Abstract
Ethnopharmacological relevance:
Nimbolide is one of hundreds of phytochemicals that have been identified within the neem tree (Azadirachta indica A. Juss). As an evergreen tree native to the Indian subcontinent, components of the neem tree have been used for millennia in traditional medicine to treat dental, gastrointestinal, urinary tract, and blood-related ailments, ulcers, headaches, heartburn, and diabetes. In modern times, natural oils and extracts from the neem tree have been found to have activities against a variety of microorganisms, including human pathogens.
Aim of the study:
Helicobacter pylori, a prevalent gastric pathogen, shows increasing levels of antibiotic resistance. Thus, there is an increasing demand for novel therapeutics to treat chronic infections. The in vitro activity of neem oil extract against H. pylori was previously characterized and found to be bactericidal. Given the numerous phytochemicals found in neem oil extract, the present study was designed to define and characterize specific compounds showing bactericidal activity against H. pylori.
Materials and methods:
Azadirachtin, gedunin, and nimbolide, which are all common in neem extracts, were tested for antimicrobial activity; the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined for nine strains of H. pylori. The specific properties of nimbolide were further characterized against H. pylori strain G27. Bactericidal kinetics, reversibility, effectiveness at low pH, and activity under bacteriostatic conditions were examined. The hemolytic activity of nimbolide was also measured. Finally, neem oil extract and nimbolide effectiveness against H. pylori biofilms were examined in comparison to common antibiotics used to treat H. pylori infection.
Results:
Nimbolide, but not azadirachtin or gedunin, were effective against H. pylori; MICs and MBCs against the nine tested strains ranged between 1.25–5 μg/mL and 2.5–10 μg/mL, respectively. Additionally, neem oil extract and nimbolide were both effective against H. pylori biofilms. Nimbolide exhibited no significant hemolytic activity at biologically relevant concentrations. The bactericidal activity of nimbolide was time- and dose-dependent, independent of active H. pylori growth, and synergistic with low pH. Furthermore, nimbolide-mediated H. pylori cell death was irreversible after exposure to high nimbolide concentrations (80 μg/mL, after 2 hours of exposure time and 40 μg/mL after 8 hours of exposure).
Conclusions:
Nimbolide has significant bactericidal activity against H. pylori, killing both free living bacterial cells as well as cells within a biofilm. Furthermore, the lack of hemolytic activity, synergistic activity at low pH and bactericidal properties even against bacteria in a state of growth arrest are all ideal pharmacological and biologically relevant properties for a potential new agent. This study underscores the potential of neem oil extract or nimbolide to be used as a future treatment for H. pylori infection.
Graphical Abstract
1. Introduction
In recent years, numerous bacterial pathogens have demonstrated increased resistance to antibiotics (Ventola, 2015). Helicobacter pylori, a Gram-negative, gastric-colonizing bacterium, is no exception to this trend (Jones et al., 2008; Savoldi et al., 2018; Vianna et al., 2016). Because chronic colonization by H. pylori is associated with an increased risk of peptic ulcers, gastritis, mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer (Yamamoto et al., 2015), symptomatic infection is usually met with aggressive treatment. Although colonization is asymptomatic for the majority of individuals, those who are colonized are at an increased risk for serious gastric diseases (Yamamoto et al., 2015); ~1% of those infected with H. pylori will develop gastric cancer (Kuipers, 1999). As such, and given that an estimated 50% of the world’s human population is colonized (Moodley et al., 2012), there are nearly one million new cases of gastric cancer diagnosed every year (Bray et al., 2018).
The most common treatment regimen for H. pylori infection is currently triple therapy (Thung et al., 2016), though newer quadruple, sequential, and concomitant therapies are also used (Kim et al., 2015). However, the efficacy of the aforementioned treatments has been diminished (De Francesco et al., 2010) by increased resistance to commonly used antibiotics like tetracycline, clarithromycin, levofloxacin, metronidazole, and amoxicillin (Gisbert et al., 2000; Kim et al., 2015; Vianna et al., 2016). Though levels of resistance differ by region and by drug, there is a general trend towards decreased sensitivity of H. pylori to all of these antibiotics. Worldwide, amoxicillin resistance is still uncommon (Pajares Garcia et al., 2007), though these rates are rising as well (Savoldi et al., 2018). The most updated reviews estimate tetracycline resistance to be around 13% (Savoldi et al., 2018). However, the number of strains resistant to clarithromycin and levofloxacin has been reported to be greater than 15% worldwide (Savoldi et al., 2018). Conversely, resistance to metronidazole in some regions can be as high as 85% (Seck et al., 2013) to 100% (Kumala and Rani, 2006). Given that eradication success rates for these first-line treatments have dropped to 70–85%, there is a pressing need for new medicinal options to combat H. pylori (Chey et al., 2007).
Research has begun to focus on phytochemicals, compounds extracted from plants (Barbieri et al., 2017), as viable alternatives to traditional antibiotics for the treatment of bacterial infections. As such, modern science is increasingly taking note of traditional medicines and folk remedies as potential sources of novel drugs for a wide range of infectious diseases (Barbieri et al., 2017; Snow Setzer et al., 2016). Studies on this topic have yielded promising results; 73% of the small molecules that were approved world-wide for treatment in the past four decades are unaltered derivatives of natural plant products (Newman and Cragg, 2016).
Recent interest has focused on the plant Azadirachta indica, which is more commonly known as the neem tree (Gupta et al., 2017). The extracts, oils, leaves, and seeds of the neem tree have been used in traditional medicine in the Indian subcontinent for at least two millennia (Kumar and Navaratnam, 2013; Nadkarni, 1954). Neem has been used to treat a variety of ailments, such as diabetes, headaches, and hypertension (Gupta et al., 2017; Nadkarni, 1954). Additionally, modern research has found that neem also shows promise in treating ulcers (Bandyopadhyay et al., 2004; Chattopadhyay et al., 2004), cancer (Hao et al., 2014), fungal infections (Al Saiqali et al., 2018), parasites (Dahiya et al., 2016), and viral infections (Parida et al., 2002). Furthermore, neem extract has also been found to have antibacterial properties against a wide range of both Gram-positive and Gram-negative bacteria (Al Akeel et al., 2017; Del Serrone et al., 2015; Fabry et al., 1998; Singaravelu et al., 2019).
Previously, our lab characterized the in vitro antibacterial activity of neem oil extract against H. pylori (Blum et al., 2019). The extract was effective against multiple strains of H. pylori and displayed synergy with low pH, suggesting that neem oil extract has potential as an alternative to traditional antibiotics (Blum et al., 2019). The compound(s) responsible for these activities and its respective mechanism of action, however, was not determined; A. indica contains more than 300 phytochemicals that have been identified to date (Gupta et al., 2017). Given that neem oil can be cytotoxic to small children (Dhongade et al., 2008; Kumar and Kumar, 2014; Sundaravalli et al., 1982), determination of which components are specifically effective against H. pylori would be advantageous.
The presence of H. pylori in biofilm-like structures in human patients has been documented (Carron et al., 2006; Coticchia et al., 2006). However, the role of biofilms in carcinogenesis has yet to be established (Rizzato et al., 2019). Biofilms are successful communities of bacteria that are known to play an important role in colonization by many human pathogens (Jamal et al., 2018). Pathogenic biofilms display an increased tolerance to antibiotics and are overall less sensitive to environmental stressors and attacks from the host immune system (Attaran et al., 2017; Bae and Jeon, 2013; de Vor et al., 2020; Hall-Stoodley et al., 2004; Mah and O’Toole, 2001; Stewart and Costerton, 2001; Yonezawa et al., 2019). For these reasons, biofilms have gained the attention of many biomedical researchers in the last few decades.
To identify a specific compound in neem oil extract that is responsible for bactericidal activity against H. pylori, we focused on three commercially available limonoids that each have established antimicrobial properties against other bacteria (Atawodi and Atawodi, 2009): azadirachtin, gedunin, and nimbolide. The bactericidal activity of all three compounds was characterized against nine different strains of H. pylori. In contrast to their bactericidal effects on other species of bacteria (Kankariya et al., 2016; Lu et al., 2019; Shah et al., 2016), azadirachtin and gedunin had no effect on H. pylori. However, nimbolide was effective against H. pylori. Further characterization revealed that the bactericidal activity of nimbolide was both time- and dose-dependent, independent of active H. pylori growth, and synergistic with low pH. Furthermore, both neem oil extract and nimbolide were more bactericidal and more effective at biofilm dispersal than amoxicillin. Importantly, we found that nimbolide-mediated cell death was irreversible at high concentrations. Finally, nimbolide showed no significant hemolytic activity. Overall, our results support future in-depth studies to assess the potential use of nimbolide to treat H. pylori infections as an effective alternative to, or in addition to, currently prescribed antibiotics.
2. Materials and Methods
2.1. Bacterial strains and growth conditions
The strains used in this study are listed in Table 1; 26695, J99, and G27 are common laboratory strains; 7.13 and SS1 are strains used in animal infection models; USU103 and UA1182 are multi-drug resistant strains. Strains were grown on horse blood agar (HBA) composed of 4% Columbia agar (Neogen Corporation), 5% defibrinated horse blood (HemoStat Laboratories, Dixon, CA), 2 mg/mL cyclodextrin (Sigma-Aldrich), and an antibiotic-antifungal cocktail composed of 10 μg/mL vancomycin (Amresco), 5 μg/mL cefsulodin (Sigma-Aldrich), 2.5 U/mL polymyxin B (Sigma-Aldrich), 5 μg/mL trimethoprim (Sigma-Aldrich), and 8 μg/mL amphotericin B (Amresco), or in liquid brucella broth (BB) (Neogen Corporation) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 10 μg/mL vancomycin. Freezing media, consisting of brain heart infusion broth (BD Biosciences) containing 10% FBS and 20% glycerol (EMD Chemicals, Inc.), was used for the creation and storage of H. pylori −80°C stock cultures.
Table 1.
Helicobacter pylori strains used in this study.
| Strain | Lab strain designation | Reference |
|---|---|---|
| G27 | DSM1 | (Baltrus et al., 2009) |
| 26695 | DSM18 | (Tomb et al., 1997) |
| HPAG1 | DSM360 | (Oh et al., 2006) |
| SS1 | DSM136 | (Lee et al., 1997) |
| J99 | DSM19 | (Alm et al., 1999) |
| 7.13 | DSM48 | (Franco et al., 2005) |
| USU101 | DSM442 | (Liu et al., 2009) |
| USU103 | DSM439 | (Makobongo et al., 2013) |
| UA1182 (ATCC 700684) | DSM1421 | (Wang et al., 1998) |
H. pylori strains were grown as previously described (Carpenter et al., 2015). Briefly, strains were cultured in gas evacuation jars at 37°C under microaerobic conditions (5% O2, 10% CO2, and 85% N2); these conditions were generated with an Anoxomat gas evacuation and replacement system (Advanced Instruments, Inc.). Liquid cultures were grown shaking at 110 RPM.
2.2. Determination of MIC and MBC
The MIC and MBC were determined as previously described (Blum et al., 2019). Briefly, H. pylori liquid cultures of the examined strains (Table 1) were inoculated from an overnight liquid culture to an optical density at 600 nm (OD600) of 0.05. One-mL aliquots were transferred to sterile 15-mm-diameter glass test tubes and azadirachtin, gedunin, or nimbolide was added to the desired final concentrations of 0.625, 1.25, 2.5, 5, 10, 20, and 40 μg/mL. Nimbolide was obtained from Cayman Chemical (batch number 0517438). The certificate of analysis for the product indicated a purity of 97.3 % by HPLC and 100% by TLC. As a solvent control, the volume of dimethyl sulfoxide (DMSO, Sigma-Aldrich) needed to achieve the highest concentration of each compound was also added to a separate culture in BB + 10% FBS plus vancomycin. An aliquot of H. pylori was retained at the start of the experiment, serially diluted in BB and plated to determine the colony-forming units (CFU) at T0. The 1-mL cultures were then incubated for 24 hours and an aliquot was also serially diluted and plated to determine CFU. After 3–4 days of growth, the CFU were quantified. Percent survival was calculated for each concentration of the three compounds with the equation T24/T0 ×100%. The MIC and MBC were calculated for each replicate and were defined as the lowest tested concentration of azadirachtin, gedunin, or nimbolide at which ≤ 100% and ≤ 0.1% of bacteria, respectively, remained after 24 hours. Limit of detection (LOD) for this assay was 500 CFU/mL. Three biological replicates were performed for each strain.
