Abstract
Neuronal apoptotic death induced by nerve growth factor (NGF) deprivation is reported to be in part mediated through a pathway that includes Rac1 and Cdc42, mitogen-activated protein kinase kinases 4 and 7 (MKK4 and -7), c-Jun N-terminal kinases (JNKs), and c-Jun. However, additional components of the pathway remain to be defined. We show here that members of the mixed-lineage kinase (MLK) family (including MLK1, MLK2, MLK3, and dual leucine zipper kinase [DLK]) are expressed in neuronal cells and are likely to act between Rac1/Cdc42 and MKK4 and -7 in death signaling. Overexpression of MLKs effectively induces apoptotic death of cultured neuronal PC12 cells and sympathetic neurons, while expression of dominant-negative forms of MLKs suppresses death evoked by NGF deprivation or expression of activated forms of Rac1 and Cdc42. CEP-1347 (KT7515), which blocks neuronal death caused by NGF deprivation and a variety of additional apoptotic stimuli and which selectively inhibits the activities of MLKs, effectively protects neuronal PC12 cells from death induced by overexpression of MLK family members. In addition, NGF deprivation or UV irradiation leads to an increase in both level and phosphorylation of endogenous DLK. These observations support a role for MLKs in the neuronal death mechanism. With respect to ordering the death pathway, dominant-negative forms of MKK4 and -7 and c-Jun are protective against death induced by MLK overexpression, placing MLKs upstream of these kinases. Additional findings place the MLKs upstream of mitochondrial cytochrome c release and caspase activation.
There has been much progress in defining the general mechanisms by which neurons and other cell types die in response to apoptotic stimuli. However, the components and order of the transducing pathways that mediate death are still not completely understood. One apoptotic pathway that appears to be particularly important in neurons includes the c-Jun N-terminal kinases (JNKs; also known as the stress-activated protein kinases) (14, 15, 22, 32, 42, 69; reviewed in reference 23). JNKs are phosphorylated and activated in response to a variety of apoptotic stimuli, including tumor necrosis factor, DNA damage, heat shock, ischemia-reperfusion, oxidative stress, hyperosmolarity, axonal injury, and loss of trophic support (20, 31, 38, 42, 55, 68, 69). Various types of evidence indicate that JNK activation plays a required role in many such paradigms of neuronal cell death. A well-defined downstream target of activated JNKs in neuronal cells that also plays an obligatory part in death is c-Jun (14, 15, 22). Neuronal c-Jun levels are elevated in response to trophic factor withdrawal, and dominant-negative forms of this transcription factor are protective against death evoked by loss of trophic support as well as by selective activation of JNKs (14, 22).
Several upstream members of the death-related JNK/c-Jun pathway have also been defined. The most distal of these are the Rho small GTPase family members Rac1 and Cdc42. Overexpression of constitutively active forms of Rac1 and Cdc42 (Rac1 V12 [mutated at position 12 to V] and Cdc42 V12) leads to activation of the JNK pathway and to death of Jurkat T lymphocytes, PC12 cells, and sympathetic neurons; conversely, overexpression of dominant-negative mutants of Cdc42 and Rac1 (Cdc42 N17 and Rac1 N17) in sympathetic neurons prevents elevation of c-Jun and death evoked by nerve growth factor (NGF) withdrawal (2, 7). Overexpression of Rac1 N17 reverses the induction of death by Cdc42 V12, whereas Cdc42 N17 has no effect on Rac1 V12-induced death, suggesting that Cdc42 lies upstream of Rac1 (2). Similar approaches have indicated that mitogen-activated protein kinase kinases 4 and 7 (MKK4 and MKK7) lie downstream of Cdc42 and Rac1 and directly upstream of the JNKs (17, 27, 45, 65, 69, 70). Finally, recent studies using constitutively active and dominant-negative constructs have implicated apoptosis signal-regulating kinase 1 (ASK1) as an additional participant in the pathway that lies between Cdc42 and the downstream MKKs and JNKs (32).
The mixed-lineage kinases (MLKs) represent an additional family that has the potential to be involved in the JNK activation pathway. MLKs have been shown to function as MKK kinases and lead to activation of JNKs via activation of MKKs (4, 9, 25, 46, 56, 63, 65). Members of the family include MLK1, MLK2 (also called MST), MLK3 (also called SPRK or PTK1), dual leucine zipper kinase (DLK; also called MUK or ZPK), and leucine zipper-bearing kinase (LZK) (12, 28, 30, 48, 57). In addition to a kinase domain, MLK family members possess one or two leucine zipper domains, and MLK1, -2, and -3, but not DLK, also have an SH3 domain and a potential Cdc42-Rac interactive binding (CRIB) motif (5, 12, 30). Constitutively active mutants of Rac1 and Cdc42 have been found to bind to and to modulate the activities of MLK2 and -3, and coexpression of MLK3 and activated Cdc42 leads to enhanced MLK3 activation, which is reported to require the presence of the MLK3 CRIB motif (4, 5, 48, 62).
Because of the widespread role of JNK activation in neuronal death signaling, we have carried out further studies to define the identities and relative order of the components of this pathway. Here, we show in particular that MLKs are mediators of JNK activation in neuronal cells.
MATERIALS AND METHODS
Materials.
Cell growth media RPMI 1640 and Dulbecco's minimal essential medium, Taq Platinum DNA polymerase, and Lipofectamine Plus were purchased from Life Technologies, Inc. (Frederick, Md.). Human recombinant NGF was kindly provided by Genentech (South San Francisco, Calif.). (−)cis-5,7-Dihydroxy-2-(2-chlorophenyl)-8[4-(3-hydroxy-1-methyl)piperidinyl]-4H-benzopyran-4-one (L86-8275; flavopiridol) was a generous gift from Peter J. Worland (National Cancer Institute). Boc-aspartyl-(ome)-fluoromethyl ketone (BAF), Ac-DEVD-pNA, and zVAD-fluoromethylketone (zVAD-fmk) were purchased from Enzyme Systems Products (Dublin, Calif.). Hoechst dye 33342, mouse NGF, and anti-mouse NGF antiserum were obtained from Sigma (St. Louis, Mo.). Phospho-AKT (Ser473) antiserum was purchased from New England Biolabs (Beverly, Mass.). DLK antiserum was purchased from StressGen (Victoria, British Columbia, Canada). MLK3 antiserum was purchased from Santa Cruz (Santa Cruz, Calif.). CEP-1347 was solubilized in dimethyl sulfoxide at a concentration of 4 mM and stored at −20°C in amber glass vials. Further dilutions were made from this stock directly into media.
RT-PCR analysis.
Expression of MLK1, MLK2, MLK3, DLK, and LZK was determined by reverse transcription (RT)-PCR analysis. Total RNA was purified using RNAzolB (TelTest Inc., Friendswood, Tex.), and Superscript II reverse transcriptase (Life Technologies) was used to synthesize cDNA. PCR assays were performed as previously described (3). The following primers were used: MLK1, 5′-AACTACGTGACCCCGCGCA-3′ and 5′-CCGCAAAATCAATTTCTAAC-3′; MLK2, 5′-TGGAGCTGGAGAGCTTCAAGAAG-3′ and 5′CAT GTCCATGTCCAGCAGTGTGG-3′; MLK3, 5′-GTCATGGAATGGCAGTGG-3′ and 5′-CACGGTCACCCTTCCTCA-3′; DLK, 5′-GAGGTAGACAGTGAAGTAGAGC-3′ and 5′-CCAATTCAGTGCTGTCACAGTC-3′; LZK, 5′-CACCAGCACATAATCCTCTCTTG-3′ and 5′-CACTGGTATTTCCCTCTTCTCC-3′; and cyclophilin, 5′-ATGGTCAACCCCACCGTGTT-3′ and 5′-CTGGTGAAGTCACCACCCT-3′. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) primers were purchased from Clontech (Palo Alto, Calif.). The identity of all PCR products was confirmed by sequencing. All RT-PCR assays were performed at least twice, except that for LZK in superior cervical ganglion (SCG) cells, which was done once. Representative results are shown.
cDNA constructs in mammalian expression vectors.
The cDNAs for MKK4b (MKK4) and MKK7β1 (MKK7) were PCR amplified from human placenta and brain, respectively, sequence verified, and inserted into pcDNA3.1 (Invitrogen, San Diego, Calif.). The cDNAs encoding the K113A mutant of MKK4 (26) and the K149A mutant of MKK7 were generated by PCR-mediated site-directed mutagenesis. A truncated cDNA of mouse MEKK1 (encoding amino acids 817 to 1493 of full-length MEKK1) was kindly provided by J. Silvio Gutkind (National Institutes of Health [NIH], Bethesda, Md.) and subcloned into the pcDNA3 vector (Invitrogen). The following constructs were generously provided as follows. Both dominant-negative and constitutively active forms of Rac1 and Cdc42 were from Alan Hall (University College London, London, United Kingdom), and both the dominant-negative form and a constitutively active form of human ASK1 (or MEKK5) were from Hidenori Ichijo (Tokyo Medical and Dental University, Tokyo, Japan). Wild-type c-Jun and dominant-negative c-Jun (TAM67 c-Jun) were from Michael J. Birrer (NIH, Rockville, Md.), and different constructs of AKT, including wild-type, active, dominant-negative, and myristylated forms, were kindly provided by Thomas Franke (Columbia University, New York, NY.). Mouse MKK7 and DLK were from Larry Holzman (University of Michigan Medical School, Ann Arbor, Mich.). Human MLK3 was kindly provided by Richard Spritz (University of Wisconsin, Madison, Wis.), and MLK1 and MLK2 were cloned from human brain cDNA. MLK2, MLK3, and DLK were subcloned into pcDNA3.1, whereas MLK1 was subcloned into pcDNA4/HisMax (Invitrogen). cDNA encoding the kinase-inactive mutant forms of MLK1 (K171A), MLK2 (K125A), MLK3 (K144R), and DLK (K155A) were generated by PCR-mediated site-directed mutagenesis. Sequences encoding the constitutively active form of human ASK1 (ΔN ASK1), Rac1 V12, Cdc42 V12, and both the wild-type and the kinase-inactive mutant forms of MLK1, MLK2, MLK3, and DLK were subcloned into pCMV.EGFP vector (Clontech).
