Abstract
Aims
This study was performed to investigate whether left atrial (LA) strain by echocardiography provides prognostic information in patients with wild‐type transthyretin amyloid cardiomyopathy (ATTRwt‐CM).
Methods and results
Among 129 patients who were diagnosed with ATTRwt‐CM at Kumamoto University Hospital from December 2002 to December 2019, 113 patients who had enough information for two‐dimensional speckle tracking echocardiography were enrolled in this study. During a median follow‐up of 668 days, 28 cardiovascular deaths occurred. Compared with patients in the non‐event group, those in the cardiovascular death group were significantly older (81.5 ± 7.4 vs. 78.1 ± 6.1 years, P < 0.01), had a lower incidence of carpal tunnel syndrome (21% vs. 47%, P < 0.05), and had a higher high‐sensitivity cardiac troponin T [0.085 (0.063–0.105) vs. 0.049 (0.036–0.079) ng/mL, P < 0.01] and B‐type natriuretic peptide concentrations [419 (239–541) vs. 271 (155–462) pg/mL, P < 0.01] and lower estimated glomerular filtration rate (41.8 ± 15.4 vs. 53.4 ± 14.6 mL/min/1.73 m2, P < 0.01). Electrocardiography showed higher rate of a V1–V3 QS pattern (52% vs. 24%, P < 0.01) and complete left bundle branch block (27% vs. 6%, P < 0.01), and echocardiography showed a significantly lower peak LA strain rate during the contraction phase (0.16 ± 0.13 vs. 0.28 ± 0.27 S−1, P < 0.05), LA strain during the reservoir phase (LASr) (5.84 ± 2.41 vs. 8.22 ± 4.05%, P < 0.01), and peak LA strain rate during the reservoir phase (0.26 ± 0.09 vs. 0.33 ± 0.15 S−1, P < 0.05) in the cardiovascular death group than in non‐event group. By contrast, conventional echocardiographic findings were not significantly different between these two groups. After adjusting for conventional predictive factors of ATTRwt‐CM (age, high‐sensitivity cardiac troponin T and B‐type natriuretic peptide concentrations, and estimated glomerular filtration rate), multivariable Cox proportional hazard analyses showed that LASr was significantly and independently associated with cardiovascular death in patients with ATTRwt‐CM (odds ratio, 0.84; 95% confidence interval, 0.72–0.98; P < 0.05). After adjusting for age and echocardiographic findings associated with cardiovascular death (LA volume index and peak LA strain rate during the contraction phase), LASr was significantly and independently associated with cardiovascular death in patients with ATTRwt‐CM (odds ratio, 0.83; 95% confidence interval, 0.70–0.98; P < 0.05). Receiver operating characteristic curve analysis showed that the area under the curve of LASr for cardiovascular death was 0.686 and that the best cut‐off value of LASr was 6.69% (sensitivity, 62.4%; specificity, 64.3%). In the Kaplan–Meier analysis, patients with low LASr (<6.69%) had a significantly higher probability of total cardiovascular death (P < 0.05) and heart failure‐related hospitalization (P < 0.05).
Conclusions
Left atrial strain during the reservoir phase provides significant prognostic value in patients with ATTRwt‐CM even after adjusting for conventional predictive factors.
Keywords: Wild‐type transthyretin amyloid cardiomyopathy, Left atrial strain during reservoir phase, Echocardiography, Two‐dimensional speckle tracking echocardiography
Introduction
Amyloid cardiomyopathy is a progressive infiltrative cardiomyopathy characterized by restrictive cardiomyopathy and various arrhythmias secondary to infiltration of the conduction system. 1 The two main types of amyloid cardiomyopathy are amyloid light‐chain amyloidosis and transthyretin (TTR) amyloidosis (ATTR). ATTR is further classified into two subtypes based on the presence or absence of a genetic mutation: mutant ATTR and wild‐type ATTR (ATTRwt). Wild‐type transthyretin amyloid cardiomyopathy (ATTRwt‐CM) is becoming increasingly recognized because of population ageing, advancements in the understanding of the disease pathobiology, and the potential benefits of emerging therapies. 2 Because ATTRwt‐CM causes repeated heart failure and cardiovascular death, 3 identification of vulnerable patients with ATTRwt‐CM at high risk of cardiovascular death is of clinical importance. Several studies have shown that the high‐sensitivity cardiac troponin T (hs‐cTnT) concentration, B‐type natriuretic peptide (BNP) concentration, and estimated glomerular filtration rate (eGFR) are useful prognostic biomarkers in patients with ATTRwt‐CM. 4 , 5 Although the hallmark of amyloid cardiomyopathy on transthoracic echocardiography is generally increased left ventricular (LV) wall thickness, 6 previous studies have demonstrated that conventional echocardiographic findings, including LV wall thickness, were not independent predictors of survival in patients with ATTR‐CM. 4 , 5 Because amyloid can infiltrate to virtually all cardiac chambers, left atrial (LA) enlargement is a common finding in patients with amyloid cardiomyopathy. 7 Two‐dimensional speckle tracking echocardiography is a robust and sensitive technique for quantitative assessment of LA function and has been proven to play an adjunctive role in the diagnosis of amyloid cardiomyopathy. 8 However, few reports have focused on the usefulness of LA function estimated by two‐dimensional speckle tracking echocardiography to predict cardiovascular death in patients with ATTRwt‐CM. Thus, the present study was performed to evaluate the utility of LA function measurement using two‐dimensional speckle tracking echocardiography to predict cardiovascular events in patients with ATTRwt‐CM.
Methods
Study population
In total, 129 patients were diagnosed with ATTRwt‐CM at Kumamoto University Hospital from December 2002 to December 2019. Of these patients, 16 were excluded because they had no transthoracic echocardiography data at diagnosis or had insufficient information for evaluation by two‐dimensional speckle tracking echocardiography (Figure 1 ). The remaining 113 patients diagnosed with ATTRwt‐CM were enrolled in this study. Baseline clinical characteristics and electrocardiographic and echocardiographic data at diagnosis were obtained while the patients were in a clinically stable condition.
Figure 1.
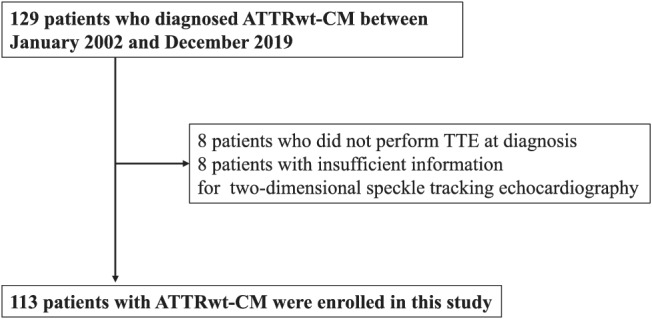
Study flow chart detailing the inclusion and exclusion criteria for the study patients. The enrolled patients were divided into two groups based on the presence or absence of cardiovascular death. ATTRwt‐CM, wild‐type transthyretin amyloid cardiomyopathy; TTE, transthoracic echocardiography.
