This systematic review and meta-analysis assesses the epidemiological associations of hearing loss and dual sensory loss with mortality among adults.
Key Points
Question
Are hearing loss and dual sensory loss associated with mortality?
Findings
In this multiadjusted observational meta-analysis of 1.2 million participants, with moderate-quality evidence overall, hearing loss was associated with 13% and 28% higher risk of all-cause and cardiovascular mortality. A dose-response association was observed, with the all-cause mortality relative hazard doubling for every 30-dB increase in audiometric thresholds; when both hearing and vision loss were present, the excess all-cause and cardiovascular mortality was even higher, at 40% and 86%.
Meaning
Hearing and dual sensory loss are associated with mortality; physicians caring for patients with hearing loss should consider its relevance to longevity.
Abstract
Importance
Hearing loss (HL) and dual sensory loss (DSL) are prevalent, disabling, and associated with numerous age-related health conditions, including dementia and frailty. To date, no evidence-based summary of their mortality risk is available.
Objective
To clarify the epidemiological associations between HL/DSL and mortality.
Data Sources
PubMed, Embase, and Cochrane Library, from inception until June 18, 2021.
Study Selection
Two blinded reviewers selected observational or interventional studies, published as full-length English articles in peer-reviewed journals, that reported the presence or severity of HL or DSL (ie, comorbid HL and vision loss), whether objectively measured or self-reported, in association with any mortality estimate, among adults 18 years and older.
Data Extraction and Synthesis
Two reviewers extracted data and evaluated study bias using the Newcastle-Ottawa Scale, following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)/Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines and a PROSPERO-registered protocol. The analysis pooled maximally adjusted estimates using mixed-effects models, measured heterogeneity using I2, investigated sources of heterogeneity using meta-regression and subgroup meta-analyses, examined and adjusted for publication bias, performed influence and cumulative meta-analyses, and assessed evidence quality using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework.
Main Outcomes and Measures
Hazard ratios (HRs) for all-cause, cardiovascular, or other mortality estimates.
Results
This review included 14 retrospective and 12 prospective observational studies (1 213 756 participants) from 3220 records. Risk of bias was low to moderate; exclusion of 3 high-risk studies did not alter conclusions. Hearing loss was associated with excess all-cause mortality (HR, 1.13; 95% CI, 1.07-1.19; I2 = 77%; n = 21; 95% prediction interval [PI], 0.93-1.37) and cardiovascular mortality (HR, 1.28; 95% CI, 1.10-1.50; I2 = 60%; n = 6; 95% PI, 0.84-1.96), while DSL was associated with larger excess risks (all-cause: HR, 1.40; 95% CI, 1.30-1.51; I2 = 34%; n = 10; 95% PI, 1.18-1.66; cardiovascular: HR, 1.86; 95% CI, 1.31-2.65; I2 = 0%; n = 2), after adjustment for demographics and comorbidities. Prespecified meta-regression sufficiently explained heterogeneity, with longer follow-up duration weakening the pooled association, leaving low (29%) residual heterogeneity. Meta-regression among audiometric studies showed a dose-response association (doubling of HR per 30-dB increase in HL). Self-reported and audiometric effect sizes were similar, with lower heterogeneity in the latter. Associations were robust to trim-and-fill adjustment for publication bias and single-study influence and cumulative meta-analyses. Associations with accident/injury, cancer, and stroke mortality were inconclusive, with only 1 to 3 studies. Overall evidence quality was moderate.
Conclusions and Relevance
In this systematic review and meta-analysis, HL and DSL were associated with excess all-cause and cardiovascular mortality. Physicians caring for patients with HL should consider its relevance to general health and longevity.
Introduction
Globally, hearing loss (HL) affects 1.57 billion people, or 20% of the population, is the third leading cause of years lived with disability, and is rapidly growing in prevalence as the population ages.1 It is a well-known cause of decreased functional capacity and mental well-being2 and has even been established as a major risk factor for dementia.3 Yet, impairment in hearing has received little priority and attention compared with vision impairment and other chronic diseases,4 particularly from the adult patient’s perspective, because the decline in auditory function is often gradual and subtle.5
Emerging evidence suggests that HL may independently predict mortality.6,7,8,9,10,11 Possible mechanisms explaining this include cardiovascular disease,12 frailty,13 falls,14 cognitive impairment, depression, and social isolation,15 which are known sequelae of HL. However, the literature is equivocal, with some studies reporting no association.16,17,18,19,20 It is also uncertain if demographics, socioeconomic status, and medical comorbidities are potentially confounding this association. To our knowledge, there has not been a systematic, evidence-based clarification of this important association.
Concurrently, HL is associated with other sensory impairments, including vision loss (VL).18,21 While previous meta-analyses have established a clear association between VL and mortality,22,23 to our knowledge, none have evaluated dual sensory loss (DSL), or comorbid HL and VL, with mortality. It is uncertain if these individuals are at higher risk of mortality than individuals with HL or VL alone and whether comorbid HL and VL may produce a synergistic (ie, larger than additive) effect on mortality.
Given the tremendous, growing burden of HL globally, there is a pressing need to clarify the presence and magnitude of the associations between HL, DSL, and mortality to inform public health priorities and patient care. In this systematic review and meta-analysis, we aimed to comprehensively pool the epidemiological associations of HL and DSL with various mortality outcomes.
Methods
This review is registered on PROSPERO (CRD42021244194) and reported in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines.24,25 The MOOSE checklist is included in eTable 1 in the Supplement.
Search Strategy
We searched 3 databases (PubMed, Embase, Cochrane Library) from inception until June 18, 2021, using search terms for HL, DSL, and mortality (eMethods in the Supplement). We also hand searched the bibliographies of included articles and relevant reviews but identified no additional relevant records.
Study Selection, Data Extraction, and Risk of Bias Grading
Two authors (F.Y.C.N. and H.J.J.M.D.S.) independently selected eligible studies (based on title and abstract, followed by full-text articles), extracted relevant data, and evaluated the risk of bias in a blinded manner, with conflicts resolved by a third author (B.K.J.T.). We included observational and interventional studies published as full-length articles in peer-reviewed journals that reported the associations of HL or DSL (ie, comorbid HL and VL) with any estimate of mortality (eg, all-cause, cardiovascular) among adults aged 18 years and older, compared with participants without HL or DSL. We accepted objective (eg, pure-tone audiometry, Snellen chart), validated subjective (eg, whisper test), or self-reported measures of HL or DSL. We excluded case reports, reviews, letters, conference abstracts, other non–full-length articles, and non–English language publications. We extracted key data (eMethods in the Supplement) from each included article. We used the Newcastle-Ottawa Scale (NOS)26 for risk of bias assessment at the study level (eTable 2 in the Supplement) as high (<5 stars), moderate (5-7 stars), or low bias (≥8 stars).
Statistical Analysis
Where 2 or more studies were available, we proceeded with our planned meta-analyses. We used mixed-effects models (eMethods in the Supplement) to pool the maximally covariate-adjusted hazard ratios (HRs) from each study. We assessed and considered between-study heterogeneity as significant if the P value of the Q test was less than 0.10, or if the I2 statistic was 50% or higher.27 Because the 95% CIs account for only the uncertainty of the mean pooled effect, but not the estimate nor uncertainty of between-study variance (τ2), we further computed the 95% prediction intervals (95% PIs) when 3 or more studies were available to estimate the potential range of true effect sizes across individual studies.28
When 10 or more studies were available, we performed additional analyses to evaluate potential sources of study heterogeneity. We prespecified various study-level characteristics as potential explanatory variables (eMethods in the Supplement), repeated our meta-analyses in subgroups, and computed a univariate random-effects meta-regression,29 in which significant effect moderators were confirmed via permutation testing with 1000 iterations.30 To explore a potential dose-response association between HL severity and all-cause mortality, we (1) repeated our meta-analyses stratified by HL severity, using only independent subgroup-level estimates from available audiometric studies, and (2) performed a post hoc univariate random-effects meta-regression using the minimum audiometric threshold in decibels (dB) as a continuous explanatory variable. To investigate small-study effects, we assessed funnel plot asymmetry both visually and using the Egger bias test,31 and imputed potentially missing studies using the trim-and-fill method (eMethods in the Supplement) if publication bias was suspected.32 To examine the influence of each study on the overall findings, we performed leave-out-one influence analyses by deleting each study in turn from our meta-analyses. To examine the stability and sufficiency of evidence as it accumulated over time, we performed a cumulative meta-analysis ranked by year published.
We conducted all analyses using R, version 4.0.3 (R Foundation for Statistical Computing) (eMethods in the Supplement). Unless otherwise specified, we considered a 2-sided P value of <.05 as statistically significant.
