Abstract
Background
A 19-year-old woman with an established diagnosis of long QT syndrome (LQTS) 2 and underlying KCNH2-mutation was referred to our centre for recurrent polymorphic ventricular tachycardia (VT) and ventricular fibrillation (VF) refractory to medical therapy and bilateral thoracic sympathectomy.
Case summary
Holter monitoring revealed a relevant premature ventricular complex (PVC) burden of two different morphologies. One PVC was originating from the left anterior fascicle, the other from the left posterior fascicle. Radiofrequency ablation resulted in complete suppression of both spontaneous PVC morphologies with a favourable clinical course over the next 2 years.
Discussion
This case presents two interesting insights: firstly, the consistent bigeminal pattern of the torsade de pointes triggering PVC. These were retrieved from the device interrogation and correlated with the pattern that was seen at the time of the procedure. Secondly, PVC morphologies suggested an origin from both the left ventricular (posterior and anterior) fascicles, which have not been described so far. This was confirmed by the preceding Purkinje potentials seen at the successful ablation sites in sinus rhythm and during PVC. Ablation of triggering PVCs causing recurrent VT/VF in LQTS 2 is feasible and effective over a mid-term follow-up.
Keywords: LQTS, PVC, Ablation, Conduction system, Case report, DAS CAM—Diploma of Advanced Studies in Cardiac Arrhythmia Management
Learning points
Premature ventricular complex (PVC) arising from the distal conduction system can trigger ventricular fibrillation in long QT syndrome (LQTS).
Ablation of triggering PVCs is feasible and effective over a mid-term follow-up in LQTS 2.
Introduction
A 19-year-old Caucasian woman with an established diagnosis of long QT syndrome (LQTS) 2 and underlying KCNH2-mutation was referred to our centre for recurrent polymorphic ventricular tachycardia (VT) and ventricular fibrillation (VF) refractory to medical therapy. Initially, presentation was in July 2015 when the patient suffered from a syncopal episode at rest with a loss of consciousness for 20 s witnessed by her mother and immediate full recovery. On the way to the cardiology clinic, the emergency doctor reported another syncope with a 3 s episode of ventricular fibrillation followed by asystole and recovery of sinus rhythm and consciousness after a few seconds. Work-up with echocardiography, cardiac magnetic resonance, and stress test were unremarkable. Electrocardiogram (ECG) revealed the suspicion of LQTS which was established thereafter by genetic testing. Bisoprolol was started at a maximum tolerated dose of 5 mg q.d. and a subcutaneous internal cardioverter-defibrillator (S-ICD) was implanted later in October 2015. After appropriate shock therapy in July 2018, medication was changed to Nadolol at a maximum tolerated dose of 40 mg q.d. and (despite being a LQTS 2) Mexiletine was added for 3 months with 150 mg b.i.d. In December 2018, she presented with repeated episodes of sustained and non-sustained polymorphic VT and VF, which were successfully terminated by appropriate shocks. An attempt to stop her electrical storms by bilateral thoracic sympathectomy in 2018 was unsuccessful, as appropriate shock therapies were frequent in the first half of 2019.
