Abstract
The function of the epidermal growth factor receptor (EGFR) family member HER4 remains unclear because its activating ligand, heregulin, results in either proliferation or differentiation. This variable response may stem from the range of signals generated by HER4 homodimers versus heterodimeric complexes with other EGFR family members. The ratio of homo- and heterodimeric complexes may be influenced both by a cell's EGFR family member expression profile and by the ligand or even ligand isoform used. To define the role of HER4 in mediating antiproliferative and differentiation responses, human breast cancer cell lines were screened for responses to heregulin. Only cells that expressed HER4 exhibited heregulin-dependent antiproliferative responses. In-depth studies of one line, SUM44, demonstrated that the antiproliferative and differentiation responses correlated with HER4 activation and were abolished by stable expression of a kinase-inactive HER4. HB-EGF, a HER4-specific ligand in this EGFR-negative cell line, also induced an antiproliferative response. Moreover, introduction and stable expression of HER4 in HER4-negative SUM102 cells resulted in the acquisition of a heregulin-dependent antiproliferative response, associated with increases in markers of differentiation. The role of HER2 in these heregulin-dependent responses was examined through elimination of cell surface HER2 signaling by stable expression of a single-chain anti-HER2 antibody that sequestered HER2 in the endoplasmic reticulum. In the cell lines with either endogenously (SUM44) or exogenously (SUM102) expressed HER4, elimination of HER2 did not alter HER4-dependent decreases in cell growth. These results suggest that HER4 is both necessary and sufficient to trigger an antiproliferative response in human breast cancer cells.
The epidermal growth factor receptor (EGFR) family has been implicated in breast cancer pathogenesis and progression (reviewed in references 13 and 39). Aberrant expression of at least two of the family members, EGFR and HER2, has been associated with poor prognosis and differential response to therapy (21, 28, 31, 44). Recently, treatment targeted against HER2 has demonstrated clinical efficacy, emphasizing the importance of members of this receptor family in breast cancer prognosis and therapy (10).
The EGFR family consists of four known members: EGFR (HER1, erbB-1), HER2 (erbB-2), HER3 (erbB-3), and HER4 (erbB-4) (reviewed in references 13, 34, and 39). The four receptors form homodimers or heterodimers upon activation by two sets of ligands, the EGF and heregulin/neuregulin families. There are several possible hetero- and homodimeric receptor combinations, which theoretically result in differential activation of multiple downstream signal transduction pathways. Additional heterogeneity results from varying phenotypic responses, depending on cell type and the duration or intensity of downstream signaling, determined in part by differences in ligand affinity, recycling, and intracellular environment, as well as other factors that govern the turnover of receptor family members (53). Because of this complexity, our understanding of EGFR family member biology is still relatively rudimentary, despite the clinical utility of biologic modifiers of EGFR and HER2.
The EGFR family members share structural and sequence similarity; there are, however, critical differences. HER2 has no known directly binding ligand but is the favored heterodimerization partner of each ligand-bound family member (50). HER3 has no significant kinase activity, unlike the other family members, but contains multiple phosphatidylinositol 3-kinase binding motifs that are phosphorylated by a heterodimeric kinase-active partner (20, 45). HER4 is more similar to EGFR and HER2 than to HER3, but it does contain a canonical phosphatidylinositol 3-kinase binding motif (14). Unlike EGFR and HER2, which have been associated with more aggressive clinical breast cancers, in several case series HER4 expression was correlated with low proliferative index and estrogen receptor expression, suggesting that HER4 may have a different impact on breast biology and cancer.
Heregulin, or Neu differentiating factor, is a member of a complex ligand family that was initially thought to be the long-sought HER2 ligand but was ultimately shown to activate HER2 through heterodimerization after binding to HER3 or HER4 (2, 7, 23, 33, 36, 54). Heregulin was also identified as a factor that caused differentiation in MDA-MB-453 human breast cancer cells (11), but its biologic effect, proliferation or differentiation, differed depending upon the cell lines used during its purification, hence the two names (heregulin and Neu differentiating factor). Subsequent work by many groups has shown that heregulin is expressed as multiple isoforms (reviewed in reference 34). Heregulin α, β 1, β 2, and β 3 were cloned, and heregulin β1 was shown to cause tyrosine phosphorylation of p185 HER2 (23). Heregulin β3 is a soluble form of heregulin, while other isoforms are at least initially membrane bound (54). A second genetic locus encodes neuregulin-2, which also causes MDA-MB-453 morphologic change but with less potency and less HER2 phosphorylation (7, 8). In general, heregulin isoforms have variable potency and receptor specificity. Heregulin α and β have different effects on mouse mammary development (26). Neuregulin 2 binds to HER3 and HER4, but there is a newly discovered third gene whose product, neuregulin 3, thus far has been found to bind to HER4 alone (56). Recently, neuregulin 4 has been identified (22).
The cell-type-specific effects of heregulin-induced proliferation or differentiation may be related to the expression, activation, and level of HER2, HER3, or HER4. Because heregulin causes HER2 tyrosine phosphorylation indirectly through its binding to HER3 and HER4, the ligand could mediate its differentiative or proliferative signal singly through HER4 or through complexes containing combinations of HER2, HER3, and HER4 (33). With this complexity of potency, receptor specificity, tissue distribution, and soluble or membrane-bound isoforms, it is not surprising that different experimental results have been obtained using different cells or isoforms. The β2 isoform of heregulin caused differentiation in MDA453 cells (1). However, the β3 isoform proved to be mitogenic in the same cell line (5). Others have demonstrated a differentiation response using the β1 isoform (9, 35). In addition, the response has been shown to be concentration dependent. In AU565 and MDA-MB-453 cells a low concentration of heregulin is mitogenic, whereas a higher concentration leads to differentiation and inhibition of cell growth (2). There are also differences in response to heregulin depending on the receptor density. In a panel of human breast cancer cell lines, level of expression of HER2 correlated with response to heregulin; cells expressing low levels of HER2 had mitogenic responses to heregulin, while cells expressing high levels of HER2 had differentiation responses (12, 17, 42). Response also depends on the cell line used and the amount of serum in the medium, as expected due to heterogenous expression of receptors and ligand (27).
HER4 can also be activated by another complex family of ligands—the EGF family. Like the heregulins, there is considerable variability in receptor activation and potency. Heparin-binding EGF (HB-EGF) binds to EGFR and HER4 (15) and induces HER4 phosphorylation in MDA-MB-453 cells. Betacellulin also binds EGFR and HER4 but not other EGFR family members (5, 37). Epiregulin activates all four EGFR family members and, in MDA-MB-453 cells (which lack appreciable EGFR), causes a differentiated phenotype (25, 38, 43). Affinity labeling and competition experiments demonstrate that epiregulin binds cooperatively to HER2-HER4, but not to HER3-HER4, heterodimers and directly binds EGFR and HER4.
Because HER4 appears to be associated with better prognostic features and can be activated by differentiation-inducing ligands, we attempted to clarify the role of HER4 by asking whether HER4 alone was necessary and sufficient to transmit an antiproliferative signal. We determined that HER4 activation by a member of the heregulin or the EGF family could transmit an antiproliferative response, and that expression of HER4 in a HER4-negative cell line was sufficient to confer an antiproliferative response. Perhaps most intriguingly, elimination of HER2 signaling did not abolish HER4-dependent antiproliferative responses in at least two distinct cell lines.
MATERIALS AND METHODS
Cell lines, tissue culture, and antibodies.
SUM44 and SUM102 cells were grown in serum-free growth factor-defined media as previously described (16, 41). SUM102 cells were derived from a microinvasive primary breast tumor, whereas SUM44 cells were derived from a metastatic pleural effusion. MDA-MB-453 cells were obtained from the American Type Culture Collection and were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. All cells were grown in a humidified incubator at 37°C with 10% CO2 and subcultured weekly, and the medium was changed three times per week. All tissue medium reagents were obtained from Sigma, except for fetal bovine serum and insulin, which were obtained from Gibco BRL.
