Abstract
Lung cancer still contributes to nearly one‐quarter cancer‐related deaths in the past decades, despite the rapid development of targeted therapy and immunotherapy in non‐small cell lung cancer (NSCLC). The development and availability of comprehensive genomic profiling make the classification of NSCLC more precise and personalized. Most treatment decisions of advanced‐stage NSCLC have been made based on the genetic features and PD‐L1 expression of patients. For the past 2 years, more than 10 therapeutic strategies have been approved as first‐line treatment for certain subgroups of NSCLC. However, some major challenges remain, including drug resistance and low rate of overall survival. Therefore, we discuss and review the therapeutic strategies of NSCLC, and focus on the development of targeted therapy and immunotherapy in advanced‐stage NSCLC. Based on the latest guidelines, we provide an updated summary on the standard treatment for NSCLC. At last, we discussed several potential therapies for NSCLC. The development of new drugs and combination therapies both provide promising therapeutic effects on NSCLC.
Keywords: combination therapy, drug resistance, immunotherapy, non‐small cell lung cancer (NSCLC), targeted therapy
Based on the development of targeted therapy and immunotherapy, the treatment strategies for NSCLC have been profoundly changed. After evaluation of the incidental finding of nodule, patients are managed to routine follow‐up or diagnosis of NSCLC. Patients with NSCLC should receive molecular testing and PD‐L1 testing to make final treatment decision. For resectable NSCLC with targetable mutations, targeted therapy as adjuvant therapy shows promising effects. Immunotherapy has also been investigated as both neoadjuvant and adjuvant therapy. For unresectable advanced NSCLC with driver gene positive, targeted therapy and immunotherapy play an essential role in anticancer treatment. This review comprehensively presents the current landscape of targeted therapy and immunotherapy in NSCLC.
1. INTRODUCTION
Lung cancer is still the most common cancer worldwide and contributes to nearly one‐quarter cancer‐related deaths in 2021, more than 80% of which are directly caused by tobacco smoking. An additional 2.7% deaths are due to second‐hand smoke. 1 As a major component of lung cancer, non‐small cell lung cancer (NSCLC) accounts for 80–85%, of which lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) are the most common subtypes. 2 The incidence of lung cancer in developed countries has been declined during recent years, whereas the incidence and mortality rates in China have been significantly increased as a result of difference in lifestyle and development of economy. 3 The mortality of lung cancer in China is estimated and may increase by approximately 40% from 2015 to 2030. 4 Thus, the public health and therapeutic strategies for lung cancer, especially NSCLC, remain critical.
Tobacco smoking is directly associated with NSCLC and there are more than 50 carcinogens in the tobacco smoke. 5 Effective tobacco control helps to decrease the incidence of lung cancer in the United State. 3 Tobacco control is assumed to be a convenient and effective measure to lower the incidence and mortality of lung cancer. Other carcinogenic factors, such as bad lifestyle, genetic mutations, and family cancer history, also contribute to lung cancer. Except for cancer prevention, lung cancer screening is also crucial in detecting early‐stage patients, of which low‐dose computed tomographic (CT) screening reduces the mortality of lung cancer. 6 Despite the high sensitivity of CT screening, high rates of false‐positive findings make the size thresholds important. According to the American National Comprehensive Cancer Network (NCCN) guidelines for NSCLC (2021), routine follow‐up by chest CT is required for low‐risk patients (i.e., patients without smoking history or other known risk factors) when the solid nodule(s) on CT ≥ 6 mm. Efficient CT screening for early detection is the second defender for fight against NSCLC. 7
Surgery is recommended for early‐stage (stage I–II) NSCLC patients, whereas more than 70% of NSCLC are diagnosed as advanced stage (stage III–IV). 8 Cytotoxic therapy, targeted therapy, and immunotherapy are essential for advanced‐stage NSCLC patients. During last decades, much progress has been made in the therapeutic strategies for advanced NSCLC, especially the development of targeted therapy and immunotherapy. NSCLC is a heterogeneous malignancy with large‐scale genomic studies profiling a diversity of driver gene mutations. Genetic features are the basis of “precision and personalized medicine.” For now, several small molecular tyrosine kinase inhibitors (TKIs), which target EGFR (epidermal growth factor receptor) mutation, rearrangements in ALK (anaplastic lymphoma kinase), fusions in ROS1(ROS proto‐oncogene 1), BRAF (v‐Raf murine sarcoma viral oncogene homolog B) V600E, NTRK (neurotrophic tyrosine receptor kinase)1/2/3 gene fusion, MET (mesenchymal‐epithelial transition) exon 14 skipping, and RET (rearranged during transfection) rearrangement, have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of driven gene mutation‐positive NSCLC patients. Because of comprehensive genomic profiling, other genetic aberrances in NSCLC, such as mutations in Kirsten rat sarcoma (KRAS), amplification of human epidermal growth factor receptor‐2 (HER2), and other genotypes of the driver genes, have been thought highly targetable and investigated in preclinical and clinical trials. 9 Of note, compared with LUAD, LUSC rarely has EGFR mutation, ALK rearrangements, or ROS1 fusions, but usually gets alterations in RTKs, CDKN2A, PTEN, MLL2, HLA‐A, NOTCH1, and RB1. 9 Immunotherapy is another treatment strategy that has significantly prolonged the survival of NSCLC patients, especially those driver gene mutation‐negative NSCLC patients. Monoclonal antibodies (mAbs) targeting programmed cell death‐1 (PD‐1), programmed cell death ligand‐1 (PD‐L1), and cytotoxic T‐lymphocyte‐associated antigen‐4 (CTLA‐4) immune checkpoints have been approved for the treatment of a variety of cancers, including NSCLC. Five immune checkpoint inhibitors (ICIs) have been approved by FDA, including nivolumab and pembrolizumab (anti‐PD‐1 antibodies), atezolizumab and durvalumab (anti‐PD‐L1 antibodies), and ipilimumab (anti‐CTLA‐4 antibody).
Despite the development of the therapeutic strategies and improved survival for NSCLC, some major concerns remain challenging, such as the resistance to targeted therapy and immunotherapy, optimal combinations of the current treatment regimens, and investigation for new potential targets. Besides, with rapid development of targeted therapy during recent years, several breakthrough TKIs have been approved clinically. Based on the clinical management of NSCLC, we provide an overview for the treatment of advanced NSCLC and focus on targeted therapy and immunotherapy. Meanwhile, the important completed and ongoing clinical trials of both targeted therapy and immunotherapy are summarized in this review.
2. CLINICAL MANAGEMENT OF NSCLC
With the introduction of surgery and cytotoxic chemotherapy, the prognosis of NSCLC patients was improved for the first time. Through the development of molecular biology, new therapies, such as antiangiogenesis therapy, targeted therapy, and immunotherapy, have yielded encouraging therapeutic effects in advanced NSCLC. Remarkable changes have been made in the treatment of NSCLC during last decades (Figure 1).
FIGURE 1.
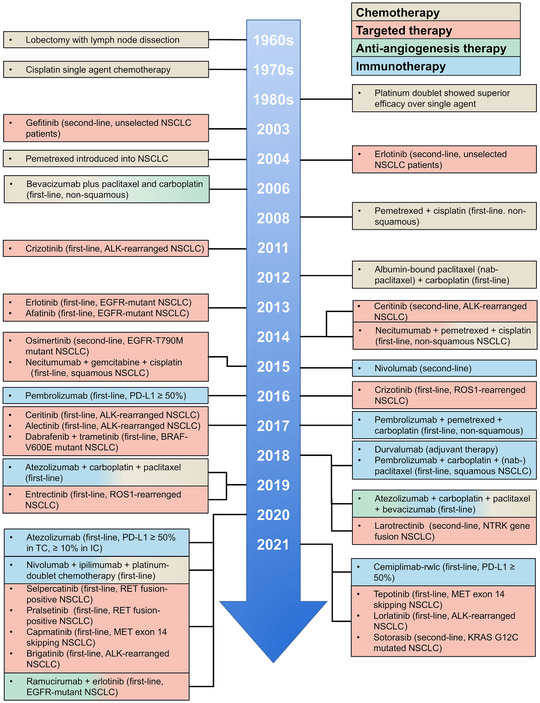
Timeline illustrating the development of treatment strategies for NSCLC. Surgery and cytotoxic chemotherapy have been introduced to NSCLC in 1960s and 1970s, for the first‐time improving prognosis of NSCLC. Antiangiogenesis therapy for nonsquamous NSCLC was approved by FDA in 2006, limited by its insufficient efficacy as monotherapy. The first‐line use of ALK TKIs and EGFR TKIs renewed the treatment strategies for NSCLC in early 2010s. Immunotherapy has developed rapidly during last 5 years. Pembrolizumab in combination with chemotherapy, approved in 2017, brings new hope for patients without targetable mutations. The combination therapies are presented in mixed colors
2.1. Molecular testing for analysis
NSCLC is a molecularly heterogeneous disease, which makes early identification of tumor genotype critical. Companion diagnostic assays are usually released by the U.S. FDA for approval of targeted agents, in order to identify essential genomic alterations before initiating therapy. 10 , 11 , 12 EGFR, ALK, and ROS1 are initially recommended as three targetable oncogenic drivers, which must be tested if the tissue is limited for next‐generation sequencing (NGS) panel. 13 NCCN recommends that all patients should be screened for EGFR, ALK, KRAS, ROS1, BRAF, NTRK1/2/3, MET, RET, and PD‐L1 expression. DNA sequencing is a traditional way to identify genomic mutations, which requires relatively enriched tumor cells. Mutation‐specific polymerase chain reaction (PCR) kits have been used in clinical laboratory for its high sensitivity with only 1–5% tumor cells. 11 However, this method is limited by its narrow spectrum of mutations. 14 , 15 Oncogenic fusion mutation can be detected by fluorescence in situ hybridization (FISH) or PCR; however, these two methods are limited by the capacity to identify fusion partners. 16 , 17 Identification of fusion partners is essential in the treatment decision making. 18 , 19 , 20 , 21 For now, NGS has become a typical molecular testing method and is able to analyze the information from DNA and RNA. 16 , 17 RNA NGS is also capable to identify the fusion partners of ALK, ROS1, RET, and NTRK. 22 , 23 , 24 , 25 Liquid biopsies, another type of detecting methods approved by the U.S. FDA, are used to detect circulating tumor DNA (ctDNA). 26 Though tissue biopsy remains the gold standard for diagnosis, liquid biopsy offers a way of continent and early diagnostic by body fluids. 27 When tumor tissue is limited, a ctDNA assay can be used to identify certain genomic mutations, including EGFR. 28 Immunohistochemistry (IHC) is a substitute for molecular testing, especially for ALK, ROS1, and NTRK. 29 , 30 For example, Ventana ALK D5F3 CDx Assay is the only IHC test approved by the U.S. FDA for ALK inhibitors. 31 , 32 Molecular testing becomes more and more important in clinical practice. Choosing the appropriate methods for a rapid diagnosis is crucial in making treatment decision for NSCLC patients.
2.2. Treatment for early‐stage NSCLC
The treatment strategy for early‐stage NSCLC is based on surgery (Figure 2). Surgery is strongly recommended for patients at stage I–II, which offers an optimal chance to fight against the disease. 7 Radical radiotherapy is another potentially curative treatment for localized NSCLC. 33 For unresectable stage II NSCLC patients, concurrent chemoradiotherapy is recommended. 34 Perioperative chemotherapy also contributed to a better survival for early‐stage patients. 35 The efficacy of postoperative cisplatin‐based chemotherapy was confirmed by a pooled analysis, especially those at stage II and III. 36 Adjuvant therapy of radiation is still under debt. In the LungArt study (IFCT‐0503, UK NCRI, and SAKK), postoperative radiation (PORT) demonstrated 3‐year disease‐free survival (DFS) of 47.1% in the PORT arm and 43.8% in the control arm among patients with resected N2‐positive NSCLC (stage III, lymph node‐positive) tumors, indicating no significant difference. 37 Meanwhile, the role of targeted therapy or immunotherapy in the treatment for early‐stage patients has not been well defined. 38 , 39 For patients with stage IIB–IIIA or high‐risk stage IB–IIA, if the diver gene mutation is ensured by molecular testing, targeted therapy could be applied as adjuvant treatment. 40 , 41 Additionally, patients with specific gene mutation show worse prognosis than patients with wild‐type genotype. 42 EGFR mutation‐positive NSCLC, targeted therapy as adjuvant treatment, has improved the survival of NSCLC patients. For instance, the 24‐month DFS is 89% in osimertinib group versus 52% in the placebo group, with reduced local relapse and metastasis. 43 Recently, immunotherapy also showed impressive therapeutic effects. Atezolizumab as adjuvant therapy significantly improved the DFS of stage II–IIIA NSCLC. 44 In 2021, atezolizumab was approved by FDA as an additional, or adjuvant, treatment for NSCLC patients (stage II–IIIA) received surgery or chemotherapy. 45 Durvalumab also showed increased 5‐year survival in unresectable stage III NSCLC patients who have not progressed after chemotherapy. 46 ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial), an ongoing large‐scale trial conducted by the National Cancer Institute, contains four important components: biomarker analysis for high‐risk resectable NSCLC (A151216), adjuvant nivolumab for NSCLC without EGFR or ALK mutation (EA5142), and adjuvant EGFR (A081105) or ALK (E4512) TKIs for NSCLC with EGFR or ALK mutation. 47 , 48 This clinical trial will provide valuable answers on ways to selecting high‐risk early‐stage NSCLC and on the efficacy of targeted therapy or immunotherapy as adjuvant therapy.
FIGURE 2.
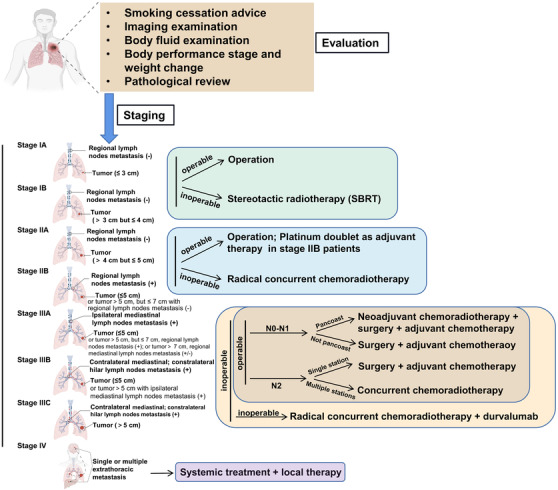
Treatment algorithm for NSCLC patients at early stage. Surgery is recommended for early‐stage NSCLC patients. For patients at stage IIA–IIIB, adjuvant therapy is required. Locally advanced or metastatic NSCLC should receive systemic therapy. The staging strategy is based on the guideline of National Comprehensive Cancer Network (NCCN) (2021) 49
2.3. Treatment for locally advanced or metastatic NSCLC (advanced NSCLC)
NSCLC patients in advanced phases usually lose the chance for surgery. For advanced NSCLC, combination chemotherapy of 4–6 cycles followed by observation is used to be treated as standard care. 50 , 51 The development of molecular biologic methods contributes to the identification of various subgroups of NSCLC. Basically, a newly diagnostic advanced NSCLC patients should receive molecular testing, PD‐L1 testing, and performance status scoring. Patients with good performance status might receive more aggressive treatment and benefit more. Around 70% of advanced NSCLC patients have a chance for targeted therapy or immunotherapy. 52 For patients without targetable mutations or negative expression of PD‐L1, the standard first‐line treatments differ from the histological types. Patients with adenocarcinoma, large cell, or NSCLC not otherwise specified should receive pembrolizumab plus carboplatin (or cisplatin) and pemetrexed, whereas patients with squamous cell carcinoma receive pembrolizumab plus carboplatin and (nab‐) paclitaxel (Figure 3). It is assumed that up to 16% of patients with squamous and 15% with nonsquamous advanced NSCLC surviving for 5 years or more, due to combination therapy with immunotherapy. 53 A comprehensive understanding of the current targeted therapy and immunotherapy is helpful in developing clinical treatments.
FIGURE 3.
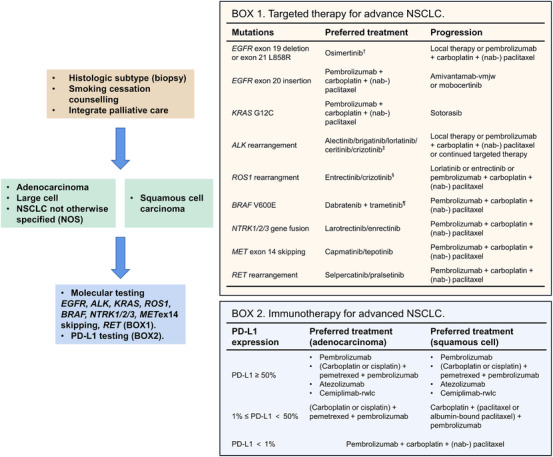
Treatment algorithm for advanced NSCLC. Advanced NSCLC consists of metastatic NSCLC and unresectable locally advanced NSCLC. EGFR, ALK, KRAS, ROS1, BRAF, NTRK1/2/3, MET, RET, and PD‐L1 expressions are included in the molecular testing. For patients without targetable mutations, pembrolizumab + carboplatin + (nab‐) paclitaxel is recommended. †For patients with EGFR exon 19 deletion or exon 21 L858R, erlotinib, afatinib, gefitinib, and dacomitinib are also recommended. However, when the disease progresses on these TKIs, patients are recommended for a second molecular testing for T790M mutation test. ‡Patients who are intolerant to crizotinib may be switched to ceritinib, alectinib, or brigatinib. §Entrectinib is recommended for patients with CNS metastasis. ¶Single‐agent vemurafenib is a treatment option if the combination of dabrafenib + trametinib is not tolerated
3. TARGETED THERAPY FOR NSCLC
Patients with advanced NSCLC benefit a lot from the development of targeted therapy. It is estimated that more than 65% of patients with advanced NSCLC have a potentially targetable genomic alteration. 52 Based on the knowledge of genomic alterations, targeted therapy becomes the first‐line treatment for selected NSCLC patients, including inhibitors for EGFR, ALK, ROS1, BRAF, NTRK1/2/3, MET, and RET (Table 1). Of note, LUSC rarely have those genomic alterations.
TABLE 1.
Targeted therapy in driver gene‐positive advanced NSCLC patients
| Target | Inhibitor | Indications | Status | Company | Key clinical trials |
|---|---|---|---|---|---|
| EGFR (first generation) | |||||
| †Geftinib (Iressa) | Advanced NSCLC with EGFR exon 19 deletion or exon 21 L858R mutations | FDA, Jul 2015 (first line) | AstraZeneca | IPASS, NCT00322452 54 | |
| Erlotinib (Tarceva) | Advanced NSCLC with EGFR exon 19 deletion or exon 21 L858R mutations | FDA, Nov 2013 (first line) | Genentech | OPTIMAL, NCT00874419 55 | |
| Icotinib (Conmana) | Advanced NSCLC with EGFR exon 19 deletion or exon 21 L858R mutations | NMPA, Nov 2014 (first line) | Beta Pharma | ICOGEN, NCT01040780 56 | |
| EGFR (second generation) | |||||
| †Afatinib (Gilotrif) | Advanced NSCLC with EGFR exon 19 deletion or exon 21 L858R mutations | FDA, Jul 2013 (first line) | Boehringer Ingelheim | LUX‐Lung 2, NCT00525148 57 ; LUX‐Lung 3, NCT00949650 58 ; LUX‐Lung 6, NCT01121393 59 | |
| †Dacomitinib (Vizimpro) | Advanced NSCLC with EGFR exon 19 deletion or exon 21 L858R mutations | FDA, Nov 2015 (first line) | Pfizer | ARCHER 1050, NCT01774721 60 | |
| EGFR (third generation) | |||||
| †Osimertinib (Tagrisso) |
|
|
AstraZeneca | FLAURA, NCT02296125 61 ; ADAURA, NCT02511106 62 | |
| Aulmonertinib (Ameile) | Advance NSCLC patients with EGFR T790M, who got disease progression on or after EGFR TKI therapy | NMPA, Mar 2020 (second line) | EQRx and Hansoh Pharma | APOLLO, NCT02981108 63 | |
| Furmonertinib (Alflutinib) | Advance NSCLC patients with EGFR T790M, who got disease progression on or after EGFR TKI therapy | NMPA, Mar 2020 (second line) | Shanghai Allist Pharmaceuticals | ALSC003, NCT03452592 64 | |
| Anti‐EGFR antibody | |||||
| Necitumumab (Portrazza) |
|
|
Eli Lilly |
|
|
| EGFR (exon 20 insertion) | |||||
| Amivantamab‐vmjw (Rybrevant) | Advanced exon 20 insertion‐positive NSCLC patients progressed after platinum‐based chemotherapy | FDA, May 2021 (second line) | Janssen Biotech | CHRYSALIS, NCT02609776 67 | |
| Mobocertinib (Exkivity) | Advanced exon 20 insertion‐positive NSCLC patients progressed after platinum‐based chemotherapy | FDA, Sep 2021 (second line) | Takeda Pharmaceuticals | Study 101, NCT02716116 68 | |
| ALK | |||||
| †Crizotinib (Xalkori) | Advanced NSCLC with ALK rearrangement | FDA, Aug 2011 (first line) | Pfizer |
PROFILE 1001, NCT00585195 69 ; PROFILE 1005, NCT00932451 70 |
|
| †Ceritinib (Zykadia) | Advanced NSCLC patients with ALK arrangements | FDA, May 2017 (first line) | Novartis | ASCEND‐4, NCT01828099 71 | |
| †Alectinib (Alecensa) | Advanced NSCLC patients with ALK arrangements | FDA, Nov 2017 (first line) | Hoffmann‐La Roche and Genentech |
ALUR, NCT02604342 72 ; ALEX, NCT02075840 73 |
|
| †Brigatinib (Alunbrig) | Advanced NSCLC patients with ALK arrangements | FDA, May 2020 (first line) | ARIAD | ALTA 1L, NCT02737501 74 | |
| †Lorlatinib (Lorbrena) | Advanced NSCLC patients with ALK arrangements | FDA, Mar 2021 (first line) | Pfizer | Study B7461001, NCT01970865 75 ; Study B7461006, NCT03052608 76 | |
| ROS1 | |||||
| †Crizotinib (Xalkori) | Advance ROS1‐positive NSCLC | FDA, Mar 2016 (first line) | Pfizer | PROFILE 1001, NCT00585195 69 | |
| †Entrectinib (Rozlytrek) | Advance ROS1‐positive NSCLC | FDA, Aug 2019 (first line) | Genentech |
STARTRK‐1, NCT02097810; STARTRK‐2, NCT02568267; ALKA‐372‐001, EudraCT number, 2012‐000148‐88 77 |
|
| BRAF | |||||
| †Dabrafenib (Tafinlar) | In combination with trametinib for advance BRAF V600E‐positive NSCLC | FDA, Jun 2017 (first line) | Novartis | BRF113928, NCT01336634 78 | |
| KRAS | |||||
| Sotorasib (Lumakras) | KRAS G12C‐mutated advanced NSCLC who have received at least one prior systemic therapy | FDA, May 2021 (second line) | Amgen | CodeBreaK 100, NCT03600883 79 | |
| RET | |||||
| †Selpercatinib (Retevmo) | Advanced RET fusion‐positive NSCLC | FDA, May 2020 (first line) | Eli Lilly | LIBRETTO‐001, NCT03157128 80 | |
| †Pralsetinib (Gavretotm) | Advanced RET fusion‐positive NSCLC | FDA, Sep 2020 (first line) | Blueprint Medicines Corporation | ARROW, NCT03037385 81 | |
| MET | |||||
| †Capmatinib (Tabrecta) | Advanced NSCLC patients with MET exon 14 skipping alterations | FDA, May 2020 (first line) | Novartis | GEOMETRY mono‐1 trial, NCT02414139 82 | |
| †Tepotinib (Tepmetko) | Advanced NSCLC patients with MET exon 14 skipping alterations | FDA, Feb 2021 (first line) | Merck KGaA | V ISION, NCT02864992 83 | |
| Crizotinib (Xalkori) | Advanced MET exon 14 skipping‐positive NSCLC patients progressed after platinum‐based chemotherapy | FDA, May 2018 (second line) | Pfizer | PROFILE 1001 study, NCT00585195 69 | |
| Savolitinib (Orpathys) | Advanced NSCLC patients with MET exon 14 skipping alterations who have progressed after or who are unable to tolerate platinum‐based chemotherapy | NMPA, Jun 2021 (second line) | HUTCHMED and AstraZeneca | NCT02897479 84 | |
| NTRK 1/2/3 | |||||
| †Entrectinib (Rozlytrek) | Solid tumors that have a neurotrophic receptor tyrosine kinase (NTRK) gene fusion without a known acquired resistance mutation | FDA, Aug 2019 (second line) | Genentech Inc |
STARTRK‐1, NCT02097810; STARTRK‐2, NCT02568267; ALKA‐372‐001, EudraCT number, 2012‐000148‐88 85 |
|
| †Larotrectinib (VITRAKVI) | Solid tumors that have a neurotrophic receptor tyrosine kinase (NTRK) gene fusion without a known acquired resistance mutation | FDA, Nov 2018 (second line) | Loxo Oncology Inc. and Bayer | LOXO‐TRK‐14001, NCT02122913 86 ; SCOUT, NCT02637687 87 ; NAVIGATE, NCT02576431 88 | |
| Repotrectinib (TPX‐0005) | Solid tumors that have a neurotrophic receptor tyrosine kinase (NTRK) gene fusion without a known acquired resistance mutation | FDA, Oct 2021 (second line) | Turning Point Therapeutics, Inc | TRIDENT‐1, NCT03093116 89 | |
Note: Inhibitors with † are recommended as first‐line treatment for advanced NSCLC with corresponding mutations, according to the NCCN guideline 2021.
