Abstract
Introduction
Clinicians and policymakers are promoting widespread use of home technology including spirometry to detect disease progression for patients with interstitial lung disease (ILD); the COVID-19 pandemic has accelerated this. Data collating clinicians’ views on the potential utility of telehealth in ILD are limited.
Aim
This survey investigated clinicians’ opinions about contemporary methods and practices used to monitor disease progression in patients with ILD using telehealth.
Methods
Clinicians were invited to participate in a cross-sectional survey (SurveyMonkey) of 13 questions designed by an expert panel. Telehealth was defined as home monitoring of symptoms and physiological parameters with regular automatic transmission of data from the patient’s home to the clinician. Data are presented as percentages of respondents.
Results
A total of 207 clinicians from 23 countries participated in the survey. A minority (81, 39%) reported using telehealth. 50% (n=41) of these respondents completed a further question about the effectiveness of telehealth. A majority of respondents (32, 70%) rated it to be quite or more effective than face-to-face visit. There were a greater number of respondents using telehealth from Europe (94, 45%) than Asia (51, 25%) and America (24%). Clinicians reported the most useful telehealth monitoring technologies as smartphone apps (59%) and wearable sensors (30%). Telehealth was most frequently used for monitoring disease progression (70%), quality of life (63%), medication use (63%) and reducing the need for in-person visits (63%). Clinicians most often monitored symptoms (93%), oxygen saturation (74%) and physical activity (72%). The equipment perceived to be most effective were spirometers (43%) and pulse oximeters (33%). The primary barriers to clinicians’ participation in telehealth were organisational structure (80%), technical challenges (63%) and lack of time and/or workload (63%). Clinicians considered patients’ barriers to participation might include lack of awareness (76%), lack of knowledge using smartphones (60%) and lack of confidence in telehealth (56%).
Conclusion
The ILD clinicians completing this survey who used telehealth to monitor patients (n=81) supported its’ clinical utility. Our findings emphasise the need for robust research in telehealth as a mode for the delivery of cost-effective healthcare services in ILD and highlight the need to assess patients’ perspectives to improve telehealth utility in patients with ILD.
Keywords: interstitial fibrosis, sarcoidosis
Key messages.
We sought to have a better understanding of the current practices of interstitial lung disease (ILD) care, the extent of use and to gain understanding of the barriers from clinicians’ perspectives that may limit the adaptation of telehealth for patients with ILD.
Our findings show a minority of ILD clinicians currently use Telehealth, but almost all these clinicians endorsed telehealth, while identifying barriers to wider implementation. Our study emphasise the need for robust research in telehealth as a mode for the delivery of cost-effective healthcare services in ILD.
The data from this study may help determine further research and policy needed to better use telehealth services.
Introduction
Interstitial lung disease (ILD) management has significantly changed during the COVID-19 pandemic and use of telehealth has increased.1 2 The British Lung Foundation and UK National Health Service recommended that patients with ILD be ‘shielded’ to prevent contracting COVID-19.3 COVID-19 impacted healthcare for patients with ILD by restricting access to diagnosis, disabling monitoring disease severity, progression and adverse effects from medication. The burden put on patients with ILD following COVID-19 is high, and there is an urgent need for a contemporary approach in ILD management.4 5 Telehealth may help overcome access barriers for patients with ILD and those unable to come to hospital in person due to lack of transportation.6 Development of home spirometry as a valid monitoring tool,7 drug monitoring and assessment of side effects,8 can support other services remotely such as local oxygen services, general practitioners and secondary care physicians, but using a combined approach with patient and care provider in tele link with specialist centre.1 8–10 Using a combined approach with patient and care provider in tele link with specialist centre will be critical for success.1 6 11–13 Monitoring symptoms and physiological parameters using remote monitoring technology may allow early detection of exacerbation, which may reduce hospitalisation and decrease healthcare costs.1 11 Although most published studies have been in patients with chronic obstructive pulmonary disease (COPD),14–17 telehealth in ILD has also been explored. Studies have shown a great potential of remotely monitoring parameters, such as heart rate, respiratory rate, oxygen level, activities and lung function using home-based technologies and equipment.6 7 10 18–25 Results have been very promising not only for e-consultation, but also for monitoring and detecting disease progression and or exacerbation.1 25
ILD is a heterogeneous group that encompasses approximately 200 different types of lung disease that may cause inflammation and/or scarring of the lung.26 27 Idiopathic pulmonary fibrosis (IPF) is specific type characterised by severe breathlessness and poor prognosis of unknown aetiology.27 28 Richeldi et al29 are the only approved antifibrotic medications, which have been shown to slow IPF progression.30 Thus, prediction of disease course and mortality is crucial for clinicians and patients. Patients with ILD, specifically those with IPF, may experience acute exacerbation or ‘flare’ that may cause severe distress and rapid disease progression.31 Additionally, the use of forced vital capacity (FVC) declines as a validated end point to identify significant treatment response in IPF necessitates large cohorts and lengthy longitudinal studies.32 Conversely, domiciliary and daily measures of symptoms and physiological parameters such as spirometry may reduce length of time, size and cost of clinical trials.7 33