This study conformed to the principles outlined in the Declaration of Helsinki. It was approved by the institutional review board and ethics committees of Kumamoto University (No. 1588). The requirement for informed consent was waived because of the low‐risk nature of this retrospective study and the inability to obtain consent directly from all patients. Instead, we extensively announced this study protocol at Kumamoto University Hospital and on our website (http://www2.kuh.kumamoto‐u.ac.jp/tyuokensabu/index.html) and gave patients the opportunity to withdraw from the study.
Diagnosis of wild‐type transthyretin amyloid cardiomyopathy
Precise diagnosis of amyloid cardiomyopathy required proof of amyloid deposition by tissue biopsy. In these days, however, non‐biopsy diagnosis of ATTR‐CM by using bone scintigraphy including 99mTc‐labelled pyrophosphate scintigraphy (99mTc‐PYP) has been established. 9 Therefore, modified Kumamoto Criteria are used to raise the pretest probability of ATTR‐CM by using inexpensive tests such as laboratory examinations, electrocardiography, and transthoracic echocardiography, followed by 99mTc‐PYP is performed for probable diagnosis of ATTRwt‐CM in Kumamoto University Hospital (Supporting Information, Figure S1 ). 10 For many patients with 99mTc‐PYP positivity, tissue biopsy is performed for definite diagnosis of ATTRwt‐CM.
The diagnosis of amyloid deposition was based on Congo red staining and apple‐green birefringence with cross‐polarized light microscopy. To confirm TTR amyloid deposition, we performed immunohistochemical staining using antibodies that react to TTR. We diagnosed ATTRwt when no mutation in the TTR gene was revealed by genetic testing (n = 84, 74%) or, if genetic testing was not performed, when the patient had no family history of amyloidosis (n = 29, 26%).
Finally, ATTR‐CM was diagnosed by (i) the presence of TTR deposition in the myocardium (n = 56, 50%), (ii) the presence of TTR deposition in extracardiac tissue with a positive finding on 99mTc‐labelled pyrophosphate scintigraphy (n = 18, 16%), or (iii) a positive finding on 99mTc‐labelled pyrophosphate scintigraphy without confirmation of pathological TTR deposition and exclusion of amyloid light‐chain amyloidosis (n = 39, 35%).
Conventional echocardiographic parameters
Conventional echocardiography was performed in patients with ATTR‐CM in stable condition using the Vivid E95 or 7 (GE Vingmed, Horten, Norway), Aplio 500 (Toshiba, Tokyo, Japan), and EPIQ 7G (Philips, Bothell, WA, USA), which were equipped with a 2.5 MHz phased‐array transducer. The chamber size, LV wall thickness, LV ejection fraction, LA volume index (LAVI), and rate between peak early diastolic velocity of LV inflow (E velocity) and peak early diastolic velocity on the septal corner of the mitral annulus (e′) (E/e′ ratio) were evaluated using standard procedures. 11 , 12 The peak early and late diastolic velocity of LV inflow (E and A velocity, respectively) and the peak systolic, early, and late diastolic velocity on the septal corner of the mitral annulus (s′, e′, and a′, respectively) were measured in the apical four‐chamber view. To minimize bias, the echocardiography reviewers were blinded to the patients' clinical history and data.
Two‐dimensional speckle tracking echocardiography
Two‐dimensional speckle tracking echocardiography was performed by one operator who was blinded to the clinical data and different from the operator who performed the conventional echocardiography. Two‐dimensional speckle tracking echocardiography was performed using a vendor‐independent software program for two‐dimensional strain analysis (TOMTEC Imaging Systems, Unterschleißheim, Germany). LV strain was assessed as follows. The regional longitudinal strain (LS), calculated from the echocardiography images in the four‐chamber, three‐chamber, and two‐chamber apical views, was determined in 16 segments of the left ventricle in accordance with the American Society for Echocardiography guidelines. 11 The LV global LS was calculated as the average LS of these 16 segments, and the relative apical LS index was estimated as the hallmark of cardiac amyloidosis on echocardiography. 13 This index was calculated as [average apical LS∕(average basal LS + average mid LS)]. To assess LA strain, the regional strain and strain rate were determined in three segments (septal, roof, and lateral) obtained from echocardiographic images in the four‐chamber apical view. 14 To evaluate LA strain components, the zero‐strain reference was defined at end‐diastole in the present study. LA reservoir function was estimated using LA strain during the reservoir phase (LASr) and the peak LA strain rate during the reservoir phase (pLASRr) during the ventricular systole phase, which represents LA filling during LV systole (Figure 2A and 2B ). LA conduit function was estimated using the peak LA strain rate during the conduit phase (pLASRcd) during the LV diastole phase (Figure 2B ). In contrast, LA pump function was estimated using the peak LA strain rate during the contraction phase (pLASRct) during the LV diastole phase (Figure 2B ). 8 , 14 Strain and strain rate are described in absolute values. Analysis of intraobserver and interobserver variability among 20 reassessed patients showed good correlations for LA strain measurements; the average intraclass correlation coefficient and 95% confidence interval (CI) were 0.97 (0.93–0.99) and 0.91 (0.79–0.96), respectively.
Figure 2.
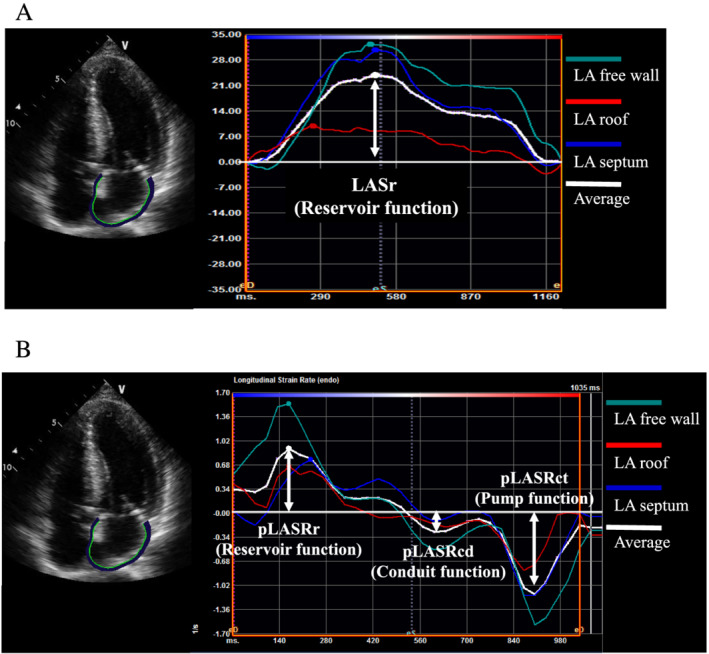
Representative example of left atrial (LA) measurement in a patient with wild‐type transthyretin amyloid cardiomyopathy. (A) LA strain during reservoir phase (LASr). (B) Peak strain rate during reservoir phase (pLASRr), peak strain rate during conduit phase (pLASRcd), and peak strain rate during contraction phase (pLASRct).