Cell culture.
PC12 cells were cultured as described previously in collagen-coated dishes with RPMI 1640 medium supplemented with 10% heat-inactivated horse serum and 5% fetal bovine serum (21). Neuronal differentiation was induced by NGF (100 ng of human recombinant protein per ml) in RPMI 1640 medium supplemented with 1% heat-inactivated donor horse serum.
Primary cultures of rat sympathetic neurons were generated from dissociated SCG of postnatal-day-1 Sprague-Dawley rats as described previously (39). The cells were plated onto collagen-coated 24-well dishes at a density of around one ganglion per well and maintained in RPMI 1640 medium supplemented with 10% heat-inactivated donor serum and 60 ng of mouse NGF per ml. A mixture of uridine and 5-fluorodeoxyuridine (10 μM each) was added on the following day to eliminate nonneuronal cells.
Transfections.
DNA used for transfection was prepared with a plasmid maxi kit (Qiagen, Valencia, Calif.). Three independent plasmid preparations were obtained, and the one with the highest transfection efficiency was used in experiments. PC12 cells were transfected with 1.0 μg of plasmid in six-well dishes and 0.5 μg of plasmid in 24-well dishes 4 to 7 days after NGF treatment or overnight after plating for naive cells using Lipofectamine Plus (Life Technologies). Six to nine hours later, medium with Lipofectamine Plus was replaced with fresh medium with NGF or with serum for neuronally differentiated or naive PC12 cells, respectively. Rat sympathetic neurons were transfected with the Helios Gene Gun system (Bio-Rad, Hercules, Calif.).
Trophic factor deprivation.
For trophic factor deprivation of PC12 cells, on day 3 after transfection, cultures were washed three times with RPMI 1640 medium, scraped (for neuronally differentiated PC12 cells) or mechanically dislodged (for naive PC12 cells) from their dishes, pelleted at low speed, and washed with serum-free medium. The spin-and-wash procedure was repeated five more times, and the cells were replated into collagen-coated 15-mm wells at approximately 2 × 105 cells per well. Trophic-factor-deprived cultures were maintained in RPMI 1640 medium, and the control nondeprived cells were supported with NGF or serum for NGF-differentiated cells or naive cells, respectively. NGF deprivation of rat sympathetic neurons was performed by washing with NGF-free medium twice and adding anti-NGF antibody (1:150 dilution), as previously described (53). Control cells were washed with serum-free medium and maintained in medium supplied with NGF and 1% horse donor serum.
Assessment of cell survival. (i) Strip counting.
From a defined time point (consistent throughout the course of the experiment), the number of healthy, nonapoptotic enhanced green fluorescent protein (EGFP)-positive cells in the same field (consisting of a strip across the diameter of each well) was assessed. The percentage of surviving cells was calculated relative to the numbers present in control wells. The numbers of transfected cells counted in control cultures were at least 400.
(ii) Apoptotic nuclei.
Nuclei were visualized by staining cells either alive, or after fixation. To stain living cells, Hoechst dye 33342 at 1 mg/ml was added to the medium to a final concentration of 1 μg/ml. Five minutes later, the medium was replaced with fresh medium without the dye. Otherwise, cells were fixed in 4% formaldehyde for 10 min and then incubated with Hoechst dye 33342 at 1 μg/ml in phosphate-buffered saline (PBS) for 5 min at room temperature without formaldehyde. The medium containing Hoechst dye was then replaced with PBS. Cells possessing condensed nuclei or fragmented chromatin were scored as apoptotic. The number of cells assessed per culture typically ranged from 100 to 200. All experiments were performed at least in triplicate, and results are reported as the means ± standard errors of the means (SEM).
Western immunoblotting.
PC12 cells were harvested in lysis buffer (10 mM Tris [pH 7.4], 1.0% Triton X-100, 0.5% Nonidet P-40, 150 mM NaCl, 20 mM sodium fluoride, 0.2 mM sodium orthovanadate, 1.0 mM EDTA, 1.0 mM EGTA, 0.2 mM phenylmethylsulfonyl fluoride). For samples subjected to phosphatase treatment, sodium orthovanadate was omitted. Cells were incubated in lysis buffer for 10 min and then scraped from the dishes and passed 10 times through a 26-gauge needle. The lysates were then centrifuged at 16,000 × g for 20 min, and the pellets were discarded. Phosphatase treatment was performed by incubating the cell extracts with 400 U of λ phosphatase for 2 h at 30°C according to the manufacturer's instructions (Biolabs). Samples (containing 200 μg of protein) were boiled in 1× sodium dodecyl sulfate sample buffer and separated by sodium dodecyl sulfate–6% polyacrylamide gel electrophoresis prior to blotting on nitrocellulose membranes (Schleicher & Schuell). The membranes were incubated in 5% fat-free milk in TBST (25 mM Tris-HCl [pH 7.4], 137 mM NaCl, 5 mM KCl, 0.7 mM CaCl2, 0.1 mM MgCl2, 0.2% [vol/vol] Tween 20) for 2 h and then with the same buffer containing various dilutions of the primary antibodies (1:200 to 1:250). Before and after incubation with secondary antibody, the membranes were washed four times for 10 min each time with TBST buffer. The proteins were detected with an appropriate secondary antibody coupled to horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin (Bur73) and visualized by enhanced chemiluminescence according to the instructions of the manufacturer (Amersham Life Science).
Immunofluorescence staining.
To detect cytochrome c, cells were fixed with 3% paraformaldehyde for 10 min, washed with PBS, and then permeabilized with 0.5% Triton X-100 and blocked with 10% goat serum for 30 min at room temperature. They were then stained with a specific monoclonal antibody against cytochrome c (clone 6H2.B4; dilution, 1:500; PharMingen, San Diego, Calif.) followed by a rhodamine-conjugated anti-mouse immunoglobulin G antibody (ImmunoPure; 1:500 dilution). To examine nuclear morphology, cells were stained with Hoechst dye (Hoechst 33342; Sigma) at 1 μg/ml.
RESULTS
MLK family members are expressed in PC12 cells and sympathetic neurons.
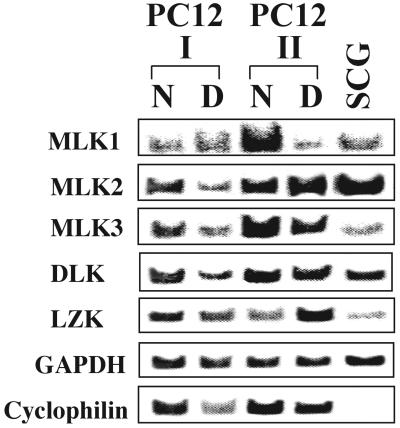
RT-PCR was used to determine expression of members of the MLK family in PC12 cells and in SCG neurons. As shown in Fig. 1, transcripts for all members of MLK family were readily detectable in both cell types. Expression in two different passages of PC12 cells was analyzed. We did not observe consistent effects of NGF on expression of any of the analyzed genes (naive versus differentiated cells). Based on approximate equivalency of GAPDH transcription levels, which served as internal controls, SCG neurons were found to express severalfold-lower levels of MLK3 and LZK than PC12 cells. The biological significance, if any, of these observations is unclear.
FIG. 1.
All five members of the MLK family are expressed in PC12 cells and SCG neurons. Total RNA was purified from naive (N) and neuronally differentiated (D) PC12 cells and rat SCG neurons. RNA was reverse transcribed, and expression levels of MLK1, MLK2, MLK3, DLK, and LZK were evaluated by PCR using specific primers as described in Materials and Methods. Expression in two different passages of PC12 cells was analyzed. GAPDH and cyclophilin served as internal controls. For each primer pair, PCR assays were performed at the same cycle number. All assays were performed at least twice, except that for LZK expression in SCGs, which was done once. Representative results are shown.
Expression of MLK family members induces apoptotic death of PC12 cells and sympathetic neurons.
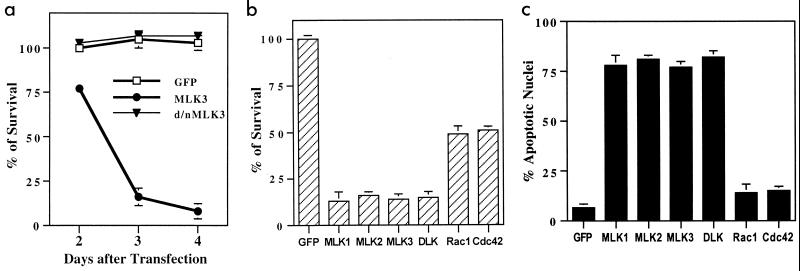
Efforts to establish a permanent line of PC12 cells expressing MLK3 under the control of the cytomegalovirus promoter proved to be unsuccessful, thus suggesting that overexpression of this protein results in cell death. To examine this issue more fully, sequences encoding MLK3 and the additional family members MLK1, MLK2, and DLK were cloned into the pCMS.EGFP vector. A full-length clone for LZK was not available at the time of this study, and so this enzyme was not included in the present experiments. The various constructs were transiently transfected into neuronally differentiated PC12 cells. The GFP signal was detectable within 1 day and was highest within 2 to 3 days following transfection. Counts of intact GFP-positive cells revealed a rapid decline in numbers within 4 days for MLK3-transfected cultures but not for empty vector and kinase-inactive controls (Fig. 2a). Similar data were obtained with MLK1, MLK2, and DLK. By day 3, cells transfected with any of the MLK family members had approximately 17% survival compared with controls (Fig. 2b). Transfection with similar levels of DNA encoding constitutively active Cdc42 and Rac1 (Cdc42 V12 and Rac1 V12) also evoked death, but in this case survival was approximately 50% by day 3 (Fig. 2b). Death was verified by staining nuclei of transfected cells with Hoechst 33342. Approximately 75% of nuclei assayed 48 h after transfection with MLKs showed apoptotic morphology, compared with around 5% in control cultures (Fig. 2c). Comparable results were achieved with naive, non-NGF-treated cultures (data not shown). In addition, both naive and neuronally differentiated PC12 cells transfected with MLKs, but not with kinase-inactive MLKs, showed additional features of apoptotic responses, including intense surface blebbing, shrunken cell bodies, and pycnotic nuclei. These findings demonstrate that overexpression of all MLK family members tested can efficiently trigger apoptotic death in neuronal cells.
