Abstract
Objective:
We investigated the effect of berberine, a natural plant product that can activate AMP-activated protein kinase (AMPK), on OA development and associated pain in mice.
Design:
Human primary knee chondrocytes were utilized to investigate how AMPK is activated by berberine. Both global knockout (KO) of AMPKα1 and congenic wild type (WT) mice were subjected to the post-traumatic OA through destabilization of medial meniscus (DMM) surgery. Two weeks after surgery, the mice were randomly divided into two groups with one group receiving berberine chloride daily via drinking water and were sacrificed at 6 and 12 weeks after surgery. OA severity was assessed by histological and histomorphometric analyses of cartilage degradation, synovitis, and osteophyte formation. OA-associated pain behavior was also determined. Immunohistochemistry (IHC) analyses were carried out to examine changes in AMPK signaling.
Results:
Berberine induced phosphorylation of AMPKα (Thr172) via liver kinase B1 (LKB1), the major upstream kinase of AMPK, in chondrocytes in vitro. Both WT and AMPKα1KO developed OA and associated pain post DMM surgery. However, treatment with berberine significantly reduced severity of OA and associated pain in WT but not AMPKα1KO mice. IHC analysis of WT DMM knee cartilage further revealed that berberine inhibited concomitant loss of expression and phosphorylation of AMPKα and expression of SIRT1 and SIRT3, suggesting an important role of activation of AMPK signaling in mediating beneficial effect of berberine.
Conclusions:
Berberine acts through AMPK to reduce joint structural damage and pain associated with post-traumatic OA in mice in vivo.
Keywords: Berberine, AMPK, osteoarthritis, cartilage, pain
Introduction
Osteoarthritis (OA) is the most common form of arthritis that causes pain and disability. Prior joint injury is one of the major risk factors for OA development. OA is a disease of whole synovial joint affecting all joints tissues (1,2). Progressive degeneration of cartilage is the central feature of OA that leads to permanent functional joint failure (1,2). Recent studies indicate that AMP-activated protein kinase (AMPK) plays a critical role in cartilage homeostasis, and dysregulation of AMPK in chondrocytes contributes to OA development (3).
AMPK, an evolutionary conserved serine/threonine protein kinase, functions as a master regulator of cellular energy balance and metabolism (4,5). AMPK is a heterotrimeric complex consisting of a catalytic α-subunit and two regulatory β- and γ-subunits (4,5). The catalytic α-subunit has 2 isoforms (α1 and α2), both of which are expressed in articular chondrocytes. However, α1 is the predominant one expressed in chondrocytes (6). Phosphorylation of a conserved threonine residue within the activation loop of the kinase domain (Thr172) of α subunit is required for the kinase activity of AMPK (4,5). AMPK is activated in response to an increase in the cellular AMP to ATP ratio by metabolic stress either increasing ATP consumption or decreasing ATP production (4,5). AMPK activity is positively regulated by upstream kinases. Our previous studies demonstrated that the liver kinase B1 (LKB1) is the primary upstream kinase that phosphorylates AMPKα Thr172 in articular chondrocytes (4,5). Notably, phosphorylation of both LKB1 and AMPKα are concurrently reduced in mouse knee cartilage in situ associated with OA and aging, in primary human knee OA chondrocytes, and in chondrocytes challenged with either inflammatory cytokines or mechanical injury in vitro (6,7). AMPK activity is also negatively regulated through dephosphorylation by protein phosphatases such as protein phosphatase 2A (PP2A) and PP2Cα (4,5). We have observed that phosphorylation of AMPKα is increased in PP2Cα knockdown chondrocytes in vitro.
AMPK can be activated by several pharmacological activators (e.g., A-769662) and a few drugs already used in the clinic (e.g., metformin), as well as a variety of natural plant products (e.g., berberine) (8). Both A-769662, a specific AMPK pharmacological activator, and metformin, the first line of medication for the treatment of type 2 diabetes, can inhibit catabolic responses induced by biomechanical injury or IL-1β or TNFα in human chondrocytes in vitro (6,9). In addition, pharmacologic activation of AMPK in chondrocytes derived from human knee OA can reverse reduced mitochondrial biogenesis capacity through induction of the NAD+-dependent protein deacetylase SIRT1 and the master regulator of mitochondrial biogenesis PPARγ co-activator 1α (PGC-1α) (10). Moreover, activation of AMPK can preserve mitochondrial DNA integrity and maintain mitochondrial function through NAD+-dependent protein deacetylase SIRT3 and the base excision DNA repair enzyme 8-oxoguanine glycosylase (OGG1) (11) and prevent excessive oxidative stress by upregulation of expression of antioxidative enzymes (12). Furthermore, in vivo studies revealed that chondrocyte-specific AMPKα1/α2 double knockout (KO) mice have accelerated OA development (13), and metformin can suppress the injury-induced OA development and progression through activation of AMPK signaling in mice (9).
Berberine is an isoquinoline alkaloid with low toxicity, which is extracted from plants such as European barberry, goldenseal, goldthread, and tree turmeric (14). It has been used as herbal remedies particularly in traditional Chinese and Ayurvedic medicine for thousands of years (14). Berberine has anti-microbial, anti-inflammatory and antioxidant properties, and has potential therapeutic effects on cardiovascular and metabolic diseases (14). The beneficial effect of berberine is mediated through multiple signaling pathways, and activation of AMPK is thought to be one of the main actions of berberine (14). Previous studies showed that berberine can suppress IL-1β-induced inflammatory responses in rat chondrocytes in vitro and inhibit cartilage degradation in post-traumatic OA modes in rats (15–18). We recently demonstrated that berberine also significantly reduced age-related spontaneous cartilage degradation in mice (12). Although berberine exhibits chondroprotective effect, the underlying mechanism is not fully understood. In addition, whether berberine has ability to alleviate OA-associated pain remains unclear. Thus, in this study, we tested the hypothesis that berberine limits OA development and relieve pain through activation of AMPK. We determined how AMPK is activated by berberine in articular chondrocytes in vitro and analyzed and compared the effect of berberine on OA severity and OA pain behavior in AMPKα1 KO and wild type (WT) mice in vivo using a post-traumatic OA model.
Materials and methods
Reagents
All chemical reagents including berberine chloride (the orally bioavailable, hydrochloride form of berberine) were obtained from Sigma-Aldrich (St. Louis, MO), unless otherwise indicated. Recombinant human IL-1/β and human MMP-3 and MMP-13 Quantikine enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN). The reagents for immunohistochemistry (IHC) staining were purchased from Vector Laboratories (Burlingame, CA). Antibodies for Western blotting include phospho-AMPKα (Thr172), total AMPKα, phosphor-LKB1 (Ser428), total LKB1, PP2A A subunit, PP2Cα subunit and SIRT3 from Cell Signaling Technology, Inc (Danvers, MA). and SIRT1 and PGC-1α from Abcam (Waltham, MA). Antibodies for IHC include phospho-AMPKα (Thr172), total AMPKα from Abcam (Waltham, MA), SIRT1 from MilliporeSigma (Carlsbad, CA), SIRT3 from Abcepta (San Diego, CA). Human LKB1 and control siRNAs was from ThermoFisher Scientific (Waltham, MA).
Studies of human and mouse articular chondrocytes
Studies were performed in compliance with institutional IRB and approved human subjects’ protocols at the VA Medical Center of San Diego. Human knee chondrocytes were isolated from knee cartilage of autopsy donors as described previously (6). Human chondrocytes were cultured in Dulbecco’s modified Eagle’s (DMEM) high glucose medium with 10% fetal bovine serum (FBS), 100 μg/ml streptomycin, and 100 IU/ml penicillin at 37°C, and no later than first passage chondrocytes were used. Mouse chondrocytes were isolated from 6–8-day-old mouse knees as described previously and cultured in the media (19). Once chondrocytes reached confluence, they were re-plated in monolayer at 2.5 × 105 cells per well in 12 well plates or 5 × 105 cells per well in 6-well plates for cell experiments. To knockdown LKB1 in human chondrocytes, cells were transfected with LKB1 and non-target control siRNAs using X-tremeGene siRNA transfection reagent (Roche) for 48 hours.
Cell viability Assay
Human chondrocytes seeded in 96 well-plate (500–0 cells/well) were treated with berberine chloride (BBR) at concentration of 5, 10, 25, 50, 100 and 200 μM for 24 hours, and cell viability was assessed using the CellTiter-Fluor™ Cell Viability Assay (Promega, Madison, WI).
Western Blotting
Cells were lysed in RIPA buffer with 2 mM sodium vanadate and protease inhibitor cocktails (Roche, Mannheim, Germany). Cell lysates (10–15 μg) were separated by gradient 4–20% SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA), probed with antibodies, exposed to SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Waltham, MA), and visualized by autoradiography.
Measurement of Nitric Oxide, MMP-3, and MMP-13 release
Chondrocytes were pre-treated with berberine chloride at indicated concentrations for 1 hour before stimulated with IL-1β (5 ng/ml) for 18 hours in the DMEM media containing 1% FBS. The supernatants (conditioned media) were collected and used to measure levels of nitric oxide (NO) and MMP-3 and MMP-13 using the Griess reaction method and ELISA, respectively.