Abbreviations: FDA, the U.S. Food and Drug administration; NMPA, Chinese National Medical Products Administration.
3.1. EGFR
There are 58 receptor tyrosine kinases (RTKs) in humans, and the epidermal growth factor receptor (EGFR, HER1, and ErbB1) is one of the first RTKs regarded as an anticancer target and brings significant improvement in the survival of NSCLC patients. 90 EGFR belongs to tyrosine kinase type I receptors family that also includes human epidermal growth factor receptor 2 (HER2 and ErbB2), HER3 (ErbB3), and HER4 (ErbB4). EGFR is normally expressed on the surface of epithelial cells and regulates cell growth, survival, invasion, and angiogenesis. 91 The EGFR gene is located on the short arm of chromosome 7 (7p11.2) and contains 28 exons and 27 introns. Mutations clustering around the ATP‐binding pocket of the tyrosine kinase domain lead to constituent, ligand‐independent activation of EGFR, which results in inappropriate activation of the antiapoptotic Ras signaling pathway and abnormal proliferation of cancer cells. 92 , 93 EGFR mutations in NSCLC occur in approximately 40% of Asian patients, about 10% of non‐Asian patients, and most of them are LUAD, young females, and nonsmoker. 94 , 95 The most common activating mutations of EGFR in NSCLC (approximately 85%) include exon 19 deletions and a point mutation on exon 21 (Leu858Arg, L858A), known as sensitizing mutations, which are responsible for oral TKIs targeting EGFR. Other mutations like exon 20 insertions and point mutations on exon 18 are less common. 92 Compared with other mutations, patients with exon 19 deletion usually benefit more from targeted therapy and show a better survival. 96 , 97 Mutations at EGFR exon 20 are a heterogeneous group, some of which are predictions for the response to EGFR TKIs, such as T790M mutation. 98 , 99 Therefore, detailed knowledge of the specific alteration is required during genomic testing of EGFR. Results from recent data showed that patients without sensitizing EGFR mutation should not receive EGFR TKIs in any line of therapy. 100
Three generations of EGFR TKIs have been used in clinical applications. First‐generation EGFR TKIs, including gefitinib, erlotinib, and icotinib, reversibly bind to EGFR and competitively inhibit the binding of ATP to tyrosine kinase domain. Gefitinib and erlotinib were first approved by the U.S. FDA for treatment of patients with advanced NSCLC (unselected), who have failed in standard chemotherapy. 101 However, the use of these two TKIs in unselected NSCLC patients remained controversial. 93 , 102 In 2009, researchers found that nonsmokers or former light smokers in East Asia benefited more from gefitinib, especially those with EGFR mutation. 54 Subsequently, several large clinical trials, including IPASS, WJTOG3405, NEJGSG002, OPTIMAL, EURTAC, and ENSURE, confirmed that gefitinib and erlotinib were superior to chemotherapy in NSCLC patients with EGFR mutations (especially sensitizing mutation, exon 19 deletion and exon 21 L858R) in terms of progression‐free survival (PFS), objective response rate (ORR), and quality of life, whereas these EGFR TKIs showed no advantage in patients without such mutation. 55 , 103 , 104 , 105 , 106 , 107 According to these clinical trials, the median PFS ranges of gefitinib and erlotinib were 9.2–10.8 and 9.7–13.7 months, respectively, whereas the median PFS of platinum‐based chemotherapy ranges from 4.6 to 6.3 months. Therefore, gefitinib and erlotinib are recommended by the U.S. FDA as the first‐line therapy for EGFR sensitizing mutation‐positive advanced NSCLC patients in 2009 and 2013, respectively. There is no significant difference between the two EGFR TKIs in the therapeutic effects based on the results of randomized phase III trials. 108 , 109 Icotinib, another first‐generation EGFR TKI, has been approved by NMPA in 2011 for the second‐line treatment of advanced NSCLC patients with EGFR mutation. The results of ICOGEN trial in China, a double‐blind, head‐to‐head phase III study containing 399 patients, showed that the median PFS of icotinib arm was 137 days as compared to that of gefitinib arm was 102 days. 56 In 2014, icotinib was recommended as first‐line treatment for advanced NSCLC with sensitizing EGFR mutations. Later, in 2020, the indication of icotinib was expanded by NMPA, as an adjuvant therapy for stage II–IIIA patients who harbor sensitizing EGFR mutation. This approval was based on the results from EVIDENCE trial, in which icotinib showed an improved median DFS of 46.9 months compared with 22.1 months in standard chemotherapy group. 110 Icotinib is now under evaluation by the U.S. FDA for the treatment of EGFR mutation‐positive NSCLC patients.
Afatinib and dacomitinib, two irreversible ERBB‐family (pan‐HER) inhibitors, are the second‐generation EGFR TKIs. Unlike the first‐generation, they bind to ATP‐binding domain of EGFR irreversibly and less selectively. Based on the results of three clinical trials, LUX‐Lung 2, LUX‐Lung 3, and LUX‐Lung 6, afatinib showed better ORR (approximately 70%) and prolonged the PFS (approximately 1 year) in advanced NSCLC patients with EGFR mutations compared with platinum‐based chemotherapy. 57 , 58 , 59 In LUX‐Lung 6, 364 patients with EGFR mutations were enrolled. The ORR in afatinib arm was 67% compared to 23% in cisplatin‐based chemotherapy arm, and the PFS was prolonged by afatinib (11 vs. 5.6 months). 59 Afatinib was initially approved by the U.S. FDA in 2013 for the treatment of advanced NSCLC patients with EGFR exon 19 deletions or exon 21 (L858R) substitution mutations and got a broadened indication as the first‐line treatment of advanced NSCLC patients with nonresistant EGFR mutations. Dacomitinib was approved by the U.S. FDA in 2018 as first‐line treatment for advanced NSCLC patients with EGFR exon 19 deletion or exon 21 L858R substitution mutations. The approval was based on a randomized, multicenter, and open‐label clinical trial (ARCHER 1050), in which the median PFS was 14.7 and 9.2 months in the dacomitinib and gefitinib arms, respectively. 60 However, because of the irreversible and wide binding to ERBB family, the second‐generation EGFR TKIs harbor both better therapeutic effects and more toxic effects than first‐generation TKIs. The advantages of the second‐generation EGFR TKIs over first‐generation EGFR TKIs are not unclear so far. 111 , 112
Nearly, all patients received first‐ or second‐generation EGFR TKIs treatment eventually acquired drug resistance, leading to disease progression. 113 Osimertinib has emerged as a third‐generation EGFR TKI and selectively and irreversible targets to original EGFR sensitizing mutations and T790M mutation. T790M is the most common second mutation causing drug resistance, which is referred to a threonine‐tomethionine substitution on codon 790 in exon 20 (T790M). 114 , 115 The third‐generation EGFR TKIs, including rociletinib, 116 PF‐06747775, 117 olmutinib (HM61713), 118 nazartinib (EGF816), 119 avitinib, 120 osimertinib (AZD9291), aulmonertinib (HS‐10296), and furmonertinib (alflutinib, AST2818), were designed to overcome the T790M mutation. Osimertinib was initially approved for the treatment of advanced NSCLC EGFR T790M mutation‐positive patients with disease progression on or after EGFR TKI therapy. Approval went through an accelerated process based on the promising results of AURA extension and AURA 2. 121 , 122 The T790M detection rates in the two trials were 64% and 63%, respectively, and the ORRs were 57% and 61%, respectively. 123 , 124 Subsequently, based on the results of two clinical trials, FLAURA (NCT02296125) and ADAURA (NCT02511106), the U.S. FDA broadened the indications of osimertinib as the first‐line treatment for advanced NSCLC patients with sensitizing EGFR mutations or as an adjuvant therapy when surgery patients with sensitizing EGFR mutations. 61 , 62 Meanwhile, osimertinib showed advantages on the ability to cross blood–brain barrier, making it possible to enter central nervous system (CNS) and kill tumor cells. 125 Aulmonertinib (HS‐10296) is the second third‐generation EGFR TKI approved in China. In March 2020, it was approved by the Chinese National Medical Products Administration (NMPA) for the treatment of advanced NSCLC patients with EGFR T790M, who got disease progression on or after EGFR TKI therapy. This approval was based on the findings of an open‐label phase II study, APOLLO, in which the median PFS and ORR of patients with progressed NSCLC harboring EGFR T790M mutation treated with aulmonertinib was 12.3 months and 68.9%. Of note, aulmonertinib induced an ORR of 61.5% in patients with CNS metastasis. 63 In 2021, the head‐to‐head phase III clinical trial (AENEAS) compared aulmonertinib with gefitinib as the first‐line treatment in advanced patients with sensitizing EGFR mutations. Aumolertinib significantly improved PFS to 19.3 months compared to 9.9 months in gefitinb arm. As for side effects, the incidence of rash or diarrhea was markedly decreased in aumolertinib arm. 126 Recently, furmonertinib, the third third‐generation of EGFR TKI, was approved by NMPA and shared the same indications with aulmonertinib. Findings of a phase IIb clinical trial were released in ASCO 2020, EGFR T790M mutation‐positive advanced NSCLC patients treated with furmonertinib showed an ORR of 74.1% and PFS of 9.6 months. 127 The ORR and PFS of patients with CNS metastasis were 66% and 11.6 months. 64 , 128 Aulmonertinib and furmonertinib are both under clinical investigation on first‐line treatment for advanced NSCLC patients harboring sensitizing EGFR mutations.
Targeted therapy strategy contains two main approaches: mAbs and small‐molecule inhibitors (SMIs). The advantages and disadvantages between mAbs and SMI have been well discussed in Ref. 90. 129 Necitumumab is a second‐generation, recombinant human IgG1 mAb that binds to EGFR, preventing receptor activation. In the phase III clinical trials (SQUIRE), necitumumab in combination with gemcitabine and cisplatin prolonged the OS and PFS of patients with advanced squamous cell lung cancer for 1.6 and 0.2 months, respectively. 66 Though the benefits were not extraordinary, based on the limited therapy for squamous cell lung cancer patients, the U.S. FDA approved necitumumab combination therapy as the first‐line treatment for advanced squamous NSCLC. However, only patients with EGFR expression (EGFR > 0) would benefit from this limited therapeutic effect. 130 Other mAbs targeting EGFR, such as cetuximab, nimotuzumab, and panitumumab, have not been approved for the treatment of NSCLC.
At present, EGFR TKIs have been approved for the treatment of sensitizing EGFR mutations, exon 19 deletions (Del19) and the L858R point mutation, which account for about 85% of observed EGFR mutations in NSCLC. 102 However, there have been about 600 types of EGFR mutations reported, of which 93% are represent in the exons 18–21, the first four exons for the expression of tyrosine kinase domain. 131 Limited by the testing methods, only some of them could be detected with high sensitivities, including G719A/S/C, Del19, S768I, exon 20 insertions (Ins20: V769_D770insASV, D770_N771insG/SVD, and H773_V774insH), T790M, L858R, and L861Q. 131 For now, relevant data on the clinical features of rare EGFR mutations have been accessed via post‐hoc analyses of clinical trials and discussed in Ref. 94. 132 The first‐generation EGFR TKIs, gefitinib and erlotinib, are less effective for G719X mutation with an ORR of 36.8% and median PFS of only 6.3 months, compared to Del19 (65.3%) and L858R (67.5%). 133 One of the second‐generation EGFR TKIs, neratinib, has shown effective inhibition by targeting G719X mutation but limited effects on Del19 or L858R. 134 A post‐hoc analysis of three clinical trials, LUX‐Lung 2, LUX‐Lung 3, and LUX‐Lung 6, revealed that afatinib was effective for three rare EGFR mutations, G719X, S786I, and L861Q. 135 Of note, patients with G719X mutation got a prolonged PFS of 13.9 months, which led to a broaden indication of afatinib by the U.S. FDA, for the treatment of advanced NSCLC patients with G719X mutation. Osimertinib as a third‐generation EGFR TKI also showed potential therapeutic effect on G719X mutation. 136 Other rare mutations, such as exon 19 insertion, 137 exon 20 insertion, 98 and EGFR Kinase domain duplication (EGFR‐KDD), 138 have been investigated by certain preclinical and clinical researches.
During the last 2 years, breakthroughs have been made in targeted therapy for EGFR exon 20 insertion, which is associated with poor prognosis. 139 In 2021, two drugs against EGFR exon 20 insertion, amivantamab‐vmjw and mobocertinib, have won the U.S. FDA approval as second‐line treatment for EGFR exon 20 insertion‐positive advanced NSCLC patients. Amivantamab‐vmjw, a bispecific mAb targeting EGFR and MET, has shown an ORR of 40% with a median response duration of 11.1 months. 140 Later, in September, the approval of mobocertinib (TAK‐788) was based on Study 101, in which mobocertinib showed an ORR of 28% with a median response duration of 17.5 months. 68
3.2. ALK
ALK is an RTK naturally expressed in human tissues, such as brain, small intestine, and testis, without complete understanding of its function, but shows strong oncogenic effects. 141 Several ligands for ALK have been identified in recent researches, such as FAM150 and heparin. 142 , 143 ALK is highly expressed in the nervous system of neonatal brain, but barely expressed in adults. 144 The expression of ALK is time and spatially controlled. ALK fusion proteins are usually found as oncogenic driver in various malignancies. The first identification of ALK as a fusion gene partner was found in anaplastic large‐cell lymphoma in 1994. 145 ALK rearrangements in NSCLC have been found in 2007 by the initial discovery of a fusion gene containing parts of the echinoderm microtubule‐associated protein‐like 4 (EML4) gene and ALK gene. 146 There are more than 20 variants of ELM4‐ALK fusion identified and EML4‐ALK, variant 1 is the most common and well‐studied one. 147 The EML4 and ALK genes are both located on chromosome 2p, and the variants of EML4‐ALK fusion depend on the fusion breakpoint in the EML4 gene, with the breakpoint in ALK is usually at exon 20. 147 Of note, fusion partners of ALK are more than ELM4, others like huntingtin‐interacting protein 1 (HIP1), 148 kinesin family member 5B (KIF5B), 149 kinesin light chain 1 (KLC1), 150 translocated promoter region (TPR), 151 and so on have also been identified in human lung cancer. ALK arrangement‐driven tumors account for about 5% of NSCLC, and most of them are Asian, men, and never/light smokers and are likely to be adenocarcinomas. 152 , 153 Unlike the predictive effects of some EGFR mutations, such as exon 19 deletion is associated with better prognosis, it is not clear whether specific genetic alteration in ALK mutation is associated with therapeutic response.
Five ALK TKIs, including crizotinib, ceritinib, alectinib, brigatinib, and loralatinib, have been approved as targeted therapy for advanced NSCLC patients who are confirmed to have ALK arrangements. 152 Crizotinib, a first‐generation ALK TKI, is an oral multiple‐target agent, targeting ALK, ROS1, and MET, and initially developed to target cMET. 154 Based on the ORRs of 50% and 61% and the PFS of 41.9 and 48.1 weeks in two single‐arm phase I and II clinical trials (expansion cohort of PROFILE 1001 and PROFILE 1005), crizotinib has been approved by the U.S. FDA in 2011 for advanced NSCLC patients with ALK rearrangements. 155
Ceritinib (LDK378) and alectinib are two second‐generation ALK TKIs approved by the U.S. FDA for NSCLC patients failed in or tolerate on crizotinib therapy. 147 They have shown an obvious advantage of penetrance into CNS compared with crizotinib. 156 Ceritinib could effectively inhibit certain ALK alteration patterns, such as L1196M, G1269A, I1171T, and S1206Y mutations, but could not overcome two crizotinib‐resistant ALK mutations, G1202R and F1174C. 157 In a randomized, open‐label, phase III clinical trial (ASCEND‐4), ceritinib, as first‐line therapy, showed an ORR of 72.5% and a prolonged median PFS of 16.6 months compared with platinum‐based chemotherapy and the median CNS response duration was 16.6 months in ceritinib arm. 71 Therefore, the U.S. FDA broadened ceritinib indication to previously untreated ALK‐positive advanced NSCLC in May 2017. Alectinib, another second‐generation ALK TKI, has shown an ORR of approximately 50% of patients failed in crizotinib therapy, with median PFS of more than 8 months. A phase III clinical trial (ALEX) revealed that patients treated with alectinib showed prolonged PFS of 26 months compared with 10 months in crizotinib group. The ORR was 79% for alectinib and 72% for crizotinib. 158
Brigatinib is a small molecular inhibitor targeting both EGFR and ALK, which has been confirmed to overcome the osimertinib‐resistant C797S mutation and expected to be the next‐generation EGFR TKI. 159 Brigatinib was initially approved for the second‐line treatment for advanced ALK‐positive NSCLC patients. 160 The first‐line efficacy of brigatinib was confirmed by a randomized phase III trial, ALTA1L. Two hundred and seventy‐five previously untreated advanced ALK‐positive NSCLC patients were enrolled in this trial. The median PFS and ORR for patients treated with brigatinib was 24 months and 74%, respectively, compared with 11.1 months and 62% for those treated with crizotinib, respectively. 161 With longer follow‐up, patients with brain metastasis have benefited more from brigatinib treatment compared with crizotinib treatment. 161 In preclinical studies, brigatinib showed potential to overcome the ceritinib‐ or alectinib‐resistance mutations, including G1202R, F1174C/V, and I1171N/T/S. 162
Recently, in March 2021, a former second‐line ALK TKI, lorlatinib, has been approved as a first‐line treatment for advanced ALK‐positive NSCLC patients. 76 Lorlatinib is a third‐generation ALK TKI and can target multiple RTKs, including ALK and ROS1, and gets ability to overcome ALK and ROS1‐resistance mutation, such as I1171T and G1202R. 163 , 164 The emergency of lorlatinib brings a breakthrough to the targeted therapy for NSCLC patients with ALK mutations. Lorlatinib has shown obvious advantages, including penetration into CNS, fewer side effects, and less drug resistance, compared with former‐generations ALK TKIs.
