Abstract
Background
The drug supply chain is global and at risk of disruption and subsequent drug shortages, especially during unanticipated events.
Objective
Our objective was to determine the impact of coronavirus disease 2019 (COVID-19) on drug purchases overall, by class, and for specific countries.
Methods
A cross-sectional time series analysis of country-level drug purchase data from August 2014 to August 2020 from IQVIA MIDAS was conducted. Standardized units per 100 population and percentage increase in units purchased were assessed from 68 countries and jurisdictions in March 2020 (when the World Health Organization declared COVID-19 a pandemic). Analyses were compared by United Nations development status and drug class. Autoregressive integrated moving average models tested the significance of changes in purchasing trends.
Results
Before COVID-19, standardized medication units per 100 population ranged from 3990 to 4760 monthly. In March 2020, there was a global 15% increase in units of drugs purchased to 5309.3 units per 100 population compared with the previous year; the increase was greater in developed countries (18.5%; P < 0.001) than in developing countries (12.8%; P < 0.0001). After the increase in March 2020, there was a correction in the global purchase rate decreasing by 4.7% (April to August 2020 rate, 21,334.6/100 population; P < 0.001). Globally, we observed high purchasing rates and large changes for respiratory medicines such as inhalers and systemic adrenergic drugs (March 2020 rate, 892.7/100 population; change from 2019, 28.5%; P < 0.001). Purchases for topical dermatologic products also increased substantially (42.2%), although at lower absolute rates (610.0/100 population in March 2020; P < 0.0001). Interestingly, purchases for systemic anti-infective agents (including antiviral drugs) increased in developing countries (11.3%; P < 0.001), but decreased in developed countries (1.0%; P = 0.06).
Conclusion
We observed evidence of global drug stockpiling in the early months of the COVID-19 pandemic, especially among developed countries. Actions toward equitable distribution of medicines through a resilient drug supply chain should be taken to increase global response to future unanticipated events, such as pandemics.
Key Points.
Background
-
•
The drug supply chain is global and at risk of disruption especially during unanticipated events such as pandemics.
-
•
During the 2019 novel coronavirus (coronavirus disease 2019 [COVID-19]) pandemic, border closings and countries prohibiting drug export potentially threatened global access to essential medicines.
-
•
Supply disruptions can lead to drug shortages, which are increasingly common and can result in worsened clinical outcomes and increased costs.
Findings
-
•
A global 15% increase in purchases occurred in March 2020 when COVID-19 was declared a pandemic but before peak global infections; the increase was greater (18%) in developed than developing countries (13%).
-
•
Drug stockpiling occurred globally in the early months of the COVID-19 pandemic, especially among developed countries. Variation in drug purchasing responses to COVID-19 by country and development status suggest an uncoordinated approach to supply chain drug distribution.
-
•
International treaties must ensure access and equitable distribution of medications similar to other resources essential to health. Actions toward a more resilient drug supply chain may increase global response to future unanticipated events, including pandemics.
Introduction
The efficient and resilient performance of the drug supply chain is important because most people worldwide use medications, taking a mean of 1.6 medications daily, with older adults taking 3 medications per day.1, 2, 3, 4 Drug suppliers and manufacturers are most often multinational corporations, providing the drug supply for many countries. Of drugs consumed in most developed countries, 90% of raw active ingredients (active pharmaceutical ingredients) are made in foreign facilities (80% in China and India).5 , 6 For 90% of drugs, all active pharmaceutical ingredients are manufactured at a single facility where a single event can disrupt production.5 Manufacturers then produce finished dosage forms from active pharmaceutical ingredients (i.e., tablet) at a different site. Concerningly, 60% of finished dosage forms are made in a single finished dosage forms manufacturing facility.5 , 6
Disruptions in the drug supply chain can lead to sudden decreases in drug supply, or drug shortages. A drug shortage is defined as a situation where a patient is unable to access an interchangeable version of a medication because of supply limitations.6 Over the past decade, the number of drug shortages has increased dramatically.6 Disruption in drug supply may arise from several causes, including manufacturing problems and recalls, sole source contracts, and demand increases. Supply disruptions that lead to shortages are a complex global issue and can be affected by geopolitical issues, trade, civil unrest, weather, and pandemics.7 , 8 Drug shortages are concerning because they have a negative impact on patient health outcomes and they result in increased health care costs.10, 11, 12, 9
The 2019 novel coronavirus (coronavirus disease 2019 [COVID-19]) pandemic, caused by the severe acute respiratory syndrome coronavirus 2, affected drug manufacturing: Chinese active pharmaceutical ingredients manufacturers closed, the European Union and Indian governments prohibited drug export, and finished dosage form disruptions in other countries were reported.13, 14, 15, 16 These issues, combined with news of patients and organizations stockpiling drugs, may have worsened the already strained drug supply chain, especially for inhalers and critical care medications used to treat patients with COVID-19.16 Before COVID-19, disruptions in the drug supply chain disproportionately affected antimicrobial agents and drugs used for central nervous system and cardiovascular indications,6 many of which are considered essential medications by the World Health Organization (WHO).17 However, the impact of the COVID-19 pandemic on purchases of all medications is unknown. Our objective was to determine the extent to which the WHO pandemic declaration affected the global drug supply overall, by class, for specific countries, and new cases of patients infected with COVID-19.
Methods
Setting
We conducted a cross-sectional time series study of global monthly pharmacy sales from August 2014 to August 2020. Data and statistical analysis were conducted in SAS version 9.4 (Cary, NC). This study was approved by the University of Pittsburgh Institutional Review Board and followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.
Data source
The current analysis was conducted in IQVIA MIDAS database (Durham, NC), which contains monthly pharmacy sales for 66 countries and 2 geographic regions (Central America [N = 6 countries] and French West Africa [N = 12 countries]) from August 2014 to August 2020. We excluded Venezuela from our analysis because of hyperinflation and unstable purchasing rates (data not indicated). Pharmacy sales are reported in standardized units overall and by sector (retail, hospital). Standardized units are defined as a single tablet/capsule, vial, or 5-mL oral liquid. On average, MIDAS captures 89.5% of all community- and hospital-based pharmacy sales in covered areas. In 2020, 73.3% of the world’s population resided in a MIDAS region (Appendix 1). Reported data are internally validated against alternate sources.18
Outcomes
Our primary outcome was global changes in monthly drug purchases per 100 population in March 2020, relative to March 2019. March 2020 was selected ex ante to be consistent with the WHO classification of the COVID-19 outbreak as a pandemic on March 11, 2020.19
Midyear population sizes were estimated using the United Nations (UN) 2019 Urbanization Prospectus. We examined both overall purchases (including all drugs) and purchases by WHO level-1 Anatomical Therapeutic Chemical (ATC1) class. For each WHO ATC1 class, we listed a sample of subclasses in Appendix 2.
Exposures
To examine whether changes in drug purchasing trends differed by economic development status, we used the UN’s 2020 World Economic Situation Prospectus to group MIDAS regions into “developed” (N = 33) and “developing” (N = 35) areas. Economies in transition were included in the developing group. This classification accounts for various aspects of a region’s total human development, including per capita gross national income, life expectancy, and educational attainment.20 Because not all regions experienced the start of their epidemics at the same time, we used publicly available data from the COVID-19 Data Repository by the Johns Hopkins University Center for Systems Science and Engineering (compiled by the University of Oxford Our World in Data Group) to calculate new COVID-19 infections per population per month. We compared these epidemic curves with the MIDAS drug purchasing trends.
