6 | 145.4 |
| 4″ | - | - | - | - | 138.2 | 140.0 | 138.1 |
| 5″ | - | - | - | - | 145.4 | 146.6 | 145.4 |
| 6″ | - | - | - | - | 108.9 | 110.4 | 108.9 |
| 7″ | - | - | - | - | 164.8 | 168.6 | 164.7 |
| Solvent | DMSO-d6 | DMSO-d6 | DMSO-d6 | DMSO-d6 | DMSO-d6 | CD3OD | DMSO-d6 |
| References | [69] | [69] | [72] | [72] | [71] | [73] | [69] |
Table 27.
The 13C-NMR spectral data of compounds 73–79 except those which have no reported 13C-NMR data.
| C | 73 | 74 | 75 | 76 | 79 |
|---|---|---|---|---|---|
| 2 | 167.7 | 174.9 | 162.3 | 163.1 | 160.6 |
| 3 | 106.3 | 105.3 | 113.2 | 111.0 | 112.4 |
| 4 | 182.0 | 182.2 | 181.9 | 181.8 | 178.5 |
| 5 | 161.1 | 160.9 | 143.1 | 146.1 | * |
| 6 | 108.1 | 106.4 | 127.0 | 128.3 | 126.5 |
| 7 | 163.1 | 163.2 | 160.1 | 161.1 | 160.8 |
| 8 | 93.4 | 93.4 | 119.4 | 121.1 | 100.6 |
| 9 | 157.1 | 157.0 | 161.3 | 157.9 | 159.0 |
| 10 | 103.0 | 103.1 | 115.8 | 114.3 | 114.6 |
| 11 | 19.9 | 32.4 | 48.7 | 48.5 | 47.8 |
| 12 | - | 19.78 | 204.4 | 204.6 | 202.4 |
| 13 | - | 19.75 | 29.8 | 30.7 | 29.8 |
| 14 | - | - | 23.3 | 23.3 | 22.5 |
| CH3O | - | - | - | 55.8 | - |
| 1′ | 70.7 | 70.7 | 75.5 | 75.6 | 71.4 |
| 2′ | 69.7 | 69.7 | 71.9 | 72.3 | 81.7 |
| 3′ | 77.6 | 77.6 | 80.0 | 79.9 | 78.2 |
| 4′ | 68.7 | 68.7 | 72.9 | 72.1 | 70.1 |
| 5′ | 81.8 | 81.7 | 79.8 | 78.5 | 81.1 |
| 6′ | 61.2 | 61.2 | 65.2 | 65.4 | 60.9 |
| 1″ | 119.7 | 119.7 | 133.6 | 128.3 | 105.1 |
| 2″ | 108.9 | 108.9 | 131.2 | 131.0 | 74.3 |
| 3″ | 145.5 | 145.4 | 116.8 | 115.3 | 76.1 |
| 4″ | 138.5 | 138.4 | 161.3 | 160.4 | 69.3 |
| 5″ | 145.5 | 145.4 | 116.8 | 115.3 | 76.1 |
| 6″ | 108.9 | 108.9 | 131.2 | 131.0 | 60.3 |
| 7″ | 165.3 | 165.4 | 146.2 | 146.2 | - |
| 8″ | - | - | 114.9 | 116.0 | - |
| 9″ | - | - | 169.2 | 169.1 | - |
| 1‴ | 119.1 | 119.0 | - | - | - |
| 2‴ | 108.7 | 108.7 | - | - | - |
| 3‴ | 145.3 | 145.3 | - | - | - |
| 4‴ | 138.4 | 138.3 | - | - | - |
| 5‴ | 145.3 | 145.3 | - | - | - |
| 6‴ | 108.7 | 108.7 | - | - | - |
| 7‴ | 164.6 | 164.4 | - | - | - |
| Solvent | DMSO-d6 | DMSO-d6 | CD3OD | CD3OD | DMSO-d6 |
| References | [71] | [71] | [74] | [74] | [78] |
* The authors missed assigning this position.
Table 28.
The 13C-NMR spectral data of compounds 80–89.
| C | 80 | 81 | 82 | 83 | 84 | 85 | 86 | 87 | 88 | 89 |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 167.0 | 169.3 | 174.4 | 173.0 | 168.2 | 167.6 | 167.2 | 174.8 | 167.7 | 174.5 |
| 3 | 107.4 | 108.7 | 105.0 | 106.2 | 108.3 | 107.8 | 107.5 | 105.4 | 108.0 | 105.6 |
| 4 | 181.9 | 184.3 | 182.2 | 181.9 | 181.9 | 182.2 | 181.9 | 182.4 | 182.2 | 182.4 |
| 5 | 160.4 | 149.4 | 160.4 | 160.4 | 160.3 | 160.8 | 160.5 | 160.7 | 161.0 | 161.0 |
| 6 | 98.4 | 94.5 | 98.5 | 98.7 | 98.3 | 97.6 | 98.3 | 97.8 | 98.0 | 97.9 |
| 7 | 162.5 | 162.8 | 162.8 | 164.2 | 162.7 | 162.2 | 162.6 | 162.3 | 162.4 | 162.6 |
| 8 | 104.3 | 105.2 | 104.1 | 104.7 | 108.4 | 103.9 | 104.0 | 102.8 | 103.9 | 102.1 |
| 9 | 156.1 | 164.6 | 156.5 | 156.6 | 158.9 | 157.1 | 156.3 | 157.2 | 157.1 | 157.2 |
| 10 | 103.5 | 106.3 | 102.0 | 103.3 | 103.8 | 102.8 | 103.5 | 104.0 | 102.0 | 104.0 |
| 11 | 19.6 | 20.3 | 32.7 | 39.1 | 42.1 | 11.0 | 19.7 | 33.1 | 20.1 | 33.1 |
| 12 | - | - | 20.0 | 27.0 | 66.5 | - | - | 19.8 | - | 20.3 |
| 13 | - | - | 19.5 | 11.4 | 45.8 | - | - | 20.3 | - | 20.1 |
| 14 | - | - | - | 17.4 | 64.1 | - | - | - | - | - |
| 15 | - | - | - | - | 23.6 | - | - | - | - | - |
| 1′ | 73.1 | 74.9 | 73.1 | 73.3 | 73.2 | 70.6 | 73.3 | 70.7 | 70.7 | 70.8 |
| 2′ | 71.0 | 72.8 | 71.2 | 71.2 | 70.3 | 72.5 | 70.0 | 72.5 | 70.1 | 70.2 |
| 3′ | 78.5 | 80.0 | 78.5 | 78.7 | 78.6 | 76.1 | 78.1 | 76.0 | 77.0 | 77.0 |
| 4′ | 70.3 | 71.7 | 70.8 | 70.9 | 71.0 | 70.8 | 70.7 | 71.2 | 68.5 | 68.9 |
| 5′ | 81.1 | 82.4 | 81.5 | 81.5 | 81.4 | 81.7 | 78.3 | 82.0 | 81.5 | 81.9 |
| 6′ | 61.3 | 62.9 | 61.8 | 61.7 | 61.5 | 61.5 | 63.8 | 61.8 | 61.0 | 61.3 |
| 1″ | - | - | - | - | - | 119.7 | 119.4 | 119.6 | 119.6 | 119.6 |
| 2″ , 6″ | - | - | - | - | - | 108.8 | 108.5 | 108.7 | 108.9 | 108.9 |
| 3″ , 5″ | - | - | - | - | - | 145.5 | 145.5 | 145.4 | 145.6 | 145.5 |
| 4″ | - | - | - | - | - | 138.8 | 138.3 | 138.3 | 138.7 | 138.7 |
| 7″ | - | - | - | - | - | 165.0 | 165.8 | 165.0 | 165.5 | 165.4 |
| 1‴ | - | - | - | - | - | - | - | - | 118.7 | 118.7 |
| 2‴ , 6‴ | - | - | - | - | - | - | - | - | 108.7 | 108.7 |
| 3‴ , 5‴ | - | - | - | - | - | - | - | - | 145.5 | 145.4 |
| 4‴ | - | - | - | - | - | - | - | - | 138.5 | 138.6 |
| 7‴ | - | - | - | - | - | - | - | - | 164.8 | 164.8 |
| OCH3 | - | 56.8 | - | - | - | - | - | - | - | - |
| Solvent | DMSO-d6 | CD3OD | DMSO-d6 | DMSO-d6 | DMSO-d6 | DMSO-d6 | DMSO-d6 | DMSO-d6 | DMSO-d6 | DMSO-d6 |
| References | [86] | [87] | [158] | [158] | [72] | [71] | [91] | [69] | [71] | [71] |
Table 29.
The 13C-NMR spectral data of compounds 91–100 except which has no reported 13C-NMR data.
| C | 91 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 100 |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 162.7 | 164.9 | 167.1 | 167.0 | 169.2 | 160.2 | 160.2 | 160.8 | 159.6 |
| 3 | 109.8 | 111.2 | 112.2 | 111.9 | 110.8 | 112.6 | 112.4 | 113.0 | 113.7 |
| 4 | 182.7 | 178.7 | 182.3 | 181.9 | 182.4 | 178.3 | 178.5 | 178.9 | 179.1 |
| 5 | 144.0 | 141.0 | 143.1 | 143.7 | 144.1 | 139.5 | 140.1 | 141.3 | 144.1 |
| 6 | 112.8 | 111.5 | 112.2 | 112.6 | 113.1 | 116.9 | 116.3 | 111.9 | 118.5 |
| 7 | 162.5 | 160.0 | 162.3 | 162.1 | 162.7 | 160.2 | 159.5 | 160.3 | 159.0 |
| 8 | 113.3 | 112.8 | 116.1 | 113.1 | 113.7 | 107.1 | 110.0 | 113.1 | 106.2 |
| 9 | 158.8 | 157.2 | 160.0 | 158.9 | 159.1 | 155.9 | 157.8 | 157.3 | 159.6 |
| 10 | 117.4 | 115.9 | 118.5 | 117.0 | 117.6 | 114.3 | 114.7 | 115.9 | 113.9 |
| 11 | 124.7 | 43.2 | 44.3 | 44.2 | 76.2 | 47.2 | 47.7 | 47.8 | 48.2 |
| 12 | 139.0 | 63.8 | 66.7 | 66.3 | 69.5 | 202.5 | 202.4 | 202.5 | 200.7 |
| 13 | 18.4 | 23.8 | 23.6 | 23.6 | 19.7 | 22.2 | 22.6 | 30.0 | 30.2 |
| 14 | 23.6 | 22.8 | 23.3 | 23.6 | 23.7 | 29.9 | 29.9 | 23.0 | 23.1 |
| 15 | 56.9 | 56.4 | - | 56.7 | 56.9 | - | - | 56.6 | - |
| 1′ | 75.1 | 73.0 | 76.0 | 74.6 | 74.9 | 80.2 | 73.5 | 72.9 | 68.1 |
| 2′ | 72.4 | 70.9 | 73.2 | 72.7 | 72.9 | 77.5 | 71.0 | 71.1 | 73.7 |
| 3′ | 80.3 | 78.8 | 80.1 | 80.0 | 80.3 | 74.8 | 78.7 | 79.1 | 73.7 |
| 4′ | 72.3 | 70.8 | 71.8 | 71.9 | 72.2 | 80.5 | 70.4 | 70.7 | 70.2 |
| 5′ | 82.9 | 81.8 | 82.7 | 82.4 | 82.6 | 68.3 | 81.5 | 81.8 | 76.6 |
| 6′ | 63.0 | 61.7 | 62.8 | 63.0 | 63.3 | 63.8 | 61.4 | 61.8 | 61.6 |
| 2 ′-OCOCH3 | - | - | - | - | - | - | - | - | 168.6, 20.7 |
| 3 ′-OCOCH3 | - | - | - | - | - | - | - | - | 169.4, 20.7 |
| 4 ′-OCOCH3 | - | - | - | - | - | - | - | - | 170.3, 20.7 |
| 6 ′-OCOCH3 | - | - | - | - | - | - | - | - | 170.6, 20.7 |
| Solvent | CD3OD | DMSO-d6 | CD3OD | CD3OD | CD3OD | DMSO-d6 | DMSO-d6 | CD3OD | CDCl3 |
| References | [80] | [159] | [160] | [82] | [160] | [94] | [94] | [96] | [93] |
Table 30.
The 13C-NMR spectral data of compounds 103–110 except which has no reported 13C-NMR data.
| C | 103 | 104 | 106 | 107 | 108 | 109 | 110 |
|---|---|---|---|---|---|---|---|
| 2 | 165.0 | 165.0 | 167.4 | 167.2 | 167.4 | 167.1 | 167.2 |
| 3 | 111.3 | 111.3 | 112.5 | 112.6 | 112.6 | 111.9 | 111.9 |
| 4 | 178.6 | 178.8 | 182.3 | 182.2 | 182.2 | 181.9 | 182.0 |
| 5 | 141.7 | 141.8 | 144.6 | 144.8 | 144.6 | 144.3 | 144.3 |
| 6 | 111.0 | 111.3 | 112.7 | 112.6 | 112.5 | 112.3 | 112.4 |
| 7 | 159.5 | 159.8 | 162.1 | 162.0 | 162.0 | 161.6 | 161.6 |
| 8 | 110.6 | 110.7 | 111.8 | 111.4 | 111.8 | 111.6 | 111.3 |
| 9 | 157.4 | 157.5 | 159.8 | 159.6 | 159.6 | 159.3 | 159.1 |
| 10 | 115.8 | 115.8 | 117.2 | 117.2 | 117.2 | 116.9 | 116.9 |
| 11 | 43.1 | 43.3 | 44.6 | 44.9 | 44.6 | 44.5 | 44.3 |
| 12 | 64.4 | 63.9 | 65.9 | 66.3 | 66.0 | 66.6 | 66.5 |
| 13 | 23.3 | 23.7 | 23.6 | 23.7 | 23.6 | 23.5 | 23.6 |
| 14 | 22.8 | 22.9 | 23.7 | 23.7 | 23.6 | 23.5 | 23.3 |
| 15 | 56.5 | 56.5 | 57.0 | 57.1 | 57.0 | 56.9 | 57.0 |
| 1′ | 70.6 | 70.6 | 72.8 | 72.8 | 72.8 | 71.9 | 71.8 |
| 2′ | 72.6 | 72.3 | 73.4 | 73.7 | 73.9 | 74.0 | 73.7 |
| 3′ | 75.7 | 75.9 | 77.7 | 77.7 | 77.9 | 77.6 | 77.4 |
| 4′ | 70.4 | 70.9 | 72.4 | 72.4 | 72.3 | 72.4 | 72.4 |
| 5′ | 81.8 | 82.0 | 83.0 | 80.1 | 82.9 | 82.7 | 82.4 |
| 6′ | 61.5 | 61.6 | 63.1 | 64.4 | 63.1 | 62.9 | 62.7 |
| 1″ | 133.9 | 125.0 | 127.0 | 127.0 | 128.2 | 135.3 | 126.7 |
| 2″ | 128.9 | 130.3 | 133.2 | 131.1 | 130.9 | 128.9 | 130.9 |
| 3″ | 128.2 | 115.8 | 115.7 | 116.9 | 115.4 | 129.7 | 116.6 |
| 4″ | 130.4 | 159.6 | 160.0 | 161.3 | 163.2 | 131.3 | 160.7 |
| 5″ | 128.2 | 115.8 | 115.7 | 116.9 | 115.4 | 129.7 | 116.6 |
| 6″ | 128.9 | 130.3 | 133.2 | 131.1 | 130.9 | 128.9 | 130.9 |
| 7″ | 144.1 | 144.4 | 145.0 | 146.6 | 146.1 | 146.1 | 146.4 |
| 8″ | 117.8 | 114.0 | 115.7 | 114.6 | 115.6 | 118.1 | 114.2 |
| 9″ | 165.0 | 165.4 | 167.6 | 168.0 | 167.8 | 167.2 | 167.8 |
| OCH3 | - | - | - | - | 55.9 | - | - |
| COCH3 | - | - | - | 173.0, 20.9 | - | - | - |
| Solvent | DMSO-d6 | DMSO-d6 | CD3OD | CD3OD | CD3OD | CD3OD | CD3OD |
| References | [99] | [159] | [80] | [100] | [100] | [82] | [82] |
Table 31.