Although there is a growing body of research1 6 7 9–11 18–20 22 34–37 focusing mainly on feasibility and reliability of in-home spirometry, further data are limited on the prevalence and utility of telehealth to detect disease exacerbation and/or progression. Telehealth utility to remotely monitor symptoms and physiological parameters may allow prediction of disease progression in ILD. Previous studies1 11 have shown that in-home monitoring of FVC was predictive for disease progression. Furthermore, a Japanese study has shown that SpO2 might predict disease prognosis.38 Real-time continuous monitoring may also allow prediction of personal trajectory and improve future care.6 18
In support of this, studies8 18 have shown the potential benefit in patients with ILD of monitoring quality-of-life, medication use and disease course.1 10 11 Telehealth was introduced as a remote solution to continue to provide necessary healthcare services and support patients with ILD. It was also expanded to provide real-time monitoring and to detect disease exacerbation and progression. Recently, Telehealth has been shown to be feasible and reliable, and one study specifically explored how telehealth successfully detected exacerbations before symptoms began.6 Despite these significant findings, research understanding clinicians’ perceptions of telehealth in patients with ILD are limited.
Our goal was to conduct a global survey to understand from healthcare professionals, what approaches of ILD care are most appropriate for telehealth and simultaneously most beneficial to patients with ILD. We approached physicians and non-physicians to better understand variability in outcomes. We hope that this would help to understand physicians’ perspectives as well as perspectives of nurses and allied health professions towards telehealth useability, effectiveness and utility. All clinicians deliver healthcare services and provide care to patients including using technologies, for example, homes, doctor’s offices, clinics and hospitals. We sought to have a better understanding of the current practices of ILD care, the extent of use and to gain understanding of the barriers from clinicians’ perspectives that may limit the adaptation of telehealth for patients with ILD.
Methods
This is a descriptive, cross-sectional survey of healthcare professionals who work with patients diagnosed with ILD throughout the world.
Study design
A 13-question web-based survey was developed by an expert panel from University College London and the University of Exeter comprised of consultant respiratory physicians, nurses and therapists. The survey was constructed using SurveyMonkey and the wording of questions, length and order were chosen carefully and divided into structured and unstructured questions. The survey consisted of multiple-choice and open questions, which are presented in online supplemental table S1. The survey content was piloted among a multidisciplinary team. The final version of the survey was approved by the research team after minor changes in accordance with the participants’ feedback and comments. Our objectives were to cover specific aspects of telehealth in ILD: Clinicians’ perspectives about the purpose, prevalence and variables monitored, type of equipment and technologies and barriers to adaption.
bmjresp-2021-001088supp001.pdf (1.8MB, pdf)
Clinicians were asked if Telehealth was used by their place of work to remotely monitor patients with ILD. Clinicians were also asked about the purpose of using telehealth at their place of work. To evaluate responders’ perceptions on Telehealth, they were also asked to rate, on a five-point Likert scale, how effective (not at all effective, not particularly effective, neither effective nor ineffective, quite effective, very effective) telehealth is compared with face-to-face care. Clinicians were asked regarding the type of equipment and technologies used at their centre to remotely monitor patients with ILD. Questions concerning barriers to both clinician and patient’s participation in telehealth were assessed. Two open-ended questions were asked on what would help clinicians to setup telehealth and if they have any further comments and suggestions regarding using telehealth programmes.
Data collection
We approached global experts throughout the world between January 2021 and Jun 2021 via email communication, explaining the aim of the project and if the survey could be distributed to their network. Healthcare professionals were sought out via Twitter, Facebook, WhatsApp and LinkedIn using a weblink. The survey was a voluntary and anonymised service evaluation survey, which requires no ethical approval according to the Research Governance Framework (2005) and health Research Authority review.
Statistical analysis
We first met with the expert panel and discussed aspects of the project. Following this, we analysed the structured questionnaires. Each question’s details were exported from SurveyMonkey to an Excel spreadsheet and reviewed. The extracted responses were analysed using descriptive statistics. The written qualitative data under each item were collected onto Nvivo software and reviewed. Following this, we grouped the comments and analysed them using thematic analysis and coding of responses. We analysed the collected data using SPSS software, V.25. We reported results as statistically significant if the p≤0.05. We used the χ2 test to assess the association between clinicians’ demographic characteristics with the use of telehealth. Geographical location was divided into seven continents (Europe, North America, Asia, Africa, South America, Antarctica and Australia).
