Abstract
Background:
ATLAS (NCT02951052), a phase 3, multicenter, open-label study, demonstrated that switching to injectable cabotegravir (CAB) with rilpivirine (RPV) long-acting dosed every 4 weeks was noninferior at week (W) 48 to continuing three-drug daily oral current antiretroviral therapy (CAR). Results from the W 96 analysis are presented.
Methods and design:
Participants completing W 52 of ATLAS were given the option to withdraw, transition to ATLAS-2M (NCT03299049), or enter an Extension Phase to continue long-acting therapy (Long-acting arm) or switch from CAR to long-acting therapy (Switch arm). Endpoints assessed at W 96 included proportion of participants with plasma HIV-1 RNA less than 50 copies/ml, incidence of confirmed virologic failure (CVF; two consecutive HIV-1 RNA ≥200 copies/ml), safety and tolerability, pharmacokinetics, and patient-reported outcomes.
Results:
Most participants completing the Maintenance Phase transitioned to ATLAS-2M (88%, n = 502/572). Overall, 52 participants were included in the W 96 analysis of ATLAS; of these, 100% (n = 23/23) and 97% (n = 28/29) in the Long-acting and Switch arms had plasma HIV-1 RNA less than 50 copies/ml at W 96, respectively. One participant had plasma HIV-1 RNA 50 copies/ml or higher in the Switch arm (173 copies/ml). No participants met the CVF criterion during the Extension Phase. No new safety signals were identified. All Switch arm participants surveyed preferred long-acting therapy to their previous daily oral regimen (100%, n = 27/27).
Conclusion:
In this subgroup of ATLAS, 98% (n = 51/52) of participants at the Extension Phase W 96 analysis maintained virologic suppression with long-acting therapy. Safety, efficacy, and participant preference results support the therapeutic potential of long-acting CAB+RPV treatment for virologically suppressed people living with HIV-1.
Keywords: antiretroviral therapy, cabotegravir, HIV-1, long-acting, rilpivirine
Introduction
To sustain viral suppression, current guideline-recommended first-line treatments for HIV-1 mandate lifelong daily adherence to oral regimens [1]. This can result in physiological, emotional, and logistical challenges for people with HIV (PWH) [2–5]. In addition, adherence to daily oral regimens can be easily disrupted by a number of factors including stigmatization concerns, pill burden, drug/food interactions, forgetfulness, depression, substance abuse, the fear of inadvertent disclosure, active lifestyles, and changes to daily routine [4,6]. Studies of PWH across diverse demographics have revealed an interest in long-acting antiretroviral therapies (ARTs) for providing an alternative to reliance on daily oral medication [7–11]. Such therapeutics have the potential to increase convenience and satisfaction by reducing dosing frequencies, helping to alleviate the multifaceted burden associated with daily oral ART [2,12].
Cabotegravir (CAB), an integrase strand transfer inhibitor, and rilpivirine (RPV), a nonnucleoside reverse transcriptase inhibitor, are two agents for which an approved monthly (United States, Canada, and EU) and every 2 months (EU) long-acting complete injectable dosing regimen has been developed [13–15]. Long-acting CAB+RPV is indicated for the treatment of HIV-1 infection in adults who are virologically suppressed (HIV-1 RNA <50 copies/ml). Regulatory approval of the monthly dosing regimen was based on two pivotal randomized phase 3 studies, ATLAS (NCT02951052) and FLAIR (NCT02938520) [16,17]. ATLAS and FLAIR demonstrated the noninferiority of intramuscular injections of long-acting CAB+RPV dosed every 4 weeks (Q4W) as a maintenance therapy vs. continuing current oral ART (CAR) in virologically suppressed PWH at W 48 at a 6% noninferiority margin [as per the Food and Drug Administration (FDA) Snapshot algorithm] [16,17]. In addition, a pooled analysis of the ATLAS and FLAIR trials demonstrated noninferiority at a stricter margin of 4% [18]. Both studies showed that the long-acting regimen was well tolerated over a 48-week period. Furthermore, participants reported greater levels of satisfaction and preference for the intramuscular therapy compared with their previous daily oral regimen [16,17]. A reduced dosing frequency for long-acting CAB+RPV of every 8 weeks (Q8W) was shown to be noninferior to Q4W dosing in the ATLAS-2M study (NCT03299049) and was the basis of the approval of the every 2 months dosing regimen [13,19].
Here, we present results from the W 96 analysis of the Extension Phase of ATLAS, constituted of a subgroup of participants initially randomized into ATLAS who did not transition to the ATLAS-2M study. We report efficacy, safety and tolerability, pharmacokinetic, and patient-reported outcome data for participants who switched from the current oral ART comparator arm (CAR arm) to long-acting CAB+RPV Q4W at the conclusion (W 52) of the Maintenance Phase (Switch arm). In addition, longer term outcomes are reported for participants already randomized to long-acting CAB+RPV Q4W at the start of the Maintenance Phase (Long-acting arm), representing 96 weeks of long-acting therapy.
