Key Points
Question
Is substantial weight loss achieved with weight loss surgery associated with improved risk and severity of COVID-19 infection in patients with obesity?
Findings
In this cohort study of 11 809 patients with obesity, the rates of positive SARS-CoV-2 test results were comparable among patients in the surgical group and control group. However, previous weight loss surgery was significantly associated with a 49% lower risk of hospitalization, 63% lower risk of need for supplemental oxygen, and 60% lower risk of severe disease during a 12-month period after contracting COVID-19 infection.
Meaning
The findings from this study show an association between weight loss achieved with surgery and improved outcomes of COVID-19 infection, suggesting that obesity can be a modifiable risk factor for the severity of COVID-19 infection.
Abstract
Importance
Obesity is an established risk factor for severe COVID-19 infection. However, it is not known whether losing weight is associated with reduced adverse outcomes of COVID-19 infection.
Objective
To investigate the association between a successful weight loss intervention and improved risk and severity of COVID-19 infection in patients with obesity.
Design, Setting, and Participants
This cohort study involved adult patients with a body mass index of 35 or higher (calculated as weight in kilograms divided by height in meters squared) who underwent weight loss surgery between January 1, 2004, and December 31, 2017, at the Cleveland Clinic Health System (CCHS). Patients in the surgical group were matched 1:3 to patients who did not have surgical intervention for their obesity (control group). The source of data was the CCHS electronic health record. Follow-up was conducted through March 1, 2021.
Exposures
Weight loss surgery including Roux-en-Y gastric bypass and sleeve gastrectomy.
Main Outcomes and Measures
Distinct outcomes were examined before and after COVID-19 outbreak on March 1, 2020. Weight loss and all-cause mortality were assessed between the enrollment date and March 1, 2020. Four COVID-19–related outcomes were analyzed in patients with COVID-19 diagnosis between March 1, 2020, and March 1, 2021: positive SARS-CoV-2 test result, hospitalization, need for supplemental oxygen, and severe COVID-19 infection (a composite of intensive care unit admission, need for mechanical ventilation, or death).
Results
A total of 20 212 patients (median [IQR] age, 46 [35-57] years; 77.6% female individuals [15 690]) with a median (IQR) body mass index of 45 (41-51) were enrolled. The overall median (IQR) follow-up duration was 6.1 (3.8-9.0) years. Before the COVID-19 outbreak, patients in the surgical group compared with control patients lost more weight (mean difference at 10 years from baseline: 18.6 [95% CI, 18.4-18.7] percentage points; P < .001) and had a 53% lower 10-year cumulative incidence of all-cause non–COVID-19 mortality (4.7% [95% CI, 3.7%-5.7%] vs 9.4% [95% CI, 8.7%-10.1%]; P < .001). Of the 20 212 enrolled patients, 11 809 were available on March 1, 2020, for an assessment of COVID-19–related outcomes. The rates of positive SARS-CoV-2 test results were comparable in the surgical and control groups (9.1% [95% CI, 7.9%-10.3%] vs 8.7% [95% CI, 8.0%-9.3%]; P = .71). However, undergoing weight loss surgery was associated with a lower risk of hospitalization (adjusted hazard ratio [HR], 0.51; 95% CI, 0.35-0.76; P < .001), need for supplemental oxygen (adjusted HR, 0.37; 95% CI, 0.23-0.61; P < .001), and severe COVID-19 infection (adjusted HR, 0.40; 95% CI, 0.18-0.86; P = .02).
Conclusions and Relevance
This cohort study found that, among patients with obesity, substantial weight loss achieved with surgery was associated with improved outcomes of COVID-19 infection. The findings suggest that obesity can be a modifiable risk factor for the severity of COVID-19 infection.
This cohort study examines weight loss outcomes from surgical and nonsurgical interventions among patients with obesity before and after the COVID-19 outbreak.
Introduction
Obesity has been recognized as a major risk factor for adverse clinical outcomes from SARS-CoV-2 infection. Obesity is associated with systemic inflammation, immune dysfunction, and a hypercoagulable state. Impaired respiratory mechanics along with coexistent pulmonary, cardiac, and metabolic disorders can also play a role in the severity of COVID-19 infection in patients with obesity.1,2,3,4,5
In patients with obesity, weight loss surgery or metabolic surgery has been found to lead to substantial and durable weight loss and improved cardiometabolic risk factors.6,7,8,9 Furthermore, data from observational studies suggest a survival benefit, amelioration of the obesity-related inflammatory state, and reduction of the risk of cardiovascular and renal adverse events after weight loss surgery.10,11,12,13,14,15,16
Although the association between obesity and poor clinical outcomes of COVID-19 has been established, a fundamental question remains unanswered. It is not known whether losing weight can improve the outcomes of COVID-19 infection in patients with obesity. If obesity is identified as a modifiable risk factor, emphasis on weight loss as a public health strategy may be beneficial during the COVID-19 pandemic and in future outbreaks of this or other infectious diseases.
In this study, we aimed to examine the association between a successful weight loss intervention and improved risk and severity of COVID-19 infection in patients with obesity. We hypothesized that, after achieving a large amount of weight loss through metabolic surgery, patients with obesity become less vulnerable to developing severe acute complications from COVID-19 infection.
Methods
This retrospective, observational, matched-cohort study of adult patients with obesity was conducted at the Cleveland Clinic Health System (CCHS). Because weight loss after metabolic surgery is generally large and durable, we constructed a cohort of patients who underwent weight loss surgery at CCHS to examine the implications of substantial weight loss for future COVID-19–related outcomes. The CCHS Institutional Review Board approved this study and waived the informed consent requirement because the study was deemed to be minimal risk research that used data collected for routine clinical practice.
The source of data was the CCHS electronic health record, using the Unified Medical Language System,17 through March 1, 2021. In the initial screening process, all CCHS patients who had at least 1 record of body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 35 or higher between January 1, 2004, and December 31, 2017, were identified (n = 675 240).
Surgical and Control Groups
All patients aged between 18 and 80 years and with a BMI between 35 and 80 who underwent either Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG) between January 1, 2004, and December 31, 2017, at CCHS were considered for enrollment in the surgical group. Any patient who met any of the following criteria was excluded: history of organ transplant, cancerous or precancerous diagnosis, alcohol use disorder or alcohol-related medical conditions, dialysis, ascites, cardiac ejection fraction less than 20%, positive HIV test result, peptic ulcer disease, and recent (within 5 days before surgery) emergency department admission (eFigure in the Supplement). The date of first weight loss surgery was considered to be the index date for patients in the surgical group.