Follow‐up and prognosis of patients with wild‐type transthyretin amyloid cardiomyopathy
Patient mortality was identified by a search of the medical records and confirmed by a questionnaire and direct contact via a telephone interview of the patient or a family member. All deaths were reviewed and divided into cardiovascular or non‐cardiovascular death. Cardiovascular death was defined as death due to worsening heart failure, a cardiovascular event, or sudden death. Non‐cardiovascular death was defined as death attributable to a non‐cardiac cause.
Statistical analysis
The cut‐off date for data collection was December 2020. Continuous variables are presented as mean ± standard deviation. Non‐normally distributed variables are presented as median (inter‐quartile range). Categorical values are presented as number (percentage). The clinical characteristics were compared between the cardiovascular death group and non‐event group using the Mann–Whitney U test or χ 2 test. We measured the degree of association between LASr and other echocardiographic findings by means of the correlation coefficient using Pearson's method. Univariate and multivariable Cox proportional hazard analyses were performed to identify the independent parameters related to cardiovascular death. The hs‐cTnT and BNP concentrations were not normally distributed, so we selected the natural logarithm (ln) TnT and ln BNP concentrations for Cox proportional hazard analyses. Variables with a P‐value of <0.05 in the univariate Cox hazard analyses model were incorporated into the multivariable Cox hazard analysis. Age, hs‐cTnT concentration, BNP concentration, and eGFR are conventional prognostic factors in patients with ATTR‐CM. 4 , 15 Therefore, these variables and LASr were forced into multivariable Model 1. Age and three echocardiographic findings (LAVI, pLASRct, and LASr) were forced into multivariable Model 2. Receiver operating characteristic (ROC) curves were constructed, and the area under the curve was calculated to assess the ability of LASr to predict cardiovascular death and determine the cut‐off value of LASr for predicting cardiovascular death. Kaplan–Meier analysis was used to determine the cumulative incidence of cardiovascular death and hospitalization for heart failure, and the log‐rank test was used to compare the incidence of cardiovascular death and hospitalization for heart failure between the high and low LASr groups. All analyses were conducted with SPSS for Windows software, Version 24.0 (IBM Corp., Armonk, NY, USA). Statistical significance was defined as P < 0.05.
Results
Clinical characteristics of patients with wild‐type transthyretin amyloid cardiomyopathy in cardiovascular death and non‐event groups
2During a median follow‐up of 668 days (25–75th percentile, 410–1136 days), 28 cardiovascular deaths (heart failure, n = 27; out‐of‐hospital sudden death, n = 1), 46 heart failure events, and 3 cerebrovascular events occurred. Table 1 shows the baseline clinical characteristics, electrocardiographic and echocardiographic findings, and treatments of all patients. Compared with patients in the non‐event group, those in the cardiovascular death group were significantly older (81.5 ± 7.4 vs. 78.1 ± 6.1 years, P < 0.01), had a lower incidence of carpal tunnel syndrome (21% vs. 47%, P < 0.05), and had a higher hs‐cTnT concentration [0.085 (0.063–0.105) vs. 0.049 (0.036–0.079) ng/mL, P < 0.01] and BNP concentration [419 (239–541) vs. 271 (155–462) pg/mL, P < 0.01] and lower eGFR (41.8 ± 15.4 vs. 53.4 ± 14.6 mL/min/1.73 m2, P < 0.01) (Table 1 ). Electrocardiography showed that the rate of a V1–3 QS pattern (52% vs. 24%, P < 0.01) and complete left bundle branch block (27% vs. 6%, P < 0.01) were significantly higher in the cardiovascular death group than non‐event group. Echocardiography showed that the rate of pLASRct (0.16 ± 0.13 vs. 0.28 ± 0.27 S−1, P < 0.05), LASr (5.84 ± 2.41 vs. 8.22 ± 4.05%, P < 0.01), and pLASRr (0.26 ± 0.09 vs. 0.33 ± 0.15 S−1, P < 0.05) were significantly lower and that the rate of mitral regurgitation (32% vs. 12%, P < 0.05) was significantly higher in the cardiovascular death group than non‐event group. Among the treatment regimens, only the rate of diuretics usage (86% vs. 64%, P < 0.05) was significantly higher in the cardiovascular death group than non‐event group.
Table 1.
