Abstract
Background
Poor cardiorespiratory fitness (CRF) is a cardinal feature of post‐treatment primary lung cancer. The most effective exercise therapy regimen to improve CRF has not been determined.
Methods
In this parallel‐group factorial randomized controlled trial, lung cancer survivors with poor CRF (below age–sex sedentary values) were randomly allocated to receive 48 consecutive supervised sessions thrice weekly of (i) aerobic training (AT)—cycle ergometry at 55% to >95% of peak oxygen consumption (VO2peak); (ii) resistance training (RT)—lower and upper extremity exercises at 50–85% of maximal strength; (iii) combination training (CT)—AT plus RT; or (iv) stretching attention control (AC) for 16 weeks. The primary endpoint was change in CRF (VO2peak, mL O2·kg−1·min−1). Secondary endpoints were body composition, muscle strength, patient‐reported outcomes, tolerability (relative dose intensity of exercise), and safety. Analysis of covariance determined change in primary and secondary endpoints from baseline to post‐intervention (Week 17) with adjustment for baseline values of the endpoint and other relevant clinical covariates.
Results
Ninety patients (65 ± 9 years; 66% female) were randomized (AT, n = 24; RT, n = 23; CT, n = 20; and AC, n = 23) of the planned n = 160. No serious adverse events were observed. For the overall cohort, the lost‐to‐follow‐up rate was 10%. Mean attendance was ≥75% in all groups. In intention‐to‐treat analysis, VO2peak increased 1.1 mL O2·kg−1·min−1 [95% confidence interval (CI): 0.0, 2.2, P = 0.04] and 1.4 mL O2·kg−1·min−1 (95% CI: 0.2, 2.5, P = 0.02) in AT and CT, respectively, compared with AC. There was no difference in VO2peak change between RT and AC (−0.1 mL O2·kg−1·min−1, 95% CI: −1.2, 1.0, P = 0.88). Favourable improvements in maximal strength and body composition were observed in RT and CT groups compared with AT and AC groups (Ps < 0.05). No between‐group changes were observed for any patient‐reported outcomes. Relative dose intensity of exercise was lower in RT and CT compared with AT (Ps < 0.05).
Conclusions
In the context of a smaller than planned sample size, AT and CT significantly improved VO2peak in lung cancer survivors; however, the tolerability‐to‐benefit ratio was superior for AT and hence may be the preferred modality to target impaired CRF in post‐treatment lung cancer survivors.
Keywords: Aerobic training, Resistance training, Combination training, Exercise capacity, Cancer survivorship
Introduction
Improvements in screening, surgical procedures, and more effective combination chemotherapeutic regimens have resulted in significant survival gains in post‐surgical lung cancer. 1 Consequently, longer‐term disease and treatment‐related morbidity and mortality are now of major clinical importance in patient‐centred, comprehensive lung cancer management. Poor cardiorespiratory fitness (CRF) is a cardinal feature in post‐treatment lung cancer survivors. Impaired CRF is a consequence of direct as well as indirect (i.e. lifestyle perturbations) adverse effects of surgery and adjuvant therapy on all organ components of the cardiopulmonary system. 2 , 3 Poor CRF is associated with poor survival from both cancer‐related and non‐cancer‐related causes as well as higher symptom burden (e.g. poor quality of life and fatigue). 4 , 5 Thus, strategies to improve CRF in lung cancer survivors are of major clinical importance. 6
Mechanistically, impaired CRF in lung cancer survivors is likely governed by both central (e.g. reduced convective O₂ transport) and peripheral (e.g. decreased diffusive O2 transport and oxidative capacity in the skeletal muscle) factors. 7 Aerobic (exercise) training (AT) is arguably the most effective method to improve CRF in healthy humans given favourable adaptations across all O2 transport components (except lung diffusion capacity 8 ). Findings from several studies indicate that AT is a safe and effective intervention for lung cancer patients 9 , 10 , 11 ; however, the improvements in CRF were modest (<15%), particularly in the post‐operative setting (~10%), despite good exercise attendance rates (≥70% of planned sessions). 12 , 13 Although less recognized, resistance training (RT) also improves CRF in older adults, possibly through improvements in peripheral limitations (e.g. diffusive O2 delivery to the mitochondria) 14 and concomitant improvements in muscle quality (e.g. strength). Hence, it appears reasonable to posit that single‐modality AT or RT will confer improvements in CRF. However, a combination training (CT) approach that effectively targets both central and peripheral limitations may confer superior improvements in CRF response among lung cancer survivors. 15 The most effective exercise therapy regimen to improve CRF has not been determined in lung cancer or any respiratory disease condition.
We conducted a randomized controlled trial (RCT) to evaluate the effects of three different exercise regimens, relative to control, in post‐treatment lung cancer survivors. 16 The primary objective was to evaluate changes in CRF. Secondary objectives were to evaluate changes in upper and lower body maximal strength, body composition, patient‐reported outcomes (PROs), safety, and tolerability.
Methods
Trial design and patients
The full methods are provided in the Supporting Information and are available elsewhere. 16 Using a parallel‐group, factorial RCT design, we evaluated AT, RT, or both (CT), relative to attention control (AC), in lung cancer survivors at Duke University Medical Center (DUMC) and Memorial Sloan‐Kettering Cancer Center (MSKCC). Eligible patients were (i) ≥1 to <10 years after completion of all definitive therapy (i.e. surgery and adjuvant radiation or chemotherapy, as applicable); (ii) age 21–80; (iii) Eastern Cooperative Oncology Group ≤1; (iv) life expectancy ≥4 months; (v) performing less than 150 min of structured moderate‐intensity exercise per week 17 ; (vi) able to complete an acceptable cardiopulmonary exercise test (CPET) and one‐repetition maximum muscular strength test; and (vii) confirmed poor CRF—that is, peak oxygen consumption (VO2peak) below age–sex‐matched inactive levels. 18 , 19 Exclusion criteria included (i) concurrent malignancy or history of other malignancy treated within the past 3 years, (ii) room air desaturation at rest ≤85%, and (iii) any absolute contraindication to CPET per guidelines. 20
Patients were randomly assigned in a 1:1:1:1 ratio to receive either one of the three exercise therapy regimens (AT, RT, or CT) or AC. The random allocation sequence was generated and implemented using REDCap with a random permuted block design. Two stratification factors were employed: (i) adjuvant chemotherapy (yes vs. no) and (ii) sex (male vs. female). Group allocation was concealed until treatment intervention was assigned. The trial primary statisticians (J. H. and S. M. T.) generated the random sequence; dedicated study coordinators enrolled patients, and the clinical research team at each centre assigned participants to treatment group. Exercise physiologists implementing the study interventions were not randomly allocated to study treatment arms. Neither patients nor exercise physiologists were blinded to group allocation. Participants and study investigators were blinded to results at pre‐randomization and post‐intervention. Study investigators were blinded to treatment allocation. All study procedures were reviewed and approved by the DUMC and MSKCC institutional review boards. All patients provided written informed consent.
