ABSTRACT
Objectives:
The aim of this article is to detect whether oral lesions affect the tongue mainly due to higher cells expressing angiotensin-converting enzyme 2 (ACE2) than in other oral sites in COVID-19 patients. Moreover, the etiology of oral lesions was evaluated either resulting from SARS-CoV-2 sequelae or from adverse effects of drugs used for COVID-19 treatment.
Materials and Methods:
One hundred and twenty-four patients were admitted to the study. All patients’ data were obtained including age and gender, laboratory testing, drug administration, respiratory and systemic conditions, signs and symptoms, and oral manifestations.
Results:
Oral manifestations were seen in 112 (90.3%) of all patients. Oral ulcers represented the most prevalent lesions in the oral cavity in 104 patients (92.8%). Lip, tongue, and labial mucosa showed the most common sites for oral ulcers. Most of oral lesions were displayed in the tongue in 96 patients (85.7%). Various medications were used in the treatment of patients.
Conclusion:
The tongue represented the most common site of oral lesions in COVID-19 patients followed by the labial mucosa. No correlation was found between the oral lesions and the drugs used for the treatment of SARS-CoV-2 infection. The systemic health and the severity of the disease were not related to the spread of the oral lesions.
KEYWORDS: Angiotensin-converting enzyme 2, coronavirus, oral lesions
INTRODUCTION
In the recent couple of years, a new coronavirus disease (COVID-19) has emerged, causing an exceptional pandemic that is being treated as an urgent threat by health authorities around the world.[1] The coronavirus-caused condition is known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is a zoonotic respiratory virus with bats and pangolins as the most likely source and transitional hosts.[2] COVID-19 is hypothesized to be transmitted by close person-to-person contact (approximately 2 m), a distance at which respiratory droplets from an infected person during coughing, sneezing, or speaking might spread to others who do not have appropriate barriers. Additional routes of transmission emerge when spit droplets fall on other surfaces, such as the ground, and persons come into touch with them,[3] with catastrophic consequences not only in medically compromised individuals, but even in otherwise healthy young people with a healthy immune system.[4]
Viral replication takes place in the upper respiratory system and subsequently disseminates throughout the body, causing serious complications.[5] Fever, shortness of breath, cough, runny nose, sputum production, muscle discomfort, headache, weariness, arthralgia, diarrhea, and conjunctivitis are some of the symptoms.[6] The average incubation time ranges from 7 to 14 days. Many patients develop mild-to-moderate diseases with upper and lower respiratory tract involvement; others have a serious disease or even have critical conditions such as respiratory failure, septic shock, or multiple organ failure.[7] After recuperation, the contagiousness can last for up to 2 or 3 weeks.[8] Susceptibility, genetics, systemic disorders, population, gender, and age are all important factors to consider when it comes to the initiation and course of viral infection. Patients with asthma or pulmonary edema are at a significant risk of death.[9]
The active receptor for SARS-CoV-2 is a metallopeptidase called angiotensin-converting enzyme 2 (ACE2), which was detected in COVID-19 patients.[10] ACE2 has been discovered in a variety of organs and tissues including type I and type II alveolar epithelial cells in nasal and oral mucosal tissues, nasopharynx, smooth muscle cells, and endothelial cells of skin and stomach vessels, obviously in the basal cell layer of epidermis and non-keratinizing squamous epithelium.[11] The levels of ACE2 in each population may also differ. As a result, the start and progress of the disease might range from subclinical signs to acute and violent consequences.[8] Because ACE2 is involved in cellular entrance, ACE2-expressing cells may act as target cells, making them vulnerable to SARS-CoV-2 infection.[12] It was discovered that mice infected with SARS-CoV-2 had lung failure as a result of ACE2 downregulation. Thus, the oral mucosa and pulmonary epithelial cells are engaged in the entry of virus, allowing it to replicate and trigger an inflammatory response.[13]
Although oral symptoms of SARS-CoV-2 infection were rare, the presence of ACE2 in the oral cavity showed that an oral infection route for SARS-CoV-2 could not be ruled out.[14] The ACE2-positive cells were found to be abundant in epithelial cells.[12] The oral tissues, based on these findings, should be considered a high-risk location for SARS-CoV-2 infection. The tongue had stronger ACE2 expression than the buccal or gingival mucosa.[14] The interaction of SARS-CoV-2 with ACE2 receptors may reduce taste bud sensitivity, resulting in defective gustatory responses.[15] Oral symptoms, on the contrary, have been postulated as a possible drug reaction that can occur during the latency phase.[16]
It is still debatable whether oral lesions could be predictably clinical manifestations rising from the direct SARS-CoV-2 infection or a systemic outcome; the probability of coinfections enhancing the COVID-19 severity, impaired immune system, and adverse reactions of medical treatment are assumed.[17]
In order to study the various routes of SARS-CoV-2 infection on the oral mucosa, we explored whether oral lesions mainly impact the tongue mucosa due to increased ACE2-expressing cell than in other oral tissues. Furthermore, the emergence of oral lesions was assessed and analyzed to determine whether it is a result of SARS-CoV-2 infection or a side effect of particular medicines used to treat COVID-19.
