Abstract
Growth faltering under 5 years of age is unacceptably high worldwide, and even more children, while not stunted, fail to reach their growth potential. The time between conception and 2 years of age is critical for development. The period from 6 to 23 months, when complementary foods are introduced, coincides with a time when growth faltering and delayed neurocognitive developments are most common. Fortunately, this is also the period when diet exercises its greatest influence. Growing up in an adverse environment, with a deficient diet, as typically seen in low‐ and middle‐income countries (LMICs), hampers growth and development of children and prevents them from realising their full developmental and economic future potential. Sufficient nutrient availability and utilisation are paramount to a child's growth and development trajectory, especially in the period after breastfeeding. This review highlights the importance of essential amino acids (EAAs) in early life for linear growth and, likely, neurocognitive development. The paper further discusses signalling through mammalian target of rapamycin complex 1 (mTORC1) as one of the main amino acid (AA)‐sensing hubs and the master regulator of both growth and neurocognitive development. Children in LMICs, despite consuming sufficient total protein, do not meet their EAA requirements due to poor diet diversity and low‐quality dietary protein. AA deficiencies in early life can cause reductions in linear growth and cognition. Ensuring AA adequacy in diets, particularly through inclusion of nutrient‐dense animal source foods from 6 to 23 months, is strongly encouraged in LMICs in order to compensate for less than optimal growth during complementary feeding.
Keywords: animal source protein, arginine, cognition, growth faltering, leucine, mTORC1, tryptophan
Key messages.
Many children in LMICs, who experience growth faltering, have inadequate levels of essential amino acids partly attributable to poor protein quality of their diets. Inclusion of animal source foods is encouraged to improve the quality of predominantly cereal/vegetal‐based diets.
Protein cannot be regarded as a single, homogeneous nutrient.
Mechanistically, inadequate intake of EAAs is likely to negatively influence linear growth and neurocognition via the mTORC1 pathway.
Further in‐depth research into AA intakes and concentrations in infants and young children presenting with varying physiological states across a wide range of geographical regions can provide valuable insights for designing appropriate nutritional strategies.
1. INTRODUCTION
Physical growth is globally recognised as the best indicator of well‐being in children as well as an accurate reflection for health inequalities faced by populations (de Onis, 2017). The unacceptably high burden of stunting or linear growth faltering is a stark sign that infants and young children across the globe are not growing well, both physically and mentally (UNICEF, 2019). In 2018, at a global level, an estimated 149 million children under 5 were stunted, having a height‐for‐age z‐score (HAZ) below −2 standard deviations (UNICEF, 2019).
Stunting is pervasively associated with and likely, in part, a cause for increased mortality, morbidity, impaired cognitive and motor development, poor school performance, poor intellectual achievement, reduced economic productivity and diminished earnings (Engle et al., 2011; Hoddinott et al., 2013). As linear growth and neurocognitive development share some of the underlying factors (e.g., suboptimal nutrition, repeated infections, inadequate access to care and lack of stimulation), it seems plausible that biological mechanisms exist that simultaneously lead to linear growth faltering and deficits in neurodevelopment in response to continued exposure to one or more of these factors (Leroy & Frongillo, 2019; Prado et al., 2019; Prado & Dewey, 2014).
Nutrient availability and/or utilisation at the cellular level plays a critical role in driving a child's trajectory of linear growth (de Onis, 2017; MAL‐ED Network Investigators, 2017) and neurocognitive development (Prado et al., 2019; Prado & Dewey, 2014). For the first 6 months of life, the World Health Organization (WHO, 2001) recommends exclusive breastfeeding to support optimal growth and development. For infants and young children aged 6–23 months, it is recommended to continue breastfeeding and complement it with foods that are diverse and sufficiently energy and nutrient dense to promote optimal growth (Bhutta, Das, et al., 2013; Horta & Victora, 2013; WHO, 2003). This is because, after 6 months, breastmilk alone is insufficient to meet a child's nutrient needs (UNICEF, 2016). The high nutritional demand coupled with limited quality and quantity of complementary foods during the first 2 years of life are key drivers of suboptimal growth, coinciding with the time of peak stunting prevalence in developing countries (Dewey & Adu‐Afarwuah, 2008; Shrimpton et al., 2001). A comprehensive exploration of the diets of 76,641 children ages 6–23 months in 42 countries from five geographic regions reported diminished diet diversity and low consumption of nutrient‐rich foods. It was found that, on average, children consumed only 2.81 out of 7 food groups in the 24 h prior to the survey and only 39% of the children consumed dairy (Choudhury et al., 2019). Among the five regions, Sub‐Saharan Africa had the lowest diet diversity, followed by South and South‐East Asia; the greatest diet diversity was seen in Latin American and the Caribbean, with on average four out of seven food groups consumed. The negative effects of these early life nutritional deficiencies on linear growth as well as neuronal development, synaptic pruning and connectivity within regions of the brain associated with both motor and attentional functioning have been reported in literature (Cusick & Georgieff, 2012; Prado & Dewey, 2014; Sudfeld et al., 2015). Until recently, the window of possible recovery from stunting or linear growth faltering was believed to be up to 24 months of age. Learnings from the COHORTS study, from rural Gambia (Prentice et al., 2013) and from the Young Lives study in Ethiopia, Peru, India and Vietnam (Fink & Rockers, 2014; Lundeen et al., 2014; Schott et al., 2013), however, suggest that possibilities for catch‐up growth may extend beyond 24 months, offering additional opportunities for interventions that may yield life cycle and intergenerational effects (Prentice et al., 2013; Suryawan et al., 2021).
A recent systematic review and meta‐analysis reported a small, but significant, positive effect of post‐natal macronutrient and micronutrient supplementation on linear growth and neurodevelopment scores (language, motor and socioemotional) in children under 5 years in low‐ and middle‐income countries (LMICs) (Prado et al., 2019). Modest effects on neurodevelopment, but not linear growth, have been reported with interventions with multiple micronutrients, whereas single micronutrient supplementation has been shown to have a small impact on linear growth but not neurodevelopment (Bhutta, Salam, & Das, 2013; Dulal et al., 2018; Prado et al., 2019; Ramakrishnan et al., 2009; Stammers et al., 2015). Overall, there is very limited evidence from intervention studies on the impact of macronutrient supplementation, and more specifically protein or amino acid (AA) supplementation, on linear growth and/or neurodevelopment in children at community level. Although no consistent benefit of additional protein on linear growth has been reported, it is important to note that these studies did not quantify protein intakes, protein quality or any biomarkers of protein status (Arsenault & Brown, 2017). The persistent global burden of linear growth faltering and delayed development in children under 5 years in LMICs suggests a need to (re‐)evaluate some fundamental dietary nutrients (Semba, Shardell, Sakr Ashour, et al., 2016) that may be lacking or inadequately represented in the dietary patterns of this vulnerable population.
In this review, we present evidence on the critical importance of protein, more specifically essential amino acids (EAAs), for linear growth and neurocognitive development in children under 5 years (0–59 months) of age in LMICs and suggest areas for future nutrition research.
2. METHODS
An exhaustive search for eligible studies was performed in Scopus, PubMed and Google Scholar. Searches were carried out without restrictions to date of publication, with main search terms including ‘infant, newborn, child’, ‘diet, dietary protein, protein quality, amino acids’, ‘protein status, amino acid status’, ‘linear growth, stunting, height’ and ‘child development, cognitive development, neurocognition’. Proper Boolean operators ‘AND’ and ‘OR’ were included to ensure comprehensiveness. This was supplemented with electronic searching of reference lists and ‘related articles’ on PubMed. Studies were eligible if their primary focus was on protein nutriture in linear growth and neurocognition in infants and young children (0–59 months) from LMIC. We excluded articles on protein in pregnancy or later in childhood, protein in medical conditions or disorders, all other forms of malnutrition and non‐LMIC populations.
3. RESULTS
3.1. EAA deficiency is common among children in developing countries
Historically, it has been assumed that children in developing countries receive adequate dietary protein (Semba, 2016). This was largely based on the reference of protein as a homogenous nutrient needed in the diet in a certain amount, not taking into account that proteins are structurally diverse macromolecules made up of various amounts of AAs that are largely not always interconvertible (Young, 2000). A steady supply of AAs is essential to ensure all body proteins can be assembled as well as execute their vital functions for optimal growth, development and health (Millward, 2003). Nine AAs (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine) cannot be synthesised in the body and need to be acquired exclusively from dietary sources (WHO & FAO, 2007). The nutritional value of the dietary protein, in effect, relates to the provision of these EAAs to the body. Therefore, assessing adequacy of protein intakes should take into account the content, pattern and bioavailability of the EAAs. Another factor often overlooked is the physiological state of the population. Infants and young children move between a healthy maintenance state, an acute routine infectious state and a rapid catch‐up growth phase after a period of infection or food insecurity, the latter two states being characterised by twofold to fourfold increased AA requirements (Reeds et al., 1994; Shivakumar et al., 2020). The relevance of these factors was reinforced in the analysis of national level data from 180 countries by Ghosh and colleagues. After correcting for EAA composition, digestibility, energy deficit and infections, the authors reported up to 50% prevalence of protein inadequacy with lysine as the primary limiting AA (Ghosh et al., 2012).
