Abstract
Aim
The purpose of this study is to study the antioxidant effect of Lactobacillus fermentum CQPC08 (CQPC08) on exercise-induced fatigue, and the beneficial intervention of GOS on CQPC08.
Methods
We use the treadmill to establish a fatigue model caused by exercise, and perform drug treatment after exercise. We tested the exhaustive exercise time of mice; investigated the changes of mice body weight, liver index, histopathology, serum biochemical indicators and mRNA expression levels of oxidative and inflammation-related genes; and assessed the potential fatigue inhibitory effect of CQPC08, and the anti-oxidation effect of the combination of GOS and CQPC08.
Results
The results suggest that CQPC08 and combination with GOS reduces fatigue-induced oxidative damage of the liver, and it decreases blood urea nitrogen (BUN), lactic acid (LA), glutamic-oxaloacetic transaminase (GOT), glutamic-pyruvic transaminase (GPT), malonaldehyde (MDA), inducible nitric oxide synthase (iNOS), tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 in serum. Higher levels of serum catalase (CAT), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) were found. Treatment with the CQPC08 and combination with GOS correlates with lower relative mRNA expression levels of neuronal NOS (nNOS), iNOS, and TNF-α, and with higher mRNA expression levels of catalase and copper/zinc (Cu/Zn) and manganese (Mn) SOD enzymes in the liver and muscles.
Conclusion
These results suggest that CQPC08 can resolve exercise-induced fatigue by improving antioxidant ability in mice, and the combination of GOS and CQPC08 enhances this ability of CQPC08.
Keywords: Lactobacillus fermentum, exercise-induced fatigue, antioxidant, GOS
Introduction
Fatigue is defined as the physiological processes of the body when it cannot continue its function to a certain extent or maintain predetermined exercise intensity.1 Studies have indicated that fatigue is not only related to the duration and intensity of exercise but also to the physical fitness, training level and muscle fiber quality of the subject and the environmental conditions.2 The exact mechanisms of fatigue, then, remain unclear. In order to provide more detail to these models, rats and mice are often selected as study subjects, and running on a treadmill or swimming are utilized as means to simulate human exercise training.3 In this study, a treadmill was used to establish a mice exercise-induced fatigue model to illustrate the anti-fatigue mechanism.
Regular physical exercise is the utmost important tool for maintaining health, but strenuous exercise and long-term exercise will cause the activated white blood cells to release a large amount of pro-inflammatory factors and free radicals, and cause muscle damage.4,5 The free radical theory of fatigue has long been considered a potentially important explanatory model.6 Oxygen free radicals are expected to increase during exercise as a result of increasing oxygen consumption and energy metabolism and declining enzymatic and non-enzymatic antioxidant activity. The resulting oxygen free radicals would directly attack and damage cell membranes; at the same time, lipid peroxides would spontaneously decompose to form more free radicals, causing free radical chain reactions and fatigue.7 Therefore, there are a variety of biological antioxidants that inhibit the production of free radicals and reactive oxygen species (ROS). These antioxidant systems are classified as preventive, capture and repair-regenerative antioxidants. Preventative antioxidants represent the first line of defense in humans, and they mainly inhibit the production of free radicals and ROS through antioxidant enzymes, including superoxide dismutases (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px).8,9 In the present study, we investigated mechanisms linking the preventative antioxidant system to fatigue induced by exercise.
Vitamin C is one of the potent reducing agents and scavenger of free radicals in biological systems, working as a scavenger of oxidizing free radicals and harmful oxygen-derived species, such as hydroxyl radical, hydrogen peroxide (H2O2), and singlet oxygen.10,11 A large number of studies have reported that vitamin C has a good antioxidant effect, and it is often used as a positive control drug in antioxidant studies. Therefore, this study also selected vitamin C as a positive control drug.12–14
Lactobacillus fermentum is one of the lactic acid bacteria commonly found in food.15 Current studies on its many probiotic characteristics focus on its adaptability in the gastrointestinal environment, its ability to degrade or even eliminate cholesterol, its antibacterial activity against harmful bacteria, and its regulation of immune responses.16 Recently, lactic acid bacteria have attracted significant attention in medical and agricultural sciences, with prospects for broad application. Studies have shown that lactic acid bacteria also have high antioxidant activity.17,18 However, there are still few studies on the improvement effect of lactic acid bacteria on exercise fatigue.
A safe, effective and non-toxic anti-fatigue factor is an important research goal, and the efficacy of Lactobacillus fermentum in the control of oxidative stress in animals suggests that it might be useful in fatigue as well. Therefore, a strain of this bacterium, CQPC08, was selected in this study to explore the impact of antioxidant effects on exercise-induced fatigue. Lactobacillus fermentum CQPC08 was isolated and identified from traditional fermented kimchi in Sichuan, China. In our previous studies, it was found that Lactobacillus fermentum CQPC08 has a protective effect on 4-nitroquinoline-1-oxide-induced tongue cancer in mice and a mitigation effect on acetate-induced oxidative stress in rats.19,20 Therefore, this study is also to investigate whether Lactobacillus fermentum CQPC08 alleviate fatigue caused by exercise via antioxidation mechanisms.
Galactooligosaccharide (GOS) is a new type of functional substance, a kind of functional oligosaccharides with natural properties, and an important prebiotic in breast milk, which will not be digested by digestive enzymes in the human body.21 Studies have shown that GOS can effectively multiply Bifidobacteria and Lactobacilli, while also inhibiting the growth of spoilage bacteria.22,23 However, there are few studies on the antioxidant effect of GOS on Lactobacillus fermentum. Therefore, this study combined GOS and CQPC08 to explore its beneficial effects on Lactobacillus fermentum.
The results of this study showed that after supplementing these mice with CQPC08, the mice’s antioxidant capacity was improved and exercise fatigue was suppressed. This study provides a theoretical basis and tools for exploring the effect of lactic acid bacteria on delaying fatigue.
Materials and Methods
Strains
A new strain of Lactobacillus was isolated and purified from naturally fermented pickles by our team in Chongqing, China. Sequence analysis of 16S rDNA and observation of colony morphology by Gram staining confirmed that it was Lactobacillus fermentum. It was deposited in the China General Microbiological Culture Collection Center (CGMCC, Beijing, China) with the preservation number 14957 and the name Lactobacillus fermentum CQPC08.
Animal Model
Establishment of a Mouse Model of Exercise-Induced Fatigue
Six-week-old Kunming male mice (n = 60, body weight 20 ± 2 g) were purchased from the experimental animal center of Chongqing Medical University. The mice were raised at 25 ± 2°C and 50 ± 5% relative humidity under 12 hr light/dark cycles, and they were fed with standard feed and water ad libitum. The padding was changed every 2 days.