An algorithm was implemented to assemble the control group from patients who were identified in the initial screening. First, patients who underwent RYGB, SG, or other less common weight loss surgical procedures were excluded. Second, each patient in the control group was randomly assigned a single date (taken from the collection of index dates of patients in the surgical group) to serve as the index date for that patient. Third, patients who met any of the following criteria at their assigned index date were excluded: (1) younger than 18 years or older than 80 years, (2) BMI lower than 35 or higher than 80, (3) exclusion criteria for patients in the surgical group, (4) less than 12 months of follow-up before the assigned index date (to increase the chance of enrolling patients who received routine care at CCHS), (5) last follow-up on or before the assigned index date, and (6) deceased on or before the assigned index date (eFigure in the Supplement).
Using this algorithm, we identified 128 258 comparable control patients to be considered for matching. In addition to establishing the primary comparison cohort, this algorithm was replicated 4 times to build 4 different control cohorts for the sensitivity analyses.
Study Outcomes
Distinct study outcomes were examined before and after the COVID-19 outbreak. Weight loss and all-cause mortality were compared between the surgical and control groups from the index date until March 1, 2020. The date March 1, 2020, corresponded with the dates of both the identification of the first cases of COVID-19 at CCHS and the launch of a COVID-19 registry to follow up all CCHS patients who were tested for SARS-CoV-2. Mortality data were obtained from a combination of the CCHS electronic health record, US Social Security Administration data, and state death indices.
We studied 4 prespecified COVID-19–related outcomes in the time-to-event analysis from March 1, 2020 (as time 0) to March 1, 2021. These 4 outcomes were (1) positive test result for SARS-CoV-2, (2) hospitalization, (3) need for supplemental oxygen, and (4) severe COVID-19 infection (defined as a composite of intensive care unit admission, need for mechanical ventilation, or death).
All COVID-19–related outcomes were extracted from the prospective CCHS Institutional Review Board–approved clinical registry. This registry contained data on all patients who underwent a reverse transcriptase–polymerase chain reaction test for SARS-CoV-2 using nasopharyngeal or oropharyngeal swab specimens within CCHS on or after March 1, 2020.
Patients who died or who did not have follow-up (ie, not available to be considered at risk for COVID-19 infection) after March 1, 2020, were not included in the analysis of COVID-19–related outcomes. Patients were followed up from March 1, 2020, until the first occurrence of either COVID-19–related outcomes or until the last known follow-up date as a censoring date (ie, last hospital discharge date or last office visit, whichever was later). A cause-specific hazard approach was applied to each outcome.
Statistical Analysis
Baseline data were reported as median (IQR) and number (percentage). Doubly robust estimation combining the propensity score and outcome regression was used to compare outcomes in the surgical and control groups. Each patient in the surgical group was matched with a propensity score by the nearest-neighbor method to 3 patients in the control group, using a logistic regression model with a logit link function based on 10 a priori–identified potential confounders. The matching variables were index date, age at index date, sex, race and ethnicity (which were obtained from electronic health records and based on patients selecting from fixed categories: Black, White, and other [including American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, and multiracial]), BMI at the index date (categorized as 35-39.9, 40-44.9, 45-49.9, 50-54.9, 55-59.9, or 60-80), smoking history (categorized as never, former, or current), presence of diabetes, Elixhauser Comorbidity Index with van Walraven weights (score range: –19 to 89, with the highest score indicating greater comorbidity burden and greater likelihood for in-hospital death), Charlson Comorbidity Index (score range: 0 to 29, with the highest score indicating maximal disease burden and predicting the risk of death), and state of residence (Ohio, Florida, or other US states).
Cumulative incidence estimates were calculated using the Kaplan-Meier method, and fully adjusted Cox proportional hazards regression models were generated for the study outcomes. Fifteen variables (Table 1) were included to adjust for potential confounding. The proportional hazards assumptions for the treatment variable were assessed by the methods of Grambsch and Therneau.18 Association was defined as the hazard ratio (HR) for COVID-19–related outcomes in the surgical group vs control group.
Table 1. Baseline Characteristics of Patients in the Surgical Group vs Control Group.
| Variable | Original cohort before COVID-19 outbreak (N = 20 212)a | At-risk cohort after COVID-19 outbreak (n = 11 809)b | ||||
|---|---|---|---|---|---|---|
| Surgical group, No. (%) | Control group, No. (%) | Standardized mean difference, %c | Surgical group, No. (%) | Control group, No. (%) | Standardized mean difference, %c | |
| All patients | 5053 (25.0) | 15 159 (75.0) | NA | 2958 (25.0) | 8851 (75.0) | NA |
| Index year, median (IQR) | 2013 (2010 to 2015) | 2013 (2010 to 2015) | 0.5 | 2013 (2011 to 2016) | 2013 (2011 to 2016) | 0.4 |
| Age, median (IQR), y | 46.0 (37.0 to 55.0) | 46.0 (34.0 to 57.0) | 1.5 | 46.0 (38.0 to 55.0) | 46.0 (34.0 to 56.0) | 5.3 |
| Sex | ||||||
| Female | 3884 (76.9) | 11 806 (77.9) | 2.4 | 2325 (78.6) | 6950 (78.5) | 0.2 |
| Male | 1169 (23.1) | 3353 (22.1) | 2.4 | 633 (21.4) | 1901 (21.5) | 0.2 |
| BMI, median (IQR) | 45.5 (41.0 to 51.6) | 45.3 (40.8 to 50.9) | 6.5 | 45.4 (40.9 to 51.4) | 45.3 (40.8 to 50.7) | 5.6 |
| BMI category | ||||||
| 35-39.9 | 1005 (19.9) | 2839 (18.7) | 2.9 | 606 (20.5) | 1654 (18.7) | 4.5 |
| 40-44.9 | 1372 (27.2) | 4410 (29.1) | 4.3 | 817 (27.6) | 2616 (29.6) | 4.3 |
