Abstract
Establishing a contextual representation of an environment places specific spatial-temporal processing demands on the mammalian hippocampus, a region showing sex-differences in processing capabilities. However, evidence for sex differences in these processing demands during contextual fear learning remains limited. Here, we examined the relationship among contextual processing, timing of footshock, and activation of the dorsal hippocampus and basolateral amygdalar nuclei (BLA) in male and female mice (C57Bl/6J). We modified the initial exposure time to the conditioning context prior to administration, or not, of a single footshock. We then quantified Fos- ir neurons activated by acquisition or retrieval of contextual fear memories in the rostral half of the dorsal CA1 (proximal – distal regions), CA3, Dentate Gyrus and basolateral amygdalar nuclei corresponding to atlas levels of the Allen Reference Atlas. In experiment 1, we found that sex differences in context elicited freezing were evident at the longest context placement-to-shock interval and that context fear retrieval with increasing contextual exposure periods increased CA1 Fos-ir in males, but not females. In experiment 2, we observed that an aversive footshock in males potentiated CA1 activation, while it downregulated CA1 activation in females. We also found that an aversive footshock independent of sex moderated Dentate Gyrus activation that normally showed increased activation with greater context exposure periods. Lastly, we identified a heightened responsiveness in BLA neurons to both footshock and length of context exposure in females compared to males. Overall, our findings suggest that sex differences in contextual fear conditioning may arise from marked sex differences in the contextual processing demands of the dorsal hippocampus subfields largely modulated by aversive outcomes.
1. Introduction
Sex differences in contextual-spatial processing can be a major contributor to observed sex differences in contextual fear conditioning (CFC) in both rats and mice (Colon et al., 2018; Keiser et al., 2017; Maren et al., 1994; Moore et al., 2010; Poulos et al., 2015; Pryce et al., 1999; Wiltgen et al., 2001). Evidence suggests the hippocampus is a key structure that underlies these sex differences, as the hippocampus is a crucial brain region in both CFC and contextual-spatial processing (Anagnostaras et al., 2001; Sanders et al., 2003). However, the hippocampus may also process information related to noxious events (Lovett-Baron et al., 2014). To further identify the processing demands of the hippocampus that contribute to sex differences in CFC, it may be important to examine patterns of hippocampal activation in male and female mice using distinct levels of contextual-spatial encoding and aversive stimulation.
CFC is a form of associative learning, where static sensory features of an environment or context are encoded and associated with an aversive event such as footshock. Most evidence of adult sex difference in CFC suggests that male rats and mice display greater CFC than females (Maren et al. 1994; Pryce et al.,1999; Wiltgen et al. 2001; Poulos et al. 2015; Colon et al., 2018), while other studies either fail to identify sex differences (Kosten et al., 2006; Dachtler et al., 2011) or have reported female mice exhibit greater CFC than males (Moore et al. 2010; Keiser et al. 2017). Studies in other animal models of spatial learning suggest that differences in the capacity to process context-spatial information (Madeira et al., 1991; Roof, 1993) may contribute to these differences. In CFC, a sufficient period of contextual processing is required prior to footshock administration, as immediate delivery of a footshock upon placement into a novel context fails to produce associative learning (Blanchard et al., 1976; Fanselow, 1986; Kiernan & Westbrook, 1993). Conversely, extending the placement-to-shock interval (PSI) or pre- exposure to the conditioning context can facilitate acquisition of CFC (Fanselow, 1986; Frankland et al., 2004). These studies generally assert that in order for CFC to occur a sufficient representation of the contextual features must be formed in the hippocampus.
Sex differences in CFC maybe partially attributed to differences in the capacity of the hippocampus to form a contextual representation of the conditioning environment (Wiltgen et al., 2001; Maren, 2013). The mouse dorsal hippocampal formation extends roughly 2.3 mm from rostral to caudal and consists of the dentate gyrus (DG), subiculum and Cornu Ammonis (CA) fields CA1, CA2, CA3. These sub-regions are anatomically situated to send and receive unique patterns of input and output among several cortical and subcortical regions (Bienkowski et al, 2018). The rostral portion of the dorsal hippocampus, which includes DG and fields CA3 and CA1, represents key nodes in contextual-spatial processing and likely store and retrieve contextual-spatial representations (Rudy & O’Reilly, 1999; Fanselow, 2000; Rudy, 2009). Male mice showed increased context exposure prior to foot shock delivery as well as increased neuronal activation in CFC and DG/CA3 and CA1, as measured by immunoreactivity of the immediate early gene Arc (Leake et al., 2017). Conversely, context fear retrieval may place greater activational demands on the CA1, CA3, and DG of male relative to female mice (Keiser et al., 2017). Interestingly, these hippocampal subregions can show sexual dimorphisms, as revealed by unique regional volumes and/or dendritic branching (Tabibnia et al., 1999) and sex-specific gene expression (Armoskus et al., 2014).
Another potential source of CFC sex differences may emerge from how the hippocampus encodes aversive outcomes. While the basolateral amygdala complex (BLA) and its footshock-related afferents are important in the association of context and aversive outcomes (Johansen et al., 2010), a recent study using a modified CFC procedure found that aversive outcomes can inhibit CA1 neuronal activity (Lovett-Baron et al., 2014). Interestingly, sex differences in stress exposure indicate that male and female rats exposed to intermittent tail-shocks show sex-specific changes in CA1 dendritic spines (Shors et al., 2001). However, we still do not know the extent to which the hippocampus is activated by aversive events such as footshock during conditioning or activated during retrieval of an aversive or non-aversive context (Fanselow, 2000; Matus-Amat et al., 2004; Zelikowsky et al., 2014; Lovett-Barron et al., 2014).
We speculate that a comprehensive and rigorous analysis of neuronal activation among the expansive subregions of the dorsal hippocampus and BLA in both male and female mice following the administration or absence of footshock and varying levels of contextual processing will reveal basic mechanistic differences in hippocampal function that underlie sex differences in CFC. Based on recent accounts of neuronal ensemble activation within the dorsal hippocampus (Tanaka et al., 2014; Krasne et al., 2015; Berneir et al., 2016; Leake et al., 2017), we hypothesize that increasing context exposure will induce an increase in neuronal activation assayed with Fos-Immunoreactivity (Fos-ir) throughout the dorsal hippocampus (DG, CA3 and CA1) and greater CFC in both male and female mice. We predict this pattern of retrieval-related activation in males and females will occur in DG and CA1 during the initial encoding of the conditioning context (Krasne et al., 2015).
To test these predictions and characterize neuronal activation in the dorsal hippocampus in male and female C57Bl/6J mice, we performed a thorough analysis of context fear acquisition and retrieval related activation for the rostral half of the dorsal hippocampus under increasing contextual-spatial processing demands. To increase the intra- and inter- experimenter reliability of regional Fos-ir counts, we employed matched Nissl counterstained coronal planes of brain sections to atlas plate level representations (Allen Reference Atlas) across rostral-caudal segments of the dorsal hippocampus subfields and basolateral amygdalar nuclei. We then identified and tabulated the total number Fos-ir cells within these aligned sections (Santarelli et al., 2018).
In experiment 1, we varied the length of context exposure time (3, 30, 152 and 240 sec) prior to the foot shock administration, we then quantified Fos-ir to re-exposure to the aversive context and assessed CFC (Fig. 1A). In experiment 2, we quantified Fos-ir in both aversive (footshock) and non-aversive contexts under varying lengths of context pre-exposure time (Fig. 1B). This approach permitted an examination of changes in hippocampal activation to the presence of aversive stimuli and whether this responsivity was sexually dimorphic. For experiments 1 and 2, coronal planes of section containing the DG, CA3 and CA1 (proximal and distal portion) of the dorsal hippocampus and basolateral amygdala complex were approximately aligned to plate levels 68–78 (Fig. 1C). Approximately −1.355 to −2.355 mm from Bregma as represented in the Allen Reference Atlas (Dong, 2008) according to visible general cytoarchitectural features observed in the Nissl stain. These results revealed a marked sex difference in the activation of the dorsal hippocampus to context exposure. Moreover, these results suggested that dorsal hippocampal activation could be modulated by the presentation of an aversive outcome.