For studies of exercise-induced fatigue, mice were randomly divided into six groups (10 in each group). The experiment was conducted for 5 weeks. During this period, the mice were fed with standard feed and water. One of the six groups, called the “normal” group, was raised in an identical environment as other mice, but this group was not subjected to exercise. Each of the other five groups was subjected to the following exercise regimen. In the first week, they were given adaptive training for 10 min every day at a running speed of 15 m/min. Beginning with the second week, a 4-week training was initiated. This training involved running 30 min per day at a speed of 30 m/min from Monday through Friday, with rest on Saturday and Sunday.3
After each exercise, the mice were treated via gavage as follows: the “Normal” and “Control” groups were treated with vehicle (0.1 mL of 0.9% normal saline daily per 10 g body weight); the “Vc” group was treated with 200 mg/kg of vitamin C in vehicle;24,25 the “CQPC” group was treated with Lactobacillus fermentum CQPC08 (1.0 × 109 CFU/mL) in vehicle;19,20 the “CQPC + GOS” group was treated with CQPC08 (1.0 × 109 CFU/mL) and 200 mg/kg GOS (Beijing Solarbio Science & Technology Co. Ltd., Beijing, China) in vehicle; and the “GOS” group was treated with 200 mg/kg GOS in vehicle.26
The protocol for these experiments was approved by the Ethics Committee of Chongqing Collaborative Innovation Center for Functional Food (201904020B, Chongqing, China). And this study strictly followed the standards of laboratory animal – guideline for ethical review of animal welfare (GB/T35892-2018) by the National Standard of the People’s Republic of China.
Exercise Exhaustion Test
After the final administration, an exercise exhaustion test was performed to analyze the exercise load. Exercise exhaustion was identified when mice could not keep up with the predetermined speed of the treadmill, and their hind limbs dragged on the belt for more than 30 s. Exercise exhaustion was noted, and exhaustion time was recorded. The overall performance was quantified by identifying high-frequency shortness of breath without obvious response after physical stimulation. The mice were ultimately sacrificed by cervical dislocation, blood was collected from the eyeballs, and the liver and skeletal muscles (soleus muscle, red, slow-twitch muscle) were dissected and separated for analysis.27,28
Hematoxylin and Eosin (HE) Staining
The liver tissue was washed with saline, and half of the right lobe was cut and fixed in a 10% formalin solution. The liver was dehydrated with an ethanol gradient, immersed and cleaned in a 1:1 xylene: ethanol (v/v) solution for approximately 30 min, embedded in paraffin, cut into 2–3 μm sections with a slicer, and fixed on a slide. HE was used to stain the cytoplasm pink or red. Morphological changes were observed using a light microscope.29
Detection of Serum Indices
The serum was separated by centrifuging the blood at 4000 rpm for 10 min at 4°C and was stored at −80°C. Serum levels of BUN, LA, GOT, GPT and the oxidative indices CAT, GSH-Px, SOD, and MDA were determined using commercially available kits according to the manufacturers’ instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
ELISA
Serum levels of TNF-α, iNOS, IL-1β and IL-6 were determined with commercially available ELISA kits according to the manufacturers’ instructions (Shanghai Enzyme Link Biotechnology Co., Ltd., Shanghai, China).
Real-Time Quantitative PCR (RT-qPCR) Assay
The expression of mRNA in the liver and skeletal muscles was quantified by a SYBR green-based RT-qPCR assay. Approximately 100 mg of liver or skeletal muscle tissue was cut into pieces to extract total RNA with Trizol (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The concentration of RNA was determined with a micro-spectrophotometer (Nano 300, Ao Sheng, Hangzhou, Zhejiang, China). A Reverse Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Baltics UAB, Lithuania, Vilnius) was used to obtain a cDNA template. Then, 10 μL SYBR Green PCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA). And 1 μL upstream primer and 1 μL downstream primer, 1 μL cDNA template and 7 μL diethyl pyrocarbonate were used to amplify the target in a StepOnePlus real-time PCR system (Thermo Fisher Scientific, Waltham, MA, USA). The conditions were as follows: pre-denaturation at 95°C for 3 min; denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 15 s, 40 cycles; the final dissolution curve was completed at 95°C for 30 s, 60°C for 30 s, and 95°C for 15 s. Finally, the relative expression level of each gene was calculated by 2−ΔΔCT, where CT is the circulation threshold. β-Actin was used as an internal reference gene.29 Table 1 shows the sequence information of primers used in this study, and Table 2 shows the conditions of each step of RT-qPCR.
Table 1.
Sequences of Primers Used in the RT-qPCR Analysis
| Gene Name | Sequence |
|---|---|
| Cu/Zn SOD | Forward: 5ʹ-AACCAGTTGTGTTGTCAGGAC-3’ |
| Reverse: 5ʹ-CCACCATGTTTCTTAGAGTGAGG-3’ | |
| Mn SOD | Forward: 5ʹ-AGACCTGCCTTACGACTATGG-3’ |
| Reverse: 5ʹ-CTCGGTGGCGTTGAGATTGTT-3’ | |
| CAT | Forward: 5ʹ-TGGCACACTTTGACAGAGAGC-3’ |
| Reverse: 5ʹ-CCTTTGCCTTGGAGTATCTGG-3’ | |
| nNOS | Forward: 5ʹ-TCCCAGTAACGGACCTCAG-3’ |
| Reverse: 5ʹ-TGCTCAACACAGGTTCTATCTCT-3’ | |
| iNOS | Forward: 5ʹ-GGAGTGACGGCAAACATGACT-3’ |
| Reverse: 5ʹ-GCCAAACTTGCTCCATGTCC-3’ | |
| TNF-α | Forward: 5ʹ-GAGGCCAAGCCCTGGTATG-3’ |
| Reverse: 5ʹ-CGGGCCGATTGATCTCAGC-3’ | |
| β-Actin | Forward: 5′-CATGTACGTTGCTATCCAGGC-3′ |
| Reverse: 5′-CTCCTTAATGTCACGCACGAT-3′ |
Abbreviations: Cu/Zn SOD, cuprozinc-superoxide dismutase; Mn SOD, manganese superoxide dismutase; CAT, catalase; nNOS, neuronal nitric oxide synthase; iNOS, inducible nitric oxide synthase; TNF-α, tumor necrosis factor-α.
Table 2.
The Conditions of Each Step of RT-qPCR
| Methods | Conditions |
|---|---|
| System of reverse transcription (Reverse Aid First Strand cDNA Synthesis Kit) | 2 μL 0.1 ng - 5 μg total RNA |
| 1 μL Oligo (dT)18 primer | |
| 9 μL Water, nuclease-free | |
| 4 μL 5× Reaction Buffer | |
| 1 μL RiboLock RNase Inhibitor (20 U/μL) | |
| 2 μL 10 mM dNTP Mix | |
| 1 μL RevertAid M-MuLV RT (200 U/μL) | |
| Total volume 20 μL | |
| Conditions of reverse transcription | Incubate for 60 min at 42°C → Terminate the reaction by heating at 70°C for 5 min |
| System of RT-PCR | 10 μL SYBR Green PCR Master Mix |
| 1 μL Upstream primer | |
| 1 μL Downstream primer | |
| 1 μL cDNA template | |
| 7 μL Diethyl pyrocarbonate | |
| Total volume 20 μL | |
| Cycle conditions of RT-qPCR | Pre-denaturation at 95°C for 3 min → denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 15 s, 40 cycles → dissolution curve was completed at 95°C for 30 s, 60°C for 30 s, and 95°C for 15 s |
Data Analysis
Serum and tissue indices of each mouse were tested in at least triplicate. Data were analyzed with IBM SPSS 22 and are expressed as means ± standard deviations (SD). The differences in the means between groups were evaluated by one-way ANOVA using Duncan’s multiple range test (MRT). Values of P < 0.05 were considered statistically significant.
