Abstract
We have examined the involvement of components of the interleukin-1 (IL-1) signaling pathway in the transactivation of gene expression by the p65 subunit of NF-κB. Transient transfection of cells with plasmids encoding wild-type MyD88, IL-1 receptor-associated kinase 1 (IRAK-1), and TRAF-6 drove p65-mediated transactivation. In addition, dominant negative forms of MyD88, IRAK-1, and TRAF-6 inhibited the IL-1-induced response. In cells lacking MyD88 or IRAK-1, no effect of IL-1 was observed. Together, these results indicate that MyD88, IRAK-1, and TRAF-6 are important downstream regulators of IL-1-mediated p65 transactivation. We have previously shown that the low-molecular-weight G protein Rac1 is involved in this response. Constitutively active RacV12-mediated transactivation was not inhibited by dominant negative MyD88, while dominant negative RacN17 inhibited the MyD88-driven response, placing Rac1 downstream of MyD88 on this pathway. Dominant negative RacN17 inhibited wild-type IRAK-1- and TRAF-6-induced transactivation, and in turn, dominant negative IRAK-1 and TRAF-6 inhibited the RacV12-driven response, suggesting a mutual codependence of Rac1, IRAK-1, and TRAF-6 in regulating this pathway. Finally, Rac1 was found to associate with the receptor complex via interactions with both MyD88 and the IL-1 receptor accessory protein. A pathway emanating from MyD88 and involving IRAK-1, TRAF-6, and Rac1 is therefore involved in transactivation of gene expression by the p65 subunit of NF-κB in response to IL-1.
Regulation of the transcription factor nuclear factor kappa B (NF-κB) following stimulation with the proinflammatory cytokine interleukin-1 (IL-1) occurs via activation of two independent pathways (28). The first and to date best-characterized pathway regulates the release of NF-κB (typically a heterodimer comprising the p50 and p65 subunits) from its inhibitory protein IκB, allowing NF-κB to translocate to the nucleus. The second pathway, which has recently been described, regulates the transactivating ability of the p65 subunit of NF-κB once it is bound to its consensus sequence (3, 17).
IL-1 signal transduction to IκB degradation has been the subject of intense investigation (21). In response to IL-1 binding to its type I IL-1 receptor (IL-1RI), a complex is formed between IL-1RI and its accessory protein (IL-1RAcP). These proteins have a region of homology in their cytoplasmic domains, the Toll/IL-1R (TIR) domain, which is characteristic of the Toll/IL-1-like receptor superfamily and is also responsible for signaling (27). The cytosolic adapter protein MyD88 (also containing a C-terminal TIR domain) interacts with IL-1RAcP via a homotypic interaction involving both TIR domains and is a key regulator of IL-1 signal transduction (5, 8, 16). The interaction of MyD88 with the receptor complex mediates the recruitment of the IL-1 receptor-associated kinases (IRAK) 1 and 2 (9, 26). Recently, a novel protein has been identified, Tollip (Toll-interacting protein), which has been shown to be involved in IRAK-1 recruitment to the receptor complex via association of Tollip with IL-1RAcP (7). Once associated with the receptor complex, IRAK-1 subsequently recruits the adapter tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF-6), an essential mediator of IL-1 signaling to NF-κB activation (10). Subsequent autophosphorylation of IRAK-1 is thought to promote dissociation of IRAK-1 from the receptor complex, thus enabling downstream signaling, resulting in activation of the IκB kinase (IKK) complex, responsible for IκB phosphorylation and subsequent ubiquitin-mediated degradation (12, 40). The upstream kinases involved in activating IKK1 and -2 are thought to belong to the mitogen-activated protein kinase (MAPK) family of kinases. NF-κB-interacting kinase (NIK) has been shown to phosphorylate and activate the IKKs when overexpressed in cells, and a role for the kinase TAK1 and its regulator TAB1, upstream of NIK, has been indicated (23). However, the involvement of NIK in regulating IKK activation in response to either TNF or IL-1 has recently been disputed (2). MEKK-1 (MAPK/ERK kinase-1) has also been shown to phosphorylate and activate the IKKs (20), and recently an adapter protein, ECSIT (evolutionarily conserved signaling intermediate in Toll pathways), has been described which regulates MEKK-1 and links TRAF-6 with MEKK-1 regulation (19).
In contrast, little is known about the signaling components involved in the pathway regulating the transactivating activity of the p65 subunit of NF-κB. Several reports have demonstrated that upon stimulation with either IL-1 or TNF, the ability of the p65 subunit of NF-κB to transactivate gene expression is enhanced, possibly as a result of phosphorylation of multiple serine residues on p65 (3, 4, 17, 36). The kinases involved in regulating this response following TNF stimulation have, to some extent, been identified. Protein kinase A has been demonstrated to phosphorylate serine 276 in the Rel homology domain of p65, and casein kinase II has been shown to be responsible for phosphorylating serine 529 in the C-terminal transactivation domain (37, 38, 41). In addition, reports have also indicated the ability of the IKKs to phosphorylate p65 on serine 536 in the transactivation domain while the protein is in the cytoplasm, adding another level of complexity to the regulation of transactivation of gene expression by p65 (30). Phosphorylation of either the Rel homology domain or the transactivation domain of p65 mediates the interaction of p65 with coactivators such as CREB-binding protein (CBP). A role for phosphatidylinositol 3-kinase (PI3K) in the events leading to phosphorylation of p65 in response to IL-1 has been demonstrated (34).
Despite the rapidly accumulating evidence for a role of various kinase pathways in regulating p65-mediated transactivation, much research is required into the signaling events regulating the kinases responsible for phosphorylating p65. Previous studies in our laboratory have demonstrated the involvement of the low-molecular-weight G protein Rac1 in regulating these events in response to IL-1 stimulation, but exactly how Rac1 mediates this signal remains to be discovered. In this study we have therefore set out to assess the involvement of key regulators of IL-1 signal transduction, namely, MyD88, IRAK-1, TRAF-6, and Rac1, in this pathway. Rac1 can be found in a complex with both IL-1RAcP and MyD88, lying downstream of MyD88 on the pathway and requiring IRAK-1 and TRAF-6 for its effect on p65-mediated transactivation of gene expression.
MATERIALS AND METHODS
Cell culture and reagents.