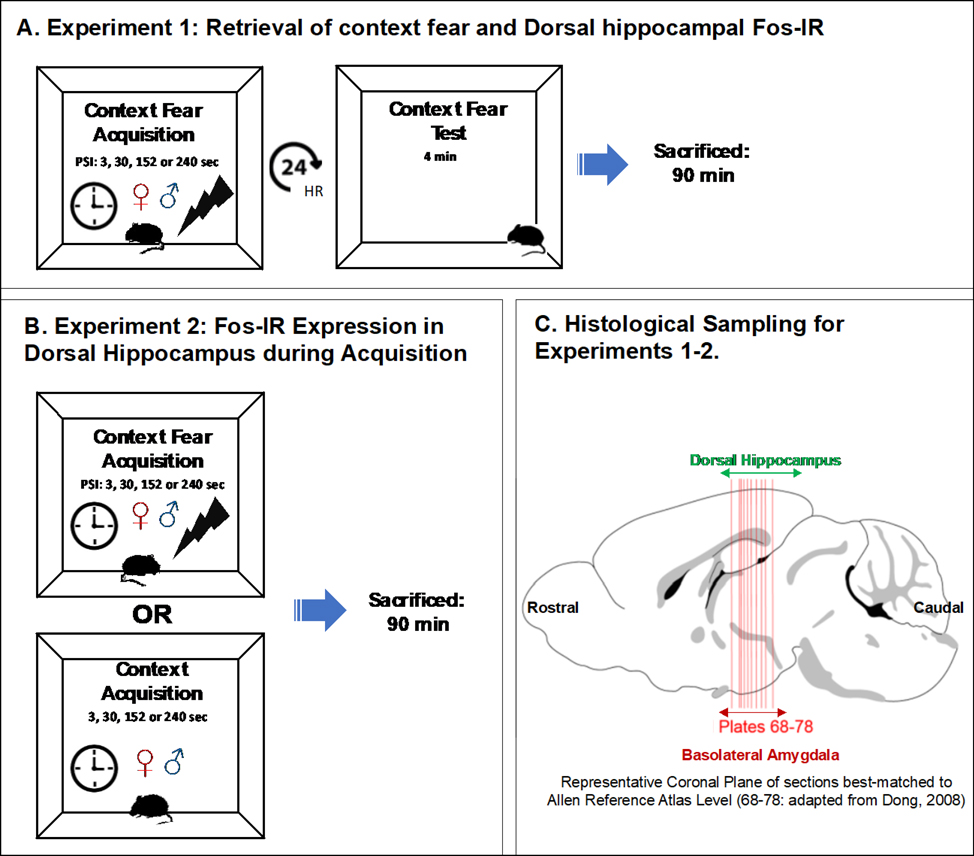
Figure 1. Experimental Design.
(A) In experiment 1, we varied the length of context exposure (3, 30, 152 or 240 sec) prior to the administration of a footshock and returned all subjects to the originally shocked context after 24 hrs. Ninety minutes following the cessation of CFC testing all subjects were sacrificed. (B) In experiment 2, we the varied lengths of context exposure (3, 30, 152 or 240 sec) which ended either with or without a footshock. Ninety minutes thereafter, all subjects were sacrificed. (C) Depicts the approximated rostral-caudal extent of coronal histological sections (containing rostral dorsal hippocampus and basolateral amygdalar nuclei) quantified for Fos-ir analysis as best-matched to plate levels of the Allen Reference Atlas (Dong, 2008).
2. Materials and Method
2.1. Subjects
61 male and 64 female 8–10 week C57Bl/6J mice were generated from a breeding colony housed in the Life Sciences Vivarium at the University at Albany, SUNY (breeders obtained from Jackson Laboratories, Bar Harbor, Maine). Mice were housed 2–3 per cage, by sex, with ad libitum access to food and water on a 14–10 hour light/dark cycle. Animal care and experiments were conducted following protocols approved by the Institutional Animal Care and Use Committee at the University of Albany, SUNY. All experimental procedures took place during the light cycle.
2.2. Apparatus
The apparatus consisted of four identical conditioning chambers measuring 30.5 × 24.1 × 21.0 cm (Med Associates Inc., VT, St. Albans). The floor of each chamber consisted of 36 stainless steel rods (29.3 × 26 × 6.1 cm) with bars 7.9mm in diameter spaced 1.8 cm apart, connected to a shock generator. Prior to conditioning and context retrieval, chambers were cleaned with 70% ethanol. Waste pans were scented with 50% Simple Green Solution and inserted under the grid floors to provide an olfactory cue. The chambers were illuminated with white fluorescent light positioned above the testing chamber A 60-dB ventilation fan located in the back of the chamber provided ambient noise. A video camera was positioned in front of the conditioning context within an external sound attenuating chamber to record behavior.
Experiment 1: Retrieval of context fear and hippocampal and BLA Fos immunoreactivity
2.3. Conditioning Procedure
All subjects were handled for 3 consecutive days for 1 minute each in a holding room adjacent to our conditioning chambers prior to training. On training day, animals were transferred in their homecage from the vivarium to the holding room where they were allowed to acclimate for 10 minutes. No exposure- home cage control animals remained in the holding room while experimental groups were placed into the conditioning chamber where they received a single footshock (1 mA, 2s) after 3, 30, 152 or 240 seconds (Fig. 1A). Mice were removed from the context immediately after delivery of the shock and brought back to the vivarium along with the no exposure group. Contextual fear memory was assessed 24 hours later by returning the mice to the training context for a 4 minute testing period and assessing the percent time freezing using Video FreezeFrame software (Med Associates). Ninety minutes after initial placement into the context animals were sacrificed (M= 23; F= 23).
2.4. Brain Tissue Collection and Processing
Animals were administered Euthanasia-III Solution and transcardially perfused with 1% potassium phosphate buffer solution and 4% paraformaldehyde (Sigma-Aldrich; MO, USA). Brains were removed, post-fixed in 4% paraformaldehyde for 24 hours, and then transferred to a 30% sucrose solution until sectioning. Brains were sliced at room temperature into 50 μm thick coronal sections and were cut via Compresstome (VF-700, Precisionary Instruments; MA, USA). Sections were collected throughout the rostral – caudal extent of the dorsal hippocampus and stored in cryo-protectant solution consisting of ethylene glycol, glycerol, and potassium phosphate buffer solution. Sections were stored at −20° C until c-Fos immunostaining procedures.
2.5. Immunohistochemistry, Nissl staining and Fos identification and Tabulation
Tissue sections were rinsed in potassium phosphate buffer solution (KPBS) three times for 10 minutes, then pretreated with 30% H2O2 for 10 minutes. After a second rinse in KPBS for 3 minutes, tissue sections were treated with non-specific antibody blocking solution consisting of KPBS, 5% Normal goat serum (Life-Technologies; CA, USA) and 1% Triton-X 100 (Sigma-Aldrich) for one hour. Tissue sections were rinsed again in KPBS, then incubated in a primary antibody solution of 2% Normal goat serum, 1% Triton-X 100 and 1:1000 rabbit polyclonal IgG c-Fos (Santa Cruz Biotechnology; CA, USA) for 48 hours at 4°C. Following three - 10 minute rinses in KPBS tissue sections were incubated in secondary antibody solution consisting of 2% Normal goat serum, 1% Triton-X 100 and 1:100 Alexa Flour 488 goat anti-rabbit IgG for 24 hours at 4°C. Tissue sections were rinsed and incubated for 10 minutes in 1% Triton-X 100 and 1% KPBS solution followed by a fifty minute incubation in Nissl stain solution – NeuroTrace 435/455 blue fluorescent Nissl stain (Invitrogen, Thermo Fisher Scientific; CA, USA). Tissue slices were then visualized and mounted onto microscope slides, cover-slipped, and scanned at 10X using a using Olympus VS-120 fluorescent Microscope. Using a Nissl channel (DAPI), sections were identified and best-matched to atlas plate levels 68 to 78 of the Allen Brain Mouse Atlas (Dong, 2008; Fig. 2 A–B). Fos positive nuclei were manually counted across the rostral - caudal extent of dorsal hippocampal and BLA plates 68–78 (Allen Brain Mouse Atlas); specifically, within CA1 (Fig. 2A: proximal-distal regions), CA3, Dentate Gyrus and basolateral amygdalar nuclei (Fig. 2B: both anterior and posterior). Criteria for establishing Fos positive expression were puncta measuring 10–20 microns in diameter and restricted within neuronal cell bodies. Total number of Fos positive cells for each region of the hippocampus and BLA are represented by the sum of Fos positive cells across all plate levels and individual plate levels (supplementary Fig. 1).
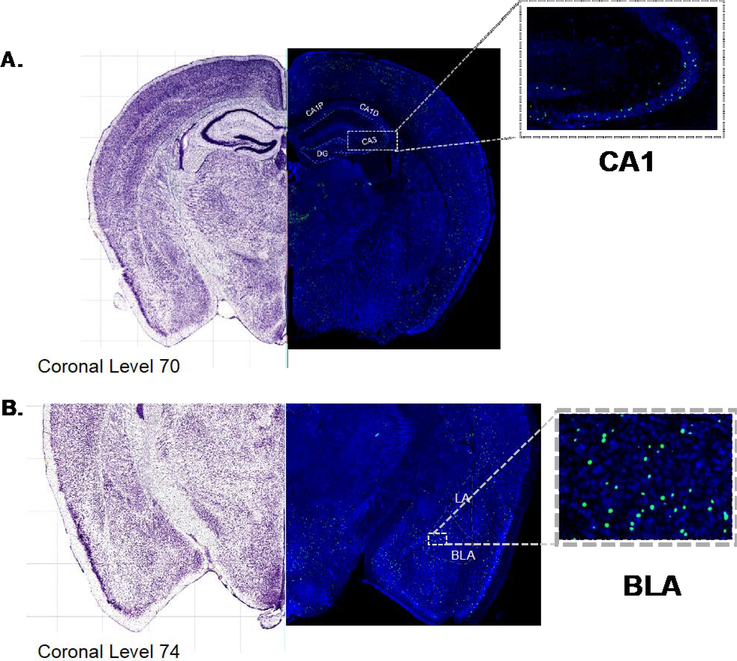
Figure 2. Representative images of (A) hippocampus and (B) BLA Fos-ir.