Methods
Study design and participants
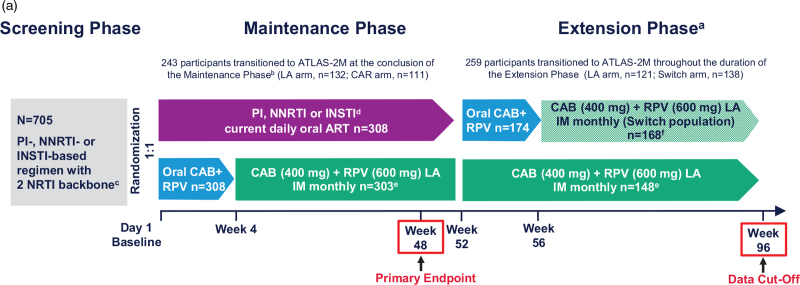
ATLAS is a randomized, multicenter, parallel-group, open-label phase 3 study evaluating the efficacy, safety, and tolerability of intramuscular long-acting CAB+RPV Q4W for the maintenance of virologic suppression following a switch from CAR in ART-experienced adults living with HIV-1 (Fig. 1a). The study design, along with the complete eligibility/exclusion criteria, has been published previously [17]. The full study protocol is available at ClinicalTrials.gov: NCT02951052. In brief, eligible participants were at least 18 years old and ART-experienced, having received uninterrupted ART without a change in medication or virologic failure for at least 6 months before screening. Participants with virologic suppression were randomized (1 : 1) to either continue CAR or switch to long-acting therapy Q4W (Long-acting arm; CAB 400 mg + RPV 600 mg, following an initial CAB 600 mg with RPV 900 mg loading dose) for the duration of the Maintenance Phase. Participants receiving long-acting therapy were given an oral lead-in of CAB 30 mg with RPV 25 mg for at least 4 weeks to assess safety and side effects before starting the intramuscular regimen.
Fig. 1.
(a) Study design.
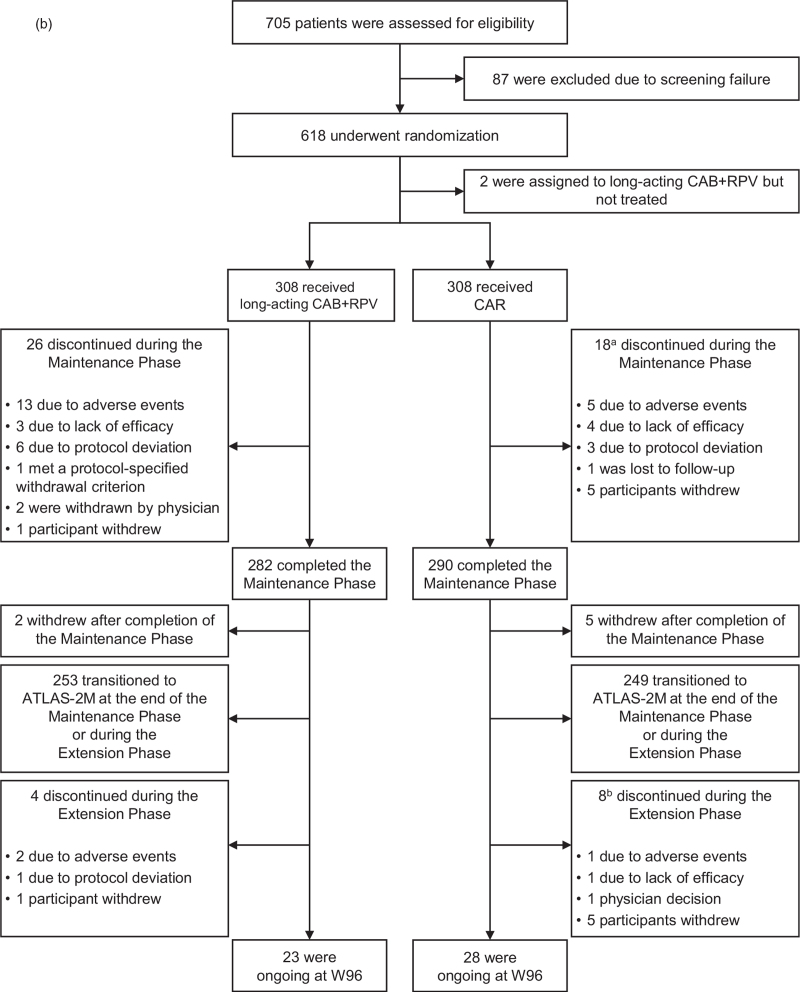
aOptional switch to long-acting CAB+RPV at W 52 for those on CAR; eligible participants in either arm could transition to the ATLAS-2M study (NCT03299049) at the conclusion of the Maintenance Phase or during the Extension Phase. bTwo patients transitioned to ATLAS-2M prior to the completion of the Maintenance Phase. cUninterrupted ART for 6 months and HIV-1 RNA less than 50 copies/ml at screening. Documented evidence of at least two HIV-1 RNA less than 50 copies/ml in the 12 months prior to screening. dINSTI-based regimen capped at 40% of enrolment; abacavir/dolutegravir/lamivudine excluded from study. eParticipants received an initial loading dose of long-acting CAB (600 mg) and long-acting RPV (900 mg) at W 4. From W 8 onwards, participants received long-acting CAB (400 mg) and long-acting RPV (600 mg) injections every 4 weeks. fParticipants received an initial loading dose of long-acting CAB (600 mg) and long-acting RPV (900 mg) at W 56. From W 60 onwards, participants received long-acting CAB (400 mg) and long-acting RPV (600 mg) injections every 4 weeks. ART, antiretroviral therapy; CAB, cabotegravir; CAR, current antiretroviral therapy; IM, intramuscular; INSTI, integrase strand transfer inhibitor; LA, long-acting; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RPV, rilpivirine. (b) Participant disposition. aIncludes two participants who deviated from protocol/transitioned to ATLAS-2M prior to the completion of the Maintenance Phase. bOne participant who discontinued at W 92 was included in the W 96 data analysis. CAB, cabotegravir; CAR, current antiretroviral therapy; LA, long-acting; RPV, rilpivirine; W, Week.