MATERIALS AND METHODS
We performed a cross-sectional study. All cases were presented with a confirmation for COVID-19 in El Beheira and Alexandria Governorate Hospitals, Egypt, between September 2, 2020 and June 10, 2021.
INCLUSION CRITERIA
The following are the inclusion criteria:
Age >18 years;
Infection with COVID-19 was verified in a laboratory using reverse transcription–polymerase chain reaction or RT–PCR.
EXCLUSION CRITERIA
The exclusion criteria are as follows:
Patients without a laboratory-confirmed diagnosis of COVID-19 infection were excluded;
Patients who had olfactory or gustatory impairment prior to infection with COVID-19;
Those who have malignant neoplasms;
Patients suffering from neurodegenerative diseases.
One hundred and twenty-four admitted patients met the inclusion criteria and were recruited in the present study. Patients’ data were collected at the time of admission and during their stay in the hospital.
Due to the deficiency of the reference time points, the initiation of the oral manifestations varied extensively among the patients. Oral lesions in all patients were reported. The site and the severity of these lesions were detected. Laboratory testing, drug administration, and respiratory and systemic conditions were documented.
Additionally, the patients were divided into not severe and severe based on clinical manifestations and oxygen supplement requirement [Table 1].
Table 1.
Severity of the disease
| Clinical classification severity [n (%)] | |
|---|---|
| I. Severe | 72 (58.1%) |
| II. Not severe | 52 (41.9%) |
Personal protective measures were used for precaution and prevention of disease transmission. These measures included wearing long-sleeved and fluid-resistant gowns, gloves, N95 masks, protective eyewear, and face shields. Sterilization of all used instruments was performed in between the patients.
Statistical descriptive analysis was conducted to determine the overall prevalence of various findings within the patients. Data were presented as frequencies and percentages. Statistical analysis was performed using the Statistics Package for Social Sciences v21.0TM software (SPSS Inc., USA). Pearson’s correlation coefficient was utilized to assess the statistical association between two variables.
RESULTS
One hundred and twenty-four COVID-19 patients [92 men (74.2%) and 32 women (25.8%)] with mean age (50.32 ± 12.47) were recruited in the current study. Table 2 shows associated general symptoms and systemic conditions. The most common symptoms were feeling tired, asthenia, breathing problems, and cough. In all patients, 92 patients (74.2%) lost both taste and smell, whereas 24 patients (19.3%) lost smell only. Additionally, dry mouth affected 104 (about 84%) of all patients.
Table 2.