| 45-49.9 | 1150 (22.8) | 3586 (23.7) | 2.1 | 663 (22.4) | 2099 (23.7) | 3.1 |
| 50-54.9 | 743 (14.7) | 2213 (14.6) | 0.3 | 434 (14.7) | 1319 (14.9) | 0.6 |
| 55-59.9 | 393 (7.8) | 1074 (7.1) | 2.6 | 230 (7.8) | 602 (6.8) | 3.7 |
| 60-80 | 390 (7.7) | 1037 (6.8) | 3.4 | 208 (7.0) | 561 (6.3) | 2.8 |
| Race and ethnicityd | ||||||
| Black | 1153 (22.8) | 3428 (22.6) | 0.5 | 780 (26.4) | 2059 (23.3) | 7.2 |
| White | 3724 (73.7) | 11 196 (73.9) | 0.4 | 2068 (69.9) | 6484 (73.3) | 7.4 |
| Othere | 176 (3.5) | 535 (3.5) | 0.3 | 110 (3.7) | 308 (3.5) | 1.3 |
| State of residence | ||||||
| Ohio | 3997 (79.1) | 12 397 (81.8) | 6.8 | 2578 (87.2) | 7645 (86.4) | 2.3 |
| Florida | 829 (16.4) | 2109 (13.9) | 7.0 | 326 (11.0) | 991 (11.2) | 0.6 |
| Other US states | 227 (4.5) | 653 (4.3) | 0.9 | 54 (1.8) | 215 (2.4) | 4.2 |
| Smoking status | ||||||
| Never | 3019 (59.7) | 9322 (61.5) | 3.6 | 1760 (59.5) | 5500 (62.1) | 5.4 |
| Former | 1889 (37.4) | 5498 (36.3) | 2.3 | 1126 (38.1) | 3170 (35.8) | 4.7 |
| Current | 145 (2.9) | 339 (2.2) | 4.0 | 72 (2.4) | 181 (2.0) | 2.6 |
| Annual income within zip code, median (IQR), $ | 51 962 (42 613 to 67 184) | 50 642 (39 814 to 66 468) | 7.0 | 51 962 (40 664 to 69 434) | 51 089 (39 850 to 67 717) | 4.6 |
| Missing data | 88 (1.7) | 171 (1.1) | NA | 24 (0.8) | 69 (0.8) | NA |
| Charlson Comorbidity Index score, median (IQR)f | 2.0 (1.0 to 3.0) | 1.0 (0 to 3.0) | 14.6 | 2.0 (1.0 to 3.0) | 2.0 (0 to 3.0) | 12.8 |
| Elixhauser Comorbidity Index score, median (IQR)g | 1.0 (−4.0 to 8.0) | 0 (−2.0 to 4.0) | 9.3 | 3.0 (−4.0 to 9.0) | 0 (−2.0 to 5.0) | 17.5 |
| eGFR, median (IQR), mL/minh | 104 (86.2 to 122) | 92.3 (74.5 to 111) | 37.2 | 104 (86.2 to 121) | 93.2 (76.0 to 112) | 32.7 |
| Missing data | 0 | 3249 (21.4) | NA | 0 | 1567 (17.7) | NA |
| Diabetes medication use | 1789 (35.4) | 4708 (31.1) | 9.2 | 1071 (36.2) | 3063 (34.6) | 3.3 |
| Antihypertensive medication use | 4283 (84.8) | 7685 (50.7) | 78.2 | 2527 (85.4) | 4823 (54.5) | 71.7 |
| Lipid-lowering medication use | 1817 (36.0) | 4530 (29.9) | 13.0 | 1086 (36.7) | 2882 (32.6) | 8.7 |
| Aspirin use | 1164 (23.0) | 3032 (20.0) | 7.4 | 744 (25.2) | 1891 (21.4) | 9.0 |
| Follow-up time, median (IQR), y | 5.9 (3.4 to 9.0) | 6.2 (3.9 to 9.0) | 7.7 | 7.5 (5.1 to 10.4) | 7.6 (5.2 to 10.2) | 0.8 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; NA, not applicable.
Baseline characteristics of patients in the original cohort (5053 in the surgical group and 15 159 in the control group) were well balanced with matching.
Baseline characteristics of patients who were at risk for COVID-19 infection between March 1, 2020, and March 1, 2021 (2958 in the surgical group and 8851 in the control group), were largely balanced. For the few remaining variables, the surgical group had a higher baseline profile (eg, higher rate of antihypertensive medication use and comorbidity scores). All baseline variables were included in the fully adjusted Cox proportional hazards regression models. The intention of the regression adjustment was to be the main method of actual statistical adjustment for comparison of study groups to control any imbalances that remained after the matching process.
Standardized mean differences (%) are the absolute value of the difference in means or proportions between the surgical group and control group divided by pooled SD. Values of 10% or less indicate appropriate matching.
Race and ethnicity were obtained from electronic health records and based on patients selecting from fixed categories.
Other category included American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, and multiracial.
Charlson Comorbidity Index score range: 0 to 29, with the highest score indicating maximal disease burden and predicting the risk of death.
Elixhauser Comorbidity Index with van Walraven weights score range: –19 to 89, with the highest score indicating greater comorbidity burden and greater likelihood for in-hospital death.
eGFR was approximated using the Modification of Diet in Renal Disease study equation.
Missing baseline values were limited to the estimated glomerular filtration rate and annual income within zip code, which were imputed using multiple imputation by chained equations to create 5 imputed data sets. Predictive mean matching was used for numeric variable imputation. Imputation-corrected variance estimates were obtained using the Rubin formula.19
A 4-knot spline interacted with treatment was used for comparing mean changes in body weight and BMI between the surgical and control groups. An unpaired, 2-tailed t test was used to analyze the mean differences between groups at the time of a positive SARS-CoV-2 test result.
A significance level of α = .05 for 2-sided comparisons was considered to be statistically significant, and 95% CIs were reported where applicable. Given that type I error was likely to occur because of multiple comparisons, the findings should be interpreted as exploratory. All statistical analysis was performed using R, version 3.5.0 (R Foundation for Statistical Computing).
Sensitivity of the HR estimates from the fully adjusted Cox proportional hazards regression models was assessed by constructing 4 additional control cohorts. These cohorts were created by repeating the algorithm of randomly assigning index dates to control patients 4 times. All analyses were performed on all 5 data sets, although the results from 1 data set were reported as the primary comparison.
Results
A total of 20 212 patients were considered for the primary comparison, including 5053 who had weight loss surgery (3348 [66.3%] underwent RYGB, and 1705 [33.7%] underwent SG) and 15 159 patients who did not undergo surgery (matched control) (eFigure in the Supplement). Overall, the original cohort comprised 15 690 female (77.6%) and 4522 male (22.4%) individuals who self-identified as having Black (4581 [22.7%]), White (14 920 [73.8%]), or other (711 [3.5%]) race and ethnicity; they had a median (IQR) age of 46 (35-57) years and a median (IQR) BMI of 45 (41-51). At baseline, 59.2% of patients (11 968) were using antihypertensive medications, 32.1% (6497) were using diabetes medications, and 31.4% (6347) were using lipid-lowering medications.