Certainty of Evidence
We evaluated the quality of pooled evidence at the outcome level (eTable 3 in the Supplement) using the Grading of Recommendations Assessment, Development and Evaluations (GRADE) framework.33
Results
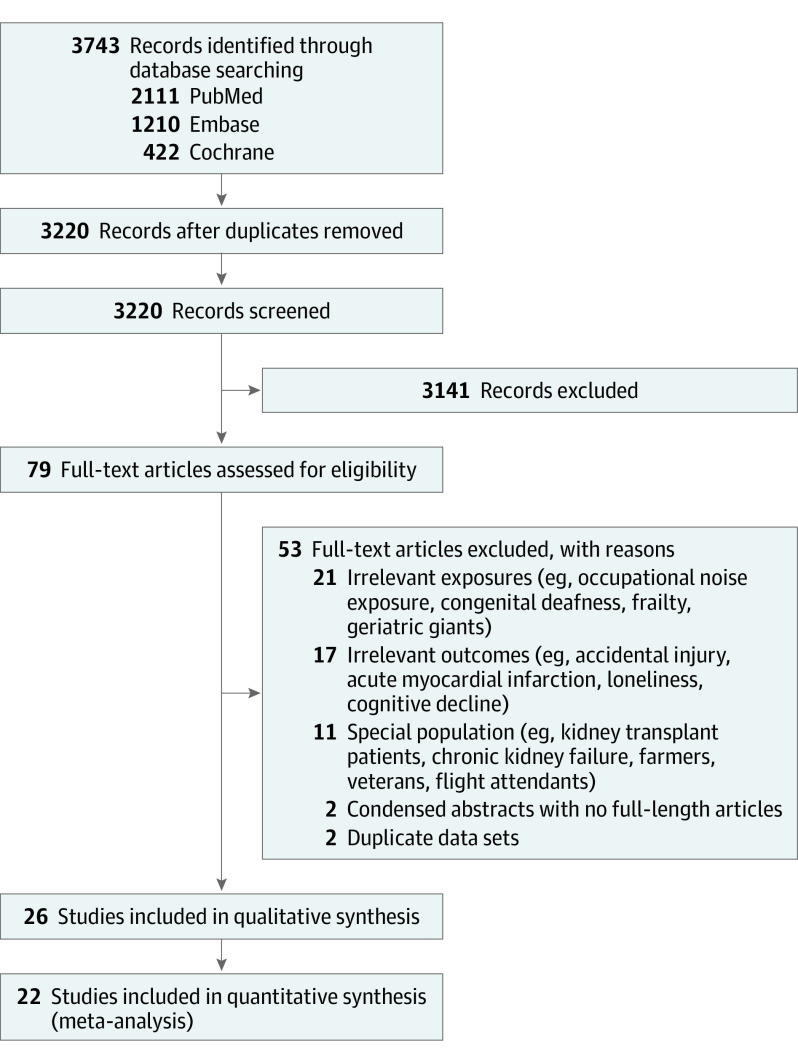
The study selection process is summarized in Figure 1. We included 26 articles from 3220 nonduplicated records after initial selection based on title and abstract and subsequent selection based on full-text articles.6,7,8,9,10,11,16,17,18,19,20,21,34,35,36,37,38,39,40,41,42,43,44,45,46,47
Figure 1. PRISMA Flow Diagram of the Study Selection Process.
Study Characteristics
Of the 26 included studies (Table),6,7,8,9,10,11,16,17,18,19,20,21,34,35,36,37,38,39,40,41,42,43,44,45,46,47 all were observational, with 13 retrospective cohorts, 12 prospective cohorts, and 1 retrospective matched cohort.46 When assessed using the NOS, 3 studies, 12 studies, and 11 studies had a high, moderate, and low risk of bias, respectively. Sensitivity analyses excluding the studies with a high risk of bias did not substantially alter our results. A total of 6, 10, 6, and 4 studies were conducted in Europe, North America, Asia, and Australasia, respectively. The mean participant age ranged from 39 to 86 years among studies. Sixteen studies, 9 studies, and 1 study investigated the associations of only HL, both HL and DSL, and only DSL with mortality, respectively. We included 22 studies in our meta-analyses.6,7,8,9,10,11,16,17,18,19,20,21,34,35,36,37,38,39,40,41,42,48
Table. Summary of Included Studies.
| Source | Study design | Sample size | Mean age, y | Male, % | Follow-up duration, median, y | Type of impairment | Impairment definition | Impairment prevalence, % | Statistical method | Covariatesa | NOS (max 9) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amieva et al,47 2018 PAQUID Study (France) | Prospective cohort | 3777 | 75.3 | 42.2 | 25 | HL | HL: self-report | HL: 48.4 | Cox proportional hazard model | Age, sex, education, number of comorbidities (hypertension, myocardial infarction, angor animi, diabetes, dyspnea, history of stroke, and smoking) | 7 |
| Hearing aid removed | |||||||||||
| Anstey et al,45 2001b Australian Longitudinal Study of Aging (Australia) | Retrospective cohort | 1947 | 78.9 | 53.4 | 5 | HL | HL: audiometry | NA | Cox proportional hazard model | Age, sex, education, self-rated health, cardiovascular diseases, neurological conditions, number of medications | 8 |
| Both ears; threshold NS | |||||||||||
| Appollonio et al,44 1995b (Italy) | Retrospective cohort | 1140 | 72.5 | 32.5 | 6 | HL | HL: whisper test | HL: 11.3 (men only) | Logistic regression | NA | 5 |
| Denney et al,6 2021 NHIS (US) | Prospective cohort | 198 902 | NA | 43.9 | 11 | HL | HL: self-report | HL: 15.5 | Cox proportional hazard model | Age, sex, race, education level, household poverty, employment status, self-rated health, marital status, household composition | 7 |
| Engdahl et al,35 2019 Nord-Trøndelag Hearing Loss Study (Norway) | Retrospective cohort | 50 462 | 50.5 | 46.8 | 17 | HL | HL: audiometry | HL: 17.9 | Cox proportional hazard model | Age, sex, smoking, alcohol use, physical activity, diabetes, resting heart rate, waist circumference, myocardial infarction, angina pectoris, stoke/brain hemorrhage, cancer, income, education, marriage, cohabiting, single and children status | 8 |
| Automatic audiometry, administered by trained audiologist | |||||||||||
| Sound-treated booth was used | |||||||||||
| HL defined as PTA of hearing thresholds >25 dB for the PTA of 4 frequencies, 0.5, 1, 2, and 4 kHz, in the better ear | |||||||||||
| Engedal,43 1996b (Norway) | Retrospective cohort | 334 | 81.7 | 23.3 | 3 | HL | HL: self-report | NA | Logistic regression | NA | 4 |
| Feeny et al,37 2012 Statistics Canada National Population Health Survey (Canada) | Retrospective cohort | 12 375 | 50.5 | 48 | 12 | HL | HL: self-report | HL: 4.7 | Cox proportional hazard model | Sociodemographic factors (age, sex, marital status, household income, education), chronic conditions associated with high risk of mortality (high blood pressure, chronic bronchitis or emphysema, diabetes, heart disease, cancer, stroke), chronic conditions possibly associated with mortality (asthma, Alzheimer disease, other dementia), conditions not associated with high risk of mortality (food allergies, other allergies, arthritis or rheumatism, back problems excluding arthritis, migraine headaches, sinusitis, epilepsy, stomach or intestinal ulcers, urinary incontinence, cataracts, glaucoma, and other long-term conditions), BMI, health behaviors (smoking, physical activity, alcohol use), psychological health and resources (psychological distress, sense of coherence, chronic stress), perceived social support | 8 |
| Fisher et al,20 2014 Age, Gene/Environment Susceptibility–Reykjavik Study (Iceland) | Prospective cohort | 4926 | 76.4 | 43.1 | 5 | HL, DSL | HL: Audiometry | HL: 25.4 DSL: 7.0 |
Cox proportional hazard model | Age, smoking status, BMI, hypertension, diabetes, systolic blood pressure, self-reported health status, cognitive status, self-reported history of falls, history of angina, record of cardiovascular event, hearing aid use | 8 |
| Conducted with Interacoustics AD229e microprocessor audiometer | |||||||||||
| Certification of examiner NS | |||||||||||
| Sound-treated booth was used | |||||||||||
| HL defined as hearing level ≥35 dB for the PTA of 4 frequencies, 0.5, 1, 2, and 4 kHz, in the better ear | |||||||||||
| Hearing aid removed | |||||||||||
| VL: ≤20/50 in the better eye assessed via an autorefractor | |||||||||||
| Genther et al,8 2015 Health et al Aging et al and Body Composition Study (US) | Prospective cohort | 1958 | 76.8 | 37.4 | 8 | HL | HL: audiometry | HL: 2.8 | Cox proportional hazard model | Age, race, sex, education, site, smoking, hypertension, diabetes, stroke, hearing aid use, pure-tone average mean, modified mini-mental state exam, gait speed, Center for Epidemiologic Studies Depression Scale score | 8 |
| Conducted with portable audiometer | |||||||||||
| Certification of examiner NS | |||||||||||
| Sound-treated booth was used | |||||||||||
| HL defined as hearing level >25 dB for the PTA of 4 frequencies, 0.5, 1, 2, and 4 kHz, in the better ear, according to WHO criteria | |||||||||||
| Hearing aid removed | |||||||||||
| Gopinath et al,39 2013 Blue Mountains Eye Study (Australia) | Retrospective cohort | 2812 | 67.4 | 43.2 | 10 | HL, DSL | HL: audiometry | HL: NS DSL: 49.7 |
Cox proportional hazard model | Age, sex, BMI, systolic blood pressure, current smoking status, poor self-rated health, walking disability, presence of hypertension and/or diabetes, history of cancer, angina, stroke and/or acute myocardial infarction, cognitive impairment | 9 |
| Conducted by audiologists in sound-treated booths | |||||||||||
| HL defined as hearing level >25 dB at the PTA of 4 frequencies, 0.5, 1, 2, and 4 kHz, in the better ear, according to WHO criteria | |||||||||||
| VL: LogMAR chart ≤20/40 in the better eye | |||||||||||
| Karpa et al,16 2010 Blue Mountains Hearing study (Australia) | Retrospective cohort | 2956 | 66.6 | 43.3 | 5 | HL | HL: audiometry | HL: 33.0 | Cox proportional hazard model | Age, sex, smoking, alcohol, hypertension, home ownership, low BMI, angina, acute myocardial infarction, walking disability, cognitive impairment, self-reported health, high serum urate | 8 |
