Abstract
Background and Purpose:
To identify the qualitative and quantitative contributions of conventional risk factors for occurrence of ischemic stroke and its key pathophysiologic subtypes among West Africans.
Methods:
The Stroke Investigative Research and Educational Network (SIREN) is a multicenter, case-control study involving 15 sites in Ghana and Nigeria. Cases include adults aged ≥18 years with ischemic stroke who were etiologically subtyped using the A-S-C-O-D classification into Atherosclerosis, Small-vessel occlusion, Cardiac pathology, Other causes and dissection. Controls were age-and-gender matched stroke-free adults. Detailed evaluations for vascular, lifestyle and psychosocial factors were performed. We used conditional logistic regression to estimate adjusted odds ratios (aOR) with 95% Confidence Interval.
Results:
There were 2,431 ischemic stroke case and stroke-free control pairs with respective mean ages of 62.2 ± 14.0 versus 60.9 ± 13.7 years. There were 1,024(42.1%) small vessel occlusions, 427(17.6%) large-artery atherosclerosis, 258(10.6%) cardio-embolic, 3(0.1%) carotid dissections and 719(29.6%) undetermined/other causes. The aOR (95%CI) for the 8 dominant risk factors for ischemic stroke were hypertension, 10.34(6.91,15.45); dyslipidemia, 5.16(3.78,7.03); diabetes mellitus, 3.44(2.60,4.56); low green vegetable consumption, 1.89(1.45,2.46); red meat consumption, 1.89(1.45,2.46); cardiac disease, 1.88(1.22,2.90); monthly income $100 or more, 1.72(1.24,2.39); and psychosocial stress, 1.62(1.18,2.21). Hypertension, dyslipidemia, diabetes were confluent factors shared by small-vessel, large-vessel and cardio-embolic subtypes. Stroke cases and stroke-free controls had a mean of 5.3 ± 1.5 versus 3.2 ± 1.0 adverse cardio-metabolic risk factors respectively (p<0.0001).
Conclusion:
Traditional vascular risk factors demonstrate important differential effect sizes with pathophysiologic, clinical and preventative implications on the occurrence of ischemic stroke among indigenous West Africans.
Keywords: Dominant, Risk Factors, Ischemic Stroke, Subtypes, West Africa
INTRODUCTION
Diverse etiologic factors are responsible for the pathophysiologic mechanisms leading to the occlusion of central nervous system blood vessels and ultimately infarctions. These etiologic factors are made up of non-modifiable and modifiable vascular risk factors which may exert differential impacts on the occurrence of ischemic stroke and its subtypes.1 The clustering of vascular risk factors at an individual host level, may contribute to the heterogeneity and overlaps in the etiology of cardiovascular diseases including ischemic strokes.2–4 Improving the accuracy of ischemic stroke etiologic phenotypes and risk factor profiling would greatly enhance our understanding of the genetic predisposition for ischemic stroke. Advances in stroke genetics so far have shown that ischemic stroke subtypes differ in their dominant causal genetic variants. Mounting lines of evidence now show that ischemic strokes exhibit polygenic hereditary patterns with contributions from an admixture of vascular risk factors with their own polygenic genetic models of inheritance interacting with environmental exposures. 5–7 In addition, information on the level of clustering of risk factors at the individual and population level for ischemic stroke would be essential for planning and implementation of primary and secondary stroke prevention interventions.
Very few research have been undertaken to provide a phenotypic characterization of ischemic strokes in sub-Saharan Africa (SSA) where the incidence, prevalence, mortality and morbidity from stroke is highest worldwide with further projected escalations in the coming decades.8,9 We therefore sought to profile the dominant risk factors for the etiologic subtypes of ischemic stroke among West Africans in Ghana and Nigeria10
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design:
The Stroke Investigative Research and Educational Network (SIREN) study is a case-control study involving 15 centers in Ghana and Nigeria. The study commenced in August 2014 and the protocol has been published elsewhere.10 In brief, stroke cases were consenting (in unconscious/aphasic subjects, consent from next of kin was obtained) adults aged ≥18 years. To be eligible for enrollment, stroke should have occurred within 8 days of current symptom onset or ‘last seen without deficit’ with neuroimaging confirmation using computerized tomography (CT) scan within 10 days of symptom onset. Stroke patients were recruited from hospitals to ensure rapid and accurate phenotyping.
Controls were consenting stroke-free adults, recruited mostly from the communities in the catchment areas of the SIREN hospitals where stroke cases resided. Community leaders were informed about the objectives of the SIREN study and permission was sought to invite interested stroke-free controls to meeting places within communities such as churches, mosques or school compounds for enrollment. All interviews, assessments and sample collection were performed within the community by trained research coordinators. Stroke-free status was ascertained using a locally pre-validated 8-item questionnaire for verifying stroke-free status (QVSFS) with 98% negative predictive value in 3 major languages spoken in West Africa (Ashanti, Hausa and Yoruba).11 We matched stroke-free controls by age (+/− 5 years), sex and ethnicity to minimize the potential confounding effect of these variables on the relationship between stroke and its risk factors. Ethnicity was defined by language spoken by participant and a specific response to a question on the ethnicity of the respondent according to family lineage. Cases were matched 1:1 to controls. Ethical approval was obtained from all study sites and informed consent was obtained from all participants.10 This study follows the STROBE checklist recommendations (Supplementary materials I).
Stroke Phenotyping:
All patients underwent a cranial CT scan, and the diagnosis of ischemic stroke was based on the historical World Health Organization criteria. Ischemic stroke was classified etiologically using the A-S-C-O-D classification into A: Atherosclerosis, S: Small-vessel occlusion, C: Cardiac pathology, O: Other causes and D: dissection.12 Each ASCOD phenotype has a sub-score of 0= disease is not present; 1= definitely a potential cause of the index stroke such as ipsilateral atherosclerotic stenosis of 50–99% of an intra- or extracranial artery for atherosclerosis; presence of atrial fibrillation or thrombus in left atrial appendage or left ventricular chamber for cardiac pathology; single subcortical infarction <20mm in diameter; 2=causality link is uncertain such as ipsilateral stenosis <50% and; 3=disease is present, but unlikely to be a direct cause of the index stroke.12
Data collection:
We collected basic demographic, socioeconomic, lifestyle and dietary data. We used validated instruments to assess physical activity, dietary practice, stress, depression, cigarette smoking, and alcohol use. These instruments were administered by an interviewer in the local dialect of participant. A uniform standard operating procedure (SOP) was employed across all study sites to obtain blood samples for HbA1c and early morning samples after overnight fast in cases (post-acute phase when fasting is feasible) and controls for quantification of fasting plasma glucose, and fasting lipid profile [total cholesterol (TC), low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C) and triglycerides (TG)].
Definition of risk factors
Hypertension: Blood pressure (BP) (average of three measurements used) was recorded at baseline and daily for 7 days or until death. The indicators of hypertension were a prior history of hypertension or cutoff of ≥140/ 90 mmHg for up to 72 hours after stroke, use of antihypertensive drugs before stroke, or >72 hours after stroke. Adjustments to systolic BP (SBP) were performed based on reported associations between pre-morbid BP and acute post-stroke BP in the Oxford Vascular Study (OXVASC).13 Definition of hypertension in controls was self-reported history of hypertension or use of antihypertensive drugs or average BP at first clinical encounter ≥140/90mmHg.14
Diabetes mellitus (DM) was defined based on history of diabetes mellitus, use of medications for DM, an HbA1c ≥6.5% or a fasting plasma glucose (FPG) levels ≥7.0mmol/l at first encounter in controls or measured after the post-acute phase in cases due to the known acute transient elevation of glucose as a stress response after stroke.15
Dyslipidemia was defined as fasting TC ≥5.2mmol/L, HDL-C ≤1.03mmol/l, TG ≥ 1.7mmol/l or LDL-C ≥ 3.4mmol/l according to National Cholesterol Education Program (NCEP) guidelines16 or use of statin prior to stroke onset.