Representative photomicrograph of coronal sections (10 x magnification) stained for Nissl (blue) and immunostained for Fos (green) and best-matched to corresponding to coronal levels from the Allen Reference Atlas (brightfield image; left). (A) dorsal hippocampus (inset CA1) (B) basolateral amygdalar nuclei (inset anterior portion of BLA).
Experiment 2: Fos-ir Expression in Dorsal Hippocampus during Acquisition
2.6. Conditioning Procedure
All subjects were handled for 3 consecutive days for 1 minute on each day prior to training. On training day, mice were randomly assigned to one of three groups: home cage controls, no shock group or shocked group. On training day all animals were transferred in their home cage to a holding room. Home cage – no exposure animals remained in the holding room during the training period (Fig. 1B). No shock treated animals were removed from their home cage and placed into the conditioning chamber and exposed to the context for either 3, 30, 152, or 240 seconds before removal from the context and then sacrificed 90 minutes after initial placement into the context. Animals in the shock treated groups received a single 1 mA, 2 second footstock following a specified placement to shock interval of either 1, 28, 150 or 238 seconds- for a total exposure time equivalent to that of the no shock group. Animals were removed from the context immediately after context exposure and sacrificed 90 minutes after initial placement into the context. Home cage control animals were removed from the holding room and sacrificed alongside the shock and no shock groups on the same day (M=38; F=41).
2.7. Brain Tissue Collection and Processing
All brain tissue collection and preparations were identical to the procedure described in experiment 1.
2.8. Immunohistochemistry, Nissl staining and Fos identification and Tabulation
All immunohistochemical, Nissl staining, Fos verification and tabulation procedures were identical to the procedure described in experiment 1.
2.9. Data Analysis for Experiments 1 and 2
Behavioral analyses of freezing were initially conducted using planned comparisons between the immediate shock (3 sec PSI) and longer PSI groups, on the basis of previous work reporting immediate shock deficits with brief PSIs (Fanselow, 1986; Wiltgen et al, 2001). Next, in groups exhibiting percent freezing levels above the 3 sec PSI group (30, 152, 240 sec PSI), we employed a 3-way repeated measures ANOVA with test minute as a within subject factor. For the analyses of Fos-ir, the sum of Fos positive cells within each region were calculated. In experiment 2, these sums were transformed into percent change from homecage controls. These values were then analyzed by univariate ANOVAs followed by post-hoc Bonferroni correction comparisons and t-tests as detailed in Table 1.
Table 1.
Statistical analysis using a sequence of analysis with ANOVAs, bonferroni corrected multiple comparisons and Pairwise comparisons follow significant ANOVAs.
| Experiment 1: Behavior | ||||||
|
| ||||||
| 3-way ANOVA | ||||||
|
| ||||||
| Variable | Univariate F | Significance | ||||
| Sex | F(1, 22) 1.910 | ns | ||||
| PSI | F(1, 22) 18.609 | p<.01 | ||||
| Time | F(3, 43) 2.252 | ns | ||||
| PSI x Sex | F(1, 22) 1.326 | ns | ||||
| Time x Sex | F(3, 43) 1.784 | ns | ||||
| Time x PSI | F(9, 45) 1.973 | ns | ||||
| Time x Sex x PSI | F(1, 45) 2.930 | p<.01 | ||||
| Sex x Time in Minutes | ||||||
| Variable | Univariate F | Significance | ||||
| Minute 1 | F(1, 12) 7.009 | p<.05 | ||||
| Minute 2 | F(1, 12) 4.046 | ns | ||||
| Minute 3 | F(1, 12) 1.878 | ns | ||||
| Minute 4 | F(1, 12) .380 | ns | ||||
|
| ||||||
| One-way ANOVA | ||||||
|
| ||||||
| MALE | ||||||
| Variable | Univariate F | Significance | ||||
| PSI | F(3, 19) 7.128 | p<.05 | ||||
| Bonferroni corrected multiple comparisons | ||||||
|
| ||||||
| 3 vs 30 | 3 vs 152 | 3 vs 240 | ||||
| p<.01 | p<.05 | p<.01 | ||||
| FEMALE | ||||||
| Variable | Univariate F | Significance | ||||
| PSI | F(3, 18) .327 | ns | ||||
| Experiment 1: Fos-ir Expression | ||||||
|
| ||||||
| Homecage Control: Average (+/−SEM) | ||||||
| DG | CA3 | CA1P | CA1D | BLA | ||
| Male | ||||||
| 212.2 (27.604) | 60.9 (12.85) | 9.6 (3.94) | 4.8 (3.38) | 15.5 (3.86) | ||
| Female | ||||||
| 242.9 (17.93) | 66.8 (19.36) | 1.13 (.718) | 2.6 (1.14) | 17.8 (7.44) | ||
| Univariate ANOVA comparisons | ||||||
|
| ||||||
| Region: CA1 distal | ||||||
| Variable | Univariate F | Significance | ||||
| SEX | F(1, 36) 2.610 | ns | ||||
| PSI | F(3, 36) 3.582 | p<.05 | ||||
| SEX x PSI | F(3, 36) 2.487 | ns | ||||
| Region: CA1 Proximal | ||||||
| SEX | F(1, 37) 14.729 | p<.01 | ||||
| PSI | F(3, 37) 5.585 | p<.01 | ||||
| SEX x PSI | F(3, 37) 3..139 | p<.05 | ||||
| Region: CA3 | ||||||
| SEX | F(1, 45) .561 | ns | ||||
| PSI | F(3, 45) 4.799 | p<.01 | ||||
| SEX x PSI | F(3, 45) .841 | ns | ||||
| Region: DG | ||||||
| SEX | F(1, 37) .113 | ns | ||||
| PSI | F(3, 37) 2.48 | ns | ||||
| SEX x PSI | F(3, 37) .669 | ns | ||||
| Region: BLA | ||||||
| SEX | F(1, 35) .282 | ns | ||||
| PSI | F(3, 35) 3.271 | p<.05 | ||||
| SEX x PSI | F(3, 35) 1.205 | ns | ||||
| Bonferroni corrected multiple comparisions | ||||||
|
| ||||||
| Variable: PSI | ||||||
| Region: CA1 distal | ||||||
| 3 vs 30 | 3 vs 152 | 3vs 240 | 30 vs 152 | 30 vs 240 | 152 vs 240 | |
| ns | ns | p<.05 | ns | ns | ns | |
| Region: CA3 | ||||||
| 3 vs 30 | 3 vs 152 | 3vs 240 | 30 vs 152 | 30 vs 240 | 152 vs 240 | |
| ns | p<.01 | p<.05 | ns | ns | ns | |
| Region: BLA | ||||||
| 3 vs 30 | 3 vs 152 | 3vs 240 | 30 vs 152 | 30 vs 240 | 152 vs 240 | |
| ns | ns | p<.05 | ns | p<.05 | ns | |
| Experiment 2 Fos-ir Expression | ||||||
|
| ||||||
| Homecage Control: Average (+/−SEM) | ||||||
| DG | CA3 | CA1P | CA1D | BLA | ||
| Male | ||||||
| 237.4 (33.896) | 75.15 (21.38) | 18.7 (6.94) | 18.25 (9.39) | 15.4 (3.04) | ||
| Female | ||||||
| 187.75 (60.27) | 96.25 (26.265) | 16.75 (9.53) | 7.58 (.43) | 13.54 (3.03) | ||
| Univariate ANOVA comparisons | ||||||
|
| ||||||