3.3. ROS1
ROS1 is an oncogenic RTK of insulin receptor family encoded by the ROS1 gene on chromosome 6q22. The biologic function of wild‐type ROS1 is not well identified and there has no specific ligand of ROS1 been found. 165 The oncogenic effects of ROS1 are based on the constitutively phosphorylated and activated by the fusions with partner genes, such as CD74 (most common), 166 FIG (fused in glioblastoma, the oncogenic effect of ROS1 rearrangements first identified), 167 SLC34A2 (so lute carrier family 34 member 2), 168 and so on. For now, 16 genes have been identified as ROS1 fusion partner genes according to Catalogue of Somatic Mutations in Cancer (COSMIC) data bese, and ROS1 arrangements have been observed in 1–2% NSCLC, most of which are adenocarcinomas, female, and never or light smokers. 24 The gold standard for ROS1 fusion detection is FISH assay. 69
The ROS1 amide acid sequence shares 49% homology with ALK in the kinase domain and 77% homology at the ATP‐binding area. 169 Almost all ALK TKIs showed activity to ROS1. Crizotinib, approved for the treatment of ALK‐positive NSCLC, is one of the two targeted agents approved by the U.S. FDA in 2016 for ROS1‐positive advanced NSCLC. This approval was based on a single‐arm study in 50 advanced NSCLC patients with ROS1 arrangements. The ORR was approximately 66% and the PFS was 18.3 months. 69 Entrectinib (Rozlytrek), the other one approved ROS1 TKI, has been simultaneously approved for the treatment of advanced ROS1‐positive NSCLC and neurotrophic tyrosine receptor kinase (NTRK) gene fusion‐positive solid tumor, including NSCLC in August 2019. The ORR of advanced NSCLC patients treated by entrectinib was 78% and the response duration was 24.6 months, with ability to penetrate blood–brain barrier. 77 These results are yielded from three clinical trials, including STARTRK‐2 (phase II), STARTRK‐1 (phase I), and ALKA‐372‐001 trials (phase I). 85
3.4. BRAF
BRAF mutations have been observed in 3–8% of NSCLC, most of which are adenocarcinomas and smokers. 170 , 171 The most common alteration of BRAF mutations (more than half) is a single‐point mutation at residue 600 of exon 15, where valine is replaced by glutamate (Val600Glu, V600E). 170 The BRAF gene encodes a threonine/serine protein kinase, which is involved in MAPK/ERK signaling pathway. V600E mutation leads to uncontrolled activation of BRAF, resulting in abnormal cell proliferation. 172 Other patterns of BRAF mutations can induce either activation or inactivation of BRAF. 173
One targeted therapy for BRAF V600E mutation‐positive advanced NSCLC has been approved by the U.S. FDA in 2017. The therapeutic effects of dabrafenib (BRAF TKI) and trametinib (MEK TKI) combination were confirmed by a nonrandomized, noncomparative, and open‐label trial. The ORRs were 63% and 61% in previously treated patients and treatment‐naive patients, respectively. However, the ORR for patients who received just single agent of dabrafenib was 27%. 174 The activation of MAPK/ERK signaling pathway is hardly blocked by BRAF inhibitor monotherapy until the addition of MEK inhibitor. 175 Therefore, the development of BRAF TKI is usually based on the combination of BRAF inhibitors and MEK inhibitors.
3.5. KRAS
KRAS is an oncogene which belongs to RAS GTPase family and in control of crucial cellular pathway, including RAF/MEK/ERKandPI3K/AKT. 176 KRAS mutations are common in NSCLC, accounting for 25% of adenocarcinomas, especially in ever/heavy smokers of western countries. 177 The genomic aberrations of KRAS lead to continuous activation of KRAS and uncontrolled cell proliferation. However, the targeted therapy for KRAS is limited and patients with KRAS mutations have poor prognosis with a median survival of 2.4 years. 178 Mutations happened in codon 12 or 13 have been studied extensively, including the most common KRAS alteration, G12C (a point mutation that guanine is replaced by cysteine). 179 Specific KRAS alterations have been well reviewed in Ref. 143. 179 Early researchers have found that MEK inhibitors (trametinib and selumetinib) showed benefits for KRAS‐positive NSCLC patients in combination with chemotherapy. 180 , 181 Recently, an inhibitor directly targeting KRAS, sotorasib, was approved by the U.S. FDA as a second‐line therapy for advanced KRAS G12C‐mutated NSCLC in May 2021. The approval for sotorasib (Lumakras) was based on the results of a single‐arm and open‐label phase I/II trial, CodeBreaK 100. KRAS G12C‐positive advanced NSCLC patients whose disease had progressed on or after chemotherapy were enrolled. The ORR was 36% with a median duration of 10 months. 182
3.6. RET
RET encodes a tyrosine kinase receptor on cell surface, which is involved in several crucial signaling pathways, including MAPK, PI3K, JAK/STAT, PKA, and PKC pathways. 183 RET arrangements (RET fusion) have been observed in 1–2% NSCLC, mostly adenocarcinomas, never/light smokers, and younger patients. 184 Numerous fusion partners of RET have been identified, among which the intron 15 of KIF5B is the most common and widely studied for targeted therapy. 185 The development of RET targeted therapy is based on multitargeted TKIs. A retrospective analysis of multicenter clinical trial containing 165 RET‐positive NSCLC patients showed that the response rates (partial or complete) to cabozantinib, vandetanib, and sunitinib were 37%, 18%, and 22%, respectively. Further responses were observed with lenvantinib and nintedanib‐treated patients. 186 Multitargeted TKIs showed limited therapeutic effects on RET‐positive NSCLC, and several RET‐specific agents have entered clinical trials. Selpercatinib (LOXO292), pralsetinib (BLU‐667), BOS172738, 187 and TPX‐0046 188 are highly selective RET targeted agents undergoing clinical trials, with the ability to penetrate into CNS and overcome the acquired resistance on multitargeted TKIs. 189 , 190 Recently, based on two phase I/II trials (NCT03157128 and NCT03037385), selpercatinib (Retevmo) and pralsetinib (Gavretotm) have been approved for RET‐positive advanced NSCLC. 80 , 81 In patients treated with selpercatinib, those previously received chemotherapy had an ORR of 64%, whereas those previously untreated had an ORR of 91%. The median duration was at least 6 months. 80 The efficacy of pralsetinib was evaluated in 114 advanced NSCLC patients. The ORRs of 87 previously treated and 27 previously untreated patients were 61% and 70%, respectively. 81
3.7. MET
The MET (mesenchymal‐epithelial transition factor) gene encodes hepatocyte growth factor receptor, the phosphorylation of which leads to the activation of several cellular signaling pathways, including MAPK, PI3K, STAT, and so on. 191 MET amplification is usually associated with EGFR TKIs resistance. MET exon 14 skipping is the most common pattern of MET mutations, which leads to increased stability and continuous activation of MET protein. 192 This mutation has been observed in 3–4% LUAD, and the median age is older than EGFR‐ or KRAS‐positive NSCLC patients. 193 After the treatment of chemotherapy, the prognosis of MET exon 14 skipping‐positive patients is much poor than driver gene‐negative patients with a median OS of 6.7 versus 11.2 months, which makes MET targeted therapy important. 194 The MET targeted therapy has made a progress on targeting MET exon 14 skipping. Crizotinib, as an MET, ALK, and ROS1 multitargeted inhibitor, was approved for the second‐line treatment of MET exon 14 skipping‐positive NSCLC patients. 195 Capmatinib (Tabrecta, 2020) and tepotinib (Tepmetko, 2021) are two MET TKIs approved by the U.S. FDA for the treatment of MET exon 14 skipping mutation‐positive advanced NSCLC. The efficacy of capmatinib was demonstrated in a phase II trial enrolling patients with confirmed MET exon 14 skipping. Previously untreated patients benefited more from capmatinib treatment with an ORR of 68% and a median duration of 12.6 months, compared with 41% and 9.7 months in previously treated patients. 82 The approval of tepotinib was based on the ORR and duration data from a phase II study, VISION. The ORR of both treatment‐naïve and previously treated patients is about 56%, with median duration of 10.8 and 11.1 months, respectively. 83 In 2021, NMPA approved the first MET TKI in China, savolitinib, which was recommended as the first‐line treatment for advanced NSCLC with MET exon 14 skipping mutation. The ORR of savolitinib‐treated group was 42.9%, with a median PFS of 6.8 months and a median OS of 12.5 months. 84
3.8. NTRK
NTRK (neurotrophic tropomyosin‐related kinases) genes 1, 2, and 3 encode tropomyosin receptor kinases (TRKs) A, B, and C, respectively. NTRK fusion has been observed in 1–2% of NSCLC patients and assumed as targetable mutations. 196 Larotrectinib, entrectinib, and repotrectinib were approved by the U.S. FDA for the treatment of NTRK gene fusion‐positive solid tumor (including NSCLC), who have progressed on their primary treatment or have no satisfactory standard therapy. 88 , 126 , 197 Approval for larotrectinib was based on the data from three multicenter, open‐label, single‐arm clinical trials, LOXO‐TRK‐14001 (NCT02122913), SCOUT (NCT02637687), and NAVIGATE (NCT02576431). 198 Whereas the approval for entrectinib was based on other three studies, ALKA, STARTRK‐1 (NCT02097810), and STARTRK‐2 (NCT02568267). 85
3.9. HER2
HER2 (ERBB2), with EGFR, HER3, and HER4, belongs to ERBB RTK family. HER2 amplification and overexpression have been observed in approximately 30% and 10% of lung cancer, respectively. 199 , 200 , 201 However, the development of HER2 targeted therapy is mainly against HER2 driving mutations, which is rare and present in about 2–3% of patients, mostly women, never/light smoker, and adenocarcinoma. 199 , 202 Like EGFR mutation, the common mutations in HER2 occur in exon 20 by insertion of DNA bases. 203 Many targeted agents, including antibodies (transtuzumab and pertuzumab), antibody–drug conjugates (ADCs), and small molecular TKIs (lapatinib, afatinib, dacomitinib, neratinib, poziotinib, and pyrotinib), have been investigated in clinical trials as monotherapy or in combination of chemotherapy. ADCs, including ado‐trastuzumab emtansine (Kadcyla) and trastuzumab‐deruxtecan (Enhertu), showed most encouraging therapeutic effects for HRE2 mutation‐positive NSCLC patients. 204 , 205 Therefore, in 2020, fam‐trastuzumab deruxtecan‐nxki (Enhertu), as second‐line treatment, was granted a breakthrough therapy designation (BTD) for the treatment of patients with advanced NSCLC. 204 , 206
3.10. VEGF/VEGFR
Besides targeting genomic alterations, inhibition tumor vascular formation by targeting angiogenic factors is also a promising and classic anticancer strategy. Vascular supply is essential for the growth and progression of solid tumor, without which tumors remain stable and localized. 207 High density of microvessels is associated with poor prognosis and metastasis of NSCLC. 208 Vascular endothelial growth factor (VEGF) and the interaction with its receptors is thought to be the most potent factor in regulating angiogenesis and is able to enhance the vascular permeability. 209 Inhibition of tumor angiogenesis is assumed as a promising therapeutic strategy. The antiangiogenic treatment for NSCLC contains mAbs, small‐molecule TKIs, and recombinant human endostatin. Of note, the efficacy of single‐agent antiangiogenic agent is limited, therefore, the recommendation of antiangiogenic treatment for NSCLC is usually based on combination therapy (discussed in combination therapy part).
Bevacizumab (Avastin, Genentech) and ramucirumab (Cyramza, Eli Lilly and Company) are two mAbs approved by the U.S. FDA for the treatment of NSCLC. Bevacizumab, which is the first antiangiogenic drug, inhibits angiogenesis through binding and neutralizing all VEGF isoforms. 210 The efficacy and safety of bevacizumab in combination with chemotherapy for advanced NSCLC was evaluated in a phase II trial, in which the addition of bevacizumab showed an increased response rate (31.5% vs. 18.8%) compared with chemotherapy alone. 211 Later, in 2006, bevacizumab in combination with carboplatin and paclitaxel was approved by the U.S. FDA for first‐line treatment of advanced nonsquamous NSCLC. This approval was based on the data from a phase III study (E4599) in which bevacizumab increased the median survival from 10.3 to 12.3 months, making an impressive improvement for NSCLC at that time. 212 In 2018, bevacizumab in combination with immunotherapy and chemotherapy was approved for first‐line treatment of nonsquamous NSCLC (discussed in combination part). Another monoclinal antibody, ramucirumab (IMC‐1121B), is a fully human IgG designed to bind extracellular VEGF‐binding domain of VEGFR‐2, resulting in the inhibition of angiogenesis. 213 Ramucirumab received first approval of the U.S. FDA in 2018, in combination with docetaxel for previously treated metastatic NSCLC by improving median survival from 9.1 to 10.5 months. 214 The U.S. FDA expanded the indication for ramucirumab in 2020 based on the results of RELAY study. Ramucirumab in combination with erlotinib significantly improved the survival of advanced NSCLC with sensitizing EGFR mutations (19.4 vs. 12.4 months) compared with erlotinib single‐agent group. 215
TKIs targeting VEGF/VEGFR, platelet‐derived growth factor/receptor (PDGF/PDGFR), fibroblast growth factor/receptor (FGF/FGFR), and c‐Kit demonstrate effects on inhibition angiogenesis, including sorafenib, sunitinib, vandetanib, nintedanib, and anlotinib. 216 , 217 , 218 , 219 However, only a few TKIs, including nintedanib and anlotinib, have shown positive anticancer effects. The indication of nintedanib for NSCLC has been approved in Europe but failed in America. 220 Anlotinib (Focus V, Chia‐Tai Tianqing Pharmaceutical and Advenchen Laboratories) harbors a broad spectrum of targets, including VEGFR 2/3, FGFR1‐4, PDGFR α/β, c‐Kit, and Ret, which is assumed to have strong effects on antiangiogenesis. 221 Anlotinib was first approved by NMPA as third‐line treatment for advanced NSCLC, which is based on the data from ALTER0302 trial. 222 , 223 This approval offered a novel approach for advanced NSCLC patients whose disease progressed after two lines treatment. In 2019, the indication of anlotinib was expanded to third‐line treatment for small cell lung cancer (SCLC), which was based on the ALTER 1202 study. 224
The angiogenesis process is also negatively regulated by endostatin, which is thought to be a potential target in antiangiogenic strategy. 225 Endostar (YH‐16), a modified recombinant human endostatin, is the only endostatin applied in clinical use for the treatment of advanced NSCLC. The approval by NMPA was based on a phase III clinical trial in which Endostar in combination with vinorelbine and cisplatin showed increased ORR of 35.4% compared with 19.5% of chemotherapy group. 226 In a phase III trial, Endostar in combination with cisplatin showed increased ORR (63% vs. 46.39%) in NSCLC with malignant hydrothorax and ascites compared with cisplatin alone. 227 However, the efficacy of Endostar still requires further validation. 228
4. MECHANISM AND REVERSE OF TARGETED THERAPY RESISTANCE
Since the first TKI, gefitinib, has been introduced to treat NSCLC in the late 1990s, the development of the therapeutic strategies for NSCLC progressed rapidly. Despite the promising effects of TKIs, unavoidable drug resistance has been observed in most patients. The increased heterogeneity within the tumor during targeted therapy is associated with poor therapeutic effects. 229 The potential mechanism includes secondary mutations, alternative activation through another pathway, and histological and phenotypic transformation. 230 , 231 Recent studies showed that late‐generation EGFR or ALK TKIs as the first‐line therapy for NSCLC with EGFR or ALK mutations presented an improve outcome. 60 , 158 , 232 Meanwhile, a repeated molecular profiling at progression is necessary for further treatment decision.
4.1. EGFR
Almost all patients have acquired resistance after first‐ or second‐generation EGFR TKIs treatment. The median duration time is less than 1 year. 113 The most common resistance (more than half) for first‐ and second‐generation EGFR TKIs is due to a secondary mutation of the gatekeeper, Thr790Met (T790M). 115 T790M mutation is referred to the point mutation at a conserved gatekeeper threonine residue within the ATP‐binding pocket, which is replaced by methionine. 233 EGFR with T790M mutation shows elevated activation either alone or in combination with primary EGFR‐sensitizing mutations in exon 19 or 21. 234 , 235 Though T790M mutation causes resistance to early‐generation EGFR TKIs, it is associated with slower tumor growth and better prognosis. 236 The third‐generation EGFR TKI, osimertinib, is responsible to overcome the T790M as well as sensitizing mutations of EGFR. However, resistance to osimertinib has also been observed in clinic. The most common secondary mutation related to osimertinib is EGFR‐C797S, 237 , 238 which occurs at the covalent binding site of osimertinib and altering osimertinib binding affinity. Other osimertinib‐related mutations, such as EGFR‐G796S/R and EGFR‐L718Q, inhibit osimertinib binding through physical interference. 239 Some less common mutations, including L792F/H, T854A, D761Y, L747S, and so on, have been also observed in patients treated with osimertinib. 239 , 240 Amplification of wild‐type EGFR also shows resistance to osimertinib. 241 The result of the clinical trial, IMPRESS, showed that doublet chemotherapy was not recommended for patients progressed after EGFR TKIs treatment. 242
Alternative pathway activation is common during the process of drug resistance of EGFR TKIs, including MAPK, PI3K/AKT, JAK‐STAT3, and SRC pathways. For example, acquisition of mutations of BRAF (G469A or V600E) in resistance to early or third generation of EGFR TKIs usually results in the reactivation of MAPK pathway. 243 , 244 The activation of JAK‐STAT3 pathway occurs at early stage of EGFR TKIs treatment. 245 However, in an early‐phase clinical trial, the ORR of combined therapy of JAK inhibitor ruxolitinib and erlotinib was less than 5% in patients with resistance to erlotinib. 246 This combined regimen might be help in early application of EGFR TKIs, but less helpful in the late resistant phase. 247 The activation of PI3K/AKT pathway and KRAS mutation, including mutations in PIKC3A and loss of PTEN, is a negative predictor of EGFR TKIs treatment. 248 , 249 Therefore, inhibitors for PI3K/AKT pathway in combination of EGFR TKIs have shown combined efficacy. 250 , 251 Of note, mutations of PI3K/AKT pathway are not common in ALK or ROS1 TKIs treatment.
4.2. ALK
The secondary ALK mutations are relatively variable in patients with drug resistance. 252 It is estimated that after a duration of around 12 months, crizotinib can induce the gatekeeper mutations, including L1196M and C1156Y. 253 L1196M is present in about 7% of patients resistant to ALK TKIs. 254 A patient with C1156Y mutation showed resistance to early‐generation ALK TKIs but response to lorlatinib, a third‐generation ALK TKI. However, upon her disease progress, a secondary mutation (L1198F) occurred, which resulted in resistance to lorlatinib, but resensitized the tumor to crizotinib. 255 Mutations like G1202R, D1203N, S1206, and amplification of ALK have shown resistance to crizotinib via physically interfering TKI binding. 252 , 256 Increasing the dose of crizotinib has been assumed to overcome the resistance caused by ALK amplification. 257 ALK‐G1202R mutation occurs in less than 2% of patients with resistance to ALK TKIs and is assumed to show resistance to almost all approved ALK TKIs. 252 , 258 The third‐generation ALK TKI, lorlatinib, has shown activity against ALK‐G1202R mutation with an ORR of 44% in a phase III clinical trial. 252 , 259 Alectinib, as a next‐generation TKI of ALK, has induced some other ALK mutations, including I1171T and V1180. 260
Activation of MAPK pathway via KRAS amplification or MEK mutation in patients with resistance to ALK TKIs was observed. 261 , 262 Combined therapy of MEK inhibitors and ALK inhibitors for NSCLC patients has been investigated in clinical trials (NCT03087448).
4.3. ROS1
ROS1 and ALK share similar structure of tyrosine kinase domains, therefore, many ROS1 mutations are structural analogues to ALK mutations. 263 However, according to the spectrum data, ROS1‐resistance mutations are less variable than ALK‐resistance mutations, indicating more therapeutic potential of crizotinib as an ROS1 TKI. Patients received crizotinib have been observed to develop gatekeeper mutation of ROS1 (L2026M). 264 ROS1‐G2032R and ROS1‐D2033N are structural analogous of ALK‐G1202R and ALK‐D1203N mutations, respectively, which show resistance to crizotinib. In a small sample study, ROS1‐G2032R is the most common mutation showing resistance to crizotinib. 265 , 266 Meanwhile, activation of MAPK pathway is critical in the mechanism of resistance to crizotinib. 267
4.4. Resistance to other TKIs
During the treatment of TKIs of RET, HER2, and MET for NSCLC patients, the medium duration is usually less than 12 months. V804L mutation of RET is a gatekeeper mutation and responsible for the resistance to cabozantinib. It is assumed that ponatinib is the most potent RET TKI to overcome the RET TKIs‐associated drug resistance. 268 An analogous mutation, HER2‐C805S, has been reported at resistance to HER2 TKI therapy in HER2‐mutated NSCLC, 269 which is assumed to affect the binding of HER2 TKIs. Activation of PI3K pathway via mutations in PIK3CA has been reported in NSCLC patients with HER2 TKIs resistance, and combined therapy of mTOR inhibitor showed therapeutic response. 270 The emergence of secondary mutations after treatment of crizotinib has been observed, including D1228N and Y1230C. 271 , 272
4.5. Off‐target resistance
Secondary mutations other than targeted gene (off‐target resistance) are also common in the process of drug resistance. During the treatment of targeted therapy, alterations of genetic characteristics are usually associated with drug resistance. In NSCLC patients who have progressed on EGFR TKIs, MET amplification occurs with 5–20% incidence and leads to resistance to early generation of EGFR TKIs. 230 , 273 Combined therapy of EGFR TKIs and MET TKIs in NSCLC patients has been investigated in clinical trials. In NSCLC patients with MET amplification and resistance to prior EGFR TKI treatment, MET inhibitor capmatinib combined with gefitinib showed the response rate of about 15%. 274 An MEK1 mutation has also been reported in a patient with resistance to ALK TKIs, who got response to an MEK inhibitor. 262 BRAF mutations (BRAF‐G469A or BRAF‐V600E) have been observed in patients with resistance to EGFR TKIs with an occurrence of 1%. 244 Amplification of HER2, belonged to the same receptor family of EGFR, has also been observed after treatment of EGFR TKIs. 275 Increased activation of EGFR has occurred in more than 40% of patients progressed on ALK inhibitor crizotinib. 253 Meanwhile, the occurrence of EML4‐ALK rearrangement is associated with resistance to EGFR TKIs. 153
Besides those targetable mutations in NSCLC, some proteins also show abnormal expression during drug resistance. Increased expression of AXL receptor tyrosine kinase (AXL) has been observed in the samples of NSCLC patients resistant to EGFR, ALK, or RET TKIs treatment, indicating that AXL TKIs might be an alternative for combination therapy in targeted therapy for NSCLC. 276 , 277 , 278 Other abnormally expressed molecules include proto‐oncogene tyrosine‐protein kinase Src (SRC), 279 insulin‐like growth factor 1 receptor (IGF1R), 280 , 281 KIT, 253 , 282 and so on.
