Abstract
Background & Aims
Tethered capsule endomicroscopy (TCE) involves swallowing small tethered pill that implements optical coherence tomography (OCT) imaging, procuring high resolution images of the whole esophagus. Here, we demonstrate and evaluate the feasibility and safety of TCE and a portable OCT imaging system in patients with Barrett’s esophagus (BE) in a multi-center (5-site) clinical study.
Methods
Untreated patients with BE as per endoscopic biopsy diagnosis were eligible to participate in the study. TCE procedures were performed in unsedated patients by either doctors or nurses. After the capsule was swallowed, the device continuously obtained 10-μm-resolution cross-sectional images as it traversed the esophagus. Following imaging, the device was withdrawn through mouth, and disinfected for subsequent reuse. BE lengths were compared to endoscopy findings when available. OCT-TCE images were compared to volumetric laser endomicroscopy (VLE) images from a patient who had undergone VLE on the same day as TCE.
Results
147 patients with BE were enrolled across all sites. 116 swallowed the capsule (79%), 95/114 (83.3%) men and 21/33 (63.6%) women (p=0.01). High-quality OCT images were obtained in 104/111 swallowers (93.7%) who completed the procedure. The average imaging duration was 5.55±1.92 minutes. A blinded comparison of maximum extent of BE measured by OCT-TCE and EGD showed a strong correlation (r=0.77–0.79). OCT-TCE images were of similar quality to those obtained by OCT-VLE.
Conclusions
The capabilities of TCE to be used across multiple sites, be administered to unsedated patients by either physicians or nurses who are not expert in OCT-TCE, and to rapidly and safely evaluate the microscopic structure of the esophagus make it an emerging tool for screening and surveillance of BE patients.
Keywords: Barrett’s esophagus, Optical coherence tomography, Tethered capsule endomicroscopy
INTRODUCTION
Barrett’s esophagus (BE) is a precursor of esophageal adenocarcinoma where normal esophageal squamous mucosa changes to metaplastic columnar mucosa in response to chronic acid reflux. Current standard of care relies on upper endoscopy with biopsy, also known as esophagogastroduodenoscopy (EGD). Since video images obtained by EGD only show the surface of the tissue at a macroscopic scale, targeting biopsies is imprecise and oftentimes diseased tissue is missed. One meta-analysis study with patients who had esophagectomies for high grade dysplasia (HGD) showed that 13 percent of the resection specimens had invasive cancer that was not detected by systematic biopsy1. Swallowable wireless video capsule endoscopy2, unsedated transnasal endoscopy3 and tethered capsule endoscopy4 do not require sedation, but like endoscopy, are limited to macroscopic visualization of the mucosal surface. Several advanced imaging techniques, including mucosal staining with vital dyes5 (chromoendoscopy), narrow band imaging6,7, magnification endoscopy8, and confocal laser endomicroscopy9 have been proposed to enhance disease detection. Although these techniques increase the contrast of mucosal features and increase diagnostic yield10, they all require sedated endoscopy, which is time consuming, expensive, and unpleasant for many patients.
Optical coherence tomography (OCT) is a cross-sectional imaging technique that uses principles of low-coherence interferometry to obtain depth-resolved, microscopic images of human tissue11. A form of OCT called volumetric laser endomicroscopy (VLE)12,13, uses a balloon probe that is inserted to the endoscope’s accessory channel. Helically scanning optics14 obtain contiguous microscopic images of a 6-cm-long segment of distal esophagus12,13. While VLE has the potential to mitigate sampling error, it is used in conjunction with sedated endoscopy.
Tethered capsule endomicroscopy (TCE), is a recently developed form of in vivo microscopy based on OCT technology15,16. Once swallowed, the TCE device obtains three-dimensional microscopic images of the superficial esophageal wall as the capsule descends the organ via gravity/peristalsis or is pulled up towards mouth using the tether. OCT-TCE does not require sedation, obtains microscopic images of the entire esophagus, and is a faster and more convenient procedure. Our group and others have successfully conducted OCT-TCE in pilot, single-center studies15–19 showing exemplary images that demonstrate the potential of this technology to improve upper GI tract diagnosis by elevating diagnostic yield, lowering costs, and bettering patient tolerance. Here, we report our experience using a next generation OCT-TCE system and device in BE patients in a multi-center clinical study (Massachusetts General Hospital, Mayo Clinic Jacksonville, Kansas City VA, Mayo Clinic Rochester and Columbia University Medical Center).
MATERIALS AND METHODS
Technology
OCT tethered capsule with a distal scanning micro-motor
The OCT-TCE optics enclosed in the capsule includes a single mode optical fiber, a glass spacer and a distal ball lens fusion spliced together, providing approximately 30μm lateral resolution. At the distal end of capsule, a right-angle prism mounted on the shaft of a micro-motor20,21 deflects the optical beam to the side of the capsule. Rotation of the shaft effectuates circumferential scanning across esophageal layers. The capsule connects to a 1-mm-diameter, 2.0-m-long, flexible tether that houses an optical fiber and electrical wires to power the micro-motor. The tether contains pad printed distance marks at 5cm intervals. The overall size of the capsule is 11mm (diameter) × 25mm (length), comparable to the size of a wireless video endoscopy capsule. A photograph of the TCE device is shown in Fig. 1A. TCE devices are multi-use; standard high-level disinfection (HLDI) is conducted between procedures.
Figure 1.