Results
Mice Body Weight and Liver Index
From the results in Table 3, it is known that the mice in the control group have the lightest weight and the lowest liver index, indicating that exercise-induced fatigue can inhibit the growth of the mice and cause liver damage. Compared with the control group, the body weight and liver index of mice in the VC, CQPC and CQPC+GOS groups were significantly improved (P<0.05). GOS treatment alone improved the body weight and liver index of mice far inferior to CQPC+GOS group and CQPC group (P<0.05). CQPC08 has a good effect on improving the growth inhibition and liver damage in mice caused by exercise fatigue. The intervention of GOS promotes the benign improvement of CQPC08 on exercise fatigue mice.
Table 3.
Effects of Lactobacillus fermentum CQPC08 and in Combination with GOS on Body Weight and Liver Indices in Mice with Exercise-Induced Fatigue (N=10/Group)
| Body Weight (g, 0 Day) | Body Weight (g, 35 Day) | Liver Weight (g) | Liver Index (%) | |
|---|---|---|---|---|
| Normal | 40.32±1.60a | 44.76±2.98a | 2.04±0.20a | 4.56±0.16a |
| Control | 39.83±2.23a | 36.74±1.48e | 1.30±0.11d | 3.53±0.21d |
| Vc | 39.00±1.29a | 40.09±1.01c | 1.70±0.05b | 4.23±0.10b |
| CQPC | 38.53±1.30a | 40.20±0.47c | 1.69±0.07b | 4.21±0.18b |
| CQPC+GOS | 39.25±1.72a | 41.82±0.44b | 1.78±0.05b | 4.26±0.07b |
| GOS | 40.39±1.93a | 38.33±0.17d | 1.53±0.03c | 3.99±0.06c |
Notes: Normal and control: vehicle (0.9% normal saline); Vc: 200 mg/kg of vitamin C in vehicle; CQPC: Lactobacillus fermentum CQPC08 (1.0 × 109 CFU/mL) in vehicle; CQPC + GOS: CQPC08 (1.0 × 109 CFU/mL) and 200 mg/kg GOS in vehicle; GOS: 200 mg/kg GOS in vehicle. a–eDifferent letters indicate that there is a significant difference between the two groups (P<0.05).
Exercise Exhaustion Time
The impact of treatment with Lactobacillus fermentum on exercise fatigue was examined through an analysis of exhaustion times following the exercise regimen. Exhaustion times are shown in Table 4. The time to exhaustion of the control group was the shortest. The groups treated with CQPC08, with or without the probiotic factor GOS, were significantly longer (P<0.05). Addition of GOS corresponded with a longer time to exhaustion, consistent with a support of Lactobacillus growth by this compound. Thus, these data are consistent with a prolonging of the exercise exhaustion time by administration of CQPC08, and this prolonging is positively impacted by co-administration of GOS.
Table 4.
Effects of Lactobacillus fermentum CQPC08 and in Combination with GOS on Exercise Exhaustion Time of Mice
| Group | Running Time (Min) |
|---|---|
| Control | 86.00±3.54e |
| Vc | 128.80±4.38c |
| CQPC | 139.60±4.34b |
| CQPC+GOS | 159.40±3.05a |
| GOS | 92.00±2.12d |
Notes: Normal and control: vehicle (0.9% normal saline); Vc: 200 mg/kg of vitamin C in vehicle; CQPC: Lactobacillus fermentum CQPC08 (1.0 × 109 CFU/mL) in vehicle; CQPC + GOS: CQPC08 (1.0 × 109 CFU/mL) and 200 mg/kg GOS in vehicle; GOS: 200 mg/kg GOS in vehicle. a–eDifferent letters indicate that there is a significant difference between the two groups (P<0.05).
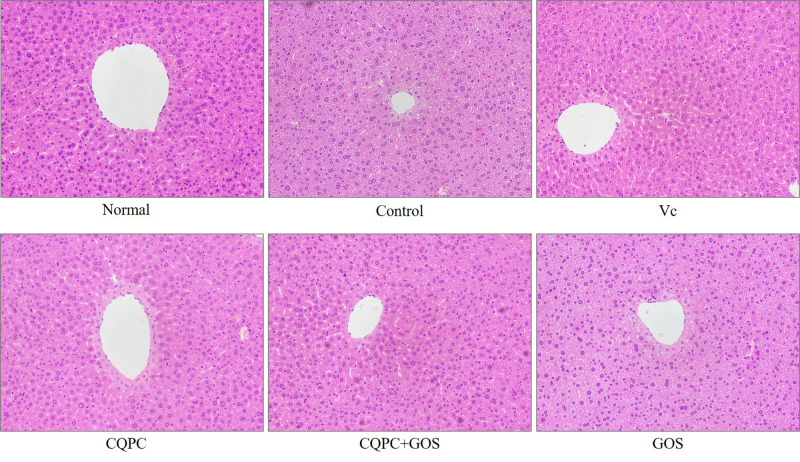
Histopathological Assessment of the Liver
The microscopic morphologies of livers stained with HE are shown in Figure 1. In the normal group, which did not undergo forced exercise, the liver lobules were clear and intact, the liver cells were arranged radially from the central vein, the liver sinuses were normal, the structures of liver cells were intact, the structures of cell nuclei were clear, and nuclei was large and round. In the control group, which underwent exercise but received only vehicle via gavage, the liver cells were disordered arrangement, and the densities of the cytoplasm were increased, the nuclei and cytoplasms were condensed, the nuclear membranes and nucleoli were broken. In the exercise group treated with CQPC08 or Vc, the liver tissue structure was similar to the normal, non-exercised, group. However, the structures of the liver lobules in the exercise group treated with GOS only was similar to those of the control group. The above results indicate that the treatment of CQPC08 improves the oxidative damage of liver tissue in mice, and the intervention of GOS promotes the good repairing effect of CQPC08.
Figure 1.
Effects of Lactobacillus fermentum CQPC08 and in combination with GOS on the liver morphology of exercise-induced fatigue mice. Magnification 200×. Normal and control: vehicle (0.9% normal saline); Vc: 200 mg/kg of vitamin C in vehicle; CQPC: Lactobacillus fermentum CQPC08 (1.0 × 109 CFU/mL) in vehicle; CQPC + GOS: CQPC08 (1.0 × 109 CFU/mL) and 200 mg/kg GOS in vehicle; GOS: 200 mg/kg GOS in vehicle.