4.6. Histological and phenotypic transformation
In a group of NSCLC patients with resistance to EGFR or ALK TKIs, histological transformation from an NSCLC to a small‐cell lung cancer histology has been observed. 230 This transformation is assumed to be associated with RB and EGFR loss. 283 Transformation to a sarcomatoid carcinoma has been reported in cases with resistance to ALK TKIs. 284 The alterations of some epithelial‐to‐mesenchymal transition‐associated molecules in tumor, including E‐cadherin, vimentin, and so on, lead to the transformation to a more invasive phenotype. 230
5. IMMUNOTHERAPY FOR NSCLC
The emergency of targeted therapy has improved the survival of certain groups of NSCLC patients; however, the 5‐year survival is still not satisfying. 1 For driver gene‐negative advanced NSCLC patients, platinum‐based chemotherapy only brings a medium PFS of 4–6 months and a medium OS of 10–12 months. 285 , 286 , 287 Since the immunotherapy was introduced in 1990s, breakthroughs have been made in anticancer therapy 288 (Table 2). In 2015, the U.S. FDA approved the first ICI, nivolumab, for the third‐line treatment of patients with squamous cell lung carcinoma. Present immunotherapy constitutes two major pathways, CTLA‐4 (cytotoxic T lymphocyte antigen 4)/B7 pathway and PD‐1 (programmed death 1)/PD‐L1 (programmed death‐ligand 1, PD‐L1) pathway. Upon the activation of T cells, the expression of immunosuppressive signaling molecules increases, including PD‐1, CTLA‐4, LAG‐3, TIM‐3, TIGIT, VISTA, and CD244. 289 These T cell coinhibitory pathways restrict the strength and duration of immune response and protect body from immune‐related damage. Tumors exploit these coinhibitory pathways and achieve immune escape. 289 ICIs increase body antitumor immune effects via blocking the immune checkpoints mentioned above. 290 Based on the data released from clinical trials, immunotherapy usually showed advantages of OS instead of PFS. For advanced NSCLC patients without EGFR or ALK mutations, immunotherapy brings promising antitumor effects and better prognosis than traditional therapy. Given the essential role of immunotherapy in anticancer treatment, ICIs are arranged to earlier stage of NSCLC as neoadjuvant or adjuvant therapy and have shown promising efficacy. 291 , 292 Though patients receive initial benefits from ICIs, most of them develop drug resistance. Combination therapy is thought to be a way to overcome this resistance. 293
TABLE 2.
Immunotherapy in driver gene‐negative advanced NSCLC patients
| Target | Checkpoint inhibitor | Indications | Status | Company | Clinical trials |
|---|---|---|---|---|---|
| PD‐1 | |||||
| Nivolumab (Opdivo) |
|
|
Bristol Mayer Squibb |
|
|
| Pembrolizumab (Keytruda) |
|
|
Merck Sharp & Dohme |
|
|
| Cemiplimab‐rwlc (Libtayo) | Advanced NSCLS whose PD‐L1 ≥ 50% (Tumor Proportion Score [TPS] ≥ 50%) | FDA, Feb 2021 (first line) | Regeneron | Study 1624, NCT03088540 302 | |
| Sintilimab (Tyvyt) |
|
NMPA, Apr 2020 (first line) NMPA, Jun 2021 (first line) |
Innovent Bio and Lilly |
|
|
| Camrelizumab (AiRuiKa) | In combination with pemetrexed and carboplatin for advanced nonsquamous NSCLC | NMPA, Jun 2020 (first line) | Hengrui Pharmaceuticals |
CTR20170322; CameL, NCT03134872 305 |
|
| Tislelizumab (BaiZeAn) | In combination with carboplatin and paclitaxel (nab‐paclitaxel) for advanced squamous NSCLC |
NMPA, Jan 2020 (first line) |
BeiGene | ||
| PD‐L1 | |||||
| Atezolizumab (Tecentriq) |
|
|
Genentech | ||
| Durvalumab (Imfinzi) | Unresectable stage III NSCLC whose disease has not progressed following concurrent platinum‐based chemotherapy and radiation therapy | FDA, Feb 2018 (adjuvant therapy) | AstraZeneca | PACIFIC, NCT02125461 292 | |
| CTLA‐4 | |||||
| Ipilimumab (Yervoy) | In combination with nivolumab and two cycles of platinum‐doublet chemotherapy for advanced NSCLC | FDA, May 2020 (first line) | Bristol‐Myers Squibb | CheckMate 9LA, NCT03215706 296 | |
Abbreviations: FDA, the U.S. Food and Drug administration; NMPA, Chinese National Medical Products Administration.
5.1. Biomarkers for immunotherapy
The expression of PD‐L1 is used to be treated as a biomarker to assess the response of patients to immunotherapy. 310 , 311 However, there is no significant correlation between PD‐L1 expression and OS of patients. 294 , 312 Combination tumor‐infiltrating lymphocytes with PD‐L1 expression have shown improved predictive effects. 313 , 314 Tumor mutational burden (TMB) is another predictive factor, which is associated with T cells activation and improved prognosis. 315 , 316 , 317 High TMB is associated with high tumor antigenicity, which correlates with the efficacy of immune therapy, regardless of PD‐L1 expression. 318 , 319 , 320 The testing for TMB is relatively expensive since it is a novel predictive marker. 321 , 322 Despite these predictive factors, combination therapy with immunotherapy still becomes the optimal choice for patients with PD‐L1 expression ≤ 1%. 323 Nowadays, intestinal commensal microbiota is also used to diagnose and predict prognosis of disease. 324 , 325 In melanoma patients received anti‐PD1 therapy, significant differences have been observed in the diversity and composition of gut microbiome between responders and nonresponders. 326 These biomarkers provide a way to predict the response of patients; however, specific treatment decision should be more precise and personalized.
5.2. PD‐1/PD‐L1 pathway
The PD‐1 molecule is mainly expressed on T/B cells, NK, and MDSCs. PD‐L1 and PD‐L2 are two ligands induced by inflammatory signals. Their interactions downregulate the activation of T cells and the production of cytokines. 327 Excessive induction of PD‐1 and expression of responsive ligands in inflammatory environment will eventually cause the T cell exhaustion. 289 Many tumors express high level of PD‐L1, including NSCLC, which indicate the blockade of PD‐1/PD‐L1 pathway is a potential therapeutic mechanism. 328 , 329 Several mAbs direct to PD‐1 (nivolumab and pembrolizumab) and PD‐L1 (atezolizumab, durvalumab, and avelumab) have been approved for clinical use.
The expression of PD‐L1 is a predictive biomarker for immunotherapy. The original selective criteria of immunotherapy for advanced NSCLC patients were set as patients with PD‐L1 expression in tumor tissue ≥ 50%. In the trial supporting nivolumab for squamous NSCLC, the outcomes were not assumed to be associated with PD‐L1 expression status. 295 In contrast, the results from KEYNOTE‐001 showed that PD‐L1 expression ≥ 50% was correlated with improved efficacy of pembrolizumab in advanced NSCLC patients. 330 However, in the later clinical trial, KEYNOTE‐042, the efficacy of pembrolizumab showed no significant difference among patients with various PD‐L1 expressions. 297 Therefore, the indication of pembrolizumab and nivolumab for advanced NSCLC patients has been expanded to those with PD‐L1 expression ≥1%. The selective criteria for atezolizumab also considered the expression of PD‐L1 in immune cells. 309 Of note, PD‐L1 expression assays differ according to the specific antibody used. 331
5.2.1. Anti‐PD‐1 monoclonal antibodies
Nivolumab (Opdivo), a human immunoglobulin G4 (IgG4) mAb targeting human PD‐1, binds PD‐1 with high affinity and blocks the interaction between PD‐1 and PD‐L1/PD‐L2. 332 , 333 Nivolumab was initially approved for the treatment of unresectable melanoma in 2014. 334 In March 2015, nivolumab was approved by the U.S. FDA for the squamous NSCLC patients, who have progressed on or after platinum‐based chemotherapy. This approval was based on the data from a phase III CheckMate 017 trial, in which nivolumab improved overall survival by 3.2 months compared with docetaxel (9.2 vs. 6.0 months). 294 Later, in another clinical trial, CheckMate 057, nivolumab also showed improved ORR and response duration of nivolumab compared to docetaxel, leading to the expanded approval for nonsquamous NSCLC patients. 295 In May 2020, based on the data from CHECKMATE‐9LA (NCT03215706), nivolumab plus ipilimumab and two cycles of chemotherapy as first‐line treatment for metastatic NSCLC patients without EGFR or ALK mutations were approved by the U.S. FDA. 296
Pembrolizumab (MK‐3475, Keytruda), a highly selective IgG4‐κ isotype mAb against PD‐1, blocks PD‐1/PD‐L1/PD‐L2 pathway via binding to PD‐1. 335 It initially received accelerated approval from the U.S. FDA for the second‐line treatment of melanoma in 2014, which was similar to nivolumab. 336 Though nivolumab was approved for advanced NSCLC patients before pembrolizumab, pembrolizumab is the first anti‐PD‐1 drug approved as first‐line treatment for advance NSCLC patients with PD‐L1 TPS ≥50%. The efficacy of pembrolizumab was evaluated in the KEYNOTE‐001 trial (NCT01295827), in which the overall ORR was 19.4% and the median duration of overall survival was 12.5 months. 330 In 2019, the indication for pembrolizumab was expanded as the first‐line treatment for advanced NSCLC patients with PD‐L1 expression (Tumor Proportion Score [TPS] ≥1%) and no EGFR or ALK mutations. This approval was based on the data from KEYNOTE‐042 (NCT02220894), in which the outcomes of patients showed no significant difference among TPS ≥1%, TPS ≥20%, and TPS ≥50%. 297
Cemiplimab‐rwlc is the third anti‐PD‐1 antibody approved by the U.S. FDA for the treatment of advanced NSCLC patients with PD‐L1 expression of at least 50%. Based on the results from Study 1624, cemiplimab‐rwlc showed significantly improved PFS (6.2 vs. 5.6 months) and OS (22.1 vs. 14.3 months) compared to those treated with platinum‐based chemotherapy. 302
There are other three anti‐PD‐1 antibodies approved by NMPA, not by the U.S. FDA, including sintilimab, camrelizumab, and tislelizumab, for the first‐line treatment of NSCLC. The indications of these three ICIs were all referred to combination with chemotherapy for advanced NSCLC with any PD‐L1 expression level. In 2020, based on the data from a phase III trial, ORIENT‐11, sintilimab in combination with pemetrexed and platinum‐based chemotherapy was approved by NMPA for the first‐line treatment of advanced nonsquamous NSCLC. 337 Later, in 2021, NMPA expanded the indication of sintilimab to first‐line treatment of advanced squamous NSCLC, in combination with gemcitabine and platinum‐based chemotherapy. This approval was based on a phase III trial, ORIENT‐12, in which the 6‐month PFS was 41.4% and ORR was 64.7%. 304
Camrelizumab is an anti‐PD‐1 antibody approved in China, which has the most indications in antitumor therapy, including classic Hodgkin lymphoma, 338 NSCLC, esophageal cancer, 339 and hepatic carcinoma. 340 In 2020, camrelizumab in combination with pemetrexed and carboplatin was approved by NMPA for the first‐line treatment of nonsquamous NSCLC patients, with a significant improved median OS of 27.9 months. 305
Tislelizumab was approved for the first‐line treatment of nonsquamous NSCLC in 2020, which was based on the data from a clinical trial, RATIONALE 304. 306 In 2021, the efficacy of tislelizumab for squamous NSCLC was evaluated in a phase III trial, BGB‐A317‐307, in which tislelizumab in combination with pemetrexed and platinum chemotherapy (either carboplatin or cisplatin) showed improved PFS (7.6 months). 307 The combined therapy was approved by NMPA for the first‐line treatment of patients with advanced squamous NSCLC in 2021.
5.2.2. Anti‐PD‐L1 monoclonal antibodies
The treatment strategy for targeting PD‐L1 is similar to anti‐PD‐1, but still has the interaction between PD‐1 and PD‐L2, which is assumed to help balance the body inflammatory response. 341 Based on this theory, the blockade of PD‐L1 is thought to be associated with reduced immune‐related toxicity, such as immune‐related pneumonitis and colitis. However, there are no sufficient data supporting the difference between PD‐1 and PD‐L1 inhibitors. 342 For now, two anti‐PD‐L1 antibodies, atezolizumab and durvalumab, have entered clinical application for NSCLC patients. Other anti‐PD‐L1 antibodies, such as BMS‐936559, avelumab, and sugemalimab, have been underestimated.
Atezolizumab (MPDL3280A), a human IgG1 mAb, is the first anti‐PD‐L1 antibody approved for NSCLC patients. In 2016, atezolizumab was initially approved as a second‐line treatment for advanced NSCLC patients whose disease has progressed after chemotherapy. 343 , 344 Later, in 2018, based on the IMpower150 trial (NCT02366143), atezolizumab in combination with bevacizumab, paclitaxel, and carboplatin was approved for the first‐line treatment of advanced nonsquamous NSCLC without EGFR or ALK mutation. 345 In 2020, the U.S. FDA expanded the indication of atezolizumab to first‐line treatment of advanced NSCLC with PD‐L1 expression ≥ 50% as a single agent. The efficacy was evaluated in the IMpower110 trial with median OS of 20.2 months compared to 13.1 months in chemotherapy arm. 309 Of note, in 2019, atezolizumab in combination with chemotherapy was approved by the U.S. FDA for the first‐line treatment of extensive‐stage SCLC, which was assumed as a breakthrough in SCLC treatment. 346
Durvalumab (MEDI4736) is a human IgG1 antibody with high affinity of PD‐L1, which was approved by the U.S. FDA as adjuvant treatment of unresectable advanced NSCLC, whose disease has not progressed following concurrent platinum‐based chemotherapy and radiation therapy. This approval was based on the data from a phase III trial, PACIFIC (NCT02125461). Advanced NSCLC completed concurrent chemotherapy and radiation and then received durvalumab or placebo. The PFS in durvalumab group was significantly improved compared with placebo arm. 292 , 347
5.3. CTLA‐4 pathway
CTLA‐4 is another inhibitory molecule expressed on activated T cells to block the excessive immune response. The responding ligands for CTLA‐4 include CD80 (B7.1) and CD86 (B7.2), which are similar to CD28, an activating signal molecule on T cell surface. 348 CTLA‐4 inhibits immune response through competitive bind to B7 ligands and blocking the interaction between CD28 and B7 ligands, resulting in decreased activation of T cells. 327 For now, only one anti‐CTLA‐4 antibody, ipilimumab, has been approved by the U.S. FDA for clinical use. Another anti‐CTLA‐4 antibody, tremelimumab, in combination with other immunotherapy has been investigated in clinical trials. 349
5.3.1. Anti‐CTLA‐4 monoclonal antibodies
Ipilimumab (BMS‐734106) is a fully humanized IgG1 mAb and was first approved in melanoma treatment with favorable outcomes. 350 The combination therapy of ipilimumab for NSCLC has been under investigation. A phase II clinical trial was conducted to evaluate the efficacy of ipilimumab in combination with chemotherapy (carboplatin and paclitaxel) in advanced NSCLC. The combination group showed improved median OS (12.2 vs. 8.3 months) and PFS (5.5 vs. 4.6 months) compared with chemotherapy group. 351 The breakthrough of ipilimumab in the treatment of NSCLC was based on the combination with nivolumab. In 2020, based on the data from a phase III clinical trial, CheckMate 227, nivolumab in combination with ipilimumab was approved by the U.S. FDA for the treatment of advanced NSCLC whose PD‐L1 expression ≥1% and without EGFR or ALK mutation. The OS of patients in immunotherapy combination arm was 17.1 versus 14.9 months in chemotherapy arm. Of note, the combination of two immunotherapy agents demonstrated more treatment‐related adverse events than single agent, with 76.7% of patients reported. 352
5.4. Other immunotherapy agents
Tiragolumab is an anti‐TIGIT mAb. The data of combination of tiragolumab with atezolizumab in CITYSCAPE study have been released at the ASCO2020 conference. Addition of tiragolumab to atezolizumab demonstrated an increased RR (37.3% vs. 20.6%) and PFS (5.6 vs. 3.9 months) versus atezolizumab alone. 353 Based on the promising efficacy, in 2021, the U.S. FDA granted tiragolumab BTD in combination with atezolizumab (Tecentriq) for the first‐line treatment of individuals with metastatic NSCLC whose tumors have PD‐L1 expression ≥50% and no EGFR or ALK genomic tumor aberrations. In late 2021, tiragolumab in combination with dabrafenib, a BRAF TKI, was approved by the U.S. FDA for the treatment of metastatic NSCLC with BRAF V600E mutation. This approval was based on the data from Study BRF113928 (NCT01336634), in which the ORRs in previously treated and untreated group were 63% and 61%, respectively, compared with 27% of dabrafenib single‐agent group. 78 Other immunotherapeutic agents targeting LAG3, IDO, CD137, and OX40 have been investigated in clinical trials. 354
6. THE COMBINATION THERAPY FOR ADVANCED NSCLC
The rapid development of targeted therapy and immunotherapy has profoundly changed the treatment strategy for NSCLC. Balancing the benefits and risks of various treatments and providing patients with best treatment with less adverse events is important for the treatment decisions. Diversity of combinations have been investigated in clinical trials.
6.1. Targeted therapy in combination with chemotherapy
There is limited evidence supporting the efficacy of combination of chemotherapy and targeted therapy. A phase II clinical trial found a PFS benefit (15.8 vs. 10.9 months) of gefitinib plus pemetrexed compared with gefitinib alone, but without statistical significance. 355 Another study, JMIT, showed both improved PFS and OS in the gefitinib plus pemetrexed and carboplatin group. 356 NEJ009 study got positive results on PFS, but the OS benefit of chemotherapy and targeted therapy combination required further validation. 357 Therefore, for those driver gene‐positive NSCLC patients, receiving more treatments during whole disease is more important than receiving combination therapy or simultaneous treatment.
6.2. Targeted therapy in combination with immunotherapy
Generally, immunotherapy is less effective than targeted therapy in NSCLC patients with targetable driver gene mutations. Several clinical trials evaluated the efficacy of immunotherapy in previously treated NSCLC patients with driver gene mutations. 295 , 298 , 343 The results showed that there was no significant improvement in OS. 358 Meanwhile, combination therapy showed increased toxicity, which resulted in the termination of some clinical trials. 359 , 360 Thus, targeted therapy in combination with immunotherapy is not the best choice for NSCLC patients with driver gene aberrations, especially for those with EGFR or ALK mutations, according to the current evidence. In AXL gene aberration‐positive NSCLC patients, bemcentinib, an AXL TKI, in combination with pembrolizumab showed significantly improved prognosis and has been granted fast track designation by the U.S. FDA in June 2021 (NCT03184558). Therefore, targeted therapy in combination with immunotherapy might be an alteration for drug development of those immature targets. However, for patients get drug resistance and progress on targeted therapy, immunotherapy plus chemotherapy and antiangiogenesis is a promising strategy to improve survival. 361 IMpower150 first showed benefits of ICIs on EGFR mutation‐positive NSCLC patients, with improved PFS (10.2 vs. 7.1 months) in atezolizumab plus bevacizumab and chemotherapy group compared with bevacizumab and chemotherapy combination group in EGFR mutation‐positive NSCLC patients. 345 Other ongoing studies, like KEYNOTE‐789, Checkmate‐722, ORIENT‐3, and TREASURE, are also investigating the therapeutic effects of immunotherapy plus chemotherapy and antiangiogenesis in EGFR mutation‐positive advanced NSCLC.
6.3. Immunotherapy in combination with chemotherapy
The combination of immunotherapy and chemotherapy is a new standard therapeutic regimen for advanced NSCLC patients, especially those without driver gene mutation. The basic theory is that increased expression of tumor antigens and PD‐L1 expression in the immunological environment induced by chemotherapy agents might enhance the therapeutic effects of immunotherapy. 362 Meanwhile, immunotherapy in combination with chemotherapy has demonstrated improved efficacy independent of PD‐L1 expression compared with chemotherapy alone. The efficacy of chemotherapy in combination with immunotherapy is apparent in both advanced squamous and nonsquamous NSCLC, especially those without EGFR or ALK mutations. Based on the data from clinical trials, different chemoimmunotherapies have been approved by the U.S. FDA for the first‐line treatment of advanced NSCLC. 363 Several anti‐PD‐1 antibodies approved by NMPA are also in regimens combined with chemotherapy (discussed in anti‐PD‐1 part).