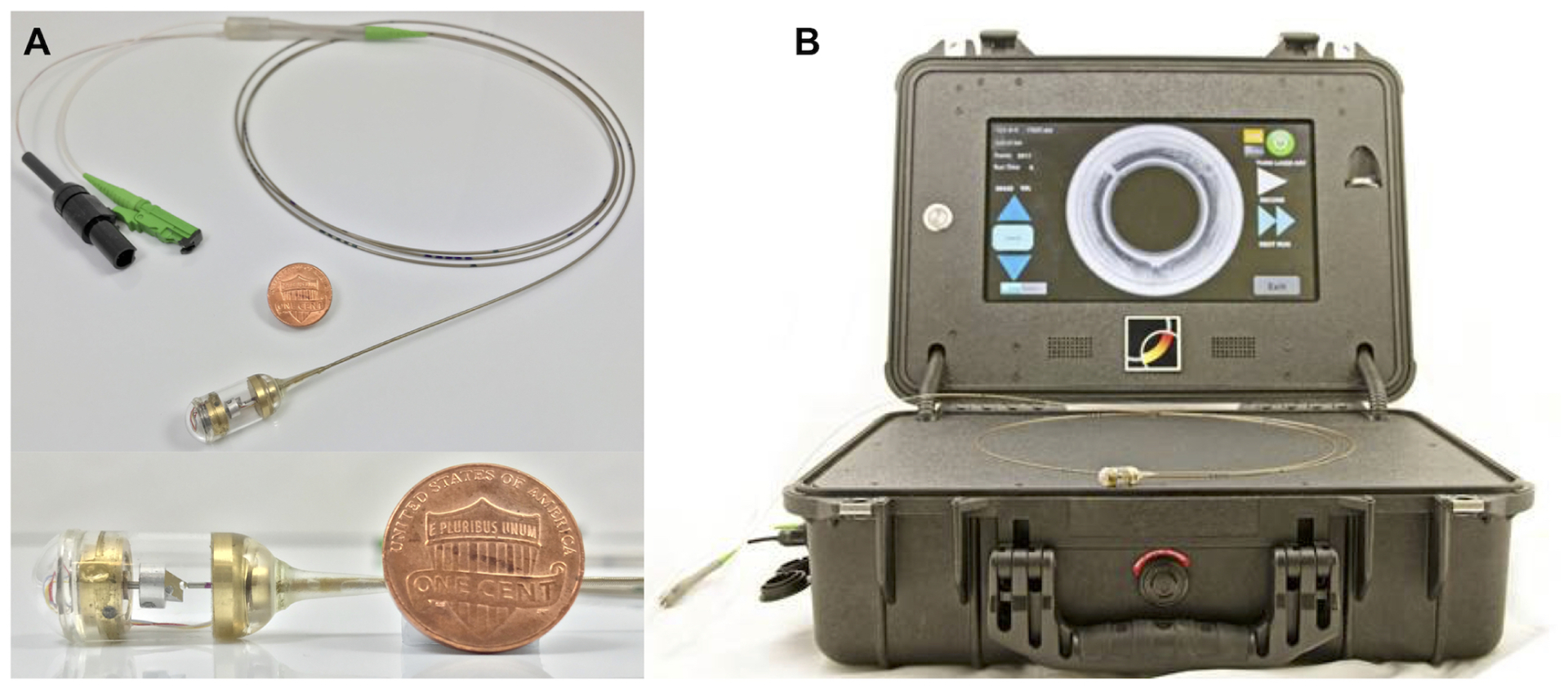
Photographs of (A) the OCT-TCE capsule with a micro-motor for distal scanning and (B) the custom-built, portable OCT imaging system.
Portable OCT imaging system
A picture of the portable OCT imaging system (size of a briefcase) is shown in Fig. 1B. Briefly, the imaging console’s laser source consisted of a commercially available swept source-based OCT imaging engine (Axsun Technologies, Inc, Billerica, MA). This OCT laser source provides an axial resolution of approximately 7μm (tissue) over a ranging depth of 5mm. The OCT engine also contains an on-board digitizers and digital signal processing electronics, which allows compressed (JPEG2000, compression ratio 7:1) OCT images to be streamed across an Ethernet bus to a mini-PC with a touchscreen monitor. The portable imaging system provides a motor control signal to the distal micro-motor of the TCE device for rotational beam scanning. Each cross-sectional OCT image consists of 2560 A-lines, acquires at a rotational rate of ~40 Hz, corresponding to a 40 frames/second (fps) cross-sectional imaging rate. The system was designed to be mass produced and easily maintained. This design allowed the imaging consoles to be rapidly manufactured, deployed to the various sites, and serviced.
Clinical study design
Patients
Patients eligible for the study had untreated, newly diagnosed BE or known BE without high grade dysplasia, intramucosal adenocarcinoma, or esophageal adenocarcinoma as per prior endoscopic biopsy diagnosis (within 9 to 15 months of baseline enrollment). Patients were over the age of 18 and capable of giving informed consent. Patients required minimal preparation, including no solid food for 4 hours prior to the procedure, and clear liquids 2 hours prior to the procedure.
Clinical procedure
All 5 sites were provided a portable OCT imaging system and at least 3 tethered capsule devices. Multiple training sessions regarding capsule and system operation, HLDI, and OCT image tissue type identification were provided to each site (See Supplementary Material and Fig. S1 for learning curves). Unsedated patients swallowed the capsule in a sitting position with an optional sip of water to facilitate swallowing the capsule. After the capsule was swallowed, patients were free to talk normally and were asked to occasionally sip water. Patients were given the option to use “Pill Glide Swallowing Spray”, a readily available over-the-counter water-based lubricating spray. When utilized, the spray was applied on the surface of capsule by capsule operator right before handing the capsule to the patient. Patients were also given the option of using an over-the-counter throat numbing spray such as “Cepacol”, “Chloraseptic” or “Topex”. When used, the spray was self-administered with the guidance of the study staff. Patients typically used the spray 5–10 minutes before the start of the procedure.
Once the capsule was swallowed, cross-sectional microscopic OCT images of the esophagus were obtained and visualized in real-time as the capsule traversed the esophagus. The capsule position was controlled manually via the tether outside of the patient’s mouth by the capsule operator. Operators were instructed to attempt to keep the tether’s translation at a constant velocity. The patient was optionally asked to take additional sips of water during the procedure to assist lower esophageal sphincter opening and capsule passage into the stomach. Typically, four passes of the esophagus were imaged, two during descent to the stomach and two pulling the tether up from the stomach towards the mouth. During each pass, similar to distance measurements that are performed during endoscopy, marks on the tether at the incisors (every 5cm) were recorded along with the real-time cross-sectional image frame number. During pull-back imaging, the TCE device was usually pulled from the gastric cardia to the 20cm tether mark at the incisors. After the imaging part was completed, the capsule operator removed the capsule from the esophagus through the mouth by gently pulling the tether. The procedural duration (the time between when the capsule was handed to the patient and the capsule was removed from patient) was recorded manually with a precision of 1 minute. The imaging duration (the time between when the capsule was swallowed and the capsule was removed from the mouth) was automatically, computer-recorded with a precision of 1 second. Following imaging, patients who swallowed the capsule successfully were given a questionnaire about the tolerability of the TCE experience and were asked if they would prefer TCE over endoscopy and whether they would recommend the procedure to other patients. If the patient was not able to swallow the capsule after five attempts, the procedure ended, and the patients were provided a separate questionnaire that focused on capsule swallowing difficulties. Capsules were then disinfected prior to reuse.