In LMICs where the diets of infants and young children are largely dominated by staple foods like rice, wheat, maize (corn), millet, sorghum, roots and tubers (Dewey, 2013; FAO, 2019; Lee, 2014), there is a high likelihood of inadequate intake of EAAs and utilisable dietary protein to support overall growth. To illustrate, Table 1 lists the dry weight percentage of protein and quantity of digestible AAs per gram of protein, based on Digestible Indispensable Amino Acid Score (DIAAS) analyses, in 21 of the most consumed protein sources around the world along with the daily protein and AA requirements for a 12‐month‐old child weighing 9.5 kg for different physiological states including environmental enteric dysfunction (EED). EED is a subclinical disorder of the gut and an important underlying cause of growth faltering, characterised by reduced villus height, increased crypt depth and lymphocyte infiltration, resulting in impaired absorption and increased small intestinal permeability (Mutasa et al., 2021). Assuming the child is consuming the minimum required amount of protein with approximately 80% coming from grain, cereal, tuber or root, and the remaining from legumes, nuts or seeds, it can be reasonably expected that the intakes of AAs will be inadequate to meet the requirements of the different physiological states. As an example, consider a few common diets, none with animal source foods: an East African diet of 80% cassava and 20% common bean will not adequately meet most of the daily EAA requirements. In contrast, a West African diet of 80% maize and 20% cowpea and a South Asian diet of 80% rice and 20% lentils will meet the AA requirements for both a healthy child and child with EED but not for catch‐up growth. Modest amounts of protein from animal source foods can address these dietary limitations as these are the best sources of EAAs and are known to have high digestibility as well as biological value. It should be noted that the FAO/WHO estimates of EAA requirements for infants are based on the assumption that human milk intake from a healthy well‐nourished mother provides optimal protein intake for an infant (WHO & FAO, 2007). For children 12–59 months of age, estimates are based on a factorial computation (Pillai & Kurpad, 2012; WHO & FAO, 2007) and reflect the physiological needs obtained under ideal conditions (Uauy, 2013). These estimates do not consider the added requirements of catch‐up growth (Pillai & Kurpad, 2012), or the high infectious disease burden common among children in developing countries (Uauy, 2013). Even a modest energy deficit, as often found in LMICs, may increase protein needs (Pillai et al., 2015; Uauy, 2013; Uauy et al., 2015). There is therefore a need to re‐evaluate the EAA requirements for infants and young children in developing countries. In LMICs, less than one in five children aged 6–23 months get animal source foods in their diets (Aguayo, 2017), whereas most children consume only one animal source food serving or less per day (White et al., 2017). Children consuming food with inadequate amounts of animal source protein are reported to have greater incidences of stunting (Arsenault & Brown, 2017; Grasgruber et al., 2016; Headey et al., 2018; Moradi, 2010). This is also confirmed in an analysis of 74,548 children aged 6–23 months from 39 Demographic and Health Surveys, which shows that children who did not consume any animal source foods (eggs, meat and dairy) had 1.44 higher odds of being stunted compared with those who reported consuming animal source foods on the day prior to the survey (Krasevec et al., 2017). Interestingly, among the various animal source foods, evidence indicates a stronger favourable role for dairy protein in promoting linear growth in children from LMICs and developed countries (Allen, 2013; Michaelsen, 2013; Thomas et al., 2020). For nutritional interventions to be effective, multiple factors, including total energy content and the micronutrient and macronutrient composition, need to be considered. Optimally, combinations of plant‐based and animal source foods could be developed to provide effective and sustainable nutritional support that is both culturally acceptable and affordable.
Table 1.
Per cent of protein and quantity of digestible amino acids per gram of protein of the most consumed protein sources around the world along with the daily protein and AA requirements
| Prot (g) | His (mg) | Ile (mg) | Leu (mg) | Lys (mg) | Thr (mg) | Val (mg) | SAA (mg) | AAA (mg) | Trp (mg) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Requirements (based on a 12‐month‐old, weight of 9.5 kg) | FAO requirement for 1–2 years old (Shivakumar et al., 2020) | 8.2 | 142.5 | 256.5 | 513.0 | 427.5 | 218.5 | 342.0 | 209.0 | 380.0 | 60.8 |
| Adapted to account for 15% loss due to EED a | 9.4 | 163.9 | 295.0 | 590.0 | 491.6 | 251.3 | 393.3 | 240.4 | 437.0 | 69.9 | |
| Catch‐up growth b (Owino et al., 2016; Semba, Shardell, Trehan, et al., 2016; Shivakumar et al., 2020) | 26.79 | 627.0 | 902.5 | 1881.0 | 1738.5 | 978.5 | 1235.0 | 836.0 | 1681.5 | 275.5 |
| Digestible AA content (mg/g protein) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prot (%) | His (mg) | Ile (mg) | Leu (mg) | Lys (mg) | Thr (mg) | Val (mg) | SAA (mg) | AAA (mg) | Trp (mg) | ||
| Cereals and grains | Maize (20316) | 5.7 | 25.6 | 30.8 | 112.8 | 21.4 | 28.6 | 43.1 | 33.3 | 78.5 | 5.5 |
| Rice (20061) | 5.4 | 24.8 | 30.8 | 66.4 | 32.0 | 28.9 | 51.5 | 38.6 | 87.5 | 10.8 | |
| Wheat (20072) | 11.6 | 21.5 | 33.4 | 63.7 | 23.6 | 26.1 | 39.7 | 38.6 | 73.0 | 11.5 | |
| Sorghum (20067) | 8.8 | 18.8 | 35.5 | 123.5 | 21.1 | 27.4 | 45.4 | 23.0 | 71.0 | 10.2 | |
| Millet (20031) | 9.8 | 18.8 | 31.6 | 100.3 | 14.4 | 21.4 | 34.1 | 30.6 | 73.0 | 10.0 | |
| Tubers or roots | Cassava (11134) | 2.1 | 14.6 | 14.9 | 23.2 | 29.8 | 16.9 | 22.6 | 26.4 | 41.9 | 12.4 |
| Sweet potatoes (11510) | 2.9 | 17.1 | 31.2 | 53.8 | 37.7 | 45.8 | 48.2 | 26.8 | 89.8 | 16.4 | |
| Yams (11602) | 4.7 | 21.3 | 28.1 | 36.8 | 38.5 | 19.9 | 24.5 | 35.3 | 44.6 | 6.4 | |
| Potato (11413) | 6.1 | 20.9 | 38.6 | 56.1 | 53.3 | 34.9 | 45.4 | 21.4 | 70.8 | 13.3 | |
| Legumes, nuts or seeds | Cowpea (16062) | 18.3 | 25.1 | 31.3 | 59.0 | 54.8 | 28.2 | 35.3 | 18.7 | 69.8 | 8.5 |
| Pigeon pea (16101) | 12.6 | 25.3 | 16.6 | 42.8 | 46.3 | 18.7 | 21.6 | 15.0 | 61.3 | 5.7 | |
| Peanuts (16087) | 19.9 | 22.7 | 29.2 | 55.7 | 21.9 | 24.3 | 34.0 | 18.6 | 83.2 | 7.0 | |
| Chickpeas (16056) | 15.6 | 21.3 | 35.1 | 56.9 | 40.4 | 27.1 | 32.1 | 17.4 | 60.0 | 7.4 | |
| Lentils (16069) | 19.7 | 28.2 | 27.9 | 54.3 | 55.4 | 25.8 | 32.4 | 15.6 | 83.9 | 6.6 | |
| Soybeans (16111) | 38.1 | 22.6 | 41.6 | 69.2 | 57.7 | 35.3 | 42.4 | 23.5 | 79.1 | 11.4 | |
| Common bean (16027) | 17.4 | 17.5 | 30.9 | 55.1 | 57.0 | 31.1 | 32.4 | 19.2 | 55.9 | 8.8 | |
| Roasted sesame (12024) | 14.6 | 25.6 | 37.4 | 67.4 | 26.3 | 34.5 | 48.6 | 47.0 | 85.4 | 17.9 | |
| Animal source foods | Chicken (05126) | 28.0 | 28.5 | 46.9 | 66.7 | 81.5 | 39.7 | 44.6 | 37.2 | 78.3 | 10.8 |
| Egg (01129) | 11.4 | 20.1 | 46.3 | 75.2 | 63.9 | 46.1 | 53.0 | 47.4 | 94.4 | 10.8 | |
| Fish (15261) | 18.3 | 19.9 | 43.1 | 72.6 | 83.8 | 43.5 | 43.5 | 36.8 | 70.3 | 9.5 | |
| Powdered whole milk (01090) | 23.4 | 34.4 | 52.6 | 93.1 | 72.2 | 41.5 | 59.6 | 32.2 | 92.7 | 13.1 | |
Note: Protein percentages were obtained from the USDA food composition database (https://fdc.nal.usda.gov); AAs are presented as DIAAS corrected values.