EL4.NOB-1 cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum, gentamicin (100 U/ml), and 2 mM l-glutamine and maintained at 37°C in a humidified atmosphere of 5% CO2. Cells were seeded at a density of 106 ml−1 for experiments and treated as indicated in the figure legends. Human embryonic kidney 293 cells and 293IL-1RI/AcP cells (40) were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum, 100 U of gentamicin per ml, and 2 mM l-glutamine and maintained at 37°C in a humidified atmosphere of 5% CO2. 293 cells stably transfected with the type I IL-1 receptor (293-RI) and 293-RI cells lacking IRAK-1 (22) were maintained in DMEM containing 10% fetal calf serum, 100 U of gentamicin (with 400 μg of geneticin [G418] per ml being added to medium for IRAK-1-deficient cells) per ml, and 2 mM l-glutamine and maintained at 37°C in a humidified atmosphere of 5% CO2. Murine embryonic fibroblasts (MEF) and MEF from MyD88 knockout mice (MyD88−/−) were grown in DMEM supplemented with 10% fetal calf serum, 100 U of gentamicin per ml, and 2 mM l-glutamine and maintained at 37°C in a humidified atmosphere of 5% CO2. Human recombinant IL-1α was a gift from the National Cancer Institute, Frederick, Md.
Plasmids.
Gal4-p65(1–551) and Gal4-p65(286–551) plasmids encoding full-length p65 subunit of NF-κB and residues 286 to 551 of p65, respectively, both fused to the Gal4 DNA-binding domain, were a kind gift from Lienhard Schmitz (German Cancer Research Center, Heidelberg, Germany) and have been described elsewhere (11, 31). The Gal-luciferase reporter gene was obtained from Stratagene. MyD88 constructs were a kind gift from Marta Muzio (Mario Negri Institute, Milan, Italy), and the plasmid encoding dominant negative IRAK-1 was a gift from Emma-Louise Cooke (Glaxo Wellcome, Stevenage, United Kingdom). The pEF expression plasmid encoding constitutively active RacV12 and dominant negative RacN17 were kind gifts from D. Cantrell (Imperial Cancer Research Fund, London, United Kingdom) and have been described elsewhere (13). The cDNA for MyD88 was cloned into the bacterial expression vector pGEX, expressed in Escherichia coli BL21(DE3) as a fusion protein with glutathione S-transferase (GST), and purified with glutathione-agarose beads (Sigma) by standard protocols.
Transient-transfection and reporter gene assays.
EL4.NOB-1 cells (7 × 106) were transfected with plasmids as indicated in the figure legends in a final volume of 0.6 ml using DEAE-dextran. Following 16 to 18 h of recovery, cells were seeded at a density of 106 viable cells (as determined by the trypan blue dye exclusion method) prior to stimulation. 293-RI and 293-IRAK−/− cells (2 × 104 per well) were seeded onto 96-well plates and transfected 24 h later with 5 ng of Gal4-p65(1–551), 25 ng of Gal-luciferase, and 40 ng of Renilla luciferase (used as an internal control) with FuGENE 6 (Roche) according to the manufacturer's recommendations. MEK and MyD88-deficient cells (2.5 × 104 per well) were seeded onto 24-well plates and transfected 24 h later with 100 ng of Gal4- p65(1–551), 200 ng of Gal-luciferase, and 200 ng of Renilla luciferase with FuGENE 6 (Roche) according to the manufacturers' recommendations. In all cases, the amount of DNA transfected was kept constant by the addition of various amounts of the appropriate empty vector plasmid. Cells were either left untreated or stimulated with IL-1 (10 ng/ml) as indicated following a period of recovery (16 to 18 h). To assay firefly luciferase activity, cells were lysed using passive lysis buffer (Promega), and luciferase activity was determined by standard procedures. Renilla luciferase acivity and β-galactosidase activity were determined by standard protocols and used to normalize firefly luciferase activity in relation to transfection efficiency.
Immunoprecipitation and Western blot analysis.
Cells were treated as described in the figure legends for the times indicated, and treatment was terminated by the addition of 5 ml of ice-cold phosphate-buffered saline (PBS). Cells were lysed on ice (30 min) in buffer containing 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% NP-40, 0.2 mM phenylmethylsulfonyl fluoride, 0.2 mM Na3VO4 and 1 μg of leupeptin per ml. Lysates were cleared by centrifugation, and following clearing for 30 min at 4°C with protein G-Sepharose (Sigma), AU1-tagged MyD88 and Flag-tagged IL-1RAcP were immunoprecipitated using 4 μg of anti-AU1 and anti-Flag M2 monoclonal antibody, respectively. The immune complexes were precipitated by incubation with protein G-Sepharose for 60 min at 4°C and washed three times with lysis buffer, and Rac1 association was detected using monoclonal anti-Rac antibody (Upstate Biotechnology) by Western blotting. Pulldowns were performed by adding 10 μg of GST or GST-MyD88 coupled to agarose beads to cleared cell lysates (prepared as described for the indicated times). Samples were incubated for 2 h at 4°C and washed three times with lysis buffer, and associated Rac1 was detected by Western blot analysis as described previously (17). Epitope-tagged RacV12 and RacN17, AU1-MyD88, and Gal4-p65(1–551) expression in EL4.NOB-1 cells was detected by Western blotting using anti-Myc (clone 9E10), anti-AU1 (Covance, Richmond, Calif.), and anti-Gal4 (obtained from Lienhard Schmitz) antibodies.
RESULTS
MyD88, IRAK-1, and TRAF-6 drive p65-mediated transactivation activity.
We have previously shown that IL-1 stimulates NF-κB transactivating activity via a pathway independent of IκB degradation. We therefore assessed the involvement of key regulators of IL-1 signal transduction (namely MyD88, IRAK-1, and TRAF-6) in this pathway using the murine thymoma cell line EL4.NOB-1, which is highly responsive to IL-1. To study the involvement of these key regulators on transactivation of gene expression by p65, we employed the Gal4-p65(1–551) trans-reporting system described previously (36). Briefly, this system employs an expression plasmid encoding the transactivation domain of the p65 subunit of NF-κB fused to the DNA-binding domain of Gal4 and a Gal4-responsive reporter plasmid, Gal-luciferase. The advantage of this assay is that Gal4-p65(1–551) is exclusively nuclear and is regulated independently of IκB, allowing the effects of various stimuli (such as IL-1) or genes of interest on transactivation by p65 to be assessed.