| Conducted by audiologist in a sound-treated booth | |||||||||||
| HL defined as hearing level >25 dB for the PTA of 4 frequencies, 0.5, 1, 2, and 4 kHz, in the better ear, according to WHO criteria | |||||||||||
| Kim et al,46 2020b Korean National Health Insurance Service–National Sample Cohort (Korea) | Retrospective matched cohort | 28 065 | NS | 54.6 | 6.9 (Mean) | HL | HL: audiometry | HL: 25.0 | Cox proportional hazard model | Age, sex, income, region of residence, hypertension, diabetes, dyslipidemia, ischemic heart disease, stroke | 9 |
| Severe HL: hearing level ≥60 dB in both ears or ≥80 dB in 1 ear and ≥40 dB in 1 ear | |||||||||||
| Profound HL: hearing level ≥90 dB in both ears | |||||||||||
| Certification of examiners and use of sound-treated booth NS | |||||||||||
| Laforge et al,18 1992 GAO-Cleveland Study (US) | Retrospective cohort | 1408 | NA | 38.2 | 1 | HL, DSL | HL: self-report | HL: 7.3 DSL: 4.3 |
Logistic regression | Age, sex | 4 |
| VL: self-report | |||||||||||
| Lam et al,36 2006 NHIS (US) | Retrospective cohort | 116 796 | NA | 45.9 | 7 (Mean) | HL, DSL | HL: self-report | HL: 10.6 DSL: 1.3 |
Cox proportional hazard model | Survey design, age, marital status, educational level, self-rated health, and number of nonocular and nonauditory conditions | 6 |
| VL: self-report | |||||||||||
| Lee et al,9 2020 Kangbuk Samsung Health Study (Korea) | Prospective cohort | 580 798 | 39.7 | 52.8 | 8.4 | HL | HL: audiometry | HL: 2.8 | Cox proportional hazard model | Age, sex, study center, year of exam, smoking status, alcohol consumption, regular exercise, BMI, education level, exposure to occupational noise, diabetes, hypertension, cancer, cardiovascular disease, medication for dyslipidemia | 9 |
| Conducted by trained audiometric technicians in sound-treated booth | |||||||||||
| HL defined as hearing level ≥25 dB at the PTA of 3 frequencies 0.5, 1, and 2 kHz, in the better ear, according to WHO criteria | |||||||||||
| Liljas et al,34 2016 British Regional Heart Study (United Kingdom) | Prospective cohort | 3981 | 72 | 100 | 10 | HL | HL: self-report | HL: 12 | Cox proportional hazard model | Age, social class, BMI, smoking, physical activity, cardiovascular diseases, hypertension, diabetes | 6 |
| Lin et al,7 2019 NHIS (US) | Retrospective cohort | 123 899 | 45.9 | 48.3 | 5 | HL | HL: self-report | HL: 13.3 | Logistic regression | Age, sex, ethnicity, race, comorbidity count (diabetes, cancer history, heart disease, stroke) | 7 |
| Liu et al,21 2016 North Carolina Established Populations for Epidemiologic Studies of the Elderly study (US) | Retrospective cohort | 3871 | 73.3 | 35.1 | 6 | DSL | HL: self-report | DSL: 4.7 | Logistic regression | Age, sex, race, education, marital status, body mass index, current or past history of smoking, depression, and a weighted health index score of 5 chronic diseases (cancer excluding skin cancer, stroke, diabetes, hypertension, myocardial infarction) | 7 |
| VL: self-report | |||||||||||
| Lopez et al,38 2011 Health in Men Study and Australian Longitudinal Study on Women’s Health (Australia) | Prospective cohort | 5354 | 78.1 | 43.7 | 6.7 (Mean) | HL | HL: self-report | HL: 30.1 | Cox proportional hazard model | NA | 6 |
| Loprinzi et al,42 2016 NHANES (US) | Prospective cohort | 1658 | 57.2 | 48.4 | 7 | HL | HL: self-report | HL: 5.1 | Cox proportional hazard model | Age, sex, race and ethnicity, HbA1c level, waist circumference, medications used to treat dizziness/balance problems | 6 |
| Mitoku et al,40 2016 Gujo City Long-term Care Insurance Database (Japan) | Retrospective cohort | 1754 | 81.9 | 34.5 | 4 (Mean) | HL, DSL | HL: self-report | HL: 23.3 DSL: 18.2 |
Cox proportional hazard model | Age, sex, level of dependency, diabetes, neurological disease, hypertension, heart disease, cerebrovascular disease, respiratory disease, musculoskeletal and connective tissue disease, eye and ear disease | 6 |
| VL: self-report | |||||||||||
| Miyawaki et al,10 2020 Komo-Ise Study (Japan) | Retrospective cohort | 9522 | 67.6 | 57.5 | 9 (Mean) | HL, DSL | HL: self-report | HL: 0.9 DSL: 0.9 |
Cox proportional hazard model | Age, sex, education years, living area, income level, marital status, primary occupation, self-rated health (self-reported histories of cancer, stroke, heart disease, diabetes, dyslipidemia and hypertension), BMI, smoking status, exercise habits, alcohol consumption, dietary patterns | 8 |
| VL: self-report | |||||||||||
| Reuben et al,17 1999 NHANES (US) | Prospective cohort | 14 317 | 65.8 | 47.5 | 10 (Mean) | HL, DSL | HL: audiometry | HL: 16.4 DSL: 4.6 |
Cox proportional hazard model | Age, sex, race, education, myocardial infarction, diabetes, hypertension, heart failure, follow-up | 9 |
| Use of sound-treated booth and certification of examiners NS | |||||||||||
| HL defined as >40 dB loss at either 1 or 2 kHz or >40-dB loss at 1 and 2 kHz in both ears | |||||||||||
| VL: ≤20/40 in the better eye; visual chart NS | |||||||||||
| Schubert et al,19 2017 Epidemiology of Hearing Loss Study (US) | Prospective cohort | 2418 | 69 | 42.2 | 12 (Mean) | HL, DSL | HL: Audiometry | HL: 50.0 DSL: 18.9 |
Cox proportional hazard model | Age, sex, education, hypertension, diabetes, cardiovascular disease, cancer, cognitive impairment, frailty, smoking, exercise, BMI, alcohol, intima media thickness, C-reactive protein, interleukin-6 | 7 |
| Conducted in sound-treated booths. Certification of examiners NS | |||||||||||
| HL defined as hearing levels >25 dB at PTA of four frequencies 0.5, 1, 2 and 4kHz in either ear. | |||||||||||
| VL: ≤20/40 in the better eye. Autorefractor. | |||||||||||
| Sun et al,11 2020 Chinese Longitudinal Healthy Longevity Survey (China) | Prospective cohort | 37 076 | 86.4 | 41.4 | NS | HL, DSL | HL: self-report | HL: 14.2 DSL: 21.0 |
Cox proportional hazard model | Age, sex, enrollment year, province, residence, ethnicity, marriage status, occupation, access to medical service, smoking status, drinking status, exercise status, ADL score, physical performance score, MMSE score, food diversity score, social activity score, chronic disease score | 6 |
| Hearing aid removed | |||||||||||
| VL: self-report | |||||||||||
| Yamada et al,41 2011 (Japan) | Prospective cohort | 1250 | NA | 54.7 | 3 | HL | HL: self-report | HL: 13.7 | Logistic regression | Age, sex, marital status, level of education, existence of support network, vision ability, depressed feeling, self-reported well-being, stroke, myocardial infarction, COPD, diabetes, rheumatoid arthritis; additional adjustment for hearing aids did not substantially change the association (HR NS) | 4 |
| Hearing aid removed |
Abbreviations: ADL, activities of daily living; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DSL, dual sensory loss; HL, hearing loss; HR, hazard ratio; MMSE, Mini-Mental State Examination; NA, not applicable; NHANES, National Health and Nutrition Examination Survey; NHIS, National Health Interview Survey; NOS, Newcastle-Ottawa Scale; NS, not stated; PTA, pure-tone average; VL, vision loss; WHO, World Health Organization.
Studies were considered to have adjusted for socioeconomic status if they adjusted for social class or for 2 or more of the following: education, poverty, income, employment status, occupation, home ownership, residence.
Excluded from meta-analyses but included in systematic review.