Interventions
Exercise therapy in all three regimens consisted of 48 individualized (one on one), supervised sessions, three times weekly for 16 consecutive weeks. Dedicated study personnel with at least bachelor's degrees in exercise science implemented the interventions and individually monitored all sessions. No informational material was provided to study participants. All intervention sessions were conducted in a dedicated exercise training facility on hospital campus at both study sites.
Aerobic training consisted of stationary cycle ergometry (Technogym Excite; Precor UBK 835) dosed at one of five different intensities (i.e. 55%, 65%, 75%, 80%, and >95%) individualized to each patient on the basis of workload (i.e. Watts) corresponding to a specific per cent of ventilatory thresholds measured during the pre‐randomization or midpoint (Week 8) CPET. Ventilatory threshold was identified from the CPET by a trained exercise physiologist defined by the following criteria: (i) drop in fraction of expired carbon dioxide oxygen content after a peak or plateau, (ii) non‐linear increase in minute ventilation, and (iii) respiratory exchange ratio between 0.98 and 1.02. All sessions were 20–60 min per session depending on intensity; interval sessions consisted of 60–120 s at VO2peak followed by 120–180 s of active recovery for 4–10 intervals per session. Dose was sequenced and continually progressed consistent with non‐linear (periodized) training. 21
Resistance training was progressively increased during an initial 2 week ramp period to perform three sets of 6–18 repetitions of 14 resistance exercises (i.e. chest press, seated row, lateral pull down, pec deck, bicep curl, triceps extension, push‐up, leg press, leg curl, leg extension, hip abduction, hip adduction, step up, and sit‐to‐stand) alternating between lower and upper extremity muscle groups for 30–60 min per session. Intensity was individualized to each patient on the basis of maximal strength corresponding to a specific percent of a one‐repetition maximum (1‐RM) measured during the pre‐randomization or midpoint (Week 8). Specifically, in Weeks 1 and 2, all three sessions per week were performed at an intensity of 50–60% of 1‐RM for 2–3 sets of 10–15 repetitions for both upper and lower body exercises. In Weeks 3–8, one session per week was 70–80% of 1‐RM for three sets of 8–10 repetitions for upper body exercise; one session per week was 60–65% of 1‐RM for three sets of 15–18 repetitions for lower body exercises; and the remaining session was a combination of the two previous days. In Weeks 9–16, one session per week was 75–85% of 1‐RM for three sets of 6–10 repetitions for upper body exercises; one session per week was 70–75% of 1‐RM for two to three sets of 10–12 repetitions for lower body exercises; and the remaining session was a combination of the two previous days.
The CT group consisted of three AT plus RT sessions per week for Weeks 1–8, and two AT plus RT and one interval AT sessions per week for Weeks 9–16. CT followed AT and RT dosing schedules for a total of 30–90 min per session. Within each session, AT was performed first followed by RT. Sequencing of AT and RT, both within each CT session and across the entire intervention period, was designed to exploit the complementary effects of each individual modality to augment CRF. Finally, AC consisted of 12–20 different stretching positions for 20–45 s per stretch following a standardized progressive individualized prescription for a total of 20–45 min per session. 22 Dose modification was permitted and performed using standardized criteria (Table S1) in all groups. Relative dose intensity (RDI) was defined as the ratio of total ‘completed’ to total ‘planned’ cumulative dose, as described previously. 22 , 23
Endpoints
The primary endpoint was change in CRF (VO2peak, mL O2·kg−1·min−1) evaluated by a symptom‐limited CPET on an electronic cycle ergometer (Corival, Lode, NI) with breath‐by‐breath expired gas analysis (ParvoMedics, TrueOne 2400, Salt Lake City, UT, USA) with 12‐lead electrocardiogram monitoring (Mac® 5000, GE Healthcare, Chicago, IL, USA). 24 All patients performed two pre‐randomization CPETs (≤30 days of each other) under similar laboratory conditions (Figure S1). If both were acceptable, the highest recorded measure was selected (the second CPET was selected in >80% of patients).
Secondary endpoints were other CPET variables, maximal muscular strength, PROs, and tolerability and safety. 23 Maximal muscular strength was assessed by 1‐RM of chest press and seated row for upper body extremity and leg press for lower body extremity and body weight and composition [weight (kg); percentage of lean and fat mass assessed via a dual‐energy X‐ray absorptiometry (Lunar DPX, General Electric) or air displacement plethysmography (Life Measurement, Inc., Concord, CA, USA)]. PROs were evaluated using quality of life [Functional Assessment of Cancer Therapy—Lung 25 that contains the subscales for physical well‐being, social well‐being, emotional well‐being, and functional well‐being that comprise the Functional Assessment of Cancer Therapy—General, 26 plus a lung cancer subscale, Functional Assessment of Chronic Illness Therapy—Fatigue Scale, 27 pain (Brief Pain Inventory), 28 and sleep quality (Pittsburgh Sleep Quality Index) 29 ] instruments. All endpoints were evaluated at pre‐randomization and repeated ≤14 days after the final intervention session (Week 17).
Tolerability was assessed by RDI (defined as the ratio of total ‘completed’ to total ‘planned’ cumulative dose) 22 and rate of lost to follow‐up (LTF; lack of completion of post‐intervention assessments), attendance (ratio of total attended to planned treatments), permanent discontinuation (treatment discontinuation prior to Week 16), treatment interruption (missing ≥3 consecutive planned sessions), dose modification [≥10% of sessions requiring modification (reduction/escalation) of intensity and/or duration], pretreatment dose modification (reduction of pretreatment session intensity), and early session termination (termination of session prior to planned duration). Safety was evaluated by the type and prevalence of serious (i.e. life‐threatening, hospitalization, significant incapacity, and important medical events) and non‐serious (e.g. knee and back pain) adverse events during CPET and exercise sessions by the dedicated exercise physiologists.