Statistical analysis
We used interventional autoregressive integrated moving average (ARIMA) models to determine whether global drug purchasing trends changed in March 2020, relative to the previous year. ARIMA models are a type of time series analysis which, unlike other methods (i.e., segmented regression), account for autocorrelation and seasonality, which are common with drug utilization.21 Thus, ARIMA models can be used to evaluate the impact of interventions or events at the population level where nonlinear trends are observed (such as the impact of the pandemic announcement on drug purchases). We hypothesized that global sales would increase at the start of the pandemic because of individual and regional stockpiling. Therefore, we fit a “pulse” intervention in the first month of the pandemic (March 2020). We also fit a “ramp” intervention to model sustained changes in drug purchases (increase, decrease, or no change) in April 2020 through August 2020. Because drug pricing trends are often autocorrelated and our data demonstrated yearly patterns, we differenced our time series by 12 months to stabilize (smooth) the variability over time. To optimize model fit and meet the assumption of stationarity, we added moving average (q) and autoregressive (p) terms as appropriate based on residual autocorrelation function, partial autocorrelation function, and white noise probability plots. The differenced series demonstrated either a seasonal moving average pattern or autocorrelation at lag 3 or 11 (Appendix 3).
Sensitivity analyses
MIDAS does not capture monthly hospital-based pharmacy sales for 20 regions (N = 3 in the developed group and N = 17 in the developing group) (Appendix 1). In sensitivity analyses, we restricted analyses to the 49 regions with both hospital-based and retail data. To account for decreases (1) in elective inpatient care and (2) initial cases of COVID-19 in China, we repeated our analyses limited to retail medication purchases and removing China, respectively.
Patient and public involvement statement
Before initiation of the research, we engaged partners from health care organizations, drug distributors, regulators, and nongovernmental organizations. These partners' were involved in identifying the research questions and medications for inclusion in our analyses.
Results
Global medication purchases
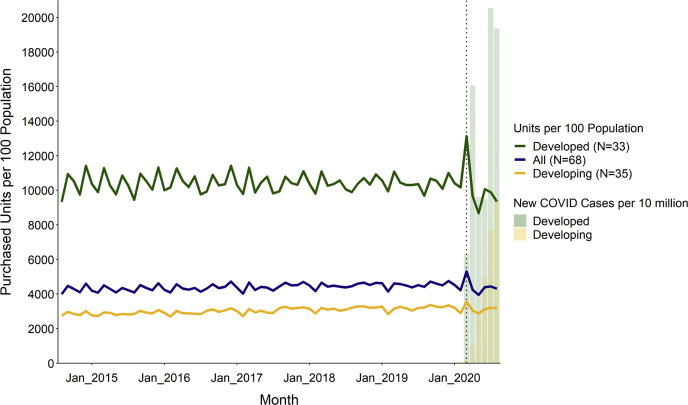
Globally, the total number of units purchased per month ranged from 217.4 billion to 304.6 billion across the study period (August 2014 to August 2020), with an average (SD) population-standardized rate of 4412.6 units (223.2) per 100 population. Before the WHO pandemic declaration, the global monthly drug purchase rate remained stable, ranging from 3990 to 4760 units per month per 100 population (217.4-271.0 billion total units) (Figure 1 ). A large increase occurred in March 2020, when COVID-19 was declared a global pandemic but before peak infection rates in the spring and summer (Figure 1). In March 2020, the global purchasing rate for all medications was 5309.3 units per 100 population (304.6 billion total units), a 15.1% increase compared with March 2019 (P < 0.001) (Table 1 ). Drug purchases for most drug classes increased globally, with absolute changes ranging from 1.8% to 42.2%, relative to 2019. Hospital solutions, sensory organ (eye and ear products), and various products (allergens, antidotes, contrast media, and radiopharmaceuticals) experienced nonsignificant decreases. Purchases for genitourinary and sex hormones (contraceptives) did not change relative to 2019 (Table 1).
Figure 1.
Global purchasing trends per 100 population, all drugs, August 2014 to August 2020. Source: authors’ analysis of MIDAS monthly sales data, August 2014 to August 2020; Johns Hopkins COVID-19 database. Note: Dotted black vertical line occurs at March 2020.
Table 1.
Changes in purchased units per 100 population, by WHO ATC1 class
| ARIMA results testing a pulse intervention in March 2020 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO ATC1 class | All regions (N = 68) |
Developed regions (N = 33) |
Developing regions (N = 35) |
|||||||||
| Units per 100 Pop. |
% change | P valuea | Units per 100 Pop. |
% change | P valuea | Units per 100 Pop. |
% change | P valuea | ||||
| March 2019 | March 2020 | March 2019 | March 2020 | March 2019 | March 2020 | |||||||
| All drugs | 4611.3 | 5309.3 | 15.1 | < 0.001 | 11081.0 | 13127.4 | 18.5 | < 0.001 | 3151.2 | 3555.6 | 12.8 | < 0.001 |
| Alimentary tract and metabolism | 755.6 | 823.0 | 8.9 | 0.008 | 1626.0 | 1840.5 | 13.2 | < 0.001 | 559.2 | 594.7 | 6.4 | 0.402 |
| Respiratory system | 694.7 | 892.7 | 28.5 | < 0.001 | 1553.4 | 2092.0 | 34.7 | < 0.001 | 500.9 | 623.7 | 24.5 | < 0.001 |
| Cardiovascular system | 621.9 | 720.4 | 15.8 | < 0.001 | 1869.2 | 2214.2 | 18.5 | < 0.001 | 340.4 | 385.4 | 13.2 | < 0.001 |
| Nervous system | 573.7 | 710.4 | 23.8 | < 0.001 | 1898.3 | 2370.0 | 24.8 | < 0.001 | 274.7 | 338.1 | 23.1 | < 0.001 |
| Sensory organs | 515.8 | 485.0 | −6.0 | 0.682 | 1206.5 | 1093.1 | −9.4 | 0.47 | 359.9 | 348.7 | −3.1 | 0.158 |
| Dermatologic preparations | 429.0 | 610.0 | 42.2 | < 0.001 | 956.4 | 1286.7 | 34.5 | < 0.001 | 310.0 | 458.2 | 47.8 | < 0.001 |
| Various | 267.0 | 266.5 | −0.2 | 0.419 | 234.1 | 273.9 | 17.0 | < 0.001 | 274.4 | 264.8 | −3.5 | 0.988 |
| Musculoskeletal system | 220.3 | 224.1 | 1.8 | 0.043 | 516.6 | 544.1 | 5.3 | 0.003 | 153.4 | 152.3 | −0.7 | 0.024 |
| Blood and blood-forming organs | 145.1 | 159.5 | 10.0 | 0.007 | 383.5 | 458.9 | 19.7 | < 0.001 | 91.2 | 92.4 | 1.2 | 0.797 |
| Anti-infective agents for systemic use | 124.9 | 127.9 | 2.4 | 0.001 | 189.0 | 210.4 | 11.3 | < 0.001 | 110.5 | 109.4 | −1.0 | 0.06 |
| Systemic hormonesb | 100.8 | 118.6 | 17.7 | < 0.001 | 248.1 | 303.3 | 22.3 | < 0.001 | 67.5 | 77.2 | 14.3 | < 0.001 |
| GU system and sex hormones | 81.6 | 85.5 | 4.8 | 0.07 | 200.5 | 218.9 | 9.2 | 0.009 | 54.8 | 55.6 | 1.5 | 0.285 |