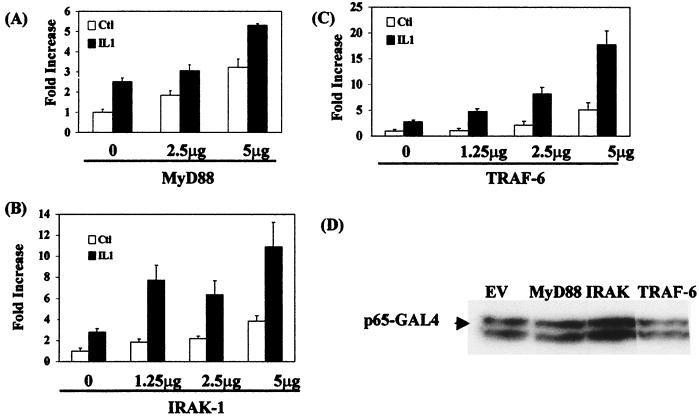
Figure 1 shows the effect of cotransfecting cells with plasmids encoding wild-type versions of MyD88, IRAK-1, or TRAF-6 together with Gal4-p65(1–551) and Gal-luciferase. Transient transfection of EL4.NOB-1 cells with 5 μg of plasmid encoding wild-type MyD88 (Fig. 1A) resulted in a threefold activation of Ga14-p65(1–551). Addition of IL-1 (10 ng/ml) to MyD88-transfected cells potentiated the response compared to that seen with IL-1 alone. Transfection of cells with 5 μg of plasmid encoding IRAK-1 (Fig. 1B) resulted in 3.8-fold activation of Gal4-p65(1–551) activity, and addition of IL-1 (10 ng/ml) to IRAK-1-transfected cells potentiated the IL-1-alone response at 1.25 μg of IRAK-1. TRAF-6 (Fig. 1C), when transiently transfected into EL4.NOB-1 cells, gave rise to an optimal fivefold response, and addition of IL-1 (10 ng/ml) to the cells resulted in a strong potentiation of the response over that seen with IL-1 alone. Expression of the plasmids encoding MyD88, IRAK-1, and TRAF-6 had little or no effect on the expression of Gal4-p65(1–551) (as shown for 5 μg of each plasmid in Fig. 1D), the Gal4-p65(1–551) fusion being consistently detected as a doublet. We also tested the ability of MyD88, TRAF-6, and IRAK-1 to activate a Gal4-p65 fusion protein missing the Rel homology domain (RHD), Gal4-p65(286–551). In each case, transient transfection of EL4-NOB.1 cells with 5 μg of MyD88, IRAK-1, and TRAF-6 drove Gal4-p65(286–551) activity two- to threefold (data not shown), indicating that these proteins could affect the transactivation domain of p65 in isolation. This, combined with the fact that all samples were corrected for the expression of the constitutive reporter gene β-galactosidase (which showed minimal changes), indicated that the effects of MyD88, IRAK-1, and TRAF-6 on the system were due to enhanced transactivation of gene expression by p65.
FIG. 1.
MyD88, IRAK-1, and TRAF-6 but not IRAK-2 drive p65-mediated transactivation of gene expression. EL4.NOB-1 cells (7 × 106) were transiently transfected with plasmids encoding wild-type (A) MyD88, (B) IRAK-1, and (C) TRAF-6 as indicated, along with 2.5 μg of β-galactosidase, 2.5 μg of Gal4-p65(1-551), and 5 μg of Gal4-luciferase. Following stimulation with IL-1 (10 ng/ml), extracts were prepared and measured for luciferase activity. Results are normalized for β-galactosidase activity and are represented as fold increase over the nonstimulated empty vector (EV) control (C+l). (D) The effect of transfecting wild-type-encoding plasmids on Gal4-p65(1–551) expression was determined by Western blotting of lysates of cells transfected with 5 μg of MyD88, IRAK-1, and TRAF-6 as indicated.
Deletion mutants of MyD88, IRAK-1, and TRAF-6 inhibit IL-1-induced p65 activity.
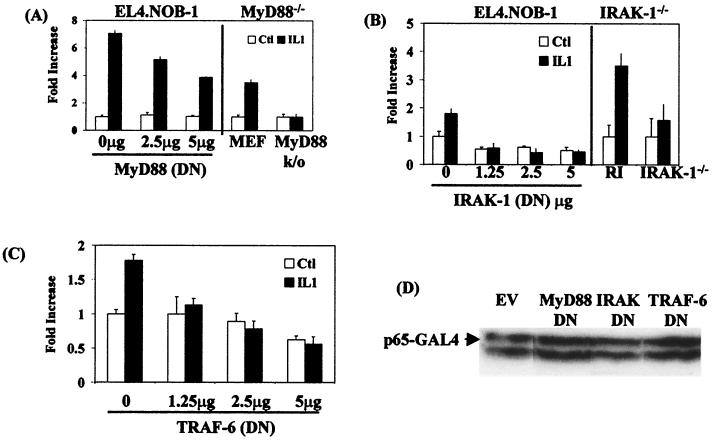
We next examined the role of MyD88, IRAK-1, and TRAF-6 in IL-1-induced transactivation of p65 more directly. To this end, dominant negative mutants of MyD88, IRAK-1, and TRAF-6 were used in cotransfection experiments with the p65 trans-reporting system, and their effects on IL-1-induced activation were assessed. Transient transfection of EL4.NOB-1 cells with dominant negative MyD88, encoding the TIR of MyD88 only, inhibited IL-1-induced Gal4-p65(1–551) activity in a dose-dependent manner (Fig. 2A, left-hand panel), decreasing the response by 50% at the highest amount of MyD88 used. In addition, induction of the response was not evident in MEF derived from MyD88-null mice (MyD88−/−), unlike cells from the parental control mice (MEF) (Fig. 2A, right-hand panel). Transfection of MyD88 into these cells strongly induced Gal4-p65(1–551) activity, which we found could not be further enhanced after stimulation with IL-1 (not shown). Transient transfection of EL4.NOB-1 cells with dominant negative IRAK-1, which encodes the death domain only of IRAK-1, completely inhibited IL-1-induced Gal4-p65(1–551) activity (Fig. 2B, left-hand panel). In experiments with 293 cells lacking IRAK-1 (IRAK−/−), IL-1 had no effect on the response, unlike the effects seen in parental 293 cells stably transfected with IL-1RI (293-RI) (Fig. 2B, right-hand panel). Transfection of IRAK-1 into these cells strongly induced Gal4-p65(1–551) activity, which we found could not be further enhanced after stimulation with IL-1 (not shown). A dominant negative mutant of TRAF-6, encoding the ring finger domain only of TRAF-6, also abolished the effect of IL-1 on Ga14-p65(1–551) activity (Fig. 2C) when transiently transfected into EL4.NOB-1 cells. Expression of the dominant negative forms of MyD88, IRAK-1, and TRAF-6 had no effect on the expression of the p65-Ga14 fusion, as shown for the highest amount of each plasmid used (Fig. 2D), and again, all results were normalized for expression of the constitutive reporter genes β-galactosidase or Renilla luciferase, which showed minimal changes. The effect of IL-1 varied somewhat between different sets of experiments, but induction of the response was always evident and was generally in the two- to threefold range, as reported by others (35). Overall, these results imply that MyD88, IRAK-1, and TRAF-6 are required for the effect of IL-1 on the transactivation of gene expression by the p65 subunit of NF-κB.