Experimental OA model in mice
All animal experiments were performed in compliance with the approved protocols from the Institutional Animal Care and Use Committee (IACUC) of the Rush University Medical Center and VA Medical Center of San Diego. AMPKα1 global knockout (KO) mice were generously provided by Dr. Benoit Viollet (INSERM, U1016, Paris, France). In this study, both AMPKα1 KO mice and congenic WT mice with C57BL/6/129 background were housed in static, polysulfone, microisolation cages and maintained on a 12:12-h light: dark cycle with caging, food and water bottles changed weekly. AMPKα1 KO mice and WT mice at 12 weeks of age were subjected to post-traumatic OA induced by destabilization of the medial meniscus (DMM) surgery. Briefly, after anesthesia, the right knee joint capsule was opened and the anteromedial meniscotibial ligament was transected as previously described (20). Sham surgeries were performed on the right knee of separate mice by opening the joint capsule in the same fashion to expose the ligament which was left intact. Two weeks after surgery, mice in the treatment group (randomly assigned by the surgeon) started receiving berberine chloride (0.1 mg/ml) via drinking water daily. Mice were euthanized at 6 and 12 weeks after surgery by carbon dioxide inhalation, and OA phenotype of knee joints were analyzed. Because male mice develop more robust and consistent OA and associated pain behaviors than female mice following DMM (21,22), only male mice were studied. Total of 96 mice (48 WT and 48 AMPKα1 KO) mice were used. Among 48 mice in each strain, 16 were in the sham-operated group as controls, 16 were in the DMM group, and 16 were in the DMM with berberine treatment group. Based on published studies, the sample sizes of mice used for the study were determined according to the design of 2 independent study groups. The sample size of 16 per group with 8 for each time point (6 and 12 weeks) were calculated with a function of the effect size of 1.4 SDs, alpha level of 0.05 and power of 80%.
Histological staining and Immunohistochemistry (IHC)
All mouse joints were fixed, decalcified, embedded in paraffin, and coronally sectioned (5 micron). For each knee joint, 9 slides at ~50-micron intervals were stained with Alcian blue/hematoxylin & orange G (AB/H&OG) for morphologic analysis. The severity of cartilage damage was assessed by analyzing all four quadrants of the joint including medial femoral (MF), medial tibial (MT), lateral femoral (LF) and lateral tibial (LT) cartilage using the OARSI score system (23) by two blinded observers. For histomorphometric measurement of articular cartilage area, the Alcian blue-positive staining areas of four quadrants were traced, and the size of each selected area was calculated using the ImageJ system (24). The final OARSI and articular cartilage area scores were average sum scores of four quadrants across the two observers. Synovitis scores were determined based on changes in synovial lining thickness and cellular density in the synovial stroma as previously described in (25). The osteophyte formation was evaluated semi-quantitatively based on both size and maturity of osteophytes (26).
For IHC staining, knee joint sections were heated at 95°C in Antigen Unmasking Solution (Vector Labs) for 15 min, and then sequentially treated with 3% H2O2, 0.5% Triton X-100, Avidin/Biotin Blocking Kit. After blocking with 10% normal goat serum for 1 h, sections were treated with primary antibodies, including total AMPKα (1:50 dilution), phospho-AMPKα (Thr172) (1:50 dilution), SIRT1 (1:100 dilution) and SIRT3 (1:100 dilution) antibody overnight at 4°C and incubated with 1/400 secondary biotinylated goat anti-rabbit or anti-mouse antibody for 30 min, followed by treatment with Vectastain Elite ABC Kit (Vector Labs, Burlingame, CA). IHC signals were revealed by ImmPACT DAB Peroxidase Substrate. The number of positive cells for each antibody was expressed as the percentage of positive-staining cells (IHC) relative to the number of cells with hematoxylin staining.
Pain behavioral assessment
To assess pain sensitivity, the mechanical allodynia test was performed by a calibrated set of von Frey filaments (North Coast Medical Inc., USA). Prior to von Frey hind paw test, the mice were allowed to accommodate for 15 minutes on a wire mesh grid. The filaments (typical force range used in mouse is from 0.04 to 6.0 g, beginning with 0.4 g) were applied to the plantar surface of the hind paw to determine the 50% force withdrawal threshold using the classical up-down iterative method as previously described (27). A response is considered positive if the animal exhibits any nocifensive behavior, including brisk paw withdrawal, licking, or shaking of the paw, either during application of the stimulus or immediately after the filament is removed. The tests were performed at 2 weeks before DMM surgery (baseline), and every other 2 weeks after DMM surgery in a blind manner and in the same order of cages of mice to be tested each time. The Laboratory Animal Behavior Observation Registration and Analysis System (LABORAS, Metris, Netherlands) was used to assess spontaneous behavior. Briefly, after animals were weighed, 4 platforms were used test 4 mice that were from the same group simultaneously for 15 hours at the same time frame from 18:00 pm to 9:00 am the next day. Four parameters including distance of locomotion, average speed of locomotion, rearing frequency, and rearing duration were evaluated.
Statistical analyses
GraphPad PRISM 8 was used for statistical analyses. All data were subjected to the normality test. For normally distributed data, one-way ANOVA with the post-hoc test (comparing > 2 groups with one independent variable) or two-way ANOVA with the post-hoc test (comparing 2 ≥ groups with 2 independent variables). For data not normally distributed, analysis was performed with Kruskal-Wallis test with multiple comparisons using Dunn’s corrections (comparing > 2 groups). The data were expressed as mean±SEM or mean with 95% confidence interval (CI). P < 0.05 was considered statistically significant.
Results
Berberine activated AMPK mediating through LKB1 in articular chondrocytes
First, we assessed chondrocyte cell viability in response to different concentrations of berberine ranging from 5 to 200 μM. Berberine at concentrations up to 100 μM did not affect cell viability (Suppl Figure 1A). As shown in Figure 1A, berberine was capable of increasing phosphorylation of AMPKα (Thr172) in primary human chondrocytes at concentrations of 5 and 25 μM. In addition, berberine at the same concentrations attenuated excessive catabolic activities induced by IL-1β in chondrocytes, demonstrated by significant inhibition of IL-1β-induced phosphorylation of NF-κB (Ser536) and release of nitric oxide (NO), MMP3 and MMP13 (Suppl Figure 1B–E). To determine if this anti-catabolic effect of berberine was mediated through AMPK, we evaluated and compared IL-1β-induced NO release in chondrocytes derived from WT and AMPKα1 KO mice in response to berberine. IL-1β-induced NO release was enhanced in AMPKα1 KO chondrocytes compared to WT chondrocytes (Suppl Figure 1F). Although IL-1β-induced NO release was significantly inhibited by berberine in both types of chondrocytes, the extent of inhibition was considerably less in AMPKα1 KO chondrocytes (40%) compared to WT chondrocytes (74%) (Suppl Figure 1F). This result indicates that AMPK, at least in part, mediated anti-catabolic effect of berberine in chondrocytes. Next, we went on to determine how AMPK is activated by berberine in chondrocytes. As shown in Figure 1A, berberine had no effect on expression of protein phosphatases PP2A and PP2Cα, which negatively regulate phosphorylation of AMPKα Thr172. In contrast, berberine was no longer able to induce phosphorylation of AMPKα Thr172 in chondrocytes with knockdown of LKB1 (Figure 1B), the major upstream kinase of AMPK, suggesting that activation of AMPK by berberine in chondrocytes requires LKB1.
Figure 1. Berberine activates AMPK via LKB1 in articular chondrocytes.