Abbreviations: AA, amino acid; AAA, aromatic AA; His, histidine; EED, environmental enteric dysfunction; Ile, isoleucine; Leu, leucine; Lys, lysine; Prot, protein; SAA, sulfur AA; Thr, threonine; Trp, tryptophan; Val, valine.
Cumulative needs based on age and malabsorption due to gut permeability and EED.
Catch‐up growth for severe wasting based on the preferred weight gain of 10 g/kg/day.
3.2. AA signatures of linear growth (faltering) and neurocognition
Although the staples and dietary practices may differ across geographic locations, recent insights from the Peru, Bangladesh and Tanzania sites of the Malnutrition and Enteric Disease (MAL‐ED) birth cohort unveil a consistent age‐related metabolic signature (in urine and plasma) for a developing infant, suggesting a common metabolic maturation process (Giallourou et al., 2020). A decline in plasma tryptophan and glutamine with age, and an increase in the urinary excretion of gut bacterial–host cometabolites of the AAs tryptophan (3‐indoxyl sulfate), tyrosine (4‐cresol sulfate and 4‐hydroxyphenylacetate) and phenylalanine (phenylacetylglutamine), was reported (Giallourou et al., 2020). Evidence suggests that these signatures diverge based on the infants' growth trajectories.
In growth‐constrained children, the aforementioned urinary metabolites are excreted in greater amounts than in their healthy peers (Mayneris‐Perxachs et al., 2016; Mayneris‐Perxachs & Swann, 2019), suggesting a metabolic immaturity, which can be seen from as early as 3 months of age until at least the second year of life (Giallourou et al., 2020). In addition, the higher excretion of N‐methyl‐2‐pyridone‐5‐carboxamide (2‐PY) and N‐methyl nicotinic acid, downstream metabolites of kynurenine, by stunted children reflects a greater oxidation of tryptophan to kynurenine (Mayneris‐Perxachs et al., 2016). Elevated kynurenine and the kynurenine:tryptophan ratio are markers for increased indoleamine 2,3‐dioxygenase 1 (IDO1) enzyme activity in response to infection and inflammation (Mayneris‐Perxachs et al., 2016).
Perturbations in AA levels in stunted compared with healthy children are corroborated by studies reporting plasma AA levels (Kosek et al., 2016; Tessema et al., 2018) or metabolic phenotypes (Kumar et al., 2018; Moreau et al., 2019; Ordiz et al., 2019; Semba, Shardell, Sakr Ashour, et al., 2016). Semba, Shardell, Sakr Ashour, et al. (2016) reported 10%–20% lower concentrations of all nine EAAs in stunted Malawian children compared with nonstunted children. Additionally, stunted children exhibited significantly lower concentrations of conditionally EAAs: arginine, glycine and glutamine (Semba, Shardell, Sakr Ashour, et al., 2016). In 9‐month‐old children in Bangladesh, poor linear growth was associated with low plasma tryptophan, valine, threonine and total EAAs (Moreau et al., 2019). At 24 months, stunted Bangladeshi children had lower levels of tryptophan, lysine, threonine, arginine, total EAAs, tryptophan/neutral AA ratio and two non‐EAAs (hydroxyproline and α‐aminobutyric acid) compared with healthy children (Kumar et al., 2018). Studies from Peru, Tanzania and Ethiopia further support the relationship between stunting and low concentrations of tryptophan (Kosek et al., 2016; Tessema et al., 2018) and lysine (Tessema et al., 2018). A direct correlation between length (height) velocity and several AAs (glycine, proline, serine, isoleucine and threonine, Ordiz et al., 2019; lysine, leucine, arginine and α‐aminobutyric acid, Moreau et al., 2019; and valine, Moreau et al., 2019; Ordiz et al., 2019) has also been suggested. When compared with an age‐matched, nonstunted German population, Malawian infants with linear growth velocity z‐score < 0 had lower concentrations of lysine, proline, tryptophan, tyrosine and valine (Ordiz et al., 2019).
There is limited understanding on the AA signatures associated with neurocognition (Moreau et al., 2019). A recent study in Bangladeshi infants showed an association for threonine with improved neurocognitive outcomes measured as full‐scale intelligence quotient (IQ), whereas kynurenine and the kynurenine:tryptophan ratio were associated with poor IQ outcomes (Moreau et al., 2019), indicative of an influence of infection and inflammation.
The low levels of EAAs in children have been attributed to poor protein quality of the diets (Giallourou et al., 2020; Semba, Shardell, Sakr Ashour, et al., 2016), reduced capacity of the gut microbiome to produce EAAs (Kumar et al., 2018) and/or functional modulation of the gut microbiome towards increased proteolytic activity (Giallourou et al., 2020; Mayneris‐Perxachs et al., 2016; Mayneris‐Perxachs & Swann, 2019). Such low concentrations of EAAs can have potential adverse consequences for child growth and development, because these processes are regulated by the AA‐sensing mammalian target of rapamycin complex 1 (mTORC1) pathway.
3.3. AAs are key drivers for mTORC1, the master regulator of growth and development
The mammalian (or mechanistic) target of rapamycin (mTOR) is a master growth regulator (Laplante & Sabatini, 2012) that functions as a signal integrator combining regulatory inputs from nutrients, growth factors, energy levels and stress signals (Efeyan et al., 2012). The mTOR regulator forms two multiprotein complexes, mTORC1 and mTORC2, which are composed of discrete protein binding partners to regulate cell growth, motility and metabolism. These complexes are sensitive to distinct stimuli, as mTORC1 is sensitive to nutrients whereas mTORC2 is regulated via phosphoinositide 3‐kinase (PI3K) and growth factor signalling. EAAs provoke a conformation of mTORC1 that allows for linear growth; without these AAs, the switch is off, and growth does not occur. When activated, mTORC1 promotes anabolic processes such as protein, lipid and nucleotide synthesis and inhibits catabolic processes such as autophagy (Aylett et al., 2016; Laplante & Sabatini, 2012). The mTORC1 pathway is exquisitely sensitive to AA availability (Figure 1) (Jewell et al., 2013; Laplante & Sabatini, 2012). In their absence, other signals, such as growth factors and energy status, cannot overcome the lack of AAs to activate mTORC1 (Bar‐Peled & Sabatini, 2014; Jewell et al., 2013). Under conditions of AA deficiency, mTORC1 is inactive, lipid and protein synthesis are suppressed and complexes involved in autophagy are upregulated (Laplante & Sabatini, 2012; Semba, Trehan, Gonzalez‐Freire, et al., 2016). The other major regulatory mechanism for AA sensing is the general control nonderepressible 2 (GCN2) pathway, activated by the lack of AAs (Lehman et al., 2015; Ye et al., 2015). GCN2 activation leads to attenuated translation of mRNAs (Bar‐Peled & Sabatini, 2014), initiation of autophagy and growth arrest (Lehman et al., 2015; Ye et al., 2015). The GCN2 pathway can inactivate and lead to sustained suppression of mTORC1 (Ye et al., 2015).
Figure 1.
