Abstract
Healthy dietary intake has been acknowledged for decades as one of the main contributors to health. More recently, the field of nutritional psychiatry has progressed our understanding regarding the importance of nutrition in supporting mental health and cognitive function. Thereby, individual nutrients, including omega-3 fatty acids and polyphenols, have been recognized to be key drivers in this relationship. With the progress in appreciating the influence of dietary fiber on health, increasingly research is focusing on deciphering its role in brain processes. However, while the importance of dietary fiber in gastrointestinal and metabolic health is well established, leading to the development of associated health claims, the evidence is not conclusive enough to support similar claims regarding cognitive function. Albeit the increasing knowledge of the impact of dietary fiber on mental health, only a few human studies have begun to shed light onto the underexplored connection between dietary fiber and cognition. Moreover, the microbiota-gut-brain axis has emerged as a key conduit for the effects of nutrition on the brain, especially fibers, that are acted on by specific bacteria to produce a variety of health-promoting metabolites. These metabolites (including short chain fatty acids) as well as the vagus nerve, the immune system, gut hormones, or the kynurenine pathway have been proposed as underlying mechanisms of the microbiota-brain crosstalk. In this minireview, we summarize the evidence available from human studies on the association between dietary fiber intake and cognitive function. We provide an overview of potential underlying mechanisms and discuss remaining questions that need to be answered in future studies. While this field is moving at a fast pace and holds promise for future important discoveries, especially data from human cohorts are required to further our understanding and drive the development of public health recommendations regarding dietary fiber in brain health.
Keywords: Fiber, cognition, microbiota-gut-brain axis, nutrition
Impact statement
Evidence regarding the significance of healthy dietary intake in mental health and cognitive functioning is accumulating. Simultaneously, increased emphasis is being placed on the microbiome in shaping brain and behavior. While literature reviews on overall dietary intake and the brain have previously been published, an overview of the state of the literature on dietary fiber and cognitive function in human populations has not been provided, partly due to the limited amount of studies that are available. However, to understand the state of the knowledge and identify future research needs, such a summary is essential. In this mini review, we synthesize these recent findings, offer compelling discussions on important questions that remain to be answered, and outline future study designs that are necessary to advance the knowledge of the field.
Introduction
You are what you eat!
This mantra, which has become engraved into the English language in the early 1940s, summarizes the fundamental influence of food in our daily lives. 1 With the isolation of the first vitamin, thiamine, in 1926, discoveries on the role of specific nutrients in bodily functions and health have accelerated and new findings are still being revealed to this day. Although dietary fiber was first defined in 1953, 2 the value of fiber in promoting health only emerged as an important concept in the late 1960s and early 1970s.3,4 While dietary fiber now is well recognized for supporting gastrointestinal, 5 immune, and metabolic health,6,7 the appreciation for its importance in cognitive function is less explored. Emerging evidence from animal studies using synthetic, extracted, or single foods high in fiber shed light on the critical involvement of fiber in cognition (e.g. exploratory behavior, recognition memory, attentional set-shifting performance),8,9 but data from human cohorts are scarce, with only a handful of observational and interventional studies being available.10–13
Undigested dietary fiber provides the major nutrient source for the human gut microbiota, the trillions of microbes (including bacteria, viruses, archaea, lower and higher eukaryotes, fungi and protozoa) 14 that inhabit the gastrointestinal tract, influencing its diversity, composition, and metabolic capacity. The impact of the gut microbiota on the brain via the microbiota-gut-brain axis has been more and more appreciated over the last decade. 15 Early evidence from animal models16,17 is progressively followed with data from human studies, demonstrating an association between the gut microbiota and cognition. 18 Additionally, intervention studies, albeit limited, 19 have begun to demonstrate that targeting the microbiota (i.e. probiotics, prebiotics) can modify cognitive abilities (e.g. cognitive flexibility, sustained attention, memory, verbal learning, social emotional cognition).20,21 Thus, with the increasing need of deciphering underlying mechanisms of the effect of dietary fiber on the brain, gut microbes as well as metabolites produced by microbial digestion of fiber (e.g. short chain fatty acids (SCFA)) have risen as major candidates.
The focus of this minireview is to compile the available evidence from the human literature regarding the association between dietary fiber and cognitive function, outline the potential pathways mediated by the gut microbiota, and discuss remaining questions and future research needs.
What is dietary fiber?
Definition and types of dietary fiber
After the term dietary fiber was first coined in 19532, its definition has evolved over the years. The definition now adopted by most countries 22 was provided in the Codex Alimentarius in 2009, defining dietary fiber as “carbohydrate polymers with ten or more monomeric units which are not hydrolyzed by the endogenous enzymes in the small intestine of humans”. 23 Due to a large proportion of dietary fibers being inaccessible to microbial degradation, more recently the term “microbiota-accessible carbohydrates” was proposed to refer to fibers that can be used by indigenous microbes. 24 Another well-studied type of dietary fiber is prebiotics, which are defined as “substrates that are selectively utilized by host microorganisms conferring a health benefit”. 25 It is important to note that while most prebiotics can be classified as dietary fibers, not all fibers can be classified as prebiotics. Likewise, other nutrients, which are not dietary fibers, e.g. polyphenols and omega-3 fatty acids, have also been described to exert “prebiotic-like” effects on the microbiota.26–29
Different types of fibers are included under the broad umbrella of dietary fiber (Box 1), which differ in their chemical properties (e.g. viscosity, solubility, and fermentability). For detailed reviews, we direct the reader to Gill et al. and Dhingra et al.5,30 These physiochemical properties are used to categorize dietary fibers, most often into soluble (e.g. pectins, β-glucans) vs. insoluble (e.g. cellulose) fiber. However, this classification has recently been challenged as it might not accurately reflect functionality. 31 A range of food sources provide dietary fiber, including whole grains, legumes, fruits, and vegetables. Thereby, the type of dietary fiber present varies by food source and usually more than one type of fiber is available in a given food.30,32 For example, wheat contains arabinoxylans (approximately 70% of total dietary fiber content), β-glucans, and cellulose, 7 whereas apples are higher in pectins as well cellulose and hemicellulose. 33 The chemical properties of the different fiber types also determine their impact on host health outcomes. Viscous fibers (e.g. β-glucan), for example, are accepted for their cholesterol-lowering effects, whereas insoluble fibers (e.g. cellulose) increase fecal bulk and reduce gut transit time, which can relieve constipation.
Box 1.
Important definitions and types of common dietary fibers.
| Carbohydrates. Organic molecules containing carbon, oxygen, and hydrogen; in the human diet, three main groups are sugars, starch, and non-starch polysaccharides. |
| Dietary fiber . Umbrella term for complex carbohydrate polymers with 10 or more monomeric units which are neither digested by the endogenous enzymes in the small intestine of humans nor absorbed; and have beneficial physiological effect; this term includes non-starch polysaccharides, oligosaccharides, lignin, resistant starch, and other plant components (i.e. gums, mucilages). |
| Added/functional fiber . Isolated and synthetic non-digestible carbohydrates with physiological benefits for humans. |
| Total dietary fiber . Sum of dietary and added/functional fiber. |
| Sugars . Monosaccharides (glucose, fructose, galactose), disaccharides (sucrose, lactose) and sugar alcohols (e.g. sorbitol, xylitol). |
| Oligosaccharides . Shorter chain complex carbohydrates of 3 to 10 (sometimes up to fifteen) monosaccharides; includes the fermentable fructooligosaccharides and galactooligosaccharides. |
| Polysaccharides . Long-chain carbohydrates of 10 or more monosaccharide units; two classes include starches and non-starch polysaccharides (i.e. dietary fiber). |
| Microbiota-accessible carbohydrates . Dietary carbohydrates that are resistant to degradation and absorption by the host and metabolically available to gut microbes. |
| Lignin . Complex polymer (not a polysaccharide) of phenylpropane units; component of plant cell walls. |
| Gums and mucilages . Not cell wall components; thick, gel-forming, highly branched non-starch polysaccharides that hold plant cell walls together; examples include guar gum and gum arabic. |
| Prebiotics . Selectively fermented ingredient that results in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health; most commonly known prebiotics are inulin, fructo- or galactooligosaccharide, or pectin. (It is worth noting that not all prebiotics are fibers and not all fibers have prebiotic capacity.) |
| Resistant starch . A form of starch that is resistant for human enzymatic digestion in the small intestine and can function as a prebiotic/dietary fiber in the large intestine; includes four forms: RS 1 “physically inaccessible”; RS 2 “ungelatinized”; RS 3 “retrograded”; RS 4 “chemically modified”; RS 5 “amylose-lipid complex”. |
| Cellulose . Major cell wall component with linearly arranged glucose units (∼10,000). |
| Hemicellulose . Complex carbohydrate containing monomers other than glucose (e.g. xylose, galactose, mannose); is smaller in size than cellulose and usually branched structured. |
| Pectin . Common cell wall polysaccharide component in fruits and vegetables; highly water soluble and almost completely metabolized by gut microbes. |
| β-glucan . A soluble, fermentable polymer of glucose with branched structure; rich food sources include oats and barley. |
| Inulin . Type of fermentable (mainly) fructose oligosaccharide with known benefits to gut microbiota and host health. |
| Arabinoxylans . Highly fermentable dietary fiber and major source of fiber in the diet; highest content can be found in rye and wheat. |
Recommended daily intakes of fiber
Guidelines on recommend daily intakes of dietary fiber vary between countries. In Ireland, recommendations are to consume 24 to 30 g per day, whereas 14 g per 1000 kcals (which is the equivalent to 28 to 35 g per day) are specified in nutrition guidelines in the United States. 34 Despite the well-documented health benefits of dietary fiber, most people in Western countries do not meet the recommended levels. In fact, levels of dietary fiber intake have been stagnantly low over the last decades. 35 Most recent data show that in the United States, an average adult consumes approximately 16 g of dietary fiber per day 36 and the average intake was reported to be between 16 to 29 g per day in Europe. 37 In the last nationwide adult nutrition survey in Ireland, the median intake was estimated at 18 g per day. 38 It is interesting to note that is has been suggested that current recommendations might actually be too low to produce significant physiological effects and levels closer to 50 g per day, as observed in rural, non-industrialized countries, might be necessary to elicit meaningful impact on host health.39,40 Thus, with increasing knowledge on the health benefits of fiber, current guidelines could undergo revisions in the future.
