Highlights
-
•
PTSD is often treated with psychotherapies based on principles of fear acquisition and extinction.
-
•
Increased AEA has resulted in enhanced extinction learning and recall among healthy adults.
-
•
These effects have not yet been comprehensively examined in a PTSD population.
-
•
Results suggest that genetic variation within the FAAH gene affects how fear learning is tuned in women with PTSD.
Keywords: Endocannabinoid system, Fatty acid amide hydrolase, fMRI, Independent component analysis, Interpersonal violence, Anandamide
Abstract
Background
Posttraumatic Stress Disorder (PTSD) is commonly treated with exposure-based cognitive therapies that are based on the principles of fear acquisition and extinction learning. Elevations in one of the major endocannabinoids (anandamide) either via inhibition of the primary degrading enzyme (fatty acid amide hydrolase; FAAH) or via a genetic variation in the FAAH gene (C385A; rs324420) has resulted in accelerated extinction learning and enhanced extinction recall among healthy adults. These results suggest that targeting FAAH may be a promising therapeutic approach for PTSD. However, these effects have not yet been comprehensively examined in a PTSD population.
Methods
The current study examined whether genetic variation in the FAAH gene (CC [n = 49] vs AA/AC [n = 36] allele carriers) influences physiological (skin conductance), cognitive (threat expectancy), and neural (network and voxel-wise activation) indices of fear acquisition and extinction learning among a sample of adult women with PTSD (N = 85).
Results
The physiological, cognitive, and neural signatures of fear acquisition and extinction learning varied as a function of whether or not individuals possess the FAAH C385A polymorphism. For instance, we report divergent responding between CC and AA/AC allele carriers to CS + vs CS- in limbic and striatum networks and overall greater activation throughout the task among AA/AC allele carriers in several regions [e.g., inferior frontal, middle frontal, parietal] that are highly consistent with a frontoparietal network involved in higher-order executive functions.
Conclusions
These results suggest that genetic variation within the FAAH gene influences physiological, cognitive, and neural signatures of fear learning in women with PTSD. In order to advance our understanding of the efficacy of FAAH inhibition as a treatment for PTSD, future clinical trials in this area should assess genetic variation in the FAAH gene in order to fully depict and differentiate the acute effects of a drug manipulation (FAAH inhibition) from more chronic (genetic) influences on fear extinction processes.
1. Introduction
Posttraumatic stress disorder (PTSD) is a highly debilitating and relatively common mental health disorder that develops in some individuals following exposure to one or more traumatic events, such as interpersonal violence (IPV; e.g., physical/sexual assault) (Olatunji et al., 2007, APA, 2013). In fact, IPV-exposure poses a greater risk for developing PTSD in comparison to other types of trauma (Kessler, 2017, Kessler et al., 1995, Resnick et al., 1993). IPV-exposure is also more commonly experienced among women compared to men (Iverson et al., 2013, Scott et al., 2018), which contributes to higher PTSD prevalence rates in women (Blanco et al., 2018, Breslau et al., 1998, Kessler et al., 2005).
Evidence-based psychotherapeutic treatment approaches for PTSD typically involves the administration of exposure-based cognitive behavioral therapies (e.g., prolonged exposure therapy, cognitive processing therapy) (Resick et al., 2002, Schnurr et al., 2007). The mechanisms of fear conditioning and extinction learning are an essential component of exposure-based therapies (Rothbaum and Davis, 2003). For instance, consistent with the fear conditioning model, a memory of a traumatic event can be conceptualized as a conditioned stimulus (CS+) capable of triggering anxiety responses (i.e., conditioned threat responses) given that the trauma memory is associated with occurrence of the traumatic event (i.e., unconditioned stimulus). Consistent with a fear extinction model, a major component of exposure-based therapies involves gradual, repeated exposure to the traumatic memory (CS+) in a safe context in order to weaken the predictive value of the CS+, which ideally weakens the ability of the trauma-related memories or reminders to elicit distress and anxiety responses (Cisler et al., 2014). Based on this understanding, fear conditioning paradigms are frequently administered in the lab, often to: 1) identify physiological, cognitive, and neural mechanisms of fear acquisition and extinction learning in those with and without clinical anxiety disorders, and 2) examine the effect of experimental manipulations on the acquisition, consolidation, or recall of extinction learning, under the notion that there may be therapeutic potential for treatments that enhance these processes (Crombie et al., 2021a, Fullana et al., 2020, Crombie et al., 2021b; Mataix-Cols et al., 2017, Tuerk et al., 2018, Zoellner et al., 2017).
Recently, there has been growing emphasis on targeting an expansive neuromodulatory system known as the endocannabinoid (eCB) system in order to enhance fear extinction learning and recall (Gunduz-Cinar et al., 2013, Hill et al., 2018, Mayo et al., 2020a, Mayo et al., 2020b, Rabinak et al., 2013, Rabinak et al., 2014, Rabinak et al., 2020). The eCB system primarily consists of receptors (cannabinoid type-1 and type-2, CB1R and CB2R, respectively) endogenous ligands known as eCBs (most notably N-arachidonoylethanolamine, anandamide, [AEA]; and 2-arachidonoylglycerol [2-AG]), and enzymes involved in the synthesis and degradation of eCBs, including fatty acid amide hydrolase (FAAH; primary degrading enzyme of AEA) and monoacylglycerol lipase (MAGL; primary degrading enzyme of 2-AG) (Katona and Freund, 2012). Early seminal studies examining the role of the eCB system in fear extinction learning demonstrated that successful extinction learning is dependent on eCB/CB1R signaling as administration of CB1R antagonists or genetic deletion of CB1Rs resulted in impaired extinction learning and increased anxiety-like behaviors (Marsicano et al., 2002). More recently, targeting the eCB system in an effort to increase AEA levels, primarily via administration of FAAH inhibitors (and via administration of CB1R agonists) has been shown to enhance extinction learning and recall in rodent models and healthy humans without a clinical anxiety disorder (Mayo et al., 2020a, Mayo et al., 2020b, Morena et al., 2014, Morena et al., 2018, Rabinak et al., 2013). More specifically, both acute and chronic administration of a FAAH inhibitor prior to fear extinction or during exposure to reminder cues has resulted in enhanced extinction and long-term consolidation of extinction memories (Mayo et al., 2021, Morena et al., 2018). Additionally, preclinical studies have reported decreased anxiety-like behavior during commonly administered behavioral tests (i.e., elevated plus maze, light/dark box) under highly aversive conditions or following stress exposure in FAAH knock-out mice (i.e., genetically altered mice without FAAH enzyme) and rodents administered a FAAH inhibitor (Haller et al., 2009, Haller et al., 2013, Hill and Patel, 2013; Kathuria et al., 2003, Moreira et al., 2009. Collectively these results suggest that targeting the eCB system, particularly via administration of an oral FAAH inhibitor (e.g., chronic daily dosing or acute administration in conjunction with exposure-based therapy in order to enhance safety learning occurring during therapy), might be a promising therapeutic approach for PTSD and potentially other fear and anxiety related disorders (see the following reviews [Mayo et al., 2021, Ney et al., 2019, Ney et al., 2021a, Ney et al., 2021c, Hill et al., 2018] for a comprehensive overview of the clinical relevance of FAAH and the eCB system in PTSD).
Interestingly, administering a FAAH inhibitor is not the only way to examine the effects of elevated AEA on fear conditioning processes - there is also a fairly common functional single nucleotide polymorphism (SNP) in the FAAH gene (C385A; rs324420) that results in lower levels of FAAH protein, and as a result, increased AEA concentrations (Mayo et al., 2020a, Sipe et al., 2002, Spagnolo et al., 2016). Interestingly, individuals that possess the FAAH C385C → A mutation exhibit elevated AEA concentrations (due to lower FAAH expression) in the amygdala and enhanced fear extinction recall neurocircuitry (i.e., enhanced regulation of amygdala by ventromedial prefrontal cortex; vmPFC), which is in contrast to women with PTSD who typically exhibit altered fear extinction neurocircuitry (e.g., amygdala hyperreactivity, vmPFC and hippocampal hyporeactivity) and poor extinction learning (Mayo et al., 2021, Mayo et al., 2020a, Green et al., 2021). Similar to FAAH inhibition, in addition to accelerated fear extinction learning (Dincheva et al., 2015), elevated AEA due to this genetic polymorphism has also been linked to decreased anxiety responses following the presentation of stress- and threat-inducing stimuli (Hariri et al., 2009) in healthy humans without a clinical anxiety disorder, and lower hyperarousal symptoms in adults with PTSD (Spagnolo et al., 2016). However, aside from one recent non-imaging investigation (Ney et al., 2021b), it remains relatively unknown whether potential genetic variations of this system (e.g., FAAH polymorphism) influence initial fear acquisition and extinction learning processes in adults with PTSD. A well-controlled laboratory study addressing this gap may yield important insight to aid in the design and development of future clinical trials examining the efficacy of FAAH inhibition as part of a treatment approach for PTSD and anxiety disorders. Therefore, the aim of the current study was to comprehensively examine whether genetic variation in the FAAH gene (i.e., C385A allele carriers) influences physiological (skin conductance responses), cognitive (threat expectancy), and neural (network and voxel-wise activation) indices of fear acquisition and extinction learning among a large sample of adult women with IPV-related PTSD.