| Antiparasitic, insecticides, and repellents | 21.8 | 22.8 | 4.9 | < 0.001 | 22.8 | 25.0 | 9.8 | < 0.001 | 21.5 | 22.3 | 3.8 | < 0.001 |
| Diagnostic agentsc | 21.2 | 22.7 | 7.2 | < 0.001 | 97.0 | 106.2 | 9.5 | < 0.001 | 4.1 | 4.0 | −2.7 | 0.716 |
| Antineoplastic and immunomodulators | 19.5 | 24.3 | 24.3 | < 0.001 | 53.0 | 63.8 | 20.3 | < 0.001 | 12.0 | 15.4 | 28.7 | < 0.001 |
| Hospital solutionsd | 18.6 | 15.8 | −14.8 | 0.134 | 26.7 | 26.7 | −0.2 | 0.281 | 16.8 | 13.4 | −20.0 | 0.043 |
| ARIMA results testing a ramp intervention in April 2020 through August 2020 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO ATC1 class | All regions (N = 68) |
Developed regions (N = 33) |
Developing regions (N = 35) |
|||||||||
| Units per 100 Pop. |
% change | P valuea | Units per 100 Pop. |
% change | P valuea | Units per 100 Pop. |
% change | P valuea | ||||
| April to August 2019 | Apr-Aug 2020 | April to August 2019 | Apr-Aug 2020 | April to August 2019 | Apr-Aug 2020 | |||||||
| All drugs | 22389.9 | 21334.6 | −4.7 | < 0.001 | 51129.2 | 47632.4 | −6.8 | < 0.001 | 15903.6 | 15435.7 | −2.9 | < 0.001 |
| Alimentary tract and metabolism | 3797.5 | 3734.1 | −1.7 | 0.048 | 7787.1 | 7327.4 | −5.9 | 0.048 | 2897.1 | 2928.0 | 1.1 | 0.048 |
| Respiratory system | 3002.8 | 2595.5 | −13.6 | < 0.001 | 6198.2 | 5305.1 | −14.4 | < 0.001 | 2281.6 | 1987.8 | −12.9 | < 0.001 |
| Cardiovascular system | 3046.0 | 3029.3 | −0.5 | < 0.001 | 9050.1 | 8841.6 | −2.3 | < 0.001 | 1690.9 | 1725.5 | 2.0 | < 0.001 |
| Nervous system | 2730.6 | 2636.7 | −3.4 | < 0.001 | 8708.7 | 8277.5 | −5.0 | < 0.001 | 1381.4 | 1371.3 | −0.7 | < 0.001 |
| Sensory organs | 2474.5 | 2159.6 | −12.7 | < 0.001 | 5139.2 | 4452.2 | −13.4 | < 0.001 | 1873.0 | 1645.3 | −12.2 | < 0.001 |
| Dermatologic preparations | 2265.2 | 2471.5 | 9.1 | < 0.001 | 4841.1 | 4795.3 | −0.9 | < 0.001 | 1683.8 | 1950.2 | 15.8 | < 0.001 |
| Various | 1357.6 | 1254.4 | −7.6 | 0.031 | 1154.2 | 1093.8 | −5.2 | 0.031 | 1403.5 | 1290.4 | −8.1 | 0.031 |
| Musculoskeletal system | 1086.0 | 996.7 | −8.2 | < 0.001 | 2505.0 | 2279.8 | −9.0 | < 0.001 | 765.7 | 708.9 | −7.4 | < 0.001 |
| Blood and blood-forming organs | 721.5 | 711.6 | −1.4 | 0.023 | 1865.6 | 1802.0 | −3.4 | 0.023 | 463.3 | 467.0 | 0.8 | 0.023 |
| Anti-infective agents for systemic use | 603.0 | 492.3 | −18.4 | < 0.001 | 803.3 | 608.6 | −24.2 | < 0.001 | 557.8 | 466.2 | −16.4 | < 0.001 |
| Systemic hormonesb | 508.5 | 499.5 | −1.8 | < 0.001 | 1174.4 | 1079.0 | −8.1 | < 0.001 | 358.2 | 369.5 | 3.2 | < 0.001 |
| GU system and sex hormones | 405.7 | 380.2 | −6.3 | < 0.001 | 972.0 | 925.0 | −4.8 | < 0.001 | 278.0 | 258.0 | −7.2 | < 0.001 |
| Antiparasitic, insecticides, and repellents | 107.9 | 103.0 | −4.5 | 0.309 | 98.9 | 78.3 | −20.8 | 0.309 | 109.9 | 108.6 | −1.2 | 0.309 |
| Diagnostic agentsc | 95.4 | 88.1 | −7.6 | 0.082 | 440.0 | 396.0 | −10.0 | 0.082 | 17.7 | 19.1 | 8.1 | 0.082 |
| Antineoplastic and immunomodulators | 94.6 | 100.2 | 6.0 | 0.309 | 257.7 | 254.8 | −1.1 | 0.309 | 57.8 | 65.6 | 13.5 | 0.309 |
| Hospital solutionsd | 93.3 | 82.1 | −12.1 | < 0.001 | 134.8 | 116.8 | −13.4 | < 0.001 | 84.0 | 74.3 | −11.6 | < 0.001 |
Abbreviations used: ARIMA, autoregressive integrated moving average; ATC1, level-1 Anatomical Therapeutic Chemical; GU, genitourinary; Pop., population; GU, genitourinary; WHO, World Health Organization.
Source: Authors’ analysis of MIDAS monthly sales data, August 2014 to August 2020.
Reported P values are for an ARIMA pulse intervention in March 2020 (top) and ARIMA ramp intervention (bottom). Bold denotes P < 0.05.
Excludes sex hormones and insulins.
There were no available data for diagnostic agents in Luxembourg for 92% of the study period. Peru has missing data for this class in 7 months.
There were no available data for hospital solutions in Switzerland across the study period. Algeria has missing data for this class in 21 months.
After the increase in March 2020, there was a correction in the global purchase rate. In April through August 2020, the global purchasing rate for all medications was 21,334.6 units per 100 population, a 4.7% decrease compared with April through August 2019 (P < 0.001) (Table 1). With the exception of antiparasitic agents, diagnostic agents, and antineoplastic agents, all other drug classes experienced significant decreases with absolute changes ranging from 0.5% to 18.4% (P < 0.048) (Table 1).
Purchases by development status
Across the study period, developed countries purchased more medication units per 100 population than developing countries (mean [SD] of 10,394.4 [631.4] and 3045.9 [184.0], respectively) (Figure 1). Both groups significantly increased their purchasing rates in March 2020, relative to previous years; however, the increase was greater in developed countries (18.5% increase to 13,127.4 units per 100 population; P < 0.001) than in developing countries (12.8% increase to 3555.6 units per 100 population; P < 0.001). After the increase, there was a significant decrease in developed (6.8% decrease) and developing (2.9% decrease) countries in April through August 2020 relative to the previous year. In developed countries, COVID-19 infections peaked in April and July 2020; we did not observe a spring peak in developing countries (Figure 1).
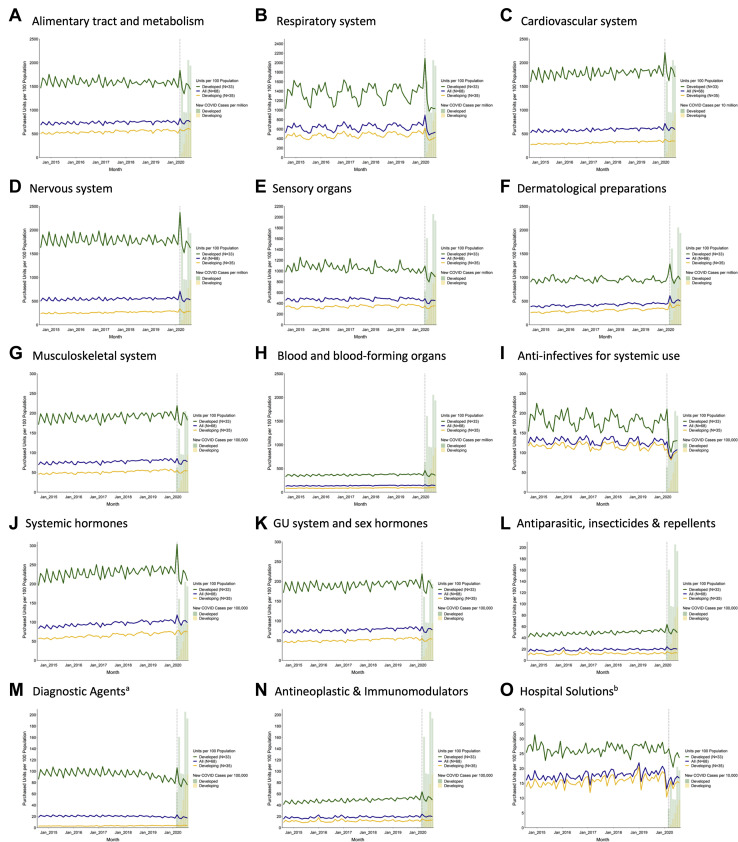
Trends over time by class overall and stratified by development status are shown in Figure 2 . Most drug classes had similar trends for developing and developed countries. Classes with agents used to treat patients with COVID-19, such as blood and blood-forming organs (anticoagulants) and anti-infective agents, had significant increases in March 2020 for developed countries whereas developing countries had nonsignificant purchases changes. Respiratory agents experienced increases in March 2020 for developing and developed countries.