FIG. 2.
Effect of dominant negative MyD88, IRAK-1, and TRAF-6 on IL-1-induced Gal4-p65(1–551) activity. EL4.NOB-1 cells (7 × 106) were transiently transfected with plasmids encoding dominant negative (DN) (A, left-hand panel) MyD88, (B, left-hand panel) IRAK-1, and (C) TRAF-6 as indicated along with 2.5 μg of β-galactosidase, 2.5 μg of Gal4-p65(1–551), and 5 μg of Gal4-luciferase. Following stimulation with IL-1 (10 ng/ml), extracts were prepared and measured for luciferase activity. Results are normalized for β-galactosidase activity and are represented as fold increase over nonstimulated empty vector (EV) control (Ctl). In addition, IL-1-induced Gal4-p65(1–551) was tested in MyD88-deficient cells derived from MyD88-null mice (A, right-hand panel) and IRAK-deficient 293 cells (B, right-hand panel). Cells (2 × 104 and 2.5 × 104 ml−1) were transfected with Gal-luciferase (200 and 25 ng, respectively), Gal4-p65(1–551) (100 and 5 ng, respectively), and constitutive Renilla luciferase (200 and 40 ng, respectively). Following stimulation with IL-1 (10 ng/ml), extracts were prepared and measured for luciferase activity. Results are normalized for Renilla luciferase activity and are represented as fold increase over nonstimulated empty vector control. (D) Effect of transfecting EL4.NOB-1 cells with dominant negative-encoding plasmids on Gal4-p65(1–551) expression was determined by Western blotting of lysates of cells transfected with 5 μg of dominant negative (DN) MyD88, IRAK-1, and TRAF-6 as indicated.
MyD88, TRAF-6, and IRAK-1 are codependent with respect to p65-mediated transactivation.
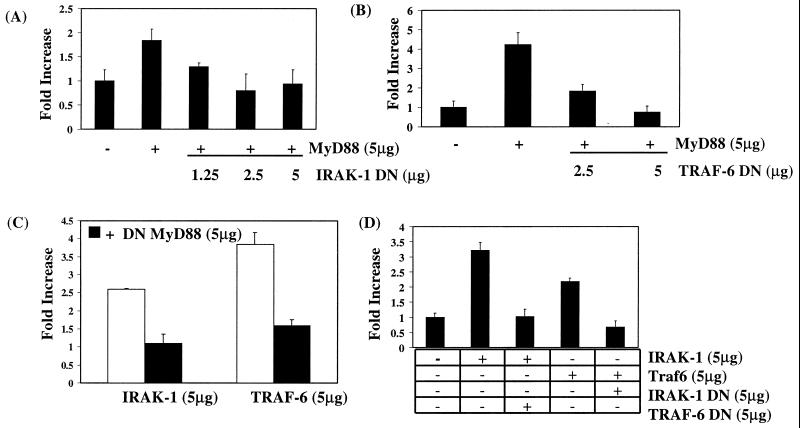
To position MyD88, IRAK-1, and TRAF-6 with respect to each other on this pathway, we cotransfected wild-type versions of each together with dominant negative MyD88, IRAK-1, and TRAF-6, as indicated. Figure 3A shows that the effect of wild-type MyD88 on Gal4-p65(1–551) activity was inhibited by cotransfection of cells with dominant negative IRAK-1, the effect being abolished at 5 μg of dominant negative IRAK-1. Similarly, dominant negative TRAF-6 abolished the MyD88-induced response (Fig. 3B). Surprisingly, dominant negative MyD88 inhibited the effect of wild-type IRAK-1 or TRAF-6 on p65-mediated transactivation (Fig. 3C). In addition, dominant negative IRAK-1 and TRAF-6 were found to repress the effects of wild-type TRAF-6 and IRAK-1, respectively (Fig. 3D). These results suggest codependency of MyD88, IRAK-1, and TRAF-6 for each other on this pathway.
FIG. 3.
MyD88, TRAF-6, and IRAK-1 are codependent with respect to p65-mediated transactivation. To position MyD88, IRAK-1, and TRAF-6 on this pathway, EL4.NOB-1 cells (7 × 106) were transiently transfected with 5 μg of plasmids encoding either wild-type or dominant negative (DN) versions of MyD88, TRAK-1, and TRAF-6 as indicated along with 2.5 μg of β-galactosidase, 2.5 μg of Gal4-p65(1–551), and 5 μg of Gal4-luciferase. Following stimulation with IL-1 (10 ng/ml), extracts were prepared and measured for luciferase activity. Results are normalized for β-galactosidase activity and are represented as fold increase over nonstimulated empty vector control.
Rac1 lies downstream of MyD88 but requires IRAK-1 and TRAF-6 on the IL-1-activated pathway to p65-mediated transactivation.