Patient and public involvement
Patients or the public were not involved in our research.
Results
A total of 207 respondents from 23 countries participated in this survey (table 1).
Table 1.
Geographical location of healthcare providers (207)
| Location | n (%) |
| Europe | 94 (45.4) |
| Asia | 51 (24.6) |
| North America | 50 (24.2) |
| Africa | 7 (3.4) |
| South America | 3 (1.4) |
| Australasia | 2 (0.9) |
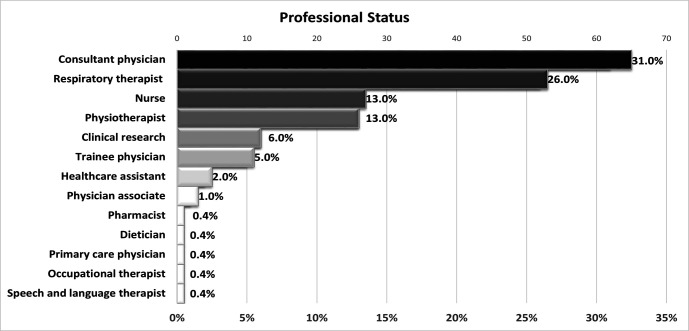
The majority of the respondents were in the age group 35–44 (67, 32%). Approximately one-third of the participants were consultant physicians (65, 31%) or equivalent, followed by respiratory therapists, physiotherapists and nurses (figure 1). χ2 showed no significant association between clinician’s demographic characteristics and the use of telehealth (table 2).
Figure 1.
Professional status of the participants.
Table 2.
Clinician’s demographic characteristics and the use of telehealth
| Is telehealth (as defined above) used by your place of work to remotely monitor ILD patients? | P value | |||
| Yes | No | |||
| What is your age group? | 18–24 | 7 | 2 | 0.244 |
| 77.8% | 22.2% | |||
| 25–34 | 19 | 23 | ||
| 45.2% | 54.8% | |||
| 35–44 | 23 | 44 | ||
| 34.3% | 65.7% | |||
| 45–54 | 18 | 30 | ||
| 37.5% | 62.5% | |||
| 55–64 | 12 | 21 | ||
| 36.4% | 63.6% | |||
| 65+ | 1 | 4 | ||
| 20.0% | 80.0% | |||
| Prefer not to say | 1 | 2 | ||
| 33.3% | 66.7% | |||
| Total | 81 | 126 | ||
| 39.1% | 60.9% | |||
| What is your professional status? | Consultant physician | 24 | 41 | 0.263 |
| 36.9% | 63.1% | |||
| Trainee physician | 2 | 9 | ||
| 18.2% | 81.8% | |||
| Nurse | 12 | 15 | ||
| 44.4% | 55.6% | |||
| Physician associate | 0 | 3 | ||
| 0.0% | 100.0% | |||
| Speech and language therapist | 0 | 1 | ||
| 0.0% | 100.0% | |||
| Occupational therapist | 1 | 0 | ||
| 100.0% | 0.0% | |||
| Physiotherapist | 12 | 14 | ||
| 46.2% | 53.8% | |||
| Healthcare assistant | 1 | 4 | ||
| 20.0% | 80.0% | |||
| Primary care physician | 0 | 1 | ||
| 0.0% | 100.0% | |||
| Dietician | 1 | 0 | ||
| 100.0% | 0.0% | |||
| Clinical research | 8 | 4 | ||
| 66.7% | 33.3% | |||
| Respiratory therapist | 20 | 33 | ||
| 37.7% | 62.3% | |||
| Pharmacist | 0 | 1 | ||
| 0.0% | 100.0% | |||
| Total | 81 | 126 | ||
| 39.1% | 60.9% | |||
| In what country do you work? | Europe | 41 | 53 | 0.332 |
| 43.6% | 56.4% | |||
| North America | 16 | 34 | ||
| 32.0% | 68.0% | |||
| South America | 0 | 3 | ||
| 0.0% | 100.0% | |||
| Australasia | 0 | 2 | ||
| 0.0% | 100.0% | |||
| Asia | 22 | 29 | ||
| 43.1% | 56.9% | |||
| Africa | 2 | 5 | ||
| 28.6% | 71.4% | |||
| Total | 81 | 126 | ||
| 39.1% | 60.9% | |||
ILD, interstitial lung disease.