Statistical analysis
This RCT was designed to accrue 160 patients (n = 40 per group). 16 Power calculations assumed a standard deviation of 3.0 mL O2·kg−1·min−1 based on prior work. 12 With a total accrual of 160 patients, 80% power was obtained under the assumption that mean change in VO2peak for the AT, RT, CT, and AC groups was 0.60, 0.60, 2.10, and 0.0 mL O2·kg−1·min−1, respectively. 16 The primary analysis used an analysis of covariance to estimate the association of study group with change from baseline to Week 17 for the primary and secondary endpoints. Comparisons between exercise regimens were for exploratory purposes only. Analyses were adjusted for baseline values of the endpoint and other variables displaying imbalance across group assignment. Hence, all analyses were adjusted for age, body mass index, and co‐morbidities [i.e. coronary artery disease, chronic obstructive pulmonary disease (COPD), and hypertension].
All analyses were conducted under the intention‐to‐treat principle. Missing values for the primary endpoint were imputed with both multiple imputation using a Monte Carlo Markov single‐chain method assuming a multivariate normal distribution and initial mean and covariance estimates derived using the expectation–maximization algorithm and last observation carried forward (LOCF). Results of both approaches were similar; thus, only data from LOCF analyses are reported. LOCF was also used for missing values in secondary endpoints. Changes between baseline and Week 17 were estimated for each patient individually; the mean change within each group was used to estimate group differences. The proportion of patients in each exercise group with a VO2peak improvement greater than the technical error (TE) was calculated as previously described 30 ; the TE was ≥1.12 mL O2·kg−1·min−1. Fisher's exact test was used to test for differences between groups. Analysis of covariance was used to estimate the association of study group and RDI with adjustment for the covariates defined previously; other tolerability measures were compared across arms with the Kruskal–Wallis test for continuous variables and Fisher's exact test for categorical variables. All analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC, USA).
Results
A total of 90 patients (56% of planned accrual goal) were randomly allocated to AT (n = 24), RT (n = 23), CT (n = 20), or AC (n = 23) (Figure 1). Recruitment was terminated early because of slow accrual. The trial was conducted at DUMC between February 2010 and May 2013, continuing at MSKCC from April 2017 to March 2018 (for a total accrual period of 4.3 years), with final post‐intervention testing conducted in July 2018. Baseline characteristics were balanced between arms (Table 1). For the overall cohort, mean pre‐randomization VO2peak was 15.8 ± 4.5 mL O2·kg−1·min−1, the equivalent of 42% and 50% below age‐matched healthy sedentary women and men, respectively. 18 , 19 Non‐protocol exercise increased in all groups with no differences between groups (P = 0.12).
Figure 1.
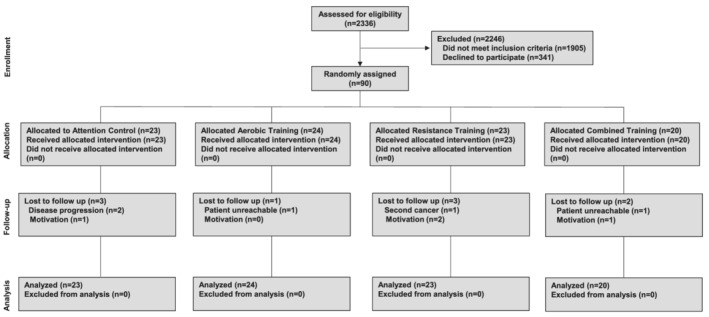
F CONSORT flow for non‐pharmacological trials.
Table 1.
Characteristics of the participants at baseline
| Characteristic | All patients (n = 90) | Aerobic training (n = 24) | Resistance training (n = 23) | Combination training (n = 20) | Attention control (n = 23) |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 65 ± 9 | 64 ± 9 | 64 ± 9 | 63 ± 11 | 67 ± 8 |
| Male, no. (%) | 31 (34) | 9 (37) | 8 (35) | 7 (35) | 7 (30) |
| BMI (kg·m−2), mean ± SD | 28 ± 6 | 26 ± 6 | 27 ± 6 | 27 ± 4 | 30 ± 6 |
| Site, no. (%) | |||||
| DUMC | 78 (87) | 20 (83) | 20 (87) | 19 (95) | 19 (83) |
| MSKCC | 12 (13) | 4 (17) | 3 (13) | 1 (5) | 4 (17) |
| Exercise (min·week−1) a , mean ± SD | 80 ± 101 | 122 ± 108 | 61 ± 128 | 71 ± 69 | 58 ± 80 |
| Smoking, no. (%) | |||||
| Never | 11 (12) | 6 (25) | 2 (9) | 1 (5) | 2 (9) |
| Former | 74 (82) | 17 (71) | 19 (83) | 17 (85) | 21 (91) |
| Current | 5 (6) | 1 (4) | 2 (9) | 2 (10) | 0 (0) |
| Disease stage, no. (%) | |||||
| I | 1 (1) | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| IA | 35 (39) | 10 (42) | 8 (35) | 9 (45) | 8 (35) |
| IB | 22 (24) | 4 (17) | 6 (26) | 5 (25) | 7 (30) |
| IIA | 10 (11) | 2 (8) | 4 (17) | 2 (10) | 2 (9) |
| IIB | 8 (9) | 3 (12) | 1 (4) | 1 (5) | 3 (13) |
| IIIA | 11 (12) | 3 (12) | 3 (13) | 2 (10) | 3 (13) |
| IIIB | 2 (2) | 0 (0) | 1 (4) | 1 (5) | 0 (0) |
| Limited stage | 1 (1) | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Adjuvant therapy, no. (%) | |||||