After completion of the Maintenance Phase, participants could choose to either withdraw, or, if eligible [HIV-1 RNA <50 copies/ml at W 48 (or upon retest by W 52), completion of at least W 52 of the ATLAS study, and plasma HIV-1 RNA <50 copies/ml at ATLAS-2M screening], transition to the ATLAS-2M study (investigating long-acting CAB+RPV Q8W vs. long-acting CAB+RPV Q4W) or enter the Extension Phase of ATLAS. Participants were also given the option to transition to ATLAS-2M throughout the Extension Phase as their study site gained approval for ATLAS-2M. Participants entering the Extension Phase of ATLAS at W 52 either continued long-acting CAB+RPV Q4W [Long-acting arm; intention-to-treat exposed (ITT-E) population] or were switched from CAR to long-acting CAB+RPV Q4W (Switch arm; Switch population). Any participant who had confirmed virologic failure (CVF; two consecutive plasma HIV-1 RNA levels ≥200 copies/ml) during the study discontinued and received oral ART. Any participant who received at least one dose of long-acting CAB+RPV in the study and discontinued for any reason entered long-term follow-up for 52 weeks. ATLAS was conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and the Declaration of Helsinki [20]. All participants provided written informed consent. The study protocol, any amendments, the informed consent, and other information that required preapproval was reviewed and approved by a national, regional, or investigational center ethics committee or institutional review board.
Endpoints and assessments
The primary endpoint was the proportion of participants with plasma HIV-1 RNA 50 copies/ml or higher at W 48 using the FDA Snapshot algorithm, and has been published previously [17]. Endpoints assessed at W 96 for the ITT-E population [Day 1 to W 96] and Extension Switch population (W 52 to W 96) included: proportion of participants with plasma HIV-1 RNA less than 50 copies/ml, changes in immunologic parameters, incidence of CVF and treatment-emergent genotypic resistance, incidence and severity of adverse events and injection site reactions (ISRs), number of discontinuations because of adverse events, CAB and RPV pharmacokinetics, and patient-reported outcomes.
Statistical analysis
Owing to the small sample size at W 96, a consequence of the large numbers of participants across both arms who elected to transition to ATLAS-2M, only descriptive analyses were performed. This analysis encompasses the results from participants’ time on study within the ATLAS Extension Phase through W 96. Therefore, the results presented also include some data (e.g. pharmacokinetic data) from participants who transitioned to ATLAS-2M after limited time in the ATLAS Extension Phase (Long-acting arm, n = 121; Switch arm, n = 138). Measurements taken at W 96 (e.g. efficacy data) only include participants present at the W 96 analysis (Long-acting arm, n = 23; Switch arm, n = 29) (i.e. exclude those who transitioned to ATLAS-2M before W 96).
Results
Participants
Baseline characteristics were comparable across arms and have been previously reported [17]. In the ITT-E population, 282 and 290 participants completed the Maintenance Phase in the long-acting and CAR arms, respectively (Fig. 1b). In total, 174 participants from the CAR arm elected to switch to long-acting CAB+RPV (Switch arm; Extension Switch population) and 148 participants in the Long-acting arm continued long-acting CAB+RPV in the Extension Phase (Long-acting arm) (Fig. 1a). At the conclusion of the Maintenance Phase, seven participants discontinued from the study (Long-acting arm, n = 2; CAR arm, n = 5), with the remainder choosing to transition to ATLAS-2 M (Long-acting arm, n = 132; CAR arm, n = 111). As participants progressed through the Extension Phase and became eligible (based on study site approval for ATLAS-2M), the majority elected to transition to ATLAS-2M (Long-acting arm, n = 121; Switch arm, n = 138). For these participants, 98% (Long-acting arm, n = 118; Switch arm, n = 137) had 24 weeks or less of exposure to CAB+RPV in the Extension Phase, with a median [interquartile range (IQR)] of 8 (4, 12) weeks for the Long-acting arm and 10 (9, 13) weeks for the Switch arm. Overall, the number of participants who withdrew for other reasons during the Extension Phase was low (Long-acting arm, n = 4; Switch arm, n = 8), of whom three discontinued because of adverse events (Long-acting arm, n = 2; Switch arm, n = 1). This left 52 participants in the ATLAS study at W 96 [Long-acting arm, n = 23; Switch arm, n = 29 (one participant who discontinued at W 92 was included in the W 96 data analysis)].