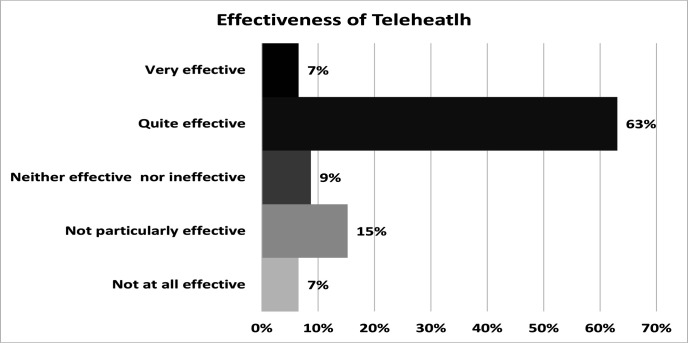
81 (39%) of respondents reported telehealth use at their place of work to monitor patients with ILD. Of these 81, only 46 (56%) respondents answered the question regarding the effectiveness of telehealth compared with face-to-face monitoring. Of these, more than two-thirds were positive and responded, ‘quite effective’ (29, 63%) and ‘very effective’ (3, 7%). Only three (7 %) of the clinicians responded that telehealth was ‘not at all effective’ (figure 2).
Figure 2.
Clinician’s opinion on effectiveness of telehealth.
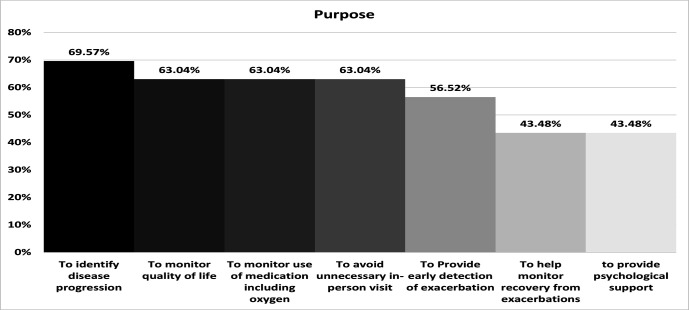
Responses to questions asking about the purpose of telehealth use are illustrated in figure 3. Of the 81 respondents with experience of telehealth 46 (57%) selected at least one answer for this question. The majority (32, 70%) reported using Telehealth to identify disease progression. The next most frequent measures were equally monitoring quality of life (29, 63%); monitoring use of medication including oxygen (29, 63%); and to avoid unnecessary in-person visits (29, 63%).
Figure 3.
Purposes given for use of telehealth.
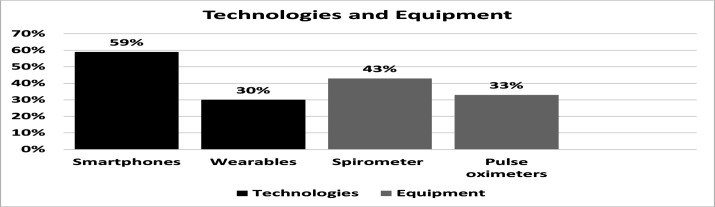
Of the 81 respondents with experience of telehealth, 46 (57%) answered this question on the equipment and technologies used to remotely monitor ILD, selecting all that applied. Of these 27 (59%) reported using a smartphone/tablet app and 14 (30%) reported using wearable sensors to monitor patients with ILD. The equipment perceived most effective were spirometers (20, 43%) and pulse oximeters (15, 33%) (figure 4).
Figure 4.
Technologies and equipment.
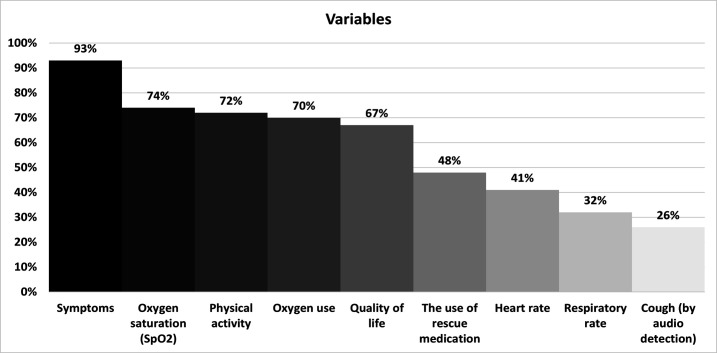
The most frequently measured variables were symptoms (43, 93 %), oxygen saturation (34, 74%) and physical activity (33, 72%). The least monitored variable reported was cough by audio detection (12, 26%) (figure 5).
Figure 5.
Variables used to monitor patients with ILD. ILD, interstitial lung disease.