| Received chemotherapy | 38 (42) | 11 (46) | 10 (43) | 8 (40) | 9 (39) |
| Received radiotherapy | 15 (16) | 5 (21) | 4 (17) | 2 (10) | 4 (17) |
| Resection degree, no. (%) | |||||
| Lobectomy | 70 (78) | 17 (71) | 20 (87) | 16 (80) | 17 (74) |
| Pneumonectomy | 5 (6) | 1 (4) | 0 (0) | 2 (10) | 2 (9) |
| Bilobectomy | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (4) |
| Wedge resection | 10 (11) | 4 (17) | 3 (13) | 1 (5) | 2 (9) |
| Segment resection | 3 (3) | 0 (0) | 0 (0) | 2 (10) | 1 (4) |
| Current medications, no. (%) | |||||
| Beta‐blockers | 14 (16) | 3 (13) | 5 (22) | 2 (10) | 4 (17) |
| ACE inhibitors | 11 (12) | 2 (8) | 2 (9) | 4 (20) | 3 (13) |
| Angiotensin receptor blockers | 7 (8) | 1 (4) | 2 (9) | 1 (5) | 3 (13) |
| Diuretic | 11 (12) | 3 (13) | 2 (9) | 3 (15) | 3 (13) |
| Aspirin/anti‐platelet | 25 (28) | 6 (25) | 8 (35) | 6 (30) | 5 (22) |
| Statins | 30 (33) | 12 (50) | 5 (22) | 8 (40) | 5 (22) |
| Calcium channel blocker | 16 (18) | 2 (8) | 6 (26) | 3 (15) | 5 (22) |
| Pre‐existing (controlled) co‐morbidities, no. (%) | |||||
| CAD | 15 (17) | 2 (8) | 5 (22) | 4 (20) | 4 (17) |
| COPD | 20 (22) | 2 (8) | 7 (30) | 7 (35) | 4 (17) |
| Arthritis | 21 (23) | 5 (21) | 4 (17) | 5 (25) | 7 (30) |
| Type 2 diabetes | 9 (10) | 1 (4) | 4 (17) | 2 (10) | 2 (9) |
| Hyperlipidaemia | 39 (43) | 12 (50) | 11 (48) | 7 (35) | 9 (39) |
| Hypertension | 45 (50) | 8 (33) | 13 (56) | 10 (50) | 14 (61) |
| Any | 66 (73) | 17 (71) | 18 (78) | 15 (75) | 16 (70) |
| Pre‐randomization VO2peak (mL O2·kg−1·min−1), mean ± SD | 15.8 ± 4.5 | 17.9 ± 5.5 | 15.2 ± 3.2 | 15.2 ± 4.5 | 14.5 ± 4.1 |
| % below age‐matched, mean ± SD | |||||
| Men | 50 ± 14 | 42 ± 17 | 55 ± 10 | 54 ± 9 | 51 ± 17 |
| Women | 42 ± 15 | 35 ± 18 | 44 ± 14 | 45 ± 14 | 46 ± 14 |
ACE, angiotensin‐converting enzyme; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DUMC, Duke University Medical Center; MSKCC, Memorial Sloan‐Kettering Cancer Center; SD, standard deviation.
All comparisons P > 0.05. Chemotherapy, radiation, and endocrine therapy rates include only those patients receiving each treatment.
Exercise defined as the total minutes of self‐reported moderate/vigorous exercise per week.
Efficacy
For the primary endpoint, VO2peak increased by 1.1 mL O2·kg−1·min−1 in the AT group [95% confidence interval (CI): 0.0, 2.2, P = 0.04] and by 1.4 mL O2·kg−1·min−1 in the CT group (95% CI: 0.2, 2.5, P = 0.02) compared with AC (Table 2). There was no difference in VO2peak change between RT and AC (−0.1 mL O2·kg−1·min−1, 95% CI: −1.2, 1.0, P = 0.88). The range of VO2peak change in the AT group was −3.3 to 6.3 mL O2·kg−1·min−1, −3.2 to 4.9 mL O2·kg−1·min−1 in RT, and −2.3 to 5.7 mL O2·kg−1·min−1 in CT, compared with −3.2 to 4.1 mL O2·kg−1·min−1 in AC (Figure 2). The proportion of patients in the AT, RT, and CT groups with a VO2peak response greater than the TE (i.e. 1.12 mL O2·kg−1·min−1) was 50%, 13%, and 50%, respectively, compared with 13% in the AC group (P = 0.01).
Table 2.
Effects on primary and secondary endpoints (intention‐to‐treat analysis)
| Variable | Parameter estimate (95% confidence interval) | ||||||
|---|---|---|---|---|---|---|---|
| AT vs. AC | P a | RT vs. AC | P a | CT vs. AC | P a | Overall P a | |
| Primary endpoint | |||||||
| VO2peak (mL O2·kg−1·min−1) | 1.1 (0, 2.2) | 0.04 | −0.1 (−1.2, 1) | 0.88 | 1.4 (0.2, 2.5) | 0.02 | 0.02 |
| Secondary endpoints | |||||||
| Resting cardiopulmonary function | |||||||
| Heart rate (b.p.m.) | 2.1 (−2.8, 7.1) | 0.39 | 2.7 (−2.4, 7.7) | 0.30 | −0.8 (−5.9, 4.3) | 0.75 | 0.48 |
| Systolic blood pressure (mmHg) | 1.7 (−5.1, 8.6) | 0.62 | 2.6 (−4.3, 9.4) | 0.46 | −1.6 (−8.7, 5.5) | 0.65 | 0.64 |
| Diastolic blood pressure (mmHg) | 0.4 (−4.3, 5.2) | 0.86 | 0.1 (−4.6, 4.8) | 0.96 | −0.6 (−5.5, 4.3) | 0.80 | 0.98 |
| Peak cardiopulmonary function | |||||||
| VO2peak (L.min−1) | 0.1 (0, 0.1) | 0.09 | 0 (−0.1, 0.1) | 0.82 | 0.1 (0, 0.2) | 0.005 | 0.02 |
| Respiratory exchange ratio | 0 (0, 0.1) | 0.19 | 0 (−0.1, 0) | 0.46 | 0 (−0.1, 0) | 0.30 | 0.10 |
| Ventilation (L.min−1) | 4.0 (0.1, 7.9) | 0.04 | −0.7 (−4.6, 3.2) | 0.73 | 4.2 (0.1, 8.2) | 0.04 | 0.02 |
| Heart rate (b.p.m.) | 7.2 (1.7, 12.8) | 0.01 | 1.4 (−4.2, 6.9) | 0.63 | 3.3 (−2.5, 9) | 0.26 | 0.06 |
| Systolic blood pressure (mmHg) | 4.2 (−5.7, 14.2) | 0.40 | 3.8 (−6.3, 13.9) | 0.46 | 4.3 (−6.5, 15) | 0.43 | 0.82 |
| Diastolic blood pressure (mmHg) | 1.7 (−3.6, 7) | 0.53 | 2.2 (−3, 7.5) | 0.40 | 3.0 (−2.7, 8.7) | 0.29 | 0.75 |
| Strength | |||||||