The distribution of baseline covariates was well balanced after matching the surgical group to the control group. However, patients in the surgical group compared with those in the control group had a relatively higher risk profile at baseline, including greater median (IQR) Charlson Comorbidity Index score (2.0 [1.0-3.0] vs 1.0 [0-3.0]; standardized mean difference, 14.6%); median (IQR) Elixhauser Comorbidity Index score (1.0 [−4.0 to 8.0] vs 0 [−2.0 to 4.0]; standardized mean difference, 9.3%); and higher rates of medication use to control hypertension (84.8% vs 50.7%; standardized mean difference, 78.2%), type 2 diabetes (35.4% vs 31.1%; standardized mean difference, 9.2%), and dyslipidemia (36.0% vs 29.9%; standardized mean difference, 13.0%). The median (IQR) follow-up time for the entire cohort was 6.1 (3.8-9.0) years, including 5.9 (3.4-9.0) years for patients in the surgical group and 6.2 (3.9-9.0) years for patients in the control group (Table 1).
Outcomes Before COVID-19 Outbreak
In the original cohort, the mean total weight loss at 10 years (before the COVID-19 outbreak) was 20.8 (95% CI, 20.6-21.0) percentage points of body weight in the surgical group and 2.3 (95% CI, 2.1-2.5) percentage points of body weight in the control group, for a mean difference of 18.6 (95% CI, 18.4-18.7) percentage points of body weight (P < .001) (Figure 1A). The mean difference in BMI change was 8.3 (95% CI, 8.2-8.4; P < .001).
Figure 1. Weight Loss and Survival Data of Patients Over 10 Years of Follow-up Before the COVID-19 Outbreak.
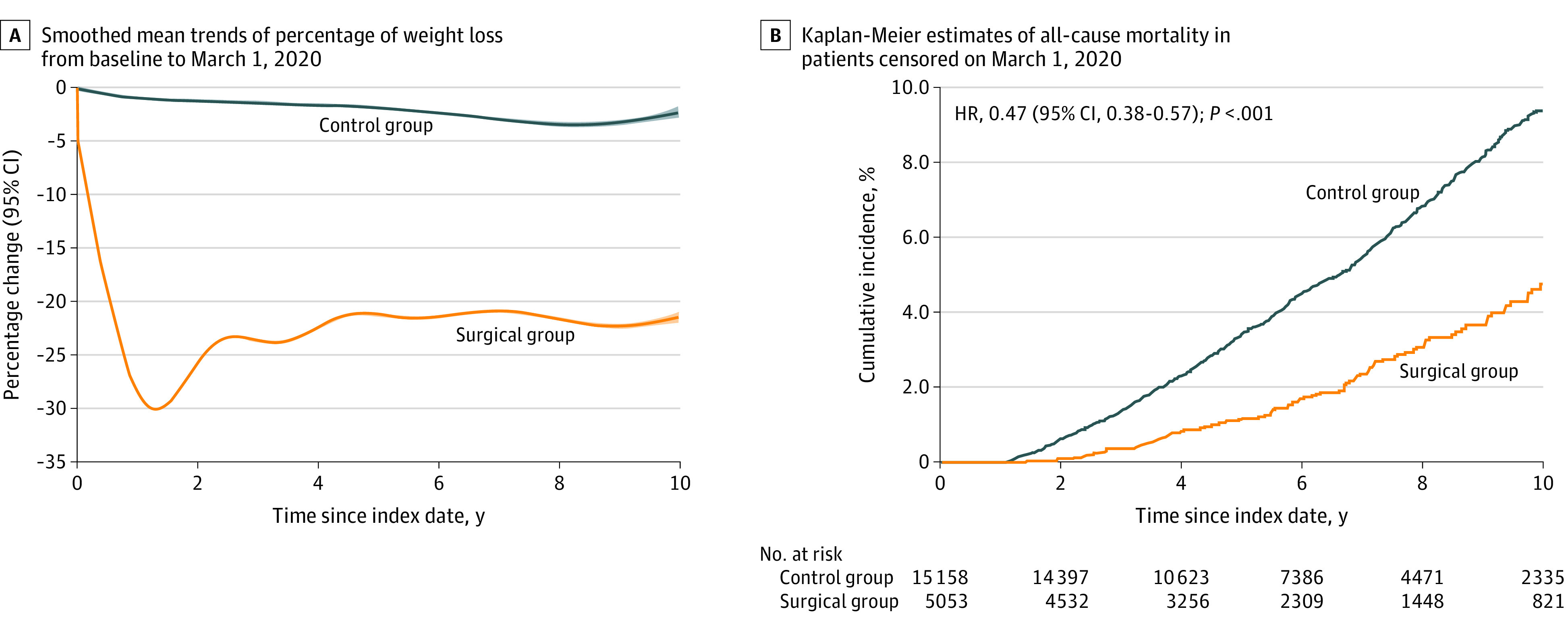
A, The mean differences in total weight loss at 10 years (censored on March 1, 2020) between surgical and control groups were estimated from a flexible regression model with a 4-knot spline on time since the index date interacted with the treatment group. B, Hazard ratios (HRs) (95% CIs) and P values were from fully adjusted Cox proportional hazards regression model that compared the relative risk of all-cause mortality in surgical vs control group (censored on March 1, 2020). All baseline variables in Table 1 were included to adjust for potential confounding.
Between the index date and March 1, 2020, 122 patients in the surgical group and 827 patients in the control group died. The cumulative incidence of all-cause mortality at 10 years was 4.7% (95% CI, 3.7%-5.7%) in the surgical group and 9.4% (95% CI, 8.7%-10.1%) in the control group, for an adjusted HR of 0.47 (95% CI, 0.38-0.57; P < .001) (Figure 1B).
Outcomes After COVID-19 Outbreak
In the at-risk cohort, 11 809 patients (including 2958 patients [25.0%] in the surgical group and 8851 patients [75.0%] in the control group) were available on March 1, 2020, for an assessment of COVID-19–related outcomes for a 12-month period. Baseline characteristics of this subgroup of patients were balanced for most characteristics. The surgical group vs the control group had a higher risk profile at the time of enrollment, such as a higher Elixhauser Comorbidity Index score (3.0 [−4.0 to 9.0] vs 0 [−2.0 to 5.0]; standardized mean difference, 17.5%), and greater use of antihypertensive medications (85.4% [2527] vs 54.5% [4823]; standardized mean difference, 71.7%) (Table 1).