Cardiac disease was defined as history of current diagnosis of atrial fibrillation, cardiomyopathy, rheumatic heart disease, or valvular heart diseases, heart failure, or ischemic heart disease.
Obesity: Participants were classified individually using either the World Health Organization (WHO) guideline cutoffs of ≥0.90 (men) and ≥0.85 (women) for WHR or ≥30kg/m2 for BMI. 17
Individuals were classified as physically active if they were regularly involved in moderate exercise (walking, cycling, or gardening) or strenuous exercise (jogging, football and vigorous swimming) for four hours or more per week.14
Dietary history included regularity of intake of food items such as meat, fish, green leafy vegetables, addition of salt at table, nuts, sugar and other local staple food items. Regular intake was defined as intake on daily, weekly or at least once in a month versus none in a month.
Alcohol use was categorized into current users (users of any form of alcoholic drinks) and none/previous users while alcohol intake was categorized as low drinkers (1–2 drinks per day for female and 1–3 drinks per day for male) and high drinkers (>2 drinks per day for female and >3 drinks per day for male. 1 drink or 1 unit of alcohol = 8g of alcohol). 14
Smoking status was defined as current smoker (individuals who smoked any tobacco in the past 12 months) or none/previous smoker.
For psychosocial risk factors, we adapted measures of depression and psychosocial stress used in the INTERSTROKE study.14 Depression combined depressed mood and a checklist of other depression symptoms experienced in the 4 weeks preceding the stroke. Psychosocial stress comprised of stress at home/work (e.g., irritability, anxiety or sleeping difficulties) and life events, experienced in the 2 weeks preceding the stroke.
Family history of cardiovascular risk/diseases was defined based on self-reported history of any of hypertension, diabetes, dyslipidemia, stroke, cardiac disease or obesity in participants’ father, mother, sibling or second degree relative.
Clustering of vascular risk factors: Risk factor combinations (score 1 or 0) for 12 dominant risk factors namely hypertension, dyslipidemia, diabetes mellitus, obesity, cardiac disease, smoking, alcohol use, perceived stress, depression, low vegetable consumption, physical inactivity and meat consumption were assessed for each stroke case and stroke-free control.
Statistical Analysis
Bivariate associations between risk factors and ischemic stroke (including pathophysiologic subtypes) were assessed using McNemar and chi-square test for paired categorical outcomes. Furthermore, we determined the adjusted associations between the risk factors and ischemic stroke and stratified by its subtypes using conditional logistic regression with adjustment for potential confounders that were not used in the matching (except baseline age which was included as a residual confounder due to the non-exact age matching). Covariates were included in the adjusted models based on careful literature review, our clinical understanding of ischemic stroke risk, and empirical evidence (significant associations observed in bivariate analyses). The final adjusted models were assessed for collinearity using variance inflation factor (VIF) and goodness of fit using residual analysis. Odds ratio (OR) and 95% confidence intervals in the final models were estimated. The adjusted Population Attributable Risks (PARs) of ischemic stroke (and subtypes) with their respective 95%CI for each exposure variable included in the best-fitted adjusted models were estimated using the AF R-package with the variance estimated via the delta method. Composite PARs for the risk factors for ischemic stroke and its etiologic subtypes were calculated using the ATTRIBRISK R package with its 95% CI computed via the bootstrap method.18 All statistical tests of hypotheses were two-sided at 5% significance level. Statistical analyses and graphics were produced with SAS 9.4 and R statistical program (version 3.4.2)
RESULTS
Stroke cases presenting to SIREN Study centers:
Between August 2014 and June 2018, approximately 7,000 clinically suspected stroke cases reported to the 15 study sites in Ghana and Nigeria out of which 4,050 (58%) had cranial CT scans performed to assess eligibility for our study. In accordance with the SIREN protocol, we excluded 780 subjects because they had had stroke symptoms lasting more than 10 days before cranial CT scan was done making it difficult for phenotyping into ischemic or hemorrhagic types or had other diagnoses. Out of the 3,285 adjudicated cases found to be eligible, there were 2,431 (74.0%) ischemic stroke cases and 854 (26.0%) intracerebral hemorrhage cases. We have previously reported on the intracerebral hemorrhage cases19, the current analysis focuses on ischemic strokes. Two (both in Ghana) of the 15 sites had dedicated stroke units.
Comparison of stroke cases with controls:
The mean age of stroke-free controls was 60.1 ± 13.8 years compared with 62.1 ± 14.0 years, p=0.87 with similar proportions of males 52.8% vs 52.8%. The control group showed several differences in other key demographic and vascular risk factor profile from stroke cases (Supplementary Table SI).
Subtypes of Ischemic strokes:
Stroke cases resided in urban areas (61.0%), semi-urban areas (29.3%) and rural settings (9.7%). According to the ASCOD etiologic classification of ischemic stroke, 1,024 (42.1%) were small vessel occlusions, 427 (17.6%) were large-artery atherosclerosis, 258 (10.6%) were of cardiac sources, 3 (0.1%) had carotid dissections and 719 (29.6%) were other causes mostly undetermined large infarctions >2.0cm in diameter. Using the clinically-based Oxfordshire Community Stroke Project classification scheme, 36.5% of ischemic stroke cases had lacunar infarction (LACI), 30.2% had partial anterior circulation infarction (PACI), 12.9% had total anterior circulation infarction (TACI), 9.3% had posterior circulation infarction (POCI) and 11.1% had no data. Among stroke cases, 272 (11.2%) reported a previous history of stroke, being 14.3%, 12.0%, 10.1% and 8.5% among those with large artery atherosclerosis, small vessel occlusion, cardio-embolic and undetermined respectively, p=0.02.
Comparative analyses of demographic and cardio-metabolic risk factor profile according to etiologic ischemic stroke subtypes:
Small vessel occlusions and cardio-embolic stroke occurred more commonly among males at 57.2% and 55.4% respectively compared with females while large-artery atherosclerosis occurred at nearly equal proportions by sex. Overall, the mean ages of participants were non-significantly different across the etiologic stroke subtypes. There were also significant differences in location of residence, by ethnicity and educational attainment but non-significant difference by income level across the ischemic stroke subtypes (Table 1).
