Ibrutinib is active both in treatment-naïve (TN) and relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) patients, including those with unmutated immunoglobulin heavy chain variable region (IGHV) genes and TP53 disruption.1-3 The acquisition of BTK or PLCg2 gene mutations conferring resistance, supports the existence of clonal evolution also under ibrutinib treatment. 4-8 Whilst it is well described that subclonal TP53 mutations undergo a positive clonal selection following chemoimmunotherapy (CIT),9-11 being the main driver of treatment failure, recent studies have suggested that this might not be the case under ibrutinib.7,12-14
In order to investigate the dynamics of major and minor TP53 mutations under ibrutinib treatment, we performed longitudinal TP53 monitoring by deep-sequencing in CLL-treated patients. Two cohorts were included: 44 TN and 14 R/R patients. A total of 216 peripheral blood (PB) samples in TN and 52 in R/R were collected at baseline and at subsequent time points during therapy. Among TN patients, 28 were males and 16 females, with a median age of 72 years (range, 54-87); they received ibrutinib plus rituximab (GIMEMA trial LLC1114), with a median ibrutinib exposure of 2.7 years (range, 1.2-3.7) and were evaluated at 6-month intervals for a median number of 5 time points (range, 3-8). R/R patients, nine males and five females, with a median age of 71 years (range, 55-80), received ibrutinib single agent after a median of 1.5 (range, 1-4) CIT lines; they were evaluated at disease progression (PD) before each line of CIT and after ibrutinib treatment (median: 4 time points; range, 2-6), with 2.5 years ibrutinib exposure (range, 2.1-3.3). Amplicon libraries, covering the entire coding region and splice sites of TP53 gene (exon 2 to 11), were prepared according to the TruSeq Custom Amplicon Low Input Library Prep kit protocol dual strand (Illumina, San Diego, CA, USA) and paired-end sequenced on a Miseq Sequencer (Illumina). A mean coverage of 8,956x was obtained; across the target region a coverage >5,000x was obtained in >80% of the sequence in 70% of the samples. Bioinformatic analysis was performed by MiSeq Reporter (Illumina) and an in-house bioinformatics pipeline. A total concordance was observed for the variants with variant allele frequency (VAF) ≥3%; variants with 1%≤VAF<3%, only identified from in-house pipeline, were manually checked on alignment files resulting from MiSeq Reporter analysis of two DNA strands, using Integrative Genomics Viewer. Variants were manually curated according to the International Agency for Research on Cancer TP53 database (http://p53.iarc.fr/). Validate polymorphism, synonymous variants and variants mapping >2 bp outside coding exons were filtered out.
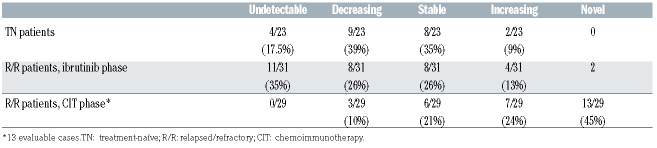
Mutations were defined major if VAF was >10% and minor if ≤10%; the latter were confirmed in an independent deep-sequencing run. VAF was corrected to cancer cell fraction (CCF) by the proportion of CD19+/CD5+ cells in each sample, assessed by flow cytometry. The limit of detection (LOD) was 1% (Online Supplementary Figure S1). A mutation was considered stable when the log2 fold-change (Log2FC) of CCF values before and after ibrutinib treatment was included between -0.5 and 0.5, decreasing or increasing if the Log2FC was <-0.5 or >0.5, respectively.
Among the 44 TN patients at baseline (T0), 27 cases (61%) resulted TP53 wild-type (WT) and 17 (39%) mutated by deep-sequencing analysis. Nine of 43 (21%) carried the del(17p) and 30 (68%) showed unmutated IGHV.
Twenty-three TP53 mutations (1.4 mutation/patient; range, 1-5) were identified: 17 (74%) were major (mean VAF 58.8%; range, 18-94.8) and six (26%) minor (mean VAF 5.3%; range, 2.1-9.2). Thirteen patients carried a sole major TP53 mutation; two cases (cases #10025, #10875) showed co-existing major and minor mutations; two cases (#9915, #10671) showed one minor mutation each and 27 none (Online Supplementary Table S1).