2. Methods and materials
The work described in this manuscript has been carried out in accordance with the latest version of The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans, and all subjects completed informed consent after the nature of procedures had been fully explained.
2.1. Methodology overview
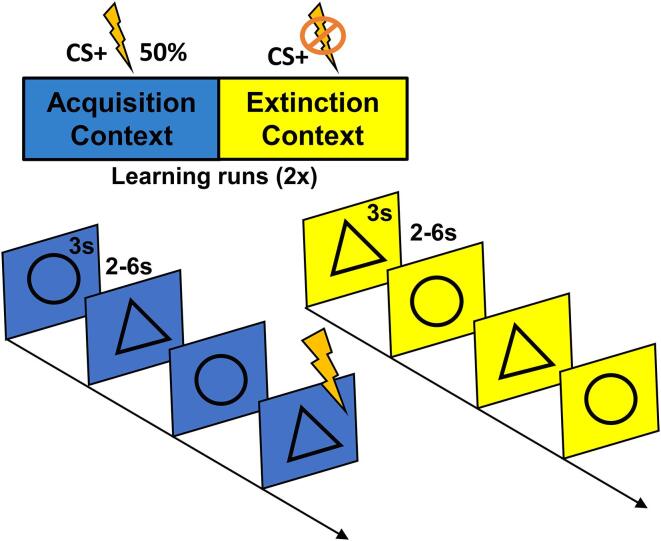
The primary study design and analyses from this project involved a 2-day imaging protocol to determine whether administration of L-DOPA (compared to placebo) delivered during the consolidation window following fear extinction learning (on day 1) reduced subsequent fear responding (on day 2) among women with PTSD (see Cisler et al., 2020 for full details and results). The methods and analyses reported in this manuscript are independent of any previously reported findings, as this study focused on the day 1 fear conditioning and extinction learning results (prior to any drug manipulation). See Fig. 1 for a depiction of the overall methodology.
Fig. 1.
Fear Acquisition and Extinction Task Structure. Participants completed the fear acquisition and fear extinction task in fMRI. The unconditioned stimulus (US) was an electric shock, which participants calibrated to an intensity level of 7/10 on a Likert scale (i.e., maximally uncomfortable but not painful). Conditioned stimuli consisted of geometric shapes (triangles and circles), each displayed for 3 s with a jittered inter-trial interval of 2–6 s. CS + shape and context color (yellow or blue backgrounds implicitly distinguishing acquisition and extinction contexts) was counterbalanced across participants. An initial baseline phase consisted of 6 presentations of each stimulus with no UCS onsets. Next, the task alternated between acquisition and extinction phases for 144 trials (156 trials including baseline), with two presentations of each phase. During the acquisition phase, each CS was presented 18 times, and the US occurred 2.5 s following CS+ onset with a 50% reinforcement schedule. No shocks were administered during the extinction phase, which presented each stimulus 18 times. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.2. Participants
Participants were recruited at two sites: the University of Arkansas for Medical Sciences (UAMS; n = 39) and the University of Wisconsin - Madison (UW; n = 46). All study procedures were approved by the respective Institutional Review Boards at UAMS and UW. Inclusion criteria consisted of female sex, age 21–50 years, and current diagnosis of PTSD related to assaultive violence exposure (Crombie et al., 2021c). PTSD diagnosis and symptom severity was assessed using the Clinician Administered PTSD Scale for DSM-5 (CAPS-5; (Weathers et al., 2013, Weathers et al., 2018)). Exclusion criteria for all participants included internal metal or other MRI contraindications, major medical disorders, and endorsement of psychotic symptoms. See the Supplementary Material for randomization procedures for the overall study, additional information on all assessments completed, and further details regarding exclusion criteria.
2.3. Genotyping
Participants provided saliva samples (Oragene, DNA Genotek Inc., Canada) from which DNA was extracted at the Max Planck Institute according to standard protocols. The FAAH C385A locus (rs324420) was called using PLINK software (PLINK 1.07; (Purcell et al., 2007)).
2.4. Fear conditioning and extinction task
The computerized fear acquisition and extinction learning task was completed by participants while in an MRI scanner. The unconditioned stimulus (US) was an electric shock (rated as maximally uncomfortable but not painful during a calibration procedure) administered via the BIOPAC STM100C module using pre-gelled electrodes placed on the left shin (i.e., fleshy portion of the mediolateral, left lower leg, directly over the tibialis anterior). Conditioned stimuli consisted of geometric shapes (triangles and circles), presented against virtual contexts (yellow or blue backgrounds implicitly distinguishing acquisition and extinction contexts), in a pseudorandomized alternating fashion. During the acquisition phase, the US occurred 2.5 s following CS + onset with a 50% reinforcement schedule. No shocks were administered during the extinction phase. The task alternated between acquisition and extinction phases, with two presentations of each phase, for a total of 156 trials (including baseline trials; see Fig. 1 for specific task details [e.g., length of trials, # of trials per phase, intertrial interval]). In order to keep participants engaged, participants’ instruction on the task was to indicate the identity of the stimulus (by selecting a button corresponding to the shape of the observed stimulus as fast as possible) and were not informed about any specific contingencies between stimuli and shocks. Threat expectancy ratings were also obtained during each learning phase. Specifically, participants were prompted to provide a 0 (not at all likely) to 10 (extremely likely) rating, pertaining to how likely they believed a shock was to follow each of the stimuli (CS+ and CS-; see Supplementary Material and Cisler et al., 2020).
2.5. Skin conductance acquisition and processing
SCR data were acquired (at both sites) on a BIOPAC MP150 Data Acquisition System using the EDA100C module with MECMRI-TRANS cable system. Data were acquired directly into BIOPAC AcqKnowledge 4.3 software at 2000 Hz (UAMS site) or 1000 Hz (UW site). SCR recording electrodes were placed on the medial portions of the thenar and hypothenar eminences of the left hand. In order to allow for the greatest range of intensity selections, amperage on the stimulation device was set to the maximum of 50 MA.
Consistent with the overall study (Cisler et al., 2020), skin conductance data was preprocessed by applying the following steps (in order): 1) a 10 ms median filter, 2) unidirectional Butterworth filter with 0.0159 Hz and 5 Hz low and high pass frequencies, and 3) downsampling to 10 Hz. Following preprocessing, skin conductance responses (SCRs) were estimated on a trial-by-trial basis by applying the well-validated forward convolution model of skin conductance responses within a GLM approach (Bach et al., 2013, Bach et al., 2018). The resulting SCRs were normalized to each individual’s max SCR to account for inter-individual differences in overall magnitude of SCR responding. The reinforced CS+ trials from the acquisition phase blocks (i.e., CS+ followed by shock) were not included in analyses to avoid any contamination of SCR responses to the stimulus with SCR responses to the shock. Consistent with previous investigations (Cisler et al., 2020, Haaker et al., 2013, Raij et al., 2018), participants whose SCR data showed excessive artifact or flat responding were removed from analyses (n = 6 from run 1, n = 10 from run 2). This percentage of data loss (6.5–10.9%) is consistent with prior fear extinction studies using SCR (Cisler et al., 2020, Crombie et al., 2021a, Garfinkel et al., 2014, Haaker et al., 2013, Raij et al., 2018).
2.6. Skin conductance analysis
Fear acquisition and extinction SCR data were analyzed with linear mixed-effects models (LMEMs), including factors for allele group (dummy coded with the CC allele group as reference) × CS (CS + vs CS-) × context (acquisition vs extinction) × time (early vs late) × run (run 1 vs run 2) interactions as well as additional covariates (z-scored) for age, education, ethnicity (dummy coded), site (UW vs UAMS), and PTSD symptom severity (CAPS total severity).
2.7. MRI imaging and data acquisition
2.8. Image preprocessing
2.9. Independent component analyses
Based on a recent shift to large-scale neural network analyses, particularly in regard to network analyses of fear learning (Ross and Cisler, 2020), our primary imaging analyses utilized independent component analysis (ICA) in order to identify large-scale networks of spatially distributed patterns of temporal coactivation. We used a model order of 35 as a tradeoff between component estimation reliability and interpretability (Cisler et al., 2020, Ray et al., 2013). ICA was implemented using GIFT in MATLAB R2019a.