Figure 2.
Global purchasing trends per 100 population, by WHO ATC1 class, August 2014 to August 2020. Source: authors’ analysis of MIDAS monthly sales data, August 2014 to August 2020. Notes: (A) There were no available data for diagnostic agents in Luxembourg for 92% of the study period. Peru has missing data for this class in 7 months. (B) There were no available data for hospital solutions in Switzerland across the study period. Algeria has missing data for this class in 21 months. Abbreviations used: ATC1, level-1 Anatomical Therapeutic Chemical; WHO, World Health Organization.
Similar decreases from April to August 2020 were observed for most classes for developing and developed countries. However, significant increases occurred from April to August 2020 for alimentary tract, cardiovascular system, dermatologic preparations, blood and blood-forming organs (which include anticoagulants), and systemic hormones (which include steroids) in developing countries, whereas these same classes experienced significant decreases in developed countries.
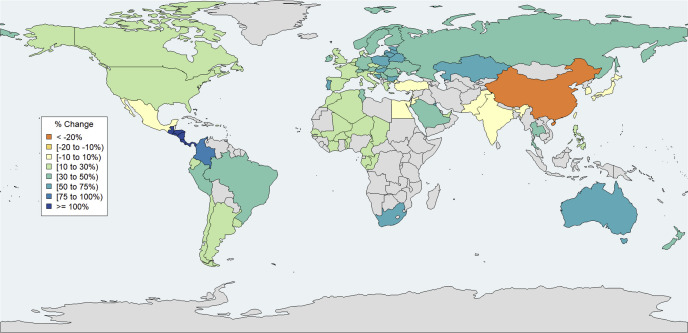
Purchases by country/jurisdiction
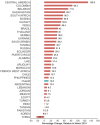
Changes in purchase rates per country in March 2020 relative to March 2019 are shown in Figure 3 and Appendix 1. China experienced the largest decrease (−23.1%), and Central America (130.8%) and Columbia (84.3%) had the largest increases. With the exception of Japan (−6.2%) and Slovenia (2.5%), all other countries that experienced decreases or no change (defined as 10% decrease to 10% increase to account for normal fluctuations in purchase patterns) were developing countries. All other developed countries experienced increases greater than or equal to 10%, with the largest relative changes in small European countries (e.g., Estonia, Hungary, Lithuania, and Bulgaria had increases ≥55%), Australia (62.1%), and New Zealand (46.9%). Canada and the United States had smaller relative increases of 22.5% and 12.1%, respectively (Appendix 4).
Figure 3.
Changes in purchased units, all drugs, by jurisdiction, March 2020 vs. March 2019. Source: authors’ analysis of MIDAS monthly sales data, August 2014 to August 2020. Notes: Individual country-level data were not available for Central America (Costa Rica, El Salvador, Honduras, Guatemala, Nicaragua, and Panama) and French West Africa (Benin, Burkina Faso, Cameroon, Chad, Congo, Gabon, Guinea, Cote d’Ivoire, Mali, Niger, Senegal, and Togo); these countries were therefore analyzed in aggregate.
Sensitivity analyses
Sensitivity analyses were conducted to assess whether our findings were robust to assumptions of selection criteria within a plausible range.
We first assessed the impact of missing data on our results by restricting our analysis to countries where retail and hospital data were available (N = 49). We observed similar overall and by-class increases in March 2020, globally (14.1% in the sensitivity analysis vs. 15.1% in the primary analysis) and within developed countries (18.3% in the sensitivity vs. 18.5% in the primary analysis) (Appendix 5). However, the overall observed increase in developing countries was lower at 9.9% (vs. 12.8% in the primary analysis).
We next restricted our analysis to retail drugs, to remove the effects of decreased elective inpatient care at the same time as the pandemic on our results. Globally, the relative increase in retail drug purchases in March 2020 was higher than the increase for hospital and retail medicines combined (18.7% in the retail-only analysis vs. 15.1% in primary analysis), especially in developing countries (18.1% vs. 12.8% in the retail-only and primary analyses, respectively) (Appendix 6). Within developing countries, retail purchases for alimentary (gastrointestinal and endocrine drugs), various (allergens antidotes, contrast, radiopharmaceuticals), anti-infective, and genitourinary drugs increased significantly, even though there were no changes for these classes in the combined hospital and retail data. Developing countries also had a substantially larger increase in purchases for antineoplastic/immunomodulating drugs in the retail setting than overall (68.2% in retail-only vs. 28.7% in primary, respectively) (Appendix 6). The trends for developed countries did not change substantially (e.g., overall increase of 19.7% vs. 18.5% in retail-only vs. primary analysis, respectively).
Our final sensitivity analysis excluded China, where drug purchase patterns likely differed because most COVID-19 cases occurred before the WHO declaration. Excluding China from the analysis demonstrated a 19.4% global increase (vs. 15.1%) in drug units purchased per 100 population (Appendix 7). This sensitivity analysis especially moderated the increase for developing countries (20.8% increase vs. 12.8% increase in our primary analysis).
Discussion
Statement of principal findings
We observed a striking global increase in drug purchases in March 2020 as the pandemic was declared. After this increase, there was a rapid decrease in April through August 2020. The March 2020 increase in drug purchases was larger in developed than developing countries and resulted in a greater subsequent decrease for developed countries. Differences in drug class trends for developing and developed countries may be due to limits in drug supply and tendency of manufacturers to sell medications to economically advantaged countries. Therefore, developing nations may be more vulnerable to disruptions because of their already-limited supply.22 Countries with drug stockpiles may have needed limited additional drug purchases for a pandemic and may explain large increases in respiratory agents and anti-infective agents. Large percentage increases in drug purchases may also be due to limited health care infrastructure. However, developed countries with well-established health care systems (e.g., Australia, Scandinavia) experienced increases greater than 50% in units purchased.
Strengths and weaknesses of the study
The IQVIA MIDAS dataset provides an unprecedented view of drug purchases from most of the world’s population, including developing and developed countries from each continent. MIDAS provides standardized sales data that allows for unique country-level comparisons over time. The data are also recent and internally validated. Although drug purchases may not reflect consumption, MIDAS is reconciled for returns and likely reflects patient use after the drug purchase date. The MIDAS dataset does not include all drug purchases for each country, and hospital data were only available for 49 countries. However, sensitivity analyses limited to countries with both hospital and retail data available did not change our overall conclusions. Although the primary analysis (excluding China because of earlier COVID occurrence) demonstrated significantly greater purchases by developed countries, including China resulted in a larger increase in developing countries (although smaller in magnitude). Our data do not account for medication supplies accessed from stockpiles, investigational products, or drugs available through emergency use authorizations, including remdesivir. Therefore, our results for medications needed to treat patients with COVID-19 (i.e., respiratory and anti-infective agents) may be underestimated.