Data collection
The study was approved by the Partners IRB (IRB 16-P000919) and the IRBs of each of the sites. Each of the four esophageal (2 down and 2 up) OCT imaging sessions were recorded to a separate file. Apart from OCT images, the medical data included patient demographics, medical history, procedure details, post-procedural questionnaires, and endoscopic and pathology reports were collected and managed using REDCap electronic data capture tools hosted at MGH22.
Data analysis
OCT images were only used for research purposes. The OCT files, collected in polar coordinates (distance, angle) were scan converted to cartesian coordinates and saved as 1024×1024 pixel movies after the procedure. OCT images were displayed in inverse gray scale, where black indicated a high OCT signal (high backscattering) and white indicated a low OCT signal (low backscattering). Tether marks and corresponding TCE frame numbers were used to calculate the imaged esophageal length as well as the capsule’s velocity throughout each esophageal scan.
Two expert OCT readers (GJT, JD) evaluated the image quality of OCT-TCE images, including ensuring adequate image sensitivity, presence of cardia and esophagus in each run, sufficient tissue in the field of view, and visualization was not hindered by intraluminal contents or other artifacts caused by system or capsule malfunction. To compare the length of BE (maximum extent, Prague M measurement23,24) measured by OCT-TCE and EGD, two readers blinded to the EGD reports identified the proximal and distal margins of BE in OCT-TCE movies with their corresponding frame number. Patients data were included in the analysis if: 1) the length of BE was indicated in EGD report, 2) at least two OCT-TCE datasets included OCT images of the distal esophagus, gastroesophageal junction, and proximal stomach, and 3) The capsule positions (tether marks at the incisors) were recorded along with their corresponding OCT-TCE frame numbers. The length of BE was calculated using the presumed velocity of the capsule, estimated by time stamps of tether mark readings. OCT-TCE images were compared to VLE images from a patient who had undergone TCE and VLE procedures on the same day (See Supplementary Materials, Fig. S3).
All statistical analyses were performed with GraphPad Prism 8 for macOS version 8.3.0 (GraphPad Software, Inc, La Jolla, CA). For datasets with a normal distribution, data were expressed as the mean ± standard deviation; for datasets that were not normally distributed, data were expressed as median with interquartile range (IQR). The outliers were identified using ROUT algorithm with coefficient of 0.5% (Q=0.5%). The flagged outliers were checked practically before being eliminated for statistical analysis. The association between gender, capsule operator, capsule lubricant or throat numbing spray and capsule swallowing rate were calculated using Chi-square test. The age and BMI difference between capsule swallowers and non-swallowers was compared with unpaired T-test. The differences in swallowing rates across all sites were compared with the Fisher’s exact test. The relationship between BE length determined by EGD and OCT-TCE was calculated using linear regression. P values ≤ 0.05 were considered statistically significant. All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
To date, a total of 147 patients with BE have been successfully enrolled across all sites (MGH: 60, Mayo Jacksonville: 27, Kansas City VA: 25, Mayo Rochester: 20, Columbia University: 15). The average age was 64.8±9.3 years old (min=33, max=83), and 114 were men (77.6%). The patient characteristics is listed in Table 1.
Table 1.
Patient demographic characteristics and medical history
| Characteristics | Number of patients | Mean ± SD or % | Range |
|---|---|---|---|
| Age at enrollment (years) | 147 | 64.8 ± 9.3 | 33 – 83 |
| Gender | |||
| Female | 33 | 22.4% | |
| Male | 114 | 77.6% | |
| Height (inches) | 147 | 68.6 ± 3.5 | 60 – 76.5 |
| Weight (pounds) | 147 | 193.7 ± 37.7 | 123 – 333.3 |
| Body mass index (BMI, kg/m 2 ) | 147 | 28.9 ± 4.6 | 19.6 – 43.3 |
| Ethnicity | |||
| Hispanic | 5 | 3.4% | |
| Non-Hispanic | 142 | 96.6% | |
| Race | |||
| Asian | 1 | 0.7% | |
| African American | 1 | 0.7% | |
| Caucasian | 145 | 98.6% | |
| Smoking history | |||
| Current smoker | 10 | 6.8% | |
| Former smoker | 69 | 46.9% | |
| Never smoked | 68 | 46.3% | |
| History of GERD | |||
| Yes | 125 | 85.0% | |
| No | 22 | 15.0% | |
| Use of proton pump inhibitors (PPI) | |||
| Yes | 125 | 85.0% | |
| No | 22 | 15.0% |
A total of 116 of 147 patients (79%) successfully swallowed the capsule (MGH: 82%, Mayo Jacksonville: 78%, Kansas City VA: 76%, Mayo Rochester: 70%, Columbia University: 87%). For data pooled across sites, sex was the only variable that was associated with statistically significant differences in swallowing rates (83.3% male, 63.6% female, p=0.01). No statistically significant associations between capsule swallowing rate and operator, the usage of capsule lubricant, or the use of throat numbing were found (p=0.20, 0.29 and 0.32, respectively). The average age and BMI of capsule swallowers was 64.8±9.0 years old and 28.8±4.6 kg/m2, respectively, while for non-swallowers it was 64.8±10.3 years old and 29.2±4.7 kg/m2, respectively. There was no statistically significant age or BMI difference between capsule swallowers and non-swallowers (p=0.98 and 0.69, respectively). The average length of BE diagnosed by endoscopy was 3.52±2.56 cm based on 72 endoscopic reports of positive swallowers where the length of BE segment was indicated, including 1 patient with a BE segment length smaller than 1cm, 44 patients between 1 to 3cm, and 27 patients greater than 3cm. 46/116 (39.7%) patients were diagnosed with a hiatal hernia. The TCE procedure was completed in 111 cases. 5 cases were terminated early because of technical issues, including 3 cases of capsule malfunction, and 2 cases of a wrong system setting. High-quality OCT images were obtained in 104 of 111 (93.7%) cases among the patients who completed the procedure, which was defined as acquiring at least one complete analyzable full scan during the procedure that typically showed distal esophagus, gastroesophageal junction, and proximal stomach. OCT-TCE images were not analyzable in 7 cases, including 6 cases of insufficient length of esophagus captured and 1 case where food particles prevented clear visualization of the esophagus.
