This randomized clinical trial examines whether care coordination delivered by an allied health liaison officer improved quality-of-life outcomes among an Australian cohort of children with chronic noncomplex medical conditions and their families.
Key Points
Question
Does care coordination improve quality-of-life outcomes in children with chronic noncomplex medical conditions (non-CMCs) and their families?
Finding
In this multicenter randomized clinical trial of 81 children, care coordination significantly improved overall child, overall family, and family functioning quality-of-life outcomes at 12 months after diagnosis.
Meaning
In this randomized clinical trial, care coordination improved the quality of life of children with non-CMCs and their families, and an orientation among health services to provide such a coordination model could lead to longer-term improved clinical outcomes.
Abstract
Importance
There is a paucity of high-quality evidence on the effect of care coordination on health-related quality of life among children with chronic noncomplex medical conditions (non-CMCs).
Objective
To examine whether care coordination delivered by an Allied Health Liaison Officer results in improved quality-of-life (QOL) outcomes for children with chronic non-CMCs and their families.
Design, Setting and Participants
This multicenter, open label, randomized clinical trial was conducted in pediatric outpatient clinics at 3 Australian hospitals with tertiary- and secondary-level pediatric care facilities. A total of 81 children with chronic non-CMCs and their families participated in the trial for a period of up to 12 months between October 2017 to October 2020. Primary care reviews were offered at 1 week, 3 months, and 6 months after diagnosis.
Interventions
Eligible children were randomized 1:1 to receive care coordination or standard care. Families of children receiving care coordination were provided access to an Allied Health Liaison Officer, who was responsible for facilitation of health care access across hospital, education, primary care, and community sectors.
Main Outcomes and Measures
The primary outcomes were scores on the Pediatric Quality of Life Inventory (PedsQL), version 4.0, and the PedsQL Family Impact Module, version 2.0, measured at 6 and 12 months. An intent-to-treat approach was used to analyze the data.
Results
Of 81 children (mean [SD] age, 8.2 [3.5] years; 55 [67.9%] male), 42 (51.9%) were randomized to care coordination and 39 (48.1%) to standard care. Compared with standard care, care coordination resulted in greater improvements in overall PedsQL scores (difference in score changes between groups, 7.10; 95% CI, 0.44-13.76; P = .04), overall PedsQL Family Impact Module scores (difference in score changes between groups, 8.62; 95% CI, 1.07-16.16; P = .03), and family functioning QOL (difference in score changes between groups, 15.83; 95% CI, 5.05-26.62; P = .004) at 12 months after diagnosis.
Conclusions and Relevance
In this randomized clinical trial, care coordination improved the quality of life of children with chronic non-CMCs and their families. Further studies should explore specific non-CMCs that may benefit most from care coordination and whether an orientation among health services to provide such a coordination model could lead to longer-term improved clinical outcomes.
Trial Registration
http://anzctr.org.au Identifier: ACTRN12617001188325
Introduction
Health services for children with special health care needs (SHCNs) are changing in high-income countries in response to significant increases in the prevalence and nature of chronic illness in children during the past 30 years.1,2,3,4,5 The prevalence of SHCNs among children is 15% to 20%,2,6,7,8 and chronic noncomplex medical conditions (non-CMCs), such as attention-deficit/hyperactivity disorder (ADHD), intellectual impairment, anxiety, and behavioral conditions, are recognized as the most prevalent among children with SHCNs.9 Children with non-CMCs experience the highest quality-of-life (QOL) burden across learning, anxiety, and behavior domains9 and often require community-based health or family support services to manage their condition. Inadequate management of non-CMCs can result in reduced QOL as well as mental health and educational difficulties.10 Longer-term consequences include the development of new chronic diseases in adulthood, criminality, and unemployment.11,12,13 Therefore, timely integrated access to medical specialists, primary care physicians, allied health, and community and educational support during childhood is vital in the management of non-CMCs.
Increasing rates of non-CMCs and reduced spending in health care support the need to adapt health care systems to meet current demands.14 Current health care systems are fragmented and inefficient in the management of chronic non-CMCs in children.15 This is likely owing to the hybrid nature of management for SHCNs, which requires multifaceted approaches that take into consideration the presence of comorbidities, mental health, social determinants of health, and family dynamics across the life span.7 One study16 found that nearly half of families of children with more complex SHCNs experienced unmet medical service needs and 33.1% experienced difficulties in accessing nonmedical services despite spending between 1 to 6 hours on care coordination per week. Caregivers and siblings of children with chronic health conditions are also more likely to have poorer mental health and health-related QOL compared with caregivers of healthy children.17,18,19 This is suboptimal given the pivotal role that families serve in the successful management of children with chronic non-CMCs.6 Thus, there is a need for better access to health care and assistance for children with chronic non-CMCs and their families that are coordinated and supported by high-quality evidence.