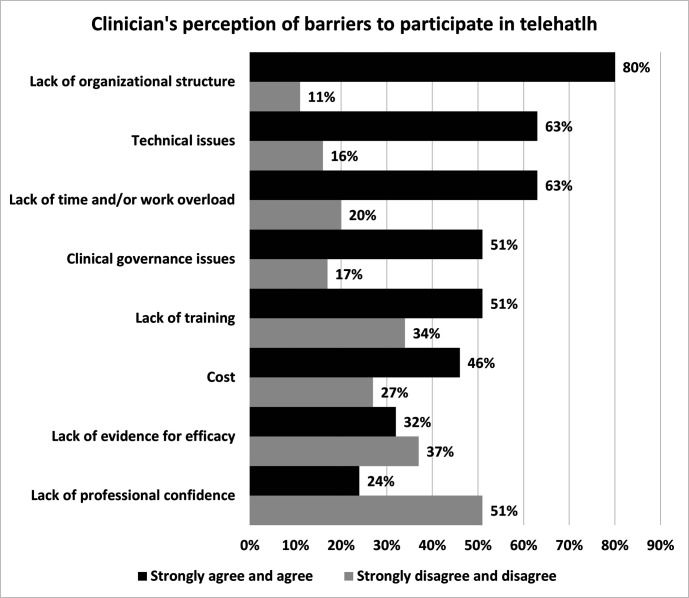
Of the 207 respondents, 142 (69%) reported perceptions of barriers that prevented wider adoption of telehealth in healthcare of patients with ILD. There was agreement (113, 80%) that the most common barrier to telehealth use was lack of organisational structure. This barrier was followed by technical issues, lack of time and/or work overload (figure 6).
Figure 6.
Clinicians' perception of barriers to adoption of telehealth.
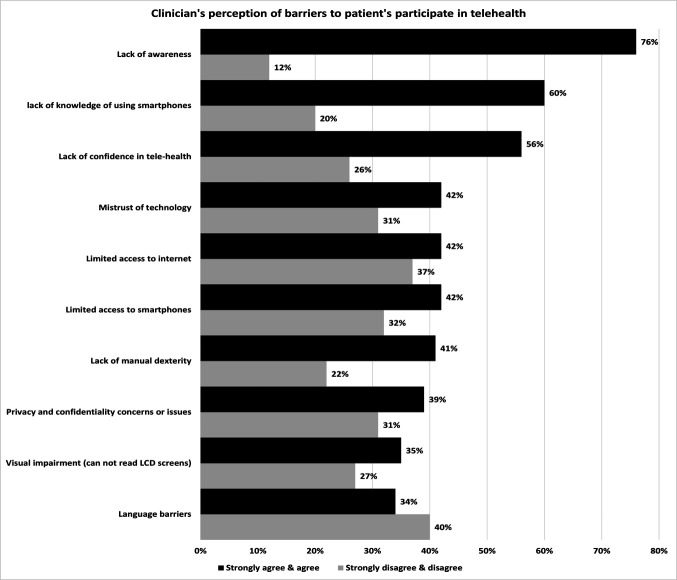
Of the 207, 142 (69%) reported their perspectives on what prevents patients from participating in telehealth (figure 7). Clinicians ‘strongly agreed’ (23, 16%) and ‘agreed’ (85, 60%) that lack of awareness was the leading barrier for wider patient participation in telehealth. This was followed by the lack of knowledge of using smartphones and the lack of confidence in telehealth. The least frequently cited barriers were language barriers and privacy and confidentiality concerns/issues.
Figure 7.
Clinicians' perception of patients’ barriers to participating in telehealth. LCD, liquid crystal display.
In addition to the main survey, and to explore other potential barriers, clinicians had the option of writing additional free-text comments or suggestions. These data were collected onto Nvivo software and analysed using thematic analysis. Almost one-third of the study respondents (142, 69%) expressed further requirements for funding, evidence, resources, support, time, training and education before widespread adoption of telehealth. We identified nine themes as follows: funding, training, resources, cost, education, administrative support, privacy, patients and confidentiality and evidence for efficacy and time (figure 8).
Figure 8.

Clinician’s comments and suggestions (the bigger the font size, the greater the frequency).
Discussion
Prior to this study, clinicians’ views about the utility of telehealth in ILD were not clear. In this global study, we explored clinician’s perspectives regarding the use of telehealth to help patients with ILD to receive healthcare services remotely. The focus of this survey was to understand clinicians’ perspective on the potential of telehealth, the type of technologies and equipment that they felt most effective, the most frequently monitored variable and the purpose of monitoring in patients with ILD. We found that 39% of the surveyed clinicians throughout the world were currently using Telehealth to remotely monitor patients with ILD. The most used technologies, equipment and variables identified by clinicians were: (1) smartphone applications and wearables, (2) home-based spirometry and pulse oximeter with data transmission and (3) symptoms and oxygen saturation. The data from this study may help determine further research and policy needed to better use telehealth services.
