Abstract
Objective
To study the association between early growth of haematoma with biomarkers of endothelial dysfunction such as leukoaraiosis (LA) and the soluble tumour necrosis factor-like weak inducer of apoptosis (sTWEAK) in patients with intracerebral haemorrhage (ICH).
Methods
This is a retrospective observational study of patients with nontraumatic ICH. Clinical and biochemical parameters were analysed. sTWEAK levels were measured by ELISA. LA was analysed in the hemisphere without haemorrhage to avoid interference with the acute injury. The main endpoint was the haematoma growth evaluated by the difference in volume between the second and the initial neuroimage. Poor functional outcome, defined as a modified Rankin Scale >2 at 3 months, was considered as secondary endpoint. Receiver operating characteristic curve analysis was performed to stablish the best cut-off for sTWEAK levels associated with haematoma growth.
Results
We included 653 patients with ICH in our analysis (71.1±11.9 years, 44% women). Haematoma growth was observed in 188 patients (28.8%). sTWEAK levels ≥5600 pg/mL predicted ICH growth with a sensitivity of 84% and a specificity of 87%. sTWEAK levels ≥5600 pg/mL and the presence of LA were associated with haematoma growth (OR: 42.46; (CI 95% 22.67 to 79.52) and OR: 2.73 (CI 95% 1.39 to 5.34), respectively). Also, the presence of LA (OR: 4.31 (CI 95% 2.89 to 6.42)) and the interaction between ICH growth and sTWEAK (OR: 2.23 (CI 95% 1.40 to 3.55)) were associated with poor functional outcome at 3 months.
Conclusion
sTWEAKs, together with the presence and grade of LA, are biomarkers able to predict ICH growth and poor functional outcome in patients with ICH.
Keywords: haemorrhage, vein, vessel wall, brain
Introduction
Intracerebral haemorrhage (ICH) accounts for 10%–15% of all strokes and is associated with poor prognosis.1 2 Leukoaraiosis (LA), also referred to as white matter lesions, has been linked to spontaneous ICH3 and also to the ICH volume in anticoagulated patients and in patients treated with fibrinolytic drugs.4 5 LA has been associated with the early growth of ICH,6 7 which is observed in 10%–30% of patients with ICH1 and is related with both early neurological deterioration and poor outcome at 3 months.8 Conversely, other studies concluded that LA is not associated with neither the ICH volume9–11 nor the ICH growth;12 13 therefore, further studies are still needed to explain the actual implication of LA in the evolution of the ICH lesion.
LA is considered a surrogate marker for small vessel disease,14 and there are several evidences in the literature that show the association of LA with endothelial dysfunction and blood brain barrier (BBB) damage.15 16 Based on these evidences, in this work, we have tested an endothelial dysfunction marker, the soluble tumour necrosis factor-like weak inducer of apoptosis (sTWEAK), as a potential biomarker associated with the presence of LA. sTWEAK is constitutively expressed by monocytes and endothelial cells and binds to the Fn14 receptor resulting in the stimulation of signalling pathways and the release of proinflammatory molecules.17–20 The TWEAK-Fn14 pathway is involved in a considerable number of pathologies, and sTWEAK is released under several inflammatory and degenerative diseases of the central nervous system such as ischaemic stroke,21 subarachnoid haemorrhage,22 traumatic brain injury,23 neuroinflammation24 and could have important implications in the progression of ICH, as demonstrated in preclinical studies.25 As such, we have recently shown the independent association between sTWEAK and LA with haemorrhagic transformation and poor outcome in patients with ischaemic stroke undergoing reperfusion therapies.26
In this work, we hypothesize that sTWEAK could act as a mediator in the early growth of haematoma in patients with ICH and with LA, and consequently may be a biomarker to predict the risk of haematoma growth in patients with ICH and with LA. In this regard, we evaluated the association of serum sTWEAK levels in patients with ICH and with different degrees of LA and in patients with no LA, in relation to early growth of haematoma and the poor functional outcome at 3 months.
Methods
Study design
This is a retrospective observational study of patients with nontraumatic ICH included consecutively and prospectively in a data bank.
The main objective of this study was to analyse the association between sTWEAK and the presence of LA with the early growth of haematoma. The secondary objectives were to analyse the association between sTWEAK and LA with the basal volume of ICH and functional outcome at 3 months.
Standard protocol approvals, registration and patient consents
Patients were treated in the emergency service of a single tertiary university hospital and later admitted to the stroke unit. All patients were treated by neurologists with special training in cerebrovascular diseases and managed according to protocols based on the Spanish and international clinical guidelines.27 28 This study was carried out in accordance with the Declaration of Helsinki of the World Medical Association (2008).
Inclusion and exclusion criteria
From January 2008 to December 2018, 1100 patients with spontaneous ICH were included in the data bank. The inclusion criteria were: (1) authorization for the anonymous use of the data for research (n=1023), (2) CT study at inclusion and between the second and fourth day (n=895), (3) hemispheric location (n=1019), (4) no ICH surgery (n=1089), (5) follow-up (face-to-face or telephone) within a minimum of 3 months (n=1066), (6) no known comorbidity with a life expectancy of less than half a year (n=996), (7) stored serum sample in the stroke biobank (n=986). The final number of included patients was 653.
Clinical and analytical variables
The severity of the neurological deficit was determined by the National Institute of Health Stroke Scale (NIHSS) on admission. Functional outcome was assessed by the modified Rankin Scale (mRS) at discharge and at 3 months±15 days. Both scales were evaluated by internationally certified neurologists and supervised by the same neurologist. Poor outcome was defined as an mRS >2 at 3 months.
Blood samples, obtained from all patients at admission, were collected in test tubes, centrifuged at 3000 g for 15 min and immediately frozen and stored at −80°C. Serum levels of sTWEAK were measured using commercial ELISA kits (Elabsciences, USA) following the manufacturer instructions. The intra and interassay coefficients of variation were 5.1% and 5.2%, respectively. All determinations were performed in a laboratory blinded to clinical and radiological data. The other laboratory tests were conducted by the Central Laboratory of the University Clinical Hospital of Santiago de Compostela and performed on fresh blood samples at the time of diagnosis or shortly after.