Association of HL and Mortality
Definitions of HL
Fifteen of the included studies (Table) assessed hearing via self-report using standardized questionnaires. In 10 studies, HL was measured objectively via audiometry, of which 6 studies defined HL as pure-tone average of hearing thresholds exceeding 25 dB for 0.5, 1, 2, and 4 kHz.8,9,16,19,39,43 Fisher et al20 and Reuben et al17 defined HL as pure-tone average of hearing thresholds exceeding 35 dB and 40 dB, respectively, for 0.5, 1, 2, and 4 kHz. Kim et al46 used higher audiometric thresholds at 60 and 90 dB. Because these were incongruent with other studies, we included them only in the subgroup meta-analysis stratified by HL severity.
Meta-analysis for All-Cause Mortality
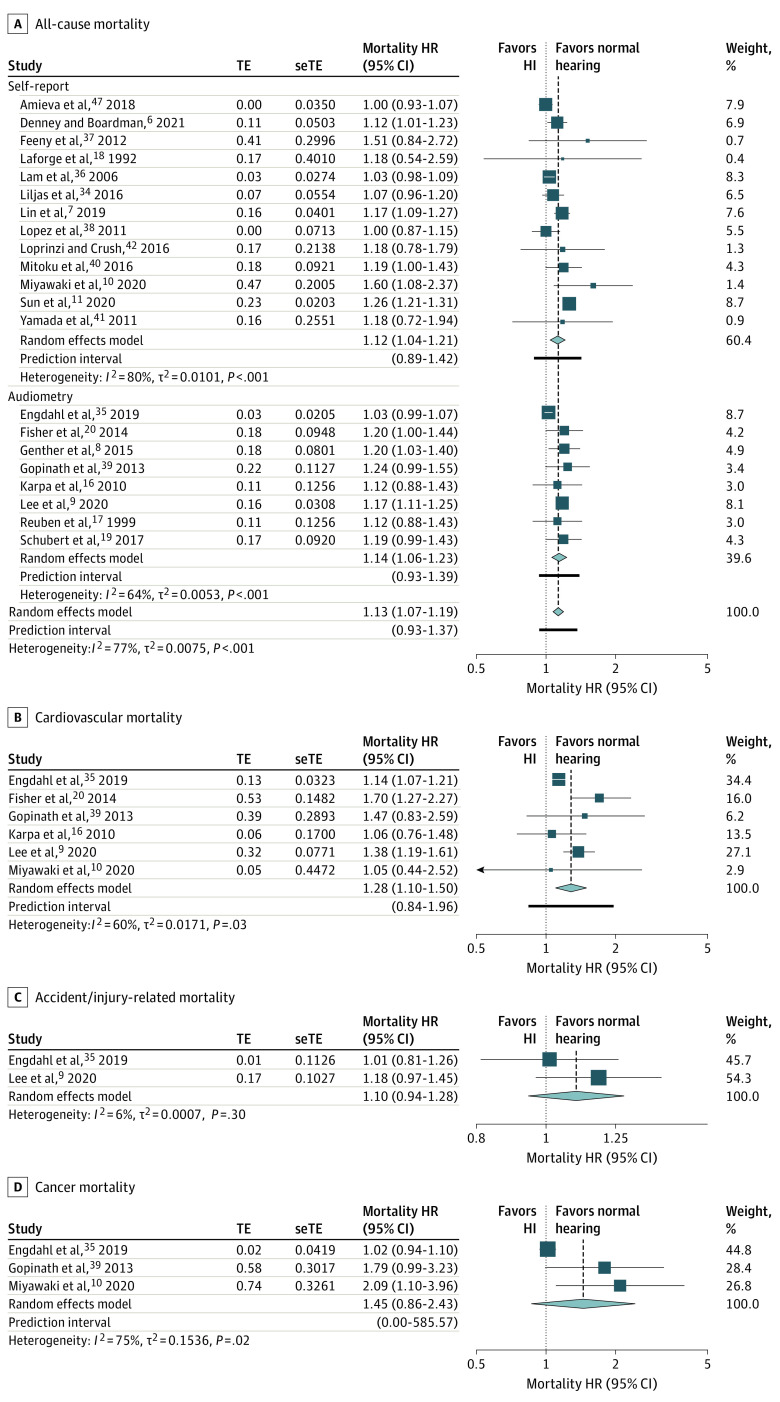
Meta-analysis for all-cause mortality included 21 studies (Figure 2A).6,7,8,9,10,11,16,17,18,19,20,34,35,36,37,38,39,40,41,42,47 Compared with participants with normal hearing, participants with HL had, on average, significantly higher pooled hazards of all-cause mortality (HR, 1.13; 95% CI, 1.07-1.19). Between-study heterogeneity was considerable (I2 = 77%), and the 95% PI (0.93-1.37) suggests that the association would hold true in most but not all individual study settings. Many of the 21 studies adjusted for covariates such as age and sex (20 studies), body mass index (8 studies), education level (11 studies), socioeconomic status (5 studies), marital status (6 studies), smoking (12 studies), alcohol (7 studies), hypertension (12 studies), diabetes (15 studies), cardiovascular disease (14 studies), stroke (9 studies), cancer (6 studies), and cognitive impairment (5 studies). Three of 21 studies adjusted for hearing aid use.8,20,41 Three other studies excluded from meta-analysis based on incompatible statistical measures all reported a significantly higher incidence rate or odds of all-cause mortality in participants with hearing impairment.43,44,45
Figure 2. Forest Plots Showing the Longitudinal Association Between Hearing Loss and (A) All-Cause Mortality, Stratified by the Method of Hearing Assessment, (B) Cardiovascular Mortality, (C) Accident/Injury-Related Mortality, and (D) Cancer Mortality.
Diamonds are the estimated pooled hazard ratio (HR) for each random-effects meta-analysis; box sizes reflect the relative weight apportioned to studies in the meta-analysis. HI indicates hearing impairment; seTE, standard error treatment effect; TE, treatment effect.
Meta-analyses for Cardiovascular, Accident/Injury-Related, and Cancer Mortality
Hearing loss was, on average, significantly associated with cardiovascular mortality (HR, 1.28; 95% CI, 1.10-1.50; I2 = 60%; n = 6; 95% PI, 0.84-1.96; Figure 2B). However, there was no significant association found between HL and accident/injury-related mortality (HR, 1.10; 95% CI, 0.94-1.28; I2 = 6%; n = 2; Figure 2C) nor cancer mortality (HR, 1.45; 95% CI, 0.86-2.43; I2 = 75%; n = 3; Figure 2D), for which only 2 to 3 studies were available. One retrospective cohort study of 2812 participants additionally reported no association with stroke mortality (HR, 1.05; 95% CI, 0.50-2.22).39
Meta-regression and Subgroup Meta-analyses
The meta-analysis of HL and all-cause mortality contained sufficient studies for further analyses. Meta-regression (eTable 4 in the Supplement) identified average follow-up duration as a significant effect moderator, accounting for 58.9% of heterogeneity and leaving low (28.5%) residual heterogeneity. The pooled HR decreased by a factor of 0.93 (95% CI, 0.88-0.98; eFigure 1 in the Supplement) per 10-year increase in follow-up. All other characteristics (mean age, percentage male, follow-up duration, exposure prevalence, number of covariates, NOS score, publication year, measurement of HL, study design, adjustment for hearing aid use, adjustment for socioeconomic status, adjustment for marital status) were not significant effect moderators.
Subgroup meta-analyses are reported in eTable 6 in the Supplement. The pooled association remained significant and similar across all subgroups, including the subgroups for self-report (HR, 1.12; 95% CI, 1.04-1.21; I2 = 79%; n = 13) and audiometry (HR, 1.14; 95% CI, 1.06-1.23; I2 = 64%; n = 8) (Figure 2A), where the former exhibited higher heterogeneity. Adjustment for socioeconomic and marital status did not influence the significance or size of the pooled association. Additional analyses showed that the pooled association remained significant on average among 3 studies that adjusted for the use of hearing aids as a covariate (HR, 1.20; 95% CI, 1.07-1.35; I2 = 0%; 95% PI, 0.56-2.55), and among 3 studies that stratified for hearing aid users (HR, 1.06; 95% CI, 1.03-1.09; I2 = 0%; 95% PI, 0.89-1.27).
Meta-analyses of audiometric studies stratified by HL severity showed a trend of higher hazards with increasing HL severity: mild HL (25-40 dB) (HR, 1.14; 95% CI, 1.07-1.22; I2 = 0%; n = 2), moderate-severe HL (≥40 dB) (HR, 1.24; 95% CI, 1.14-1.35; I2 = 0%; n = 4), severe HL (60-90 dB) (HR, 4.07; 95% CI, 3.71-4.46; n = 1), and profound HL (≥90 dB) (HR, 4.22; 95% CI, 3.52-5.05; n = 1). On meta-regression using audiometric thresholds (dB) as a continuous explanatory variable (eFigure 2 in the Supplement), the HR doubled (2.05; 95% CI, 1.45-2.90; P < .001) for every 30-dB increase in audiometric HL.
