Abstract
The principle of ketogenic diet (KD) is restriction of carbohydrates to a maximum of 5–10% of the total daily caloric intake, aiming at shifting body metabolism toward ketone bodies. Different studies suggested promising results of KD to help patients to lose weight, to reduce insulin requirements in diabetes, to supplement cancer protocols, to treat neurological conditions and to optimize control of metabolic and cardiovascular diseases. However, literature about the anti-inflammatory properties of KD in rheumatic diseases is still limited. The beneficial effects of weight loss in patients with inflammatory arthritis can be explained by biomechanical and biochemical factors. Obesity is associated with macrophage activation and production of pro-inflammatory cytokines including TNF-α, IL-1b, and IL-6. The clinical effect of KD may be primarily attributed to improvement of insulin sensitivity. Insulin resistance is associated with an increase of TNF-α, IL-1α, IL-1β, IL-6, and leptin. Moreover, reduction of body's adipose tissue and weight loss account for part of the anti-inflammatory effects and for the impact of KD on cardiovascular health. In rheumatoid arthritis, fasting was shown to be effective in reducing disease symptoms, possibly through the production of β-hydroxybutyrate (BHB), the main ketone body. BHB may exert inhibitory effects also on IL-17 and intermittent fasting improved the clinical manifestations of psoriatic arthritis. In ankylosing spondylitis, current literature doesn't allow to draw conclusion about the effects of KD. Future prospective studies will be needed to elucidate the potential beneficial effects of KD on specific domains and clinical outcomes in patients with inflammatory arthritis.
Keywords: ketogenic, diet, inflammatory, arthritis, rheumatoid, psoriatic, ankylosing spondylitis, cardiovascular
Introduction
Ketogenic diet (KD) is characterized by marked carbohydrate restriction, usually to <50 grams a day, and not in a single meal. In a standard KD, carbohydrates should represent about 5–10% of the total daily caloric intake, while the rest of macronutrients consists of proteins (20%) and fats (70–75%) (1). The concept of KD was proposed for the first time in 1921 as a substitute for fasting (2). In the early 20th century, before the introduction of anti-epileptic drugs, fasting was the method of choice to manage epilepsy (3). But fasting, although efficient, cannot be maintained for a long period of time. Therefore, in 1921, Dr. Wilder proposed KD as a suitable method to induce a metabolic state similar to fasting, through the production of ketone bodies, but without caloric restriction. This method was widely used as a treatment for epilepsy during the fourth and fifth decade of 20th century but then dramatically decreased when new anti-epileptic drugs were introduced. KD experienced a reemergence in recent years as a means for weight loss and the physiological concepts behind the dietary regimen gained new scientific interest (3). In the present mini review, we summarize available literature regarding the potential role, pathophysiology and clinical implications of KD in inflammatory arthritis.
Literature review was limited to published primary research, including basic science, cohort studies, intervention and observational trials, and review articles indexed in PubMed.
The following search terms were used: “ketogenic diet” AND “arthritis” OR “rheumatoid arthritis” OR “psoriatic arthritis” OR “ankylosing spondylitis.” As the intent of the review was narrative, inclusion was based on relevance, as deemed so by the authors, to one of the three subcategories of interest: (1) ketogenic diet in rheumatoid arthritis (RA); (2) ketogenic diet in psoriatic arthritis (PsA); (3) ketogenic diet in ankylosing spondylitis (AS). Additionally, articles reporting the effects of KD on cardiovascular health in patients with rheumatic diseases were considered relevant.
Physiological Effects of Ketogenic Diet
The balance between formation (ketogenesis) and degradation (ketolysis) controls circulating levels of ketone bodies, in a process mainly regulated by the secretion of insulin and glucagon (4). Among the important physiological changes induced by KD there is insulin reduction (5) and the hormonal changes caused by KD, with decreased insulin and increased glucagon levels, favor gluconeogenesis. Under conditions of marked carbohydrate restriction, the body primary energy source switches from glucose toward ketones and fatty acids which are obtained from dietary fat and proteins but also from endogenous sources such as glycogen and adipose stores through lipolysis (6). The accelerated mobilization rate of fatty acids from adipose tissue leads to conversion of acetyl-CoA into ketone bodies, in a process known as ketogenesis (7). Ketogenesis takes place primarily in the liver, which can produce ketone bodies in two ways (8). The first way is the oxidation of fatty acids to acetyl-CoA, which are the building blocks of the ketone bodies, or by conversion of amino acids directly into ketone bodies (9). This results in the synthesis of β-hydroxybutyrate (BHB), acetoacetate and acetone. BHB is the primary and most abundant ketone body found in bloodstream. However, the liver cannot use ketone bodies due to the lack of the enzyme succinyl CoA:3-ketoacid CoA transferase. Therefore, ketone bodies are utilized as a fuel by extra-hepatic tissues, thus sparing glucose metabolism. In these circumstances, ketone bodies replace most of the glucose required by the brain, while liver gluconeogenesis provides the limited amount of energy needed by glucose-dependent tissues such as red blood cells, retina and renal medulla (7).
The mean time for achieving nutritional ketosis is generally 3 days, although longer periods up to 10 days have been documented (10). During the first 3 days, the ketosis induction phase, the patient may experience adverse effects including headache, nausea, asthenia, fatigue, constipation. These effects tend to disappear at the end of the induction phase (10). A different type of adverse effects may be seen in long-duration KD, including gastroesophageal reflux disease, uric acid increase, electrolyte imbalance and hyperlipidemia (11).
Clinical Effects of the Ketogenic Diet
Insulin reduction and improvement of insulin sensitivity contribute to the clinical effects of KD. Insulin resistance is an impaired response to insulin stimulation of target tissues, primarily liver, muscle and adipose tissue (12). Insulin signal is conducted through complex intracellular mechanisms regulated by multiple kinase enzymes (13). One of the most prominent applications of KD is for the purpose of weight loss (14, 15) and a meta-analysis of 60 dietary trials of weight loss suggested insulin reduction as a primum movens of weight loss (16). Another clinical manifestation of KD related to glucose metabolism and insulin regulation can be observed in patients diagnosed with glucose transporter 1 (GLUT1) mutation, which have impaired absorption of glucose from the blood, leading to a reduced supply of nutrients to the brain that manifests as epilepsy. In these patients, KD caused a significant improvement of seizure frequency (17). Furthermore, KD has been evaluated as part of cancer treatment protocols in brain, colon, breast, and lung tumors. These studies resulted in beneficial effects when used alongside chemotherapy, radiotherapy, or both (9). Additionally, KD can be used in the setting of Alzheimer's disease, with an increase in cognitive function (18), and therapeutic effects have been proposed also for neurologic conditions characterized by substantial motor dysfunction (19).