Dietary fiber and the gut microbiota
The chemical properties of dietary fiber also determine the degree of its impact on the microbiota. Thereby, it has been suggested that only about 30% of dietary fiber from grain products is available for microbial metabolism, whereas 75% to 90% of fibers from fruit and vegetables were estimated to be metabolized by the gut microbiota.41,42 Likewise, microbes differ in their enzymatic capacity to metabolize the complex linkages of dietary fiber, with some species being able to degrade a wide range of these polysaccharides (generalists) while others are specialized on only a few different ones (specialists).43–45
The impact of dietary fiber on microbiota composition and function has been extensively studied and reviewed.39,46,47 Typically, a diet high in fiber has been associated with a “healthy” microbiota, such as increased microbial diversity, 48 although results can be inconsistent. 49 Furthermore, several studies have demonstrated a bloom in beneficial microbes, such as bifidobacteria and lactobacilli, in response to high dietary fiber intake,49–51 although these strain specific changes can be distinct based on the fiber type. Resistant starch consumption increased the abundance of Bifidobacterium, Faecalibacterium, Eubacterium, while decreasing some Ruminococcus strains52,53 and insoluble non-fermentable fiber such as cellulose, can be degraded by cellulose-metabolizing microbes such as Ruminococcus and Fibrobacter. 54
Due to dietary fiber serving as the main substrate for microbial fermentation, microbial metabolite production is also influenced by fiber intake, most notably SCFAs (acetate, propionate, butyrate). Thereby, specific metabolites are produced by specialized microbes. 55 Interestingly, a recent study in pigs demonstrated that a microbiota-accessible fiber elicited both targeted and secondary metabolic shifts in the microbiota, 56 suggesting that metabolic exchanges and cross-feeding also contribute to metabolite production in response to dietary fiber. Although insoluble fibers are usually considered to be less metabolizable by the gut microbes, changes in enzymatic pathways and metabolite production, such as linoleic acid, nicotinate and nicotinamide, glycerophospholipid, glutathione, sphingolipid, as well as valine, leucine, and isoleucine metabolic pathways were observed after cellulose intake in animal models.57,58
Although some generalizations regarding the impact of dietary fiber on higher microbial diversity, composition (especially the bloom of beneficial microbes) and enzymatic capacity can be made, large interindividual variations are often observed. These differences in responses could be attributed to intrinsic factors of the host (e.g. genetics)59,60 as well as habitual diet or the baseline microbiota composition. For example, a baseline microbiota composition harboring the enzymatic capacity able of degrading dietary fiber (e.g. Prevotella) could be an important predictor of an individual’s response to a dietary intervention.61,62 Likewise, individuals with a lower habitual fiber intake could potentially have the highest gain from a fiber intervention.63,64 More studies investigating how the microbiota responds to dietary fiber and which factors could predict the directionality of the response are warranted.
Dietary fiber and cognition: What do we know?
The impact of dietary fiber on the brain has been investigated in relation to mental health, brain function, and cognitive performance (Box 2). Regarding mental health, the importance of adequate and healthy dietary intake has long been appreciated65,66 and large cohort studies have demonstrated that high diet quality and healthy dietary patterns are associated with reduced symptoms of depression.67,68 More recently, adequate dietary fiber intake (often a hallmark of diet quality) has also emerged as an important factor in supporting mental well-being by lowering odds of developing depression. 69 While less data are available regarding the association between dietary fiber intake and brain structure, results seem to suggest that dietary patterns with higher fiber intake might be associated with better brain integrity (larger total brain volume and less white matter damage) in older adults 70 and animal models (white matter microstructural integrity). 71 In terms of cognitive function, the importance of diet quality and overall healthy dietary patterns across the lifespan has also been acknowledged. Thereby, better maternal diet quality including adequate intake of dietary fiber can have positive effects on neurodevelopment in children of elementary school age or younger. 72 In children and adolescents, better food quality (that is consumption of healthier foods, including whole grains, fruits, and vegetables) was associated with improvements in executive functioning (e.g. inhibitory control, working memory), but also attention and episodic memory. 73 On the other hand, poor dietary quality (e.g. intake of sugar-sweetened beverages, processed food) correlated with poorer executive function. 73 On the other extreme of life, in elderly populations, healthy dietary patterns, such as the Mediterranean or MIND (Mediterranean-Dietary Approach to Stop Hypertension Intervention for Neurodegenerative Delay) diet and consumption of a dietary pattern characterized by high intakes of vegetables and fruits have been associated with healthy aging and reduced cognitive decline as well as alleviated cognitive impairment.74–77 The combined intake of fruits and vegetables has been shown to improve performance in attention, memory, executive functions, speed of processing, and global cognition (Mini-Mental State Exam). 78 Despite the growing knowledge on the impact of dietary patterns and food groups on cognitive abilities, whether these benefits are associated with the dietary fiber content or can be attributed to other beneficial nutrients present in these foods, such as polyphenols, unsaturated fatty acids or vitamins, cannot be determined from these studies.
Box 2.
Definitions of terms relating to the brain.
| Brain function . Umbrella term to all functions pertaining to the brain. |
| Brain structure . Refers to the anatomy of the brain. The three main structural divisions are the cerebrum (divided into four lobes (frontal, parietal (middle), occipital (back) and temporal (sides)), brainstem (divided into medulla, pons and midbrain), and cerebellum. The two types of tissues that make up the brain are the grey and white matter. |
| Mental health . According to the World Health Organization, mental health is defined as “a state of well-being in which the individual realizes his or her own abilities, can cope with the normal stresses of life, can work productively and fruitfully, and is able to contribute to his or her community”. Includes emotional, psychological, and social well-being. |
| Cognition . Process of acquiring knowledge and processing information; different types of cognitive processes include attention, learning, memory, problem-solving, or decision making. |
Evidence from observational studies
To better understand which nutrients influence cognition, studies have begun to individually correlate nutrient intake with cognitive outcomes. For example, evidence suggests that the benefits of omega-3 fatty acids on cognitive functions include improved attention. 79 The associations between dietary fiber and cognition studies are summarized in Table 1. In children, higher intakes of insoluble and total fiber (as measured by a three-day food record) were related to better cognitive control involving attentional inhibition. 11 Interestingly, these associations might be gender specific, as suggested by studies reporting significant correlations between dietary fiber intake or diet quality scores and non-verbal reasoning in boys, but not in girls.13,83 Although this is an intriguing observation, differences could also be attributed to known gender differences in cognitive function; thus, this correlation warrants further investigation. Additionally, while these studies hint at the important role adequate fiber intake plays in healthy development during childhood, a recent systematic review concluded that low to very low evidence for the relationship between childhood fiber intake and subsequent health outcomes, including cognitive function, is available. 80
Table 1.
Human study data on the association between fiber and cognition.