Proliferation assays.
Cells were plated into six-well plates at a density of 5 × 104 to 5 × 105 cells per well and grown in the appropriate medium with or without recombinant heregulin β1 (gift from Amgen) or HB-EGF (R&D) for 6 to 7 days, or three medium changes. Cells were trypsinized and counted with a hemocytometer.
Quantitative PCR.
Total RNA was isolated using the guanidinium isothiocyanate-based RNeasy kit (Qiagen) and was treated with RNAse-free DNAse (Ambion) to prevent nonspecific priming of the PCRs. HER4-specific 5′-3′ oligonucleotides and an intervening fluorescent dye-labeled probe were designed using Primer Express software (ABI/Perkin Elmer). The nonextendable HER4 probe was synthesized and labeled with 5′ FAM (6-carboxyfluorescein) reporter and 3′ TAMRA (6-carboxy-tetramethyl-rhodamine) quencher dyes (Integrated DNA Technologies), followed by high-pressure liquid chromatography purification. Real-time fluorescence quantitative PCR was performed with the ABI PRISM 7700 (PE Bio). Full-length HER4 mRNA was in vitro transcribed using MEGAscript (Ambion) and used as a positive control and absolute quantitation standard for the assays. Similarly transcribed constructs for HER1, HER2, and HER3 were used as negative controls. Amplifications of twofold serial dilutions of full-length HER4 RNA were used to construct standard linear curves that permit us to routinely and accurately measure from 200 copies to 90 million template copies of HER4 mRNA. Ten nanograms of total RNA isolated from the cell lines was assayed in triplicate for HER4 expression levels.
Immunoprecipitation and immunoblot analysis.
Cells were washed with cold phosphate-buffered saline and lysed in lysis buffer containing 20 mM HEPES (pH 7.3), 50 mM sodium fluoride, 10% glycerol, 1% Triton X-100, 5 mM EDTA, and 0.5 M NaCl supplemented with the tyrosine phosphatase inhibitor sodium orthovanadate (1 mM) and the protease inhibitors aprotinin(6 μg/ml) and leupeptin(10 μg/ml). Nuclei and insoluble material were removed by centrifugation at 13,000 × g for 10 min at 4°C. Receptor proteins were precipitated with various antibodies [HER2, clone 9G6.10, mouse monoclonal antibody (Neomarkers, Inc.); HER3, (c-17)G, goat polyclonal antibody (Santa Cruz); HER4, polyclonal rabbit antisera raised against recombinant gluthathione S-transferase fusion protein containing the C-terminal 80 amino acids of HER4] and protein A/G or protein A agarose beads (Santa Cruz) for 3 h at 4°C. Immune complexes were washed three times with lysis buffer and denatured in sodium dodecyl sulfate sample buffer. Protein samples were separated on a sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis gel and were electrophoretically transferred to a Sequi-blot polyvinylidene difluoride membrane (Bio-Rad). After blocking with 3% cold fish gelatin (Sigma), the membrane was probed overnight at 4°C with antiphosphotyrosine antibody (RC20; Transduction Laboratories), washed three times with Tris-buffered saline–Tween, and detected with an enhanced chemiluminescence detection kit (Amersham Life Sciences).
Neutral lipid detection.
Cells were grown on glass coverslips in appropriate media with or without heregulin or HB-EGF for 1 week and then fixed with 10% neutral buffered formalin for 10 min. After a 60% isopropyl alcohol rinse, they were stained with Sudan IV solution (10 g of Sudan IV, 500 ml of acetone, 500 ml of 70% ethyl alcohol) for 4 min, followed by 60% isopropyl alcohol and distilled water rinses. Cells were then stained with Gill's hematoxylin (Fisher Scientific) for 1 min, rinsed in distilled water, and then stained with lithium carbonate (47 g of lithium carbonate, 3,500 ml of distilled water) till blue (about 30 s). After thorough rinsing in distilled water, slides were mounted in Aquamount for direct microscopic visualization of red-staining lipid droplets. Alternatively, staining of neutral lipid droplets in the cellular cytoplasm was done as described previously (40). In brief, the cells suspended in phosphate-buffered saline (106 cells/ml) were incubated for 5 min with Nile red (final concentration, 100 ng/ml) at room temperature. The yellow fluorescence of Nile red-stained neutral lipids droplets was analyzed with a FACScan (Becton Dickinson) using linear amplifiers.
cDNA constructs and clones.
Full-length human HER4 cDNA was created from three PCR fragments amplified from MDA-MB-453 cells. The fragments were recombined into the pLXSN retroviral vector (29), and the resulting full-length cDNA was sequenced in its entirety. The kinase-dead HER4 construct was created by site-directed mutagenesis, changing lysine to alanine in the 751 position and abolishing the ability to bind ATP. The construct was entirely sequenced and cloned into pLXSN. The 5R construct is a HER2 single-chain antibody with an endoplasmic reticulum (ER)-targeting sequence cloned into the pBABEpuro vector (19).
Creation of cell lines stably expressing introduced constructs.
For production of retrovirus using the above cDNAs in pLXSN, the amphotropic packaging cell line PA317 was plated at 5 × 105 cells per 60-mm dish and then transfected with 20 μg of retroviral DNA using 2 M CaCl2 precipitation as previously described (32). Viral supernatants were collected after 60 h of incubation, the last 48 h at 37°C with addition of sodium butyrate as described previously (30). Viral supernatants were filtered through a 0.45-μm-pore-size syringe filter, and 1 ml of viral supernatant was added with 8 μg of Polybrene per ml to recipient cells which had been plated at 7 × 105 cells per 100-mm dish the day before. After 48 h of incubation, cells were placed in medium containing G418 (0.3 mg/ml for SUM102 and 0.5 mg/ml for SUM44). G418-resistant, puromycin-resistant, or G418- and puromycin-resistant cells were pooled, and expression of the cDNA product was confirmed by Western blotting or reverse transcription-PCR (RT-PCR).
RESULTS
Proliferative response of human breast cancer cell lines to heregulin.
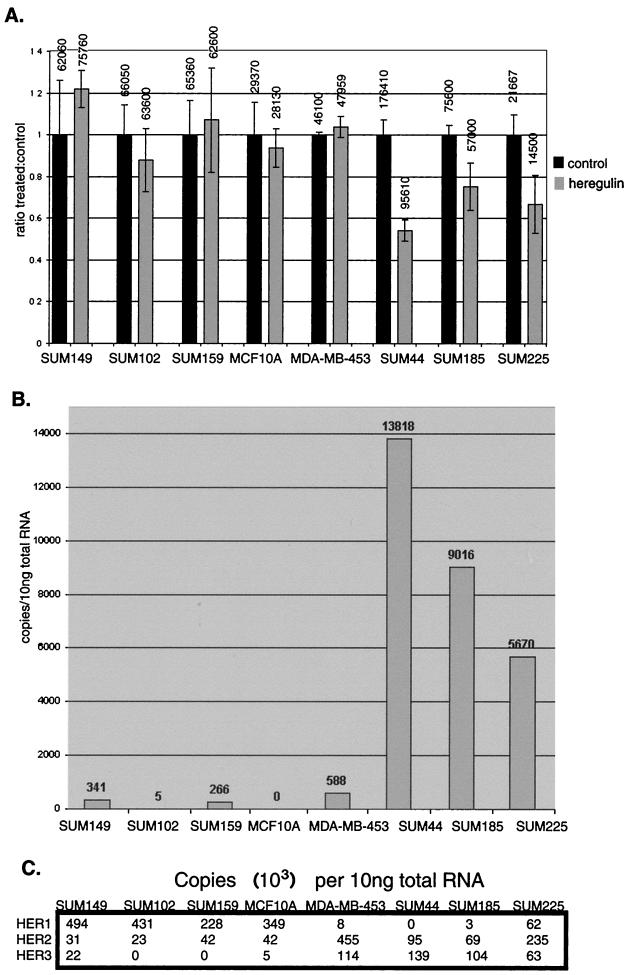
Heregulin has been shown to cause alternatively a mitogenic or an antimitogenic effect under various experimental conditions. To determine the range of this effect, and to select appropriate cell lines for study of the differentiative effects of heregulin, we characterized the proliferative response to heregulin of a panel of human breast cancer cell lines, many of which had not previously been evaluated for their heregulin response. The cell lines differ in their EGFR family member expression (Fig. 1B and C) and their exogenous ligand requirements. Many grow under growth factor-defined, serum-free conditions, allowing evaluation of the effect of heregulin without the confounding factor of undefined serum growth factors and without subjecting the cells to serum starvation. Three cell lines demonstrated a significant growth inhibitory response to heregulin: SUM44, SUM185, and SUM225 (Fig. 1A). The MDA-MB-453 cells obtained from the American Type Culture Collection were only minimally responsive to heregulin and in fact grew slowly under the conditions tested. As discussed below, they appeared to have constitutive activation of HER4, which precluded their use for evaluation of ligand dependence. MCF10A cells, which have been shown to have a proliferative response to heregulin when grown in serum-containing medium or when starved of insulin or EGF, did not demonstrate a proliferative effect when heregulin was added to defined medium containing EGF and insulin.