A summary of the results from procedure and post procedural questionnaires is provided in Table 2. There were no adverse events associated with TCE procedure. The results showed that the majority (85.2%) stated that they likely preferred TCE over endoscopy, and 96.6% of patients would recommend TCE if it was approved for clinical use. The reusable TCE device was used 4.2±3.8 times averaged across all participating sites. Histograms of capsule number of swallow attempts, procedural duration, imaging duration, and post procedural questionnaire results are shown in Fig. S2.
Table 2.
Summary of results of procedure and post procedural questionnaire.
| Procedural results | No. of patients | % | Comments |
|---|---|---|---|
| Registered Nurses | 72 | 49.0% | |
| Usage of capsule lubricant | 142 | 96.6% | |
| Usage of throat numbing spray | 55 | 42.0% | Of 131 patients who were provided throat numbing spray option |
| Capsule swallower | 116 | 79% | |
| Completed OCT image acquisition | 111 | 95.7% | Of 116 capsule swallowers |
| High quality analyzable OCT images | 104 | 93.7% | Of 111 completed image acquisition |
| Median | Interquartile range | Comments | |
| No. of capsule swallowing attempts | 1 | 1 – 2 | |
| Mean ± SD | Lower 95% CI of mean | Upper 95% CI of mean | |
| Procedural duration (min) | 7.98± 2.93 | 7.40 | 8.56 |
| Imaging duration (min) | 5.55 ± 1.92 | 5.17 | 5.93 |
| Length of esophagus imaged (cm) | 21.69 ± 5.90 | 21.12 | 22.27 |
| Average traverse speed (mm/s) | 4.16 ± 2.31 | 3.93 | 4.38 |
| Post procedural questionnaire | Median | Interquartile range | Scale |
| Overall level of discomfort | 2 | 1 – 4 | 0 = none 10 = severe |
| Would you prefer it over endoscopy | 1 | 1 – 2 | 1 = extremely likely 2 = somewhat likely 3 = somewhat unlikely 4 = extremely unlikely |
| Would you recommend it if it is approved for clinical usage | 1 | 1 – 1 | 1 = definitely 2 = probably 3 = probably not 4 = definitely not |
Factors that interfered with the ability to swallow the capsule was assessed for patients who could not swallow the capsule. The major factors included the capsule size (n=22) and the presence of a tether (n=19). 13 non-swallowers reported an extraordinary strong gag reflux and 11 patients stated that fear of gagging also interfered with their ability to swallow the capsule. 5 patients stated that the throat numbing was insufficient, and 1 patient stated that the lubrication of the capsule was inadequate.
After the procedures, all datasets were sent to the study coordinating center (MGH), where the quality of the OCT-TCE images were assessed by readers who were experts in OCT. Images acquired had an average of 95.5% (95% CI: 94.1–96.8%) visible esophageal mucosa in the field of view based on OCT-TCE movies from 15% randomly selected patients who swallowed the capsule. Figure 2 shows example OCT-TCE images of healthy squamous of esophagus obtained from a study patient. The full thickness esophageal architecture including the epithelium, lamina propria/muscularis mucosa (LP/MM), submucosa (SM), muscularis propria (MM), myenteric plexus (MYP) and adventitia were seen.
Figure 2.
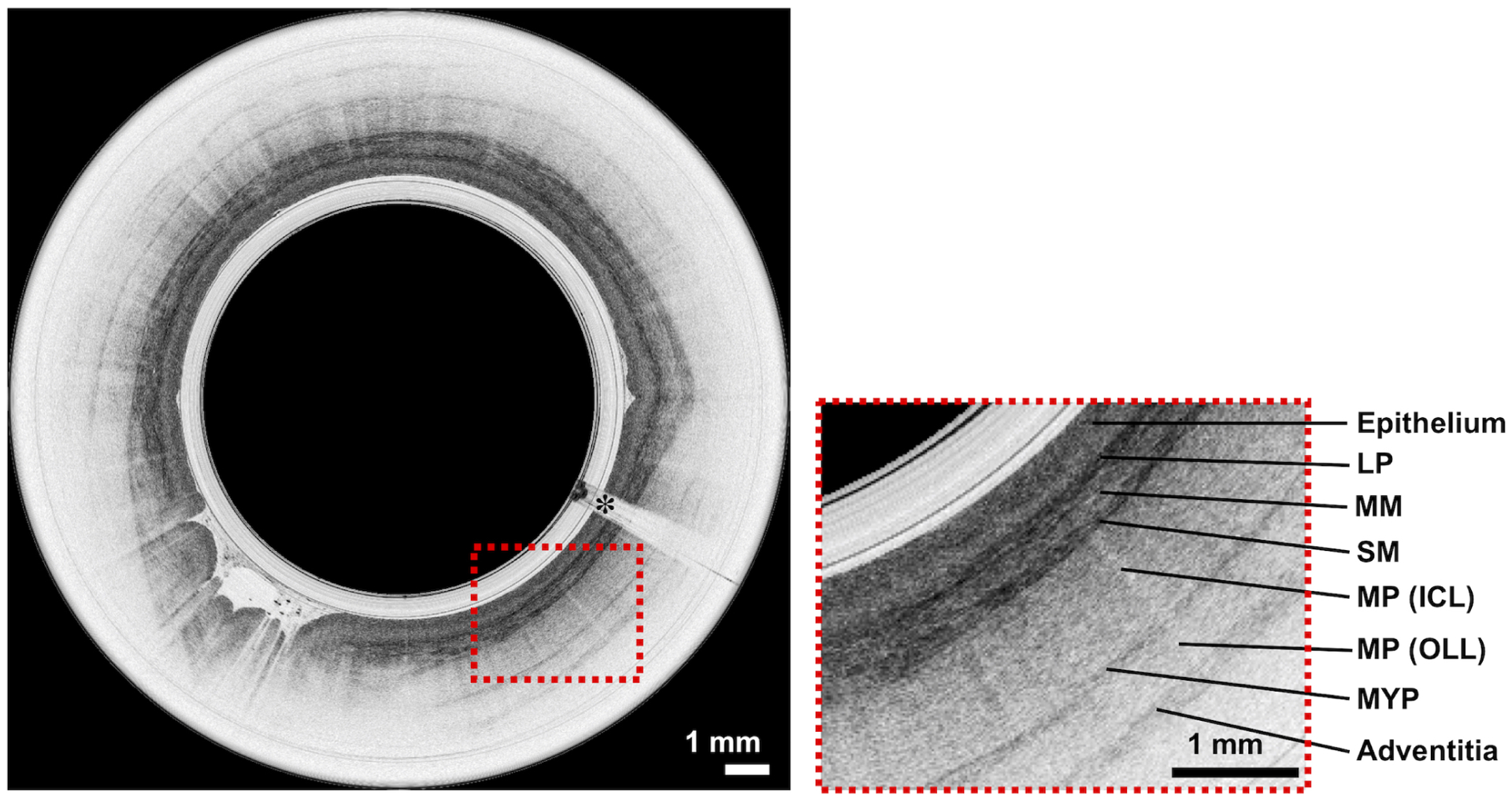
Representative surface and subsurface OCT-TCE microscopic images of esophagus obtained by TCE, showing layered tissue structure in the esophageal wall. Esophageal TCE images show epithelium, LP/MM, SM, inner circular layer of muscularis propria (MP-ICL), outer longitudinal layer of muscularis propria (MP-OLL), myenteric plexus (MYP) and adventitia. Scale bars, 1 mm. The asterisks indicate the image shadows caused by electrical wires that power up the distal micro-motor.