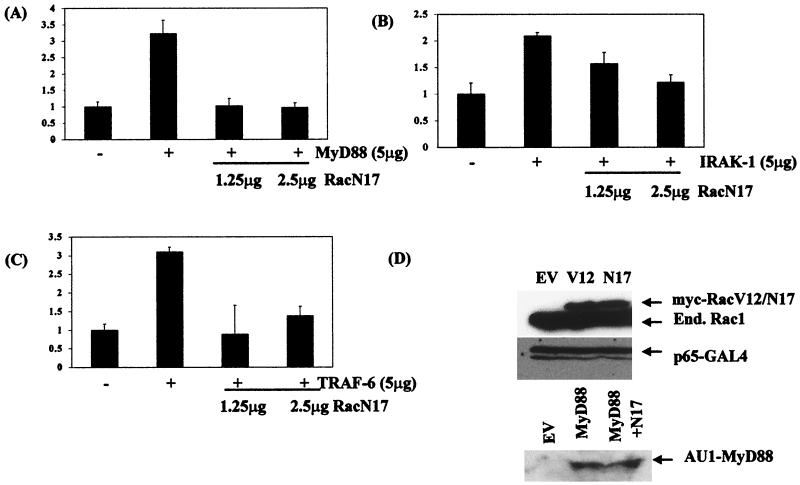
We have previously shown that the low-molecular-weight G protein Rac1 is required for the transactivation response of the p65 subunit of NF-κB in IL-1-treated cells, with a dominant negative mutant of Rac1, RacN17, inhibiting IL-1-induced Gal4-p65(1–551) activity in transient-transfection experiments in a dose-dependent manner. We therefore examined the involvement of Rac1 using dominant negative RacN17 in combination with plasmids encoding wild-type MyD88, IRAK-1, or TRAF-6. The ability of wild-type MyD88 to transactivate p65-mediated gene expression was inhibited by cotransfection of cells with dominant negative RacN17 (Fig. 4A). In a similar manner, both IRAK-1- and TRAF-6-induced Gal4-p65(1–551) activity was inhibited by RacN17 (Fig. 4B and C, respectively). Controls to determine the specificity of the effects shown here and in Fig. 3 included normalization of the levels of luciferase induced with the constitutive reporter gene β-galactosidase (which always showed minimal alterations) and also immunoblotting for transfected proteins to check that their expression was unaltered, as shown for MyD88 and Gal4-p65(1–551) in RacV12- and RacN17-transfected cells (Fig. 4D).
FIG. 4.
Dominant negative RacN17 inhibits MyD88-, IRAK-1-, and TRAF-6-driven Gal4-p65(1–551) activity. EL4.NOB-1 cells (7 × 106) were transiently transfected with 5 μg of plasmids encoding (A) MyD88, (B) IRAK-1, and (C) TRAF-6 along with 1.25 or 2.5 μg of dominant negative (DN) Rac1 as indicated, 2.5 μg of β-galactosidase, 2.5 μg of Gal4-p65(1–551), and 5 μg of Gal4-luciferase. Following stimulation with IL-1 (10 ng/ml), extracts were prepared and measured for luciferase activity. Results are normalized for β-galactosidase activity and are represented as fold increase over nonstimulated empty vector (EV) control. (D) The effect of cotransfecting EL4.NOB-1 cells with RacV12 and RacN17 on Gal4-p65(1–551) and AU1-tagged MyD88 (in the case of RacN17) expression was determined by Western blotting of lysates from cells which had been transfected with 5 μg of RacV12, RacN17, or AU1-MyD88 and 2.5 μg of Gal4-p65(1–551) as indicated. End., endogenus.
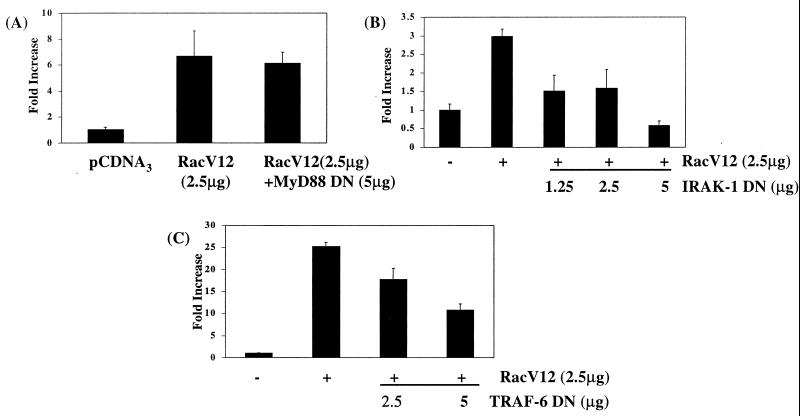
To confirm the involvement of Rac1 and to position it on this pathway, the ability of dominant negative MyD88, IRAK-1, and TRAF-6 to inhibit constitutively active RacV12-induced Gal4-p65(1–551) activation was determined. Consistent with Rac1's lying downstream of MyD88, dominant negative MyD88 was unable to inhibit RacV12-induced p65-mediated transactivation (Fig. 5A). In contrast, dominant negative IRAK-1 and TRAF-6 inhibited the RacV12-induced response, indicating that they both play a critical role in regulating the effects of RacV12 on this pathway (Fig. 5B and C, respectively).
FIG. 5.
Dominant negative (DN) IRAK-1 and TRAF-6 but not MyD88 inhibit constitutively active RacV12-induced transactivation by p65. EL4.NOB-1 cells (7 × 106) were transiently transfected with plasmids encoding dominant negative (A) MyD88, (B) IRAK-1, and (C) TRAF-6 as indicated along with 2.5 μg of RacV12, 2.5 μg of β-galactosidase, 2.5 μg of Gal4-p65(1–551), and 5 μg of Gal4-luciferase. Following stimulation with IL-1 (10 ng/ml), extracts were prepared and measured for luciferase activity. Results are normalized for β-galactosidase activity and are represented as fold increase over nonstimulated empty vector control.
Rac1 is detected in a complex with MyD88 and IL-1RAcP.