FIG. 1.
(A) Proliferative response of human breast cancer cell lines to heregulin. Cells were plated at a density of 5 × 104 to 5 × 105 cells per well, depending on plating efficiency and growth rate, in six-well plates and grown in the presence or absence of 10 ng of heregulin β1 per ml for three medium changes (7 days; approximately three doublings), and the number of cells was counted. The ratio of number of cells grown in the presence versus the absence of heregulin is shown, with the number of cells (average of three experiments) listed at the top of each column. Error bars represent standard deviations of at least three experiments. SUM185 (P = 0.03), SUM225 (P = 0.03), and SUM44 (P = 0.0009) cells demonstrated a statistically significant (by Student's t test) heregulin-dependent antiproliferative effect, with the effect in SUM44 cells being most pronounced. (B) HER4 mRNA levels as determined by quantitative PCR. Total RNA was extracted and reverse transcribed, and quantitative PCR was performed with the ABI PRISM 7700 using HER4-specific fluorescence-labeled oligonucleotide probes, as described in Materials and Methods. (C) HER1, -2, and -3 mRNA levels as determined by quantitative PCR. Quantitative PCR was performed using HER1-, HER2-, and HER3-specific probes as described above. The abundance of message of the other EGFR family members is usually much higher than that of HER4.
To correlate the antiproliferative response with HER4 expression, mRNA levels from these cell lines were evaluated by quantitative PCR using the ABI PRISM 7700 (Fig. 1B and C). The three cell lines that demonstrated an antiproliferative response to heregulin all expressed HER4, while the cell lines that lacked an antiproliferative response to heregulin did not, or expressed very low levels. Thus, HER4 expression correlated with an antiproliferative response to heregulin.
Receptor tyrosine phosphorylation in response to heregulin stimulation.
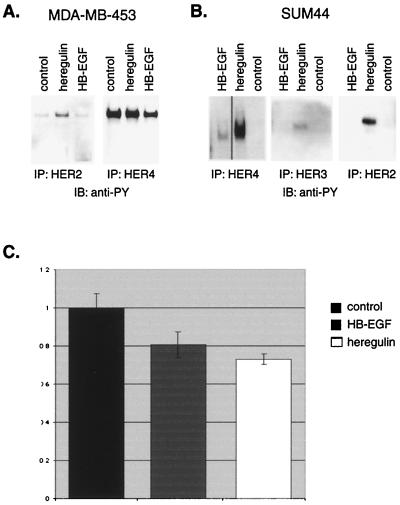
Previous work (1) led us to examine heregulin-dependent tyrosine phosphorylation of the EGFR family members in MDA-MB-435 cells, which have a high level of HER2 expression (Fig. 1C) and modest HER4 mRNA levels as measured by quantitative PCR. Despite low levels of HER4 message, substantial, constitutive HER4 tyrosine phosphorylation was observed, which was not further increased by heregulin treatment (Fig. 2A). In contrast, the low-level constitutive HER2 tyrosine phosphorylation was further induced by heregulin. Thus, any antiproliferative effect mediated by HER4 was already near the maximum, and in fact this clone of MDA-MB-453 cells proliferates slowly even in the absence of heregulin, displaying the flattened morphology with prominent vacuolization and the high cytoplasm-to-nucleus ratio typical of the differentiated phenotype described for MDA-MB-453 cells. Therefore, despite clear induction of HER2 phosphorylation by heregulin, there was no positive or negative proliferative change, and this clone was not useful for ligand-dependent studies.
FIG. 2.
Receptor tyrosine phosphorylation in response to heregulin stimulation. Cells were treated with 10 ng of heregulin β1 or 100 ng of HB-EGF per ml for 30 min or left untreated. Cell lysates were immunoprecipitated (IP) with antibodies to HER2, HER3, or HER4 and immunoblotted with antiphosphotyrosine (anti-PY) antibody RC20. (A) Heregulin induced tyrosine phosphorylation of HER2 in MDA-MB-453 cells, but these cells demonstrated constitutively phosphorylated HER4, which was not further induced by heregulin. (B) Heregulin induced tyrosine phosphorylation of HER2, HER3, and HER4 but HB-EGF induced tyrosine phosphorylation of only HER4 in SUM44 cells. (C) Antiproliferative response to HB-EGF. SUM44 cells were plated at a density of 5 × 105 cells per well in six-well plates and grown in the presence or absence of 10 ng of heregulin B1 or 100 ng of HB-EGF per ml for three medium changes (7 days), and the number of cells was counted. The ratio of number of cells grown in the presence versus the absence of ligand is shown. Error bars represent standard deviations of at least three experiments. Like heregulin, HB-EGF caused a significant antiproliferative effect, although the effect of HB-EGF was not as great as that of heregulin.
In contrast, SUM44 cells demonstrated a consistent antiproliferative response to heregulin. Without heregulin treatment there was no HER2 or HER4 activation (Fig. 2B). As anticipated, since it is a ligand for both HER3 and HER4 that can also dimerize with and activate HER2, heregulin induced tyrosine phosphorylation of HER2, HER3, and HER4.
HER4 tyrosine phosphorylation and antiproliferative response to HB-EGF.
Since heregulin induces HER2, HER3, and HER4 tyrosine phosphorylation, any or all could be responsible for the antiproliferative response. Therefore, the effect of a ligand that would bind specifically to HER4 was examined. HB-EGF binds to EGFR and HER4 but not directly to HER2 or HER3 (15). As anticipated, when SUM44 cells were treated with HB-EGF, HER4 became tyrosine phosphorylated, but, in contrast to results with heregulin, HER2 and HER3 were not (Fig. 2B). SUM44 cells do not express EGFR. HB-EGF-induced HER4 tyrosine phosphorylation was not as robust as that resulting from heregulin stimulation. The consequence of HB-EGF-dependent HER4 tyrosine phosphorylation in SUM44 cells was antiproliferative, although to a lesser degree than heregulin (Fig. 2C). The attenuated antiproliferative effect of HB-EGF correlated with the lower levels of HER4 tyrosine phosphorylation (Fig. 2B). Thus, activation of HER4 alone correlates with an antiproliferative effect in response to HER4 ligands. In MDA-MB-453 cells, HB-EGF did not induce HER2 or HER4 tyrosine phosphorylation above the baseline constitutive activation (Fig. 2B) and did not slow growth of these cells (data not shown).
Differentiation in response to HER4 activation.