Representative OCT-TCE images of BE without dysplasia are shown in Fig. 3(A,B). Images of non-dysplastic BE were devoid of squamous layering or pit and crypt architecture, had an irregular mucosal surface, and heterogeneous backscattering, consistent with previously published BE diagnostic criteria25,26. Figure 3(C,D) depicts OCT-TCE images of dysplastic BE; dysplasia was confirmed by histology of biopsies obtained in their subsequent EGD procedures. OCT-TCE images of dysplastic BE showed glandular atypia (Fig. 3C), lack of epithelial surface maturation (Fig. 3D), also consistent with previously validated OCT criteria for dysplastic BE27.
Figure 3.
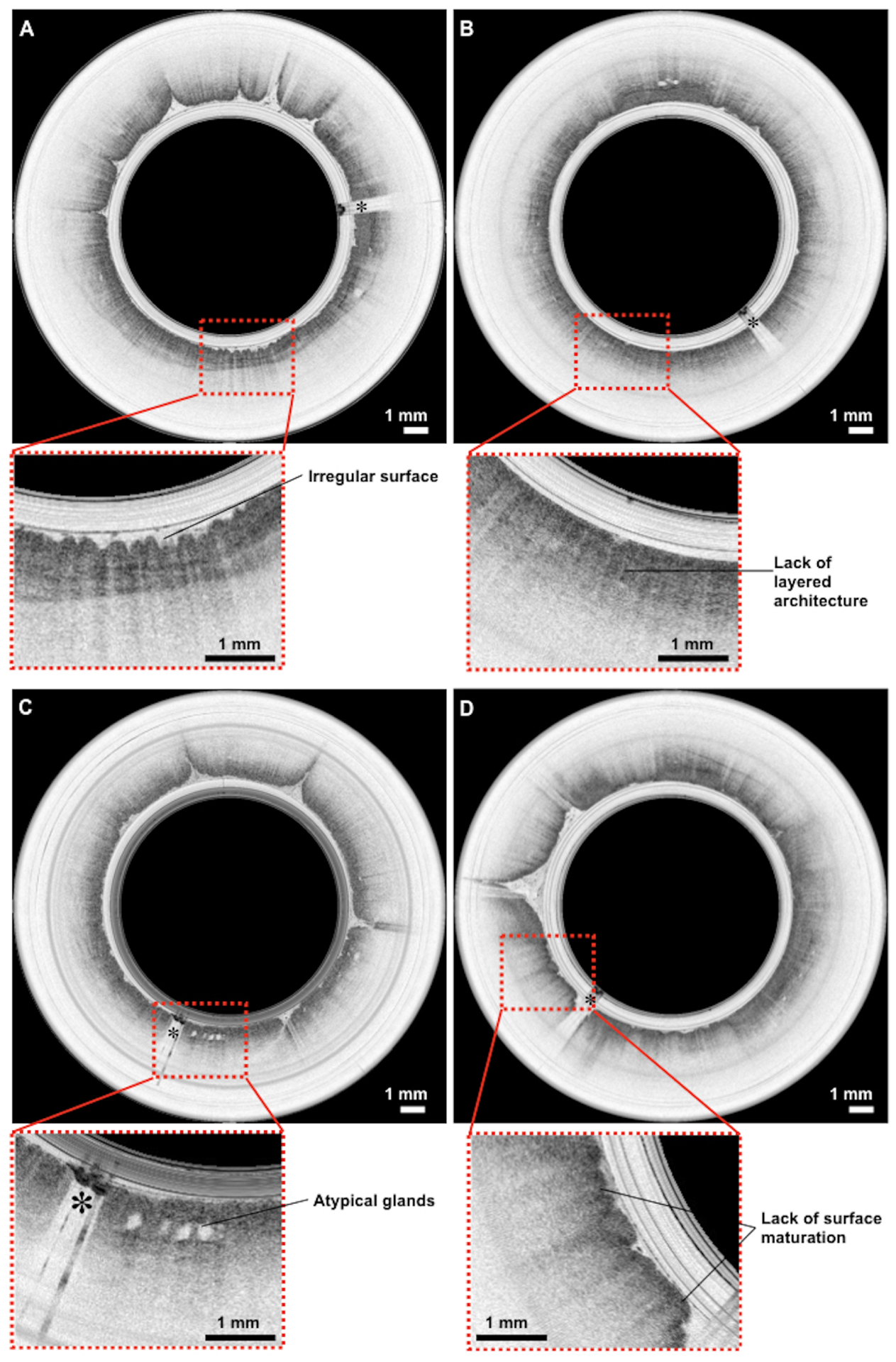
Representative OCT-TCE images of biopsy proven BE with and without dysplasia from different patients. OCT-TCE features consistent with non-dysplastic BE included an irregular surface (A) and a lack of layered squamous architecture or pit and crypt pattern, and heterogeneous scattering (B)25,26. For dysplastic BE, OCT-TCE features were consistent with previously published criteria27, including glandular architectural atypia (C) and lack of epithelial surface maturation (D), manifested as high superficial backscattering. Scale bar, 1 mm. The asterisks indicate the image shadows caused by electrical wires that power the distal micro-motor.