Ketogenic Diet and Systemic Inflammation
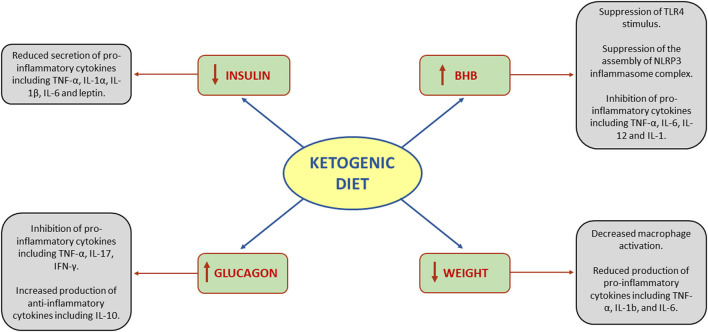
Systemic inflammation is regulated by the production of pro- and anti-inflammatory cytokines. Alterations in the balance of these mediators results in reduction or increase in systemic inflammation (20). The effects of KD on systemic inflammation are related to three main drivers: (1) insulin reduction, (2) BHB synthesis, (3) glucagon increase (21). Additionally, since insulin reduction leads to weight loss, all the anti-inflammatory effects of weight loss should be taken into account as well (Figure 1).
Figure 1.
Effects of ketogenic diet on systemic inflammation. BHB, beta-hydroxybutyrate; IFN-γ, interferon gamma; IL, interleukin; NLRP3, NOD-, LRR-, and pyrin domain-containing protein 3; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor-alpha.
Insulin
Insulin and chronic hyperinsulinemia are associated with an increase of the pro-inflammatory cytokines TNF-α, IL-1α, IL-1β, IL-6, and leptin (22). Leptin is a unique cytokine due to its adipose-derived origin. Adipose tissue is considered not only as the body's energy reservoir, but also as an endocrine tissue that can produce proinflammatory cytokines (23). Furthermore, weight loss is a relevant part of the anti-inflammatory effects of KD and reduction of body's adipose tissue has an influence on the secretion of hormones such as leptin (24).
β-Hydroxybutyrate
BHB has a double effect on NLRP3 inflammasome complex (NIC). NIC is a protein complex involved in monocyte-induced inflammation (25). When activated, NIC functions as an inducer of caspase 1, which cleaves pro-IL-1β. After being cleaved, pro-IL1β becomes functional IL-1β (26). The activation of NIC requires two steps: first the stimulus from Toll-like receptor 4 (TLR4) that promotes the synthesis of NIC proteins, and then a second step which is their assembly. BHB suppresses TLR4 stimulus and the assembly of NIC individual proteins (27). Furthermore, BHB functions as a ligand to hydroxycarboxylic acid receptors (HCAr). HCAr, also known as GPR109a, is a G protein-coupled receptor predominantly expressed in adipose and immune cells (28). Activation of HCAr suppresses pro-inflammatory cytokine production, including TNF-α, IL-6, IL-12, and IL-1 (29).
Glucagon
The increase in glucagon is a direct effect of the decrease in insulin. Nevertheless, glucagon is also a potent hormone that affects many systems, including the immune system (30). Glucagon exerts its effect by activating the cyclic adenosine monophosphate (cAMP) pathway. The effects of cAMP activation vary between tissues and even between cells of the same tissue. In dendritic cells, the increase in cAMP suppresses the release of pro-inflammatory mediators including TNF-α, IL-17, IFN-γ, and promotes anti-inflammatory cytokine production such as IL-10 (31). T cells have shown to reduce proliferation and production of IL-2 (31) and to increase production of IL-4 and IL-5, both interleukins that promote Th2 differentiation (32). In smooth muscle cells, cAMP inhibits proliferation and suppresses the release of cytokines such as IL-1ß and IL-8 (30).
Weight Loss
The beneficial effects of weight reduction can be explained by biomechanical and biochemical issues. Weight loss reduces the load exerted on joints (33). Increased adipose tissue has been associated with local and systemic inflammation (23). Obesity is implicated in macrophage activation and production of pro-inflammatory cytokines including TNF-α, IL-1b, and IL-6 (34). As mentioned previously, insulin reduction precedes weight loss (16). Therefore, insulin reduction can be considered a benefit of weight loss. Part of insulin pro-inflammatory effect is exerted through inhibition of anti-inflammatory cytokines production (35). KD, besides the potential anti-inflammatory properties, is also the best non-surgical treatment for weight reduction. A meta-analysis of 53 studies including 68.128 participants suggested that higher-fat, low-carbohydrate diet, was the best intervention to achieve the weight loss and weight maintenance targets (36). In RA, it is important to distinguish between intentional and unintentional weight loss. Intentional weight loss is beneficial in improving the clinical aspects of RA, while unintentional weight loss can worsen the manifestation of RA (37). A retrospective analysis of electronic medical records of 178 patients diagnosed with RA demonstrated obese and overweight patients who achieved weight reduction exceeding 5 Kg had a three-fold higher probability to experience improvement in RA symptoms in comparison with patients who did not succeed in losing weight (38). Similar results were observed in PsA, where several disease activity parameters improved after weight loss treatment with very low energy diet, in a dose responder manner (39).
Ketogenic Diet in Rheumatoid Arthritis
Several studies have been conducted to investigate the role of dietary interventions in RA (40–44). Available evidence suggests the potentially beneficial effects of anti-inflammatory diets on disease activity (45). Products such as red meat, salt or high-fat diet may trigger inflammation, while fruit, vegetables and fish may exert an anti-inflammatory action (46, 47). Nevertheless, there is no specific dietary recommendation in RA.
A systematic review of 70 dietary studies revealed that fasting, omega 3 and vitamin D3 significantly reduced RA symptoms (48). Fasting and calorie restriction, in particular, were associated with improvement of RA activity, with stronger effects on subjective symptoms (49).