| Region: CA1 distal | ||||||
| Variable | Univariate F | Significance | ||||
| SEX | F(1, 61) 19.568 | p<.01 | ||||
| CONTEXT EXPSOURE (CE) | F(3, 61) 3.419 | p<.05 | ||||
| SHOCK | F(1, 61) 1.759 | ns | ||||
| SEX x CE | F(3, 61) .567 | ns | ||||
| SEX x SHOCK | F(1, 61) 6.387 | p<.05 | ||||
| CE x SHOCK | F(3, 61) .705 | ns | ||||
| SEX x CE x SHOCK | F(3, 61) .126 | ns | ||||
| Region: CA1 proximal | ||||||
| Variable | Univariate F | Significance | ||||
| SEX | F(1, 61) 15.660 | p<.01 | ||||
| CONTEXT EXPSOURE (CE) | F(3, 61) 8.391 | p<.01 | ||||
| SHOCK | F(1, 61) .161 | ns | ||||
| SEX x CE | F(3, 61) 2.026 | ns | ||||
| SEX x SHOCK | F(1, 61) 12.191 | p<.01 | ||||
| CE x SHOCK | F(3, 61) 4.163 | p<.05 | ||||
| SEX x CE x SHOCK | F(3, 61) .674 | ns | ||||
| Region: CA3 | ||||||
| Variable | Univariate F | Significance | ||||
| SEX | F(1, 61) 14.222 | p<.01 | ||||
| CONTEXT EXPSOURE (CE) | F(3, 61) 2.626 | ns | ||||
| SHOCK | F(1, 61) 1.718 | ns | ||||
| SEX x CE | F(3, 61) 2.178 | ns | ||||
| SEX x SHOCK | F(1, 61) .452 | ns | ||||
| CE x SHOCK | F(3, 61) 2.523 | ns | ||||
| SEX x CE x SHOCK | F(3, 61) .704 | ns | ||||
| Region: DG | ||||||
| Variable | Univariate F | Significance | ||||
| SEX | F(1, 61) 9.099 | p<.01 | ||||
| CONTEXT EXPSOURE (CE) | F(3, 61) 3.251 | p<.05 | ||||
| SHOCK | F(1, 61) .005 | ns | ||||
| SEX x CE | F(3, 61) 1.147 | ns | ||||
| SEX x SHOCK | F(1, 61) 3.133 | ns | ||||
| CE x SHOCK | F(3, 61) 4.423 | p<.01 | ||||
| SEX x CE x SHOCK | F(3, 61) .861 | ns | ||||
| Region: BLA | ||||||
| Variable | Univariate F | Significance | ||||
| SEX | F(1, 61) 19.272 | p<.01 | ||||
| CONTEXT EXPSOURE (CE) | F(3, 61) 3.319 | p<.05 | ||||
| SHOCK | F(1, 61) 1.419 | ns | ||||
| SEX x CE | F(3, 61) 5.481 | p<.01 | ||||
| SEX x SHOCK | F(1, 61) 6.136 | p<.05 | ||||
| CE x SHOCK | F(3, 61) 1.131 | ns | ||||
| SEX x CE x SHOCK | F(3, 61) 4.934 | p<.01 | ||||
| Region: CA1 proximal | ||||||
| Variable: SEX x SHOCK | ||||||
|
| ||||||
|---|---|---|---|---|---|---|
| t-test: fold increase in Fos-IR neurons under shocked conditions in males and females compared to no shock | ||||||
|
| ||||||
| t(48) = 3.401, p<.01 | ||||||
| Region: CA1 distal | ||||||
| Variable: SEX x SHOCK | ||||||
|
| ||||||
| t-test: fold increase in Fos-IR neurons under shocked conditions in males and females compared to no shock | ||||||
|
| ||||||
| t(35) = 2.564, p<.05 | ||||||
| Region: DG | ||||||
| Variable: CE x SHOCK | ||||||
| 3 vs 30 | 3 vs 152 | 3vs 240 | 30 vs 152 | 30 vs 240 | 152 vs 240 | |
| Shock | ns | ns | ns | ns | ns | ns |
| No Shock | ns | ns | p<.05 | ns | p<.05 | ns |
| Region: BLA | ||||||
| Variable | Univariate F | Significance | ||||
| SEX | F(1, 59) 19.272 | p<.01 | ||||
| CONTEXT EXPSOURE (CE) | F(3, 59) 3.319 | p<.05 | ||||
| SHOCK | F(1, 59) 1.419 | ns | ||||
| SEX x CE | F(3, 59) 5.481 | p<.01 | ||||
| SEX x SHOCK | F(1, 59) 6.136 | p<.01 | ||||
| CE x SHOCK | F(3, 59) 1.131 | ns | ||||
| SEX x CE x SHOCK | F(3, 59) 4.934 | p<.05 | ||||
| Variable: SEX x SHOCK @ PSI 30 sec | ||||||
|
| ||||||
| t-test: fold increase in Fos-ir neurons under shocked conditions in males and females compared to no shock | ||||||
|
| ||||||
| t(9) = .348, p >.05 | ||||||
| Variable: SEX x SHOCK @ PSI 152 sec | ||||||
|
| ||||||
| t-test: fold increase in Fos-ir neurons under shocked conditions in males and females compared to no shock | ||||||
|
| ||||||
| t(8) = 6.784, p <.05 | ||||||
3. Results
Experiment 1: Retrieval of Context Fear and Dorsal Hippocampal Fos-ir
3.1. Context Fear Retrieval Test
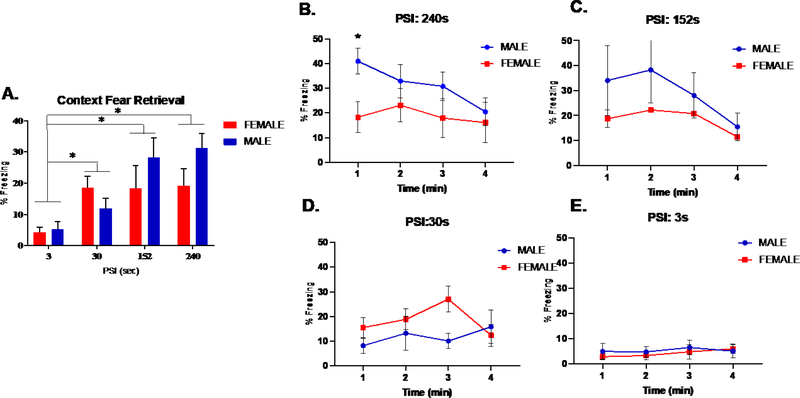
Figure 3A depicts the percentage of time spent freezing for male and female mice during a 4 min context fear retrieval test after conditioning with 4 placement-to-shock intervals (PSI). Since prior evidence indicates that brief PSIs of less than 20 sec produces an immediate shock deficit in context fear conditioning (Fanselow, 1986; Wiltgen et al, 2001), planned comparisons were conducted to determine if PSI of 30 sec and greater yielded learning over our immediate shock group (3 sec). These comparisons revealed significant learning at 30 (p<.01), 152 (p<.05), and 240 sec PSIs (p<.01). We then analyzed these data for context fear retrieval using a 3-way analysis of variance (ANOVA) for Sex (male and female), PSI (30, 152, 240 sec) as between subject factors and Test Minute (1–4) as a within subject factor (Fig. 3B–E). The ANOVA detected a 3-way interaction between Sex, PSI, and Test Minute (F(1, 45) 2.930, p<.01). To further determine the source of this interaction, follow up ANOVAs were performed at each test minute and revealed a Sex x PSI interaction at minute 1 (F(1, 12) 7.009, p<.05) but not at other test minutes (see Table 1). Post hoc comparisons with Bonferroni corrections confirmed that freezing at minute 1 was greater in males than females for 240 (p<.05), but not 152 sec PSI (p=.07) (Fig. 3B–C). Taken together, these results indicate context fear retrieval as measured by freezing increases with conditioning utilizing longer PSIs and that sex differences in freezing were observed at the longest PSI. Next, we sought to determine how the retrieval of context fear associations established under these distinct PSIs corresponded to dorsal hippocampal activation.
Figure 3. Freezing Behavior during Context Fear Retrieval.
(A) Overall freezing during a 4 minute test of CFC. Planned comparison identified significant levels of freezing in 240, 152 and 30 sec groups relative to the 3 sec PSI group (p < .05) (B-E) Freezing during each test minute of the 4 minute test of CFC. 3-way analysis of variance (ANOVA) for Sex and PSI (30, 152, 240 sec) as between subject factors and Test Minute (1–4) as a within subject factor identified a significant sex difference at minute 1 for (B) 240 (p<.05), but not (C) 152 (p=.07) sec, (D) 30 sec, or (E) 3 sec PSI; n=54, 6–9 animals per group. * denotes significant difference between males and females at minute 1, while colored * denotes significant difference within sex.