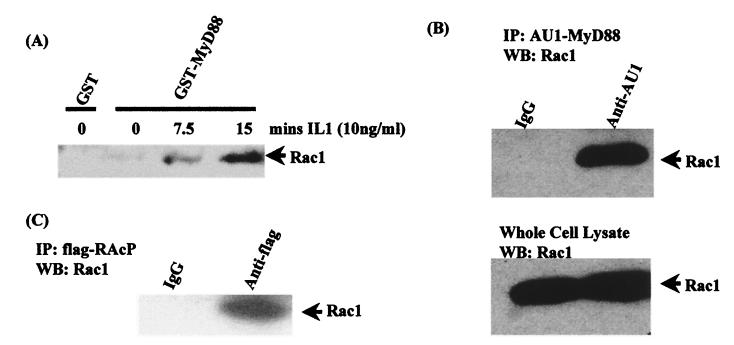
Finally, we sought biochemical evidence for Rac1's interacting with the IL-1 receptor complex. Lysates from EL4.NOB-1 cells treated with IL-1 (10 ng/ml) for various times were incubated with an immobilized GST-MyD88 fusion protein, and the ability of endogenous Rac1 to bind to GST-MyD88 was determined by Western blot analysis with an anti-Rac antibody. Figure 6A demonstrates that while GST-MyD88 could interact with endogenous Rac1 in lysates from untreated cells, stimulation with IL-1 increased the amount of associated Rac1 in a time-dependent manner, with a strong association being detected at 15 min (lane 4). GST alone did not associate with Rac1 in lysates from IL-1-treated cells (data not shown). This experiment suggests an increased association between Rac1 and MyD88 upon stimulation with IL-1, with activated Rac1 associating more strongly with MyD88. The ability of Rac1 to associate with MyD88 was confirmed by overexpressing AU1-tagged wild-type MyD88 in the human embryonic kidney cell line 293T, which transfects to a high degree of efficiency. Immunoprecipitation of AU1-tagged MyD88, followed by Western blotting for endogenous Rac1, confirmed the ability of Rac1 to associate with the adapter protein MyD88 (Fig. 6B). The interaction of endogenous Rac1 with the IL-1 receptor complex was also demonstrated in 293 cells that had been stably transfected with Flag-tagged IL-1RAcP. Following immunoprecipitation of Flag-tagged IL-1RAcP, the detection of endogenous Rac1 in the immunoprecipitated complex clearly demonstrates that Rac1 associates with the receptor complex (Fig. 6C).
FIG. 6.
Rac1 associates with MyD88 and IL-1RAcP. (A) Lysates from IL-1-treated EL4.NOB-1 cells were incubated with 10 μg of GST-MyD88. (B) Overexpressed AU1-tagged MyD88 was immunoprecipitated (IP) from 293 cells (transfected with 10 μg of AU1-MyD88) using a monoclonal antibody against AU1. (C) Flag-tagged IL1-RAcP was immunoprecipitated from 293 cells stably transfected with IL-1RI and IL-1RAcP using a monoclonal antibody against Flag. In each case association of endogenous Rac1 was detected by Western blot (WB) analysis using an anti-Rac1 antibody.
DISCUSSION
To date, the pathway activated by IL-1 resulting in enhanced transactivation by the p65 subunit of NF-κB has not been characterized. The key role of MyD88, IRAK-1, and TRAF-6 in IL-1 signal transduction suggested that these proteins may also be key regulators of this pathway. By using plasmids encoding either wild-type or inactive mutants of MyD88, IRAK-1, and TRAF-6 and by testing cells from MyD88-null mice and 293 cells lacking IRAK-1, we clearly demonstrated the importance of these proteins in regulating the signal from the IL-1 receptor complex to p65-mediated transactivation. Transient transfection of EL4.NOB-1 cells with plasmids encoding these proteins demonstrated that in each case the wild-type protein induced Gal4-p65(1–551) activity, with IL-1 causing a further stimulation. Dominant negative MyD88, IRAK-1, and TRAF-6 all inhibited IL-1-induced Gal4-p65(1–551) activity, and in addition, no response to IL-1 was seen in cells lacking either MyD88 or IRAK-1, clearly pointing to the importance of these three proteins in the pathway.
Previous studies on the involvement of MyD88, IRAK-1, and TRAF-6 in IL-1 signal transduction have demonstrated that MyD88 interacts with the receptor complex via a homotypic interaction via its TIR domain and that IRAK-1 and TRAF-6 are subsequently recruited to the activated complex (reviewed in reference 27) (http://stke.sciencemag.org/cgi/content/full/OC_sigtrans;2000/44/re1). MyD88 was originally isolated as an early transcript in IL-6-induced differentiating myeloid cells and was postulated to be a mediator of macrophage differentiation. The similarities between the Toll/IL-1 receptor superfamily and the C-terminal domain of MyD88 subsequently suggested a role for MyD88 in signal transduction pathways, and further investigation demonstrated its importance in IL-1-mediated signaling to NF-κB activation. In addition, dominant negative versions of IRAK-1 and TRAF-6 were found to inhibit MyD88-induced activation of NF-κB, demonstrating that IRAK-1 and TRAF-6 lie downstream of MyD88 on the pathway leading to NF-κB activation (8). The autophosphorylating activity of IRAK-1 disrupts the interaction of IRAK-1 with the death domain of MyD88, with the result that the interaction of IRAK-1 with the receptor complex is extremely transient.
Our data suggest a codependence between MyD88, IRAK-1, and TRAF-6 on this separate pathway regulating p65-mediated transactivation. Cotransfection of cells with wild-type MyD88 and either dominant negative IRAK-1 or TRAF-6 completely inhibited the ability of MyD88 to transactivate gene expression. In addition, dominant negative MyD88 inhibited the effect of wild-type IRAK-1- and TRAF-6. Similar experiments using wild-type and dominant negative versions of IRAK-1 and TRAF-6 indicated that IRAK-1 and TRAF-6 are also codependent. These results imply that a multiprotein complex involving MyD88, IRAK-1, and TRAF-6 is required for this pathway and that the presence of a dominant negative version of any of these will disrupt signaling. This is in contrast to results observed using an NF-κB-dependent reporter gene, which places MyD88 upstream of IRAK-1 and TRAF-6 (8). The basis for this difference is unclear. Importantly, we and others have shown that dominant negative MyD88, IRAK-1, and TRAF-6 block both NF-κB-linked reporter genes and the induction of endogenous genes that are NF-κB dependent, such as that encoding IL-2 (6, 14, 18). Although the effect of these dominant negative mutants will involve inhibition of IκB phosphorylation and subsequent degradation, an additional effect on p65-mediated transactivation of gene expression can also be concluded from our study here.