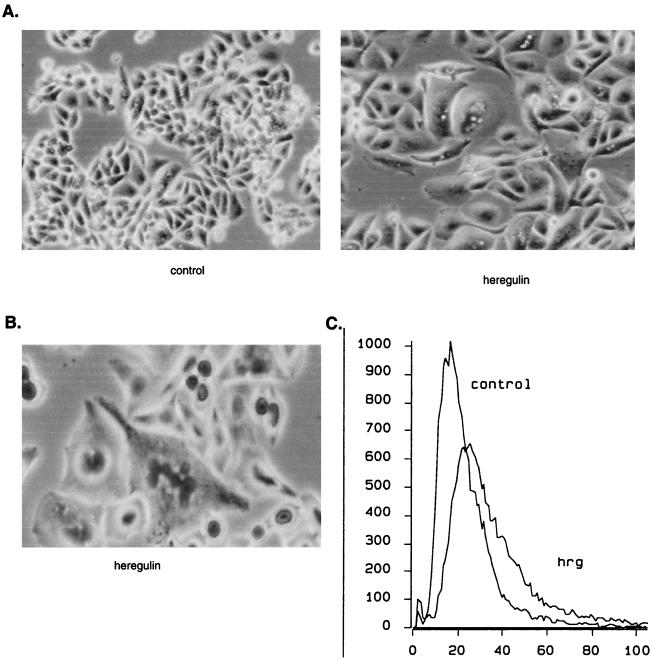
Decreased proliferation is one of the phenotypic changes that occur with differentiation of human breast cancer cells, but decreased proliferation may occur without differentiation. Therefore, we looked to see whether other phenotypic changes occurred with heregulin or HB-EGF stimulation. With differentiation, breast epithelial cells change morphology, becoming more flattened with higher cytoplasm-to-nucleus ratios. They produce milk proteins, which in human cells can be measured by detecting an increase in neutral lipids. SUM44 cells were treated with heregulin and examined for morphologic changes and neutral lipid production as measured by Sudan IV staining. Over a 2- to 6-day heregulin treatment, a proportion of the SUM44 cells underwent clear morphologic changes consistent with differentiation (Fig. 3A). These and other cells without such significant morphologic changes produced neutral lipid droplets (Fig. 3B). In order to better evaluate the percentage of cells that underwent these differentiative changes, we quantified neutral lipid-producing cells by fluorescence-activated cell sorting (FACS) analysis using Nile red (Figure 3C). Heregulin clearly induced morphologic changes and neutral lipid production in SUM44 cells.
FIG. 3.
Differentiation changes in SUM44 cells in response to heregulin. SUM44 cells were grown in the presence or absence of 10 ng of heregulin β1 per ml for 1 week and photographed live (A) or after staining with Sudan IV, a neutral lipid stain (B). In the presence of heregulin, cells become larger and flattened, with prominent vacuolization. Sudan IV staining demonstrates lipid droplet formation in heregulin-treated cells. (C) To quantify the extent of neutral lipid production, cells were stained with a fluorescent neutral lipid stain, Nile red, and analyzed by FACS. Treatment with heregulin induces accumulation of neutral lipids, as evidenced by a shift of the curve toward higher-intensity staining in the heregulin (hrg)-treated cells.
Attenuation of antiproliferative response to heregulin by expression of kinase-inactive HER4.
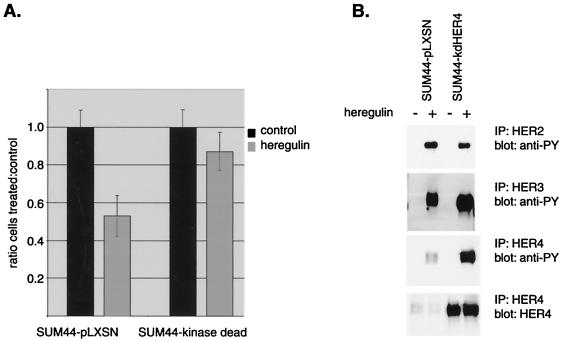
Heregulin causes tyrosine phosphorylation of HER2 and HER3 as well as HER4 in SUM44 cells and induces differentiation changes. HB-EGF induces tyrosine phosphorylation of only HER4 in these cells and induces antiproliferative changes, suggesting that HER4 alone is responsible for transmitting the antiproliferative signal seen in response to both ligands. To further support the role of HER4 in transmitting an antiproliferative signal, we attempted to block the antiproliferative response to heregulin by interfering with HER4 activation. A kinase-inactive HER4 construct (kdHER4) that in other receptor contexts acts as a dominant negative was created by site-directed mutagenesis and introduced into SUM44 cells by retroviral infection. Selection of kdHER4- or vector-expressing cells was performed with the antibiotic G418. Cells expressing kdHER4 demonstrated increased proliferation compared with vector control cells, suggesting that kdHER4 was counteracting a growth inhibitory signal. In addition, expression of kinase-dead HER4 (but not vector) in SUM44 cells blocked the heregulin-dependent antiproliferative response (Fig. 4A). The effects of kinase-dead HER4 expression on HER2, HER3, and HER4 tyrosine phosphorylation are shown in Fig. 4B. Expression of kinase-dead HER4 did not interfere with ligand-induced HER2 or HER3 tyrosine phosphorylation. There was an apparent increase in HER4 phosphorylation, presumably due to phosphorylation of the kinase-dead receptor, which is expressed at high levels. This may result from HER2-kdHER4 heterodimers, with the HER2 providing the kinase, as occurs with EGF-dependent EGFR tyrosine phosphorylation of kinase-dead HER2. Regardless, it is clear that heregulin-dependent HER2, HER3, and HER4 tyrosine phosphorylation is insufficient to send the full HER4 signal in cells overexpressing expressing kdHER4; i.e., the antiproliferation response is attenuated. The explanation for attenuation of the HER4 signal presumably lies in the lack of specific tyrosine phosphorylation sites on HER4 phosphorylated by HER2 or, perhaps more intriguingly, the absence of activated HER4 kinase domain-engaging specific substrates (even soluble non-SH2 domain-containing substrates) that trigger the antiproliferation signal; we are currently investigating these hypotheses.
FIG. 4.
(A) Introduction of a kinase-dead HER4 construct in SUM44 cells. Full-length HER4 containing a mutation in the ATP binding domain which renders it kinase dead (kdHER4) was expressed in SUM44 cells after retroviral infection and selection with G418 (expression was confirmed by RT-PCR). SUM44-kdHER4 cells or vector control cells were plated at a density of 105 cells per well in six-well plates and grown in the presence or absence of 10 ng of heregulin β1 per ml for three medium changes (7 days), and the number of cells was counted. Error bars represent standard deviations of at least three experiments. Control cells demonstrated significant growth inhibition in response to heregulin, but this response was attenuated in cells expressing kinase-dead HER4. (B) Ligand-induced receptor phosphorylation in vector control cells or cells expressing kdHER4. Cells were treated with 10 ng of heregulin β1 per ml for 10 min, immunoprecipitated (IP) with HER2, HER3, or HER4, electrophoresed, and blotted with antiphosphotyrosine (anti-PY). Expression of kinase-dead HER4 did not affect HER2 or HER3 phosphorylation. There was an apparent increase in HER4 phosphorylation, presumably due to endogenous phosphorylation by heterodimeric partners of the kinase-dead receptor, which is expressed at high levels.
Expression of HER4 in HER4-negative cells: acquisition of antiproliferative and differentiation capability.