FIG. 2.
Induction of apoptosis in neuronal PC12 cells by overexpression of MLK family members. (a) Transient expression of wild-type MLK3 but not the kinase-inactive form (d/n) induces cell death. Neuronally differentiated PC12 cells were transfected with empty cloning vector (pCMS.EGFP) or with DNA encoding full-length MLK3 or the kinase-inactive form of MLK3 and assessed for cell viability in the presence of NGF at 2, 3, and 4 days after transfection. The percentage of surviving cells was calculated by normalizing to the numbers of cells transfected with pCMS.EGFP at 2 days after transfection. The data are the means of counts from three wells ± SEM, and similar results were obtained in three additional independent experiments. (b) Overexpression of MLK family members or Rac1 V12 and Cdc42 V12 kills neuronal PC12 cells. Cells were transfected with either empty cloning vector (pCMS.EGFP) or constructs encoding different MLK family members, Rac1 V12, or Cdc42 V12. Three days after transfection, numbers of transfected cells were counted in three replicate wells as described in Materials and Methods. (c) Induction of apoptosis in neuronally differentiated PC12 cells by the expression of different MLK family members and constitutive active forms of Rac1 and Cdc42. Cells were transfected with either empty cloning vector (pCMS.EGFP) or constructs encoding different MLK family members, Rac1 V12, or Cdc42 V12. Three days after transfection, the percentages of apoptotic nuclei were determined by scoring per condition at least 100 Hoechst 33242-stained nuclei of cells expressing EGFP. The values in panels b and c are the means of four independent experiments plus SEM.
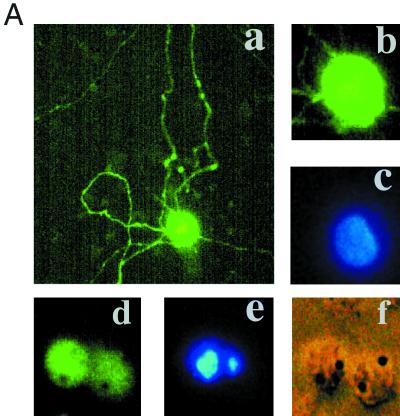
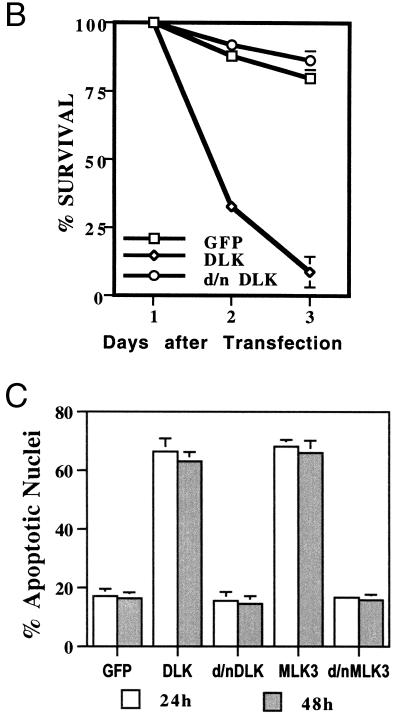
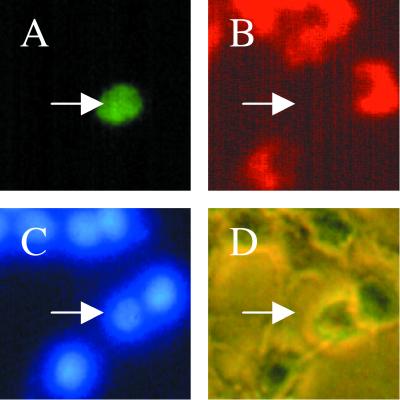
To examine whether comparable effects might occur in cultures of sympathetic neurons, the normal counterparts of neuronal PC12 cells, a biolistic device (Helios Gene Gun system) was used to transfect them with pCMS.EGFP-MLK3 or -DLK. As illustrated in Fig. 3A, DLK induced apparent apoptotic death with cell shrinkage, loss of neurites, and appearance of broken or condensed nuclei. The same response was observed for MLK3 (data not shown). This effect was confirmed and quantified by counting GFP-positive cells (Fig. 3B) or nuclei with apoptotic morphology (Fig. 3C).
FIG. 3.
Expression of DLK or MLK3 induces sympathetic neuronal apoptosis. (A) Representative immunofluorescence photomicrographs of cells transfected with control vector, wild-type MLK3, or DLK (data shown here are for DLK; the same results were obtained with MLK3). Sympathetic neurons cultured for 2 days in the presence of NGF were transfected with control vector (pCMS.EGFP) (a to c) or pCMS.EGFP-DLK (d to f). Forty-eight hours later, nuclei of the living cells were stained with Hoechst 33242 as described in Materials and Methods. Normal nuclei are stained blue (c), while nuclei of cells undergoing apoptosis appear light blue (e) because of the intensified signal due to shrinking or condensation of nuclei. Magnifications, ×200 (a) and ×600 (b to f). (B) Sympathetic neurons (prepared as for panel A) were transfected with control vector (pCMS.EGFP) or DNA encoding wild-type or dominant-negative (d/n) forms of DLK. At 24, 48, and 72 h later, numbers of cells with EGFP signal in the same well were counted and the percentages of surviving cells were assessed as described in Materials and Methods. The values are the means for three wells ± SEM, and similar results were obtained in two additional independent experiments. (C) Sympathetic neurons were prepared and transfected as for panel B. Twenty-four hours later, living cells were stained as in panel A, and the proportions of apoptotic nuclei in transfected cells were assessed immediately and 24 h later. The results are the means of three independent experiments plus SEM.
MLK family members induce death of neuronal PC12 cells by acting upstream both of MKK4 and -7 and c-Jun and of cytochrome c release and caspase activation.
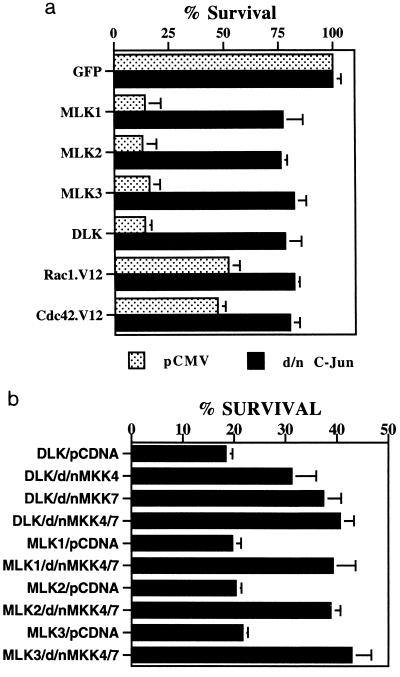
DLK has been shown to act upstream of MKK7, and MLK2 and MLK3 have been shown to act upstream of both MKK4 and MKK7 (9, 46, 56, 63), which, in turn, lie upstream of JNKs and of c-Jun in the apoptotic pathway activated by NGF deprivation (14, 15, 22). To assess whether MLK3 and other MLK family members might also evoke death in neuronal PC12 cells by activating this pathway, we coexpressed each of them with dominant-negative forms of MKK4, MKK7, and c-Jun. As shown in Fig. 4, the dominant-negative constructs suppressed death in each case. However, the dominant-negative forms of MKK4 and MKK7, even when transfected together, only partially suppressed death evoked by overexpression of MLKs and did so to a significantly lesser degree than the dominant-negative form of c-Jun. This raises the possibility that MKK4 and MKK7 may not be the only MKKs that transduce signals from MLKs to c-Jun or that the dominant-negative MKKs are inefficient with respect to suppression of JNK activation.
FIG. 4.
The MLK family is upstream of MKK4 and -7 and c-Jun in the apoptotic pathway. (a) A dominant-negative form of c-Jun suppresses death induced by overexpression of Rac1 V12 and Cdc42 V12 as well as by the MLK family. pCMS.EGFP as well as DNA-encoding MLK family members, Rac1 V12, and Cdc42 V12 (all in the pCMS.EGFP vector) were cotransfected with either pCMV vector as a control or the dominant-negative (d/n) form of c-Jun (pCMV-TAM67 c-Jun) as described in Materials and Methods. Three days after transfection, cell numbers were determined as described in Materials and Methods. Cell numbers in cultures transfected with pCMS.EGFP-pCDNA3 and pCMS.EGFP-pCMV were defined as 100% survival, and cell numbers in the other transfected cultures were normalized accordingly. (b) Dominant-negative (d/n) forms of MKK4 and MKK7 suppress death induced by MLK expression. pCMS.EGFP and constructs encoding different MLK family members were cotransfected with either pCDNA3 vector or dominant-negative MKK4 and/or dominant-negative MKK7 (both in pCDNA3) as described in Materials and Methods. In those cotransfected with both dominant-negative MKK4 and dominant-negative MKK7, the amounts of dominant-negative MKK4 and dominant-negative MKK7 DNA were equal and the total amounts of both together (1.25 μg/well) were equal to five times the amount of DNA (0.25 μg/well) with which they were cotransfected. Three days after transfection, cell numbers were counted as described in Materials and Methods. Numbers of cells transfected with pCMS.EGFP-pCDNA3 were defined as 100%, and survival in cultures with other transfections were normalized to these values. The values are the means from three independent experiments plus SEM.