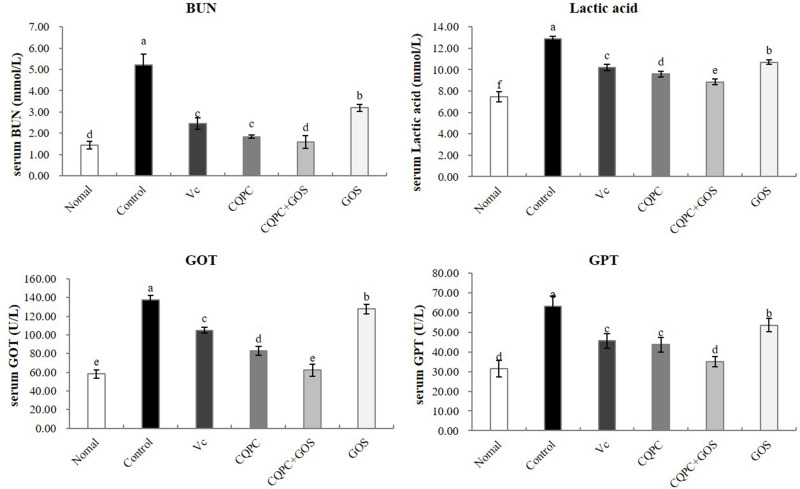
Serum Levels of BUN, Lactic Acid, GOT and GPT
As shown in Figure 2, the serum levels of BUN, LA, GOT and GPT were all highest in the control group, which underwent forced exercise but were treated with vehicle. The serum levels of BUN, LA, GOT and GPT were lowest in the normal group, which was not subjected to forced exercise; those levels were higher only in the CQPC+GOS group relative to the normal group and they were significantly lower than in the model group (P<0.05). The serum levels of BUN, LA, GOT and GPT in the CQPC group were only higher than those in the CQPC+GOS group. Thus, CQPC08 effectively inhibits the occurrence of BUN, LA, GOT and GPT in mice with induced exercise fatigue, and it also enhances anti-fatigue capacity in a manner that is improved by co-administration of GOS.
Figure 2.
The effect of Lactobacillus fermentum CQPC08 and in combination with GOS on the serum levels of BUN, lactic acid, GOT and GPT of exercise-induced fatigue mice. Normal and control: vehicle (0.9% normal saline); Vc: 200 mg/kg of vitamin C in vehicle; CQPC: Lactobacillus fermentum CQPC08 (1.0 × 109 CFU/mL) in vehicle; CQPC + GOS: CQPC08 (1.0 × 109 CFU/mL) and 200 mg/kg GOS in vehicle; GOS: 200 mg/kg GOS in vehicle. a–eDifferent letters indicate that there is a significant difference between the two groups (P<0.05).
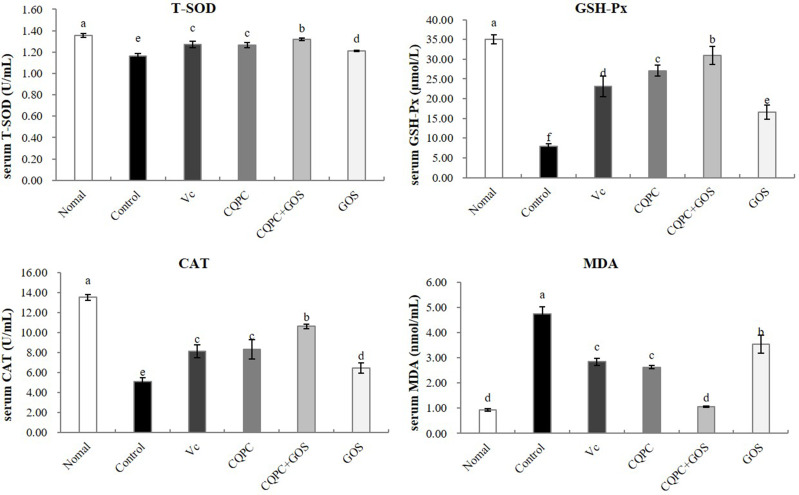
Serum Levels of Oxidation Index CAT, GSH-Px, T-SOD and MDA
Oxidative damage caused by exercise can lead to alterations in several serum chemicals and enzymes of the antioxidant system, such as T-SOD, GSH-Px, CAT and MDA, so these concentrations can serve as markers of oxidative stress. As shown in Figure 3, The level of MDA in the serum was lowest in the exercised group treated with normal, while those of CAT, GSH-Px and T-SOD were the highest. Compared with the control group, the contents of T-SOD, CAT and GSH-Px in the serum of mice in the Vc group, CQPC group and CQPC+GOS group were significantly increased (P<0.05), and MDA was significantly decreased (P<0.05). The content of the CQPC+GOS group was similar to that of the normal group, which suggests that treatment with CQPC+GOS restored oxidative markers to unexercised levels. The content of oxidation indicators in the serum of mice in the GOS group was closer to that of the control group. Thus, treatment with the CQPC08 seems to mitigate exercise-induced oxidative stress, and the intervention of GOS improves the antioxidant effect of CQPC08.
Figure 3.
The effect of Lactobacillus fermentum CQPC08 and in combination with GOS on the serum levels of oxidation index CAT, GSH-Px, T-SOD and MDA of exercise-induced fatigue mice. Normal and control: vehicle (0.9% normal saline); Vc: 200 mg/kg of vitamin C in vehicle; CQPC: Lactobacillus fermentum CQPC08 (1.0 × 109 CFU/mL) in vehicle; CQPC + GOS: CQPC08 (1.0 × 109 CFU/mL) and 200 mg/kg GOS in vehicle; GOS: 200 mg/kg GOS in vehicle. a–eDifferent letters indicate that there is a significant difference between the two groups (P<0.05).
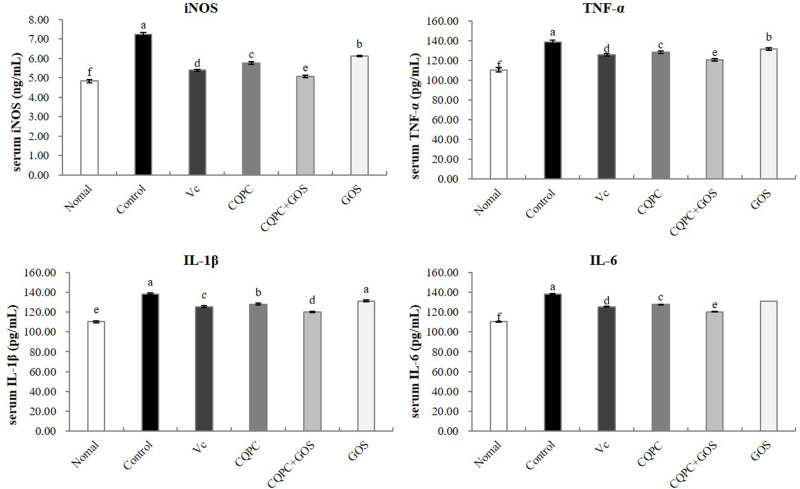
Serum Levels of Inflammatory Cytokines
Inflammation is another potential mediator of exercise-induced fatigue, and it can be analyzed through the detection of inflammation-associated molecules. As illustrated in Figure 4, the serum levels of the pro-inflammatory cytokines iNOS, TNF-α, IL-1β and IL-6 are the highest in the control group, which was subjected to exercise without bacterial treatment, and the lowest in the normal group. The only group with serum levels of inflammatory factors that are similar to that of the normal, non-exercise, group is the CQPC+GOS group. In the serum of mice in the CQPC group, the levels of pro-inflammatory factors were comparable to those in the VC group (P>0.05), while the GOS group was only lower than the control group (P<0.05). This result indicates that CQPC08 effectively inhibits the inflammatory response induced by exercise-induced fatigue, and that the anti-inflammatory effect is promoted by GOS.
Figure 4.