Table 1:
Comparison of demographic, clinical and vascular risk characteristics according to Ischemic stroke subtypes
| Characteristic | Large-artery atherosclerosis N=427 | Small vessel occlusion N=1024 | Cardio-embolism N=258 | Undetermined etiology N=722 | Total N=2431 | P-value |
|---|---|---|---|---|---|---|
| Gender, Male, n (%) | 215(50.4) | 586(57.2) | 143(55.4) | 340(47.1) | 1284(52.8) | <0.001 |
| Age, mean ± SD | 63.2 ±13.9 | 62.11 ±13.3 | 61.9 ± 15.7 | 61.7 ± 14.3 | 62.1 ± 14.0 | 0.326 |
| Age categories, n (%) | ||||||
| <30 | 4(0.9) | 16(1.6) | 11(4.3) | 21(2.9) | 52(2.1) | <0.001 |
| 30–49 | 63(14.8) | 133(13.0) | 47(18.2) | 112(15.5) | 355(14.6) | <0.001 |
| 50–69 | 204(47.8) | 552(53.9) | 103(39.9) | 351(48.6) | 1210(49.8) | <0.001 |
| >=70 | 154(36.1) | 317(31.0) | 97(37.6) | 235(32.6) | 803(33.0) | <0.001 |
| Age >65years, n (%) | 186(43.6) | 400(39.1) | 120(45.6) | 277(38.4) | 983(40.4) | 0.057 |
| Domicile | ||||||
| Rural, n (%) | 44(10.30) | 77(7.52) | 26(10.08) | 74(10.25) | 221(9.09) | 0.007 |
| Semi-urban, n (%) | 149(34.39) | 310(0.27) | 71(27.52) | 183(25.35) | 713(29.33) | |
| Urban, n (%) | 232(54.33) | 632(61.72) | 161(62.40) | 457(63.30) | 1482(60.96) | |
| Monthly Income >$100, n (%) | 186(43.56) | 400(39.06) | 120(46.51) | 277(38.37) | 983(40.44) | 0.057 |
| Education, (some) n (%) | 327(76.58) | 813(79.39) | 217(84.11) | 541(74.93) | 1898(78.7) | 0.021 |
| Ethnicity | ||||||
| Country, Ghana, n (%) | 104(24.4) | 330(32.2) | 152(58.9) | 149(20.6) | 735(30.2) | <0.001 |
| Akan | 82(19.2) | 224(21.9) | 106(41.1) | 112(15.5) | 524(21.6) | 0.08 |
| Gal/Adange | 6(1.4) | 47(4.6) | 24(9.3) | 15(2.1) | 92(3.8) | |
| Ewe | 6(1.4) | 41(4.0) | 13(5.0) | 10(1.4) | 70(1.9) | |
| others | 10(2.3) | 18(1.8) | 9(3.5) | 12(1.7) | 49(2.0) | |
| Country, Nigeria, n (%) | 318(74.5) | 688(67.2) | 106(41.1) | 568(78.7) | 1680(69.1) | <0.001 |
| Yoruba | 177(41.5) | 357(34.9) | 56(21.7) | 287(39.8) | 877(36.1) | 0.01 |
| Igbo | 14(3.3) | 45(4.4) | 4(1.6) | 39(5.4) | 102(4.2) | |
| Hausa | 80(18.7) | 159(15.5) | 27(10.5) | 172(23.8) | 438(18.0) | |
| Others | 47(11.0) | 127(12.4) | 19(7.4) | 70(9.7) | 263(10.8) | |
| Hypertension, n (%) | 398(93.2) | 962(94.0) | 245(95.0) | 674(93.4) | 2279(93.8) | 0.993 |
| Dyslipidemia, n (%) | 362(84.8) | 866(84.6) | 229(88.8) | 605(83.8) | 2062(84.8) | 0.003 |
| Diabetes, n (%) | 182(42.6) | 435(42.5) | 100(38.8) | 283(39.2) | 1000(41.1) | 0.420 |
| Cardiac Disease, n (%) | 40(9.4) | 92(9.0) | 96(37.2) | 108(15.0) | 336(13.8) | <0.001 |
| HDL-Cholesterol, mg/dl, mean ± SD | 42.6 ±18.0 | 46.2 ±16.8 | 48.0 ± 19.6 | 47.7 ± 19.3 | 46.3 ± 18.2 | <0.001 |
| HDL-Cholesterol ≤ 18.54 mg/dl, n (%) | 175(41.0) | 332(32.4) | 81(31.4) | 228(31.6) | 816(33.6) | <0.001 |
| LDL-Cholesterol, mg/dl, mean ± SD | 121.2 ± 53.6 | 120.2 ± 51.4 | 125.3 ± 51.8 | 120.6 ± 48.6 | 121.0 ± 51.0 | 0.595 |
| LDL-Cholesterol ≥ 61.2 mg/dl, n (%) | 129(30.2) | 320(32.3) | 103(39.9) | 235(32.6) | 787(32.4) | 0.198 |
| LDL/HDL ratio, mean ± SD | 3.4 ± 2.2 | 2.9 ± 1.6 | 3.0 ± 2.1 | 2.9 ± 1.7 | 3.0 ± 1.8 | <0.001 |
| LDL/HDL ratio >2.96, n (%) | 159(37.2) | 318(31.1) | 85(33.0) | 223(30.9) | 785(32.3) | 0.009 |
| LDL/HDL ratio by thirds: | ||||||
| ≤ 2.00 | 87(20.4) | 254(24.8) | 67(26.0) | 193(26.7) | 601(24.7) | 0.065 |
| 2.01 – 2.96 | 86(20.1) | 257(25.1) | 69(26.7) | 182(25.2) | 594(24.4) | |
| ≥ 2.97 | 159(37.2) | 318(31.1) | 221(85.7) | 598(82.8) | 1980(81.5) | |
| Total Cholesterol, mmol/l, mean ± SD | 186.8 ± 60.5 | 190.8 ± 57.1 | 190.4 ± 55.6 | 191.8 ± 58.0 | 190.3 ± 57.8 | 0.634 |
| Total Cholesterol ≥ 93.6 mg/dl, n(%) | 132(30.9) | 341(33.3) | 98(38.0) | 265(36.7) | 836(34.4) | 0.377 |
| Triglyceride, mg/dl, mean ± SD | 121.5 ± 70.8 | 124.6 ± 70.3 | 123.6 ± 87.5 | 146.6 ± 105.3 | 130.6 ±84.9 | <0.001 |
| Triglyceride ≥ 30.6 mg/dl, n (%) | 86(20.1) | 203(19.8) | 46(17.8) | 203(28.1) | 538(22.1) | <0.001 |
| Waist-to-hip Ratio, mean ± SD | 0.6 ± 0.5 | 0.6 ± 0.5 | 0.5 ± 0.5 | 0.6 ±0.5 | 0.6 ± 0.5 | 0.054 |
| Waist-to-hip Ratio raised, n (%) | 335(78.5) | 815(79.6) | 186(72.1) | 548(75.9) | 1884(77.5) | 0.203 |
| Waist-to-hip Ratio by thirds: | ||||||
| ≤ .90 | 101(23.7) | 231(22.6) | 71(27.5) | 157(21.8) | 560(23.0) | 0.015 |
| .91 – .96 | 161(37.7) | 357(34.9) | 83(32.2) | 210(29.1) | 811(33.4) | |
| ≥.97+ | 136(31.9) | 366(35.7) | 79(30.6) | 283(39.2) | 864(35.5) | |
| WHR**, Lowest vs highest thirds, n (%) | 136(31.9) | 366(35.7) | 79(30.6) | 283(39.2) | 864(35.5) | 0.054 |
| WHR**, 1st vs 2nd+3rd thirds, n (%) | 297(69.6) | 723(70.6) | 162(62.8) | 493(68.3) | 1675(68.9) | 0.230 |
| BMI*** (kg/m2), mean ± SD | 27.2± 5.1 | 26.6 ± 5.4 | 26.9± 5.6 | 26.6 ± 5.1 | 26.7 ± 5.3 | 0.264 |
| BMI*** >30kg/m2, n (%) | 75(17.6) | 192(18.8) | 52(20.2) | 111(15.4) | 430(17.7) | 0.347 |
| Physical inactivity, n (%) | 6(1.4) | 44(4.3) | 21(8.1) | 43(6.0) | 114(4.7) | <0.001 |
| Tobacco use in past 12 months, n (%) | 8(1.9) | 28(2.7) | 10(3.9) | 24(3.3) | 70(2.9) | 0.379 |
| Tobacco (any use), n (%) | 34(8.0) | 107(10.5) | 28(10.9) | 65(9.0) | 234(9.6) | 0.458 |