Initiation of apoptotic death in response to various stimuli, including NGF deprivation, requires release of cytochrome c from mitochondria (11; reviewed in references 6 and 10). Recent findings reveal that activation of the JNK pathway can cause cytochrome c release and that apoptotic stimuli fail to release cytochrome c in JNK null cells (64). Accordingly, to determine whether death evoked by MLKs is propagated in this manner, neuronal PC12 cells were transfected with MLK3 or DLK and immunostained 30 h later with anti-cytochrome c antibodies. As illustrated in Fig. 5, many such cells, but none of the control cells, showed total loss or diminution of cytochrome c staining. The loss of cytochrome c staining may reflect its rapid degradation after cytochrome c is released into the cytoplasm, as reported elsewhere (49, 59). In a few cells, a low level of diffuse cytochrome c staining was detected, which presumably reflects translocation to the cytoplasm. All cells with apoptotic nuclei (as judged by the staining pattern with the Hoechst dye) lost cytochrome c staining. Some of the cells that lost the cytochrome c signal still had normal Hoechst dye-stained nuclei, suggesting, as anticipated (59), that cytochrome c release occurs before the effects of MLK family expression on nuclear morphology.
FIG. 5.
Overexpression of MLK family members induces cytochrome c release. MLK3 and DLK were transfected into neuronally differentiated PC12 cells (data shown here are for DLK; the same results were obtained with MLK3). Thirty hours later, cells were fixed and immunostained with anti-cytochrome c as described in Materials and Methods. All panels show the same field; arrows point to a cell that has an EGFP signal. (A) Cell transfected with pCMS.EGFP-DLK which shows the EGFP signal. (B) Immunostaining with antibodies against cytochrome c. (C) Cells stained with Hoechst dye 33342. (D) Phase-contrast photomicrograph. Magnification, ×400.
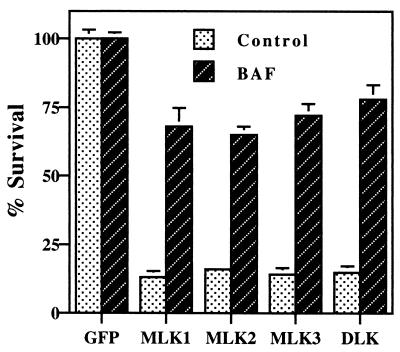
To assess the role of caspases in death evoked by MLK family members, transfected cultures of neuronally differentiated PC12 cells were treated with or without BAF, a general caspase inhibitor, and the transfected cells were subsequently scored for survival and proportion of apoptotic nuclei. BAF (25 μM) provided significant protection in each case (Fig. 6). Another caspase inhibitor, zVAD-fmk (50 μM), also provided comparable protection from MLK3, whereas the less general inhibitor Ac-DEVD-pNA (10 μM) had little effect (data not shown). Taken together, the above findings suggest a scheme in which MLK family members elicit death by a downstream pathway that includes MKK4 and -7, c-Jun, cytochrome c release, and activation of caspases.
FIG. 6.
Death induced by MLK family expression can be suppressed by the general caspase inhibitor BAF. pCMS.EGFP as well as constructs encoding MLK family members MLK1, -2, and -3 and DLK were transfected into neuronal PC12 cells as described in Materials and Methods, and half of the cultures were pretreated and maintained with 25 μM BAF. Three days after transfection, cell numbers were determined as described in Materials and Methods. Numbers of cells transfected with pCMS.EGFP or pCMS.EGFP/BAF were each defined as 100%, and the other transfections were normalized to them. The values are the means of three independent experiments plus SEM.
The Cdk inhibitor flavopiridol (1 μM) provides long-term rescue from apoptotic death evoked by NGF deprivation or DNA damage (52). Flavopiridol had no effect on death caused by overexpression of MLKs (data not shown), indicating that the JNK/c-Jun death pathway does not involve or require Cdks or other cell cycle components downstream of Cdks.
Kinase-inactive forms of MLK family members suppress death evoked by overexpression of MLKs.
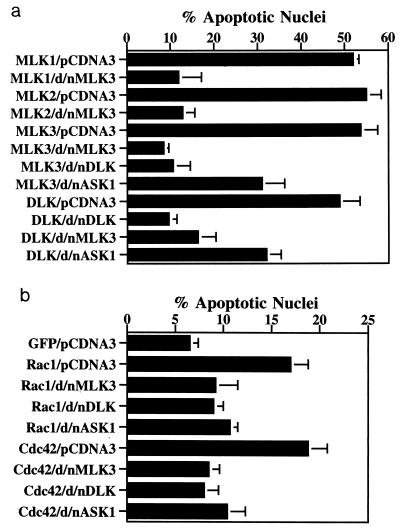
Because MLK family members can homodimerize as well as form complexes with other proteins (41, 65, 67), we tested whether kinase-inactive forms would act as dominant-negative suppressors of death. A kinase-inactive form of MLK3 (dominant-negative MLK3, MLK3 K144R) was cotransfected into neuronal PC12 cells along with wild-type MLK1, MLK2, MLK3, or DLK. In each case, death was strongly suppressed (Fig. 7a). A kinase-inactive DLK (dominant-negative DLK, DLK K152A) also effectively blocked death elicited by MLK3 (Fig. 7a). These findings indicate not only that kinase-inactive MLK forms can act as dominant-negative forms to suppress death caused by overexpression of their own wild-type forms but also that they can do so for other members of the MLK family. Thus, MLK-induced death appears to require dimerization and/or association with one or more shared binding partners.
FIG. 7.
Relationships between MLKs, Rac1/Cdc42, and ASK1. (a) Death induced by MLK overexpression can be suppressed significantly by dominant-negative (d/n) forms of other family members and partially by the dominant-negative form of ASK1. pCMS.EGFP or DNA encoding MLK family members in the pCMS.EGFP vector were cotransfected with either pCDNA3 or dominant-negative MLK3 or dominant-negative ASK1 constructs in the pCDNA3 vector. MLK3 was also cotransfected with dominant-negative DLK as described in Materials and Methods. Three days after transfection, the proportions of apoptotic nuclei were determined as described in Materials and Methods. The values are the means of three independent experiments plus SEM. (b) Death induced by Rac1 V12 and Cdc42 V12 can be suppressed by dominant-negative (d/n) forms of MLKs as well as by dominant-negative ASK1. pCMS.EGFP and Rac1 V12 and Cdc42 V12 DNAs in the pCMS.EGFP vector were cotransfected with either the pCDNA3 vector or dominant-negative MLK3, dominant-negative DLK, or dominant-negative ASK1 constructs (each in the latter vector) as described in Materials and Methods. Three days after transfection, the proportions of apoptotic nuclei were determined as described in Materials and Methods. Because the results from cotransfection with pCMS.EGFP-pCDNA3 and pCMS.EGFP cotransfected with dominant-negative MLK3, dominant-negative DLK, or dominant-negative ASK1 DNA were not apparently different from each other, only results obtained with pCMS.EGFP-pCDNA3 are shown. The values are the means of three independent experiments plus SEM.
Rac1 and Cdc42 have been implicated as components that lie upstream of MKK4 and -7 and JNK in the JNK signaling pathway (17, 65). Moreover, there is evidence that Rac1 and Cdc42 bind to and enhance activation of MLK2 and -3 (4, 48, 62). If Rac1 and Cdc42 act upstream of MLKs in our system, then death elicited by constitutively active forms of these GTPases should be suppressed by dominant-negative MLKs. The data in Fig. 7b indicate that this is the case, thus supporting a scheme in which MLKs mediate the apoptotic actions of Rac1 and Cdc42.
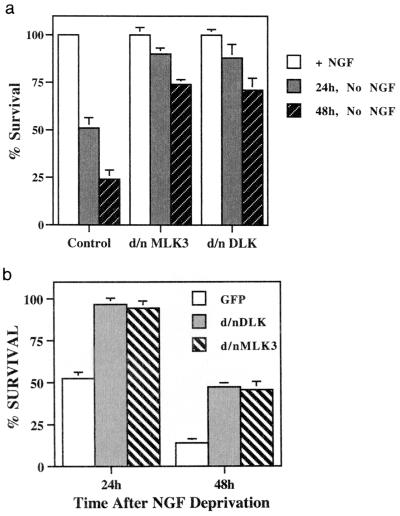
Dominant-negative forms of MLKs protect PC12 cells and sympathetic neurons from death elicited by trophic deprivation.
The above data indicate that all known MLK family members are present in PC12 cells and sympathetic neurons, that overexpression of MLKs can stimulate apoptotic neuronal death, and that such death is suppressed by dominant-negative MLKs. We therefore determined whether dominant-negative MLKs would protect against death induced by trophic factor withdrawal. Neuronal PC12 cells and sympathetic neurons were transfected with DNA encoding dominant-negative MLK3 or dominant-negative DLK cloned into the pCMS.EGFP vector and then deprived of NGF. In each case, the dominant-negative forms provided a significant level of protection from death (Fig. 8). The kinase-inactive forms of MLK1 and MLK2 also provided similar protection for neuronal PC12 cells deprived of NGF (data not shown).
FIG. 8.