The effect of Lactobacillus fermentum CQPC08 and in combination with GOS on the serum levels of inflammatory cytokines iNOS, TNF-α, IL1β and IL-6 of exercise-induced fatigue mice. Normal and control: vehicle (0.9% normal saline); Vc: 200 mg/kg of vitamin C in vehicle; CQPC: Lactobacillus fermentum CQPC08 (1.0 × 109 CFU/mL) in vehicle; CQPC + GOS: CQPC08 (1.0 × 109 CFU/mL) and 200 mg/kg GOS in vehicle; GOS: 200 mg/kg GOS in vehicle. a–eDifferent letters indicate that there is a significant difference between the two groups (P<0.05).
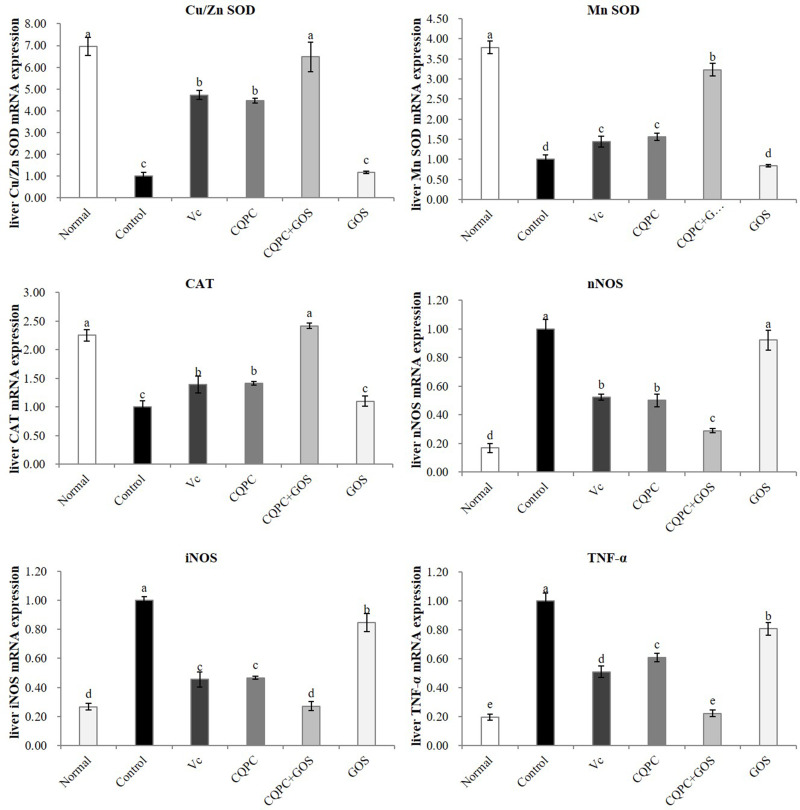
Expression of Oxidation- and Inflammation-Associated Genes in the Liver
As shown in Figure 5, the liver expression levels of mRNAs encoding the antioxidant enzymes Cu/Zn SOD, Mn SOD and CAT were the lowest in the control group and the highest in the normal group. The CQPC+GOS group was similar to the normal group, and the levels in the CQPC group are significantly higher than those in the control group (P<0.05) and are close to those in the Vc group. The expression of the GOS group was closer to that of the control group. On the other hand, the levels of nNOS, iNOS and TNF-α mRNAs were the highest in the control group; it was the lowest in the normal group. The expression level of the CQPC+GOS group was similar to that of the normal group, and was significantly lower than that of the Vc and CQPC groups (P<0.05). The expression of mRNA in the CQPC group is significantly lower than that of the model group (P<0.05), close to that of the Vc group. The activation of expression of mRNA for antioxidant enzymes and reduction of mRNA for proinflammatory cytokines suggest mechanisms by which CQPC08 exerts its positive effect on liver damage induced by exercise-induced fatigue in mice and might explain how GOS promotes this positive effect.
Figure 5.
The effect of Lactobacillus fermentum CQPC08 and in combination with GOS on the mRNA expression of oxidation- and inflammation-associated genes in the liver. Normal and control: vehicle (0.9% normal saline); Vc: 200 mg/kg of vitamin C in vehicle; CQPC: Lactobacillus fermentum CQPC08 (1.0 × 109 CFU/mL) in vehicle; CQPC + GOS: CQPC08 (1.0 × 109 CFU/mL) and 200 mg/kg GOS in vehicle; GOS: 200 mg/kg GOS in vehicle. a–eDifferent letters indicate that there is a significant difference between the two groups (P<0.05).
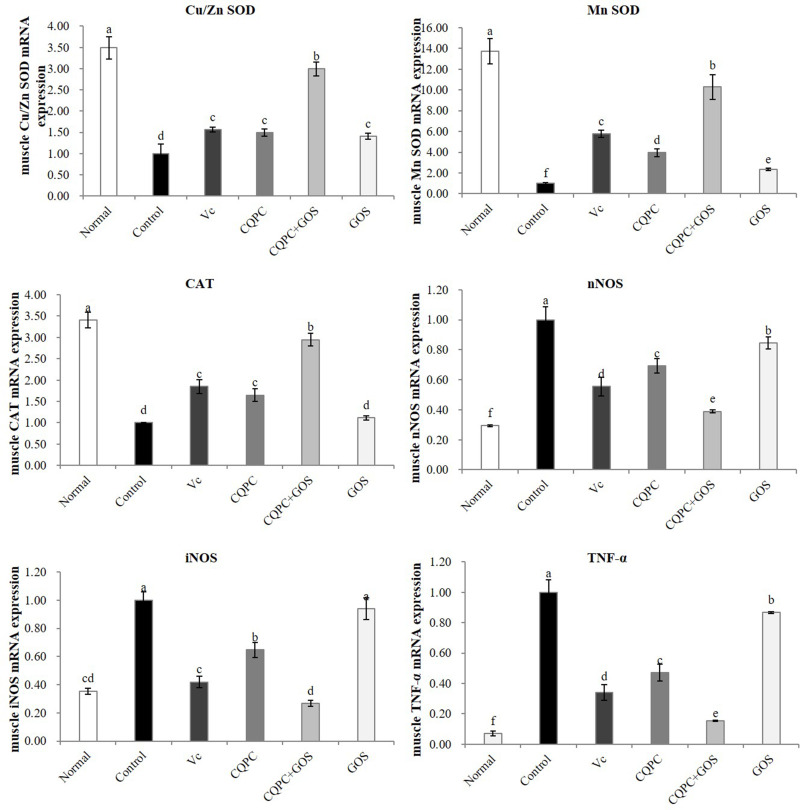
Expression of Oxidation- and Inflammation-Associated Genes in Skeletal Muscle
Similar to liver, we endeavored to analyze expression of relevant genes in the skeletal muscle of the various mouse groups. As shown in Figure 6, the mRNA expression levels of Cu/Zn SOD, Mn SOD and CAT in the skeletal muscles of the control exercise group are the lowest, and those of the normal group are the highest, and the CQPC + GOS group is closest to the normal group, suggesting that treatment with Lactobacillus and GOS supports the activity of the antioxidant system. On the contrary, the mRNA expressions of nNOS, iNOS and TNF-α are the highest in the control group and the lowest in the normal group, and those of the CQPC + GOS group are closest to the normal group. The mRNA expression levels of nNOS, iNOS and TNF-α in the CQPC group are slightly higher than those in the CQPC + GOS group but significantly lower than those in the control group and GOS group (P<0.05), suggesting that Lactobacillus itself has a significant impact on antioxidants. Similarly, the mRNA expression levels of Cu/Zn SOD, Mn SOD and CAT in the CQPC group are close to those in the Vc group. Thus, CQPC08 may have a positive effect on the skeletal muscle injury caused by exercise-induced fatigue by enhancing the antioxidation process and lowering the inflammatory response. GOS improves these effects, and these effects would result in the reduction of the free radical injury caused by fatigue. They would also protect natural antioxidants in the body and would maintain an appropriate balance of inflammatory factors.