The 13C-NMR spectral data of compounds 111–122 except which has no reported 13C-NMR data.
| C | 111 | 112 | 113 | 114 | 115 | 116 | 117 | 119 | 120 | 121 | 122 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 167.2 | 167.6 | 169.7 | 160.3 | 163.0 | 160.1 | 163.1 | 160.6 | 164.0 | 167.6 | 166.8 |
| 3 | 112.1 | 112.2 | 110.5 | 1125 | 113.8 | 112.5 | 111.0 | 112.5 | 112.4 | 112.2 | 112.2 |
| 4 | 182.2 | 182.3 | 182.4 | 178.6 | 182.1 | 178.4 | 181.8 | 178.6 | 182.0 | 182.3 | 182.3 |
| 5 | 143.5 | 144.6 | 144.8 | 141.0 | 144.7 | 140.4 | 146.1 | 141.8 | 144.9 | 144.6 | 144.8 |
| 6 | 116.3 | 112.6 | 112.7 | 115.8 | 112.2 | 115.7 | 128.3 | 111.5 | 113.1 | 112.6 | 112.7 |
| 7 | 161.3 | 162.0 | 162.1 | 159.l | 162.1 | 159.4 | 161.1 | 159.7 | 162.3 | 161.0 | 162.2 |
| 8 | 110.0 | 111.9 | 111.8 | 110.2 | 112.0 | 110.7 | 121.1 | 110.8 | 111.6 | 111.9 | 111.9 |
| 9 | 160.4 | 159.6 | 159.4 | 158.3 | 159.6 | 157.9 | 157.9 | 157.4 | 159.5 | 159.6 | 159.6 |
| 10 | 117.0 | 117.3 | 117.5 | 114.8 | 117.1 | 114.8 | 114.3 | 115.6 | 117.6 | 117.2 | 117.3 |
| 11 | 44.3 | 44.6 | 75.7 | 48.1 | 49.1 | 47.8 | 48.5 | 47.9 | 98.6 | 44.6 | 44.6 |
| 12 | 66.7 | 66.8 | 69.2 | 202.4 | 204.7 | 202.3 | 204.6 | 202.1 | 203.7 | 66.8 | 66.8 |
| 13 | 23.5 | 23.6 | 19.7 | 30.4 | 29.9 | 29.6 | 30.7 | 29.6 | 23.7 | 23.7 | 24.0 |
| 14 | 23.5 | 23.6 | 23.7 | 22.7 | 23.7 | 22.6 | 23.3 | 22.7 | 25.0 | 23.6 | 23.5 |
| 15 | - | 57.1 | 57.1 | - | 57.1 | - | 55.8 | 55.5 | 57.2 | 57.1 | 57.1 |
| 1′ | 73.0 | 72.1 | 72.8 | 70.2 | 72.1 | 73.3 | 75.6 | 70.4 | 72.6 | 72.6 | 72.6 |
| 2′ | 74.0 | 74.0 | 74.1 | 72.3 | 73.9 | 70.8 | 72.3 | 72.2 | 74.3 | 74.1 | 73.6 |
| 3′ | 77.9 | 77.9 | 77.8 | 76.0 | 77.9 | 78.5 | 79.9 | 75.8 | 78.0 | 77.8 | 77.6 |
| 4′ | 72.1 | 72.7 | 72.3 | 70.2 | 72.6 | 70.4 | 72.1 | 70.6 | 71.9 | 78.3 | 78.1 |
| 5′ | 82.8 | 82.9 | 83.0 | 81.8 | 82.8 | 78.4 | 78.5 | 81.9 | 83.3 | 82.9 | 82.9 |
| 6′ | 63.1 | 63.1 | 63.1 | 61.8 | 63.3 | 64.8 | 65.4 | 61.5 | 63.5 | 63.0 | 63.0 |
| 1″ | 128.3 | 127.5 | 135.7 | 125 I | 127.0 | 125.0 | 128.3 | 125.5 | 135.7 | 129.8 | 129.7 |
| 2″ | 131.0 | 115.1 | 129.2 | 1301 | 130.8 | 130.3 | 131.0 | 111.1 | 129.3 | 130.8 | 132.6 |
| 3″ | 116.7 | 149.9 | 130.1 | 115.8 | 116.7 | 115.7 | 115.3 | 147.9 | 130.1 | 118.0 | 116.7 |
| 4″ | 161.2 | 146.6 | 131.6 | 159.6 | 161.3 | 159.7 | 160.4 | 149.2 | 131.6 | 163.8 | 159.7 |
| 5″ | 116.7 | 116.6 | 130.1 | 115.8 | 116.7 | 115.7 | 115.3 | 115.5 | 130.1 | - | - |
| 6″ | 131.0 | 123.0 | 129.2 | 1301 | 130.8 | 130.3 | 131.0 | 122.9 | 129.3 | - | - |
| 7″ | 146.2 | 147.1 | 146.4 | 144.4 | 145.3 | 144.9 | 146.2 | 144.6 | 146.3 | 146.0 | 144.7 |
| 8″ | 115.8 | 114.5 | 118.4 | 114.1 | 115.1 | 114.0 | 116.0 | 114.2 | 118.5 | 116.5 | 117.6 |
| 9″ | 168.0 | 168.2 | 167.4 | 165.4 | 168.1 | 166.7 | 169.1 | 165.4 | 167.4 | 167.8 | 167.7 |
| OCH3 | 56.1 | - | - | - | - | - | - | 56.3 | - | - | - |
| 1‴ | - | - | - | - | - | - | - | - | - | 101.9 | 101.7 |
| 2‴ | - | - | - | - | - | - | - | - | - | 74.8 | 74.9 |
| 3‴ | - | - | - | - | - | - | - | - | - | 72.1 | 72.6 |
| 4‴ | - | - | - | - | - | - | - | - | - | 71.3 | 71.2 |
| 5‴ | - | - | - | - | - | - | - | - | - | 78.0 | 77.9 |
| 6‴ | - | - | - | - | - | - | - | - | - | 62.5 | 62.4 |
| Solvent | CD3OD | CD3OD | CD3OD | DMSO-d6 | DMSO-d6 | DMSO-d6 | CD3OD | DMSO-d6 | CD3OD | CD3OD | CD3OD |
| References | [80] | [82] | [160] | [161] | [162] | [163] | [74] | [164] | [81] | [160] | [160] |
Table 32.
The 13C-NMR spectral data of compounds 123–129 except which has no reported 13C-NMR data.
| C | 123 | 124 | 126 | 127 | 128 | 129 |
|---|---|---|---|---|---|---|
| 2 | 167.7 | 168.3 | 171.3 | 170.7 | 167.7 | 169.9 |
| 3 | 108.0 | 108.5 | 105.6 | 105.5 | 108.3 | 108.4 |
| 4 | 182.2 | 182.4 | 184.3 | 183.0 | 182.7 | 182.4 |
| 5 | 158.8 | 157.8 | 160.2 | 158.1 | 159.5 | 159.6 |
| 6 | 110.4 | 111.1 | 112.5 | 112.8 | 107.4 | 105.2 |
| 7 | 162.3 | 163.1 | 163.8 | 161.0 | 162.9 | 163.1 |
| 8 | 93.2 | 90.5 | 94.4 | 93.0 | 92.8 | 92.9 |
| 9 | 156.1 | 156.6 | 157.8 | 156.1 | 156.7 | 156.4 |
| 10 | 103.3 | 104.4 | 106.8 | 105.6 | 103.4 | 103.8 |
| 11 | 19.9 | 19.9 | 61.6 | 60.0 | 18.8 | 60.0 |
| OCH3 | - | 56.5 | - | - | - | - |
| 1′ | 20.5 | 20.5 | 22.1 | 20.5 | 26.4 | 26.4 |
| 2′ | 126.9 | 126.4 | 128.7 | 124.6 | 79.9 | 79.8 |
| 3′ | 131.8 | 132.3 | 132.9 | 134.1 | 143.7 | 143.7 |
| 4′ | 66.1 | 66.1 | 68.4 | 61.0 | 114.2 | 114.2 |
| 5′ | 21.2 | 21.3 | 21.9 | 20.0 | 15.2 | 15.2 |
| 1″ | 101.5 | 101.6 | 102.7 | 100.1 | 99.4 | 99.4 |
| 2″ | 73.6 | 73.6 | 75.3 | 73.2 | 73.6 | 73.6 |
| 3″ | 76.9 | 77.0 | 78.3 | 76.7 | 76.3 | 76.3 |
| 4″ | 70.2 | 70.2 | 71.8 | 67.7 | 70.2 | 70.2 |
| 5″ | 77.0 | 77.0 | 77.9 | 77.0 | 76.7 | 76.7 |
| 6″ | 61.1 | 61.1 | 62.9 | 60.1 | 61.2 | 61.2 |
| Solvent | DMSO-d6 | DMSO-d6 | CD3OD | CD3OD | CD3OD | CD3OD |
| References | [104] | [104] | [106] | [60] | [60] | [60] |
Table 33.
The 13C-NMR spectral data of compounds 130–139.
| C | 130 | 131 | 132 | 133 | 134 | 135 | 136 | 137 | 138 | 139 |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 170.7 | 167.6 | 170.7 | 168.3 | 171.4 | 168.9 | 165.0 | 171.0 | 171.5 | 171.8 |
| 3 | 105.5 | 107.4 | 104.9 | 108.1 | 105.5 | 113.9 | * | 110.4 | 110.7 | 110.7 |
| 4 | 183.0 | 181.8 | 181.9 | 182.5 | 182.5 | 176.6 | * | 179.3 | 180.4 | 180.6 |
| 5 | 158.8 | 158.8 | 158.9 | 159.5 | 159.5 | 132.6 | * | 106.6 | 119.2 | 126.2 |
| 6 | 111.4 | 97.6 | 97.8 | 98.3 | 98.4 | 111.4 | 99.3 | 150.7 | 126.2 | 126.6 |
| 7 | 162.1 | 159.9 | 160.1 | 160.6 | 160.8 | 158.2 | 162.9 | 153.1 | 122.1 | 135.6 |
| 8 | 93.3 | 107.5 | 107.4 | 108.1 | 108.2 | 103.6 | 94.8 | 106.9 | 147.8 | 119.3 |
| 9 | 156.3 | 153.8 | 153.5 | 154.4 | 154.1 | 163.4 | * | 149.8 | 149.0 | 158.0 |
| 10 | 105.7 | 104.3 | 104.8 | 105.0 | 105.4 | 112.7 | * | 116.2 | 125.0 | 124.3 |
| 11 | 60.0 | 19.5 | 59.3 | 20.2 | 59.5 | 127.9 | 121.6 | 102.2 | 141.4 | 136.2 |
| 1′ | 28.2 | 20.3 | 20.3 | 21.0 | 20.9 | 131.9 | 128.5 | 74.3 | 129.5 | 114.0 |
| 2′ | 74.6 | 120.5 | 120.4 | 121.1 | 121.0 | 115.7 | 115.7 | 78.1 | 129.6 | 150.7 |
| 3′ | 147.9 | 134.9 | 134.9 | 135.6 | 135.7 | 157.1 | 161.5 | 71.5 | 127.4 | 146.4 |
| 4′ | 109.5 | 65.9 | 65.8 | 66.5 | 66.5 | 115.7 | 115.7 | 78.0 | 129.6 | 118.1 |
| 5′ | 16.6 | 13.1 | 13.1 | 13.8 | 13.7 | 131.9 | 128.5 | 62.8 | 129.5 | 122.0 |
| 6′ | - | - | - | - | - | 39.3 | 39.1 | - | 33.8 | 33.6 |
| 7′ | - | - | - | - | - | 86.3 | 37.5 | - | 37.0 | 37.1 |
| 8′ | - | - | - | - | - | 127.9 | 121.6 | 102.2 | 141.4 | 136.2 |
| 1″ | 101.2 | 100.1 | 100.1 | 100.7 | 100.7 | 101.8 | 100.3 | 131.5 | 103.0 | 102.9 |
| 2″ | 73.6 | 72.9 | 72.9 | 73.5 | 73.5 | 74.6 | * | 133.0 | 75.0 | 74.9 |
| 3″ | 76.5 | 76.7 | 76.7 | 76.7 | 76.7 | 78.4 | * | 116.3 | 78.2 | 77.8 |
| 4″ | 69.8 | 69.2 | 69.2 | 69.8 | 69.8 | 71.2 | * | 164.1 | 71.3 | 71.3 |
| 5″ | 77.1 | 76.1 | 76.1 | 77.3 | 77.3 | 78.2 | * | 116.3 | 78.2 | 78.1 |
| 6″ | 61.1 | 60.2 | 60.2 | 60.8 | 60.8 | 62.5 | * | 120.9 | 62.5 | 62.4 |
| 7″ | - | - | - | - | - | - | - | 33.9 | - | - |
| 8″ | - | - | - | - | - | - | - | 37.1 | - | - |
| OCH3 | - | - | - | - | - | - | - | 6-OCH3 56.3 4″-OCH3 55.1 |
- | 56.6 |
| Solvent | CD3OD | DMSO-d6 | DMSO-d6 | DMSO-d6 | DMSO-d6 | CD3OD | DMSO-d6 | DMSO-d6 | CD3OD | CD3OD |
| References | [60] | [108] | [108] | [109] | [109] | [110] | [111] | [112] | [113] | [114] |
* The authors missed assigning these positions.