Care coordination for children with SHCNs is advocated worldwide20,21,22,23 and has been shown to improve clinical, patient-satisfaction, QOL, and treatment-adherence outcomes for children with asthma, autism spectrum disorder (ASD), and ADHD.10,24,25,26,27 Similarly, multiple studies of various designs have shown that care coordination improves access to care28,29,30 and parents’ perceptions of caregivers and the care provided for children with SHCNs.29,30,31,32,33 However, other studies found that longer-term care coordination demonstrated no benefits for health-related QOL in children with medical complexity33 and no changes in parent self-esteem, parenting stress, or family function in families of children who have SHCNs.34 The different findings on the effectiveness of care coordination are likely attributable to the heterogeneous population of children with SHCNs in previous studies and the relatively few studies with high-quality research designs (ie, randomized clinical trials).29,32,33,35,36,37
Caregivers of children with chronic health conditions have poorer health-related QOL compared with caregivers of healthy children.17,18,19 Previous studies of care coordination in mixed populations of children with CMCs and non-CMCs have not shown benefits in direct health outcomes such as QOL and psychological distress.32,36 Of interest, in a heterogeneous cohort of children with SHCNs, only children with non-CMCs and those from families with fewer coping resources benefited from coordinated pediatric home care and showed improvements in child psychological status, family outcomes, and family functioning.38 This provides proof of concept that care coordination may improve QOL in children with non-CMCs. To our knowledge, no studies have evaluated the effect of care coordination on health-related QOL outcomes exclusively among children with non-CMCs. Thus, we conducted a multicenter, randomized clinical trial to examine whether care coordination delivered by an Allied Health Liaison Officer (AHLO) improves health-related QOL for children with non-CMCs. We hypothesized that children with non-CMCs who received care coordination would have better health-related QOL than would those who received standard care.
Methods
This multicenter, open label, randomized clinical trial (ACTRN12617001188325) was conducted between October 2017 to October 2020 in pediatric outpatient clinics at 3 Australian public hospitals with tertiary- and secondary-level pediatric care facilities (Queensland Children’s Hospital, Gold Coast University Hospital, and Caboolture Hospital). The trial protocol is given in Supplement 1 and has previously been published.39 A tertiary pediatric care facility was added to the trial when we received an additional grant in July 2019. The trial was approved by Children’s Health Queensland, the Department of Human Services, and the Queensland Government Department of Education and Training ethics review committees. Parents or guardians provided written informed consent. The trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Children were recruited from pediatric outpatient clinics. Children aged 0 to 16 years with a newly diagnosed chronic health condition without complexity were eligible for inclusion. Chronic health conditions without complexity were defined as any health condition diagnosed by a general pediatrician that was expected to last more than 6 months, produce consequences for the child’s QOL, and require community-based health or family support services.40 Children were excluded if they had an acute medical condition that required urgent intervention and/or had a CMC for which hospital-based multidisciplinary teams provided coordinated care (eg, cystic fibrosis, cancer).
Randomization, Allocation, and Blinding
Patients were randomly assigned 1:1 using a permuted block design (with a block size of 2) to receive integrated care coordination or standard care. Intervention was randomly allocated to enrolled participants from concealed, sequentially numbered opaque envelopes immediately after informed consent was obtained. Blinding in this trial was not possible owing to the nature of care coordination. The research assistant was blinded to group allocation for all participants for the purposes of independent collection of measures at the 1-week, 3-month, 6-month, and 12-month telephone review time points.
Interventions
Integrated Care Coordination
Participants and their families who received integrated care coordination were provided access to an AHLO who was employed by and accountable to the hospital sector. There was 1 AHLO for the 3 sites. The AHLO was a speech pathologist with clinical experience in the multidisciplinary assessment and intervention of pediatric neurodevelopmental conditions. The AHLO was responsible for communication with the children and their caregivers to ascertain their priorities in health care access across hospital, education, primary care, and community sectors for the duration of their participation in the trial. Frequency of communication varied among participants and was determined by the individual preferences of each caregiver. Examples of activities undertaken by the AHLO included coordination of multidisciplinary team meetings in the primary care and education sectors, assistance with health literacy and advocacy (eg, navigation of different funding schemes and support, service linkages, assistance with completion of forms, and provision of health literacy brokerage across sectors and internal to the hospital), and coordination of health care appointment scheduling.
Combined primary care reviews with the AHLO, general practitioner, and participants were offered at standard time points of 1 week, 3 months, and 6 months after diagnosis. Regular reviews with the primary care general practitioner were conducted to facilitate long-term relationships for participants in the primary care sector and allow for appropriate initiation and monitoring of individualized health management plans. Further details are given in the trial protocol (Supplement 1).39
Standard Care
Participants and their families who received standard care were not provided access to an AHLO. Caregivers independently sought assistance with management of their child’s chronic condition using standard systems and resources across the primary care, education, community, and hospital sectors. The frequency of reviews with a primary care physician was individually determined by each caregiver with no standardized time points in the participant’s health care experience.
Outcome Measures
Primary outcomes were overall pediatric and family QOL, measured by the Pediatric Quality of Life Inventory (PedsQL) Generic Core Scales, version 4.0,41 and the PedsQL Family Impact Module, version 2.0, respectively.42 Secondary outcomes included child behaviors, measured by the Child Behavior Checklist43; caregiver levels of stress, measured by the Subjective Units of Distress Scale (SUDS)44; and the caregiver’s locus of control, measured by the Rotter Locus of Control Scale.45 Secondary outcome measures included the number of federally funded primary care appointments, specialist appointments, hospital admissions, and days absent from school. These measures were extracted from administrative databases encompassing the national health services Medicare Benefits Schedule, state education system (Education Queensland), and state health systems (Queensland Health, Hospital-Based Corporate Information Systems). Primary caregiver unwell days were also recorded based on caregiver report at 1 week, 3 months, 6 months, and 12 months after diagnosis. The cost of the care coordination intervention was estimated as the AHLO’s time spent on care coordination activities based on a detailed activity log that was prospectively completed by the AHLO throughout the trial period. A within-trial cost-effectiveness analysis was planned, and these results are planned to be reported in a subsequent article.