2.3. Bactericidal kinetics of nimbolide
The bactericidal kinetics were determined as previously described (Blum et al., 2019). Briefly, an overnight liquid culture of H. pylori G27 in BB + 10% FBS plus vancomycin was subcultured to an OD600 of 0.05 and 1-mL aliquots were transferred to 15-mm-diameter glass test tubes. Nimbolide was then added to the desired concentration (0–160 μg/mL). The same volume of DMSO found in the highest concentration of nimbolide was used as a solvent control. After 1, 2, 4, 6, 8, 22, and 24 hours of culture, an aliquot was removed, serially diluted, and plated to determine CFU. After 3–4 days of growth, CFU were enumerated. LOD for this assay was 500 CFU/mL. Three biological replicates were performed.
2.4. Nimbolide activity at low pH
As described previously (Blum et al., 2019), the pH of liquid media (BB + 10% FBS plus vancomycin) was adjusted to pH 4.6 by the addition of 6M hydrochloric acid. The pH-adjusted media was filter-sterilized with a 0.22 μm filter (Corning, Inc.). An overnight liquid culture of H. pylori G27 was used to inoculate fresh media at an OD600 of 0.05 with H. pylori cells that were pelleted and then resuspended in pH-adjusted media. A culture of cells resuspended in unadjusted media was used as a control. One-mL aliquots of culture were transferred to glass test tubes and nimbolide was added to 0, 2.5, 10, or 40 μg/mL. After 2, 4, 6, 8, and 24 hours of culture, an aliquot was removed, serially diluted, and plated to determine CFU. LOD for this assay was 500 CFU/mL. Four biological replicates were performed.
2.5. Effect of growth arrest on bactericidal activity
The assay was performed similarly to that described for neem oil extract (Blum et al., 2019). An overnight liquid culture of H. pylori G27 was subcultured to an OD600 of 0.05 in BB + 10% FBS plus vancomycin with or without 10 μg/mL chloramphenicol. One-mL aliquots were transferred to glass test tubes and nimbolide was added to 0, 2.5, 10, or 40 μg/mL. After 2, 4, 6, 8, and 24 hours of culture, an aliquot was removed, serially diluted, and plated to determine CFU. LOD for this assay was 500 CFU/mL. Three biological replicates were performed.
2.6. Reversibility of nimbolide exposure
The assay was performed as previously described (Blum et al., 2019). Briefly, an overnight liquid culture of strain G27 was subcultured to an OD600 of 0.05 and 1-mL aliquots were transferred to glass test tubes. Nimbolide was added to 0, 10, 40, or 80 μg/mL. After 2, 4, 6, 8, and 24 hours of culture, an aliquot was removed, serially diluted, and plated to determine CFU. After plating for CFU, at the 2-, 4-, 6-, or 8-hour time points, the culture was transferred to a 1.5-mL Eppendorf tube and washed 3 times by repeated centrifugation at 2000×g for 2 min, removal of supernatant, and resuspension in fresh media. After the third wash, the cells were transferred to a fresh glass test tube. Then, an aliquot was removed to measure the remaining CFU before the culture was returned to the incubator. Viable CFU were determined for the remaining time points as described above. LOD for this assay was 500 CFU/mL. Three biological replicates were performed.
2.7. Hemolytic activity of nimbolide
The assay was performed as previously published (Blum et al., 2019). Briefly, centrifugation for 5 min at 2700×g and resuspension in phosphate-buffered saline (PBS) was repeated three times in order to wash horse red blood cells (RBC, Hemostat) before resuspension to 10% hematocrit. 200-μL aliquots of serial 2-fold dilutions of nimbolide in PBS and were transferred to a flat-bottomed 96-well microtiter plate. Fifty microliters of 10% hematocrit RBCs were added to the microtiter plate to yield a final concentration of 2% hematocrit. Nimbolide concentrations were calculated to achieve final concentrations of 1.25 to 160 μg/mL after addition of RBCs. RBCs were resuspended in distilled water as a positive control for hemolysis. PBS was used to measure background hemolysis and the same volume of DMSO found in the highest concentration of nimbolide was included to measure solvent-induced lysis. The plate was centrifuged at 3452×g for 5 min after 1 hour of incubation at 37°C shaking at 220 RPM. Two hundred microliters of the resulting supernatant were transferred to a new flat-bottomed 96-well microtiter plate. Hemoglobin release was measured at 540 nm with a BioTek Synergy HTX Multi-Mode Reader (program Gen5, version 3.00). Percent hemolysis was calculated as: [(Abs540 in nimbolide − Abs540 in PBS) / (Abs540 in water)] × 100%. Six biological replicates were performed.
2.8. Effect of neem extract, nimbolide, and antibiotics against biofilms
For these assays, neem oil extract, nimbolide, amoxicillin, metronidazole, or clarithromycin was used to treat pre-formed H. pylori biofilms. Neem oil extract was isolated as previously described (Blum et al., 2019). Briefly, 5 mL of certified organic neem oil from Essential Wholesale & Labs (Portland, OR; Cat. 111 No. 122–136A) was combined with 50 mL of diethyl ether and 50 mL of aqueous methanol in a separatory funnel. The solution was allowed to separate overnight and then the aqueous methanol phase was eluted into several conical tubes. Following thorough drying, the extract was dissolved in either methanol or ethanol, the insoluble material was removed by centrifugation, and the soluble fraction was retained. The supernatant was transferred to Eppendorf tubes, dried, and weighed. The extract was then dissolved in methanol at a concentration of 20 mg/mL and stored at −80°C. Biofilm quantification assays were performed as described previously with slight modifications (Windham et al., 2018). BB + 10% FBS supplemented with vancomycin was inoculated with an OD-controlled overnight liquid culture to an OD600 of 0.1; 1 mL was then added per well into a 24-well tissue culture-treated plate (Corning). The plates were grown at 37°C in a microaerobic environment with shaking at 110 RPM for 72 hours. At the 72-hour time point, neem oil extract, nimbolide, amoxicillin, metronidazole, or clarithromycin was added at the concentrations listed in Figures 7 and 8. The biofilms were grown for another 24 hours, at which point the wells were divided into two groups for each compound: biomass and CFU. For the biomass wells, the media was removed and wells were washed with PBS twice before drying and methanol fixation (J. T. Baker). 1% Gram’s crystal violet solution (Sigma-Aldrich), achieved by dilution with water, was then added to each well; an empty control well was used as a blank. The plates were incubated for 15 min at room temperature. Wells were then washed with distilled water three times and then air dried for 5 min at 37°C. Crystal violet was solubilized via incubation with differentiation solution (Sigma-Aldrich) for 15 minutes. Solubilized crystal violet solution was read at an absorbance of 590 (OD590) for each sample as a measure of biomass.
Fig. 7.
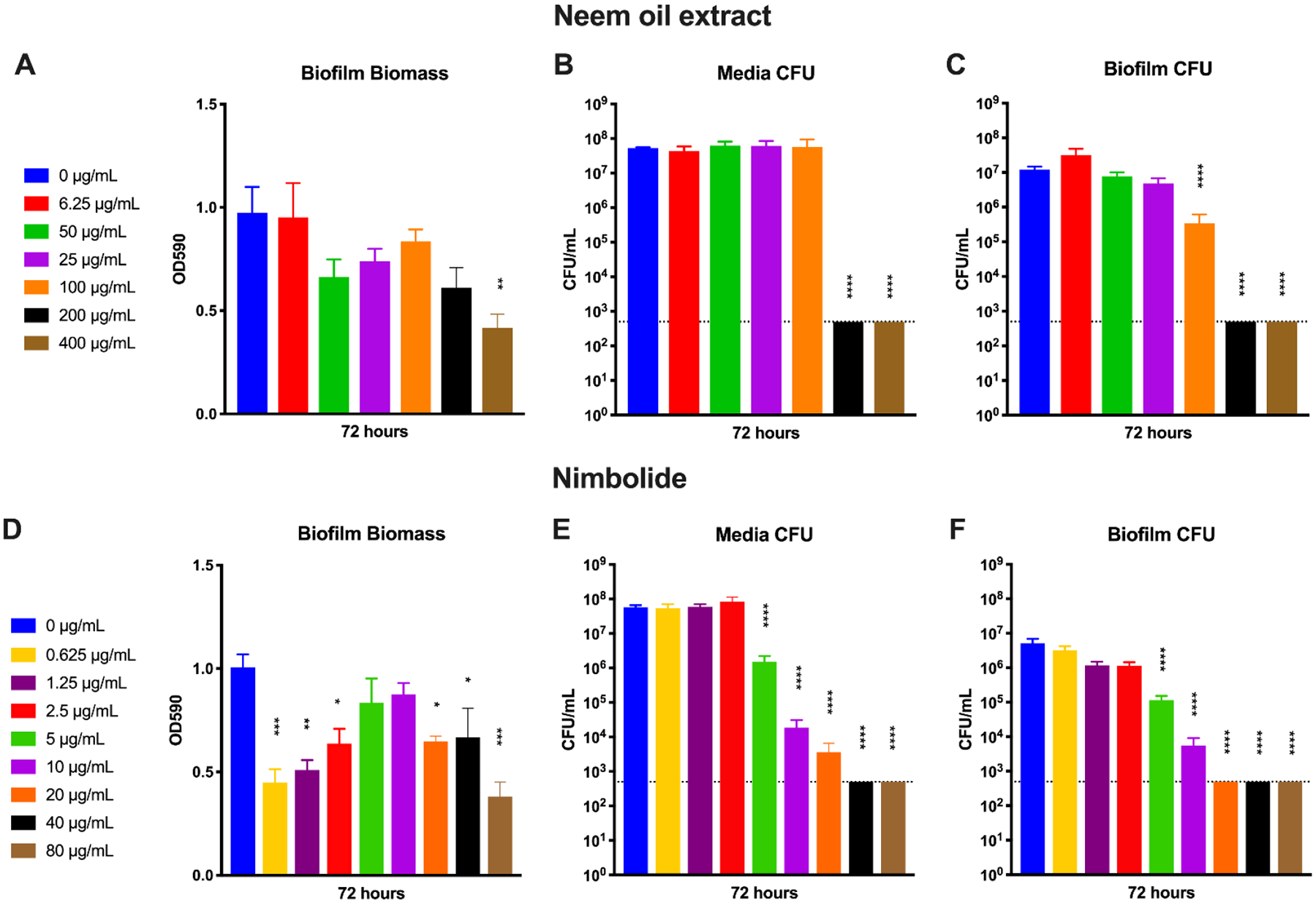
Activity of neem oil extract and nimbolide against H. pylori biofilms. An overnight culture of H. pylori G27 was diluted to an OD600 of 0.1 in fresh media and 1 mL was added to each well of a 24-well plate. The plate was incubated for 72 hours before neem oil extract was added at concentrations from 6.25 μg/mL to 400 μg/mL (A–C) or nimbolide was added at concentrations from 0.625 μg/mL to 80 μg/mL (D–F). The biofilms were grown for another 24 hours before biomass (A and D), media CFU (B and E), and biofilm CFU (C and F) were quantified using crystal violet staining or plating for CFU/mL. Untreated (0 μg/mL) wells were used as controls. Four biological replicates were performed. Mean and standard error of the mean (SEM) are graphed. The limit of detection (LOD, 500 CFU/mL) is indicated by the horizontal dotted line. A one-way ANOVA followed by Dunnett’s test for multiple comparisons was performed. **** = p < 0.0001, *** = p < 0.001, **= p < 0.005, and * = p < 0.05 for the indicated samples as compared to the untreated control sample.
Fig. 8.