OCT-TCE provided data that was consistent with that obtained by consecutive endoscopy. We assessed BE length (maximum extent) from 40 BE patients who had analyzable OCT-TCE datasets and EGD reports. OCT-TCE and EGD measurements of BE extent showed a strong correlation (r=0.77–0.79, Fig. 4), which is comparable with OCT-TCE results previously published from a single-center study (r=0.77–0.78)18. TCE interobserver correlation was very strong (r=0.91). Of the 104 patients in this study who had analyzable OCT-TCE datasets, 1 patient had a biopsy with LGD and 1 patient had a biopsy showing focal HGD. Both patients with biopsy-proven dysplastic BE had OCT features consistent with dysplasia27, as determined by OCT-TCE readers blinded to biopsy results. An additional 6 patients had biopsies that were read as IND. Of these patients, 4 showed OCT-TCE evidence of dysplasia27.
Figure 4.
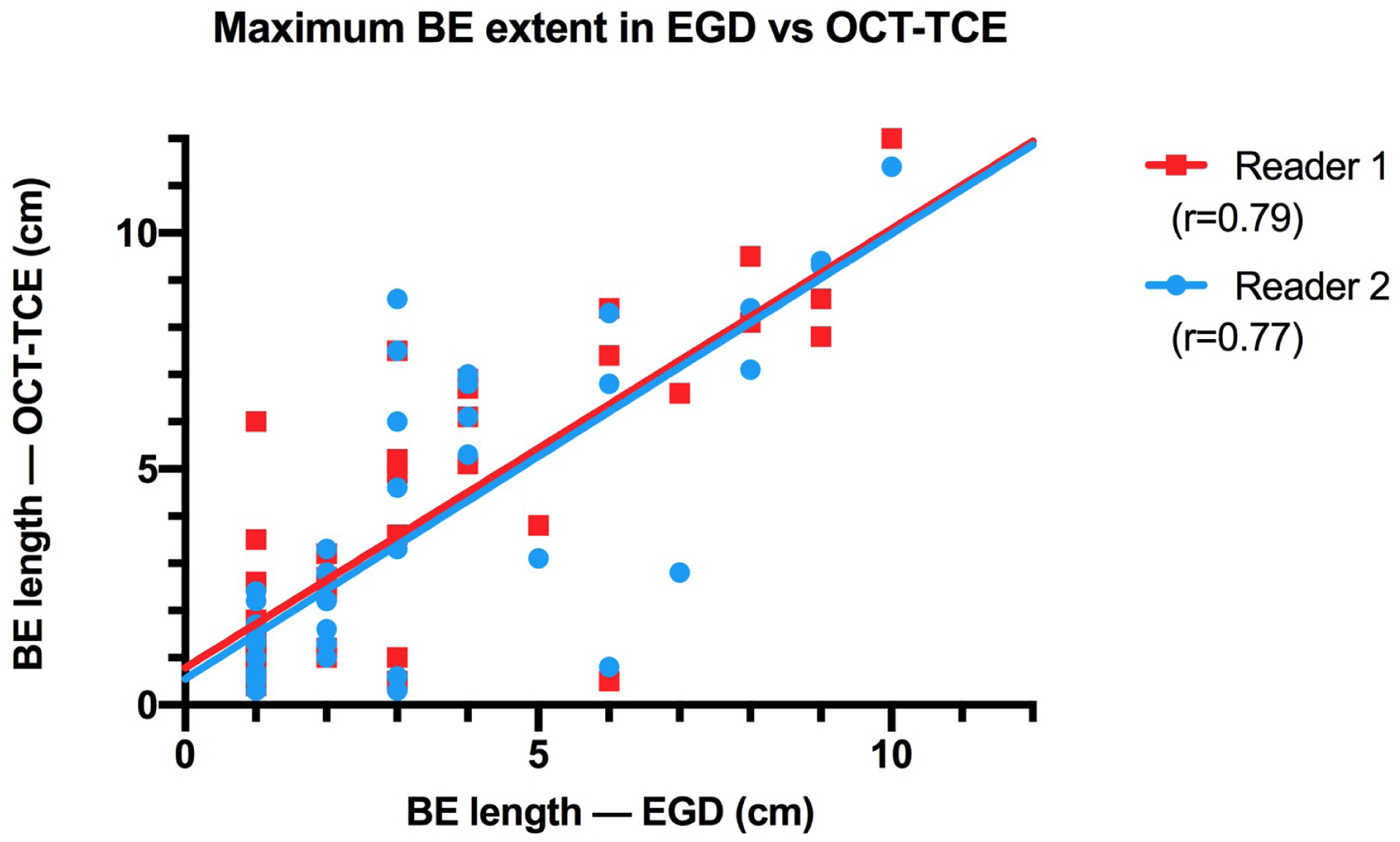
Scatter plot of maximum BE extent measured by EGD vs OCT-TCE.
DISCUSSION
In this paper, we have demonstrated the feasibility, safety, and patient tolerability of OCT-TCE in a multi-center clinical study. The device was customized for external multi-site use by making the imaging system robust and portable, and by including a micro-motor inside the capsule to scan the OCT light beam. Findings showed that this TCE technology can be effectively used across multiple sites to evaluate the esophagus, retaining all of the advantages seen previously in other TCE pilot studies. Results, including BE length and dysplasia, were consistent with those obtained on subsequent EGD. In this multi-center study, after a comprehensive training tutorial, the TCE procedure was performed equally well in the hands of physicians and nurses. This feature may mitigate some of the cost and time associated with other procedures that require physician operation. The device is also reusable; the average number of times the capsule was used here (4.2±3.8) was similar to that reported in a previously published single center study (4±3 times)18.
Compared to prior studies that had successful TCE swallowing rates between 85% to 92%16–18, we found the overall successful swallowing rate of this study (79%) was slightly lower. Unlike the single center studies that were done in a small number of subjects by investigators who had developed the technology, here we sent the devices to sites that were naïve to TCE development and operation. Additionally, previously published studies16–18 included healthy volunteers and other types of subjects, which may also affect the overall swallowing rate. Swallowing rates were lower in some sites whereas in others they were higher (range 70%−87%), and the differences in swallowing rates between sites were not statistically significant (p=0.75). Nonetheless, averaged over all sites, the swallowing rate was near 80%, and the procedure was widely accepted and well tolerated by BE patients. In the questionnaire from non-swallowers, feedback indicated that the capsule size and the existence of a tether were the two major factors that interfered with swallowing. Reducing the capsule size and changing the material/diameter of the tether may improve swallowing rates and comfort.
Identification of BE tissue and dysplasia has been previously validated by OCT histopathologic correlative studies25–27. Using these same criteria, we were able to identify healthy squamous and BE tissues in the OCT-TCE images. One limitation of the current study is a lack of biopsy confirmation for the TCE findings. Such a study could be facilitated by incorporating TCE-based laser cautery marking (similar to VLE real-time targeting28) that has been recently reported21 and uses the same imaging and capsule technology described here. Future studies are merited to validate this technology using standard endoscopic and histologic findings.