2.10. Fear conditioning and extinction imaging task network analyses
Of the 35 networks, we identified 9 functional networks that were responsive to the task (i.e., significant main effect of CS, context, time, and/or run at p < 0.05) and are thought to be related to learning or PTSD (i.e., excluding 26 networks that represented either motion artifact, CSF, or networks that were non-responsive to the task or were not of interest for this study [e.g., motor and visual cortex]; See Fig. 2 and Supplementary Fig. 1). Each network’s timecourse was regressed onto the corresponding task design matrix (calculated with AFNI’s 3dDeconvolve) in order to characterize functional activation of the network (further details in supplement). For our group-level analyses, beta coefficients defining functional activation for each network for each participant were then compared between groups using LMEMs, in which the beta coefficients were regressed onto the dummy-coded allele groups (CC allele vs AC/AA allele) × CS × context × time × run interactions as well as covariates for age, education, ethnicity, site, and PTSD symptom severity. Bonferroni correction was used to control for alpha inflation (i.e., p < 0.0056). Matlab R2019a was used for these analyses.
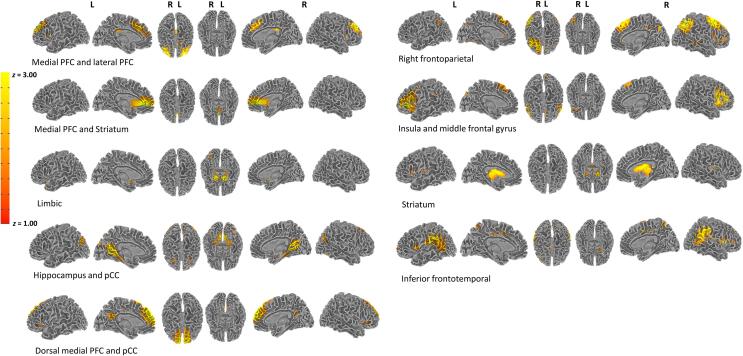
Fig. 2.
Networks of interest from the Independent Component Analysis (ICA) conducted on task data. The ICA used a model order of 35, and 9 of these components were selected as networks of interest after removing components attributed to artifact (e.g., CSF, head motion) and networks of non-interest (e.g., visual and motor networks). Labeling of each network was based on peak loading coordinates obtained in AFNI.
2.11. Voxelwise activation analyses
Given that univariate analyses have been the primary focus of prior fear extinction investigations, we also presented secondary neural results from standard voxelwise general linear models that used identical design matrices as the ICA network analyses. Second-level voxelwise analyses used identical LMEMs as the ICA network analyses. The second-level analysis was masked with a group-level gray matter mask defined from individual subjects’ segmented anatomical scans. Voxelwise comparisons used cluster-thresholding (Eklund et al., 2016) to control for multiple comparisons (AFNIs 3dClustSim), in which a voxel-level uncorrected p < 0.001 was used with a cluster threshold of k ≥ 18 to achieve a corrected p < 0.05 (Cisler et al., 2020).
3. Results
3.1. Participant characteristics
There were no significant (ps = 0.141 to 0.988) differences between groups (CC allele n = 49, AA/AC allele n = 36) for age, education, race, PTSD symptoms and symptom severity, depressive symptoms, alcohol, cannabis, and nicotine use, and percent receiving psychological or pharmacological treatment (see Table 1).
Table 1.
Participant characteristics.
| AA/AC (n = 36) | CC (n = 49) | t-statistic | p-value | |
|---|---|---|---|---|
| Variable | ||||
| Age (yrs) | 34.64 ± 9.84 | 34.22 ± 8.10 | 0.213 | 0.832 |
| Education (yrs) | 15.29 ± 2.40 | 15.27 ± 2.51 | 0.054 | 0.957 |
| Race | −0.152 | 0.879 | ||
| # (% Caucasian) | 27 (75.00) | 36 (73.47) | ||
| # (% Black/African-American) | 6 (16.67) | 9 (18.37) | ||
| # (% Hispanic/Latino) | 1 (2.78) | 1 (2.04) | ||
| # (% other) | 2 (5.56) | 3 (6.12) | ||
| Psychological Treatment* - # receiving (%) | 18 (51.43) | 31 (63.27) | −1.073 | 0.283 |
| Medication - # receiving (%) | 19 (52.78) | 31 (63.27) | −0.960 | 0.337 |
| Smokers (nicotine) - # of regular consumers (%) | 15 (41.67) | 17 (34.69) | 0.647 | 0.518 |
| Alcohol - # of regular consumers (%) | 24 (66.67) | 29 (59.18) | 0.694 | 0.488 |
| Current Anxiety - # diagnosed (%) | 24 (66.67) | 35 (71.43) | −0.462 | 0.644 |
| Current MDD - # diagnosed (%) | 6 (16.67) | 11 (22.45) | −0.648 | 0.517 |
| Depression (BDI-II) | 23.71 ± 11.99 | 21.35 ± 11.42 | 0.917 | 0.362 |
| PTSD symptoms and severity (CAPS-5) | ||||
| Cluster B: Re-experiencing/intrusive | ||||
| Symptom severity | 10.72 ± 4.31 | 10.31 ± 3.38 | 0.499 | 0.619 |
| Number of symptoms | 3.39 ± 1.50 | 3.39 ± 1.26 | 0.004 | 0.997 |
| Cluster C: Avoidance | ||||
| Symptom severity | 5.17 ± 1.84 | 5.33 ± 1.66 | −0.418 | 0.677 |
| Number of symptoms | 1.53 ± 0.65 | 1.61 ± 0.57 | −0.634 | 0.528 |
| Cluster D: Negative alterations in cognition/mood | ||||
| Symptom severity | 14.28 ± 5.38 | 15.80 ± 4.83 | −1.365 | 0.176 |
| Number of symptoms | 4.19 ± 1.97 | 4.98 ± 1.96 | −1.820 | 0.072 |
| Cluster E: Marked alterations in arousal/reactivity | ||||
| Symptom severity | 10.92 ± 4.12 | 10.45 ± 3.54 | 0.561 | 0.576 |
| Number of symptoms | 3.17 ± 1.78 | 3.31 ± 1.40 | −0.404 | 0.687 |
| Total PTSD symptoms | ||||
| Symptom severity | 41.08 ± 12.06 | 41.88 ± 10.49 | −0.324 | 0.747 |
| Number of symptoms | 13.81 ± 2.90 | 14.16 ± 2.58 | −0.599 | 0.551 |
| Self-reported PTSD symptom severity (PCL-5) | 41.54 ± 13.80 | 43.96 ± 15.33 | −0.742 | 0.460 |
Note. Values listed as M ± SD unless otherwise listed. There were no significant differences between groups for all variables. Parametric data was analyzed between groups using independent samples t-tests. Nominal data (race, medication, psychological treatment) was analyzed with Wilcoxon Rank Sum tests and therefore reported test statistics are Z-values. For # receiving psychological treatment, n = 35 in the AA/AC group. PTSD = Posttraumatic Stress Disorder; MDD = Major Depressive Disorder; BDI-II = Beck Depression Inventory; CAPS-5 = Clinician Administered PTSD Scale for DSM-V; PCL-5 = PTSD Checklist for DSM-V.
3.2. Impact of FAAH genetic variation on physiological measures of fear responding (SCR)
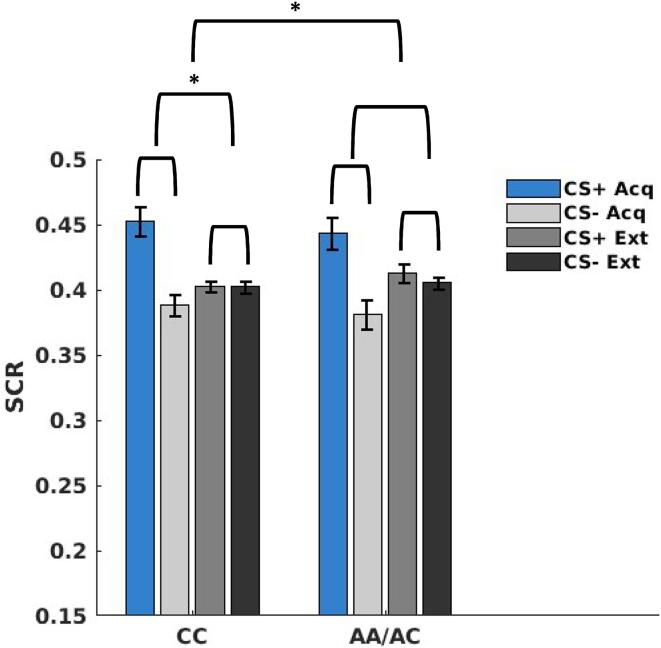
As expected, analyses of SCR data indicated that fear acquisition and extinction learning successfully occurred in both groups (see Supplementary Material). In terms of primary outcomes of interest (i.e., group main effect or interactions), there was a significant context × group interaction (t(1146) = -2.33, p = 0.019). Simple effect testing indicated a significant main effect of context (t(674) = 4.39, p = < 0.001) for the CC allele group, but not the AA/AC allele group (t(467) = 0.67, p = .500), suggesting greater responding to the acquisition context compared to the extinction context (i.e., greater differentiation between contexts) for CC allele carriers (see Fig. 3) but not AA allele carriers. See Supplementary Material for complete SCR LMEM results.
Fig. 3.