Strengths and weaknesses in relation to other studies
There are limited data on the global distribution of medications. Wealthier countries have higher rates of medicine use.1 Although developing and emerging markets account for most of medication growth in recent years, per-capita use still lags behind wealthier countries.1 International assessments of antibiotic purchases before the COVID-19 pandemic found that low- and middle-income countries (LMICs) had a large increase in antibiotics with slight decreases by high-income countries.23A subsequent study suggests that LMICs have less access to newer antibiotics with effectiveness against multidrug-resistant pathogens even though multidrug resistance was more prevalent in LMICs.24 To the best of our knowledge, our analysis is the first international comparison of drug supply in aggregate and for all therapeutic categories. Importantly, we provide the first evidence of the impact of a global pandemic on drug purchases and supply.
Meaning of the study
We believe our results provide important insights on the global distribution of drugs. Our results reflect drug purchases during a global pandemic and may be relevant to future unanticipated events affecting the global drug supply chain. Worldwide increases for most drugs likely caused substantial pressure on the drug supply chain. There was variation by country and economic status, which suggests an uncoordinated approach to supply chain drug distribution. Medications are essential for health in all countries and are a global resource. As observed with COVID-19, border closings and countries prohibiting drug export potentially threatened global access to essential medicines.13, 14, 15, 16 Important lessons from this pandemic highlight the need for global action. International treaties must ensure access and equitable distribution of medications similar to other resources essential to health.
Unanswered questions and future research
Future research is needed on the extent to which inequitable drug distribution affects patient outcomes and access to first-line medications. The impact of international laws (e.g., patent laws) and country-specific policies on drug supply and shortages is unknown. In addition, identifying solutions to improve resiliency of the drug supply chain is urgently needed, especially during unanticipated events.
Conclusion
A significant increase in drug purchases occurred in March 2020 when the WHO declared COVID-19 a pandemic. However, a large variation was observed across countries, with developed countries increasing their purchases of drugs to a higher extent than developing countries. The equitable distribution of medicines through a resilient drug supply chain is essential for reducing the global burden of disease, improving the health and productivity of communities. Therefore, global action should be taken to ensure equitable distribution of drugs.
Biographies
Katie J. Suda, PharmD, MS, FCCP, Professor, Center for Health Equity Research and Promotion, VA Pittsburgh Healthcare System, Pittsburgh, PA; Division of General Internal Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA
Katherine Callaway Kim, MPH, Graduate Research Assistant, Division of General Internal Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA; and Department of Health Policy and Management, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA
Inmaculada Hernandez, PharmD, PhD, Associate Professor, Division of Clinical Pharmacy, University of California San Diego, La Jolla, CA
Walid F. Gellad, MD, MPH, Professor, Center for Health Equity Research and Promotion, VA Pittsburgh Healthcare System, Pittsburgh, PA: and Division of General Internal Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA
Scott Rothenberger, PhD, Assistant Professor, Division of General Internal Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA
Allen Campbell, BS, Director, IQVIA, Plymouth Meeting, PA
Lisa Malliart, PhD, Professor, Department of Industrial Engineering, University of Pittsburgh Swanson School of Engineering, Pittsburgh, PA
Mina Tadrous, PharmD, PhD, Assistant Professor, Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON, Canada; and Women’s College Research Institute, Women’s College Hospital, Toronto, ON, Canada
Footnotes
Disclosures: Inmaculada Hernandez is a consultant at Pfizer and BMS. Allen Campbell is an employee at IQVIA. All other authors declare no relevant conflicts of interest or financial relationships.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Transparency declaration: Dr. Suda affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; no important aspects of the study have been omitted; and discrepancies from the originally proposed study methods have been explained.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs, the U.S. government, or of IQVIA or any of its affiliated entities. The statements, findings, conclusions, views, and opinions contained and expressed in this publication are based in part on data obtained under license from IQVIA as part of the IQVIA Institute’s Human Data Science Research Collaborative.
ORCID Katie J. Suda: http://orcid.org/0000-0002-8977-1850
Appendix
Appendix 1.
List of MIDAS Regions summarizes the population coverage of the MIDAS SMART dataset in 2020
| Geographic Group | Included Regionsa | 2020 Mid-Year Population (millions) | % of 2020 Global Population |
|---|---|---|---|
| North America – Developed | Canada, USA | 357.4 | 4.6% |
| North America – Developing | Puerto Rico | 3.7 | 0.05% |
| Europe - Developed | Austria, Belgium, Bulgaria, Croatia, Czech Rep., Denmark, Estonia∗, Finland, France, Germany, Greece∗, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg∗, Netherlands, Norway, Poland, Portugal, Romania, Slovak Rep., Slovenia, Spain, Sweden, Switzerland, UK | 523.4 | 6.7% |
| Europe - Developing | Belarus, Bosnia, Kazakhstan, Russian Fed., Serbia, Turkey, Ukraine | 311.6 | 4.0% |
| Latin America - Developing | Argentina∗, Brazil, Central America∗,b, Chile∗, Colombia∗, Ecuador∗, Mexico, Peru∗, Uruguay∗ | 565.9 | 7.3% |
| Middle East and Africa - Developing | Algeria∗, Egypt∗, French West Africa∗,c, Jordan∗, Kuwait∗, Lebanon∗, Morocco∗, Saudi Arabia, South Africa, Tunisia, UAE∗ | 523.6 | 6.7% |
| Asia Pacific - Developed | Australia, Japan, New Zealand | 156.7 | 2.0% |
| Asia Pacific – Developing | China, India, Korea, Pakistan∗, Philippines, Taiwan, Thailand | 3270.5 | 42.0% |
Source: MIDAS, UN 2018 Population Prospectus
Asterisk denotes a region for which only retail pharmacy sales were available.
Central America included Costa Rica, El Salvador, Honduras, Guatemala, Nicaragua & Panama.
French West Africa included Benin, Burkina Faso, Cameroon, Chad, Congo, Gabon, Guinea, Cote d’Ivoire, Mali, Niger, Senegal, Togo.
Appendix 2.
Example subclasses included in each ATC1 class defined by the WHO describes subclasses within the WHO ATC1 drug classes
| WHO ATC1 Class | Example drug classes |
|---|---|
| Alimentary tract and metabolism | Drugs for peptic ulcer and gastro-esophageal reflux disease, antiemetics, laxatives, antiobesity, antidiabetics, anabolic agents |
| Respiratory system | Respiratory inhalers, systemic adrenergics, cough and cold products |
| Cardiovascular system | Cardiac glycosides, antiarrhythmics, antihypertensives, diuretics, peripheral vasodilators, lipid modifying agents |
| Nervous system | Anesthetics (general and local), opioids, salicylate analgesics, antimigraine, antiepileptics, anti-parkinson drugs, psycholeptics, psychoanaleptics |
| Sensory organs | Opthamological and ontological agents |
| Dermatological preparations | Predominately topical agents including antimicrobials, antipruritics, acne products, wound preparations |
| Various | Allergens, antidotes, contrast media, radiopharmaceuticals |
| Musculoskeletal system | Antiinflammatory and antirheumatic products (systemic and topical), muscle relaxants, antigout products, bone disease agents |
| Blood and blood-forming organs | Antithrombotics, antihemorrhagics, antianemics, and blood substitutes |
| Anti-infectives for systemic use | Antibacterials, antimycotics, antivirals, vaccines |
| Systemic hormonesa | Corticosteroids, thyroid products, pituitary and hypothalamic agents |
| GU system and sex hormones | Gynecological products, contraceptives, urologicals (includes drugs for benign prostatic hypertrophy) |
| Antiparasitic, insecticides & repellents | Antiprotozoals, antihelmintics, ectoparasiticides |
| Diagnostic Agents | Urine tests, diagnostics agents for diabetes and other diseases |
| Antineoplastic and immunomodulators | Antineoplastics, endocrine therapy, immunostimulants, immunosuppresants |
| Hospital Solutions | Normal saline, dextrose in water |
Appendix 3.