| Alcohol (current user), n (%) | 49(11.5) | 152(14.8) | 44(17.1) | 87(12.1) | 332(13.7) | 0.092 |
| Alcohol (any use), n (%) | 112(26.2) | 336(32.8) | 101(39.2) | 167(23.1) | 716(29.5) | <0.001 |
| Alcohol use categories: | ||||||
| Never Use | 304(71.2) | 672(65.6) | 155(60.1) | 537(74.4) | 1668(68.6) | <0.001 |
| Ever Low Use | 61(14.3) | 186(18.2) | 56(21.7) | 83(11.5) | 386(15.9) | |
| Ever High Use | 1(0.2) | 23(2.3) | 7(2.7) | 16(2.2) | 47(1.9) | |
| Stress, n (%) | 71(16.6) | 197(19.2) | 48(18.6) | 108(15.0) | 424(17.4) | 0.119 |
| Cancer, n (%) | 1(0.2) | 7(0.7) | 1(0.4) | 6(0.8) | 15(0.6) | 0.002 |
| Depression, n (%) | 19(4.5) | 93(9.1) | 27(10.5) | 39(5.4) | 177(7.3) | 0.001 |
| Family history of CVD, n (%) | 150(35.1) | 397(38.8) | 107(41.5) | (33.8) | 898(36.9) | 0.060 |
| Adding salt at table, n (%) | 16(3.8) | 73(7.1) | 18(7.0) | 38(5.3) | 145(6.0) | 0.093 |
| Green vegetable consumption, n (%) | 93(21.8) | 225(22.0) | 45(17.4) | 202(28.0) | 265(10.9) | 0.013 |
| Whole grains consumption, n (%) | 301(70.5) | 768(75.0) | 194(75.2) | 574(79.5) | 1857(76.4) | 0.875 |
| Legumes consumption, n (%) | 261(61.1) | 632(61.7) | 150(58.1) | 428(59.3) | 1471(60.5) | 0.119 |
| Fruit consumption, n (%) | 294(68.9) | 778(75.9) | 201(77.9) | 575(79.6) | 1848(76.0) | 0.042 |
| Sugar consumption or otherwise, n (%) | 69(16.6) | 282(27.5) | 66(25.6) | 201(27.8) | 618(25.4) | 0.002 |
| Meat consumption or otherwise, n (%) | 261(61.1) | 650(63.5) | 157(60.9) | 519(71.9) | 1587(65.3) | 0.012 |
| Fish consumption or otherwise, % | 319(74.7) | 838(81.8) | 223(86.4) | 621(86.0) | 2001(82.3) | 0.060 |
| Investigations | ||||||
| Electrocardiography | 211 (49.4) | 612 (59.8) | 187 (72.5) | 457 (63.3) | 1467 (60.3) | <0.0001 |
| Echocardiography | 163 (38.2) | 438 (42.8) | 148 (57.4) | 273 (37.8) | 1022 (42.0) | <0.0001 |
| Carotid Doppler | 186 (43.6) | 387 (37.8) | 114 (44.2) | 243 (33.7) | 930 (38.3) | 0.01 |
| Cranial CT scan | 427 (100.0) | 1024 (100.0) | 258 (100.0) | 722 (100.0) | 2431 (100.0) | |
| Previous stroke, n (%) | 61(14.3) | 123(12.0) | 26(10.1) | 62(8.6) | 272(11.2) | 0.018 |
P-value for analysis of variance comparing mean across Ischemic stroke types
Approximately 94% of cases had hypertension, 78% had elevated waist-to-hip ratio, 41% had diabetes mellitus, 17% reported having psycho-social stress prior to stroke onset and 10% reported tobacco use but there were no significant proportional differences between the four etiologic subtypes of ischemic stroke as shown in Table 1. The prevalence of dyslipidemia among cases was 84.8% with no significant differences among the subtypes of stroke although lipid sub-fractions showed some differences. The LDL-cholesterol-to-HDL-cholesterol ratio, an indicator of pro-atherogenic risk, showed a graded and significant trend being highest among those with large-artery atherosclerosis, followed by cardio-embolic and finally small vessel disease with the ratios being 3.4 ± 2.2, 3.0 ± 2.1 and 2.9 ± 1.6, p<0.001 respectively. Furthermore, the overall frequency of behavioral and clinical-psychological characteristics reported among ischemic stroke cases were: regular consumption green vegetables (23.2%), fruits (76.0%), meat (65.3%), sugar (25.4%), family history of Cardiovascular Disease (36.9%), alcohol use (29.5%), cardiac disease (13.8%), cancer (0.6%), physical inactivity (4.7%) and depression (7.3%) with significant statistical differences for trend within the 4 etiologic sub-groups.
Risk factors for ischemic stroke and its subtypes:
The 8 dominant risk factors for ischemic strokes with adjusted Odds Ratios were systemic hypertension 10.34(6.91,15.45), dyslipidemia 5.16(3.78,7.03), diabetes mellitus 3.44(2.60,4.56), low green vegetable consumption 1.89(1.45,2.46), red meat consumption 1.89(1.45,2.46), cardiac disease 1.88(1.22,2.90), monthly income of $100 1.72(1.24,2.39) and psychosocial stress 1.62(1.18,2.21). Hypertension, dyslipidemia and diabetes mellitus were three (3) confluent independent factors shared by small-vessel disease, large-artery atherosclerosis and cardio-embolic ischemic stroke subtypes but demonstrated differential effect sizes relative to stroke-free controls (Table 2). For instance, the effect size of hypertension is highest among SVOs at 13.98 (7.18–27.22) followed by 8.24(2.08–32.61) among cardio-embolic strokes and 6.87 (2.75–17.19) among those with large artery atherosclerosis. Stress, regular meat consumption and low vegetable consumption are independent factors shared by SVO and LAA but not cardioembolic strokes. Overall, eight factors contributed a composite PAR of 90.56% (95% CI: 86.44–94.67%) for ischemic stroke occurrence. The PARs for stroke subtypes are shown in Table 2. Figure 1 shows Forest plots of adjusted odds ratios and population attributable risks for ischemic stroke and its subtypes and Figure 2 for dose-response associations between ischemic stroke and income, salt intake, meat intake and vegetable intake categories.
Table 2.