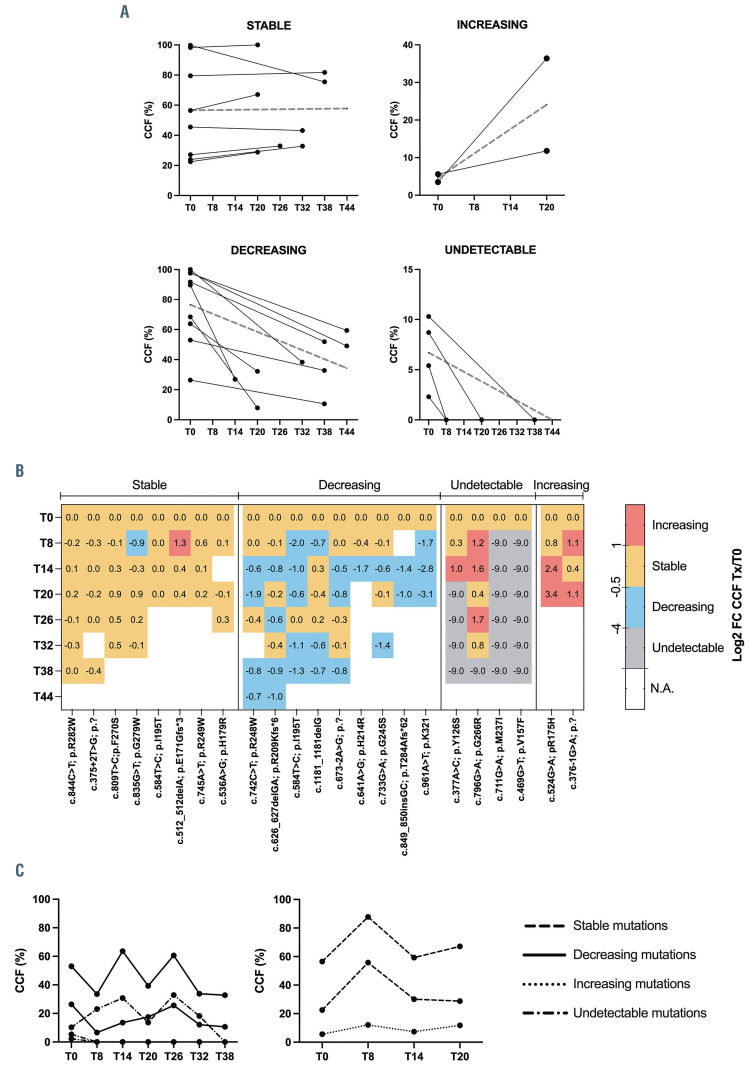
According to the CCF, after 2.7 years from ibrutinib treatment, nine of 23 (39%) major mutations decreased, eight of 23 (35%) major mutations persisted stable, four of 23 (17%) (1 major and 3 minor) were undetectable and two of 23 (9%) minor mutations increased (Figure 1A and B; Table 1; Online Supplementary Table S1). Novel TP53 mutations emerged during treatment neither in TP53 mutated nor WT patients.
Thus, TP53 mutated TN patients followed two main patterns: i) major TP53 mutations persisting major from T0 with a stable CCF (6 patients); ii) major TP53 mutations persisting major from T0 with a decreasing CCF (7 patients). In addition, in one patient (#10671) a minor TP53 mutation at T0 showed an increasing CCF, becoming major and in another patient a minor TP53 mutation resulted undetectable; in the two cases with a complex mutational architecture, TP53 mutations dynamics is shown in Figure 1C. No TP53 mutation was detected in 27 patients over time. Most patients are still on therapy, including those who showed an increase of TP53 mutation CCF (#10671, #10875) at month +20 (T20) who are still on therapy at T36 and T30, respectively. Two discontinued due to PD (#9795, #10239).
Among TN patients, seven TP53 mutated and 11 WT with a measurable PB residual disease (CD19+/CD5+ >20%) at T20 (n=3), T26 (n=2), T32 (n=7), T38 (n=4) and T44 (n=2), were analyzed by next-generation sequencing for BTK and PLCg2 mutations and resulted WT for both genes, including patients with PD (Online Supplementary Table S1).
Table 1.
Dynamics of TP53 mutations in treatment-naïve under ibrutinib and relapsed/refractory patients under ibrutinib and chemoimmunotherapy.
Figure 1.
Dynamics of TP53 mutations under ibrutinib therapy in treatment-naïve patients. (A) Each line corresponds to the cancer cell fraction (CCF) of each mutation. Dashed lines represent the median CCF. (B) Heatmap with the CCF log2-fold-change (Log2FC) for each mutation. Color code indicates the trend of TP53 mutation over time: decrease (<-0.5), increase (>1), stable (-0.5 and 1). (C) Dynamics of TP53 mutations in the 2 treatment-naïve cases with co-existing major and minor TP53 mutations at time zero (T0).