Between March 1, 2020, and March 1, 2021, 206 patients in the surgical group and 578 patients in the control group had a positive SARS-CoV-2 test result. The cumulative incidence of contracting COVID-19 infection was 9.1% (95% CI, 7.9%-10.3%) in the surgical group and 8.7% (95% CI, 8.0%-9.3%) in the control group, with an adjusted HR of 1.03 (95% CI, 0.87-1.22; P = .71) (Figure 2A).
Figure 2. Cumulative Incidence Estimates for 4 COVID-19–Related Outcomes.
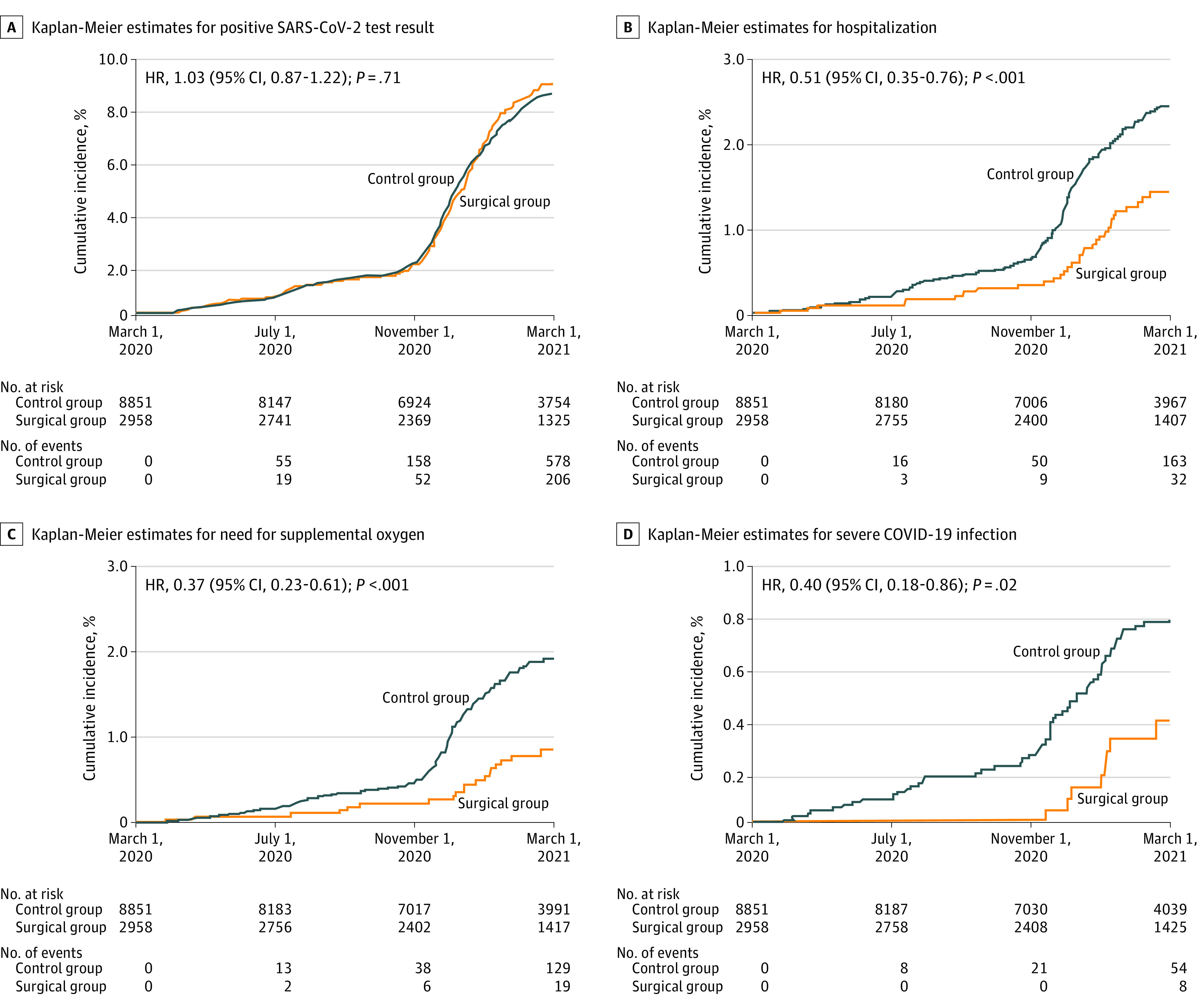
Kaplan-Meier estimates of 4 prespecified COVID-19–related outcomes involved 11 809 patients between March 1, 2020, and March 1, 2021. Hazard ratios (HRs) (95% CIs) and P values were from fully adjusted Cox proportional hazards regression models that compared the relative instantaneous risk of each outcome in surgical vs control group. All baseline variables in Table 1 were included to adjust for potential confounding.
At the time of a positive SARS-CoV-2 test result, the mean body weight of patients was 108.4 kg in the surgical group and 128.4 kg in the control group, for a mean difference of 20.0 kg (95% CI, 15.2-24.7 kg; P < .001). The BMI was 38.3 in the surgical group and 46.3 in the control group, for a mean difference of 7.9 (95% CI, 6.4-9.5; P < .001). In addition, the mean level of glycated hemoglobin at the time of COVID-19 diagnosis was 6.0% in the surgical group and 7.2% in the control group, for a mean difference of 1.2% (95% CI, 0.8%-1.6%; P < .001).
In the surgical group, after contracting SARS-CoV-2, 32 patients were hospitalized, 19 patients required supplemental oxygen, 8 patients developed the composite outcome of severe COVID-19 infection, and 2 patients died. In the control group, 163 patients were hospitalized, 129 patients required supplemental oxygen, 54 patients developed severe COVID-19 infection, and 21 patients died. Among patients with a positive SARS-CoV-2 test result, the crude rates in the surgical vs control group were 15.5% vs 28.2% for hospitalization, 9.2% vs 22.3% for need for supplemental oxygen, 3.9% vs 9.3% for severe COVID-19 infection, and 1.0% vs 3.6% for death (Table 2). Weight loss surgery was associated with a 49% lower risk of hospitalization (adjusted HR, 0.51; 95% CI, 0.35-0.76; P < .001) (Figure 2B), 63% lower risk of need for supplemental oxygen (adjusted HR, 0.37; 95% CI, 0.23-0.61; P < .001) (Figure 2C), and 60% lower risk of severe COVID-19 infection (adjusted HR, 0.40; 95% CI, 0.18-0.86; P = .02) (Figure 2D). The proportional hazard assumption was satisfied for the study outcomes.