Timeline
| Day of admission | Diagnostic work-up with unremarkable electrocardiogram, echocardiography, cardiac magnetic resonance, and stress test. |
| Suspicion of long QT syndrome 2 established by genetic testing. | |
| Beta-blockers established | |
| Four months later | Subcutaneous internal cardioverter-defibrillator implantation |
| Three years later | Appropriate shock therapies for polymorphic ventricular tachycardia |
| Mexiletine added | |
| Five months later | Electrical storm |
| Bilateral thoracic sympathectomy | |
| Seven months later | Ablation therapy in July 2019 |
| Two years later | Latest follow-up in July unremarkable |
Case presentation
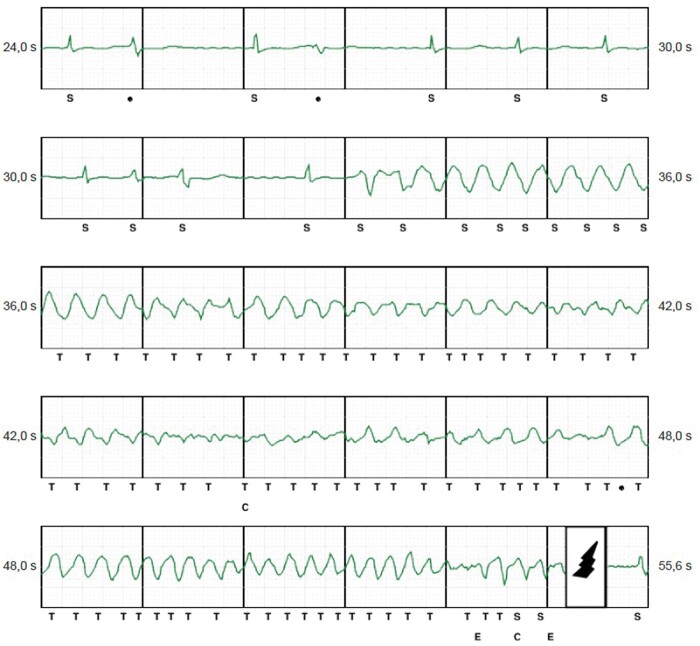
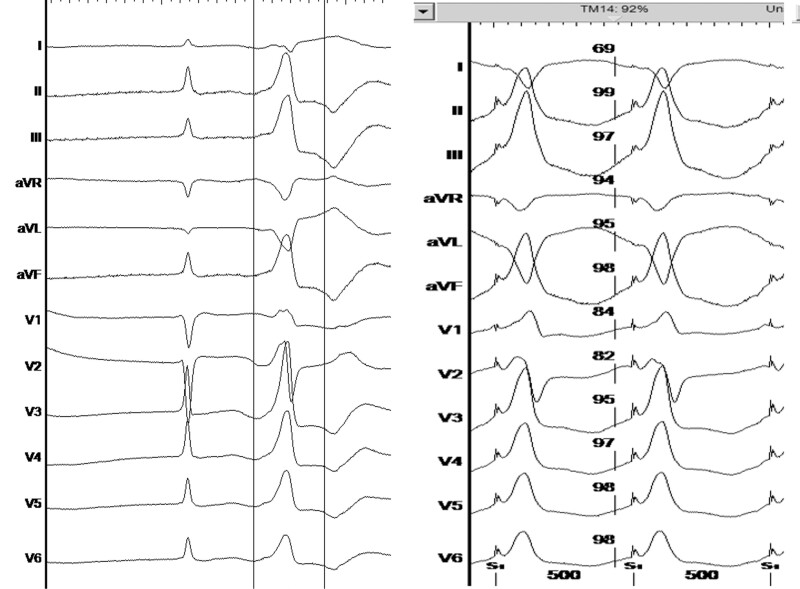
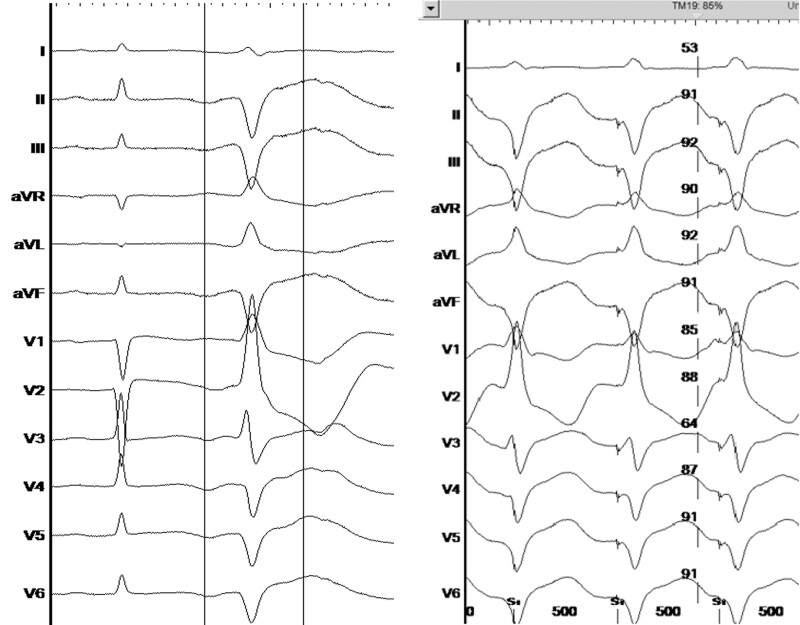
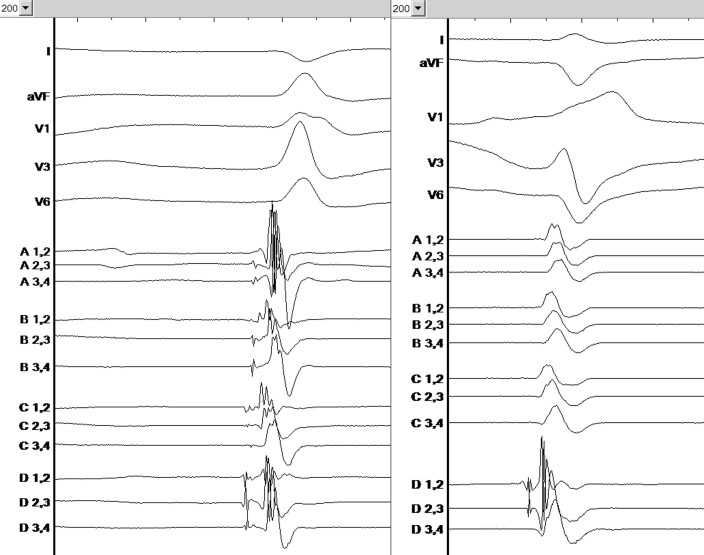
Interrogation of the ICD revealed frequent episodes of torsade de pointes like VT initiated by premature ventricular complexes (PVC, Figure 1). The ambulatory Holter monitoring revealed a PVC burden of 3657 (4%) per 24 h with two distinct dominant morphologies. One PVC had right bundle branch morphology with inferior axis suggesting that the origin was from the left anterior fascicle (Figure 2) and was the dominant morphology. The second PVC had a right bundle branch morphology with superior axis (Figure 3) suggesting an origin from the left posterior fascicle. A decision was made to perform an invasive electrophysiology study to map and ablate the triggering PVC.
Figure 1.
Subcutaneous internal cardioverter-defibrillator episode with ventricular extrasystoles triggering ventricular fibrillation.
Figure 2.
Ventricular premature beat—left anterior fascicle morphology, (left panel: spontaneous ventricular premature beat, right panel: pace matching, revealing 92% pace match in the Bard system).
Figure 3.
Ventricular premature beat—left posterior fascicle morphology, (left panel: spontaneous ventricular premature beat, right panel: pace matching, revealing 85% pace match in the Bard system).