Final Fitted ARIMA Models provides the model specifications for the main ARIMA analyses presented in Table 1
| WHO ATC1 Class | All Regions (N=68) | Developed Regions (N=33) | Developing Regions (N=35) |
|---|---|---|---|
| All Drugs | MA model w/autocorrelation at lags 1 & 12. [AR (p=0, d=12, q=1,12)]. Specified w/o intercept. | ||
| Alimentary tract & metabolism | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. | Differenced model at lag 12. [AR (p=0, d=12, q=0)]. | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. |
| Blood and blood-forming organs | Differenced model at lag 12. [AR (p=0, d=12, q=0)]. | MA model w/autocorrelation at lags 1 & 12. [AR (p=0, d=12, q=1,12)]. Specified w/o intercept. | Differenced model at lag 12. [AR (p=0, d=12, q=0)]. |
| Cardiovascular system | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. | Differenced model at lag 12. [AR (p=0, d=12, q=0)]. | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. |
| Dermatological preparations | Moving average model accounting for autoregression at lags 1 & 12. [AR (p=0, d=12, q=1,12)]. Specified w/o intercept. | Differenced model at lag 12. [AR (p=0, d=12, q=0)]. | Moving average model accounting for autoregression at lags 1 & 12. [AR (p=0, d=12, q=1,12)]. Specified w/o intercept. |
| GU system and sex hormones | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. | Differenced model at lag 12. [AR (p=0, d=12, q=0)]. | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. |
| Systemic hormonesa | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. | MA model w/autocorrelation at lags 1 & 12. [AR (p=0, d=12, q=1,12)]. Specified w/o intercept. | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. |
| Anti-infectives for systemic use | MA model w/autocorrelation at lags 1 & 12. [AR (p=0, d=12, q=1,12)]. Specified w/o intercept. | ||
| Hospital Solutions | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. | ||
| Antineoplastic and immunomodulators | AR model w/autocorrelation at lag 11. [AR (p=11, d=12, q=0)]. | ||
| Musculoskeletal system | MA model w/autocorrelation at lags 1 & 12. [AR (p=0, d=12, q=1,12)]. Specified w/o intercept. | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. | |
| Nervous system | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. | MA model w/autocorrelation at lags 1 & 12. [AR (p=0, d=12, q=1,12)]. Specified w/o intercept. | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. |
| Antiparasitic, insecticides & repellents | AR model w/autocorrelation at lag 11. [AR (p=11, d=12, q=0)]. | ||
| Respiratory system | MA model w/autocorrelation at lags 1 & 12. [AR (p=0, d=12, q=1,12)]. Specified w/o intercept. | ||
| Sensory organs | MA model w/autocorrelation at lags 1 & 12. [AR (p=0, d=12, q=1,12)]. Specified w/o intercept. | ||
| Diagnostic Agents | MA model w/autocorrelation at lags 1 & 12. [AR (p=0, d=12, q=1,12)]. Specified w/o intercept. | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. | |
| Various | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. | Differenced model at lag 12. [AR (p=0, d=12, q=0)]. | AR model w/autocorrelation at lag 3. [AR (p=3, d=12, q=0)]. |
Source: Authors’ analysis of MIDAS Monthly Sales Data, August 2014-August 2020.
Source: Authors’ analysis of MIDAS Monthly Sales Data, August 2014-August 2020.
Abbreviations: ATC, Anatomical Therapeutic Chemical; AR, autoregressive; MA, moving average; p, number of autoregressive terms; d, number of nonseasonal differences needed for stationarity; q, number of lagged forecast errors in the prediction equation.
Excludes sex hormones and insulins.
Appendix 4.
Changes in Purchased Units per 100 Population, March 2020 vs. March 2019, by Jurisdiction shows country-level changes in units purchased per 100 population in March 2020, relative to March 2019
| A. Developed Regions (N=33) | B. Developing Regions (N=35) |
|---|---|
 |
 |
Source: Authors’ analysis of MIDAS Monthly Sales Data, August 2014-August 2020.
Source: Authors’ analysis of MIDAS Monthly Sales Data, August 2014-August 2020.
Abbreviations: ATC, Anatomical Therapeutic Chemical; AR, autoregressive; MA, moving average; p, number of autoregressive terms; d, number of nonseasonal differences needed for stationarity; q, number of lagged forecast errors in the prediction equation.
Appendix 5.
Changes in Purchased Units per 100 Population, by ATC1 Class, March 2020 vs. March 2019, Excluding Regions without Available Hospital Data presents a sensitivity analysis, excluding regions without hospital data
| WHO ATC1 Class | All Regions (N=49) |
Developed Regions (N=30) |
Developing Regions (N=19) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units per 100 Pop. |
% Change | p-val.a | Units per 100 Pop. |
% Change | p-val.a | Units per 100 Pop. |
% Change | p-val.a | ||||
| Mar. 2019 | Mar. 2020 | Mar. 2019 | Mar. 2020 | Mar. 2019 | Mar. 2020 | |||||||
| All Drugs | 4840.8 | 5521.4 | 14.1 | <0.001 | 11118.2 | 13147.8 | 18.3 | <0.001 | 3030.5 | 3331.1 | 9.9 | <0.001 |
| Alimentary tract and metabolism | 774.1 | 841.4 | 8.7 | 0.008 | 1631.9 | 1846.1 | 13.1 | <0.001 | 526.8 | 552.9 | 5.0 | 0.452 |
| Respiratory system | 708.6 | 917.4 | 29.5 | <0.001 | 1547.6 | 2085.7 | 34.8 | <0.001 | 466.7 | 581.8 | 24.7 | <0.001 |
| Cardiovascular system | 685.7 | 794.0 | 15.8 | <0.001 | 1875.3 | 2222.8 | 18.5 | <0.001 | 342.7 | 383.7 | 12.0 | <0.001 |
| Nervous system | 616.5 | 753.6 | 22.2 | <0.001 | 1904.2 | 2373.3 | 24.6 | <0.001 | 245.1 | 288.4 | 17.7 | <0.001 |
| Sensory organs | 525.5 | 484.9 | -7.7 | 0.864 | 1219.8 | 1103.5 | -9.5 | 0.448 | 325.3 | 307.3 | -5.5 | 0.381 |
| Dermatological preparations | 436.8 | 587.4 | 34.5 | <0.001 | 964.1 | 1280.6 | 32.8 | <0.001 | 284.7 | 388.3 | 36.4 | <0.001 |
| Various | 322.7 | 319.8 | -0.9 | 0.536 | 239.4 | 279.7 | 16.8 | <0.001 | 346.7 | 331.3 | -4.5 | 0.856 |
| Musculoskeletal system | 213.1 | 218.2 | 2.4 | 0.046 | 516.2 | 543.3 | 5.3 | 0.003 | 125.7 | 124.8 | -0.8 | 0.593 |
| Blood and blood-forming organs | 150.9 | 168.2 | 11.5 | 0.003 | 380.7 | 456.1 | 19.8 | <0.001 | 84.6 | 85.5 | 1.1 | 0.801 |
| Anti-infectives for systemic use | 126.6 | 127.5 | 0.7 | 0.006 | 189.8 | 211.8 | 11.6 | <0.001 | 108.4 | 103.3 | -4.7 | 0.435 |
| Systemic hormonesb | 110.3 | 129.5 | 17.4 | <0.001 | 247.8 | 302.3 | 22.0 | <0.001 | 70.6 | 79.8 | 13.1 | 0.005 |
| GU system and sex hormones | 84.0 | 88.7 | 5.6 | 0.089 | 200.6 | 219.3 | 9.3 | 0.008 | 50.4 | 51.2 | 1.6 | 0.552 |
| Antiparasitic, insecticides & repellents | 17.9 | 18.7 | 4.4 | 0.198 | 23.1 | 25.4 | 9.9 | 0.005 | 16.3 | 16.7 | 2.2 | <0.001 |
| Diagnostic Agentsc | 23.7 | 25.9 | 9.2 | <0.001 | 98.2 | 107.6 | 9.6 | <0.001 | 2.2 | 2.4 | 9.6 | 0.983 |
| Antineoplastic and immunomodulators | 22.8 | 28.2 | 23.9 | <0.001 | 53.1 | 63.9 | 20.2 | <0.001 | 14.0 | 18.0 | 28.3 | <0.001 |
| Hospital Solutionsd | 21.8 | 18.2 | -16.6 | 0.087 | 26.8 | 26.6 | -0.4 | 0.277 | 20.4 | 15.8 | -22.6 | 0.034 |
Source: Authors’ analysis of MIDAS Monthly Sales Data, August 2014-August 2020.