Other tethered capsule devices have recently been developed that detect BE using a sponge that collect cells scraped off the esophageal mucosal surface of unsedated patients, these cells are then sent off for molecular analysis that detects biomarkers indicative of BE or dysplasia29–31. The format of these devices is similar to OCT-TCE technology described here and thus patient preparation, case utilization, and procedural times are comparable. Devices differ in interpretation, as the cells from sampling sponges are sent to clinical pathology labs, providing a gold standard result32. Sampling sponges are further along in their validation, with multiple studies showing good sensitivities/specificities compared to endoscopic biopsy31,33,34. In contrast, OCT-TCE images are currently interpreted by expert readers, which is a limited resource. Thus, it is critical to develop automated image analysis algorithms to diagnose BE from OCT-TCE images in real time and this research is ongoing35–37. Studies with other forms of OCT12,13 have shown good sensitivity and specificity for BE13,38, but the accuracy of OCT-TCE has not yet been directly demonstrated. Both technologies offer analysis of the entire relevant portion of the esophagus, but OCT-TCE has the additional advantage of being able to localize BE anatomically, which may be useful for follow up treatment or to assess features such as the extent of BE (Fig. 4) or dysplasia. In addition, OCT-TCE provides information below the mucosal surface, thus allowing the detection of BE/dysplasia depth39 or other anomalies such as buried BE and submucosal BE extension40,41.
This study describes the extensibility of this technology to be utilized in multiple sites that are not expert in OCT-TCE, providing an understanding of the feasibility of OCT-TCE for broader utilization beyond the expert labs and for screening in general. Our experience with OCT-TCE technology in this multi-center clinical study demonstrates that this technology is safe and feasible for depth-resolved microscopic imaging of the entire esophagus in BE patients. High quality microscopic images of the entire esophageal wall were obtained in majority of the cases (93.7%) across the different sites, which is consistent with that reported in prior TCE studies16–18. The high image quality, lack of sedation, ease of use, short procedure time, and patient acceptance of OCT-TCE procedure suggest that this technology has the potential to become a useful surveillance and screening tool for BE.
Supplementary Material
IMPLICATIONS FOR PATIENT CARE.
Owing to the merits of lack of sedation, ease of use, short procedure time, better patient comfort and high quality images, OCT-TCE technology is an emerging tool for screening and surveillance of BE patients.
ACKNOWLEDGEMENTS
The authors acknowledge Sohaila Ayad, Sebastian Vetu, Eboni Smith for capsule fabrication; Justin Palermo, Alfred Kyrollos, Anita Chung, Krysta Calnan, Omair Shakil, Hany Osman, Tiffany Mangels, April Higbee, Chandra Dasari, Frances Cayer, Bryan Linn, James Allen, Lori Lutzke and Griselda Compress for facilitating the human studies approval processes, patient enrollment, data management and assistance in clinical procedures; Katie Cressman for assistance in the fabrication process. The authors also thank each of the sites’ GI units for their cooperation in this study. This study is supported by NIH grant R01CA184102, the John and Dottie Remondi Family Foundation, the Mike and Sue Hazard Family, and the MGH Research Scholars Program.
FINDINGS
OCT-TCE is a safe and feasible procedure to be performed across multiple sites for rapidly obtaining depth-resolved microscopic images of the entire esophagus. It is capable of being administered to unsedated patients by either physicians or nurses, and has high patient acceptance.
Abbreviations:
- BE
Barrett’s esophagus
- BMI
body mass index
- CI
confidence interval
- EGD
esophagogastroduodenoscopy
- GERD
gastroesophageal reflux disease
- HGD
high grade dysplasia
- HLDI
high-level disinfection
- IND
indefinite for dysplasia
- IQR
interquartile range
- Kansas City VA
Kansas City Veterans Administration
- LGD
low grade dysplasia
- LP
lamina propria
- MGH
Massachusetts General Hospital
- MM
muscularis mucosa
- MP-ICL
inner circular layer of muscularis propria
- MP-OLL
outer longitudinal layer of muscularis propria
- MYP
myenteric plexus
- OCT
optical coherence tomography
- PPI
proton pump inhibitors
- SM
submucosa
- TCE
tethered capsule endomicroscopy
- VLE
volumetric laser endomicroscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Massachusetts General Hospital has a licensing arrangement with NinePoint Medical. Dr. Tearney has the rights to receive royalties from this licensing arrangement. Dr. Tearney receives sponsored research from CN USA Biotech Holdings, Boston Scientific, and iLumen, Inc.
Clinical trial registry website and trial number: https://clinicaltrials.gov/ct2/show/NCT02994693?term=Tethered+capsule+endomicroscopy&draw=2&rank=3
https://clinicaltrials.gov/ct2/show/NCT03459339?term=Tethered+capsule+endomicroscopy&draw=2&rank=1
BACKGROUND
Tethered capsule endomicroscopy obtains high resolution images of the entire esophagus. We evaluate the feasibility and safety of OCT-TCE in patients with Barrett’s esophagus in a multi-center study.