| 1‐RM leg press (lb) | 5.3 (−15.1, 25.7) | 0.61 | 24.2 (4.1, 44.4) | 0.02 | 16.5 (−4.4, 37.3) | 0.12 | 0.08 |
| 1‐RM chest press (lb) | −0.6 (−13.4, 12.3) | 0.93 | 20.4 (8.2, 32.6) | <0.001 | 6.0 (−6.6, 18.7) | 0.34 | 0.003 |
| 1‐RM seated row (lb) | −7.6 (−15.6, 0.5) | 0.06 | 7.7 (0.1, 15.3) | 0.04 | 3.7 (−4.2, 11.5) | 0.36 | 0.001 |
| Body weight and composition | |||||||
| Weight (kg) | 1.1 (0, 2.3) | 0.06 | 0.6 (−0.6, 1.7) | 0.33 | 0.2 (−0.9, 1.4) | 0.71 | 0.25 |
| Lean body mass (%) | −1.0 (−2.8, 0.9) | 0.29 | 1.6 (−0.2, 3.4) | 0.08 | 1.1 (−0.8, 3) | 0.25 | 0.03 |
| Fat mass (%) | 1.0 (−0.9, 2.8) | 0.30 | −1.6 (−3.4, 0.2) | 0.08 | −1.1 (−3, 0.8) | 0.24 | 0.03 |
| PROs | |||||||
| FACT‐L (0–136) | 2.5 (−2.9, 7.9) | 0.36 | −2.0 (−7.1, 3.2) | 0.46 | −0.4 (−5.9, 5.2) | 0.90 | 0.42 |
| FACT‐G total (0–108) | 1.7 (−4.3, 7.8) | 0.57 | −0.8 (−6.6, 5.1) | 0.80 | −1.1 (−7.4, 5.1) | 0.72 | 0.79 |
| Physical well‐being (0–28) | 0 (−1.4, 1.4) | 0.98 | 0.1 (−1.3, 1.5) | 0.92 | −1.2 (−2.6, 0.3) | 0.11 | 0.27 |
| Social well‐being (0–28) | 1.4 (−0.9, 3.7) | 0.22 | −0.7 (−3, 1.5) | 0.52 | 0.8 (−1.6, 3.2) | 0.50 | 0.27 |
| Emotional well‐being (0–24) | −0.7 (−2.7, 1.3) | 0.50 | −0.8 (−2.7, 1.2) | 0.45 | −0.3 (−2.4, 1.7) | 0.75 | 0.87 |
| Functional well‐being (0–28) | 2.0 (−0.4, 4.5) | 0.10 | −1.1 (−3.4, 1.3) | 0.37 | 1.0 (−1.4, 3.5) | 0.41 | 0.06 |
| FACIT‐Fatigue (0–52) | −1.7 (−3.8, 0.4) | 0.11 | 0.1 (−2, 2.2) | 0.93 | −0.5 (−2.7, 1.7) | 0.64 | 0.32 |
| Pain (0–10) | −0.3 (−0.8, 0.3) | 0.37 | 0.2 (−0.4, 0.7) | 0.52 | −0.3 (−0.8, 0.3) | 0.39 | 0.34 |
| Sleep (0–28) | −1.1 (−2.6, 0.4) | 0.16 | 0.3 (−1.2, 1.9) | 0.66 | −0.8 (−2.4, 0.8) | 0.33 | 0.22 |
AC, attention control; ANCOVA, analysis of covariance; AT, aerobic training; CT, combination training; FACIT, Functional Assessment of Chronic Illness Therapy; FACT‐G, Functional Assessment of Cancer Therapy—General; FACT‐L, Functional Assessment of Cancer Therapy—Lung; PROs, patient‐reported outcomes; RM, repetition maximum; RT, resistance training.
Data presented as parameter estimate (95% confidence interval) as estimated from ANCOVA model with outcome of change adjusted for baseline value and age, body mass index, and co‐morbidities (coronary artery disease, chronic obstructive pulmonary disease, and hypertension).
P‐value from ANCOVA model with outcome of change adjusted for baseline factors.
Figure 2.
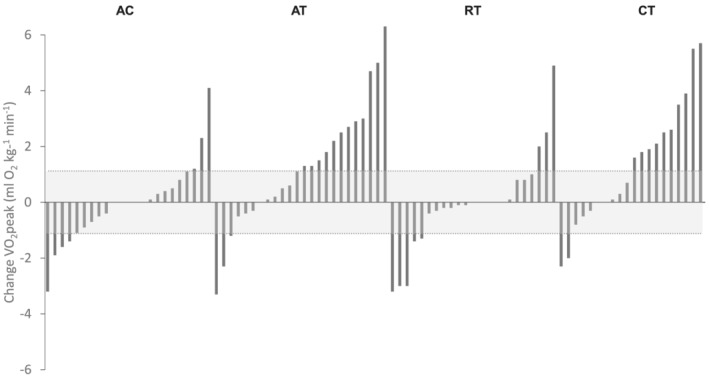
Waterfall plots for change in cardiorespiratory fitness. The technical error for VO2peak (Δ1.12 mL O2·kg−1·min−1) is illustrated by the shaded area. A change in cardiorespiratory fitness greater than technical error is classified as a meaningful response. AC, attention control; AT, aerobic training; CT, combination training; RT, resistance training.
For secondary endpoints, in comparison with AC, AT was associated with significant improvements in several other cardiopulmonary endpoints including ventilation and peak heart rate (Ps < 0.05) (Table 2). Single‐modality RT led to significant improvements in upper and lower extremity maximal strength compared with AC (Ps < 0.05), while increases in lean mass and reductions in fat mass compared with AC approached significance (Ps = 0.08) (Table 2). No between‐group changes were observed for any PROs (Table 2). Exploratory comparisons between exercise regimens revealed both AT and CT were superior to RT for improvements in several peak cardiopulmonary endpoints, whereas RT‐containing regimens were superior to AT for improvements in maximal muscle, strength, and body composition (Table S2).
Tolerability and safety
Relative dose intensity of exercise was significantly lower in RT (−24%; 95% CI: −40, −5, P = 0.003) and CT (−30%, 95% CI: −46, −14, P < 0.001) groups compared with AT. LTF was 4%, 13%, and 10% in AT, RT, and CT, respectively, compared with 13% in AC (P = 0.73), whereas mean attendance was ≥75% in all groups (P = 0.35) (Table 3). Median attendance was 90% (range: 4–100%). Rates of permanent discontinuation were 4%, 22%, and 25% in AT, RT, and CT, respectively (P = 0.10), whereas rates of dose interruption were 33%, 44%, and 70% in AT, RT, and CT, respectively (P = 0.05). The most common specified reason for dose modification was arthralgia (Table S3). Arthralgia events were more common in RT‐containing exercise regimens, specifically 65% and 60% in RT and CT groups, respectively, compared with 13% in AT and AC groups (P < 0.001; Table S4). No serious adverse events were observed during CPET procedures or any exercise training sessions.
Table 3.