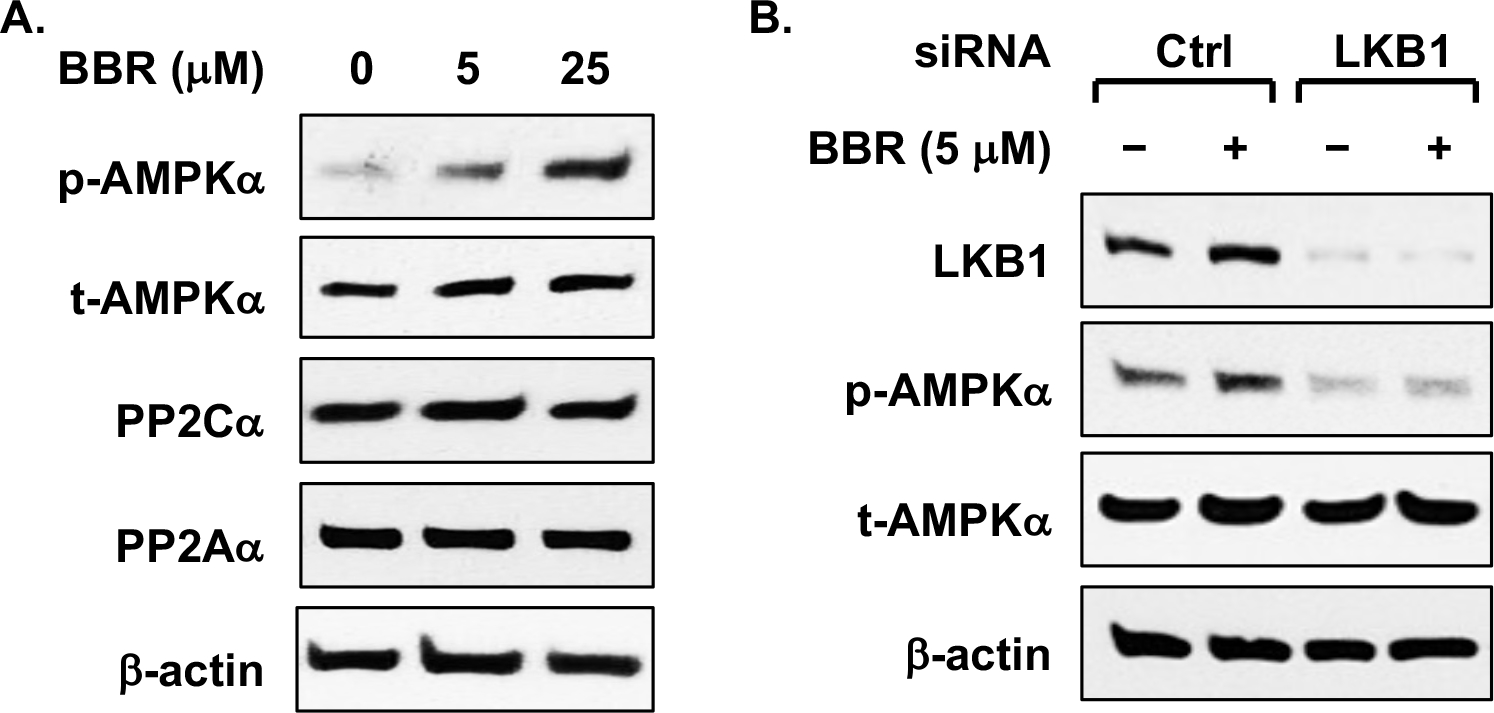
Primary human chondrocytes were (A) treated with berberine chloride (BBR) at 5 and 25 μM for 18 hours or (B) transfected with LKB1 and negative control siRNAs for 48 hours before the cells were treated with berberine chloride at 5 μM for 18 hours. Phosphorylation of AMPKα (Thr172) and expression of AMPKα, LKB1 and PP2Cα, PP2Aα were examined by Western blot analysis. β-actin was included as a loading control. Data representative of 3 independent experiments in 3 different donors of human chondrocytes.
Berberine limited cartilage damage in post-traumatic knee OA in mice, at least in part, via AMPK and downstream SIRT1 and SIRT3 signaling
To determine the effect of berberine on OA development and progression, we performed the DMM surgery in both WT and AMPKα1 KO mice. Both strains of mice were randomly divided into 2 groups with one group starting the berberine chloride (BBR) treatment daily via drinking water at 2-weeks post-surgery. The knee joints of these mice were collected at 6- and 12-weeks post-surgery and subjected to histological assessment of cartilage degeneration based on the OARSI scores and articular cartilage area scores (the detailed sub-scale scores shown in Supplemental Table I and II). As expected, cartilage degradation in the DMM operated knee joints was seen in WT mice without berberine treatment, which progressed from mild to severe with mean OARSI scores of 3.75 (95% CI 3.08 to 4.22) and 8.81 (95% CI 7.51 to 10.1) for 6- and 12-weeks post-surgery, respectively (Figure 2B). In comparison, cartilage degradation in those WT mice received berberine treatment was lessened, evidenced by a significant lower mean OARSI scores, which were 1.94 (95% CI: 1.25 to 2.62) and 5.75 (95% CI: 4.59 to 6.91) for 6- and 12-weeks post-surgery, respectively (Figure 2A and 2B). There was little cartilage degradation in the sham-surgery control groups of both strains of mice (data not shown). Notably, AMPKα1 KO mice after DMM surgery displayed the similar extent of cartilage degradation to WT mice (Figure 2B). However, berberine was no longer able to inhibit cartilage degradation in AMPKα1 KO mice (Figure 2B). The difference in the OARSI scores of DMM plus berberine group between WT and AMPKα1 KO mice was statistically significant (Figure 2B). Quantitative histomorphometric analysis of the Alcian blue stained articular cartilage showed cartilage areas in both WT and AMPKα1 KO mice were significantly and progressively reduced after DMM surgery (Figure 2C). Although berberine treatment appeared to slightly increase the cartilage area scores in WT but not AMPKα1 KO mice at both time points, the differences were not statistically significant (Figure 2C). IHC analysis of WT mice revealed decreased phosphorylation of AMPKα (Thr172) and concomitantly reduced expression of SIRT1 and SIRT3 in situ in the DMM knee cartilage, which were significantly reversed by berberine (Figure 3). Stimulation of human OA chondrocytes with berberine in vitro exhibited increased phosphorylation of AMPKα (Thr172), correlated with increased NAD+/NADH ratio and expression of NAD+-dependent SIRT1 and SIRT3, as well as downstream targets PGC-1α and OGG1(Supplemental Figure 2). Collectively, these data suggest that berberine can limit OA development and progression in post-traumatic OA in mice, and it does so, at least in part, through AMPK and downstream SIRT1 and SIRT3 signaling.
Figure 2. Berberine protected mice from cartilage damage in an AMPK-dependent manner in a post-traumatic OA model in mice.
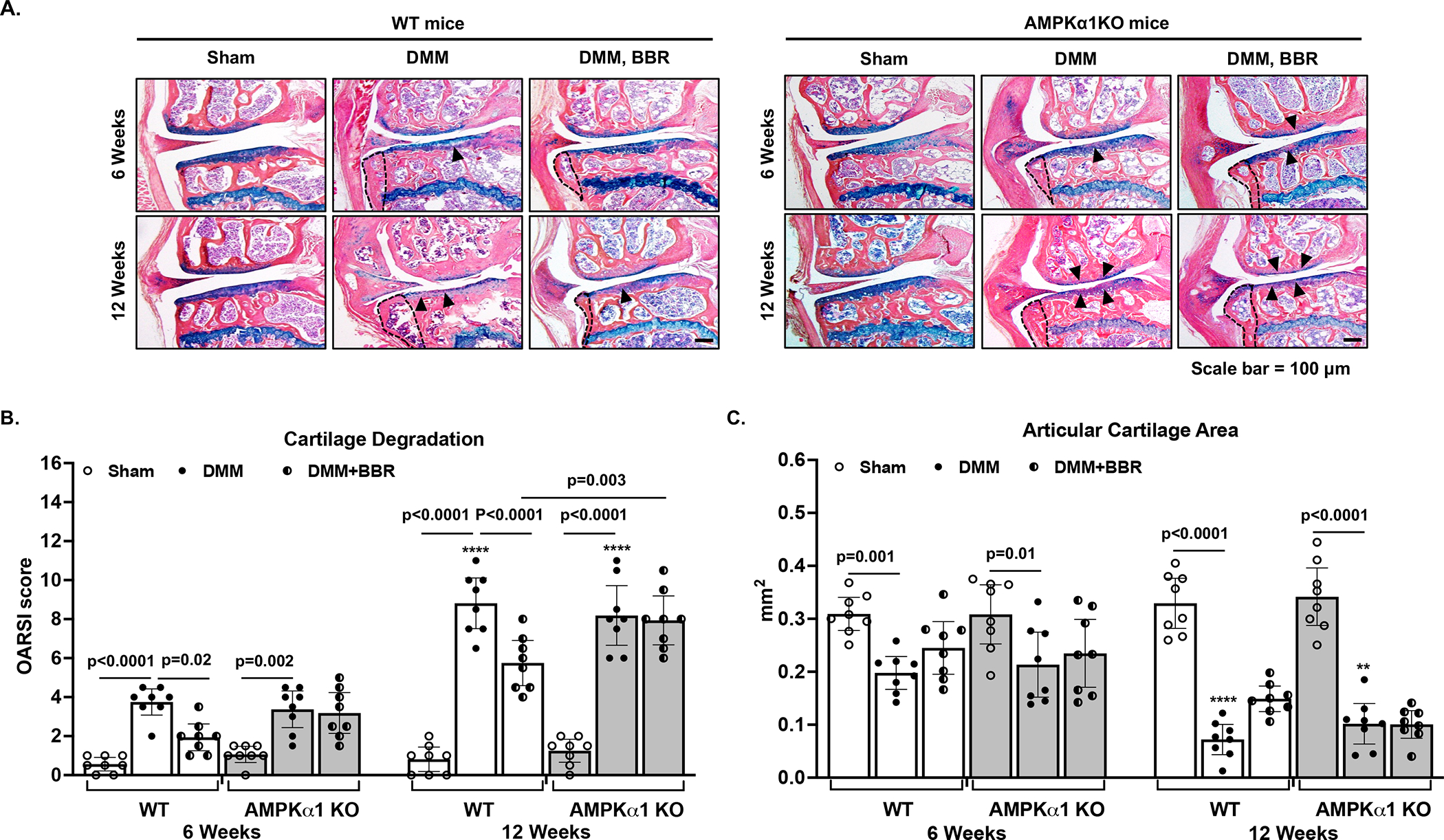
Both WT and AMPKα1 KO mice were subjected to the DMM surgery to induce OA development. Sham surgery was used as a control. Two weeks after the surgery, mice in the treatment group started to receive berberine chloride (BBR) via drinking water daily. At 6 and 12 weeks after the DMM surgery, mice were sacrificed and histological analysis of mouse knee sections (A) and assessment of cartilage damage (indicated by black arrowheads) including cartilage degradation (B) and cartilage area (C) were performed as described in the Methods (n=8 for each time point per group). Statistical analysis was performed using Two-way ANOVA with Sidak’s multiple comparisons test (multiple comparisons between sham and DMM, sham and DMM+BBR, and DMM and DMM+BBR at each time point) in B and C. The data were expressed as mean with 95% CI. Only p values with significance were indicated in the figures. ****p<0.0001, **p<0.002, comparing with each respective condition at 6 weeks.