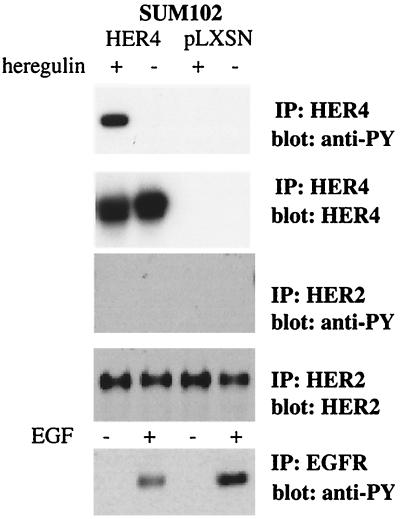
It is possible that some unique characteristic of SUM44 cells resulted in the detection of a HER4-dependent antiproliferative response. Therefore, a second model cell system was sought. SUM102 is a primary human breast cancer cell line that does not demonstrate a proliferative or antiproliferative response to heregulin (Fig. 1A), nor does it exhibit heregulin-dependent differentiation (not shown). SUM102 cells do not express HER4 (Fig. 1B). Therefore, to determine whether expression of HER4 in a HER4-negative cell line would be sufficient to induce an antiproliferative and/or differentiation response to heregulin, SUM102 cells were infected with retrovirus containing full-length HER4 or vector alone and selected for neomycin resistance. The resistant colonies grew slowly but yielded several lines. Vector-infected control cells do not express HER4, while SUM102-HER4 lines stably express HER4 that is tyrosine phosphorylated in response to heregulin (Fig. 5). EGFR phosphorylation in response to EGF is unaffected by HER4 expression. SUM102 cells express very low levels of HER2 (Fig. 1C), which is not appreciably phosphorylated in response to heregulin whether or not HER4 is expressed. SUM102 cells do not express HER3.
FIG. 5.
Stably infected SUM102 cells express HER4 that is activated by heregulin. Full-length HER4 was stably expressed in SUM102 cells, a HER4-negative human breast cancer cell line, by retroviral infection and selection for G418 resistance. HER4 expression was confirmed by Western blotting using HER4 antiserum. Vector expression was confirmed in control cells by RT-PCR of neomycin-resistant cells (data not shown). Tyrosine phosphorylation of HER4 and HER2 in response to heregulin stimulation was measured by immunoprecipitation (IP) with antibody to HER4 or HER2 and Western blotting with antiphosphotyrosine (anti-PY). Phosphorylation of EGFR in response to EGF stimulation was similarly examined. In SUM102-HER4 lines, HER4 is not constitutively activated but is activated in response to ligand. There is no appreciable phosphorylation of HER2 in either HER4-expressing or wild-type cells, and EGFR phosphorylation in response to EGF is not altered by HER4 expression.
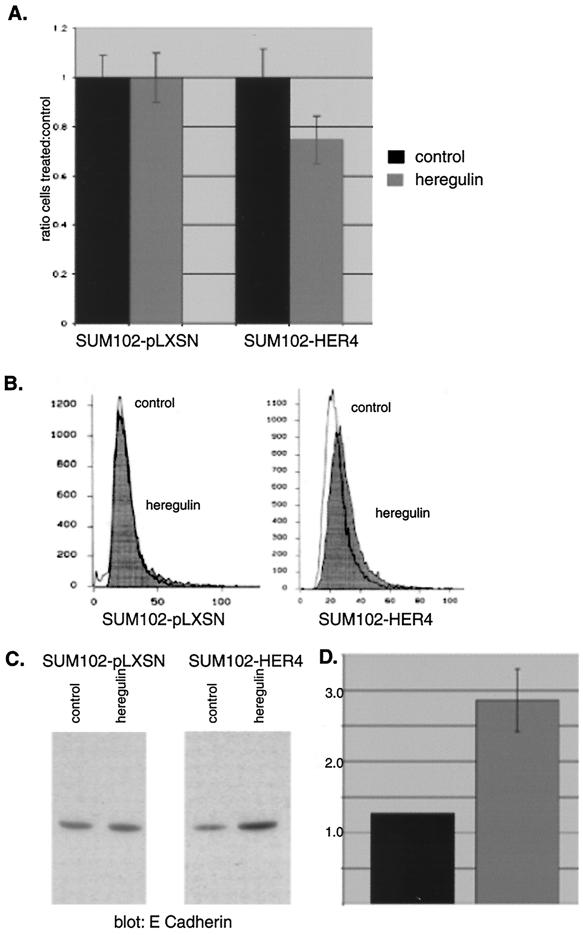
While neither parental SUM102 cells nor SUM102-pLXSN vector control cells demonstrated an antiproliferative or differentiation response to heregulin, SUM102-HER4 exhibited slowed growth in response to heregulin (Fig. 6A). In addition, SUM102-HER4 cells demonstrated increased neutral lipid production when treated with heregulin, while the parental SUM102 cells (data not shown) and SUM102-pLXSN control cells (Fig. 6B) did not. Thus, expression of HER4 provided SUM102 cells with both antiproliferative and differentiative responses to heregulin, suggesting that HER4 is essential for the differentiation response.
FIG. 6.
SUM102 antiproliferative and differentiative response to heregulin with and without HER4. (A) Antiproliferative response. SUM102-HER4 or vector control cells were plated at a density of 5 × 105 cells per well in six-well plates and grown in the presence or absence of 10 ng of heregulin β1 per ml for three medium changes (7 days), and the number of cells was counted. The ratio of number of cells grown in the presence versus the absence of ligand is shown. Error bars represent standard deviations of at least three experiments. SUM102-HER4 cells are growth inhibited with heregulin, to an extent comparable to that of SUM44 cells. Wild-type (Fig. 1A) and vector control SUM102 cells do not have an antiproliferative response to HER4. (B) Neutral lipid production. SUM102 cells expressing vector or HER4 were treated with 10 ng of heregulin per ml for 4 to 6 days and stained with Nile red. The intensity of staining was measured by flow cytometry, and histograms of control and heregulin-treated cells were overlaid. SUM102-HER4 cells have increased neutral lipid staining when treated with heregulin, comparable to SUM44 cells, while HER4-negative control cells do not. (C and D) E cadherin expression. (C) SUM102-pLXSN vector control cells or SUM102-HER4 cells were treated with 10 ng of heregulin per ml for 4 to 6 days and lysed, and Western blotting was performed with anti-E cadherin antibody. (D) Densitometry of E cadherin expression by Western blot. Values are intensities (fold), and standard deviations of at least three experiments are shown by the error bars. SUM102-HER4 but not SUM102-pLXSN demonstrated increased E cadherin expression in response to heregulin.
To further confirm that SUM102-HER4 cells were undergoing differentiation changes upon heregulin stimulation, we evaluated the expression of E cadherin, whose expression has been correlated with differentiation changes in a number of systems (reviewed in reference 51). Heregulin induced a 2.5-fold increase in expression of E cadherin in SUM102-HER4 cells but not in control cells (Fig. 6C), and this was quantified by densitometry (Fig. 6D). Thus, heregulin induces an antiproliferative response only in SUM102 cells that express HER4. The antiproliferative response is paralleled by differentiation changes, including neutral lipid production and increased E cadherin expression.
Removal of HER2 does not abolish the heregulin-dependent antiproliferative response.
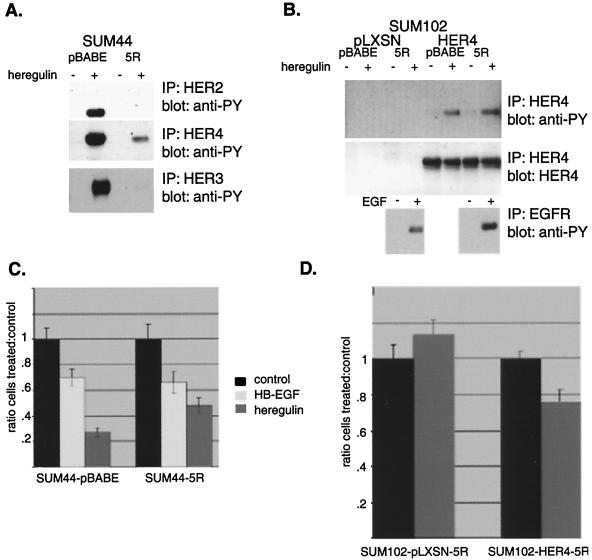
Our results in both cell lines suggested that HER4 plays a necessary role in mediating an antiproliferative and differentiation signal, but they do not answer a central question; does HER2 contribute to this response? To determine this, the capacity for HER2 signaling was removed from both SUM44 and SUM102-HER4 cells by abolishing HER2 cell surface expression. This was accomplished by sequestering HER2 in the ER by expressing single-chain anti-HER2 antibody containing an ER-targeting sequence (19). This cDNA construct, 5R, was introduced into cells after having been packaged as an amphotrophic retrovirus. Selection of infected SUM102-pLXSN, SUM102-HER4, and SUM44 cells by puromycin resistance resulted in cell lines expressing 5R in addition to HER4. This resulted in a loss of membrane-localized HER2, as determined by immunohistochemistry (data not shown), and completely abolished heregulin-dependent HER2 tyrosine phosphorylation (Fig. 7A). Consistent with reports that expression of 5R can reduce heregulin-induced HER4 phosphorylation (4), there was a reduction in heregulin-induced HER4 tyrosine phosphorylation in SUM44 cells. The HER2 single-chain ER-tagged antibody also virtually abolished heregulin-induced HER3 phosphorylation in SUM44 cells. In SUM102-HER4 cells, expression of the 5R construct did not appreciably dampen phosphorylation of the exogenously expressed HER4 (Fig. 7B), possibly because this HER4 is expressed at high levels compared with the endogenous levels of HER4 seen in SUM44 cells, and there is essentially no detectable HER2 activation in the parental line (Fig. 5). The 5R construct did not affect the ability of EGF to induce phosphorylation of EGFR. SUM102 cells do not demonstrate appreciable HER2 phosphorylation in response to heregulin (Fig. 5) or express HER3 (Fig. 1C).