Abbreviations: ATC, Anatomical Therapeutic Chemical; Pop., population; p-val., p-value; GU, genito-urinary.
Reported p-values are for an ARIMA pulse intervention in March 2020. Bold denotes p-value < 0.05.
Excludes sex hormones and insulins.
There was no available data for diagnostic agents in Luxembourg for 92% of the study period. Peru was missing data for this class in 7 months.
There was no available data for hospital solutions in Switzerland across the study period. Algeria was missing data for this class in 21 months.
Appendix 6.
Changes in Purchased Units per 100 Population, by ATC1 Class, March 2020 vs. March 2019, Restricting to Retail Purchases presents a sensitivity analysis, limiting data to the retail sector
| WHO ATC1 Class | All Regions (N=68) |
Developed Regions (N=33) |
Developing Regions (N=35) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units per 100 Pop. |
% Change | p-val.a | Units per 100 Pop. |
% Change | p-val.a | Units per 100 Pop. |
% Change | p-val.a | ||||
| Mar. 2019 | Mar. 2020 | Mar. 2019 | Mar. 2020 | Mar. 2019 | Mar. 2020 | |||||||
| All Drugs | 3932.2 | 4666.7 | 18.7 | <0.001 | 9841.0 | 11782.4 | 19.7 | <0.001 | 2571.3 | 3038.0 | 18.1 | <0.001 |
| Alimentary tract and metabolism | 649.8 | 726.9 | 11.9 | <0.001 | 1458.4 | 1670.2 | 14.5 | <0.001 | 463.6 | 511.0 | 10.2 | 0.001 |
| Respiratory system | 650.0 | 852.1 | 31.1 | <0.001 | 1449.6 | 1950.4 | 34.5 | <0.001 | 465.9 | 600.7 | 28.9 | <0.001 |
| Cardiovascular system | 534.0 | 630.2 | 18.0 | <0.001 | 1713.3 | 2048.1 | 19.5 | <0.001 | 262.4 | 305.7 | 16.5 | <0.001 |
| Nervous system | 497.6 | 631.4 | 26.9 | <0.001 | 1686.0 | 2135.4 | 26.7 | <0.001 | 223.9 | 287.2 | 28.3 | <0.001 |
| Sensory organs | 463.7 | 441.8 | -4.7 | 0.546 | 1089.4 | 995.1 | -8.7 | 0.50 | 319.6 | 315.2 | -1.4 | 0.06 |
| Dermatological preparations | 357.2 | 514.2 | 43.9 | <0.001 | 715.6 | 1011.7 | 41.4 | <0.001 | 274.6 | 400.3 | 45.8 | <0.001 |
| Various | 139.3 | 173.0 | 24.2 | <0.001 | 194.0 | 232.1 | 19.7 | <0.001 | 126.7 | 159.5 | 25.9 | <0.001 |
| Musculoskeletal system | 204.6 | 209.4 | 2.4 | 0.008 | 478.1 | 504.2 | 5.5 | 0.004 | 141.6 | 141.9 | 0.2 | 0.007 |
| Blood and blood-forming organs | 121.1 | 135.9 | 12.2 | <0.001 | 339.7 | 411.4 | 21.1 | <0.001 | 70.8 | 72.9 | 2.9 | 0.895 |
| Anti-infectives for systemic use | 97.6 | 103.5 | 6.1 | <0.001 | 152.2 | 168.0 | 10.3 | <0.001 | 85.0 | 88.7 | 4.4 | 0.001 |
| Systemic hormonesb | 89.5 | 107.9 | 20.5 | <0.001 | 227.2 | 279.9 | 23.2 | <0.001 | 57.8 | 68.5 | 18.4 | <0.001 |
| GU system and sex hormones | 72.9 | 77.9 | 6.8 | 0.003 | 186.2 | 204.5 | 9.9 | 0.006 | 46.8 | 48.9 | 4.5 | 0.002 |
| Antiparasitic, insecticides & repellents | 21.4 | 22.2 | 3.4 | <0.001 | 21.9 | 22.6 | 3.2 | 0.03 | 21.3 | 22.1 | 3.4 | <0.001 |
| Diagnostic Agentsc | 18.9 | 20.5 | 8.7 | <0.001 | 85.9 | 96.3 | 12.1 | <0.001 | 3.5 | 3.2 | -7.9 | 0.265 |
| Antineoplastic and immunomodulators | 12.4 | 17.4 | 39.6 | <0.001 | 40.3 | 48.9 | 21.5 | <0.001 | 6.0 | 10.2 | 68.2 | <0.001 |
| Hospital Solutionsd | 2.0 | 2.3 | 15.0 | <0.001 | 3.4 | 3.8 | 13.1 | 0.008 | 1.7 | 2.0 | 16.0 | <0.001 |
Source: Authors’ analysis of MIDAS Monthly Sales Data, August 2014-August 2020.
Abbreviations: ATC, Anatomical Therapeutic Chemical; Pop., population; p-val., p-value; GU, genito-urinary.
Reported p-values are for an ARIMA pulse intervention in March 2020. Bold denotes p-value < 0.05.
Excludes sex hormones and insulins.
There was no available data for diagnostic agents in Luxembourg for 92% of the study period. Peru was missing data for this class in 7 months.
There was no available data for hospital solutions in Switzerland across the study period. Algeria was missing data for this class in 21 months.
Appendix 7.
Changes in Purchased Units per 100 Population, by ATC1 Class, March 2020 vs. March 2019, Excluding China presents a sensitivity analysis, excluding China
| WHO ATC1 Class | All Regions (N=67) |
Developed Regions (N=33) |
Developing Regions (N=34) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units per 100 Pop. |
% Change | p-val.a | Units per 100 Pop. |
% Change | p-val.a | Units per 100 Pop. |
% Change | p-val.a | ||||
| Mar. 2019 | Mar. 2020 | Mar. 2019 | Mar. 2020 | Mar. 2019 | Mar. 2020 | |||||||
| All Drugs | 5529.4 | 6602.0 | 19.4 | <0.001 | 11081.0 | 13127.4 | 18.5 | <0.001 | 3716.8 | 4489.4 | 20.8 | <0.001 |
| Alimentary tract and metabolism | 918.5 | 1025.2 | 11.6 | <0.001 | 1626.0 | 1840.5 | 13.2 | <0.001 | 687.4 | 761.2 | 10.7 | 0.001 |
| Respiratory system | 896.3 | 1175.1 | 31.1 | <0.001 | 1553.4 | 2092.0 | 34.7 | <0.001 | 681.7 | 878.2 | 28.8 | <0.001 |
| Cardiovascular system | 753.9 | 888.1 | 17.8 | <0.001 | 1869.2 | 2214.2 | 18.5 | <0.001 | 389.8 | 458.8 | 17.7 | <0.001 |
| Nervous system | 735.4 | 919.8 | 25.1 | <0.001 | 1898.3 | 2370.0 | 24.8 | <0.001 | 355.7 | 450.3 | 26.6 | <0.001 |
| Sensory organs | 644.1 | 618.8 | -3.9 | 0.433 | 1206.5 | 1093.1 | -9.4 | 0.47 | 460.5 | 465.3 | 1.0 | 0.034 |
| Dermatological preparations | 556.8 | 802.6 | 44.1 | <0.001 | 956.4 | 1286.7 | 34.5 | <0.001 | 426.4 | 645.9 | 51.5 | <0.001 |
| Various | 123.0 | 181.7 | 47.7 | <0.001 | 234.1 | 273.9 | 17.0 | <0.001 | 86.7 | 151.9 | 75.1 | <0.001 |
| Musculoskeletal system | 284.6 | 291.6 | 2.5 | 0.03 | 516.6 | 544.1 | 5.3 | 0.003 | 208.8 | 209.8 | 0.50 | 0.008 |
| Blood and blood-forming organs | 172.0 | 194.4 | 13.0 | <0.001 | 383.5 | 458.9 | 19.7 | <0.001 | 102.9 | 108.8 | 5.7 | 0.884 |
| Anti-infectives for systemic use | 129.5 | 146.6 | 13.2 | <0.001 | 189.0 | 210.4 | 11.3 | <0.001 | 110.0 | 125.9 | 14.4 | 0.002 |
| Systemic hormonesb | 125.1 | 149.7 | 19.6 | <0.001 | 248.1 | 303.3 | 22.3 | <0.001 | 85.0 | 99.9 | 17.6 | <0.001 |
| GU system and sex hormones | 101.3 | 108.5 | 7.1 | 0.008 | 200.5 | 218.9 | 9.2 | 0.009 | 69.0 | 72.8 | 5.5 | 0.012 |
| Antiparasitic, insecticides & repellents | 28.9 | 30.4 | 5.0 | <0.001 | 22.8 | 25.0 | 9.8 | 0.005 | 31.0 | 32.1 | 3.8 | <0.001 |
| Diagnostic Agentsc | 28.2 | 30.2 | 7.2 | <0.001 | 97.0 | 106.2 | 9.5 | <0.001 | 5.7 | 5.6 | -2.0 | 0.783 |
| Antineoplastic and immunomodulators | 20.2 | 27.3 | 35.1 | <0.001 | 53.0 | 63.8 | 20.3 | <0.001 | 9.5 | 15.5 | 62.9 | <0.001 |
| Hospital Solutionsd | 11.4 | 12.1 | 6.0 | 0.018 | 26.7 | 26.7 | -0.2 | 0.281 | 6.5 | 7.4 | 14.8 | 0.012 |
Source: Authors’ analysis of MIDAS Monthly Sales Data, August 2014-August 2020.