3.2. DORSAL HIPPOCAMPAL FOS-IR
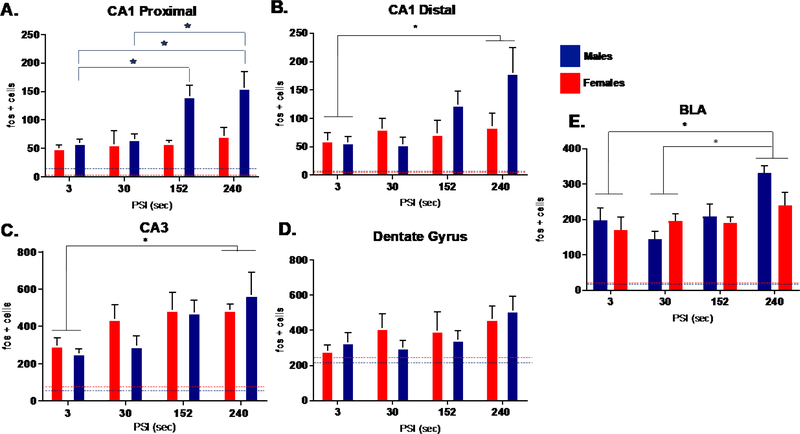
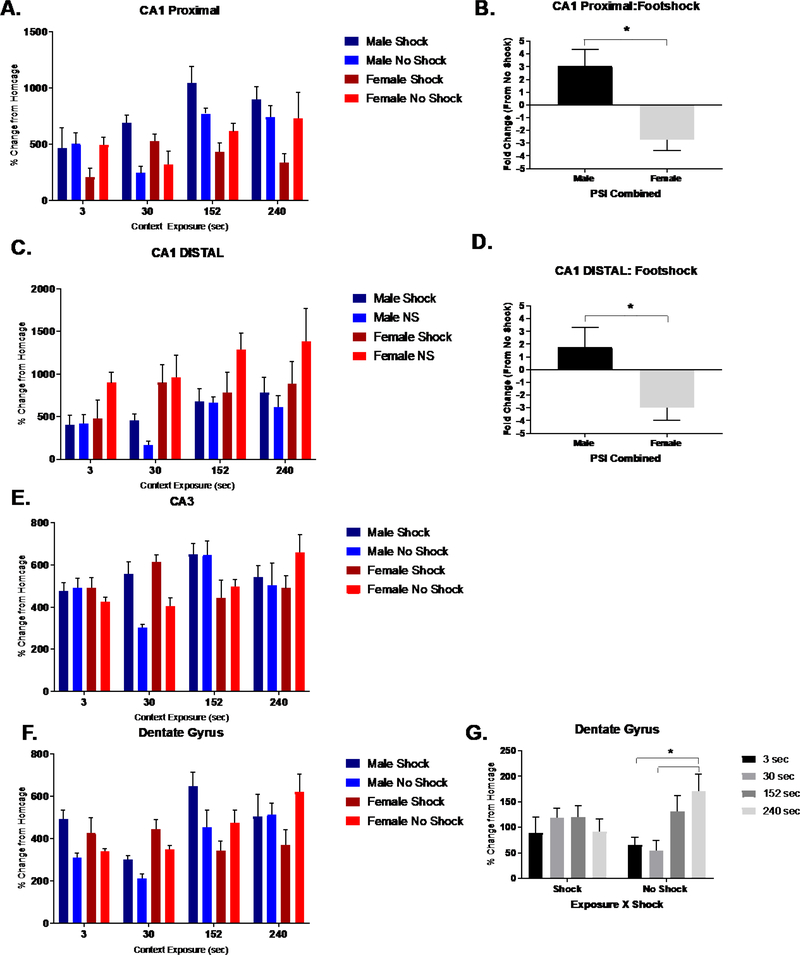
We investigated dorsal hippocampal activation in three regional subfields, including the proximal and distal CA1, CA3, and dentate gyrus verified by the cytoarchitectural features using Nissl stain (Fig. 2A). We found that Fos-ir in proximal CA1 of males, but not females, increased with context fear retrieval encoded with longer PSIs (Fig. 4A). The number of CA1 proximal Fos immunoreactive cells was analyzed using a factorial ANOVA for Sex and PSI. Using an ANOVA, we detected an interaction between Sex and PSI (F (3, 36) = 3.139, p<.01. This was followed by separate one-way ANOVAs for each sex, which revealed a main effect of PSI in males (F (3, 19) = 7.128, p< .05), but females (F (3, 18) = .327, p> .05). In males, Bonferroni corrected post hoc tests confirmed that context fear retrieval resulting from longer PSIs (240 and 152 sec) produced greater Fos-ir compared to shorter PSIs (3 sec: p < .05; p < .01; 30 sec: p < .01).
Figure 4. Experiment 1: Dorsal Hippocampus (A-D) and BLA (E) Fos – ir retrieval related activation plotted across placement to shock interval in males and females.
Experiment 1: Mean number of Fos – ir cells in response to context fear retrieval in males (blue) and females (red) plotted across placement to shock interval. Blue (male) and red (female) dotted lines represent mean number of Fos-ir cells in homecage control groups. (A) CA1 proximal Fos – ir cells revealed a significant Sex by PSI interaction. Follow-up univariate ANOVA identified greater immunoreactivity with increasing PSIs in males (p <.05) but not females (p >.05) (B) CA1 distal revealed no significant interaction between Sex and PSI; significant main effect of PSI [240 > 3] (C) CA3 revealed a significant main effect of PSI; [240 > 3] (D) DG revealed no significant interaction or main effect. (E) Basolateral amygdalar nuclei revealed a significant main effect of PSI [240 > 3, 30] (n= 5–6 animals per group). * denotes significant difference across PSI.
While no significant interactions were detected in either distal CA1, CA3, or dentate gyrus (Fig. 4D) (p>.05), we demonstrated overall main effects of PSI for (Fig. 4B) distal CA1 (F (3, 36) = 3.582, p< .05) and (Fig. 4C) CA3 (F (3, 37) = 4.799, p< .01). Post hoc comparisons revealed that context fear retrieval resulting from longer PSIs (240s) produced greater Fos-ir compared to shorter PSI (3 sec). While overall Fos activation in portions of CA1 and CA3 increased with retrieval of contextual fear association established under longer PSIs, this pattern within proximal CA1 was only evident in males.
3.3. BASOLATERAL AMYGDALAR – NUCLEI FOS-IR
Nissl stained sections were used to verify the general boundaries of the basolateral amygdala (Fig. 2B), a region crucial for associative fear memory storage. This region showed that context fear memories acquired with longer PSIs produced greater Fos-ir, independent of sex (Fig. 4E). This result was supported by a significant main effect of PSI (F (3, 35) = 5.944, p< .05) followed by post hoc comparisons, where subjects conditioned with the longest PSI significantly differed in Fos- ir from those conditioned with shorter PSIs [PSI 3 < 240s (p < .01); PSI 30 < PSI 240s (p < .05)]. We detected no significant interaction between PSI and Sex (p>.05). These results while consistent with the overall role of the BLA in the formation and retrieval of associative fear memories, suggest combined Fos-ir counts from anterior and posterior BLA subnuclei as assessed here, may only be evident under the longest PSI.
Experiment 2: Acquisition of Context Fear and Dorsal Hippocampal Fos- ir
3.4. DORSAL HIPPOCAMPAL FOS-IR
As experiment 1 established that retrieval of an associative context fear memory resulted in a sex-specific increase in Fos- ir within a dorsal portion of CA1, we next sought to examine activation of the dorsal hippocampus under learning conditions that independently support aversive and non-aversive contextual memories in male and female mice. So, we assessed hippocampal and BLA activation resulting from conditioning to both a neutral (un-shocked) and aversive (shocked) contexts under varying context encoding time. For CA1 proximal (Fig. 5A), we analyzed % change in Fos-ir (from homecage) using a 3-way ANOVA for Sex (male or female), Shock (shock or no) and Context Exposure (3, 30. 152, or 240 sec). The ANOVA detected a two-way interaction of Context Exposure x Shock (F (3, 61) = 4.163, p< .01) and Sex x Shock (F (3, 61) = 12.191, p< .01), suggesting exposure to shock differentially effects Fos- ir into both a sex and context exposure dependent manner. A follow up test assessing the fold change in Fos- ir to footshock confirmed that proximal CA1 neurons in males and females respond uniquely to this aversive US (p<.01; Fig. 5B). Post hoc comparisons within non-shock mice revealed that Fos-ir within the longest context exposure group significantly differed from those of shorter context exposure groups (30 (p< .01) and 152 sec (p< .01)).
Figure 5. Experiment 2: Hippocampal Fos – ir acquisition related activation.
Experiment 2: Mean percent change (from homecage subjects) in the number of dorsal hippocampal Fos – ir cells in response to context or context fear acquisition in males and females plotted across context exposure times. (A,C,E,F) Fos – ir activation in shocked and non-shocked groups. (B,D) CA1 proximal and distal of males and females were oppositely impacted by footshock exposure. Fold change in Fos-ir to footshock revealed significant sex differences (*p<.05) in CA1 proximal (B) and distal (D) regions of dorsal hippocampus. (E) No significant interactions in CA3 region were identified. (G) DG revealed a significant PSI x Condition interaction (p <.01). * denotes significant difference in across PSI in non-shocked groups (p<.05). n=85; 3–6 animals per group.