During Ramadan, Muslims refrain from eating and drinking from dawn to sunset. It results in a month of intermittent fasting. Studying the effects of fasting during Ramadan, Su et al. (50) observed non-significantly higher DAS-28 scores before than during Ramadan and significant improvement in morning stiffness and functional disability. Current literature suggests the benefit of fasting in treatment of RA (49–52) and, as previously mentioned, the metabolic state induced by fasting can be considered similar to KD. On this basis, it is conceivable a role for KD in RA but the available knowledge outlines little efficacy (53, 54). Fraser et al. (54) found that fasting, but not KD, significantly decreased serum IL-6 levels and improved disease activity in RA patients. However, in these studies KD was protracted only for 7 days, in order to reproduce the effects of fasting. It has been demonstrated that longer periods are needed to have a response on pain control (55) and to negatively affect oxidative stress (56). It is possible that the tested period of treatment was too short to obtain significant results in RA.
KD may affect RA in several different ways. First, BHB suppresses macrophages and neutrophils' synthesis of IL-1 by inhibiting NIC and thus reducing TNF-α (24, 57). Secondly, BHB has been shown to suppress proinflammatory interleukins including IL-1, IL-12, and IL-6 by activation of HCAr (29). Furthermore, BHB may inhibit the release of IL-1β and IL-18 mediated by NLRP3, contributing to the anti-inflammatory role of KD (58).
Ketogenic Diet in Psoriatic Arthritis
PsA is associated with several metabolic abnormalities. Insulin resistance, hypertension, diabetes, and hyperuricemia are common comorbidities defining the spectrum of the systemic psoriatic disease (59). Literature about the effects of nutritional interventions in PsA is limited (60, 61), with no specific dietary indication for PsA patients. In psoriasis, it has been shown that a ketogenic nutritional regimen led to significant improvement in disease activity indices (62). Through the assessment of nuclear magnetic medicine metabolomic profile, it was also possible to demonstrate a marked amelioration in biochemical parameters indicative of metabolites related to psoriasis (62). It has been suggested that, in psoriatic patients, KD may facilitate weight loss and modulate systemic inflammation resulting in a quick response to systemic therapy (63–65).
We could not retrieve studies about KD in PsA but some information can be derived from a study conducted on PsA patients during the Ramadan fasting (66). Adawi et al. demonstrated that intermittent fasting improved the clinical manifestation of PsA, including PsA disease activity scores, enthesitis and dactylitis. Furthermore, the patients' improvement was independent of changes in the patients' weight (66). PsA has been strongly associated with Th17 and IL-17A increase (67). In addition to the aforementioned KD effects, BHB induces the production of IL-10 via dendritic HCAr, resulting in an inhibitory effect on Th17 (68, 69).
Ketogenic Diet in Ankylosing Spondylitis
Similar to RA and PsA, there is little evidence that specific dietary interventions influence the activity of AS. Macfarlane et al. (70) conducted a systematic review of 16 publications, including 10 full-text articles, to investigate which dietary regimen induced the best clinical results in AS. The authors concluded that reduction in starch intake, exclusion of dairy products, consumption of fish and fish oil or probiotic supplementation did not improve AS symptoms.
Patients affected by AS and, in general, by axial spondyloarthritis (axSpA), are characterized by an increased cardiovascular risk (71–73). Mediterranean diet has been shown to exert a protective role on cardiovascular morbidity (74, 75). When the impact of Mediterranean diet was investigated in axSpA patients, both the subjective perception of pain, acute phase reactants and disease activity improved after 6 months of nutritional intervention (76).
One of the key pathogenetic mechanisms of AS is the impairment of immunomodulatory function of regulatory T cells, resulting in enhanced IL-17 and other pro-inflammatory cytokines production, with proliferation of pro-inflammatory T cell subsets (77). This process leads to inflammation in the enthesis and ileum of patients with active disease (78). Th17 differentiation is facilitated by TGF-β and IL-6, while IL-23 is determinant to stabilize and maintain TH17 activation and secretion of pro-inflammatory cytokines (79).
Interestingly, KD alters the gut microbiome, with ketone bodies directly inhibiting the growth of gut bacteria. Data obtained from mice models suggest that, reducing the colonization levels of gut bacteria, KD may mediate the lack of intestinal Th17 induction (80). KD can thus induce changes in host metabolites. The alteration of gut microbiota may have downstream consequences for immune cells, reducing levels of intestinal TH17 cells (80). Moreover, BHB was shown to affect microbial-mediated immunomodulation in addition to its ability to inhibit the NLRP3 inflammasome with consequent anti-inflammatory effects (80).
Cardiovascular Risk in Rheumatic Diseases and the Effects of Ketogenic Diet
In RA, the risk of cardiovascular diseases has been reported to be higher than in the general population, in particular for stroke, heart failure, myocardial infarction and atrial fibrillation (81–84). Patients with RA also have increased mortality rate independently from the presence of other cardiovascular risk factors (85). Additionally, high prevalence of cardiovascular comorbidities and increased mortality related to cardiovascular diseases has been demonstrated in patients with PsA and AS (71, 86–90). Robust evidence outlines a pivotal role of inflammation and immune system dysregulation in the pathogenesis of atherosclerosis and endothelial damage (91–93). Biologic and non-biologic anti-rheumatic therapies may exert a protective role on cardiovascular outcomes in patients with rheumatic diseases (94) and optimizing the control of disease activity has been associated with reduction of cardiovascular events in RA, PsA and AS (95–98). Assessment and management of cardiovascular risk factors is therefore essential in the follow-up of patients with rheumatic diseases. Although no study analyzed the effects of KD on cardiovascular health of individuals with inflammatory arthritis, the potential applications of KD in modulating cardiovascular risk factors and outcomes have been extensively investigated in non-rheumatic patients. A systematic review and meta-analysis of clinical trials carried out to study the efficacy of low-carbohydrate diet on major cardiovascular risk factors demonstrated significant reduction in body weight, BMI, abdominal circumference, blood pressure, plasma triglycerides, fasting plasma glucose, glycated hemoglobin, plasma insulin and plasma C-reactive protein, along with an increase in HDL-cholesterol (99). An overall beneficial impact of low-carbohydrate diet on cardiovascular health was therefore observed, although a possible duration effect was suggested, with benefits that decrease over time. However, other reviews found controversial results, with no or little difference in changes of cardiovascular risk factors with KD, rising also questions about prolonged adherence to the dietary regimen (14, 100). In conclusion, current literature suggests that KD might be associated with improvement of cardiovascular risk factors, mainly driven by weight loss possibility but further studies are needed to evaluate the long-term effects of KD on cardiovascular outcomes and also to assess which is the optimal macronutrients composition (101).