In our survey, healthcare professionals could be divided into two groups; clinicians who used telehealth and those who do not. One group (81, 39%) reported using telehealth and believed it to be generally effective. This percentage is similar to other estimates obtained from clinicians where it was shown that 42% started using e-health in patients with ILD during COVID-19 pandemic.39 Our findings support the results of Nakshbandi et al39 and show that most clinicians who use telehealth believe it to be quite effective in patients with ILD. Further support is seen in a physician-respondent survey by Walia et al.40 The survey shows a significant shift towards telehealth training and that the quality of telehealth is similar to face-to-face visits.40 However, our study varies from previous studies primarily in identifying the methods and practices that are most effective to detect deteriorations and/or exacerbations in patients with ILD using telehealth. Overall, clinicians with experience in use of telehealth view it as a helpful solution to provide care for patients with ILD. In particular, 70% of respondents reported using telehealth to identify disease progression. Previous studies1 11 reaffirmed that home-based monitoring facilitates prediction of disease progression. Russell et al1 suggested that the rate of change in FVC of home-based spirometry is predictive of disease progression as measured at hospital visits. Furthermore, Veit et al11 found that measuring FVC using home-based spirometry predicts disease progression.
Sixty-three per cent (63%) reported using telehealth equally to monitor: quality of life; use of medication, including oxygen; and to avoid unnecessary in-person visits. A 24-week RCT by Moor et al18 investigating the potential benefit of telehealth monitoring programme reported that patients had better-tailored treatment decisions, and better insights into the effects of medication by seeing their monitored physiological parameters. Another study by Broos et al8 suggested using in-home monitoring to evaluate response to therapy. Interestingly, 57% reported using telehealth to provide early detection of exacerbation. A prior pilot study of 10 subjects by Moor et al6 demonstrated the potential of telehealth to detect exacerbation in patients with ILD 2 days earlier than symptoms using home-based spirometry measurements. Finally, 43% reported using telehealth to provide psychological support. Aghdam et al12 used the K-BILD questionnaire to evaluate health-related quality of life and found that the King's Brief Interstitial Lung Disease Questionnaire (K-BILD) psychological domain score was higher in the in-home monitoring patients. Interestingly, oxygen saturation and physical activity were highly reported to be monitored in patients with ILD. These two variables were investigated before and have shown promising results.41 We believe that telehealth may potentially provide a valuable way of supporting both physiological and psychological needs of patients with ILD.
Although most respondents believe telehealth in patients with ILD to be quite effective, they also acknowledged barriers to wider adoption of telehealth in patients wih ILD. The second group was not using telehealth and reported their main barriers to telehealth adaption. These barriers include factors like lack of organisational structure (80%), technical issues (63%) and lack of time and/or workload (63%). A previous study by Nakshbandi et al39 reported similar challenges to telehealth adaptation. Similarly, Walia et al.40 reported challenges like lack of organisational support, inadequate telehealth technology and training. Despite advances in telehealth technology and ease of useability, technical issues were seen as one of the main barriers to adaptation of telehealth in patients with ILD.39 Maher et al35 raised similar concerns related to technical issues with home-based spirometry data variability. However, a potential solution to technical issues and barriers reported by clinicians lies in helpdesk services, training and administrative support.11 18
The healthcare professionals involved in our survey felt that patients’ barriers to participation in telehealth were mainly lack of awareness (76%), lack of knowledge of using smartphones (60%) and lack of confidence in telehealth (56%). Edward et al36 used video-based training with patients with ILD and reported that patients who were involved in a 2-week telehealth trial reported that they were happy and wanted to continue. Several studies provided training for patients at the site10 and reported high adherence.1 9 35 Moor et al6 provided standardised instructions for home spirometry and an online platform and reported that (80%) of patients were highly satisfied and found it to be easy.19 Furthermore, A 24-week randomised control trial collected data from patients with ILD and found that patients felt ‘reassured and involved in their healthcare’.18
A limitation to this study is that we did not explore patients’ perspectives, which would benefit from furthering research.
Conclusion
Our findings show a minority of ILD clinicians currently use Telehealth, but almost all these clinicians endorsed telehealth, while identifying barriers to wider implementation.