Neuroimaging studies
All neuroimaging studies were supervised by the same neuroradiologist who was blinded to this study. The images of LA were stratified according to the Fazekas scale.29 LA was analysed in the initial CT scan in the hemisphere without ICH to avoid interference with the acute injury. The volumes were determined using the ABC/2 method30 until 2016 and through automated planimetric method afterwards. The ICH growth (difference in volume between the second and the initial neuroimage) was categorised in ≤33% versus >33% for its sensitivity and specificity according to the literature.31 32
Statistical method
We have described the variables, either categorical (described with frequency and percentage) or continuous (represented as mean±SD or median and IQR (25th and 75th percentiles)). The adjustment to normal distribution was determined with the Kolmogorov-Smirnov test with the Lilliefors correction. χ2 tests were performed to determine differences in the variables between patients with and without ICH early growth. A receiver operating characteristic (ROC) curve was performed to stablish a cut-off point for sTWEAK levels to predict haematoma growth. Logistic regression analyses were performed to identify those variables independently associated with haematoma growth, initial haematoma volume and poor functional outcome after adjustment by those variables with statistical significance in the bivariate analysis. These results were shown as ORs with 95% CI. A p value <0.05 was considered statistically significant in all analyses. All statistical analyses were conducted in SPSS V.21.0 (IBM, USA).
Results
We analysed a sample of 1100 patients with ICH admitted in a tertiary hospital between January 2008 and December 2018. Among them, 653 were included in our study, with an average age of 71.1±11.9 years and 44% women. We did not find significant differences with the excluded patients in any of the clinical or analytical variables analysed (online supplemental table S1). This analysis demonstrates the lack of bias in the selection of admitted patients, despite the retrospective nature of this study.
svn-2020-000684supp001.pdf (53.6KB, pdf)
Association between haematoma growth with sTWEAK levels and LA
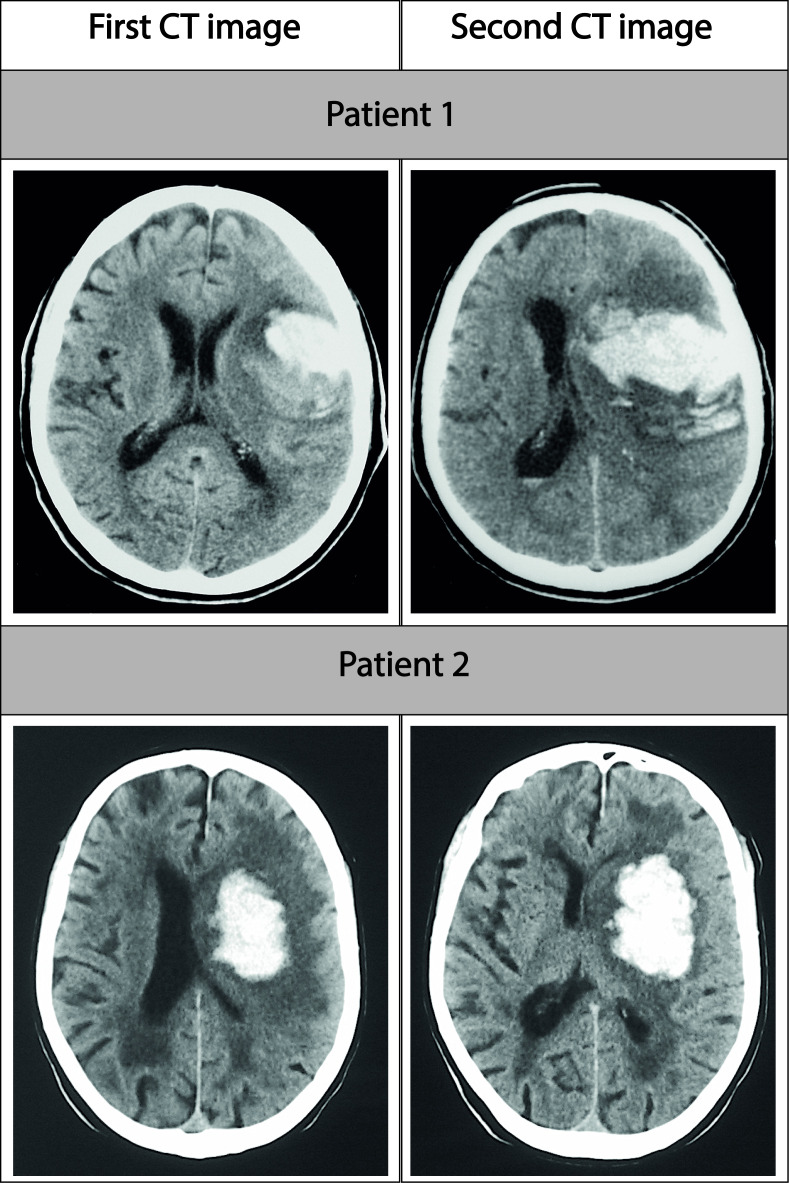
Haematoma growth, defined as an increase of more than 33% in the haematoma volume measured in a second neuroimage, was observed in 188 patients (28.8%). Figure 1 shows a representative example of haematoma growth in three different patients. Taking into consideration that the average latency time (or the time from the ICH symptom onset to the first scan) was 233±208 min, the percentage of patients with ICH growth fits the probability predicted by mathematical models.1 We compared the clinical and analytical variables between patients with and without ICH growth, and significant differences were found in several parameters (listed in table 1) including, among others, the presence and degree of LA (p<0.0001) and the basal volume of ICH (p<0.0001).
Figure 1.
Representative CT images of early haematoma growth for two patients with grade II leukoaraiosis.
Table 1.