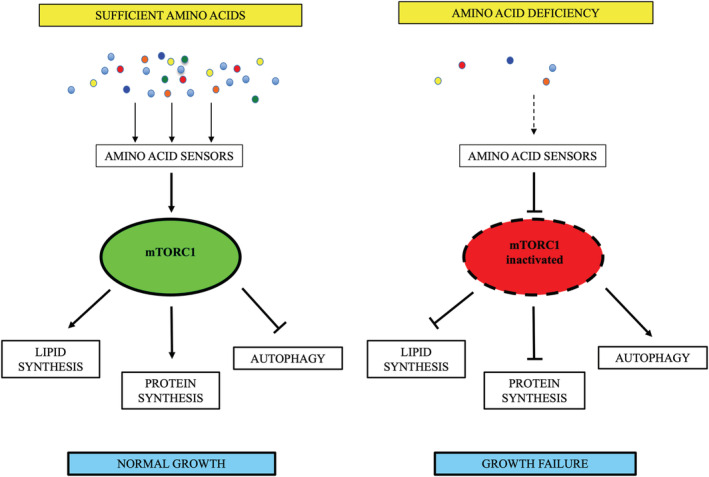
Amino acid sensing by mammalian target of rapamycin complex 1 (mTORC1)
mTORC1 plays an important role in the most rapid phase of childhood growth and development (Figure 2). The elongation of tubular bones occurs at the growth plate through endochondral ossification, a process in which cartilage is replaced by bone (Mackie et al., 2011). The rate of chondrogenesis determines the height of children (Baron et al., 2015) and is critically controlled by mTORC1 (Chen & Long, 2014; Phornphutkul et al., 2008). mTORC1 stimulates chondrocyte growth, synthesis of extracellular matrix and transition of chondrocytes to a hypertrophic state (Kim et al., 2009), whereas direct mTORC1 inhibition with rapamycin reduces growth of long bones (Phornphutkul et al., 2009). Furthermore, mTORC1 regulates osteoblast differentiation, proliferation and bone formation (Chen et al., 2014; Chen & Long, 2015) and plays a role in skeletal muscle growth (Goodman et al., 2015; Semba, Trehan, Gonzalez‐Freire, et al., 2016). In addition, mTORC1 promotes myelination in both the central and peripheral nervous systems (Lebrun‐Julien et al., 2014; Norrmén et al., 2014; Wahl et al., 2014), regulates neuronal development (Lipton & Sahin, 2014) and is crucial in defining neuronal polarity, axon guidance, dendrite arborisation and gliogenesis (Garza‐Lombó & Gonsebatt, 2016; Graber et al., 2013; Switon et al., 2017; Takei & Nawa, 2014). The activation of mTORC1 is essential for synaptic plasticity that underlies processes involved in learning and memory (Switon et al., 2017). mTORC1 has also been shown to play a role in several other brain functions, such as regulation of food uptake and circadian entrainment (Lipton & Sahin, 2014).
Figure 2.
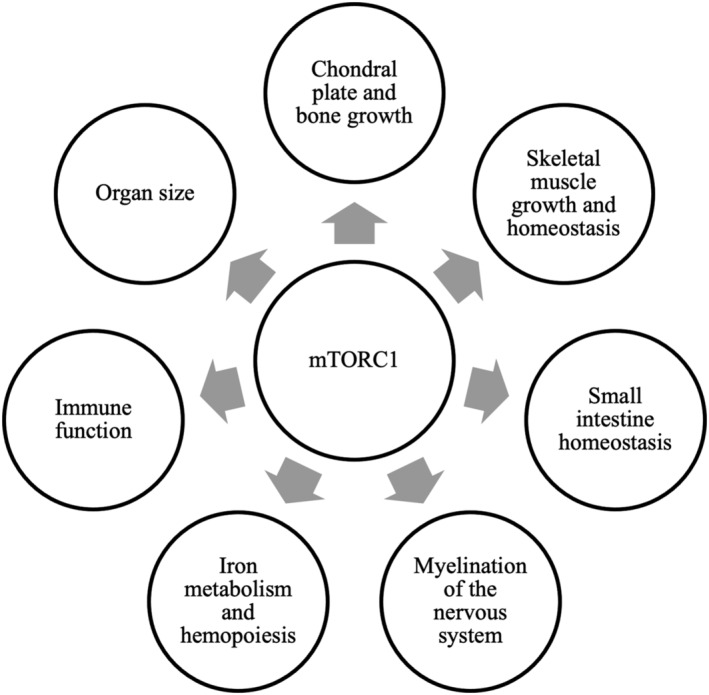
Biological processes regulated by mammalian target of rapamycin complex 1 (mTORC1)
Somewhat paradoxically, the specific AAs—the most effective stimuli—required for the activation of mTORC1 are least understood (Jewell et al., 2013). Limited available evidence suggests that the withdrawal of the EAAs leucine and arginine is as efficient as total AA removal in downregulating mTORC1 signalling (Avruch et al., 2009). Additionally, glutamine is required for extracellular leucine to activate mTOR (Nicklin et al., 2009), and glutamine metabolism has recently been shown to control mTORC1 (Jewell et al., 2013). Dietary lysine increases mTORC1 signalling in skeletal muscle and suppresses autophagy (Sato et al., 2015). Tryptophan activates mTORC1 and the expression of tight junction proteins that maintain gut barrier function (Wang et al., 2015). Leucine activates mTORC1 in neurons after influx through system L amino acid transporter (LAT). Uptake of arginine by cationic amino acid transporters CAT1 and CAT3 has also been reported to activate mTORC1 in neurons (Huang et al., 2007). In the central nervous system, neurotransmitters (such as glutamate and serotonin), neuromodulators and hormones have been reported to activate mTORC1 (Takei & Nawa, 2014); the importance of various AAs as precursors for brain neurotransmitters is well established.
As growth and neurocognitive development are dependent on the same regulatory pathway, controlled by common stimuli (i.e., AAs) (Jewell et al., 2013; Semba, Trehan, Gonzalez‐Freire, et al., 2016), effects on linear growth can occur in tandem with cognitive effects (Uauy et al., 2015). If there are nutritional deficiencies during this vulnerable phase, linear growth will falter (Uauy et al., 2015) and cognitive development as well as cognitive functioning can be affected (Uauy et al., 2015; Wu, 2010).
3.4. Other nutrients essential for healthy growth and development
Recent studies implicate an association of a broader range of nutritional metabolites, than only AAs, with linear growth and neurocognition, suggesting the involvement of multiple metabolic pathways. Studies from Malawi (Semba, Shardell, Sakr Ashour, et al., 2016) and Bangladesh (Moreau et al., 2019) show a significant association of phosphatidylcholines, sphingomyelins and hydroxy‐sphingomyelins with growth outcomes. Phosphatidylcholines, the most abundant phospholipid in mammalian cell membrane, constitute the dominant phospholipids circulating in plasma and are critical for cell membrane structure and linear growth of bones (Fagone & Jackowski, 2013; Li et al., 2014; Watkins et al., 2003). Although the mechanism by which sphingomyelins contribute to stunting is not well understood (Moreau et al., 2019), their involvement, along with phosphatidylcholines, in chondrogenesis may explain the association seen with linear growth faltering (Baron et al., 2015). Sphingomyelins, a major lipid component of myelin, are critical for myelination of the central nervous system (Semba, Shardell, Sakr Ashour, et al., 2016), which may account for the observed association with neurocognitive outcomes in the study of Moreau et al. (2019).
The synthesis of sphingomyelins depends on phosphatidylcholines, which are synthesised in the CDP–choline pathway that begins with the absorption of dietary choline in the small intestine (Semba, Shardell, Sakr Ashour, et al., 2016). Choline also serves as a precursor for the neurotransmitter acetylcholine (Zeisel & Da Costa, 2009). A recent study reports lower levels of choline in the serum of stunted Malawian children (Semba, Shardell, Sakr Ashour, et al., 2016). The lower excretion of betaine and dimethylglycine seen in undernourished Brazilian children further supports the observation of disturbances in choline metabolism with stunting (Mayneris‐Perxachs et al., 2016).
Animal source foods, which are often lacking in the diets in LMICs (Aguayo, 2017; Dewey, 2013; FAO, 2019; Lee, 2014; White et al., 2017), represent the richest source of choline (Zeisel & Da Costa, 2009).
4. DISCUSSION
The first 1000 days of life are critical for child development. This crucial period is by no means homogeneous. Different phases can be identified (e.g., pregnancy, exclusive breastfeeding [0–6 months of age] and complementary feeding [6–24 months of age]) that are all characterised by different growth requirements and growth determinants. Therefore, each phase requires a unique approach in ensuring optimal growth and development (Park et al., 2019). Unfortunately, globally, stunting remains a serious problem in children under the age of 5, and a sizeable number of children who do not meet the stunting threshold still fail to reach proper growth and development milestones. A growing body of work suggests that children experiencing growth faltering have inadequate levels of EAAs and other diverse metabolic perturbations. This is partly attributed to the poor protein quality of the LMIC diets, and inclusion of animal source foods—the richest sources of EAAs—is therefore encouraged to improve the quality of the predominantly cereal/vegetal‐based local diets. Beside EAAs, animal source foods are sources of other essential macronutrients (energy, high‐quality protein and fatty acids) and micronutrients (such as zinc, iron, iodine, magnesium, calcium, B‐vitamins, vitamin A and vitamin D) (Dror & Allen, 2011; Penny, 2012) that supply building blocks as well as regulate the processes involved in growth and development (Table 2). Not all animal source foods contain the same nutrients and, thus, diet diversity is important. An analysis of 130,432 children aged 6–23 months from 49 countries confirms the strong protective advantage of consuming multiple animal source foods over a single source against stunting (Headey et al., 2018). In a systematic review, Dror and Allen (2011) show the link between animal source food consumption and cognitive function among undernourished children in low‐income settings.
Table 2.