Statistical Analysis
Assuming 5% significance, 80% power, and 50% attrition, we determined that a total sample size of 112 was needed to detect a mean (SD) difference of 15 (20) units between the change in primary outcome measures before and after the intervention in the integrated care coordination group compared with the standard care group (ie, a difference-in-differences estimate). An unplanned blinded preliminary analysis was completed at the original study closure date of April 2019 to help support further grant funding to continue recruitment given slower than anticipated recruitment rates after the original planned data collection period. Further funding was received for the trial to extend recruitment for another 18 months, including the addition of a tertiary center. The trial ceased in October 2020, as planned. To assess the missingness mechanism, the Little test for data missing completely at random was used. Missing completely at random means that missingness does not depend on the values of the variables in the data set subject to analysis. Because the P value for the Little test was not significant at the 1-sided level of <.05, the null hypothesis that missingness was missing completely at random was not rejected.46
All statistical analyses were conducted with Stata, version 16.0 (StataCorp LLC). Frequency counts and percentages were used to describe study data for categorical variables, and means and SDs were used to describe continuous data. All outcome analyses used an intent-to-treat approach in which available follow-up data from participants were included in the analyses. As the missingness mechanism, missing completely at random was not rejected by the Little test; generalized estimating equations with a gaussian family, an identity link, and an unstructured working correlation matrix were used to quantify the intervention effect attributable to the correlational nature of within-patient variability. A sensitivity analysis was also undertaken to compare results from this approach with those of a mixed effects modeling approach (eTable 2 in Supplement 2) that assumed that the missing mechanism was missing at random. In addition to the intervention effect, each model included an interaction effect with time (baseline, 6 months, and 12 months). To control for confounding and ensure all models were parsimonious, covariates were retained in the modeling if they were found to produce a univariable 2-sided P value <.05. The presence of multicollinearity and model goodness of fit were assessed by the variance inflation factor and quantile-quantile plots, respectively. For each model, unadjusted and adjusted regression coefficients with 95% CIs were calculated. Postestimation analyses were performed using the margins and marginsplot commands in Stata to produce unadjusted and adjusted predictive means. Statistical significance for all 2-sided tests was set at P < .05. The number of missed school days and caregiver days spent feeling unwell was analyzed using bootstrapping with 1000 replications. Data were censored at the last contact date to reflect the time point at which data collection occurred, with monthly estimates derived and then standardized to reflect a 12-month period. Bootstrapped means were reported along with 95% CIs calculated using the percentile method.
Results
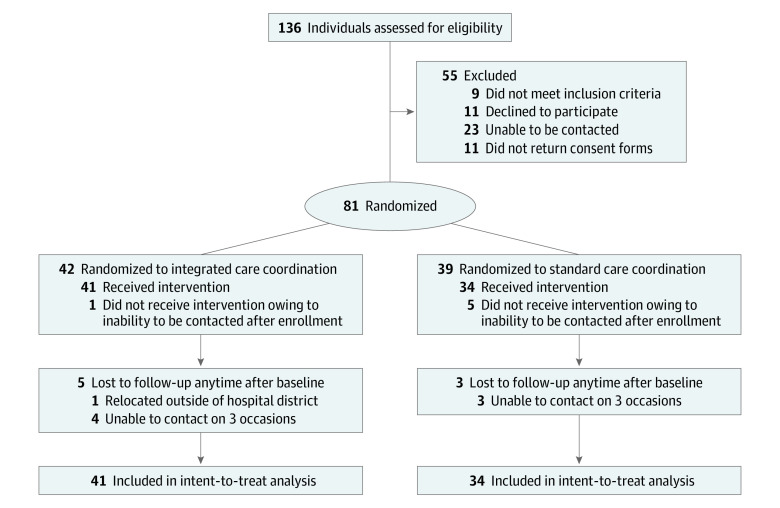
A total of 137 children were screened for eligibility, and 81 children were randomized between October 2017 and October 2020 (Figure): 42 (51.9%) to receive integrated coordination care and 39 (48.1%) to receive standard care. Attrition rates were less than 15% in both groups. The mean (SD) age of the 81 participants was 8.2 (3.5) years, and 55 (67.9%) were male.
Figure. CONSORT Participant Flow Diagram.
Of the 81 children, 47 (58.0%) had a new diagnosis of ADHD and 34 (44.4%) had at least 1 additional coexisting chronic health condition. Baseline characteristics were similar between the 2 groups (Table 1). Of the 81 children randomized, 57 (70.4%) provided data at all 3 time points, 13 (16.0%) provided data at the first 2 time points, and 5 (6.2%) provided data only at baseline. All of these children were included in the data analyses. However, 6 children provided no data after enrollment; therefore, the sample included in the data analysis was reduced from 81 to 75.
Table 1. Baseline Characteristics of Children and Their Caregivers Enrolled in the Trial by Treatment Group.