Risk factors of Ischemic stroke and its pathophysiologic subtypes
| Risk factor | All Ischemic strokes | Small-vessel ischemic stroke | Large-vessel atherosclerotic stroke | Cardio-embolic stroke | ||||
|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | PAR (95% CI) | Adjusted OR (95% CI) | PAR (95% CI) | Adjusted OR (95% CI) | PAR (95% CI) | Adjusted OR (95% CI) | PAR (95% CI) | |
| Age | 0.60(0.35,1.01) | −25.6(−61.8,10.7) | 0.38(0.17–0.85) | −61.6(−14.5 – 22.7) | 1.78(0.43–7.29) | 15.6(−13.3 –44.4) | 1.00(0.16–6.25) | −21.0(−108.2–107.8) |
| Educational attainment | 1.30(0.92,1.82) | 18.9(−2.6,40.5) | 1.90(1.09–3.33) | 39.7(14.3–65.1) | 1.56(0.70–3.47) | 29.9(−12.8–72.7) | 1.44(0.36–5.73) | 26.1(−92.8–14.0) |
| Hypertension | 10.34(6.91,15.45) | 85.3(81.2,89.5) | 13.98(7.18–27.22) | 88.8(83.8–93.9) | 6.87(2.75–17.19) | 79.6(65.9–93.2) | 8.24(2.08–32.61) | 80.9(65.5–96.4) |
| Dyslipidemia | 5.16(3.78,7.03) | 71.0(65.5,76.6) | 8.49(4.89–14.73) | 79.3 (72.7–85.8) | 2.66(1.26–5.62) | 53.1(27.4–78.7) | 4.71(1.61–13.82) | 68.4(47.1–89.7) |
| Diabetes mellitus | 3.44(2.60,4.56) | 29.5(25.6,33.4) | 3.71(2.43–5.66) | 30.7(25.0–36.3) | 4.68(2.09–10.44) | 32.5(24.6–40.5) | 2.57(1.00–6.68) | 25.2(7.2–43.2) |
| Obesity | 0.77(0.59,1.02) | −6.6(−14.6,1.3) | 0.72(0.47–1.09) | −8.7(−20.2–3.1) | 0.84(0.37–1.94) | −4.7(−32.3–22.9) | 0.44(0.16–1.16) | −35.2(−97.7–27.3) |
| Cigarette smoking | 1.79(0.75,4.26) | 1.3(−0.2,2.8) | 1.14(0.33–3.94) | 0.3(−1.9–2.5) | 1.53(0.13–18.44) | 1.0(−3.3–5.3) | 17.6(0.15–20.71) | 1.7(0.4–2.9) |
| Stress | 1.62(1.18,2.21) | 9.0(4.3,13.6) | 1.79(1.09–2.94) | 10.6(3.5–17.7) | 1.75(0.77–3.96) | 10.3(−0.9–21.5) | 0.59(0.19–1.84) | −21.1(−68.4–26.1) |
| Cardiac disease | 1.88(1.22,2.90) | 5.7(2.5,8.8) | 0.77(0.38–1.56) | −2.1(−9.8–5.7) | 1.67(0.56–5.05) | 4.2(−3.8–12.1) | 16.46(3.46–78.18) | 34.6(28.6–40.6) |
| Alcohol | 1.12(0.77,1.61) | 1.7(−4.0,7.4) | 0.83(0.53–1.31) | −7.2(−27.0–12.6) | 0.78(0.35–1.73) | −9.0(−43.2–25.2) | 1.47(0.53–4.05) | 12.4(−16.7–41.5) |
| Meat consumption | 1.89(1.45,2.46) | 33.5(23.2,43.9) | 1.82(1.19–2.79) | 31.3(15.6–47.0) | 2.26(1.13–4.51) | 41.3(20.5–62.1) | 1.75(0.67–4.59) | 27.2(−6.0–60.3) |
| Low Vegetable consumption | 2.11(1.59,2.80) | 16.4(11.9,20.8) | 2.44(1.53–3.91) | 16.8(11.1–22.5) | 1.93(0.95–3.90) | 14.1(2.7–25.5) | 2.05(0.74–5.71) | 14.0(0.2–27.7) |
| Physical inactivity | 0.65(0.32,1.29) | −1.5(−4.9,1.8) | 0.66(0.22–2.00) | −1.5(−6.4–3.5) | 0.29(0.04–2.29) | 2.8(−11.4–5.9) | 1.07(0.18 –6.53) | 0.6(−7.5–8.2) |
| Salt intake | 1.54(0.94,2.53) | 2.3(0.3,4.4) | 1.78(0.90–3.55) | 3.9(0.3–7.5) | 0.76(0.09–6.66) | −0.9(−9.3–7.4) | 4.69(0.78–28.34) | 6.2(2.3–10.2) |
| Income | 1.72(1.24,2.39) | 10.8(5.8,15.9) | -- | -- | -- | -- | -- | -- |
| Composite PAR | 90.6(86.4,94.7) | 88.1(81.0–95.2) | 89.2(78.6–99.9) | 85.3(63.9–106.6) | ||||
Note: The analyses were adjusted for all the factors included in the table
Figure 1.
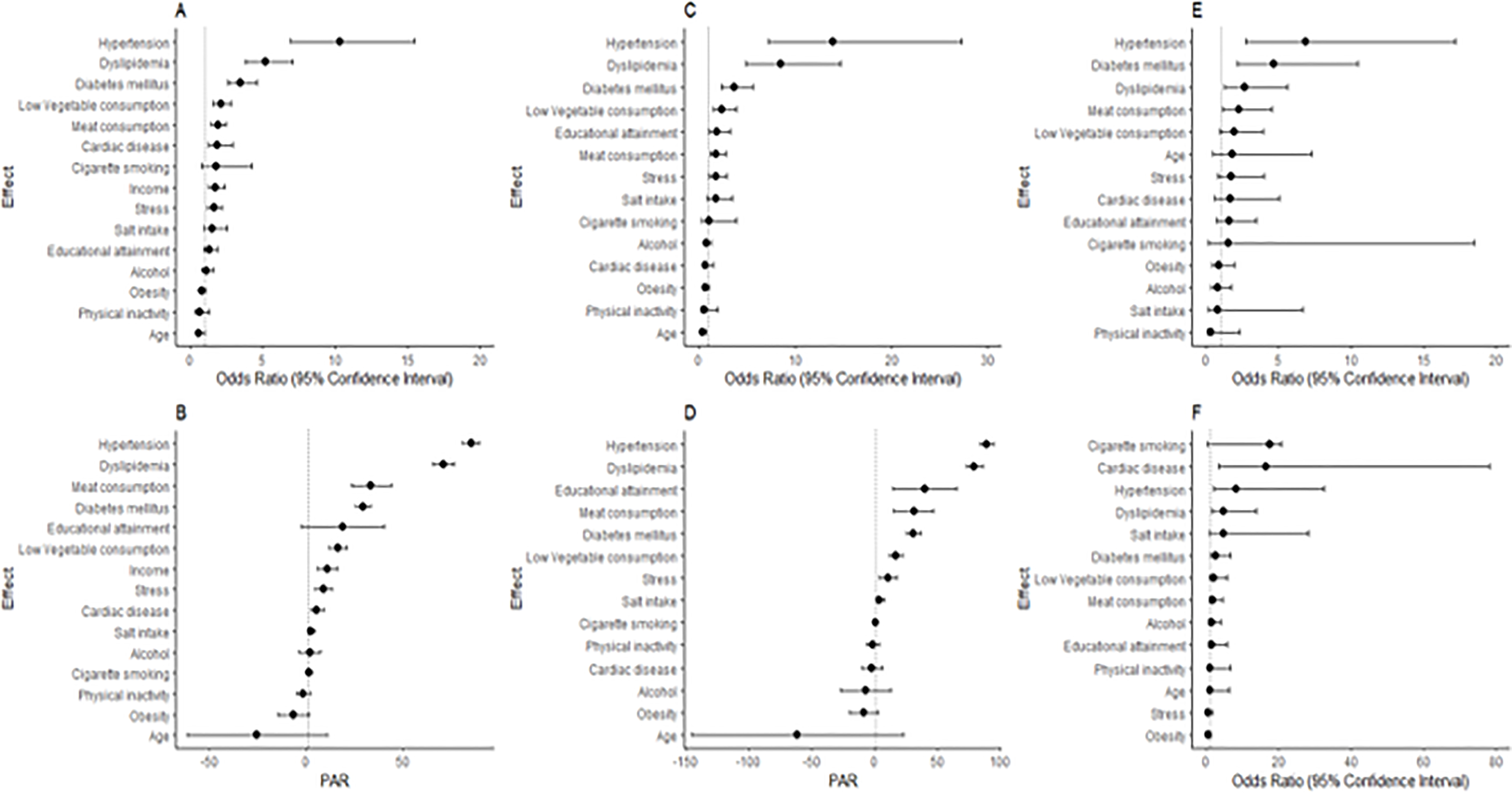
Forest plots showing risk factors of Ischemic stroke and subtypes among West Africans. (A) Odds ratios and 95% CI of risk factors, (B) PARs and their 95% CI for all ischemic stroke; (C) and (D) show odds ratios and PARs respectively for small-vessel occlusion; (E) shows odds ratios for large-vessel atherosclerotic ischemic strokes; (F) show odds ratios for Cardio-embolic stroke.