The effect of dominant negative MyD88 was unlikely to be nonspecific. We have found that it is unable to inhibit IRAK-1-induced p38 MAPK, activation (E. M. Palsson and L. A. J. O'Neill, unpublished observations) and, more importantly for this study, RacV12-induced transactivation by p65. We have previously implicated the low-molecular-weight G protein Rac1 in the IL-1 pathway regulating the transactivation of p65-mediated gene expression (17). That Rac1 may be involved at the level of the receptor complex and its immediate signaling processes was suggested from previous studies that had shown an interaction between Rac1 and a GST–IL-1RI fusion protein (33). Our data indicate that Rac1 lies downstream of MyD88 on this pathway as, along with the lack of effect of dominant negative MyD88 on constitutively active RacV12-induced p65-mediated transactivation, dominant negative RacN17 inhibited the ability of wild-type MyD88 to drive Gal4-p65(1–551) activation in cotransfection experiments. While dominant negative RacN17 totally prevented IRAK-1- and TRAF-6-mediated activation of Gal4-p65(1–551) activity, the dominant negative mutants of IRAK-1 and TRAF-6 also inhibited RacV12-induced activation of this response. Our data argue for a role for Rac1 in mediating IRAK-1- and TRAF-6-induced Gal4-p65(1–551) activity and in addition suggest that IRAK-1, TRAF-6, and Rac1 are mutually codependent for their activity, as dominant negative versions of any of these signaling components prevent the ability of the other two proteins to drive p65-mediated transactivation. IRAK-1 and TRAF-6, however, also require MyD88, while Rac1 does not. Ultimately, however, all four proteins (MyD88, IRAK-1, TRAF-6, and Rac1) are key participants in the signaling pathway recruited by IL-1, which mediates transactivation by p65.
We used a number of approaches to assess possible interactions of Rac1 with components of the IL-1 receptor complex and found that Rac1 associated with both a GST-MyD88 fusion protein in pulldown assays and overexpressed AU1-tagged MyD88 following immunoprecipitation. Treatment of cells with IL-1 increased the amount of Rac1 associating with MyD88. It is likely that MyD88 associates more strongly with active Rac1, as indicated by our results. Constitutively active RacV12 does not require MyD88 for its effect on p65, implying that the role of MyD88 might be to recruit and activate Rac1, whose effects then require IRAK-1 and TRAF-6. Apart from MyD88, endogenous Rac1 was found to associate with the IL-1RAcP when it was immunoprecipitated from 293 cells stably transfected with Flag-tagged IL-1RAcP, although this association may be via MyD88. We were unable to test for interaction with endogenous IL-1RAcP, as its level of expression was too low in cells. In contrast to Singh et al. (33), we were unable to coprecipitate Rac1 with Myc-tagged IL-1RI which was stably transfected into 293 cells (unpublished observations). This indicated that the interaction of Rac1 with the IL-1 receptor complex specifically involves MyD88 and IL-1RAcP rather than IL-1RI. How IL-1 activates Rac1 and the significance of the interaction of Rac1 with the receptor complex are unclear. However, taken together, our data point to a clear role for Rac1 in IL-1 signal transduction pathways, with Rac1 associating with a complex involving MyD88 upon activation by IL-1.
The presence of Rac1 in the IL-1 receptor complex may be linked to its role as a critical regulator of the actin cytoskeletal network in cells (reviewed in reference 15). The IL-1 receptor complex has been shown to localize to focal adhesion points at the cell membrane, similar to focal complexes which require Rac1 for their formation (29). The localization of IL-1RI in focal adhesion complexes is required for NF-κB activation and p42/p44 MAPK activation, and IRAK-1 has recently been shown to be recruited into such complexes during the activation of p42/p44 MAPK (24). We have previously shown that p42/p44 MAPK is required for the transactivation pathway activated by IL-1 via Rac1, although the target for this kinase is not known (17). The kinases that are responsible for the transactivating effect of p65 have not been fully characterized, although recently a role for protein kinase A and casein kinase II in response to TNF stimulation has been demonstrated (38, 41). Interestingly, researchers have shown that casein kinase II associates constitutively with IκB–NF-κB complexes in the cytoplasm and that the presence of IκB prevents phosphorylation of p65. Thus, degradation of IκB is the key signal that regulates p65 phosphorylation and hence transactivation. How the components involved in the IL-1 response link to this process is currently under investigation. Sizemore et al. have demonstrated a clear role for PI3K and Akt in the phosphorylation and activation of p65 in response to IL-1 (34). Since Rac1 is a regulator of PI3K, we suggest that the mechanism by which IL-1 activates PI3K involves Rac1. Furthermore, a recent paper by Arbibe et al. has demonstrated that TLR2, which like IL-1RI and IL-1RAcP has a TIR domain, promotes p65-mediated transactivation via Rac1 and PI3K (1). Taken together with the results of Sizemore et al. and the results presented here, it is likely that TIR domain-containing receptors (several of which have been shown to activate NF-κB, including TLR4 [25] and TLR9 [32]) promote p65-mediated transactivation via a pathway involving MyD88, IRAK-1, TRAF-6, Rac1, PI3K, and Akt. The p65-proximal kinases activated following IL-1 stimulation have yet to be definitively identified and are currently under investigation in our laboratory. In addition, it is possible that these processes regulate phosphorylation of coactivators such as CBP/p300 and pCAF, which are required for p65-mediated transactivation.
In conclusion, our results clearly indicate that a signaling complex involving MyD88, IRAK-1, TRAF-6, and Rac1 is required for p65-dependent transactivation of NF-κB in response to IL-1. The requirement for Rac1 in the MyD88, IRAK-1, and TRAF-6 response and its association with MyD88 in the receptor complex suggest that Rac1 may be the additional component necessary for these proteins to engage with this pathway rather than that leading to IκB phosphorylation, which is Rac1 independent (17).
ACKNOWLEDGMENTS
We thank Osamu Takeuchi and Shizuo Akira (Department of Host Defense, Research Institute for Microbial Diseases, Osaka University) for kindly providing us with MEF from MyD88-null mice.