Table 2. Event Rates, Cumulative Incidence Estimates, and Hazard Ratios in Patients in the Surgical vs Control Group.
| COVID-19–related outcome | Surgical group (n = 2958) | Control group (n = 8851) | HR (95% CI)b | P valueb | ||||
|---|---|---|---|---|---|---|---|---|
| Event rate, No. (%)a | Crude rate in patients with COVID-19, % | Cumulative incidence at 12 mo (95% CI), % | Event rate, No. (%)a | Crude rate in patients with COVID-19, % | Cumulative incidence at 12 mo (95% CI), % | |||
| Positive SARS-CoV-2 test result | 206 (7.0) | NA | 9.1 (7.9-10.3) | 578 (6.5) | NA | 8.7 (8.0-9.3) | 1.03 (0.87-1.22) | .71 |
| Hospitalization | 32 (1.1) | 15.5 | 1.4 (0.9-1.9) | 163 (1.8) | 28.2 | 2.4 (2.1-2.8) | 0.51 (0.35-0.76) | <.001 |
| Need for supplemental oxygen | 19 (0.6) | 9.2 | 0.8 (0.5-1.2) | 129 (1.5) | 22.3 | 1.9 (1.6-2.3) | 0.37 (0.23-0.61) | <.001 |
| Severe COVID-19 infectionc | 8 (0.3) | 3.9 | 0.4 (0.1-0.7) | 54 (0.6) | 9.3 | 0.8 (0.6-1.0) | 0.40 (0.18-0.86) | .02 |
| Death | 2 (0.1) | 1.0 | 0.1 (0-0.3) | 21 (0.2) | 3.6 | 0.3 (0.2-0.4) | 0.27 (0.06-1.20) | .09 |
Abbreviations: HR, hazard ratio; NA, not applicable.
Events occurred in a 12-month period, from March 1, 2020, to March 1, 2021, starting from the first COVID-19 diagnosis in the Cleveland Clinic Health System in March 2020.
HRs (95% CIs) and P values were from adjusted Cox proportional hazards regression models that compared the relative instantaneous risk of each outcome for the surgical vs control group. All baseline variables in Table 1 were adjusted for potential confounding.
Composite of intensive care unit admission, need for mechanical ventilation, or death.
Sensitivity Analysis
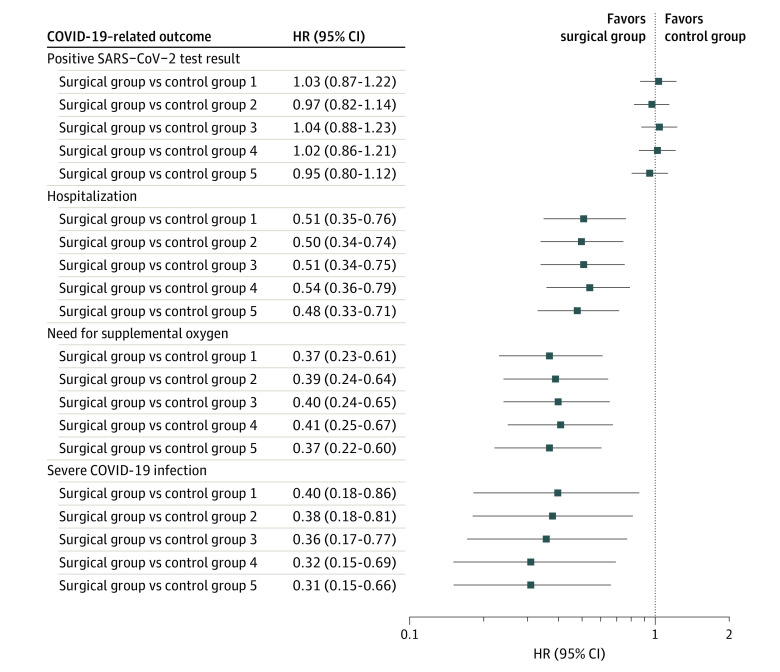
The adjusted HRs and 95% CIs for the treatment variable obtained from 4 additional data sets for COVID-19–related outcomes are shown in Figure 3. Overall, the differences in HRs, which compared the risk of all COVID-19–related outcomes in patients in the surgical group vs control group, were negligible between the 4 additional cohorts and the estimates reported from the primary comparison.
Figure 3. Sensitivity Analysis.
The surgical index dates were randomly assigned (5 times) to patients in the control group, and 5 data sets were created with the matching ratio of 1:3. The fully adjusted Cox proportional hazards regression models were run on each of the 4 COVID-19–related outcomes, and the hazard ratios (HRs) and 95% CIs for the treatment variable were obtained for each outcome in 5 data sets. Control group 1 represents the primary group who did not undergo surgery and whose data were reported in the article. The consistency of results in the sensitivity analysis supports the robustness of the findings.
Discussion
This cohort study observed that previous substantial weight loss that was achieved by surgery in patients with a BMI of 35 or higher was associated with improved outcomes for patients who subsequently developed COVID-19 infection. Specifically, undergoing weight loss surgery was associated with a 49% lower risk for hospitalization, 63% lower risk of need for supplemental oxygen, and 60% lower risk of severe COVID-19 infection. Given the observational nature of the study and relatively low number of infections, these findings should be considered as hypothesis-generating and not conclusive. Nonetheless, the consistency of the sensitivity analyses results and the large observed magnitude of the risk reductions support the robustness of the findings. The study did not find an association between previous weight loss surgery and risk of contracting SARS-CoV-2 infection.
Numerous studies have already established an association between obesity and severe COVID-19 infection.1,2,3,4,5 Almost all of the available studies enrolled a single cohort of patients in which obesity was identified in multivariable analyses as an independent risk factor for poor COVID-19 outcomes. Such a study design can only demonstrate that obesity is a major risk factor for severe COVID-19 infection. However, such studies cannot ascertain whether obesity is a modifiable risk factor for poor COVID-19 outcomes and cannot provide evidence that losing weight can reduce these adverse outcomes.