Nutrients (other than amino acids) provided by animal source foods that are essential for healthy growth and development
| Nutrient(s) | Function |
|---|---|
| Essential fatty acids (long‐chain ω‐3 fatty acid and DHA) | Constituent of cell membrane phospholipids; influences membrane protein function, lipid rafts and intracellular signalling; highly concentrated in grey matter of the brain and photoreceptors in the retina; accumulates at a very high rate during the first 2 years of life. |
| Calcium | Major structural element in bones and teeth; plays a role in mediating the constriction and relaxation of blood vessels, nerve impulse transmission, muscle contraction and the secretion of hormones like insulin; involved in regulation of protein function, including enzymes, optimising their activity. |
| Zinc | Constituent of over 200 metalloenzymes in the human body; important catalyst for metabolic processes linked to linear growth and cognitive development; present in synaptic vesicles and has a role in neurotransmission mediated by glutamate and GABA. |
| Iodine | Required for the synthesis of thyroid hormones that regulate a number of physiological processes, including growth, development, metabolism and reproduction, as well as for myelination of the central nervous system. |
| Iron | Essential component of hundreds of proteins and enzymes involved in various aspects of cellular metabolism, including those associated with oxygen transport and storage, electron transport and energy generation; needed for proper development of myelin sheaths; required cofactor for neurotransmitter synthesis. |
| Magnesium | Involved in over 300 metabolic reactions; critical roles in energy production, cell signalling, and fatty acid and protein synthesis; structural role in bone, cell membrane and chromosomes. |
| Vitamin A | Essential for visual development and acuity; important for immunity and defence against infections. |
| B‐vitamins | Roles in nerve cell myelination, neurotransmitter synthesis and regulation of gene expression in the central nervous system. |
| Vitamin D | Essential for bone mineralisation through the regulation of calcium and phosphorous homeostasis. |
EAA requirements for infants and young children are based on the estimated physiological needs under ideal conditions but do not consider the extra requirements for catch‐up growth or the burden of infectious disease (Pillai & Kurpad, 2012; Uauy, 2013; WHO & FAO, 2007).
There is therefore a need to re‐evaluate the EAA requirements for infants and young children in developing countries. In‐depth research characterising AA intakes and concentrations in infants and young children presenting with varying physiological states across a wide range of geographical regions can provide valuable insights for designing appropriate nutritional guidance and complementary feeding in order to promote optimal growth and neurocognitive development, particularly in children in LMICs.
Here, we present evidence that EAAs influence linear growth and, likely, neurocognition via the mTORC1 pathway and for the importance of high‐quality (animal source) protein during complementary feeding to prevent or compensate suboptimal growth. In many LMIC populations, there is an overlap in timing when faltering in linear growth begins and periods of rapid brain development occur. The continued exposure to a nutritionally deprived environment during this vulnerable period may have detrimental consequences on both linear growth and neurocognition (Leroy & Frongillo, 2019). Although linear growth retardation is not the only factor negatively impacting children's ability to reach their full potential, it is certainly a significant modifiable one. If and how it is linked to neurocognitive development in young children via the mTORC1 pathway needs further substantiation. It is clear however that the AA‐sensing mTORC1 pathway is a master regulator of overall growth and development and can be activated by AAs and a myriad of other signals, including cytokines (e.g., tumour necrosis factor α), growth factors (e.g., insulin‐like growth factor 1 [IGF1]) and cellular energy status.
Although animal source foods, rich in EAAs, significantly increase the nutritional quality of the diet of young children in LMICs, other dietary and environmental factors need to be addressed as well to resolve the complex problem of malnutrition.
CONFLICTS OF INTEREST
JG and RB are employees of FrieslandCampina. PP was an employee of FrieslandCampina at the time of submission.
CONTRIBUTIONS
PP, RS, MM, EU and RB conceptualised the manuscript. PP wrote the first draft of the manuscript. RS, MM, EU, RB, BKP, NR, JG, RS and TTN provided critical comments, reviewed and edited the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Science Impact (Winnipeg, Canada) for post‐editing the manuscript.
There are no financial or contractual agreements pertaining to this publication. The findings and conclusions contained within are those of the authors and do not necessarily reflect the position of FrieslandCampina.
Parikh, P. , Semba, R. , Manary, M. , Swaminathan, S. , Udomkesmalee, E. , Bos, R. , Poh, B. K. , Rojroongwasinkul, N. , Geurts, J. , Sekartini, R. , & Nga, T. T. (2021). Animal source foods, rich in essential amino acids, are important for linear growth and development of young children in low‐ and middle‐income countries. Maternal & Child Nutrition, 18:e13264. 10.1111/mcn.13264
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the USDA food composition database at https://fdc.nal.usda.gov.
REFERENCES
- Aguayo, V. M. (2017). Complementary feeding practices for infants and young children in South Asia. A review of evidence for action post‐2015. Maternal & Child Nutrition, 13(Suppl 2), e12439. 10.1111/mcn.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, L. (2013). Comparing the value of protein sources for maternal and child nutrition. Food and Nutrition Bulletin, 34(2), 263–266. 10.1177/156482651303400223 [DOI] [PubMed] [Google Scholar]
- Arsenault, J. E. , & Brown, K. H. (2017). Effects of protein or amino‐acid supplementation on the physical growth of young children in low‐income countries. Nutrition Reviews, 75(9), 699–717. 10.1093/nutrit/nux027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch, J. , Long, X. , Ortiz‐Vega, S. , Rapley, J. , Papageorgiou, A. , & Dai, N. (2009). Amino acid regulation of TOR complex 1. American Journal of Physiology ‐ Endocrinology and Metabolism, 296(4), E592–E602. 10.1152/ajpendo.90645.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylett, C. H. S. , Sauer, E. , Imseng, S. , Boehringer, D. , Hall, M. N. , Ban, N. , & Maier, T. (2016). Architecture of human mTOR complex 1. Science, 351(6268), 48–52. 10.1126/science.aaa3870 [DOI] [PubMed] [Google Scholar]
- Baron, J. , Sävendahl, L. , De Luca, F. , Dauber, A. , Phillip, M. , Wit, J. M. , & Nilsson, O. (2015). Short and tall stature: A new paradigm emerges. Nature Reviews Endocrinology, 11(12), 736–746. 10.1038/nrendo.2015.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar‐Peled, L. , & Sabatini, D. M. (2014). Regulation of mTORC1 by amino acids. Trends in Cell Biology, 24(7), 400–406. 10.1016/j.tcb.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutta, Z. A. , Das, J. K. , Rizvi, A. , Gaffey, M. F. , Walker, N. , Horton, S. , Webb, P. , Lartey, A. , & Black, R. E. (2013). Evidence‐based interventions for improvement of maternal and child nutrition: What can be done and at what cost? The Lancet, 382(9890), 452–477. 10.1016/S0140-6736(13)60996-4 [DOI] [PubMed] [Google Scholar]
- Bhutta, Z. A. , Salam, R. A. , & Das, J. K. (2013). Meeting the challenges of micronutrient malnutrition in the developing world. British Medical Bulletin, 106(1), 7–17. 10.1093/bmb/ldt015 [DOI] [PubMed] [Google Scholar]
- Chen, J. , & Long, F. (2014). mTORC1 signaling controls mammalian skeletal growth through stimulation of protein synthesis. Development (Cambridge), 141(14), 2848–2854. 10.1242/dev.108811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , & Long, F. (2015). MTORC1 signaling promotes osteoblast differentiation from preosteoblasts. PLoS ONE, 10(6), e0130627. 10.1371/journal.pone.0130627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Tu, X. , Esen, E. , Joeng, K. S. , Lin, C. , Arbeit, J. M. , Rüegg, M. A. , Hall, M. N. , Ma, L. , & Long, F. (2014). WNT7B promotes bone formation in part through mTORC1. PLoS Genetics, 10(1), e1004145. 10.1371/journal.pgen.1004145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury, S. , Headey, D. D. , & Masters, W. A. (2019). First foods: Diet quality among infants aged 6–23 months in 42 countries. Food Policy, 88, 101762. 10.1016/j.foodpol.2019.101762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick, S. E. , & Georgieff, M. K. (2012). Nutrient supplementation and neurodevelopment timing is the key. Archives of Pediatrics and Adolescent Medicine, 166(5), 481–482. 10.1001/archpediatrics.2012.199 [DOI] [PubMed] [Google Scholar]