(a) Dominant-negative (d/n) forms of MLK3 and DLK protect neuronal PC12 cells from NGF deprivation-induced apoptosis. pCMS.EGFP or DNA encoding dominant-negative forms of MLK3 and DLK in the pCMS.EGFP vector were transfected into neuronal PC12 cells. Two days after transfection, NGF deprivation was performed as described in Materials and Methods. NGF was added back to half of the cultures. Surviving cells were counted 24 and 48 h later. Values from wells with NGF were used as controls for each transfection and defined as 100% survival, and values from cultures without NGF were normalized accordingly. (b) Dominant-negative (d/n) forms of MLK3 and DLK protect rat sympathetic neurons from NGF deprivation-induced cell death. pCMS.EGFP alone or containing DNA encoding dominant-negative MLK3 or DLK was transfected into rat sympathetic neurons, and NGF deprivation was performed as described in Materials and Methods. NGF was added back to half the cultures. Numbers of transfected cells were determined 24 and 48 h after NGF deprivation as described in Materials and Methods. Numbers from cultures with NGF were used as controls for each transfection and defined as 100% survival, and values from NGF deprivation cultures were normalized to them. Values are the means from three replicate cultures plus SEM. Similar results were obtained in two additional independent experiments.
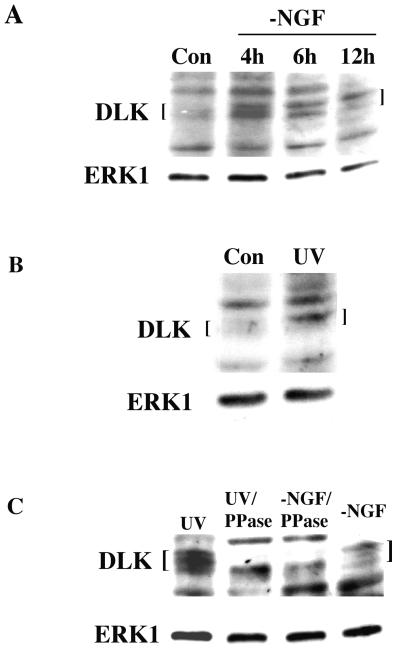
Endogenous DLK is regulated by NGF deprivation and UV exposure.
We next addressed whether apoptotic stimuli affect endogenous MLKs. We were unable to reliably detect endogenous MLK1 or -2 by Western immunoblotting of PC12 cell protein. It is unclear whether this reflects the absence of expression of such proteins (in contrast to the presence of the corresponding transcripts), the quality of the available antisera, or low levels of MLK1 and -2 expression. In contrast, Western immunoblotting revealed endogenous MLK3 and DLK in naive and neuronally differentiated PC12 cells. Due to its clearer resolution, we focused on the response of DLK to apoptotic stimuli. As illustrated in Fig. 9A, withdrawal of NGF from neuronally differentiated PC12 cells results in both an increase in DLK protein levels and an enhanced signal of the most slowly migrating form relative to that in control cells. The increases in levels were apparent within 4 h of NGF deprivation, and the shift to the most slowly migrating form was progressive over time up to 12 h of withdrawal. An increased level and upward mobility shift of DLK protein was also observed in extracts of neuronal PC12 cells after 4 h of UV treatment at a dose that induces apoptosis (Fig. 9B). These times are consistent with those previously reported for elevations of JNK activity evoked by NGF deprivation (42, 69) and UV exposure (42, 53). The decreased electrophoretic mobility of DLK associated with apoptotic stimuli appeared to be due to enhanced phosphorylation in that it was abolished by treatment of cell extracts with phosphatase (Fig. 9C). There is evidence that MLK3 is activated by phosphorylation (24), and it therefore seems likely that the increases in levels and phosphorylation of endogenous DLK induced by apoptotic stimuli reflect its activation.
FIG. 9.
Elevation of DLK levels and phosphorylation in response to NGF deprivation and UV treatment. (A) DLK response to NGF deprivation (−NGF). PC12 cells were treated with NGF for 7 days, washed with serum-free medium without NGF, replated, and collected 0 (control [Con]), 4, 6, and 12 h later. Cell extracts were prepared and analyzed by Western immunoblotting as described in Materials and Methods. DLK antiserum recognized several species at approximately 95 kDa. This recognition was blocked by the peptide used for preparation of the antiserum, and the fastest-migrating of the recognized species comigrated with the kinase-inactive form of DLK overexpressed in CHO cells (data not shown). The membranes in this and other panels in this figure were stripped and reprobed with ERK1 antiserum. (B) DLK response to UV irradiation. Neuronally differentiated PC12 cells were either left untreated (Con) or exposed to UV light (650 J/m2). Four hours later, cell extracts were prepared and subjected to Western immunoblotting with antisera against DLK and ERK1. (C) Multiple electrophoretic forms of DLK are generated by phosphorylation. Extracts of neuronally differentiated PC12 cells exposed to UV light or deprived of NGF (−NGF) as described above were treated with or without phosphatase (PPase) as described in Materials and Methods. The extracts were then subjected to Western immunoblotting with antisera against DLK and ERK1 as probes.
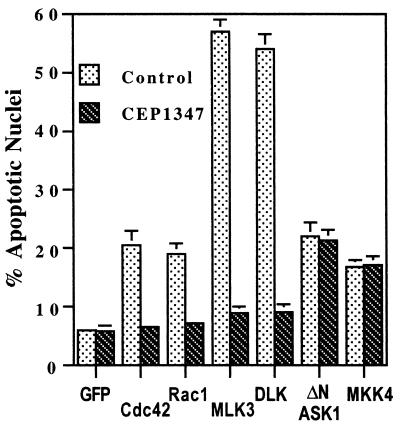
CEP-1347 protects neuronal PC12 cells from death evoked by MLKs and upstream members of the JNK pathway.
CEP-1347 is an indolocarbazole that effectively protects neurons and neuronal PC12 cells from a variety of apoptotic stimuli, including trophic factor deprivation (42, 43, 54, 58). Mechanistic studies reveal that CEP-1347 selectively blocks activation of the JNK pathway in living cells and that in in vitro assays it inhibits the kinase activity of MLK family members but not of a variety of other kinases (42a). These CEP-1347 activities all occur in a similar concentration range (50 to 200 nM). Such findings suggest that in neuronal cells, in which MLKs are activated in response to apoptotic stimuli, CEP-1347 provides protection from death by blocking MLK activity and, consequently, activation of JNKs. We reasoned that if this is the case, then CEP-1347 should protect neuronal cells from death evoked by MLKs and upstream components of the JNK pathway but not from death evoked by downstream pathway members. Neuronally differentiated PC12 cells were pretreated with 200 nM CEP-1347 for 2 h and then transfected with MLK family members or with Rac1 V12 or Cdc42 V12. In all cases, the compound provided a significant protection from death comparable to that achieved with BAF (Fig. 10). In contrast, when similar experiments were carried out with the constitutively active form of ASK1 (ΔN ASK1) and MKK4, there was no protection from death (Fig. 10).
FIG. 10.
Death induced by MLK family members is suppressed by the JNK pathway inhibitor CEP-1347 (200 nM). pCMS.EGFP vector and DNA encoding MLK family members, Rac1 V12, Cdc42 V12, and ΔN ASK1 in pCMS.EGFP were transfected into neuronal PC12 cells. Percentages of apoptotic nuclei were determined as for Fig. 6. The values are means of three independent experiments plus SEM.
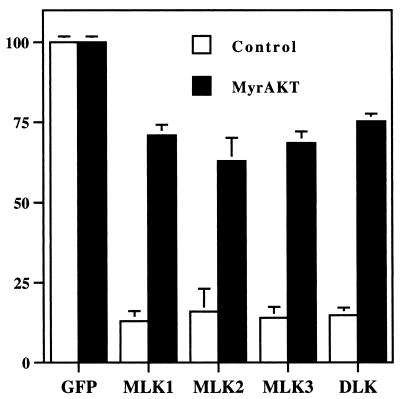
Myr-AKT protects neuronal cells from death evoked by MLKs.
AKT is an important component of the signaling pathways by which trophic factors such as NGF protect neuronal cells from death (13, 66; reviewed in reference 18). Presumably, AKT acts in part by suppressing activation of the MLKs or other components of the JNK pathway when NGF is present. To determine whether AKT might act downstream of the MLKs, we cotransfected MLK family members together with myristylated AKT (myr-AKT). Myr-AKT appears to be constitutively activated when expressed in cells (19). The data depicted in Fig. 11 show that myr-AKT effectively suppressed death caused by overexpression of MLK, family members. Cotransfection with wild-type AKT also suppressed death (data not shown).
FIG. 11.
Apoptotic death induced by MLK family members is suppressed by myr-AKT. pCMS.EGFP and DNA encoding MLK family members in pCMS.EGFP were cotransfected with either pCMV6 (control) vector or pCMV6-MyrAKT (MyrAKT) as described in Materials and Methods. Three days after transfection, surviving transfected cells were counted. Cell numbers for pCMS.EGFP-pCMV6 and pCMS.EGFP-pCMV6-MyrAKT were defined as 100% survival, and the cell numbers for other transfections were normalized accordingly. The values are the means from three independent experiments plus SEM.
The role of ASK1 in the JNK apoptotic pathway.
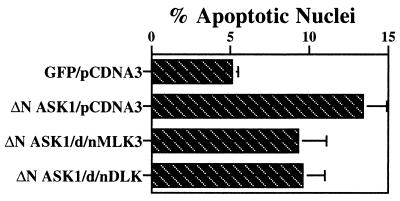
ASK1 is identified as an MKK kinase that can activate the JNK and p38 signaling cascades (29). It was recently reported that overexpression of ΔN ASK1 evokes death of neuronal PC12 cells and sympathetic neurons and that a dominant-negative ASK1 partially protects such cells from death induced by NGF withdrawal (32). We confirmed that ΔN ASK1 evokes death when transfected into neuronal PC12 cells; however, the extent of death was considerably less than that achieved with comparable amounts of cDNA encoding MLKs (Fig. 10). Moreover, in contrast to its protective effects against death evoked by activated Rac1 and Cdc42, MLKs, or NGF withdrawal, CEP-1347 had little, if any, effect on the extent of death promoted by ΔN ASK1 (Fig. 10). This finding indicates that ASK1 is neither a target of CEP-1347 nor upstream of the MLKs.