Publication Bias, Influence Analyses, and Cumulative Meta-analysis
Visual inspection, though not Egger bias (intercept = −0.44; 95% CI, −1.46 to 0.58; t = −0.85; P = .42), suggested possible funnel plot asymmetry. Trim-and-fill imputed 2 missing studies (eFigure 3 in the Supplement) with minimal change to the pooled effect size (HR, 1.12; 95% CI, 1.07-1.18; I2 = 76%; n = 21 + 2; 95% PI, 0.93-1.36). Leave-out-one influence analysis (eFigure 4 in the Supplement) showed that no single study drastically changed the pooled effect size, while cumulative meta-analysis (eFigure 5 in the Supplement) showed a significant and stable pooled effect size since 2014.
Quality of Evidence
Using the GRADE framework (eTable 3 in the Supplement), we judged the certainty of evidence for each outcome as the following: all-cause, high quality; cardiovascular, low quality; and cancer and accident/injury-related mortality, very low quality. For all-cause mortality, we upgraded 1 level for the dose-response association, and another level for the large effect size for severe and profound hearing loss; downgrading was not warranted because heterogeneity was explained, and trim-and-fill analysis showed a robust association.
Association of DSL and Mortality
Definitions of DSL
Dual sensory loss was defined as concomitant HL and VL. Among the 10 studies that studied DSL (Table),10,11,17,18,19,20,21,36,39,40 6 studies measured vision loss via self-report, while the remaining 4 classified vision loss objectively as having a visual acuity of worse than 20/40 (3 studies) or 20/50 (1 study) in the better eye.
Meta-analyses
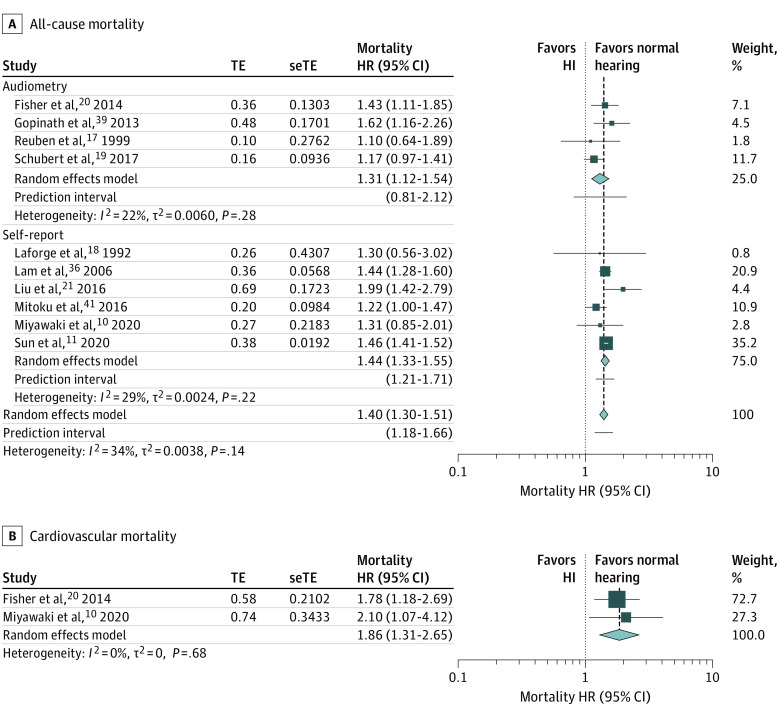
Compared with participants with normal hearing and vision, participants with DSL had a significantly higher pooled hazards of all cause-mortality (HR, 1.40; 95% CI, 1.30-1.51; I2 = 34%; n = 10; 95% PI, 1.18-1.66; Figure 3A) and cardiovascular mortality (HR, 1.86; 95% CI, 1.31-2.65; I2 = 0%; n = 2; Figure 3B), with low heterogeneity in the former and none in the latter. The PI for all-cause mortality does not cross the null, indicating that this association can be observed in at least 95% of individual study settings. Many of the 10 studies adjusted for important covariates, such as age (10 of 10), sex (8 of 10), body mass index (5 of 10), education level (6 of 10), socioeconomic status (2 of 10), smoking history (6 of 10), alcohol use (3 of 10), and major comorbidities, such as hypertension (5 of 10), diabetes (5 of 10), cardiovascular disease (7 of 10), cognitive impairment (4 of 10), stroke (4 of 10), and cancer (3 of 10).
Figure 3. Forest Plots Showing the Longitudinal Association Between Dual Sensory Loss and (A) All-Cause Mortality, Stratified by the Method of Hearing and Vision Assessment, and (B) Cardiovascular Mortality.
Diamonds are the estimated pooled hazard ratio (HR) for each random-effects meta-analysis; box sizes reflect the relative weight apportioned to studies in the meta-analysis. HI indicates hearing impairment; seTE, standard error treatment effect; TE, treatment effect.
Meta-regression and Subgroup Meta-analyses
The meta-analysis of DSL and all-cause mortality was eligible for further analyses because it included sufficient studies. Meta-regression of prespecified study-level characteristics showed that none were significant effect moderators (eTable 5 in the Supplement). The pooled association remained significant and similar across the various subgroup meta-analyses (eTable 6 in the Supplement). Adjustment for socioeconomic status and marital status did not influence the significance or size of the pooled association.
Publication Bias, Influence Analyses, and Cumulative Meta-analysis
Egger bias (intercept = 0.38; 95% CI, 0.33-0.44; t = −0.85; P = .42) and visual inspection suggested no funnel plot asymmetry, and trim-and-fill imputed no missing studies (eFigure 6 in the Supplement). Leave-out-one influence analysis (eFigure 7 in the Supplement) showed that no single study drastically changed the pooled effect size, while cumulative meta-analysis (eFigure 8 in the Supplement) showed a significant and stable pooled effect size since 2006.
GRADE Quality of Evidence
Using the GRADE framework (eTable 3 in the Supplement), we judged the certainty of evidence for each outcome as the following: all-cause, moderate quality; and cardiovascular mortality, low quality.
Discussion
In this systematic review and meta-analysis of 26 observational studies comprising a combined cohort of 1 213 756 participants, HL was associated with 13% and 28% increased pooled hazards of all-cause and cardiovascular mortality, while DSL was associated with a higher excess risk of 40% and 86%, respectively. A dose-response association between HL and all-cause mortality was suggested by meta-regression among audiometric studies, with the relative hazard doubling for every 30-dB increase in HL. The associations were robust to qualitative and quantitative assessments of publication bias, as well as single-study influence and cumulative meta-analyses.
To the best of our knowledge, this is the first systematic review and meta-analysis, and the most comprehensive evidence-based synthesis to date, on the associations of HL and DSL with mortality. Our findings are consistent with recent meta-analyses on the association of VL and mortality, which report 30% increased hazards of both all-cause and cardiovascular mortality.22,23,49 While HL is associated with a lower excess risk of all-cause mortality (13%) than VL (30%), both are associated with similar excess cardiovascular mortality (28%-30%). When both HL and VL are present (ie, DSL), the combined associations with all-cause (40%) and cardiovascular (86%) mortality are larger than either HL or VL alone. The current level of precision does not allow us to infer whether a synergistic (ie, larger than additive) association of HL and VL with mortality is present.
Several mechanisms may explain the association between HL and mortality (Figure 4). First, we must consider the possibility that the association may be partially confounded by shared comorbidities. Numerous pathological processes, such as hypertension, diabetes mellitus, stroke, neurodegenerative decline, and systemic inflammation, increase the risk of HL via microvascular ischemia, inflammatory damage, and neuronal degeneration involving the cochlea, auditory nerve, or central auditory pathways50,51,52,53,54; these diseases are also known risk factors for cardiovascular and all-cause mortality. It is thus possible that these diseases may have caused both HL and cardiovascular mortality, with HL manifesting earlier. Though most included studies had adjusted for cardiovascular risk factors and disease as covariates, we cannot exclude the possibility of residual confounding.
Figure 4. Schematic Representation of Some Possible Mechanisms Accounting for the Association Between Hearing Loss and Mortality.