Figure 6.
The effect of Lactobacillus fermentum CQPC08 and in combination with GOS on the mRNA expression of oxidation- and inflammation-associated genes in the skeletal muscle. Normal and control: vehicle (0.9% normal saline); Vc: 200 mg/kg of vitamin C in vehicle; CQPC: Lactobacillus fermentum CQPC08 (1.0 × 109 CFU/mL) in vehicle; CQPC + GOS: CQPC08 (1.0 × 109 CFU/mL) and 200 mg/kg GOS in vehicle; GOS: 200 mg/kg GOS in vehicle. a–eDifferent letters indicate that there is a significant difference between the two groups (P<0.05).
Discussion
Vigorous activity increases the production of free radicals that can lead to exercise-induced fatigue or even injury or some diseases.30 Among the various free radicals, oxygen free radicals have the closest relationship with exercise. An increase of oxygen free radicals during exercise, then, is an important factor leading to exercise-induced fatigue.31 Studies have shown that in the case of strenuous exercise or long-term exercise, the antioxidant capacity of mice decreases, free radicals increase in the body, and cause oxidative damage to the liver.4 In this study, the strain CQPC08 in particular has been proven to scavenge oxygen free radicals, reduce liver cell damage caused by free radicals, support liver cell integrity, and to inhibit oxidative damage caused by exercise. In addition, the combination of CQPC08 and GOS has enhenced these capabilities.
Fatigue exercise inhibits the growth of mice and reduces liver index.32 This study shows that CQPC08 can effectively promote the growth and liver index of mice, and the combination of CQPC and GOS has a good effect.
The time from running to exhaustion is a common indicator reflecting exercise ability, and improvement of exercise ability is the most powerful macro-reflection of anti-fatigue ability.33 Our study confirmed that the CQPC08 significantly prolongs the time from running to exhaustion.
The liver is a key organ that is a target of lipid peroxidation, and it is also a place where free radicals and lipid peroxides are easily produced.34 Our study confirmed that the CQPC08 effectively resolves the oxidative damage of the liver cell caused by exercise-induced fatigue.
BUN is the final product of protein and amino acid catabolism, that is positively correlated with body function, fatigue degree and load, and is typically used as an indicator to evaluate the amount of exercise. Fatigue exercise makes the metabolism of glycogen and fat insufficient for energy needs, so the body must rely on protein decomposition for its energy supply.35 Blood lactic acid (LA) is an important indicator of aerobic metabolism and fatigue.36 Under normal circumstances, the lactic acid produced during exercise will be decomposed, then fatigue will be resolved. Fatigue exercise, however, will lead to relative hypoxia and accelerated glycolysis, and thus excess lactic acid will be produced and will leech out into the bloodstream.37 At the same time, pure mechanical traction-induced muscle tissue damage can occur, resulting in leakage of enzymes, including GPT and GOT, into the blood.38 GPT and GOT mainly exist in the liver and muscle, and their activity is directly related to the metabolism of protein in the liver and muscle, and their activity in the blood indirectly reflects the extent of liver and skeletal muscle damage.39 Studies have shown that after vigorous exercise on mice, the levels of BUN, lactic acid, GOT and GPT in the serum of mice are significantly down-regulated.35–39 While, the results in this study showed that after administrating CQPC08, the serum levels of BUN, lactic acid, GOT and GPT of exercise-fatigued mice were significantly down-regulated, which may be due to a slowing down of the utilization of protein as an energy source during exercise. Thereby reducing the fatigue caused by exercise.
During high-intensity exercise, the production of free radicals in the body increases, which is also one of the causes of exercise-induced fatigue.40 Antioxidant enzymes are an important line of defense to protect cells and tissues from excessive free radical damage. There are many types of antioxidant enzymes in humans, including CAT, GSH-Px, and SOD.41 CAT and GSH-Px catalyze the decomposition of hydrogen peroxide in the body, and scavenge the products of peroxygenation stress, so as to inhibit oxidative stress.42 SOD produces oxygen and hydrogen peroxide through a disproportionation reaction of oxygen free radicals, and effectively scavenges superoxide anion free radicals, so as to reduce the production of more toxic hydroxyl radicals.43 Cu/Zn SOD and Mn-SOD both inhibit the free radicals that are produced during exercise, and they play a role in reducing exercise fatigue.44 Lipid peroxidation product MDA can damage the structures of cell membranes, leading to cell swelling and necrosis. It is often used to indirectly reflect the changes of free radical metabolism and the degree of tissue peroxidative damage.45 Some research reports pointed out that strenuous exercise caused a decrease in the expression of SOD1 (Cu/Zn SOD), CAT, and GPx1 (GSH-Px) in mice.4 Similarly, our results show that exercise-induced fatigue correlates with the production of markers of free radicals in the serum, liver and skeletal muscles, and thus leads to oxidative stress. CQPC08, though, enhances the activities of CAT, GSH-Px, SOD, Cu/Zn SOD and Mn SOD, reduces the level of MDA, improves the ability of free radical scavengers, enhances the defense against lipid peroxidation, and plays a role in protecting the body. In addition, GOS promotes the specific antioxidant capacity of CQPC08, regulates oxidative stress and stimulates the expression of antioxidant enzymes.
Inflammation can promote oxidative stress,46 so we also analyzed the effect of CQPC08 on exercise-induced fatigue mice by detecting the expression of inflammatory cytokines and related genes. nNOS is distributed on the membranes of fast muscle fibers. Studies have shown that the expression of nNOS is significantly up-regulated after rigorous or long-duration exercise, and the expression of nNOS is reduced after recovery from exercise exhaustion.47 iNOS catalyzes the production of NO, which is continuously produced in skeletal muscle, at a low level in a resting state, and at a high level during skeletal muscle contraction.48 The expression of iNOS is induced by endotoxins and various cytokines, such as TNF-α and IL-1β. TNF-α and IL-1β are the initiating factors of the cascade of inflammatory mediators. TNF-α induces fever and apoptosis by producing IL-1β and IL-6, thereby inducing inflammation.49 IL-1β can attract neutrophils and causes the release of inflammatory mediators.50 IL-6 is a pleiotropic cytokine, and its receptor is widely expressed in liver cells. Studies have shown that the amount of IL-6 is positively correlated with the degree of liver damage.51 Therefore, the expression of iNOS, nNOS, TNF-α, IL1β and IL-6 reflects the degree of fatigue after exercise.46 In addition, because intense exercise increases blood supply of muscle, hypoperfusion of internal organs is induced, which results in exercise-induced organ damage. Organ damage induced by ischemia and trauma leads to systemic inflammatory responses, which result in multiple organ damage.52–54 In damaged tissues, the expression of inflammatory cytokines such as IL-1β, IL-6 and TNF-α and leukocyte infiltration are observed.55–57 In the present study, CQPC08 was found to significantly down-regulate the expression of iNOS, nNOS, TNF-α, IL1β and IL-6 consistent with its capability of reducing the inflammatory response and oxidative stress caused by exercise fatigue and improving the ability of free radical scavenging in mice. And the combination of GOS and CQPC08 enhances these capabilities.