Discussion
KD is a well-established treatment option used since the 1920s for drug-resistant epilepsy (102). Emerging evidence suggests an adjuvant role of KD in cancer treatment (9, 103) and a possible applicability also in other conditions such as Alzheimer's disease (18) and Parkinson's disease (104). However, the most significant results of KD have been obtained in treating obesity, with robust evidence showing improvement in body weight and reduction in levels of cholesterol, triglycerides and blood glucose (4, 14, 105). Moreover, a role of KD in the reduction of cardiovascular risk has been proposed (99).
Obesity has been proposed as an environmental factor promoting onset and evolution of autoimmune diseases through a direct involvement of adipokines in their pathogenesis (106). Common mechanistic pathways are shared by obesity and rheumatic conditions (107) and the assessment of obesity status and of possible therapeutic interventions is of relevance when establishing a treatment plan for patients with rheumatic and musculoskeletal diseases (108). Extensive experimental and clinical research suggests that being overweight or obese impacts not only disease activity but also several aspects of the life of patients living with inflammatory arthritis (109). Weight loss improves outcomes in patients with RA (37, 38, 44, 49), PsA (39, 61, 64) and AS (110, 111). Besides inflammatory arthritis, metabolic, and eating disorders were suggested to facilitate the occurrence of early clues of connective tissue disorders (112) and obesity was shown to worsen musculoskeletal symptoms also in patients with fibromyalgia (113). Promoting weight loss strategies may be part of the treatment for overweight and obese patients with fibromyalgia but evidence regarding the efficacy of KD in this setting is lacking. Obesity is also a known risk factor for development and progression of osteoarthritis (114). Weight-loss programs were shown to ameliorate osteoarthritis symptoms (115), with exercise and diet therapy leading to significant improvements (116). Low-carbohydrate diet reduced pain intensity in individuals with knee osteoarthritis (117) but, again, the efficacy of a therapeutic KD on different domains of osteoarthritis such as symptoms, pain relief, physical function and health-related quality of life has not been evaluated.
In conclusion, literature about the effects of KD on disease activity and patient reported outcomes in inflammatory arthritis is extremely limited. Evidence derived from fasting studies suggests a mild beneficial effect. Since fasting and KD induce a similar metabolic state, a potential efficacy of KD could be assumed but the available data do not allow to draw conclusions. Future prospective, population-based and adequately powered studies of dietary intervention are required to determine whether KD plays a role in the treatment strategy of patients with rheumatic musculoskeletal diseases.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by progetto 5x1000 anno 2019 (redditi 2018) and project 5x1000 year 2019 (income 2018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a shared affiliation with several of the authors JC, LM, VB, LL, RM, and FU at time of review.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Shilpa J, Mohan V. Ketogenic diets: boon or bane? Indian J Med Res. (2018) 148:251–3. 10.4103/ijmr.IJMR_1666_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JM. Ketogenic diet: Old treatment, new beginning. Clin Neurophysiol Pract. (2017) 2:161–2. 10.1016/j.cnp.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheless JW. History of the ketogenic diet. Epilepsia. (2008) 49(Suppl. 8):3–5. 10.1111/j.1528-1167.2008.01821.x [DOI] [PubMed] [Google Scholar]
- 4.Drabińska N, Wiczkowski W, Piskuła MK. Recent advances in the application of a ketogenic diet for obesity management. Trends Food Sci Technol. (2021) 110:28–38. 10.1016/j.tifs.2021.01.080 [DOI] [Google Scholar]
- 5.Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. (2020) 10:38. 10.1038/s41387-020-00142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahill GF, Jr. Starvation in man. N Engl J Med. (1970) 282:668–75. 10.1056/NEJM197003192821209 [DOI] [PubMed] [Google Scholar]
- 7.Manninen AH. Metabolic effects of the very-low-carbohydrate diets: misunderstood “villains” of human metabolism. J Int Soc Sports Nutr. (2004) 1:7–11. 10.1186/1550-2783-1-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mcgarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. (1980) 49:395–420. 10.1146/annurev.bi.49.070180.002143 [DOI] [PubMed] [Google Scholar]
- 9.Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer - Where do we stand? Mol Metab. (2020) 33:102–21. 10.1016/j.molmet.2019.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey C, Schofield GM, Williden M. The use of nutritional supplements to induce ketosis and reduce symptoms associated with keto-induction: a narrative review. PeerJ. (2018) 6:e4488. 10.7717/peerj.4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergqvist AG. Long-term monitoring of the ketogenic diet: Do's and Don'ts. Epilepsy Res. (2012) 100:261–6. 10.1016/j.eplepsyres.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 12.Freeman AM, Pennings N. Insulin resistance. In: StatPearls. Treasure Island, FL: StatPearls Publishing; (2021). [PubMed] [Google Scholar]
- 13.Tripolino C, Ciaffi J, Pucino V, Ruscitti P, Van Leeuwen N, Borghi C, et al. Insulin signaling in arthritis. Front Immunol. (2021) 12:672519. 10.3389/fimmu.2021.672519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bueno NB, De Melo IS, De Oliveira SL, Da Rocha Ataide T. Very-low-carbohydrate ketogenic diet vs. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. (2013) 110:1178–87. 10.1017/S0007114513000548 [DOI] [PubMed] [Google Scholar]
- 15.Castellana M, Conte E, Cignarelli A, Perrini S, Giustina A, Giovanella L, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. (2020) 21:5–16. 10.1007/s11154-019-09514-y [DOI] [PubMed] [Google Scholar]