Baseline clinical characteristics, electrocardiographic findings, echocardiographic findings, and treatment of ATTRwt‐CM patients in this study
| Cardiovascular death group (n = 28) | Non‐event group (n = 85) | P‐value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age at diagnosis (years) | 81.5 ± 7.4 | 78.1 ± 6.1 | <0.01 |
| Male sex, n (%) | 24 (86) | 70 (84) | 0.78 |
| Body mass index (kg/m2) | 23.8 ± 7.6 | 23.0 ± 3.0 | 0.68 |
| Systolic blood pressure (mmHg) | 114.2 ± 19.0 | 120.0 ± 17.9 | 0.33 |
| Diastolic blood pressure (mmHg) | 65.1 ± 13.7 | 70.3 ± 13.4 | 0.14 |
| Medical history | |||
| Hypertension, n (%) | 13 (46) | 51 (60) | 0.21 |
| Diabetes mellitus, n (%) | 4 (14) | 22 (26) | 0.21 |
| Dyslipidaemia, n (%) | 7 (25) | 31 (36) | 0.27 |
| Prior myocardial infarction, n (%) | 1 (4) | 3 (4) | 0.99 |
| Atrial fibrillation, n (%) | 15 (54) | 37 (44) | 0.36 |
| Carpal tunnel syndrome, n (%) | 6 (21) | 40 (47) | <0.05 |
| Laboratory findings | |||
| hs‐cTnT (ng/mL) | 0.085 (0.063–0.105) | 0.049 (0.036–0.079) | <0.01 |
| BNP (pg/mL) | 419 (239–541) | 271 (155–462) | <0.05 |
| eGFR (mL/min/1.73 m2) | 41.8 ± 15.4 | 53.4 ± 14.6 | <0.01 |
| Electrocardiographic findings | |||
| Pacing rhythm, n (%) | 2 (7) | 5 (6) | 0.81 |
| V1–V3 QS pattern, n (%) | 14 (52) | 20 (24) | <0.01 |
| Low voltage, n (%) | 13 (50) | 25 (31) | 0.08 |
| CLBBB, n (%) | 7 (27) | 5 (6) | <0.01 |
| CRBBB, n (%) | 7 (27) | 18 (22) | 0.60 |
| Echocardiographic findings | |||
| LAVI (mL/m2) | 73.5 ± 38.8 | 62.9 ± 19.8 | 0.42 |
| IVSTd (mm) | 16.5 ± 3.1 | 15.3 ± 2.4 | 0.10 |
| LVPWTd (mm) | 15.9 ± 2.9 | 15.5 ± 2.8 | 0.43 |
| LVEF (%) | 49.5 ± 11.0 | 52.5 ± 10.5 | 0.15 |
| E/A ratio | 1.76 ± 0.95 | 1.73 ± 1.37 | 0.48 |
| E‐wave velocity (cm/s) | 74.0 ± 18.8 | 78.9 ± 22.5 | 0.40 |
| A‐wave velocity (cm/s) | 48.2 ± 22.9 (n = 14) | 58.8 ± 29.3 (n = 49) | 0.18 |
| e′ velocity (cm/s) | 3.91 ± 1.14 | 4.01 ± 1.22 | 0.77 |
| a′ velocity (cm/s) | 4.30 ± 1.56 (n = 14) | 4.99 ± 2.22 (n = 49) | 0.37 |
| s′ velocity (cm/s) | 4.00 ± 1.44 | 4.24 ± 1.28 | 0.23 |
| E/e′ ratio | 21.2 ± 8.2 | 20.5 ± 6.9 | 0.65 |
| Aortic stenosis, n (%) | 1 (4) | 10 (12) | 0.21 |
| Mitral regurgitation, n (%) | 9 (32) | 10 (12) | <0.05 |
| Tricuspid regurgitation, n (%) | 8 (29) | 16 (19) | 0.27 |
| TRPG (mmHg) | 28.7 ± 9.7 | 27.7 ± 10.0 | 0.65 |
| Pericardial effusion, n (%) | 10 (36) | 24 (28) | 0.45 |
| LV‐GLS (%) | 10.0 ± 2.9 | 10.5 ± 3.2 | 0.31 |
| RapLSI | 1.23 ± 0.64 | 1.03 ± 0.39 | 0.21 |
| LASr (%) | 5.84 ± 2.41 | 8.22 ± 4.05 | <0.01 |
| pLASRr (S−1) | 0.26 ± 0.09 | 0.33 ± 0.15 | <0.05 |
| pLASRcd (S−1) | 0.30 ± 0.18 (n = 14) | 0.34 ± 0.18 (n = 49) | 0.30 |
| pLASRct (S−1) | 0.16 ± 0.13 (n = 14) | 0.28 ± 0.27 (n = 49) | <0.05 |
| Treatments | |||
| ACEI or ARB, n (%) | 13 (46) | 40 (47) | 0.95 |
| MRA, n (%) | 8 (29) | 29 (34) | 0.59 |
| Beta‐blocker, n (%) | 7 (25) | 26 (31) | 0.57 |
| Diuretics, n (%) | 24 (86) | 54 (64) | <0.05 |
| PMI, n (%) | 3 (11) | 9 (11) | 0.99 |
| ICD, n (%) | 3 (11) | 8 (9) | 0.84 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ATTRwt‐CM, wild‐type transthyretin amyloid cardiomyopathy; BNP, B‐type natriuretic peptide; CLBBB, complete left bundle branch block; CRBBB, complete right bundle branch block; eGFR, estimated glomerular filtration rate; hs‐cTnT, high‐sensitivity cardiac troponin T; ICD, implantable cardioverter defibrillator; IVSTd, interventricular septal thickness in diastole; LASr, left atrial strain during reservoir phase; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LV‐GLS, left ventricular global longitudinal strain; LVPWTd, left ventricular posterior wall thickness in diastole; MRA, mineralocorticoid receptor antagonist; pLASRcd, peak strain rate during conduit phase; pLASRct, peak strain rate during contraction phase; pLASRr, peak strain rate during reservoir phase; PMI, pacemaker implantation; RapLSI, relative apical longitudinal strain index; TRPG, transtricuspid pressure gradient.
The P‐values were obtained by Mann–Whitney U test or χ 2 test.
Correlation between left atrial strain during the reservoir phase and other echocardiographic findings
Table 2 showed the correlation between LASr and other echocardiographic findings in patients with ATTRwt‐CM. Various echocardiographic findings were significantly correlated with LASr. Especially pLASRr was strongly correlated with LASr (R = 0.90, P < 0.01).
Table 2.
Correlation between LASr and other echocardiographic findings
| Variables | R | P‐value |
|---|---|---|
| LAVI per 1 mL/m2 | 0.34 | <0.01 |
| IVSTd per 1 mm | 0.28 | <0.01 |
| LVPWTd per 1 mm | 0.31 | <0.01 |
| LVEF per 1% | 0.31 | <0.01 |
| E‐wave velocity per 1 cm/s | −0.14 | 0.13 |
| A‐wave velocity per 1 cm/s | 0.40 | <0.01 |
| E/A ratio | −0.31 | 0.01 |
| e′ velocity per 1 cm/s | 0.11 | 0.24 |
| a′ velocity per 1 cm/s | 0.58 | <0.01 |
| s′ velocity per 1 cm/s | 0.45 | <0.01 |
| E/e′ ratio per 1 | −0.18 | 0.05 |
| TRPG per 1 mmHg | −0.17 | 0.07 |
| LV‐GLS per 1% | 0.56 | <0.01 |
| RapLSI per 1 | −0.09 | 0.37 |
| pLASRr per 1 S−1 | 0.90 | <0.01 |
| pLASRcd per 1 S−1 | 0.48 | <0.01 |
| pLASRct per 1 S−1 | 0.66 | <0.01 |
IVSTd, interventricular septal thickness in diastole; LASr, left atrial strain during reservoir phase; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LV‐GLS, left ventricular global longitudinal strain; LVPWTd, left ventricular posterior wall thickness in diastole; pLASRcd, peak strain rate during conduit phase; pLASRct, peak strain rate during contraction phase; pLASRr, peak strain rate during reservoir phase; RapLSI, relative apical longitudinal strain index; TRPG, transtricuspid pressure gradient.
P‐value was obtained by using Pearson's method.