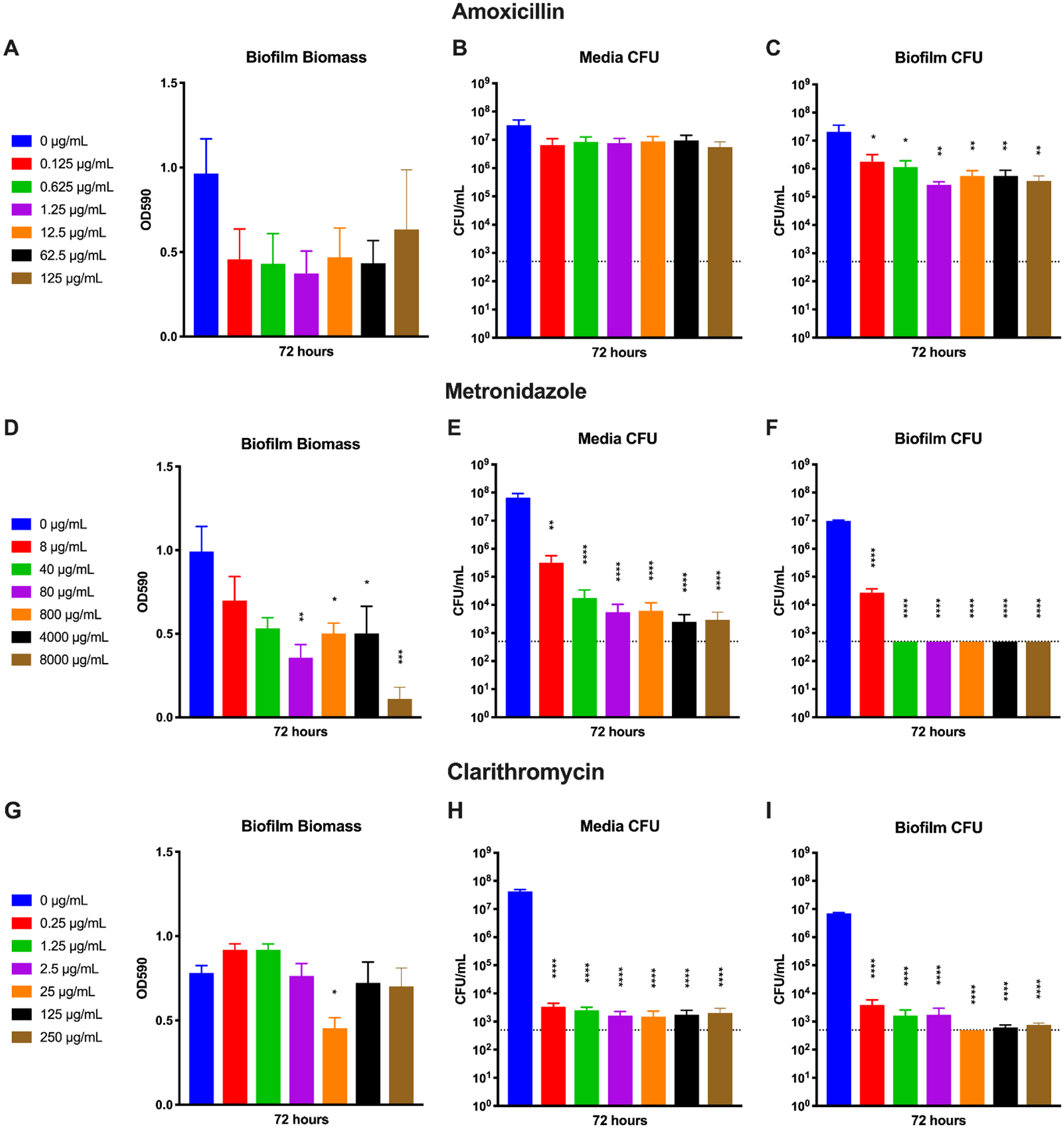
Activity of amoxicillin, metronidazole, and clarithromycin against H. pylori biofilms. An overnight culture of H. pylori G27 was diluted to an OD600 of 0.1 in fresh media and 1 mL was added to each well of a 24-well plate. The plate was incubated for 72 hours before amoxicillin was added at concentrations from 0.125 μg/mL to 125 μg/mL (A–C), metronidazole was added at concentrations from 8 μg/mL to 8000 μg/mL (D–F), or clarithromycin was added at concentrations from 0.25 μg/mL to 250 μg/mL (G–I). The biofilms were grown for another 24 hours before biomass (A, D, and G), media CFU (B, E, and H), and biofilm CFU (C, F, and I) were quantified using crystal violet staining or plating for CFU/mL. Untreated (0 μg/mL) wells were used as controls. Four biological replicates were performed. Mean and standard error of the mean (SEM) are graphed. The limit of detection (LOD, 500 CFU/mL) is indicated by the horizontal dotted line. A one-way ANOVA followed by Dunnett’s test for multiple comparisons was performed. **** = p < 0.0001, *** = p < 0.001, **= p < 0.005, and * = p < 0.05 for the indicated samples as compared to the untreated control sample.
The second set of wells was used to quantify CFU. First, the media was removed to collect the planktonic bacteria. To prevent cellular aggregates, samples were supplemented with 2 μM EDTA and sonicated for 10 min in a benchtop water bath (Branson 1510 ultrasonic cleaner). Samples were then diluted and plated onto HBA plates for later enumeration. Next, the same set of wells were washed twice with 2 ml of warm BB + 10% FBS to remove any remaining planktonic cells. To remove the cells from the surface of the polystyrene plate for biofilm CFU enumeration, the wells were then filled with 1 ml of BB + 10% FBS supplemented with 200 μg/ml proteinase K (Sigma-Aldrich) and incubated for 1 hour. The 1-mL cultures were then supplemented with 2 μM EDTA and sonicated for 10 min in the benchtop water bath to further break up cellular aggregates. These samples were then diluted and plated onto HBA plates for later enumeration. LOD for this assay was 500 CFU/mL. The data shown represent results from four biologically independent experiments.
2.9. Statistical analyses
All CFU data were log-transformed for analysis. All statistical analyses were performed with GraphPad Prism (version 8.0.2). Except where noted, all possible comparisons were performed with the complete data set in each experiment. One-way ANOVAs were used to compare data to a single control sample. Two-way ANOVAs were used for multiple comparisons and were followed by Dunnett’s correction. Two-way ANOVAs with pre-planned comparisons of interest at the indicated time points were carried out on the data sets in Figures 3 and 4, followed by manual Bonferroni adjustments.
Fig. 3.
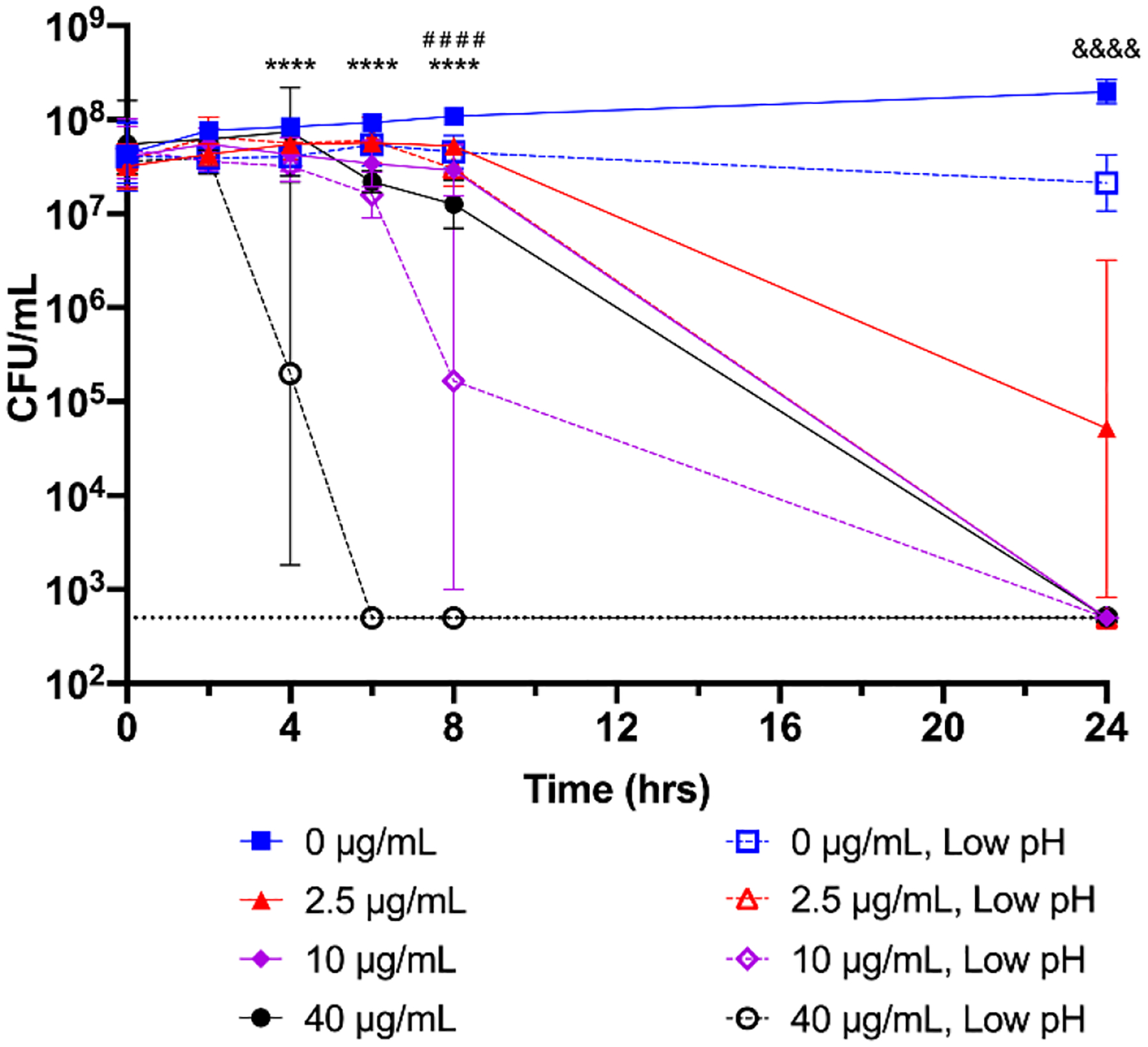
Bactericidal activity of nimbolide at low pH. An overnight culture of H. pylori G27 was diluted to an OD600 of 0.05 in standard growth media (pH ~7.0, closed symbols) or in media adjusted to pH 4.6 (open symbols). Nimbolide was added to 0, 2.5, 10, or 40 μg/mL and bacterial survival was measured at 0, 2, 4, 6, 8, and 24 h by plating for CFU/mL. The geometric mean of three biological replicates is plotted. The geometric standard deviation is indicated by the error bars; where the bars would be shorter than the height of the symbol, the error bars are not visible. The limit of detection (LOD, 500 CFU/mL) is indicated by the horizontal dotted line. Four pre-planned comparisons of interest were performed using a two-way ANOVA followed by a Bonferroni adjustment. A given concentration of nimbolide was compared to the same concentration at low pH; **** = p < 0.0001 for 40 μg/mL at the indicated time points; # # # # = p < 0.0001 for 10 μg/mL at 8 h; &&&& = p < 0.0001 for 2.5 μg/mL at 24 h.
Fig. 4.