Efficacy
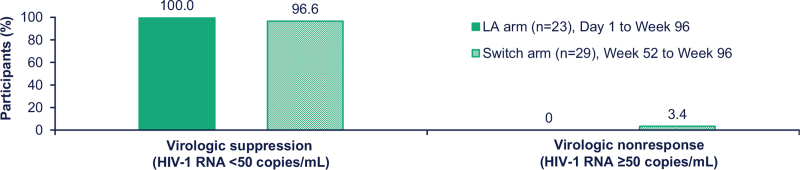
At W 96, long-acting CAB+RPV maintained virologic suppression (HIV-1 RNA <50 copies/ml) in most participants across both treatment arms. In the Long-acting arm, comprising participants randomized to long-acting CAB+RPV at the beginning of the Maintenance Phase, 100% (n = 23/23) of participants included in the W 96 analysis maintained virologic suppression. In the Switch arm, constituting participants originally randomized to CAR at the beginning of the Maintenance Phase, 97% of participants (n = 28/29) included in the W 96 analysis maintained virologic suppression (Fig. 2); the participant with HIV-1 RNA 50 copies/ml or higher had a viral load of 173 copies/ml at the W 96 data analysis and was placed on atazanavir, emtricitabine, and tenofovir and entered Long-term follow-up. No participant in either treatment arm met the CVF criterion during the Extension Phase up to the 96-week timepoint. At W 96, the mean [standard deviation (SD)] CD4+ cell count change from Baseline in the long-acting arm and Extension Baseline (W 52) in the Switch arm was −5.7 (167.6) cells/μl (n = 23) and −33.6 (145.3) cells/μl (n = 29), respectively.
Fig. 1 (Continued).
(a) Study design.
aOptional switch to long-acting CAB+RPV at W 52 for those on CAR; eligible participants in either arm could transition to the ATLAS-2M study (NCT03299049) at the conclusion of the Maintenance Phase or during the Extension Phase. bTwo patients transitioned to ATLAS-2M prior to the completion of the Maintenance Phase. cUninterrupted ART for 6 months and HIV-1 RNA less than 50 copies/ml at screening. Documented evidence of at least two HIV-1 RNA less than 50 copies/ml in the 12 months prior to screening. dINSTI-based regimen capped at 40% of enrolment; abacavir/dolutegravir/lamivudine excluded from study. eParticipants received an initial loading dose of long-acting CAB (600 mg) and long-acting RPV (900 mg) at W 4. From W 8 onwards, participants received long-acting CAB (400 mg) and long-acting RPV (600 mg) injections every 4 weeks. fParticipants received an initial loading dose of long-acting CAB (600 mg) and long-acting RPV (900 mg) at W 56. From W 60 onwards, participants received long-acting CAB (400 mg) and long-acting RPV (600 mg) injections every 4 weeks. ART, antiretroviral therapy; CAB, cabotegravir; CAR, current antiretroviral therapy; IM, intramuscular; INSTI, integrase strand transfer inhibitor; LA, long-acting; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RPV, rilpivirine. (b) Participant disposition. aIncludes two participants who deviated from protocol/transitioned to ATLAS-2M prior to the completion of the Maintenance Phase. bOne participant who discontinued at W 92 was included in the W 96 data analysis. CAB, cabotegravir; CAR, current antiretroviral therapy; LA, long-acting; RPV, rilpivirine; W, Week.
Fig. 2.
Efficacy outcomes based on observed viral load values at W 96 analysis.
LA, long-acting.
Safety and tolerability
Safety data collected during the Extension Phase for the Long-acting and Switch arms were comparable with those collected for the Long-acting arm during the Maintenance Phase, with respect to the type of overall adverse events, non-ISR adverse events, and common nonserious adverse events (Table 1 and Supplemental Digital Content Table S1, an overview of common nonserious adverse events). Overall, 105 and 79 participants in the Switch arm reported adverse events and drug-related adverse events, respectively. Four Switch arm participants reported Grade 3 or above adverse events [Grade 3 injection site pain (n = 3) and Grade 4 increased lipase (n = 1)]. One new participant in the Long-acting arm reported an adverse event during the Extension Phase as compared with the Maintenance Phase. No new participants in the Long-acting arm reported a drug-related adverse event during the Extension Phase. No serious adverse event was considered related of long-acting CAB+RPV treatment. During the Extension Phase, two participants in the Long-acting arm discontinued because of adverse events, which were identified as acute hepatitis B (Grade 3, considered not related to study treatment) and fear (Grade 1, considered related to study treatment). One participant in the Switch arm discontinued because of two adverse events of injection site pain. No deaths were reported during the Extension Phase in either treatment arm.
Table 1.
Adverse event overview.
| Maintenance phase [17] | Extension phasea | |||
| Outcome, n (%) | LA arm (Day 1 to W 52) n = 308 | CAR arm (Day 1 to W 52) n = 308 | LA armb (W 52 to W 96)c | Switch arm (W 52 to W 96)d |
| Any AEs | 294 (95) | 220 (71) | 1 | 105 |
| Excluding ISRs | 264 (86) | 220 (71) | 3 | 76 |
| Any Grade 3 to 4 AEs | 35 (11) | 23 (7) | 2 | 7 |
| Excluding ISRs | 25 (8) | 23 (7) | 2 | 5 |
| Any drug-related AEs | 255 (83) | 8 (3) | 0 | 79 |
| Drug-related Grade 3 to 4 AEs | 14 (5) | 1 (<1) | 0 | 4e |
| Any serious AEsf | 13 (4) | 14 (5) | 2 | 2 |
| Fatal serious AEs | 0 | 1 (<1)g | 0 | 0 |
| AEs leading to withdrawal | 14 (5) | 5 (2) | 2h | 1i |
AE, adverse event; CAB, cabotegravir; CAR, current antiretroviral therapy; ISR, injection site reaction; LA, Long-acting; RPV, rilpivirine.
Percentage was not calculated because of the declining population/denominator over time.
Values represent the number of new participants with adverse events in the Long-acting arm during the Extension Phase.
One hundred and forty-eight participants entered the Extension Phase; however, this number declined throughout the study, leaving 23 participants at the W 96 analysis.