Skin conductance responses (SCR) to conditioned stimuli (CS+ and CS-) during acquisition and extinction phases (collapsed across runs) for each group (CC vs AA/AC). CC allele carriers exhibited greater responding (p < 0.001) to the acquisition context compared to the extinction context (i.e., greater differentiation between contexts), which is contrast to AA/AC allele carriers (p = .500).
3.3. Impact of FAAH genetic variation on cognitive measures of fear responding (Threat expectancy Ratings)
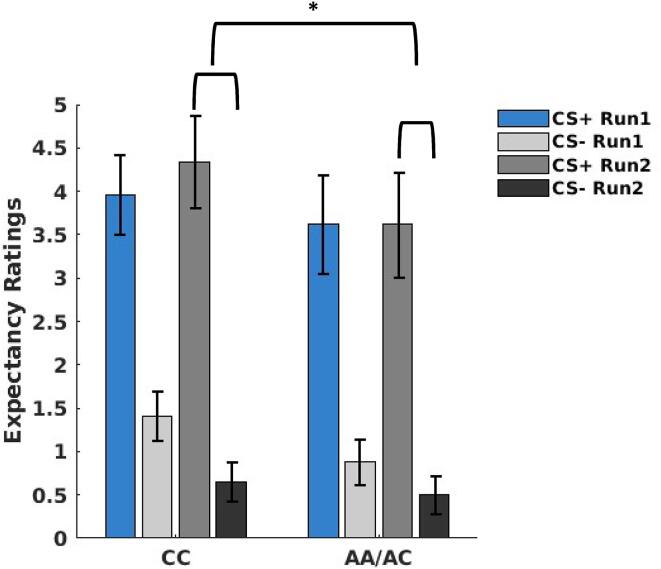
As expected, analyses of threat expectancy data indicated that fear acquisition and extinction learning successfully occurred in both groups (see Supplementary Material). In terms of primary outcomes of interest (i.e., group main effect or interactions), there was a significant CS × run × group interaction (t(1227) = −1.99, p = 0.046). Simple effect testing indicated a significant CS × group interaction (t(611) = -2.03, p = 0.042) during run 2, and a significant CS × run interaction (t(715) = 4.31, p < 0.001) for the CC allele group, but not the AA allele group (t(507) = 1.39, p = 0.164). These results suggest the greater threat expectancy to the CS during run 2 for the CC allele group was driven by increased expectancies to the CS + in the CC allele carriers compared to the AA/AC allele carriers (see Fig. 4). See Supplementary Material for complete threat expectancy LMEM results.
Fig. 4.
Threat expectancy ratings to conditioned stimuli (CS+ and CS-) during run 1 and run 2 (collapsed across context) for each group (CC vs AA/AC). Greater responding to the CS during run 2 for the CC allele group was driven by increased responding to the CS+ in the CC allele carriers (p < 0.001) compared to the AA/AC allele carriers (p = 0.164).
3.4. Impact of FAAH genetic variation on functional networks during fear acquisition and extinction
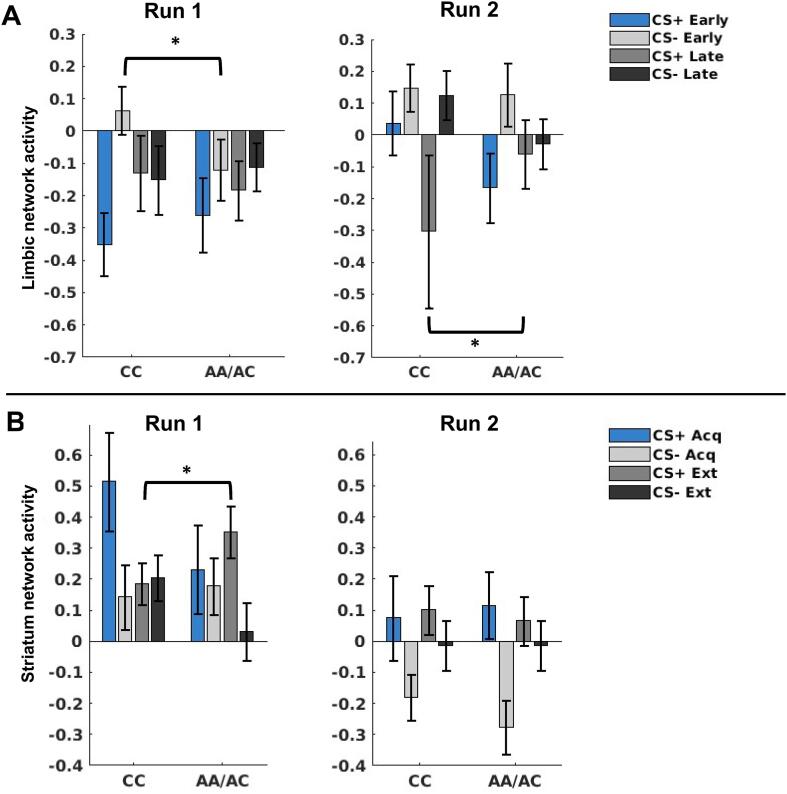
After controlling for multiple comparisons, two functional networks (with peak loadings in regions consistent with a labeling of these networks as limbic [x = -21, y = 7, z = 6] and striatum [x = -10, y = -16, z = -1]) demonstrated a significant effect with FAAH allele groups (see Supplementary Material for complete LMEM results for non-significant networks). The omnibus LMEM for the limbic network (including amygdala, hippocampus, and basal ganglia) demonstrated a significant CS × time × run × group interaction (t(1069) = 2.80, p = 0.005). We probed this interaction by analyzing effects separately for each factor (CS, time, run, and group) involved in the interaction. Post hoc tests demonstrated that the interaction was attributable to differential group responses to the CS + and CS- depending on the timing of the trials and the run. Specifically, the CC allele carriers exhibited greater responding to the CS- during the early trials of run 1 (t(533) = 2.21, p = 0.027), whereas the AA/AC allele carriers exhibited greater responding to the CS + during the late trials of run 2 (t(531) = 2.06, p = 0.039; see Fig. 5A and Supplementary Material for complete LMEM results).
Fig. 5.
Mean functional activation of the Limbic (A) and Striatum (B) networks across groups to each task condition separately for each run. In the limbic network, the CC allele carriers exhibited greater responding (p = 0.027), to the CS- during the early trials of run 1, whereas the AA/AC allele carriers exhibited greater responding (p = 0.039) to the CS+ during the late trials of run 2 (A); in the striatum network, the AA/AC allele carriers exhibited greater responding to the CS+ during extinction run 1 compared to the CC allele carriers (B).
The omnibus LMEM for the striatum network demonstrated a significant CS × context × run × group interaction (t(1069) = 2.80, p = 0.005). We probed this interaction by analyzing effects separately for each factor (CS, context, run, and group) involved in the interaction. Post hoc tests demonstrated that the interaction was attributable to greater responding to the CS + during extinction run 1 for the AA/AC allele carriers (t(531) = 2.23, p = 0.026). Additionally, although non-significant, the CC allele carriers exhibited greater responding to the CS- in extinction run 1 (t(533) = -1.69, p = 0.091; see Fig. 5B and Supplementary Material for complete LMEM results).
As detailed in the Supplementary Material, although seven other networks were responsive to the task (i.e., significant main effect of CS, context, time, and/or run at p < 0.05), there were non-significant group main effects or interactions in the medial and lateral PFC (CS × time × run × group: t(1069) = 2.71, p = 0.006), medial PFC and striatum (time × run × group: t(1069) = −2.55, p = 0.010), dorsal medial PFC and posterior cingulate cortex (pCC)(CS × context × run × group: t(1069) = 2.68, p = 0.007), hippocampus and pCC (run × group: t(1069) = 2.61, p = 0.009), right frontoparietal (group: t(1069) = 2.57, p = 0.010), and inferior frontal temporal (group: t(1069) = −2.04, p = 0.040) networks.
3.5. Impact of FAAH genetic variation on voxelwise activation during fear acquisition and extinction
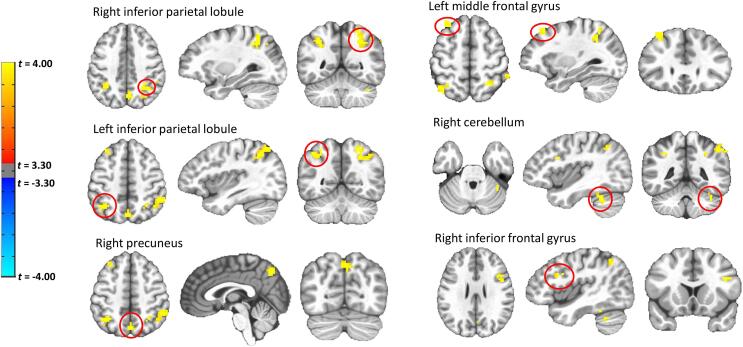
The voxelwise LMEMs indicated a significant group main effect for six significant clusters (controlling for voxel wise comparisons), indicating overall greater activation (in the left and right inferior parietal lobes, right precuneus, left middle frontal gyrus, right cerebellum, and right inferior frontal gyrus) during the task for the AA/AC allele carriers compared to the CC allele carriers (see Fig. 6). See Supplementary Material for additional data (i.e., regions, coordinates, cluster sizes). In contrast to our network level analyses, our voxelwise analyses revealed that there were no significant group interaction effects.