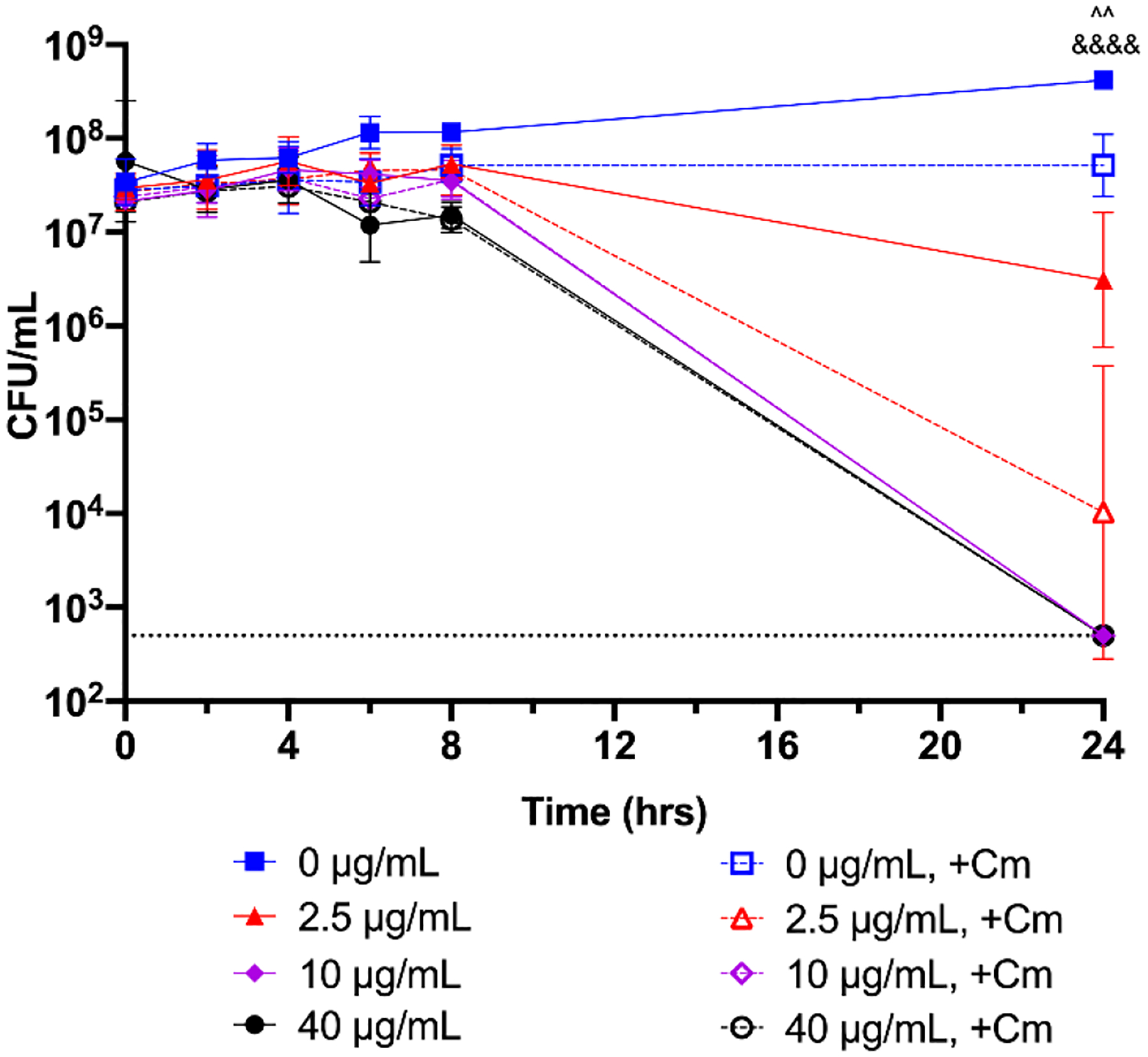
Bactericidal activity of nimbolide under bacteriostatic conditions. An overnight culture of H. pylori G27 was diluted to an OD600 of 0.05 and 10 μg/mL of chloramphenicol (+Cm, open symbols) or nothing was added (closed symbols). Nimbolide was then added to 0, 2.5, 10, or 40 μg/mL and bacterial survival was measured at 0, 2, 4, 6, 8, and 24 h by plating for CFU/mL. The geometric mean of three biological replicates is plotted. The geometric standard deviation is indicated by the error bars; where the bars would be shorter than the height of the symbol, the error bars are not visible. The limit of detection (LOD, 500 CFU/mL) is indicated by the horizontal dotted line. Four pre-planned comparisons of interest were performed using a two-way ANOVA followed by a Bonferroni adjustment. A given concentration of nimbolide was compared to the same concentration in the presence of Cm; ^^ = p < 0.01 for 0 μg/mL at 24 h; &&&& = p < 0.0001 for 2.5 μg/mL at 24 h.
3. Results
3.1. Nimbolide is effective against H. pylori, but azadirachtin and gedunin are not.
Neem oil extract was previously found to be bactericidal against H. pylori (Blum et al., 2019). In order to identify compounds responsible for this bactericidal activity, three commercially available phytochemicals were examined: azadirachtin, gedunin, and nimbolide. These compounds were chosen because they were easily available and because each has been demonstrated to have both in vitro (Braga et al., 2020; Kankariya et al., 2016; Sarkar et al., 2016) and in vivo antimicrobial activity against other pathogens (da Silva et al., 2021; Habila et al., 2011; Kumar et al., 2012; Misra et al., 2011). Thus, each was assayed for activity against H. pylori.
Neither azadirachtin nor gedunin had any effect on H. pylori G27 viability at the concentrations tested (Figure 1). In contrast, not only did nimbolide kill G27 in a dose-dependent manner (Figure 1), but it also killed eight other tested strains (Table 2). MICs ranged from 1.25–2.5 μg/mL and MBCs ranged from 2.5–10 μg/mL for the panel of standard lab strains, animal-colonizing strains, and multi-drug resistant strains. Of note, though USU103 and UA1182 are multi-drug resistant (Makobongo et al., 2013; Wang et al., 1998), both strains were sensitive to nimbolide. The bactericidal kinetics of nimbolide activity were next assessed using strain G27 (Figure 2). At concentrations of 5 μg/mL and higher, the cultures experienced population loss to below the limit of detection by 22 hours. As expected, only a slight decrease in viable cells was observed for 2.5 μg/mL nimbolide, which represents the MIC for G27 (Table 2). Together, these results indicate that nimbolide shows activity against numerous H. pylori strains and suggest that nimbolide is a major bactericidal component of neem oil extract.
Fig. 1.
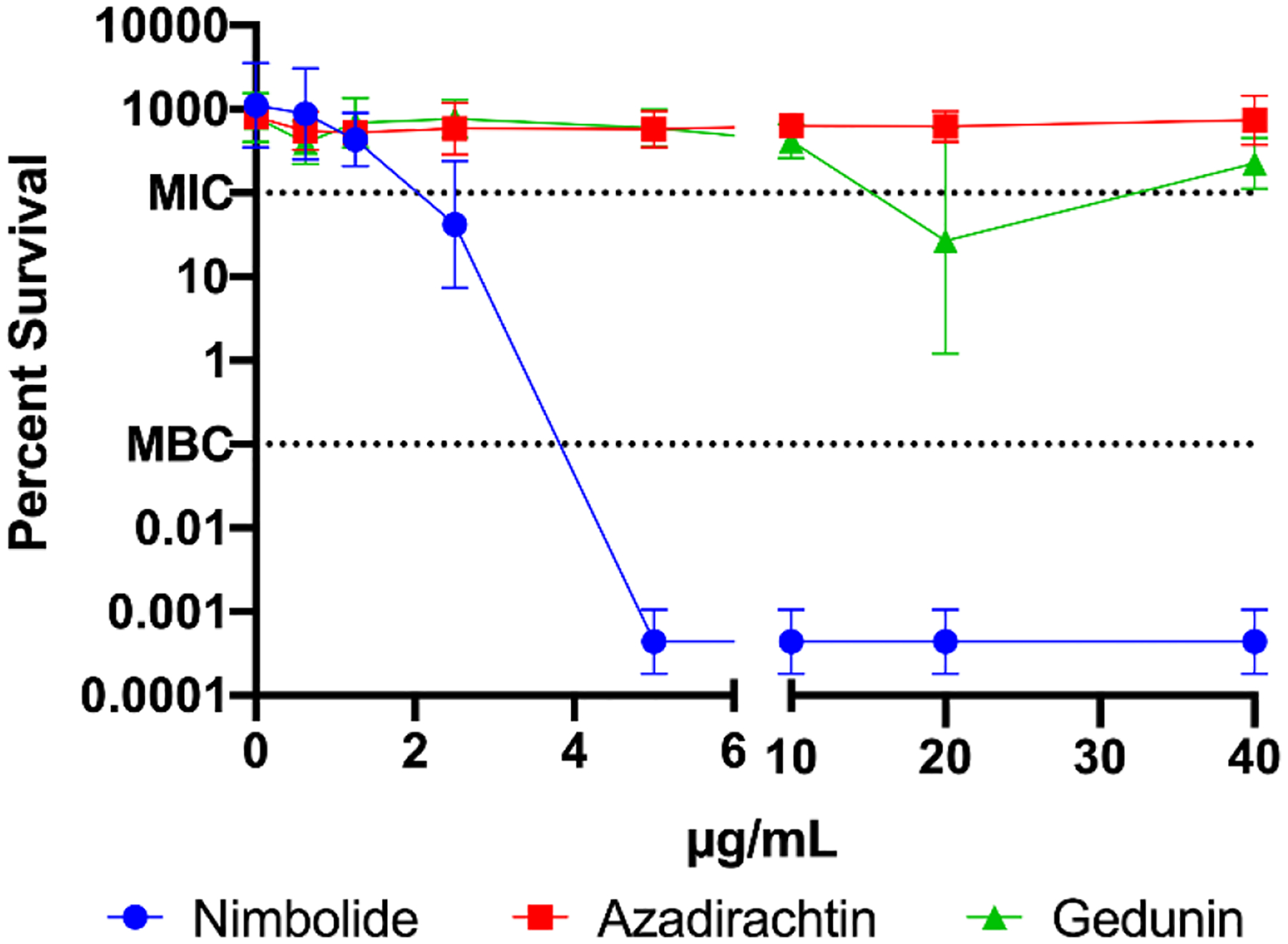
Bactericidal activity of nimbolide, azadirachtin, and gedunin against H. pylori strain G27. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the three compounds were determined. H. pylori G27 cultures were diluted to an OD600 of 0.05 in fresh media and exposed to either nimbolide, azadirachtin, or gedunin at the indicated concentrations for 24 hours. To determine CFU, bacteria were plated at 0 (T0) and 24 (T24) hours. The equation T24/T0 × 100% was used to calculate percent survival. The geometric mean of three biological replicates is plotted. The geometric standard deviation is indicated by the error bars; where the bars would be shorter than the height of the symbol, the error bars are not visible. The MIC (100% survival) and MBC (0.1% survival) are indicated using dashed lines.
Table 2.
Antibacterial activity of nimbolide.
| H. pylori strain | Nimbolide concentration (μg/mL) | |
|---|---|---|
| MIC | MBC | |
| G27 | 2.5 | 5 |
| 26695 | 1.25 | 10 |
| J99 | 1.25 | 5 |
| 7.13 | 2.5 | 5 |
| SS1 | 2.5* | 5 |
| HPAG1 | 2.5 | 10* |
| USU101 | 2.5* | 10 |
| USU103 | 2.5 | 5 |
| UA1182 | 2.5 | 10 |
range was not within 2-fold over the 3 biological replicates; where this is the case, the highest obtained MIC or MBC is reported
Fig. 2.
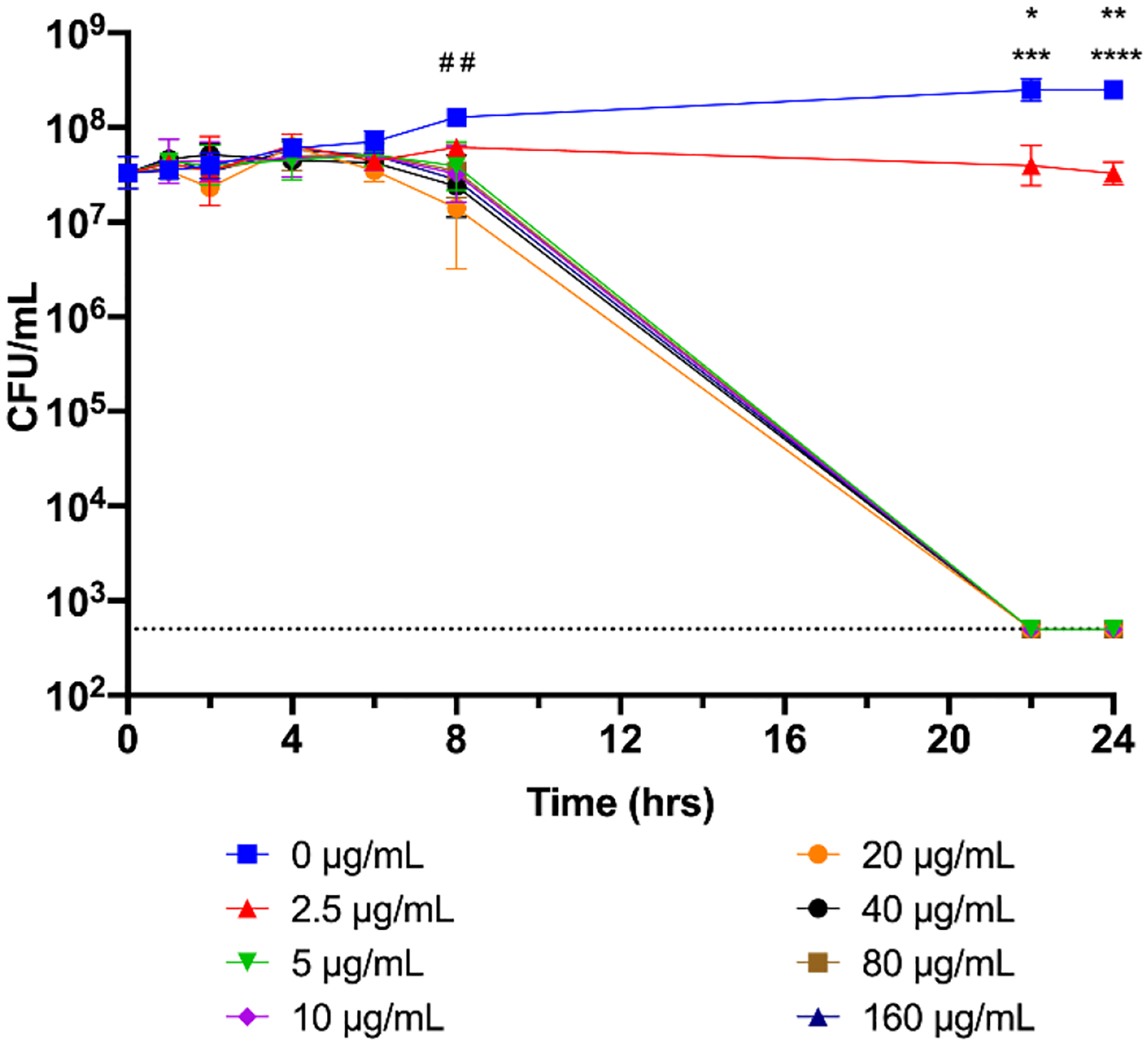
Bactericidal kinetics of nimbolide. An overnight culture of H. pylori G27 was diluted to an OD600 of 0.05 and nimbolide or DMSO was added; the same volume of DMSO found in the highest concentration of nimbolide was used as a solvent control. Survival at 0, 1, 2, 4, 6, 8, 22, and 24 h was assayed by plating for CFU/mL. The geometric mean of three biological replicates is plotted. The geometric standard deviation is indicated by the error bars; where the bars would be shorter than the height of the symbol, the error bars are not visible. The limit of detection (LOD, 500 CFU/mL) is indicated by the horizontal dotted line. A two-way ANOVA followed by Dunnett’s test for multiple comparisons was performed using the complete temporal data set. # # indicates p < 0.01 for 160 μg/mL nimbolide as compared to 0 μg/mL at 8 h; * = p < 0.05 for 2.5 μg/mL vs 0 μg/mL at 22 h; *** = p <0.001 for 5, 10, 20, 40, 80, and 160 μg/mL vs 0 μg/mL at 22 h; ** = p < 0.01 for 2.5 μg/mL vs 0 μg/mL at 24 h; **** = p < 0.0001 for 5, 10, 20, 40, 80, and 160 μg/mL vs 0 μg/mL at 24 h.