- de Onis, M. (2017). Child growth and development. In Bloem M. W., de Pee S., & Taren D. (Eds.), Nutrition and health in a developing world (pp. 119–141). Springer. 10.1007/978-3-319-43739-2_6 [DOI] [Google Scholar]
- Dewey, K. G. (2013). The challenge of meeting nutrient needs of infants and young children during the period of complementary feeding: An evolutionary perspective. Journal of Nutrition, 143(12), 2050–2054. 10.3945/jn.113.182527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey, K. G. , & Adu‐Afarwuah, S. (2008). Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal & Child Nutrition, 4(SUPPL.1), 24–85. 10.1111/j.1740-8709.2007.00124.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror, D. K. , & Allen, L. H. (2011). The importance of milk and other animal‐source foods for children in low‐income countries. Food and Nutrition Bulletin, 32(3), 227–243. 10.1177/156482651103200307 [DOI] [PubMed] [Google Scholar]
- Dulal, S. , Liégeois, F. , Osrin, D. , Kuczynski, A. , Manandhar, D. S. , Shrestha, B. P. , Sen, A. , Saville, N. , Devakumar, D. , & Prost, A. (2018). Does antenatal micronutrient supplementation improve children's cognitive function? Evidence from the follow‐up of a double‐blind randomised controlled trial in Nepal. BMJ Global Health, 3(1), e000527. 10.1136/bmjgh-2017-000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan, A. , Zoncu, R. , & Sabatini, D. M. (2012). Amino acids and mTORC1: From lysosomes to disease. Trends in Molecular Medicine, 18(9), 524–533. 10.1016/j.molmed.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle, P. L. , Fernald, L. C. H. , Alderman, H. , Behrman, J. , O'Gara, C. , Yousafzai, A. , De Mello, M. C. , Hidrobo, M. , Ulkuer, N. , Ertem, I. , & Iltus, S. (2011). Strategies for reducing inequalities and improving developmental outcomes for young children in low‐income and middle‐income countries. The Lancet, 378(9799), 1339–1353. 10.1016/S0140-6736(11)60889-1 [DOI] [PubMed] [Google Scholar]
- Fagone, P. , & Jackowski, S. (2013). Phosphatidylcholine and the CDP–choline cycle. Biochimica et Biophysica Acta ‐ Molecular and Cell Biology of Lipids, 1831(3), 523–532. 10.1016/j.bbalip.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . (2019). Dynamic development, shifting demographics, changing diets: The story of the rapidly evolving food system in Asia and the Pacific and why it is constantly on the move. http://www.fao.org/3/I8499EN/i8499en.pdf
- Fink, G. , & Rockers, P. C. (2014). Childhood growth, schooling, and cognitive development: Further evidence from the Young Lives study. American Journal of Clinical Nutrition, 100(1), 182–188. 10.3945/ajcn.113.080960 [DOI] [PubMed] [Google Scholar]
- Garza‐Lombó, C. , & Gonsebatt, M. E. (2016). Mammalian target of rapamycin: Its role in early neural development and in adult and aged brain function. Frontiers in Cellular Neuroscience, 10(JUN), 157. 10.3389/fncel.2016.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, S. , Suri, D. , & Uauy, R. (2012). Assessment of protein adequacy in developing countries: Quality matters. British Journal of Nutrition, 108(SUPPL. 2), S77–S87. 10.1017/S0007114512002577 [DOI] [PubMed] [Google Scholar]
- Giallourou, N. , Fardus‐Reid, F. , Panic, G. , Veselkov, K. , McCormick, B. J. J. , Olortegui, M. P. , Ahmed, T. , Mduma, E. , Yori, P. P. , Mahfuz, M. , Svensen, E. , Ahmed, M. M. M. , Colston, J. M. , Kosek, M. N. , & Swann, J. R. (2020). Metabolic maturation in the first 2 years of life in resource‐constrained settings and its association with postnatal growths. Science Advances, 6(15), eaay5969. 10.1126/sciadv.aay5969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, C. A. , Hornberger, T. A. , & Robling, A. G. (2015). Bone and skeletal muscle: Key players in mechanotransduction and potential overlapping mechanisms. Bone, 80, 24–36. 10.1016/j.bone.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber, T. E. , McCamphill, P. K. , & Sossin, W. S. (2013). A recollection of mTOR signaling in learning and memory. Learning and Memory, 20(10), 518–530. 10.1101/lm.027664.112 [DOI] [PubMed] [Google Scholar]
- Grasgruber, P. , Sebera, M. , Hrazdíra, E. , Cacek, J. , & Kalina, T. (2016). Major correlates of male height: A study of 105 countries. Economics and Human Biology, 21, 172–195. 10.1016/j.ehb.2016.01.005 [DOI] [PubMed] [Google Scholar]
- Headey, D. , Hirvonen, K. , & Hoddinott, J. (2018). Animal sourced foods and child stunting. American Journal of Agricultural Economics, 100(5), 1302–1319. 10.1093/ajae/aay053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddinott, J. , Behrman, J. R. , Maluccio, J. A. , Melgar, P. , Quisumbing, A. R. , Ramirez‐Zea, M. , Stein, A. D. , Yount, K. M. , & Martorell, R. (2013). Adult consequences of growth failure in early childhood. American Journal of Clinical Nutrition, 98(5), 1170–1178. 10.3945/ajcn.113.064584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta, B. L. , & Victora, C. G. (2013). Long‐term health effects of breastfeeding: A systematic review. In World Health Organization (Vol. 129, Issues 8–9). http://www.ncbi.nlm.nih.gov/pubmed/20960419 [Google Scholar]
- Huang, Y. , Kang, B. N. , Tian, J. , Liu, Y. , Luo, H. R. , Hester, L. , & Snyder, S. H. (2007). The cationic amino acid transporters CAT1 and CAT3 mediate NMDA receptor activation‐dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway. Journal of Neuroscience, 27(3), 449–458. 10.1523/JNEUROSCI.4489-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell, J. L. , Russell, R. C. , & Guan, K. L. (2013). Amino acid signalling upstream of mTOR. Nature Reviews Molecular Cell Biology, 14(3), 133–139. 10.1038/nrm3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. S. , Ke, Y. W. , Auyeung, V. , Chen, Q. , Gruppuso, P. A. , & Phornphutkul, C. (2009). Leucine restriction inhibits chondrocyte proliferation and differentiation through mechanisms both dependent and independent of mTOR signaling. American Journal of Physiology ‐ Endocrinology and Metabolism, 296(6), E1374–E1382. 10.1152/ajpendo.91018.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek, M. N. , Mduma, E. , Kosek, P. S. , Lee, G. O. , Svensen, E. , Pan, W. K. Y. , Olortegui, M. P. , Bream, J. H. , Patil, C. , Asayag, C. R. , Sanchez, G. M. , Caulfield, L. E. , Gratz, J. , & Yori, P. P. (2016). Plasma tryptophan and the kynurenine‐tryptophan ratio are associated with the acquisition of statural growth deficits and oral vaccine underperformance in populations with environmental enteropathy. American Journal of Tropical Medicine and Hygiene, 95(4), 928–937. 10.4269/ajtmh.16-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasevec, J. , An, X. , Kumapley, R. , Bégin, F. , & Frongillo, E. A. (2017). Diet quality and risk of stunting among infants and young children in low‐ and middle‐income countries. Maternal & Child Nutrition, 13(Suppl 2), e12430. 10.1111/mcn.12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, M. , Ji, B. , Babaei, P. , Das, P. , Lappa, D. , Ramakrishnan, G. , Fox, T. E. , Haque, R. , Petri, W. A. , Bäckhed, F. , & Nielsen, J. (2018). Gut microbiota dysbiosis is associated with malnutrition and reduced plasma amino acid levels: Lessons from genome‐scale metabolic modeling. Metabolic Engineering, 49, 128–142. 10.1016/j.ymben.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante, M. , & Sabatini, D. M. (2012). MTOR signaling in growth control and disease. Cell, 149(2), 274–293. 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun‐Julien, F. , Bachmann, L. , Norrmén, C. , Trötzmüller, M. , Köfeler, H. , Rüegg, M. A. , Hall, M. N. , & Suter, U. (2014). Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. Journal of Neuroscience, 34(25), 8432–8448. 10.1523/JNEUROSCI.1105-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D. E. R. (2014). Children's protein consumption in Southeast Asia: Consideration of quality as well as quantity of children's protein consumption in Southeast Asia. Wharton Research Scholars, 115. https://repository.upenn.edu/wharton_research_scholars/115 [Google Scholar]