| Characteristic | Children and caregivers, No./total No. (%) | |
|---|---|---|
| Care coordination (n = 42) | Standard care (n = 39) | |
| Children | ||
| Age, mean (SD), y | 8.6 (3.6) | 7.8 (3.4) |
| Sex | ||
| Female | 14/42 (33.3) | 12/39 (30.8) |
| Male | 28/42 (66.7) | 27/39 (69.2) |
| New diagnosisa | ||
| Attention-deficit/hyperactivity disorder | 24/42 (57.1) | 23/39 (59.0) |
| Autism spectrum disorder | 9/42 (21.4) | 11/39 (28.2) |
| Otherb | 9/42 (21.4) | 5/39 (12.8) |
| School levela | ||
| Child care | 9/39 (23.1) | 14/35 (40.0) |
| Primary | 21/39 (53.9) | 16/35 (45.7) |
| Secondary | 9/39 (23.1) | 5/35 (14.3) |
| Caregivers | ||
| Highest educational level | ||
| Primary | 9/42 (21.4) | 3/34 (8.8) |
| Secondary | 16/42 (38.1) | 12/34 (35.3) |
| Technical and further education | 16/42 (38.1) | 15/34 (44.1) |
| Tertiary | 1/42 (2.4) | 4/34 (11.8) |
| Employment status | ||
| Employed | 19/42 (45.2) | 21/34 (61.8) |
| Not employed | 23/42 (54.8) | 13/34 (38.2) |
| Self-identified mental health condition | ||
| No | 19/42 (45.2) | 17/34 (50.0) |
| Yes | 23/42 (54.8) | 17/34 (50.0) |
| Sitea | ||
| Caboolture Hospital | 25/42 (59.5) | 21/39 (53.8) |
| Gold Coast University Hospital | 12/42 (28.6) | 13/39 (33.3) |
| Queensland Children’s Hospital | 5/42 (11.9) | 5/39 (12.8) |
Percentages may not sum to 100% owing to rounding.
Other diagnoses included global developmental delay, oppositional defiant disorder, adjustment disorder, and generalized anxiety disorder.
Protocol Adherence
Within the care coordination group, 41 children (97.6%) attended an appointment with the AHLO and their general practitioner 1 week after diagnosis per the trial protocol. After caregiver agreement for the necessity of care management, 11 children (26.2%) and 9 children (21.4%) attended organized appointments with their community general practitioner at 3 and 6 months after diagnosis, respectively. Advocacy for referral acceptance by community agencies and ongoing management was the most common family-initiated request for assistance at 3 months (26 children [61.9%]) and 6 months (18 children [42.9%]) after diagnosis. Assistance with coordination of medical and allied health appointments across hospital and community facilities was the most common family-initiated request at 12 months after diagnosis (19 children [46.3%]).
Primary Outcome
At 6 months after diagnosis, there were no significant intergroup differences in improvement of any of the QOL scores (Table 2). However, at 12 months, indicative of a temporal and beneficial influence on QOL scores, all differences between groups increased. Specifically, compared with the standard care group, the care coordination group had a higher mean total PedsQL score (65.36 [95% CI, 60.27-70.46] vs 61.57 [95% CI, 57.39-65.74]; difference: 7.10 [95% CI, 0.44-13.76]; P = .04), total PedsQL Family Impact Module score (57.99 [95% CI, 53.26-62.72] vs 49.93 [95% CI, 43.42-56.45]; difference: 8.62 [95% CI, 1.07-16.16]; P = .03), and family functioning subscale score (60.45 [95% CI, 55.16-65.17] vs 45.43 [95% CI, 36.88-53.98]; difference: 15.83; 95% CI, 5.05-26.62; P < .01) at 12 months. No significant intergroup differences were found for the physical functioning subscale (difference, 5.83; 95% CI, –4.04 to 15.69; P = .25), psychosocial subscale (difference, 5.25; 95% CI, –1.59 to 12.09; P = .13), and caregiver subscale (difference, 5.88; 95% CI, –2.90 to 14.66; P = .19) (Table 2). Adjusted score estimates were found to be comparable with unadjusted score estimates (eTable 1 in Supplement 2), thus indicating minimal confounding by model covariates.
Table 2. Comparison of Adjusted Predicted Mean Quality of Life Scores Between the Care Coordination and Standard Care Groups.
| Variable | Score, mean (SD) | Difference in change scores between groups, mean (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Care coordination | Standard care | |||||||||
| Baseline | 6 mo | 12 mo | Baseline | 6 mo | 12 mo | 6 mo vs baseline | P value | 12 mo vs baseline | P value | |
| PedsQL | ||||||||||
| Total scorea | 50.46 (15.02) | 62.49 (17.64) | 65.36 (16.32) | 53.77 (18.80) | 60.19 (15.02) | 61.57 (12.20) | 5.60 (−0.29 to 11.50) | .06 | 7.10 (0.44 to 13.76) | .04 |
| Physical functioning subscale scoreb | 60.45 (21.62) | 73.48 (22.61) | 78.20 (21.25) | 65.62 (22.98) | 73.60 (16.98) | 77.55 (15.68) | 5.06 (−3.40 to 13.52) | .24 | 5.83 (−4.04 to 15.69) | .25 |
| Psychosocial subscale scorec | 45.08 (15.81) | 56.29 (18.46) | 57.06 (16.01) | 45.56 (17.63) | 52.83 (18.01) | 52.29 (13.45) | 3.94 (−3.28 to 11.10) | .28 | 5.25 (−1.59 to 12.09) | .13 |
| PedsQL Family Impact Module | ||||||||||
| Total scored | 45.52 (17.06) | 54.49 (20.57) | 57.99 (17.07) | 46.07 (23.58) | 48.00 (19.27) | 49.93 (21.03) | 7.04 (−0.61 to 14.68) | .07 | 8.62 (1.07 to 16.16) | .03 |
| Caregiver subscale scoree | 48.58 (17.83) | 57.34 (20.86) | 60.96 (18.60) | 46.15 (25.37) | 49.28 (21.21) | 52.65 (22.28) | 5.62 (−2.61 to 13.85) | .18 | 5.88 (−2.90 to 14.66) | .19 |
| Family functioning subscale scoref | 43.80 (23.57) | 55.77 (24.24) | 60.45 (18.34) | 44.61 (26.11) | 47.48 (19.75) | 45.43 (25.24) | 9.10 (−1.37 to 19.57) | .09 | 15.83 (5.05 to 26.62) | .004 |
Abbreviation: PedsQL, Pediatric Quality of Life Inventory.