Figure 3. Berberine exerted chondroprotective effect through activation of AMPK signaling via SIRT1 and SIRT3 in mice.
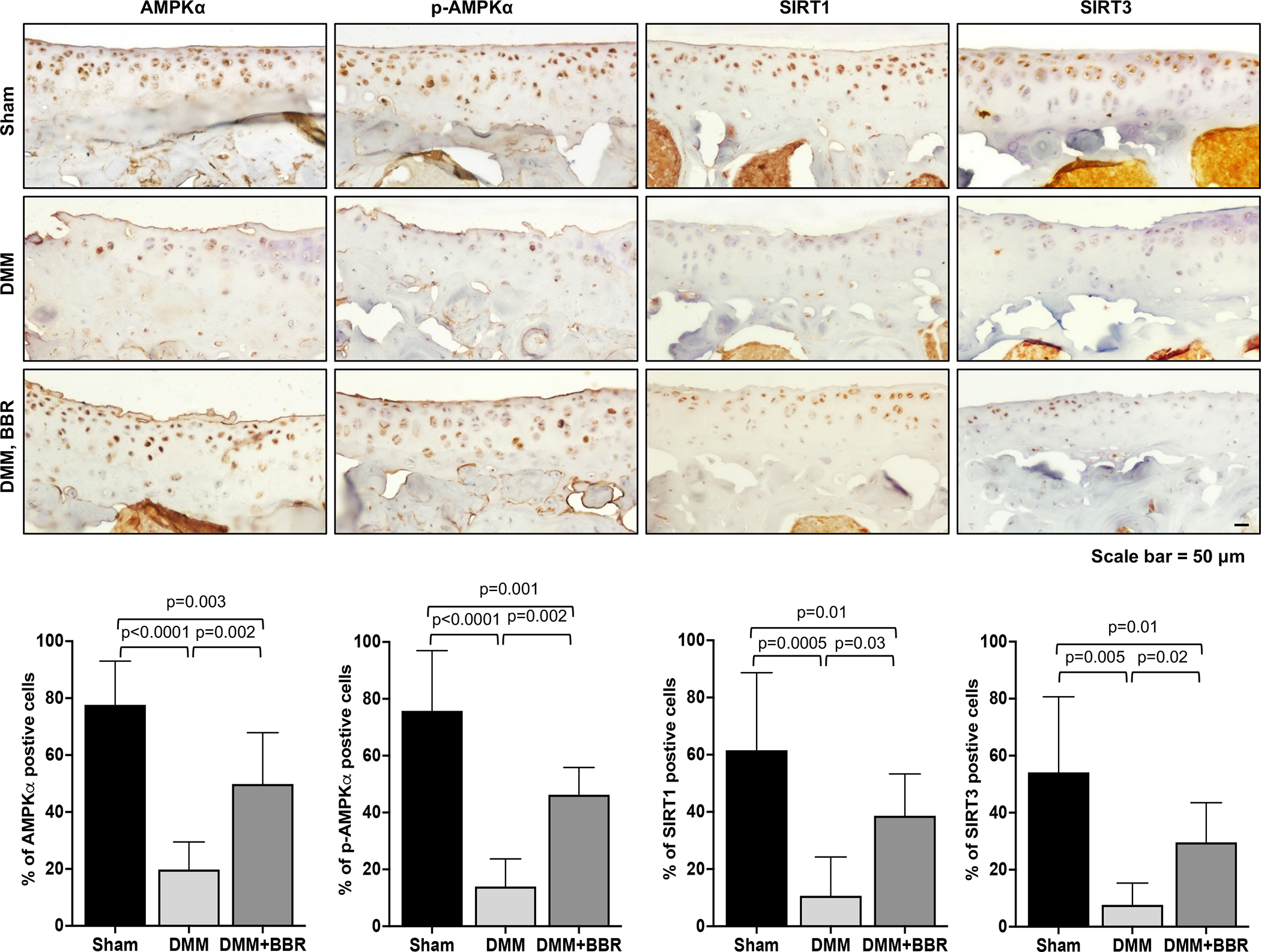
Mouse knee sections from the sham control and DMM-operated mice (WT) with and without berberine chloride (BBR) treatment (as described in the figure legend in Figure 2) were used for IHC analysis. A. Representative IHC images of phosphorylated AMPKα (Thr172), total AMPKα, SIRT1 and SIRT3. B. The cells present in the non-calcified regions of femoral and tibial cartilage were subjected to further analysis. Cells with positive staining for phosphorylated and total AMPKα, SIRT1 and SIRT3 were quantified (n=3). One-way ANOVA with Tukey’s multiple comparisons test was used for statistical analysis (comparing between the sham and DMM and DMM+BBR groups). The data were expressed as mean with 95% CI. Only p values with significance were indicated in the figures.
Berberine restricted progression of synovitis and osteophyte formation associated with post-traumatic OA in mice
Synovial hyperplasia was observed in the medial compartment of knees of WT mice at 6 and 12 weeks after DMM surgery (H&E images shown in Suppl Figure 3), indicated by considerably increased mean synovitis scores, which were 4.38 (95% CI 3.41 to 5.34) and 5.5 (95% CI 4.5 to 6.5), respectively (Figure 4A). Similar results were observed in AMPKα1 KO mice (Figure 4A). Berberine treatment significantly lower synovitis scores in WT (mean difference between the DMM and DMM+BBR groups, 1.56) but not AMPKα1 KO mice (mean difference between the DMM and DMM+BBR groups, 0.69) at 12 weeks after DMM surgery (Figure 4A). Osteophyte formation, reflected by markedly increased osteophyte size (Figure 2A, indicated in black dotted line), was observed in the tibial region of knee joints of both WT and AMPKα1 KO mice at 6 weeks after DMM surgery (Figure 4B) with mean scores of 1.13 (95% CI 0.21 to 1.66) and 1.69 (95% CI 0.61 to 2.26) for WT and AMPKα1 KO mice, respectively, which progressed to be more prominent at 12 weeks after DMM surgery with mean scores of 2.56 (95% CI 1.53 to 2.97) and 2.56 (95% CI 1.42 to 3.08) for WT and AMPKα1 KO mice, respectively. Similar results on osteophyte maturity were seen (Figure 4C). Treatment with berberine significantly reduced osteophyte size scores at 12 weeks after DMM surgery in WT (mean difference between the DMM and DMM+BBR groups, 0.75) but not AMPKα1 KO mice (mean difference between the DMM and DMM+BBR, 0.125). Berberine also reduced osteophyte maturity scores at 12 weeks after DMM surgery in WT but not AMPKα1 KO mice (Figure 4C), but the difference was not statistically significant, suggesting limited effect of berberine on osteophyte maturity. Taken together, berberine can reduce pathological changes of synovial and subchondral bone tissues associated with post-traumatic OA, which at least in part mediated through AMPK.
Figure 4. Berberine restricted progression of synovitis and osteophyte formation associated with post-traumatic OA in mice.