Among the 14 R/R patients, prior to ibrutinib treatment four patients (29%) resulted TP53 WT and ten (71%) mutated by deep-sequencing analysis. Four of 11 (37%) carried the del(17p) and seven (50%) showed unmutated IGHV. Thirty-one TP53 mutations (3.1 mutation/ patient; range, 1-11) were identified: 11 (35.5%) were major (mean VAF 31.9%; range, 10.5-78.8) and 20 (64.5%) minor (mean VAF 2.9%; range 1-6.8). Five patients carried one or two major mutations (#8271, #5708, #3547, #9225, #7458), three cases showed a complex mutational architecture (#5717, #8353 and #3425); two patients showed only minor mutations (3 in #3546 and 11 in #8540, respectively); four patients had no mutations (Online Supplementary Table S2). As expected, before ibrutinib, the TP53 mutational load of R/R patients was higher and more complex than that of TN patients. In the R/R patients, eight of 31 (26%) TP53 mutations (4 major and 4 minor) decreased, eight of 31 (26%) (4 major and 4 minor) persisted stable, 11 of 31 (35%) (2 major and 9 minor) were undetectable, four of 31 (13%) (2 major in #7458 and #8353; 2 minor in #3546) increased in three patients, and two novel minor mutations emerged in two cases already TP53-mutated prior to ibrutinib treatment (cases #5717, #3425) (Table 1; Online Supplementary Table S2). No novel TP53 mutations arose in TP53 WT patients over time under ibrutinib. In R/R patients, because of the more complex mutational profile of each patient we could not identify patient-related patterns, rather we documented different dynamics of different TP53 mutations within the same patient (Online Supplementary Table S2), possibly due to a different sensitivity to the drug, as suggested.12,13 Among R/R patients, four TP53-mutated and two WT continued ibrutinib (#8540, #6856, #3547, #9225, #5717, #6123), four discontinued ibrutinib for adverse events (#5708, #8353, #7458, #3380), one shifted to venetoclax (#3425), three died (#3546, #8991, #8271).
No significant changes were observed in the mean CCF of TP53 mutations before and after ibrutinib: 60.7% versus 43.1% and 20.5% versus 20.2%, in TN and R/R patients, respectively. On the contrary, the lymphocyte count decreased significantly after ibrutinib treatment in TP53-mutated patients from both cohorts: from 40.7x109/L (range: 4.9-132.2x109/L) to 11.2x109/L (range, 1.2-135.7x109/L) (P=0.018, Mann-Whitney test) and from 39.7x109/L (range, 1.5-99.0x109/L) to 7.1x109/L (range, 1.4-18.9x109/L) (P=0.034), respectively. The decrease in lymphocytosis in the presence of a stable TP53 mutations CCF proves the effectiveness of ibrutinib both on TP53-mutated and WT CLL cells, regardless of previous therapies, at least during the first years of treatment.
In 13 of the 14 R/R patients, TP53 mutations were retrospectively evaluated by deep sequencing also before each line of CIT. At the first evaluated time point, three patients were mutated (2 with minor and 1 with one major mutation) and ten resulted WT; of the latter, six acquired major or minor TP53 mutations over time. Overall, among the nine mutated cases, 29 mutations were identified with the following dynamics: 13 (45%) (3 major and 10 minor) novel mutations emerged, seven (24%) minor mutations increased, six (21%) (3 major and 3 minor) persisted stable and three (10%) (1 major and 2 minor) decreased. The decrease in CCF for the latter was from 84.44% to 46%, from 3.3% to 2.01% and from 4.69% to 1.83%, respectively. No mutation was undetectable. While the increased and novel mutations were significantly more common during the CIT phase (20/29 vs. 6/33, during CIT and ibrutinib, respectively; P<0.0001, Fisher’s exact test), the decreased and undetectable mutations were more frequent under ibrutinib treatment (3/29 vs. 19/33, during CIT and ibrutinib, respectively; P<0.0001) (Table 1).
In the present study, in TP53-mutated TN and R/R CLL patients, ibrutinib appears to decrease the major and minor mutations’ numerosity and complexity, since most mutations decreased (39% and 24%) or were undetectable (17% and 34%) and one third of mutations remained stable. On the other hand, a small proportion of TP53 mutations (9%, 2 minor, in TN; 13%, 2 major and 2 minor, in R/R cases) increased in CCF under ibrutinib treatment, although without clear clinical consequences with the current follow-up. We observed no association between the dynamics of TP53 mutations and the type of mutation, or the exon involved, neither the type of karyotype (data not shown) nor the presence of del(17p) (8 TN with vs. 9 without del(17p), decreased/undetectable vs. increased/novel mutations, P=0.46; 4 R/R with vs. 3 without del(17p), P=1 at Fisher’s exact test).