| Results | ||||||
|---|---|---|---|---|---|---|
| Cohort | Study type | Type of fiber | Cognition | Microbiome | Other notable | References |
| Observational studies | ||||||
| Elderly (65–90 years of age)n = 260Spain | Cross-sectional | Dietary fiber (7-day weighed food record | Subjects with lower errors on Pfeiffer’s Mental Status Questionnaire (PMSQ) had higher intakes of dietary fiber. | Not reported | Associations with other foods and nutrients were observed (e.g. lower intake of saturated fatty acids, and greater total food, fruit, vegetables, folate, vitamin C, iron, and zinc) | 81 |
| Elderly women (n = 4809) France | Cross-sectional analysis from large longitudinal cohort | Dietary fiber (diet history questionnaire) | Increased odds of cognitive decline (assessed by observed cognitive deterioration scale) with decrease intake of total and soluble fiber | Not reported | Association was also observed for omega-3 fatty acids | 82 |
| Elderly (>65 years of age) n = 178Spain | Cross-sectional | 7-day food record | Less errors made on short portable Mental Status Questionnaire (assesses short- and long-term memory, orientation, knowledge of current events and calculation capacity) with increased fiber intake | Not reported | Many associations between better cognitive capacity scores and foods and nutrients (e.g. cereal, eggs, oils, fats, fish and vegetable consumption, carbohydrates, PUFAs, riboflavin, vitamin C, D, and E) | 12 |
| Children 7–9 years of agen = 65United States | Cross-sectional form longitudinal cohort | Dietary fiber (assessed with 3-day food record) | Positive association between congruent response accuracy* and insoluble fiber and total fiber; positive association between incongruent response accuracy* and insoluble fiber, pectins, and total fiber*in modified Eriksen flanker task to assess attentional inhibition | Not reported | None | 11 |
| Adults (average age 50 years) n = 3231 (56% female, 45% male) United States | Prospective cohort study (dietary assessment in young adulthood and cognitive performance in midlife) | Dietary and crude fiber (study specific diet history questionnaire) | Fiber intake was associated with better cognitive performance in verbal learning and memory (Rey Auditory Verbal learning test) and psychomotor speed, sustained attention and working memory (Digit symbol substitution test) *cognitive assessment in middle age | Not reported | Profound association between intake of fruits and vegetables high in fiber and cognitive performance; Lycopene and β-carotene also associated with better cognitive performance | 93 |
| Adults (55–65 years of age) n = 617, 49% men, 51% women) Australia | Data from wellbeing, eating and exercise for a long life study (prospective, population-based longitudinal study) | Fiber foods in food frequency questionnaire | Consumption of higher fiber or multigrain bread types was associated with better cognitive performance compared to white breadAssessed by brief, 13-item test(Telephone Interview for Cognitive Status Modified) of global cognition | Not reported | Past higher dietary variety (in women) and recent diet quality and fluid consumption (in men) was associated with better cognitive function | 92 |
| Children (6–8 years of age) n= 487 (250 boys, 237 girlsFinland | Cross-sectional from larger study | Dietary fiber (4-day food record) | Higher scores in non-verbal reasoning (Raven’s Colored Progressive Matrices) with higher total and soluble fiber intake in boys | Not reported | Insoluble fiber was not associated with cognitive outcome; No associations were found in girls | 13 |
| Fiber-containing foods intervention studies | ||||||
| High school students (15–17 years of age) n = 28 | Randomized, controlled, parallel, single-blinded study9 weeks | Mixed grain diet (providing 13.4 g of dietary fiber) provided 3 times per day | Improvements in mental-fatigue test (specifically sustained attention) after mixed grain diet | Not reported | Higher change in plasma BDNF levels in mixed grain vs. regular diet | 85 |
| Healthy older adults (50–70 years of age) n = 40 (30 women, 10 men) Sweden | Randomized, controlled, cross over design, 5 weeks | Berry beverage (11 g of fiber, also 795 mg of total polyphenols) | Better performance in working memory test (assessed by verbal working memory test) 30 min after standardized breakfast in berry beverage group | Not reported | Reduction in total and LDL cholesterol | 94 |
| 38 healthy middle-aged adults (52–70 years of age) n = 38 (30 women, 8 men) Sweden | Cross over, randomized design, 3 days | Rye-based breads (whole grain rye kernel/flour mixture supplemented with resistant starch type 2); Total dietary fiber 40.9 g/day | No differences in cognitive performance (verbal working memory and selective attention test) | Not reported; But, increased plasma SCFA (acetate, butyrate, total) and breath hydrogen | Improvements in mood measures (valence (unpleasantness-pleasantness) and activation (quietness-excitement) on Swedish Core Affect Scale); Increased insulin sensitivity and fasting gut hormones; Decreased in fasting inflammatory marker IL-1βPositive correlation between insulin sensitivity and working memory and negative correlation between markers of glucose regulation and mood variables | 97 |
| Isolated fiber/prebiotic intervention studies | ||||||
| Healthy volunteers (18–45 years of age); n = 45 (22 males, 23 females) Great Britain | Randomized trial3 weeks | Fructooligosaccharide (FOS) or Bimuno-galactooligosaccharide (B-GOS) (5.5 g/day) | Decreased attentional vigilance to negative vs. positive information (improved emotional information processing) after B-GOS | Not reported | Lower cortisol awakening response after B-GOS; Significant changes were only observed after B-GOS supplementation, but not FOS | 88 |
| Healthy adults (19–30 years (28 females, 19 males) n = 47 Great Britain | Double blind placebo controlled, cross over design One dose | Oligofructose-enriched inulin (5 g one dose) | Greater accuracy in recognition memory task and improved recall performance (improvements in memory tasks) Improved subjective mood | Not reported | Less indigestion and hunger after inulin supplementation | 89 |
| Middle-aged adults (45–60 years of age) n = 73 (26 male, 47 female) Australia and New Zealand | Randomized, placebo-controlled double-blind design, 1 dose, testing 30 min after ingestion | Non-starch polysaccharide (Ambrotose complex) | Improvements in recognition and working memory | Not reported | No changes in subjective levels of anxiety, alertness, contentedness, and calmness | 84 |
| Elderly participants (66–90 years of age) n = 50 (15 men, 35 women) Spain | Randomized, placebo-controlled, double-blind design, 13 weeks | Inulin plus FOS (7.5 g) | No significant effect on cognitive performance (mini mental state examination). | Not reported | Improvement in frailty criteria (exhaustion and handgrip strength) | 90 |
| Patients with psychosis (18–60 years old) n = 39 Great Britain | Randomized trial3 weeks | B-GOS (5.5 g/day), 12 weeks | Enhanced cognitive performance (specifically executive functioning) | Not reported | No effect on plasma metabolic or immune markers | 91 |
| Healthy female adults (18–40 years of age) n = 18Ireland | Randomized, cross over design4 weeks | Polydextrose (12.5 g/day) | Improvements in cognitive flexibility and sustained attention | Increase in Ruminiclostridium 5 | None | 20 |
BDNF: brain-derived neurotrophic factor; IBS: irritable bowel syndrome; FOS: fructooligosaccharide; XOS: xylo-oligosaccharide; SCFA: short chain fatty acids.
A few studies have also investigated the impact of dietary fiber intake in other age groups. In an Australian middle-aged cohort, habitual consumption of higher fiber or multigrain products was associated with better cognitive performance. 92 However, this study included a brief, 13-item telephone interview screening for global cognitive function, which is recognized as a major limitation in this study. Among older populations, higher habitual intakes of dietary fiber correlated with better cognitive capacity and reduced cognitive decline.12,81,82,92 Good dietary practices in earlier life, including adequate dietary fiber intake, could also be crucial for later cognitive abilities, as was demonstrated in a recent study. This prospective study reported that higher intake of dietary fiber in young adulthood was associated with later better cognitive performance in verbal learning and memory, psychomotor speed, sustained attention and working memory in middle age. 93
Evidence from interventional studies
Most human interventional studies investigating the efficacy of dietary fiber to improve cognition have used prebiotic supplementations. 87 In healthy participants, a decrease of attentional vigilance to negative (versus positive) information was observed after a three-week supplementation with B-immuno-galactooligosaccharide 88 and our lab recently showed that polydextrose led to subtle improvements in cognitive flexibility and sustained attention. 20 Likewise, improvements in recognition memory were observed after one acute dose of oligofructose-enriched inulin 89 and non-starch polysaccharide 84 in healthy adults. Although there is an increase in human intervention studies investigating the cognitive enhancing effects of prebiotics, a recent systematic review and meta-analysis concluded that the currently available evidence does not support the use of prebiotics to influence cognitive measures. 19
Besides using isolated dietary fibers or prebiotics, other ways to study the impact of dietary fiber on the brain is through the use of high fiber foods. A small number of intervention studies have used single high fiber foods to investigate whether cognitive performance could be improved through increased fiber intake, yielding inconsistent results. For example, a nine-week supplementation with mixed-grain product containing 13.4 g of total dietary fiber in high-school students (age range 15–17 years of age) improved performance in sustained attention (continuous performance test). 85 Likewise, better performance on a working memory task was observed after an intervention with a berry beverage. 94 However, the berry beverage also contained a significant amount of polyphenols, and given the known impact of polyphenols on the brain86,95,96 it is not possible to decipher whether the benefits on cognitive performance in this study could be attributed to the fiber or polyphenol content of the study product. On the other hand, a short-term (three days) intervention with rye-based bread did not change working memory and attention. 97
By reviewing the available literature, a gap in studies investigating the impact of dietary fiber on cognition was identified. Most studies to date focus on mental health (particularly depression) in relation to dietary fiber intake. While knowledge on the association between dietary fiber and cognition is growing, only a handful of studies (especially those focusing on dietary fiber alone) are available to date. While current marketing strategies include the promotion of dietary fiber for improving cognition, scientific evidence for these claims are lacking. Thereby, both studies examining the general association between fiber and cognition as well as other specific research questions remain to be addressed.
Candidate mechanisms for the impact of fiber on cognition
With the now widely accepted concept of the microbiota-brain communication, 15 researchers are focusing on exploring how microbes exactly exert their influence on the brain. Both microbiota-dependent as well as microbiota-independent effects of dietary fiber on host health have been described. 98
Microbiota-independent mechanisms
Independently of the gut microbes, dietary fiber can interact with enterocytes and support epithelial barrier function, gut homeostasis, and the intestinal immune response. For example, it has been demonstrated that dietary fiber can promote the assembly of tight junction proteins or intestinal epithelial cell proliferation and differentiation through AMP-activated protein kinase, epidermal growth factor receptors, or toll-like receptor dependent mechanisms. 98 Likewise, dietary fiber can regulate cytokine and chemokine production and release from intestinal epithelial cells as well as act on intestinal dendritic cells, macrophages, monocytes and mast cells, thereby promoting the development of the intestinal immune system. 98 This microbiota-independent immune modulation of dietary fiber was demonstrated in an animal study in which supplementation with resistant starch resulted in reduced macrophage expression in the adipose tissue and improved insulin sensitivity. 99
Microbiota-dependent mechanisms
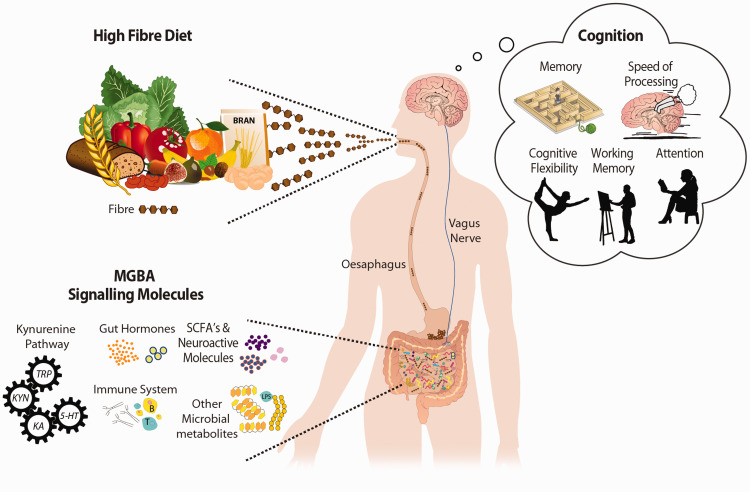
While these microbiota-independent effects might have local health benefits, microbiota-dependent mechanisms could be more relevant to the distant brain modulating properties associated with high fiber intake. Specifically, the well-documented growth in beneficial gut commensals (e.g. Bifidobacterium, Lactobacillus, Akkermansia muciniphila), inhibition of potential pathogens (e.g. some Clostridium species, Enterococcus, Escherichia), as well as the production of microbial metabolites (e.g. SCFAs) could play important roles. Other potential mechanisms include immune, endocrine, neuronal, or vagal routes. These potential pathways involved in the impact of dietary fiber on cognition are illustrated in Figure 1 and are summarized in the sections below.