- 16.Wiebe N, Ye F, Crumley ET, Bello A, Stenvinkel P, Tonelli M. Temporal associations among body mass index, fasting insulin, and systemic inflammation: a systematic review and meta-analysis. JAMA Netw Open. (2021) 4:e211263. 10.1001/jamanetworkopen.2021.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang M, Park SH, De Vivo DC, Monani UR. Therapeutic strategies for glucose transporter 1 deficiency syndrome. Ann Clin Transl Neurol. (2019) 6:1923–32. 10.1002/acn3.50881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer's disease. Alzheimers Dement (N Y). (2018) 4:28–36. 10.1016/j.trci.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veyrat-Durebex C, Reynier P, Procaccio V, Hergesheimer R, Corcia P, Andres CR, et al. How can a ketogenic diet improve motor function? Front Mol Neurosci. (2018) 11:15. 10.3389/fnmol.2018.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaffer U, Wade RG, Gourlay T. Cytokines in the systemic inflammatory response syndrome: a review. HSR Proc Intensive Care Cardiovasc Anesth. (2010) 2:161–75. [PMC free article] [PubMed] [Google Scholar]
- 21.Dupuis N, Curatolo N, Benoist JF, Auvin S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. (2015) 56:e95–e8. 10.1111/epi.13038 [DOI] [PubMed] [Google Scholar]
- 22.Shi J, Fan J, Su Q, Yang Z. Cytokines and abnormal glucose and lipid metabolism. Front Endocrinol. (2019) 10:703. 10.3389/fendo.2019.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg AH, Scherer PE. Adipose tissue, inflammation, cardiovascular disease. Circ Res. (2005) 96:939–49. 10.1161/01.RES.0000163635.62927.34 [DOI] [PubMed] [Google Scholar]
- 24.Wieser V, Moschen AR, Tilg H. Inflammation, cytokines and insulin resistance: a clinical perspective. Arch Immunol Ther Exp (Warsz). (2013) 61:119–25. 10.1007/s00005-012-0210-1 [DOI] [PubMed] [Google Scholar]
- 25.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. (2019) 20:3328. 10.3390/ijms20133328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao L, Kitani A, Strober W, Fuss IJ. The role of NLRP3 and IL-1β in the pathogenesis of inflammatory bowel disease. Front Immunol. (2018) 9:2566. 10.3389/fimmu.2018.02566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg EL, Asher JL, Molony RD, Shaw AC, Zeiss CJ, Wang C, et al. β-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. (2017) 18:2077–87. 10.1016/j.celrep.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graff EC, Fang H, Wanders D, Judd RL. Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2. Metabolism. (2016) 65:102–13. 10.1016/j.metabol.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 29.Zandi-Nejad K, Takakura A, Jurewicz M, Chandraker AK, Offermanns S, Mount D, et al. The role of HCA2 (GPR109A) in regulating macrophage function. FASEB J. (2013) 27:4366–74. 10.1096/fj.12-223933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Insuela DBR, Carvalho VF. Glucagon and glucagon-like peptide-1 as novel anti-inflammatory and immunomodulatory compounds. Eur J Pharmacol. (2017) 812:64–72. 10.1016/j.ejphar.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 31.Raker VK, Becker C, Steinbrink K. The cAMP pathway as therapeutic target in autoimmune and inflammatory diseases. Front Immunol. (2016) 7:123. 10.3389/fimmu.2016.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacour M, Arrighi J-F, Müller KM, Cariberg C, Saurat JH, Hauser C. cAMP up-regulates IL-4 and IL-5 production from activated CD4+ T cells while decreasing IL-2 release and NF-AT induction. Int Immunol. (1994) 6:1333–43. 10.1093/intimm/6.9.1333 [DOI] [PubMed] [Google Scholar]
- 33.Messier SP, Gutekunst DJ, Davis C, Devita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. (2005) 52:2026–32. 10.1002/art.21139 [DOI] [PubMed] [Google Scholar]
- 34.Ferrante AW, Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. (2007) 262:408–14. 10.1111/j.1365-2796.2007.01852.x [DOI] [PubMed] [Google Scholar]
- 35.Han JM, Patterson SJ, Speck M, Ehses JA, Levings MK. Insulin inhibits IL-10-mediated regulatory T cell function: implications for obesity. J Immunol. (2014) 192:623–9. 10.4049/jimmunol.1302181 [DOI] [PubMed] [Google Scholar]
- 36.Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2015) 3:968–79. 10.1016/S2213-8587(15)00367-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker JF, England BR, Mikuls TR, Sayles H, Cannon GW, Sauer BC, et al. Obesity, weight loss, and progression of disability in rheumatoid arthritis. Arthritis Care Res (Hoboken). (2018) 70:1740–7. 10.1002/acr.23579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreps DJ, Halperin F, Desai SP, Zhang ZZ, Losina E, Olson AT, et al. Association of weight loss with improved disease activity in patients with rheumatoid arthritis: A retrospective analysis using electronic medical record data. Int J Clin Rheumtol. (2018) 13:1–10. 10.4172/1758-4272.1000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klingberg E, Bilberg A, Björkman S, Hedberg M, Jacobsson L, Forsblad-D'elia H, et al. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: an interventional study. Arthritis Res Ther. (2019) 21:17. 10.1186/s13075-019-1810-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haugen M, Kjeldsen-Kragh J, Nordvåg BY, Førre O. Diet and disease symptoms in rheumatic diseases–results of a questionnaire based survey. Clin Rheumatol. (1991) 10:401–7. 10.1007/BF02206660 [DOI] [PubMed] [Google Scholar]
- 41.Kjeldsen-Kragh J, Haugen M, Borchgrevink CF, Laerum E, Eek M, Mowinkel P, et al. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet. (1991) 338:899–902. 10.1016/0140-6736(91)91770-U [DOI] [PubMed] [Google Scholar]
- 42.Hafström I, Ringertz B, Spångberg A, Von Zweigbergk L, Brannemark S, Nylander I, et al. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: the effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology (Oxford). (2001) 40:1175–9. 10.1093/rheumatology/40.10.1175 [DOI] [PubMed] [Google Scholar]