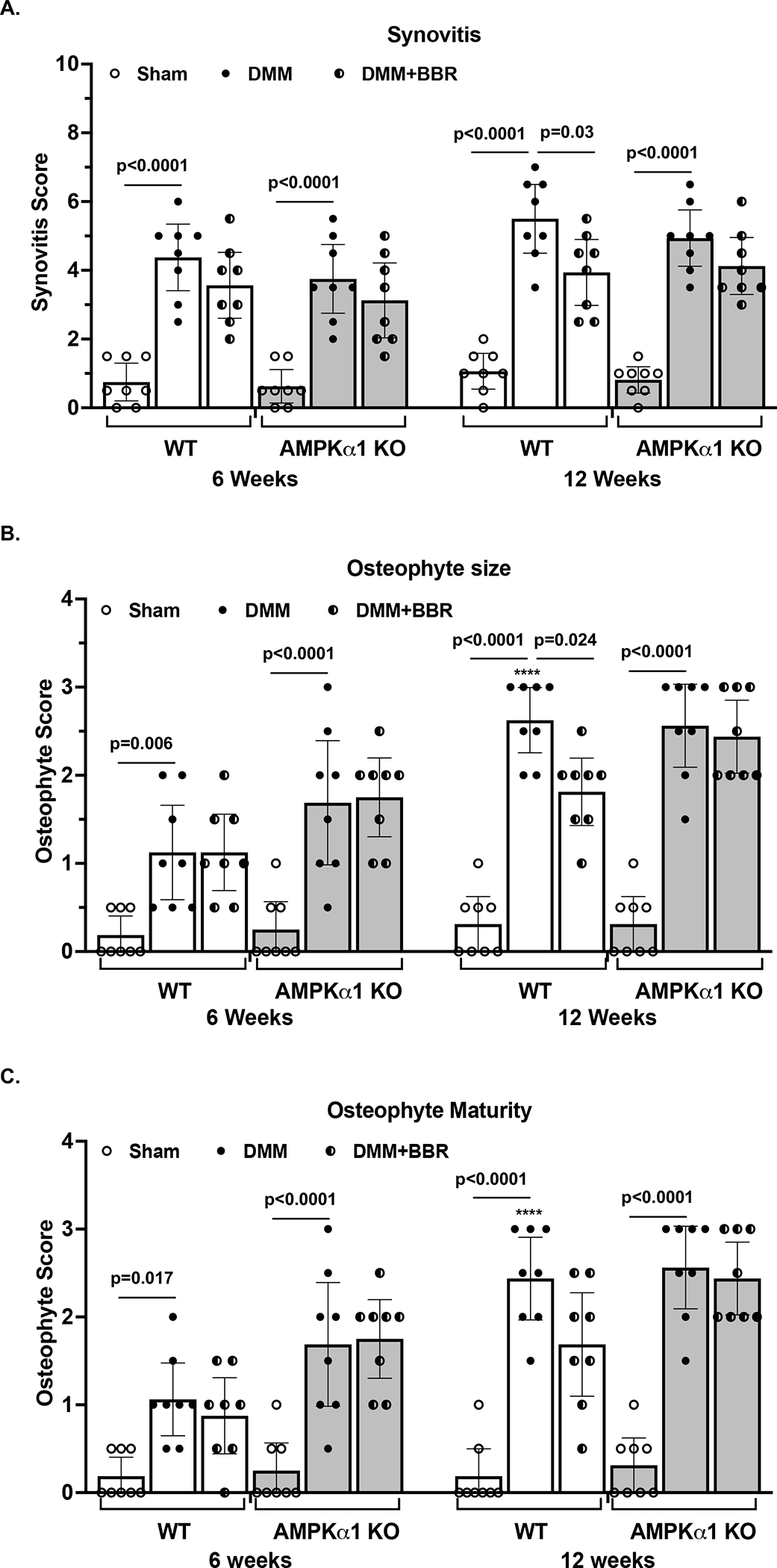
Synovitis (A) and osteophyte formation including both size (B) and maturity (C) were evaluated semi-quantitatively as described in the Methods in the knees of both WT and AMPKα1 KO mice with and without berberine chloride (BBR) treatment after DMM surgery (n=8 for each time point per group). Statistical analysis was performed using Two-way ANOVA with Sidak’s multiple comparisons test (multiple comparisons between sham and DMM, sham and DMM+BBR, and DMM and DMM+BBR at each time point). The data were expressed as mean with 95% CI. Only p values with significance were indicated in the figures. ****p<0.0001, comparing with each respective condition at 6 weeks.
Berberine reduced pain associated with post-traumatic OA via AMPK in mice
To examine the effect of berberine on pain associated with post-traumatic OA, we analyzed pain sensitivity by evaluating mechanical allodynia with the von Frey test in all mice at 2, 4, 6, 8 and 10 weeks after DMM surgery. Paw withdraw response threshold was markedly decreased in both WT and AMPKα1 KO mice 2 weeks after DMM surgery, which was sustained in the rest of study period (Figure 5A and 5B). Notably, WT but not AMPKα1KO mice received berberine treatment exhibited significantly increased paw withdraw response threshold starting at 4 weeks after DMM surgery, which was 2 weeks after initiation of berberine treatment. (Figure 5A and 5B). Area under the curve (AUC) values for von Frey paw withdrawal threshold during the entire time course further supported the ability of berberine to improve pain sensitivity induced by DMM in WT but not AMPKα1 KO mice, as a significant mean difference between the DMM+BBR and DMM groups was only observed in WT mice (Figure 5C and 5D). We also examined spontaneous activity using the Laboratory Animal Behavior Observation, Registration and Analysis System (LABORAS) in both WT and AMPKα1KO mice at 2, 6 and 10 weeks after DMM surgery. As shown in Figure 6, both WT and AMPKα1KO mice without berberine treatment showed reduced spontaneous activities, evidenced by prominently and progressively decreased travel distance (A and B) and average walking speed (C and D). Rearing frequency (E and F) and rearing duration (D and G) were also reduced in these mice, although they were not statistically significant. Treatment with berberine significantly improved spontaneous activities in WT but not AMPKα1KO mice (A-G). These data suggest that berberine can reduce pain associated with post-traumatic OA in mice in an AMPK-dependent manner.
Figure 5. AMPK mediated the effect of berberine on relieving pain through reduced pain sensitivity in post-traumatic OA.
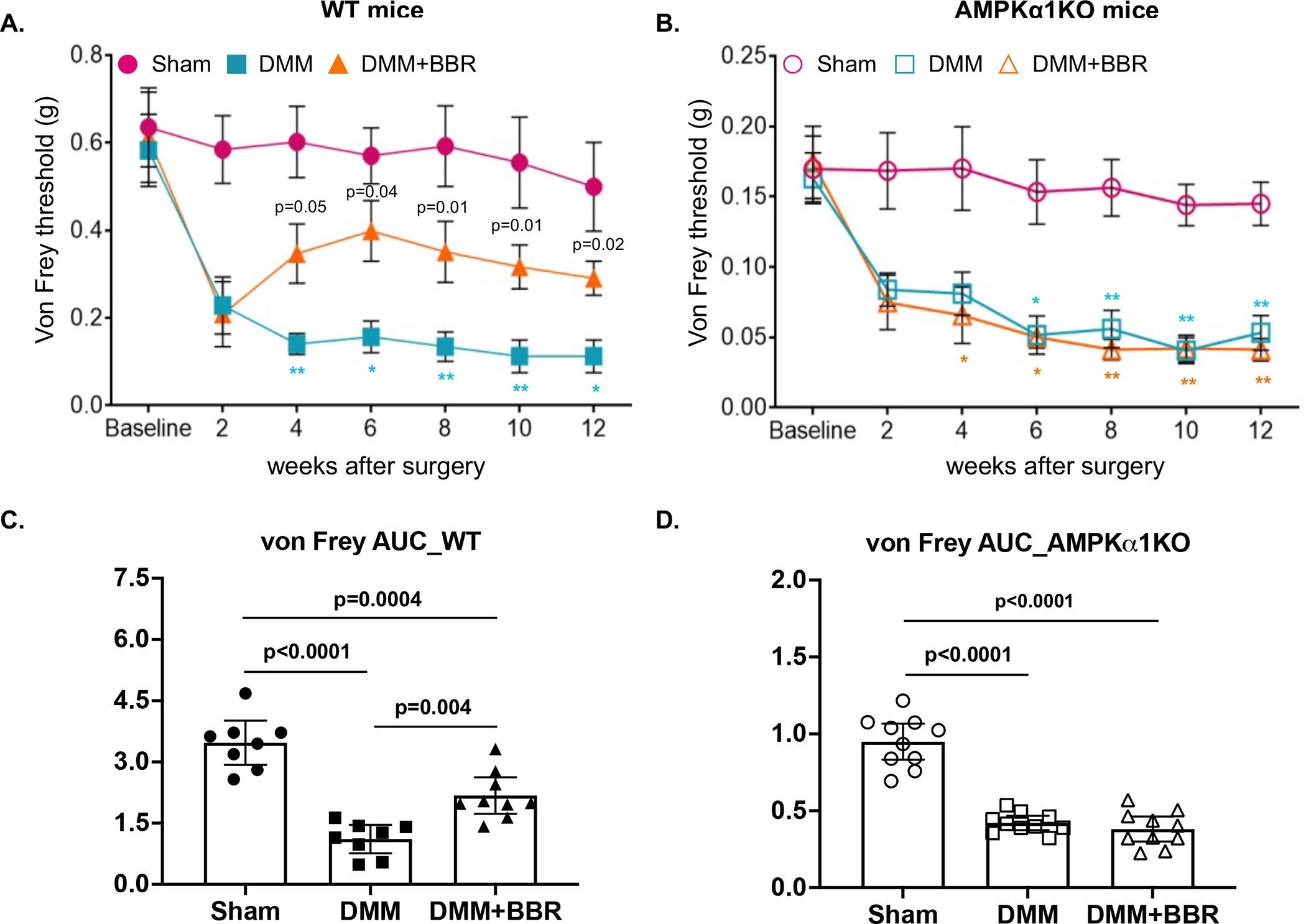
The von Frey test was performed to assess pain sensitivity biweekly after DMM surgery in WT and AMPKα1 KO mice (A and B) with and without berberine chloride (BBR) treatment (mice with sham surgery were included as control) as described in the Methods (n=8 for each time point per group). Area under curve (AUC) was used to represent the pain sensitivity for the entire time course (C and D). Statistical analysis was conducted using Kruskal-Wallis test with Dunn’s multiple comparisons for A and B (comparing DMM with DMM+BBR, DMM with sham, or DMM+BRR with sham at each time point) and one-way ANOVA with Tukey’s multiple comparisons test for C and D. The data were expressed as mean±SEM for A and B and mean with 95% CI for C and D. Only p values with significance were indicated in the figures. Exact p values shown in A represent the comparisons between DMM and DMM+BBR. P values shown in symbols “*” and “** indicating p<0.03 and **p<0.002 respectively for comparisons between DMM and sham in A and B (blue font) or between DMM+BBR and sham in B (orange font).