Table 34.
The 13C-NMR spectral data of compounds 140–148.
| C | 140 | 141 | 142 | 143 | 144 | 145 | 146 | 147 | 148 |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 147.2 | 146.9 | 147.3 | 89.9 | 89.0 | 92.9 | 92.1 | 92.3 | 88.2 |
| 3 | 106.1 | 105.0 | 104.9 | 24.3 | 28.3 | 28.1 | 27.9 | 28.0 | 30.1 |
| 4 | 153.7 | 160.5 | 159.2 | 165.5 | 164.5 | 160.3 | 165.3 | 156.3 | 158.5 |
| 5 | 177.2 | 185.9 | 184.7 | 176.9 | 176.4 | 184.7 | 176.6 | 176.6 | 182.9 |
| 6 | 109.7 | 107.8 | 107.2 | 111.9 | 109.8 | 109.5 | 110.9 | 111.4 | 107.2 |
| 7 | 163.7 | 169.0 | 168.7 | 163.3 | 164.0 | 169.7 | 162.7 | 162.6 | 166.7 |
| 9 | 95.8 | 92.0 | 92.0 | 94.8 | 95.2 | 90.4 | 94.0 | 94.2 | 88.8 |
| 10 | 157.8 | 156.1 | 154.5 | 159.9 | 160.1 | 168.3 | 159.7 | 165.4 | 166.6 |
| 11 | 117.0 | 114.3 | 113.4 | 118.7 | 117.2 | 111.3 | 118.1 | 118.5 | 108.7 |
| 12 | 152.8 | 106.9 | 106.3 | 112.3 | 111.4 | 106.6 | 112.5 | 112.8 | 102.9 |
| 13 | 155.6 | 155.7 | 155.2 | 156.4 | 157.7 | 157.9 | 156.1 | 159.9 | 158.6 |
| 14 | 65.7 | 67.8 | 66.3 | 21.4 | 20.7 | 20.8 | 66.4 | 66.8 | 66.1 |
| 4-OCH3 | 61.9 | - | - | - | * | - | 60.8 | 61.0 | - |
| 1′ | 103.0 | 104.4 | 104.4 | 77.3 | 74.3 | 79.7 | 70.8 | 70.8 | 143.7 |
| 2′ | 74.0 | 75.0 | 74.2 | 23.4 | 24.1 | 24.3 | 26.0 | 26.2 | 115.5 |
| 3′ | 77.2 | 77.9 | 77.6 | 22.3 | 23.5 | 23.0 | 25.6 | 25.8 | 15.7 |
| 4′ | 70.6 | 71.6 | 70.9 | - | - | - | - | - | - |
| 5′ | 77.1 | 78.0 | 76.7 | - | - | - | - | - | - |
| 6′ | 61.7 | 68.7 | 69.5 | - | - | - | - | - | - |
| 1″ | - | 111.1 | 103.3 | 98.9 | 100.1 | 99.5 | 104.0 | 104.4 | 102.8 |
| 2″ | - | 77.2 | 74.4 | 75.1 | 74.6 | 75.6 | 74.8 | 75.0 | 73.7 |
| 3″ | - | 80.6 | 77.4 | 78.8 | 78.6 | 78.5 | 78.4 | 78.3 | 76.7 |
| 4″ | - | 74.9 | 70.9 | 71.3 | 71.4 | 72.0 | 71.4 | 71.3 | 70.3 |
| 5″ | - | 65.5 | 77.7 | 77.1 | 78.2 | 76.8 | 78.1 | 75.7 | 76.9 |
| 6″ | - | - | 61.9 | 62.4 | 63.1 | 68.5 | 62.6 | 64.5 | 61.4 |
| 1‴ | - | - | - | - | - | 111.2 | - | 126.5 | - |
| 2‴ | - | - | - | - | - | 78.6 | - | 111.4 | - |
| 3‴ | - | - | - | - | - | 81.0 | - | 149.0 | - |
| 4‴ | - | - | - | - | - | 75.6 | - | 151.2 | - |
| 5‴ | - | - | - | - | - | 66.2 | - | 116.8 | - |
| 6‴ | - | - | - | - | - | - | - | 123.9 | - |
| 7‴ | - | - | - | - | - | - | - | 145.9 | - |
| 8‴ | - | - | - | - | - | - | - | 115.1 | - |
| 9‴ | - | - | - | - | - | - | - | 167.8 | - |
| 3‴-OCH3 | - | - | - | - | - | - | - | 55.9 | - |
| Solvent | DMSO-d6, C6D6 | CD3OD | DMSO-d6 | CDCl3, CD3OD | CDCl3, CD3OD | CD3OD | C5D5N | C5D5N | CD3OD |
| References | [117] | [118] | [109] | [122] | [122] | [119] | [121] | [132] | [60] |
* The authors missed assigning this position.
Table 35.
The 13C-NMR spectral data of compounds 149–155.
| C | 149 | 150 | 151 | 152 | 153 | 154 | 155 |
|---|---|---|---|---|---|---|---|
| 2 | 167.4 | 169.5 | 162.7 | 167.6 | 167.6 | 170.9 | 168.7 |
| 3 | 108.7 | 108.0 | 111.1 | 107.5 | 107.4 | 105.6 | 108.6 |
| 4 | 182.7 | 184.2 | 175.3 | 181.9 | 181.8 | 182.5 | 182.6 |
| 5 | 160.0 | 160.9 | 160.7 * | 158.6 | 158.5 | 159.2 | 160.3 |
| 6 | 104.1 | 105.0 | 104.9 | 103.1 | 103.1 | 103.8 | 102.5 |
| 7 | 159.6 | 160.4 | 152.7 * | 163.5 | 163.3 | 164.1 | 164.3 |
| 8 | 94.9 | 95.8 | 91.1 | 109.8 | 109.9 | 110.5 | 105.8 |
| 9 | 156.3 | 157.7 | 157.8 | 152.9 | 152.7 | 153.1 | 155.0 |
| 10 | 104.4 | 105.0 | 107.7 | 105.5 | 105.4 | 106.5 | 106.1 |
| 2′ | 78.4 | 79.3 | 76.6 | 69.6 | 69.6 | 70.2 | 74.2 |
| 3′ | 74.3 | 75.0 | 72.4 | 135.2 | 135.0 | 135.8 | 73.5 |
| 4′ | 22.3 | 22.7 | 22.4 | 124.7 | 124.7 | 125.2 | 132.7 |
| 5′ | - | - | - | 20.6 | 20.3 | 20.9 | 117.3 |
| 1″ | 102.4 | 102.0 | 100.4 | 101.2 | 100.9 | 101.9 | 103.9 |
| 2″ | 74.9 | 74.9 | ** | 72.8 | 72.6 | 73.3 | 73.7 |
| 3″ | 78.4 | 78.1 | 76.9 | 76.8 | 76.1 | 76.8 | 76.5 |
| 4″ | 71.8 | 71.9 | 70.2 | 70.1 | 69.4 | 70.1 | 70.9 |
| 5″ | 78.4 | 75.0 | 76.9 | 76.6 | 75.2 | 76.6 | 76.0 |
| 6″ | 63.0 | 68.8 | 61.3 | 60.6 | 67.7 | 61.6 | 68.5 |
| 1‴ | - | 111.0 | - | - | 102.7 | - | 103.4 |
| 2‴ | - | 77.9 | - | - | 72.8 | - | 73.7 |
| 3‴ | - | 80.5 | - | - | 76.1 | - | 76.9 |
| 4‴ | - | 75.0 | - | - | 69.4 | - | 70.0 |
| 5‴ | - | 65.7 | - | - | 75.9 | - | 77.0 |
| 6‴ | - | - | - | - | 60.4 | - | 61.1 |
| 2a | 20.1 | 20.4 | 19.0 | 19.4 | 19.2 | 59.7 | 20.1 |
| 2’a | 22.3 | 22.4 | 21.2 | - | - | - | - |
| 2’b | 25.7 | 26.1 | 25.2 | - | - | - | - |
| 3’a | - | - | - | 69.6 | 69.6 | 70.2 | 73.0 |
| 7-OCH3 | - | - | 56.0 | - | - | - | - |
| Solvent | CDCl3 | CD3OD | DMSO-d6 | DMSO-d6 | DMSO-d6 | DMSO-d6 | DMSO-d6 |
| References | [121] | [119] | [123] | [108] | [108] | [108] | [109] |
* Interchangeable data. ** The authors missed the assignment of this position.
Table 36.
The 13C-NMR spectral data of compounds 157–165.
| C | 157 | 158 | 159 | 160 | 161 | 162 | 163 | 164 | 165 |
|---|---|---|---|---|---|---|---|---|---|
|
Chromone
moiety |
|||||||||
| 2′ | 92.4 | 92.2 | 92.2 | 92.2 | 92.2 | 92.2 | 92.2 | 92.2 | 92.2 |
| 3′ | 27.9 | 27.8 | 27.8 | 27.8 | 27.8 | 27.8 | 27.8 | 27.8 | 27.8 |
| 4′ | 156.3 | 156.2 | 156.2 | 156.2 | 156.2 | 156.2 | 156.2 | 156.2 | 156.1 |
| 5′ | 176.2 | 176.2 | 176.2 | 176.2 | 176.2 | 176.2 | 176.3 | 176.3 | 176.3 |
| 6′ | 111.5 | 110.9 | 111.1 | 110.9 | 110.9 | 110.8 | 111.1 | 111.2 | 111.1 |
| 7′ | 162.1 | 162.5 | 162.4 | 162.4 | 162.7 | 162.5 | 162.6 | 162.6 | 162.5 |
| 9′ | 94.1 | 94.0 | 94.0 | 94.0 | 94.1 | 94.0 | 94.0 | 94.1 | 94.0 |
| 10′ | 165.4 | 165.2 | 165.2 | 165.2 | 165.2 | 165.2 | 165.2 | 165.2 | 165.2 |
| 11′ | 118.5 | 118.4 | 118.4 | 118.4 | 118.4 | 118.4 | 118.4 | 118.4 | 118.4 |
| 12′ | 112.9 | 112.8 | 112.8 | 112.5 | 112.8 | 112.8 | 112.8 | 112.8 | 112.7 |
| 13′ | 159.9 | 159.8 | 159.8 | 159.7 | 159.8 | 159.7 | 159.8 | 159.9 | 159.8 |
| 14′ | 66.3 | 66.3 | 66.5 | 66.2 | 66.4 | 66.2 | 66.5 | 66.3 | 66.5 |
| 4-OCH3 | 60.9 | 60.9 | 60.9 | 60.8 | 60.9 | 60.9 | 60.9 | 60.9 | 60.8 |
| 1″ | 70.6 | 70.6 | 70.6 | 70.6 | 70.6 | 70.6 | 70.6 | 70.7 | 70.6 |
| 2″ | 26.0 | 26.1 | 26.1 | 26.1 | 26.1 | 26.1 | 26.1 | 26.2 | 26.1 |
| 3″ | 25.6 | 25.7 | 25.7 | 25.6 | 25.7 | 25.7 | 25.6 | 25.6 | 25.7 |
| Glu-1 | 101.7 | 104.1 | 104.2 | 104.1 | 104.1 | 104.0 | 104.2 | 103.9 | 104.3 |
| Glu-2 | 74.5 | 74.9 | 74.8 | 74.8 | 74.9 | 74.9 | 74.9 | 74.8 | 74.9 |
| Glu-3 | 79.1 | 78.3 | 78.1 | 78.4 | 78.4 | 78.4 | 78.4 | 78.2 | 78.1 |
| Glu-4 | 69.3 | 71.2 | 71.4 | 71.4 | 71.5 | 71.3 | 71.5 | 72.0 | 71.9 |
| Glu-5 | 78.4 | 76.9 | 75.4 | 77.1 | 77.1 | 76.7 | 77.0 | 76.8 | 76.7 |
| Glu-6 | 62.4 | 63.5 | 64.2 | 62.7 | 62.7 | 62.8 | 62.6 | 62.9 | 62.7 |
|
Triterpene
moiety |
|||||||||
| 1 | 31.8 | 31.9 | 31.9 | 32.3 | 30.3 | 32.0 | 27.4 | 31.9 | 31.9 |
| 2 | 29.8 | 29.8 | 30.0 | 30.0 | 29.5 | 30.0 | 29.8 | 29.6 | 29.8 |
| 3 | 87.8 | 88.0 | 88.3 | 88.4 | 88.1 | 88.3 | 88.3 | 88.0 | 88.0 |
| 4 | 41.1 | 40.3 | 41.2 | 41.2 | 41.2 | 41.2 | 40.7 | 41.1 | 41.1 |
| 5 | 47.1 | 47.0 | 47.4 | 47.4 | 42.6 | 47.4 | 43.8 | 47.0 | 46.9 |
| 6 | 20.5 | 20.4 | 20.8 | 20.9 | 21.7 | 20.8 | 22.0 | 20.4 | 20.3 |
| 7 | 25.6 | 25.8 | 25.9 | 26.4 | 113.2 | 26.3 | 114.0 | 25.8 | 25.7 |
| 8 | 46.0 | 45.9 | 47.1 | 49.0 | 149.1 | 47.3 | 148.6 | 45.8 | 46.4 |
| 9 | 19.8 | 19.9 | 19.2 | 19.9 | 21.1 | 19.5 | 27.6 | 20.0 | 20.5 |
| 10 | 26.5 | 26.6 | 26.6 | 26.5 | 28.2 | 26.4 | 29.1 | 26.6 | 27.0 |
| 11 | 36.7 | 36.6 | 26.3 | 26.4 | 25.4 | 26.1 | 63.2 | 36.7 | 36.4 |
| 12 | 77.2 | 76.8 | 31.4 | 34.0 | 33.9 | 33.3 | 48.3 | 77.2 | 76.9 |
| 13 | 48.8 | 48.7 | 42.2 | 41.8 | 41.2 | 46.6 | 45.4 | 48.7 | 48.8 |
| 14 | 47.2 | 47.4 | 45.2 | 46.5 | 49.8 | 45.1 | 48.1 | 47.8 | 47.8 |
| 15 | 43.4 | 43.4 | 50.8 | 82.5 | 80.4 | 42.8 | 45.2 | 43.8 | 45.6 |
| 16 | 70.7 | 72.8 | 218.6 | 103.0 | 103.3 | 72.5 | 114.5 | 71.1 | 74.6 |
| 17 | 55.7 | 55.6 | 60.4 | 60.5 | 60.3 | 51.4 | 61.0 | 56.8 | 52.4 |
| 18 | 13.5 | 13.5 | 18.8 | 20.3 | 22.5 | 20.5 | 20.7 | 13.5 | 12.9 |
| 19 | 29.9 | 30.7 | 30.0 | 30.7 | 28.2 | 30.1 | 18.7 | 29.8 | 30.0 |
| 20 | 26.9 | 25.4 | 29.1 | 27.6 | 27.5 | 34.2 | 23.7 | 26.0 | 24.6 |
| 21 | 21.1 | 20.8 | 19.9 | 21.4 | 21.6 | 17.3 | 19.6 | 21.1 | 25.7 |
| 22 | 41.5 | 38.6 | 40.3 | 33.6 | 33.6 | 86.2 | 37.8 | 41.9 | 106.0 |
| 23 | 104.2 | 102.9 | 173.4 | 74.2 | 74.4 | 109.6 | 71.7 | 101.9 | 152.9 |
| 24 | 83.1 | 212.4 | - | 80.5 | 80.4 | 77.3 | 88.5 | 77.7 | 75.9 |
| 25 | 79.1 | 35.0 | - | 76.4 | 76.5 | 83.5 | 76.4 | 81.3 | 78.2 |
| 26 | 19.4 | 19.2 | - | 21.6 | 21.6 | 27.3 | 23.2 | 23.7 | 22.3 |
| 27 | 29.8 | 19.7 | - | 24.1 | 24.1 | 24.5 | 20.2 | 24.9 | 23.1 |
| 28 | 19.4 | 19.4 | 19.6 | 11.8 | 18.2 | 19.4 | 27.4 | 19.5 | 20.7 |
| 29 | 25.6 | 25.6 | 25.6 | 25.5 | 25.6 | 25.6 | 25.8 | 25.6 | 25.6 |
| 30 | 15.2 | 15.2 | 15.2 | 15.5 | 14.2 | 15.3 | 14.5 | 15.2 | 15.2 |
| Xyl-1 | 107.4 | 107.5 | 107.5 | 107.4 | 107.4 | 107.4 | 107.4 | 107.5 | 107.5 |
| Xyl-2 | 75.5 | 75.5 | 75.5 | 75.5 | 75.5 | 75.5 | 75.5 | 75.5 | 75.5 |
| Xyl-3 | 78.5 | 78.6 | 78.5 | 78.5 | 78.5 | 78.5 | 78.5 | 78.5 | 78.5 |
| Xyl-4 | 71.1 | 71.0 | 71.1 | 71.1 | 71.2 | 71.1 | 71.1 | 71.2 | 71.1 |
| Xyl-5 | 67.0 | 67.0 | 67.1 | 67.0 | 67.0 | 67.0 | 67.0 | 67.0 | 67.0 |
| COCH3 | 170.4 21.5 |
170.4 21.5 |
- | 171.0 21.1 |
171.0 21.1 |
- | - | 170.5 21.6 |
170.6 21.1 |
| Solvent | C5D5N | C5D5N | C5D5N | C5D5N | C5D5N | C5D5N | C5D5N | C5D5N | C5D5N |
| References | [130] | [130] | [130] | [130] | [130] | [130] | [130] | [136] | [136] |
Table 37.