- Lehman, S. L. , Cerniglia, G. J. , Johannes, G. J. , Ye, J. , Ryeom, S. , & Koumenis, C. (2015). Translational upregulation of an individual p21Cip1 transcript variant by GCN2 regulates cell proliferation and survival under nutrient stress. PLoS Genetics, 11(6), e1005212. 10.1371/journal.pgen.1005212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy, J. L. , & Frongillo, E. A. (2019). Perspective: What does stunting really mean? A critical review of the evidence. Advances in Nutrition, 10(2), 196–204. 10.1093/advances/nmy101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Wu, G. , Van Der Veen, J. N. , Hermansson, M. , & Vance, D. E. (2014). Phosphatidylcholine metabolism and choline kinase in human osteoblasts. Biochimica et Biophysica Acta ‐ Molecular and Cell Biology of Lipids, 1841(6), 859–867. 10.1016/j.bbalip.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Lipton, J. O. , & Sahin, M. (2014). The neurology of mTOR. Neuron, 84(2), 275–291. 10.1016/j.neuron.2014.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeen, E. A. , Behrman, J. R. , Crookston, B. T. , Dearden, K. A. , Engle, P. , Georgiadis, A. , Penny, M. E. , & Stein, A. D. (2014). Growth faltering and recovery in children aged 1–8 years in four low‐ and middle‐income countries: Young Lives. Public Health Nutrition, 17(9), 2131–2137. 10.1017/S1368980013003017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie, E. J. , Tatarczuch, L. , & Mirams, M. (2011). The skeleton: A multi‐functional complex organ. The growth plate chondrocyte and endochondral ossification. Journal of Endocrinology, 211(2), 109–121. 10.1530/JOE-11-0048 [DOI] [PubMed] [Google Scholar]
- MAL‐ED Network Investigators . (2017). Childhood stunting in relation to the pre‐ and postnatal environment during the first 2 years of life: The MAL‐ED longitudinal birth cohort study. PLoS Medicine, 14(10), e1002408. 10.1371/journal.pmed.1002408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayneris‐Perxachs, J. , Lima, A. A. M. , Guerrant, R. L. , Leite, Á. M. , Moura, A. F. , Lima, N. L. , Soares, A. M. , Havt, A. , Moore, S. R. , Pinkerton, R. , & Swann, J. R. (2016). Urinary N‐methylnicotinamide and β‐aminoisobutyric acid predict catch‐up growth in undernourished Brazilian children. Scientific Reports, 6, 19780. 10.1038/srep19780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayneris‐Perxachs, J. , & Swann, J. R. (2019). Metabolic phenotyping of malnutrition during the first 1000 days of life. European Journal of Nutrition, 58(3), 909–930. 10.1007/s00394-018-1679-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelsen, K. F. (2013). Cow's milk in the prevention and treatment of stunting and wasting. Food and Nutrition Bulletin, 34(2), 249–251. 10.1177/156482651303400219 [DOI] [PubMed] [Google Scholar]
- Millward, D. J. (2003). An adaptive metabolic demand model for protein and amino acid requirements. British Journal of Nutrition, 90(2), 249–260. 10.1079/bjn2003924 [DOI] [PubMed] [Google Scholar]
- Moradi, A. (2010). Nutritional status and economic development in sub‐Saharan Africa, 1950–1980. Economics and Human Biology, 8(1), 16–29. 10.1016/j.ehb.2009.12.002 [DOI] [PubMed] [Google Scholar]
- Moreau, G. B. , Ramakrishnan, G. , Cook, H. L. , Fox, T. E. , Nayak, U. , Ma, J. Z. , Colgate, E. R. , Kirkpatrick, B. D. , Haque, R. , & Petri, W. A. (2019). Childhood growth and neurocognition are associated with distinct sets of metabolites. eBioMedicine, 44, 597–606. 10.1016/j.ebiom.2019.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutasa, K. , Ntozini, R. , Mbuya, M. N. N. , Rukobo, S. , Govha, M. , Majo, F. D. , Tavengwa, N. , Smith, L. E. , Caulfield, L. , Swann, J. R. , Stoltzfus, R. J. , Moulton, L. H. , Humphrey, J. H. , Gough, E. K. , & Prendergast, A. J. (2021). Biomarkers of environmental enteric dysfunction are not consistently associated with linear growth velocity in rural Zimbabwean infants. The American Journal of Clinical Nutrition, 113(5), 1185–1198. 10.1093/ajcn [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin, P. , Bergman, P. , Zhang, B. , Triantafellow, E. , Wang, H. , Nyfeler, B. , Yang, H. , Hild, M. , Kung, C. , Wilson, C. , Myer, V. E. , MacKeigan, J. P. , Porter, J. A. , Wang, Y. K. , Cantley, L. C. , Finan, P. M. , & Murphy, L. O. (2009). Bidirectional transport of amino acids regulates mTOR and autophagy. Cell, 136(3), 521–534. 10.1016/j.cell.2008.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmén, C. , Figlia, G. , Lebrun‐Julien, F. , Pereira, J. A. , Trötzmüller, M. , Köfeler, H. C. , Rantanen, V. , Wessig, C. , van Deijk, A. L. F. , Smit, A. B. , Verheijen, M. H. G. , Rüegg, M. A. , Hall, M. N. , & Suter, U. (2014). mTORC1 controls PNS myelination along the mTORC1‐RXRγ‐SREBP‐lipid biosynthesis axis in Schwann cells. Cell Reports, 9(2), 646–660. 10.1016/j.celrep.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Ordiz, M. I. , Semba, R. D. , Moaddel, R. , Rolle‐Kampczyk, U. , von Bergen, M. , Herberth, G. , Khadeer, M. , Röder, S. , & Manary, M. J. (2019). Serum amino acid concentrations in infants from Malawi are associated with linear growth. Current Developments in Nutrition, 3(10), nzz100. 10.1093/cdn/nzz100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owino, V. , Ahmed, T. , Freemark, M. , Kelly, P. , Loy, A. , Manary, M. , & Loechl, C. (2016). Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics, 138(6), e20160641. 10.1542/peds.2016-0641 [DOI] [PubMed] [Google Scholar]
- Park, J. J. H. , Fang, M. L. , Harari, O. , Dron, L. , Siden, E. G. , Majzoub, R. , Jeziorska, V. , Thorlund, K. , Mills, E. J. , & Bhutta, Z. A. (2019). Association of early interventions with birth outcomes and child linear growth in low‐income and middle‐income countries. Bayesian network meta‐analyses of randomized clinical trials. JAMA Network Open, 2(7), e197871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny, M. E. (2012). Micronutrients in the treatment of stunting and moderate malnutrition. Nestle Nutrition Institute Workshop Series, 70, 11–21. 10.1159/000337388 [DOI] [PubMed] [Google Scholar]
- Phornphutkul, C. , Lee, M. , Voigt, C. , Wu, K. Y. , Ehrlich, M. G. , Gruppuso, P. A. , & Chen, Q. (2009). The effect of rapamycin on bone growth in rabbits. Journal of Orthopaedic Research, 27(9), 1157–1161. 10.1002/jor.20894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phornphutkul, C. , Wu, K. Y. , Auyeung, V. , Chen, Q. , & Gruppuso, P. A. (2008). mTOR signaling contributes to chondrocyte differentiation. Developmental Dynamics, 237(3), 702–712. 10.1002/dvdy.21464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai, R. R. , Elango, R. , Ball, R. O. , Kurpad, A. V. , & Pencharz, P. B. (2015). Lysine requirements of moderately undernourished school‐aged Indian children are reduced by treatment for intestinal parasites as measured by the indicator amino acid oxidation technique. Journal of Nutrition, 145(5), 954–959. 10.3945/jn.114.208439 [DOI] [PubMed] [Google Scholar]
- Pillai, R. R. , & Kurpad, A. V. (2012). Amino acid requirements in children and the elderly population. British Journal of Nutrition, 108(SUPPL. 2), S44–S49. 10.1017/S0007114512002401 [DOI] [PubMed] [Google Scholar]
- Prado, E. L. , & Dewey, K. G. (2014). Nutrition and brain development in early life. Nutrition Reviews, 72(4), 267–284. 10.1111/nure.12102 [DOI] [PubMed] [Google Scholar]
- Prado, E. L. , Larson, L. M. , Cos, K. , Bettencourt, K. , Kubes, J. N. , & Shankar, A. H. (2019). Do effects of early life interventions on linear growth correspond to effects on neurobehavioural development? A systematic review and meta‐analysis. Lancet, 7, e1398–e1413. [DOI] [PubMed] [Google Scholar]
- Prentice, A. M. , Ward, K. A. , Goldberg, G. R. , Jarjou, L. M. , Moore, S. E. , Fulford, A. J. , & Prentice, A. (2013). Critical windows for nutritional interventions against stunting. American Journal of Clinical Nutrition, 97(5), 911–918. 10.3945/ajcn.112.052332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan, U. , Nguyen, P. , & Martorell, R. (2009). Effects of micronutrients on growth of children under 5 y of age: Meta‐analyses of single and multiple nutrient interventions. American Journal of Clinical Nutrition, 89(1), 191–203. 10.3945/ajcn.2008.26862 [DOI] [PubMed] [Google Scholar]