To determine whether ASK1 might interact directly with MLKs or share common binding partners with MLKs, we cotransfected dominant-negative MLKs with ΔN ASK1. As shown in Fig. 12, death caused by ΔN ASK1 overexpression was partially suppressed by dominant-negative MLK3 and dominant-negative DLK. In the converse experiment, a kinase-inactive dominant-negative ASK1 construct also partially blocked death evoked by MLK3 and DLK; however, it was not as effective in this regard as dominant-negative MLK family members (Fig. 7a). In contrast, dominant-negative ASK1 was as effective as dominant-negative MLK3 and dominant-negative DLK in suppressing death evoked by activated Rac1 and Cdc42 (Fig. 7b).
FIG. 12.
Cell death induced by overexpression of ΔN ASK1 is partially suppressed by dominant-negative (d/n) forms of MLK3 and DLK. pCMS.EGFP (GFP) and pCMS.EGFP-ΔNASK1 (ΔN ASK1) were cotransfected with either pCDNA3 vector or constructs encoding dominant-negative MLK3 or dominant-negative DLK (in the pCDNA3 vector) as described in Materials and Methods. Three days after transfection, the proportions of apoptotic nuclei were assessed as described in Materials and Methods. Because the results for pCMS.EGFP-pCDNA3 and pCMS.EGFP cotransfected with dominant-negative MLK3 or dominant-negative DLK were not apparently different from one other, only the results for pCMS.EGFP-pCDNA3 are shown. The values are the means from three independent experiments plus SEM.
DISCUSSION
Although several elements in the apoptotic signaling pathway that lead to JNK and c-Jun activation have been defined, the molecules that couple Rac1 and Cdc42 to activation of MKK4 and MKK7 in this pathway are less well understood. In this light, the drug CEP-1347 provided both a clue and a challenge. That is, since CEP-1347 blocks JNK activation (42) and appears to inhibit neither JNKs, MKKs, Rac1, nor Cdc42 directly (42a), there is another implied constituent that must be in the pathway and that is sensitive to the drug. The data provided here implicate MLK family members in this role.
Multiple forms of evidence indicate a role for the MLK family in the neuronal apoptotic JNK/c-Jun pathway. First, transcripts for all members of the MLK family are expressed in both naive and neuronally differentiated PC12 cells and in sympathetic neurons. Moreover, at least two members of the family (MLK3 and DLK) were found here to be present at the protein level in PC12 cells. Multiple members of the MLK family are also present in brain (12, 44, 57) (data not shown). Second, overexpression of different members of the MLK family, including MLK1, MLK2, MLK3, and DLK, induced significant cell death in both neuronally differentiated and naive PC12 cells. In addition, the two family members tested (DLK and MLK3) were also potent inducers of apoptosis in cultured sympathetic neurons. This process was mediated by activation of the JNK pathway, as indicated by its blockade by coexpression of dominant-negative MKK4, dominant-negative MKK7, or dominant-negative c-Jun. These observations are consistent with past findings in nonneuronal cells demonstrating that MLK2, MLK3, and DLK activate the JNK pathway though the activation of MKK7 and/or SEK1 (MKK4) and that expression of inactive SEK1 inhibits basal and MLK3-activated JNK activity (4, 9, 25, 46, 56, 62, 63, 65). Third, dominant-negative forms of MLK family members effectively protected neuronally differentiated PC12 cells and sympathetic neurons from apoptosis induced by NGF deprivation. Fourth, apoptotic stimuli led to both elevated levels and apparent activation of endogenous DLK in neuronal PC12 cells. Lastly, CEP-1347, which protects sympathetic neurons and neuronally differentiated PC12 cells from death evoked by NGF deprivation as well as a number of other insults (42), also protected the cells from death elicited by overexpression of MLK family members. In contrast, the compound did not protect against death caused by overexpression of MKK4 or ΔN ASK1. This is consistent with recent findings identifying MLK family members as specific targets of CEP-1347 (42a).
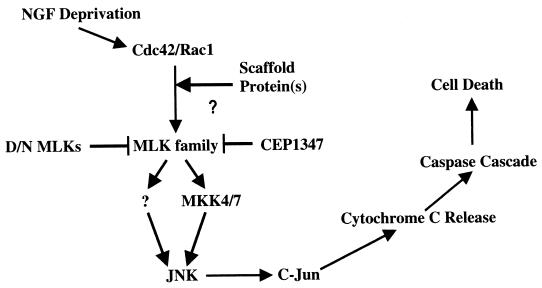
Our use of various JNK/c-Jun pathway constructs as well as of CEP-1347 permitted us to verify the order of the constituents of this pathway. Rac1 and Cdc42 have been implicated as acting upstream of MKK4 and -7, JNKs, and c-Jun (1, 2, 8, 17, 27, 32, 47, 51, 62, 65). Consistent with prior studies, our observations that dominant-negative MLKs and CEP-1347 suppress death evoked by constitutively activated forms of Rac1 and Cdc42 also place these GTPases upstream of the MLKs. Furthermore, as anticipated, blockade of MLK-induced death by dominant-negative MKK4 and -7 as well as by dominant-negative c-Jun positions MLKs upstream of MKK4 and/or MKK7. A scheme summarizing this pathway is shown in Fig. 13.
FIG. 13.
Scheme for the MLK-dependent pathway for apoptotic neuronal death. NGF deprivation of neuronal cells induces the activation of Cdc42 and Rac1, which in turn activate the MLK family members, very likely with the mediation of a scaffold protein(s). Activated MLKs phosphorylate and activate MKK4 and -7 (and possibly other MKK family members) which, in turn, leads to the phosphorylation and activation of JNK and c-Jun subsequently. The activated JNK pathway induces the release of cytochrome c, which activates the caspase cascade and thus leads to cell death. CEP-1347 protects both NGF deprivation and expression of MLK-induced death. The protection is presumably due to its inhibition of the activity of MLK family.
Cytoplasmic cytochrome c is known to cause neuronal death (11, 36, 49). It was recently reported that JNK null cells exhibit a defect in the mitochondrial death signaling mechanism, including the failure to release cytochrome c in response to apoptotic stimuli (64). This indicated that JNK activity is upstream of mitochondrial events in the death pathway. Our observation that MLK3 or DLK overexpression leads to loss of cytochrome c staining is consistent with this interpretation. The finding that cell death induced by overexpression of MLK family members can be prevented by general caspase inhibitors further suggests that MLKs act upstream of caspase activation.
JNK/c-Jun pathway activation has been found to elevate transcription of FAS ligand in some systems, and this has been suggested to contribute, in turn, to apoptotic death (40). We observed in neuronal PC12 cells that a dominant-negative form of FADD, which should interfere with FAS signaling, provided only a very limited protection from death elicited by MLK3 or DLK overexpression (Z. Xu, unpublished results). This suggests that regulated pathways other than those involving FAS and FADD are likely to play more major roles in death evoked by MLK or JNK activation. Alternatively, it is possible that FAS ligand may not depend completely on FADD to kill cells in our system.
What are the possible mechanisms by which MLK family members might be activated and by which they, in turn, phosphorylate their targets? In addition, what does the capacity, observed here, of various MLK dominant-negative forms to block death caused by other MLK family members tell us about these mechanisms? Overexpressed MLK3 and DLK can form homodimers through leucine zipper interactions, leading to autophosphorylation and activation, and such dimerization is a prerequisite for subsequent activation of JNKs (41, 44, 50). Vacratsis and Gallo (65) recently reported that constitutively activated Cdc42 fully activates a monomeric MLK3 leucine zipper mutant in terms of both autophosphorylation and histone phosphorylation activity and induces the same in vivo phosphorylation pattern as that of wild-type MLK3. However, this catalytically active monomeric MLK3 mutant is unable to stimulate JNK activation because it fails to phosphorylate one of the two activating phosphorylation sites, Thr258, of MKK4. These studies indicate that zipper-mediated MLK3 oligomerization is not required for MLK3 activation by Cdc42 but instead is critical for proper interaction and phosphorylation of a downstream target(s). This further suggests that one mechanism by which MLK and DLK dominant-negative forms act is by interfering with homodimerization required for substrate interaction.
In addition to blocking death caused by overexpression of their own wild-type counterparts, dominant-negative MLK3 and dominant-negative DLK also suppressed death evoked by other family members. One potential explanation is that various family members act in a cascade. For instance, on the basis that dominant-negative DLK suppresses MLK3-induced JNK1 activation, it was suggested that DLK functions downstream of MLK3 (60). However, in apparent disagreement with this possibility, we observed that dominant-negative MLK3 also effectively suppresses death evoked by DLK. A second model is that MLK3 and DLK form heterodimers whose function can be blocked by dominant-negative forms of either partner. Consistent with this, the two proteins can be coimmunoprecipitated (60). However, Nihalani et al. (50) recently provided evidence that MLK3 and DLK do not form heterodimers.
A third model, which is also consistent with coimmunoprecipitation, is that the MLK3 and DLK compete for binding to a common intermediary protein. For instance, it is possible that MLK3 and DLK (and their dominant-negative forms) compete for interaction with upstream activators such as Cdc42 and Rac1 and/or with the downstream kinases MKK4 and MKK7. For example, there is evidence that MLK2 and MLK3 associate with the activated (GTP-bound) forms of Rac1 and Cdc42 (48). Moreover, coexpression of activated Cdc42 with MLK3 leads to a substantial increase in MLK3 dimerization as well as altered MLK3 serine/threonine phosphorylation (4, 41). However, unlike the case of the PAK family of protein kinases, the activation of MLK3 by Cdc42 cannot be recapitulated in an in vitro system using purified, recombinant proteins (4). Such studies thus suggest that an additional component is required for MLK3 activation by Cdc42. Good candidates for this role would be scaffold proteins, such as JIP1 or POSH. The multidomain protein POSH binds to the constitutively active form of Rac1, which is known to regulate the activity of MLKs, while JIP1 binds to MLKs and additional components of the JNK pathway and appears to be capable of activating MLKs (50, 61, 67). Thus, it is attractive to consider the possibility that dominant-negative MLKs may act, at least in part, by competing for binding to a common scaffold protein that is required for activation of the JNK death pathway.