General symptoms and systemic conditions
| General symptoms [n (%)] | |
|---|---|
| I. No symptoms | 12 (9.7%) |
| II. Symptomatic: | 112 (90.3%) |
| 1. Feeling tired | 24 (21.4% of symptomatic patients) |
| 2. Asthenia | 84 (75% of symptomatic patients) |
| 3. Breath problems | 84 (75% of symptomatic patients) |
| 4. Cough | 84 (75% of symptomatic patients) |
| 5. Abdominal symptoms | 16 (14.3% of symptomatic patients) |
| A. Asthenia+ breath difficulty+ cough | 56 (50% of symptomatic patients) |
| B. Asthenia+ breath difficulty+ cough+ abdominal problems | 8 (7.1% of symptomatic patients) |
| C. Felt tired+ asthenia+ breath difficulty+ cough | 4 (3.6% of symptomatic patients) |
| D. Felt tired only | 12 (10.7% of symptomatic patients) |
| E. Felt tired+ abdominal problems | 4 (3.6% of symptomatic patients) |
| F. Asthenia+ breath difficulty | 12 (10.7% of symptomatic patients) |
| G. Felt tired+ asthenia+ breath difficulty+ cough+ abdominal problems | 4 (3.6% of symptomatic patients) |
| H. Cough only | 12 (10.7% of symptomatic patients) |
| Medical condition [n (%)] | |
| I. Medically free | 68 (54.8%) |
| II. Medically compromised | 56 (45.2%) |
| 1. Hypertension | 16 (28.5% of medically compromised patients) |
| 2. Diabetic | 52 (92.8% of medically compromised patients) |
| 3. Renal disease | 4 (7.1% of medically compromised patients) |
| 4. Liver disease | 4 (7.1% of medically compromised patients) |
| 5. Cardiac | 8 (14.2% of medically compromised patients) |
| A. Diabetic only | 40 (71.4% of medically compromised patients) |
| B. Hypertensive+ diabetic+ hepatic+ cardiac | 4 (7.1% of medically compromised patients) |
| C. Hypertensive+ cardiac | 4 (7.1% of medically compromised patients) |
| D. Hypertensive+ diabetic+ renal+ cardiac | 4 (7.1% of medically compromised patients) |
| E. Hypertensive+ diabetic | 4 (7.1% of medically compromised patients) |
| Loss of sensation [n (%)] | |
| I. Loss of taste and smell | 92 (74.2%) |
| II. Loss of smell only | 24 (19.3%) |
| Salivary gland [n (%)] | |
| I. Dry mouth | 104 (83.9%) |
| II. Not affected | 20 (16.1%) |
All medications prescribed to the patients are displayed in Table 3. It represents the treatment guidelines for COVID-19. All patients received zithrocin, iverzine, vitamin C, and zinc. About 90% of the patients received anticoagulant therapy. Prednisolone was required for 76 patients. Antibacterial drugs were needed in about 70% of the patients. Other drugs were used according to the treatment requirements for the patient.
Table 3.
Medications prescribed to the patients
| Medication [n (%)] | ||
|---|---|---|
| I. | Zithrocin | 124 (100%) |
| II. | Iverzine | 124 (100%) |