Figure 1.
Candidate mechanisms for the impact of dietary fiber on cognitive functions. While the mechanisms underlying the benefit of fiber on cognition have not been established, several microbiota-dependent pathways could be proposed. Certain microbes can degrade dietary fiber, supporting their growth and the production of metabolites (e.g. short chain fatty acids (SCFAs)). These microbes themselves and their metabolites can regulate neurochemistry relevant to cognitive processes, stimulate neurotransmitter production, and influence neuronal function. Likewise, gut hormones (e.g. ghrelin, peptide YY) with neuroactive potential can be affected by microbes and microbial metabolites. Another important pathway whereby dietary fiber exerts influence on cognition could be immune mediated, through the kynurenine pathway or the vagus nerve.
Short chain fatty acids
As the end-product of microbial fermentation of dietary fiber, SCFAs appear to be the most obvious route of communication between the gut microbiota and the brain that could be mediated by dietary fiber intake. Indeed, a wide variety of potential mechanisms whereby SCFAs exert influence on brain functioning have been proposed. First and foremost, SCFAs support intestinal barrier integrity and regulate gastrointestinal immune cells, 100 which positively modulates the peripheral immune system and ultimately protects against neuroinflammation. Protection against infiltration by neurotoxic factors is also provided through the enhancing effect of SCFAs on blood–brain barrier (BBB) integrity through increasing occludin expression. 101 Additionally, SCFAs can modulate the concentrations of neurotransmitters (e.g. serotonin, glutamate, γ-aminobutyric acid (GABA))102,103 and neurotrophic factors, 104 thereby regulating the growth and excitability of neurons and synapses and influencing cognitive processes such as learning and memory. 104 Lastly, SCFAs modulate the hypothalamus-pituitary-adrenal (HPA) axis, the main neuroendocrine regulator of stress responses in mammals, which was demonstrated in a recent human trial in which SCFAs attenuated cortisol response to psychosocial stress. 105 Other routes through which SCFAs manipulate microbial communication to the brain include neuronal, vagal or other humoral pathways, which are further outlined below.
Neurotransmitters and neurotrophic factors
Early evidence using germ-free mice demonstrated that the microbiota does not only fundamentally impact host behavior, but also regulates neurochemistry and brain structure.17,106,107 Other animal studies reporting correlations between the abundance of certain bacterial species and concentrations of molecules important for cognitive processes (e.g. brain-derived neurotrophic factor (BDNF), a key molecule for memory formation) 108 provided additional evidence that gut microbes could be involved in neurochemical aspects relevant to cognitive function. Additionally, different neurotransmitters and receptor functions that have been implicated in cognitive processes (e.g. serotonergic, dopaminergic, cholinergic, adrenergic system)109,110 can be directly or indirectly influenced by the microbiota. The neuroactive potential of the human gut microbiota resulted in the construction of so-called “gut brain modules”, 111 demonstrating the capability of microbes to produce neurotransmitters from dietary sources.112,113 Although these neurotransmitters cannot directly be produced from dietary fiber, bacterial species which are known neurotransmitter producers also respond to dietary fiber. For example, Lactobacillus, a beneficial microbe that grows under high fiber intake, showed positive correlations with mRNA expression of serotonin receptors in juvenile rats 114 and another Lactobacillus strain (L. casei) improved serotonin biosynthesis in healthy young adults. 115
More importantly, a recent small-scale human study observing associations between the gut microbiota, processing speed and mental flexibility as well as changes in fecal and plasma glutamate metabolism 116 and a rye-kernel-based bread intervention resulting in increased plasma BDNF levels in a human cohort, 117 could suggest that dietary fiber modulates the microbial influence on neurochemistry. Likewise, BDNF is involved in synaptic plasticity, 118 the activity-dependent change in strength or efficacy of synaptic transmission, suggesting another indirect avenue by which fiber-modulation of the gut microbiota could impact cognitive processes.
Gut hormones
Gut microbes can directly produce and SCFAs can stimulate the secretion of gut hormones, such as glucagon-peptide 1 (GLP-1), peptide YY (PYY), and ghrelin.119,120 Recent research has determined that the function of these gut hormones can go beyond local regulation in the gastrointestinal tract. 121 For example, these gut-derived hormones can influence synaptic activity and formation and contribute to cognitive functions, such as learning and memory formation.122,123 Mechanisms of action could include direct effects in the brain as some hormones were shown to cross the BBB and reach the hypothalamus or hippocampus124,125 or signalling through receptors that have been identified in various brain regions and vagal afferent terminals.126–128 Dietary fiber could thereby mediate the production of gut hormones by the microbiota as shown in a human study reporting that highly fermentable prebiotics resulted in increased satiety, reduced hunger and changes in appetite though microbiota-elicited changes in GLP-1 and PYY. 129
The immune system
The gut microbiota plays a fundamental role in training and regulating the immune system, an association that could also be of importance in cognition,130–132 as inflammation has been linked to cognitive impairment and decline. 133 Dietary fiber could, thereby, alleviate cognitive dysfunction through its anti-inflammatory potential via the gut microbiota, support of gut barrier integrity or increasing the abundance of bacterial strains, such as Bifidobacterium or Lactobacillus, and the concentration of SCFAs, which have anti-inflammatory properties.134,135 A recent animal study revealed that dietary cellulose exerts anti-inflammatory effects through maturation of the gut microbiota 136 and a short-term dietary fiber intervention increased circulating anti-inflammatory SCFAs and decreased pro-inflammatory chemokines and cytokines in patients with rheumatoid arthritis. 137 Direct evidence for the underlying immune pathway in cognitive benefits of dietary fiber were presented recently in an animal model where microbiota-accessible carbohydrates prevented neuroinflammation and cognitive decline in a high-fat, fiber-deficient diet-induced obese mice model. 138 Another study highlighted that supplementation with a B-GOS mixture attenuated neuroinflammation and cognitive impairment in a rat model of abdominal surgery. 139 Furthermore, immune mediated pathways have been suggested to underlie the impact of the gut microbiota on neurogenesis.140,141
Kynurenine pathway
Kynurenine is a metabolite of tryptophan metabolism. Although the most well-known bioactive molecule derived from tryptophan is probably serotonin, it has been estimated that 90% of available tryptophan feeds into the kynurenine pathway. 142 Kynurenines can be produced in various tissues, including the liver by the enzyme tryptophan dioxygenase and the brain through indoleamine 2,3-dioxygenase. 143 Importantly, the kynurenine pathway has been implicated in a range of neurobiological functions, including cognition.144,145 The microbiota has been implicated in regulating the kynurenine pathway, by metabolizing dietary derived tryptophan, but also by independently producing tryptophan and other kynurenine metabolites 146 In this regard, the use of prebiotics and especially probiotic was recently found in a systematic review to modulate the kynurenine pathway. 147 Numerous mechanisms (e.g. neuroendocrine or immune system) were identified by which the gut microbiota can regulate the kynurenine pathway. 146
Kynurenine has the ability to cross the BBB and be metabolized into neuroactive products, including kynurenic (neuroprotective) and quinolinic (neurotoxic) acid. These metabolites in turn can impact cholinergic, glutaminergic, or dopaminergic neurotransmission,148,149 important mechanisms for cognitive processes such as memory consolidation 150 or spatial working memory. 151 Exactly how dietary fiber could modulate the kynurenine pathway is unclear, but it could be proposed that dietary modulation of gut microbes which either impact the availability of circulating kynurenine and modulate distribution in the CNS or exert anti-inflammatory properties could be an important link. It has been shown that immune system and kynurenine metabolism are tightly connected, 146 with inflammation disrupting the kynurenine pathway and consequently neurotransmission. Thus, a bloom in anti-inflammatory microbes and their metabolites (e.g. SCFA) through dietary fiber could normalize the disrupted kynurenine pathway in inflammatory states. Indirect evidence that an increase in beneficial microbes could positively modulate cognition through the kynurenine pathway was provided by a probiotic (Lactobacillus plantarum 299v) supplementation study which decreased kynurenine concentration and improved measures of attention and episodic verbal learning and memory in depressed patients. 152 Whether dietary fiber could have similar effects remains to be determined.
The vagus nerve
Landmark studies reporting that behavioral effects could be abolished after animals undergo vagotomy provide direct evidence for vagal signal transduction from the gut microbiota to the brain.153,154 Importantly, the vagus nerve can be activated by fiber-responsive gut microbes (including symbionts such as L. rhamnosus or B. longum154,155) and SCFAs.105,119 This vagus nerve activation could enhance aspects of cognition, as demonstrated in a human cohort that showed improved recognition memory after undergoing vagal stimulation. 156 However, results need to be reproduced to establish whether this vagal stimulation modulates cognitive processes. Furthermore, the potential of dietary fiber to influence the vagus nerve through the microbiota was demonstrated in a recent animal study, in which potato-resistant starch inhibited vagal remodeling associated with a high-fat diet-induced microbiota. 157 Lastly, vagal activation could, also stimulate neurogenesis and BDNF expression. 158 Neurogenesis, especially in the hippocampus, was suggested to play an integral part in learning and memory, 159 indicating that the vagus nerve could be involved in the microbial influence on cognitive processes. Taken together, these findings suggest an indirect pathway through the microbiota and vagus nerve by which dietary fiber influences cognition.
With the increase in studies investigating the influence of dietary fiber on brain function, other, previously unknown mechanisms, are likely to be unraveled. Certainly, a multitude of intertwined pathways (both microbiota-dependent and independent) are probable to emerge as underling avenues of the fiber-brain cross talk.