With a prolonged follow-up of more than 2 years, up to 44 months, our data add to the initial findings of a general stability of TP53 subclones over the early treatment period and support the notion that there is no specific positive selection of TP53 mutations under ibrutinib.7,14 Emergence of novel mutations proved exceptional events, mainly limited to R/R patients that display from the beginning a more complex mutational architecture, suggesting a potential influence of the previous CIT.13
However, in the long-term TP53 disrupted CLL patients tend to experience an inferior outcome.12,13 This can be due to an intrinsic genomic instability and a greater possibility of acquiring additional mutations conferring drug resistance and more frequent relapses in the long-term.7 Moreover, in vitro apoptosis and inhibition of proliferation are inferior in TP53 mutated than in WT cells exposed to ibrutinib, pointing to different mechanisms of cell fitness control in addition to the BCR pathway, 15 that can make the difference over time.
In conclusion, in TP53-mutated CLL patients ibrutinib in any line of therapy decreases the TP53 complexity at least within the first years of treatment and it does not exert a positive selective pressure on pre-existing TP53 mutated clones, unlike CIT. In TP53 WT patients, ibrutinib never induced the emergence of novel TP53 mutations after >2 years of exposure. These findings reinforce a broader use of a BTK inhibitor rather than CIT in the management of CLL, particularly for patients with an unfavorable genetic profile or with R/R disease.
Supplementary Material
Funding Statement
Funding: the authors wish to thank the Associazione Italiana per la Ricerca sul Cancro (AIRC), Metastases Special Program, N° 21198, Milan, Italy (RF); Progetti di Rilevante Interesse Nazionale (PRIN) 2015ZMRFEA (RF, AC).
References
- 1.O'Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment- naïve and relapsed/refractory chronic lymphocytic leukemia: a 5- year experience. Blood. 2018;131(17):1910-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munir T, Brown JR, O'Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of firstline ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woyach JA, Ruppert AS, Guinn D, et al. BTKC481S- mediated resistance to ibrutinib in chronic lymphocytic leukemia. J Clin Oncol. 2017; 35(13):1437-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger JA, Landau DA, Taylor-Weiner A, et al. Clonal evolution in patients with chronic lymphocytic leukaemia developing resistance to BTK inhibition. Nat. Commun. 2016;7:11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn IE, Underbayev C, Albitar A, et al. Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood. 2017; 129(11):1469-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landau DA, Sun C, Rosebrock D, et al. The evolutionary landscape of chronic lymphocytic leukemia treated with ibrutinib targeted therapy. Nat Commun. 2017;8(1):2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanagal-Shamanna R, Jain P, Patel KP, et al. Targeted multigene deep sequencing of Bruton tyrosine kinase inhibitor-resistant chronic lymphocytic leukemia with disease progression and Richter transformation. Cancer. 2019;125(4):559-574. [DOI] [PubMed] [Google Scholar]
- 9.Rossi D, Khiabanian H, Spina V, et al. Clinical impact of small TP53 mutated subclones in chronic lymphocytic leukemia. Blood. 2014;123(14):2139-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malcikova J, Stano-Kozubik K, Tichy B, et al. Detailed analysis of therapy- driven clonal evolution of TP53 mutations in chronic lymphocytic leukemia. Leukemia. 2015;29(4):877-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadeu F, Delgado J, Royo C, et al. Clinical impact of clonal and subclonal TP53, SF3B1, BIRC3, NOTCH1, and ATM mutations in chronic lymphocytic leukemia. Blood. 2016;127(17):2122-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadri S, Lee J, Fitzpatrick C, et al. Clonal evolution underlying leukemia progression and Richter transformation in patients with ibrutinibrelapsed CLL. Blood Adv. 2017;1(12):715-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gángó A, Alpár D, Galik B, et al. Dissection of subclonal evolution by temporal mutation profiling in chronic lymphocytic leukemia patients treated with ibrutinib. Int J Cancer. 2020;146(1):85-93. [DOI] [PubMed] [Google Scholar]
- 14.Malcikova J, Pavlova S, Kunt Vonkova B, et al. Low-burden TP53 mutations in CLL: Clinical impact and clonal evolution within the context of different treatment options. Blood. 2021. May 4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarini A, Peragine N, Messina M, et al. Unravelling the suboptimal response of TP53-mutated chronic lymphocytic leukaemia to ibrutinib. Br J Haematol. 2019;184(3):392-396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.