Fig. 6.
Voxelwise LME results for group (CC vs AA/AC) main effect. AA/AC allele carriers exhibited overall greater (p < 0.05) activation (in the left and right inferior parietal lobes, right precuneus, left middle frontal gyrus, right cerebellum, and right inferior frontal gyrus) during the task compared to the CC allele carriers. Voxelwise comparisons used cluster-thresholding to control for multiple comparisons (AFNIs 3dClustSim), in which a voxel-level uncorrected p < 0.001 was used with a cluster threshold of k ≥ 18 to achieve a corrected p < 0.05.
4. Discussion
The main finding from this study is that although fear acquisition and extinction learning successfully occurred (based on physiological and cognitive indices) regardless of genetic variation in the FAAH gene, the neural, physiological, and cognitive signatures of fear acquisition and extinction learning varied as a function of whether the FAAH C385A allele (which typically results in elevated AEA due to reduced FAAH activity) was present. Neural evidence in support of this notion was apparent based on divergent responding between CC vs AA/AC allele carriers within two networks (labeled as limbic and striatum based on peak loadings) putatively involved in fear acquisition and extinction learning (Delgado et al., 2008, Fullana et al., 2016, Fullana et al., 2018, Sehlmeyer et al., 2009, Suarez-Jimenez et al., 2020), along with high levels of CB1R expression (Katona and Freund, 2012, Glass et al., 1997, Herkenham et al., 1990, Howlett et al., 1990). Specifically, this divergent responding between groups varied depending on the type of CS (CS+ vs CS-) presented. For instance, during the first run of the extinction context, the CC allele carriers exhibited greater activation within the striatum network during the presentation of the CS- stimuli, whereas the AA allele carriers exhibited greater activation to the CS+. Similar divergent responding to the CS+ vs CS- was also evident in the limbic network as CC allele carriers exhibited greater activation to the CS- during the early trials of run 1, whereas the AA allele carriers exhibited greater activation to the CS+ during the late trials of run 2. Collectively, these results suggest that within the limbic network, the CC allele carriers initially exhibit greater activation following the presentation of a safety stimulus (CS-) early on during the first run of the task, in comparison to the AA allele carriers. Upon initial exposure to the extinction context (where no shock occurred regardless of CS presentation), the CC allele carriers similarly exhibit greater activation within the striatum network to the same stimuli (CS-) that was safe in both contexts, whereas the AA/AC allele carriers exhibited greater activation to a stimulus (CS+) that previously predicted shock in a different context (i.e., acquisition context). As the task progressed into a second learning run, while there was no longer evidence for divergent responding between allele carriers within the striatum network, the AA/AC allele carriers continued to exhibit greater activation within the limbic network to the CS+ during the late trials. In other words, these results suggest that FAAH genetic variation may affect how fear learning is tuned, with AA/AC allele carriers’ processing tuned specifically to the CS+, and CC allele carriers’ processing tuned to the CS-. Clearly, an important consideration for future research involves the clinical implications of such an effect - in other words, is the observed divergent responding to the CS+ vs CS- less (or more) adaptive for one group of individuals (i.e., CC vs AA/AC carriers). For instance, based on the finding that CC allele carriers exhibited greater responding to the CS- in the limbic network during the early trials of run 1, it could be argued that CC allele carriers exhibit a slower rate of safety learning in comparison to the AA/AC allele carriers (with presumably higher AEA levels), suggesting a beneficial role of elevated AEA in promoting accelerated safety learning. Moreover, the increased responding within the striatum network to the CS+ during the first extinction run for AA/AC allele carriers may be an adaptive response, partially due to the neuromodulatory role of the eCB system in regulating dopamine release within mesolimbic dopamine system (Cheer et al., 2004, Lupica and Riegel, 2005). Recent research has demonstrated that the absence of an aversive stimulus during extinction learning is encoded as rewarding (i.e., increased dopamine signaling), which is in contrast to a traditional reward learning task during which decreased dopamine signaling might be expected when an organism experiences less reward than predicted (Kalisch et al., 2019, Zhang et al., 2020). As such, it may be the case that elevated AEA within the aforementioned reward circuitry exerts a modulatory role that promotes the release of dopamine and an accompanying greater negative prediction error signal to a stimulus (CS+) that previously signaled threat in the acquisition run immediately preceding the first extinction run. These are clearly speculative hypothesis that cannot be addressed with the current study – but highlight the importance of further determining the clinical relevance of the aforementioned findings.
In addition to our functional network analyses, which allowed for an examination of coordinated responses throughout the brain, supplementary univariate analyses also provided evidence for divergent responding for FAAH C385A carriers. Specifically, the AA/AC allele carriers exhibited greater activation throughout the task in several regions (e.g., inferior frontal, middle frontal, parietal, etc.) that are highly consistent with a frontoparietal network involved in higher-order executive functions (Smith et al., 2009, Witt et al., 2021). As such, future research should aim to determine whether the beneficial effects of increased AEA (e.g., due to FAAH inhibition) on fear learning processes (e.g., enhanced extinction recall) are in part due to increased frontoparietal activity during fear acquisition and extinction learning. Although speculative based on limited existing data, future research should also probe the clinical relevance of this effect by examining whether AA/AC allele carriers perform better on tasks that specifically assess elements of the frontoparietal network (i.e., tasks assessing complex problem solving, sustained attention and working memory). However, it is also important to highlight that the observed increased frontoparietal activity was specific to isolated regions from our univariate analyses, and such effects were not present when examining the networks as defined by ICA. It is also worth distinguishing that the increased overall activity for AA/AC allele carriers within the frontoparietal network was not specific to any elements of the fear acquisition and extinction task, which is in contrast to the significant group interaction effects with our network analyses that involved design elements of the task (e.g., CS, context, time).
Consistent with a recent investigation among healthy adults (Zabik et al., 2021), our physiological indices of fear learning suggest that both groups (CC vs AA/AC allele carriers) exhibited differential responding to the CS+ vs CS- during acquisition and decreased (and non-differential) responding to CS during extinction, suggesting successful acquisition and extinction learning occurred. However, as was the case with the neuroimaging outcomes, the physiological and cognitive signatures of fear acquisition and extinction learning varied as a function of genetic variation within the FAAH C385 gene. For instance, it appears that the significant context × group interaction for SCR indicating greater distinction between the acquisition and extinction contexts for CC allele carriers was largely driven by similar levels of responding among the CS- in the acquisition context and the CS+ and CS- in the extinction context for CC allele carriers (i.e., greater responding to the CS+ in acquisition context drove the effect). Given that the AA/AC allele carriers exhibited differentiation between CS+ and CS- in the acquisition context, and lower levels of responding to the CS+ and CS- in the extinction context (i.e., expected results), it remains unknown whether the observed context × group interaction possesses any significant clinical implications. In contrast to SCR, the CS × run × group interaction for the threat expectancy ratings appears to be driven by increased responding to the CS+ during the second run of the task for CC allele carriers. Although assessing various outcomes provides a comprehensive overview, these findings highlight that physiological and cognitive assessments of fear learning are measuring distinct and different, though related, processes. For example, recent modeling work suggest that SCR measures a mixture of uncertainty and threat value of the stimulus, whereas pupil dilation measures threat value alone (Tzovara et al., 2018). As such, convergence across these measures is not necessarily expected and divergent cognitive and physiological findings have been reported in several recent investigations (Cisler et al., 2020, Crombie et al., 2021a, Esser et al., 2020; Taschereau-Dumouchel et al., 2019). These data further demonstrate the need for research to specifically probe the various ways of assessing fear learning in order to be more precise in making inferences about differences in fear learning (e.g., associated with genetic or clinical individual differences or as outcome variables in clinical trials) (Ehlers et al., 2020, Lonsdorf et al., 2017).
Despite significant group differences for physiological, cognitive, and neuroimaging (network analyses and voxelwise activation) outcomes, genetic variation in the FAAH gene had no influence on the percentage of individuals with an anxiety or depression diagnosis or depressive symptoms. This finding is not entirely surprising as previous research in non-clinical adults (Dincheva et al., 2015, Hariri et al., 2009, Mayo et al., 2020a, Mayo et al., 2020b) and pre-clinical models (Bluett et al., 2014) suggest a protective role of AEA on stress reactivity and stress-induced increases in anxiety, as opposed to general anxiety levels. However, there is one preclinical report of reduced anxiety-like behavior following administration of a FAAH inhibitor (Kathuria et al., 2003). Also, there were no differences in PTSD symptoms or symptom severity between groups. The only other study examining the influence of the FAAH C385A variant on PTSD symptoms reported significantly lower hyperarousal symptoms (but not avoidance, re-experiencing or PSTD severity symptoms) for AA/AC carriers, although it should be noted that study involved a smaller sample of treatment seeking men and women with a comorbid alcohol use disorder (Spagnolo et al., 2016).