One hundred and seventy-four participants entered the Extension Phase; however, this number declined throughout the study, leaving 29 participants at the W 96 analysis.
Grade 3 injection site pain (n = 3) and Grade 4 increased lipase (n = 1).
No serious adverse events were classified as related to CAB+RPV.
Death was because of a methamphetamine overdose and was considered not related to the study treatment.
Includes acute hepatitis B and fear.
Injection site pain.
Injection site reactions
Among 2627 injections administered during the Extension Phase (Long-acting arm, n = 1363; Switch arm, n = 1264), 392 ISRs were recorded (Long-acting arm, n = 154; Switch arm, n = 238) (Table 2). Most ISRs were Grade 1 (mild) or Grade 2 (moderate) in severity [Long-acting arm, 100% (n = 154/154); Switch arm, 99% (n = 235/238)], except for three single Grade 3 adverse events of injection site pain in the Switch arm; none were considered serious by investigators. The most frequently occurring ISR event was injection site pain [Long-acting arm, 78% of ISR events (n = 120/154); Switch arm, 87% of ISR events (n = 207/238)]; all other ISRs had an incidence of less than 5% in each arm, with the exception of injection site induration [Long-acting arm, 5% of ISR events (n = 8/154); Switch arm, 6% of ISR events (n = 14/238)]. No participants in the Long-acting arm discontinued because of reasons related to injections during the Extension Phase. Two participants in the Switch arm withdrew for reasons related to injections: one because of two Grade 2 events of injection site pain and another because of intolerability of injections.
Table 2.
Injection site reactions.
| Maintenance phase | Extension phase | ||
| LA arm (Day 1 to W 52) n = 308a | LA armb (W 52 to W 96) | Switch armc (W 52 to W 96) | |
| Number of injections | 6978 | 1363 | 1264 |
| ISR events | 1460 | 154 | 238 |
| Pain,dn (% of injections) | 1208 (17.3) | 120 (8.8) | 207 (16.4) |
| Induration, n (% of injections) | 54 (<1) | 8 (<1) | 14 (1.1) |
| Nodule, n (% of injections) | 54 (<1) | 7 (<1) | 3 (<1) |
| Swelling, n (% of injections) | 48 (<1) | 2 (<1) | 4 (<1) |
| Erythema, n (% of injections) | 38 (<1) | 2 (<1) | 0 |
| Pruritus, n (% of injections) | 12 (<1) | 14 (1.0) | 3 (<1) |
| Grade ≥3 (severe) ISR eventse | 21 | 0 | 3 |
| Median duration of ISRs (days) | 3 | 3 | 3 |
| Number of participant withdrawals because of ISRs or injection intolerability | 4 | 0 | 2 |
ISR, injection site reaction; LA, long-acting.
Three hundred and three participants received injection.
One hundred and forty-eight participants entered the Extension Phase; however, this number declined throughout the study, leaving 23 participants at the W 96 analysis.
One hundred and seventy-four participants entered the Extension Phase; however, this number declined throughout the study, leaving 29 participants at the W 96 analysis.
Only the six most frequent ISRs are listed.
There were no Grade 4 or 5 ISR events.
Laboratory evaluations and vital signs
The incidence values of clinical laboratory abnormalities in the Extension Phase are detailed in Supplemental Digital Content Table S2, which summarizes maximum emergent chemistry toxicities. Two participants in the Extension Phase (both arm) had alanine aminotransferase elevations to at least three times the upper limit of the normal range, both of which met protocol-defined liver-related stopping criteria. Both participants were found to have viral hepatitis; one participant tested positive for acute hepatitis E and the other participant had acute hepatitis B. In the Long-acting and Switch arms, median (IQR) bodyweight increased throughout the Extension Phase: a weight gain of 2.1 (−1.0, 5.0) kg from Baseline to W 96 occurred in the Long-acting arm (n = 23); a weight gain of 1.1 (0.0, 3.1) kg from W 52 to W 96 occurred in the Switch arm (n = 29). Similarly, increases in median (IQR) BMI were also recorded [Long-acting arm, 0.7 (−0.4, 1.5) kg/m2 increase from Baseline; Switch arm, 0.3 (0, 1.1) kg/m2 increase from Extension Baseline].
Pharmacokinetic analysis
In both the Switch and Long-acting arms, median plasma CAB and RPV trough concentrations remained above their respective protein binding-adjusted 90% inhibitory concentration (PA-IC90: CAB, 0.166 μg/ml; RPV, 12.0 ng/ml) throughout the Extension Phase (Supplemental Digital Content Figure S1, which illustrates median plasma CAB and RPV trough concentrations collected throughout the study). For the Switch arm, median W 96 CAB and RPV trough concentrations after 40 weeks of intramuscular injections were 1.7-fold and 1.6-fold higher, respectively, than at W 60, 4 weeks following the initial intramuscular loading dose [CAB: W 60, 1.41 μg/ml (n = 127); W 96, 2.42 μg/ml (n = 24); RPV: W 60, 42.9 ng/ml (n = 127); W 96, 66.7 ng/ml (n = 24)]. For the Long-acting arm, the median W 96, CAB trough concentration after 92 weeks of intramuscular injections was comparable with that observed in the Switch arm [Long-acting arm, 2.56 μg/ml (n = 19); Switch arm, 2.42 μg/ml (n = 24)], consistent with an achievement of steady state after ∼44 weeks of injections. For RPV, the median W 96 plasma trough concentration in the Long-acting arm was higher than that in the Switch arm [Long-acting arm, 109.0 ng/ml (n = 19); Switch arm, 66.7 ng/ml (n = 24)]. This is consistent with limited further accumulation in the second year of injections, in line with the reported 28-week half-life of long-acting RPV [21].