Clinical variables, biochemical parameters and neuroimaging values of patients classified according to early haematoma growth
| Early haematoma growth | P value | ||
| No (N=465) | Yes (N=188) | ||
| Age, years | 71.5±11.6 | 70.2±12.5 | 0.200 |
| Women, % | 48.4 | 43 | 0.061 |
| Previous Rankin scale | 1(0, 1) | 0(0, 1) | 0.415 |
| Latency time, min | 237.2±207.4 | 223.5±212.2 | 0.49 |
| Wake-up stroke, % | 5.6 | 4.8 | 0.848 |
| Arterial hypertension, % | 70.5 | 70.7 | 0.919 |
| Diabetes, % | 21.9 | 21.8 | 0.931 |
| Smoking, % | 8.8 | 11.7 | 0.305 |
| Enolism, % | 13.5 | 18.6 | 0.115 |
| Hyperlipidaemia, % | 41.1 | 39.4 | 0.725 |
| Peripheral arterial disease, % |
4.5 | 6.4 | 0.328 |
| Atrial fibrillation, % | 16.6 | 36.2 | <0.0001 |
| Ischaemic heart disease, % |
9.2 | 12.2 | 0.254 |
| Heart failure, % | 2.4 | 5.9 | 0.032 |
| Previous antiaggregants, % | 17 | 12.8 | 0.194 |
| Previous anticoagulants, % | 11.6 | 38.3 | <0.0001 |
| Axillary temperature at admission, °C | 36.6±0.8 | 36.9±0.7 | 0.003 |
| Glucose, mg/dL | 141.3±49.2 | 136.8±46.7 | 0.297 |
| Glycosylated haemoglobin, % | 5.9±1.0 | 5.9±0.9 | 0.85 |
| Leucocytes, ×103/mL | 9.2±3.2 | 8.9±3.3 | 0.272 |
| Haemoglobin, g/dL | 13.6±4.7 | 13.1±3.3 | 0.319 |
| Platelets, ×103/mL | 212.1±71.7 | 209.8±80.6 | 0.422 |
| Fibrinogen, mg/d | 448.3±103.2 | 432.5±99.9 | 0.101 |
| LDL cholesterol, mg/dL | 108.9±34.6 | 112.5±36.9 | 0.354 |
| HDL cholesterol, mg/dL | 39.7±21.3 | 36.6±17.6 | 0.152 |
| Triglycerides, mg/dL | 104.3±51.9 | 104.6±48.2 | 0.947 |
| Sedimentation rate, mm | 30.5±22.8 | 24.9±19.7 | 0.004 |
| Leukoaraiosis, % | 57.8 | 85.1 | <0.0001 |
| Degree of leukoaraiosis | <0.0001 | ||
| Grade I, % | 68.6 | 31.4 | |
| Grade II, % | 62 | 38 | |
| Grade III, % | 46.8 | 53.2 | |
| Basal volume ICH, mL | 44.7±38.8 | 29.6±28.8 | <0.0001 |
| Location of the ICH | 0.018 | ||
| Lobar, % | 43.9 | 35.5 | |
| Deep, % | 56.1 | 66.5 | |
| Ventricular/subarachnoid contamination, % | 19.4 | 17.6 | 0.659 |
| NIHSS at admission | 14 [10, 19] | 13 [9, 17] | <0.0001 |
| Rankin scale at 3 months | 3 [1, 6] | 3 [2, 6] | 0.007 |
| Poor outcome at 3 months, % | 59.8 | 71.3 | 0.007 |
| Etiopathogenesis | <0.0001 | ||
| Hypertensive, % | 57.8 | 36.7 | |
| Amyloid, % | 14.4 | 4.3 | |
| Antiplatelet drugs/anticoagulants, % | 11.4 | 39.9 | |
| Indeterminate/others, % | 16.3 | 19.1 | |
HDL, High Density Lipoprotein; ICH, intracerebral haemorrhage; LDL, Low Density Lipoprotein; NIHSS, National Institute of Health Stroke Scale.
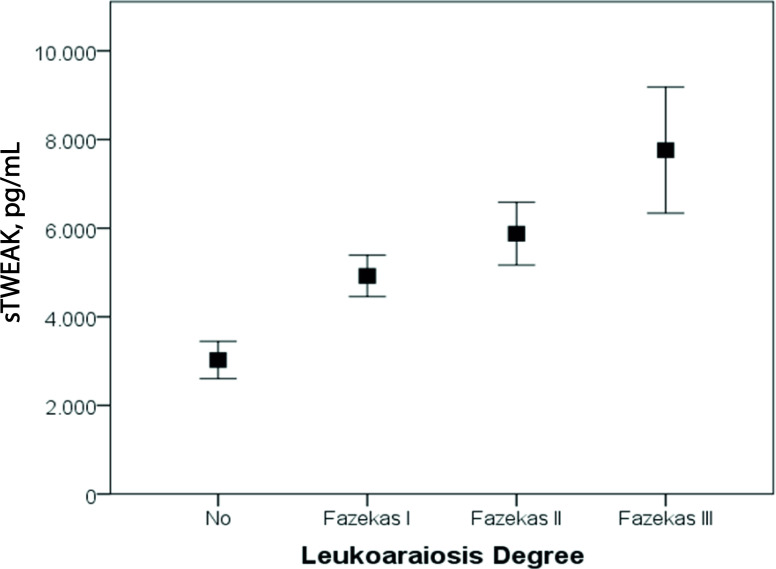
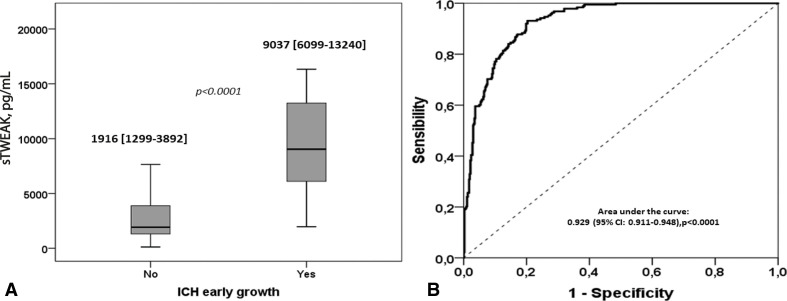
In agreement with our previous study, in this cohort of patients, we found a correlation between sTWEAK levels and the degree of LA (figure 2, Spearman’s rho=0.321; p<0.001). sTWEAK levels were analysed in relation to the ICH early growth (figure 3A). It was observed that sTWEAK levels were significantly lower in those patients without ICH growth compared with patients with ICH growth (1916 (1299 to 3892) versus 9037 (6099 to 13240) pg/mL, p<0.0001). Patients with ICH with no LA had significantly lower levels of sTWEAK compared with patients with LA (1894 (1167 to 3715) vs 4838 (1877 to 8207) pg/mL, p<0.001).