The 13C-NMR spectral data of compounds 166 and 167.
| C | 166 | 167 |
|---|---|---|
| 1 | 98.1 | 98.1 |
| 3 | 154.5 | 154.5 |
| 4 | 105.6 | 105.6 |
| 5 | 28.7 | 28.6 |
| 6 | 30.0 | 29.9 |
| 7 | 75.1 | 75.0 |
| 8 | 133.3 | 133.3 |
| 9 | 43.9 | 43.8 |
| 10 | 121.1 | 121.1 |
| 11 | 168.6 | 168.5 a |
| 1′ | 99.8 | 99.7 |
| 2′ | 74.8 | 74.7 b |
| 3′ | 77.9 | 77.8 c |
| 4′ | 71.6 | 71.2 d |
| 5′ | 78.5 | 78.4 e |
| 6′ | 62.7 | 62.4 f |
| 2″ | 167.5 | 168.1 a |
| 3″ | 118.2 | 118.6 |
| 4″ | 181.6 | 181.8 |
| 5″ | 163.5 | 163.1 |
| 6″ | 100.2 | 101.1 |
| 7″ | 166.2 | 164.9 |
| 8″ | 94.7 | 95.7 |
| 9″ | 159.2 | 158.7 |
| 10″ | 104.9 | 106.6 |
| -CH3 | 18.9 | 19.0 |
| 1‴ | - | 101.6 |
| 2‴ | - | 74.8 b |
| 3‴ | - | 77.9 c |
| 4‴ | - | 71.6 d |
| 5‴ | - | 78.5 e |
| 6‴ | - | 62.7 f |
| NMR | CD3OD | CD3OD |
| References | [43] | [43] |
a−f Values with the same superscript are interchangeable.
Table 38.
The 13C-NMR spectral data of compounds 168–180 except those which have no reported 13C-NMR data.
| C | 168 | 169 | 170 | 171 | 172 | 178 | 179 | 180 |
|---|---|---|---|---|---|---|---|---|
| 2 | 163.7 | 159.3 | 168.0 | 159.6 | 167.5 | 170.2 | 166.3 | 166.3 |
| 3 | 108.7 | 108.8 | 109.7 | 109.9 | 110.0 | 110.8 | 110.0 | 110.0 |
| 4 | 179.2 | 179.5 | 178.9 | 178.8 | 179.0 | 179.0 | 178.7 | 178.8 |
| 4a | 125.8 | 126.2 | 125.9 * | 126.4 | 125.9 * | 125.8 * | 125.8 * | 126.5 |
| 5 | 149.6 | 149.6 | 149.9 | 150.0 | 149.8 | 150.1 | 149.7 | 149.9 |
| 6 | 125.4 | 125.4 | 125.8 | 125.9 | 125.9 * | 125.8 * | 125.9 | 126.0 |
| 6a | 137.0 | 137.2 | 137.0 | 137.0 | 137.4 | 137.2 | 137.3 | 136.3 |
| 7 | 183.0 | 183.3 | 183.2 | 183.5 | 183.3 | 183.1 | 183.1 | 181.5 |
| 7a | 125.8 | 127.3 | 126.3 * | 126.5 | 126.4 * | 126.1 * | 126.2 * | 130.7 |
| 8 | 140.0 | 140.6 | 139.7 | 140.0 | 140.1 | 139.9 | 140.2 | 119.3 |
| 9 | 133.0 | 132.6 | 132.9 | 132.2 | 133.1 | 133.3 | 133.1 | 133.2 |
| 10 | 138.4 | 138.5 | 137.8 | 137.0 | 138.5 | 138.6 | 138.6 | 140.7 |
| 11 | 159.7 | 163.9 | 159.7 | 167.6 | 159.9 | 159.9 | 159.8 | 159.5 |
| 11a | 116.0 | 115.8 | 116.1 | 116.0 | 116.2 | 116.0 | 116.1 | 116.1 |
| 12 | 188.1 | 188.0 | 187.9 | 187.7 | 188.1 | 188.3 | 188.0 | 187.7 |
| 12a | 118.9 | 118.9 | 119.1 | 119.1 | 119.2 | 118.9 | 119.2 | 119.8 |
| 12b | 155.7 | 155.8 | 156.0 | 156.0 | 156.2 | 155.8 | 156.1 | 156.2 |
| 13 | 24.0 | 24.1 | 24.3 | 24.2 | 24.1 | 24.2 | 24.1 | 24.1 |
| 14 | 127.2 | 125.9 | 57.6 | 60.3 | 59.1 | 75.6 | 57.7 | 57.7 |
| 15 | 12.1 | 15.0 | 13.8 | 14.9 | 14.5 | 23.5 | 14.5 | 14.4 |
| 16 | 134.2 | 134.2 | 62.0 | 61.7 | 61.6 | 71.7 * | 63.9 | 63.9 |
| 17 | 14.9 | 12.1 | 14.1 | 123.3 | 123.3 | 127.4 | 55.4 | 55.4 |
| 18 | - | - | - | 134.1 | 134.0 | 130.2 | 51.8 | 51.8 |
| 19 | - | - | - | 14.4 | 13.8 | 13.5 | 17.2 | 17.2 |
| 2′ | 77.3 | 77.7 | 77.2 | 77.6 | 77.3 | 77.2 | 77.3 | 67.9 |
| 3′ | 71.9 | 71.8 | 71.3 | 70.6 | 71.9 | 71.7 * | 71.9 | 71.0 |
| 4′ | 67.4 | 67.5 | 67.8 | 68.1 | 67.4 | 67.3 * | 67.4 | 57.7 |
| 5′ | 28.3 | 41.0 | 28.8 | 38.8 | 28.4 | 28.4 | 28.3 | 35.0 |
| 6′ | 75.2 | 75.4 | 74.8 | 74.0 | 75.2 | 75.0 | 75.2 | 68.2 |
| 7′ | 18.9 | 18.9 | 18.8 | 18.5 | 18.9 | 18.9 | 18.9 | 17.5 |
| 8′ | - | - | - | - | - | - | - | 13.2 |
| 4′-N(CH3)2 | 40.4 | 40.5 | 40.3 | 40.4 | 40.4 | 40.3 | 40.4 | 37.1 |
| 2″ | 67.2 | 69.8 | 67.5 | 69.8 | 67.2 | 67.4 * | 67.3 | - |
| 3″ | 70.8 | 76.4 | 70.3 | 74.5 | 70.9 | 70.7 | 70.9 | - |
| 4″ | 57.4 | 57.6 | 57.6 | 59.1 | 57.3 | 57.9 | 57.3 | - |
| 5″ | 33.6 | 28.6 | 33.3 | 30.4 | 33.6 | 33.3 | 33.7 | - |
| 6″ | 69.5 | 64.9 | 69.4 | 65.7 | 69.7 | 69.6 | 69.6 | - |
| 7″ | 17.6 | 15.0 | 17.4 | 15.1 | 17.7 | 17.6 | 17.6 | - |
| 8″ | 12.3 | 13.7 | 13.1 | 13.9 | 12.3 | 12.6 | 12.3 | - |
| 3″-COCH3 | - | 170.6 21.3 |
- | 171.0 21.5 |
- | - | - | - |
| 4″-N(CH3)2 | 36.8 | 39.3 | 36.9 | 38.8 | 36.8 | 36.8 | 36.8 | - |
| Solvent | CDCl3 | CDCl3 | CDCl3 | CDCl3 | CDCl3 | CDCl3 | CDCl3 | CDCl3 |
| References | [165] | [140] | [141] | [140] | [142] | [143] | [165] | [146] |
* Interchangeable values.
Table 39.
The 13C-NMR spectral data of compounds 181–183.
| C | 181 | 182 | 183 |
|---|---|---|---|
| 2 | 168.4 | 176.4 | 176.5 |
| 3 | 112.0 | 111.1 | 111.3 |
| 4 | 180.3 | 181.3 | 178.4 |
| 4a | 126.1 | 126.0 | 124.7 |
| 5 | 150.4 | 150.4 | 149.6 |
| 6 | 121.3 | 121.0 | 121.2 |
| 6a | 138.3 | 138.1 | 139.0 |
| 7 | 182.7 | 182.7 | 182.4 |
| 7a | 133.2 | 133.2 | 133.2 |
| 8 | 120.1 | 120.0 | 119.8 |
| 9 | 135.4 | 135.5 | 135.6 |
| 10 | 137.4 | 137.4 | 137.0 |
| 11 | 161.1 | 161.0 | 161.3 |
| 11a | 118.1 | 118.0 | 118.0 |
| 12 | 189.1 | 189.2 | 189.2 |
| 12a | 122.0 | 121.9 | 121.6 |
| 12b | 157.7 | 157.4 | 156.9 |
| 13 | 70.8 | 70.8 | 175.3 |
| 14 | 61.2 | 77.7 | 77.7 |
| 15 | 63.7 | 72.6 | 72.6 |
| 16 | 20.2 | 23.9 | 23.9 |
| 17 | 13.7 | 17.0 | 17.1 |
| 1′ | 98.9 | 98.9 | - |
| 2′ | 37.2 | 37.2 | - |
| 3′ | 58.3 | 58.4 | - |
| 4′ | 71.2 | 71.1 | - |
| 5′ | 70.4 | 70.4 | - |
| 6′ | 17.2 | 17.2 | - |
| 7′ | 24.6 | 24.5 | - |
| 1″ | 70.2 | 70.1 | 70.3 |
| 2″ | 28.0 | 27.7 | 27.4 |
| 3″ | 65.2 | 65.1 | 64.9 |
| 4″ | 75.1 | 75.0 | 75.2 |
| 5″ | 72.6 | 72.6 | 72.3 |
| 6″ | 18.1 | 18.2 | 18.3 |
| 3″-N(CH3)2 | 43.2 41.7 |
43.3 41.4 |
42.5 42.5 |
| 1‴ | 101.4 | 101.4 | 101.4 |
| 2‴ | 33.4 | 33.4 | 33.4 |
| 3‴ | 66.6 | 66.6 | 66.6 |
| 4‴ | 72.0 | 72.0 | 72.0 |
| 5‴ | 69.5 | 69.5 | 69.5 |
| 6‴ | 17.5 | 17.5 | 17.5 |
| Solvent | CD3OD | CD3OD | CD3OD |
| References | [147] | [147] | [147] |
Table 40.