KeyNote 189 is the first clinical trial demonstrating the promising therapeutic effects of chemoimmunotherapy compared with chemotherapy alone in nonsquamous NSCLC, in which pembrolizumab in combination with platinum‐based chemotherapy significantly improved the OS and PFS of NSCLC patients without EGFR or ALK mutations. 300 Another study, IMpower 130, evaluated the therapeutic effects of platinum‐based chemotherapy in combination with atezolizumab, an anti‐PD‐L1 antibody, in advanced nonsquamous NSCLC. Improve OS (18.6 vs. 13.9 months) and PFS (7 vs. 5.5 months) were observed in the combination group compared with chemotherapy alone. 364 Chemoimmunotherapy in combination with antiangiogenic agent for the treatment of nonsquamous NSCLC has also been approved by the U.S. FDA based on the data from IMpower 150 study. Carboplatin–paclitaxel–atezolizumab in combination with bevacizumab, an antiangiogenic antibody, has demonstrated an improved OS and PFS. 308
Studies also have been performed in squamous NSCLC and demonstrated promising outcomes. KeyNote 407 is the first phase III study to make a change in the standard treatment for squamous NSCLC. Carboplatin–paclitaxel or abraxane in combination with pembrolizumab demonstrated a better OS (15.9 vs. 11.3 months) compared with chemotherapy alone. 301 The efficacy of combination therapy containing anti‐PD‐L1 inhibitor has also been evaluated in another phase III clinical trial, IMpower131. Carboplatin–nab–paclitaxelin in combination with atezolizumab has showed improved PFS (6.5 vs. 5.6 months) compared with chemotherapy alone. 365
6.4. Anti‐PD‐1/PD‐L1 in combination with anti‐CTLA 4
The efficacy of combination of anti‐PD‐1/PD‐L1 pathway with anti‐CTLA4 has been under clinical investigation. As mentioned above, nivolumab in combination with ipilimumab has been approved by the U.S. FDA for the first‐line treatment of advanced NSCLC. 352 , 366 Patients in nivolumab and ipilimumab combination group showed increased RR (response rate) of 45% versus 26.9% in chemotherapy group. However, due to high incidence of adverse events, withdrawals of dual immunotherapy are common. 367 The efficacy of durvalumab in combination with tremelimumab, another anti‐CTLA‐4 antibody, versus chemotherapy has been investigated in ARCTIC study. 368 Though this combination showed an increase in OS (11.5 vs. 8.7 months), when compared with combination of anti‐PD‐1/PD‐L1 and chemotherapy, the survival data were not satisfying.
6.5. Combination with antiangiogenesis strategy
There are two antiangiogenic drugs approved by the U.S. FDA for the treatment of NSCLC. Bevacizumab in combination with chemotherapy (carboplatin and paclitaxel) was initially approved by the U.S. FDA in 2006, for the treatment of advanced nonsquamous NSCLC. 369 In patients with EGFR mutation, the PFS in erlotinib plus bevacizumab group was 16 months compared with 9.7 months in erlotinib single‐agent group. 370 In another phase III study, NEJ026, erlotinib in combination with bevacizumab improved PFS from 13.3 to 16.9 months. 371 However, the benefit of OS requires further validation. In the NEJ026 study, combination therapy (bevacizumab plus erlotinib) also showed an increase in adverse events. 372 The efficacy of antiangiogenesis in combination with immunotherapy has been confirmed in the IMpower150 study. Atezolizumab plus carboplatin plus paclitaxel plus bevacizumab (ACPB) regimen significantly improved the PFS and OS in advanced NSCLC patients, regardless of PD‐L1 expression and EGFR or ALK genetic alteration status. 308 Based on the results of IMpower150, ACPB regimen was approved by the U.S. FDA in 2018 for the first‐line treatment of advanced nonsquamous NSCLC with no EGFR or ALK genomic tumor aberrations. In 2020, the U.S. FDA approved the first anti‐VEGFR and EGFR–TKI combination as first‐line treatment for advanced NSCLC. Ramucirumab (Cyramza), a VEGFR2 antagonist, in combination with erlotinib showed improved survival for patients with EGFR exon 19 deletion and L858R mutations. But patients in combination group also demonstrated high incidence (72%) of grade 3–4 treatment‐emergent adverse events. 215
7. OTHER PROMISING THERAPIES FOR NSCLC
7.1. HER2 and HER3
HER2 and HER3 belong to an RTK family that includes EGFR (ERBB1), HER2 (ERBB2/NEU), HER3 (ERBB3), and HER4 (ERBB4). In contrast to EGFR, no ligand has been identified for HER2 and only one ligand, heregulin (HRG), for HER3 has been identified. Still, they are involved in receptor interaction and promote the dimerization of all ERBB family components. 373 , 374 EGFR TKIs are thought to have potential effects on the mutations of other ERBB family members, which requires further validation. 375 Afatinib, a second‐generation EGFR TKI targeting ERBB family, did not show therapeutic benefit in NSCLC with HER2 mutations. 376 Mobocertinib (TAK788) is dual TKI targeting EGFR insertion and HER2 mutation, which is approved for the treatment of EGFR exon 20 insertion mutations. Mobocertinib is granted a BTD from the U.S. FDA for NSCLC with EGFR or HER2 exon 20 insertion mutations, based on the data from a phase II trial. 68 Despite the approval of fam‐trastuzumab deruxtecan‐nxki (Enhertu), other inhibitor targeting HER2 showed promising effects on NSCLC. Poziotinib, an oral irreversible pan‐HER TKI, demonstrated a promising ORR of 35.1% in HER2‐mutated NSCLC in a phase II trial, ZENTITH20‐2. 377 The safety and effects of another irreversible pan‐HER TKI, pyrotinib, have been investigated in a phase II study and showed tolerable response with an ORR of 31.7%. 378
The aberrant activation of HER3 is observed in NSCLC and more than 80% of NSCLC patients with EGFR mutation express HER3. 379 Upregulating HER3 in cancer cells is also associated with resistant to EGFR TKIs. 380 Patritumab deruxtecan (U3‐1402, HER3‐DXd) is an ADC agent consists of an HER3‐targeted antibody and a topoisomerase I inhibitor. HER3‐DXd has shown the most promising effects and tolerable safety in NSCLC with EGFR mutations in a phase I clinical trial, with a disease control rate of 70% and median duration of 6.9 months. 381 The efficacy of HER3‐DXd will be confirmed in a phase II trial, HERTHENA‐Lung01 (NCT04619004). Other mAbs targeting HER3, including patritumab and lumretuzumab, are under evaluation in early clinical trials. 382 , 383
7.2. PIK3CA
The phosphoinositide 3‐kinase (PI3K) pathway regulates multiple cellular biologic process. The aberrant activation of PI3K/AKT/mTOR signaling is common in different cancer types. 384 The phosphatidylinositol‐4,5‐bisphosphate 3‐kinase, catalytic subunit alpha (PIK3CA) gene encodes the catalytic subunit alpha of PIK3. However, the mutations of PIK3CA are not common in lung cancer (mainly observed in LUSC), by contrast amplification of which is more prevalent. 385 PIK3CA mutations are associated with worse OS and PFS in patients with NSCLC. 386 Targeting PI3K strategies contain selective PI3K inhibitors and PI3K/mTOR inhibitors. Taselisib, buparlisib, voxtalisib, and PX‐866 are PI3K inhibitors under investigation of phase II clinical trial as monotherapy or in combination with other treatments. 387 , 388 , 389 In one study, the addition of PX‐866 showed an increased ORR (6% vs. 0%) compared with docetaxel single agent, whereas with no improvement in PFS or OS (NCT01204099). Apitolisib is a dual PI3K/mTOR inhibitor, the efficacy of which was evaluated in a single‐arm phase Ib trial in combination with carboplatin and paclitaxel and bevacizumab (NCT01301716). Based on the current knowledge, more evidence is required to confirm the therapeutic effects of targeting PI3K.
7.3. Epigenetic therapy
Epigenetic alteration is one of the cancer hallmarks, which is mainly referred to DNA methylation, histone deacetylation, and noncoding RNAs (ncRNAs). Epigenetic drugs have made breakthroughs in hematologic malignancies and are approved by the U.S. FDA, including HDAC inhibitors for cutaneous T cell lymphoma (vorinostat and romidepsin), DNMT inhibitors for myelodysplastic syndrome (5‐azacytidine and decitabine), and JAK1/2 inhibitor for myelofibrosis (ruxolitinib). The approval of tazemetostat, an EZH2 inhibitor, for epithelioid sarcomas is a milestone in epigenetic anticancer therapy for solid tumor. 390
DNA hypermethylation happens during early stage of carcinogenesis and has been widely studied. 391 , 392 DNA methyltransferase inhibitor (DNMTi) is capable to reverse the hypermethylation of DNA, especially the hypermethylation of tumor suppressor. Azacytidine and decitabine are two common DNMTi used in clinical trials. 393 Deacetylation by histone deacetylase (HDAC) leads to tumor suppressor silencing. HDAC inhibitors (HDACi) bind to the catalytic region of HDAC and prevent tumor suppressor silencing. Common HDAC inhibitors include trichostatin A, SAHA, depsipeptide, and valproic acid. ncRNA‐targeted therapy through miRNA is another potential treatment, which is widely investigated in clinical trials. 394 ncRNAs, including long noncoding RNAs (lncRNAs), short micro‐RNAs, and circular RNAs (circRNAs), are involved in tumorigenesis and tumor progression. 395 For example, miR‐34a mimic and miR‐16 mimic are currently being tested in phase I clinical trial for multiple solid tumors, including NSCLC. 396 , 397 Inhibition of lncRNAs and circRNAs has also been investigated in clinical trials. However, the disadvantages of RNA‐based therapy are apparent, such as off‐target effects, which are urgently required to overcome. Epigenetic therapy can also be used to resensitize cancer cells with resistance to other treatments, for example, resistance to TKIs. 398
The treatment strategy through regulation of epigenetic events is usually based on combination therapy. 399 , 400 Epigenetic therapy in combination with targeted therapy, chemotherapy, and immunotherapy has been investigated in clinical trials. Early trials, such as azacitidine plus erlotinib and decitabine plus cisplatin, showed unsatisfied therapeutic effects. 401 , 402 In a phase II study, though entinostat with erlotinib did not show therapeutic advantages, this therapy improved OS in advanced NSCLC patients with high level of E‐cadherin. 403 This indicated that identification of epigenetic biomarkers is important for application of epigenetic therapy. A recent clinical trial compared pembrolizumab plus azacitidine versus pembrolizumab alone. The combined therapy showed an ORR of 20% versus 14% with median PFS 2.9 versus 4.0 months. Importantly, the incidence of grade≥ 3 toxicity in combination arm was much higher than single‐agent arm (78% vs. 55%). 404 HDAC inhibitors combined with DNMT inhibitors have also been investigated in clinical trials. A phase II clinical trial combining azacitidine and entinostat showed an OS of 6.4 months in all enrolled advanced NSCLC patients but 10.4 months in “methylation marker”‐positive patients, including APC, RASSF1A, CDH13, and CDKN2A. 399 Given the current evidence for epigenetic therapy, more studies are required to confirm the therapeutic effects in NSCLC.
8. DISCUSSION AND FUTURE PERSPECTIVE
Surgery provides the best chance to cure the disease, but most NSCLC patients present with advanced‐stage disease. Cytotoxic chemotherapy had held a leading role in the treatment of advanced NSCLC until the first time TKIs became the first‐line treatment in certain subgroups of NSCLC patients. During the last decade, targeted therapy and immunotherapy have changed the treatment strategies for NSCLC, especially those in late phases of this disease. Based on the features of genetic alterations and PD‐L1 expression, clinicians make the final treatment decision more personalized. However, major challenges still remain, including identifying new targetable genetic alterations, developing new drugs and effective drug combinations, overcoming drug resistance, and discovering better biomakers to predict therapeutic response and prognosis of patients. Combination therapy could be an effective strategy in overcoming TKI resistance by targeting the native genomic mutation and the secondary alterations. However, the use of combination therapies increases the drug‐associated toxicity, which requires strict validation. To determine further treatment, molecular testing is not only essential for initiation of treatment, but also necessary at the time that disease progresses.
Currently, a variety of treatment strategies are available for NSCLC. Choosing appropriate and personalized combination therapy for patients is challenging for clinicians. Of note, patients not only benefit from the release of new drugs, but also benefit from new use or combos of the existing drugs.
CONFLICT OF INTERESTS
The authors declare no competing interests.
ETHICS STATEMENT
Ethics approval was not required for this review.
AUTHOR CONTRIBUTIONS
Yuan Cheng: conceptualization, methodology, and writing. Tao Zhang: Figure preparation. Qing Xu: conceptualization and reviewing.
ACKNOWLEDGMENTS
This work is supported by the National Natural Science Foundation of China, Grant/Award Number: 82102896. Figure 2 is created via BioRender software (BioRender.com) and we appreciated the help from it.
Cheng Y, Zhang T, Xu Q. Therapeutic advances in non‐small cell lung cancer: Focus on clinical development of targeted therapy and immunotherapy. MedComm. 2021;2:692–729. 10.1002/mco2.105
DATA AVAILABILITY STATEMENT
The datasets in this study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359‐E386. [DOI] [PubMed] [Google Scholar]
- 3. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 4. Martín‐Sánchez JC, Lunet N, González‐Marrón A, et al. Projections in breast and lung cancer mortality among women: a Bayesian analysis of 52 countries worldwide. Cancer Res. 2018;78(15):4436‐4442. [DOI] [PubMed] [Google Scholar]
- 5. Smith CJ, Perfetti TA, Rumple MA, Rodgman A, Doolittle DJ. “IARC Group 2A Carcinogens” reported in cigarette mainstream smoke. Food Chem Toxicol. 2000;38(4):371‐383. [DOI] [PubMed] [Google Scholar]
- 6. National Lung Screening Trial Research Team , Aberle DR, Adams AM, et al. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med. 2011;365(5):395‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ettinger DS, Wood DE, Aisner DL, et al. NCCN guidelines insights: non‐small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19(3):254‐266. [DOI] [PubMed] [Google Scholar]
- 8. Baker S, Dahele M, Lagerwaard FJ, Senan S. A critical review of recent developments in radiotherapy for non‐small cell lung cancer. Radiat Oncol. 2016;11(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cancer Genome Atlas Research Network . Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gregg JP, Li T, Yoneda KY. Molecular testing strategies in non‐small cell lung cancer: optimizing the diagnostic journey. Transl Lung Cancer Res. 2019;8(3):286‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn. 2018;20(2):129‐159. [DOI] [PubMed] [Google Scholar]
- 12. Wu YL, Planchard D, Lu S, et al. Pan‐Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non‐small‐cell lung cancer: a CSCO‐ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(2):171‐210. [DOI] [PubMed] [Google Scholar]
- 13. Halmos B. Molecular testing in lung cancer: where to draw the line. Arch Pathol Lab Med. 2018;142(7):787‐789. [DOI] [PubMed] [Google Scholar]
- 14. Iyevleva AG, Mitiushkina NV, Karaseva NA, et al. Lung carcinomas with EGFR exon 19 insertions are sensitive to gefitinib treatment. J Thorac Oncol. 2014;9(4):e31‐e33. [DOI] [PubMed] [Google Scholar]
- 15. Coleman N, Woolf D, Welsh L, et al. EGFR exon 20 insertion (A763_Y764insFQEA) mutant NSCLC is not identified by Roche Cobas version 2 tissue testing but has durable intracranial and extracranial response to osimertinib. J Thorac Oncol. 2020;15(10):e162‐e165. [DOI] [PubMed] [Google Scholar]
- 16. Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next‐generation sequencing. Nat Med. 2014;20(12):1479‐1484. [DOI] [PubMed] [Google Scholar]
- 17. Lam SW, Cleton‐Jansen AM, Cleven A, et al. Molecular analysis of gene fusions in bone and soft tissue tumors by anchored multiplex PCR‐based targeted next‐generation sequencing. J Mol Diagn. 2018;20(5):653‐663. [DOI] [PubMed] [Google Scholar]
- 18. Oxnard GR, Hu Y, Mileham KF, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M‐positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4(11):1527‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McLeer‐Florin A, Duruisseaux M, Pinsolle J, et al. ALK fusion variants detection by targeted RNA‐next generation sequencing and clinical responses to crizotinib in ALK‐positive non‐small cell lung cancer. Lung Cancer. 2018;116:15‐24. [DOI] [PubMed] [Google Scholar]
- 20. Yoh K, Seto T, Satouchi M, et al. Vandetanib in patients with previously treated RET‐rearranged advanced non‐small‐cell lung cancer (LURET): an open‐label, multicentre phase 2 trial. Lancet Respir Med. 2017;5(1):42‐50. [DOI] [PubMed] [Google Scholar]
- 21. Drilon A, Fu S, Patel MR, et al. A phase I/Ib trial of the VEGFR‐sparing multikinase RET inhibitor RXDX‐105. Cancer Discov. 2019;9(3):384‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davies KD, Le AT, Sheren J, et al. Comparison of molecular testing modalities for detection of ROS1 rearrangements in a cohort of positive patient samples. J Thorac Oncol. 2018;13(10):1474‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non‐small‐cell lung cancer. N Engl J Med. 2010;363(18):1693‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vendrell JA, Taviaux S, Béganton B, et al. Detection of known and novel ALK fusion transcripts in lung cancer patients using next‐generation sequencing approaches. Sci Rep. 2017;7(1):12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trombetta D, Sparaneo A, Fabrizio FP, Muscarella LA. Liquid biopsy and NSCLC. Lung Cancer Manag. 2016;5(2):91‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rolfo C, Mack PC, Scagliotti GV, et al. Liquid biopsy for advanced non‐small cell lung cancer (NSCLC): a statement paper from the IASLC. J Thorac Oncol. 2018;13(9):1248‐1268. [DOI] [PubMed] [Google Scholar]
- 28. Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol. 2018;36(9):911‐919. [DOI] [PubMed] [Google Scholar]
- 29. Marchiò C, Scaltriti M, Ladanyi M, et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann Oncol. 2019;30(9):1417‐1427. [DOI] [PubMed] [Google Scholar]
- 30. Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol. 2018;13(3):323‐358. [DOI] [PubMed] [Google Scholar]
- 31. Conde E, Rojo F, Gómez J, et al. Molecular diagnosis in non‐small‐cell lung cancer: expert opinion on ALK and ROS1 testing. J Clin Pathol. 2021;2021:207490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garrido P, Conde E, de Castro J, et al. Updated guidelines for predictive biomarker testing in advanced non‐small‐cell lung cancer: a National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin Transl Oncol. 2020;22(7):989‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walls GM, Hanna GG, Qi F, et al. Predicting outcomes from radical radiotherapy for non‐small cell lung cancer: a systematic review of the existing literature. Front Oncol. 2018;8:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dudani S, Leighl NB, Ho C, et al. Approach to the non‐operative management of patients with stage II non‐small cell lung cancer (NSCLC): a survey of Canadian Medical and Radiation Oncologists. Lung Cancer. 2016;94:74‐80. [DOI] [PubMed] [Google Scholar]
- 35. NSCLC Meta‐analysis Collaborative Group . Preoperative chemotherapy for non‐small‐cell lung cancer: a systematic review and meta‐analysis of individual participant data. Lancet. 2014;383(9928):1561‐1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pignon J‐P, Tribodet H, Giorgio V, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552‐3559. [DOI] [PubMed] [Google Scholar]
- 37. Pechoux CL. An international randomized trial, comparing post‐operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non‐small cell lung cancer (NSCLC) and mediastinal N2 involvement: primary end‐point analysis of LungART. Ann Oncol. 2020;31(4):S1142‐S1215. [Google Scholar]
- 38. Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB–IIIA non‐small‐cell lung cancer (RADIANT): a randomized, double‐blind, phase III trial. J Clin Oncol. 2015;33(34):4007‐4014. [DOI] [PubMed] [Google Scholar]
- 39. Hirsch FR, Bunn PA Jr. Adjuvant TKIs in NSCLC: what can we learn from RADIANT. Nat Rev Clin Oncol. 2015;12(12):689‐690. [DOI] [PubMed] [Google Scholar]
- 40. Gabay C, Russo A, Raez LE, Rolfo Cervetto C. Adjuvant therapy in non‐small cell lung cancer: is targeted therapy joining the standard of care. Expert Rev Anticancer Ther. 2021;21(11):1229‐1235. [DOI] [PubMed] [Google Scholar]
- 41. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR‐mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open‐label, phase 3 study. Lancet Oncol. 2018;19(1):139‐148. [DOI] [PubMed] [Google Scholar]
- 42. Jones GD, Caso R, Tan KS, et al. KRAS G12C mutation is associated with increased risk of recurrence in surgically resected lung adenocarcinoma. Clin Cancer Res. 2021;27(9):2604‐2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR‐mutated non‐small‐cell lung cancer. N Engl J Med. 2020;383(18):1711‐1723. [DOI] [PubMed] [Google Scholar]
- 44. Roche Pivotal Phase III Study Shows Roche's Tecentriq Helped People with Early Lung Cancer Live Longer without their Disease Returning. 2021. https://www.roche.com/media/releases/med‐cor‐2021‐03‐22.htm. Accessed 22 March 2021.
- 45. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non‐small‐cell lung cancer (IMpower010): a randomised, multicentre, open‐label, phase 3 trial. Lancet. 2021;398(10308):1344‐1357. [DOI] [PubMed] [Google Scholar]
- 46. Spigel DR, Faivre‐Finn C, Gray JE, et al. Five‐year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: an update from the PACIFIC trial. J Clin Oncol. 2021;39(15):8511. [Google Scholar]
- 47. Sands J, Mandrekar SJ, Geoffrey R, et al. ALCHEMIST: adjuvant targeted therapy or immunotherapy for high‐risk resected NSCLC. J Clin Oncol. 2020;38(15_suppl):TPS9077. [Google Scholar]
- 48. Govindan R, Mandrekar SJ, Gerber DE, et al. ALCHEMIST trials: a golden opportunity to transform outcomes in early‐stage non‐small cell lung cancer. Clin Cancer Res. 2015;21(24):5439‐5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. National Comprehensive Cancer Network Non‐Small Cell Lung Cancer (Version 5. 2021). https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 15 June 2021.