Figure 2.
Variation of the serum sTWEAK levels in patients with ICH with different leukoaraiosis degrees. ICH, intracerebralhaemorrhage; sTWEAK, soluble tumour necrosisfactor-like weak inducer of apoptosis.
Figure 3.
(A) Median values of serum sTWEAK levels measured in patients without and with early growth of ICH. Mann-Whitney test was used to evaluate the p value. (B) ROC curve analysis illustrating the ability of sTWEAK to diagnose early ICH growth. ICH, intracerebral haemorrhage; ROC, receiver operating characteristic; sTWEAK, soluble tumour necrosis factor-like weakinducer of apoptosis.
Next, a ROC analysis was carried out to define the best cut-off for sTWEAK levels to predict haematoma growth, obtaining an area under the curve of 0.929 (CI 95% 0.911 to 0.948, p<0.0001). The optimal sTWEAK operating point from the ROC curve corresponded to 5600 pg/mL, with a sensitivity of 84% and a specificity of 87% (figure 3B). This value was, therefore, taken as a reference for the subsequent multivariate analysis.
The association of sTWEAK and LA with the haematoma growth was evaluated with a logistic regression analysis (table 2). sTWEAK levels ≥5600 pg/mL were associated with haematoma growth (OR: 42.46 (CI 95% 22.67 to 79.52)) as well as the presence of LA (OR: 2.73 (CI 95% 1.39 to 5.34)) and the ICH basal volume (OR: 0.96 (CI 95% 0.95 to 0.97)). A second logistic regression model was carried out to evaluate the association of haematoma growth with the three degrees of LA severity in relation with the sTWEAK levels. Here, a similar association was observed between haematoma growth and sTWEAK levels (OR: 46.63 (CI 95% 24.18 to 89.9)) and ICH basal volume (OR: 0.96 (CI 95% 0.94 to 0.97)); however, we found differences regarding the three grades of LA, since grade I LA was not associated haematoma growth while grades II and III LA were associated with LA with higher OR values (OR: 3.42 (CI 95% 1.59 to 7.35), and 7.79 (CI 95% 2.75 to 22.08), respectively).
Table 2.
Logistic regression model for factors related with early haematoma growth
| Unadjusted | Adjusted* | |||||
| OR | 95% CI | P | OR | 95% CI | P | |
| sTWEAK ≥5600 pg/mL | 29.29 | 18.54 to 46.30 | <0.0001 | 42.46 | 22.67 to 79.52 | <0.0001 |
| ICH basal volume | 0.98 | 0.98 to 0.99 | <0.0001 | 0.96 | 0.95 to 0.97 | <0.0001 |
| Leukoaraiosis | 4.16 | 2.68 to 6.48 | <0.0001 | 2.73 | 1.39 to 5.34 | 0.003 |
| Unadjusted | Adjusted† | |||||
| OR | 95% CI | P | OR | 95% CI | P | |
| sTWEAK ≥5600 pg/mL | 29.29 | 18.54 to 46.30 | <0.0001 | 46.63 | 24.18 to 89.9 | <0.0001 |
| ICH basal volume | 0.98 | 0.98 to 0.99 | <0.0001 | 0.96 | 0.94 to 0.97 | <0.0001 |
| Leukoaraiosis | ||||||
| No LA | Ref. | – | – | Ref | – | – |
| Grade I | 3.2 | 1.94 to 5.29 | <0.0001 | 1.55 | 0.7 to 3.43 | 0.275 |
| Grade II | 4.24 | 2.61 to 7.06 | <0.0001 | 3.42 | 1.59 to 7.35 | 0.002 |
| Grade III | 7.97 | 4.21 to 15.06 | <0.0001 | 7.79 | 2.75 to 22.08 | <0.0001 |
*Adjusted by atrial fibrillation, heart failure, previous anticoagulants, temperature at 183 admissions, sedimentation rate, deep ICH, NIHSS at admission, ICH for anticoagulants, basal ICH volume, presence of LA and sTWEAK ≥5600 pg/mL.
†Adjusted by the same variables; the presence of LA was replaced by the three grades of LA.
ICH, intracerebral haemorrhage; LA, leukoaraiosis; NIHSS, National Institute of Health Stroke Scale; sTWEAK, soluble tumour necrosis factor-like weak inducer of apoptosis.
Association between the basal volume of ICH with sTWEAK levels and LA
The association of sTWEAK levels and the presence of LA were analysed in relation with the ICH basal volumes measured at admission. We did not find a correlation between sTWEAK and ICH volumes (p=0.231). In addition, we did not find significant differences in basal ICH volume neither between patients without and with LA (p=0.163) nor among patients with different degrees of LA (p=0.539).