Figure 6. AMPK mediated the effect of berberine on relieving pain through improving spontaneous activities in post-traumatic OA.
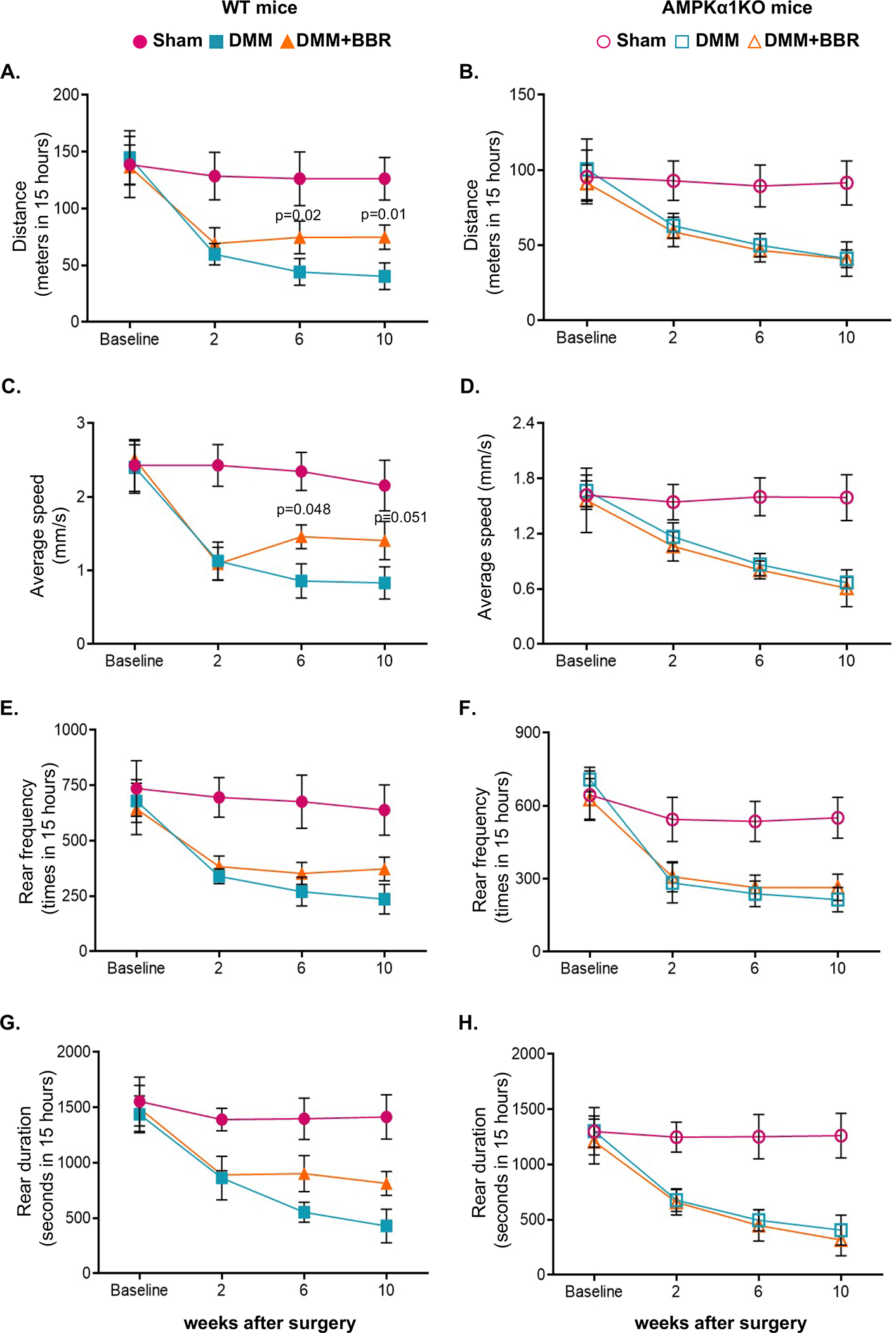
The LABORAS system was used to evaluate spontaneous pain biweekly after DMM surgery in WT and AMPKα1 KO mice with and without berberine chloride (BBR) treatment (mice with sham surgery were included as controls) as described in the Methods (n=8 for each time point per group), which included travel distance (A, B), average walking speed (C, D), rear frequency (E, F), and rearing duration (G, H). Sham control group were included only for reference. Statistical analysis was conducted using Two-way ANOVA with Sidak’s multiple comparisons test for A, C, E, G, and Kruskal-Wallis test with Dunn’s multiple comparisons for B, D. F, H (comparing DMM with DMM+BBR at each time point). The data were expressed as mean±SEM. Only p values with significance were indicated in the figures.
Discussion
To date, OA disease modifying therapies are not yet available. The conventional treatment for OA such as using steroids or non-steroids anti-inflammatory (NSAIDs) drugs provides relatively short-term and non-sustainable relief of the symptoms. In addition, they fail to modify disease progression. Given the evidence suggesting that some natural products including berberine possess anti-inflammatory and antioxidant properties, the potential of using naturally derived compounds as a therapeutic strategy for OA treatment has drawn increased attention (28). Although preclinical studies have showed that berberine chloride, a botanical-derived isoquinoline alkaloid, can limit cartilage degradation (15–18), the underlying mechanism of chondroprotection is not fully understood. Because activation of AMPK is considered as a major molecular action of berberine (14), in this study, we investigated how AMPK is activated in articular chondrocytes in vitro and determined if AMPK is required for chondroprotective effect of berberine in mice in vivo. In addition, we investigated if berberine has ability to relieve OA-associated pain in mice.
We previously observed that phosphorylation of AMPKα (Thr172) is decreased in human knee OA chondrocyte/cartilage and in mouse knee cartilage associated with OA and aging (6,7). This is correlated with reduced phosphorylation of LKB1, which is shown to be the major upstream kinase of AMPK in articular chondrocytes (6,7). In this study, we found that activation of AMPK in chondrocytes by berberine requires LKB1, as phosphorylation of AMPKα was impaired in LKB1 knockdown chondrocytes. In addition, we demonstrated that anti-catabolic effect of berberine in chondrocytes in response to inflammatory cytokine IL-1β was at least in part mediated through AMPK activation. Since activation of AMPK can inhibit mitogen-activated protein kinases (MAPK) signaling induced by cytokines (29), the previous findings that anti-inflammatory and anti-catabolic ability of berberine via inhibition of MAPKs (15) are likely AMPK-dependent. Berberine is also shown to have anti-apoptotic effect in chondrocytes via activation of AMPK and suppression of p38 MAPK (16).
Chondroprotective effect of berberine has been demonstrated in a surgically induced rat OA models (15–18). We previously observed that age-related cartilage degeneration was suppressed by berberine in C57BL/6 mice (12). In this study, we revealed that berberine can also limit progression of cartilage degeneration at least in part through AMPK in a post-traumatic OA model. This was evidenced by significant less cartilage degradation in WT but not AMPKα1 KO mice received berberine treatment after DMM surgery. Notably, there were no differences in the extent of cartilage degradation induced by DMM surgery between WT and AMPKα1 KO mice. This may be explained by the compensatory effect of AMPKα2 for the loss of AMPKα1 in AMPKα1 KO mice. A recent study demonstrated that there was no difference in cartilage degradation between chondrocyte-specific AMPKα1 KO and WT mice in an injury-induced OA model (30). There was a compensatory increase in AMPKα2 expression in chondrocytes isolated from chondrocyte-specific AMPKα1 KO mice, indicating the feedback role of AMPKα2 expression after AMPKα1 loss (30). In comparison, chondrocyte-specific AMPKα1 and AMPKα2 double knockout mice were shown to have accelerated cartilage degradation, compared to the WT control mice in both injury-induced and age-related OA models (13). Our previous studies implicate that chondroprotective effect of AMPK activation is largely due to preventing mitochondrial dysfunction by preserving mitochondrial biogenesis capacity through SIRT1 and the master regulator of mitochondrial biogenesis PGC-1α and promoting DNA repair through SIRT3 and a base excision DNA repair enzyme OGG1 (10–12). Indeed, treatment of human OA chondrocytes with berberine in vitro resulted in increased NAD+/NADH ratio and expression of NAD+-dependent SIRT1 and SIRT3 and their downstream targets PGC-1α and OGG1, correlated with increased phosphorylation of AMPKα (Thr172). IHC analysis also confirmed that berberine significantly inhibited concurrent loss of phosphorylation of AMPKα and expression of SIRT1 and SIRT3 in the DMM knee cartilage. These results indicate that berberine may also signal through our previously identified AMPK-SIRT1-PGC-1α and AMPK-SIRT3-OGG1 signaling pathways to exert its chondroprotective effect in post-traumatic OA.