3.2. Nimbolide shows a synergistic effect with low pH.
H. pylori colonizes the highly acidic environment of the stomach (Levi et al., 1989; Walsh et al., 1975). Therefore, to determine whether low pH affects the ability of nimbolide to kill H. pylori, bactericidal activity was assayed in both standard media conditions (pH ~7.0) and in acidic conditions (pH 4.6). At pH 7.0, incubation with 10 μg/mL and 40 μg/mL of nimbolide (2x and 8x the MBC, respectively) resulted in no recoverable CFU by 24 hours (Figure 3). However, at low pH, the rate of nimbolide-dependent killing was significantly enhanced; synergistic activity was present at all three tested concentrations, with no bacteria recovered after 24 hours of exposure to 2.5 μg/mL and 10 μg/mL nimbolide. This effect was even more pronounced with 40 μg/mL of nimbolide; no CFU were recovered after 6 hours of exposure to this concentration at low pH. Thus, in contrast to other antibiotics whose activity is reduced in acidic conditions (Baudoux et al., 2007; Erah et al., 1997; Falagas et al., 1997; Marcus et al., 2012; Scott et al., 2016), nimbolide, similar to neem oil extract (Blum et al., 2019), shows increased activity at low pH.
3.3. Nimbolide maintains effectiveness in bacteriostatic conditions.
Since many antibiotics target metabolic processes (Murima et al., 2014) and are ineffective against slower-growing bacteria (Eng et al., 1991; Pontes and Groisman, 2019), the activity of nimbolide was assessed upon bacterial growth arrest, which was induced by the addition of 10 μg/mL chloramphenicol (Cm). This concentration of Cm prevented H. pylori growth without affecting survival (Figure 4). At the three concentrations of nimbolide assayed (1x, 5x, and 16x the MIC), a significant difference was only observed at the 24-hour time point for 2.5 μg/mL of nimbolide in bacteriostatic conditions. At this time point, bactericidal activity was actually increased in the presence of chloramphenicol; however, the survivability of H. pylori at this concentration (2.5 μg/mL, the MIC) varied between replicates. The individual kinetics for the other concentrations were essentially identical at the other time points, regardless of the presence or absence of chloramphenicol (Figure 4). Thus, nimbolide activity against H. pylori does not require active bacterial growth.
3.4. The effect of nimbolide is not reversible at high concentrations and after extended exposure.
A desirable pharmacokinetic quality for antibiotics is the post-antibiotic effect (Spivey, 1992), which is defined as the period of time after removal of an antibiotic when there is no growth of the target organism (Zhanel et al., 1991). To test whether nimbolide possesses such an effect, bacteria were incubated with nimbolide for 2, 4, 6, or 8 hours before the culture was washed to remove the compound (Figure 5). Removal of 10 μg/mL nimbolide after 2, 4, 6, or 8 hours of exposure did not result in any observable killing of H. pylori by the 24-hour time point (Figure 5A). Thus, regardless of exposure time, the bacteria were able to recover from this low-dose challenge as compared to the unwashed control. Similarly, removal of 40 μg/mL of nimbolide after 2, 4, or 6 hours of exposure allowed for a near-complete recovery. At this concentration, however, the H. pylori population was decreased by approximately one and a half logs at the 8-hour time point and continued to die even after nimbolide was removed (Figure 5B). Notably, H. pylori was unable to survive challenge with 80 μg/mL of nimbolide, regardless of the length of exposure (Figure 5C); the number of bacteria was below the limit of detection by the end of the assay. Taken together, these data indicate that extended exposure times and/or higher concentrations of nimbolide exert an irreversible bactericidal effect on H. pylori.
Fig. 5.
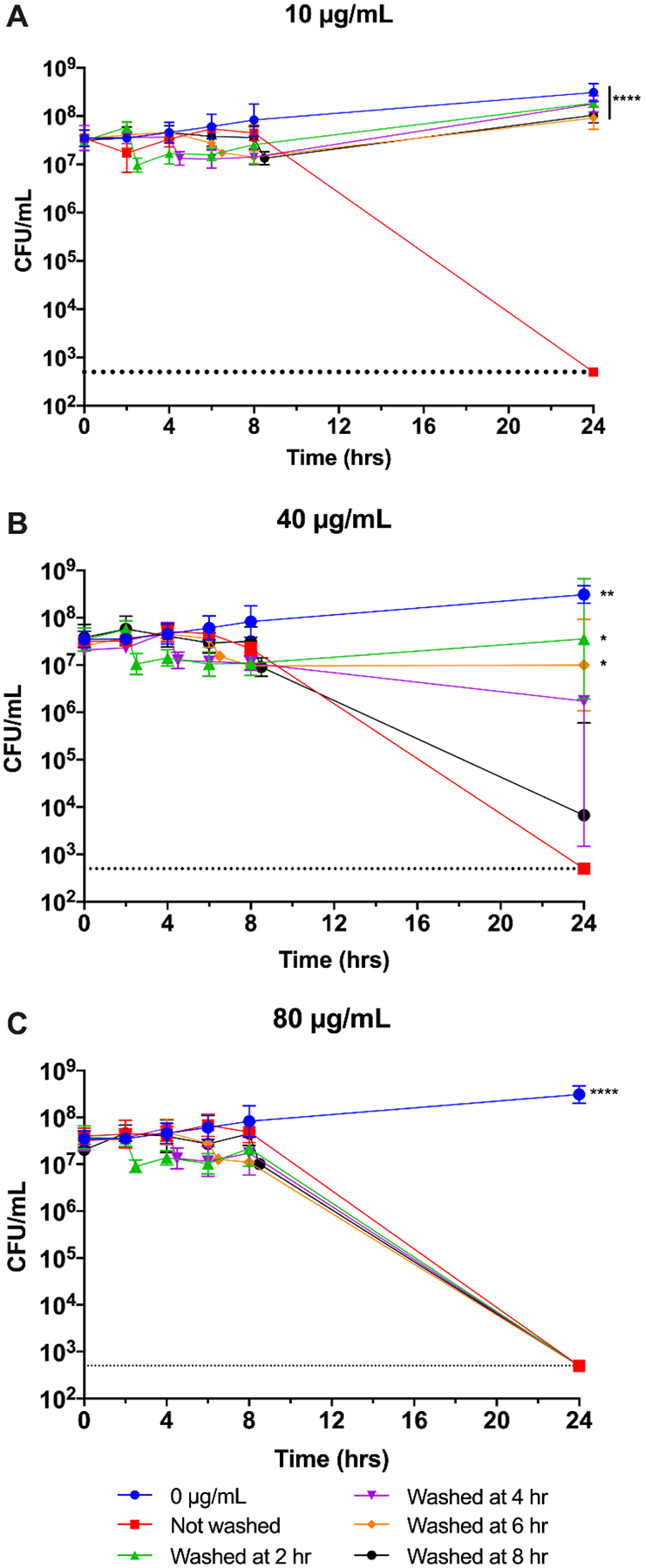
Reversibility of nimbolide. An overnight culture of H. pylori G27 was diluted to an OD600 of 0.05 and nimbolide was added to 10 μg/mL (A), 40 μg/mL (B), or 80 μg/mL (C). H. pylori grown without nimbolide present (0 μg/mL) was also assayed and is graphed in all three panels for comparison. Survival was measured at 0, 2, 4, 6, 8, and 24 h by plating for CFU/mL. Nimbolide was removed from the indicated cultures by washing at 2, 4, 6 or 8 h. The remaining cells were measured by plating for CFU/mL, and the samples were returned to the incubator to be sampled as normal for the remainder of the experiment. The geometric mean of three biological replicates is plotted. The geometric standard deviation is indicated by the error bars; where the bars would be shorter than the height of the symbol, the error bars are not visible. The limit of detection (LOD, 500 CFU/mL) is indicated by the horizontal dotted line. A two-way ANOVA followed by Dunnett’s test for multiple comparisons was performed at 24 h. **** = p < 0.0001 for the indicated conditions as compared to Not washed; ** = p < 0.01 for 0 μg/mL vs Not washed; * = p < 0.05 for Washed at 2 hr and Washed at 6 hr vs Not washed at 24 h.
3.5. Nimbolide is non-hemolytic.
In order to appeal as a potential therapeutic, a compound must not damage host cells. As an initial measure of possible mammalian cellular toxicity, the hemolytic activity of nimbolide was determined by incubation with red blood cells for 1 hour (Figure 6). Distilled water was used to achieve maximum hemolysis and DMSO was used as a control for solvent-induced lysis. Even at 160 μg/mL, well above the MBC determined for H. pylori (Table 2), minimal hemolytic activity was observed (Figure 6). Thus, nimbolide shows minimal host cell damage in this assay.
Fig. 6.