Similar to proximal CA1, we found that the distal portion of the CA1 in both male and female mice were differentially affected by footshock exposure (Fig. 5C). This result was supported by a significant interaction of Shock x Sex (F (1, 61) = 6.387, p< .05). A follow-up test assessing the fold change in Fos-ir to footshock confirmed that distal CA1 neurons in males and females differentially respond to an aversive US (Fig. 5D). This result is consistent with proximal CA1. No significant interactions were detected within CA3 (p>.05), however we did find a significant main effect of sex, such that males showed higher Fos-ir compared to females (p>.05) no significant main effect of PSI was detected (Fig. 5E). In the dentate gyrus, we identified an interaction between Context Exposure x Shock (F (4, 61) 4.423, p<.01; Fig. 5F). Post hoc comparisons within non-shock animals revealed that the longest context exposure group (240 sec) significantly differed from 3 (p< .01) and 30 (p< .01) sec context exposure groups, which were not evident in shocked groups (Fig. 5G).
3.5. BASOLATERAL AMYGDALAR NUCLEI FOS-IR
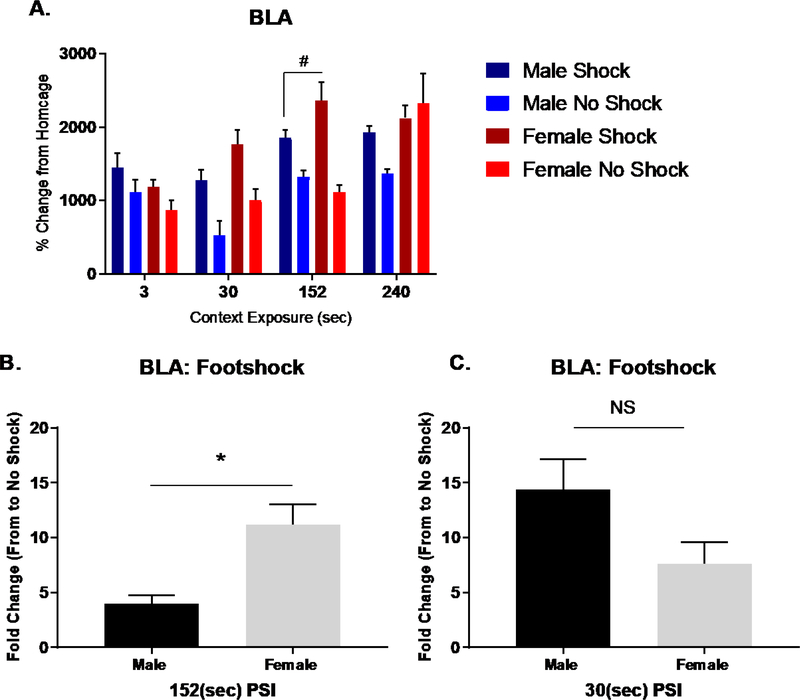
While BLA Fos-ir increased with retrieval of context fear established with longer PSIs, we found that conditioning produced a 3-way interaction between Sex x Context Exposure x Shock (F (3, 60) 4.423, p<.01) (Fig. 6A). To further parse out the contributions of Shock x Sex at each Context Exposure period, we performed separate 2-way ANOVAs. These analyses detected an interaction for Sex x Shock at context exposure periods of 30 (F (1, 15) 5.932, p<.01) and 152 sec (F (1, 16) 6.655, p<.05). For these periods, follow up tests assessing the fold change in Fos-ir to footshock indicated that the BLA in females was more responsive than males at 152 sec (Fig. 6B) 30 sec PSIs (Fig. 6C). While we detected no interactions at 3 and 240 sec, we did identify a main effect of Shock at 3 sec (F (1, 15) 4.773, p<.05) and a main effect of Sex at 240 sec (F (1, 60) 8.137, p<.01). This main effect of Sex at 240 sec arose from greater Fos-ir in females than males. Collectively, these results support prior evidence that the BLA of female mice show greater activation than male mice (Keiser et al., 2017).
Figure 6. Experiment 2 - Basolateral Amygdala Fos – ir retrieval related activation.
Experiment 2 – Mean percent change (from homecage subjects) in the number of basolateral amygdalar nuclei Fos – ir cells in response to context or context fear acquisition in males and females plotted across context exposure times. (A) Fos – ir activation in shocked and non-shocked groups. (B) Fold change in Fos-ir to footshock revealed significant sex difference in BLA at 152 sec PSI (p<.05) but not at (C) 30 sec PSI (ns).
4. Discussion
Here, we examined whether adult sex differences in contextual fear conditioning arise from unique capacities in contextual-spatial processing and dorsal hippocampal activation by modifying the initial time spent in the conditioning context in the presence or absence of a footshock. Though our sex difference in conditioning was specifically evident at the 240 sec PSI (experiment 1), we identified an overall sex-specific response in the CA1 region to an aversive outcome, in which a single footshock induced a net increase of Fos-ir in males and a decrease in females (experiment 2). This main finding is consistent with the results of experiment 1, where activation of the proximal CA1 region during context fear retrieval was evident in males, but not females. Independent of sex, we observed a similar pattern of activation in CA3 and distal CA1. Finally, we identified sex differences in basolateral amygdalar nuclei activation. Taken together, we provide evidence that context fear conditioning in male and female mice may arise from differences in how footshock impacts dorsal hippocampal-dependent contextual processing. We believe these results will more broadly shed light upon sex differences in the processing demands of the hippocampus and form the foundation to spur new theories of hippocampal function.
4.1. Behavior
Our behavioral results indicate that successful encoding of the conditioning context is dependent on the length of time spent in the context prior to the footshock administration, consistent with previous results (Wiltgen et al., 2001, Frankland et al., 2004; Leake et al., 2017). In both males and females, immediate shock (3 sec) upon placement into the conditioning chamber induced little or no freezing. Increasing context exposure produced greater freezing during retrieval. A cursory glance of fear retrieval in males and females suggests freezing in males increased with longer PSIs, while freezing in females plateaued after 30 sec PSI. Although this pattern of results slightly diverges from a prior report (Wiltgen et al., 2001), where conditioning in female mice plateaued at 180 sec PSI, we posit differences in our procedure such as length of post shock exposure likely account for these differences. Such differences are noted in Bernier et al (2015).
4.2. DH Activation to CFC Retrieval
A major finding that we observed describes greater CA1 neuronal activation after context fear retrieval with longer placement to shock intervals in male but not female mice. Examining CA1 IEG expression only in males, Leake et al., (2017) found a similar PSI-dependent increase in CA1 Arc expression. Here, we found that both males and females engage the dorsal hippocampus to retrieve contextual-spatial memories, but functional differences in hippocampal activation between the sexes exist that largely revolve around retrieval-dependent recruitment of proximal CA1 in males, but not females. Consistent with these findings, Kudo et al., (2004) found that the entire CA1 from male rats expressed greater phosphorylated CREB-immunoreactivity compared to females with context fear retrieval. This result is consistent with Keiser et al., (2017) that measured Fos-ir in mice.
It is well-established that proximal and distal segments of the CA1 have distinct functions and make unique connections within the parahippocampal circuit (Witter et al., 2000; Naber et al., 2001; Knierim et al., 2014; Nakazawa et al., 2016). The proximal CA1 is crucial for encoding information related to one’s position in space and retrieving contextual memories, while the distal CA1 likely encodes detailed information about the environment (Knierim et al., 2014; Nakazawa et al., 2016). Taken together, these functional differences suggest that proximal and distal segments of CA1 possess independent functions requiring subregional analysis for reporting CA1 activation (Nakazawa et al., 2016).
4.3. DH Activation to CFC Acquisition
Differential activation of the hippocampus may present a plausible explanation for previously reported sex differences in context fear memory and contextual-spatial processing (Maren et al., 1994, Kudo et al., 2004, Gresack et al., 2009, Keiser et al, 2017). Here, we modified the total time subjects were exposed to the conditioning chamber in the presence or absence of a footshock. We revealed that the proximal and distal regions of CA1 produced a net increase in males and decrease in females of Fos-ir to footshock. These Fos-ir patterns were independent of context exposure time. This sex-specific response of CA1 neurons during conditioning corresponds to our current findings that context fear retrieval induced CA1 proximal activation in males but not females. Our current results suggest that previously reported sex differences in context fear conditioning may partially result from a sex distinct responsiveness of CA1 neurons to footshock. In dentate gyrus, increased context exposure time resulted in increased Fos-ir. However, this effect was disrupted following footshock. These findings are consistent with previous work demonstrating that DG neurons respond to cues used to discriminate distinct contexts (Rangel et al., 2014). While changes in DG activation in response to specifically aversive stimuli remains understudied, we speculate that local information processing in the dentate gyrus likely includes aversive cues, in addition to the known olfactory and spatial cues.