Treatment tolerability
| Variable | All (n = 90) | AT (n = 24) | RT (n = 23) | CT (n = 20) | AC (n = 23) | P |
|---|---|---|---|---|---|---|
| Lost to follow‐up, no. (%) a | 9 (10) | 1 (4) | 3 (13) | 2 (10) | 3 (13) | 0.73 |
| Attendance, %, mean ± SD a | 79 ± 27 | 86 ± 19 | 77 ± 32 | 75 ± 31 | 76 ± 24 | 0.35 |
| Permanent discontinuation, no. (%) b | 16 (18) | 1 (4) | 5 (22) | 5 (25) | 5 (22) | 0.10 |
| Dose interruption, no. (%) b | 32 (36) | 8 (33) | 10 (44) | 14 (70) | 0 (0) | 0.05 |
| Dose modification, no. (%) b | 52 (58) | 16 (67) | 18 (78) | 18 (90) | 0 (0) | 0.17 |
| Pretreatment dose modification, no. (%) b | 40 (44) | 5 (21) | 18 (78) | 17 (85) | 0 (0) | <0.001 |
| Early session termination, no. (%) b | 25 (28) | 12 (50) | — | 13 (65) | 0 (0) | 0.32 |
AC, attention control; AT, aerobic training; CT, combination training; RT, resistance training; SD, standard deviation.
Lost to follow‐up indicates lack of completion of follow‐up assessments at post‐intervention; attendance indicates ratio of total number of attended to planned treatments; permanent discontinuation indicates permanent discontinuation of treatment prior to Week 16; treatment interruption indicates missing ≥3 consecutive sessions; dose modification indicates ≥10% of sessions requiring modification (reduction/escalation) of intensity or duration; pretreatment dose modification indicates reduction of pretreatment session intensity; early session termination indicates early termination of planned session duration; relative dose intensity indicates the ratio of total ‘completed’ to total ‘planned’ cumulative dose. All variables are collectively counted as one entity in the same patient unless otherwise indicated.
P values obtained from Fisher's exact test or Kruskal–Wallis test for differences across all groups.
P values obtained from Fisher's exact test, Kruskal–Wallis test, or χ 2 test for AT vs. RT vs. CT.
Discussion
In this trial involving lung cancer survivors with poor CRF, we evaluated the effects and tolerability of three different exercise regimens relative to AC on CRF. Our results failed to support the primary hypothesis that CT would confer the largest improvements in CRF. AT and CT both lead to significant improvements in VO2peak in lung cancer survivors compared with AC. Single‐modality RT was poorly tolerated and did not augment CRF but did improve maximal strength and body composition. Our findings should be interpreted with caution given that the planned total accrual goal was not achieved, increasing the risk of type 1 and type 2 errors. Our results are therefore exploratory and should be considered hypothesis generating.
The present trial is the first to evaluate exercise therapy in longer‐term lung cancer survivors, with most prior studies conducted in the immediate post‐surgical period. For instance, Edvardsen et al. 31 reported that supervised CT thrice weekly for 20 weeks was associated with a 4.1 mL O2·kg−1·min−1 increase in VO2peak compared with a 0.9 mL O2·kg−1·min−1 increase in the control group in 61 lung cancer patients 4–6 weeks following pulmonary resection. A subsequent meta‐analysis including four RCTs (representing 135 patients; mean sample size, n = 57 per study) 32 reported that exercise training increased VO2peak, on average, by 2.97 mL O2·kg−1·min−1 compared with control. A meta‐analysis of 16 RCTs (representing 779 patients) in patients with COPD reported similar findings. 33 Several factors may contribute to the smaller effects of exercise therapy on CRF observed in the present trial compared with prior work [e.g. one CPET at baseline, setting (immediate vs. >1 year after surgery), and non‐intention‐to‐treat analyses]. Perhaps the most important of these is the conduct of two pre‐randomization CPETs to minimize learning effects; all prior studies have conducted only one pre‐randomization CPET. 34 The magnitude of improvement in VO2peak across the two CPETs is consistent with our previous observations in other cancer settings 34 , 35 and indicates that learning effects may contribute to the reported magnitude of CRF improvement in prior exercise‐oncology trials. 33 , 34
Our trial is also the first to conduct a four‐arm trial evaluating the effects of single‐modality AT, RT, or both in comparison with usual care in any respiratory disease. The lack of larger CRF improvement with CT (compared with usual care) vs. the effects of single‐modality AT are similar to those previously reported in at least three exercise RCTs in COPD. 36 , 37 , 38 Further, AT had several advantages over CT including a higher RDI and requiring 30–60 min·week−1 less time compared with CT. These factors support AT as the preferred modality to target impaired CRF in post‐treatment lung cancer survivors. Nevertheless, the notion that the single‐modality AT prescription tested in this trial is the ‘optimal’ exercise prescription to improve CRF in lung cancer survivors is likely imprudent. 39 Indeed, findings of the present trial corroborate our prior work in various oncology settings demonstrating that CRF response to exercise therapy prescribed at standard doses not only displays considerable heterogeneity but also more importantly confers relatively modest CRF improvements for most patients. 22 , 40 As a potential alternative approach, we posit that stratification of patients with a common but heterogeneous condition into homogeneous subgroups will guide targeted exercise therapy that vary in the configuration of basic exercise prescription parameters (e.g. modality, intensity, length, and sequence) to augment CRF response. 39
Three additional findings of the present trial are also noteworthy. First, none of the three exercise regimens were associated with improvements in any PROs in comparison with AC. Meta‐analyses and systematic reviews conclude that exercise therapy confers significant improvements in several PROs in cancer survivors. 41 Prior work, however, has classically compared exercise therapy to a non‐intervention control group. 34 Thus, whether the reported findings reflected an exercise‐specific effect or were a consequence of social interaction inherent to supervised exercise therapy interventions is unclear. In corroboration of our prior work also employing AC groups, 22 , 40 social interaction appears to contribute significantly to the reported favourable effects of exercise on PROs in the oncology setting. Second, despite no change in CRF, RT‐containing regimens improved maximal strength and body composition compared with AT and AC. These findings support the notion that exercise therapy should be designed to target the primary endpoint of interest. 39 For instance, in view of prior work indicating that RT results in favourable changes in muscle function and lean mass in lung cancer patients, 31 , 42 , 43 RT may be the most appropriate intervention for patients with or at high risk of cachexia or sarcopenia. 44
Finally, comprehensive evaluation of exercise therapy safety and tolerability in the present trial is novel and important. Evaluation of tolerability in prior exercise‐oncology trials is limited to reporting of LTF and attendance. 34 However, these metrics provide limited insight and could lead to erroneous conclusions regarding exercise tolerability in a given setting. 22 As illustrated in the present study, rates of LTF and attendance were within a range considered acceptable in exercise trials, yet more detailed metrics adapted from drug trials (e.g. RDI) reveal that AT was well tolerated whereas RT‐containing regimens were not, primarily due to arthralgia, fatigue, and other non‐specific reasons. To our knowledge, only one trial in the oncology setting has evaluated RT tolerability using ‘drug trial’ metrics. Fairman et al. 45 reported dose interruption and modification rates of 51% and 85% in 47 men with advanced prostate cancer receiving single‐modality RT (three times weekly for 12 weeks). Further investigation of the tolerability‐to‐benefit ratio of RT‐containing exercise regimens is required, particularly in older, more frail cancer populations.