The 13C-NMR spectral data of compounds 184–192.
| C | 184 | 185 | 186 | 187 | 188 | 189 | 190 | 191 | 192 |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 167.5 | 167.4 | 167.0 | 167.0 | 165.7 | 165.7 | 167.5 | 169.3 | 169.3 |
| 3 | 111.1 | 110.9 | 110.9 | 110.8 | 111.3 | 111.3 | 111.1 | 109.1 | 109.1 |
| 4 | 180.2 | 180.0 | 179.4 | 179.4 | 178.5 | 178.6 | 180.2 | 182.4 | 182.5 |
| 4a | 126.6 | 126.4 | 126.5 | 126.4 | 126.3 | 126.3 | 126.6 | 113.3 | 113.3 |
| 5 | 149.2 | 149.1 | 149.4 | 149.2 | 148.2 | 148.2 | 149.3 | 166.7 | 166.7 |
| 6 | 122.5 | 122.4 | 122.9 | 122.8 | 124.1 | 124.0 | 122.5 | 110.7 | 110.7 |
| 6a | 137.2 | 137.1 | 137.0 | 136.9 | 136.9 | 136.9 | 137.2 | 139.8 | 139.9 |
| 7 | 181.2 | 181.0 | 181.2 | 181.2 | 181.5 | 181.4 | 181.2 | 180.8 | 180.8 |
| 7a | 130.3 | 130.2 | 130.4 | 130.3 | 130.5 | 130.5 | 130.5 | 130.6 | 130.4 |
| 8 | 119.8 | 119.7 | 119.7 | 119.7 | 119.5 | 119.5 | 119.8 | 119.4 | 119.5 |
| 9 | 133.6 | 133.7 | 133.4 | 133.6 | 133.3 | 133.5 | 133.7 | 132.4 | 132.8 |
| 10 | 141.3 | 141.1 | 141.0 | 140.9 | 141.0 | 140.9 | 140.7 | 140.2 | 140.9 |
| 11 | 159.1 | 159.2 | 159.3 | 158.9 | 159.3 | 159.3 | 159.4 | 159.1 | 159.1 |
| 11a | 115.8 | 115.6 | 115.9 | 115.7 | 115.9 | 115.9 | 115.9 | 115.6 | 115.4 |
| 12 | 186.9 | 186.9 | 187.1 | 186.9 | 187.5 | 187.4 | 186.9 | 186.2 | 186.3 |
| 12a | 121.8 | 121.6 | 121.6 | 121.6 | 120.8 | 120.8 | 121.8 | 112.3 | 112.4 |
| 12b | 156.8 | 156.7 | 156.6 | 156.5 | 156.1 | 156.1 | 156.8 | 156.6 | 156.6 |
| 13 | 80.9 | 80.0 | 79.0 | 78.9 | 48.3 | 48.2 | 80.9 | - | - |
| 14 | 59.8 | 59.7 | 59.8 | 59.7 | 59.8 | 59.7 | 59.8 | 60.0 | 60.0 |
| 15 | 19.7 | 19.6 | 19.8 | 19.7 | 20.0 | 19.9 | 19.6 | 19.6 | 19.7 |
| 16 | 62.7 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.7 | 62.7 | 62.7 |
| 17 | 13.4 | 13.3 | 13.4 | 13.3 | 13.5 | 13.4 | 13.3 | 13.2 | 13.2 |
| 18 | 170.5 | 170.4 | 170.9 | 170.9 | 170.4 | 170.4 | 170.5 | - | - |
| 19 | 52.6 | 52.5 | 52.4 | 52.3 | 52.3 | 52.3 | 52.6 | - | - |
| 2′ | 73.8 | 73.8 | 74.9 | 74.9 | 74.3 | 74.2 | 73.8 | - | - |
| 3′ | 68.9 | 68.9 | 68.5 | 68.2 | 68.8 | 68.8 | 69.0 | - | - |
| 4′ | 80.2 | 80.1 | 74.8 | 74.9 | 81.5 | 81.4 | 80.2 | - | - |
| 5′ | 67.9 | 67.9 | 26.1 | 26.0 | 68.0 | 67.9 | 68.0 | - | - |
| 6′ | 73.7 | 73.6 | 70.3 | 70.2 | 73.9 | 74.0 | 73.7 | - | - |
| 7′ | 14.1 | 14.0 | 14.7 | 14.7 | 14.7 | 14.6 | 14.0 | - | - |
| 4′-OCH3 | 57.9 | 57.8 | 55.6 | 55.5 | 57.1 | 57.1 | 58.0 | - | - |
| 2″ | 70.3 | 70.7 | 70.2 | 70.8 | 70.3 | 70.8 | 70.0 | 70.0 | 70.7 |
| 3″ | 77.7 | 82.6 | 77.8 | 82.7 | 77.8 | 82.8 | 76.0 | 77.3 | 82.8 |
| 4″ | 54.9 | 58.1 | 55.1 | 58.1 | 54.9 | 58.1 | 51.7 | 56.1 | 58.1 |
| 5″ | 40.3 | 44.7 | 40.4 | 44.7 | 40.4 | 44.8 | 44.9 | 38.5 | 44.8 |
| 6″ | 62.1 | 62.2 | 62.3 | 62.2 | 62.2 | 62.3 | 62.3 | 63.6 | 62.3 |
| 7″ | 14.7 | 13.5 | 14.8 | 13.5 | 14.7 | 13.6 | 14.1 | 15.4 | 13.6 |
| 8″ | 24.1 | 14.0 | 23.8 | 13.9 | 24.2 | 14.1 | 32.6 | 22.6 | 14.0 |
| 4″-N(CH3)n | 27.9 | 40.3 | 27.8 | 40.3 | 28.1 | 40.4 | - | 27.2 | 40.3 |
| 1‴ | 93.4 | 94.4 | 93.7 | 94.5 | 93.4 | 94.5 | 93.3 | 94.5 | 94.5 |
| 2‴ | 30.8 | 31.1 | 30.9 | 31.1 | 30.9 | 31.1 | 30.8 | 31.1 | 31.1 |
| 3‴ | 74.8 | 74.9 | 74.8 | 74.9 | 74.9 | 75.0 | 74.8 | 75.0 | 75.0 |
| 4‴ | 72.1 | 72.1 | 72.1 | 72.0 | 72.2 | 72.2 | 72.2 | 71.5 | 72.2 |
| 5‴ | 65.4 | 65.0 | 65.7 | 65.0 | 65.4 | 65.1 | 65.4 | 66.6 | 65.0 |
| 6‴ | 17.7 | 17.6 | 17.7 | 17.6 | 17.8 | 17.7 | 17.8 | 17.5 | 17.7 |
| 3‴-OCH3 | 55.9 | 56.1 | 56.1 | 56.1 | 55.9 | 56.2 | 56.0 | 56.4 | 56.1 |
| Solvent | CDCl3 | CDCl3 | CDCl3 | CDCl3 | CDCl3 | CDCl3 | CDCl3 | CDCl3 | CDCl3 |
| References | [149] | [149] | [149] | [149] | [137] | [137] | [137] | [137] | [137] |
Figure 25.
Representative guide figure for numbering of chromone glycosides attached to different substituents.
6. Conclusions
Chromone glycosides are one of the most important classes of secondary metabolites. In this review, we summarized 192 naturally occurring chromone glycosides with their sources, reported activities, and spectroscopic features. Basically, they were categorized into several classes: chromone-O-glycosides including compounds 1–59, among them, four chromanone glycosides (60–63), chromone-C-glycosides including compounds 64–122, prenyl and isoprenyl chromone glycosides including compounds 123–134, phenyl ethyl chromone glycosides including compounds 135–139, furano-chromone glycosides including compounds 140–148, pyrano-chromone glycosides including compounds 149–151, oxepino-chromone glycosides including compounds 152–155, Pyrido-chromone glycoside including compound 156, furanochromones with cycloartane triterpenes including compounds 157–165, glycoside derivatives of chromones with secoiridoids including compounds 166 and 167, and chromone alkaloids aminoglycosides including compounds 168–192. Diverse bioactivities were discovered for most of the reported chromone glycosides. Several chromone glycosides show potent biological activities as anti-viral, acetylcholinesterase inhibition, anti-tumor, anti-inflammatory, etc. This review directs the attention for further deep investigation of chromone glycosides for drug discovery.
Abbreviations
| All | Allose |
| Api | Apiose |
| Ara | Arabinose |
| Caf | Caffeic acid |
| Cin | Cinnamic acid |
| Cou | Coumaric acid |
| Fer | Ferulic acid |
| Gall | Gallic acid |
| Glu | Glucose |
| Rha | Rhamnose |
| Xyl | Xylose |
Author Contributions
Y.A.: conceptualization, data collection, writing, reviewing. M.E., A.O. and M.S.: data collection, writing, reviewing. K.S.: supervision, reviewing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Herath H.M.T.B., Jacob M., Wilson A.D., Abbas H.K., Nanayakkara N.P.D. New secondary metabolites from bioactive extracts of the fungus Armillaria tabescens. Nat. Prod. Res. 2013;27:1562–1568. doi: 10.1080/14786419.2012.738206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi S., Park Y.-I., Lee S.-K., Kim J.-E., Chung M.-H. Aloesin inhibits hyperpigmentation induced by UV radiation. Clin. Exp. Dermatol. 2002;27:513–515. doi: 10.1046/j.1365-2230.2002.01120.x. [DOI] [PubMed] [Google Scholar]
- 3.Machado N.F.L., Marques M.P.M. Bioactive Chromone Derivatives-Structural Diversity. Curr. Bioact. Compd. 2010;6:76–89. doi: 10.2174/157340710791184859. [DOI] [Google Scholar]
- 4.Daniel M. Medicinal Plants: Chemistry and Properties. Science Publishers; Hauppauge, NY, USA: 2006. [Google Scholar]
- 5.Mander L., Liu H.-W. Comprehensive Natural Products II: Chemistry and Biology. Volume 1. Elsevier; Amsterdam, The Netherlands: 2010. [Google Scholar]
- 6.Dewick P.M. Medicinal Natural Products: A Biosynthetic Approach. John Wiley & Sons; Hoboken, NJ, USA: 2002. [Google Scholar]
- 7.Daily A., Seligmann O., Nonnenmacher G., Fessler B., Wong S., Wagner H. New chromone, coumarin, and coumestan derivatives from Mutisia acuminata var. hirsuta. Planta Med. 1988;54:50–52. doi: 10.1055/s-2006-962334. [DOI] [PubMed] [Google Scholar]
- 8.Tschesche R., Delhvi S., Sepulveda S., Breitmaier E. Eucryphin, a new chromone rhamnoside from the bark of Eucryphia cordifolia. Phytochemistry. 1979;18:867–869. doi: 10.1016/0031-9422(79)80032-1. [DOI] [Google Scholar]
- 9.Li Z., Li D., Owen N.L., Len Z., Cao Z., Yi Y. Structure determination of a new chromone glycoside by 2D INADEQUATE NMR and molecular modeling. Magn. Reson. Chem. 1996;34:512–517. doi: 10.1002/(SICI)1097-458X(199607)34:7<512::AID-OMR912>3.0.CO;2-8. [DOI] [Google Scholar]
- 10.Feng Z., Wang Y., Zhang P. Chemical constituents of Rhododendron ovatum Planch. Yaoxue Xuebao. 2005;40:150–152. [PubMed] [Google Scholar]
- 11.Kuo L.-M.Y., Zhang L.-J., Huang H.-T., Lin Z.-H., Liaw C.-C., Cheng H.-L., Lee K.-H., Morris-Natschke S.L., Kuo Y.-H., Ho H.-O. Antioxidant Lignans and Chromone Glycosides from Eurya japonica. J. Nat. Prod. 2013;76:580–587. doi: 10.1021/np3007638. [DOI] [PubMed] [Google Scholar]
- 12.Chen G., Jin H.Z., Li X.F., Zhang Q., Shen Y.H., Yan S.K., Zhang W.D. A new chromone glycoside from Rhododendron spinuliferum. Arch. Pharm. Res. 2008;31:970–972. doi: 10.1007/s12272-001-1253-y. [DOI] [PubMed] [Google Scholar]
- 13.Han L.K., Ninomiya H., Taniguchi M., Baba K., Kimura Y., Okuda H. Norepinephrine-augmenting lipolytic effectors from Astilbe thunbergii rhizomes. J. Nat. Prod. 1998;61:1006–1011. doi: 10.1021/np980107o. [DOI] [PubMed] [Google Scholar]
- 14.Sumiyoshi M., Kimura Y. Enhancing effects of a chromone glycoside, eucryphin, isolated from Astilbe rhizomes on burn wound repair and its mechanism. Phytomedicine. 2010;17:820–829. doi: 10.1016/j.phymed.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Liao Y.Y. A new chromone glycoside from leaves of Salix matsudana. Chinese Tradit. Herb. Drugs. 2014;24:2887–2889. [Google Scholar]
- 16.Tane P., Ayafor J.F., Sondengam B.L., Connolly J.D. Chromone glycosides from Schumanniophyton magnificum. Phytochemistry. 1990;29:1004–1007. doi: 10.1016/0031-9422(90)80071-N. [DOI] [PubMed] [Google Scholar]
- 17.Kumar V., Gupta M., Gandhi S.G., Bharate S.S., Kumar A., Vishwakarma R.A., Bharate S.B. Anti-inflammatory chromone alkaloids and glycoside from Dysoxylum binectariferum. Tetrahedron Lett. 2017;58:3974–3978. doi: 10.1016/j.tetlet.2017.09.005. [DOI] [Google Scholar]
- 18.Al-Taweel A., Alqasoumi S., Alam P., Abdel-Kader M. Densitometric-high-performance thin-layer chromatographic estimation of diosmin, hesperidin, and ascorbic acid in pharmaceutical formulations. J. Planar Chromatogr. Mod. TLC. 2013;26:336–342. doi: 10.1556/JPC.26.2013.4.8. [DOI] [Google Scholar]
- 19.Fawzy G.A., Al-Taweel A.M., Perveen S., Khan S.I., Al-Omary F.A. Bioactivity and chemical characterization of Acalypha fruticosa Forssk. growing in Saudi Arabia. Saudi Pharm. J. 2017;25:104–109. doi: 10.1016/j.jsps.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sueyoshi E., Yu Q., Matsunami K., Otsuka H. Staphylosides A and B: Two new chromone diglucosides from leaves of Staphylea bumalda DC. Heterocycles. 2008;76:845–849. [Google Scholar]
- 21.Elgorashi E.E., Coombes P.H., Mulholland D.A., Van Staden J. Isoeugenitol, a cyclooxygenase-1 inhibitor from Gethyllis ciliaris. South African J. Bot. 2007;73:156–158. doi: 10.1016/j.sajb.2006.06.008. [DOI] [Google Scholar]
- 22.Han L., Zheng F., Zhang Y., Liu E., Li W., Xia M., Wang T., Gao X. Triglyceride accumulation inhibitory effects of new chromone glycosides from Drynaria fortunei. Nat. Prod. Res. 2015;29:1703–1710. doi: 10.1080/14786419.2014.998216. [DOI] [PubMed] [Google Scholar]
- 23.Isaka M., Haritakun R., Supothina S., Choowong W., Mongkolsamrit S. N-Hydroxypyridone alkaloids, chromone derivatives, and tetrahydroxanthones from the scale-insect pathogenic fungus Orbiocrella sp. BCC 33248. Tetrahedron. 2014;70:9198–9203. doi: 10.1016/j.tet.2014.10.029. [DOI] [Google Scholar]
- 24.Ma L., Ma S., Wei F., Lin R., But P.P., Lee S.H., Lee S.F. Uncinoside A and B, two new antiviral chromone glycosides from Selaginella uncinata. Chem. Pharm. Bull. (Tokyo) 2003;51:1264–1267. doi: 10.1248/cpb.51.1264. [DOI] [PubMed] [Google Scholar]
- 25.Arakawa Y., Chiji H., Izawa M. Structural elucidation of two new chromones isolated from glasswort (Salicornia europaea L.) Agric. Biol. Chem. 1983;47:2029–2033. [Google Scholar]