Cox proportional hazard analysis for cardiovascular death in patients with wild‐type transthyretin amyloid cardiomyopathy
The univariate and multivariable Cox proportional hazard analysis results for cardiovascular death are shown in Tables 3 and 4 , respectively. In the univariate Cox proportional hazard analysis, 10 variables were identified as significant predictors of cardiovascular death: age, history of carpal tunnel syndrome, ln TnT concentration, ln BNP concentration, eGFR, low voltage on the electrocardiogram, and LAVI, LASr, pLASRr, and pLASRct on echocardiography. Considering the internal correlation of LASr with pLASRr, we excluded pLASRr from the multivariable Cox proportional hazard analysis. After adjusting for conventional predictive factors of ATTRwt‐CM (age, hs‐cTnT concentration, BNP concentration, and eGFR), LASr was significantly and independently associated with cardiovascular death in patients with ATTRwt‐CM (odds ratio, 0.84; 95% CI, 0.72–0.98; P < 0.05) (Model 1 in Table 4 ). After adjusting for age and echocardiographic findings associated with cardiovascular death (LAVI and pLASRct), LASr was still significantly and independently associated with cardiovascular death in patients with ATTRwt‐CM (odds ratio, 0.83; 95% CI, 0.70–0.98; P < 0.05) (Model 2 in Table 4 ).
Table 3.
Univariate Cox proportional hazards model for cardiovascular death
| Univariate analysis | ||
|---|---|---|
| OR (95% CI) | P‐value | |
| Age per 1 year | 1.12 (1.04–1.21) | <0.01 |
| Male sex (yes) | 2.01 (0.67–6.05) | 0.22 |
| Body mass index per 1 kg/m2 | 1.01 (0.91–1.11) | 0.89 |
| Hypertension (yes) | 0.83 (0.39–1.76) | 0.63 |
| Diabetes mellitus (yes) | 0.65 (0.22–1.91) | 0.43 |
| Dyslipidaemia (yes) | 0.65 (0.27–1.54) | 0.33 |
| Prior myocardial infarction (yes) | 5.15 (0.60–44.06) | 0.14 |
| Atrial fibrillation (yes) | 1.97 (0.92–4.24) | 0.08 |
| Carpal tunnel syndrome (yes) | 0.35 (0.14–0.88) | <0.05 |
| ln TnT per 1 | 5.04 (2.65–9.58) | <0.01 |
| ln BNP per 1 | 3.40 (1.89–6.13) | <0.01 |
| eGFR per 1 mL/min/1.73 m2 | 0.93 (0.91–0.96) | <0.01 |
| V1–V3 QS pattern (yes) | 1.79 (0.84–3.83) | 0.13 |
| Low voltage (yes) | 2.80 (1.21–6.48) | <0.05 |
| CLBBB (yes) | 1.84 (0.76–4.46) | 0.17 |
| CRBBB (yes) | 1.15 (0.48–2.76) | 0.76 |
| LAVI per 1 mL/m2 | 1.02 (1.00–1.03) | <0.01 |
| IVSTd per 1 mm | 1.00 (0.88–1.13) | 0.99 |
| LVPWTd per 1 mm | 0.98 (0.86–1.12) | 0.73 |
| LVEF per 1% | 0.97 (0.94–1.00) | 0.08 |
| E‐wave velocity per 1 cm/s | 1.00 (0.98–1.02) | 0.86 |
| A‐wave velocity per 1 cm/s | 0.99 (0.96–1.01) | 0.27 |
| E/A ratio | 1.15 (0.70–1.89) | 0.59 |
| e′ velocity per 1 cm/s | 0.82 (0.57–1.19) | 0.29 |
| a′ velocity per 1 cm/s | 0.79 (0.60–1.06) | 0.11 |
| s′ velocity per 1 cm/s | 0.78 (0.56–1.08) | 0.14 |
| E/e′ ratio per 1 | 1.05 (0.99–1.14) | 0.12 |
| TRPG per 1 mmHg | 1.01 (0.97–1.05) | 0.55 |
| Aortic stenosis (yes) | 0.65 (0.09–4.88) | 0.67 |
| Mitral regurgitation (yes) | 1.72 (0.77–3.84) | 0.19 |
| Tricuspid regurgitation (yes) | 1.60 (0.69–3.68) | 0.27 |
| Pericardial effusion (yes) | 1.43 (0.66–3.13) | 0.37 |
| LV‐GLS per 1% | 0.90 (0.79–1.01) | 0.09 |
| RapLSI per 1 | 1.70 (0.99–2.92) | 0.06 |
| LASr per 1% | 0.82 (0.72–0.94) | <0.01 |
| pLASRr per 1 S−1 | 0.03 (0.00–0.69) | <0.05 |
| pLASRcd per 1 S−1 | 0.15 (0.01–3.13) | 0.22 |
| pLASRct per 1 S−1 | 0.09 (0.01–0.92) | <0.05 |
BNP, B‐type natriuretic peptide; CI, confidence interval; CLBBB, complete left bundle branch block; CRBBB, complete right bundle branch block; eGFR, estimated glomerular filtration rate; IVSTd, interventricular septal thickness in diastole; LASr, left atrial strain during reservoir phase; LAVI, left atrial volume index; ln, natural logarithm; LVEF, left ventricular ejection fraction; LV‐GLS, left ventricular global longitudinal strain; LVPWTd, left ventricular posterior wall thickness in diastole; OR, odds ratio; pLASRcd, peak strain rate during conduit phase; pLASRct, peak strain rate during contraction phase; pLASRr, peak strain rate during reservoir phase; RapLSI, relative apical longitudinal strain index; TnT, troponin T; TRPG, transtricuspid pressure gradient.
P‐value was obtained by the univariate Cox hazard analyses model.
Table 4.
Multivariable Cox proportional hazards model for cardiovascular death
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Age per 1 year | 1.15 (1.06–1.25) | <0.01 | 1.12 (1.03–1.21) | <0.01 |
| ln hs‐cTnT per 1 | 3.75 (1.34–10.50) | <0.05 | ||
| ln BNP per 1 | 2.67 (1.23–5.80) | <0.05 | ||
| eGFR per 1 mL/min/1.73 m2 | 0.99 (0.95–1.03) | 0.63 | ||
| LAVI per 1 mL/m2 | 1.00 (0.99–1.02) | 0.78 | ||
| pLASRct per 1 S−1 | 0.81 (0.05–14.29) | 0.89 | ||
| LASr per 1% | 0.84 (0.72–0.98) | <0.05 | 0.83 (0.70–0.98) | <0.05 |
BNP, B‐type natriuretic peptide; CI, confidence interval; eGFR, estimated glomerular filtration rate; hs‐cTnT, high‐sensitivity cardiac troponin T; LASr, LA strain during reservoir phase; LAVI, left atrial volume index; ln, natural logarithm; OR, odds ratio; pLASRct, peak strain rate during contraction phase.
P‐value was obtained by the multivariate Cox hazard analysis.