REFERENCES
- 1.Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high-grade dysplasia in Barrett’s esophagus overestimated?. Clin Gastroenterol Hepatol 2008; 6(2):159–164. [DOI] [PubMed] [Google Scholar]
- 2.Eliakim R, Yassin K, Shlomi I, et al. A novel diagnostic tool for detecting esophageal pathology: the PillCam esophageal video capsule. Aliment Pharmacol Ther 2004;20:1083–9. [DOI] [PubMed] [Google Scholar]
- 3.Cho S, Arya N, Swan K, et al. Unsedated transnasal endoscopy: a Canadian experience in daily practice. Can J Gastroenterol 2008; 22: 243–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez FC, Akins R, Shaukat M. Screening of Barrett’s esophagus with string-capsule endoscopy: a prospective blinded study of 100 consecutive patients using histology as the criterion standard. Gastrointest Endosc 2008;68:25–31. [DOI] [PubMed] [Google Scholar]
- 5.Wong Kee Song LM, Adler DG, Chand B, et al. Chromoendoscopy. Gastrointest Endosc 2007;66:639–49 [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, Hawes RH, Bansal A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: a prospective, international, randomized controlled trial. Gut. 2013;62:15–21. [DOI] [PubMed] [Google Scholar]
- 7.Wolfsen HC, Crook JE, Krishna M, et al. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett’s Esophagus. Gastroenterology. 2008;135:24–31. [DOI] [PubMed] [Google Scholar]
- 8.Li HY, Ge ZZ, Fujishiro M, et al. Current clinical applications of magnifying endoscopy with narrow band imaging in the stoach. Diagn Ther Endosc. 2012;2012:271914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace MB, Meining A, Canto MI, et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010;31:548–52 [DOI] [PubMed] [Google Scholar]
- 10.Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta-analysis and systematic review. Clin Gastroenterol Hepatol 2013;11(12.e2):1562–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang D, Swanson EA, Lin CP, et al. Optical Coherence Tomography. Science 1991;254:1178–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfsen HC, Sharma P, Wallace MB, et al. Safety and feasibility of volumetric laser endomicroscopy in patients with Barrett’s esophagus (with videos). Gastrointest Endosc 2015;82(4):631–40. [DOI] [PubMed] [Google Scholar]
- 13.Alshelleh M, Inamdar S, McKinley M, et al. Incremental yield of dysplasia detection in Barrett’s esophagus using volumetric laser endomicroscopy with and without laser marking compared with a standardized random biopsyprotocol. Gastrointest Endosc 2018;88(1):35–42. [DOI] [PubMed] [Google Scholar]
- 14.Suter MJ, Vakoc BJ, Yachimski PS, et al. Comprehensive microscopy of the esophagus in human patients with optical frequency domain imaging. Gastrointest Endosc 2008;68(4):745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gora MJ, Sauk JS, Carruth RW, et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat Med 2013;19(2):238–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gora MJ, Sauk JS, Carruth RW, et al. Imaging the upper gastrointestinal tract in unsedated patients using tethered capsule endomicroscopy. Gastroenterology 2013;145(4):723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gora MJ, Simmons LH, Quénéhervé L, et al. Tethered capsule endomicroscopy: from bench to bedside at a primary care practice. J Biomed Opt 2016;21(10):104001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gora MJ, Quénéhervé L, Carruth RW, et al. Tethered capsule endomicroscopy for unsedated microscopic imaging of the esophagus, stomach, and duodenum in humans (with video), Gastrointest Endosc 2018;88(5.e3):830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang K, Ahsen OO, Lee HC, et al. Volumetric Mapping of Barrett’s Esophagus and Dysplasia With en face Optical Coherence Tomography Tethered Capsule. Am J Gastroenterol 2016;111(11):1664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang K, Traverso G, Lee HC, et al. Ultrahigh speed en face OCT capsule for endoscopic imaging. Biomed Opt Express 2015;6(4):1146–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang CP, Dong J, Ford T, et al. Optical coherence tomography-guided laser marking with tethered capsule endomicroscopy in unsedated patients. Biomed Opt Express 2019;10(3):1207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol 2016;111(1):30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014;63:7–42. [DOI] [PubMed] [Google Scholar]
- 25.Poneros JM, Brand S, Bouma BE, et al. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology 2001;120(1):7–12. [DOI] [PubMed] [Google Scholar]
- 26.Evans JA, Bouma BE, Bressner J, et al. Identifying intestinal metaplasia at the squamocolumnar junction by using optical coherence tomography. Gastrointest Endosc 2007;65(1):50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans JA, Poneros JM, Bouma BE, et al. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett’s esophagus. Clin Gastroenterol Hepatol 2006;4(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swager AF, Groof AJD, Meijer SL, et al. Feasibility of laser marking in Barrett’s esophagus with volumetric laser endomicroscopy: first-in-man pilot study. Gastrointest Endosc 2017;86(3): 464–472. [DOI] [PubMed] [Google Scholar]
- 29.Ross-Innes CS, Debiram-Beecham I, O’Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of Trefoil Factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case-control study. PLoS Med 2015;12(1):e1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyer PG, Taylor WR, Johnson ML, et al. Highly discriminant methylated DNA markers for the non-endoscopic detection of Barrett’s esophagus. Am J Gastroenterol 2018;113(8):1156–1166. [DOI] [PubMed] [Google Scholar]
- 31.Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018;10(424),eaao5848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chettouh H, Mowforth O, Galeano-Dalmau N, et al. Methylation panel is a diagnostic biomarker for Barrett’s oesophagus in endoscopic biopsies and non-endoscopic cytology specimens. Gut 2018;67(11):1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzgerald RC, di Pietro M, O’Donovan M, et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: a multicenter, pragmatic, randomised controlled trial. Lancet 2020;396(10247):333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iyer PG, Taylor WR, Johnson ML, et al. Accurate nonendoscopic detection of Barrett’s Esophagus by Methylated DNA markers: a multisite case control study. Am J Gastroenterol 2020;115(8):1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ughi GJ, Gora MJ, Swager A-F, et al. Automated segmentation and characterization of esophageal wall in vivo by tethered capsule optical coherence tomography endomicroscopy. Biomed Opt. Express 2016;7(2):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Struyvenberg M, de Groof AJ, Fonolla R, et al. Prospective development and validation of a volumetric laser endomicroscopy computer algorithm for detection of Barrett’s neoplasia. Gastrointest Endosc 2020;In Press. [DOI] [PubMed] [Google Scholar]
- 37.Swager AF, van der Sommen F, Klomp SR, et al. Computer-aided detection of early Barrett’s neoplasia using volumetric laser endomicroscopy. Gastrointest Endosc 2017;86(5):839–846. [DOI] [PubMed] [Google Scholar]
- 38.Leggett CL, Gorospe EC, Chan DK, et al. Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett’s esophagus. Gastrointest Endosc 2016;83(5):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levink IJM, Tearney GJ, Erler NS, et al. Barrett’s epithelial thickness, assessed by volumetric laser endomicroscopy, is associated with response to radiofrequency ablation. Clin Gastroenterol Hepatol 2020;In Press [DOI] [PubMed] [Google Scholar]
- 40.Swager AF, Boerwinkel DF, de Bruin DM, et al. Detection of buried Barrett’s glands after radiofrequency ablation with volumetric laser endomicroscopy. Gastrointest Endosc 2016;83(1):80–88 [DOI] [PubMed] [Google Scholar]
- 41.Leggett CL, Gorospe E, Owens VL, et al. Volumetric laser endomicroscopy detects subsquamous Barrett’s adenocarcinoma. Am J Gastroenterol 2014;109(2):298–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