- 26.Chen X.-Q., Zan K., Liu H., Yang J., Lai M.-X., Wang Q. Triterpenes and flavonoids from Ilex hainanensis Merr. (Aquifoliaceae) Biochem. Syst. Ecol. 2009;37:678–682. doi: 10.1016/j.bse.2009.08.006. [DOI] [Google Scholar]
- 27.Simon A., Chulia A.J., Kaouadji M., Delage C. Quercetin 3-[triacetylarabinosyl(1→6)galactoside] and chromones from Calluna vulgaris. Phytochemistry. 1994;36:1043–1045. doi: 10.1016/S0031-9422(00)90488-6. [DOI] [PubMed] [Google Scholar]
- 28.Cui C.B., Tezuka Y., Kikuchi T., Nakano H., Tamaoki T., Park J.H. Constituents of a fern, Davallia mariessi Moore. IV. Isolation and structures of a novel norcarotane sesquiterpene glycoside, a chromone glucuronide, and two epicatechin glycosides. Chem. Pharm. Bull. (Tokyo) 1992;40:2035–2040. doi: 10.1248/cpb.40.2035. [DOI] [Google Scholar]
- 29.Li X., Yu M., Meng D., Li Z., Zhang L. A new chromone glycoside from Polygonum capitatum. Fitoterapia. 2007;78:506–509. doi: 10.1016/j.fitote.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Murata T., Selenge E., Suganuma K., Asai Y., Batkhuu J., Yoshizaki F. Chromone acyl glucosides and an ayanin glucoside from Dasiphora parvifolia. Phytochem. Lett. 2013;6:552–555. doi: 10.1016/j.phytol.2013.07.004. [DOI] [Google Scholar]
- 31.Yu J., Song X., Wang D., Wang X., Wang X. Five new chromone glycosides from Scindapsus officinalis (Roxb.) Schott. Fitoterapia. 2017;122:101–106. doi: 10.1016/j.fitote.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Cisowski W. 2-Methyl-5,7-dihydroxychromone glucoside from Ammi visnaga Lamarck. Pol. J. Chem. 1986;60:837–840. [Google Scholar]
- 33.Wang Y.-B., Huang R., Zhang H.-B., Li L. Chromone glycosides from Knoxia corymbosa. J. Asian Nat. Prod. Res. 2006;8:663–670. doi: 10.1080/10286020500246303. [DOI] [PubMed] [Google Scholar]
- 34.Ghosal S., Kumar Y., Singh S., Ahad K. Biflorin, a chromone-C-glucoside from Pancratium biflorum. Phytochemistry. 1983;22:2591–2593. doi: 10.1016/0031-9422(83)80172-1. [DOI] [Google Scholar]
- 35.Ghosal S., Singh S., Bhagat M.P., Kumar Y. Three chromones from bulbs of Pancratium biflorum. Phytochemistry. 1980;21:2943–2946. doi: 10.1016/0031-9422(80)85074-6. [DOI] [Google Scholar]
- 36.Ibrahim S., Mohamed G., Shaala L., Youssef D. Non-alkaloidal compounds from the bulbs of the Egyptian plant Pancratium maritimum. Z. Naturforsch. C. 2014;69:92–98. doi: 10.5560/znc.2013-0111. [DOI] [PubMed] [Google Scholar]
- 37.Kozowska V., Zheleva A., Pangarova T. Proceedings of the International Conference of Chemistry and Biotechnology of Biologically Active Natural Products, Budapest, Hungary, 15–19 August 1983. Volume 3. Bulgarian Academy of Sciences; Sofia, Bulgaria: 1981. 7-(β-d-Glucopyranosyloxy)-5-hydroxy-2-methyl chromone from Peucedanum austriacum; pp. 114–116. [Google Scholar]
- 38.Gujral V.K., Gupta S.R., Verma K.S. A new chromone glucoside from Tecomella undulata. Phytochemistry. 1979;18:181–182. doi: 10.1016/S0031-9422(00)90945-2. [DOI] [Google Scholar]
- 39.Brown R.T., Blackstock W.P., Chapple C.L. Isolation of 5,7-dihydroxy-2-methylchromone and its 7-O-glycosides from Adina rubescens. J. Chem. Soc. Perkin Trans. 1975:1776–1778. doi: 10.1039/p19750001776. [DOI] [Google Scholar]
- 40.Peng B., Bai R.-F., Li P., Han X.-Y., Wang H., Zhu C.-C., Zeng Z.-P., Chai X.-Y. Two new glycosides from Dryopteris fragrans with anti-inflammatory activities. J. Asian Nat. Prod. Res. 2016;18:59–64. doi: 10.1080/10286020.2015.1121853. [DOI] [PubMed] [Google Scholar]
- 41.Chen X.-Q., Li Y., Cheng X., Wang K., He J., Pan Z.-H., Li M.-M., Peng L.-Y., Xu G., Zhao Q.-S. Polycyclic Polyprenylated Acylphloroglucinols and Chromone O-Glucosides from Hypericum henryi subsp. uraloides. Chem. Biodivers. 2010;7:196–204. doi: 10.1002/cbdv.200900009. [DOI] [PubMed] [Google Scholar]
- 42.An R.B., Jeong G.S., Beom J.-S., Sohn D.H., Kim Y.C. Chromone glycosides and hepatoprotective constituents of Hypericum erectum. Arch. Pharm. Res. 2009;32:1393–1397. doi: 10.1007/s12272-009-2008-1. [DOI] [PubMed] [Google Scholar]
- 43.Itoh A., Tanahashi T., Nagakura N., Nishi T. Two chromone-secoiridoid glycosides and three indole alkaloid glycosides from Neonauclea sessilifolia. Phytochemistry. 2003;62:359–369. doi: 10.1016/S0031-9422(02)00541-1. [DOI] [PubMed] [Google Scholar]
- 44.Abe F., Yamauchi T. Megastigmanes and flavonoids from the leaves of Scorodocarpus borneensis. Phytochemistry. 1993;33:1499–1501. doi: 10.1016/0031-9422(93)85120-G. [DOI] [Google Scholar]
- 45.Gujral V.K., Gupta S.R., Verma K.S. Structure of undulatoside-B, a new chromone glycoside from Tecomella undulata. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 1979;17B:40–41. [Google Scholar]
- 46.Yoshimitsu H., Nishida M., Hashimoto F., Tanaka M., Sakata Y., Okawa M., Nohara T. Chromone and flavonol glycosides from Delphinium hybridum cv. “Belladonna Casablanca”. J. Nat. Med. 2007;61:334–338. doi: 10.1007/s11418-007-0142-y. [DOI] [Google Scholar]
- 47.Shang Z.-P., Meng J.-J., Zhao Q.-C., Su M.-Z., Luo Z., Yang L., Tan J.-J. Two new chromone glycosides from Drynaria fortunei. Fitoterapia. 2013;84:130–134. doi: 10.1016/j.fitote.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka N., Jia Y., Niwa K., Imabayashi K., Tatano Y., Yagi H., Kashiwada Y. Phloroglucinol derivatives and a chromone glucoside from the leaves of Myrtus communis. Tetrahedron. 2018;74:117–123. doi: 10.1016/j.tet.2017.11.044. [DOI] [Google Scholar]
- 49.Yi J., Zhang G., Li B. Studies on the chemical constituents of Pseudotsuga sinensis. Yao Xue Xue Bao. 2002;37:352–354. [PubMed] [Google Scholar]
- 50.Tanaka N., Kashiwada Y., Nakano T., Shibata H., Higuchi T., Sekiya M., Ikeshiro Y., Takaishi Y. Chromone and chromanone glucosides from Hypericum sikokumontanum and their anti-Helicobacter pylori activities. Phytochemistry. 2009;70:141–146. doi: 10.1016/j.phytochem.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Huneck S., Jakupovic J., Follmann G. The final structures of the lichen chromones galapagin, lobodirin, mollin, and roccellin. Zeitschrift Naturforsch. B. 1992;47:449–451. doi: 10.1515/znb-1992-0327. [DOI] [Google Scholar]
- 52.Li X., Niu L.-T., Meng W.-T., Zhu L.-J., Zhang X., Yao X.-S. Matteuinterins A-C, three new glycosides from the rhizomes of Matteuccia intermedia. J. Asian Nat. Prod. Res. 2020;22:225–232. doi: 10.1080/10286020.2019.1681410. [DOI] [PubMed] [Google Scholar]
- 53.Hu L., Chen N.-N., Hu Q., Yang C., Yang Q.-S., Wang F.-F. An Unusual Piceatannol Dimer from Rheum austral D. Don with Antioxidant Activity. Molecules. 2014;19:11453–11464. doi: 10.3390/molecules190811453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao H., Wang Z., Cheng J., Li R., Wang Z. New chromone glucoside from roots of rumex gmelini. Tianran Chanwu Yanjiu Yu Kaifa. 2009;21:189–191. [Google Scholar]
- 55.Singh J. Photochemical investigations on Cassia multijuga leaves. Isolation and characterization of new chromone glycosides. Pol. J. Chem. 1981;55:1181–1183. [Google Scholar]
- 56.Singh J. Two chromone glycosides from Cassia multijuga. Phytochemistry. 1982;21:1177–1179. doi: 10.1016/S0031-9422(00)82449-8. [DOI] [Google Scholar]
- 57.Kashiwada Y., Nonaka G.I., Nishioka I. Chromone glucosides from rhubarb. Phytochemistry. 1990;29:1007–1009. doi: 10.1016/0031-9422(90)80072-O. [DOI] [Google Scholar]
- 58.Mou L.-Y., Wei M., Wu H.-Y., Hu L.-J., Li J.-L., Li G.-P. O-beta-d-Glucopyranosyl-2-methylchromone, a new chromone glycoside from the Tibetan medicine plant of Swertia punicea Hemsl. Nat. Prod. Res. 2020:1–9. doi: 10.1080/14786419.2020.1777123. [DOI] [PubMed] [Google Scholar]
- 59.Ahmad V., Ullah F., Hussain J., Farooq U., Zubair M., Khan M., Choudhary M. Tyrosinase inhibitors from Rhododendron collettianum and their structure-activity relationship (SAR) studies. Chem. Pharm. Bull. (Tokyo) 2004;52:1458–1461. doi: 10.1248/cpb.52.1458. [DOI] [PubMed] [Google Scholar]
- 60.Kim S.B., Ahn J.H., Han S.B., Hwang B.Y., Kim S.Y., Lee M.K. Anti-adipogenic chromone glycosides from Cnidium monnieri fruits in 3T3-L1 cells. Bioorganic Med. Chem. Lett. 2012;22:6267–6271. doi: 10.1016/j.bmcl.2012.07.103. [DOI] [PubMed] [Google Scholar]
- 61.Liang H., Zhao Y.Y., Zhang R.Y. A new chromone glycoside from Bupleurum chinense. Chinese Chem. Lett. 1998;9:69–70. [Google Scholar]
- 62.Lu T.S., Yi Y.H., Mao S.L., Zhou D.Z., Xu Q.Z., Tang H.F., Zhang S.Y. A new chromone glycoside from Cassia siamea Lam. Chinese Chem. Lett. 2001;12:703–704. [Google Scholar]
- 63.Tanaka Y., Honma D., Tamura M., Yanagida A., Zhao P., Shoji T., Tagashira M., Shibusawa Y., Kanda T. New chromanone and acylphloroglucinol glycosides from the bracts of hops. Phytochem. Lett. 2012;5:514–518. doi: 10.1016/j.phytol.2012.05.003. [DOI] [Google Scholar]
- 64.Barash I., Karr A.L., Jr., Strobel G.A. Isolation and characterization of stemphylin, a chromone glucoside from Stemphylium botrysum. Plant Physiol. 1975;55:646–651. doi: 10.1104/pp.55.4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Do Nascimento B.O., da Silva Neto O.C., Teodoro M.T.F., de Oliveira Silva E., Guedes M.L.S., David J.M. Macrolobin: A new unusual C-glycoside chromone from Macrolobium latifolium and its anticholinesterase and antimicrobial activities. Phytochem. Lett. 2020;39:124–127. doi: 10.1016/j.phytol.2020.08.002. [DOI] [Google Scholar]
- 66.Iswaldi I., Arráez-Román D., Rodríguez-Medina I., Beltrán-Debón R., Joven J., Segura-Carretero A., Fernández-Gutiérrez A. Identification of phenolic compounds in aqueous and ethanolic rooibos extracts (Aspalathus linearis) by HPLC-ESI-MS (TOF/IT) Anal. Bioanal. Chem. 2011;400:3643–3654. doi: 10.1007/s00216-011-4998-z. [DOI] [PubMed] [Google Scholar]
- 67.Lee H.-H., Shin J.-S., Lee W.-S., Ryu B., Sik Jang D., Lee K.-T. Biflorin, Isolated from the Flower Buds of Syzygium aromaticum L., Suppresses LPS-Induced Inflammatory Mediators via STAT1 Inactivation in Macrophages and Protects Mice from Endotoxin Shock. J. Nat. Product. 2016;79:711–720. doi: 10.1021/acs.jnatprod.5b00609. [DOI] [PubMed] [Google Scholar]
- 68.Ryu B., Woo J.-H., Kim H.M., Choi J.-H., Jang D.S. A new acetophenone glycoside from the flower buds of Syzygium aromaticum (cloves) Fitoterapia. 2016;115:46–51. doi: 10.1016/j.fitote.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 69.Satake T., Kamiya K., Saiki Y., Hama T., Fujimoto Y., Endang H., Umar M. Chromone C-glycosides from Baeckea frutescens. Phytochemistry. 1998;50:303–306. doi: 10.1016/S0031-9422(98)00513-5. [DOI] [Google Scholar]
- 70.Kamiya K., Satake T. Chemical constituents of Baeckea frutescens leaves inhibit copper-induced low-density lipoprotein oxidation. Fitoterapia. 2010;81:185–189. doi: 10.1016/j.fitote.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 71.Ito H., Kasajima N., Tokuda H., Nishino H., Yoshida T. Dimeric Flavonol Glycoside and Galloylated C-Glucosylchromones from Kunzea ambigua. J. Nat. Prod. 2004;67:411–415. doi: 10.1021/np030367s. [DOI] [PubMed] [Google Scholar]
- 72.Wang S., Feng K., Han L., Fang X., Zhang Y., Yu H., Pang X. Glycosidic compounds from Cassia obtusifolia seeds and their inhibitory effects on OATs, OCTs and OATPs. Phytochem. Lett. 2019;32:105–109. doi: 10.1016/j.phytol.2019.05.010. [DOI] [Google Scholar]