Receiver operating characteristic curve analysis for cardiovascular death
Receiver operating characteristic curve analysis was performed to determine the optimal LASr cut‐off value for predicting cardiovascular death in patients with ATTRwt‐CM. As shown in Figure 3 , the area under the ROC curve of LASr for cardiovascular death was 0.686. We also found that the best cut‐off value of LASr was 6.69% (sensitivity, 62.4%; specificity, 64.3%).
Figure 3.
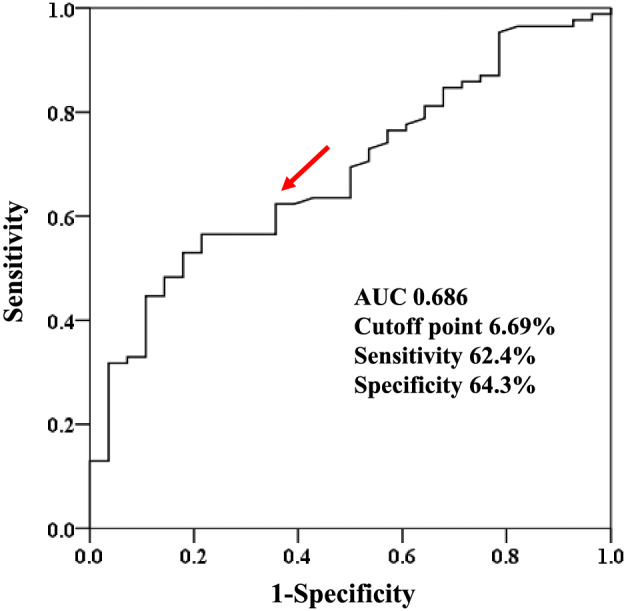
Receiver operating characteristic curve analysis of left atrial strain during reservoir phase for predicting cardiovascular death. Red arrow indicates cut‐off point. AUC, area under the curve.
Follow‐up of patients with high and low left atrial strain during the reservoir phase values
We divided the patients with ATTRwt‐CM into a high LASr group (≥6.69%, n = 63) and low LASr group (<6.69%, n = 50) using the best cut‐off value of LASr estimated by the ROC curve analysis. Kaplan–Meier analysis demonstrated a significantly higher probability of total cardiovascular death (P < 0.05 by log‐rank test) (Figure 4 A ) and heart failure‐related hospitalization (P < 0.05 by log‐rank test) (Figure 4 B ) in patients with high than low LASr.
Figure 4.
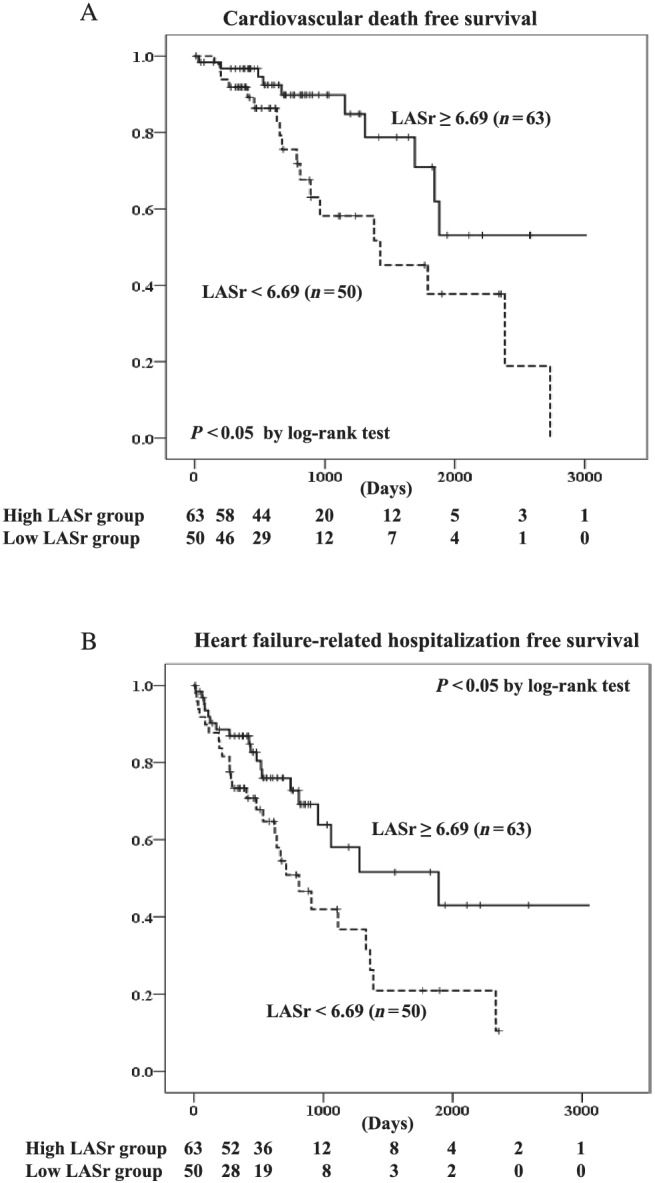
Kaplan–Meier curves of (A) cardiovascular death and (B) heart failure‐related hospitalization in patients with wild‐type transthyretin amyloid cardiomyopathy with high or low left atrial strain during the reservoir phase (LASr).
Discussion
This is the first study to reveal the usefulness of LA strain by two‐dimensional speckle tracking echocardiography to predict cardiovascular death in patients with ATTRwt‐CM.
Although a recent study has shown that the transthyretin stabilizer tafamidis is associated with reductions in all‐cause death, cardiovascular‐related hospitalizations, functional capacity, and quality of life, 16 the usefulness of tafamidis in advanced cases has not been clarified. Therefore, it is critical to identify vulnerable patients with ATTR‐CM at high risk of cardiovascular death. The present study showed the usefulness of LA function to predict cardiovascular death in patients with ATTRwt‐CM. However, LV systolic and diastolic function parameters, such as the LV ejection fraction and E/e′ ratio, were not associated with cardiovascular death in the present study. In addition, LV global LS, which can be used to evaluate subtle regional and global LV dysfunction, 17 also had no association with cardiovascular death. These results indicate that LV function indicators are not significant predictors of cardiovascular death in patients with ATTRwt‐CM. Amyloid deposition occurs not only in the left ventricle but also in the left atrium and right ventricle, 18 indicating that ATTR‐CM induces multi‐atrioventricular dysfunction. Therefore, echocardiographic findings representing LV function might have a weak effect on the prognosis in patients with ATTRwt‐CM. In contrast, LA dysfunction is usually correlated with greater impairment of LV diastolic function because higher LV filling pressure leads to deterioration of LA function as a result of haemodynamic overload and mechanical stretching of the LA wall. 19 Therefore, both direct amyloid infiltration and restrictive LV function with high filling pressures are important causes of LA dysfunction in patients with ATTRwt‐CM. This might explain why LA dysfunction had a strong effect on the prognosis in patients with ATTRwt‐CM in the present study. Huntjens et al. also revealed prognostic utility of both atrial and ventricular strain imaging in patients with cardiac amyloidosis. 20 However, these reports enrolled patients with various types of amyloid cardiomyopathy. Because the prognosis depends on the type of amyloid cardiomyopathy, 21 specific evaluations should be performed according to each type of amyloid cardiomyopathy. The present study included only patients with ATTRwt‐CM and revealed the prognostic impact of LA function in these patients.