Berberine also significantly decreased synovitis and osteophyte scores in WT but not AMPKα1 KO mice at 12 weeks after DMM surgery, suggesting that it could restrict progression of synovial hyperplasia and osteophyte formation associated with post-traumatic OA at least in part through AMPK. Synovitis is known to associate with more rapid progression of cartilage loss in human OA joints (31). Synovial macrophages are found to contribute both directly and indirectly to OA progression through the induction of inflammatory mediators, growth factors and proteinases, resulting in enhanced cartilage degeneration and osteophyte formation (32). Since AMPK activation promotes macrophage polarization from pro-inflammatory to anti-inflammatory phenotype (33), the effect of berberine in reducing synovitis and osteophyte formation may result from AMPK activation in synovial macrophages, which warrants further investigation.
We noticed that the inhibitory effect of berberine on cartilage degradation, synovitis and osteophyte formation was limited in the present study. There are a couple of possible reasons. First, only one dose of berberine was used in the study. Berberine was given to mice through drinking water at 0.1 mg/ml, which was about 13.3–16 mg/kg/day based on the average adult male mouse body weight at 25–30 grams and the average daily consumption of water at 4 ml. This was slightly lower than the typical dose used in human. The common dosage of oral berberine supplement used in human is 1500 mg/day, which is about 18.75–20 mg/kg/day based on the average adult human body weight at 60–80 kg. A future dose-response study is needed to determine the optimal dosage of berberine that efficiently limit OA development in the DMM mode. Second, chondrocyte apoptosis in the weight bearing area of mouse knee cartilage can occur prior to week 2 after DMM surgery (34), leading to progressive loss of chondrocytes. In addition, a recent mouse study demonstrated that expression of pro-inflammatory genes can be detected in the mouse synovium at 3 days after DMM surgery, and synovial hyperplasia is evident as early as 1 week after DMM surgery (35). It is known that chondrocyte apoptosis can be promoted by inflammation (36). These findings suggest that time of initiating therapies may be critical in the DMM model (34). In our study, berberine treatment started at 2 weeks after DMM surgery. The viable chondrocyte population in cartilage may have already been reduced, which would affect the efficacy of berberine in inhibiting cartilage matrix degradation. Histological evidence of synovial inflammation (observed 1 week after DMM surgery) appears to be earlier than cartilage degradation and osteophyte formation occurring at 4 and 8 weeks respectively after surgery in mice (35), implicating importance of early intervention of OA through targeting synovium after joint injury. Future studies of the impact of different treatment initiation times on the efficacy of berberine in the DMM model may be necessary to identify a therapeutic window for berberine to suppress synovial inflammation, cartilage degradation, and osteophyte formation most efficiently in post-traumatic OA in mice.
AMPK is recently emerged as a novel pain target (37,38). AMPK activators are capable of alleviating pain in a variety of preclinical pain models through inhibition of signaling pathways known to promote changes in the function and phenotype of peripheral nociceptive neurons and promote chronic pain (37,38). In our previous study, we found that metformin can effectively relieve OA pain in an AMPK-dependent manner in mice after DMM surgery (9). Increased excitability of peripheral sensory neurons in the dorsal root ganglia (DRG) is known to associate with pain hypersensitivity resulted from injury (39). Metformin significantly upregulated expression of phosphorylated and total AMPKα expression in DRG and suppressed pain hypersensitivity caused by DMM surgery through reduction of expression of membrane-associated transient receptor potential ankyrin 1 (TRPA1), an ion channel widely recognized as a chemical and thermal sensor that plays vital roles in pain transduction (9). In this study, we found that berberine was also able to relieve OA-associated pain in an AMPK-dependent manner, supported by significantly reduced pain sensitivity and increased spontaneous activities in berberine-treated WT but not AMPKα1 KO mice after DMM surgery. Berberine is likely to inhibit pain sensitivity through activation of AMPK and inhibit TRPA1 in DRG, although we did not directly test that.
It is known that synovitis is associated with neuropathy-like pain sensitization in knee OA patients (40). In addition, histological evidence of synovitis is temporally associated with the onset and persistence of allodynia in the mouse DMM model (41). In our current study, we saw a correlation between synovitis (histological score) and reduced pain sensitization (paw withdraw threshold) at 6 and 12 weeks after DMM surgery. Berberine only significantly reduced synovitis in WT mice at 12 weeks (but not 6 weeks) after DMM surgery, despite that it considerably improved pain sensitization at both time points. Interestingly, the beneficial effect of some therapies on pain sensitization do not correlate with improvement of histological evidence of synovitis in mouse DMM model (41), indicating that histopathology is not sensitive enough to detect subtle differences in synovial inflammation. A recent study comparing cartilage degeneration and pain in male and female mice in the DMM surgery-induced OA revealed that joint damage develops comparably in both female and male mice after DMM although it progresses less in females (42), which agreed with the previous findings (21). However, there was a subtle sex difference in pain behaviors and analgesic efficacy of an antagonist of transient receptor potential cation channel subfamily V member 1 TRPV1(42), which cooperates with TRPA1 to mediate the detection of noxious stimuli in primary afferent sensory neurons (43). Since only male mice were used in the current study, whether berberine has the similar effect on limiting joint damage and relieving pain in female mice remains to be determined.
In summary, this study demonstrated that activation of AMPK by berberine can limit OA progression by reducing cartilage degradation, synovitis and osteophyte formation and improve pain sensitization in mice in a post-traumatic OA model. Taken together with our previous finding that berberine cam also limit aging-associated cartilage degradation, we conclude that berberine has a potential to be used as a disease modifying therapeutic agent for OA treatment.
Supplementary Material
Supplemental Figure 1. AMPK, at least in part, mediated anti-catabolic effect of berberine in chondrocytes. Primary human chondrocytes were treated with berberine chloride (BBR) at concentration of 5, 10, 25, 50, 100 and 200 μM for 24 hours, and cell viability was assessed using Promega CellTiter-Fluor™ Cell Viability Assay (A). Primary human knee chondrocytes were pre-treated with BBR at 5 and 25 μM for 1 hour before stimulated with IL-1β (5 ng/ml) for 2 hours (B) and 18 hours (C-E). Phosphorylated (Ser536) and total p65 NF-κB expression was examined by Western blot analysis (B). Release of NO, MMP3 and MMP13 were measured by the Griess reaction method and ELISA analysis of the conditioned media (C-E). Primary mouse knee chondrocytes isolated from 6–8 days of WT and AMPKα1KO mice were pre-treated with BBR at 25 μM for 1 hour before stimulated with IL-1β (5 ng/ml) for 18 hours. NO release was measured from the conditioned media by the Griess reaction method (F). One-way ANOVA with Dunn’s multiple comparisons test (comparing BBR at each concentration with no treatment) was used for the statistical analysis in A (n=4 biological replicates).Two-way ANOVA with Tukey’s multiple comparisons test (comparing IL-1β-treated with no treatment control at each BBR concentration, and IL-1β+BBR at 5 or 25 μM with IL-1β without BBR, biological replicates n=4 for B-E and n=3 for F) was used for the statistical analysis in B-F. Only p values with significance were indicated in the figures. *p<0.0001 comparing BBR at 200 μM with no BBR in A.
Supplemental Figure 2. Activation of AMPK in chondrocytes by berberine increased NAD+-dependent SIRT1 and SIRT3 and their downstream targets. Primary human knee chondrocytes were stimulated with berberine chloride (BBR, 5 μM) for 18 hours. Intracellular levels of NAD and NADH were measured using the NADH/NAD Quantification Kit (BioVision), and NAD/NADH ratio was determined (A). Expression of phosphorylated and total AMPKα, SIRT1, SIRT3, PGC-1α and OGG1 were examined by Western blot analysis (B). Statistical analysis was performed using the student t-test. The data were expressed as mean±SD for A.
Supplemental Figure 3. Effect of berberine on changes in synovial tissues. Knee sections of WT and AMPKα1 KO mice from the sham control, DMM and DMM+BBR groups were subjected to H&E staining. Representative images of medial compartment of knees for all conditions were shown in the figure. Medial femoral and tibial synovial tissue changes were evident (boxes with black dotted lines).
Acknowledgements
We would like to acknowledge Dr. Benoit Viollet (INSERM, U1016, Paris, France) for providing us with AMPKα1 global knockout (KO) mice.
Role of the funding source:
This work was supported by the Department of Veterans Affairs Merit Review grant 1I01BX002234 (PI: Liu-Bryan) and the National Institutes of Health (NIH) Grant R01AR070222 (PI: Chen).