Patient-reported outcomes
At W 96, HIV Treatment Satisfaction Questionnaire status version (HIVTSQs) total treatment satisfaction scores for Long-acting arm participants remained high and were comparable with W 24 and W 44, with numerical improvements from Baseline observed across all timepoints [mean HIVTSQs total score (SD): Baseline (n = 302), 55.25 (9.14); W 24 (n = 290), 61.80 (6.65); W 44 (n = 282), 61.80 (5.31); W 96 (n = 27), 61.33 (8.06)]. Numerical improvements in total treatment satisfaction at W 96 were also observed for participants in the Switch arm [mean HIVTSQs total score (SD): Extension Baseline (n = 174), 54.66 (10.72); W 96 (n = 35), 59.20 (12.77)].
At W 96, 100% (n = 27/27) of participants in the Switch arm who responded to the treatment satisfaction questionnaire selected long-acting CAB+RPV as their preferred regimen compared with the daily oral treatment that they had received during the Maintenance Phase.
Discussion
Long-acting CAB+RPV was found to provide durable virologic suppression with a favorable safety profile over a period of ∼2 years. Providing PWH with broader therapeutic choices, including an effective ART regimen with much less frequent administration, is inherently valuable to clinicians to enable prescribing of therapies best suited to individual PWH needs. The reduction in dosing frequency facilitated by injectable long-acting therapies may help to alleviate some of the multifaceted burden associated with daily oral ART, including stigmatization, high pill burden, drug/food interactions, and the fear of inadvertent disclosure [4,6]. The present study, along with the longer term results reported from the phase 3 FLAIR and phase 2b LATTE-2 studies [22,23], provides further evidence supporting the durability of intramuscular long-acting CAB+RPV Q4W for the maintenance of virologic suppression in PWH.
Long-acting CAB+RPV maintained virologic suppression in 98% (n = 51/52) of participants present at the W 96 analysis, constituting participants who switched from CAR at the beginning of the Extension Phase as well as those who had been receiving long-acting therapy throughout the 52-week Maintenance Phase (representing ∼2 years of long-acting therapy). This, in combination with the 96-week data collected from the FLAIR study [23], supports the longer term therapeutic potential of intramuscular long-acting CAB+RPV treatment. Notably, no participant in either the continued arm or the Switch arm met the CVF criterion during the Extension Phase. This is consistent with the results from W 96 of FLAIR, at which no participants receiving long-acting treatment met the CVF criterion between the W 48 and W 96 data analyses [23], as well as the primary W 48 analysis for ATLAS and FLAIR, at which the occurrence of CVF was low (∼1%) [18]. Notably, the rate of CVF for participants receiving long-acting therapy was similar to that of the oral comparator regimen and consistent with phase 3 switch studies evaluating contemporary oral ARTs [18,24,25]. In a post hoc analysis across the ATLAS, FLAIR, and ATLAS-2M studies, the occurrence of CVF was indicated to be multifactorial, whereby an increased risk of CVF was associated with the presence of at least two baseline factors of: proviral resistance-associated mutations to RPV, HIV-1 subtype A6/A1, and/or BMI equal to or greater than 30 kg/m2[26].
With respect to safety and tolerability, data collected for participants in the Switch arm during the Extension Phase were comparable with those collected for the Long-acting arm during the Maintenance Phase. No longer term safety signals were identified in the arm; however, the low number of participants at the W 96 analysis (Long-acting arm, n = 23; Switch arm, n = 29) limits the robustness of this conclusion. Nevertheless, as most participants transitioned to ATLAS-2M, any longer term safety signals would have likely been captured in the primary clinical analysis of ATLAS-2M [19]. During the Extension Phase, the frequency and severity of ISRs in the Switch arm were consistent with the results observed in the Long-acting arm during the Maintenance Phase. Notably, relative to the number of injections, fewer ISRs were observed in the Extension Phase than the Maintenance Phase in the Long-acting arm. This may reflect a gradual increase in injection tolerance in the second year of long-acting therapy, a pattern mirrored in the results from other longer term studies of long-acting CAB+RPV that demonstrated a reduction in ISRs over time, with fewer withdrawals because of injection-related reasons in the second year of treatment [23].
The pharmacokinetic profile of Switch arm participants during the Extension Phase was consistent with Long-acting arm participants during the Maintenance Phase through W 48 and participants receiving long-acting CAB+RPV in the first 48 weeks of FLAIR [16,17]. The longer term pharmacokinetic profile of Long-acting arm participants suggested an achievement of steady state for CAB after ∼44 weeks. For RPV, concentrations appear to plateau in the second year, with only limited further accumulation of RPV in the second year of injections. This is consistent with the half-life of RPV and has been observed in other longer term studies evaluating long-acting CAB+RPV [21,23].