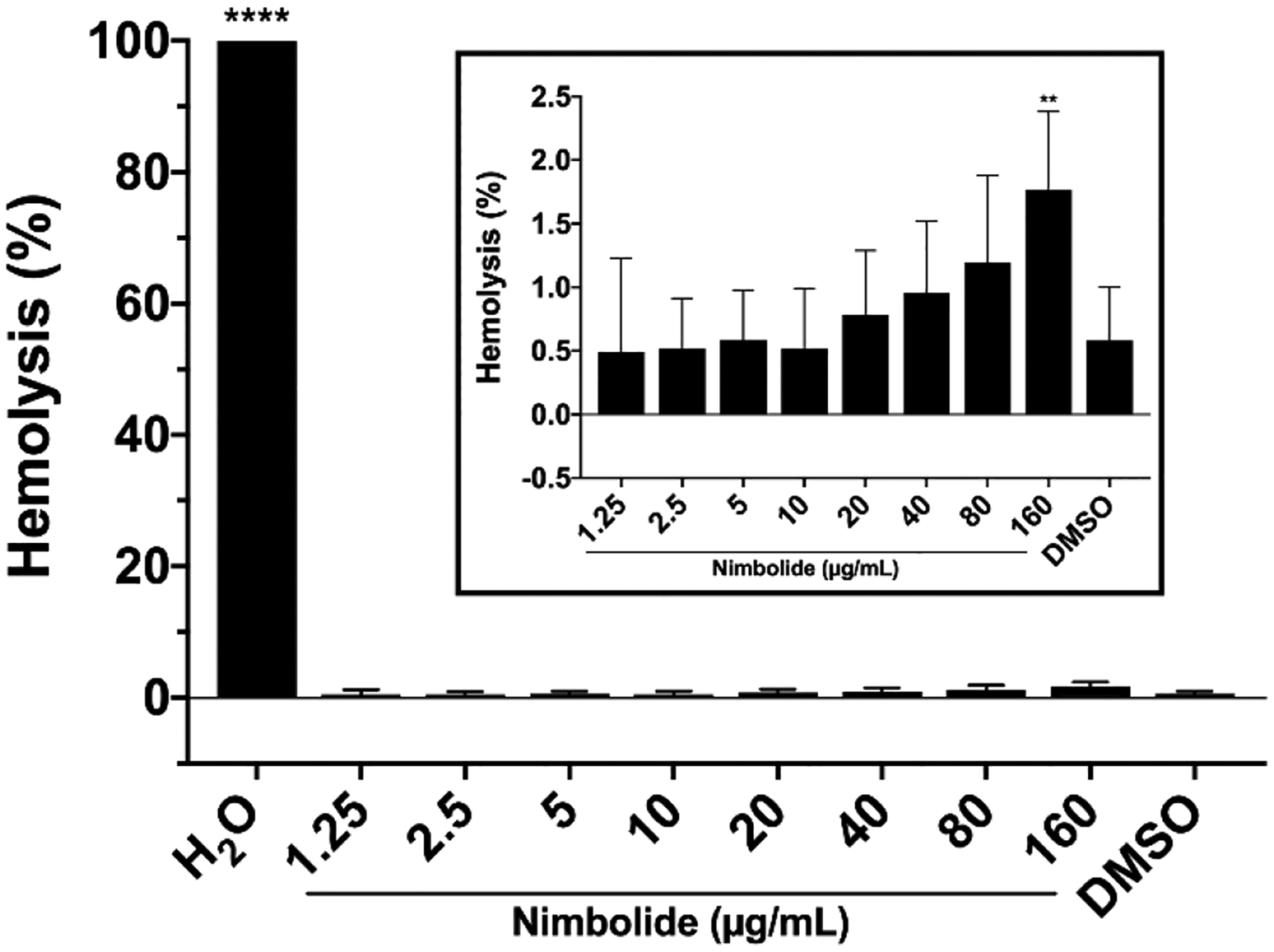
Hemolytic activity of nimbolide. Horse red blood cells (RBCs) were incubated for 1 hr at 37 °C with shaking in nimbolide diluted in PBS. RBCs were incubated in PBS to determine background absorbance, in distilled water (H2O) as a control for 100% hemolysis, or with the amount of DMSO present in the highest concentration of nimbolide to measure solvent-induced lysis. After incubation, the microtiter plate was centrifuged to pellet intact RBCs and the supernatants were transferred to a new microtiter plate. The absorbance of the supernatant was measured at 540 nm. The equation [(Abs540 in nimbolide − Abs540 in PBS) / (Abs540 in water)] × 100% was used to calculate percent hemolysis. Mean and standard deviation are graphed. Six biological replicates were performed. Inset: nimbolide and DMSO samples were re-graphed with an expanded y-axis for clarity. A one-way ANOVA followed by Dunnett’s test for multiple comparisons was performed. **** = p < 0.0001 and ** = p < 0.002 for the indicated samples as compared to the DMSO-treated control sample.
3.6. Neem and nimbolide disperse and kill H. pylori biofilms.
Biofilms have been established as an alternative growth mode that is highly advantageous to many pathogens (Jamal et al., 2018), including H. pylori (Hathroubi et al., 2018; Servetas et al., 2018; Windham et al., 2018). Biofilms can provide protection against the immune system or increase tolerance to antibiotics (Hall-Stoodley et al., 2004; Stewart and Costerton, 2001) due to the decreased metabolic activity of bacteria deep within the biofilm (Wan et al., 2018). Indeed, the tolerance of biofilm cells to antibiotics can increase over a thousand-fold (Macia et al., 2014; Olsen, 2015). Therefore, any potential therapeutic should ideally eradicate both free-living and biofilm modes of growth. To determine the efficacies of neem oil extract and nimbolide against pre-formed H. pylori biofilms, assays were performed with increasing concentrations of each compound (Figure 7). Neem oil extract showed a general trend of biofilm dispersal, though this was only statistically significant at the highest concentration (Figure 7A). In contrast, though this effect did not appear to be dose-dependent at the tested concentrations, most of the tested concentrations of nimbolide significantly dispersed biofilm biomass (Figure 7D). Furthermore, both neem extract and nimbolide killed the H. pylori cells within the media in a dose-dependent manner (Figures 7B and 7E). Importantly, neem oil extract eradicated the cells of the biofilm to below the limit of detection at the two highest concentrations tested (Figure 7C). Nimbolide was even more effective than neem oil (Figure 7F); CFU could not be recovered from the biofilm at nimbolide concentrations of 20 μg/mL or higher. Notably, this concentration is not much higher than the MBC of H. pylori in liquid culture (Table 2), which further adds to the attractiveness of nimbolide as a potential antibacterial compound.
Finally, to draw further conclusions about the possible effectiveness of neem oil extract and nimbolide as therapeutics, antibiotics commonly used as therapies for H. pylori infection were also assayed against H. pylori biofilms for comparison (Figure 8); the concentration represented by the red bar indicates the antibiotic breakpoint in each case (Chey et al., 2017). Amoxicillin treatment did not produce a statistically significant decrease in biomass (Figure 8A), nor was there an appreciable difference in viable CFU taken from the media (Figure 8B). Amoxicillin treatment did cause a statistically significant decrease in biofilm CFU (Figure 8C), though even at 1000x the MIC for amoxicillin, only a log of killing occurred. In contrast, nimbolide eliminated the biofilm CFU at only 10x the MIC (Figure 7C). Metronidazole was effective at dispersal of the biofilm at concentrations of 80 μg/mL or more (Figure 8D) and significantly killed cells within the media at all concentrations tested (Figure 8E). Additionally, treatment with metronidazole eliminated recoverable CFU from the biofilm at concentrations of 5x the MIC and higher (Figure 8F). Finally, clarithromycin did not consistently disperse the biomass at concentrations up to 1000x the MIC (Figure 8G). Even though clarithromycin efficiently killed cells in the media (Figure 8H) and in the biofilm (Figure 8I), neither of the latter metrics consistently dropped below the limit of detection. Taken together, nimbolide appeared more effective than amoxicillin in the treatment of H. pylori biofilms.
4. Discussion
The rise of antibiotic resistance in H. pylori has been declared a high priority for public health-related research (Jones et al., 2008; Savoldi et al., 2018; Vianna et al., 2016; WHO, 2017). The prevalence of this bacterial pathogen, alongside the increasing frequency of multidrug-resistant strains (Chey et al., 2017), only further emphasize the importance of novel therapeutics for this organism. To aid in this search, we previously characterized the in vitro bactericidal activity of neem oil extract against H. pylori (Blum et al., 2019). Herein, the commercially available phytochemicals azadirachtin, gedunin, and nimbolide, each derivatives of neem with known antimicrobial activities in vitro (Braga et al., 2020; Kankariya et al., 2016; Sarkar et al., 2016) and in vivo (da Silva et al., 2021; Habila et al., 2011; Kumar et al., 2012; Misra et al., 2011), were tested to determine if any of these three compounds showed antibacterial activity against H. pylori. While neither gedunin nor azadirachtin had an effect against H. pylori, nimbolide was bactericidal (Figure 1) and possessed activity against a number of strains, including those that are multi-drug resistant (Table 2). This effect was both concentration- and time-dependent (Figure 2), as well as synergistic with low pH (Figures 3). Importantly, the bactericidal activity was independent of the H. pylori growth state (Figure 4). Furthermore, at higher concentrations or with longer exposure, this effect was irreversible (Figures 5). Additionally, our biofilm results (Figures 7 and 8) imply that both nimbolide and neem oil extract are more effective at killing H. pylori biofilms than amoxicillin. Importantly, neem and nimbolide have mild to no effect on normal human cells (Hao et al., 2014; Kashif et al., 2019), and our hemolysis results support this fact (Blum et al., 2019) (Figure 6).
While our original goal was to identify specific compounds that have anti-H. pylori activity, it is important to note that the concentrations of the many phytochemicals that are found within neem oil extracts vary depending on the extraction method and part of the neem tree that is used. For example, nimbolide was found to be the compound in highest abundance within an ethyl acetate extraction of neem leaves (Santos et al., 2018). Similarly, another group used high performance thin-layer chromatography with extracts from several different parts of the neem tree and determined that the nimbolide content ranged from ~0.003% to <0.3% (Rout and Mishra, 2014). Even though nimbolide possesses strong anti-H. pylori activity (Figure 1), whether it is the only phytochemical within neem extract that possesses this activity is currently unclear. Indeed, many of the hundreds of phytochemicals present in neem extracts may have similar activities. As such, further detailed analyses will be needed to determine the relative contribution of individual phytochemicals to the antibacterial activity of the parent plant extract.
Although H. pylori resistance to first-line antibiotics is increasing (Savoldi et al., 2018), there have been no reports of resistance to neem or nimbolide in any field thus far. Our preliminary data suggest that the rate of resistance to nimbolide is less than 1.4×10−10 when cells were challenged with 10 μg/mL of the compound (data not shown). Thus, resistance to nimbolide appears to be an infrequent event. Future studies to assess the ability of nimbolide to synergize with other antibiotics used to treat H. pylori infection will be of keen interest.
Although there are a multitude of studies that describe the anti-cancer mechanisms of nimbolide (Bodduluru et al., 2014; Liu et al., 2015; Mitra and Bhattacharya, 2020; Wang et al., 2016; Zhang et al., 2016), the antimicrobial mechanisms of this compound have yet to be explored and are currently unknown. During cancer treatment and as an anti-inflammatory treatment in the context of various diseases (Anchi et al., 2021; Bansod and Godugu, 2021; Shu et al., 2021; Wang et al., 2016), nimbolide is believed to inhibit the growth of cancer cells via generation of reactive oxygen species (ROS) (Liu et al., 2015). Thus, perhaps nimbolide-induced ROS are responsible for the antimicrobial activity; this possibility remains to be explored. However, we note that it has been hypothesized that other neem extracts, and even nimbolide, have many different effects on cancer cells due to a large number of potential targets (Gupta et al., 2010; Gupta et al., 2017), which may make it difficult to pin down a specific mechanism of killing.
In this vein, it is possible that nimbolide targets numerous biological processed in H. pylori. Other members of the terpenoid class of phytochemicals have established antibacterial mechanisms that include membrane damage, inhibition of oxygen uptake, alteration of oxidative phosphorylation, and modulation of efflux pumps (Mahizan et al., 2019). On the other hand, the growth-independent and irreversible effects at higher concentrations of nimbolide could suggest a DNA-damaging mechanism. Furthermore, it is also possible that the biofilm-disruptive activity of nimbolide may be due to its interaction with proteinaceous components of the H. pylori extracellular matrix (Lahiri et al., 2021; Sarkar et al., 2016). Overall, there is still much to be defined in terms of the potential antimicrobial mechanism(s) of nimbolide and other phytochemicals. Some of these unknowns may be elucidated through future studies that test nimbolide in combination with antibiotics that have established mechanisms of action. However, we do note that the antibacterial activity of nimbolide against multidrug-resistant pathogens has begun to be investigated; one group found nimbolide to have membrane-damaging and biofilm-disrupting activities when used to treat the Gram-positive Staphylococcus aureus in vitro (Sarkar et al., 2016).
En masse, the results described herein suggest that nimbolide could serve as an attractive alternative treatment for H. pylori. To reinforce this possibility, future studies will assess the ability of nimbolide, alone and in combination with established antibiotic regimens, to clear in vivo H. pylori infections from the established small animal models for this pathogen. Additionally, subsequent work will seek to elucidate the antibacterial mechanisms of nimbolide, as well as thoroughly explore the potential for resistance development to these compounds.
Highlights:
Nimbolide has significant bactericidal activity against Helicobacter pylori
This activity is synergistic with low pH and independent of growth state
Nimbolide results in a post-antibiotic effect and is non-hemolytic
Neem oil extract and nimbolide kill cells within established H. pylori biofilms
Acknowledgments
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Uniformed Services University or the Department of Defense (DoD). H. pylori research in the Merrell lab is supported by funding from the NIH (R21AI156357) and the U.S. DoD. We would like to acknowledge Dr. Cara Olsen for assistance with statistical analyses.