Association between the functional outcome of patients with ICH with serum sTWEAK levels and LA
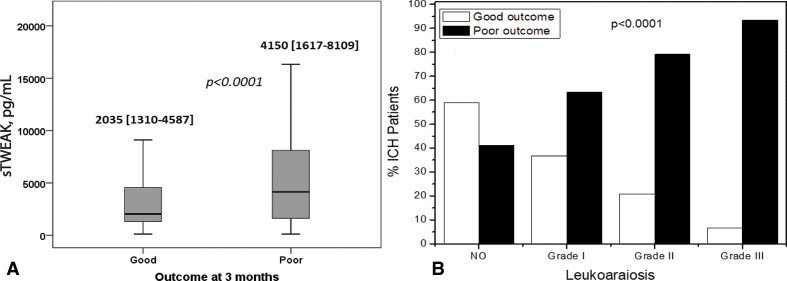
Finally, we analysed the association between the functional outcome of patients with ICH and with the sTWEAK levels and the degree of LA (figure 4A). Poor functional outcome was defined as an mRS >2 evaluated at 3 months after hospital discharge. Patients with poor outcome showed higher levels of sTWEAK than patients with good outcome (4150 (1617 to 8109) vs 2035 (1310 to 4558) pg/mL, p<0.0001). In addition, the presence and degree of LA also had a significant influence on the outcome of patients, as illustrated in figure 4B.
Figure 4.
(A) Median values of serum sTWEAK levels measured in patients with good and poor functional outcome at 3 months. Mann-Whitney test was used to evaluate the p value. ((B) Percentage of patients with good and poor outcome at 3 months in relation with the grade of leukoaraiosis. Kruskal-Wallis test was used to evaluate the p value. sTWEAK, soluble tumour necrosis factor-like weakinducer of apoptosis.
Analogous to the analysis performed for haematoma growth, a bivariate analysis was carried out using the functional outcome as the dependent variable (table 3). Next, we carried out a logistic regression analysis using the clinical outcome as the dependent variable. The clinical and analytical variables that showed significant differences between patients with good and poor outcomes were included in the analysis, and the results were adjusted using the presence of LA or the degree of LA (table 4). In this case, the model showed that the presence of LA was associated with poor outcome (OR: 4.31 (CI 95% 2.89 to 6.42)). In this model, we observed that neither sTWEAK levels ≥5600 pg/mL (OR: 1.57 (CI 95% 0.87 to 2.83)) nor the early haematoma growth OR: 1.61 (CI 95% 0.88 to 2.94)) was associated with poor outcome; however, the interaction of both parameters was significantly associated with poor outcome (OR: 2.23 (CI 95% 1.40 to 3.55)). The second logistic regression model including the three degrees of LA showed a similar association between the clinical outcome with the three degrees of LA, being lower for patients with grade I LA (OR: 2.63 (CI 95% 2.66 to 4.14)), compared with grade II (OR: 6.05 (CI 95% 3.67 to 9.98)) and grade III LA (OR: 17.03 (CI 95% 5.72 to 50.68)). The interaction between sTWEAK and early haematoma growth associated with poor outcome was also observed in the second logistic regression model.
Table 3.
Clinical variables, biochemical parameters and neuroimaging values of patients classified according to the clinical outcome at 3 months
| Outcome at 3 months | |||
| Good (242) | Poor (411) | P value | |
| Age, years | 67.9±12.5 | 72.8±10.9 | <0.0001 |
| Women, % | 45.5 | 43.1 | 0.568 |
| Previous Rankin scale | 0 (0, 1) | 1 (0, 1) | 0.015 |
| Latency time, min | 251.6±216.3 | 221.1±203.5 | 0.113 |
| Wake-up stroke, % | 5.4 | 5.4 | 1 |
| Arterial hypertension, % | 69.4 | 71.3 | 0.657 |
| Diabetes, % | 19.4 | 23.4 | 0.281 |
| Smoking, % | 12 | 8.3 | 0.132 |
| Enolism, % | 14 | 15.6 | 0.651 |
| Hyperlipidaemia, % | 43 | 39.2 | 0.364 |
| Peripheral arterial disease, % | 3.7 | 5.8 | 0.27 |
| Atrial fibrillation, % | 14.9 | 26.5 | 0.001 |
| Ischaemic heart disease, % | 7 | 11.9 | 0.059 |
| Heart failure, % | 2.1 | 4.1 | 0.183 |
| Previous antiaggregants, % | 14.9 | 16.3 | 0.658 |
| Previous anticoagulants, % | 11.2 | 24.1 | <0.0001 |
| Axillary temperature at admission, °C | 36.5±0.7 | 36.8±0.8 | <0.0001 |
| Glucose, mg/dL | 128.2±45.7 | 146.6±48.8 | <0.0001 |
| Glycosylated haemoglobin, % | 5.8±0.9 | 5.9±1.0 | 0.286 |
| Leucocytes, ×103/mL | 8.6±2.7 | 9.4±3.4 | 0.001 |
| Haemoglobin, g/dL | 13.2±3.9 | 12.9±6.5 | 0.407 |
| Platelets, ×103/mL | 210.9±75.8 | 207.5±91.7 | 0.514 |
| Fibrinogen, mg/dL | 436.2±89.6 | 447.9±109.9 | 0.203 |
| LDL cholesterol, mg/dL | 114.8±33.3 | 106.9±36.4 | 0.032 |
| HDL cholesterol, mg/dL | 37.4±16.8 | 39.4±22.1 | 0.346 |
| Triglycerides, mg/dL | 104.3±50.1 | 104.5±51.3 | 0.98 |
| Sedimentation rate, mm | 25.0±17.6 | 31.2±24.1 | 0.001 |
| Leukoaraiosis, % | 45.5 | 77.6 | <0.0001 |
| Degree of leukoaraiosis | <0.0001 | ||
| Grade I, % | 28.5 | 29 | |
| Grade II, % | 15.8 | 34.5 | |
| Grade III, % | 1.7 | 14.1 | |
| Basal volume ICH, mL | 25.6±20.8 | 49.0±41.2 | <0.0001 |
| Location of the ICH | 0.458 | ||
| Lobar, % | 38.8 | 42.1 | |
| Deep, % | 61.2 | 57.9 | |
| Ventricular/subarachnoid contamination, % | 13.6 | 21.9 | 0.01 |
| NIHSS at admission | 10 [6, 14] | 16 [13, 20] | <0.0001 |
| Etiopathogenesis | <0.0001 | ||
| Hypertensive, % | 56.2 | 49.1 | |
| Amyloid, % | 11.2 | 11.7 | |
| Antiplatelet drugs/anticoagulants, % | 11.6 | 24.3 | |
| Indeterminate/others, % | 21.1 | 14.8 | |
| sTWEAK (cat) ≥5600 pg/mL, % | 18.7 | 41.7 | <0.0001 |
| Early haematoma growth % | 21.1 | 33.3 | 0.001 |
HDL, High Density Lipoprotein; ICH, intracerebral haemorrhage; LDL, Low Density Lipoprotein; NIHSS, National Institute of Health Stroke Scale; sTWEAK, soluble tumour necrosis factor-like weak inducer of apoptosis.