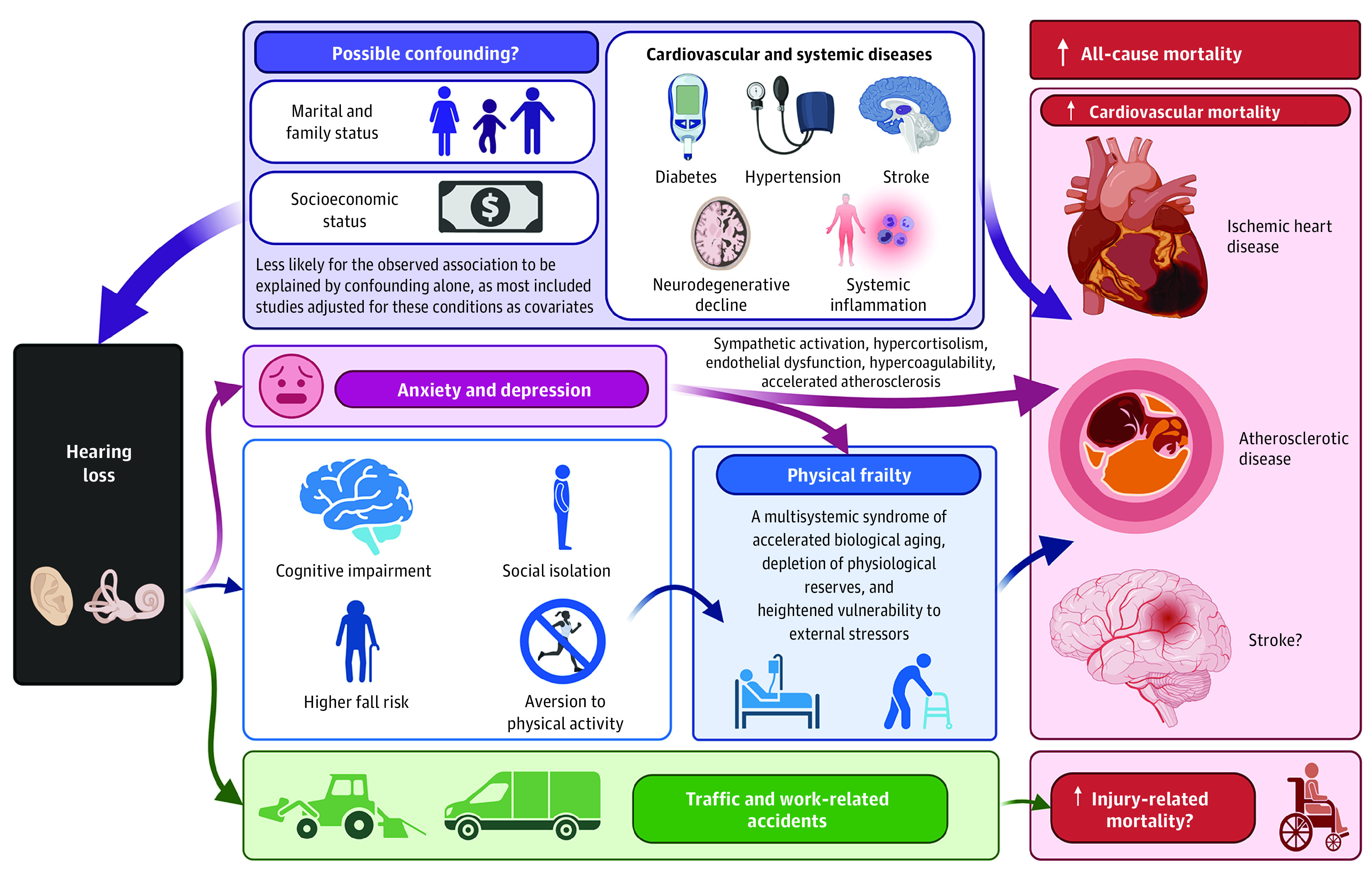
Created with BioRender.com.
Similarly, socioeconomic and marital status may reasonably be confounding the observed associations. Blue-collar workers and persons with low educational attainment are more likely to develop incident HL,55 possibly owing to occupational noise and ototoxic chemical exposure.56 They also have poorer access to health care, which may delay treatment for HL and other health conditions.57 Marital and family status—in particular, being divorced or childless—have also been reported to moderate the mortality risk associated with HL,35 possibly because the reduced need for interaction within the household provides less impetus to prevent hearing decline. These individuals may also have poorer health status and microvascular comorbidities that could increase the risk of both HL and mortality.58 However, in our meta-analyses, socioeconomic and marital status were not found to be significant effect moderators based on meta-regression, and the pooled effect remained significant among studies that adjusted for these variables. This suggests that the observed associations are unlikely to be explained by confounding from socioeconomic or marital status alone.
Instead, we may also consider possible associated mechanisms between HL and mortality (Figure 4). A possible consequence of HL is physical frailty,13 which may be mediated by aversion to physical activity, frequent falls, depression, anxiety, cognitive impairment, and social isolation,12,14,59,60,61,62 as reviewed elsewhere.13 In turn, frailty—a multisystemic syndrome of accelerated biological aging, depletion of physiological reserves, and heightened vulnerability to external stressors—is a strong independent predictor of cardiovascular and all-cause mortality across various settings.63 Furthermore, HL is known to be adversely associated with mental health, such as through increased risk of depression and anxiety disorders.64 These disorders are established risk factors for the incidence and progression of cardiovascular disease and mortality,65,66 possibly via sympathetic activation, hypercortisolism, endothelial dysfunction, hypercoagulability, and accelerated atherosclerosis.66,67 Therefore, a causal relationship of HL with cardiovascular mortality and other disease-related mortality remains possible, and frailty and mental health disorders should be investigated as potential mediators of these associations. Additionally, HL is a safety hazard and could in theory increase accident/injury-related mortality. For instance, drivers with HL have a higher risk of traffic accidents,68 while workers with occupational noise–induced HL experience more frequent work-related accidents.69 Further studies are required to validate the association between HL and accident/injury-related mortality, given that our meta-analysis of only 2 studies was likely underpowered.
Overall, our observational meta-analyses provide insufficient evidence for causal conclusions, but minimally show that HL and DSL are early risk markers for mortality. Because only 3 included studies considered hearing aid use and we found no relevant randomized clinical trials, it is unknown if hearing interventions may mitigate the excess mortality associated with HL. Regardless, this study adds to the growing evidence base suggesting that HL and VL are not isolated problems but may be relevant to general health and longevity. Physicians treating patients with HL should be aware of the dose-response association with all-cause and cardiovascular mortality, particularly when vision is also impaired, and encourage patients to follow up regularly with their primary care and cardiovascular physicians for their chronic health conditions.
Strengths and Limitations
The strengths of our study lie in the large number of systematically included studies from diverse settings, which increases the generalizability of our findings. We applied a rigorous prespecified protocol of systematic searching, bias assessment, and quality grading according to international guidelines. Only 3 of 26 included studies had a high risk of bias, and their exclusion did not change our findings. We pooled maximally adjusted estimates to best account for potential confounders within the limits of existing literature. Heterogeneity was adequately explained by meta-regression, with longer follow-up duration weakening the observed association. The weakening of predictor variables over long follow-up durations is an expected phenomenon and may be explained by unmeasured changes in comorbidities over time, age-related variability in predictor strength, selective mortality, and other reasons, as described elsewhere.70 Our findings were robust to subgroup, meta-regression, influence, cumulative, and small-study analyses. We found no evidence of publication bias.
Nonetheless, several limitations should be acknowledged. First, though our meta-analyses of longitudinal studies demonstrate a clear temporal sequence, the observational nature of the included studies does not permit causal conclusions because residual confounding cannot be excluded. Our meta-analyses were also limited by the wide variation in the number and choice of covariates among the included studies, which could have biased our findings. Nonetheless, meta-regression did not identify the number of covariates as a significant effect moderator, and most studies consistently adjusted for sociodemographic factors, lifestyle factors, and key comorbidities, such as cardiovascular disease. Indeed, some studies could even have overadjusted their statistical models by including variables that may lie on the causal pathway toward mortality, which would have created a negative bias. Second, more than half of the studies in our meta-analyses assessed for HL via self-report, which may be subject to bias. We had planned in our a priori protocol to accept self-reported measurements of HL but conduct sensitivity analyses, because upfront exclusion could omit potentially important information from the systematic review. As expected, heterogeneity was higher in the self-reported subgroup than in the objectively assessed subgroup meta-analysis. However, the stratified pooled effect sizes were very similar (Figure 2A and Figure 3A), and meta-regression did not identify the method of assessment as a significant effect moderator; thus, the inclusion of self-reported HL and DSL is less likely to have substantially biased our findings. Third, the current level of precision of pooled effect in this meta-analysis implies that the true average excess all-cause mortality associated with HL and DSL may range between 7% to 19% and 30% to 51%, respectively, based on the 95% CI. While the true effect is potentially as small as 7%, this may be sufficient to be clinically relevant. Fourth, although we assessed the associations with various subtypes of mortality, such as cardiovascular, accident/injury-related, cancer, and stroke mortality, the latter 3 subtypes only had between 1 and 3 included studies, which is insufficient to draw firm conclusions. Fifth, our meta-regression of audiometric HL severity, which provides some evidence for a dose-response association, was conducted only in a subset of studies with available data and was not prespecified because we had not anticipated the availability of such data. In meta-regression, aggregate/ecological bias and confounding cannot be excluded, and linearity is assumed. Nevertheless, clinical plausibility and a large effect size are consistent with the likelihood of a true dose-response association. Sixth, none of the studies accounted for the duration and trajectory of HL after baseline assessment; hence, we were unable to examine HL progression in relation to mortality. Finally, we studied HL at the level of the impairment rather than its subtypes (conductive/sensorineural) or underlying cause (eg, noise-induced HL). These require further investigation in relation to mortality. Nonetheless, as age-related HL is estimated to account for nearly 80% of the burden of adult-onset HL,71 and most included studies had a mean participant age of 60 years or older, our findings are mostly representative of age-related hearing loss.