- 73.Han A.-R., Paik Y.-S. Antioxidant and prolyl endopeptidase inhibitory capacities of chromone C-glucosides from the clove buds (Syzygium aromaticum) J. Appl. Biol. Chem. 2012;55:195–198. doi: 10.3839/jabc.2012.031. [DOI] [Google Scholar]
- 74.Rehman N.U., Hussain H., Khiat M., Al-Riyami S.A., Csuk R., Khan H.Y., Abbas G., Al-Thani G.S., Green I.R., Al-Harrasi A. ChemInform Abstract: Aloeverasides A and B: Two Bioactive C-Glucosyl Chromones from Aloe vera Resin. Helv. Chim. 2016;47 doi: 10.1002/chin.201652403. [DOI] [Google Scholar]
- 75.Rehman N.U., Al-Riyami S.A., Hussain H., Ali A., Khan A.L., Al-Harrasi A. Secondary metabolites from the resins of Aloe vera and Commiphora mukul mitigate lipid peroxidation. Acta Pharm. 2019;69:433–441. doi: 10.2478/acph-2019-0027. [DOI] [PubMed] [Google Scholar]
- 76.Kahramanoglu I., Chen C., Chen J., Wan C. Chemical constituents, antimicrobial activity, and food preservative characteristics of Aloe vera gel. Agronomy. 2019;9:831. doi: 10.3390/agronomy9120831. [DOI] [Google Scholar]
- 77.Singh M., Singh J. Chemical examination of the seeds of Cassia spectabilis. Zeitschrift Naturforsch. B. 1984;39B:1425–1426. doi: 10.1515/znb-1984-1020. [DOI] [Google Scholar]
- 78.Agrawal A., Singh J. Glycosides of two xanthones and a chromone from roots of Chrozophora prostrata. Phytochemistry. 1988;27:3692–3694. doi: 10.1016/0031-9422(88)80803-3. [DOI] [Google Scholar]
- 79.Jong-Anurakkun N., Bhandari M.R., Hong G., Kawabata J. α-Glucosidase inhibitor from Chinese aloes. Fitoterapia. 2008;79:456–457. doi: 10.1016/j.fitote.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 80.Lv L., Yang Q.-Y., Zhao Y., Yao C.-S., Sun Y., Yang E.-J., Song K.-S., Mook-Jung I., Fang W.-S. BACE1 (beta-secretase) inhibitory chromone glycosides from Aloe vera and Aloe nobilis. Planta Med. 2008;74:540–545. doi: 10.1055/s-2008-1074496. [DOI] [PubMed] [Google Scholar]
- 81.Kim J.H., Yoon J.Y., Yang S.Y., Choi S.K., Kwon S.J., Cho I.S., Jeong M.H., Ho Kim Y., Choi G.S. Tyrosinase inhibitory components from Aloe vera and their antiviral activity. J. Enzyme Inhib. Med. Chem. 2017;32:78–83. doi: 10.1080/14756366.2016.1235568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okamura N., Hine N., Harada S., Fujioka T., Mihashi K., Yagi A. Three chromone components from Aloe vera leaves. Phytochemistry. 1996;43:495–498. doi: 10.1016/0031-9422(96)00306-8. [DOI] [Google Scholar]
- 83.Wen J., Shi H.-M., Tu P.-F. Chemical constituents of Abrus mollis Hance. Biochem. Syst. Ecol. 2006;34:177–179. doi: 10.1016/j.bse.2005.08.007. [DOI] [Google Scholar]
- 84.Goodger J.Q.D., Seneratne S.L., Nicolle D., Woodrow I.E. Foliar Essential Oil Glands of Eucalyptus Subgenus Eucalyptus (Myrtaceae) Are a Rich Source of Flavonoids and Related Non-Volatile Constituents. PLoS ONE. 2016;11:e0155568. doi: 10.1371/journal.pone.0151432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Al-Sayed E., Hamid H.A., Abu El Einin H.M. Molluscicidal and antischistosomal activities of methanol extracts and isolated compounds from Eucalyptus globulus and Melaleuca styphelioides. Pharmaceut. Biol. 2014;52:698–705. doi: 10.3109/13880209.2013.865240. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y., Chen Y. Isobiflorin, a chromone C-glucoside from cloves (Eugenia caryophyllata) Phytochemistry. 1997;45:401–403. doi: 10.1016/S0031-9422(96)00836-9. [DOI] [Google Scholar]
- 87.Gao H.Y., Wang H.Y., Li G.Y., Du X.W., Zhang X.T., Han Y., Huang J., Li X.X., Wang J.H. Constituents from Zhuyeqing Liquor and their inhibitory effects on nitric oxide production. Phytochem. Lett. 2014;7:150–155. doi: 10.1016/j.phytol.2013.11.011. [DOI] [Google Scholar]
- 88.Gao W.-N., Luo J.-G., Kong L.-Y. Quality evaluation of Hypericum japonicum by using high-performance liquid chromatography coupled with photodiode array detector and electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2009;23:1022–1030. doi: 10.1002/bmc.1218. [DOI] [PubMed] [Google Scholar]
- 89.Luo G., Zhou M., Ye Q., Mi J., Fang D., Zhang G., Luo Y. Phenolic derivatives from Hypericum japonicum. Nat. Prod. Commun. 2015;10:1934578X1501001224. doi: 10.1177/1934578X1501001224. [DOI] [PubMed] [Google Scholar]
- 90.Santos S.A.O., Villaverde J.J., Freire C.S.R., Domingues R.M.M., Neto C.P., Silvestre A.J.D. Phenolic composition and antioxidant activity of Eucalyptus grandis, E. urograndis (E. grandis × E. urophylla) and E. maidenii bark extracts. Ind. Crops Prod. 2012;39:120–127. doi: 10.1016/j.indcrop.2012.02.003. [DOI] [Google Scholar]
- 91.Tanaka T., Orii Y., Nonaka G., Nishioka I. Tannins and related compounds. CXXIII. Chromone, acetophenone and phenylpropanoid glycosides and their galloyl and/or hexahydroxydiphenoyl esters from the leaves of Syzygium aromaticum Merr. et Perry. Chem. Pharm. Bull. (Tokyo) 1993;41:1232–1237. doi: 10.1248/cpb.41.1232. [DOI] [Google Scholar]
- 92.Yuan A., Kang S., Qin L., Ruan B., Fan Y. Chemical constituents of the leaves of Chinese aloe (Aloe vera var. chinensis) Zhongcaoyao. 1994;25:339–341. [Google Scholar]
- 93.Che Q.-M., Akao T., Hattori M., Kobashi K., Namba T. Metabolism of aloesin and related cmpounds by human intestinal bacteria: A bacterial cleavage of the C-glucosyl bond and the subsequent reduction of the acetonyl side chain. Chem. Pharm. Bull. 1990;39:704–708. doi: 10.1248/cpb.39.704. [DOI] [PubMed] [Google Scholar]
- 94.Park M.K., Park J.H., Shin Y.G., Kim W.Y., Lee J.H., Kim K.H. Neoaloesin A: A new C-glucofuranosyl chromosome from Aloe barbadensis. Planta Med. 1996;62:363–365. doi: 10.1055/s-2006-957907. [DOI] [PubMed] [Google Scholar]
- 95.Lucini L., Pellizzoni M., Pellegrino R., Molinari G.P., Colla G. Phytochemical constituents and in vitro radical scavenging activity of different Aloe species. Food Chem. 2015;170:501–507. doi: 10.1016/j.foodchem.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 96.Bisrat D., Dagne E., Van Wyk B.-E., Viljoen A. Chromones and anthrones from Aloe marlothii and Aloe rupestris. Phytochemistry. 2000;55:949–952. doi: 10.1016/S0031-9422(00)00328-9. [DOI] [PubMed] [Google Scholar]
- 97.Conner J.M., Gray A.I., Reynolds T., Waterman P.G. Anthrone and chromone components of Aloe cremnophila and A. jacksonii leaf exudates. Phytochemistry. 1990;29:941–944. doi: 10.1016/0031-9422(90)80051-H. [DOI] [Google Scholar]
- 98.Wang W., Cuyckens F., Van den Heuvel H., Apers S., Pieters L., Steenkamp V., Stewart M.J., Luyckx V.A., Claeys M. Structural characterization of chromone C-glucosides in a toxic herbal remedy. Rapid Commun. Mass Spectrom. 2003;17:49–55. doi: 10.1002/rcm.875. [DOI] [PubMed] [Google Scholar]
- 99.Hutter J.A., Salman M., Stavinoha W.B., Satsangi N., Williams R.F., Streeper R.T., Weintraub S.T. Antiinflammatory C-Glucosyl Chromone from Aloe barbadensis. J. Nat. Prod. 1996;59:541–543. doi: 10.1021/np9601519. [DOI] [PubMed] [Google Scholar]
- 100.Zhong J.S., Huang Y.Y., Zhang T.H., Liu Y.P., Ding W.J., Wu X.F., Xie Z.Y., Luo H.B., Wan J.Z. Natural phosphodiesterase-4 inhibitors from the leaf skin of Aloe barbadensis Miller. Fitoterapia. 2015;100:68–74. doi: 10.1016/j.fitote.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 101.Lee S., Do S.G., Kim S.Y., Kim J., Jin Y., Lee C.H. Mass spectrometry-based metabolite profiling and antioxidant activity of Aloe vera (Aloe barbadensis Miller) in different growth stages. J. Agric. Food Chem. 2012;60:11222–11228. doi: 10.1021/jf3026309. [DOI] [PubMed] [Google Scholar]
- 102.Rehman N.U., Hussain H., Khiat M., Khan H.Y., Abbas G., Green I.R., Al-Harrasi A. Bioactive chemical constituents from the resin of Aloe vera. Zeitschrift für Naturforsch. B. 2017;72:955–958. doi: 10.1515/znb-2017-0117. [DOI] [Google Scholar]
- 103.Yagi A., Kanbara T., Morinobut N. Inhibition of Mushroom-Tyrosinase by Aloe Extract. Planta Med. 1987;53:515–517. doi: 10.1055/s-2006-962798. [DOI] [PubMed] [Google Scholar]
- 104.Baba K., Kawanishi H., Taniguchi M., Kozawa M. Chromone glucosides from Cnidium japonicum. Phytochemistry. 1993;35:221–225. doi: 10.1016/S0031-9422(00)90538-7. [DOI] [Google Scholar]
- 105.Kimura Y., Sumiyoshi M., Taniguchi M., Baba K. Antitumor actions of a chromone glucoside cnidimoside A isolated from Cnidium japonicum. J. Nat. Med. 2008;62:308–313. doi: 10.1007/s11418-008-0242-3. [DOI] [PubMed] [Google Scholar]
- 106.Zhao J., Zhou M., Liu Y., Zhang G., Luo Y. Chromones and coumarins from the dried fructus of Cnidium monnieri. Fitoterapia. 2011;82:767–771. doi: 10.1016/j.fitote.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 107.Cisowski W., Grimshaw J. Glucoside of chromone from Angelica archangelica L. and Archangelica litoralis Fries. herbs. Pol. J. Chem. 1988;62:135–141. [Google Scholar]
- 108.Kopp B., Kubelka E., Reich C., Robien W., Kubelka W. 4H-Chromenone Glycosides from Eranthhis hyernalis (L.) SALISBU. Helv. Chim. Acta. 1991;74:611–616. doi: 10.1002/hlca.19910740318. [DOI] [Google Scholar]
- 109.Kuroda M., Uchida S., Watanabe K., Mimaki Y. Chromones from the tubers of Eranthis cilicica and their antioxidant activity. Phytochemistry. 2009;70:288–293. doi: 10.1016/j.phytochem.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 110.Bernhardt M., Shaker K.H., Elgamal M.H.A., Seifert K. The New Bishomoflavone Ononin and Its Glucoside from Ononis vaginalis. Zeitschrift für Naturforsch. C. 2000;55:516–519. doi: 10.1515/znc-2000-7-806. [DOI] [Google Scholar]
- 111.Ibrahim S.R.M. New 2-(2-Phenylethyl)chromone Derivatives from the Seeds of Cucumis melo L var. reticulatus. Nat. Prod. Commun. 2010;5:403–406. doi: 10.1177/1934578X1000500313. [DOI] [PubMed] [Google Scholar]
- 112.Quan L., Zhang J., Wu Y. One Novel 2-(2-Phenylethyl)Chromone Glycoside from Aquilaria sinensis. Chem. Nat. Compd. 2017;53:635–637. doi: 10.1007/s10600-017-2078-0. [DOI] [Google Scholar]
- 113.Liu X., Zhang B., Yang L., Yu G., Wang Z. Two new chromones and a new flavone glycoside from Imperata cylindrical. Zhongguo Tianran Yaowu. 2013;11:77–80. doi: 10.3724/SP.J.1009.2013.00077. [DOI] [Google Scholar]
- 114.Shao H., Mei W.-L., Kong F.-D., Dong W.-H., Li W., Zhu G.-P., Dai H.-F. A new 2-(2-phenylethyl)chromone glycoside in Chinese agarwood “Qi-Nan” from Aquilaria sinensis. J. Asian Nat. Prod. Res. 2017;19:42–46. doi: 10.1080/10286020.2016.1187602. [DOI] [PubMed] [Google Scholar]
- 115.Zaher A., Boufellous M., Ouhssine M., Bourkhiss B. Phytochemical Screening Of An Umbelliferae: Ammi visnaga L. (Lam.) In The Region Of Sidi Slimane- North-West Of Morocco. J. Mater. Environ. Sci. 2019;10:995–1002. [Google Scholar]
- 116.Stevens T.J., Jones B.W., Vidmar T.J., Moran J.J., Manni D.S., Day C.E. Hypocholesterolemic effect of khellin and khelloside in female cynomolgus monkeys. Arzneimittelforschung. 1985;35:1257–1260. [PubMed] [Google Scholar]
- 117.Günaydin K., Beyazit N. The chemical investigations on the ripe fruits of Ammi visnaga (Lam.) Lamarck growing in Turkey. Nat. Prod. Res. 2004;18:169–175. doi: 10.1080/14786410310001608091. [DOI] [PubMed] [Google Scholar]