| III. | Zinc, vitamin C | 124 (100%) |
| IV. | Prednisolone | 76 (61.3%) |
| V. | Remdesiv ir | 28 (22.6%) |
| VI. | Anticoagulant | 112 (90.3%) |
| VII. | Antihypertensive | 16 (12.9%) |
| VIII. | Antibacterial | 88 (70.9%) |
| IX. | Foradil | 16 (12.9%) |
| X. | Colchicine or hydroxychloroquine | 16 (12.9%) |
| XI. | Acetylcysteine | 24 (19.3%) |
| XII. | Silymarin | 4 (3.2%) |
Oral manifestations were seen in 112 (90.3%) of all patients. About 62% of oral manifestations were asymptomatic. The symptomatic oral manifestations were detected whether painful (21.4%) or burning sensation (14.3%).
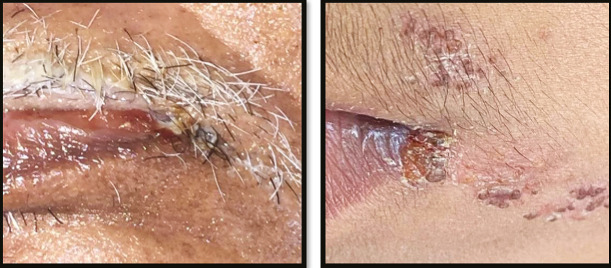
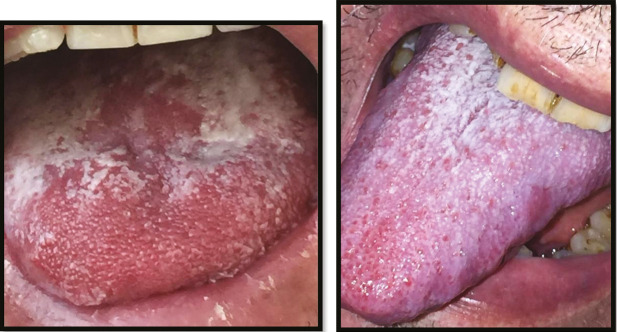
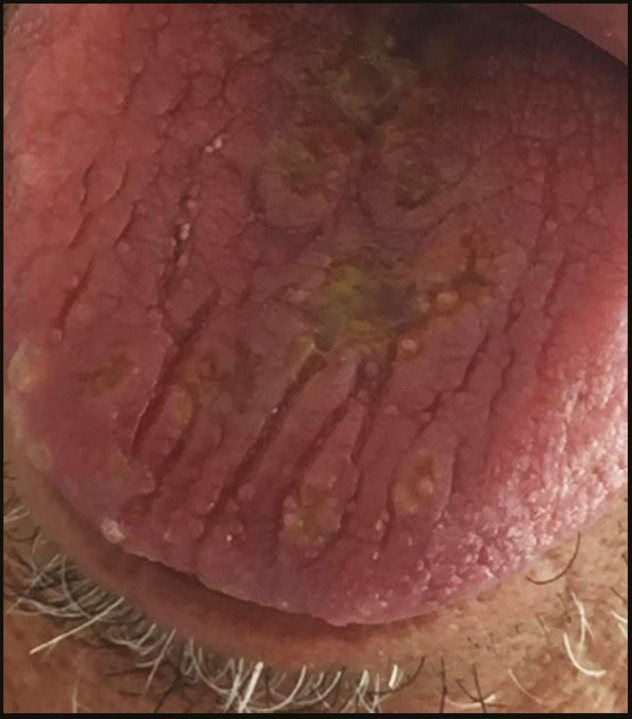
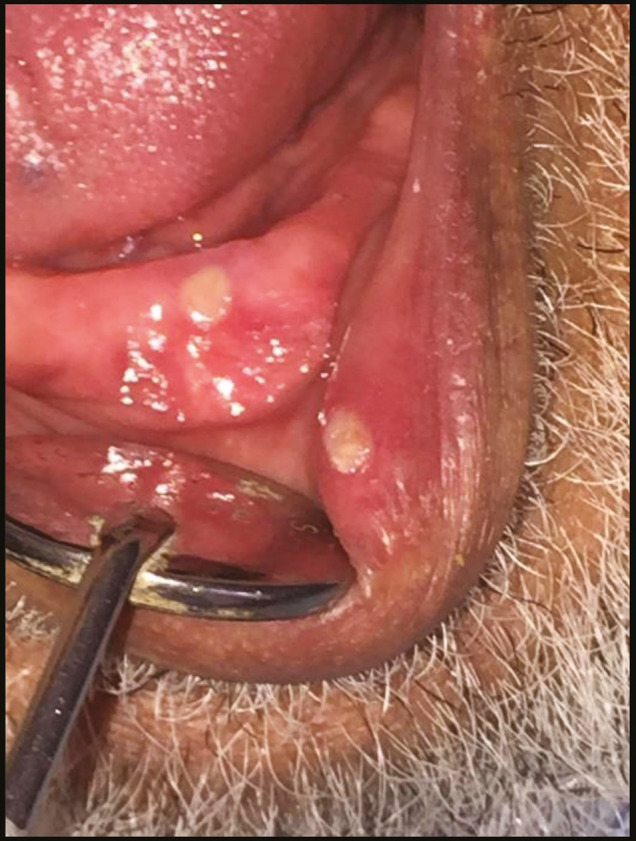
There were various oral lesions but oral ulcers represented the most prevalent lesions in the oral cavity in 104 patients (92.8%). Most of oral ulcers were aphthous-like ulcers covered with pseudo-membrane. Lip, tongue, and labial mucosa showed the most common sites for oral ulcers (42.3%, 38.5%, and 34.6%, respectively) [Figure 1]. Regarding lip ulcers, they were hemorrhagic ulcers with crust [Figure 2]. Candida infection followed oral ulcers, which represented 42.8% of all oral lesions with 50% displayed in the tongue [Figure 3]. Whole mouth Candida and the mucosa represented 25% and 16.7%, respectively, of Candida infection. Other lesions were observed as hyperpigmentation (25%) which was shown mostly in the floor of the mouth (57.1%) [Figure 4], tongue coating (10.7%), atrophy of the tongue (17.8%), petechiae (17.8%), herpes (7.14%), and white lesions on the tongue (7.14%). Most of the oral lesions were displayed in the tongue in 96 patients (85.7%) like ulcers only (29.2% of lesions of the tongue), atrophy only (20.8% of lesions of the tongue), or a combination of lesions (12.5% tongue ulcer + tongue Candida, 12.5% tongue ulcer + tongue coating) [Figure 5]. This is followed by lesions in the labial mucosa in 76 patients (67.8%) and then the buccal mucosa in 60 patients (53.6%). Moreover, 52 patients (46.4%) suffered from lesions in the lip, whereas 44 patients (39.28%) experienced lesions of the floor of the mouth [Figure 6]. In contrast, lesions of the gingiva represented the least site affected by the oral lesions [Table 4]. Furthermore, there was no significant correlation between oral manifestations and severity or medical conditions. Because all patients received zithrocin, iverzine, vitamin C, and zinc, the correlation between oral manifestations and these drugs cannot be computed. However, there was no significant correlation between oral manifestations and all other drugs taken by the patients [Table 5].