Limitations
The most important limitation is that the planned accrual was not achieved. This significantly impacts interpretability of trial results. Generalizability of our findings may be limited by the low participation rate. The reasons for non‐participation (and therefore slow accrual) were mainly related to the inconvenience (e.g. travel distance) of facility‐based supervised exercise sessions. This approach was selected to maximize exercise treatment fidelity and safety/tolerability. Clearly, however, design and testing of ‘site‐less’ telemedicine solutions that enable exercise sessions and potentially other study procedures to be conducted remotely may address major barriers to the rigorous conduct of exercise training investigations in clinical populations. 46 , 47 Second, our findings are limited to unfit lung cancer survivors and do not generalize to patients with normal CRF with different exercise training tolerability and response. Third, we tested the effects of intensely monitored exercise regimens that were carefully individualized, quantified, and sequenced; thus, the feasibility, safety, and efficacy of exercise regimens will likely differ when implemented under different conditions. Other limitations include the short length of intervention.
Conclusions
In the context of a smaller than planned sample size, AT and CT significantly improved VO2peak in lung cancer survivors; however, the tolerability‐to‐benefit ratio was superior for AT and hence may be the preferred modality to target impaired CRF in post‐treatment lung cancer survivors.
Funding
This study was supported by a research grant from the National Cancer Institute at the National Institutes of Health (R01‐CA138624 to L.W.J.) and grants from AKTIV Against Cancer and the Memorial Sloan‐Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Author contributions
L.W.J. obtained the funding. L.W.J. coordinated the study and is the guarantor. L.W.J., J.E.H., P.S.D., and N.D.E. designed the study. J.M.S., C.C., J.N.H., K.J.S., T.N., E.E., M.G.M., and L.W.J. collected the data. S.M.T. and J.E.H. analysed the data. L.W.J. wrote the first substantial draft of the article and is the guarantor. All authors critically revised the manuscript. All authors read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Conflict of interest
L.W.J. has stock ownership in Pacylex, Inc. Other authors report no conflict of interest.
Supporting information
Table S1. Aerobic Training Dose Modification Guidelines.
Table S2. Comparison of Exercise Treatment Modalities on Primary and Secondary End points.
Table S3. Reasons for Exercise Dose Modifications.
Table S4. Adverse Events.
Figure S1. Comparison of VO2peak Between the Two Cardiopulmonary Exercise Tests Conducted at Pre‐Randomization.
Supplementary References.
Supplementary Methods
Acknowledgements
We would like to especially thank the study participants and their families. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle. 48
Scott J. M., Thomas S. M., Herndon J. E. II, Douglas P. S., Yu A. F., Rusch V., Huang J., Capaci C., Harrison J. N., Stoeckel K. J., Nilsen T., Edvardsen E., Michalski M. G., Eves N. D., and Jones L. W. (2021) Effects and tolerability of exercise therapy modality on cardiorespiratory fitness in lung cancer: a randomized controlled trial, Journal of Cachexia, Sarcopenia and Muscle, 12, 1456–1465, 10.1002/jcsm.12828
Trial registration: Clinicaltrials.gov identifier: NCT01068210.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Nezu K, Kushibe K, Tojo T, Takahama M, Kitamura S. Recovery and limitation of exercise capacity after lung resection for lung cancer. Chest 1998;113:1511–1516. [DOI] [PubMed] [Google Scholar]
- 3. Pelletier C, Lapointe L, LeBlanc P. Effects of lung resection on pulmonary function and exercise capacity. Thorax 1990;45:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones LW, Watson D, Herndon JE 2nd, Eves ND, Haithcock BE, Loewen G, et al. Peak oxygen consumption and long‐term all‐cause mortality in nonsmall cell lung cancer. Cancer 2010;116:4825–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brunelli A, Pompili C, Salati M, Refai M, Berardi R, Mazzanti P, et al. Preoperative maximum oxygen consumption is associated with prognosis after pulmonary resection in stage I non‐small cell lung cancer. Ann Thorac Surg 2014;98:238–242. [DOI] [PubMed] [Google Scholar]
- 6. Avancini A, Sartori G, Gkountakos A, Casali M, Trestini I, Tregnago D, et al. Physical activity and exercise in lung cancer care: will promises be fulfilled? Oncologist 2020;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Mieghem W, Demedts M. Cardiopulmonary function after lobectomy or pneumonectomy for pulmonary neoplasm. Respir Med 1989;83:199–206. [DOI] [PubMed] [Google Scholar]
- 8. Jones NL, Killian KJ. Exercise limitation in health and disease. N Engl J Med 2000;343:632–641. [DOI] [PubMed] [Google Scholar]
- 9. Stefanelli F, Meoli I, Cobuccio R, Curcio C, Amore D, Casazza D, et al. High‐intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non‐small‐cell lung cancer undergoing lobectomy. Eur J Cardiothorac Surg 2013;44:e260–e265. [DOI] [PubMed] [Google Scholar]
- 10. Hwang CL, Yu CJ, Shih JY, Yang PC, Wu YT. Effects of exercise training on exercise capacity in patients with non‐small cell lung cancer receiving targeted therapy. Support Care Cancer 2012;20:3169–3177. [DOI] [PubMed] [Google Scholar]
- 11. Licker M, Karenovics W, Diaper J, Frésard I, Triponez F, Ellenberger C, et al. Short‐term preoperative high‐intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol 2017;12:323–333. [DOI] [PubMed] [Google Scholar]
- 12. Jones LW, Eves ND, Peterson BL, Garst J, Crawford J, West MJ, et al. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical nonsmall cell lung cancer patients: a pilot study. Cancer 2008;113:3430–3439. [DOI] [PubMed] [Google Scholar]
- 13. Jones LW, Peddle CJ, Eves ND, Haykowsky MJ, Courneya KS, Mackey JR, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer 2007;110:590–598. [DOI] [PubMed] [Google Scholar]
- 14. Hepple RT, Mackinnon SLM, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on VO2peak and the capillary supply to skeletal muscle. J Appl Physiol (1985) 1997;82:1305–1310. [DOI] [PubMed] [Google Scholar]
- 15. Skeletal muscle dysfunction in chronic obstructive pulmonary disease: a statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med 1999;159:S1–S40. [DOI] [PubMed] [Google Scholar]