Most participants, regardless of the treatment they received during the ATLAS study (Long-acting CAB+RPV Q4W or CAR), elected to transition to ATLAS-2M (investigating Long-acting CAB+RPV Q8W vs. Q4W) after completing the Maintenance Phase. Such willingness to switch to, or continue, intramuscular long-acting CAB+RPV treatment suggests a strong preference for long-acting therapy over oral standard of care. This is further supported by data collected at the W 96 analysis, at which all Switch arm participants responding to the treatment satisfaction questionnaire (100%, n = 27/27) indicated a preference for long-acting CAB+RPV therapy over their previous daily oral regimen. High participant preference for, and satisfaction with, longer term long-acting therapy over 96 weeks of treatment has been reported in other studies investigating long-acting CAB+RPV, such as the phase 3 FLAIR study and the phase 2b LATTE-2 study [16,22,23].
Whilst long-acting CAB+RPV treatment could be beneficial to those with adherence issues with oral ARTs, this needs to be specifically examined in such populations. Further clarity in this regard will be provided by the ongoing LATITUDE study (NCT03635788), which is currently investigating intramuscular long-acting CAB+RPV in a population with historic suboptimal adherence [27].
Limitations
One limitation of the current analysis is participant attrition throughout the Extension Phase, primarily because of the decision of study participants to move from ATLAS to the ATLAS-2M study after 48 weeks. As a result, the residual sample restricted the ability to perform statistical analyses and should be taken into consideration whenever interpreting the reported data. Several other studies with larger populations are ongoing to confirm the longer term efficacy and safety of long-acting CAB+RPV. Additionally, because of the nature of injectable therapies, ATLAS is conducted as an open-label trial. Thus, some patient-reported results may be subject to bias. Finally, conclusions on treatment satisfaction are limited to patients who are willing to consider injectable therapy, reflecting the enrolled trial population, and, therefore, cannot be generalized to all PWH.
Conclusions
After the end of the randomized ATLAS Maintenance Phase, 99% (n = 565/572) of study participants chose to continue or switch to long-acting therapy in ATLAS (n = 63) or ATLAS-2M (n = 502), with few participants (n = 7) choosing to withdraw entirely. In the Switch arm, intramuscular long-acting CAB+RPV maintained virologic suppression over 48 weeks in all but one participant switching from CAR, with no participants meeting the CVF criterion. In the long-acting arm, long-acting CAB+RPV effectively maintained virologic suppression in all remaining participants over a period of 96 weeks, with no new safety signals identified and no participants meeting the CVF criterion in the second year of treatment. The results must be interpreted in the context of the low number of participants present at the W 96 data analysis. Taken together, these longer term efficacy, safety, and tolerability data complement the positive results collected at W 48 and support patient preference for, and the therapeutic potential of, long-acting CAB+RPV treatment for virologically suppressed PWH.
Acknowledgements
We thank everyone who has contributed to the success of the study: all study participants and their families, and the clinical investigators and their staff.
The Antiretroviral Therapy as Long-Acting Suppression (ATLAS) study (NCT02951052) is funded by ViiV Healthcare and Janssen Pharmaceuticals.
Professional medical writing and editorial assistance was provided by Euan Paul and Niamh Mortimer at SciMentum (Nucleus Global) and funded by ViiV Healthcare.
S.S., T.L., L.V.Z., N.P., M.S., E.M., and A.S. participated in the collection and interpretation of the data. P.B., J.O.H., C.M.H., K.H., S.L.F., C.L.T., V.C., H.C., R.V.S., S.V., D.A.M., K.Y.S., K.V., and W.R.S. were responsible for the development, conception, and data analysis of the study. All authors contributed to the review and development of the manuscript.
Data sharing: data sharing requests will be considered by the management group upon written request to the corresponding author. Deidentified participant data or other prespecified data will be available subject to a written proposal and a signed data sharing agreement.
Conflicts of interest
S.S. reports grants from ViiV Healthcare, during the conduct of the study. T.L. received funding of studies from GlaxoSmithKline, Janssen-Cilag, Merck, Sharp & Dohme, AbbVie, Gilead Sciences, Heidelberg ImmunoTherapeutics. L.V.Z., N.P., and E.M. have nothing to disclose. M.S. received honoraria as an advisor or lecturer, as well as funding of studies, from GlaxoSmithKline, Janssen-Cilag, Merck, Sharp & Dohme, Novartis, and ViiV Healthcare. A.S. received honoraria as an advisor or lecturer, as well as funding of studies, from GlaxoSmithKline, Janssen-Cilag, Merck, Sharp & Dohme, and ViiV Healthcare. H.C., R.V.S., S.V., and K.V. are employees and stockholders of Janssen, Pharmaceutical Companies of Johnson & Johnson. P.B., C.M.H., C.L.T., V.C., K.Y.S., and W.R.S. are employees of ViiV Healthcare and stockholders of GlaxoSmithKline. J.O.H., K.H., and S.L.F. are employees and stockholders of GlaxoSmithKline. D.A.M. was an employee of ViiV Healthcare and stockholder of GlaxoSmithKline during the conduct of the study and is now an employee of Brii Biosciences.