Abbreviations: ATC, Anatomical Therapeutic Chemical; Pop., population; p-val., p-value; GU, genito-urinary.
Reported p-values are for an ARIMA pulse intervention in March 2020. Bold denotes p-value < 0.05.
Excludes sex hormones and insulins.
There was no available data for diagnostic agents in Luxembourg for 92% of the study period. Peru was missing data for this class in 7 months.
There was no available data for hospital solutions in Switzerland across the study period. Algeria was missing data for this class in 21 months.
References
- 1.Ghotkar O., Harbrow H., Nass D., et al. Global medicine spending and usage trends: outlook to 2024. IQVIA Institute for Human Data Science. https://heatinformatics.com/sites/default/files/images-videosFileContent/global-medicine-spending-and-usage-trends.pdf Available at:
- 2.Barat I., Andreasen F., Damsgaard E.M. The consumption of drugs by 75-year-old individuals living in their own homes. Eur J Clin Pharmacol. 2000;56(6–7):501–509. doi: 10.1007/s002280000157. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman D.W., Kelly J.P., Rosenberg L., Anderson T.E., Mitchell A.A. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 4.Rozenfeld S., Fonseca M.J.M., Acurcio F.A. Drug utilization and polypharmacy among the elderly: a survey in Rio de Janeiro City, Brazil. Rev Panam Salud Publica. 2008;23(1):34–43. doi: 10.1590/s1020-49892008000100005. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration Report | Drug shortages: root causes and potential solutions. https://www.fda.gov/drugs/drug-shortages/report-drug-shortages-root-causes-and-potential-solutions Available at:
- 6.Lenihan K. Identifying the root causes of drug shortages and finding enduring solutions. https://healthpolicy.duke.edu/sites/default/files/2020-02/presentation_slides__0.pdf Available at:
- 7.Bruinen de Bruin Y., Lequarre A.S., McCourt J., et al. Initial impacts of global risk mitigation measures taken during the combatting of the COVID-19 pandemic. Saf Sci. 2020;128:104773. doi: 10.1016/j.ssci.2020.104773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M., Jie F. Managing supply chain uncertainty and risk in the pharmaceutical industry. Health Serv Manage Res. 2020;33(3):156–164. doi: 10.1177/0951484819845305. [DOI] [PubMed] [Google Scholar]
- 9.Vail E., Gershengorn H.B., Hua M., Walkey A.J., Rubenfeld G., Wunsch H. Association between US norepinephrine shortage and mortality among patients with septic shock. JAMA. 2017;317(14):1433–1442. doi: 10.1001/jama.2017.2841. [DOI] [PubMed] [Google Scholar]
- 10.Metzger M.L., Billett A., Link M.P. The impact of drug shortages on children with cancer — the example of mechlorethamine. N Engl J Med. 2012;367(26):2461–2463. doi: 10.1056/NEJMp1212468. [DOI] [PubMed] [Google Scholar]
- 11.Phuong J.M., Penm J., Chaar B., Oldfield L.D., Moles R. The impacts of medication shortages on patient outcomes: a scoping review. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0215837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caulder C.R., Mehta B., Bookstaver P.B., Sims L.D., Stevenson B. Impact of drug shortages on health system pharmacies in the Southeastern United States. Hosp Pharm. 2015;50(4):279–286. doi: 10.1310/hpj5004-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edney A. Virus likely curtailed China drug output, raising shortage fears. https://www.bloomberg.com/news/articles/2020-03-25/virus-likely-curtailed-china-drug-output-raising-shortage-fears Available at:
- 14.Balfour H. COVID-19 update: coronavirus and the pharmaceutical supply chain. https://www.europeanpharmaceuticalreview.com/article/116145/covid-19-update-coronavirus-and-the-pharmaceutical-supply-chain/ Available at:
- 15.Chatterjee P. Indian pharma threatened by COVID-19 shutdowns in China. Lancet. 2020;395(10225):675. doi: 10.1016/S0140-6736(20)30459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowland C., Slater J. Spikes in demand from coronavirus patients are creating shortages of asthma drugs and sedatives for ventilator patients. https://www.washingtonpost.com/business/2020/04/12/drug-ventilator-shortage-coronavirus/ Available at:
- 17.World Health Organization WHO model list of essential medicines - 21st list. 2019. https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06 Available at:
- 18.Yuan Y. 2019 ACTS annual report: statistical quality assurance applied to IQVIA’s information offerings. https://www.iqvia.com/-/media/iqvia/pdfs/library/publications/2019-acts-annual-report.pdf Available at:
- 19.Ghebreyesus T.A. WHO Director-General’s opening remarks at the mission briefing on COVID-19 - 12 March 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-mission-briefing-on-covid-19---12-march-2020 Available at:
- 20.United Nations Department of Economic and Social Affairs World economic situation and prospects 2020. https://www.un.org/development/desa/dpad/wp-content/uploads/sites/45/WESP2020_FullReport.pdf Available at:
- 21.Schaffer A.L., Dobbins T.A., Pearson S.A. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: a guide for evaluating large-scale health interventions. BMC Med Res Methodol. 2021;21(1):58. doi: 10.1186/s12874-021-01235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jbaily A., Feldhaus I., Bigelow B., et al. Toward health system strengthening in low- and middle-income countries: insights from mathematical modeling of drug supply chains. BMC Health Serv Res. 2020;20(1):776. doi: 10.1186/s12913-020-05549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein E.Y., Van Boeckel T.P., Martinez E.M., et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115(15):E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein E.Y., Tseng K.K., Pant S., Laxminarayan R. Tracking global trends in the effectiveness of antibiotic therapy using the Drug Resistance Index. BMJ Glob Health. 2019;4(2) doi: 10.1136/bmjgh-2018-001315. [DOI] [PMC free article] [PubMed] [Google Scholar]