Two‐dimensional speckle tracking echocardiography is a useful technique to investigate advanced atrial functional components such as the reservoir function, conduit function, and contraction function, which are otherwise difficult to investigate noninvasively. 22 , 23 The present study revealed that LASr was independent and significantly associated with cardiovascular death in patients with ATTRwt‐CM, indicating the usefulness of estimating the LA reservoir function to evaluate the prognosis in patients with ATTRwt‐CM. In contrast, pLASRct was not significantly associated with cardiovascular death, and both the A‐wave velocity and a′ velocity, conventional echocardiographic measurements of LA contraction function, 24 , 25 were not associated with cardiovascular death in patients with ATTRwt‐CM. Amyloid deposition in the left ventricle leads to diastolic dysfunction in the early phase, and LV systolic function is relatively preserved in the late phase. 26 Amyloid deposition occurs not only in the left ventricle but also in the left atrium. 18 Therefore, as with the left ventricle, amyloid deposition might impair LA reservoir function prior to LA contractive function. Thus, we speculated that LA reservoir function was relatively important for the prognosis of patients with ATTRwt‐CM. Nochioka et al. 8 reported that LA conduit function did not differ significantly between patients with amyloid cardiomyopathy and control patients. Therefore, LA conduit function is thought to be a compensatory mechanism in response to impairment of LA reservoir and contraction function, and this might explain why pLASRcd was not significantly associated with cardiovascular death in the present study. Although LA volume is correlated with cardiovascular morbidity and mortality in various pathological conditions, including amyloid cardiomyopathy, 7 , 27 , 28 LAVI was not associated with cardiovascular death in the present study. The LA volume is influenced by various factors, such as diastolic function, the LV filling pressure, and loading conditions. 29 , 30 In contrast, strain analysis using speckle tracking is a direct measurement of intrinsic LA myocardial deformation, which is independent of loading conditions 31 , 32 and geometric assumptions. 22 We speculate that these points might be the reasons why LA function evaluated by two‐dimensional speckle tracking echocardiography was more important than LA volume to predict cardiovascular death in patients with ATTRwt‐CM.
Gillmore et al. previously reported that the combination of eGFR and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) was useful to evaluate prognosis in patients with ATTR‐CM. 5 Therefore, it is important to evaluate the usefulness of LASr for additional ability on combination of eGFR and NT‐proBNP. However, we could not evaluate it because the number of patients in our present study was relatively small. However, we revealed that LASr was significantly and independently associated with cardiovascular death after adjusting for conventional predictive factors including BNP and eGFR. Thus, LASr might have additional ability to stratify prognosis on top of combination of eGFR and NT‐proBNP. Further multicentre prospective studies with more patients are needed to validate the usefulness of LASr for prognostic value in patients with ATTRwt‐CM.
Study limitations
This study had several limitations. First, it included a small number of patients and was performed at a single centre. Second, our results may be typical for Asian patients with ATTRwt‐CM because all patients in our present study were Japanese. Third, we obtained echocardiographic images using several ultrasound vendors. The two‐dimensional speckle tracking echocardiography analysis was performed with TOMTEC Image‐Arena™ (vendor‐independent) software. Although there are significant correlations in the LS values analysed using vendor‐independent software for paired images obtained from different ultrasound vendors, 33 inter‐vendor variability still might have affected our study results.
Despite these limitations, our study is unique and the first to demonstrate the importance of LA function estimated by two‐dimensional speckle tracking echocardiography in patients with ATTRwt‐CM. We believe that our results have significant value in the clinical setting.
Conclusions
Left atrial strain during the reservoir phase estimated by two‐dimensional speckle tracking echocardiography provides significant prognostic value in patients with ATTRwt‐CM even after adjusting for conventional predictive factors.
Conflict of interest
None declared.
Funding
This study was supported in part by a Grant‐in‐Aid for Scientific Research of the Japan Society for the Promotion of Science (Reference Number 20K08476 to H.U.).
Ethics statement
This study was approved by the institutional review board and ethics committee of Kumamoto University (Reference Number 1588).
Supporting information
Figure S1. Diagnostic strategy for ATTR‐CM using 99mTc‐PYP scintigraphy, hs‐cTnT, LV Posterior wall thickness, QRS, and RapLSI in Kumamoto University Hospital. Abbreviations: hs‐cTnT, high‐sensitivity cardiac troponin T; LV, left ventricular; RapLSI, relative apical longitudinal strain index; 99mTc‐PYP, 99mTc‐labeled pyrophosphate; TTR, transthyretin.
Acknowledgement
We thank Angela Morben, DVM, ELS, from Edanz Group (https://en‐author‐services.edanz.com/ac), for editing a draft of this manuscript.
Oike, F. , Usuku, H. , Yamamoto, E. , Yamada, T. , Egashira, K. , Morioka, M. , Nishi, M. , Komorita, T. , Hirakawa, K. , Tabata, N. , Yamanaga, K. , Fujisue, K. , Hanatani, S. , Sueta, D. , Arima, Y. , Araki, S. , Takashio, S. , Oda, S. , Misumi, Y. , Kawano, H. , Matsushita, K. , Ueda, M. , Matsui, H. , and Tsujita, K. (2021) Prognostic value of left atrial strain in patients with wild‐type transthyretin amyloid cardiomyopathy. ESC Heart Failure, 8: 5316–5326. 10.1002/ehf2.13621.