Conclusions and future research needs
While data from animal studies are accumulating, demonstrating the influence of dietary fibers on cognitive abilities, definite evidence supporting translatability of these findings into human populations is lacking. Emerging evidence from observational and interventional studies has started to elucidate the impact of dietary fiber on cognition, but more data are needed to support conclusions to be drawn at this stage. Given the low fiber consumption in Western societies, understanding the long-term implications on cognitive function is a necessary area of investigation.
Most of the available evidence provided has focused on the relationship between fiber intake at the extremes of life, highlighting the need for studies investigating the impact of dietary fiber across different age groups. Considering the importance of dietary fiber on overall health and existing evidence regarding mental health, it is undoubtful that these studies will generate data that establishes detrimental consequences of low habitual dietary fiber intake on cognitive outcomes. Therefore, another question that needs attention is whether these adverse effects can be salvaged by switching to a high fiber diet. Whole-diet nutritional interventions have shown improvements in some cognitive domains across different age ranges, but additional studies are vital to elucidate the role of dietary fiber. Additionally, based on recent discussions, pinpointing the amount of fiber necessary to elicit cognitive benefits will be important for the development of dietary guidelines. Similarly, the degree to which the different types of dietary fiber can influence cognition and whether certain aspects of cognition are more prone to be manipulated by dietary fiber needs to be addressed. In human cohorts, observational data indicated that especially intake of fiber from vegetables, fruit, or seaweed was associated with decreased odds of depressive symptoms, whereas this association was not observed for other fibers, for example from cereals.160–162 Thereby, microbial accessibility (i.e. fermentability) could be an important factor in determining the cognitive influence of a dietary fiber. Lastly, most studies to date investigating the relationship between dietary fiber and cognition have not investigated potential underlying mechanisms, including the role of the gut microbiota. Therefore, future research should include outcomes that allow for the investigation of mechanisms, including immune pathways and microbial metabolites.
Certainly, addressing these research questions in human populations will not come without challenges. For example, measuring cognitive improvements in a healthy population with normal baseline cognitive function can be difficult due to ceiling effects. Thus, selection of appropriate cognitive tests that are sensitive to changes even in non-clinical populations and robust to repeated measures (e.g. alternative validated forms) is essential. Thereby, increasing tasks demands, going beyond assessing overall cognitive function, and dissecting the underlying cognitive processes (for example, in a working memory tasks determining if participants have difficulties or improve in maintenance or manipulation processes) will be important considerations. Furthermore, an interdisciplinary science approach bringing together nutrition and cognition scientists and adding neuroimaging techniques is important in order to have a stronger exploration of the specificity of effects on cognitive processes that underlie more broad overall cognitive functions. Likewise, investigating cohorts with cognitive deficits may be warranted as targeting the microbiota to improve cognition could hold promise for future intervention strategies. 163 In the context of malnutrition, which is often associated with poor cognitive function, 65 a microbiota-directed complimentary foods intervention that included some fiber sources improved neurodevelopment in malnourished children. 164 Lastly, although the health benefits of consuming adequate dietary fiber apply to all individuals, high variability in metabolic or gastrointestinal responses is often observed, which could be attributed to individual’s genetics, microbiota, or other lifestyle factors. Thus, it will be crucial to understand determining factors that could predict an individual’s cognitive response to dietary fiber intake.
Despite many opportunities for improving mental health and cognitive functioning being connected to adequate dietary fiber intake, more research needs to be done to clearly establish causality, understand the role of different fiber types, and decipher mechanistic relationships. Many health claims have been associated with a high fiber diet and for some diseases (e.g. gastrointestinal disorders), dietary fiber interventions are a common clinical approach for the management of symptoms. 5 Previously, the role of dietary habits in supporting mental health has been emphasized 165 and it has been recommended that patients with mental health problems should be encouraged to consume diets high with content of grains and fibers. 166 However, more high-quality data on the relationship between fiber and brain are required to establish evidence-based health claims and to develop therapeutic interventions for diseases associated with cognitive dysfunction.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Kenneth J. O’Riordan for his assistance with figure design and comments on the manuscript.
AUTHORS’ CONTRIBUTIONS: KB, CC, and JFC reviewed the literature, wrote and approved the final version of the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JFC has been an invited speaker at meetings organized by Mead Johnson, Friesland, Neuropharmex, Yakult, and Alkermes, has been a consultant for Nestle, and has received research funding from Mead Johnson, Cremo, Nutricia, and DuPont. All other authors report no conflict of interest.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: APC Microbiome Ireland is a research center funded by Science Foundation Ireland (SFI), through the Irish Government’s National Development Plan [grant number 12/RC/2273 P2]. KB has received a postdoctoral fellowship from the Irish Research Council [grant number GOIPD/2019/33]. The Cryan Laboratory is also funded by the Saks-Kavanaugh Foundation.
ORCID iD: John F Cryan https://orcid.org/0000-0001-5887-2723
References
- 1.Lindlahr VH. You are what you eat. [By Victor H. Lindlahr]. ■: National Nutrition Society, Incorporated, 1942.
- 2.Hipsley EH. Dietary “fibre” and pregnancy toxaemia. Br Med J 1953; 2:420–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkitt D, Walker A, Painter N. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet 1972; 2:1408–12 [DOI] [PubMed] [Google Scholar]
- 4.Cummings JH, Engineer A. Denis burkitt and the origins of the dietary fibre hypothesis. Nutr Res Rev 2018; 31:1–15 [DOI] [PubMed] [Google Scholar]
- 5.Gill SK, Rossi M, Bajka B, Whelan K. Dietary fibre in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol 2021; 18:101–16 [DOI] [PubMed] [Google Scholar]
- 6.Anderson JW, Baird P, Davis RH, Jr, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev 2009; 67:188–205 [DOI] [PubMed] [Google Scholar]
- 7.Prasadi NVP, Joye IJ. Dietary fibre from whole grains and their benefits on metabolic health. Nutrients 2020; 12:3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming SA, Monaikul S, Patsavas AJ, Waworuntu RV, Berg BM, Dilger RN. Dietary polydextrose and galactooligosaccharide increase exploratory behavior, improve recognition memory, and alter neurochemistry in the young pig. Nutr Neurosci 2019; 22:499–512 [DOI] [PubMed] [Google Scholar]
- 9.Gronier B, Savignac HM, Di Miceli M, Idriss SM, Tzortzis G, Anthony D, Burnet PWJ. Increased cortical neuronal responses to NMDA and improved attentional set-shifting performance in rats following prebiotic (B-GOS((R))) ingestion. Eur Neuropsychopharmacol 2018; 28:211–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassevoort KM, Lin AS, Khan NA, Hillman CH, Cohen NJ. Added sugar and dietary fiber consumption are associated with creativity in preadolescent children. Nutr Neurosci 2020; 23:791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan NA, Raine LB, Drollette ES, Scudder MR, Kramer AF, Hillman CH. Dietary fiber is positively associated with cognitive control among prepubertal children. J Nutr 2015; 145:143–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vizuete AA, Robles F, Rodríguez-Rodríguez E, López-Sobaler AM, Ortega RM. Association between food and nutrient intakes and cognitive capacity in a group of institutionalized elderly people. Eur J Nutr 2010; 49:293–300 [DOI] [PubMed] [Google Scholar]
- 13.Sehrish N, Venäläinen T, Eloranta A-M, Erkkilä AT, Jalkanen H, Lindi V, Lakka TA, Haapala EA. Associations of dietary carbohydrate and fatty acid intakes with cognition among children. Publ Health Nutr 2020; 23:1657–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome 2015; 3:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. The microbiota-gut-brain axis. Physiol Rev 2019; 99:1877–2013 [DOI] [PubMed] [Google Scholar]
- 16.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry 2014; 19:146–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 2011; 108:3047–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tooley KL. Effects of the human gut microbiota on cognitive performance, brain structure and function: a narrative review. Nutrients 2020; 12:3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marx W, Scholey A, Firth J, D'Cunha NM, Lane M, Hockey M, Ashton MM, Cryan JF, O'Neil A, Naumovski N, Berk M, Dean OM, Jacka F. Prebiotics, probiotics, fermented foods and cognitive outcomes: a meta-analysis of randomized controlled trials. Neurosci Biobehav Rev 2020; 118:472–84 [DOI] [PubMed] [Google Scholar]
- 20.Berding K, Long-Smith CM, Carbia C, Bastiaanssen TFS, van de Wouw M, Wiley N, Strain CR, Fouhy F, Stanton C, Cryan JF, Dinan TG. A specific dietary fibre supplementation improves cognitive performance-an exploratory randomised, placebo-controlled, crossover study. Psychopharmacology 2021; 238:149–63 [DOI] [PubMed] [Google Scholar]