- 50. Masters GA, Temin S, Azzoli CG, et al. Systemic therapy for stage IV non‐small‐cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(30):3488‐3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: new biological insights and recent therapeutic advances. CA Cancer J Clin. 2011;61(2):91‐112. [DOI] [PubMed] [Google Scholar]
- 52. Tsao AS, Scagliotti GV, Bunn PA Jr, et al. Scientific advances in lung cancer 2015. J Thorac Oncol. 2016;11(5):613‐638. [DOI] [PubMed] [Google Scholar]
- 53. Gettinger S, Horn L, Jackman D, et al. Five‐year follow‐up of nivolumab in previously treated advanced non‐small‐cell lung cancer: results from the CA209‐003 study. J Clin Oncol. 2018;36(17):1675‐1684. [DOI] [PubMed] [Google Scholar]
- 54. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947‐957. [DOI] [PubMed] [Google Scholar]
- 55. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735‐742. [DOI] [PubMed] [Google Scholar]
- 56. Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non‐small‐cell lung cancer (ICOGEN): a randomised, double‐blind phase 3 non‐inferiority trial. Lancet Oncol. 2013;14(10):953‐961. [DOI] [PubMed] [Google Scholar]
- 57. Yang JC, Shih JY, Su WC, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX‐Lung 2): a phase 2 trial. Lancet Oncol. 2012;13(5):539‐548. [DOI] [PubMed] [Google Scholar]
- 58. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327‐3334. [DOI] [PubMed] [Google Scholar]
- 59. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): an open‐label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213‐222. [DOI] [PubMed] [Google Scholar]
- 60. Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first‐line treatment for patients with EGFR‐mutation‐positive non‐small‐cell lung cancer (ARCHER 1050): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2017;18(11):1454‐1466. [DOI] [PubMed] [Google Scholar]
- 61. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med. 2020;382(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 62. Wu YL, Herbst RS, Mann H, Rukazenkov Y, Marotti M, Tsuboi M. ADAURA: phase III, double‐blind, randomized study of osimertinib versus placebo in EGFR mutation‐positive early‐stage NSCLC after complete surgical resection. Clin Lung Cancer. 2018;19(4):e533‐e536. [DOI] [PubMed] [Google Scholar]
- 63. Lu S, Wang Q & Zhang G et al. A multicenter, open‐label, single‐arm, phase II study: the third generation EGFR tyrosine kinase inhibitor almonertinib for pretreated EGFR T790M‐positive locally advanced or metastatic non‐small cell lung cancer (APOLLO). AACR Annual Meeting 2020. 2020: Abstract CT190.
- 64. Shi Y, Hu X, Zhang S, et al. Efficacy, safety, and genetic analysis of furmonertinib (AST2818) in patients with EGFR T790M mutated non‐small‐cell lung cancer: a phase 2b, multicentre, single‐arm, open‐label study. Lancet Respir Med. 2021;9(8):829‐839. [DOI] [PubMed] [Google Scholar]
- 65. Paz‐Ares L, Mezger J, Ciuleanu TE, et al. Necitumumab plus pemetrexed and cisplatin as first‐line therapy in patients with stage IV non‐squamous non‐small‐cell lung cancer (INSPIRE): an open‐label, randomised, controlled phase 3 study. Lancet Oncol. 2015;16(3):328‐337. [DOI] [PubMed] [Google Scholar]
- 66. Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first‐line therapy in patients with stage IV squamous non‐small‐cell lung cancer (SQUIRE): an open‐label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763‐774. [DOI] [PubMed] [Google Scholar]
- 67. Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR exon 20 insertion‐mutated non‐small‐cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391‐3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhou C, Ramalingam SS, Kim TM, et al. Treatment outcomes and safety of mobocertinib in platinum‐pretreated patients with EGFR exon 20 insertion‐positive metastatic non‐small cell lung cancer: a phase 1/2 open‐label nonrandomized clinical trial. JAMA Oncol. 2021:e214761 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med. 2014;371(21):1963‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Blackhall F, Ross Camidge D, Shaw AT, et al. Final results of the large‐scale multinational trial PROFILE 1005: efficacy and safety of crizotinib in previously treated patients with advanced/metastatic ALK‐positive non‐small‐cell lung cancer. ESMO Open. 2017;2(3):e000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Soria JC, Tan D, Chiari R, et al. First‐line ceritinib versus platinum‐based chemotherapy in advanced ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐4): a randomised, open‐label, phase 3 study. Lancet. 2017;389(10072):917‐929. [DOI] [PubMed] [Google Scholar]
- 72. Novello S, Mazières J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib‐pretreated anaplastic lymphoma kinase (ALK)‐positive non‐small‐cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018;29(6):1409‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression‐free survival data for patients with treatment‐naive advanced ALK‐positive non‐small‐cell lung cancer in the ALEX study. Ann Oncol. 2020;31(8):1056‐1064. [DOI] [PubMed] [Google Scholar]
- 74. Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK‐positive non‐small‐cell lung cancer. N Engl J Med. 2018;379(21):2027‐2039. [DOI] [PubMed] [Google Scholar]
- 75. Shaw AT, Solomon BJ, Chiari R, et al. Lorlatinib in advanced ROS1‐positive non‐small‐cell lung cancer: a multicentre, open‐label, single‐arm, phase 1–2 trial. Lancet Oncol. 2019;20(12):1691‐1701. [DOI] [PubMed] [Google Scholar]
- 76. Shaw AT, Bauer TM, de Marinis F, et al. First‐line lorlatinib or crizotinib in advanced ALK‐positive lung cancer. N Engl J Med. 2020;383(21):2018‐2029. [DOI] [PubMed] [Google Scholar]
- 77. Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion‐positive non‐small‐cell lung cancer: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):261‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Planchard D, Kim TM, Mazieres J, et al. Dabrafenib in patients with BRAF(V600E)‐positive advanced non‐small‐cell lung cancer: a single‐arm, multicentre, open‐label, phase 2 trial. Lancet Oncol. 2016;17(5):642‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Drilon A, Oxnard GR, Tan D, et al. Efficacy of selpercatinib in RET fusion‐positive non‐small‐cell lung cancer. N Engl J Med. 2020;383(9):813‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gainor JF, Curigliano G, Kim DW, et al. Pralsetinib for RET fusion‐positive non‐small‐cell lung cancer (ARROW): a multi‐cohort, open‐label, phase 1/2 study. Lancet Oncol. 2021;22(7):959‐969. [DOI] [PubMed] [Google Scholar]
- 82. Wolf J, Seto T, Han JY, et al. Capmatinib in MET exon 14‐mutated or MET‐amplified non‐small‐cell lung cancer. N Engl J Med. 2020;383(10):944‐957. [DOI] [PubMed] [Google Scholar]
- 83. Paik PK, Felip E, Veillon R, et al. Tepotinib in non‐small‐cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lu S, Fang J, Li X, et al. Once‐daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non‐small‐cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single‐arm, open‐label, phase 2 study. Lancet Respir Med. 2021;9(10):1154‐1164. [DOI] [PubMed] [Google Scholar]
- 85. Doebele RC, Drilon A, Paz‐Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion‐positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):271‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hong DS, Bauer TM, Lee JJ, et al. Larotrectinib in adult patients with solid tumours: a multi‐centre, open‐label, phase I dose‐escalation study. Ann Oncol. 2019;30(2):325‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open‐label, phase 1/2 study. Lancet Oncol. 2018;19(5):705‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion‐positive cancers in adults and children. N Engl J Med. 2018;378(8):731‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Drilon A, Ou SI, Cho BC, et al. Repotrectinib (TPX‐0005) is a next‐generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent‐front mutations. Cancer Discov. 2018;8(10):1227‐1236. [DOI] [PubMed] [Google Scholar]
- 90. Mendelsohn J, Masui H, Goldenberg A. Anti‐epidermal growth factor receptor monoclonal antibodies may inhibit A431 tumor cell proliferation by blocking an autocrine pathway. Trans Assoc Am Physicians. 1987;100:173‐178. [PubMed] [Google Scholar]
- 91. Lemmon MA, Schlessinger J, Ferguson KM. The EGFR family: not so prototypical receptor tyrosine kinases. Cold Spring Harb Perspect Biol. 2014;6(4):a020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169‐181. [DOI] [PubMed] [Google Scholar]
- 93. Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497‐1500. [DOI] [PubMed] [Google Scholar]
- 94. Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339‐346. [DOI] [PubMed] [Google Scholar]
- 95. Tsao AS, Tang XM, Sabloff B, et al. Clinicopathologic characteristics of the EGFR gene mutation in non‐small cell lung cancer. J Thorac Oncol. 2006;1(3):231‐239. [DOI] [PubMed] [Google Scholar]
- 96. Wang Y, Li RQ, Ai YQ, et al. Exon 19 deletion was associated with better survival outcomes in advanced lung adenocarcinoma with mutant EGFR treated with EGFR‐TKIs as second‐line therapy after first‐line chemotherapy: a retrospective analysis of 128 patients. Clin Transl Oncol. 2015;17(9):727‐736. [DOI] [PubMed] [Google Scholar]
- 97. Yoon HY, Ryu JS, Sim YS, et al. Clinical significance of EGFR mutation types in lung adenocarcinoma: a multi‐centre Korean study. PLoS One. 2020;15(2):e0228925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non‐small cell lung cancer. Signal Transduct Target Ther. 2019;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Vasconcelos P, Gergis C, Viray H, et al. EGFR‐A763_Y764insFQEA is a unique exon 20 insertion mutation that displays sensitivity to approved and in‐development lung cancer EGFR tyrosine kinase inhibitors. JTO Clin Res Rep. 2020;1(3):100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jassem J, Dziadziuszko R. EGFR inhibitors for wild‐type EGFR NSCLC: to use or not to use?. Lancet Oncol. 2013;14(10):916‐917. [DOI] [PubMed] [Google Scholar]
- 101. Blackhall F, Ranson M, Thatcher N. Where next for gefitinib in patients with lung cancer. Lancet Oncol. 2006;7(6):499‐507. [DOI] [PubMed] [Google Scholar]
- 102. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129‐2139. [DOI] [PubMed] [Google Scholar]
- 103. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121‐128. [DOI] [PubMed] [Google Scholar]
- 104. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open‐label, first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non‐small‐cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866‐2874. [DOI] [PubMed] [Google Scholar]
- 105. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239‐246. [DOI] [PubMed] [Google Scholar]
- 106. Wu YL, Zhou C, Liam CK, et al. First‐line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer: analyses from the phase III, randomized, open‐label, ENSURE study. Ann Oncol. 2015;26(9):1883‐1889. [DOI] [PubMed] [Google Scholar]
- 107. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380‐2388. [DOI] [PubMed] [Google Scholar]
- 108. Urata Y, Katakami N, Morita S, et al. Randomized phase III study comparing gefitinib with erlotinib in patients with previously treated advanced lung adenocarcinoma: WJOG 5108L. J Clin Oncol. 2016;34(27):3248‐3257. [DOI] [PubMed] [Google Scholar]
- 109. Yang JJ, Zhou Q, Yan HH, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non‐small cell lung cancer with EGFR mutations. Br J Cancer. 2017;116(5):568‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. He J, Su C, Liang W, et al. Icotinib versus chemotherapy as adjuvant treatment for stage II–IIIA EGFR‐mutant non‐small‐cell lung cancer (EVIDENCE): a randomised, open‐label, phase 3 trial. Lancet Respir Med. 2021;9(9):1021‐1029. [DOI] [PubMed] [Google Scholar]
- 111. Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first‐line treatment of patients with EGFR mutation‐positive non‐small‐cell lung cancer (LUX‐Lung 7): a phase 2B, open‐label, randomised controlled trial. Lancet Oncol. 2016;17(5):577‐589. [DOI] [PubMed] [Google Scholar]
- 112. Paz‐Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation‐positive advanced non‐small‐cell lung cancer: overall survival data from the phase IIb LUX‐Lung 7 trial. Ann Oncol. 2017;28(2):270‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non‐small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12(3 Pt 1):839‐844. [DOI] [PubMed] [Google Scholar]
- 114. Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR‐mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17(6):1616‐1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non‐small‐cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786‐792. [DOI] [PubMed] [Google Scholar]
- 116. Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR‐mutated non‐small‐cell lung cancer. N Engl J Med. 2015;372(18):1700‐1709. [DOI] [PubMed] [Google Scholar]
- 117. Husain H, Martins RG, Goldberg SB. 1358PFirst‐in‐human phase I study of PF‐06747775, a third‐generation mutant selective EGFR tyrosine kinase inhibitor (TKI) in metastatic EGFR mutant NSCLC after progression on a first‐line EGFR TKI. Ann Oncol. 2017;28;mdx380. [Google Scholar]
- 118. Park K, Janne PA, Yu C. 412OA global phase II study of olmutinib (HM61713) in patients with T790M‐positive NSCLC after failure of first‐line EGFR‐TKI. Ann Oncol. 2017;28:mdx671. [Google Scholar]
- 119. Kim D‐W, Tan DSW, Aix SP, et al. Preliminary phase II results of a multicenter, open‐label study of nazartinib (EGF816) in adult patients with treatment‐naïve EGFR‐mutant non‐small cell lung cancer (NSCLC). J Clin Oncol. 2018;36:9094. [Google Scholar]
- 120. Wang H, Zhang L, Hu P, et al. Penetration of the blood–brain barrier by avitinib and its control of intra/extra‐cranial disease in non‐small cell lung cancer harboring the T790M mutation. Lung Cancer. 2018;122:1‐6. [DOI] [PubMed] [Google Scholar]
- 121. Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M‐positive advanced non‐small‐cell lung cancer: AURA study phase II extension component. J Clin Oncol. 2017;35(12):1288‐1296. [DOI] [PubMed] [Google Scholar]
- 122. Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met‐positive advanced non‐small‐cell lung cancer (AURA2): a multicentre, open‐label, single‐arm, phase 2 study. Lancet Oncol. 2016;17(12):1643‐1652. [DOI] [PubMed] [Google Scholar]
- 123. Jenkins S, Yang JC, Jänne PA, et al. EGFR mutation analysis for prospective patient selection in two phase II registration studies of osimertinib. J Thorac Oncol. 2017;12(8):1247‐1256. [DOI] [PubMed] [Google Scholar]
- 124. Khozin S, Weinstock C, Blumenthal GM, et al. Osimertinib for the treatment of metastatic EGFR T790M mutation‐positive non‐small cell lung cancer. Clin Cancer Res. 2017;23(9):2131‐2135. [DOI] [PubMed] [Google Scholar]
- 125. Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M‐positive advanced non‐small‐cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36(26):2702‐2709. [DOI] [PubMed] [Google Scholar]
- 126. Lu S, Dong X, Jian H, et al. Randomized phase III trial of aumolertinib (HS‐10296, Au) versus gefitinib (G) as first‐line treatment of patients with locally advanced or metastatic non‐small cell lung cancer (NSCLC) and EGFR exon 19 del or L858R mutations (EGFRm). J Clin Oncol. 2021;39(suppl 15):Abstract 9013. [Google Scholar]
- 127. Shi Y, Hu X, Zhang S, et al. Efficacy and safety of alflutinib (AST2818) in patients with T790M mutation‐positive NSCLC: a phase IIb multicenter single‐arm study. ASCO. 2020;38:Abstract 9602. [Google Scholar]
- 128. Shi Y, Hu X, Zhang S, et al. Efficacy and safety of alflutinib (AST2818) in patients with T790M mutation‐positive NSCLC: a phase IIb multicenter single‐arm study. J Clin Oncol. 2020;38:9602. [Google Scholar]
- 129. Imai K, Takaoka A. Comparing antibody and small‐molecule therapies for cancer. Nat Rev Cancer. 2006;6(9):714‐727. [DOI] [PubMed] [Google Scholar]
- 130. Paz‐Ares L, Socinski MA, Shahidi J, et al. Correlation of EGFR‐expression with safety and efficacy outcomes in SQUIRE: a randomized, multicenter, open‐label, phase III study of gemcitabine‐cisplatin plus necitumumab versus gemcitabine‐cisplatin alone in the first‐line treatment of patients with stage IV squamous non‐small‐cell lung cancer. Ann Oncol. 2016;27(8):1573‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci. 2016;107(9):1179‐1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non‐small cell lung cancer. Semin Cancer Biol. 2020;61:167‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kobayashi Y, Togashi Y, Yatabe Y, et al. EGFR exon 18 mutations in lung cancer: molecular predictors of augmented sensitivity to afatinib or neratinib as compared with first‐ or third‐generation TKIs. Clin Cancer Res. 2015;21(23):5305‐5313. [DOI] [PubMed] [Google Scholar]
- 134. Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan‐ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non‐small‐cell lung cancer. J Clin Oncol. 2010;28(18):3076‐3083. [DOI] [PubMed] [Google Scholar]
- 135. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non‐small‐cell lung cancer harbouring uncommon EGFR mutations: a combined post‐hoc analysis of LUX‐Lung 2, LUX‐Lung 3, and LUX‐Lung 6. Lancet Oncol. 2015;16(7):830‐838. [DOI] [PubMed] [Google Scholar]
- 136. Cho JH, Lim SH, An HJ, et al. Osimertinib for patients with non‐small‐cell lung cancer harboring uncommon EGFR mutations: a multicenter, open‐label, phase II trial (KCSG‐LU15‐09). J Clin Oncol. 2020;38(5):488‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Su J, Zhong W, Zhang X, et al. Molecular characteristics and clinical outcomes of EGFR exon 19 indel subtypes to EGFR TKIs in NSCLC patients. Oncotarget. 2017;8(67):111246‐111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wang J, Li X, Xue X, et al. Clinical outcomes of EGFR kinase domain duplication to targeted therapies in NSCLC. Int J Cancer. 2019;144(11):2677‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Naidoo J, Sima CS, Rodriguez K, et al. Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: clinical outcomes and response to erlotinib. Cancer. 2015;121(18):3212‐3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Park K, Haura EB, Natasha B, et al. Amivantamab in EGFR exon 20 insertion–mutated non–small‐cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391‐3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pulford K, Lamant L, Espinos E, et al. The emerging normal and disease‐related roles of anaplastic lymphoma kinase. Cell Mol Life Sci. 2004;61(23):2939‐2953. [DOI] [PubMed] [Google Scholar]
- 142. Murray PB, Lax I, Reshetnyak A, et al. Heparin is an activating ligand of the orphan receptor tyrosine kinase ALK. Sci Signal. 2015;8(360):ra6. [DOI] [PubMed] [Google Scholar]
- 143. Reshetnyak AV, Murray PB, Shi X, et al. Augmentor α and β (FAM150) are ligands of the receptor tyrosine kinases ALK and LTK: hierarchy and specificity of ligand–receptor interactions. Proc Natl Acad Sci U S A. 2015;112(52):15862‐15867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Iwahara T, Fujimoto J, Wen D, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14(4):439‐449. [DOI] [PubMed] [Google Scholar]
- 145. Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non‐Hodgkin's lymphoma. Science. 1994;263(5151):1281‐1284. [DOI] [PubMed] [Google Scholar]
- 146. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature. 2007;448(7153):561‐566. [DOI] [PubMed] [Google Scholar]
- 147. Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res. 2015;21(10):2227‐2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Fang DD, Zhang B, Gu Q, et al. HIP1‐ALK, a novel ALK fusion variant that responds to crizotinib. J Thorac Oncol. 2014;9(3):285‐294. [DOI] [PubMed] [Google Scholar]
- 149. Takeuchi K, Choi YL, Togashi Y, et al. KIF5B‐ALK, a novel fusion oncokinase identified by an immunohistochemistry‐based diagnostic system for ALK‐positive lung cancer. Clin Cancer Res. 2009;15(9):3143‐3149. [DOI] [PubMed] [Google Scholar]
- 150. Togashi Y, Soda M, Sakata S, et al. KLC1‐ALK: a novel fusion in lung cancer identified using a formalin‐fixed paraffin‐embedded tissue only. PLoS One. 2012;7(2):e31323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Choi YL, Lira ME, Hong M, et al. A novel fusion of TPR and ALK in lung adenocarcinoma. J Thorac Oncol. 2014;9(4):563‐566. [DOI] [PubMed] [Google Scholar]
- 152. Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non‐small cell lung cancer. Clin Cancer Res. 2013;19(15):4273‐4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Shaw AT, Yeap BY, Mino‐Kenudson M, et al. Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol. 2009;27(26):4247‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumor activity of PF‐2341066, a novel inhibitor of anaplastic lymphoma kinase and c‐Met, in experimental models of anaplastic large‐cell lymphoma. Mol Cancer Ther. 2007;6(12 Pt 1):3314‐3322. [DOI] [PubMed] [Google Scholar]
- 155. Kazandjian D, Blumenthal GM, Chen HY, et al. FDA approval summary: crizotinib for the treatment of metastatic non‐small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19(10):e5‐e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Gainor JF, Ou SH, Logan J, Borges LF, Shaw AT. The central nervous system as a sanctuary site in ALK‐positive non‐small‐cell lung cancer. J Thorac Oncol. 2013;8(12):1570‐1573. [DOI] [PubMed] [Google Scholar]
- 157. Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non‐small cell lung cancer. Cancer Discov. 2014;4(6):662‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK‐positive non‐small‐cell lung cancer. N Engl J Med. 2017;377(9):829‐838. [DOI] [PubMed] [Google Scholar]
- 159. Wang Y, Yang N, Zhang Y, et al. Effective treatment of lung adenocarcinoma harboring EGFR‐activating mutation, T790M, and cis‐C797S triple mutations by brigatinib and cetuximab combination therapy. J Thorac Oncol. 2020;15(8):1369‐1375. [DOI] [PubMed] [Google Scholar]
- 160. Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib‐refractory anaplastic lymphoma kinase‐positive non‐small‐cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490‐2498. [DOI] [PubMed] [Google Scholar]
- 161. Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK inhibitor‐naive advanced ALK‐positive NSCLC: final results of phase 3 ALTA‐1L trial. J Thorac Oncol. 2021;16:2091‐2108. [DOI] [PubMed] [Google Scholar]
- 162. Huang WS, Liu S, Zou D, et al. Discovery of brigatinib (AP26113), a phosphine oxide‐containing, potent, orally active inhibitor of anaplastic lymphoma kinase. J Med Chem. 2016;59(10):4948‐4964. [DOI] [PubMed] [Google Scholar]
- 163. Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)‐7‐amino‐12‐fluoro‐2,10,16‐trimethyl‐15‐oxo‐10,15,16,17‐tetrahydro‐2H‐8,4‐(metheno)pyrazolo[4,3‐h][2,5,11]‐benzoxadiazacyclotetradecine‐3‐carbonitrile (PF‐06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c‐ros oncogene 1 (ROS1) with preclinical brain exposure and broad‐spectrum potency against ALK‐resistant mutations. J Med Chem. 2014;57(11):4720‐4744. [DOI] [PubMed] [Google Scholar]