FIG. 7.
The antiproliferative effect of heregulin persists even after removal of HER2 signaling. SUM44 cells and SUM102-pLXSN or SUM102-HER4 cells were infected with retrovirus containing vector alone or containing the anti-HER2 ER-tagged single-chain antibody 5R. After selection in G418, removal of HER2 from the membrane by 5R was confirmed by immunohistochemistry, demonstrating loss of HER2 membrane immunoreactivity in both (SUM44 and SUM102) 5R-containing lines (data not shown). (A) Tyrosine phosphorylation of HER2-4 in response to heregulin in SUM44 derivatives. Cells containing the 5R construct did not demonstrate heregulin-dependent HER2 tyrosine phosphorylation, as opposed to vector control cells, indicating that 5R effectively eliminates HER2 signaling in these cells. The 5R construct also inhibited heregulin-induced HER3 phosphorylation and dampened HER4 phosphorylation. IP, immunoprecipitation; anti-PY, antiphosphotyrosine. (B) Tyrosine phosphorylation of HER4 and EGFR in response to ligand stimulation in SUM102 derivatives. The 5R construct did not affect HER4 or EGFR ligand-induced phosphorylation. (C) Antiproliferative response of SUM44-5R cells. SUM44 vector control pBABE and 5R expressing cells were treated with heregulin or HB-EGF, and the proliferative response was measured as described in Materials and Methods and for Fig. 1. The absence of HER2 signaling did not alter the growth inhibitory responses of heregulin and HB-EGF. (D) Antiproliferative response to SUM102-HER4 cells. SUM102-HER4 cells or vector control cells containing 5R were treated with heregulin. Sequestration of HER2 and removal of HER2 tyrosine phosphorylation did not abolish the antiproliferative effect, and SUM102-5R cells which do not contain HER4 did not demonstrate an antiproliferative effect.
SUM44 cells expressing the pBABE vector exhibited both HB-EGF- and heregulin-dependent antiproliferative responses. Again, heregulin was more potent. Introduction of 5R and elimination of HER2 signaling did not block either ligand-dependent antiproliferative response in SUM44 cells (Fig. 7C). In the SUM102-pLXSN cells, which do not express HER4, sequestration of HER2 did not change the lack of antiproliferative response to heregulin (Fig. 7D). Furthermore, in SUM102-HER4 cells, which had acquired an antiproliferative response to heregulin by virtue of HER4 expression, sequestration of HER2 did not abolish this response. Thus, unlike HER4, HER2 is not necessary for the antiproliferative response in cells with either endogenous (SUM44) or exogenously expressed (SUM102-HER4) HER4.
DISCUSSION
In our studies of HER4 in human breast cancer cells, we found clear antiproliferative and differentiative responses to heregulin in SUM44 cells. This response correlated with heregulin-induced HER4 tyrosine phosphorylation and was induced by another HER4 ligand, HB-EGF, which activates HER4 but not the other EGFR family members in this cell line. In addition, overexpression of kinase-dead HER4 obliterated this response. The only other cell lines that demonstrated growth suppression upon treatment with heregulin, SUM185 and SUM225, also exhibited HER4 expression. HER4-negative cells did not show a heregulin-dependent antiproliferative response. To further confirm the involvement of HER4 in mediating an antiproliferative and differentiative response, we expressed HER4 in HER4-negative SUM102 cells. HER4-expressing SUM102 cells acquired an antiproliferative and differentiative response upon HER4 activation. Thus, HER4 can mediate antiproliferative and differentiative signals in human breast cancer cells.
Activation of HER4 and HER2 has been associated with a range of responses, including growth stimulation and suppression, as well as stimulation of expression of differentiation markers. The outcome depends upon the cell type, the complement of EGFR family members expressed, the level of HER2 expression, the ligand (and even the ligand isoform), and the presence of other growth factors or serum. Our aim was to specifically investigate the role of HER4 in the antiproliferative and/or differentiative response and to prove, to the extent possible, that HER4 activation alone was necessary and or sufficient to produce this response.
We hypothesized that if any EGFR family member was primarily responsible for the antiproliferative and differentiative response, HER4 was the likely candidate, since HER4 has been implicated in differentiation developmental responses in a number of settings. In the endometrium, HER4 expression and expression of HER4 ligands are increased during the secretory phase, suggesting a role in endometrial maturation (46). HER4 is critical for cardiac and neural development, as HER4 knockout mice are nonviable due to impaired cardiac and neural development (6, 18). In the mouse mammary gland, a carboxy-terminal deletion mutation of HER4 impairs postpartum lobuloalveolar development due to a lack of terminal differentiation (24). Consistent with a role in antiproliferation and differentiation, in human breast cancers HER4 expression is associated with low histological grade (47). This is in contrast to HER2, which is often associated with tumors with poorer prognostic features and outcome.
The complicated nature of EGFR family member interactions makes it difficult to discern the contribution of each member to the differentiation response. For example, the differentiation response to heregulin has alternately been attributed to HER2 and HER4, since heregulin can activate both receptors. We first implicated HER4 by using a ligand, HB-EGF, that does not activate HER2. To more definitely eliminate the contribution of HER2, we used single-chain antibodies that sequester HER2 in the ER. The antiproliferative response to heregulin was not abolished with HER2 loss. Our studies demonstrate that HER4 can mediate an antiproliferative signal but do not rule out a contribution from HER2 to a differentiative signal. This is consistent with the findings of others. In MCF7 cells, removal of surface HER2 affected heregulin-induced morphologic differentiation changes. However, HER2 was not required for heregulin effects on proliferation (4). Antisense HER2 expressed in AU565 cells caused cells to proliferate more slowly and abolished the antiproliferative and differentiation response to high concentrations of heregulin without affecting the proliferative response to low concentrations of heregulin (55). In AU565 cells, HER2 inhibitory antibodies induce differentiation, suggesting that removal of HER2 may enable a HER4 differentiation signal to predominate (3). HER4 agonist antibodies can induce a differentiation response, which is partially reversed by HER4 antagonist antibodies, but this is also seen with HER2 (9).
However, some studies of EGFR family member activation in 32D mouse myeloid cells support a proliferative function for HER4, since cells expressing HER4 alone or in combination with EGFR demonstrated a mitogenic response to stimulation with EGF or epiregulin (43, 49). Furthermore, downregulation of exogenously expressed HER4 by ribozymes decreased proliferation, suggesting that HER4 was mediating proliferative as opposed to antiproliferative or differentiative responses (48). However, others found that 32D cells expressing both HER2 and HER4 were growth stimulated by HB-EGF, whereas those expressing only HER4 had a growth inhibitory response, suggesting that HER4 may be involved in proliferative or antiproliferative signals, depending on presence of HER2 (52). Similarly, we have found that activation of an EGFR-HER4 chimera induced an antiproliferative response in 32D cells (data not shown).