- 21.Lew LC, Hor YY, Yusoff NAA, Choi SB, Yusoff MSB, Roslan NS, Ahmad A, Mohammad JAM, Abdullah M, Zakaria N, Wahid N, Sun Z, Kwok LY, Zhang H, Liong MT. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebo-controlled study. Clin Nutr 2019; 38:2053–64 [DOI] [PubMed] [Google Scholar]
- 22.Jones JM. CODEX-aligned dietary fiber definitions help to bridge the 'fiber gap'. Nutr J 2014; 13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alimentarius C. Guidelines on nutrition labelling CAC/GL 2-1985 as last amended 2010 . Joint FAO/WHO food standards programme, secretariat of the codex alimentarius commission. Rome: FAO, 2010 [Google Scholar]
- 24.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 2014; 20:779–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017; 14:491–502 [DOI] [PubMed] [Google Scholar]
- 26.Watson H, Mitra S, Croden FC, Taylor M, Wood HM, Perry SL, Spencer JA, Quirke P, Toogood GJ, Lawton CL, Dye L, Loadman PM, Hull MA. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018; 67:1974–83 [DOI] [PubMed] [Google Scholar]
- 27.Costantini L, Molinari R, Farinon B, Merendino N. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci 2017; 18:2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duenas M, Munoz-Gonzalez I, Cueva C, Jimenez-Giron A, Sanchez-Patan F, Santos-Buelga C, Moreno-Arribas MV, Bartolome B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res Int 2015; 2015:850902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma G, Chen Y. Polyphenol supplementation benefits human health via gut microbiota: a systematic review via meta-analysis. J Funct Foods 2020; 66:103829 [Google Scholar]
- 30.Dhingra D, Michael M, Rajput H, Patil RT. Dietary fibre in foods: a review. J Food Sci Technol 2012; 49:255–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams BA, Mikkelsen D, Flanagan BM, Gidley MJ. Dietary fibre”: moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J Anim Sci Biotechnol 2019; 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lunn J, Buttriss J. Carbohydrates and dietary fibre. Nutr Bulletin 2007; 32:21–64 [Google Scholar]
- 33.Koutsos A, Lima M, Conterno L, Gasperotti M, Bianchi M, Fava F, Vrhovsek U, Lovegrove JA, Tuohy KM. Effects of commercial apple varieties on human gut microbiota composition and metabolic output using an in vitro colonic model. Nutrients 2017; 9:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephen AM, Champ MM-J, Cloran SJ, Fleith M, van Lieshout L, Mejborn H, Burley VJ. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev 2017; 30:149–90 [DOI] [PubMed] [Google Scholar]
- 35.King DE, Mainous IIA, Lambourne CA. Trends in dietary fiber intake in the United States, 1999-2008. J Acad Nutr Diet 2012; 112:642–48 [DOI] [PubMed] [Google Scholar]
- 36.McGill CR, Devareddy L. Ten-year trends in fiber and whole grain intakes and food sources for the United States population: national health and nutrition examination survey 2001–2010. Nutrients 2015; 7:1119–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.EFSA Panel on Dietetic Products N, Allergies. Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J 2010; 8:1462 [Google Scholar]
- 38.Bannon S, Walton J, Flynn A. The national adult nutrition survey: dietary fibre intake of Irish adults. Proc Nutr Soc 2011; 70:E113 [Google Scholar]
- 39.Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018; 23:705–15 [DOI] [PubMed] [Google Scholar]
- 40.O'Keefe SJD. The need to reassess dietary fiber requirements in healthy and critically ill patients. Gastroenterol Clin North Am 2018; 47:219–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nyman M, Asp NG, Cummings J, Wiggins H. Fermentation of dietary fibre in the intestinal tract: comparison between man and rat. Br J Nutr 1986; 55:487–96 [DOI] [PubMed] [Google Scholar]
- 42.Wisker E, Daniel M, Rave G, Feldheim W. Fermentation of non-starch polysaccharides in mixed diets and single fibre sources: comparative studies in human subjects and in vitro. Br J Nutr 1998; 80:253–61 [PubMed] [Google Scholar]
- 43.Scott KP, Martin JC, Duncan SH, Flint HJ. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol 2014; 87:30–40 [DOI] [PubMed] [Google Scholar]
- 44.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol 2011; 9:e1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012; 3:289–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Q, Liang Q, Balakrishnan B, Belobrajdic DP, Feng QJ, Zhang W. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients 2020; 12:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson AS, Koller KR, Ramaboli MC, Nesengani LT, Ocvirk S, Chen C, Flanagan CA, Sapp FR, Merritt ZT, Bhatti F, Thomas TK, O'Keefe SJD. Diet and the human gut microbiome: an international review. Dig Dis Sci 2020; 65:723–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McBurney MI, Davis C, Fraser CM, Schneeman BO, Huttenhower C, Verbeke K, Walter J, Latulippe ME. Establishing what constitutes a healthy human gut microbiome: state of the science, regulatory considerations, and future directions. J Nutr 2019; 149:1882–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.So D, Whelan K, Rossi M, Morrison M, Holtmann G, Kelly JT, Shanahan ER, Staudacher HM, Campbell KL. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr 2018; 107:965–83 [DOI] [PubMed] [Google Scholar]
- 50.Tangestani H, Emamat H, Ghalandari H, Shab-Bidar S. Whole grains, dietary fibers and the human gut microbiota: a systematic review of existing literature. Recent Pat Food Nutr Agric 2020; 11:235–48 [DOI] [PubMed] [Google Scholar]
- 51.Christensen EG, Licht TR, Kristensen M, Bahl MI. Bifidogenic effect of whole-grain wheat during a 12-week energy-restricted dietary intervention in postmenopausal women. Eur J Clin Nutr 2013; 67:1316–21 [DOI] [PubMed] [Google Scholar]
- 52.Martínez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One 2010; 5:e15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alfa MJ, Strang D, Tappia PS, Graham M, Van Domselaar G, Forbes JD, Laminman V, Olson N, DeGagne P, Bray D, Murray BL, Dufault B, Lix LM. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin Nutr 2018; 37:797–807 [DOI] [PubMed] [Google Scholar]
- 54.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol 2008; 6:121–31 [DOI] [PubMed] [Google Scholar]
- 55.Peredo-Lovillo A, Romero-Luna HE, Jiménez-Fernández M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res Int 2020; 136:109473. [DOI] [PubMed] [Google Scholar]
- 56.Michalak L, Gaby JC, Lagos L, La Rosa SL, Hvidsten TR, Tétard-Jones C, Willats WGT, Terrapon N, Lombard V, Henrissat B, Dröge J, Arntzen MØ, Hagen LH, Øverland M, Pope PB, Westereng B. Microbiota-directed fibre activates both targeted and secondary metabolic shifts in the distal gut. Nat Commun 2020; 11:5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim Y, Hwang SW, Kim S, Lee YS, Kim TY, Lee SH, Kim SJ, Yoo HJ, Kim EN, Kweon MN. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes 2020; 11:944–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berer K, Martinez I, Walker A, Kunkel B, Schmitt-Kopplin P, Walter J, Krishnamoorthy G. Dietary non-fermentable fiber prevents autoimmune neurological disease by changing gut metabolic and immune status. Sci Rep 2018; 8:10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fenwick PH, Jeejeebhoy K, Dhaliwal R, Royall D, Brauer P, Tremblay A, Klein D, Mutch DM. Lifestyle genomics and the metabolic syndrome: a review of genetic variants that influence response to diet and exercise interventions. Crit Rev Food Sci Nutr 2019; 59:2028–39 [DOI] [PubMed] [Google Scholar]
- 60.Lampe JW, Navarro SL, Hullar MA, Shojaie A. Inter-individual differences in response to dietary intervention: integrating omics platforms towards personalised dietary recommendations. Proc Nutr Soc 2013; 72:207–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab 2015; 22:971–82 [DOI] [PubMed] [Google Scholar]
- 62.Hjorth MF, Roager HM, Larsen TM, Poulsen SK, Licht TR, Bahl MI, Zohar Y, Astrup A. Pre-treatment microbial prevotella-to-bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int J Obes 2018; 42:580–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jefferson A, Adolphus K. The effects of intact cereal grain fibers, including wheat bran on the gut microbiota composition of healthy adults: a systematic review. Front Nutr 2019; 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Healey G, Murphy R, Butts C, Brough L, Whelan K, Coad J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br J Nutr 2018; 119:176–89 [DOI] [PubMed] [Google Scholar]
- 65.O’Neil A, Quirk SE, Housden S, Brennan SL, Williams LJ, Pasco JA, Berk M, Jacka FN. Relationship between diet and mental health in children and adolescents: a systematic review. Am J Public Health 2014; 104:e31–e42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in Middle age. Br J Psychiatry 2009; 195:408–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nanri A, Kimura Y, Matsushita Y, Ohta M, Sato M, Mishima N, Sasaki S, Mizoue T. Dietary patterns and depressive symptoms among Japanese men and women. Eur J Clin Nutr 2010; 64:832–39 [DOI] [PubMed] [Google Scholar]
- 68.Jacka FN, Mykletun A, Berk M, Bjelland I, Tell GS. The association between habitual diet quality and the common mental disorders in community-dwelling adults: the Hordaland health study. Psychosom Med 2011; 73:483–90 [DOI] [PubMed] [Google Scholar]
- 69.Fatahi S, Matin SS, Sohouli MH, Găman M-A, Raee P, Olang B, Kathirgamathamby V, Santos HO, Guimarães NS, Shidfar F. Association of dietary fiber and depression symptom: a systematic review and meta-analysis of observational studies. Complement Ther Med 2020; 56:102621. [DOI] [PubMed] [Google Scholar]
- 70.Prinelli F, Fratiglioni L, Kalpouzos G, Musicco M, Adorni F, Johansson I, Marseglia A, Xu W. Specific nutrient patterns are associated with higher structural brain integrity in dementia-free older adults. Neuroimage 2019; 199:281–88 [DOI] [PubMed] [Google Scholar]