Footnotes
Contributors: MA, JRH, JP, A-MR: conception, design, distributed the survey, data acquisition, analysis, interpretation and approval of final version. MA contributed conceptualisation, distributed the survey, data collection, formal analysis, project administration, wrote original draft, reviewed and edited the manuscript; JSA reviewed and edited the manuscript. MA, JRH, JP, A-MR are guarantors
Funding: This work was supported by Department of Respiratory Therapy, Faculty of Medical Rehabilitation Sciences, King Abdulaziz University, Jeddah, Saudi Arabia through the Saudi Arabian Cultural Bureau in London.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants but N/A exempted this study Participants gave informed consent to participate in the study before taking part.
References
- 1.Russell A-M, Adamali H, Molyneaux PL, et al. Daily home spirometry: an effective tool for detecting progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2016;194:989–97. 10.1164/rccm.201511-2152OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakshbandi G, Moor CC, Wijsenbeek MS. Home monitoring for patients with ILD and the COVID-19 pandemic. Lancet Respir Med 2020;8:1172–4. 10.1016/S2213-2600(20)30452-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foundation BL . Coronavirus and COVID-19, 2020. Available: https://www.blf.org.uk/support-for-you/coronavirus/what-is-social-shielding
- 4.Wong AW, Fidler L, Marcoux V, et al. Practical considerations for the diagnosis and treatment of fibrotic interstitial lung disease during the coronavirus disease 2019 pandemic. Chest 2020;158:1069–78. 10.1016/j.chest.2020.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med 2020;8:807–15. 10.1016/S2213-2600(20)30225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moor CC, Wapenaar M, Miedema JR, et al. A home monitoring program including real-time wireless home spirometry in idiopathic pulmonary fibrosis: a pilot study on experiences and barriers. Respir Res 2018;19:105. 10.1186/s12931-018-0810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johannson KA, Vittinghoff E, Morisset J, et al. Home monitoring improves endpoint efficiency in idiopathic pulmonary fibrosis. Eur Respir J 2017;50:1602406. 10.1183/13993003.02406-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broos CE, Wapenaar M, Looman CWN, et al. Daily home spirometry to detect early steroid treatment effects in newly treated pulmonary sarcoidosis. Eur Respir J 2018;51:1702089. 10.1183/13993003.02089-2017 [DOI] [PubMed] [Google Scholar]
- 9.Noth I, Cottin V, Chaudhuri N, et al. Home spirometry in patients with idiopathic pulmonary fibrosis: data from the INMARK trial. Eur Respir J 2021;58:2001518. 10.1183/13993003.01518-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moor CC, van Leuven SI, Wijsenbeek MS, et al. Feasibility of online home spirometry in systemic sclerosis–associated interstitial lung disease: a pilot study. Rheumatology 2021;60:2467–71. 10.1093/rheumatology/keaa607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veit T, Barnikel M, Crispin A, et al. Variability of forced vital capacity in progressive interstitial lung disease: a prospective observational study. Respir Res 2020;21:270. 10.1186/s12931-020-01524-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aghdam MRF, Vodovnik A, Hameed RA. Role of telemedicine in multidisciplinary team meetings. J Pathol Inform 2019;10:35. 10.4103/jpi.jpi_20_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demartines N, Battegay E, Liebermann J, et al. [Telemedicine: perspectives and multidisciplinary approach]. Schweiz Med Wochenschr 2000;130:314–23. [PubMed] [Google Scholar]
- 14.Hurst JR, Donaldson GC, Quint JK, et al. Domiciliary pulse-oximetry at exacerbation of chronic obstructive pulmonary disease: prospective pilot study. BMC Pulm Med 2010;10:52. 10.1186/1471-2466-10-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Rajeh AM, Aldabayan YS, Aldhahir A, et al. Once daily versus overnight and symptom versus physiological monitoring to detect exacerbations of chronic obstructive pulmonary disease: pilot randomized controlled trial. JMIR Mhealth Uhealth 2020;8:e17597. 10.2196/17597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah SA, Velardo C, Farmer A, et al. Exacerbations in chronic obstructive pulmonary disease: identification and prediction using a digital health system. J Med Internet Res 2017;19:e69. 10.2196/jmir.7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu F, Burt J, Chowdhury T, et al. Specialty COPD care during COVID-19: patient and clinician perspectives on remote delivery. BMJ Open Respir Res 2021;8:e000817. 10.1136/bmjresp-2020-000817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moor CC, Mostard RLM, Grutters JC, et al. Home monitoring in patients with idiopathic pulmonary fibrosis. A randomized controlled trial. Am J Respir Crit Care Med 2020;202:393–401. 10.1164/rccm.202002-0328OC [DOI] [PubMed] [Google Scholar]
- 19.Moor CC, Gür-Demirel Y, Wijsenbeek MS. Feasibility of a comprehensive home monitoring program for sarcoidosis. J Pers Med 2019;9:23. 10.3390/jpm9020023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moor CC, van Manen MJG, Tak NC, et al. Development and feasibility of an eHealth tool for idiopathic pulmonary fibrosis. Eur Respir J 2018;51:1702508. 10.1183/13993003.02508-2017 [DOI] [PubMed] [Google Scholar]
- 21.Moor K, Johannson KAM, Maher TM. eHealth and Home-Monitoring of patients with interstitial lung diseases; worldwide experiences and perspectives. TP27 TP027 diffuse parenchymal lung disease: patient characteristics and outcomes.