- Reeds, P. J. , Fjeld, C. R. , & Jahoor, F. (1994). Do the differences between the amino acid compositions of acute‐phase and muscle proteins have a bearing on nitrogen loss in traumatic states? Journal of Nutrition, 124(6), 906–910. 10.1093/jn/124.6.906 [DOI] [PubMed] [Google Scholar]
- Sato, T. , Ito, Y. , & Nagasawa, T. (2015). Dietary l‐lysine suppresses autophagic proteolysis and stimulates Akt/mTOR signaling in the skeletal muscle of rats fed a low‐protein diet. Journal of Agricultural and Food Chemistry, 63(37), 8192–8198. 10.1021/acs.jafc.5b03811 [DOI] [PubMed] [Google Scholar]
- Schott, W. B. , Crookston, B. T. , Lundeen, E. A. , Stein, A. D. , & Behrman, J. R. (2013). Periods of child growth up to age 8 years in Ethiopia, India, Peru and Vietnam: Key distal household and community factors. Social Science and Medicine, 97, 278–287. 10.1016/j.socscimed.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba, R. D. (2016). The rise and fall of protein malnutrition in global health. Annals of Nutrition and Metabolism, 69(2), 79–88. 10.1159/000449175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba, R. D. , Shardell, M. , Sakr Ashour, F. A. , Moaddel, R. , Trehan, I. , Maleta, K. M. , Ordiz, M. I. , Kraemer, K. , Khadeer, M. A. , Ferrucci, L. , & Manary, M. J. (2016). Child stunting is associated with low circulating essential amino acids. eBioMedicine, 6, 246–252. 10.1016/j.ebiom.2016.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba, R. D. , Shardell, M. , Trehan, I. , Moaddel, R. , Maleta, K. M. , Ordiz, M. I. , Kraemer, K. , Khadeer, M. , Ferrucci, L. , & Manary, M. J. (2016). Metabolic alterations in children with environmental enteric dysfunction. Scientific Reports, 6, 28009. 10.1038/srep28009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba, R. D. , Trehan, I. , Gonzalez‐Freire, M. , Kraemer, K. , Moaddel, R. , Ordiz, M. I. , Ferrucci, L. , & Manary, M. J. (2016). Perspective: The potential role of essential amino acids and the mechanistic target of rapamycin complex 1 (mTORC1) pathway in the pathogenesis of child stunting. Advances in Nutrition, 7(5), 853–865. 10.3945/an.116.013276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar, N. , Jackson, A. A. , Courtney‐Martin, G. , Elango, R. , Ghosh, S. , Hodgkinson, S. , Xipsiti, M. , Lee, W. T. K. , Kurpad, A. V. , & Tomé, D. (2020). Protein quality assessment of follow‐up formula for young children and ready‐to‐use therapeutic foods: Recommendations by the FAO Expert Working Group in 2017. Journal of Nutrition, 150(2), 195–201. 10.1093/jn/nxz250 [DOI] [PubMed] [Google Scholar]
- Shrimpton, R. , Victora, C. G. , de Onis, M. , Lima, R. C. , Blössner, M. , & Clugston, G. (2001). Worldwide timing of growth faltering: Implications for nutritional interventions. Pediatrics, 107(5), e75. 10.1542/peds.107.5.e75 [DOI] [PubMed] [Google Scholar]
- Stammers, A. L. , Lowe, N. M. , Medina, M. W. , Patel, S. , Dykes, F. , Pérez‐Rodrigo, C. , Serra‐Majam, L. , Nissensohn, M. , & Moran, V. H. (2015). The relationship between zinc intake and growth in children aged 1–8 years: A systematic review and meta‐analysis. European Journal of Clinical Nutrition, 69(2), 147–153. 10.1038/ejcn.2014.204 [DOI] [PubMed] [Google Scholar]
- Sudfeld, C. R. , McCoy, D. C. , Danaei, G. , Fink, G. , Ezzati, M. , Andrews, K. G. , & Fawzi, W. W. (2015). Linear growth and child development in low‐ and middle‐income countries: A meta‐analysis. Pediatrics, 135(5), e1266–e1275. 10.1542/peds.2014-3111 [DOI] [PubMed] [Google Scholar]
- Suryawan, A. , Jalaludin, M. Y. , Poh, B. K. , Sanusi, R. , Tan, V. M. H. , Geurts, J. M. , & Muhardi, L. (2021). Malnutrition in early life and its neurodevelopmental and cognitive consequences: A scoping review. Nutrition Research Reviews, 1–41. 10.1017/S0954422421000159 [DOI] [PubMed] [Google Scholar]
- Switon, K. , Kotulska, K. , Janusz‐Kaminska, A. , Zmorzynska, J. , & Jaworski, J. (2017). Molecular neurobiology of mTOR. Neuroscience, 341, 112–153. 10.1016/j.neuroscience.2016.11.017 [DOI] [PubMed] [Google Scholar]
- Takei, N. , & Nawa, H. (2014). mTOR signaling and its roles in normal and abnormal brain development. Frontiers in Molecular Neuroscience, 7(APR), 28. 10.3389/fnmol.2014.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessema, M. , Gunaratna, N. S. , Brouwer, I. D. , Donato, K. , Cohen, J. L. , McConnell, M. , Belachew, T. , Belayneh, D. , & Groote, H. D. (2018). Associations among high‐quality protein and energy intake, serum transthyretin, serum amino acids and linear growth of children in Ethiopia. Nutrients, 10(11), 1776. 10.3390/nu10111776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, T. , Singh, M. , Swaminathan, S. , & Kurpad, A. V. (2020). Age‐related differences in height gain with dairy protein and micronutrient supplements in Indian primary school children. Asia Pacific Journal of Clinical Nutrition, 29(2), 355–362. 10.6133/apjcn.202007_29(2).0018 [DOI] [PubMed] [Google Scholar]
- Uauy, R. (2013). Keynote: Rethinking protein. Food and Nutrition Bulletin, 34(2), 228–231. 10.1177/156482651303400213 [DOI] [PubMed] [Google Scholar]
- Uauy, R. , Kurpad, A. , Tano‐Debrah, K. , Otoo, G. E. , Aaron, G. A. , Toride, Y. , & Ghosh, S. (2015). Role of protein and amino acids in infant and young child nutrition: Protein and amino acid needs and relationship with child growth. Journal of Nutritional Science and Vitaminology, 61, S192–S194. 10.3177/jnsv.61.S192 [DOI] [PubMed] [Google Scholar]
- UNICEF . (2016). From the first hour of life: Making the case for improved infant and young child feeding everywhere. In Unicef. United Nations Children's Fund. [Google Scholar]
- UNICEF . (2019). The state of the world's children 2019. In Children, food and nutrition: Growing well in a changing world. United Nations. [Google Scholar]
- Wahl, S. E. , McLane, L. E. , Bercury, K. K. , Macklin, W. B. , & Wood, T. L. (2014). Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. Journal of Neuroscience, 34(13), 4453–4465. 10.1523/JNEUROSCI.4311-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Ji, Y. , Wu, G. , Sun, K. , Sun, Y. , Li, W. , Wang, B. , He, B. , Zhang, Q. , Dai, Z. , & Wu, Z. (2015). l‐Tryptophan activates mammalian target of rapamycin and enhances expression of tight junction proteins in intestinal porcine epithelial cells. Journal of Nutrition, 145(6), 1156–1162. 10.3945/jn.114.209817 [DOI] [PubMed] [Google Scholar]
- Watkins, B. A. , Li, Y. , Lippman, H. E. , & Feng, S. (2003). Modulatory effect of omega‐3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 68(6), 387–398. 10.1016/S0952-3278(03)00063-2 [DOI] [PubMed] [Google Scholar]
- White, J. M. , Bégin, F. , Kumapley, R. , Murray, C. , & Krasevec, J. (2017). Complementary feeding practices: Current global and regional estimates. Maternal & Child Nutrition, 13(Suppl 2), e12505. 10.1111/mcn.12505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2001). The Optimal Duration of Exclusive Breastfeeding: Report of An Expert Consultation. In World Health Organization (Issue March). http://www.who.int/nutrition/publications/infantfeeding/WHO_NHD_01.09/en/ [Google Scholar]
- WHO . (2003). Implementing the global strategy for infant and young child feeding, meeting report 2003. World Health Organization. [Google Scholar]
- WHO , & FAO . (2007). Protein and amino acid requirements in human nutrition: Report of a joint FAO/WHO/UNU expert consultation (WHO Technical Report Series 935). In Nutrition abstracts and reviews (Vol. 35). https://www.who.int/nutrition/publications/nutrientrequirements/WHO_TRS_935/en/ [Google Scholar]
- Wu, G. (2010). Functional amino acids in growth, reproduction, and health. Advances in Nutrition, 1(1), 31–37. 10.3945/an.110.1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, J. , Palm, W. , Peng, M. , King, B. , Lindsten, T. , Li, M. O. , Koumenis, C. , & Thompson, C. B. (2015). GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes and Development, 29(22), 2331–2336. 10.1101/gad.269324.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, V. R. (2000). Protein and amino acids. In Nutrition and immunology (pp. 49–64). Humana Press. [Google Scholar]
- Zeisel, S. H. , & Da Costa, K. A. (2009). Choline: An essential nutrient for public health. Nutrition Reviews, 67(11), 615–623. 10.1111/j.1753-4887.2009.00246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available in the USDA food composition database at https://fdc.nal.usda.gov.