To date, the majority of research focused on the impact of elevated AEA levels (often due to FAAH genetic variation or administration of FAAH inhibitor) on fear conditioning processes has primarily reported on accelerated extinction learning and enhanced recall, with the majority of investigations (all but Ney et al., 2021b) being conducted in healthy individuals without a clinical diagnosis such as PTSD (Dincheva et al., 2015, Mayo et al., 2020a, Mayo et al., 2020b, Zabik et al., 2021). Given that this is the first functional neuroimaging study to examine a clinical population (PTSD), and due to differences in task design, our ability to directly compare and contrast our findings with the existing literature is limited. However, while the current study provided evidence suggesting that genetic variation in the FAAH gene affects the tuning of fear learning (i.e., divergent responding to CS+ vs CS-), we did not find evidence for accelerated extinction learning among AA/AC allele carriers suggesting that this genetic variation may only exhibit an enhanced extinction learning effect in individuals without a diagnosis of PTSD. Given the design, the current study cannot address whether genetic variation within the FAAH gene enhances extinction recall among women with PTSD. This remains an important and clinically relevant question to be addressed with future research.
Although this study provided a comprehensive examination of the effects of FAAH genetic variation on fear acquisition and extinction learning in adults with PTSD, the current study is not withstanding limitations. While this study provided important information surrounding the neural, physiological, and cognitive signatures of fear acquisition and extinction learning among women with PTSD possessing the FAAH C385A variant, we only examined women with PTSD stemming from a specific type of trauma exposure (i.e., interpersonal violence). While this resulted in a homogenous sample, future research with both men and women experiencing PTSD due to other types of trauma (and other anxiety disorders in general) is warranted. Relatedly, this study did not examine a control group (i.e., healthy controls or trauma-exposed adults without PTSD), which prevents us from understanding if the observed effects of FAAH genetic variation would be similar in other populations. Additionally, this study did not have a direct measure of AEA concentrations, and as a result, we can only presume that AEA concentrations were higher in the AA/AC allele carriers. However, prior preclinical and clinical research have consistently documented higher AEA in animals and humans with the FAAH C385A variant. Perhaps more of a limitation is the fact that there is often up to a 10x increase in AEA concentrations (Mayo et al., 2020a) following administration of a FAAH inhibitor (a likely avenue for future clinical trials), which is in contrast to the FAAH C385A variant, which typically results in a 30% increase in AEA concentrations (Dincheva et al., 2015, Spagnolo et al., 2016) in comparison to CC carriers. Nonetheless, the current study’s findings which focused solely on genetic variation within the FAAH gene provided important considerations for the design and development of future clinical trials with FAAH inhibitors. Specifically, at a very minimum, it is recommended that future trials in this area assess the contributions of genetic variation of the FAAH gene on fear conditioning outcomes and treatment efficacy following administration of FAAH inhibitors. This study also tested fear acquisition and extinction in the same day (with two runs each) with clear distinctions between contexts (i.e., distinct colored backgrounds for acquisition [e.g., blue] and extinction [e.g., yellow] contexts). It is possible that a two-day design without context cues (i.e., different colored backgrounds) clearly (albeit implicitly) defining extinction from acquisition, would have yielded different effects (i.e., accelerated extinction learning in the AA/AC carriers as has been observed in non-clinical populations). Relatedly, given that this was a secondary analysis of a clinical trial attempting to pharmacologically modulate fear extinction recall, we were unable to examine the impact of FAAH gene variation on extinction recall. Primary outcomes of interest in future clinical trials will undoubtedly focus on extinction recall and memory consolidation, due to the fact that the return of fear (assessed during extinction recall tests) is believed to be among the most significant barriers to successfully treating PTSD with exposure-based CBT (Craske et al., 2008, Craske et al., 2014, Vervliet et al., 2013).
Funding
Portions of this work were supported by the National Institutes of Health [MH108753, MH119132], and the Brain and Behavior Foundation. The funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The authors have nothing to disclose.
CRediT authorship contribution statement
Kevin M. Crombie: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Software, Writing – original draft, Supervision. Anthony A. Privratsky: Investigation, Writing – review & editing. Chloe M. Schomaker: Visualization, Writing – review & editing. Mickela Heilicher: Visualization, Writing – review & editing. Marisa C. Ross: Investigation, Writing – review & editing. Anneliis Sartin-Tarm: Investigation, Writing – review & editing. Kyrie Sellnow: Investigation, Writing – review & editing. Elisabeth B. Binder: Resources, Writing – review & editing. G. Andrew James: Writing – review & editing. Josh M. Cisler: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Software, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102922.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- American Psychiatric Association . American Psychiatric Publishing; Washington DC: 2013. Diagnostic and statistical manual of mental disorders, Fifth edition (DSM-5) [Google Scholar]
- Bach D.R., Friston K.J., Dolan R.J. An improved algorithm for model-based analysis of evoked skin conductance responses. Biol. Psychol. 2013;94(3):490–497. doi: 10.1016/j.biopsycho.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach D.R., Castegnetti G., Korn C.W., Gerster S., Melinscak F., Moser T. Psychophysiological modeling: current state and future directions. Psychophysiology. 2018;55(11) doi: 10.1111/psyp.13209. [DOI] [PubMed] [Google Scholar]
- Blanco C., Hoertel N., Wall M.M., Franco S., Peyre H., Neria Y., Helpman L., Limosin F. Toward understanding sex differences in the prevalence of Posttraumatic Stress Disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry. 2018;79(2) doi: 10.4088/JCP.16m11364. [DOI] [PubMed] [Google Scholar]
- Bluett R.J., Gamble-George J.C., Hermanson D.J., Hartley N.D., Marnett L.J., Patel S. Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl. Psychiatry. 2014;4(7):e408. doi: 10.1038/tp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N., Kessler R.C., Chilcoat H.D., Schultz L.R., Davis G.C., Andreski P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit Area Survey of Trauma. Arch. Gen. Psychiatry. 1998;55(7):626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Cheer J.F., Wassum K.M., Heien M.L.A.V., Phillips P.E.M., Wightman R.M. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J. Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler, J.M., Steele, J.S., Lenow, J.K., Smitherman, S., Everett, B., Messias, E., Kilts, C.D., 2014. Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: an exploratory fMRI study. J. Psychiatr. Res. 48, 47-55. https://doi.org/j.jpsychires.2013.09.013. [DOI] [PMC free article] [PubMed]
- Cisler J.M., Privratsky A.A., Sartin-Tarm A., Sellnow K., Ross M., Weaver S., Hahn E., Herringa R.J., James G.A., Kilts C.D. L-DOPA and consolidation of fear extinction learning among women with posttraumatic stress disorder. Transl. Psychiatry. 2020;10:287. doi: 10.1038/s41398-020-00975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske M.G., Kircanski K., Zelikowsky M., Mystkowski J., Chowdhury N., Baker A. Optimizing inhibitory learning during exposure therapy. Behav. Res. Ther. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Craske M.G., Treanor M., Conway C., Zbozinek T., Vervliet B. Maximizing exposure therapy: an inhibitory learning approach. Behav. Res. Ther. 2014;58:10–23. doi: 10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie K.M., Sartin-Tarm A., Sellnow K., Ahrenholtz R., Lee S., Matalamaki M., Adams T.G., Cisler J.M. Aerobic exercise and consolidation of fear extinction learning among women with posttraumatic stress disorder. Behav. Res. Ther. 2021;142:103867. doi: 10.1016/j.brat.2021.103867. [DOI] [PubMed] [Google Scholar]
- Crombie, K.M., Sartin-Tarm, A., Sellnow, K., Ahrenholtz, R., Lee, S., Matalamaki, M., Almassi, N.E., Hillard, C.J., Koltyn, K.F., Adams, T.G., Cisler, J.M., 2021b. Exercise-induced increases in Anandamide and BDNF during extinction consolidation contribute to reduced threat following reinstatement: Preliminary evidence from a randomized controlled trial. Psychoneuroendocrinology 132, 105355. https://doi.org/10.1016j.psyneuen.2021.105355. [DOI] [PMC free article] [PubMed]