Moreover, the mechanism by which GOS promotes the anti-fatigue effect of CQPC08 may be attributed to the promoting effect of GOS on the growth of Lactobacillus fermentum CQPC08, because GOS cannot be absorbed in the body, but it can promote the growth of beneficial bacteria. Studies have shown that GOS regulates the immune system by selectively promoting the number and growth of some intestinal bacteria such as Bifidobacteria and Lactobacilli.58 In addition, studies have pointed out that the addition of GOS to the diet increases the number of Lactobacilli and Bifidobacteria in the feces, and at the same time improves the growth performance of weaned piglets, and improves the immunity and antioxidant capacity of weaned piglets.59 Our research has also obtained similar results. The extra addition of GOS has further improved the antioxidant capacity of mice that were originally increased under the action of Lactobacillus fermentum CQPC08.
Conclusion
In this study, we established a model of exercise-induced fatigue in mice and demonstrated that CQPC08 and its combination with GOS improves the antioxidant capacity of mice by regulating oxidative stress, so as to reduce the damage of exercise-induced fatigue. The results showed that CQPC08 and its combination with GOS can reduce the degree of liver oxidative damage, down-regulates the serum levels of BUN, lactic acid, GOT, GPT, MDA, iNOS, TNF-α, IL-1β and IL-6, and the relative mRNA expression of nNOS, iNOS and TNF-α in the liver and skeletal muscles, and up-regulates serum levels of CAT, GSH-Px and T-SOD, and the relative mRNA expression of Cu/Zn SOD, Mn SOD and CAT in the liver and muscle. In short, CQPC08 and its combination with GOS on alleviating exercise-induced fatigue in mice by improving antioxidant capacity, and elaborated its mechanism, so as to provide reference for the future study and development of food borne antioxidants to support the health of athletes.
Acknowledgments
This research was funded by Natural Science Foundation of Liaoning Province (2020-MS-037); Science and Technology Research Project of Chongqing Municipal Education Commission (KJQN202001616); Chongqing Social Science Planning and Cultivation Project (2020PY30).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ament W, Verkerke GJ. Exercise and fatigue. Sports Med. 2009;39(5):389–422. doi: 10.2165/00007256-200939050-00005 [DOI] [PubMed] [Google Scholar]
- 2.Wan J, Qin Z, Wang P, et al. Muscle fatigue: general understanding and treatment. Exp Mol Med. 2017;49(10):e384–e384. doi: 10.1038/emm.2017.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poole DC, Copp SW, Colburn TD, et al. Guidelines for animal exercise and training protocols for cardiovascular studies. Am J Physiol Heart C. 2020;318(5):H1100–H1138. doi: 10.1152/ajpheart.00697.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruhee RT, Ma S, Suzuki K. Protective effects of sulforaphane on exercise-induced organ damage via inducing antioxidant defense responses. Antioxidants. 2020;9(2):136. doi: 10.3390/antiox9020136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki K, Tominaga T, Ruhee RT, et al. Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants. 2020;9(5):401. doi: 10.3390/antiox9050401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava KK, Kumar R. Stress, oxidative injury and disease. Indian J Clin Biochem. 2015;30(1):3–10. doi: 10.1007/s12291-014-0441-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118. doi: 10.4103/0973-7847.70902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos-Sánchez NF, Salas-Coronado R, Villanueva-Cañongo C, et al. Antioxidant Compounds and Their Antioxidant Mechanism. London, UK: IntechOpen; 2019. doi: 10.5772/intechopen.85270 [DOI] [Google Scholar]
- 9.Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153(1–3):83–104. doi: 10.1016/s0300-483x(00)00306-1 [DOI] [PubMed] [Google Scholar]
- 10.Hacısevki A. An overview of ascorbic acid biochemistry. J Fac Pharm Ankara Univ. 2009;38(3):233–255. doi: 10.1501/Eczfak_0000000528 [DOI] [Google Scholar]
- 11.Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta. 2002;1569(1–3):1–9. doi: 10.1016/S0304-4165(01)00235-5 [DOI] [PubMed] [Google Scholar]
- 12.Rouhier N, Lemaire SD, Jacquot JP. The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation. Annu Rev Plant Biol. 2008;59:143–166. doi: 10.1146/annurev.arplant.59.032607.092811 [DOI] [PubMed] [Google Scholar]
- 13.Bindhumol V, Chitra KC, Mathur PP. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology. 2003;188(2–3):117–124. doi: 10.1016/S0300-483X(03)00056-8 [DOI] [PubMed] [Google Scholar]
- 14.Duarte TL, Lunec J. When is an antioxidant not an antioxidant? A review of novel actions and reactions of Vitamin C. Free Radic Res. 2005;39(7):671–686. doi: 10.1080/10715760500104025 [DOI] [PubMed] [Google Scholar]
- 15.Çataloluk O, Gogebakan B. Presence of drug resistance in intestinal lactobacilli of dairy and human origin in Turkey. FEMS Microbiol Lett. 2004;236(1):7–12. doi: 10.1111/j.1574-6968.2004.tb09620.x [DOI] [PubMed] [Google Scholar]
- 16.Ao X, Pu B, Cai Y. Research progress of Lactobacillus fermentum and its probiotic characteristics. J Food Sci Biotechnol. 2015;2:1211127. [Google Scholar]
- 17.Mikelsaar M, Zilmer M. Lactobacillus fermentum ME-3–an antimicrobial and antioxidative probiotic. Microb Ecol Health Dis. 2009;21(1):1–27. doi: 10.1080/08910600902815561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang AN, Yi XW, Yu HF, et al. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing–finishing pigs. J Appl Microbiol. 2009;107(4):1140–1148. doi: 10.1111/j.1365-2672.2009.04294.x [DOI] [PubMed] [Google Scholar]
- 19.Liu B, Zhang J, Yi R, et al. Preventive effect of Lactobacillus fermentum CQPC08 on 4-nitroquineline-1-oxide induced tongue cancer in C57BL/6 mice. Foods. 2019;8(3):93. doi: 10.3390/foods8030093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long X, Sun F, Wang Z, et al. Lactobacillus fermentum CQPC08 protects rats from lead-induced oxidative damage by regulating the Keap1/Nrf2/ARE pathway. Food Funct. 2021;12(13):6029–6044. doi: 10.1039/D1FO00589H [DOI] [PubMed] [Google Scholar]
- 21.Krumbeck JA, Rasmussen HE, Hutkins RW, et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome. 2018;6(1):1–16. doi: 10.1186/s40168-018-0494-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohajeri MH, Brummer RJM, Rastall RA, et al. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. 2018;57(1):1–14. doi: 10.1007/s00394-018-1703-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerry RG, Patra JK, Gouda S, et al. Benefaction of probiotics for human health: a review. J Food Drug Anal. 2018;26(3):927–939. doi: 10.1016/j.jfda.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Gendy KS, Aly NM, Mahmoud FH, et al. The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem Toxicol. 2010;48(1):215–221. doi: 10.1016/j.fct.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 25.Aly N, Kawther ELG, Mahmoud F, et al. Protective effect of vitamin C against chlorpyrifos oxidative stress in male mice. Pestic Biochem Phys. 2010;97(1):7–12. doi: 10.1016/j.pestbp.2009.11.007 [DOI] [Google Scholar]