Adjusted for the mental health status of the caregiver.
Adjusted for the caregiver’s relationship to the child.
Adjusted for the mental health status of the caregiver.
Adjusted for the caregiver’s relationship to the child and the mental health status of the caregiver.
Adjusted for the caregiver’s relationship to the child, the educational status of the caregiver, the employment status of the caregiver, and the mental health status of the caregiver.
Adjusted for the caregiver’s relationship to the child and the mental health status of the caregiver.
Secondary Outcomes
There were no significant differences between groups in caregiver locus of control, caregiver distress, and child behaviors at the 6- and 12-month time points. Children in the care coordination group experienced fewer missed school days (mean, −15.98 days; 95% CI, −47.92 to 3.92 days) during a 12-month period, and their primary caregivers had more days spent feeling unwell (mean, 3.24 days; 95% CI, −14.44 to 20.14 days) compared with children and caregivers in the standard care group (Table 3). The cost of AHLO time in delivering the intervention was estimated to be $AU 715 (95% CI, $AU 629 to $AU 790) (1 AUD = 0.74 USD [2021]) per participant in the care coordination group, based on a mean of 10.0 hours spent on care coordination activities per participant over 12 months.
Table 3. Summary of Bootstrapped Means and 95% CIs for Missed School Days and Caregiver Sick Days During 12 Months.
| Outcome | Time, mean (95% CI), d | ||
|---|---|---|---|
| Standard care | Care coordination | Difference | |
| Missed school days owing to illness | 23.98 (5.30 to 55.18) | 8.00 (4.30 to 12.29) | −15.98 (−47.92 to 3.92) |
| Caregiver days spent feeling unwell | 22.73 (11.80 to 35.93) | 25.97 (15.35 to 37.94) | 3.24 (−14.44 to 20.14) |
Discussion
To our knowledge, this is the first multicenter randomized clinical trial of care coordination designed to measure QOL improvements in children with non-CMCs. Care coordination significantly improved some but not all primary outcome measures at 12 months. There were significant improvements in overall child QOL, overall family QOL, and family functioning QOL for children and their families in the care coordination group compared with those in the standard care group. However, there were no significant intergroup differences for several subscale scores at 12 months and for all outcomes at 6 months. Likewise, there were no significant differences between the groups for all the secondary outcomes.
In this trial, both groups had improved overall child QOL scores at 12 months after diagnosis when interpreted using a minimal clinically meaningful difference cutoff of 4.5.41 However, the greatest improvements were seen in the care coordination group, in which the mean overall child QOL score (65.36; 95% CI, 60.27-70.46) was similar to the mean (SD) score of 82.29 (15.55) seen in healthy children in previous studies.41 The baseline overall family QOL and family functioning scores in the trial cohort were similar to those for families of children with CMCs in one study42 but lower than those for families of healthy children in another study.47 Only children and families who received care coordination demonstrated improvements that approached similar values seen within normative samples.41 These findings show that the benefits of care coordination for children with non-CMCs may be more pronounced than the benefits seen for children with CMCs. Studies32,36 have demonstrated that care coordination for children with CMCs does not alter QOL outcomes. In contrast, our study found that care coordination improved QOL for children with non-CMCs. The difference in findings is likely attributable to the inclusion of 2 different clinical populations whose access to health care resources may have differed in management intensity based on medical condition complexity.
The improvements in QOL outcomes based on care coordination by an AHLO in this study were comparable with those found in other studies of children with non-CMCs such as ADHD and ASD.26,27 A recent cluster randomized clinical trial in children with comorbid ADHD reported improvements in symptom reduction and QOL at 12 months when care managers and primary care physicians worked collaboratively.26 Similarly, Parker and colleagues27 described a pediatric ASD case study that emphasized the importance of the development of patient- and family-centered goals and the use of existing health, community, and educational resources to improve outcomes for children with ASD. The results of those studies, in addition to those in our trial, provide empirical evidence that (1) supports the important role that care coordination has in the effective management of non-CMCs and (2) compliments the literature that highlights caregiver perspectives on the lack of communication between health care professionals and the lack of knowledge about how to access available intervention programs for children with ADHD and/or ASD.48,49
Organizational health literacy skills are required to navigate health systems across the care continuum for successful maternal and child health outcomes.50 The improved QOL outcomes for children and their caregivers found in this study may be explained by the health literacy–informed communication strategies completed by an AHLO that support organizational and parental health literacy across sectors.51 Communication that may have contributed to organization health literacy included the tracking and reporting of communication failures, obtaining and incorporating feedback on health information from all stakeholders involved in care, and assistance in finding the right allied health and community support services.52 Similarly, communication that may have assisted caregiver health literacy included assistance in explaining the condition and helping caregivers understand risks and choices in available treatments and services as well as coordination of medical appointments. Given that reduced health literacy among parents is likely associated with unmet health care needs and reduced adherence to health management plans,53,54 more targeted interventions toward health literacy for non-CMCs that are formally measured in future studies are needed.55
Limitations
This study has limitations. Children and their caregivers were not blinded to group allocation, and this may have influenced their responses. Instead, the research assistant who completed the relevant questionnaires at each time point and the data analysts were blinded to group allocation until the completion of the trial. Preliminary analyses were not originally planned but were completed to help support further grant funding to continue recruitment given slower than anticipated recruitment rates at the original study closure date of April 2019. It is likely that a type II error contributed to the nonsignificant data found for some outcomes given that the planned sample size was not reached. Nevertheless, because the current trial reached its planned study closure date and stopped, the results were unlikely to be biased.56 In addition, generalizability of the use of an AHLO is likely limited to general pediatric outpatient clinics within the public health care system in Australia. Translational research studies of interventions implementing the types of activities performed by the AHLO are required to determine the effectiveness of care coordination in other public and private health care settings. In addition, because 5 children in the standard care group did not receive the intervention compared with only 1 child in the integrated coordination care group, the results may be biased owing to differential attrition. However, because an intention-to-treat analysis was conducted and attrition rates were less than 15% in both groups, it is unlikely that the internal validity of the study was substantially weakened.57
Conclusions
This randomized clinical trial found that care coordination helped to improve some but not all QOL scores for children with non-CMCs. Owing to the heterogeneity of conditions considered to be non-CMCs, further studies are required that focus on specific conditions to better understand which children with non-CMCs would benefit most from care coordination. Larger studies and a health economic evaluation of care coordination are needed to consolidate findings and determine whether a cost-effective implementation across general pediatric clinics can be sustained.