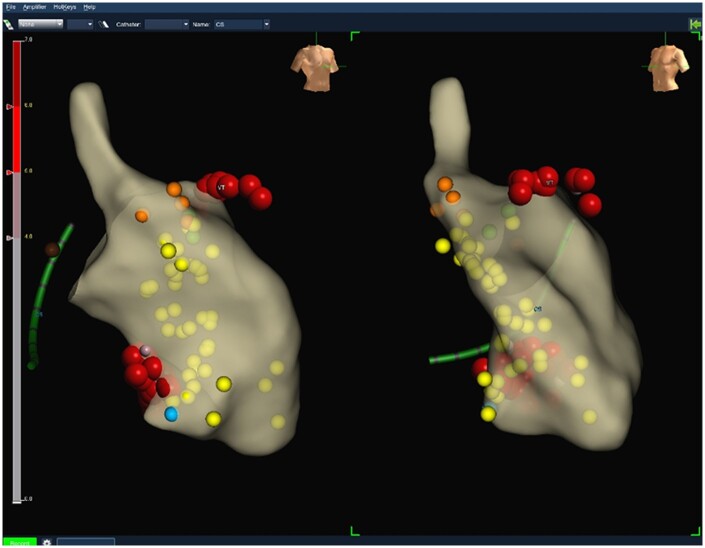
Using a 3D electro-anatomical mapping system (EnSite Precision, Abbott), the left ventricle was mapped and the Purkinje potentials of both left-sided fascicles were tagged (Figure 4). As under general anaesthesia only a few spontaneous PVC were present, pace mapping was mainly utilized to narrow down the region of interest. Best pace matches were obtained at the distal insertion of the left anterior (94%) and the left posterior (85%) fascicles (Figures 2 and 3, right panels). The earliest activation of both spontaneous PVC was identified to be at the distal portions of both left ventricular fascicles and at least 38 ms ahead of the PVC QRS-onset. Using both retrograde and transseptal approach radiofrequency energy was applied at 45–50 W at the very distal left anterior and posterior fascicular regions targeting areas with identifiable Purkinje potentials directly at local QRS-onset (Figure 5). Ablation at these sites produced runs of idioventricular rhythm with QRS morphology mimicking the PVC that initiated the torsade de pointes.
Figure 4.
Electro-anatomical map of left ventricle; orange dots—His potential, yellow dots—Purkinje potentials, red dots—ablation points, blue dot—good pace match for left posterior fascicle morphology; green dots—reasonable pace match for left anterior fascicle morphology; left side—right anterior oblique view, right side—left anterior oblique view.
Figure 5.
Purkinje potentials at ablation areas—left panel: left anterior fascicle morphology, right panel: left posterior fascicle morphology.
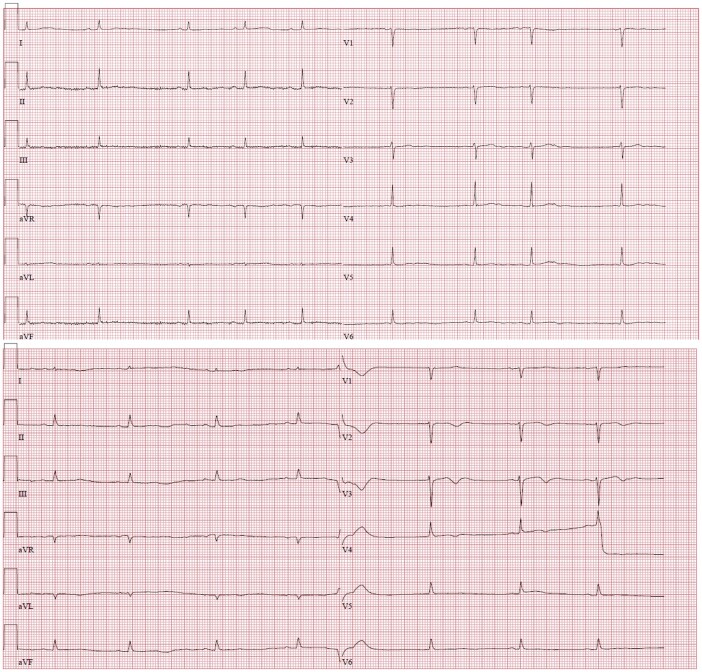
Radiofrequency ablation resulted in the complete suppression of both spontaneous PVC morphologies. The surface ECG after ablation at the distal Purkinje fibres remained rather unchanged (Figure 6).
Figure 6.
Twelve-lead electrocardiograms in 25 mm/s speed before and after ablation.
Over a follow-up period of 24 months after the procedure, the patient has fared well with a marked reduction of PVC burden from 3657 in 24 h (4%) to 66 PVCs in 24 h (0.08%) and only one single episode of VT with appropriate shock shortly after ablation.