- 43.Tedeschi SK, Frits M, Cui J, Zhang ZZ, Mahmoud T, Iannaccone C, et al. Diet and rheumatoid arthritis symptoms: survey results from a rheumatoid arthritis Registry. Arthritis Care Res (Hoboken). (2017) 69:1920–5. 10.1002/acr.23225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forsyth C, Kouvari M, D'cunha NM, Georgousopoulou EN, Panagiotakos DB, Mellor DD, et al. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: a systematic review of human prospective studies. Rheumatol Int. (2018) 38:737–47. 10.1007/s00296-017-3912-1 [DOI] [PubMed] [Google Scholar]
- 45.Vadell AKE, Bärebring L, Hulander E, Gjertsson I, Lindqvist HM, Winkvist A. Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA)-a randomized, controlled crossover trial indicating effects on disease activity. Am J Clin Nutr. (2020) 111:1203–13. 10.1093/ajcn/nqaa019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. (2014) 14:404. 10.1007/s11882-013-0404-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliviero F, Spinella P, Fiocco U, Ramonda R, Sfriso P, Punzi L. How the Mediterranean diet and some of its components modulate inflammatory pathways in arthritis. Swiss Med Wkly. (2015) 145:w14190. 10.4414/smw.2015.14190 [DOI] [PubMed] [Google Scholar]
- 48.Philippou E, Petersson SD, Rodomar C, Nikiphorou E. Rheumatoid arthritis and dietary interventions: systematic review of clinical trials. Nutr Rev. (2021) 79:410–28. 10.1093/nutrit/nuaa033 [DOI] [PubMed] [Google Scholar]
- 49.Khanna S, Jaiswal KS, Gupta B. Managing rheumatoid arthritis with dietary interventions. Front Nutr. (2017) 4:52. 10.3389/fnut.2017.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su VX, Azahar NA, Jeans Y, Abdullah MNH, Mohamed Said MS, Shaharir SS, et al. AB0244 Retrospective study on effects of ramadhan month fasting on rheumatoid arthritis patients. Ann Rheum Dis. (2013) 72:A861. 10.1136/annrheumdis-2013-eular.256728850992 [DOI] [Google Scholar]
- 51.Udén AM, Trang L, Venizelos N, Palmblad J. Neutrophil functions and clinical performance after total fasting in patients with rheumatoid arthritis. Ann Rheum Dis. (1983) 42:45–51. 10.1136/ard.42.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hafström I, Ringertz B, Gyllenhammar H, Palmblad J, Harms-Ringdahl M. Effects of fasting on disease activity, neutrophil function, fatty acid composition, and leukotriene biosynthesis in patients with rheumatoid arthritis. Arthritis Rheum. (1988) 31:585–92. 10.1002/art.1780310502 [DOI] [PubMed] [Google Scholar]
- 53.Fraser DA, Thoen J, Bondhus S, Haugen M, Reseland JE, Djøseland O, et al. Reduction in serum leptin and IGF-1 but preserved T-lymphocyte numbers and activation after a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. (2000) 18:209–14. [PubMed] [Google Scholar]
- 54.Fraser DA, Thoen J, Djøseland O, Førre O, Kjeldsen-Kragh J. Serum levels of interleukin-6 and dehydroepiandrosterone sulphate in response to either fasting or a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. (2000) 18:357–62. [PubMed] [Google Scholar]
- 55.Ruskin DN, Suter TA, Ross JL, Masino SA. Ketogenic diets and thermal pain: dissociation of hypoalgesia, elevated ketones, and lowered glucose in rats. J Pain. (2013) 14:467–74. 10.1016/j.jpain.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. (2010) 40:238–44. 10.1016/j.nbd.2010.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Longo R, Peri C, Cricrì D, Coppi L, Caruso D, Mitro N, et al. Ketogenic diet: a new light shining on old but gold biochemistry. Nutrients. (2019) 11:2497. 10.3390/nu11102497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tedeschi SK, Costenbader KH. Is there a role for diet in the therapy of rheumatoid arthritis? Curr Rheumatol Rep. (2016) 18:23. 10.1007/s11926-016-0575-y [DOI] [PubMed] [Google Scholar]
- 59.Tripolino C, Ciaffi J, Ruscitti P, Giacomelli R, Meliconi R, Ursini F. Hyperuricemia in psoriatic arthritis: epidemiology, pathophysiology, clinical implications. Front Med. (2021) 8:737573. 10.3389/fmed.2021.737573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ford AR, Siegel M, Bagel J, Cordoro KM, Garg A, Gottlieb A, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. (2018) 154:934–50. 10.1001/jamadermatol.2018.1412 [DOI] [PubMed] [Google Scholar]
- 61.Caso F, Navarini L, Carubbi F, Picchianti-Diamanti A, Chimenti MS, Tasso M, et al. Mediterranean diet and Psoriatic Arthritis activity: a multicenter cross-sectional study. Rheumatol Int. (2020) 40:951–8. 10.1007/s00296-019-04458-7 [DOI] [PubMed] [Google Scholar]
- 62.Castaldo G, Pagano I, Grimaldi M, Marino C, Molettieri P, Santoro A, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. (2021) 20:1509–21. 10.1021/acs.jproteome.0c00646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castaldo G, Galdo G, Rotondi Aufiero F, Cereda E. Very low-calorie ketogenic diet may allow restoring response to systemic therapy in relapsing plaque psoriasis. Obes Res Clin Pract. (2016) 10:348–52. 10.1016/j.orcp.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 64.Barrea L, Megna M, Cacciapuoti S, Frias-Toral E, Fabbrocini G, Savastano S, et al. Very low-calorie ketogenic diet (VLCKD) in patients with psoriasis and obesity: an update for dermatologists and nutritionists. Crit Rev Food Sci Nutr. (2020) 1–17. 10.1080/10408398.2020.1818053 [DOI] [PubMed] [Google Scholar]
- 65.Castaldo G, Rastrelli L, Galdo G, Molettieri P, Rotondi Aufiero F, Cereda E. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. (2020) 74:110757. 10.1016/j.nut.2020.110757 [DOI] [PubMed] [Google Scholar]
- 66.Adawi M, Damiani G, Bragazzi NL, Bridgewood C, Pacifico A, Conic RRZ, et al. The impact of intermittent fasting (ramadan fasting) on psoriatic arthritis disease activity, enthesitis, and dactylitis: a multicentre study. Nutrients. (2019) 11:601. 10.3390/nu11030601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. (2018) 391:2273–84. 10.1016/S0140-6736(18)30830-4 [DOI] [PubMed] [Google Scholar]