- 26.Suh MG, Bae GY, Jo K, et al. Photoprotective effect of dietary Galacto-Oligosaccharide (GOS) in hairless mice via regulation of the MAPK signaling pathway. Molecules. 2020;25(7):1679. doi: 10.3390/molecules25071679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro B, Kuang S. Evaluation of muscle performance in mice by treadmill exhaustion test and whole-limb grip strength assay. Bio-Protoc. 2017;7(8). doi: 10.21769/BioProtoc.2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma S, Huang Q, Tominaga T, et al. An 8-week ketogenic diet alternated interleukin-6, ketolytic and lipolytic gene expression, and enhanced exercise capacity in mice. Nutrients. 2018;10(11):1696. doi: 10.3390/nu10111696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan Y, Wang H, Tan F, et al. Lactobacillus plantarum KFY02 enhances the prevention of CCl4-induced liver injury by transforming geniposide into genipin to increase the antioxidant capacity of mice. J Func Foods. 2020;73:104128. doi: 10.1016/j.jff.2020.104128 [DOI] [Google Scholar]
- 30.Simioni C, Zauli G, Martelli AM, et al. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9(24):17181. doi: 10.18632/oncotarget.24729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamura T, Muraoka I. Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants. 2018;7(9):119. doi: 10.3390/antiox7090119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu YJ, Huang WC, Chiu CC, et al. Capsaicin supplementation reduces physical fatigue and improves exercise performance in mice. Nutrients. 2016;8(10):648. doi: 10.3390/nu8100648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai G, Wang Y, Wu J, et al. Study on the recognition of exercise intensity and fatigue on runners based on subjective and objective information. In: Healthcare. Vol. 7. No. 4. Multidiscip Digit Publishing I; 2019:150. doi: 10.3390/healthcare7040150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Repetto M, Semprine J, Boveris A. Lipid peroxidation: chemical mechanism, biological implications and analytical determination. Lipid Peroxid. 2012;1:3–30. doi: 10.5772/45943 [DOI] [Google Scholar]
- 35.Xie Q, Sun Y, Cao L, et al. Antifatigue and antihypoxia activities of oligosaccharides and polysaccharides from Codonopsis pilosula in mice. Food Funct. 2020;11(7):6352–6362. doi: 10.1039/D0FO00468E [DOI] [PubMed] [Google Scholar]
- 36.Theofilidis G, Bogdanis GC, Koutedakis Y, et al. Monitoring exercise-induced muscle fatigue and adaptations: making sense of popular or emerging indices and biomarkers. Sports. 2018;6(4):153. doi: 10.3390/sports6040153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni W, Gao T, Wang H, et al. Anti-fatigue activity of polysaccharides from the fruits of four Tibetan plateau indigenous medicinal plants. J Ethnopharmacol. 2013;150(2):529–535. doi: 10.1016/j.jep.2013.08.055 [DOI] [PubMed] [Google Scholar]
- 38.Skugor S, Holm HJ, Bjelland AK, et al. Nutrigenomic effects of glucosinolates on liver, muscle and distal kidney in parasite-free and salmon louse infected Atlantic salmon. Parasit Vector. 2016;9(1):1–17. doi: 10.1186/s13071-016-1921-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim NI, Kim SJ, Jang JH, et al. Changes in fatigue recovery and muscle damage enzymes after deep-sea water thalassotherapy. App Sci. 2020;10(23):8383. doi: 10.3390/app10238383 [DOI] [Google Scholar]
- 40.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex J Med. 2018;54(4):287–293. doi: 10.1016/j.ajme.2017.09.001 [DOI] [Google Scholar]
- 42.Ma X, Deng D, Chen W. Inhibitors and Activators of SOD, GSH‐Px, and CAT. Enzyme Inhibitors Activators. 2017;29:207. doi: 10.5772/65936 [DOI] [Google Scholar]
- 43.Muradian KK, Utko NA, Fraifeld V, et al. Superoxide dismutase, catalase and glutathione peroxidase activities in the liver of young and old mice: linear regression and correlation. Arch Gerontol Geriat. 2002;35(3):205–214. doi: 10.1016/S0167-4943(02)00025-0 [DOI] [PubMed] [Google Scholar]
- 44.Steinbacher P, Eckl P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules. 2015;5(2):356–377. doi: 10.3390/biom5020356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gil P, Fariñas F, Casado A, et al. Malondialdehyde: a possible marker of ageing. Gerontology. 2002;48(4):209–214. doi: 10.1159/000058352 [DOI] [PubMed] [Google Scholar]
- 46.Biswas SK. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;2016:1–9. doi: 10.1155/2016/5698931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Percival JM. nNOS regulation of skeletal muscle fatigue and exercise performance. Biophyl Rev. 2011;3(4):209–217. doi: 10.1007/s12551-011-0060-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terrando N, Monaco C, Ma D, et al. Tumor necrosis factor-α triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci. 2010;107(47):20518–20522. doi: 10.1073/pnas.1014557107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. CSH Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki K. Characterization of exercise-induced cytokine release, the impacts on the body, the mechanisms and modulations. Int J Sports Exerc Med. 2019;5:1–13. doi: 10.23937/2469-5718/1510122 [DOI] [Google Scholar]
- 53.Suzuki K. Cytokine response to exercise and its modulation. Antioxidants. 2018;7(1):17. doi: 10.3390/antiox7010017 [DOI] [Google Scholar]
- 54.Suzuki K, Nakaji S, Yamada M, et al. Systemic inflammatory response to exhaustive exercise. Cytokine Kinet Exerc Immunol. 2002;8:6–48. [PubMed] [Google Scholar]
- 55.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1083–G1088. doi: 10.1152/ajpgi.00568.2005 [DOI] [PubMed] [Google Scholar]
- 56.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7(4):189–200. doi: 10.1038/nrneph.2011.16 [DOI] [PubMed] [Google Scholar]
- 57.Luissint AC, Parkos CA, Nusrat A. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology. 2016;151(4):616–632. doi: 10.1053/j.gastro.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeurink PV, Esch BCV, Rijnierse A, et al. Mechanisms underlying immune effects of dietary oligosaccharides. Am J Clin Nutr. 2013;98(2):572S–577S. doi: 10.3945/ajcn.112.038596 [DOI] [PubMed] [Google Scholar]
- 59.Xing Y, Li K, Xu Y, et al. Effects of galacto-oligosaccharide on growth performance, feacal microbiota, immune response and antioxidant capability in weaned piglets. J Appl Anim Res. 2020;48(1):63–69. doi: 10.1080/09712119.2020.1732394 [DOI] [Google Scholar]