Figure 2:
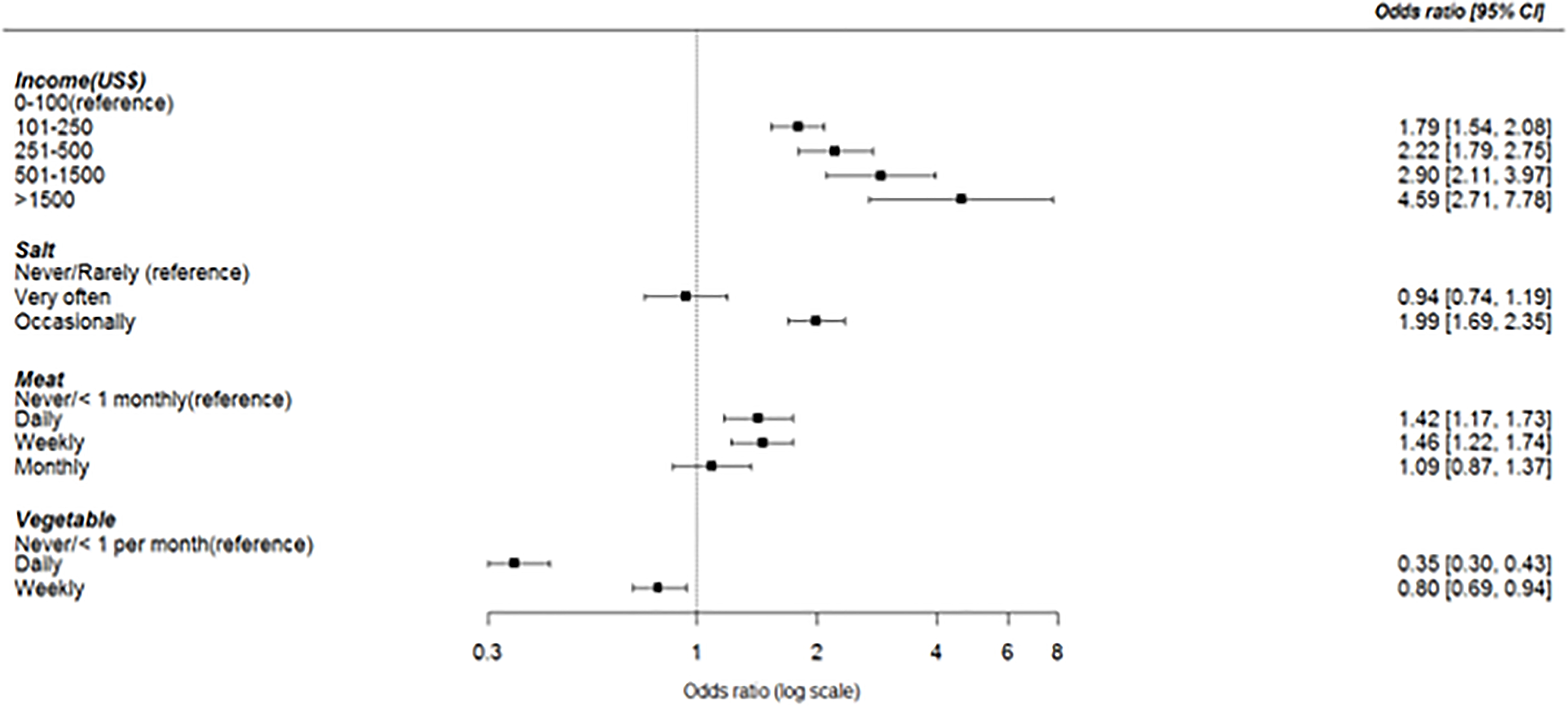
Dose-response associations between income level, salt intake, meat intake and vegetable consumption with occurrence of ischemic stroke. Adjusted odds ratio with 95% Confidence intervals are presented.
Clustering of vascular risk factors among individuals with ischemic stroke:
At the individual participant level, each ischemic stroke case had a mean (± SD) cluster of 5.3 ± 1.5 risk factors out of 12 potential factors shown in Table SII and Figure 3.
Figure 3.
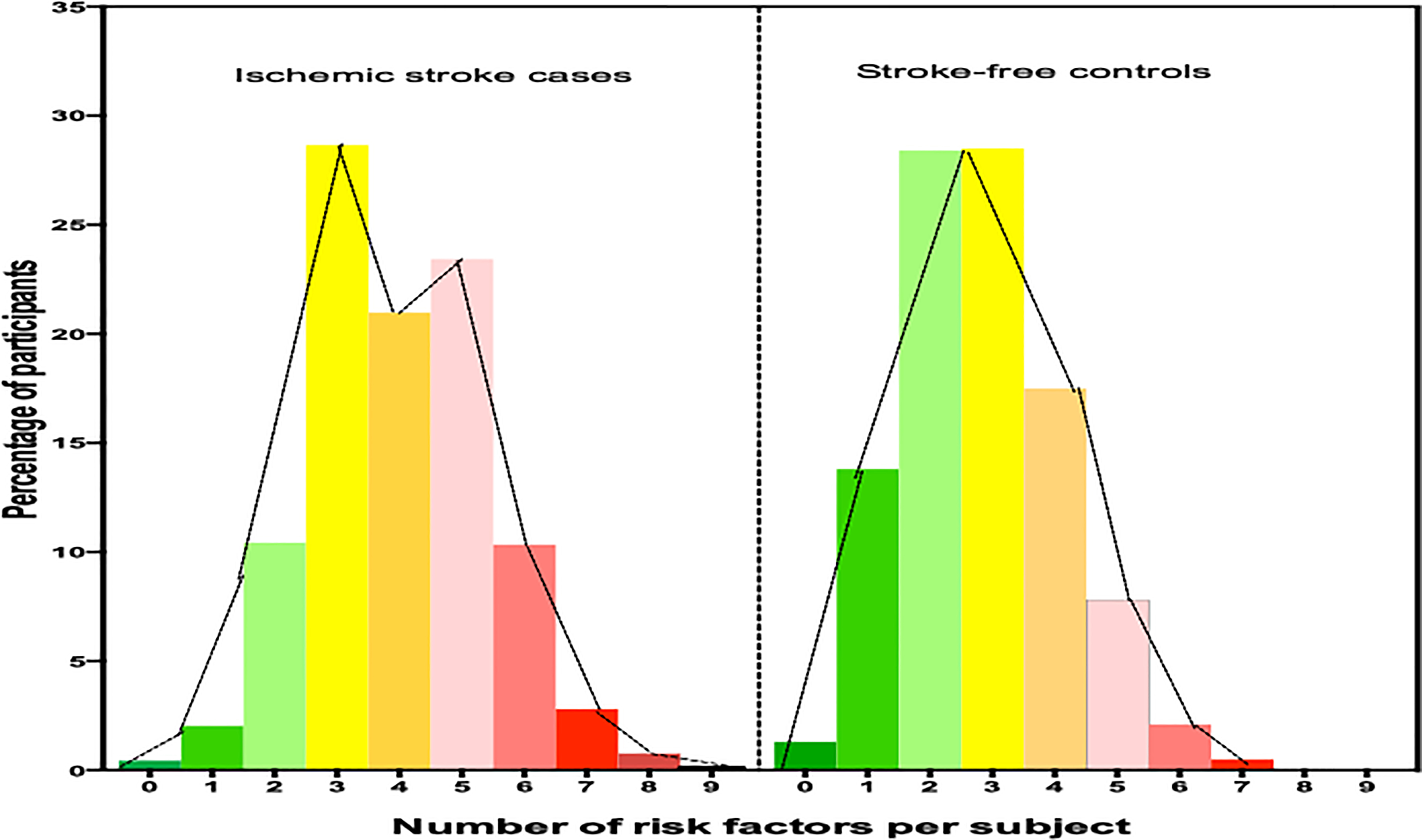
Comparison of number of vascular risk factors per ischemic stroke cases versus stroke-free controls.