Table 4.
Logistic regression model for factors related with poor clinical outcome at 3 months
| Unadjusted | Adjusted* | |||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| sTWEAK ≥5600 pg/mL | 3.12 | 2.13 to 4.55 | <0.0001 | 1.57 | 0.87 to 2.83 | 0.129 |
| ICH basal volume | 1.03 | 1.03 to 1.04 | <0.0001 | 1.04 | 1.03 to 1.05 | <0.0001 |
| Leukoaraiosis | 4.16 | 2.92 to 5.86 | <0.0001 | 4.31 | 2.89 to 6.42 | <0.0001 |
| ICH growth | 1.87 | 1.29 to 2.71 | 0.001 | 1.61 | 0.88 to 2.94 | 0.123 |
| sTWEAK×ICH growth | 2.23 | 1.40 to 3.55 | 0.001 | |||
| Unadjusted | Adjusted† | |||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| sTWEAK ≥5600 pg/mL | 3.12 | 2.13 to 4.55 | <0.0001 | 1.67 | 0.93 to 2.99 | 0.086 |
| ICH basal volume | 1.03 | 1.03 to 1.04 | <0.0001 | 1.03 | 1.03 to 1.04 | <0.0001 |
| Leukoaraiosis | ||||||
| No LA | Ref. | – | – | Ref | – | – |
| Grade I | 1.43 | 1.06 to 1.92 | 0.019 | 2.63 | 1.66 to 4.14 | <0.0001 |
| Grade II | 2.47 | 1.79 to 3.39 | <0.0001 | 6.05 | 3.67 to 9.98 | <0.0001 |
| Grade III | 3.07 | 1.89 to 4.97 | <0.0001 | 17.03 | 5.72 to 50.68 | <0.0001 |
| ICH growth | 1.87 | 1.29 to 2.71 | 0.001 | 1.49 | 0.82 to 2.73 | 0.193 |
| sTWEAK×ICH growth | 2.01 | 1.27 to 3.23 | 0.003 | |||
*Adjusted by age, previous mRS, atrial fibrillation, previous anticoagulants, temperature at admission, glucose, leucocytes, C reactive protein, LDL cholesterol, sedimentation rate, basal ICH volume, presence of LA, ICH growth and sTWEAK ≥5600 pg/mL.
†Adjusted by the same variables; the presence of LA was replaced by the three grades of LA.
HDL, High Density Lipoprotein; ICH, intracerebral haemorrhage; LA, leukoaraiosis; LDL, Low Density Lipoprotein; mRS, modified Rankin Scale; sTWEAK, soluble tumour necrosis factor-like weak inducer of apoptosis.
Discussion
The goals of this study were to demonstrate the association between the presence of LA and the high levels of sTWEAK in patients with ICH and with the early growth of haematoma and poor functional outcome at 3 months. In this work, we observed a relation between sTWEAK and LA, in agreement with our previous studies in patients with ischaemic stroke undergoing reperfusion treatments.26
Our results demonstrate the association between LA and early haematoma growth in ICH and also suggest that this relation is mediated by sTWEAK, a soluble cytokine that is expressed in many inflammatory and degenerative diseases of the central nervous system.19 24 The association of sTWEAK or the TWEAK/Fn14 pathway with neuronal cell death and oedema progression has been reported before. In this regard, previous studies have reported the presence of elevated sTWEAK levels in the serum of patients with ischaemic stroke, suggesting its potential application as a blood biomarker able to detect ischaemic stroke and responsiveness to therapeutic intervention.33 34 Regarding haemorrhagic stroke, although preclinical studies demonstrated that sTWEAK contributes to cerebral endothelial dysfunction and oedema progression in animal models of ICH,25 the association of sTWEAK with the progression of the lesion has not been demonstrated yet. In our study, we observed a remarkable increase in the risk of developing early ICH growth in patients with high sTWEAK levels. Moreover, sTWEAK serum levels were able to predict the development of early ICH growth with a sensitivity and specificity of 85%, highlighting the value of sTWEAK as a reliable biomarker for ICH growth.
The relationship between LA and ICH growth is still not well understood and the clinical data available so far are still controversial. While some authors have reported a direct association between LA and ICH growth,6 other studies have showed the opposite, no relation between the haematoma expansion and LA.12 13 In our series, we found a direct association of early ICH growth with the presence of LA in patients with ICH, being this relationship higher for patients with and advanced LA (grades II and III). An important factor that should be taken into consideration when evaluating ICH growth is the time from ICH symptom onset to baseline scan, since the probabilities to observe ICH growth decrease over time. Our data fit the predicted percentage of patients with ICH growth, according to the mathematical models,1 and the association observed between LA and ICH growth was not influenced by the time between the symptom’s onset and the time to hospital arrival and ICH image. Furthermore, our multivariate regression model showed that the association of the ICH growth and the poor outcome could be mediated by sTWEAK in patients with LA. Here, we have demonstrated the association between serum sTWEAK levels and LA, which could be a consequence of cerebral endothelial dysfunction,35 36 so it was expected to observe an association between LA and ICH growth independent on sTWEAK levels. The increase in the OR values of sTWEAK after including LA in the model could be derived from the inflammatory response that triggers the expression of sTWEAK and the subsequent release of other cytokines such as metalloproteases and interleukins,17 25 37 with deleterious consequences in the clinical outcome of patients, as discussed next.