Figure 1.
Irregular ulcers covered with pseudo-membrane on the dorsal surface of the tongue
Figure 2.
Commissural cheilitis with bleeding, crust, and pus located in the commissure
Figure 3.
Candida infection on the dorsal surface of the tongue
Figure 4.
Hyperpigmentation in the floor of the mouth
Figure 5.
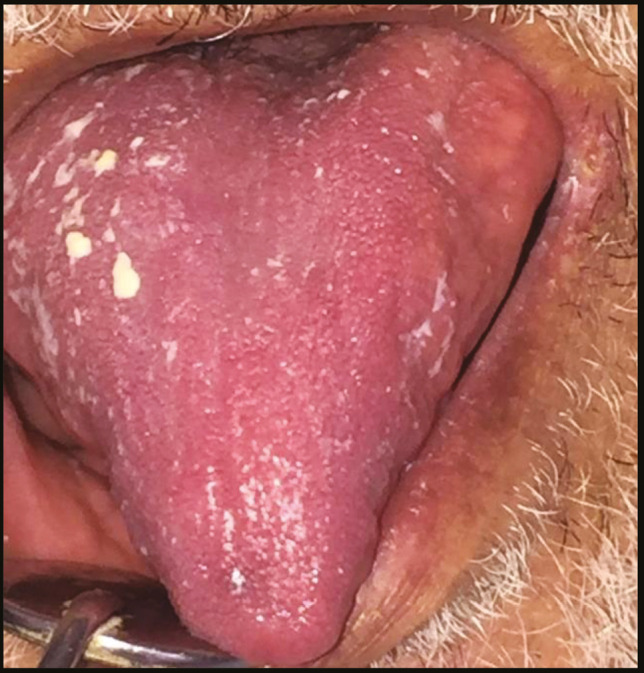
Atrophy of the dorsal surface of the tongue with white Candida infection distributed mainly on the right lateral side
Figure 6.
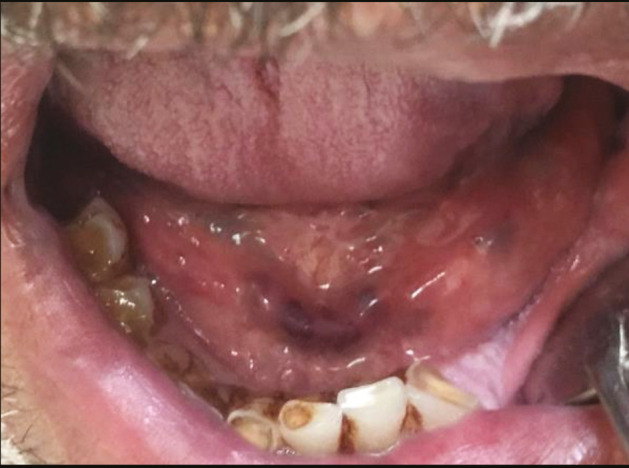
Aphthous-like ulcer in the labial mucosa and the floor of the mouth
Table 4.