Trial Protocol
eTable 1. Comparison of Unadjusted Predicted Mean Quality-of-Life Scores Between Care Coordination and Standard Care Groups
eTable 2. Sensitivity Analysis
Data Sharing Statement
References
- 1.Parner ET, Thorsen P, Dixon G, et al. A comparison of autism prevalence trends in Denmark and Western Australia. J Autism Dev Disord. 2011;41(12):1601-1608. doi: 10.1007/s10803-011-1186-0 [DOI] [PubMed] [Google Scholar]
- 2.Perrin JM, Anderson LE, Van Cleave J. The rise in chronic conditions among infants, children, and youth can be met with continued health system innovations. Health Aff (Millwood). 2014;33(12):2099-2105. doi: 10.1377/hlthaff.2014.0832 [DOI] [PubMed] [Google Scholar]
- 3.Van Cleave J, Gortmaker SL, Perrin JM. Dynamics of obesity and chronic health conditions among children and youth. JAMA. 2010;303(7):623-630. doi: 10.1001/jama.2010.104 [DOI] [PubMed] [Google Scholar]
- 4.Wolfe I, Thompson M, Gill P, et al. Health services for children in western Europe. Lancet. 2013;381(9873):1224-1234. doi: 10.1016/S0140-6736(12)62085-6 [DOI] [PubMed] [Google Scholar]
- 5.McPherson M, Arango P, Fox H, et al. A new definition of children with special health care needs. Pediatrics. 1998;102(1 Pt 1):137-140. doi: 10.1542/peds.102.1.137 [DOI] [PubMed] [Google Scholar]
- 6.Bethell CD, Newacheck PW, Fine A, et al. Optimizing health and health care systems for children with special health care needs using the life course perspective. Matern Child Health J. 2014;18(2):467-477. doi: 10.1007/s10995-013-1371-1 [DOI] [PubMed] [Google Scholar]
- 7.Cohen E, Berry JG, Sanders L, Schor EL, Wise PH. Status complexicus? The emergence of pediatric complex care. Pediatrics. 2018;141(suppl 3):S202-S211. doi: 10.1542/peds.2017-1284E [DOI] [PubMed] [Google Scholar]
- 8.Raphael JL, Rueda A, Lion KC, Giordano TP. The role of lay health workers in pediatric chronic disease: a systematic review. Acad Pediatr. 2013;13(5):408-420. doi: 10.1016/j.acap.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig BM, Hartman JD, Owens MA, Brown DS. Prevalence and losses in quality-adjusted life years of child health conditions: a burden of disease analysis. Matern Child Health J. 2016;20(4):862-869. doi: 10.1007/s10995-015-1874-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culpepper L, Fried R. Attention-deficit/hyperactivity disorder in a chronic care paradigm. Postgrad Med. 2013;125(4):78-86. doi: 10.3810/pgm.2013.07.2680 [DOI] [PubMed] [Google Scholar]
- 11.Kent AL. Developmental origins of health and adult disease: what should neonatologists/paediatricians be considering about the long-term health of their patients? J Paediatr Child Health. 2012;48(9):730-734. doi: 10.1111/j.1440-1754.2012.02541.x [DOI] [PubMed] [Google Scholar]
- 12.Das D, Cherbuin N, Butterworth P, Anstey KJ, Easteal S. A population-based study of attention deficit/hyperactivity disorder symptoms and associated impairment in middle-aged adults. PLoS One. 2012;7(2):e31500-e31500. doi: 10.1371/journal.pone.0031500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenstein P, Halldner L, Zetterqvist J, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367(21):2006-2014. doi: 10.1056/NEJMoa1203241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins J, Agrawal R. Protecting rights of children with medical complexity in an era of spending reduction. Pediatrics. 2018;141(suppl 3):S242-S249. doi: 10.1542/peds.2017-1284I [DOI] [PubMed] [Google Scholar]
- 15.Schindler CA, Pordes ES, Finkenbinder SD, Lee KJ. Safety in children with medical complexity: our canaries in the coal mine? Curr Treat Options Pediatr. 2019;5(2):165-182. doi: 10.1007/s40746-019-00159-2 [DOI] [Google Scholar]
- 16.Kuo DZ, Cohen E, Agrawal R, Berry JG, Casey PH. A national profile of caregiver challenges among more medically complex children with special health care needs. Arch Pediatr Adolesc Med. 2011;165(11):1020-1026. doi: 10.1001/archpediatrics.2011.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohn LN, Pechlivanoglou P, Lee Y, et al. Health outcomes of parents of children with chronic illness: a systematic review and meta-analysis. J Pediatr. 2020;218:166-177.e2. doi: 10.1016/j.jpeds.2019.10.068 [DOI] [PubMed] [Google Scholar]
- 18.Hatzmann J, Heymans HSA, Ferrer-i-Carbonell A, van Praag BMS, Grootenhuis MA. Hidden consequences of success in pediatrics: parental health-related quality of life—results from the Care Project. Pediatrics. 2008;122(5):e1030-e1038. doi: 10.1542/peds.2008-0582 [DOI] [PubMed] [Google Scholar]
- 19.Pickles DM, Lihn SL, Boat TF, Lannon C. A roadmap to emotional health for children and families with chronic pediatric conditions. Pediatrics. 2020;145(2):e20191324. doi: 10.1542/peds.2019-1324 [DOI] [PubMed] [Google Scholar]