The secondary objectives of this study were to analyse the association of LA and sTWEAK with both ICH basal volume and the clinical outcome at 3 months. We did not find an association between LA and ICH basal volume. Our results are in agreement with the previous studies,9–13 although some authors have found an association between LA and ICH volume in anticoagulated patients and in patients treated with fibrinolytics.4 5 Regarding the clinical outcome at 3 months, we observed an association between both LA and sTWEAK with a poor prognosis in ICH, which hindered the influence of the basal ICH volume over the outcome at 3 months. sTWEAK is known to activate the Fn14 receptor in endothelial cells, triggering the release of several proinflammatory cytokines, matrix metalloproteases (MMP9) and the leucocyte adhesion.24 These mechanisms can exacerbate the deleterious effect of the ICH and increase bleeding; indeed, the activation of the TWEAK-Fn14 signalling pathway has a pro-inflammatory effect that could be blocked during the recruitment phase of immune cells across the BBB,37 which could yield new therapeutic options for patients with ICH.
This work has the common limitations of retrospective studies, and there is a lack of a validation cohort to support the predictive value of sTWEAK in haematoma growth. Besides, there are no previous preclinical data supporting the molecular mechanisms involved in the interactions of sTWEAK with haematoma growth. Experimental studies to confirm the observed relationships are still necessary. Another weakness is the analysis of the soluble levels of sTWEAK and not the TWEAK-Fn14 association, which could have more implications in the development of ICH. Also, we only have evaluated the association of LA and sTWEAK with the evolution of the ICH volume, while other neuroimage markers of haematoma growth could have some implications in the sTWEAK levels. The strength of this work is the unbiased selection of patients, the high number of included patients and the blinded analysis of sTWEAK.
In conclusion, our study shows that sTWEAK and LA are biomarkers with capacity to predict haematoma growth and poor functional outcome in patients with ICH.
Footnotes
Contributors: Conception and design of the study (PH, RI, JC). Data acquisition and analysis (AdS-C, PAG.). Clinical data acquisition and analysis (MR-Y, JMP ILD, JC). Handled funding and supervision (FC, TS, JC). Statistical analysis (PH and JC). Manuscript drafting (PH, RI, JC). Critical revision for important intellectual content (FC, TS, JC). Supervision (PH, RI, JC). All authors reviewed and approved the manuscript.
Funding: This study was partially supported by grants from the Spanish Ministry of Science and Innovation (SAF2017-84267-R), Xunta de Galicia (Axencia Galega de Innovación (GAIN): IN607A2018/3), Instituto de Salud Carlos III (ISCIII) (PI17/00540, PI17/01103), Spanish Research Network on Cerebrovascular Diseases RETICS-INVICTUS PLUS (RD16/0019) and by the European Union FEDER program. T. Sobrino (CPII17/00027), F. Campos (CPII19/00020) are recipients of research contracts from the Miguel Servet Program (Instituto de Salud Carlos III). The sponsors did not participate in the study design, collection, analysis, or interpretation of the data, in writing the report, or in the decision to submit the paper for publication.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. All data are available within the text of the manuscript. Further anonymized data could be made available to qualified investigators upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Study was approved by the Clinical Research Ethics Committee of Galicia (registration codes 2019/616 and 2016/399). Informed consent was obtained from patients or their relatives at the time of inclusion in the registry, authorising the anonymous use of data for further studies.
References
- 1. Al-Shahi Salman R, Frantzias J, Lee RJ, et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol 2018;17:885–94. 10.1016/S1474-4422(18)30253-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iglesias-Rey R, Rodríguez-Yáñez M, Arias S, et al. Inflammation, edema and poor outcome are associated with hyperthermia in hypertensive intracerebral hemorrhages. Eur J Neurol 2018;25:1161–8. 10.1111/ene.13677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vagal V, Venema SU, Behymer TP, et al. White matter lesion severity is associated with intraventricular hemorrhage in spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2020;29:104661. 10.1016/j.jstrokecerebrovasdis.2020.104661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith EE, Rosand J, Knudsen KA, et al. Leukoaraiosis is associated with warfarin-related hemorrhage following ischemic stroke. Neurology 2002;59:193–7. 10.1212/wnl.59.2.193 [DOI] [PubMed] [Google Scholar]
- 5. Curtze S, Haapaniemi E, Melkas S, et al. White matter lesions double the risk of Post-Thrombolytic intracerebral hemorrhage. Stroke 2015;46:2149–55. 10.1161/STROKEAHA.115.009318 [DOI] [PubMed] [Google Scholar]
- 6. Lou M, Al-Hazzani A, Goddeau Richard P. Relationship between white-matter hyperintensities and hematoma volume and growth in patients with intracerebral hemorrhage. Stroke 2010;41:34–40. 10.1161/STROKEAHA.109.564955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen X, Jin Y, Chen J, et al. Relationship between white matter hyperintensities and hematoma volume in patients with intracerebral hematoma. Aging Dis 2018;9:999–1009. 10.14336/AD.2018.0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leira R, Dávalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology 2004;63:461–7. 10.1212/01.wnl.0000133204.81153.ac [DOI] [PubMed] [Google Scholar]
- 9. Caprio FZ, Maas MB, Rosenberg NF, et al. Leukoaraiosis on magnetic resonance imaging correlates with worse outcomes after spontaneous intracerebral hemorrhage. Stroke 2013;44:642–6. 10.1161/STROKEAHA.112.676890 [DOI] [PubMed] [Google Scholar]