- 68.Ni FF, Li CR, Liao JX, Wang GB, Lin SF, Xia Y, et al. The effects of ketogenic diet on the Th17/Treg cells imbalance in patients with intractable childhood epilepsy. Seizure. (2016) 38:17–22. 10.1016/j.seizure.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 69.Offermanns S. Hydroxy-carboxylic acid receptor actions in metabolism. Trends Endocrinol Metab. (2017) 28:227–36. 10.1016/j.tem.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 70.Macfarlane TV, Abbood HM, Pathan E, Gordon K, Hinz J, Macfarlane GJ. Relationship between diet and ankylosing spondylitis: a systematic review. Eur J Rheumatol. (2018) 5:45–52. 10.5152/eurjrheum.2017.16103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Papagoras C, Voulgari PV, Drosos AA. Atherosclerosis and cardiovascular disease in the spondyloarthritides, particularly ankylosing spondylitis and psoriatic arthritis. Clin Exp Rheumatol. (2013) 31:612–20. [PubMed] [Google Scholar]
- 72.Papagoras C, Markatseli TE, Saougou I, Alamanos Y, Zikou AK, Voulgari PV, et al. Cardiovascular risk profile in patients with spondyloarthritis. Joint Bone Spine. (2014) 81:57–63. 10.1016/j.jbspin.2013.03.019 [DOI] [PubMed] [Google Scholar]
- 73.Ciaffi J, Mele G, Mancarella L, Brusi V, Lisi L, Faranda Cordella J, et al. Prevalence of type 2 and type 1 diabetes in psoriatic arthritis: an Italian Study. JCR J Clin Rheumatol. (2021). 10.1097/RHU.0000000000001706 [DOI] [PubMed] [Google Scholar]
- 74.Sleiman D, Al-Badri MR, Azar ST. Effect of Mediterranean diet in diabetes control and cardiovascular risk modification: a systematic review. Front Public Health. (2015) 3:69. 10.3389/fpubh.2015.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asamudo EU, Okolo CA. Book review: the prevention of cardiovascular disease through the mediterranean diet. Front Physiol. (2019) 10:52. 10.3389/fphys.2019.00052 [DOI] [Google Scholar]
- 76.Ometto F, Ortolan A, Farber D, Lorenzin M, Dellamaria G, Cozzi G, et al. Mediterranean diet in axial spondyloarthritis: an observational study in an Italian monocentric cohort. Arthritis Res Ther. (2021) 23:219. 10.1186/s13075-021-02600-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends Immunol. (2011) 32:603–11. 10.1016/j.it.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mcginty J, Brittain N, Kenna TJ. Looking beyond Th17 cells: a role for Tr1 cells in ankylosing spondylitis? Front Immunol. (2020) 11:608900. 10.3389/fimmu.2020.608900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jain R, Chen Y, Kanno Y, Joyce-Shaikh B, Vahedi G, Hirahara K, et al. Interleukin-23-induced transcription factor blimp-1 promotes pathogenicity of T helper 17 cells. Immunity. (2016) 44:131–42. 10.1016/j.immuni.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. (2020) 181:1263–75.e1216. 10.1016/j.cell.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jagpal A, Navarro-Millán I. Cardiovascular co-morbidity in patients with rheumatoid arthritis: a narrative review of risk factors, cardiovascular risk assessment and treatment. BMC Rheumatol. (2018) 2:10. 10.1186/s41927-018-0014-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Semb AG, Ikdahl E, Wibetoe G, Crowson C, Rollefstad S. Atherosclerotic cardiovascular disease prevention in rheumatoid arthritis. Nat Rev Rheumatol. (2020) 16:361–79. 10.1038/s41584-020-0428-y [DOI] [PubMed] [Google Scholar]
- 83.Valero-Jaimes JA, López-González R, Martín-Martínez MA, García-Gómez C, Sánchez-Alonso F, Sánchez-Costa JT, et al. Body mass index and disease activity in chronic inflammatory rheumatic diseases: results of the cardiovascular in rheumatology (Carma) project. J Clin Med. (2021) 10:382. 10.3390/jcm10030382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lo Gullo A, Rodríguez-Carrio J, Aragona CO, Dattilo G, Zito C, Suárez A, et al. Subclinical impairment of myocardial and endothelial functionality in very early psoriatic and rheumatoid arthritis patients: association with vitamin D and inflammation. Atherosclerosis. (2018) 271:214–22. 10.1016/j.atherosclerosis.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 85.Argnani L, Zanetti A, Carrara G, Silvagni E, Guerrini G, Zambon A, et al. Rheumatoid arthritis and cardiovascular risk: retrospective matched-cohort analysis based on the RECORD study of the Italian Society for Rheumatology. Front Med. (2021) 8:745601. 10.3389/fmed.2021.745601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han C, Robinson DW Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV, et al. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis. J Rheumatol. (2006) 33:2167–72. [PubMed] [Google Scholar]
- 87.Jamnitski A, Symmons D, Peters MJ, Sattar N, Mcinnes I, Nurmohamed MT. Cardiovascular comorbidities in patients with psoriatic arthritis: a systematic review. Ann Rheum Dis. (2013) 72:211–6. 10.1136/annrheumdis-2011-201194 [DOI] [PubMed] [Google Scholar]
- 88.Haroon NN, Paterson JM, Li P, Inman RD, Haroon N. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann Intern Med. (2015) 163:409–16. 10.7326/M14-2470 [DOI] [PubMed] [Google Scholar]
- 89.Kibari A, Cohen AD, Gazitt T, Bitterman H, Lavi I, Feldhamer I, et al. Cardiac and cardiovascular morbidities in patients with psoriatic arthritis: a population-based case control study. Clin Rheumatol. (2019) 38:2069–75. 10.1007/s10067-019-04528-y [DOI] [PubMed] [Google Scholar]
- 90.Verhoeven F, Prati C, Demougeot C, Wendling D. Cardiovascular risk in psoriatic arthritis, a narrative review. Joint Bone Spine. (2020) 87:413–8. 10.1016/j.jbspin.2019.12.004 [DOI] [PubMed] [Google Scholar]
- 91.Bordy R, Totoson P, Prati C, Marie C, Wendling D, Demougeot C. Microvascular endothelial dysfunction in rheumatoid arthritis. Nat Rev Rheumatol. (2018) 14:404–20. 10.1038/s41584-018-0022-8 [DOI] [PubMed] [Google Scholar]