The objective of the current study was to examine the association between a successful weight loss intervention and improved COVID-19–related outcomes in patients with obesity, and not to replicate the already established association between obesity and worse COVID-19 outcomes. Two separate cohorts of patients with obesity were constructed, carefully matched at baseline on multiple factors such as BMI, and were followed up for a median of 7.6 years. The surgical group lost 18.6% more body weight over time and experienced better outcomes after contracting SARS-CoV-2 infection. Despite the limitations of retrospective studies (eg, residual measured or unmeasured confounders, including unknown confounders given that COVID-19 is a new disease; control selection bias; loss to follow-up bias; misclassification bias; or differential information bias), we believe this study provides reasonably strong evidence that obesity is a modifiable risk factor for severe COVID-19 infection and that a successful weight loss intervention can play a role in improved COVID-19–related outcomes. Because conducting clinical trials in this setting is not feasible, the findings of this study represent the best available evidence on the implications of a successful weight loss intervention for COVID-19 outcomes.
To examine the association between substantial weight loss and future COVID-19–related outcomes, it was necessary to enroll patients who underwent weight loss surgery many years before the COVID-19 outbreak. This time gap allowed for adequate time to observe the benefits of weight loss surgery for body weight, cardiometabolic risk factors, and obesity-related comorbidities. In this matched analysis of patients with moderate to severe obesity who were enrolled between 2004 and 2017, patients lost a large amount of weight after weight loss surgery and their risk for all-cause non–COVID-19 mortality was decreased by 53% before the beginning of the COVID-19 outbreak in March 2020. Previous research found that large weight loss that was achieved with weight loss surgery was associated with improvement in cardiometabolic, structural, hemodynamic, inflammatory, and coagulation abnormalities as well as survival.5,6,7,8,9,10,11,12,13,14,15,16,20,21
At the time of COVID-19 diagnosis, patients in the surgical group achieved a 20.0-kg lower body weight and better glycemic control. COVID-19 infection is a proinflammatory and prothrombotic disease process that may be favorably altered by surgically induced weight loss through the amelioration of obesity-mediated hyperinflammation, hypercoagulopathy, and metabolic derangements.20,21,22 Furthermore, improvement in the mechanics of breathing and lung physiological functions; better glycemic control; reduction in hypertension level; and lower risk of other coexistent medical conditions such as cardiac disease, sleep apnea, and kidney function may play a role in observed outcomes.22,23,24,25,26 Although the exact underlying mechanisms remain uncertain, these data suggest that patients with a history of weight loss surgery were generally healthier at the time of contracting SARS-CoV-2, which may be associated with better clinical outcomes. With limited therapies for COVID-19 infection, patients with substantial and sustained weight loss were likely physically and physiologically better equipped to cope with an infection that has the potential for multiorgan involvement.22,23,24
The COVID-19 pandemic was a defining event that highlighted the major health consequences of obesity and the vulnerabilities of patients with excessive adipose tissue. The findings of this study emphasize the need to address obesity in an expeditious and comprehensive manner.27,28,29,30 As a complex, chronic, progressive, and costly disease that can affect many organ systems, obesity requires a multidisciplinary and individualized management approach (including lifestyle and behavioral interventions, pharmacotherapy, and weight loss surgery) that is based on the patient’s condition.22 The results of this study should not be misinterpreted as demonstrating the superiority of weight loss surgery over nonsurgical treatment of obesity because the control group consisted of patients with obesity and was not exclusively composed of patients who were actively pursuing behavioral interventions or weight loss medications for their obesity. Findings of this study support the ability to reverse some of the health consequences of obesity in the setting of COVID-19, demonstrating that a successful weight loss approach can be a protective factor in the decreased risk of severe COVID-19 complications.
Although data from several observational studies indicate a higher risk of contracting SARS-CoV-2 and the viral test positivity in patients with obesity,1,2 the current study found a comparable risk for both surgical and control groups. The absence of an association with rates of infection (ie, overlapping of the Kaplan-Meier curves in Figure 2A and a nonsignificant adjusted HR of 1.03) may indirectly suggest a low risk of bias in the current analysis by indicating that the rates of COVID-19 diagnosis were similar in the 2 groups. However, 3 severity-related outcomes were statistically different between the 2 groups.
Limitations
This study has several limitations. First, although we used doubly robust estimation that combined the propensity score and outcome regression to compare outcomes in the surgical and control groups, residual measured or unmeasured confounders could have altered the findings of this cohort study. Second, 11 809 patients were available on March 1, 2020, to be assessed for COVID-19 outcomes. Nonetheless, the baseline characteristics were balanced for most variables in this subgroup of surgical and control patients. For variables with some imbalance, the surgical group actually had a higher risk profile at baseline. Furthermore, the regression adjustment was intended to be the main method of actual statistical adjustment to control any imbalances that existed among the cohorts of patients who were at risk for COVID-19 infection. Third, a relatively small number of events generated wide CIs for these outcomes. Nonetheless, the statistical comparisons between the 2 groups for all prespecified COVID-19 severity-related outcomes consistently reached significance both in the primary comparison and the sensitivity analyses. Given the small number of events and large number of factors included in the Cox proportional hazards regression models, the fully adjusted results should be interpreted with caution.
Fourth, in contrast to patients in the surgical group whose index (or surgery) dates were constant, the patients in the control group were randomly assigned index dates from their counterparts in the surgical group. Including the index date variable in both propensity matching and statistical adjustment and then repeating the random assignment of index dates to control patients for 4 additional times as part of the sensitivity analysis can potentially limit the uncertainty of this approach. Fifth, although the analyses were not adjusted for different COVID-19 therapies that patients received, there is no reason to expect that patients in the surgical group, who were generally healthier than those in the control group at the time of COVID-19 infection, received more effective therapies at CCHS than did the control group. Sixth, although all patients who had a positive SARS-CoV-2 test result received daily telephone calls for 2 weeks to monitor their disease progression, some patients may have been admitted to a hospital outside CCHS, and therefore their outcomes were not captured in the CCHS electronic health record. Seventh, the current study was not designed to identify the underlying mechanisms that were associated with the favorable outcomes in the surgical group. Considering these limitations, the observed associations may be attributed to differences other than treatment assignment (weight loss surgery vs no surgery), and causality cannot be assumed.