Our studies conclusively support a role for HER4, in the absence of HER2, as a mediator of an antiproliferative and differentiative response in human breast cancer cell lines. Further investigations are under way to determine the downstream signal transduction pathways involved in HER4 signaling.
ACKNOWLEDGMENTS
This work was supported by P50CA58223 National Cancer Institute Breast Cancer SPORE, Breast Cancer Research Foundation, K08CA83753, and Department of Defense DAMD17-96-1-6015.
We thank Dominic Moore for statistical assistance, Lynn Dressler for immunohistochemical confirmation of HER2 sequestration by 5R, and Mark Day for helpful discussion.
REFERENCES
- 1.Bacus S S, Gudkov A V, Zelnick C R, Chin D, Stern R, Stancovski I, Peles E, Ben-Baruch N, Farbstein H, Lupu R, et al. Neu differentiation factor (heregulin) induces expression of intercellular adhesion molecule 1: implications for mammary tumors. Cancer Res. 1993;53:5251–5261. [PubMed] [Google Scholar]
- 2.Bacus S S, Huberman E, Chin D, Kiguchi K, Simpson S, Lippman M, Lupu R. A ligand for the erbB-2 oncogene product (gp30) induces differentiation of human breast cancer cells. Cell Growth Differ. 1992;3:401–411. [PubMed] [Google Scholar]
- 3.Bacus S S, Stancovski I, Huberman E, Chin D, Hurwitz E, Mills G B, Ullrich A, Sela M, Yarden Y. Tumor-inhibitory monoclonal antibodies to the HER-2/Neu receptor induce differentiation of human breast cancer cells. Cancer Res. 1992;52:2580–2589. [PubMed] [Google Scholar]
- 4.Beerli R R, Graus-Porta D, Woods-Cook K, Chen X, Yarden Y, Hynes N E. Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell specific and displays a differential requirement for ErbB-2. Mol Cell Biol. 1995;15:6496–6505. doi: 10.1128/mcb.15.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beerli R R, Hynes N E. Epidermal growth factor-related peptides activate distinct subsets of ErbB receptors and differ in their biological activities. J Biol Chem. 1996;271:6071–6076. doi: 10.1074/jbc.271.11.6071. [DOI] [PubMed] [Google Scholar]
- 6.Carraway K L., III Involvement of the neuregulins and their receptors in cardiac and neural development. Bioessays. 1996;18:263–266. doi: 10.1002/bies.950180403. [DOI] [PubMed] [Google Scholar]
- 7.Carraway K L, III, Weber J L, Unger M J, Ledesma J, Yu N, Gassmann M, Lai C. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature. 1997;387:512–516. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- 8.Chang H, Riese II D J, Gilbert W, Stern D F, McMahan U J. Ligands for ErbB-family receptors encoded by a neuregulin-like gene. Nature. 1997;387:509–512. doi: 10.1038/387509a0. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Levkowitz G, Tzahar E, Karunagaran D, Lavi S, Ben-Baruch N, Leitner O, Ratzkin B J, Bacus S S, Yarden Y. An immunological approach reveals biological differences between the two NDF/heregulin receptors. ErbB-3 and ErbB-4. J Biol Chem. 1996;271:7620–7629. [PubMed] [Google Scholar]
- 10.Cobleigh M A, Vogel C L, Tripathy D, Robert N J, Scholl S, Fehrenbacher L, Wolter J M, Paton V, Shak S, Lieberman G, Slamon D J. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 11.Culouscou J M, Plowman G D, Carlton G W, Green J M, Shoyab M. Characterization of a breast cancer cell differentiation factor that specifically activates the HER4/p180erbB4 receptor. J Biol Chem. 1993;268:18407–18410. [PubMed] [Google Scholar]
- 12.Daly J M, Jannot C B, Beerli R R, Graus-Porta D, Maurer F G, Hynes N E. Neu differentiation factor induces ErbB2 down-regulation and apoptosis of ErbB2-overexpressing breast tumor cells. Cancer Res. 1997;57:3804–3811. [PubMed] [Google Scholar]
- 13.Earp H S, Dawson T L, Li X, Yu H. Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implications for breast cancer research. Breast Cancer Res Treat. 1995;35:115–132. doi: 10.1007/BF00694752. [DOI] [PubMed] [Google Scholar]
- 14.Elenius K, Choi C J, Paul S, Santiestevan E, Nishi E, Klagsbrun M. Characterization of a naturally occurring ErbB4 isoform that does not bind or activate phosphatidyl inositol 3-kinase. Oncogene. 1999;18:2607–2615. doi: 10.1038/sj.onc.1202612. [DOI] [PubMed] [Google Scholar]
- 15.Elenius K, Paul S, Allison G, Sun J, Klagsbrun M. Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. EMBO J. 1997;16:1268–1278. doi: 10.1093/emboj/16.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ethier S P, Mahacek M L, Gullick W J, Frank T S, Weber B L. Differential isolation of normal luminal mammary epithelial cells and breast cancer cells from primary and metastatic sites using selective media. Cancer Res. 1993;53:627–635. [PubMed] [Google Scholar]
- 17.Giani C, Casalini P, Pupa S M, De Vecchi R, Ardini E, Colnaghi M I, Giordano A, Menard S. Increased expression of c-erbB-2 in hormone-dependent breast cancer cells inhibits cell growth and induces differentiation. Oncogene. 1998;17:425–432. doi: 10.1038/sj.onc.1201954. [DOI] [PubMed] [Google Scholar]
- 18.Golding J P, Trainor P, Krumlauf R, Gassmann M. Defects in pathfinding by cranial neural crest cells in mice lacking the neuregulin receptor ErbB4. Nat Cell Biol. 2000;2:103–109. doi: 10.1038/35000058. [DOI] [PubMed] [Google Scholar]
- 19.Graus-Porta D, Beerli R R, Hynes N E. Single-chain antibody-mediated intracellular retention of ErbB-2 impairs Neu differentiation factor and epidermal growth factor signaling. Mol Cell Biol. 1995;15:1182–1191. doi: 10.1128/mcb.15.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guy P M, Platko J V, Cantley L C, Cerione R A, Carraway K L., III Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci USA. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haffty B G, Brown F, Carter D, Flynn S. Evaluation of HER-2 neu oncoprotein expression as a prognostic indicator of local recurrence in conservatively treated breast cancer: a case-control study. Int J Radiat Oncol Biol Phys. 1996;35:751–757. doi: 10.1016/0360-3016(96)00150-2. [DOI] [PubMed] [Google Scholar]
- 22.Harari D, Tzahar E, Romano J, Shelly M, Pierce J H, Andrews G C, Yarden Y. Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene. 1999;18:2681–2689. doi: 10.1038/sj.onc.1202631. [DOI] [PubMed] [Google Scholar]
- 23.Holmes W E, Sliwkowski M X, Akita R W, Henzel W J, Lee J, Park J W, Yansura D, Abadi N, Raab H, Lewis G D, et al. Identification of heregulin, a specific activator of p185erbB2. Science. 1992;256:1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 24.Jones F E, Welte T, Fu X Y, Stern D F. ErbB4 signaling in the mammary gland is required for lobuloalveolar development and Stat5 activation during lactation. J Cell Biol. 1999;147:77–88. doi: 10.1083/jcb.147.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komurasaki T, Toyoda H, Uchida D, Morimoto S. Epiregulin binds to epidermal growth factor receptor and ErbB-4 and induces tyrosine phosphorylation of epidermal growth factor receptor, ErbB-2, ErbB-3 and ErbB-4. Oncogene. 1997;15:2841–2848. doi: 10.1038/sj.onc.1201458. [DOI] [PubMed] [Google Scholar]