- 16. Jones LW, Eves ND, Kraus WE, Potti A, Crawford J, Blumenthal JA, et al. The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design. BMC Cancer 2010;10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self‐report: a concurrent validity study. Can J Public Health 1986;77:359–362. [PubMed] [Google Scholar]
- 18. Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age‐related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta‐analysis. J Appl Physiol (1985) 1997;83:160–165. [DOI] [PubMed] [Google Scholar]
- 19. American College of Sports Medicine . ACSM's Guidelines for Graded Exercise Testing and Prescription, 10th ed. Philadelphia: Wolters Kluwer (Lippincott Williams & Wilkins); 2018. p 226–267. [Google Scholar]
- 20. American Thoracic Society , American College of Chest Physicians . ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211–277. [DOI] [PubMed] [Google Scholar]
- 21. Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW. A framework for prescription in exercise‐oncology research. J Cachexia Sarcopenia Muscle 2015;6:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scott JM, Iyengar NM, Nilsen TS, Michalski M, Thomas SM, Herndon J 2nd, et al. Feasibility, safety, and efficacy of aerobic training in pretreated patients with metastatic breast cancer: a randomized controlled trial. Cancer 2018;124:2552–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nilsen TS, Scott JM, Michalski M, Capaci C, Thomas S, Herndon JE 2nd, et al. Novel methods for reporting of exercise dose and adherence: an exploratory analysis. Med Sci Sports Exerc 2018;50:1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 25. Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy—Lung (FACT‐L) quality of life instrument. Lung Cancer 1995;12:199–220. [DOI] [PubMed] [Google Scholar]
- 26. Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570–579. [DOI] [PubMed] [Google Scholar]
- 27. Lai JS. Item banking to improve, shorten and computerize self‐reported fatigue: an illustration of steps to create a core item bank from the FACIT‐Fatigue Scale. Qual Life Res 2003;12:485–501. [DOI] [PubMed] [Google Scholar]
- 28. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129–138. [PubMed] [Google Scholar]
- 29. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 30. Ross R, de Lannoy L, Stotz PJ. Separate effects of intensity and amount of exercise on interindividual cardiorespiratory fitness response. Mayo Clin Proc 2015;90:1506–1514. [DOI] [PubMed] [Google Scholar]
- 31. Edvardsen E, Skjønsberg OH, Holme I, Nordsletten L, Borchsenius F, Anderssen SA. High‐intensity training following lung cancer surgery: a randomised controlled trial. Thorax 2015;70:244–250. [DOI] [PubMed] [Google Scholar]
- 32. Sommer MS, Staerkind M, Christensen J, Vibe‐Petersen J, Larsen K, Pedersen J, et al. Effect of postsurgical rehabilitation programmes in patients operated for lung cancer: a systematic review and meta‐analysis. J Rehabil Med 2018;50:236–245. [DOI] [PubMed] [Google Scholar]
- 33. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;2:CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta‐analysis. J Clin Oncol 2018;36:2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scott JM, Hornsby WE, Lane A, Kenjale AA, Eves ND, Jones L. Reliability of maximal cardiopulmonary exercise testing in men with prostate cancer. Med Sci Sports Exerc 2015;47:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bernard S, Whittom F, Leblanc P, Jobin J, Belleau R, Bérubé C, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:896–901. [DOI] [PubMed] [Google Scholar]
- 37. Covey MK, Collins EG, Reynertson SI, Dilling DF. Resistance training as a preconditioning strategy for enhancing aerobic exercise training outcomes in COPD. Respir Med 2014;108:1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rinaldo N, Bacchi E, Coratella G, Vitali F, Milanese C, Rossi A, et al. Effects of combined aerobic‐strength training vs fitness education program in COPD patients. Int J Sports Med 2017;38:1001–1008. [DOI] [PubMed] [Google Scholar]
- 39. Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation 2018;137:1176–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scott JM, Thomas SM, Peppercorn JM, Herndon JE 2nd, Douglas PS, Khouri MG, et al. Effects of exercise therapy dosing schedule on impaired cardiorespiratory fitness in patients with primary breast cancer: a randomized controlled trial. Circulation 2020;141:560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta‐analysis of 34 RCTs. Cancer Treat Rev 2017;52:91–104. [DOI] [PubMed] [Google Scholar]
- 42. Quist M, Adamsen L, Rørth M, Laursen JH, Christensen KB, Langer SW. The impact of a multidimensional exercise intervention on physical and functional capacity, anxiety, and depression in patients with advanced‐stage lung cancer undergoing chemotherapy. Integr Cancer Ther 2015;14:341–349. [DOI] [PubMed] [Google Scholar]
- 43. Salhi B, Huysse W, Van Maele G, Surmont VF, Derom E, van Meerbeeck JP. The effect of radical treatment and rehabilitation on muscle mass and strength: a randomized trial in stages I–III lung cancer patients. Lung Cancer 2014;84:56–61. [DOI] [PubMed] [Google Scholar]
- 44. Grande AJ, Silva V, Maddocks M. Exercise for cancer cachexia in adults: executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle 2015;6:208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fairman CM, Nilsen TS, Newton RU, Taaffe DR, Spry N, Joseph D, et al. Reporting of resistance training dose, adherence, and tolerance in exercise oncology. Med Sci Sports Exerc 2020;52:315–322. [DOI] [PubMed] [Google Scholar]
- 46. Topol EJ. A decade of digital medicine innovation. Sci Transl Med 2019;11:eaaw7610. [DOI] [PubMed] [Google Scholar]
- 47. Scott JM, Dolan LB, Norton L, Charles JB, Jones LW. Multisystem toxicity in cancer: lessons from NASA's countermeasures program. Cell 2019;179:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Aerobic Training Dose Modification Guidelines.
Table S2. Comparison of Exercise Treatment Modalities on Primary and Secondary End points.
Table S3. Reasons for Exercise Dose Modifications.
Table S4. Adverse Events.
Figure S1. Comparison of VO2peak Between the Two Cardiopulmonary Exercise Tests Conducted at Pre‐Randomization.
Supplementary References.
Supplementary Methods