Spread of oral lesions
| Oral manifestations [n (%)] | |
|---|---|
| I. Absence | 12 (9.7%) |
| II. Presence: | 112 (90.3%) |
| a) Asymptomatic | 72 (62.3% of the oral manifestation) |
| b) Painful | 24 (21.4% of the oral manifestation) |
| c) Burning sensation | 16 (14.3% of the oral manifestation) |
| Oral lesions [n (%)], N=112 (100%) | |
| I. Herpes | 8 (7.14%) |
| II. White lesions | 8 (7.14%) |
| III. Ulcers | 104 (92.8%) |
| a) Perioral ulcers | 4 (3.9% of ulcers) |
| b) Ulcers in whole mouth | 12 (11.5% of ulcers) |
| c) Lip ulcers | 44 (42.3% of ulcers) |
| d) Tongue ulcers | 40 (38.5% of ulcers) |
| e) Labial mucosa ulcers | 36 (34.6% of ulcers) |
| f) Buccal mucosa ulcers | 8 (7.7% of ulcers) |
| g) Gingival ulcer | 4 (3.9% of ulcers) |
| h) Floor of mouth ulcers | 8 (7.7% of ulcers) |
| IV. Candida | 48 (42.8%) |
| a) Whole mouth Candida | 12 (25% of Candida) |
| b) Tongue Candida | 24 (50% of Candida) |
| c) Mucosa Candida | 8 (16.7% of Candida) |
| V. Hyperpigmentation | 28 (25%) |
| a) Tongue hyperpigmentation | 4 (14.3% of hyperpigmentation) |
| b) Floor of mouth hyperpigmentation | 16 (57.1% of hyperpigmentation) |
| c) Lip hyperpigmentation | 8 (28.6% of hyperpigmentation) |
| VI. Tongue coating | 12 (10.7%) |
| VII. Atrophic tongue | 20 (17.8%) |
| VIII. Petechiae | 20 (17.8%) |
| I. Lesions of the tongue | 96 (85.7%) |
| a) Tongue ulcer only | 28 (29.2% of lesions of the tongue) |
| b) Tongue ulcer + tongue Candida | 12 (12.5% of lesions of the tongue) |
| c) Tongue ulcers+ tongue coating | 12 (12.5% of lesions of the tongue) |
| d) Tongue Candida only | 12 (12.5% of lesions of the tongue) |
| e) Atrophic tongue only | 20 (20.8% of lesions of the tongue) |
| f) Tongue hyperpigmentation only | 4 (4.1% of lesions of the tongue) |
| g) White lesion only | 8 (8.3% of lesions of the tongue) |
| II. Lesions of the labial mucosa | 76 (67.8%) |
| a) Petechiae only | 12 (15.8% of lesions of the labial mucosa) |
| b) Ulcer only | 32 (42.1% of lesions of the labial mucosa) |
| c) Candida only | 12 (15.8% of lesions of the labial mucosa) |
| d) Ulcer+ Candida | 8 (10.5% of lesions of the labial mucosa) |
| e) Herpes+ Candida | 4 (5.3% of lesions of the labial mucosa) |
| f) Ulcer+ petechiae | 4 (5.3% of lesions of the labial mucosa) |
| g) Herpes+ ulcer | 4 (5.3% of lesions of the labial mucosa) |
| III. Lesions of the buccal mucosa | 60 (53.6%) |
| a) Petechiae only | 20 (33.3% of lesions of the buccal mucosa) |
| b) Ulcer only | 8 (13.3% of lesions of the buccal mucosa) |
| c) Candida only | 16 (26.7% of lesions of the buccal mucosa) |
| d) White lesion only | 8 (13.3% of lesions of the buccal mucosa) |
| e) Ulcer+ Candida | 8 (13.3% of lesions of the buccal mucosa) |
| IV. Lesions of the gingiva | 16 (14.3%) |
| a) Ulcer only | 16 (100% of lesions of the gingiva) |
| V. Lesions of the floor of mouth | 44 (39.28%) |
| a) Ulcer only | 16 (36.3% of lesions of the floor of mouth) |
| b) Candida only | 8 (18.2% of lesions of the floor of mouth) |
| c) Hyperpigmentation only | 12 (27.3.3% of lesions of the floor of mouth) |
| d) Candida+ hyperpigmentation | 4 (9.1% of lesions of the floor of mouth) |
| e) Ulcer+Candida | 4 (9.1% of lesions of the floor of mouth) |
| VI. Lesions of the lip | 52 (46.4%) |
| a) Ulcer only | 36 (69.2% of symptomatic group) |
| b) Hyperpigmentation only | 8 (15.4% of symptomatic group) |
| c) Herpes+ ulcer | 8 (15.4% of symptomatic group) |
Table 5.