Conclusions
In this multiadjusted observational meta-analysis of 1 213 756 participants with moderate-quality evidence overall, HL was associated with 13% and 28% higher risk of all-cause and cardiovascular mortality, respectively. We observed a dose-response association, with all-cause mortality hazard doubling for every 30-dB increase in audiometric thresholds. When both HL and VL were present, the excess all-cause and cardiovascular mortality was even higher, at 40% and 86%. It is presently unknown if hearing interventions may mitigate the excess mortality associated with HL. Nonetheless, this study adds to the growing evidence base suggesting that hearing and vision loss are not isolated problems but may be reflective of general health and longevity. Physicians treating patients with HL should be aware of this dose-response association, particularly when vision is also impaired, and encourage patients to follow up regularly with their primary care and cardiovascular physicians for their chronic health conditions.
eMethods.
eFigure 1. Bubble plot for random-effects meta-regression of log(HRs) against average follow-up duration (in years) for the longitudinal association of hearing loss with all-cause mortality.
eFigure 2. Bubble plot for random-effects meta-regression of log(HRs) against minimum audiometric thresholds (in decibels, dB) for the longitudinal association of hearing loss with all-cause mortality.
eFigure 3. Contour-enhanced funnel plot for the longitudinal association of hearing loss with all-cause mortality, with missing studies imputed via the trim-and-fill method.
eFigure 4. Leave-out-one influence analysis of the longitudinal association between hearing loss and all-cause mortality.
eFigure 5. Cumulative meta-analysis, by year published, of the longitudinal association between hearing loss and all-cause mortality.
eFigure 6. Contour-enhanced funnel plot for the longitudinal association of dual sensory loss with all-cause mortality, with missing studies imputed via the trim-and-fill method.
eFigure 7. Leave-out-one influence analysis of the longitudinal association between dual sensory loss and all-cause mortality.
eFigure 8. Cumulative meta-analysis, by year published, of the longitudinal association between dual sensory loss and all-cause mortality.
eTable 1. MOOSE Checklist.
eTable 2. Evaluation of risk of bias using the Newcastle-Ottawa Scale (NOS) for cohort studies.
eTable 3. Evaluation of quality of pooled evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.
eTable 4. Random-effects meta-regression of log(HRs) against potential effect moderators (continuous and categorical study-level characteristics) for the longitudinal association of hearing loss with all-cause mortality.
eTable 5. Random-effects meta-regression of log(HRs) against potential effect moderators (continuous and categorical study-level characteristics) for the longitudinal association of dual sensory loss with all-cause mortality.
eTable 6. Meta-analyses in subgroups, stratified by categorical study-level characteristics for the longitudinal associations of hearing loss or dual sensory loss each with all-cause mortality.
References
- 1.Haile LM, Kamenov K, Briant PS, et al. ; GBD 2019 Hearing Loss Collaborators . Hearing loss prevalence and years lived with disability, 1990-2019: findings from the Global Burden of Disease Study 2019. Lancet. 2021;397(10278):996-1009. doi: 10.1016/S0140-6736(21)00516-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chia EM, Wang JJ, Rochtchina E, Cumming RR, Newall P, Mitchell P. Hearing impairment and health-related quality of life: the Blue Mountains Hearing Study. Ear Hear. 2007;28(2):187-195. doi: 10.1097/AUD.0b013e31803126b6 [DOI] [PubMed] [Google Scholar]
- 3.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018;144(2):115-126. doi: 10.1001/jamaoto.2017.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ta NH. ENT in the context of global health. Ann R Coll Surg Engl. 2019;101(2):93-96. doi: 10.1308/rcsann.2018.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yueh B, Collins MP, Souza PE, et al. Long-term effectiveness of screening for hearing loss: the screening for auditory impairment—which hearing assessment test (SAI-WHAT) randomized trial. J Am Geriatr Soc. 2010;58(3):427-434. doi: 10.1111/j.1532-5415.2010.02738.x [DOI] [PubMed] [Google Scholar]
- 6.Denney JT, Boardman JD. Hearing impairment, household composition, marital status, and mortality among US adults. J Gerontol B Psychol Sci Soc Sci. 2021;76(1):201-208. doi: 10.1093/geronb/gbz157 [DOI] [PubMed] [Google Scholar]
- 7.Lin HW, Mahboubi H, Bhattacharyya N. Hearing difficulty and risk of mortality. Ann Otol Rhinol Laryngol. 2019;128(7):614-618. doi: 10.1177/0003489419834948 [DOI] [PubMed] [Google Scholar]
- 8.Genther DJ, Betz J, Pratt S, et al. ; Health ABC Study . Association of hearing impairment and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2015;70(1):85-90. doi: 10.1093/gerona/glu094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee W, Chang Y, Shin H, Ryu S. Hearing loss and risk of overall, injury-related, and cardiovascular mortality: the Kangbuk Samsung Health Study. J Clin Med. 2020;9(5):E1415. doi: 10.3390/jcm9051415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyawaki A, Kobayashi Y, Kawachi I. Self-reported hearing/visual loss and mortality in middle-aged and older adults: findings from the Komo-Ise cohort, Japan. J Epidemiol. 2020;30(2):67-73. doi: 10.2188/jea.JE20180198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J, Li L, Sun J. Sensory impairment and all-cause mortality among the elderly adults in China: a population-based cohort study. Aging (Albany NY). 2020;12(23):24288-24300. doi: 10.18632/aging.202198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gispen FE, Chen DS, Genther DJ, Lin FR. Association between hearing impairment and lower levels of physical activity in older adults. J Am Geriatr Soc. 2014;62(8):1427-1433. doi: 10.1111/jgs.12938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan BKJ, Man REK, Gan ATL, et al. Is sensory loss an understudied risk factor for frailty? a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2020;75(12):2461-2470. doi: 10.1093/gerona/glaa171 [DOI] [PubMed] [Google Scholar]
- 14.Jiam NT-L, Li C, Agrawal Y. Hearing loss and falls: A systematic review and meta-analysis. Laryngoscope. 2016;126(11):2587-2596. doi: 10.1002/lary.25927 [DOI] [PubMed] [Google Scholar]
- 15.Dawes P, Emsley R, Cruickshanks KJ, et al. Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PLoS One. 2015;10(3):e0119616. doi: 10.1371/journal.pone.0119616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karpa MJ, Gopinath B, Beath K, et al. Associations between hearing impairment and mortality risk in older persons: the Blue Mountains Hearing Study. Ann Epidemiol. 2010;20(6):452-459. doi: 10.1016/j.annepidem.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 17.Reuben DB, Mui S, Damesyn M, Moore AA, Greendale GA. The prognostic value of sensory impairment in older persons. J Am Geriatr Soc. 1999;47(8):930-935. doi: 10.1111/j.1532-5415.1999.tb01286.x [DOI] [PubMed] [Google Scholar]
- 18.Laforge RG, Spector WD, Sternberg J. The relationship of vision and hearing impairment to one-year mortality and functional decline. J Aging Health. 1992;4(1):126-148. doi: 10.1177/089826439200400108 [DOI] [Google Scholar]
- 19.Schubert CR, Fischer ME, Pinto AA, et al. Sensory impairments and risk of mortality in older adults. J Gerontol A Biol Sci Med Sci. 2017;72(5):710-715. doi: 10.1093/gerona/glw036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher D, Li CM, Chiu MS, et al. Impairments in hearing and vision impact on mortality in older people: the AGES-Reykjavik Study. Age Ageing. 2014;43(1):69-76. doi: 10.1093/ageing/aft122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu PL, Cohen HJ, Fillenbaum GG, Burchett BM, Whitson HE. Association of co-existing impairments in cognition and self-rated vision and hearing with health outcomes in older adults. Gerontol Geriatr Med. 2016;2:2. doi: 10.1177/2333721415623495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng Yin Ling C, Seshasai S, Chee ML, et al. Visual impairment, major eye diseases, and mortality in a multi-ethnic Asian population and a meta-analysis of prospective studies. Am J Ophthalmol. 2021;231:88-100. doi: 10.1016/j.ajo.2021.04.026 [DOI] [PubMed] [Google Scholar]
- 23.Ehrlich JR, Ramke J, Macleod D, et al. Association between vision impairment and mortality: a systematic review and meta-analysis. Lancet Glob Health. 2021;9(4):e418-e430. doi: 10.1016/S2214-109X(20)30549-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(n71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. Accessed November 23, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 28.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 29.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559-1573. doi: 10.1002/sim.1187 [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23(11):1663-1682. doi: 10.1002/sim.1752 [DOI] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 33.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liljas AE, Wannamethee SG, Whincup PH, et al. Hearing impairment and incident disability and all-cause mortality in older British community-dwelling men. Age Ageing. 2016;45(5):662-667. doi: 10.1093/ageing/afw080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engdahl B, Idstad M, Skirbekk V. Hearing loss, family status and mortality—findings from the HUNT study, Norway. Soc Sci Med. 2019;220:219-225. doi: 10.1016/j.socscimed.2018.11.022 [DOI] [PubMed] [Google Scholar]