Abbreviations:
- CFU
colony-forming units
- BB
brucella broth
- FBS
fetal bovine serum
- HBA
horse blood agar
- DMSO
dimethyl sulfoxide
- LOD
limit of detection
- MALT
mucosa-associated lymphoid tissue
- ROS
reactive oxygen species
- MIC
minimum inhibitory concentration
- MBC
minimum bactericidal concentration
- OD
optical density
- PBS
phosphate-buffered saline
- RBC
red blood cell
- RPM
revolutions per minute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of compounds studied: Neem oil extract (Blum et al., 2018), nimbolide (Cayman Chemical), azadirachtin (Sigma), gedunin (Cayman Chemical)
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al Akeel R, Mateen A, Janardhan K, Gupta VC, 2017. Analysis of anti-bacterial and anti oxidative activity of Azadirachta indica bark using various solvents extracts. Saudi J Biol Sci 24(1), 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Saiqali M, Tangutur AD, Banoth C, Bhukya B, 2018. Antimicrobial and anticancer potential of low molecular weight polypeptides extracted and characterized from leaves of Azadirachta indica. Int J Biol Macromol 114, 906–921. [DOI] [PubMed] [Google Scholar]
- Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, Carmel G, Tummino PJ, Caruso A, Uria-Nickelsen M, Mills DM, Ives C, Gibson R, Merberg D, Mills SD, Jiang Q, Taylor DE, Vovis GF, Trust TJ, 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397(6715), 176–180. [DOI] [PubMed] [Google Scholar]
- Anchi P, Swamy V, Godugu C, 2021. Nimbolide exerts protective effects in complete Freund’s adjuvant induced inflammatory arthritis via abrogation of STAT-3/NF-kappaB/Notch-1 signaling. Life Sci 266, 118911. [DOI] [PubMed] [Google Scholar]
- Atawodi SE, Atawodi JC, 2009. Azadirachta indica (neem): a plant of multiple biological and pharmacological activities. Phytochemistry Reviews 8(3), 601–620. [Google Scholar]
- Attaran B, Falsafi T, Ghorbanmehr N, 2017. Effect of biofilm formation by clinical isolates of Helicobacter pylori on the efflux-mediated resistance to commonly used antibiotics. World J Gastroenterol 23(7), 1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J, Jeon B, 2013. Increased emergence of fluoroquinolone-resistant Campylobacter jejuni in biofilm. Antimicrob Agents Chemother 57(10), 5195–5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltrus DA, Amieva MR, Covacci A, Lowe TM, Merrell DS, Ottemann KM, Stein M, Salama NR, Guillemin K, 2009. The complete genome sequence of Helicobacter pylori strain G27. J Bacteriol 191(1), 447–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay U, Biswas K, Sengupta A, Moitra P, Dutta P, Sarkar D, Debnath P, Ganguly CK, Banerjee RK, 2004. Clinical studies on the effect of Neem (Azadirachta indica) bark extract on gastric secretion and gastroduodenal ulcer. Life Sci 75(24), 2867–2878. [DOI] [PubMed] [Google Scholar]
- Bansod S, Godugu C, 2021. Nimbolide ameliorates pancreatic inflammation and apoptosis by modulating NF-kappaB/SIRT1 and apoptosis signaling in acute pancreatitis model. Int Immunopharmacol 90, 107246. [DOI] [PubMed] [Google Scholar]
- Barbieri R, Coppo E, Marchese A, Daglia M, Sobarzo-Sanchez E, Nabavi SF, Nabavi SM, 2017. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol Res 196, 44–68. [DOI] [PubMed] [Google Scholar]
- Baudoux P, Bles N, Lemaire S, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F, 2007. Combined effect of pH and concentration on the activities of gentamicin and oxacillin against Staphylococcus aureus in pharmacodynamic models of extracellular and intracellular infections. J Antimicrob Chemother 59(2), 246–253. [DOI] [PubMed] [Google Scholar]
- Blum FC, Singh J, Merrell DS, 2019. In vitro activity of neem (Azadirachta indica) oil extract against Helicobacter pylori. J Ethnopharmacol 232, 236–243. [DOI] [PubMed] [Google Scholar]
- Bodduluru LN, Kasala ER, Thota N, Barua CC, Sistla R, 2014. Chemopreventive and therapeutic effects of nimbolide in cancer: the underlying mechanisms. Toxicol In Vitro 28(5), 1026–1035. [DOI] [PubMed] [Google Scholar]
- Braga TM, Rocha L, Chung TY, Oliveira RF, Pinho C, Oliveira AI, Morgado J, Cruz A, 2020. Biological Activities of Gedunin-A Limonoid from the Meliaceae Family. Molecules 25(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6), 394–424. [DOI] [PubMed] [Google Scholar]
- Carpenter BM, West AL, Gancz H, Servetas SL, Pich OQ, Gilbreath JJ, Hallinger DR, Forsyth MH, Merrell DS, Michel SL, 2015. Crosstalk between the HpArsRS two-component system and HpNikR is necessary for maximal activation of urease transcription. Front Microbiol 6, 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carron MA, Tran VR, Sugawa C, Coticchia JM, 2006. Identification of Helicobacter pylori biofilms in human gastric mucosa. J Gastrointest Surg 10(5), 712–717. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay I, Nandi B, Chatterjee R, Biswas K, Bandyopadhyay U, Banerjee RK, 2004. Mechanism of antiulcer effect of Neem (Azadirachta indica) leaf extract: effect on H+-K+-ATPase, oxidative damage and apoptosis. Inflammopharmacology 12(2), 153–176. [DOI] [PubMed] [Google Scholar]
- Chey WD, Leontiadis GI, Howden CW, Moss SF, 2017. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol 112(2), 212–239. [DOI] [PubMed] [Google Scholar]
- Chey WD, Wong BC, Practice Parameters Committee of the American College of, G., 2007. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 102(8), 1808–1825. [DOI] [PubMed] [Google Scholar]
- Coticchia JM, Sugawa C, Tran VR, Gurrola J, Kowalski E, Carron MA, 2006. Presence and density of Helicobacter pylori biofilms in human gastric mucosa in patients with peptic ulcer disease. J Gastrointest Surg 10(6), 883–889. [DOI] [PubMed] [Google Scholar]
- da Silva DF, Amaral JC, Carlos RM, Ferreira AG, Forim MR, Fernandes JB, da Silva M, Filho HDC, de Souza AA, 2021. Octahedral ruthenium and magnesium naringenin 5-alkoxide complexes: NMR analysis of diastereoisomers and in-vivo antibacterial activity against Xylella fastidiosa. Talanta 225, 122040. [DOI] [PubMed] [Google Scholar]
- Dahiya N, Chianese G, Abay SM, Taglialatela-Scafati O, Esposito F, Lupidi G, Bramucci M, Quassinti L, Christophides G, Habluetzel A, Lucantoni L, 2016. In vitro and ex vivo activity of an Azadirachta indica A.Juss. seed kernel extract on early sporogonic development of Plasmodium in comparison with azadirachtin A, its most abundant constituent. Phytomedicine 23(14), 1743–1752. [DOI] [PubMed] [Google Scholar]
- De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Ierardi E, Zullo A, 2010. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis 19(4), 409–414. [PubMed] [Google Scholar]
- de Vor L, Rooijakkers SHM, van Strijp JAG, 2020. Staphylococci evade the innate immune response by disarming neutrophils and forming biofilms. FEBS Lett 594(16), 2556–2569. [DOI] [PubMed] [Google Scholar]
- Del Serrone P, Toniolo C, Nicoletti M, 2015. Neem (Azadirachta indica A. Juss) Oil to Tackle Enteropathogenic Escherichia coli. Biomed Res Int 2015, 343610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhongade RK, Kavade SG, Damle RS, 2008. Neem oil poisoning. Indian Pediatr 45(1), 56–57. [PubMed] [Google Scholar]
- Eng RH, Padberg FT, Smith SM, Tan EN, Cherubin CE, 1991. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob Agents Chemother 35(9), 1824–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erah PO, Goddard AF, Barrett DA, Shaw PN, Spiller RC, 1997. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother 39(1), 5–12. [DOI] [PubMed] [Google Scholar]
- Fabry W, Okemo PO, Ansorg R, 1998. Antibacterial activity of East African medicinal plants. J Ethnopharmacol 60(1), 79–84. [DOI] [PubMed] [Google Scholar]
- Falagas ME, McDermott L, Snydman DR, 1997. Effect of pH on in vitro antimicrobial susceptibility of the Bacteroides fragilis group. Antimicrob Agents Chemother 41(9), 2047–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M, Whitehead R, Gaus K, O’Brien DP, Romero-Gallo J, Peek RM Jr., 2005. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA 102(30), 10646–10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert JP, Gonzalez L, Calvet X, Garcia N, Lopez T, Roque M, Gabriel R, Pajares JM, 2000. Proton pump inhibitor, clarithromycin and either amoxycillin or nitroimidazole: a meta-analysis of eradication of Helicobacter pylori. Aliment Pharmacol Ther 14(10), 1319–1328. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Prasad S, Reuter S, Kannappan R, Yadav VR, Ravindran J, Hema PS, Chaturvedi MM, Nair M, Aggarwal BB, 2010. Modification of cysteine 179 of IkappaBalpha kinase by nimbolide leads to down-regulation of NF-kappaB-regulated cell survival and proliferative proteins and sensitization of tumor cells to chemotherapeutic agents. J Biol Chem 285(46), 35406–35417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Prasad S, Tyagi AK, Kunnumakkara AB, Aggarwal BB, 2017. Neem (Azadirachta indica): An indian traditional panacea with modern molecular basis. Phytomedicine 34, 14–20. [DOI] [PubMed] [Google Scholar]
- Habila N, Humphrey NC, Abel AS, 2011. Trypanocidal potentials of Azadirachta indica seeds against Trypanosoma evansi. Vet Parasitol 180(3–4), 173–178. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P, 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2(2), 95–108. [DOI] [PubMed] [Google Scholar]
- Hao F, Kumar S, Yadav N, Chandra D, 2014. Neem components as potential agents for cancer prevention and treatment. Biochim Biophys Acta 1846(1), 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathroubi S, Servetas SL, Windham I, Merrell DS, Ottemann KM, 2018. Helicobacter pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol Mol Biol Rev 82(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, Nawaz MA, Hussain T, Ali M, Rafiq M, Kamil MA, 2018. Bacterial biofilm and associated infections. J Chin Med Assoc 81(1), 7–11. [DOI] [PubMed] [Google Scholar]
- Jones KR, Cha JH, Merrell DS, 2008. Who’s Winning the War? Molecular Mechanisms of Antibiotic Resistance in Helicobacter pylori. Curr Drug ther 3(3), 190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankariya AR, Patel AR, Kunte SS, 2016. The effect of different concentrations of water soluble azadirachtin (neem metabolite) on Streptococcus mutans compared with chlorhexidine. J Indian Soc Pedod Prev Dent 34(2), 105–110. [DOI] [PubMed] [Google Scholar]
- Kashif M, Hwang Y, Kim WJ, Kim G, 2019. In-vitro Morphological Assessment of Apoptosis Induced by Nimbolide; A Limonoid from Azadirachta Indica (Neem Tree). Iran J Pharm Res 18(2), 846–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Choi DJ, Chung JW, 2015. Antibiotic treatment for Helicobacter pylori: Is the end coming? World J Gastrointest Pharmacol Ther 6(4), 183–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers EJ, 1999. Review article: exploring the link between Helicobacter pylori and gastric cancer. Aliment Pharmacol Ther 13 Suppl 1, 3–11. [DOI] [PubMed] [Google Scholar]
- Kumala W, Rani A, 2006. Patterns of Helicobacter pylori isolate resistance to fluoroquinolones, amoxicillin, clarithromycin and metronidazoles. Southeast Asian J Trop Med Public Health 37(5), 970–974. [PubMed] [Google Scholar]
- Kumar S, Kumar N, 2014. Neem oil poisoning as a cause of toxic encephalopathy in an infant. Indian J Pediatr 81(9), 955. [DOI] [PubMed] [Google Scholar]
- Kumar S, Raman RP, Kumar K, Pandey PK, Kumar N, Mohanty S, Kumar A, 2012. In vitro and in vivo antiparasitic activity of Azadirachtin against Argulus spp. in Carassius auratus (Linn. 1758). Parasitol Res 110(5), 1795–1800. [DOI] [PubMed] [Google Scholar]