- 26.Krane I M, Leder P. NDF/heregulin induces persistence of terminal end buds and adenocarcinomas in the mammary glands of transgenic mice. Oncogene. 1996;12:1781–1788. [PubMed] [Google Scholar]
- 27.Lewis G D, Lofgren J A, McMurtrey A E, Nuijens A, Fendly B M, Bauer K D, Sliwkowski M X. Growth regulation of human breast and ovarian tumor cells by heregulin: evidence for the requirement of ErbB2 as a critical component in mediating heregulin responsiveness. Cancer Res. 1996;56:1457–1465. [PubMed] [Google Scholar]
- 28.Mendelsohn J, Fan Z. Epidermal growth factor receptor family and chemosensitization. J Natl Cancer Inst. 1997;89:341–343. doi: 10.1093/jnci/89.5.341. [DOI] [PubMed] [Google Scholar]
- 29.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–982. , 984–986, 989–9890. [PMC free article] [PubMed] [Google Scholar]
- 30.Olsen J C, Sechelski J. Use of sodium butyrate to enhance production of retroviral vectors expressing CFTR cDNA. Hum Gene Ther. 1995;6:1195–1202. doi: 10.1089/hum.1995.6.9-1195. [DOI] [PubMed] [Google Scholar]
- 31.Paik S, Hazan R, Fisher E R, Sass R E, Fisher B, Redmond C, Schlessinger J, Lippman M E, King C R. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8:103–112. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- 32.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peles E, Bacus S S, Koski R A, Lu H S, Wen D, Ogden S G, Levy R B, Yarden Y. Isolation of the neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992;69:205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- 34.Peles E, Yarden Y. Neu and its ligands: from an oncogene to neural factors. Bioessays. 1993;15:815–824. doi: 10.1002/bies.950151207. [DOI] [PubMed] [Google Scholar]
- 35.Pinkas-Kramarski R, Shelly M, Guarino B C, Wang L M, Lyass L, Alroy I, Alimandi M, Kuo A, Moyer J D, Lavi S, Eisenstein M, Ratzkin B J, Seger R, Bacus S S, Pierce J H, Andrews G C, Yarden Y, Alamandi M. ErbB tyrosine kinases and the two neuregulin families constitute a ligand-receptor network. Mol Cell Biol. 1998;18:6090–6101. doi: 10.1128/mcb.18.10.6090. . (Erratum, 18:7602.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plowman G D, Green J M, Culouscou J M, Carlton G W, Rothwell V M, Buckley S. Heregulin induces tyrosine phosphorylation of HER4/p180erbB4. Nature. 1993;366:473–475. doi: 10.1038/366473a0. [DOI] [PubMed] [Google Scholar]
- 37.Riese D J, II, Bermingham Y, van Raaij T M, Buckley S, Plowman G D, Stern D F. Betacellulin activates the epidermal growth factor receptor and erbB-4, and induces cellular response patterns distinct from those stimulated by epidermal growth factor or neuregulin-beta. Oncogene. 1996;12:345–353. [PubMed] [Google Scholar]
- 38.Riese D J, II, Komurasaki T, Plowman G D, Stern D F. Activation of ErbB4 by the bifunctional epidermal growth factor family hormone epiregulin is regulated by ErbB2. J Biol Chem. 1998;273:11288–11294. doi: 10.1074/jbc.273.18.11288. [DOI] [PubMed] [Google Scholar]
- 39.Riese D J, II, Stern D F. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 40.Rodes J F, Berreur-Bonnenfant J, Tremolieres A, Brown S C. Modulation of membrane fluidity and lipidic metabolism in transformed rat fibroblasts induced by the sesquiterpenic hormone farnesylacetone. Cytometry. 1995;19:217–225. doi: 10.1002/cyto.990190305. [DOI] [PubMed] [Google Scholar]
- 41.Sartor C I, Dziubinski M L, Yu C L, Jove R, Ethier S P. Role of epidermal growth factor receptor and STAT-3 activation in autonomous proliferation of SUM-102PT human breast cancer cells. Cancer Res. 1997;57:978–987. [PubMed] [Google Scholar]
- 42.Sepp-Lorenzino L, Eberhard I, Ma Z, Cho C, Serve H, Liu F, Rosen N, Lupu R. Signal transduction pathways induced by heregulin in MDA-MB-453 breast cancer cells. Oncogene. 1996;12:1679–1687. [PubMed] [Google Scholar]
- 43.Shelly M, Pinkas-Kramarski R, Guarino B C, Waterman H, Wang L M, Lyass L, Alimandi M, Kuo A, Bacus S S, Pierce J H, Andrews G C, Yarden Y. Epiregulin is a potent pan-ErbB ligand that preferentially activates heterodimeric receptor complexes. J Biol Chem. 1998;273:10496–10505. doi: 10.1074/jbc.273.17.10496. [DOI] [PubMed] [Google Scholar]
- 44.Slamon D J, Clark G M, Wong S G, Levin W J, Ullrich A, McGuire W L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 45.Soltoff S P, Carraway III K L, Prigent S A, Gullick W G, Cantley L C. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol. 1994;14:3550–3558. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srinivasan R, Benton E, McCormick F, Thomas H, Gullick W J. Expression of the c-erbB-3/HER-3 and c-erbB-4/HER-4 growth factor receptors and their ligands, neuregulin-1 alpha, neuregulin-1 beta, and betacellulin, in normal endometrium and endometrial cancer. Clin Cancer Res. 1999;5:2877–2883. [PubMed] [Google Scholar]
- 47.Srinivasan R, Gillett C E, Barnes D M, Gullick W J. Nuclear expression of the c-erbB-4/HER-4 growth factor receptor in invasive breast cancers. Cancer Res. 2000;60:1483–1487. [PubMed] [Google Scholar]
- 48.Tang C K, Concepcion X Z, Milan M, Gong X, Montgomery E, Lippman M E. Ribozyme-mediated down-regulation of ErbB-4 in estrogen receptor-positive breast cancer cells inhibits proliferation both in vitro and in vivo. Cancer Res. 1999;59:5315–5322. [PubMed] [Google Scholar]
- 49.Tang C K, Goldstein D J, Payne J, Czubayko F, Alimandi M, Wang L M, Pierce J H, Lippman M E. ErbB-4 ribozymes abolish neuregulin-induced mitogenesis. Cancer Res. 1998;58:3415–3422. [PubMed] [Google Scholar]
- 50.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin B J, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vleminckx K, Kemler R. Cadherins and tissue formation: integrating adhesion and signaling. Bioessays. 1999;21:211–220. doi: 10.1002/(SICI)1521-1878(199903)21:3<211::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 52.Wang L M, Kuo A, Alimandi M, Veri M C, Lee C C, Kapoor V, Ellmore N, Chen X H, Pierce J H. ErbB2 expression increases the spectrum and potency of ligand-mediated signal transduction through ErbB4. Proc Natl Acad Sci USA. 1998;95:6809–6814. doi: 10.1073/pnas.95.12.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waterman H, Sabanai I, Geiger B, Yarden Y. Alternative intracellular routing of ErbB receptors may determine signaling potency. J Biol Chem. 1998;273:13819–13827. doi: 10.1074/jbc.273.22.13819. [DOI] [PubMed] [Google Scholar]
- 54.Wen D, Peles E, Cuppies R, Siggs S, Bacus S, Luo Y, Trail G, Hu S, Silbiger S, ben Levy R, Koski R, Lu H, Yarden Y. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- 55.Yoo J Y, Hamburger A W. Changes in heregulin beta1 (HRGbeta1) signaling after inhibition of ErbB-2 expression in a human breast cancer cell line. Mol Cell Endocrinol. 1998;138:163–171. doi: 10.1016/s0303-7207(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 56.Zhang D, Sliwkowski M X, Mark M, Frantz G, Akita R, Sun Y, Hillan K, Crowley C, Brush J, Godowski P J. Neuregulin-3 (NRG3): a novel neural tissue-enriched protein that binds and activates ErbB4. Proc Natl Acad Sci USA. 1997;94:9562–9567. doi: 10.1073/pnas.94.18.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]