Previous presentation: HIV Drug Therapy Glasgow Virtual Congress; 5–8 October 2020.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1. U.S. Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. 2020. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/15/virologic-failure. [Accessed 25 February 2020] [Google Scholar]
- 2.Kerrigan D, Mantsios A, Gorgolas M, Montes ML, Pulido F, Brinson C, et al. Experiences with long acting injectable ART: a qualitative study among PLHIV participating in a phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One 2018; 13:e0190487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swindells S, Flexner C, Fletcher CV, Jacobson JM. The critical need for alternative antiretroviral formulations, and obstacles to their development. J Infect Dis 2011; 204:669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shubber Z, Mills EJ, Nachega JB, Vreeman R, Freitas M, Bock P, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med 2016; 13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Los Rios P, Young B, Marcotullio S, Punekar Y, Koteff J, Ustianowski A, et al. 1329. Experiences and emotional challenges of antiretroviral treatment (ART)—findings from the Positive Perspectives Study. Open Forum Infect Dis 2019; 6: (Suppl 2): S481. [Google Scholar]
- 6.Tseng A, Seet J, Phillips EJ. The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future. Br J Clin Pharmacol 2015; 79:182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dandachi D, Dang BN, Lucari B, Swindells S, Giordano TP. Acceptability and preferences for long-acting antiretroviral formulations among people with HIV infection. AIDS Care 2020; 33:801–809. [DOI] [PubMed] [Google Scholar]
- 8.Williams J, Sayles HR, Meza JL, Sayre P, Sandkovsky U, Gendelman HE, et al. Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomedicine (Lond) 2013; 8:1807–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weld ED, Rana MS, Dallas RH, Camacho-Gonzalez AF, Ryscavage P, Gaur AH, et al. Interest of youth living with HIV in long-acting antiretrovirals. J Acquir Immune Defic Syndr 2019; 80:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerrigan D, Sanchez Karver T, Muraleetharan O, Savage V, Mbwambo J, Donastorg Y, et al. ‘A dream come true’: perspectives on long-acting injectable antiretroviral therapy among female sex workers living with HIV from the Dominican Republic and Tanzania. PLoS One 2020; 15:e0234666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philbin MM, Parish CL, Kinnard EN, Reed SE, Kerrigan D, Alcaide ML, et al. Multisite study of women living with HIV's perceived barriers to, and interest in, long-acting injectable antiretroviral therapy. J Acquir Immune Defic Syndr 2020; 84:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Amico R, Margolis DA. Long-acting injectable therapy: an emerging paradigm for the treatment of HIV infection. Curr Opin HIV AIDS 2020; 15:13–18. [DOI] [PubMed] [Google Scholar]
- 13. ViiV Healthcare. Vocabria summary of product characteristics. EU. 2021. [Google Scholar]
- 14. ViiV Healthcare. Vocabria (cabotegravir tablets) and Cabenuva (cabotegravir and rilpivirine extended release injectable suspensions) Product Monograph. Canada. 2020. [Google Scholar]
- 15. ViiV Healthcare. Cabotegravir extended-release injectable suspension; rilpivirine extended-release injectable suspension (Cabenuva) Prescribing Information. US. 2021. [Google Scholar]
- 16.Orkin C, Arasteh K, Górgolas Hernández-Mora M, Pokrovsky V, Overton ET, Girard PM, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382:1124–1135. [DOI] [PubMed] [Google Scholar]
- 17.Swindells S, Andrade-Villanueva JF, Richmond GJ, Rizzardini G, Baumgarten A, Masiá M, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382:1112–1123. [DOI] [PubMed] [Google Scholar]
- 18.Rizzardini G, Overton ET, Orkin C, Swindells S, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting injectable cabotegravir + rilpivirine for HIV maintenance therapy: week 48 pooled analysis of phase 3 ATLAS and FLAIR Trials. J Acquir Immune Defic Syndr 2020; 85:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overton ET, Richmond G, Rizzardini G, Jaeger H, Orrell C, Nagimova F, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2 M), 48-week results: a randomised, multicentre, open-label, phase 3b, noninferiority study. Lancet 2021; 396:1994–2005. [DOI] [PubMed] [Google Scholar]
- 20.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 21.Ford S, Crauwels H, Han K, Rossenu S, Zhang F, Huang JO, et al. Cabotegravir and rilpivirine PK following long-acting HIV treatment discontinuation. Abstract presented at: Conference on Retroviruses and Opportunistic Infections (CROI); 8–11 March 2020; Boston, Massachusetts, USA. [Google Scholar]
- 22.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, Eron JJ, Yazdanpanah Y, Podzamczer D, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, noninferiority trial. Lancet 2017; 390:1499–1510. [DOI] [PubMed] [Google Scholar]
- 23.Orkin C, Oka S, Philibert P, Brinson C, Bassa A, Gusev D, et al. Long-acting cabotegravir + rilpivirine for treatment in adults with HIV 1 infection: week 96 results of the randomized, open-label, phase 3 FLAIR study. Lancet HIV 2021; 8:e185–e196. [DOI] [PubMed] [Google Scholar]
- 24.Cahn P, Madero JS, Arribas JR, Antinori A, Ortiz R, Clarke AE, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, noninferiority, phase 3 trials. Lancet 2019; 393:143–155. [DOI] [PubMed] [Google Scholar]
- 25.Llibre JM, Hung CC, Brinson C, Castelli F, Girard PM, Kahl LP, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, noninferiority SWORD-1 and SWORD-2 studies. Lancet 2018; 391:839–849. [DOI] [PubMed] [Google Scholar]
- 26.Cutrell AG, Schapiro JM, Perno CF, Kuritzkes DR, Quercia R, Patel P, et al. Exploring predictors of HIV-1 virologic failure to long-acting cabotegravir and rilpivirine: a multivariable analysis. AIDS 2021; 35:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ClinicalTrials.gov. The LATITUDE study: long-acting therapy to improve treatment success in daily life ( NCT03635788). Available at: https://www.clinicaltrials.gov/ct2/show/NCT03635788. [Accessed 18 March 2020] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.