- 10. Yu Z, Zheng J, Guo R, et al. Prognostic significance of leukoaraiosis in intracerebral hemorrhage: a meta-analysis. J Neurol Sci 2019;397:34–41. 10.1016/j.jns.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 11. Tveiten A, Ljøstad U, Mygland Åse, et al. Leukoaraiosis is associated with short- and long-term mortality in patients with intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2013;22:919–25. 10.1016/j.jstrokecerebrovasdis.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 12. Boulouis G, van Etten ES, Charidimou A, et al. Association of key magnetic resonance imaging markers of cerebral small vessel disease with hematoma volume and expansion in patients with lobar and deep intracerebral hemorrhage. JAMA Neurol 2016;73:1440–7. 10.1001/jamaneurol.2016.2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sykora M, Herweh C, Steiner T. The association between leukoaraiosis and poor outcome in intracerebral hemorrhage is not mediated by hematoma growth. J Stroke Cerebrovasc Dis 2017;26:1328–33. 10.1016/j.jstrokecerebrovasdis.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 14. Lambert C, Benjamin P, Zeestraten E, et al. Longitudinal patterns of leukoaraiosis and brain atrophy in symptomatic small vessel disease. Brain 2016;139:1136–51. 10.1093/brain/aww009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Topakian R, Barrick TR, Howe FA, et al. Blood-Brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry 2010;81:192–7. 10.1136/jnnp.2009.172072 [DOI] [PubMed] [Google Scholar]
- 16. Hassan A, Hunt BJ, O’Sullivan M. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain 2003;126:424–32. [DOI] [PubMed] [Google Scholar]
- 17. Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov 2008;7:411–25. 10.1038/nrd2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wajant H. The TWEAK-Fn14 system as a potential drug target. Br J Pharmacol 2013;170:748–64. 10.1111/bph.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Potrovita I, Zhang W, Burkly L, et al. Tumor necrosis factor-like weak inducer of apoptosis-induced neurodegeneration. J Neurosci 2004;24:8237–44. 10.1523/JNEUROSCI.1089-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Echeverry R, Wu F, Haile WB, et al. The cytokine tumor necrosis factor-like weak inducer of apoptosis and its receptor fibroblast growth factor-inducible 14 have a neuroprotective effect in the central nervous system. J Neuroinflammation 2012;9:45. 10.1186/1742-2094-9-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Comertpay E, Vural S, Eroğlu O. The diagnostic value of sTWEAK in acute ischemic stroke. Balkan Med J 2020;37:336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dai JX, Cai JY, Sun J. Serum soluble tumor necrosis factor-like weak inducer of apoptosis is a potential biomarker for outcome prediction of patients with aneurysmal subarachnoid hemorrhage. Clin Chim Acta 2020;510:354–9. [DOI] [PubMed] [Google Scholar]
- 23. Tang B, Zhong Z, Qiu Z, et al. Serum soluble TWEAK levels in severe traumatic brain injury and its prognostic significance. Clinica Chimica Acta 2019;495:227–32. 10.1016/j.cca.2019.04.070 [DOI] [PubMed] [Google Scholar]
- 24. Boulamery A, Desplat-Jégo S. Regulation of neuroinflammation: what role for the tumor necrosis factor-like weak inducer of Apoptosis/Fn14 pathway? Front Immunol 2017;8:1534. 10.3389/fimmu.2017.01534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang J, Dong B, Hao J. Lncrna Snhg3 contributes to dysfunction of cerebral microvascular cells in intracerebral hemorrhage rats by activating the TWEAK/Fn14/STAT3 pathway. Life sciences 2019;237:116929. [DOI] [PubMed] [Google Scholar]
- 26. da Silva-Candal A, Pérez-Mato M, Rodríguez-Yáñez M, et al. The presence of leukoaraiosis enhances the association between sTWEAK and hemorrhagic transformation. Ann Clin Transl Neurol 2020;7:2103–14. 10.1002/acn3.51171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez-Yanez M, Castellanos M, Freijo MM. Clinical practice guidelines in intracerebral haemorrhage. Neurologia 2013;28:236–49. [DOI] [PubMed] [Google Scholar]
- 28. Hemphill JC, Greenberg SM, Anderson CS. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke 2015;46:2032–60. [DOI] [PubMed] [Google Scholar]
- 29. Ferguson KJ, Cvoro V, MacLullich AMJ. Visual rating scales of white matter hyperintensities and atrophy: comparison of computed tomography and magnetic resonance imaging. J Stroke Cerebrovasc Dis 2018;27:1815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sims JR, Gharai LR, Schaefer PW. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 2009;72:2104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al-Shahi Salman R, Frantzias J, Lee RJ. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. The Lancet Neurology 2018;17:885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dowlatshahi D, Demchuk AM, Flaherty ML. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology 2011;76:1238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Inta I, Frauenknecht K, Dörr H, et al. Induction of the cytokine TWEAK and its receptor Fn14 in ischemic stroke. J Neurol Sci 2008;275:117–20. 10.1016/j.jns.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 34. Augello CJ, Noll JM, Distel TJ, et al. Identification of novel blood biomarker panels to detect ischemic stroke in patients and their responsiveness to therapeutic intervention. Brain Res 2018;1698:161–9. 10.1016/j.brainres.2018.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wen J, Doerner J, Weidenheim K, et al. Tnf-Like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice. J Autoimmun 2015;60:40–50. 10.1016/j.jaut.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yepes M. Tweak and Fn14 in the neurovascular unit. Front Immunol 2013;4:367. 10.3389/fimmu.2013.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stephan D, Sbai O, Wen J, et al. Tweak/Fn14 pathway modulates properties of a human microvascular endothelial cell model of blood brain barrier. J Neuroinflammation 2013;10:9. 10.1186/1742-2094-10-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000684supp001.pdf (53.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. All data are available within the text of the manuscript. Further anonymized data could be made available to qualified investigators upon reasonable request.