- Kumar VS, Navaratnam V, 2013. Neem (Azadirachta indica): prehistory to contemporary medicinal uses to humankind. Asian Pac J Trop Biomed 3(7), 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri D, Nag M, Dutta B, Mukherjee I, Ghosh S, Dey A, Banerjee R, Ray RR, 2021. Catechin as the Most Efficient Bioactive Compound from Azadirachta indica with Antibiofilm and Anti-quorum Sensing Activities Against Dental Biofilm: an In Vitro and In Silico Study. Appl Biochem Biotechnol 193(6), 1617–1630. [DOI] [PubMed] [Google Scholar]
- Lee A, O’Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF, 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112(4), 1386–1397. [DOI] [PubMed] [Google Scholar]
- Levi S, Beardshall K, Swift I, Foulkes W, Playford R, Ghosh P, Calam J, 1989. Antral Helicobacter pylori, hypergastrinaemia, and duodenal ulcers: effect of eradicating the organism. BMJ 299(6714), 1504–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Merrell DS, Semino-Mora C, Goldman M, Rahman A, Mog S, Dubois A, 2009. Diet synergistically affects Helicobacter pylori-induced gastric carcinogenesis in nonhuman primates. Gastroenterology 137(4), 1367–1379 e1361–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JF, Hou CH, Lin FL, Tsao YT, Hou SM, 2015. Nimbolide Induces ROS-Regulated Apoptosis and Inhibits Cell Migration in Osteosarcoma. Int J Mol Sci 16(10), 23405–23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XF, Lin PC, Zi JC, Fan XN, 2019. [Limonoids from seeds of Azadirachta indica and their antibacterial activity]. Zhongguo Zhong Yao Za Zhi 44(22), 4864–4873. [DOI] [PubMed] [Google Scholar]
- Macia MD, Rojo-Molinero E, Oliver A, 2014. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin Microbiol Infect 20(10), 981–990. [DOI] [PubMed] [Google Scholar]
- Mah TF, O’Toole GA, 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9(1), 34–39. [DOI] [PubMed] [Google Scholar]
- Mahizan NA, Yang SK, Moo CL, Song AA, Chong CM, Chong CW, Abushelaibi A, Lim SE, Lai KS, 2019. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules 24(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makobongo MO, Einck L, Peek RM Jr., Merrell DS, 2013. In vitro characterization of the anti-bacterial activity of SQ109 against Helicobacter pylori. PLoS One 8(7), e68917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus EA, Inatomi N, Nagami GT, Sachs G, Scott DR, 2012. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther 36(10), 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Verma M, Mishra SK, Srivastava S, Lakshmi V, Misra-Bhattacharya S, 2011. Gedunin and photogedunin of Xylocarpus granatum possess antifilarial activity against human lymphatic filarial parasite Brugia malayi in experimental rodent host. Parasitol Res 109(5), 1351–1360. [DOI] [PubMed] [Google Scholar]
- Mitra T, Bhattacharya R, 2020. Phytochemicals modulate cancer aggressiveness: A review depicting the anticancer efficacy of dietary polyphenols and their combinations. J Cell Physiol 235(11), 7696–7708. [DOI] [PubMed] [Google Scholar]
- Moodley Y, Linz B, Bond RP, Nieuwoudt M, Soodyall H, Schlebusch CM, Bernhoft S, Hale J, Suerbaum S, Mugisha L, van der Merwe SW, Achtman M, 2012. Age of the association between Helicobacter pylori and man. PLoS Pathog 8(5), e1002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murima P, McKinney JD, Pethe K, 2014. Targeting bacterial central metabolism for drug development. Chem Biol 21(11), 1423–1432. [DOI] [PubMed] [Google Scholar]
- Nadkarni KM, 1954. Dr. K.M. Nadkarni’s Indian materia medica: With Ayurvedic, Unani-Tibbi, Siddha, allopathic, homeopathic, naturopathic & home remedies, appendices & indexes, Third ed. Bombay: Popular Prakashan Private Ltd. [Google Scholar]
- Newman DJ, Cragg GM, 2016. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod 79(3), 629–661. [DOI] [PubMed] [Google Scholar]
- Oh JD, Kling-Backhed H, Giannakis M, Xu J, Fulton RS, Fulton LA, Cordum HS, Wang C, Elliott G, Edwards J, Mardis ER, Engstrand LG, Gordon JI, 2006. The complete genome sequence of a chronic atrophic gastritis Helicobacter pylori strain: evolution during disease progression. Proc Natl Acad Sci USA 103(26), 9999–10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I, 2015. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis 34(5), 877–886. [DOI] [PubMed] [Google Scholar]
- Pajares Garcia JM, Pajares-Villarroya R, Gisbert JP, 2007. [Helicobacter pylori infection: antibiotic resistance]. Rev Esp Enferm Dig 99(2), 63–70. [DOI] [PubMed] [Google Scholar]
- Parida MM, Upadhyay C, Pandya G, Jana AM, 2002. Inhibitory potential of neem (Azadirachta indica Juss) leaves on dengue virus type-2 replication. J Ethnopharmacol 79(2), 273–278. [DOI] [PubMed] [Google Scholar]
- Pontes MH, Groisman EA, 2019. Slow growth determines nonheritable antibiotic resistance in Salmonella enterica. Sci Signal 12(592). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzato C, Torres J, Kasamatsu E, Camorlinga-Ponce M, Bravo MM, Canzian F, Kato I, 2019. Potential Role of Biofilm Formation in the Development of Digestive Tract Cancer With Special Reference to Helicobacter pylori Infection. Front Microbiol 10, 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout KK, Mishra SK, 2014. Development of a sensitive HPTLC method for quantification of nimbolide in Azadirachta indica and its dosage form. J Chromatogr Sci 52(9), 1089–1094. [DOI] [PubMed] [Google Scholar]
- Santos KS, Barbosa AM, Freitas V, Muniz AVCS, Mendonça MC, Calhelha RC, Ferreira ICFR, Franceschi E, Padilha FF, Oliveira MBPP, Dariva C, 2018. Antiproliferative Activity of Neem Leaf Extracts Obtained by a Sequential Pressurized Liquid Extraction. Pharmaceuticals 11(3), 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P, Acharyya S, Banerjee A, Patra A, Thankamani K, Koley H, Bag PK, 2016. Intracellular, biofilm-inhibitory and membrane-damaging activities of nimbolide isolated from Azadirachta indica A. Juss (Meliaceae) against meticillin-resistant Staphylococcus aureus. J Med Microbiol 65(10), 1205–1214. [DOI] [PubMed] [Google Scholar]
- Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E, 2018. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 155(5), 1372–1382 e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DR, Sachs G, Marcus EA, 2016. The role of acid inhibition in Helicobacter pylori eradication. F1000Res 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seck A, Burucoa C, Dia D, Mbengue M, Onambele M, Raymond J, Breurec S, 2013. Primary antibiotic resistance and associated mechanisms in Helicobacter pylori isolates from Senegalese patients. Ann Clin Microbiol Antimicrob 12, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servetas SL, Doster RS, Kim A, Windham IH, Cha JH, Gaddy JA, Merrell DS, 2018. ArsRS-Dependent Regulation of homB Contributes to Helicobacter pylori Biofilm Formation. Front Microbiol 9, 1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Venkataraghavan K, Choudhary P, Mohammad S, Trivedi K, Shah SG, 2016. Evaluation of antimicrobial effect of azadirachtin plant extract (Soluneem ()) on commonly found root canal pathogenic microorganisms (viz. Enterococcus faecalis) in primary teeth: A microbiological study. J Indian Soc Pedod Prev Dent 34(3), 210–216. [DOI] [PubMed] [Google Scholar]
- Shu X, Hu Y, Huang C, Wei N, 2021. Nimbolide ameliorates the streptozotocin-induced diabetic retinopathy in rats through the inhibition of TLR4/NF-kappaB signaling pathway. Saudi J Biol Sci 28(8), 4255–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaravelu S, Sankarapillai J, Sasidharn Chandrakumari A, Sinha P, 2019. Effect of Azadirachta indica Crude Bark Extracts Concentrations against Gram-Positive and Gram-Negative Bacterial Pathogens. J Pharm Bioallied Sci 11(1), 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow Setzer M, Sharifi-Rad J, Setzer WN, 2016. The Search for Herbal Antibiotics: An In-Silico Investigation of Antibacterial Phytochemicals. Antibiotics 5(3), 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivey JM, 1992. The postantibiotic effect. Clin Pharm 11(10), 865–875. [PubMed] [Google Scholar]
- Stewart PS, Costerton JW, 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358(9276), 135–138. [DOI] [PubMed] [Google Scholar]
- Sundaravalli N, Raju BB, Krishnamoorthy KA, 1982. Neem oil poisoning. Indian J Pediatr 49(398), 357–359. [DOI] [PubMed] [Google Scholar]
- Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, Valasek MA, 2016. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 43(4), 514–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, Nelson K, Quackenbush J, Zhou L, Kirkness EF, Peterson S, Loftus B, Richardson D, Dodson R, Khalak HG, Glodek A, McKenney K, Fitzegerald LM, Lee N, Adams MD, Hickey EK, Berg DE, Gocayne JD, Utterback TR, Peterson JD, Kelley JM, Cotton MD, Weidman JM, Fujii C, Bowman C, Watthey L, Wallin E, Hayes WS, Borodovsky M, Karp PD, Smith HO, Fraser CM, Venter JC, 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388(6642), 539–547. [DOI] [PubMed] [Google Scholar]
- Ventola CL, 2015. The antibiotic resistance crisis: part 1: causes and threats. P T 40(4), 277–283. [PMC free article] [PubMed] [Google Scholar]
- Vianna JS, Ramis IB, Ramos DF, A VONG, Silva PE, 2016. Drug Resistance in Helicobacter Pylori. Arq Gastroenterol 53(4), 215–223. [DOI] [PubMed] [Google Scholar]
- Walsh JH, Richardson CT, Fordtran JS, 1975. pH dependence of acid secretion and gastrin release in normal and ulcer subjects. J Clin Invest 55(3), 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan N, Wang H, Ng CK, Mukherjee M, Ren D, Cao B, Tang YJ, 2018. Bacterial Metabolism During Biofilm Growth Investigated by (13)C Tracing. Front Microbiol 9, 2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Jiang Q, Taylor DE, 1998. Genotypic characterization of clarithromycin-resistant and -susceptible Helicobacter pylori strains from the same patient demonstrates existence of two unrelated isolates. J Clin Microbiol 36(9), 2730–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Phan DD, Zhang J, Ong PS, Thuya WL, Soo R, Wong AL, Yong WP, Lee SC, Ho PC, Sethi G, Goh BC, 2016. Anticancer properties of nimbolide and pharmacokinetic considerations to accelerate its development. Oncotarget 7(28), 44790–44802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics.
- Windham IH, Servetas SL, Whitmire JM, Pletzer D, Hancock REW, Merrell DS, 2018. Helicobacter pylori Biofilm Formation Is Differentially Affected by Common Culture Conditions, and Proteins Play a Central Role in the Biofilm Matrix. Appl Environ Microbiol 84(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Fujisaki J, Omae M, Hirasawa T, Igarashi M, 2015. Helicobacter pylori-negative gastric cancer: characteristics and endoscopic findings. Dig Endosc 27(5), 551–561. [DOI] [PubMed] [Google Scholar]
- Yonezawa H, Osaki T, Hojo F, Kamiya S, 2019. Effect of Helicobacter pylori biofilm formation on susceptibility to amoxicillin, metronidazole and clarithromycin. Microb Pathog 132, 100–108. [DOI] [PubMed] [Google Scholar]
- Zhanel GG, Hoban DJ, Harding GK, 1991. The postantibiotic effect: a review of in vitro and in vivo data. DICP 25(2), 153–163. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ahn KS, Kim C, Shanmugam MK, Siveen KS, Arfuso F, Samym RP, Deivasigamanim A, Lim LH, Wang L, Goh BC, Kumar AP, Hui KM, Sethi G, 2016. Nimbolide-Induced Oxidative Stress Abrogates STAT3 Signaling Cascade and Inhibits Tumor Growth in Transgenic Adenocarcinoma of Mouse Prostate Model. Antioxid Redox Signal 24(11), 575–589. [DOI] [PubMed] [Google Scholar]