References
- 1. Izumiya Y, Takashio S, Oda S, Yamashita Y, Tsujita K. Recent advances in diagnosis and treatment of cardiac amyloidosis. J Cardiol 2018; 71: 135–143. [DOI] [PubMed] [Google Scholar]
- 2. Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis 2010; 52: 347–361. [DOI] [PubMed] [Google Scholar]
- 3. Castano A, Drachman BM, Judge D, Maurer MS. Natural history and therapy of TTR‐cardiac amyloidosis: emerging disease‐modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev 2015; 20: 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural history of wild‐type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol 2016; 68: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 5. Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez‐Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez‐Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J 2018; 39: 2799–2806. [DOI] [PubMed] [Google Scholar]
- 6. Mohty D, Damy T, Cosnay P, Echahidi N, Casset‐Senon D, Virot P, Jaccard A. Cardiac amyloidosis: updates in diagnosis and management. Arch Cardiovasc Dis 2013; 106: 528–540. [DOI] [PubMed] [Google Scholar]
- 7. Falk RH, Quarta CC. Echocardiography in cardiac amyloidosis. Heart Fail Rev 2015; 20: 125–131. [DOI] [PubMed] [Google Scholar]
- 8. Nochioka K, Quarta CC, Claggett B, Roca GQ, Rapezzi C, Falk RH, Solomon SD. Left atrial structure and function in cardiac amyloidosis. Eur Heart J Cardiovasc Imaging 2017; 18: 1128–1137. [DOI] [PubMed] [Google Scholar]
- 9. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133: 2404–2412. [DOI] [PubMed] [Google Scholar]
- 10. Usuku H, Takashio S, Yamamoto E, Kinoshita Y, Nishi M, Oike F, Marume K, Hirakawa K, Tabata N, Oda S, Misumi Y, Ueda M, Kawano H, Kaikita K, Matsushita K, Ando Y, Matsui H, Tsujita K. Usefulness of relative apical longitudinal strain index to predict positive (99m) tc‐labeled pyrophosphate scintigraphy findings in advanced‐age patients with suspected transthyretin amyloid cardiomyopathy. Echocardiography 2020; 37: 1774–1783. [DOI] [PubMed] [Google Scholar]
- 11. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the european Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 12. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the european Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 13. Phelan D, Collier P, Thavendiranathan P, Popovic ZB, Hanna M, Plana JC, Marwick TH, Thomas JD. Relative apical sparing of longitudinal strain using two‐dimensional speckle‐tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012; 98: 1442–1448. [DOI] [PubMed] [Google Scholar]
- 14. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D'Hooge J, Donal E, Fraser AG, Marwick T, Mertens L, Popescu BA, Sengupta PP, Lancellotti P, Thomas JD, Voigt JU. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two‐dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018; 19: 591–600. [DOI] [PubMed] [Google Scholar]
- 15. Yamada T, Takashio S, Arima Y, Nishi M, Morioka M, Hirakawa K, Hanatani S, Fujisue K, Yamanaga K, Kanazawa H, Sueta D, Araki S, Usuku H, Nakamura T, Suzuki S, Yamamoto E, Ueda M, Kaikita K, Tsujita K. Clinical characteristics and natural history of wild‐type transthyretin amyloid cardiomyopathy in Japan. ESC Heart Fail 2020; 7(5): 2829–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 17. Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 2016; 37: 1196–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation 2005; 112: 2047–2060. [DOI] [PubMed] [Google Scholar]
- 19. Guan Z, Zhang D, Huang R, Zhang F, Wang Q, Guo S. Association of left atrial myocardial function with left ventricular diastolic dysfunction in subjects with preserved systolic function: a strain rate imaging study. Clin Cardiol 2010; 33: 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huntjens PR, Zhang KW, Soyama Y, Karmpalioti M, Lenihan DJ, Gorcsan J 3rd. Prognostic utility of echocardiographic atrial and ventricular strain imaging in patients with cardiac amyloidosis. JACC Cardiovasc Imaging 2021; 14: 1508–1519. [DOI] [PubMed] [Google Scholar]
- 21. Ng B, Connors LH, Davidoff R, Skinner M, Falk RH. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain‐associated amyloidosis. Arch Intern Med 2005; 165: 1425–1429. [DOI] [PubMed] [Google Scholar]
- 22. Cameli M, Caputo M, Mondillo S, Ballo P, Palmerini E, Lisi M, Marino E, Galderisi M. Feasibility and reference values of left atrial longitudinal strain imaging by two‐dimensional speckle tracking. Cardiovasc Ultrasound 2009; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Bella G, Minutoli F, Madaffari A, Mazzeo A, Russo M, Donato R, Zito C, Aquaro GD, Piccione MC, Pedri S, Vita G, Pingitore A, Carerj S. Left atrial function in cardiac amyloidosis. J Cardiovasc Med (Hagerstown) 2016; 17: 113–121. [DOI] [PubMed] [Google Scholar]
- 24. Manning WJ, Silverman DI, Katz SE, Riley MF, Doherty RM, Munson JT, Douglas PS. Temporal dependence of the return of atrial mechanical function on the mode of cardioversion of atrial fibrillation to sinus rhythm. Am J Cardiol 1995; 75: 624–626. [DOI] [PubMed] [Google Scholar]
- 25. Oike F, Yamamoto E, Sueta D, Tokitsu T, Usuku H, Nishihara T, Takae M, Fujisue K, Arima Y, Kanazawa H, Ito M, Hanatani S, Araki S, Takashio S, Sakamoto K, Suzuki S, Kawano H, Soejima H, Kaikita K, Tsujita K. Clinical significance of diastolic late mitral annular velocity in heart failure with preserved ejection fraction. Int J Cardiol 2020; 316: 145–151. [DOI] [PubMed] [Google Scholar]
- 26. Montecucco C, Gobbi G, Perlini S, Rossi S, Grandi AM, Caporali R, Finardi G. Impaired diastolic function in active rheumatoid arthritis. relationship with disease duration. Clin Exp Rheumatol 1999; 17: 407–412. [PubMed] [Google Scholar]
- 27. Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population‐based study. J Am Coll Cardiol 2003; 41: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 28. Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation 2003; 107: 2207–2212. [DOI] [PubMed] [Google Scholar]
- 29. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006; 47: 2357–2363. [DOI] [PubMed] [Google Scholar]
- 30. Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 2002; 90: 1284–1289. [DOI] [PubMed] [Google Scholar]
- 31. Boyd AC, Richards DA, Marwick T, Thomas L. Atrial strain rate is a sensitive measure of alterations in atrial phasic function in healthy ageing. Heart 2011; 97: 1513–1519. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Q, Yip GW, Yu CM. Approaching regional left atrial function by tissue Doppler velocity and strain imaging. Europace 2008; 10: iii62–iii69. [DOI] [PubMed] [Google Scholar]
- 33. Nagata Y, Takeuchi M, Mizukoshi K, Wu VC, Lin FC, Negishi K, Nakatani S, Otsuji Y. Intervendor variability of two‐dimensional strain using vendor‐specific and vendor‐independent software. J Am Soc Echocardiogr 2015; 28: 630–641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Diagnostic strategy for ATTR‐CM using 99mTc‐PYP scintigraphy, hs‐cTnT, LV Posterior wall thickness, QRS, and RapLSI in Kumamoto University Hospital. Abbreviations: hs‐cTnT, high‐sensitivity cardiac troponin T; LV, left ventricular; RapLSI, relative apical longitudinal strain index; 99mTc‐PYP, 99mTc‐labeled pyrophosphate; TTR, transthyretin.