DISCUSSION
We present here an account of the proximate etiologic mechanisms and associated risk factors of ischemic stroke among Ghanaians and Nigerians resident in West Africa. Upon deploying the ASCOD scheme of classification, the proportions with SVO was 42%, 17% were large-artery atherosclerosis, 11% were of cardiac sources, and 30% were undetermined or other causes. The higher preponderance of SVO and lower frequency of cardio-embolic stroke in our study is in accord with data from other developing countries such as Pakistan20 and China21 but contrasts with data from high-income countries such as the United Kingdom 22 (see Supplementary figure I). Data from this study and those of others support a growing notion that the relative frequency distribution of ischemic stroke subtypes follows geographic or ecological trends with potential racial or genetic underpinnings. In support of the latter, SVOs was twice more common among Africans in UK22 and US 23 ischemic stroke cohorts than Caucasians who had a greater propensity towards cardio-embolic and large-artery atherosclerotic diseases.24 The genetic architecture of the different ischemic stroke subtypes is now the pursuit of several research groups and consortia.5−7
Nearly a third of all ischemic strokes did not have a definitive etiology, a proportion comparable with several other studies.13, 20–23 We were able to conduct a rigorous evaluation of cardio-metabolic and behavioral modifiable vascular risk factors in relation to ischemic stroke occurrence and its pathophysiologic subtypes. Eight out of an ensemble of 13 modifiable vascular risk factors assessed were associated with ischemic stroke among West Africans. These are hypertension, dyslipidemia, regular meat consumption, diabetes mellitus, low green vegetable consumption, psychosocial stress, higher monthly income and cardiac disease, a list largely in consonance with our previous report25 and reports from the Global Burden of Disease26 and INTERSTROKE studies.14
However, the analyses in the present study extend these findings further by reporting on the effect size of risk factors on the occurrence of ischemic stroke sub-types. For instance, although hypertension was found to be the eminent precursory factor associated with ischemic stroke, its effect reverberated through all ischemic stroke subtypes but at different effect sizes by stroke subtype. The effect sizes of hypertension and dyslipidemia was highest among those with SVO, suggesting that elevations in blood pressure and perturbations in serum lipid transport are crucial in orchestrating arteriolosclerosis of deep perforating arteries and microatheroma propagation as principal mechanisms in SVO. Dyslipidemia exhibited an approximately 2-fold step-wise decline across the subtypes with adjusted odds ratio of 8.49 (4.89 – 14.73) for SVOs, 4.71 (1.61 −13.82) for cardio-embolic stroke, and 2.66 (1.26 – 5.62) for large-artery atherosclerotic disease. However, lipid sub-fraction analyses revealed that while mean serum LDL-cholesterol concentration remained even across stroke subtypes, HDL-cholesterol concentration being lowest in large artery atherosclerosis would favor atherogenesis in this stroke subtype. In addition, large artery atherosclerotic strokes had the highest effect size for diabetes mellitus which engenders a pathophysiologic milieu for accelerated atherosclerosis. Thus hypertension, dyslipidemia and diabetes mellitus are three dominant factors for ischemic stroke occurrence in Africa and are intermediary factors for different ischemic stroke subtypes depending on their effect sizes. Notably, the population attributable risk of hypertension and dyslipidemia exceeded even that of cardiac disease for cardio-embolic strokes. These observations are intriguing because the mean age of study participants across the stroke subtypes were non-significantly different.
Behavioral and lifestyle factors such as regular meat consumption and low green leafy vegetable intake though independently associated with SVO and LAA occurrence may mediate their effects via the more established traditional risk factors. Similarly, acute and chronic psychosocial stress provokes physiological alterations such as activation of sympathetic nervous system, hypothalamic-pituitary-adrenal axis and vagal nerve withdrawal leading to hemo-concentration, endothelial dysfunction, pro-inflammatory and hypercoagulable states.27 Such patho-biologic disturbances induced by stress has been found to play major role in the occurrence of acute coronary syndrome in patients with stable coronary atherosclerotic heart disease.28 In our study, significant associations were observed between psychosocial stress and SVO and LAA but not with cardio-embolic strokes. Although having a cancer was not significantly associated with ischemic stroke occurrence in our models, we noted that the highest frequency of cancers was reported among ischemic stroke of undetermined etiology. Cancer-associated stroke is pathophysiologically linked with coagulopathy, radiation therapy, chemotherapy infections, direct tumor effects and invasive procedures.29
Overall, each stroke case had a mean of five (5) modifiable vascular risk factors compared with a mean of three (3) among stroke free controls. This suggests a greater clustering of vascular risk among stroke cases to be addressed by secondary prevention strategies among those who survive a stroke. Nearly, 11% of all stroke cases in our cohort reported a previous history of stroke suggestive of recurrent strokes. An emerging theme from our data is that, for primodial or primary prevention at a population level, cardiovascular health promotion and education in Africa will need to emphasize a broad-based screening, detection and control of several behavioral and traditional risk factors most notably hypertension, dyslipidemia and dysglycemia.
Limitations and strengths:
We sought in this case-control study to assess associations and quantify effect sizes and population attributable risks of risk factors but not to establish causality of these risk factors with ischemic stroke. Our inability to fully investigate all stroke cases, in particular with arterial and echocardiographic investigations and prolonged arrhythmia monitoring, potentially led to an underestimation of atherosclerotic and cardiac stroke subtypes. Furthermore, subjects with alterations in consciousness or dysphasia had their lifestyle and behavioral history assessed from valid proxies (by spouses or first-degree relatives). Our previous reports and analysis suggest that the associations observed were in the same direction as those assessed directly.25 Our stroke cases were not consecutively enrolled but included only those meeting study eligibility criteria. Systematic differences, for instance, according to demographic and vascular risk factors were not assessed between those who were eligible and those ineligible. Furthermore, of the 11.2% with a previous clinical history of stroke based on self-report, none had neuro-radiological evidence of the first stroke to help corroborate previous stroke diagnosis due often to the pervasive lack of medical records or non-presentation to hospital after onset of stroke symptoms in our settings.
An important strength of our study is the utilization of active community engagement activities before, during and after recruitment of stroke cases and controls to mitigate presentation bias to enhance the generalizability of our findings. However, we do concede that mild or fatal ischemic stroke cases resident in the communities and rural settings may have been missed.
Implications of our findings and future direction:
Given the enormous and rising burden of stroke and post-stroke mortality and morbidity in Africa, aggressive public health interventions aimed at improving the control of hypertension30 and other vascular risk factors at the population level are urgently needed to stem the rising tide of CVDs.