Only one adequate S-ICD shock occurred 3 weeks after ablation. Since then, the patient remained arrhythmia free on beta-blocker therapy over a follow-up period of more than 24 months with a markedly reduced burden of PVCs.
Discussion
Ventricular fibrillation triggering PVC that falls within the vulnerable period of the QT-interval has been reported in a broad spectrum of patients including patients with LQTS.1 Catheter ablation for treatment of recurrent PVC triggered VF was first described by Haissaguerre et al.2 in 2002 in patients with structurally normal hearts. Since then the approach has been reported to be feasible in eliminating VF by targeting PVC triggers in patient with long QT and Brugada syndromes.1 There have been few reports to date on catheter ablation for VF in patients with LQTS.1,3
The case presented is a female with a mutation in the KCNH2 which is reported to be associated with an increased risk of cardiac events,4 as evidenced by her recurrent events refractory to medical treatment, sympathectomy, and requiring multiple ICD shocks. The PVC burden with two distinct morphologies provided the rationale to catheter ablation as a further treatment to ameliorate her events and symptoms.
This case presents the following interesting insights:
Firstly, the consistent pattern of the torsade de pointes triggering PVC. These were retrieved from the device interrogation and Holter ECG and correlated with the pattern that was seen at the time of the procedure. Secondly, PVC morphologies suggested an origin from both the left ventricular (posterior and anterior) fascicles, which have not been described so far. This was confirmed by the preceding Purkinje potentials seen at the successful ablation sites in sinus rhythm and during PVC.
The case presented with identifiable triggering PVC initiating torsade de pointes. Ablation strategy was to target the ablation sites with identification of Purkinje potentials, pace mapping, and activation mapping which resulted in the endpoint of the abolition of targeted PVC. Pace mapping was performed with our standard settings of 10 V and 2 ms. Thus, differences in the paced QRS morphologies and the spontaneous VPBs could also be due to not directly capturing the fascicles but the surrounding myocardial tissue.
The case highlights a unique presentation of bigeminal PVC pattern and localization to both left ventricular fascicles. The localization of the triggering PVC by multiple techniques to localize successful ablation sites has so far resulted in a good outcome. Ablation of triggering PVCs causing recurrent VT/VF in LQTS 2 is feasible and effective over a mid-term follow-up.
Lead author biography

The lead author is clinical electrophysiologist in four clinical centres in Austria, mainly delivering ablations and device implants. He mainly published in the areas of ablation therapy for atrial fibrillation and ventricular tachycardia. He is an Associate Professor at the Medical University of Innsbruck and Visiting Professor at the Medical University of Vienna.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case including images and associated text has been obtained from the patient in line with COPE guidelines.
Conflict of interest: none declared.
Funding: None declared.
Supplementary Material
DAS CAM—This case was presented as part of the Diploma of Advanced Studies in Cardiac Arrhythmia Management at Maastricht (a joint program of Maastricht University, Maastricht University Medical Center, EHRA, and the European Heart Academy of the ESC).
References
- 1. Haïssaguerre M, Extramiana F, Hocini M, Cauchemez B, Jaïs P, Cabrera JA. et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation 2003;108:925–928. [DOI] [PubMed] [Google Scholar]
- 2. Haïssaguerre M, Shoda M, Jaïs P, Nogami A, Shah DC, Kautzner J. et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation 2002;106:962–967. [DOI] [PubMed] [Google Scholar]
- 3. Haïssaguerre M, Shah DC, Jaïs P, Shoda M, Kautzner J, Arentz T. et al. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet 2002;359:677–678. [DOI] [PubMed] [Google Scholar]
- 4. Migdalovich D, Moss AJ, Lopes CM, Costa J, Ouellet G, Barsheshet A. et al. Mutation and gender-specific risk in type 2 long QT syndrome: implications for risk stratification for life-threatening cardiac events in patients with long QT syndrome. Heart Rhythm 2011;8:1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.