4.4. BLA Activation to CFC
We also examined Fos-ir in the BLA during both retrieval and acquisition as a function of varying context exposure. Consistent with previous proposals, memories of contextual stimuli are formed within the hippocampal subfields, and the BLA encodes and maintains an associative context-shock fear memory (Fendt & Fanselow, 1999; Fanselow, 2000; Maren, 2001; Reijmers et al., 2007). Following retrieval, we found that BLA activation did not vary as a function of sex. We found the BLA was activated based on variations in the length of context exposure, as longer PSIs induced greater Fos-ir. However, acquisition produced a three-way interaction between sex, length of context exposure, and the presence or absence of an aversive stimuli. At PSIs that support conditioning (30, 152, and 240 sec), we found the BLA was not only responsive to footshock, but also produced greater overall Fos-ir in females than males. However, it was only at the 152 sec context exposure period that this sex difference was driven by footshock. These results are consistent with a previous study that demonstrated heightened responsiveness of BLA neurons and increased dendritic spines in females compared to males (Blume et al., 2017). Taken together, our results suggest that contextual fear conditioning in males and females induces a sex-dependent activation in dorsal CA1 to footshock and to some extent the BLA.
4.5. Conclusions
Based on our results, we propose that previously reported sex differences in contextual fear conditioning may largely result from differences in how dorsal hippocampal CA1 in males and females respond to footshock presentations. In a previous study that did not use sex as a variable, calcium-imaging of somatostatin-positive CA1 interneurons during a modified fear conditioning demonstrated increased activity to an aversive airpuff (Lovett-Baron et al., 2014). This heightened activity may provide inhibitory influence to pyramidal neurons, as reported here, in females. Hippocampal long-term potentiation (LTP) in male and female rats reveals that high frequency theta stimulation of the performant path in males induced a robust and persistent potentiation compared to females (Maren et al., 1994). Conversely, studies exploring the effects of acute stress using a repeated tail shock procedure identified sex-specific-changes in hippocampal neuronal morphology and functioning. Female rats showed an attenuation in both hippocampal CA1 dendritic spines and subsequent hippocampal-dependent trace eye conditioning, while male rats exhibited increases in dendritic spines and conditioning (Wood & Shores, 1998; Wood et al., 2001; Shors et al., 2001,2004). Consistent with these findings, our results indicate that an aversive footshock in males upregulates CA1 activation, while a footshock downregulated CA1 activation in females. Collectively, this up-regulation in males may promote better contextual processing, while this down-regulation in females may diminish contextual learning.
The general supposition that the dorsal hippocampus processes aversive footshock components of the environment is still a matter of debate. Other studies have failed to find evidence of the DH processing of additional information other than contextual spatial information (Fanselow, 2000; Matus-Amat et al., 2004; Zelikowsky et al., 2014). Differences in methodology and analyzing the DH may contribute to these conflicting findings.
In summary, these experiments suggest that males and females differentially recruit the dorsal hippocampus during both the processing and retrieval of contextual information, particularly within CA1 neurons. Further investigations will determine how differences between males and females utilize differential CA1 activation to modulate contextual encoding as well as to elucidate the underlying mechanisms (e.g. molecular, hormonal, and/ or neural connection differences) that triggers sex differences in hippocampal activation. Functional differences in hippocampal and BLA neural ensemble and network activation and recruitment between sexes may drive the behavioral differences observed in contextual fear conditioning. Our proposed framework may also shed light upon sex differences observed in the onset and prevalence of psychopathological disorders such as in Post-Traumatic Stress Disorder (PTSD), where deficits in contextual processing have been proposed to contribute to the development of this disorder (Milad et al., 2009; Maren et al., 2013). Reduced CA1 DH activation in females may serve as a contributing factor for greater susceptibility to developing this disorder. More work will uncover how reduced recruitment of CA1 neuronal ensembles under aversive conditions, may ultimately lead us to a greater understanding of the etiology of sex differences in fear related disorders.
Supplementary Material
Support:
National Institute of Mental Health R01/MH114961 (AMP), State University of New York, University at Albany, Startup Funds (AMP).
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Acheson DT, Gresack JE, & Risbrough VB (2012). Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology, 62(2), 674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Arruda-Carvalho M, Josselyn SA, & Frankland PW (2012). Ontogeny of contextual fear memory formation, specificity, and persistence in mice. Learning & Memory, 19(12), 598–604. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing [Google Scholar]
- Armoskus C, Mota T, Moreira D, & Tsai HW (2014). Effects of prenatal testosterone exposure on sexually dimorphic gene expression in the neonatal mouse cortex and hippocampus. Journal of steroids & hormonal science, 5(3), 1000139. [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, & Fanselow MS (1999). Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. The Journal of neuroscience, 19(3), 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, & Fanselow MS (2001). Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus, 11(1), 8–17. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, & McDonald RJ (1999). Discriminative fear conditioning to context expressed by multiple measures of fear in the rat. Behavioural brain research, 101(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, & McDonald RJ (2000). Amygdala, hippocampus and discriminative fear conditioning to context. Behavioural brain research, 108(1), 1–19. [DOI] [PubMed] [Google Scholar]
- Biedenkapp JC, & Rudy JW (2004). Context memories and reactivation: constraints on the reconsolidation hypothesis. Behavioral neuroscience, 118(5), 956. [DOI] [PubMed] [Google Scholar]
- Bienkowski MS, Bowman I, Song MY, Gou L, Ard T, Cotter K, … & Azam S (2018). Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks. Nature neuroscience, 21(11), 1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC (1970). Species-specific defense reactions and avoidance learning. Psychological review, 77(1), 32. [Google Scholar]
- Blanchard RJ, Fukunaga KK, & Blanchard DC (1976). Environmental control of defensive reactions to footshock. Bulletin of the Psychonomic Society, 8(2), 129–130. [Google Scholar]
- Blume SR, Freedberg M, Vantrease JE, Chan R, Padival M, Record MJ & Rosenkranz JA (2017). Sex-and estrus-dependent differences in rat basolateral amygdala. Journal of Neuroscience, 37(44), 10567–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon L, Odynocki N, Santarelli A, & Poulos AM (2018). Sexual differentiation of contextual fear responses. Learning & Memory, 25(5), 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumas S, Halley H, Francés B, & Lassalle JM (2005). Encoding, consolidation, and retrieval of contextual memory: differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learning & memory, 12(4), 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachtler J, Fox KD, & Good MA (2011). Gender specific requirement of GluR1 receptors in contextual conditioning but not spatial learning. Neurobiology of learning and memory, 96(3), 461–467. [DOI] [PubMed] [Google Scholar]
- Davis S, Bozon B, & Laroche S (2003). How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning?. Behavioural brain research, 142(1), 17–30. [DOI] [PubMed] [Google Scholar]
- Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, … & Hen R (2014). Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron, 83(1), 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW (2008). The Allen reference atlas: A digital color brain atlas of the C57Bl/6J male mouse. John Wiley & Sons Inc. [Google Scholar]
- Dong HW, Swanson LW, Chen L, Fanselow MS, & Toga AW (2009). Genomic– anatomic evidence for distinct functional domains in hippocampal field A1. Proceedings of the National Academy of Sciences, 106(28), 11794–11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS (1986). Associative vs topographical accounts of the immediate shock-freezing deficit in rats: implications for the response selection rules governing species-specific defensive reactions. Learning and Motivation, 17(1), 16–39. [Google Scholar]
- Fanselow MS (1990). Factors governing one-trial contextual conditioning. Animal Learning & Behavior, 18(3), 264–270. [Google Scholar]
- Fanselow MS, & LeDoux JE (1999). Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron, 23(2), 229–232. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (2000). Contextual fear, gestalt memories, and the hippocampus. Behavioural brain research, 110(1), 73–81. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, & Poulos AM (2005). The neuroscience of mammalian associative learning. Annu. Rev. Psychol, 56, 207–234. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, & Dong HW (2010). Are the dorsal and ventral hippocampus functionally distinct structures?. Neuron, 65(1), 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, & Silva AJ (1998). The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behavioral neuroscience, 112(4), 863. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA, Anagnostaras SG, Kogan JH, Takahashi E, & Silva AJ (2004). Consolidation of CS and US representations in associative fear conditioning. Hippocampus, 14(5), 557–569. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, & Fanselow MS (2001). Cholinergic modulation of pavlovian fear conditioning: effects of intrahippocampal scopolamine infusion. Hippocampus, 11(4), 371–376. [DOI] [PubMed] [Google Scholar]