- 71.Torres-Velázquez M, Sawin EA, Anderson JM, Yu JJ. Refractory diet-dependent changes in neural microstructure: implications for microstructural endophenotypes of neurologic and psychiatric disease. Magn Reson Imaging 2019; 58:148–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borge TC, Aase H, Brantsaeter AL, Biele G. The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: a systematic review and meta-analysis. BMJ Open 2017; 7:e016777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen JF, Gorski MT, Gruber S, Kurdziel L, Rimm EB. The effect of healthy dietary consumption on executive cognitive functioning in children and adolescents: a systematic review. Br J Nutr 2016; 116:989–1000 [DOI] [PubMed] [Google Scholar]
- 74.Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol 2018; 17:1006–15 [DOI] [PubMed] [Google Scholar]
- 75.Gardener SL, Rainey-Smith SR. The role of nutrition in cognitive function and brain ageing in the elderly. Curr Nutr Rep 2018; 7:139–49 [DOI] [PubMed] [Google Scholar]
- 76.Yin Z, Chen J, Zhang J, Ren Z, Dong K, Kraus VB, Wang Z, Zhang M, Zhai Y, Song P. Dietary patterns associated with cognitive function among the older people in underdeveloped regions: finding from the NCDFaC study. Nutrients 2018; 10:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gehlich KH, Beller J, Lange-Asschenfeldt B, Köcher W, Meinke MC, Lademann J. Fruit and vegetable consumption is associated with improved mental and cognitive health in older adults from non-Western developing countries. Public Health Nutr 2019; 22:689–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nurk E, Refsum H, Drevon CA, Tell GS, Nygaard HA, Engedal K, Smith AD. Cognitive performance among the elderly in relation to the intake of plant foods. The Hordaland health study. Br J Nutr 2010; 104:1190–201 [DOI] [PubMed] [Google Scholar]
- 79.Fontani G, Corradeschi F, Felici A, Alfatti F, Migliorini S, Lodi L. Cognitive and physiological effects of omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur J Clin Invest 2005; 35:691–9 [DOI] [PubMed] [Google Scholar]
- 80.Reynolds AN, Diep Pham HT, Montez J, Mann J. Dietary fibre intake in childhood or adolescence and subsequent health outcomes: a systematic review of prospective observational studies. Diabetes Obes Metab 2020; 22:2460–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ortega RM, Requejo AM, Andrés P, López-Sobaler AM, Quintas ME, Redondo MR, Navia B, Rivas T. Dietary intake and cognitive function in a group of elderly people. Am J Clin Nutr 1997; 66:803–9 [DOI] [PubMed] [Google Scholar]
- 82.Vercambre MN, Boutron-Ruault MC, Ritchie K, Clavel-Chapelon F, Berr C. Long-term association of food and nutrient intakes with cognitive and functional decline: a 13-year follow-up study of elderly French women. Br J Nutr 2009; 102:419–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haapala EA, Eloranta A-M, Venäläinen T, Schwab U, Lindi V, Lakka TA. Associations of diet quality with cognition in children – the physical activity and nutrition in children study. Br J Nutr 2015; 114:1080–87 [DOI] [PubMed] [Google Scholar]
- 84.Best T, Howe P, Bryan J, Buckley J, Scholey A. Acute effects of a dietary non-starch polysaccharide supplement on cognitive performance in healthy middle-aged adults. Nutr Neurosci 2015; 18:76–86 [DOI] [PubMed] [Google Scholar]
- 85.Chung YC, Park CH, Kwon HK, Park YM, Kim YS, Doo JK, Shin DH, Jung ES, Oh MR, Chae SW. Improved cognitive performance following supplementation with a mixed-grain diet in high school students: a randomized controlled trial. Nutrition 2012; 28:165–72 [DOI] [PubMed] [Google Scholar]
- 86.Donoso F, Ramírez VT, Golubeva AV, Moloney GM, Stanton C, Dinan TG, Cryan JF. Naturally derived polyphenols protect against corticosterone-induced changes in primary cortical neurons. Int J Neuropsychopharmacol 2019; 22:765–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Desmedt O, Broers VJ, Zamariola G, Pachikian B, Delzenne N, Luminet O. Effects of prebiotics on affect and cognition in human intervention studies. Nutr Rev 2019; 77:81–95 [DOI] [PubMed] [Google Scholar]
- 88.Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PW. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015; 232:1793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith AP, Sutherland D, Hewlett P. An investigation of the acute effects of oligofructose-enriched inulin on subjective wellbeing, mood and cognitive performance. Nutrients 2015; 7:8887–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buigues C, Fernández-Garrido J, Pruimboom L, Hoogland AJ, Navarro-Martínez R, Martínez-Martínez M, Verdejo Y, Mascarós MC, Peris C, Cauli O. Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. Int J Mol Sci 2016; 17:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kao AC, Safarikova J, Marquardt T, Mullins B, Lennox BR, Burnet PW. Pro-cognitive effect of a prebiotic in psychosis: a double blind placebo controlled cross-over study. Schizophr Res 2019; 208:460–1 [DOI] [PubMed] [Google Scholar]
- 92.Milte CM, Ball K, Crawford D, McNaughton SA. Diet quality and cognitive function in mid-aged and older men and women. BMC Geriatr 2019; 19:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mao X, Chen C, Xun P, Daviglus ML, Steffen LM, Jacobs DR, Van Horn L, Sidney S, Zhu N, Qin B, He K. Intake of vegetables and fruits through young adulthood is associated with better cognitive function in midlife in the US general population. J Nutr 2019; 149:1424–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nilsson A, Salo I, Plaza M, Björck I. Effects of a mixed berry beverage on cognitive functions and cardiometabolic risk markers; a randomized cross-over study in healthy older adults. PLoS One 2017; 12:e0188173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bastianetto S, Menard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta 2015; 1852:1195–201 [DOI] [PubMed] [Google Scholar]
- 96.Godos J, Castellano S, Ray S, Grosso G, Galvano F. Dietary polyphenol intake and depression: results from the Mediterranean healthy eating, lifestyle and aging (MEAL) study. Molecules 2018; 23:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sandberg JC, Björck IME, Nilsson AC. Impact of rye-based evening meals on cognitive functions, mood and cardiometabolic risk factors: a randomized controlled study in healthy middle-aged subjects. Nutr J 2018; 17:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cai Y, Folkerts J, Folkerts G, Maurer M, Braber S. Microbiota‐dependent and‐independent effects of dietary fibre on human health. Br J Pharmacol 2020; 177:1363–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bindels LB, Segura Munoz RR, Gomes-Neto JC, Mutemberezi V, Martínez I, Salazar N, Cody EA, Quintero-Villegas MI, Kittana H, de los Reyes-Gavilán CG, Schmaltz RJ, Muccioli GG, Walter J, Ramer-Tait AE. Resistant starch can improve insulin sensitivity independently of the gut microbiota. Microbiome 2017; 5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khoshbin K, Camilleri M. Effects of dietary components on intestinal permeability in health and disease. Am J Physiol Gastrointest Liver Physiol 2020; 319:G589–G608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li H, Sun J, Wang F, Ding G, Chen W, Fang R, Yao Y, Pang M, Lu ZQ, Liu J. Sodium butyrate exerts neuroprotective effects by restoring the blood-brain barrier in traumatic brain injury mice. Brain Res 2016; 1642:70–78 [DOI] [PubMed] [Google Scholar]
- 102.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, Carling D, Swann JR, Gibson G, Viardot A, Morrison D, Louise Thomas E, Bell JD. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 2014; 5:3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol 2003; 284:R1269–76 [DOI] [PubMed] [Google Scholar]
- 104.Moris G, Vega J. Neurotrophic factors: basis for their clinical application. Neurologia 2003; 18:18–28 [PubMed] [Google Scholar]
- 105.Dalile B, Vervliet B, Bergonzelli G, Verbeke K, Van Oudenhove L. Colon-delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: a randomized, placebo-controlled trial. Neuropsychopharmacol 2020; 45:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 2011; 23:255–64, e119 [DOI] [PubMed] [Google Scholar]
- 107.Luczynski P, Whelan SO, O'Sullivan C, Clarke G, Shanahan F, Dinan TG, Cryan JF. Adult microbiota‐deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci 2016; 44:2654–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jørgensen BP, Hansen JT, Krych L, Larsen C, Klein AB, Nielsen DS, Josefsen K, Hansen AK, Sørensen DB. A possible link between food and mood: dietary impact on gut microbiota and behavior in BALB/c mice. PLoS One 2014; 9:e103398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Robbins TW. Neurochemistry of cognition. Oxford Textbook Cogn Neurol Dement 2016; 91:91–101 [Google Scholar]
- 110.Handra C, Coman OA, Coman L, Enache T, Stoleru S, Sorescu A-M, Ghită I, Fulga I. The connection between different neurotransmitters involved in cognitive processes. Farmacia 2019; 67:193–201 [Google Scholar]
- 111.Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, Raes J. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 2019; 4:623–32 [DOI] [PubMed] [Google Scholar]
- 112.Portune KJ, Beaumont M, Davila A-M, Tomé D, Blachier F, Sanz Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: the two sides of the coin. Trends Food Sci Technol 2016; 57:213–32 [Google Scholar]
- 113.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015; 161:264–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mika A, Gaffney M, Roller R, Hills A, Bouchet CA, Hulen KA, Thompson RS, Chichlowski M, Berg BM, Fleshner M. Feeding the developing brain: juvenile rats fed diet rich in prebiotics and bioactive milk fractions exhibit reduced anxiety-related behavior and modified gene expression in emotion circuits. Neurosci Lett 2018; 677:103–09 [DOI] [PubMed] [Google Scholar]
- 115.Kato-Kataoka A, Nishida K, Takada M, Suda K, Kawai M, Shimizu K, Kushiro A, Hoshi R, Watanabe O, Igarashi T, Miyazaki K, Kuwano Y, Rokutan K. Fermented milk containing Lactobacillus casei strain shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef Microbes 2016; 7:153–6 [DOI] [PubMed] [Google Scholar]