- 118.Liu Y.R., Wu Z.J., Li C.T., Xi F.M., Sun L.N., Chen W.S. Heracleifolinosides A-F, new triterpene glycosides from cimicifuga heracleifolia, and their inhibitory activities against hypoxia and reoxygenation. Planta Med. 2013;79:301–307. doi: 10.1055/s-0032-1328174. [DOI] [PubMed] [Google Scholar]
- 119.Ma S.Y., Shi L.G., Gu Z.B., Wu Y.L., Wei L.B., Wei Q.Q., Gao X.L., Liao N. Two New Chromone Glycosides from the Roots of Saposhnikovia divaricata. Chem. Biodivers. 2018;15:2–7. doi: 10.1002/cbdv.201800253. [DOI] [PubMed] [Google Scholar]
- 120.Jiang Y., Guo F., Chen L., Xu L., Zhang W., Liu B. The antitumor activity of naturally occurring chromones: A review. Fitoterapia. 2019;135:114–129. doi: 10.1016/j.fitote.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 121.Sasaki H., Taguchi H., Endo T., Yosioka I. The constituents of Ledebouriella seseloides Wolff. I. Structures of three new chromones. Chem. Pharm. Bull. 1982;3:3555–3562. doi: 10.1248/cpb.30.3555. [DOI] [Google Scholar]
- 122.Urbagarova B.M., Shults E.E., Taraskin V.V., Radnaeva L.D., Petrova T.N., Rybalova T.V., Frolova T.S., Pokrovskii A.G., Ganbaatar J. Chromones and coumarins from Saposhnikovia divaricata (Turcz.) Schischk. Growing in Buryatia and Mongolia and their cytotoxicity. J. Ethnopharmacol. 2020;261:112517. doi: 10.1016/j.jep.2019.112517. [DOI] [PubMed] [Google Scholar]
- 123.Lemmich J. Monoterpene, chromone and coumarin glucosides of Diplolophium buchananii. Phytochemistry. 1995;38:427–432. doi: 10.1016/0031-9422(94)00599-O. [DOI] [Google Scholar]
- 124.Shao Z., Zhang Y., Jiang K., CHEN J., Zuo B. Chemical constituents from the roots of Sphallerocarpus gracilis. Nat. Prod. Res. Dev. 2003;15:196–198. [Google Scholar]
- 125.Yan Z., Yang X., Wu J., Su H., Chen C., Chen Y. Qualitative and quantitative analysis of chemical constituents in traditional Chinese medicinal formula Tong-Xie-Yao-Fang by high-performance liquid chromatography/diode array detection/electrospray ionization tandem mass spectrometry. Anal. Chim. Acta. 2011;691:110–118. doi: 10.1016/j.aca.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 126.Semwal R.B., Semwal D.K., Combrinck S., Viljoen A. Health benefits of chromones: Common ingredients of our daily diet. Phytochem. Rev. 2020;19:761–785. doi: 10.1007/s11101-020-09681-w. [DOI] [Google Scholar]
- 127.Khalil N., Bishr M., Desouky S., Salama O. Ammi visnaga L., a potential medicinal plant: A review. Molecules. 2020;25:301. doi: 10.3390/molecules25020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.An R.-B., Park B.-Y., Kim J.-H., Kwon O.-K., Lee J.-K., Min B.-S., Ahn K.-S., Oh S.-R., Lee H.-K. Coumarins and chromones from Angelica genuflexa. Nat. Prod. Sci. 2005;11:79–84. [Google Scholar]
- 129.Baba K., Hata K., Kimura Y., Matsuyama Y., Kozawa M. Chemical studies of Angelica japonica A. Gray. I. On the constituents of the ethyl acetate extract of the root. Chem. Pharm. Bull. (Tokyo) 1981;29:2565–2570. doi: 10.1248/cpb.29.2565. [DOI] [Google Scholar]
- 130.Shi Q.-Q., Lu J., Peng X.-R., Li D.-S., Zhou L., Qiu M.-H. Cimitriteromone A–G, Macromolecular Triterpenoid–Chromone Hybrids from the Rhizomes of Cimicifuga foetida. J. Org. Chem. 2018;83:10359–10369. doi: 10.1021/acs.joc.8b01466. [DOI] [PubMed] [Google Scholar]
- 131.Sun J., Su X., Zhang Z., Hu D., Hou G., Zhao F., Sun J., Cong W., Wang C., Li H. Separation of three chromones from Saposhnikovia divaricata using macroporous resins followed by preparative high-performance liquid chromatography. J. Sep. Sci. 2021;44:3287–3294. doi: 10.1002/jssc.202100345. [DOI] [PubMed] [Google Scholar]
- 132.Lu L., Chen J.C., Li Y., Qing C., Wang Y.Y., Nian Y., Qiu M.H. Studies on the constituents of Cimicifuga foetida collected in guizhou province and their cytotoxic activities. Chem. Pharm. Bull. 2012;60:571–577. doi: 10.1248/cpb.60.571. [DOI] [PubMed] [Google Scholar]
- 133.Wang H., Xu Y., Yuan Z. Isolation and identification of chemical constituents of roots of Glehnia littoralis. J. Shenyang Pharm. Univ. 2011;28:530–534. [Google Scholar]
- 134.Zheng M., Jin W., Son K.-H., Chang H.-W., Kim H.-P., Bae K.-H., Kang S.-S. The constituents isolated from Peucedanum japonicum Thunb. and their cyclooxygenase (COX) inhibitory activity. Korean J. Med. Crop Sci. 2005;13:75–79. [Google Scholar]
- 135.Akunyili D.N., Akubue P.I. Schumanniofoside, the antisnake venom principle from the stem bark of Schumanniophyton magnificum Harms. J. Ethnopharmacol. 1986;18:167–172. doi: 10.1016/0378-8741(86)90028-0. [DOI] [PubMed] [Google Scholar]
- 136.Shi Q.-Q., Gao Y., Lu J., Zhou L., Qiu M.-H. Two new triterpenoid-chromone hybrids from the rhizomes of Actaea cimicifuga L.(syn. Cimicifuga foetida L.) and their cytotoxic activities. Nat. Prod. Res. 2020:1–7. doi: 10.1080/14786419.2020.1775228. [DOI] [PubMed] [Google Scholar]
- 137.Brill G.M., Jackson M., Whittern D.N., Buko A.M., Hill P., Chen R.H., Mcalpine J.B. Altromycins E, F, G, H and I; additional novel components of the altromycin complex. J. Antibiot. (Tokyo) 1994;47:1160–1164. doi: 10.7164/antibiotics.47.1160. [DOI] [PubMed] [Google Scholar]
- 138.Kanda N. A New Antitumor Antibiotic, Kidamycin. I Isolation, Purification and Properties of Kidamycin. J. Antibiot. (Tokyo) 1971;24:599–606. doi: 10.7164/antibiotics.24.599. [DOI] [PubMed] [Google Scholar]
- 139.Kondo S., Wakashiro T., Hamada M., Maeda K., Takeuchi T., Umezawa H. Isolation and characterization of a new antibiotic, neopluramycin. J. Antibiot. (Tokyo) 1970;23:354–359. doi: 10.7164/antibiotics.23.354. [DOI] [PubMed] [Google Scholar]
- 140.Kondo S., Miyamoto M., Naganawa H., Takeuchi T., Umezawa H. Structures of pluramycin A and neopluramycin. J. Antibiot. (Tokyo) 1977;30:1143–1145. doi: 10.7164/antibiotics.30.1143. [DOI] [PubMed] [Google Scholar]
- 141.Byrne K.M., Gonda S.K., Hilton B.D. Largomycin FII chromophore component 4, a new pluramycin antibiotic. J. Antibiot. (Tokyo) 1985;38:1040–1049. doi: 10.7164/antibiotics.38.1040. [DOI] [PubMed] [Google Scholar]
- 142.Nadig H., Sèquin U. Isolation and Structure Elucidation of Some Components of the Antitumor Antibiotic Mixture ‘Rubiflavin'. Helv. Chim. Acta. 1987;70:1217–1228. doi: 10.1002/hlca.19870700427. [DOI] [Google Scholar]
- 143.Nadig H., Séquin U., Bunge R.H., Hurley T.R., Murphey D.B., French J.C. Isolation and structure of a new antibiotic related to rubiflavin A. Helv. Chim. Acta. 1985;68:953–957. doi: 10.1002/hlca.19850680419. [DOI] [Google Scholar]
- 144.Schmitz H., Crook K.E., Jr., Bush J.A. Hedamycin, a new antitumor antibiotic. I. Production, isolation, and characterization. Antimicrob. Agents Chemother. 1966;6:606–612. doi: 10.1128/AAC.6.5.606. [DOI] [PubMed] [Google Scholar]
- 145.Séquin U., Bedford C.T., Chung S.K., Scott A.I. The structure of the antibiotic hedamycin. I. Chemical, physical and spectral properties. Helv. Chim. Acta. 1977;60:896–906. doi: 10.1002/hlca.19770600320. [DOI] [PubMed] [Google Scholar]
- 146.Sato Y., Watabe H.-O., Nakazawa T., Shomura T., Yamamoto H., Sezaki M., Kondo S. Ankinomycin, a potent antitumor antibiotic. J. Antibiot. (Tokyo) 1989;42:149–152. doi: 10.7164/antibiotics.42.149. [DOI] [PubMed] [Google Scholar]
- 147.Vertesy L., Barbone F.P., Cashmen E., Decker H., Ehrlich K., Jordan B., Knauf M., Schummer D., Segeth M.P., Wink J. Pluraflavins, potent antitumor antibiotics from Saccharothrix sp. DSM 12931. J. Antibiot. (Tokyo) 2001;54:718–729. doi: 10.7164/antibiotics.54.718. [DOI] [PubMed] [Google Scholar]
- 148.Jackson M., Karwowski J.P., Theriault R.J., Hardy D.J., Swanson S.J., Barlow G.J., Tillis P.M., McAlpine J.B. Altromycins, novel pluramycin-like antibiotics I. Taxonomy of the producing organism, fermentation and antibacterial activity. J. Antibiot. (Tokyo) 1990;43:223–228. doi: 10.7164/antibiotics.43.223. [DOI] [PubMed] [Google Scholar]
- 149.Brill G.M., Mcalpine J.B., Whittern D.N., Buko A.M. Altromycins, novel pluramycin-like antibiotics II. Isolation and elucidation of structure. J. Antibiot. (Tokyo) 1990;43:229–237. doi: 10.7164/antibiotics.43.229. [DOI] [PubMed] [Google Scholar]
- 150.Gallivan J.B. Spectroscopic studies on some chromones. Can. J. Chem. 1970;48:3928–3936. doi: 10.1139/v70-658. [DOI] [Google Scholar]
- 151.Griffiths P.J.F., Ellis G.P. Benzopyrones—VI: The ultraviolet absorption spectra of chromone and 2-substituted chromones. Spectrochim. Acta Part A Mol. Spectrosc. 1972;28:707–713. doi: 10.1016/0584-8539(72)80039-4. [DOI] [Google Scholar]
- 152.Staunton J. In: In Comprehensive Organic Chemistry. Barton D., Ollis W.D., editors. Pegamon Press; Oxford, UK,: 1979. [Google Scholar]
- 153.Nchinda A. Chemical Studies of Selected Chromone Derivatives. Rhodes University; Makhanda, South Africa: 2001. [Google Scholar]
- 154.Dey P.M. Methods in Plant Biochemistry. Academic Press; Cambridge, MA, USA: 2012. [Google Scholar]
- 155.Markham K.R., Mabry T.J. The Flavonoids. Springer; Boston, MA, USA: 1975. Ultraviolet-Visible and Proton Magnetic Resonance Spectroscopy of Flavonoids; pp. 45–77. [Google Scholar]
- 156.Murray R.D.H., McCabe P.H. Infrared studies of chromones—I: Carbonyl and hydroxyl regions. Tetrahedron. 1969;25:5819–5837. doi: 10.1016/S0040-4020(01)83090-8. [DOI] [Google Scholar]
- 157.Liu S., Zhu T., Qiu Y., Qi W., Wu H., Cai B., Lin J. Chromones and tannins from the fruit of Euscaphis japonica var. wupingensis. BioResources. 2019;14:5355–5364. doi: 10.15376/biores.14.3.5355-5364. [DOI] [Google Scholar]
- 158.Wu Q.-L., Wang S.-P., Du L.-J., Zhang S.-M., Yang J.-S., Xiao P.-G. Chromone glycosides and flavonoids from Hypericum japonicum. Phytochemistry. 1998;49:1417–1420. doi: 10.1016/S0031-9422(98)00106-X. [DOI] [PubMed] [Google Scholar]
- 159.Speranza G., Dadà G., Lunazzi L., Phytochemistry P.G. A C-glucosylated 5-methylchromone from Kenya aloe. Phytochemistry. 1986;25:2219–2222. doi: 10.1016/0031-9422(86)80095-4. [DOI] [Google Scholar]
- 160.Okamura N., Hine N., Tateyama Y., Nakazawa M., Fujioka T., Mihashi K. Five chromones from Aloe vera leaves. Phytochemistry. 1998;49:219–223. doi: 10.1016/S0031-9422(97)01071-6. [DOI] [Google Scholar]
- 161.Speranza G., Gramatica P., Dadá G., Manitto P. Aloeresin C, a bitter C, O-diglucoside from Cape Aloe. Phytochemistry. 1985;24:1571–1573. doi: 10.1016/S0031-9422(00)81068-7. [DOI] [Google Scholar]
- 162.Rauwald H.W., Maucher R., Dannhardt G., Kuchta K. Dihydroisocoumarins, Naphthalenes, and Further Polyketides from Aloe vera and A. plicatilis: Isolation, Identification and Their 5-LOX/COX-1 Inhibiting Potency. Molecules. 2021;26:4223. doi: 10.3390/molecules26144223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Van Heerden F.R., Viljoen A.M., Van Wyk B.E. 6′-O-Coumaroylaloesin from Aloe castanea—A taxonomic marker for Aloe section Anguialoe. Phytochemistry. 2000;55:117–120. doi: 10.1016/S0031-9422(00)00252-1. [DOI] [PubMed] [Google Scholar]
- 164.Holzapfel C.W., Wessels P.L., Van Wyk B.E., Marais W., Portwig M. Chromone and aloin derivatives from Aloe broomii, A. Africana and A. speciosa. Phytochemistry. 1997;45:97–102. doi: 10.1016/S0031-9422(96)00799-6. [DOI] [Google Scholar]
- 165.Séquin U., Furukawa M. The structure of the antibiotic hedamycin—III: 13C NMR spectra of hedamycin and kidamycin. Tetrahedron. 1978;34:3623–3629. doi: 10.1016/0040-4020(78)88440-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.