- 92.Crowson CS, Rollefstad S, Ikdahl E, Kitas GD, Van Riel P, Gabriel SE, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. (2018) 77:48–54. 10.1136/annrheumdis-2017-211735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lo Gullo A, Aragona CO, Scuruchi M, Versace AG, Saitta A, Imbalzano E, et al. Endothelial progenitor cells and rheumatic disease modifying therapy. Vascul Pharmacol. (2018) 108:8–14. 10.1016/j.vph.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 94.Bartoloni E, Alunno A, Valentini V, Luccioli F, Valentini E, La Paglia GMC, et al. Targeting inflammation to prevent cardiovascular disease in chronic rheumatic diseases: myth or reality? Front Cardiovasc Med. (2018) 5:177. 10.3389/fcvm.2018.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roubille C, Richer V, Starnino T, Mccourt C, Mcfarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. (2015) 74:480–9. 10.1136/annrheumdis-2014-206624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruscitti P, Cipriani P, Masedu F, Romano S, Berardicurti O, Liakouli V, et al. Increased cardiovascular events and subclinical atherosclerosis in rheumatoid arthritis patients: 1 year prospective single centre study. PLoS One. (2017) 12:e0170108. 10.1371/journal.pone.0170108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ursini F, Leporini C, Bene F, D'angelo S, Mauro D, Russo E, et al. Anti-TNF-alpha agents and endothelial function in rheumatoid arthritis: a systematic review and meta-analysis. Sci Rep. (2017) 7:5346. 10.1038/s41598-017-05759-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Atzeni F, Nucera V, Galloway J, Zoltán S, Nurmohamed M. Cardiovascular risk in ankylosing spondylitis and the effect of anti-TNF drugs: a narrative review. Expert Opin Biol Ther. (2020) 20:517–24. 10.1080/14712598.2020.1704727 [DOI] [PubMed] [Google Scholar]
- 99.Santos FL, Esteves SS, Da Costa Pereira A, Yancy WS Jr, Nunes JPL. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. (2012) 13:1048–66. 10.1111/j.1467-789X.2012.01021.x [DOI] [PubMed] [Google Scholar]
- 100.Naude CE, Schoonees A, Senekal M, Young T, Garner P, Volmink J. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: a systematic review and meta-analysis. PLoS One. (2014) 9:e100652. 10.1371/journal.pone.0100652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kosinski C, Jornayvaz FR. Effects of ketogenic diets on cardiovascular risk factors: evidence from animal and human studies. Nutrients. (2017) 9:517. 10.3390/nu9050517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D'Andrea Meira I, Romão TT, Pires Do Prado HJ, Krüger LT, Pires MEP, Da Conceição PO. Ketogenic diet and epilepsy: what we know so far. Front Neurosci. (2019) 13:5. 10.3389/fnins.2019.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li J, Zhang H, Dai Z. Cancer treatment with the ketogenic diet: a systematic review and meta-analysis of animal studies. Front Nutr. (2021) 8:594408. 10.3389/fnut.2021.594408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP. Low-fat versus ketogenic diet in Parkinson's disease: a pilot randomized controlled trial. Mov Disord. (2018) 33:1306–14. 10.1002/mds.27390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dashti HM, Mathew TC, Hussein T, Asfar SK, Behbahani A, Khoursheed MA, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. (2004) 9:200–5. [PMC free article] [PubMed] [Google Scholar]
- 106.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. (2014) 13:981–1000. 10.1016/j.autrev.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 107.Nikiphorou E, Fragoulis GE. Inflammation, obesity and rheumatic disease: common mechanistic links. A narrative review. Ther Adv Musculoskelet Dis. (2018) 10:157–67. 10.1177/1759720X18783894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ciaffi J, Gigliotti PE, Festuccia G, Borlandelli E, Facchini G, Chiaravalloti A, et al. Can chest imaging be used to draw information about body mass index and obesity status? Obes Res Clin Pract. (2021) 15:187–90. 10.1016/j.orcp.2021.01.006 [DOI] [PubMed] [Google Scholar]
- 109.Ciaffi J, Brusi V, Lisi L, Mancarella L, D'onghia M, Quaranta E, et al. Living with arthritis: a “training camp” for coping with stressful events? A survey on resilience of arthritis patients following the COVID-19 pandemic. Clin Rheumatol. (2020) 39:3163–70. 10.1007/s10067-020-05411-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen C-H, Chen H-A, Liu C-H, Liao H-T, Chou C-T, Chen C-H. Association of obesity with inflammation, disease severity and cardiovascular risk factors among patients with ankylosing spondylitis. Int J Rheum Dis. (2020) 23:1165–74. 10.1111/1756-185X.13912 [DOI] [PubMed] [Google Scholar]
- 111.Liew JW, Gianfrancesco MA, Heckbert SR, Gensler LS. The relationship between body mass index, disease activity, and exercise in ankylosing spondylitis. Arthritis Care Res (Hoboken). (2021). 10.1002/acr.24565 [DOI] [PubMed] [Google Scholar]
- 112.Ciaffi J, Ajasllari N, Mancarella L, Brusi V, Meliconi R, Ursini F. Nailfold capillaroscopy in common non-rheumatic conditions: a systematic review and applications for clinical practice. Microvasc Res. (2020) 131:104036. 10.1016/j.mvr.2020.104036 [DOI] [PubMed] [Google Scholar]
- 113.D'Onghia M, Ciaffi J, Lisi L, Mancarella L, Ricci S, Stefanelli N, et al. Fibromyalgia and obesity: a comprehensive systematic review and meta-analysis. Semin Arthritis Rheum. (2021) 51:409–424. 10.1016/j.semarthrit.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 114.Lementowski PW, Zelicof SB. Obesity and osteoarthritis. Am J Orthop (Belle Mead NJ). (2008) 37:148–51. 10.2519/jospt.2007.37.3.86 [DOI] [PubMed] [Google Scholar]
- 115.Riecke BF, Christensen R, Christensen P, Leeds AR, Boesen M, Lohmander LS, et al. Comparing two low-energy diets for the treatment of knee osteoarthritis symptoms in obese patients: a pragmatic randomized clinical trial. Osteoarthritis Cartilage. (2010) 18:746–54. 10.1016/j.joca.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 116.Bliddal H, Leeds AR, Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons - a scoping review. Obes Rev. (2014) 15:578–86. 10.1111/obr.12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Strath LJ, Jones CD, Philip George A, Lukens SL, Morrison SA, Soleymani T, et al. The effect of low-carbohydrate and low-fat diets on pain in individuals with knee osteoarthritis. Pain Med. (2020) 21:150–60. 10.1093/pm/pnz022 [DOI] [PMC free article] [PubMed] [Google Scholar]