Conclusions
Among patients with obesity, substantial weight loss achieved with surgery, compared with no surgery, was associated with a significantly lower risk of hospitalization, need for supplemental oxygen, and severe disease after contracting COVID-19 infection. The findings of this study suggest that obesity can be a modifiable risk factor for the severity of COVID-19 infection.
eFigure. Identification of Eligible Patients for Inclusion
References
- 1.Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):e13128. doi: 10.1111/obr.13128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected—obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021;17(3):135-149. doi: 10.1038/s41574-020-00462-1 [DOI] [PubMed] [Google Scholar]
- 3.Gao M, Piernas C, Astbury NM, et al. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9(6):350-359. doi: 10.1016/S2213-8587(21)00089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Siqueira JVV, Almeida LG, Zica BO, Brum IB, Barceló A, de Siqueira Galil AG. Impact of obesity on hospitalizations and mortality, due to COVID-19: A systematic review. Obes Res Clin Pract. 2020;14(5):398-403. doi: 10.1016/j.orcp.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Földi M, Farkas N, Kiss S, et al. ; KETLAK Study Group . Obesity is a risk factor for developing critical condition in COVID-19 patients: a systematic review and meta-analysis. Obes Rev. 2020;21(10):e13095. doi: 10.1111/obr.13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mingrone G, Panunzi S, De Gaetano A, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2021;397(10271):293-304. doi: 10.1016/S0140-6736(20)32649-0 [DOI] [PubMed] [Google Scholar]
- 7.Schauer PR, Bhatt DL, Kirwan JP, et al. ; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641-651. doi: 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikramuddin S, Korner J, Lee WJ, et al. Lifestyle intervention and medical management with vs without Roux-en-Y gastric bypass and control of hemoglobin A1c, LDL cholesterol, and systolic blood pressure at 5 years in the Diabetes Surgery Study. JAMA. 2018;319(3):266-278. doi: 10.1001/jama.2017.20813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen RV, Pereira TV, Aboud CM, et al. Effect of gastric bypass vs best medical treatment on early-stage chronic kidney disease in patients with type 2 diabetes and obesity: a randomized clinical trial. JAMA Surg. 2020;155(8):e200420. doi: 10.1001/jamasurg.2020.0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien R, Johnson E, Haneuse S, et al. Microvascular outcomes in patients with diabetes after bariatric surgery versus usual care: a matched cohort study. Ann Intern Med. 2018;169(5):300-310. doi: 10.7326/M17-2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324(9):879-887. doi: 10.1001/jama.2020.12567 [DOI] [PubMed] [Google Scholar]
- 12.Aminian A, Zajichek A, Arterburn DE, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA. 2019;322(13):1271-1282. doi: 10.1001/jama.2019.14231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297-2304. doi: 10.1001/jama.2014.5988 [DOI] [PubMed] [Google Scholar]
- 14.Fisher DP, Johnson E, Haneuse S, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. 2018;320(15):1570-1582. doi: 10.1001/jama.2018.14619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlsson LMS, Sjöholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383(16):1535-1543. doi: 10.1056/NEJMoa2002449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Syn NL, Cummings DE, Wang LZ, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021;397(10287):1830-1841. doi: 10.1016/S0140-6736(21)00591-2 [DOI] [PubMed] [Google Scholar]
- 17.Milinovich A, Kattan MW. Extracting and utilizing electronic health data from Epic for research. Ann Transl Med. 2018;6(3):42. doi: 10.21037/atm.2018.01.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 19.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 20.Bhatt DL, Aminian A, Kashyap SR, et al. Cardiovascular biomarkers after metabolic surgery versus medical therapy for diabetes. J Am Coll Cardiol. 2019;74(2):261-263. doi: 10.1016/j.jacc.2019.04.058 [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Zhang J, Liu W, Chen X, Liu Z, Zhou Z. Improvements in humoral immune function and glucolipid metabolism after laparoscopic sleeve gastrectomy in patients with obesity. Surg Obes Relat Dis. 2019;15(9):1455-1463. doi: 10.1016/j.soard.2019.05.021 [DOI] [PubMed] [Google Scholar]
- 22.Aminian A, Fathalizadeh A, Tu C, et al. Association of prior metabolic and bariatric surgery with severity of coronavirus disease 2019 (COVID-19) in patients with obesity. Surg Obes Relat Dis. 2021;17(1):208-214. doi: 10.1016/j.soard.2020.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iannelli A, Bouam S, Schneck AS, et al. The impact of previous history of bariatric surgery on outcome of COVID-19. A nationwide medico-administrative French study. Obes Surg. 2021;31(4):1455-1463. doi: 10.1007/s11695-020-05120-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aminian A, Tu C. Association of bariatric surgery with clinical outcomes of SARS-CoV-2 infection: a systematic review and meta-analysis in the initial phase of COVID-19 pandemic. Obes Surg. 2021;31(6):2419-2425. doi: 10.1007/s11695-020-05213-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubino F, Cohen RV, Mingrone G, et al. Bariatric and metabolic surgery during and after the COVID-19 pandemic: DSS recommendations for management of surgical candidates and postoperative patients and prioritisation of access to surgery. Lancet Diabetes Endocrinol. 2020;8(7):640-648. doi: 10.1016/S2213-8587(20)30157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aminian A, Kermansaravi M, Azizi S, et al. Bariatric surgical practice during the initial phase of COVID-19 outbreak. Obes Surg. 2020;30(9):3624-3627. doi: 10.1007/s11695-020-04617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Executive Council of ASMBS . Safer through surgery: American Society for Metabolic and Bariatric Surgery statement regarding metabolic and bariatric surgery during the COVID-19 pandemic. Surg Obes Relat Dis. 2020;16(8):981-982. doi: 10.1016/j.soard.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belanger MJ, Hill MA, Angelidi AM, Dalamaga M, Sowers JR, Mantzoros CS. Covid-19 and disparities in nutrition and obesity. N Engl J Med. 2020;383(11):e69. doi: 10.1056/NEJMp2021264 [DOI] [PubMed] [Google Scholar]
- 29.Townsend MJ, Kyle TK, Stanford FC. Outcomes of COVID-19: disparities in obesity and by ethnicity/race. Int J Obes (Lond). 2020;44(9):1807-1809. doi: 10.1038/s41366-020-0635-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dicker D, Bettini S, Farpour-Lambert N, et al. Obesity and COVID-19: the two sides of the coin. Obes Facts. 2020;13(4):430-438. doi: 10.1159/000510005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Identification of Eligible Patients for Inclusion