- 164. Baglivo S, Ricciuti B, Ludovini V, et al. Dramatic response to lorlatinib in a heavily pretreated lung adenocarcinoma patient harboring G1202R mutation and a synchronous novel R1192P ALK point mutation. J Thorac Oncol. 2018;13(8):e145‐e147. [DOI] [PubMed] [Google Scholar]
- 165. Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta. 2009;1795(1):37‐52. [DOI] [PubMed] [Google Scholar]
- 166. Facchinetti F, Rossi G, Bria E, et al. Oncogene addiction in non‐small cell lung cancer: focus on ROS1 inhibition. Cancer Treat Rev. 2017;55:83‐95. [DOI] [PubMed] [Google Scholar]
- 167. Birchmeier C, Sharma S, Wigler M. Expression and rearrangement of the ROS1 gene in human glioblastoma cells. Proc Natl Acad Sci U S A. 1987;84(24):9270‐9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190‐1203. [DOI] [PubMed] [Google Scholar]
- 169. Song A, Kim TM, Kim DW, et al. Molecular changes associated with acquired resistance to crizotinib in ROS1‐rearranged non‐small cell lung cancer. Clin Cancer Res. 2015;21(10):2379‐2387. [DOI] [PubMed] [Google Scholar]
- 170. Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29(15):2046‐2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non‐small cell lung cancer. Clin Cancer Res. 2013;19(16):4532‐4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Dankner M, Rose A, Rajkumar S, Siegel PM, Watson IR. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene. 2018;37(24):3183‐3199. [DOI] [PubMed] [Google Scholar]
- 173. Dagogo‐Jack I, Martinez P, Yeap BY, et al. Impact of BRAF mutation class on disease characteristics and clinical outcomes in BRAF‐mutant lung cancer. Clin Cancer Res. 2019;25(1):158‐165. [DOI] [PubMed] [Google Scholar]
- 174. Planchard D, Smit EF, Groen H, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E‐mutant metastatic non‐small‐cell lung cancer: an open‐label, phase 2 trial. Lancet Oncol. 2017;18(10):1307‐1316. [DOI] [PubMed] [Google Scholar]
- 175. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877‐1888. [DOI] [PubMed] [Google Scholar]
- 176. Takashima A, Faller DV. Targeting the RAS oncogene. Expert Opin Ther Targets. 2013;17(5):507‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small‐cell lung cancer: meta‐analyses by ethnicity and histology (mutMap). Ann Oncol. 2013;24(9):2371‐2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998‐2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Román M, Baraibar I, López I, et al. KRAS oncogene in non‐small cell lung cancer: clinical perspectives on the treatment of an old target. Mol Cancer. 2018;17(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Jänne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS‐mutant advanced non‐small‐cell lung cancer: a randomised, multicentre, placebo‐controlled, phase 2 study. Lancet Oncol. 2013;14(1):38‐47. [DOI] [PubMed] [Google Scholar]
- 181. Gandara DR, Leighl N, Delord JP, et al. A phase 1/1b study evaluating trametinib plus docetaxel or pemetrexed in patients with advanced non‐small cell lung cancer. J Thorac Oncol. 2017;12(3):556‐566. [DOI] [PubMed] [Google Scholar]
- 182. Mullard A. Amgen overcomes historically undruggable target, with FDA nod for first KRAS inhibitor. Nat Rev Drug Discov. 2021;20(7):496. [DOI] [PubMed] [Google Scholar]
- 183. Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005;16(4‐5):441‐467. [DOI] [PubMed] [Google Scholar]
- 184. Michels S, Scheel AH, Scheffler M, et al. Clinicopathological characteristics of RET rearranged lung cancer in European patients. J Thorac Oncol. 2016;11(1):122‐127. [DOI] [PubMed] [Google Scholar]
- 185. Ferrara R, Auger N, Auclin E, Besse B. Clinical and translational implications of RET rearrangements in non‐small cell lung cancer. J Thorac Oncol. 2018;13(1):27‐45. [DOI] [PubMed] [Google Scholar]
- 186. Gautschi O, Milia J, Filleron T, et al. Targeting RET in patients with RET‐rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 2017;35(13):1403‐1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Schoffski P, Aftimos PG, Massard C, et al. A phase I study of BOS172738 in patients with advanced solid tumors with RET gene alterations including non‐small cell lung cancer and medullary thyroid cancer. J Clin Oncol. 2019;37(15):TPS3162. [Google Scholar]
- 188. Drilon A, Rogers E, Zhai D, et al. 506P ‐ TPX‐0046 is a novel and potent RET/SRC inhibitor for RET‐driven cancers. Ann Oncol. 2019;30(Suppl. 5):v190‐v191. [Google Scholar]
- 189. Nakaoku T, Kohno T, Araki M, et al. A secondary RET mutation in the activation loop conferring resistance to vandetanib. Nat Commun. 2018;9(1):625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET‐altered cancers. Ann Oncol. 2018;29(8):1869‐1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Liu D, Zhong M, Zhan D, Zhang Y, Liu S. Roles of the HGF/Met signaling in head and neck squamous cell carcinoma: focus on tumor immunity (Review). Oncol Rep. 2020;44(6):2337‐2344. [DOI] [PubMed] [Google Scholar]
- 192. Kong‐Beltran M, Seshagiri S, Zha J, et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res. 2006;66(1):283‐289. [DOI] [PubMed] [Google Scholar]
- 193. Awad MM, Oxnard GR, Jackman DM, et al. MET exon 14 mutations in non‐small‐cell lung cancer are associated with advanced age and stage‐dependent MET genomic amplification and c‐Met overexpression. J Clin Oncol. 2016;34(7):721‐730. [DOI] [PubMed] [Google Scholar]
- 194. Gow CH, Hsieh MS, Wu SG, Shih JY. A comprehensive analysis of clinical outcomes in lung cancer patients harboring a MET exon 14 skipping mutation compared to other driver mutations in an East Asian population. Lung Cancer. 2017;103:82‐89. [DOI] [PubMed] [Google Scholar]
- 195. Drilon A, Clark JW, Weiss J, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med. 2020;26(1):47‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Doebele RC, Davis LE, Vaishnavi A, et al. An oncogenic NTRK fusion in a patient with soft‐tissue sarcoma with response to the tropomyosin‐related kinase inhibitor LOXO‐101. Cancer Discov. 2015;5(10):1049‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Marcus L, Donoghue M, Aungst S, et al. FDA approval summary: entrectinib for the treatment of NTRK gene fusion solid tumors. Clin Cancer Res. 2021;27(4):928‐932. [DOI] [PubMed] [Google Scholar]
- 198. Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion‐positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Pillai RN, Behera M, Berry LD, et al. HER2 mutations in lung adenocarcinomas: a report from the Lung Cancer Mutation Consortium. Cancer. 2017;123(21):4099‐4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Wang SE, Narasanna A, Perez‐Torres M, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10(1):25‐38. [DOI] [PubMed] [Google Scholar]
- 201. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31(16):1997‐2003. [DOI] [PubMed] [Google Scholar]
- 203. Shigematsu H, Takahashi T, Nomura M, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65(5):1642‐1646. [DOI] [PubMed] [Google Scholar]
- 204. Smit EF, Nakagawa K, Nagasaka M, et al. Trastuzumab deruxtecan (T‐DXd; DS‐8201) in patients with HER2‐mutated metastatic non‐small cell lung cancer (NSCLC): interim results of DESTINY‐Lung01. J Clin Oncol. 2020;38(15):9504. [Google Scholar]
- 205. Li BT, Shen R, Buonocore D, et al. Ado‐trastuzumab emtansine for patients with HER2‐mutant lung cancers: results from a phase II basket trial. J Clin Oncol. 2018;36(24):2532‐2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Tsurutani J, Iwata H, Krop I, et al. Targeting HER2 with trastuzumab deruxtecan: a dose‐expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 2020;10(5):688‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Herbst RS, Fidler IJ. Angiogenesis and lung cancer: potential for therapy. Clin Cancer Res. 2000;6(12):4604‐4606. [PubMed] [Google Scholar]
- 208. Meert AP, Paesmans M, Martin B, et al. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta‐analysis. Br J Cancer. 2002;87(7):694‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669‐676. [DOI] [PubMed] [Google Scholar]
- 210. Presta LG, Chen H, O'Connor SJ, et al. Humanization of an anti‐vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57(20):4593‐4599. [PubMed] [Google Scholar]
- 211. Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non‐small‐cell lung cancer. J Clin Oncol. 2004;22(11):2184‐2191. [DOI] [PubMed] [Google Scholar]
- 212. Sandler A, Gray R, Perry MC, et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med. 2006;355(24):2542‐2550. [DOI] [PubMed] [Google Scholar]
- 213. Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC‐1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor‐2. J Clin Oncol. 2010;28(5):780‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second‐line treatment of stage IV non‐small‐cell lung cancer after disease progression on platinum‐based therapy (REVEL): a multicentre, double‐blind, randomised phase 3 trial. Lancet. 2014;384(9944):665‐673. [DOI] [PubMed] [Google Scholar]
- 215. Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR‐mutated, advanced non‐small‐cell lung cancer (RELAY): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655‐1669. [DOI] [PubMed] [Google Scholar]
- 216. Hall RD, Le TM, Haggstrom DE, Gentzler RD. Angiogenesis inhibition as a therapeutic strategy in non‐small cell lung cancer (NSCLC). Transl Lung Cancer Res. 2015;4(5):515‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Paz‐Ares L, Hirsh V, Zhang L, et al. Monotherapy administration of sorafenib in patients with non‐small cell lung cancer (MISSION) trial: a phase III, multicenter, placebo‐controlled trial of sorafenib in patients with relapsed or refractory predominantly nonsquamous non‐small‐cell lung cancer after 2 or 3 previous treatment regimens. J Thorac Oncol. 2015;10(12):1745‐1753. [DOI] [PubMed] [Google Scholar]
- 218. Baggstrom MQ, Socinski MA, Wang XF, et al. Maintenance sunitinib following initial platinum‐based combination chemotherapy in advanced‐stage IIIB/IV non‐small cell lung cancer: a randomized, double‐blind, placebo‐controlled phase III study‐CALGB 30607 (Alliance). J Thorac Oncol. 2017;12(5):843‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second‐line treatment for patients with advanced non‐small‐cell lung cancer (ZODIAC): a double‐blind, randomised, phase 3 trial. Lancet Oncol. 2010;11(7):619‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Hanna NH, Kaiser R, Sullivan RN, et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non‐small cell lung cancer (LUME‐Lung 2): a randomized, double‐blind, phase III trial. Lung Cancer. 2016;102:65‐73. [DOI] [PubMed] [Google Scholar]
- 221. Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi‐target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Han B, Li K, Zhao Y, et al. Anlotinib as a third‐line therapy in patients with refractory advanced non‐small‐cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer. 2018;118(5):654‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Zhou M, Chen X, Zhang H, et al. China National Medical Products Administration approval summary: anlotinib for the treatment of advanced non‐small cell lung cancer after two lines of chemotherapy. Cancer Commun (Lond). 2019;39(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Cheng Y, Wang Q, Li K, et al. Anlotinib vs placebo as third‐ or further‐line treatment for patients with small cell lung cancer: a randomised, double‐blind, placebo‐controlled phase 2 study. Br J Cancer. 2021;125(3):366‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Abdollahi A, Hahnfeldt P, Maercker C, et al. Endostatin's antiangiogenic signaling network. Mol Cell. 2004;13(5):649‐663. [DOI] [PubMed] [Google Scholar]
- 226. Sun Y, Wang J, Liu Y, et al. Results of phase III trial of rh‐endostatin (YH‐16) in advanced non‐small cell lung cancer (NSCLC) patients. J Clin Oncol. 2005;23(16):7138. [Google Scholar]
- 227. Shukui Q, Liuqing Y, Jun L, et al. Intra‐pleural injection of recombinant human endostatin and/or cisplatin in treatment of malignant hydrothorax and ascites: a multicenter randomized controlled trial. Chin Clin Oncol. 2017;22(3):193‐202. [Google Scholar]
- 228. Rong B, Yang S, Li W, Zhang W, Ming Z. Systematic review and meta‐analysis of Endostar (rh‐endostatin) combined with chemotherapy versus chemotherapy alone for treating advanced non‐small cell lung cancer. World J Surg Oncol. 2012;10:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Suda K, Murakami I, Sakai K, et al. Heterogeneity in resistance mechanisms causes shorter duration of epidermal growth factor receptor kinase inhibitor treatment in lung cancer. Lung Cancer. 2016;91:36‐40. [DOI] [PubMed] [Google Scholar]
- 230. Sequist LV, Waltman BA, Dias‐Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299‐311. [DOI] [PubMed] [Google Scholar]
- 232. Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib as first‐line treatment of EGFR mutation‐positive advanced non‐small‐cell lung cancer. J Clin Oncol. 2018;36(9):841‐849. [DOI] [PubMed] [Google Scholar]
- 233. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Mulloy R, Ferrand A, Kim Y, et al. Epidermal growth factor receptor mutants from human lung cancers exhibit enhanced catalytic activity and increased sensitivity to gefitinib. Cancer Res. 2007;67(5):2325‐2330. [DOI] [PubMed] [Google Scholar]
- 235. Vikis H, Sato M, James M, et al. EGFR‐T790M is a rare lung cancer susceptibility allele with enhanced kinase activity. Cancer Res. 2007;67(10):4665‐4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Gaut D, Sim MS, Yue Y, et al. Clinical implications of the T790M mutation in disease characteristics and treatment response in patients with epidermal growth factor receptor (EGFR)‐mutated non‐small‐cell lung cancer (NSCLC). Clin Lung Cancer. 2018;19(1):e19‐e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Yu HA, Tian SK, Drilon AE, et al. Acquired resistance of EGFR‐mutant lung cancer to a T790M‐specific EGFR inhibitor: emergence of a third mutation (C797S) in the EGFR tyrosine kinase domain. JAMA Oncol. 2015;1(7):982‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non‐small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21(6):560‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Ou SI, Cui J, Schrock AB, et al. Emergence of novel and dominant acquired EGFR solvent‐front mutations at Gly796 (G796S/R) together with C797S/R and L792F/H mutations in one EGFR (L858R/T790M) NSCLC patient who progressed on osimertinib. Lung Cancer. 2017;108:228‐231. [DOI] [PubMed] [Google Scholar]
- 240. Chiba M, Togashi Y, Bannno E, et al. Efficacy of irreversible EGFR‐TKIs for the uncommon secondary resistant EGFR mutations L747S, D761Y, and T854A. BMC Cancer. 2017;17(1):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Kim TM, Song A, Kim DW, et al. Mechanisms of acquired resistance to AZD9291: a mutation‐selective, irreversible EGFR inhibitor. J Thorac Oncol. 2015;10(12):1736‐1744. [DOI] [PubMed] [Google Scholar]
- 242. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR‐mutation‐positive non‐small‐cell lung cancer after progression on first‐line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16(8):990‐998. [DOI] [PubMed] [Google Scholar]
- 243. Ho CC, Liao WY, Lin CA, Shih JY, Yu CJ, Yang JC. Acquired BRAF V600E mutation as resistant mechanism after treatment with osimertinib. J Thorac Oncol. 2017;12(3):567‐572. [DOI] [PubMed] [Google Scholar]
- 244. Ohashi K, Sequist LV, Arcila ME, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A. 2012;109(31):E2127‐E2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Blakely CM, Pazarentzos E, Olivas V, et al. NF‐κB‐activating complex engaged in response to EGFR oncogene inhibition drives tumor cell survival and residual disease in lung cancer. Cell Rep. 2015;11(1):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Yu HA, Perez L, Chang Q, et al. A phase 1/2 trial of ruxolitinib and erlotinib in patients with EGFR‐mutant lung adenocarcinomas with acquired resistance to erlotinib. J Thorac Oncol. 2017;12(1):102‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ, Settleman J. Drug resistance via feedback activation of Stat3 in oncogene‐addicted cancer cells. Cancer Cell. 2014;26(2):207‐221. [DOI] [PubMed] [Google Scholar]
- 248. Sos ML, Koker M, Weir BA, et al. PTEN loss contributes to erlotinib resistance in EGFR‐mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009;69(8):3256‐3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Ludovini V, Bianconi F, Pistola L, et al. Phosphoinositide‐3‐kinase catalytic alpha and KRAS mutations are important predictors of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in patients with advanced non‐small cell lung cancer. J Thorac Oncol. 2011;6(4):707‐715. [DOI] [PubMed] [Google Scholar]
- 250. Tan DS‐W, Lim KH, Meng W, et al. A phase Ib safety and tolerability study of a pan class I PI3K inhibitor buparlisib (BKM120) and gefitinib in EGFR TKI‐resistance NSCLC. J Clin Oncol. 2013;31(15):8107. [Google Scholar]
- 251. Price KA, Azzoli CG, Krug LM, et al. Phase II trial of gefitinib and everolimus in advanced non‐small cell lung cancer. J Thorac Oncol. 2010;5(10):1623‐1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first‐ and second‐generation ALK inhibitors in ALK‐rearranged lung cancer. Cancer Discov. 2016;6(10):1118‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK‐rearranged lung cancers. Sci Transl Med. 2012;4(120):120ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Choi YL, Soda M, Yamashita Y, et al. EML4‐ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363(18):1734‐1739. [DOI] [PubMed] [Google Scholar]
- 255. Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N Engl J Med. 2016;374(1):54‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non‐small cell lung cancer. Clin Cancer Res. 2012;18(5):1472‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Katayama R, Khan TM, Benes C, et al. Therapeutic strategies to overcome crizotinib resistance in non‐small cell lung cancers harboring the fusion oncogene EML4‐ALK. Proc Natl Acad Sci U S A. 2011;108(18):7535‐7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Heuckmann JM, Hölzel M, Sos ML, et al. ALK mutations conferring differential resistance to structurally diverse ALK inhibitors. Clin Cancer Res. 2011;17(23):7394‐7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Solomon BJ, Bauer TM, Felip E, et al. Safety and efficacy of lorlatinib (PF‐06463922) from the dose‐escalation component of a study in patients with advanced ALK+ or ROS1+ non‐small cell lung cancer (NSCLC). J Clin Oncol. 2016;34(15):9009. [Google Scholar]
- 260. Katayama R, Friboulet L, Koike S, et al. Two novel ALK mutations mediate acquired resistance to the next‐generation ALK inhibitor alectinib. Clin Cancer Res. 2014;20(22):5686‐5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Hrustanovic G, Olivas V, Pazarentzos E, et al. RAS‐MAPK dependence underlies a rational polytherapy strategy in EML4‐ALK‐positive lung cancer. Nat Med. 2015;21(9):1038‐1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Crystal AS, Shaw AT, Sequist LV, et al. Patient‐derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346(6216):1480‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Facchinetti F, Loriot Y, Kuo MS, et al. Crizotinib‐resistant ROS1 mutations reveal a predictive kinase inhibitor sensitivity model for ROS1‐ and ALK‐rearranged lung cancers. Clin Cancer Res. 2016;22(24):5983‐5991. [DOI] [PubMed] [Google Scholar]
- 264. McCoach CE, Le AT, Gowan K, et al. Resistance mechanisms to targeted therapies in ROS1+ and ALK+ non‐small cell lung cancer. Clin Cancer Res. 2018;24(14):3334‐3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Gainor JF, Friboulet L, Yoda S, et al. Frequency and spectrum of ROS1 resistance mutations in ROS1‑positive lung cancer patients progressing on crizotinib. J Clin Oncol. 2016;34(15):9072. [Google Scholar]
- 266. Drilon A, Somwar R, Wagner JP, et al. A novel crizotinib‐resistant solvent‐front mutation responsive to cabozantinib therapy in a patient with ROS1‐rearranged lung cancer. Clin Cancer Res. 2016;22(10):2351‐2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Cargnelutti M, Corso S, Pergolizzi M, et al. Activation of RAS family members confers resistance to ROS1 targeting drugs. Oncotarget. 2015;6(7):5182‐5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Huang Q, Schneeberger VE, Luetteke N, et al. Preclinical modeling of KIF5B‐RET fusion lung adenocarcinoma. Mol Cancer Ther. 2016;15(10):2521‐2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Kosaka T, Tanizaki J, Paranal RM, et al. Response heterogeneity of EGFR and HER2 exon 20 insertions to covalent EGFR and HER2 inhibitors. Cancer Res. 2017;77(10):2712‐2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. MA04.02 Neratinib ± Temsirolimus in HER2‐mutant lung cancers: an international, randomized phase II study. J Thorac Oncol. 2017;12(1):S358‐S359. [Google Scholar]
- 271. Heist RS, Sequist LV, Borger D, et al. Acquired resistance to crizotinib in NSCLC with MET exon 14 skipping. J Thorac Oncol. 2016;11(8):1242‐1245. [DOI] [PubMed] [Google Scholar]
- 272. Ou SI, Young L, Schrock AB, et al. Emergence of preexisting MET Y1230C mutation as a resistance mechanism to crizotinib in NSCLC with MET exon 14 skipping. J Thorac Oncol. 2017;12(1):137‐140. [DOI] [PubMed] [Google Scholar]
- 273. Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039‐1043. [DOI] [PubMed] [Google Scholar]
- 274. Wu Y‐L, Yang JC, Kim D‐W, et al. Safety and efficacy of INC280 in combination with gefitinib (gef) in patients with EGFR‐mutated (mut), MET‐positive NSCLC: a single‐arm phase lb/ll study. J Clin Oncol. 2014;32(15):8017. [Google Scholar]
- 275. Takezawa K, Pirazzoli V, Arcila ME, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR‐mutant lung cancers that lack the second‐site EGFRT790M mutation. Cancer Discov. 2012;2(10):922‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Zhang Z, Lee JC, Lin L, et al. Activation of the AXL kinase causes resistance to EGFR‐targeted therapy in lung cancer. Nat Genet. 2012;44(8):852‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Wilson FH, Johannessen CM, Piccioni F, et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell. 2015;27(3):397‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Nelson‐Taylor SK, Le AT, Yoo M, et al. Resistance to RET‐inhibition in RET‐rearranged NSCLC is mediated by reactivation of RAS/MAPK signaling. Mol Cancer Ther. 2017;16(8):1623‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009;14(7):667‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Lovly CM, McDonald NT, Chen H, et al. Rationale for co‐targeting IGF‐1R and ALK in ALK fusion‐positive lung cancer. Nat Med. 2014;20(9):1027‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin‐like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006;66(20):10100‐10111. [DOI] [PubMed] [Google Scholar]