One question that is not currently resolved by our experiments is whether all or only a subset of MLK family members participate in the JNK apoptotic pathway. All appear to be expressed in the neuronal cell systems studied here, and all tested members of the family elicited death upon overexpression. This would suggest that multiple family members may indeed contribute to JNK activation and thereby to the death process.
Another kinase that has been raised as a potential upstream component of the JNK apoptotic pathway in neurons is ASK1. Overexpression of ΔN ASK1 evokes death of neuronal PC12 cells and sympathetic neurons, apparently by activation of the JNK/c-Jun pathway, and dominant-negative ASK1 partially protects such cells from death caused by NGF withdrawal (32). We observed that death stimulated by active ASK1, in contrast to death evoked by NGF deprivation and other apoptotic stimuli, was not blocked by CEP-1347 and that dominant-negative ASK1 partially suppressed death caused by MLK3 and DLK overexpression. Two main interpretations of these findings are currently possible. One is that ASK1 lies downstream of MLKs in the JNK pathway. However, our observation that dominant-negative MLK3 and dominant-negative DLK reciprocally inhibit death evoked by constitutively active ASK1 is inconsistent with this possibility. The other interpretation is that ASK1 is not a major player in the JNK death pathway activated by NGF withdrawal and that the protective effects of dominant-negative ASK1 overexpression reflect nonspecific competition with MLKs for interaction with Rac1, Cdc42, scaffold proteins, or downstream targets.
We noted that death promoted by MLK family members and other elements of the JNK pathway was suppressed by coexpression of a constitutively active form of AKT (Fig. 11 and data not shown). AKT plays an important role in mediating antiapoptotic activities of growth factors and appears to do so in part by phosphorylating and affecting the activities of a number of proapoptotic proteins, including caspase 9, BAD, IκB kinase, GSK3β, and FKHR (16; reviewed in references 33 to 35). Although AKT was recently reported to inhibit the Rac1 signal transduction pathway (37), an additional mechanism must be invoked in the present studies, since it also provided protection from elements that are downstream of Rac1 in the JNK death pathway. This indicates that AKT must suppress the JNK-dependent death mechanism not only upstream or at the level of Rac1 activation but also at some point downstream of MLKs (which appear to lack consensus sites for AKT phosphorylation). Although the identity of the downstream AKT-sensitive element(s) is unknown, it is of interest that many known substrates seem unlikely. Rat (the species used in our studies) caspase 9 lacks the consensus site for phosphorylation by AKT, and there is no current evidence that BAD is involved in death evoked by NGF deprivation. Also, GSK3β and FKHR do not appear to be likely downstream targets of the JNK pathway. This raises the possibility that downstream of the JNK pathway there lies an additional novel AKT substrate that regulates survival and death.
ACKNOWLEDGMENTS
We thank Michael J. Birrer, Thomas Franke, J. Silvio Gutkind, Alan Hall, Larry Holzman, Hidenori Ichijo, and Richard Spritz for plasmid constructs; James M. Angelastro, David X. Liu, and Jaya Padmanabhan (Columbia University) for helpful advice; Sheryl L. Meyer, Steve Trusko, and Chrysanthe Spais (Cephalon Inc.) for providing molecular reagents; and Claudine Bitel (Columbia University) for technical aid.
This work was supported in part by grants from the NIH-NINDS and Blanchette Rockefeller Foundation (L.A.G.).
REFERENCES
- 1.Bagrodia S, Derijard B, Davis R J, Cerione R A. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 2.Bazenet C E, Mota M A, Rubin L L. The small GTP-binding protein Cdc42 is required for nerve growth factor withdrawal-induced neuronal death. Proc Natl Acad Sci USA. 1998;95:3984–3989. doi: 10.1073/pnas.95.7.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat R V, DiRocco R, Marcy V R, Flood D G, Zhu Y, Dobrzanski P, Siman R, Scott R, Contreras P C, Miller M. Increased expression of IL-1 beta converting enzyme in hippocampus after ischemia: selective localization in microglia. J Neurosci. 1996;16:4146–4154. doi: 10.1523/JNEUROSCI.16-13-04146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bock B C, Vacratsis P O, Qamirani E, Gallo K A. Cdc42-induced activation of the mixed-lineage kinase SPRK in vivo. Requirement of the Cdc42/Rac interactive binding motif and changes in phosphorylation. J Biol Chem. 2000;275:14231–14241. doi: 10.1074/jbc.275.19.14231. [DOI] [PubMed] [Google Scholar]
- 5.Burbelo P D, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Yang J, Jones D P. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta. 1998;1366:139–149. doi: 10.1016/s0005-2728(98)00109-1. [DOI] [PubMed] [Google Scholar]
- 7.Chuang T H, Hahn K M, Lee J D, Danley D E, Bokoch G M. The small GTPase Cdc42 initiates an apoptotic signaling pathway in Jurkat T lymphocytes. Mol Biol Cell. 1997;8:1687–1698. doi: 10.1091/mbc.8.9.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 9.Cuenda A, Dorow D S. Differential activation of stress-activated protein kinase kinases SKK4/MKK7 and SKK1/MKK4 by the mixed-lineage kinase-2 and mitogen-activated protein kinase kinase (MKK) kinase-1. Biochem J. 1998;333:11–15. doi: 10.1042/bj3330011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desagher S, Martinou J C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 11.Deshmukh M, Johnson E M., Jr Evidence of a novel event during neuronal death: development of competence-to-die in response to cytoplasmic cytochrome c. Neuron. 1998;21:695–705. doi: 10.1016/s0896-6273(00)80587-5. [DOI] [PubMed] [Google Scholar]
- 12.Dorow D S, Devereux L, Tu G F, Price G, Nicholl J K, Sutherland G R, Simpson R J. Complete nucleotide sequence, expression, and chromosomal localisation of human mixed-lineage kinase 2. Eur J Biochem. 1995;234:492–500. doi: 10.1111/j.1432-1033.1995.492_b.x. [DOI] [PubMed] [Google Scholar]
- 13.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 14.Eilers A, Whitfield J, Babij C, Rubin L L, Ham J. Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J Neurosci. 1998;18:1713–1724. doi: 10.1523/JNEUROSCI.18-05-01713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estus S, Zaks W J, Freeman R S, Gruda M, Bravo R, Johnson E M., Jr Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994;127:1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eves E M, Xiong W, Bellacosa A, Kennedy S G, Tsichlis P N, Rosner M R, Hay N. Akt, a target of phosphatidylinositol 3-kinase, inhibits apoptosis in a differentiating neuronal cell line. Mol Cell Biol. 1998;18:2143–2152. doi: 10.1128/mcb.18.4.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foltz I N, Gerl R E, Wieler J S, Luckach M, Salmon R A, Schrader J W. Human mitogen-activated protein kinase kinase 7 (MKK7) is a highly conserved c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) activated by environmental stresses and physiological stimuli. J Biol Chem. 1998;273:9344–9351. doi: 10.1074/jbc.273.15.9344. [DOI] [PubMed] [Google Scholar]
- 18.Franke T F, Kaplan D R, Cantley L C. P13K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 19.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 20.Garrington T P, Johnson G L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 21.Greene L A, Tischler A S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ham J, Babij C, Whitfield J, Pfarr C M, Lallemand D, Yaniv M, Rubin L L. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 23.Ham J, Eilers A, Whitfield J, Neame S J, Shah B. c-Jun and the transcriptional control of neuronal apoptosis. Biochem Pharmacol. 2000;60:1015–1021. doi: 10.1016/s0006-2952(00)00372-5. [DOI] [PubMed] [Google Scholar]
- 24.Hehner S P, Hofmann T G, Ushmorov A, Dienz O, Wing-Lan Leung I, Lassam N, Scheidereit C, Droge W, Schmitz M L. Mixed-lineage kinase 3 delivers CD3/CD28-derived signals into the IκB kinase complex. Mol Cell Biol. 2000;20:2556–2568. doi: 10.1128/mcb.20.7.2556-2568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirai S, Katoh M, Terada M, Kyriakis J M, Zon L I, Rana A, Avruch J, Ohno S. MST/MLK2, a member of the mixed lineage kinase family, directly phosphorylates and activates SEK1, an activator of c-Jun N-terminal kinase/stress-activated protein kinase. J Biol Chem. 1997;272:15167–15173. doi: 10.1074/jbc.272.24.15167. [DOI] [PubMed] [Google Scholar]
- 26.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 27.Holland P M, Suzanne M, Campbell J S, Noselli S, Cooper J A. MKK7 is a stress-activated mitogen-activated protein kinase kinase functionally related to hemipterous. J Biol Chem. 1997;272:24994–24998. doi: 10.1074/jbc.272.40.24994. [DOI] [PubMed] [Google Scholar]
- 28.Holzman L B, Merritt S E, Fan G. Identification, molecular cloning, and characterization of dual leucine zipper bearing kinase. A novel serine/threonine protein kinase that defines a second subfamily of mixed lineage kinases. J Biol Chem. 1994;269:30808–30817. [PubMed] [Google Scholar]
- 29.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 30.Ing Y L, Leung I W, Heng H H, Tsui L C, Lassam N J. MLK-3: identification of a widely-expressed protein kinase bearing an SH3 domain and a leucine zipper-basic region domain. Oncogene. 1994;9:1745–1750. [PubMed] [Google Scholar]