- 20.Council on Children with Disabilities and Medical Home Implementation Project Advisory Committee . Patient- and family-centered care coordination: a framework for integrating care for children and youth across multiple systems. Pediatrics. 2014;133(5):e1451-e1460. doi: 10.1542/peds.2014-0318 [DOI] [PubMed] [Google Scholar]
- 21.Dewan T, Cohen E. Children with medical complexity in Canada. Paediatr Child Health. 2013;18(10):518-522. doi: 10.1093/pch/18.10.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zurynski Y, Altman L, Breen C, Woolfenden S. Care coordination for children with chronic and complex conditions in Australia: significant benefits for patients and their families. Int J Integr Care. 2018;18(s1):113. doi: 10.5334/ijic.s1113 [DOI] [Google Scholar]
- 23.Janamian T, Jackson CL, Glasson N, Nicholson C. A systematic review of the challenges to implementation of the patient-centred medical home: lessons for Australia. Med J Aust. 2014;201(3)(suppl):S69-S73. doi: 10.5694/mja14.00295 [DOI] [PubMed] [Google Scholar]
- 24.Britto MT, Vockell AL, Munafo JK, et al. Improving outcomes for underserved adolescents with asthma. Pediatrics. 2014;133(2):e418-e427. doi: 10.1542/peds.2013-0684 [DOI] [PubMed] [Google Scholar]
- 25.Hamburger R, Berhane Z, Gatto M, Yunghans S, Davis RK, Turchi RM. Evaluation of a statewide medical home program on children and young adults with asthma. J Asthma. 2015;52(9):940-948. doi: 10.3109/02770903.2014.999282 [DOI] [PubMed] [Google Scholar]
- 26.Kolko DJ, Hart JA, Campo J, et al. Effects of collaborative care for comorbid attention deficit hyperactivity disorder among children with behavior problems in pediatric primary care. Clin Pediatr (Phila). 2020;59(8):787-800. doi: 10.1177/0009922820920013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker ML, Diamond RM, Del Guercio AD. Care coordination of autism spectrum disorder: a solution-focused approach. Issues Ment Health Nurs. 2020;41(2):138-145. doi: 10.1080/01612840.2019.1624899 [DOI] [PubMed] [Google Scholar]
- 28.Boudreau AA, Perrin JM, Goodman E, Kurowski D, Cooley WC, Kuhlthau K. Care coordination and unmet specialty care among children with special health care needs. Pediatrics. 2014;133(6):1046-1053. doi: 10.1542/peds.2013-2174 [DOI] [PubMed] [Google Scholar]
- 29.Farmer JE, Clark MJ, Drewel EH, Swenson TM, Ge B. Consultative care coordination through the medical home for CSHCN: a randomized controlled trial. Matern Child Health J. 2011;15(7):1110-1118. doi: 10.1007/s10995-010-0658-8 [DOI] [PubMed] [Google Scholar]
- 30.Palfrey JS, Sofis LA, Davidson EJ, Liu J, Freeman L, Ganz ML; Pediatric Alliance for Coordinated Care . The Pediatric Alliance for Coordinated Care: evaluation of a medical home model. Pediatrics. 2004;113(5)(suppl):1507-1516. [PubMed] [Google Scholar]
- 31.Turchi RM, Berhane Z, Bethell C, Pomponio A, Antonelli R, Minkovitz CS. Care coordination for CSHCN: associations with family-provider relations and family/child outcomes. Pediatrics. 2009;124(suppl 4):S428-S434. doi: 10.1542/peds.2009-1255O [DOI] [PubMed] [Google Scholar]
- 32.Lawson KA, Bloom SR, Sadof M, Stille C, Perrin JM. Care coordination for children with special health care needs: evaluation of a state experiment. Matern Child Health J. 2011;15(7):993-1000. doi: 10.1007/s10995-010-0660-1 [DOI] [PubMed] [Google Scholar]
- 33.Looman WS, Antolick M, Cady RG, Lunos SA, Garwick AE, Finkelstein SM. Effects of a telehealth care coordination intervention on perceptions of health care by caregivers of children with medical complexity: a randomized controlled trial. J Pediatr Health Care. 2015;29(4):352-363. doi: 10.1016/j.pedhc.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trute B, Hiebert-Murphy D, Wright A. Family-centred service coordination in childhood health and disability services: the search for meaningful service outcome measures. Child Care Health Dev. 2008;34(3):367-372. doi: 10.1111/j.1365-2214.2008.00819.x [DOI] [PubMed] [Google Scholar]
- 35.Antonelli R, McAllister JW, Popp J. Making care coordination a critical component of the pediatric health system: a multidisciplinary framework. The Commonweath Fund. 2009:1277. [Google Scholar]
- 36.Looman WS, Hullsiek RL, Pryor L, Mathiason MA, Finkelstein SM. Health-related quality of life outcomes of a telehealth care coordination intervention for children with medical complexity: a randomized controlled trial. J Pediatr Health Care. 2018;32(1):63-75. doi: 10.1016/j.pedhc.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caskey R, Moran K, Touchette D, et al. Effect of comprehensive care coordination on Medicaid expenditures compared with usual care among children and youth with chronic disease: a randomized clinical trial. JAMA Netw Open. 2019;2(10):e1912604-e1912604. doi: 10.1001/jamanetworkopen.2019.12604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jessop DJ, Stein REK. Who benefits from a pediatric home care program? Pediatrics. 1991;88(3):497-505. [PubMed] [Google Scholar]