Conclusion:
Small vessel occlusion is the dominant ischemic stroke subtype among West Africans. Traditional vascular risk factors demonstrate important differential effect sizes with pathophysiologic, clinical and preventative implications on the occurrence of ischemic stroke among indigenous West African populations. Strategic and sustained efforts targeting multiple cardio-metabolic factors at both individual and population levels may yield the greatest dividends for primary and secondary prevention of stroke among indigenous Africans.
Supplementary Material
Source of Funding
The SIREN (Stroke Investigative Research and Education Networks) and SIBS Genomics studies were funded by the National Institutes of Health grant U54 HG007479 and R01NS107900 under the H3Africa initiative.
Non standard acronyms and abbreviations
- CVD
Cardiovascular disease
- CT scan
Computerized Tomography scan
- LAA
Large artery atherosclerotic disease
- SVO
Small Vessel Occlusion
- SIREN
Stroke Investigative Research and Educational Networks
Footnotes
Disclosures: None
REFERENCES
- 1.Mohr JP, Sacco RL. Classification of ischemic stroke. In: Barnett HJM, Stein BM, Mohr JP, Yatsu FM, eds. Stroke: Pathophysiology, Diagnosis, and Management. New York, NY: Churchill Livingstone, Inc; 1992:271–283. [Google Scholar]
- 2.Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 3.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yusuf HR, Giles WH, Croft JB, Anda RF, Casper ML. Impact of multiple risk factor profiles on determining cardiovascular disease risk. Prev Med 1998;27:1–9. [DOI] [PubMed] [Google Scholar]
- 5.Holliday EG, Traylor M, Malik R, Bevan S, Falcone G, Hopewell JC, Cheng YC, Cotlarciuc I, Bis JC, Boerwinkle E, et al. Genetic overlap between diagnostic subtypes of ischemic stroke. Stroke 2015; 46(3):615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik R, Traylor M, Pulit SL, Bevan S, Hopewell JC, Holliday EG, Zhao W, Abrantes P, Amouyel P, Attia JR, et al. Low-frequency and common genetic variation in ischemic stroke: The metastroke collaboration. Neurology. 2016;86:1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neurology Working Group of the Cohorts for H; Aging Research in genomic epidemiology C; Stroke Genetics N; International Stroke Genetics C. Identification of additional risk loci for stroke and small vessel disease: A meta-analysis of genomic-wide association studies. Lancet Neurology. 2016;15:695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owolabi MO, Arulogun O, Melikam S, Adeoye AM, Akarolo-Anthony S, Akinyemi R, Arnett D, Tiwari H, Gebregziabher M, Jenkins C, et al. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc J Afr. 2015;26(2 Suppl 1):S27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarfo FS, Awuah DO, Nkyi C, Akassi J, Opare-Sem OK, Ovbiagele B. Recent patterns and predictors of neurological mortality among hospitalized patients in Central Ghana. J Neurol Sci. 2016;363:217–24. [DOI] [PubMed] [Google Scholar]
- 10.Akpalu A, Sarfo FS, Ovbiagele B, Akinyemi R, Gebregziabher M, Obiako R, Owolabi L, Sagoe K, Jenkins C, Arulogun O, et al. Phenotyping stroke in sub-Saharan Africa: Stroke Investigative Research and Education Network (SIREN) Phenomics protocol. Neuroepidemiology. 2015;45(2):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarfo F, Gebregziabher M, Ovbiagele B, Akinyemi R, Owolabi L, Obiako R, Akpa O, Armstrong K, Akpalu A, Adamu S, et al. Multilingual validation of the Questionnaire for verifying stroke-free status in West Africa. Stroke. 2016;47(1):167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. New approach to stroke subtyping: the A-S-C-O (phenotypic) classification of stroke. Cerebrovasc Dis 2009; 27:502–08. [DOI] [PubMed] [Google Scholar]
- 13.Fischer U, Conney MT, Bull LM, Silver LE, Chalmers J, Anderson CS, Mehta Z, Rothwell PM. Acute post-stroke blood pressure relative to premorbid levels in intracerebral hemorrhage versus major ischemic stroke: a population-based study. Lancet Neurol 2014; 13:374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnel MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016; 388 (10046): 761–75. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of high blood cholesterol in adults (Adult treatment panel). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high cholesterol in adults (Adult Treatment Panel III). Circulation 2002;106:3143–21. [PubMed] [Google Scholar]
- 17.World Health Organization. Waist circumference and waist-hip ratio. Report of a WHO expert consultation. 2008. http://www.who.int/nutrition/publications/obesity/WHO_report_waistcircumference_and_waisthip_ratio/en/. Assessed May 27, 2017.
- 18.Llorca J, Delgado- Rodriguez M. A comparison of several procedures to estimate the confidence interval for attributable risk in case-control studies. Statistics in Medicine 2000; 19:1089–1099. [DOI] [PubMed] [Google Scholar]
- 19.Sarfo FS, Ovbiagele B, Gebregziabher M, Akpa O, Akpalu A, Wahab K, Ogbole G, Akinyemi R, Obiako R, Komolafe M, et al. Unraveling the risk factors for spontaneous intracerebral hemorrhage among West Africans. Neurology. 2020. March 10;94(10):e998–e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syed NA, Khealani BA, Ali S, Hasan A, Akhtar N, Brohi H, Mozaffar T, Ahemed N, Hameed A, Baig SM, et al. Ischemic stroke subtypes in Pakistan: the Aga Khan University Stroke Data Bank. J Pak Med Assoc 2003;53:584–588. [PubMed] [Google Scholar]
- 21.Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese versus white populations: a systematic review. Neurology 2013;81:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart JA, Dundas R, Howard RS, Rudd AG, Wolfe CD. Ethnic differences in incidence of stroke: prospective study with stroke register. BMJ 1999;318:967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005;111:1327–1331. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamurthi RV, Barker-Collo S, Parag V, Parmar P, Witt E, Jones A, Mahon S, Anderson CS, Barber PA, Feigin VL. Stroke incidence by major pathological type and ischemic subtypes in the Auckland Regional Community Stroke Studies. Stroke. 2018;49:3–10. [DOI] [PubMed] [Google Scholar]
- 25.Owolabi MO, Sarfo F, Akinyemi R, Gebregziabher M, Akpa O, Akpalu A, Wahab K, Obiako R, Owolabi L, Ovbiagele B, et al. Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): a case-control study. Lancet Global Health. 2018; 6(4):e436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, Mensah GA, Norrving B, Shiue I, Ng M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016; 15(9):913–924. [DOI] [PubMed] [Google Scholar]
- 27.Austin AW, Wissmann T, von Kanel R. Stress and hemostasis: an update. Semin Thromb Hemost 2013;39:902–12. [DOI] [PubMed] [Google Scholar]
- 28.Wittstein IS, Thiemann DR, Lima JAC, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352:539–48. [DOI] [PubMed] [Google Scholar]
- 29.Dardiotis E, Aloizou AM, Markoula S, Siokas V, Tsarouhas K, Tzanakakis G, Libra M, Kyritsis AP, Brotis AG, Aschner M, et al. Cancer-associated stroke: Pathophysiology, detection and management (Review). Int J Oncol. 2019. March; 54(3): 779–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarfo FS, Treiber F, Gebregziabher M, Adamu S, Nichols M, Singh A, Obese V, Sarfo-Kantanka O, Sakyi A, Adu-Darko N, et al. Phone-based intervention for blood pressure control among Ghanaian stroke survivors: a pilot randomized controlled trial. Int J Stroke. 2019;14(6):630–638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