- Crombie K.M., Ross M.C., Letkiewicz A.M., Sartin-Tarm A., Cisler J.M. Differential relationships of PTSD symptom clusters with cortical thickness and grey matter volumes among women with PTSD. Sci. Rep. 2021;11:1825. doi: 10.1038/s41598-020-80776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R., Li J., Schiller D., Phelps E.A. The role of the striatum in aversive learning and aversive prediction errors. Philos. Trans. R Soc. Lond. B Biol. Sci. 2008;363(1511):3787–3800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincheva I., Drysdale A.T., Hartley C.A., Johnson D.C., Jing D., King E.C., Ra S., Gray J.M., Yang R., DeGruccio A.M., Huang C., Cravatt B.F., Glatt C.E., Hill M.N., Casey B.J., Lee F.S. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat. Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M.R., Nold J., Kuhn M., Klingelhöfer-Jens M., Lonsdorf T.B. Revisiting potential associations between brain morphology, fear acquisition and extinction through new data and a literature review. Sci. Rep. 2020;10:19894. doi: 10.1038/s41598-020-76683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. USA. 2016;113(28):7900–79005. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser R., Fuss J., Haaker J. Initial evidence for pharmacological modulation of observational threat learning by the GABAergic noradrenergic systems in humans. Behav. Res. Ther. 2020;129:103605. doi: 10.1016/j.brat.2020.103605. [DOI] [PubMed] [Google Scholar]
- Fullana M., Dunsmoor J.E., Schruers K.R.J., Savage H.S., Bach D.R., Harrison B.J. Human fear conditioning: From neuroscience to the clinic. Behav. Res. Ther. 2020;124:103528. doi: 10.1016/j.brat.2019.103528. [DOI] [PubMed] [Google Scholar]
- Fullana M.A., Harrison B.J., Soriano-Mas C., Vervliet B., Cardoner N., Àvila-Parcet A., Radua J. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol. Psychiatry. 2016;21(4):500–508. doi: 10.1038/mp.2015.88. [DOI] [PubMed] [Google Scholar]
- Fullana M.A., Albajes-Eizagirre A., Soriano-Mas C., Vervliet B., Cardoner N., Benet O., Radua J., Harrison B.J. Fear extinction in the human brain: a meta-analysis of fMRI studies in healthy participants. Neurosci. Biobehav. Rev. 2018;88:16–25. doi: 10.1016/j.neubiorev.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Garfinkel S.N., Abelson J.L., King A.P., Sripada R.K., Wang X., Gaines L.M., Liberzon I. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J. Neurosci. 2014;34(40):13435–13443. doi: 10.1523/JNEUROSCI.4287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M., Faull R.L.M., Dragunow M. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77(2):299–318. doi: 10.1016/S0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Green D.G.J., Kim J., Kish S.J., Tyndale R.F., Hill M.N., Strafella A.P., Tong J., McCluskey T., Westwood D.J., Houle S., Lobaugh N.J., Boileau I. Fatty acid amide hydrolase binding is inversely correlated with amygdalar functional connectivity: a combined positron emission tomography and magnetic resonance imaging study in healthy individuals. J. Psychiatry Neurosci. 2021;46(2):E238–E246. doi: 10.1503/jpn.200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O., MacPherson K.P., Cinar R., Gamble-George J., Sugden K., Williams B., Godlewski G., Ramikie T.S., Gorka A.X., Alapafuja S.O., Nikas S.P., Makriyannis A., Poulton R., Patel S., Hariri A.R., Caspi A., Moffitt T.E., Kunos G., Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol. Psychiatry. 2013;18(7):813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker J., Gaburro S., Sah A., Gartmann N., Lonsdorf T.B., Meier K., Singewald N., Pape H.-C., Morellini F., Kalisch R. Single dose of L-dopa makes extinction memories context-independent and prevents the return of fear. Proc. Natl. Acad. Sci. USA. 2013;110(26):E2428–E2436. doi: 10.1073/pnas.1303061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J., Barna I., Barsvari B., Gyimesi Pelczer K., Yasar S., Panlilio L.V., Goldberg S. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology. 2009;204(4):607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J., Goldberg S.R., Pelczer K.G., Aliczki M., Panlilio L.V. The effects of anandamide signaling enhanced by the FAAH inhibitor URB597 on coping styles in rats. Psychopharmacology. 2013;230(3):353–362. doi: 10.1007/s00213-013-3161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Gorka A., Hyde L.W., Kimak M., Halder I., Ducci F., Ferrell R.E., Goldman D., Manuck S.B. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol. Psychiatry. 2009;66(1):9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M., Lynn A.B., Little M.D., Johnson M.R., Melvin L.S., de Costa B.R., Rice K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Campolongo P., Yehuda R., Patel S. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. 2018;43(1):80–102. doi: 10.1038/npp.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Patel S. Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biol. Mood Anxiety Disord. 2013;3(1):19. doi: 10.1186/2045-5380-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett A.C., Bidaut-Russell M., Devane W.A., Melvin L.S., Johnson M.R., Herkenham M. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13(10):420–423. doi: 10.1016/0166-2236(90)90124-S. [DOI] [PubMed] [Google Scholar]
- Iverson K.M., McLaughlin K.A., Gerber M.R., Dick A., Smith B.N., Bell M.E., Cook N., Mitchell K.S. Exposure to interpersonal violence and its association with psychiatric mordibidity in a U.S. National Sample: A Gender Comparison. Psychol. Violence. 2013;3(3):273–287. doi: 10.1037/a0030956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R., Gerlicher A.M.V., Duvarci S. A dopaminergic basis for fear extinction. Trends Cogn. Sci. 2019;23(4):274–277. doi: 10.1016/j.tics.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Kathuria S., Gaetani S., Fegley D., Valiño F., Duranti A., Tontini A., Mor M., Tarzia G., Rana G.L., Calignano A., Giustino A., Tattoli M., Palmery M., Cuomo V., Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003;9(1):76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Katona I., Freund T.F. Multiple functions of endocannabinoid signaling in the brain. Annu. Rev. Neurosci. 2012;35(1):529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., et al. Trauma and PTSD in the WHO World Mental Health Surveys. Eur. J. Psychotraumatol. 2017;8(5):1353383. doi: 10.1080/20008198.2017.1353383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Merikangas K.R., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Lonsdorf T.B., Menz M.M., Andreatta M., Fullana M.A., Golkar A., Haaker J., Merz C.J. Don’t fear “fear conditioning”: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci. Biobehav. Rev. 2017;77:247–285. doi: 10.1016/j.neurobiorev.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Lupica C.R., Riegel A.C. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48(8):1105–1116. doi: 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Marsicano G., Wotjak C.T., Azad S.C., Bisogno T., Rammes G., Cascio M.G., Hermann H., Tang J., Hofmann C., Zieglgänsberger W., Di Marzo V., Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D., Fernández de la Cruz L., Monzani B., Rosenfield D., Andersson E., Pérez-Vigil A., Frumento P., de Kleine R.A., Difede JoAnn, Dunlop B.W., Farrell L.J., Geller D., Gerardi M., Guastella A.J., Hofmann S.G., Hendriks G.-J., Kushner M.G., Lee F.S., Lenze E.J., Levinson C.A., McConnell H., Otto M.W., Plag J., Pollack M.H., Ressler K.J., Rodebaugh T.L., Rothbaum B.O., Scheeringa M.S., Siewert-Siegmund A., Smits J.A.J., Storch E.A., Ströhle A., Tart C.D., Tolin D.F., van Minnen A., Waters A.M., Weems C.F., Wilhelm S., Wyka K., Davis M., Rück C., Altemus M., Anderson P., Cukor J., Finck C., Geffken G.R., Golfels F., Goodman W.K., Gutner C., Heyman I., Jovanovic T., Lewin A.B., McNamara J.P., Murphy T.K., Norrholm S., Thuras P. D-cycloserine augmentation of exposure-based cognitive behavior therapy for anxiety, obsessive-compulsive, and posttraumatic stress disorders: a systematic review and meta-analysis of individual participant data. JAMA Psychiatry. 2017;74(5):501. doi: 10.1001/jamapsychiatry.2016.3955. [DOI] [PubMed] [Google Scholar]
- Mayo L.M., Asratian A., Lindé J., Morena M., Haataja R., Hammar V., Augier G., Hill M.N., Heilig M. Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: a randomized, controlled experimental medicine trial. Biol. Psychiatry. 2020;87(6):538–547. doi: 10.1016/j.biopsych.2019.07.034. [DOI] [PubMed] [Google Scholar]
- Mayo L.M., Asratian A., Lindé J., Holm L., Nätt D., Augier G., Stensson N., Vecchiarelli H.A., Balsevich G., Aukema R.J., Ghafouri B., Spagnolo P.A., Lee F.S., Hill M.N., Heilig M. Protective effects of elevated anandamide on stress and fear-related behaviors: translational evidence from humans and mice. Mol. Psychiatry. 2020;25(5):993–1005. doi: 10.1038/s41380-018-0215-1. [DOI] [PubMed] [Google Scholar]
- Mayo, L.M., Rabinak, C.A., Hill, M.N., Heilig, M., 2021. Targeting the endocannabinoid system in the treatment of posttraumatic stress disorder: a promising case of preclinical-clinical translation? Biol. Psychiatry, online ahead of print. https://doi.org/10.1016/j.biopsych.2021.07.019. [DOI] [PMC free article] [PubMed]