- Galea S, Nandi A, & Vlahov D (2005). The epidemiology of post-traumatic stress disorder after disasters. Epidemiologic reviews, 27(1), 78–91. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Schafe GE, Orr PT, & Frick KM (2009). Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal- regulated kinase activation. Neuroscience, 159(2), 451–467. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, & McGaugh JL (2001). Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. The Journal of Neuroscience, 21(14), 5089–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, & Everitt BJ (2001). Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. Journal of Neuroscience, 21(6), 2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA (2006). Elemental representations of stimuli in associative learning. Psychological review, 113(3), 584. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Rosenberg JS, & Kesner RP (2008). The role of the dentate gyrus, CA3a, b, and CA3c for detecting spatial and environmental novelty. Hippocampus, 18(10), 1064–1073. [DOI] [PubMed] [Google Scholar]
- Ji J, & Maren S (2008). Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learning & Memory, 15(4), 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, & Fanselow MS (1992). Modality-specific retrograde amnesia of fear. Science, 256(5057), 675–677 [DOI] [PubMed] [Google Scholar]
- Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, & Tronson NC (2017). Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology, 42(2), 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, & Deadwyler SA (2003). Experience-dependent regulation of the immediate- early gene arc differs across brain regions. The Journal of Neuroscience, 23(16), 6443–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry, 62(6), 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, & Hen R (2013). Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron, 77(5), 955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, & Hen R (2014). Add neurons, subtract anxiety. Scientific American, 311(1), 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MJ, & Westbrook RF (1993). Effects of exposure to a to-be-shocked environment upon the rat’s freezing response: Evidence for facilitation, latent inhibition, and perceptual learning. The Quarterly Journal of Experimental Psychology Section B, 46(3b), 271–288. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP, & Deshmukh SS (2014). Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local–global reference frames. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1635), 20130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Lee HJ, & Kim JJ (2006). Early life stress impairs fear conditioning in adult male and female rats. Brain research, 1087(1), 142–150. [DOI] [PubMed] [Google Scholar]
- Krasne FB, Cushman JD, & Fanselow MS (2015). A Bayesian context fear learning algorithm/automaton. Frontiers in behavioral neuroscience, 9, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubik S, Miyashita T, & Guzowski JF (2007). Using immediate-early genes to map hippocampal subregional functions. Learning & Memory, 14(11), 758–770. [DOI] [PubMed] [Google Scholar]
- Kudo K, Qiao CX, Kanba S, & Arita J (2004). A selective increase in phosphorylation of cyclic AMP response element-binding protein in hippocampal CA1 region of male, but not female, rats following contextual fear and passive avoidance conditioning. Brain research, 1024(1), 233–243. [DOI] [PubMed] [Google Scholar]
- Leake J, Zinn R, Corbit L, & Vissel B (2017). Dissociation between complete hippocampal context memory formation and context fear acquisition. Learning & Memory, 24(4), 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K, & Milad MR (2012). Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biology of mood & anxiety disorders, 2(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barron M, Kaifosh P, Kheirbek MA, Danielson N, Zaremba JD, Reardon TR, & Losonczy A (2014). Dendritic inhibition in the hippocampus supports fear learning. Science, 343(6173), 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MD, Sousa N, & Paula-Barbosa MM (1991). Sexual dimorphism in the mossy fiber synapses of the rat hippocampus. Experimental Brain Research, 87(3), 537–545. [DOI] [PubMed] [Google Scholar]
- Malkani S, & Rosen JB (2000). Differential expression of EGR-1 mRNA in the amygdala following diazepam in contextual fear conditioning. Brain research, 860(1), 53–63. [DOI] [PubMed] [Google Scholar]
- Maren S, De Oca B, & Fanselow MS (1994). Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain research, 661(1), 25–34. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS (1997). Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol. Learn. Mem. 67, 142–149 [DOI] [PubMed] [Google Scholar]
- Maren S (2001). Is there savings for pavlovian fear conditioning after neurotoxic basolateral amygdala lesions in rats?. Neurobiology of learning and memory, 76(3), 268–283. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, & Liberzon I (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience, 14(6), 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, & Rudy JW (2004). The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. Journal of Neuroscience, 24(10), 2431–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Morrison JH (2013). The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron, 79(1), 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, & Zinbarg R (2006). A contemporary learning theory perspective on the etiology of anxiety disorders: it’s not what you thought it was. American psychologist, 61(1), 10. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, … & Rauch SL (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological psychiatry, 66(12), 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MD, Cushman J, Chandra D, Homanics GE, Olsen RW, & Fanselow MS (2010). Trace and contextual fear conditioning is enhanced in mice lacking the α4 subunit of the GABAA receptor. Neurobiology of learning and memory, 93(3), 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber PA, Lopes da Silva FH, & Witter MP (2001). Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus, 11(2), 99–104. [DOI] [PubMed] [Google Scholar]
- Nadel L, & Willner J (1980). Context and conditioning: A place for space. Physiological Psychology, 8(2), 218–228. [Google Scholar]
- Nakazawa Y, Pevzner A, Tanaka KZ, & Wiltgen BJ (2016). Memory retrieval along the proximodistal axis of CA1. Hippocampus, 26(9), 1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD & Soliman F (2012). Altered fear learning across development in both mouse and human. Proceedings of the National Academy of Sciences, 109(40), 16318–16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Lehmann J, & Feldon J (1999). Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacology Biochemistry and Behavior, 64(4), 753–759. [DOI] [PubMed] [Google Scholar]
- Poulos AM, Zhuravka I, Long V, Gannam C, & Fanselow M (2015). Sensitization of fear learning to mild unconditional stimuli in male and female rats. Behavioral neuroscience, 129(1), 62. [DOI] [PubMed] [Google Scholar]
- Rangel LM, Alexander AS, Aimone JB, Wiles J, Gage FH, Chiba AA, & Quinn LK (2014). Temporally selective contextual encoding in the dentate gyrus of the hippocampus. Nature communications, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP, & Fanselow MS (2005). Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neuroscience & Biobehavioral Reviews, 29(8), 1207–1223. [DOI] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, & Mayford M (2007). Localization of a stable neural correlate of associative memory. Science, 317(5842), 1230–1233. [DOI] [PubMed] [Google Scholar]
- Roof RL (1993). The dentate gyrus is sexually dimorphic in prepubescent rats: testosterone plays a significant role. Brain research, 610(1), 148–151. [DOI] [PubMed] [Google Scholar]
- Rudy JW, & O’Reilly RC (1999). Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral neuroscience, 113(5), 867. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, & Matus-Amat P (2004). Understanding contextual fear conditioning: insights from a two-process model. Neuroscience & Biobehavioral Reviews, 28(7), 675–685. [DOI] [PubMed] [Google Scholar]
- Rudy JW (2009). Context representations, context functions, and the parahippocampal– hippocampal system. Learning & memory, 16(10), 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders Matthew J., Wiltgen Brian J., and Fanselow Michael S.. “The place of the hippocampus in fear conditioning.” European journal of pharmacology 463.1–3 (2003): 217–223. [DOI] [PubMed] [Google Scholar]
- Santarelli AJ, Khan AM, & Poulos AM (2018). Contextual fear retrieval-induced Fos expression across early development in the rat: An analysis using established nervous system nomenclature ontology. Neurobiology of learning and memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM (2015). Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiology of stress, 1, 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Smithuis J, Stamatakis AM, Jennings JH, Kantak PA, Ung RL, & Stuber GD (2014). Inhibition of projections from the basolateral amygdala to the entorhinal cortex disrupts the acquisition of contextual fear. Frontiers in behavioral neuroscience, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Zörner B, Zacher C, Sadovska G, Herdegen T, & Gass P (2003). Memory retrieval after contextual fear conditioning induces c-Fos and JunB expression in CA1 hippocampus. Genes, Brain and Behavior, 2(1), 3–10. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, & Rudy JW (1989). Configural association theory: The role of the hippocampal formation in learning, memory, and amnesia. Psychobiology, 17(2), 129–144. [Google Scholar]
- Tabibnia G, Cooke BM, & Breedlove SM (1999). Sex difference and laterality in the volume of mouse dentate gyrus granule cell layer. Brain research, 827(1), 41–45. [DOI] [PubMed] [Google Scholar]
- Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, & Wiltgen BJ (2014). Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron, 84(2), 347–354. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Behne NS, & Fanselow MS (2001). Sex differences, context preexposure, and the immediate shock deficit in Pavlovian context conditioning with mice. Behavioral neuroscience, 115(1), 26. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, & Fanselow MS (2006). Context fear learning in the absence of the hippocampus. The Journal of neuroscience, 26(20), 5484–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlsson MG, Parivash SN & Silva AJ (2010). The hippocampus plays a selective role in the retrieval of detailed contextual memories. Current Biology, 20(15), 1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, & Van Haeften T (2000). Anatomical organization of the parahippocampal-hippocampal network. Annals of the New York Academy of Sciences, 911(1), 1–24. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Hersman S, Chawla MK, Barnes CA, & Fanselow MS (2014). Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. Journal of Neuroscience, 34(25), 8462–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.