- 22.Maher T, Cottin V, Russell A-M, et al. Correlation between home and clinic spirometry in subjects with IPF: results from the INMARK trial. European Respiratory Journal 2019;54:PA1318. [Google Scholar]
- 23.Marcoux V, Wang M, Burgoyne SJ, et al. Mobile health monitoring in patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2019;16:1327–9. 10.1513/AnnalsATS.201904-335RL [DOI] [PubMed] [Google Scholar]
- 24.Bahmer T, Kirsten A-M, Waschki B, et al. Clinical correlates of reduced physical activity in idiopathic pulmonary fibrosis. Respiration 2016;91:497–502. 10.1159/000446607 [DOI] [PubMed] [Google Scholar]
- 25.Althobiani MA, Evans RA, Alqahtani JS, et al. Home monitoring of physiology and symptoms to detect interstitial lung disease exacerbations and progression: a systematic review. ERJ Open Res 2021:00441-2021–2021. 10.1183/23120541.00441-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikolasch TA, Porter JC. Transbronchial cryobiopsy in the diagnosis of interstitial lung disease: a cool new approach. Respirology 2014;19:623–4. 10.1111/resp.12320 [DOI] [PubMed] [Google Scholar]
- 27.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikolasch TA, Garthwaite HS, Porter JC. Update in diagnosis and management of interstitial lung disease . Clin Med 2017;17:146–53. 10.7861/clinmedicine.17-2-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. New England Journal of Medicine 2014;370:2071–82. 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 30.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (capacity): two randomised trials. Lancet 2011;377:1760–9. 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 31.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international Working Group report. Am J Respir Crit Care Med 2016;194:265–75. 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 32.Win T, Screaton NJ, Porter JC, et al. Pulmonary 18F-FDG uptake helps refine current risk stratification in idiopathic pulmonary fibrosis (IPF). Eur J Nucl Med Mol Imaging 2018;45:806–15. 10.1007/s00259-017-3917-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spagnolo P, Maher TM. Clinical trial research in focus: why do so many clinical trials fail in IPF? Lancet Respir Med 2017;5:372–4. 10.1016/S2213-2600(17)30122-4 [DOI] [PubMed] [Google Scholar]
- 34.Moor K, Visser L, Aerts J, et al. Diurnal variation in forced vital capacity in patients with fibrotic interstitial lung disease using home spirometry: the Diva study. European Respiratory Journal 2019;54:PA2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2020;8:147–57. 10.1016/S2213-2600(19)30341-8 [DOI] [PubMed] [Google Scholar]
- 36.Edwards C, Costello E, Cassidy N, et al. Use of the patientMpower APP with home-based spirometry to monitor the symptoms and impact of fibrotic lung conditions: longitudinal observational study. JMIR Mhealth Uhealth 2020;8:e16158. 10.2196/16158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranjan Y, Althobiani M, Jacob J, et al. Remote assessment of lung disease and impact on physical and mental health (RALPMH): protocol for a prospective observational study. JMIR Res Protoc 2021;10:e28873. 10.2196/28873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takei R, Yamano Y, Kataoka K, et al. Pulse oximetry saturation can predict prognosis of idiopathic pulmonary fibrosis. Respir Investig 2020;58:190–5. 10.1016/j.resinv.2019.12.010 [DOI] [PubMed] [Google Scholar]
- 39.Nakshbandi G, Moor CC, Johannson KA, et al. Worldwide experiences and opinions of healthcare providers on eHealth for patients with interstitial lung diseases in the COVID-19 era. ERJ Open Res 2021;7:00405-2021–2021. 10.1183/23120541.00405-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walia B, Shridhar A, Arasu P, et al. Us physicians' perspective on the sudden shift to telehealth: survey study. JMIR Hum Factors 2021;8:e26336. 10.2196/26336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drent M, Elfferich M, Breedveld E, et al. Benefit of wearing an activity Tracker in sarcoidosis. J Pers Med 2020;10:97. 10.3390/jpm10030097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2021-001088supp001.pdf (1.8MB, pdf)
Data Availability Statement
Data are available upon reasonable request.