Pearson’s correlation coefficient test between oral manifestations and Covid-19 patients
| Pearson’s correlation | Sig. (two-tailed) | |
|---|---|---|
| Oral manifestations Severity |
0.38 | 0.03 (NS) |
| Oral manifestations Medical condition |
0.29 | 0.10 (NS) |
| Oral manifestations Xithrone |
a | a |
| Oral manifestations Iverzine |
a | a |
| Oral manifestations Zinc and Vit C |
a | a |
| Oral manifestations Prednisolone |
0.41 | 0.02 (NS) |
| Oral manifestations Remdesivir |
0.17 | 0.34 (NS) |
| Oral manifestations Anticoagulant |
0.26 | 0.15 (NS) |
| Oral manifestations Antihypertensive |
0.12 | 0.49 (NS) |
| Oral manifestations Antibacterial |
0.27 | 0.14 (NS) |
| Oral manifestations Foradil |
0.12 | 0.49 (NS) |
| Oral manifestations Colchicine or hydroxychloroquine |
0.12 | 0.49 (NS) |
| Oral manifestations Acetylcysteine |
−0.39 | 0.02 (NS) |
| Oral manifestations Silymarin |
0.06 | 0.74 (NS) |
aCannot be computed because at least one of the variables is constant
NS = non-significant correlation
DISCUSSION
By the end of 2019, a new coronavirus caused a pneumonia outbreak in Wuhan, China, reinforcing the threat that coronaviruses offer to public health. COVID-19 affects the immune system, generating significant variations in response reactions that might turn against the host, resulting in autoimmune damage, especially to the connective tissue of the lungs. The infection pathways and pathophysiology of COVID-19, however, are still unknown.[18]
The present study demonstrated the spread of oral manifestations in 124 COVID-19 patients. About 90% of the patients represented oral lesions, remarkably oral ulcers, despite the severity of the disease. The most common sites of oral lesions were the tongue and the labial mucosa. In addition, dry mouth was observed in most of the patients. This confirms that this could be related to the distribution of ACE2 receptors, which are highly expressed in the salivary glands. Moreover, ACE2-positive cells are enriched in epithelial cells and the oral mucosa, which express the ACE2 higher in tongue than other oral sites. SARS-CoV-2 utilizes ACE2 receptors to access the cells causing inflammatory reactions in related organs and tissues, such as the tongue mucosa and salivary glands, which leads to oral manifestations.[14]
This affects the functioning of salivary glands, taste/smell sensations, and oral mucosa integrity, interfering with dynamic oral environment also by exerting influence on microbiota balance.[19,20]
Vardhana and Wolchok[21] reported that there is a high production of inflammatory cytokines due to SARS-CoV-2 infection, which activates multiple pathways of innate immunity. Raised levels of tumor necrosis factor (TNF) can cause neutrophil chemotaxis to the oral mucosa and the formation of aphthous-like lesions in COVID-19 patients. Stress and immunosuppression as a result of SARS-CoV-2 infection could also be factors in the development of these lesions in COVID-19 patients.[22] Oral candidiasis was the second most oral manifestation detected. This could be attributed to the drop in the medical condition, long-term antibiotic therapy, and deterioration in the oral hygiene.[1,22,23]
Oral hyperpigmentation represented 25% of oral manifestations. This could be related to higher levels of inflammatory cytokines such as interleukin-1 (IL-1), TNF-α, and prostaglandins.[4,23]
In our study, about 45% of the patients were only systemically compromised. The oral lesions were detected in more than 90% of the patients enrolled in the study no matter what the severity of the disease is. Additionally, not all patients received the same protocol of treatment. This was contradicted by studies which hypothesized that oral lesions are secondary lesions resulting from the worsening of systemic health or due to medications used for treatments for COVID-19.[24] Besides, Wu et al.[25] found that a lack of mouth care due to external ventilation as in medically compromised and severe cases can result in the deterioration of oral health rapidly. This could be attributed to the course of the SARS-CoV-2 infection, which adversely affects the immune response and leads to high release of inflammatory mediators that are responsible to the development of many oral lesions.
Many approaches to improving immune responses are necessary. COVID-19 patients are treated with a variety of medicines. Instead of fighting the virus directly, they use anti-inflammatories, immuno-modulatory, and/or monoclonal antibodies to regulate the immune response associated with COVID-19 infection, despite the fact that no medications have been proven to be helpful in the treatment of SARS-CoV-2 infection.[17]
Prednisolone was taken by almost 60% of the patients for modulation of the immune response in the majority of severe inflammatory disorders.[4] Antiviral treatment using iverzine (ivermectin) was taken which can provide a course of action to decrease the viral load, the severity, and unfavorable clinical outcomes.[26] It was reported that less than 2% have side effects affecting the oral mucosa from antiviral treatment.[27] On the contrary, antibiotics significantly affect the microbial balance.[28] This could be correlated to the Candida infection presented in less than 50% of the patients. Remdesivir and chloroquine/hydroxychloroquine were prescribed for a small percentage of the patients for potential treatment of severe COVID-19 complications.[29,30]
Within the limitation of this study, it was concluded that the tongue represents the most common site of oral lesions in COVID-19 patients due to high expression of ACE2 on the oral mucosa. Oral lesions seemed to be not correlated to the drugs used for the treatment of SARS-CoV-2. Spread of oral lesions in COVID-19 patients is detected despite the systemic health and the severity of the disease.
FINANCIAL SUPPORT AND SPONSORSHIP
No financial support or sponsorship received, this study is self-funded.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS CONTRIBUTIONS
All authors contributed in the study design, data collection and analysis, study writing, and revision process.