REFERENCES
- 1.Arbibe L, Mira J, Teusch N, Kline L, Guha M, Mackman N, Godowski P J, Ulevitch R J, Knaus U G. Toll-like receptor 2-mediated NF-kappaB activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 2.Baud V, Liu Z-G, Bennett B, Suzuki N, Xia Y, Karin M. Signalling by proinflammatory cytokines: oligomerisation of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999;13:1297–1308. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann M, Hart L, Lindsay M, Barnes P J, Newton R. IkappaBalpha degradation and nuclear factor-kappaB DNA binding are insufficient for interleukin-1beta and tumor necrosis factor-alpha-induced kappaB-dependent transcription: requirement for an additional activation pathway. J Biol Chem. 1998;273:6607–6610. doi: 10.1074/jbc.273.12.6607. [DOI] [PubMed] [Google Scholar]
- 4.Bird T A, Schooley K, Dower S K, Hagen H, Virca G D. Activation of nuclear transcription factor NF-kappaB by interleukin-1 is accompanied by casein kinase IL-mediated phosphorylation of the p65 subunit. J Biol Chem. 1997;272:32606–32612. doi: 10.1074/jbc.272.51.32606. [DOI] [PubMed] [Google Scholar]
- 5.Bonnert T P, Garka K E, Parnet P, Sonoda G, Testa J R, Sims J E. The cloning and characterisation of human MyD88: a member of the IL-1 receptor related family. FEBS Lett. 1997;402:81–84. doi: 10.1016/s0014-5793(96)01506-2. [DOI] [PubMed] [Google Scholar]
- 6.Bowie A, Kiss-Toth E, Symons J A, Smith G L, Dower S K, O'Neill L A J. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc Natl Acad Sci USA. 2000;97:10162–10167. doi: 10.1073/pnas.160027697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns K, Clatworthy J, Martin L, Martinon F, Plumpton C, Maschera B, Lewis A, Ray K, Tschopp J, Volpe F. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat Cell Biol. 2000;2:346–351. doi: 10.1038/35014038. [DOI] [PubMed] [Google Scholar]
- 8.Burns K, Martinon F, Esslinger C, Pahl H, Schneider P, Bodmer J L, Di Marco F, French L, Tschopp J. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 9.Cao Z, Henzel W J, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 10.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel D V. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 11.De Bosscher K, Schmitz M L, Vanden Berghe W, Plaisance S, Fiers W, Haegeman G. Glucocorticoid-mediated repression of nuclear factor-kappaB-dependent transcription involves direct interference with transactivation. Proc Natl Acad Sci USA. 1997;94:13504–13509. doi: 10.1073/pnas.94.25.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 13.Genot E, Cleverley S, Henning S, Cantrell D. Multiple p21ras effector pathways regulate nuclear factor of activated T cells. EMBO J. 1996;15:3923–3933. [PMC free article] [PubMed] [Google Scholar]
- 14.Greene C, O'Neill L. Interleukin-1 receptor-associated kinase and TRAF-6 mediate the transcriptional regulation of interleukin-2 by interleukin-1 via NFκB but unlike interleukin-1 are unable to stabilise interleukin-2 mRNA. Biochim Biophys Acta. 1999;1451:109–121. doi: 10.1016/s0167-4889(99)00079-8. [DOI] [PubMed] [Google Scholar]
- 15.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 16.Hultmark D. Macrophage differentiation marker MyD88 is a member of the Toll/IL-1 receptor family. Biochem Biophys Res Commun. 1994;199:144–146. doi: 10.1006/bbrc.1994.1206. [DOI] [PubMed] [Google Scholar]
- 17.Jefferies C A, O'Neill L A J. Rac1 regulates interleukin 1-induced nuclear factor κB activation in an inhibitory protein κBα-independent manner by enhancing the ability of the p65 subunit to transactivate gene expression. J Biol Chem. 2000;275:3114–3120. doi: 10.1074/jbc.275.5.3114. [DOI] [PubMed] [Google Scholar]
- 18.Knop J, Wesche H, Lang D, Martin M U. Effects of overexpression of IL-1 receptor-associated kinase on NFkappaB activation, IL-2 production and stress-activated protein kinases in the murine T cell line EL4. Eur J Immunol. 1998;28:3100–3109. doi: 10.1002/(SICI)1521-4141(199810)28:10<3100::AID-IMMU3100>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Kopp E, Medzhitov R, Carothers J, Xiao C, Douglas I, Janeway C A, Ghosh S. ECSIT is a evolutionarily conserved intermediate in the Toll/IL-1 signal transduction pathway. Genes Dev. 1999;13:2059–2071. doi: 10.1101/gad.13.16.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Karin M. Signalling pathways leading to nuclear factor-kappa B activation. Methods Enzymol. 2000;319:273–279. doi: 10.1016/s0076-6879(00)19027-5. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Commane M, Burns C, Vithalani K, Cao Z, Stark G R. Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol Cell Biol. 1999;19:4643–4652. doi: 10.1128/mcb.19.7.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling L, Cao Z, Goeddel D V. NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacGillivray M K, Cruz T F, McCulloch C A G. The recruitment of the interleukin-1 receptor-associated kinase (IRAK) into focal adhesion complexes is required for IL-1 beta-induced ERK activation. J Biol Chem. 2000;275:23509–23515. doi: 10.1074/jbc.M003186200. [DOI] [PubMed] [Google Scholar]
- 25.Medzhitov R, Preston Hurlburt P, Janeway C A., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 26.Muzio M, Ni J, Feng P, Dixit V M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 27.O'Neill L A J, Dinarello C A. The interleukin 1/Toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today. 2000;21:206–209. doi: 10.1016/s0167-5699(00)01611-x. [DOI] [PubMed] [Google Scholar]
- 28.Perkins N D. Achieving transcriptional specificity with NF-kappa B. Int J Biochem Cell Biol. 1997;29:1433–1448. doi: 10.1016/s1357-2725(97)00088-5. [DOI] [PubMed] [Google Scholar]
- 29.Qwarnstrom E E, Page R C, Gillis S, Dower S K. Binding, internalization and intracellular localization of interleukin-1 beta in human diploid fibroblasts. J Biol Chem. 1988;263:8261–8269. [PubMed] [Google Scholar]
- 30.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz M L, Baeuerie P A. The p65 subunit is responsible for the strong transactivating potential of NF-kappa B. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnare M, Holt A C, Takeda K, Akira S, Medzhitov R. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr Biol. 2000;10:1139–1142. doi: 10.1016/s0960-9822(00)00700-4. [DOI] [PubMed] [Google Scholar]
- 33.Singh R, Wang B, Shirvaikar A, Khan S, Kamat S, Schelling J R, Konieczkowski M, Sedor J R. The IL-1 receptor and Rho directly associate to drive cell activation in inflammation. J Clin Investig. 1999;103:1561–1570. doi: 10.1172/JCI5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sizemore N, Leung S, Stark G R. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz M L, Fiers W, Haegeman G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 36.Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz M L, Fiers W, Haegeman G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 37.Wang D, Baldwin A S. Activation of nuclear factor-kappaB-dependent transcription by tumor necrosis factor-alpha is mediated through phosphorylation of RelA/p65 on serine 529. J Biol Chem. 1998;273:29411–29416. doi: 10.1074/jbc.273.45.29411. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Westerheide S D, Hanson J L, Baldwin A S. TNFalpha-induced phosphorylation of RelA/p65 on serine 529 by casein kinase II. J Biol Chem. 2000;275:38127–38130. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 39.Wesche H, Henzel W J, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 40.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 41.Zhong H, Voll R E, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]