Footnotes
Competing interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 2012;64:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol 2007;213:626–34. [DOI] [PubMed] [Google Scholar]
- 3.Liu-Bryan R. Inflammation and intracellular metabolism: new targets in OA. Osteoarthritis Cartilage 2015;23:1835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev 2009;89:1025–78. [DOI] [PubMed] [Google Scholar]
- 5.Garcia D, Shaw RJ. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 2017;66:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terkeltaub R, Yang B, Lotz M, Liu-Bryan R. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1β and tumor necrosis factor α. Arthritis Rheum 2011;63:1928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petursson F, Husa M, June R, Lotz M, Terkeltaub R, Liu-Bryan R. Linked decreases in liver kinase B1 and AMP-activated protein kinase activity modulate matrix catabolic responses to biomechanical injury in chondrocytes. Arthritis Res Ther 2013;15:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardie DG. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes 2013;62:2164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Zhang B, Liu WX, Lu K, Pan H, Wang T, et al. Metformin limits osteoarthritis development and progression through activation of AMPK signaling. Ann Rheum Dis 2020;79:635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X, Petursson F, Viollet B, Lotz M, Terkeltaub R, Liu-Bryan R. Peroxisome proliferator-activated receptor γ coactivator 1α and FOXO3a mediate chondroprotection by AMP-activated Protein Kinase. Arthritis Rheum 2014;66:3073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Zhao X, Lotz M, Terkeltaub R, Liu-Bryan R. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor γ coactivator 1α. Arthritis Rheumatol 2015;67:2141–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen LY, Wang Y, Terkeltaub R, Liu-Bryan R. Activation of AMPK-SIRT3 signaling is chondroprotective by preserving mitochondrial DNA integrity and function. Osteoarthritis Cartilage 2018;26:1539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou S, Lu W, Chen L, Ge Q, Chen D, Xu Z, et al. AMPK deficiency in chondrocytes accelerated the progression of instability-induced and ageing-associated osteoarthritis in adult mice. Sci Rep 2017;7:43245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng X, Sureda A, Jafari S, Memariani Z, Tewari D, Annunziata G, et al. Berberine in cardiovascular and metabolic diseases: from mechanisms to therapeutics. Theranostics 2019;9:1923–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, He P, Hou Y, Chen S, Xiao Z, Zhan J, et al. Berberine inhibits the interleukin-1 beta-induced inflammatory response via MAPK downregulation in rat articular chondrocytes. Drug Dev Res 2019;80:637–45. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Liu SQ, Yu L, He B, Wu SH, Zhao Q, et al. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis 2015;20:1187–99. [DOI] [PubMed] [Google Scholar]
- 17.Zhao H, Zhang T, Xia C, Shi L, Wang S, Zheng X, et al. Berberine ameliorates cartilage degeneration in interleukin-1β-stimulated rat chondrocytes and in a rat model of osteoarthritis via Akt signalling. J Cell Mol Med 2014;18:283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu PF, Chen WP, Tang JL, Bao JP, Wu LD. Protective effects of berberine in an experimental rat osteoarthritis model. Phytother Res 2011;25:878–85. [DOI] [PubMed] [Google Scholar]
- 19.Cecil DL, Terkeltaub R. Transamidation by transglutaminase 2 transforms S100A11 Calgranulin into a catabolic cytokine for chondrocytes. J. Immunol 2008;180:8378–8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 2007; 15:1061–1069. [DOI] [PubMed] [Google Scholar]
- 21.Ma HL, Blanchet TJ, Peluso D, Hopkins B, Morris EA, Glasson SS. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage. 2007;15:695–700. [DOI] [PubMed] [Google Scholar]
- 22.Malfait AM, Ritchie J, Gil AS, Austin JS, Hartke J, Qin W, et al. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthr Cartil. 2010;18:572–80. [DOI] [PubMed] [Google Scholar]
- 23.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 2010;18 Suppl 3:S17–23. [DOI] [PubMed] [Google Scholar]
- 24.Rasband WS, ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997–2018. [Google Scholar]
- 25.Lewis JS, Hembree WC, Furman BD, Tippets L, Cattel D, Huebner JL, Little D, DeFrate LE, Kraus VB, Guilak F, Olson SA. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis Cartilage. 2011;19:864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 28.Henrotin Y, Mobasheri A. Natural products for promoting joint health and managing osteoarthritis. Curr Rheumatol Rep 2018;20:72. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Yoon MY, Choi SL, Kang I, Kim SS, Kim YS, et al. Effects of stimulation of AMP-activated protein kinase on insulin-like growth factor 1- and epidermal growth factor-dependent extracellular signal-regulated kinase pathway.The Journal of biological chemistry.2001;276:19102–19110. [DOI] [PubMed] [Google Scholar]
- 30.Yang C, Li Z, Lai P, Bai X, Jin D. Chondrocyte-Specific Ablation of AMPKα1 Does Not Affect Bone Development or Pathogenesis of Osteoarthritis in Mice. DNA Cell Biol. 2016;35:156–62. [DOI] [PubMed] [Google Scholar]
- 31.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Cai D, Bai X. Macrophages regulate the progression of osteoarthritis. Osteoarthritis Cartilage. 2020;28:555–561. [DOI] [PubMed] [Google Scholar]
- 33.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haase T, Sunkara V, Kohl B, Meier C, Bußmann P, Becker J et al. Discerning the spatio-temporal disease patterns of surgically induced OA mouse models. PLOS ONE 2019;14: e0213734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao L, Zhang S, Zhao L, Chang X, Han L, Huang J et al. Acute Synovitis after Trauma Precedes and is Associated with Osteoarthritis Onset and Progression. Int J Biol Sci. 2020;16:970–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis and Rheumatism. 2001; 44: 1237–47. [DOI] [PubMed] [Google Scholar]
- 37.Asiedu MN, Dussor G, Price TJ. Targeting AMPK for the alleviation of pathological pain. Exp Suppl 2016;107:257–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price TJ, Das V, Dussor G. Adenosine monophosphate-activated protein kinase (AMPK) activators for the prevention, treatment and potential reversal of pathological pain. Curr Drug Targets 2016;17:908–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker SM, Beggs S, Baccei ML. Persistent changes in peripheral and spinal nociceptive processing after early tissue injury. Exp Neurol 2016;275 Pt2:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neogi T, Guermazi A, Roemer F, Nevitt MC, Scholz J, Arendt-Nielsen L et al. Association of joint inflammation with pain sensitization in knee osteoarthritis: the multicenter osteoarthritis study. Arthritis Rheumatol. 2016;68:654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shu CC, Zaki S, Ravi V, Schiavinato A, Smith MM, Little CB. The relationship between synovial inflammation, structural pathology, and pain in post-traumatic osteoarthritis: differential effect of stem cell and hyaluronan treatment. Arthritis Res Ther. 2020;22:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang HS, Park IY, Hong JI, Kim JR, Kim HA. Comparison of joint degeneration and pain in male and female mice in DMM model of osteoarthritis. Osteoarthritis Cartilage 2021;29:728–38. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br J Pharmacol 2012;166:510–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. AMPK, at least in part, mediated anti-catabolic effect of berberine in chondrocytes. Primary human chondrocytes were treated with berberine chloride (BBR) at concentration of 5, 10, 25, 50, 100 and 200 μM for 24 hours, and cell viability was assessed using Promega CellTiter-Fluor™ Cell Viability Assay (A). Primary human knee chondrocytes were pre-treated with BBR at 5 and 25 μM for 1 hour before stimulated with IL-1β (5 ng/ml) for 2 hours (B) and 18 hours (C-E). Phosphorylated (Ser536) and total p65 NF-κB expression was examined by Western blot analysis (B). Release of NO, MMP3 and MMP13 were measured by the Griess reaction method and ELISA analysis of the conditioned media (C-E). Primary mouse knee chondrocytes isolated from 6–8 days of WT and AMPKα1KO mice were pre-treated with BBR at 25 μM for 1 hour before stimulated with IL-1β (5 ng/ml) for 18 hours. NO release was measured from the conditioned media by the Griess reaction method (F). One-way ANOVA with Dunn’s multiple comparisons test (comparing BBR at each concentration with no treatment) was used for the statistical analysis in A (n=4 biological replicates).Two-way ANOVA with Tukey’s multiple comparisons test (comparing IL-1β-treated with no treatment control at each BBR concentration, and IL-1β+BBR at 5 or 25 μM with IL-1β without BBR, biological replicates n=4 for B-E and n=3 for F) was used for the statistical analysis in B-F. Only p values with significance were indicated in the figures. *p<0.0001 comparing BBR at 200 μM with no BBR in A.
Supplemental Figure 2. Activation of AMPK in chondrocytes by berberine increased NAD+-dependent SIRT1 and SIRT3 and their downstream targets. Primary human knee chondrocytes were stimulated with berberine chloride (BBR, 5 μM) for 18 hours. Intracellular levels of NAD and NADH were measured using the NADH/NAD Quantification Kit (BioVision), and NAD/NADH ratio was determined (A). Expression of phosphorylated and total AMPKα, SIRT1, SIRT3, PGC-1α and OGG1 were examined by Western blot analysis (B). Statistical analysis was performed using the student t-test. The data were expressed as mean±SD for A.
Supplemental Figure 3. Effect of berberine on changes in synovial tissues. Knee sections of WT and AMPKα1 KO mice from the sham control, DMM and DMM+BBR groups were subjected to H&E staining. Representative images of medial compartment of knees for all conditions were shown in the figure. Medial femoral and tibial synovial tissue changes were evident (boxes with black dotted lines).