- 282. Dziadziuszko R, Le AT, Wrona A, et al. An activating KIT mutation induces crizotinib resistance in ROS1‐positive lung cancer. J Thorac Oncol. 2016;11(8):1273‐1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small‐cell lung cancer. Nat Commun. 2015;6:6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Kobayashi Y, Sakao Y, Ito S, et al. Transformation to sarcomatoid carcinoma in ALK‐rearranged adenocarcinoma, which developed acquired resistance to crizotinib and received subsequent chemotherapies. J Thorac Oncol. 2013;8(8):e75‐e78. [DOI] [PubMed] [Google Scholar]
- 285. Rosell R, Karachaliou N. Large‐scale screening for somatic mutations in lung cancer. Lancet. 2016;387(10026):1354‐1356. [DOI] [PubMed] [Google Scholar]
- 286. Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab‐paclitaxel in combination with carboplatin versus solvent‐based paclitaxel plus carboplatin as first‐line therapy in patients with advanced non‐small‐cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055‐2062. [DOI] [PubMed] [Google Scholar]
- 287. Paz‐Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non‐small‐cell lung cancer. J Clin Oncol. 2013;31(23):2895‐2902. [DOI] [PubMed] [Google Scholar]
- 288. McNutt M. Cancer immunotherapy. Science. 2013;342(6165):1417. [DOI] [PubMed] [Google Scholar]
- 289. Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21(4):687‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non‐small‐cell lung cancer (NADIM): an open‐label, multicentre, single‐arm, phase 2 trial. Lancet Oncol. 2020;21(11):1413‐1422. [DOI] [PubMed] [Google Scholar]
- 292. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med. 2017;377(20):1919‐1929. [DOI] [PubMed] [Google Scholar]
- 293. Błach J, Wojas‐Krawczyk K, Nicoś M, Krawczyk P. Failure of immunotherapy—the molecular and immunological origin of immunotherapy resistance in lung cancer. Int J Mol Sci. 2021;22(16):9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373(2):123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373(17):1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Paz‐Ares L, Ciuleanu TE, Cobo M, et al. First‐line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non‐small‐cell lung cancer (CheckMate 9LA): an international, randomised, open‐label, phase 3 trial. Lancet Oncol. 2021;22(2):198‐211. [DOI] [PubMed] [Google Scholar]
- 297. Mok T, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819‐1830. [DOI] [PubMed] [Google Scholar]
- 298. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387(10027):1540‐1550. [DOI] [PubMed] [Google Scholar]
- 299. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: a randomised, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol. 2016;17(11):1497‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Gandhi L, Rodríguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078‐2092. [DOI] [PubMed] [Google Scholar]
- 301. Paz‐Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med. 2018;379(21):2040‐2051. [DOI] [PubMed] [Google Scholar]
- 302. Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first‐line treatment of advanced non‐small‐cell lung cancer with PD‐L1 of at least 50%: a multicentre, open‐label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592‐604. [DOI] [PubMed] [Google Scholar]
- 303. Shi Y, Wu L, Yu X, et al. Retraction notice to “30MO ORIENT‐3: a randomized, open‐label, phase III study of sintilimab versus docetaxel in previously treated advanced/metastatic squamous non‐small cell lung cancer (sqNSCLC)”: [Annals of Oncology Volume 31, Supplement 7, December 2020, Page S1428]. Ann Oncol. 2021;32(4):576. [DOI] [PubMed] [Google Scholar]
- 304. Zhou C, Wu L, Fan Y, et al. Sintilimab plus platinum and gemcitabine as first‐line treatment for advanced or metastatic squamous NSCLC: results from a randomized, double‐blind, phase 3 trial (ORIENT‐12). J Thorac Oncol. 2021;16(9):1501‐1511. [DOI] [PubMed] [Google Scholar]
- 305. Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy‐naive patients with advanced non‐squamous non‐small‐cell lung cancer (CameL): a randomised, open‐label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9(3):305‐314. [DOI] [PubMed] [Google Scholar]
- 306. Lu S, Wang J, Yu Y, et al. Tislelizumab plus chemotherapy as first‐line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): a randomized phase 3 trial. J Thorac Oncol. 2021;16(9):1512‐1522. [DOI] [PubMed] [Google Scholar]
- 307. Wang J, Lu S, Yu X, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first‐line treatment for advanced squamous non‐small‐cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(5):709‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288‐2301. [DOI] [PubMed] [Google Scholar]
- 309. Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first‐line treatment of PD‐L1‐selected patients with NSCLC. N Engl J Med. 2020;383(14):1328‐1339. [DOI] [PubMed] [Google Scholar]
- 310. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor‐based immunotherapy. Lancet Oncol. 2016;17(12):e542‐e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism‐driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Carbone DP, Reck M, Paz‐Ares L, et al. First‐line nivolumab in stage IV or recurrent non‐small‐cell lung cancer. N Engl J Med. 2017;376(25):2415‐2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Tumeh PC, Harview CL, Yearley JH, et al. PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175(2):313‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Rousseau B, Foote MB, Maron SB, et al. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N Engl J Med. 2021;384(12):1168‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Alexandrov LB, Nik‐Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69‐74. [DOI] [PubMed] [Google Scholar]
- 318. Fang W, Ma Y, Yin JC, et al. Comprehensive genomic profiling identifies novel genetic predictors of response to anti‐PD‐(L)1 therapies in non‐small cell lung cancer. Clin Cancer Res. 2019;25(16):5015‐5026. [DOI] [PubMed] [Google Scholar]
- 319. Pennell NA, Arcila ME, Gandara DR, West H. Biomarker testing for patients with advanced non‐small cell lung cancer: real‐world issues and tough choices. Am Soc Clin Oncol Educ Book. 2019;39:531‐542. [DOI] [PubMed] [Google Scholar]
- 320. Proto C, Ferrara R, Signorelli D, et al. Choosing wisely first line immunotherapy in non‐small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treat Rev. 2019;75:39‐51. [DOI] [PubMed] [Google Scholar]
- 321. Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol. 2019;16(6):341‐355. [DOI] [PubMed] [Google Scholar]
- 322. Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non‐small cell lung cancer (NSCLC). Cancer. 2020;126(2):260‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open‐label, phase 2 KEYNOTE‐158 study. Lancet Oncol. 2020;21(10):1353‐1365. [DOI] [PubMed] [Google Scholar]
- 324. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science. 2018;359(6371):91‐97. [DOI] [PubMed] [Google Scholar]
- 325. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti‐PD‐1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science. 2018;359(6371):97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539‐573. [DOI] [PubMed] [Google Scholar]
- 328. Zou W, Chen L. Inhibitory B7‐family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467‐477. [DOI] [PubMed] [Google Scholar]
- 329. Meng M. Clin Cancer Res. 2004 Sep 15; 10(18 Pt 2):6371S‐6376S. Urol Oncol. 2017;35(5):313‐314. [DOI] [PubMed] [Google Scholar]
- 330. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med. 2015;372(21):2018‐2028. [DOI] [PubMed] [Google Scholar]
- 331. Kerr KM, Hirsch FR. Programmed death ligand‐1 immunohistochemistry: friend or foe. Arch Pathol Lab Med. 2016;140(4):326‐331. [DOI] [PubMed] [Google Scholar]
- 332. Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti‐PD‐1 antibody nivolumab, BMS‐936558, and in vivo toxicology in non‐human primates. Cancer Immunol Res. 2014;2(9):846‐856. [DOI] [PubMed] [Google Scholar]
- 333. Sundar R, Cho BC, Brahmer JR, Soo RA. Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol. 2015;7(2):85‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Raedler LA. Opdivo (Nivolumab): second PD‐1 inhibitor receives FDA approval for unresectable or metastatic melanoma. Am Health Drug Benefits. 2015;8(Spec Feature):180‐183. [PMC free article] [PubMed] [Google Scholar]
- 335. Najjar YG, Kirkwood JM. Pembrolizumab: Pharmacology and Therapeutics Review. 2014.
- 336. Barone A, Hazarika M, Theoret MR, et al. FDA approval summary: pembrolizumab for the treatment of patients with unresectable or metastatic melanoma. Clin Cancer Res. 2017;23(19):5661‐5665. [DOI] [PubMed] [Google Scholar]
- 337. Yang Y, Zhou H, Zhang L. Response to letter to the editor: efficacy and safety of sintilimab plus pemetrexed and platinum as first‐line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double‐blind, phase 3 study (ORIENT‐11). J Thorac Oncol. 2020;15(12):e191‐e192. [DOI] [PubMed] [Google Scholar]
- 338. Nie J, Wang C, Liu Y, et al. Addition of low‐dose decitabine to anti‐PD‐1 antibody camrelizumab in relapsed/refractory classical Hodgkin lymphoma. J Clin Oncol. 2019;37(17):1479‐1489. [DOI] [PubMed] [Google Scholar]
- 339. Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator's choice of chemotherapy as second‐line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open‐label, phase 3 study. Lancet Oncol. 2020;21(6):832‐842. [DOI] [PubMed] [Google Scholar]
- 340. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open‐label, parallel‐group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571‐580. [DOI] [PubMed] [Google Scholar]
- 341. Philips GK, Atkins M. Therapeutic uses of anti‐PD‐1 and anti‐PD‐L1 antibodies. Int Immunol. 2015;27(1):39‐46. [DOI] [PubMed] [Google Scholar]
- 342. Haile ST, Dalal SP, Clements V, Tamada K, Ostrand‐Rosenberg S. Soluble CD80 restores T cell activation and overcomes tumor cell programmed death ligand 1‐mediated immune suppression. J Immunol. 2013;191(5):2829‐2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): a multicentre, open‐label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837‐1846. [DOI] [PubMed] [Google Scholar]
- 345. Socinski MA, Nishio M, Jotte RM, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first‐line metastatic nonsquamous NSCLC. J Thorac Oncol. 2021;16(11):1909‐1924. [DOI] [PubMed] [Google Scholar]
- 346. Horn L, Mansfield AS, Szczęsna A, et al. First‐line atezolizumab plus chemotherapy in extensive‐stage small‐cell lung cancer. N Engl J Med. 2018;379(23):2220‐2229. [DOI] [PubMed] [Google Scholar]
- 347. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342‐2350. [DOI] [PubMed] [Google Scholar]
- 348. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Subudhi SK, Siddiqui BA, Aparicio AM, et al. Combined CTLA‐4 and PD‐L1 blockade in patients with chemotherapy‐naïve metastatic castration‐resistant prostate cancer is associated with increased myeloid and neutrophil immune subsets in the bone microenvironment. J Immunother Cancer. 2021;9(10):e002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first‐line treatment in stage IIIB/IV non‐small‐cell lung cancer: results from a randomized, double‐blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046‐2054. [DOI] [PubMed] [Google Scholar]
- 352. Hellmann MD, Paz‐Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non‐small‐cell lung cancer. N Engl J Med. 2019;381(21):2020‐2031. [DOI] [PubMed] [Google Scholar]
- 353. Rodriguez‐Abreu D, Johnson ML, Hussein MA, et al. Primary analysis of a randomized, double‐blind, phase II study of the anti‐TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first‐line (1L) treatment in patients with PD‐L1‐selected NSCLC (CITYSCAPE). J Clin Oncol. 2020;38(15):9503. [Google Scholar]
- 354. De Giglio A, Di Federico A, Nuvola G, Deiana C, Gelsomino F. The landscape of immunotherapy in advanced NSCLC: driving beyond PD‐1/PD‐L1 inhibitors (CTLA‐4, LAG3, IDO, OX40, TIGIT, vaccines). Curr Oncol Rep. 2021;23(11):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Cheng Y, Murakami H, Yang PC, et al. Randomized phase II trial of gefitinib with and without pemetrexed as first‐line therapy in patients with advanced nonsquamous non‐small‐cell lung cancer with activating epidermal growth factor receptor mutations. J Clin Oncol. 2016;34(27):3258‐3266. [DOI] [PubMed] [Google Scholar]
- 356. Han B, Jin B, Chu T, et al. Combination of chemotherapy and gefitinib as first‐line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: a randomized controlled trial. Int J Cancer. 2017;141(6):1249‐1256. [DOI] [PubMed] [Google Scholar]
- 357. Hosomi Y, Morita S, Sugawara S, et al. Gefitinib alone versus gefitinib plus chemotherapy for non‐small‐cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38(2):115‐123. [DOI] [PubMed] [Google Scholar]
- 358. Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non‐small cell lung carcinoma: a systematic review and meta‐analysis. JAMA Oncol. 2018;4(2):210‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Oxnard GR, Yang JC, Yu H, et al. TATTON: a multi‐arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR‐mutant lung cancer. Ann Oncol. 2020;31(4):507‐516. [DOI] [PubMed] [Google Scholar]
- 360. Yang JC, Shepherd FA, Kim DW, et al. Osimertinib plus durvalumab versus osimertinib monotherapy in EGFR T790M‐positive NSCLC following previous EGFR TKI therapy: CAURAL brief report. J Thorac Oncol. 2019;14(5):933‐939. [DOI] [PubMed] [Google Scholar]
- 361. Reck M, Mok T, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non‐small‐cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open‐label phase 3 trial. Lancet Respir Med. 2019;7(5):387‐401. [DOI] [PubMed] [Google Scholar]
- 362. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune‐based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale‐based combined treatments against cancer. Cell Death Differ. 2014;21(1):15‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Mielgo‐Rubio X, Uribelarrea EA, Cortés LQ, Moyano MS. Immunotherapy in non‐small cell lung cancer: update and new insights. J Clin Transl Res. 2021;7(1):1‐21. [PMC free article] [PubMed] [Google Scholar]
- 364. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab‐paclitaxel chemotherapy compared with chemotherapy alone as first‐line treatment for metastatic non‐squamous non‐small‐cell lung cancer (IMpower130): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20(7):924‐937. [DOI] [PubMed] [Google Scholar]
- 365. Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in combination with carboplatin and nab‐paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15(8):1351‐1360. [DOI] [PubMed] [Google Scholar]
- 366. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093‐2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first‐line treatment for advanced non‐small‐cell lung cancer (CheckMate 012): results of an open‐label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Planchard D, Yokoi T, McCleod MJ, et al. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clin Lung Cancer. 2016;17(3):232‐236.e1. [DOI] [PubMed] [Google Scholar]
- 369. Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first‐line treatment of advanced/metastatic recurrent nonsquamous non‐small cell lung cancer. Oncologist. 2007;12(6):713‐718. [DOI] [PubMed] [Google Scholar]
- 370. Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141‐151. [DOI] [PubMed] [Google Scholar]
- 371. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR‐positive advanced non‐squamous non‐small‐cell lung cancer (NEJ026): interim analysis of an open‐label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625‐635. [DOI] [PubMed] [Google Scholar]
- 372. Kawashima Y, Fukuhara T, Saito H, et al. Bevacizumab plus erlotinib versus erlotinib alone in Japanese patients with advanced, metastatic, EGFR‐mutant non‐small‐cell lung cancer (NEJ026): overall survival analysis of an open‐label, randomised, multicentre, phase 3 trial. Lancet Respir Med. 2021. 10.1016/S2213-2600(21)00166-1. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 373. Wei XW, Gao X, Zhang XC, et al. Mutational landscape and characteristics of ERBB2 in non‐small cell lung cancer. Thorac Cancer. 2020;11(6):1512‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Ogiso H, Ishitani R, Nureki O, et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110(6):775‐787. [DOI] [PubMed] [Google Scholar]
- 375. Yonesaka K, Hirotani K, Kawakami H, et al. Anti‐HER3 monoclonal antibody patritumab sensitizes refractory non‐small cell lung cancer to the epidermal growth factor receptor inhibitor erlotinib. Oncogene. 2016;35(7):878‐886. [DOI] [PubMed] [Google Scholar]
- 376. Dziadziuszko R, Smit EF, Dafni U, et al. Afatinib in NSCLC with HER2 mutations: results of the prospective, open‐label phase II NICHE trial of European Thoracic Oncology Platform (ETOP). J Thorac Oncol. 2019;14(6):1086‐1094. [DOI] [PubMed] [Google Scholar]
- 377. Socinski M. LBA60 ‐ ZENITH20, a multinational, multi‐cohort phase II study of poziotinib in NSCLC patients with EGFR or HER2 exon 20 insertion mutations. Ann Oncol. 2020;31(4):S1142‐S1215. [Google Scholar]
- 378. Gao G, Li X, Wang Q, et al. Single‐arm, phase II study of pyrotinib in advanced non‐small cell lung cancer (NSCLC) patients with HER2 exon 20 mutation. J Clin Oncol. 2019;37(15):9089. [Google Scholar]
- 379. Scharpenseel H, Hanssen A, Loges S, et al. EGFR and HER3 expression in circulating tumor cells and tumor tissue from non‐small cell lung cancer patients. Sci Rep. 2019;9(1):7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Yonesaka K. HER2‐/HER3‐targeting antibody‐drug conjugates for treating lung and colorectal cancers resistant to EGFR inhibitors. Cancers (Basel). 2021;13(5):1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Janne PA, Baik C, Su WC, et al. Efficacy and safety of patritumab deruxtecan (HER3‐DXd) in EGFR inhibitor‐resistant, EGFR‐mutated non‐small cell lung cancer. Cancer Discov. 2021. 10.1158/2159-8290.CD-21-0715. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Bensch F, Lamberts LE, Smeenk MM, et al. 89Zr‐lumretuzumab PET imaging before and during HER3 antibody lumretuzumab treatment in patients with solid tumors. Clin Cancer Res. 2017;23(20):6128‐6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Forster MD, Dillon MT, Kocsis J, et al. Patritumab or placebo, with cetuximab plus platinum therapy in recurrent or metastatic squamous cell carcinoma of the head and neck: a randomised phase II study. Eur J Cancer. 2019;123:36‐47. [DOI] [PubMed] [Google Scholar]
- 384. Liu XL, Zhang XT, Meng J, et al. ING5 knockdown enhances migration and invasion of lung cancer cells by inducing EMT via EGFR/PI3K/Akt and IL‐6/STAT3 signaling pathways. Oncotarget. 2017;8(33):54265‐54276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. [DOI] [PubMed] [Google Scholar]
- 386. Wang Y, Wang Y, Li J, Li J, Che G. Clinical significance of PIK3CA gene in non‐small‐cell lung cancer: a systematic review and meta‐analysis. Biomed Res Int. 2020;2020:3608241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Tan AC. Targeting the PI3K/Akt/mTOR pathway in non‐small cell lung cancer (NSCLC). Thorac Cancer. 2020;11(3):511‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Vansteenkiste JF, Canon JL, De Braud F, et al. Safety and efficacy of buparlisib (BKM120) in patients with PI3K pathway‐activated non‐small cell lung cancer: results from the phase II BASALT‐1 study. J Thorac Oncol. 2015;10(9):1319‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Hoy SM. Tazemetostat: first approval. Drugs. 2020;80(5):513‐521. [DOI] [PubMed] [Google Scholar]
- 391. Hatzimichael E, Crook T. Cancer epigenetics: new therapies and new challenges. J Drug Deliv. 2013;2013:529312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Mehta A, Dobersch S, Romero‐Olmedo AJ, Barreto G. Epigenetics in lung cancer diagnosis and therapy. Cancer Metastasis Rev. 2015;34(2):229‐241. [DOI] [PubMed] [Google Scholar]
- 393. Tang M, Xu W, Wang Q, Xiao W, Xu R. Potential of DNMT and its epigenetic regulation for lung cancer therapy. Curr Genomics. 2009;10(5):336‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Huang D, Cui L, Ahmed S, et al. An overview of epigenetic agents and natural nutrition products targeting DNA methyltransferase, histone deacetylases and microRNAs. Food Chem Toxicol. 2019;123:574‐594. [DOI] [PubMed] [Google Scholar]
- 395. Yi J, Li S, Wang C, et al. Potential applications of polyphenols on main ncRNAs regulations as novel therapeutic strategy for cancer. Biomed Pharmacother. 2019;113:108703. [DOI] [PubMed] [Google Scholar]
- 396. van Zandwijk N, Pavlakis N, Kao S, et al. MesomiR 1: a phase I study of TargomiRs in patients with refractory malignant pleural mesothelioma (MPM) and lung cancer (NSCLC). Ann Oncol. 2015;26(2):ii16. [Google Scholar]
- 397. Beg MS, Brenner AJ, Sachdev J, et al. Phase I study of MRX34, a liposomal miR‐34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. 2017;35(2):180‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Tang J, Salama R, Gadgeel SM, Sarkar FH, Ahmad A. Erlotinib resistance in lung cancer: current progress and future perspectives. Front Pharmacol. 2013;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Juergens RA, Wrangle J, Vendetti FP, et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non‐small cell lung cancer. Cancer Discov. 2011;1(7):598‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Schiffmann I, Greve G, Jung M, Lübbert M. Epigenetic therapy approaches in non‐small cell lung cancer: update and perspectives. Epigenetics. 2016;11(12):858‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Schwartsmann G, Schunemann H, Gorini CN, et al. A phase I trial of cisplatin plus decitabine, a new DNA‐hypomethylating agent, in patients with advanced solid tumors and a follow‐up early phase II evaluation in patients with inoperable non‐small cell lung cancer. Invest New Drugs. 2000;18(1):83‐91. [DOI] [PubMed] [Google Scholar]
- 402. Bauman J, Verschraegen C, Belinsky S, et al. A phase I study of 5‐azacytidine and erlotinib in advanced solid tumor malignancies. Cancer Chemother Pharmacol. 2012;69(2):547‐554. [DOI] [PubMed] [Google Scholar]
- 403. Witta SE, Jotte RM, Konduri K, et al. Randomized phase II trial of erlotinib with and without entinostat in patients with advanced non‐small‐cell lung cancer who progressed on prior chemotherapy. J Clin Oncol. 2012;30(18):2248‐2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Levy BP, Giaccone G, Besse B, et al. Randomised phase 2 study of pembrolizumab plus CC‐486 versus pembrolizumab plus placebo in patients with previously treated advanced non‐small cell lung cancer. Eur J Cancer. 2019;108:120‐128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets in this study are available from the corresponding author on reasonable request.