- 39.Frakking TT, Waugh J, Teoh H-J, et al. Integrated children’s clinic care (ICCC) versus a self-directed care pathway for children with a chronic health condition: a multi-centre randomised controlled trial study protocol. BMC Pediatr. 2018;18(1):72. doi: 10.1186/s12887-018-1034-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Halloran J, Miller GC, Britt H. Defining chronic conditions for primary care with ICPC-2. Fam Pract. 2004;21(4):381-386. doi: 10.1093/fampra/cmh407 [DOI] [PubMed] [Google Scholar]
- 41.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800-812. doi: 10.1097/00005650-200108000-00006 [DOI] [PubMed] [Google Scholar]
- 42.Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL Family Impact Module: preliminary reliability and validity. Health Qual Life Outcomes. 2004;2:55-55. doi: 10.1186/1477-7525-2-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles. University of Vermont; 2001. [Google Scholar]
- 44.Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80(1):1-28. doi: 10.1037/h0092976 [DOI] [PubMed] [Google Scholar]
- 45.Norman SB. Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE): Patient workbook therapist guide by Sudie E. Back, Edna B. Foa, Therese K. Killeen, Katherine L. Mills, Maree Teesson, Bonnie Dansky Cotton, Kathleen M. Carroll & Kathleen T. Brady. Oxford University Press, 2014ISBN: 978-0-19-933451-3, 192 pp. Drug Alcohol Rev. 2016;35(1):117-118. doi: 10.1111/dar.12300 [DOI] [Google Scholar]
- 46.Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83(404):1198-1202. doi: 10.1080/01621459.1988.10478722 [DOI] [Google Scholar]
- 47.Medrano GR, Berlin KS, Hobart Davies W. Utility of the PedsQL™ family impact module: assessing the psychometric properties in a community sample. Qual Life Res. 2013;22(10):2899-2907. doi: 10.1007/s11136-013-0422-9 [DOI] [PubMed] [Google Scholar]
- 48.Coussens M, Van Driessen E, De Baets S, et al. Parents’ perspectives on participation of young children with attention deficit hyperactivity disorder, developmental coordination disorder, and/or autism spectrum disorder: a systematic scoping review. Child Care Health Dev. 2020;46(2):232-243. doi: 10.1111/cch.12735 [DOI] [PubMed] [Google Scholar]
- 49.Hurt L, Langley K, North K, et al. Understanding and improving the care pathway for children with autism. Int J Health Care Qual Assur. 2019;32(1):208-223. doi: 10.1108/IJHCQA-08-2017-0153 [DOI] [PubMed] [Google Scholar]
- 50.Vamos CA, Thompson EL, Griner SB, Liggett LG, Daley EM. Applying organizational health literacy to maternal and child health. Matern Child Health J. 2019;23(5):597-602. doi: 10.1007/s10995-018-2687-7 [DOI] [PubMed] [Google Scholar]
- 51.Morrison AK, Glick A, Yin HS. Health literacy: implications for child health. Pediatr Rev. 2019;40(6):263-277. doi: 10.1542/pir.2018-0027 [DOI] [PubMed] [Google Scholar]
- 52.Eigelbach B. Ten suggested health literacy attributes of a health care organization. J Consum Health internet. 2017;21(2):201-208. doi: 10.1080/15398285.2017.1311606 [DOI] [Google Scholar]
- 53.Fong HF, Rothman EF, Garner A, et al. Association between health literacy and parental self-efficacy among parents of newborn children. J Pediatr. 2018;202:265-271.e3. doi: 10.1016/j.jpeds.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 54.Paschal AM, Mitchell QP, Wilroy JD, Hawley SR, Mitchell JB. Parent health literacy and adherence-related outcomes in children with epilepsy. Epilepsy Behav. 2016;56:73-82. doi: 10.1016/j.yebeh.2015.12.036 [DOI] [PubMed] [Google Scholar]
- 55.Keim-Malpass J, Letzkus LC, Kennedy C. Parent/caregiver health literacy among children with special health care needs: a systematic review of the literature. BMC Pediatr. 2015;15(1):92-92. doi: 10.1186/s12887-015-0412-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Psaty BM, Rennie D. Stopping medical research to save money: a broken pact with researchers and patients. JAMA. 2003;289(16):2128-2131. doi: 10.1001/jama.289.16.2128 [DOI] [PubMed] [Google Scholar]
- 57.Dumville JC, Torgerson DJ, Hewitt CE. Reporting attrition in randomised controlled trials. BMJ. 2006;332(7547):969-971. doi: 10.1136/bmj.332.7547.969 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Comparison of Unadjusted Predicted Mean Quality-of-Life Scores Between Care Coordination and Standard Care Groups
eTable 2. Sensitivity Analysis
Data Sharing Statement