- 31.Ip Y T, Davis R J. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 32.Kanamoto T, Mota M, Takeda K, Rubin L L, Miyazono K, Ichijo H, Bazenet C E. Role of apoptosis signal-regulating kinase in regulation of the c-Jun N-terminal kinase pathway and apoptosis in sympathetic neurons. Mol Cell Biol. 2000;20:196–204. doi: 10.1128/mcb.20.1.196-204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kandel E S, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan D R, Miller F D. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 35.Kops G J, Burgering B M. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J Mol Med. 1999;77:656–665. doi: 10.1007/s001099900050. [DOI] [PubMed] [Google Scholar]
- 36.Krajewski S, Krajewska M, Ellerby L M, Welsh K, Xie Z, Deveraux Q L, Salvesen G S, Bredesen D E, Rosenthal R E, Fiskum G, Reed J C. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci USA. 1999;96:5752–5757. doi: 10.1073/pnas.96.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon T, Kwon D Y, Chun J, Kim J H, Kang S S. Akt protein kinase inhibits Rac1-GTP binding through phosphorylation at serine 71 of Rac1. J Biol Chem. 2000;275:423–428. doi: 10.1074/jbc.275.1.423. [DOI] [PubMed] [Google Scholar]
- 38.Kyriakis J M, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 39.Lee V M, Shelanski M L, Greene L A. Characterization of antisera raised against cultured rat sympathetic neurons. Neuroscience. 1980;5:2239–2245. doi: 10.1016/0306-4522(80)90140-2. [DOI] [PubMed] [Google Scholar]
- 40.Le-Niculescu H, Bonfoco E, Kasuya Y, Claret F X, Green D R, Karin M. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol Cell Biol. 1999;19:751–763. doi: 10.1128/mcb.19.1.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung I W, Lassam N. Dimerization via tandem leucine zippers is essential for the activation of the mitogen-activated protein kinase kinase kinase, MLK-3. J Biol Chem. 1998;273:32408–32415. doi: 10.1074/jbc.273.49.32408. [DOI] [PubMed] [Google Scholar]
- 42.Maroney A C, Finn J P, Bozyczko-Coyne D, O'Kane T M, Neff N T, Tolkovsky A M, Park D S, Yan C Y, Troy C M, Greene L A. CEP-1347 (KT7515), an inhibitor of JNK activation, rescues sympathetic neurons and neuronally differentiated PC12 cells from death evoked by three distinct insults. J Neurochem. 1999;73:1901–1912. [PubMed] [Google Scholar]
- 42a.Maroney, A. C., J. P. Finn, T. J. Connors, J. T. Durkin, T. Angeles, G. Gessner, Z. Xu, S. L. Meyer, M. J. Savage, L. A. Greene, R. W. Scott, and J. L. Vaught. CEP-1347 (KT7515), a synthetic inhibitor of the mixed lineage kinase family. J. Biol. Chem., in press. [DOI] [PubMed]
- 43.Maroney A C, Glicksman M A, Basma A N, Walton K M, Knight E, Jr, Murphy C A, Bartlett B A, Finn J P, Angeles T, Matsuda Y, Neff N T, Dionne C A. Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J Neurosci. 1998;18:104–111. doi: 10.1523/JNEUROSCI.18-01-00104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mata M, Merritt S E, Fan G, Yu G G, Holzman L B. Characterization of dual leucine zipper-bearing kinase, a mixed lineage kinase present in synaptic terminals whose phosphorylation state is regulated by membrane depolarization via calcineurin. J Biol Chem. 1996;271:16888–16896. doi: 10.1074/jbc.271.28.16888. [DOI] [PubMed] [Google Scholar]
- 45.Mazars A, Tournigand C, Mollat P, Prunier C, Ferrand N, Bourgeade M F, Gespach C, Atfi A. Differential roles of JNK and Smad2 signaling pathways in the inhibition of c-Myc-induced cell death by TGF-beta. Oncogene. 2000;19:1277–1287. doi: 10.1038/sj.onc.1203420. [DOI] [PubMed] [Google Scholar]
- 46.Merritt S E, Mata M, Nihalani D, Zhu C, Hu X, Holzman L B. The mixed lineage kinase DLK utilizes MKK7 and not MKK4 as substrate. J Biol Chem. 1999;274:10195–10202. doi: 10.1074/jbc.274.15.10195. [DOI] [PubMed] [Google Scholar]
- 47.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 48.Nagata K, Puls A, Futter C, Aspenstrom P, Schaefer E, Nakata T, Hirokawa N, Hall A. The MAP kinase kinase kinase MLK2 co-localizes with activated JNK along microtubules and associates with kinesin superfamily motor KIF3. EMBO J. 1998;17:149–158. doi: 10.1093/emboj/17.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neame S J, Rubin L L, Philpott K L. Blocking cytochrome c activity within intact neurons inhibits apoptosis. J Cell Biol. 1998;142:1583–1593. doi: 10.1083/jcb.142.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nihalani D, Merritt S, Holzman L B. Identification of structural and functional domains in mixed lineage kinase dual leucine zipper-bearing kinase required for complex formation and stress-activated protein kinase activation. J Biol Chem. 2000;275:7273–7279. doi: 10.1074/jbc.275.10.7273. [DOI] [PubMed] [Google Scholar]
- 51.Olson M F, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 52.Park D S, Morris E J, Stefanis L, Troy C M, Shelanski M L, Geller H M, Greene L A. Multiple pathways of neuronal death induced by DNA-damaging agents, NGF deprivation, and oxidative stress. J Neurosci. 1998;18:830–840. doi: 10.1523/JNEUROSCI.18-03-00830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park D S, Stefanis L, Yan C Y I, Farinelli S E, Greene L A. Ordering the cell death pathway. Differential effects of BCL2, an interleukin-1-converting enzyme family protease inhibitor, and other survival agents on JNK activation in serum/nerve growth factor-deprived PC12 cells. J Biol Chem. 1996;271:21898–21905. doi: 10.1074/jbc.271.36.21898. [DOI] [PubMed] [Google Scholar]
- 54.Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto A M, Walton K M, Ylikoski J. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20:43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 56.Rana A, Gallo K, Godowski P, Hirai S, Ohno S, Zon L, Kyriakis J M, Avruch J. The mixed lineage kinase SPRK phosphorylates and activates the stress-activated protein kinase activator, SEK-1. J Biol Chem. 1996;271:19025–19028. doi: 10.1074/jbc.271.32.19025. [DOI] [PubMed] [Google Scholar]
- 57.Sakuma H, Ikeda A, Oka S, Kozutsumi Y, Zanetta J P, Kawasaki T. Molecular cloning and functional expression of a cDNA encoding a new member of mixed lineage protein kinase from human brain. J Biol Chem. 1997;272:28622–28629. doi: 10.1074/jbc.272.45.28622. [DOI] [PubMed] [Google Scholar]
- 58.Saporito M S, Brown E R, Carswell S, DiCamillo A M, Miller M S, Murakata C, Neff N T, Vaught J L, Haun F A. Preservation of cholinergic activity and prevention of neuron death by CEP-1347/KT-7515 following excitotoxic injury of the nucleus basalis magnocellularis. Neuroscience. 1998;86:461–472. doi: 10.1016/s0306-4522(98)00059-1. [DOI] [PubMed] [Google Scholar]
- 59.Stefanis L, Park D S, Friedman W J, Greene L A. Caspase-dependent and -independent death of camptothecin-treated embryonic cortical neurons. J Neurosci. 1999;19:6235–6247. doi: 10.1523/JNEUROSCI.19-15-06235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka S, Hanafusa H. Guanine-nucleotide exchange protein C3G activates JNK1 by a ras-independent mechanism. JNK1 activation inhibited by kinase negative forms of MLK3 and DLK mixed lineage kinases. J Biol Chem. 1998;273:1281–1284. doi: 10.1074/jbc.273.3.1281. [DOI] [PubMed] [Google Scholar]
- 61.Tapon N, Nagata K, Lamarche N, Hall A. A new rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-kappaB signalling pathways. EMBO J. 1998;17:1395–1404. doi: 10.1093/emboj/17.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teramoto H, Coso O A, Miyata H, Igishi T, Miki T, Gutkind J S. Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J Biol Chem. 1996;271:27225–27228. doi: 10.1074/jbc.271.44.27225. [DOI] [PubMed] [Google Scholar]
- 63.Tibbles L A, Ing Y L, Kiefer F, Chan J, Iscove N, Woodgett J R, Lassam N J. MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J. 1996;15:7026–7035. [PMC free article] [PubMed] [Google Scholar]
- 64.Tournier C, Hess P, Yang D D, Xu J, Turner T K, Nimnual A, Bar-Sagi D, Jones S N, Flavell R A, Davis R J. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 65.Vacratsis P O, Gallo K A. Zipper-mediated oligomerization of the mixed lineage kinase SPRK/MLK-3 is not required for its activation by the GTPase cdc 42 but is necessary for its activation of the JNK pathway. Monomeric SPRK L410P does not catalyze the activating phosphorylation of Thr258 of murine mitogen-activated protein kinase kinase 4. J Biol Chem. 2000;275:27893–27900. doi: 10.1074/jbc.M002858200. [DOI] [PubMed] [Google Scholar]
- 66.Vaillant A R, Mazzoni I, Tudan C, Boudreau M, Kaplan D R, Miller F D. Depolarization and neurotrophins converge on the phosphatidylinositol 3-kinase-Akt pathway to synergistically regulate neuronal survival. J Cell Biol. 1999;146:955–966. doi: 10.1083/jcb.146.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitmarsh A J, Cavanagh J, Tournier C, Yasuda J, Davis R J. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- 68.Whitmarsh A J, Davis R J. Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem Sci. 1998;23:481–485. doi: 10.1016/s0968-0004(98)01309-7. [DOI] [PubMed] [Google Scholar]
- 69.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 70.Yamauchi J, Kaziro Y, Itoh H. Differential regulation of mitogen-activated protein kinase kinase 4 (MKK4) and 7 (MKK7) by signaling from G protein beta gamma subunit in human embryonal kidney 293 cells. J Biol Chem. 1999;274:1957–1965. doi: 10.1074/jbc.274.4.1957. [DOI] [PubMed] [Google Scholar]