- 116.Palomo-Buitrago ME, Sabater-Masdeu M, Moreno-Navarrete JM, Caballano-Infantes E, Arnoriaga-Rodríguez M, Coll C, Ramió L, Palomino-Schätzlein M, Gutiérrez-Carcedo P, Pérez-Brocal V, Simó R, Moya A, Ricart W, Herance JR, Fernández-Real JM. Glutamate interactions with obesity, insulin resistance, cognition and gut microbiota composition. Acta Diabetol 2019; 56:569–79 [DOI] [PubMed] [Google Scholar]
- 117.Sandberg JC, Björck IME, Nilsson AC. Increased plasma brain-derived neurotrophic factor 10.5 h after intake of whole grain rye-based products in healthy subjects. Nutrients 2018; 10:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol 2014; 220:223–50 [DOI] [PubMed] [Google Scholar]
- 119.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 2019; 16:461–78 [DOI] [PubMed] [Google Scholar]
- 120.Sun LJ, Li JN, Nie YZ. Gut hormones in microbiota-gut-brain cross-talk. Chin Med J 2020; 133:826–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lach G, Schellekens H, Dinan TG, Cryan JF. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 2018; 15:36–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci 2006; 9:381–8 [DOI] [PubMed] [Google Scholar]
- 123.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 2003; 9:1173–9 [DOI] [PubMed] [Google Scholar]
- 124.Stengel A, Goebel M, Wang L, Tache Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: role as regulators of food intake and body weight. Peptides 2010; 31:357–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 2008; 9:568–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 2002; 418:650–4 [DOI] [PubMed] [Google Scholar]
- 127.Rasmussen BA, Breen DM, Lam TK. Lipid sensing in the gut, brain and liver. Trends Endocrinol Metab 2012; 23:49–55 [DOI] [PubMed] [Google Scholar]
- 128.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY3–36 and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal–brainstem–hypothalamic pathway. Brain Res 2005; 1044:127–31 [DOI] [PubMed] [Google Scholar]
- 129.Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr 2004; 92:521–6 [DOI] [PubMed] [Google Scholar]
- 130.Beilharz JE, Kaakoush NO, Maniam J, Morris MJ. The effect of short-term exposure to energy-matched diets enriched in fat or sugar on memory, gut microbiota and markers of brain inflammation and plasticity. Brain Behav Immun 2016; 57:304–13 [DOI] [PubMed] [Google Scholar]
- 131.Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, Giampieri E, Jennings A, Candela M, Turroni S, Zoetendal EG, Hermes GDA, Elodie C, Meunier N, Brugere CM, Pujos-Guillot E, Berendsen AM, De Groot L, Feskins EJM, Kaluza J, Pietruszka B, Bielak MJ, Comte B, Maijo-Ferre M, Nicoletti C, De Vos WM, Fairweather-Tait S, Cassidy A, Brigidi P, Franceschi C, O'Toole PW. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 2020; 69:1218–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leigh SJ, Kaakoush NO, Bertoldo MJ, Westbrook RF, Morris MJ. Intermittent cafeteria diet identifies fecal microbiome changes as a predictor of spatial recognition memory impairment in female rats. Transl Psychiatry 2020; 10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leigh SJ, Morris MJ. Diet, inflammation and the gut microbiome: mechanisms for obesity-associated cognitive impairment. Biochim Biophys Acta Mol Basis Dis 2020; 1866:165767. [DOI] [PubMed] [Google Scholar]
- 134.Zhai S, Qin S, Li L, Zhu L, Zou Z, Wang L. Dietary butyrate suppresses inflammation through modulating gut microbiota in high-fat diet-fed mice. FEMS Microbiol Lett 2019; 366:fnz153. [DOI] [PubMed] [Google Scholar]
- 135.Swann OG, Kilpatrick M, Breslin M, Oddy WH. Dietary fiber and its associations with depression and inflammation. Nutr Rev 2020; 78:394–411 [DOI] [PubMed] [Google Scholar]
- 136.Fischer F, Romero R, Hellhund A, Linne U, Bertrams W, Pinkenburg O, Eldin HS, Binder K, Jacob R, Walker A. Dietary cellulose induces anti-inflammatory immunity and transcriptional programs via maturation of the intestinal microbiota. Gut Microbes 2020; 12:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dürholz K, Hofmann J, Iljazovic A, Häger J, Lucas S, Sarter K, Strowig T, Bang H, Rech J, Schett G. Dietary short-term fiber interventions in arthritis patients increase systemic SCFA levels and regulate inflammation. Nutrients 2020; 12:3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shi H, Wang Q, Zheng M, Hao S, Lum JS, Chen X, Huang XF, Yu Y, Zheng K. Supplement of microbiota-accessible carbohydrates prevents neuroinflammation and cognitive decline by improving the gut microbiota-brain axis in diet-induced obese mice. J Neuroinflammation 2020; 17:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang XD, Wang LK, Wu HY, Jiao L. Effects of prebiotic galacto-oligosaccharide on postoperative cognitive dysfunction and neuroinflammation through targeting of the gut-brain axis. BMC Anesthesiol 2018; 18:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O'Leary OF. Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry 2015; 78:e7–9 [DOI] [PubMed] [Google Scholar]
- 141.Möhle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, Wolf SA. Ly6C(hi) monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep 2016; 15:1945–56 [DOI] [PubMed] [Google Scholar]
- 142.Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med 2006; 8:1. [DOI] [PubMed] [Google Scholar]
- 143.Savitz J. The kynurenine pathway: a finger in every pie. Mol Psychiatry 2020; 25:131–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H, Chen L, Ning Y. Cross-sectional relationship between kynurenine pathway metabolites and cognitive function in major depressive disorder. Psychoneuroendocrinology 2019; 101:72–79 [DOI] [PubMed] [Google Scholar]
- 145.Solvang SEH, Nordrehaug JE, Tell GS, Nygård O, McCann A, Ueland PM, Midttun Ø, Meyer K, Vedeler CA, Aarsland D. The kynurenine pathway and cognitive performance in community-dwelling older adults. The Hordaland health study. Brain Behav Immun 2019; 75:155–62 [DOI] [PubMed] [Google Scholar]
- 146.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017; 112:399–412 [DOI] [PubMed] [Google Scholar]
- 147.Purton T, Staskova L, Lane MM, Dawson SL, West M, Firth J, Clarke G, Cryan JF, Berk M, O'Neil A, Dean O, Hadi A, Honan C, Marx W. Prebiotic and probiotic supplementation and the tryptophan-kynurenine pathway: a systematic review and meta analysis. Neurosci Biobehav Rev 2021; 123:1–13 [DOI] [PubMed] [Google Scholar]
- 148.Szalardy L, Zadori D, Toldi J, Fulop F, Klivenyi P, Vecsei L. Manipulating kynurenic acid levels in the brain–on the edge between neuroprotection and cognitive dysfunction. Curr Top Med Chem 2012; 12:1797–806 [PubMed] [Google Scholar]
- 149.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 2012; 13:465–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blake MG, Krawczyk MC, Baratti CM, Boccia MM. Neuropharmacology of memory consolidation and reconsolidation: insights on central cholinergic mechanisms. J Physiol Paris 2014; 108:286–91 [DOI] [PubMed] [Google Scholar]
- 151.Kim JS., Levin ED. Nicotinic, muscarinic and dopaminergic actions in the ventral hippocampus and the nucleus accumbens: effects on spatial working memory in rats. Brain Res 1996; 725:231–40 [DOI] [PubMed] [Google Scholar]
- 152.Rudzki L, Ostrowska L, Pawlak D, Malus A, Pawlak K, Waszkiewicz N, Szulc A. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019; 100:213–22 [DOI] [PubMed] [Google Scholar]
- 153.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 2004; 558:263–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011; 108:16050–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil 2011; 23:1132–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 1999; 2:94–8 [DOI] [PubMed] [Google Scholar]
- 157.Klingbeil EA, Cawthon C, Kirkland R, de La Serre CB. Potato-Resistant starch supplementation improves microbiota dysbiosis, inflammation, and gut-brain signaling in high fat-fed rats. Nutrients 2019; 11:2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.O’Leary OF, Ogbonnaya ES, Felice D, Levone BR, Conroy LC, Fitzgerald P, Bravo JA, Forsythe P, Bienenstock J, Dinan TG. The vagus nerve modulates BDNF expression and neurogenesis in the hippocampus. Eur Neuropsychopharmacol 2018; 28:307–16 [DOI] [PubMed] [Google Scholar]
- 159.Oomen CA, Bekinschtein P, Kent BA, Saksida LM, Bussey TJ. Adult hippocampal neurogenesis and its role in cognition. Wiley Interdiscip Rev Cogn Sci 2014; 5:573–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Miki T, Eguchi M, Kurotani K, Kochi T, Kuwahara K, Ito R, Kimura Y, Tsuruoka H, Akter S, Kashino I, Kabe I, Kawakami N, Mizoue T. Dietary fiber intake and depressive symptoms in Japanese employees: the Furukawa nutrition and health study. Nutrition 2016; 32:584–9 [DOI] [PubMed] [Google Scholar]
- 161.Xu H, Li S, Song X, Li Z, Zhang D. Exploration of the association between dietary fiber intake and depressive symptoms in adults. Nutrition 2018; 54:48–53 [DOI] [PubMed] [Google Scholar]
- 162.Kim CS, Byeon S, Shin DM. Sources of dietary fiber are differently associated with prevalence of depression. Nutrients 2020; 12:2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Long-Smith C, O'Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota-gut-brain axis: new therapeutic opportunities. Annu Rev Pharmacol Toxicol 2020; 60:477–502 [DOI] [PubMed] [Google Scholar]
- 164.Gehrig JL, Venkatesh S, Chang HW, Hibberd MC, Kung VL, Cheng J, Chen RY, Subramanian S, Cowardin CA, Meier MF, O'Donnell D, Talcott M, Spears LD, Semenkovich CF, Henrissat B, Giannone RJ, Hettich RL, Ilkayeva O, Muehlbauer M, Newgard CB, Sawyer C, Head RD, Rodionov DA, Arzamasov AA, Leyn SA, Osterman AL, Hossain MI, Islam M, Choudhury N, Sarker SA, Huq S, Mahmud I, Mostafa I, Mahfuz M, Barratt MJ, Ahmed T, Gordon JI. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science 2019; 365:eaau4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Adan RAH, van der Beek EM, Buitelaar JK, Cryan JF, Hebebrand J, Higgs S, Schellekens H, Dickson SL. Nutritional psychiatry: towards improving mental health by what you eat. Eur Neuropsychopharmacol 2019; 29:1321–32 [DOI] [PubMed] [Google Scholar]
- 166.Dinan TG, Stanton C, Long-Smith C, Kennedy P, Cryan JF, Cowan CSM, Cenit MC, van der Kamp JW, Sanz Y. Feeding melancholic microbes: MyNewGut recommendations on diet and mood. Clin Nutr 2019; 38:1995–2001 [DOI] [PubMed] [Google Scholar]