- Moreira F.A., Grieb M., Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract. Res. Clin. Endocrinol. Metab. 2009;23(1):133–144. doi: 10.1016/j.beem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Morena M., Berardi A., Colucci P., Palmery M., Trezza V., Hill M.N., Campolongo P. Enhancing endocannabinoid neurotransmission augments the efficacy of extinction training and ameliorates traumatic stress-induced behavioral alterations in rats. Neuropsychopharmacology. 2018;43(6):1284–1296. doi: 10.1038/npp.2017.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M., Roozendaal B., Trezza V., Ratano P., Peloso A., Hauer D., Atsak P., Trabace L., Cuomo V., McGaugh J.L., Schelling G., Campolongo P. Endogenous cannabinoid release within prefrontal-limbic pathways affects memory consolidation of emotional training. Proc. Natl. Acad. Sci. USA. 2014;111(51):18333–18338. doi: 10.1073/pnas.1420285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney L.J., Matthews A., Bruno R., Felmingham K.L. Cannabinoid interventions for PTSD: where to next? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;93:124–140. doi: 10.1016/j.pnpbp.2019.03.017. [DOI] [PubMed] [Google Scholar]
- Ney L.J., Akhurst J., Bruno R., Laing P.A.F., Matthews A., Felmingham K.L. Dopamine, endocannabinoids and their interaction in fear extinction and negative affect in PTSD. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;105:110118. doi: 10.1016/j.pnpbp.2020.110118. [DOI] [PubMed] [Google Scholar]
- Ney L.J., Matthews A., Hsu C.-M., Zuj D.V., Nicholson E., Steward T., Nichols D., Graham B., Harrison B., Bruno R., Felmingham K. Cannabinoid polymorphisms interact with plasma endocannabinoid levels to predict fear extinction learning. Depress Anxiety. 2021;38(10):1087–1099. doi: 10.1002/da.23170. [DOI] [PubMed] [Google Scholar]
- Ney L.J., Crombie K.M., Mayo L.M., Felmingham K.L., Bowser T., Matthews A. Translation of animal endocannabinoid models of PTSD mechanisms to humans: where to next? Neurosci. Biobehav. Rev. 2021;132:76–91. doi: 10.1016/j.neurobiorev.2021.11.040. [DOI] [PubMed] [Google Scholar]
- Olatunji B.O., Cisler J.M., Tolin D.F. Quality of life in the anxiety disorders: a meta-analytic review. Clin. Psychol. Rev. 2007;27(5):572–581. doi: 10.1016/j.cpr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., Maller J., Sklar P., de Bakker P.I.W., Daly M.J., Sham P.C. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak C.A., Angstadt M., Lyons M., Mori S., Milad M.R., Liberzon I., Phan K.L. Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiol. Learn Mem. 2014;113:125–134. doi: 10.1016/j.nlm.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak C.A., Angstadt M., Sripada C.S., Abelson J.L., Liberzon I., Milad M.R., Phan K.L. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64:396–402. doi: 10.1016/j.neuropharm.2012.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak C.A., Blanchette A., Zabik N.L., Peters C., Marusak H.A., Iadipaolo A., Elrahal F. Cannabinoid modulation of corticolimbic activation to threat in trauma-exposed adults: a preliminary study. Psychopharmacology. 2020;237(6):1813–1826. doi: 10.1007/s00213-020-05499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raij T., Nummenmaa A., Marin M.-F., Porter D., Furtak S., Setsompop K., Milad M.R. Prefrontal cortex stimulation enhances fear extinction memory in humans. Biol. Psychiatry. 2018;84(2):129–137. doi: 10.1016/j.biopsych.2017.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K.L., McKay D.R., Fox P.M., Riedel M.C., Uecker A.M., Beckmann C.F., Smith S.M., Fox P.T., Laird A.R. ICA model order selection of task co-activation networks. Front Neurosci. 2013;7:237. doi: 10.3389/fnins.2013.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resick P.A., Nishith P., Weaver T.L., Astin M.C., Feuer C.A. A comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. J. Consult. Clin. Psychol. 2002;70:867–879. doi: 10.1037/0022-006X.70.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick H.S., Kilpatrick D.G., Dansky B.S., Saunders B.E., Best C.L. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J. Consult. Clin. Psychol. 1993;61(6):984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Ross M.C., Cisler J.M. Altered large-scale functional brain organization in posttraumatic stress disorder: a comprehensive review of univariate and network-level neurocircuitry models of PTSD. Neuroimage Clin. 2020;27:102319. doi: 10.1016/j.nicl.2020.102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum B.O., Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann. N. Y. Acad. Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Schnurr P.P., Friedman M.J., Engel C.C., Foa E.B., Shea M.T., Chow B.K., Resick P.A., Thurston V., Orsillo S.M., Haug R., Turner C., Bernardy N. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA. 2007;297:820–830. doi: 10.1001/jama.297.8.820. [DOI] [PubMed] [Google Scholar]
- Scott K.M., et al. Post-traumatic stress disorder associated with sexual assault among women in the WHO World Mntal Health Surveys. Psychol. Med. 2018;48(1):155–167. doi: 10.1017/S0033291717001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C., Schöning S., Zwitserlood P., Pfleiderer B., Kircher T., Arolt V., Konrad C., Gendelman H.E. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4(6):e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe J.C., Chiang K., Gerber A.L., Beutler E., Cravatt B.F. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc. Natl. Acad. Sci. USA. 2002;99(12):8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo P.A., Ramchandani V.A., Schwandt M.L., Kwako L.E., George D.T., Mayo L.M., Hillard C.J., Heilig M. FAAH gene variation moderates stress response and symptom severity in patients with posttraumatic stress disorder and comorbid alcohol dependence. Alcohol Clin. Exp. Res. 2016;40(11):2426–2434. doi: 10.1111/acer.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Jimenez B., Albajes-Eizagirre A., Lazarov A., Zhu X., Harrison B.J., Radua J., Neria Y., Fullana M.A. Neural signatures of conditioning, extinction learning, and extinction recall in posttraumatic stress disorder: a meta-analysis of functional magnetic resonance imaging studies. Psychol. Med. 2020;50(9):1442–1451. doi: 10.1017/S0033291719001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschereau-Dumouchel V., Kawato M., Lau H. Multivoxel pattern analysis reveals dissociations between subjective fear and its physiological correlates. Mol. Psychiatry. 2019;25(10):2342–2354. doi: 10.1038/s41380-019-0520-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk P.W., Wangelin B.C., Powers M.B., Smits J.A.J., Acierno R., Myers U.S., Orr S.P., Foa E.B., Hamner M.B. Augmenting treatment efficiency in exposure therapy for PTSD: a randomized double-blind placebo-controlled trial of yohimbine HCl. Cogn. Behav. Ther. 2018;47(5):351–371. doi: 10.1080/16506073.2018.1432679. [DOI] [PubMed] [Google Scholar]
- Tzovara A., Korn C.W., Bach D.R., Gershman S.J. Human Pavlovian fear conditioning conforms to probabilistic learning. PLoS Comput. Biol. 2018;14(8):e1006243. doi: 10.1371/journal.pcbi.1006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B., Craske M.G., Hermans D. Fear extinction and relapse: state of the art. Annu. Rev. Clin. Psychol. 2013;9(1):215–248. doi: 10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- Weathers, F.W., Blake, D.D., Schnurr, P.P., Kaloupek, D.G., Marx, B.P., Keane, T.M. 2013. The clinician-administered PTSD Scale for DSM-5 (CAPS-5). Interview available from the national center for PTSD at www.ptsd.va.gov.
- Weathers F.W., Bovin M.J., Lee D.J., Sloan D.M., Schnurr P.P., Kaloupek D.G., Keane T.M., Marx B.P. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol. Assess. 2018;30:383–395. doi: 10.1037/pas0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt, S.T., van Ettinger-Veenstra, H., Salo, T., Riedel, M.C., Laird, A.R., 2021. What executive function network is that? an image-based meta-analysis of network labels. Brain Topogr., https://doi.org/10.1007/s10548-021-00847-z. [DOI] [PubMed]
- Zabik N.L., Iadipaolo A.S., Marusak H.A., Peters C., Burghardt K., Rabinak C.A. A common genetic variant in fatty acid amide hydrolase is linked to alterations in fear extinction neural circuitry in a racially diverse, nonclinical sample of adults. J. Neurosci. Res. 2021 doi: 10.1002/jnr.24860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Kim J., Tonegawa S. Amygdala reward neurons form and store fear extinction memory. Neuron. 2020;105(6):1077–1093. doi: 10.1016/j.neuron.2019.12.025. [DOI] [PubMed] [Google Scholar]
- Zoellner L.A., Telch M., Foa E.B., Farach F.J., McLean C.P., Gallop R., Bluett E.J., Cobb A., Gonzalez-Lima F. Enhancing Extinction learning in posttraumatic stress disorder with brief daily imaginal exposure and methylene blue: a randomized controlled trial. J. Clin. Psychiatry. 2017;78(7):e782–e789. doi: 10.4088/JCP.16m10936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.