ETHICAL POLICY AND INSTITUTIONAL REVIEW BOARD STATEMENT
The protocol of the present study was reviewed and approved by the Ethics Committee of Faculty of Dentistry, Kafrelsheikh University (No. DK/04/20) and was registered at ClinicalTrial.gov (NCT04917549). Our study protocol followed the Helsinki Principles Declaration stated in 1975.
PATIENT DECLARATION OF CONSENT
All the patients had signed an informed consent and it is available upon request.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
No materials from other sources were used.
CLINICAL TRIAL REGISTRATION
This clinical trial was registered under a clinical trial registration number NCT04917549.
ACKNOWLEDGEMENTS
Not applicable.
REFERENCES
- 1.Díaz Rodríguez M, Jimenez Romera A, Villarroel M. Oral manifestations associated with COVID-19. Oral Dis. 2020 doi: 10.1111/odi.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng JL, Huang C, Zhang GJ, Liu DW, Li P, Lu CY, et al. [Epidemiological characteristics of novel coronavirus pneumonia in Henan] Zhonghua Jie He Hu Xi Za Zhi. 2020;43:327–31. doi: 10.3760/cma.j.cn112147-20200222-00148. [DOI] [PubMed] [Google Scholar]
- 3.Kwok YL, Gralton J, McLaws ML. Face touching: A frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112–4. doi: 10.1016/j.ajic.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M, et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020;42:1560–9. doi: 10.1002/hed.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–1. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: A systematic review and clinical perspective. Eur Respir J. 2020;55:2001009. doi: 10.1183/13993003.01009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–20. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 11.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoVinfection. Front Med. 2020;14:185–92. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, et al. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441–51. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariz BALA, Brandão TB, Ribeiro ACP, Lopes MA, Santos-Silva AR. New insights for the pathogenesis of COVID-19-related dysgeusia. J Dent Res. 2020;99:1206. doi: 10.1177/0022034520936638. [DOI] [PubMed] [Google Scholar]
- 16.Sakaida T, Tanimoto I, Matsubara A, Nakamura M, Morita A. Unique skin manifestations of COVID-19: Is drug eruption specific to COVID-19? J Dermatol Sci. 2020;99:62–4. doi: 10.1016/j.jdermsci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dziedzic A, Wojtyczka R. The impact of coronavirus infectious disease 19 (COVID-19) on oral health. Oral Dis. 2021;27:703–6. doi: 10.1111/odi.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92:2249. doi: 10.1002/jmv.26234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovato A, de Filippis C, Marioni G. Upper airway symptoms in coronavirus disease 2019 (COVID-19) Am J Otolaryngol. 2020;41:102474. doi: 10.1016/j.amjoto.2020.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabino-Silva R, Jardim ACG, Siqueira WL. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin Oral Investig. 2020;24:1619–21. doi: 10.1007/s00784-020-03248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med. 2020;217:e20200678. doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, De Paula RM, Cembranel AC, Santos-Silva AR, et al. Oral mucosal lesions in a COVID-19 patient: New signs or secondary manifestations? Int J Infect Dis. 2020;97:326–8. doi: 10.1016/j.ijid.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corchuelo J, Ulloa FC. Oral manifestations in a patient with a history of asymptomatic COVID-19: Case report. Int J Infect Dis. 2020;100:154–7. doi: 10.1016/j.ijid.2020.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2020;27(Suppl. 3):710–12. doi: 10.1111/odi.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–43. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, et al. HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax. 2004;59:252–6. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Center for Biotechnology Information 2021. PubChem Compound Summary for CID 392622, Ritonavir. https://pubchem.ncbi.nlm.nih.gov/compound/Ritonavir.
- 28.Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr T, Andersen MH, et al. Procalcitonin and Survival Study Group. Invasive Candida infections and the harm from antibacterial drugs in critically ill patients: Data from a randomized, controlled trial to determine the role of ciprofloxacin, piperacillin-tazobactam, meropenem, and cefuroxime. Crit Care Med. 2015;43:594–602. doi: 10.1097/CCM.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 29.Mahase E. Covid-19: What treatments are being investigated? Br Med J. 2020;368:m1252. doi: 10.1136/bmj.m1252. [DOI] [PubMed] [Google Scholar]
- 30.Sayburn A. Covid-19: Trials of four potential treatments to generate “robust data” of what works. Br Med J. 2020;368:m1206. doi: 10.1136/bmj.m1206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.