- 36.Lam BL, Lee DJ, Gómez-Marín O, Zheng DD, Caban AJ. Concurrent visual and hearing impairment and risk of mortality: the National Health Interview Survey. Arch Ophthalmol. 2006;124(1):95-101. doi: 10.1001/archopht.124.1.95 [DOI] [PubMed] [Google Scholar]
- 37.Feeny D, Huguet N, McFarland BH, Kaplan MS, Orpana H, Eckstrom E. Hearing, mobility, and pain predict mortality: a longitudinal population-based study. J Clin Epidemiol. 2012;65(7):764-777. doi: 10.1016/j.jclinepi.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez D, McCaul KA, Hankey GJ, et al. Falls, injuries from falls, health related quality of life and mortality in older adults with vision and hearing impairment—is there a gender difference? Maturitas. 2011;69(4):359-364. doi: 10.1016/j.maturitas.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 39.Gopinath B, Schneider J, McMahon CM, Burlutsky G, Leeder SR, Mitchell P. Dual sensory impairment in older adults increases the risk of mortality: a population-based study. PLoS One. 2013;8(3):e55054. doi: 10.1371/journal.pone.0055054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitoku K, Masaki N, Ogata Y, Okamoto K. Vision and hearing impairments, cognitive impairment and mortality among long-term care recipients: a population-based cohort study. BMC Geriatr. 2016;16:112. doi: 10.1186/s12877-016-0286-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada M, Nishiwaki Y, Michikawa T, Takebayashi T. Impact of hearing difficulty on dependence in activities of daily living (ADL) and mortality: a 3-year cohort study of community-dwelling Japanese older adults. Arch Gerontol Geriatr. 2011;52(3):245-249. doi: 10.1016/j.archger.2010.04.023 [DOI] [PubMed] [Google Scholar]
- 42.Loprinzi PD, Crush E. Sensory impairment, functional balance and physical activity with all-cause mortality. J Phys Act Health. 2016;13(9):980-987. doi: 10.1123/jpah.2015-0692 [DOI] [PubMed] [Google Scholar]
- 43.Engedal K. Mortality in the elderly—a 3-year follow-up of an elderly community sample. Int J Geriatr Psychiatry. 1996;11(5):467-471. doi: [DOI] [Google Scholar]
- 44.Appollonio I, Carabellese C, Magni E, Frattola L, Trabucchi M. Sensory impairments and mortality in an elderly community population: a six-year follow-up study. Age Ageing. 1995;24(1):30-36. doi: 10.1093/ageing/24.1.30 [DOI] [PubMed] [Google Scholar]
- 45.Anstey KJ, Luszcz MA, Giles LC, Andrews GR. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychol Aging. 2001;16(1):3-11. doi: 10.1037/0882-7974.16.1.3 [DOI] [PubMed] [Google Scholar]
- 46.Kim SY, Min C, Kim HJ, et al. Mortality and cause of death in hearing loss participants: a longitudinal follow-up study using a national sample cohort. Otol Neurotol. 2020;41(1):25-32. doi: 10.1097/MAO.0000000000002429 [DOI] [PubMed] [Google Scholar]
- 47.Amieva H, Ouvrard C, Meillon C, Rullier L, Dartigues JF. Death, depression, disability, and dementia associated with self-reported hearing problems: a 25-year study. J Gerontol A Biol Sci Med Sci. 2018;73(10):1383-1389. doi: 10.1093/gerona/glx250 [DOI] [PubMed] [Google Scholar]
- 48.Li H, Chen J, Wang X, He M, Zhang Z, Cen Y. Nodal induced by hypoxia exposure contributes to dacarbazine resistance and the maintenance of stemness in melanoma cancer stem–like cells. Oncol Rep. 2018;39(6):2855-2864. doi: 10.3892/or.2018.6387 [DOI] [PubMed] [Google Scholar]
- 49.Hong K. Visual impairment and mortality: systematic review and meta-analysis with data from the EPIC-Norfolk Eye Study [abstract]. Invest Ophthalmol Vis Sci. 2019;60(9):3639. [Google Scholar]
- 50.Kim MB, Zhang Y, Chang Y, et al. Diabetes mellitus and the incidence of hearing loss: a cohort study. Int J Epidemiol. 2017;46(2):717-726. doi: 10.1093/ije/dyw243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin BM, Curhan SG, Wang M, Eavey R, Stankovic KM, Curhan GC. Hypertension, diuretic use, and risk of hearing loss. Am J Med. 2016;129(4):416-422. doi: 10.1016/j.amjmed.2015.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nash SD, Cruickshanks KJ, Zhan W, et al. Long-term assessment of systemic inflammation and the cumulative incidence of age-related hearing impairment in the epidemiology of hearing loss study. J Gerontol A Biol Sci Med Sci. 2014;69(2):207-214. doi: 10.1093/gerona/glt075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bamiou DE. Hearing disorders in stroke. Handb Clin Neurol. 2015;129:633-647. doi: 10.1016/B978-0-444-62630-1.00035-4 [DOI] [PubMed] [Google Scholar]
- 54.Gates GA, Anderson ML, Feeney MP, McCurry SM, Larson EB. Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 2008;134(7):771-777. doi: 10.1001/archotol.134.7.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cruickshanks KJ, Tweed TS, Wiley TL, et al. The 5-year incidence and progression of hearing loss: the epidemiology of hearing loss study. Arch Otolaryngol Head Neck Surg. 2003;129(10):1041-1046. doi: 10.1001/archotol.129.10.1041 [DOI] [PubMed] [Google Scholar]
- 56.Campo P, Morata TC, Hong O. Chemical exposure and hearing loss. Dis Mon. 2013;59(4):119-138. doi: 10.1016/j.disamonth.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Driscoll AK, Bernstein AB. Health and access to care among employed and unemployed adults: United States, 2009-2010. NCHS Data Brief. 2012;(83):1-8. [PubMed] [Google Scholar]
- 58.Ebrahim S, Wannamethee G, McCallum A, Walker M, Shaper AG. Marital status, change in marital status, and mortality in middle-aged British men. Am J Epidemiol. 1995;142(8):834-842. doi: 10.1093/oxfordjournals.aje.a117723 [DOI] [PubMed] [Google Scholar]
- 59.Lawrence BJ, Jayakody DMP, Bennett RJ, Eikelboom RH, Gasson N, Friedland PL. Hearing loss and depression in older adults: a systematic review and meta-analysis. Gerontologist. 2020;60(3):e137-e154. doi: 10.1093/geront/gnz009 [DOI] [PubMed] [Google Scholar]
- 60.Cosh S, Naël V, Carrière I, et al. Bidirectional associations of vision and hearing loss with anxiety: prospective findings from the Three-City Study. Age Ageing. 2018;47(4):582-589. doi: 10.1093/ageing/afy062 [DOI] [PubMed] [Google Scholar]
- 61.Tay T, Wang JJ, Kifley A, Lindley R, Newall P, Mitchell P. Sensory and cognitive association in older persons: findings from an older Australian population. Gerontology. 2006;52(6):386-394. doi: 10.1159/000095129 [DOI] [PubMed] [Google Scholar]
- 62.Shukla A, Harper M, Pedersen E, et al. Hearing loss, loneliness, and social isolation: a systematic review. Otolaryngol Head Neck Surg. 2020;162(5):622-633. doi: 10.1177/0194599820910377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh M, Stewart R, White H. Importance of frailty in patients with cardiovascular disease. Eur Heart J. 2014;35(26):1726-1731. doi: 10.1093/eurheartj/ehu197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi: 10.1017/S0033291718003409 [DOI] [PubMed] [Google Scholar]
- 65.Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35(21):1365-1372. doi: 10.1093/eurheartj/eht462 [DOI] [PubMed] [Google Scholar]
- 66.Celano CM, Daunis DJ, Lokko HN, Campbell KA, Huffman JC. Anxiety disorders and cardiovascular disease. Curr Psychiatry Rep. 2016;18(11):101-101. doi: 10.1007/s11920-016-0739-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dhar AK, Barton DA. Depression and the link with cardiovascular disease. Front Psychiatry. 2016;7:33-33. doi: 10.3389/fpsyt.2016.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ivers RQ, Mitchell P, Cumming RG. Sensory impairment and driving: the Blue Mountains Eye Study. Am J Public Health. 1999;89(1):85-87. doi: 10.2105/AJPH.89.1.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Picard M, Girard SA, Simard M, Larocque R, Leroux T, Turcotte F. Association of work-related accidents with noise exposure in the workplace and noise-induced hearing loss based on the experience of some 240,000 person-years of observation. Accid Anal Prev. 2008;40(5):1644-1652. doi: 10.1016/j.aap.2008.05.013 [DOI] [PubMed] [Google Scholar]
- 70.Meinow B, Kåreholt I, Parker MG, Thorslund M. The effect of the duration of follow-up in mortality analysis: the temporal pattern of different predictors. J Gerontol B Psychol Sci Soc Sci. 2004;59(3):S181-S189. doi: 10.1093/geronb/59.3.S181 [DOI] [PubMed] [Google Scholar]
- 71.Dobie RA. The burdens of age-related and occupational noise-induced hearing loss in the United States. Ear Hear. 2008;29(4):565-577. doi: 10.1097/AUD.0b013e31817349ec [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Bubble plot for random-effects meta-regression of log(HRs) against average follow-up duration (in years) for the longitudinal association of hearing loss with all-cause mortality.
eFigure 2. Bubble plot for random-effects meta-regression of log(HRs) against minimum audiometric thresholds (in decibels, dB) for the longitudinal association of hearing loss with all-cause mortality.
eFigure 3. Contour-enhanced funnel plot for the longitudinal association of hearing loss with all-cause mortality, with missing studies imputed via the trim-and-fill method.
eFigure 4. Leave-out-one influence analysis of the longitudinal association between hearing loss and all-cause mortality.
eFigure 5. Cumulative meta-analysis, by year published, of the longitudinal association between hearing loss and all-cause mortality.
eFigure 6. Contour-enhanced funnel plot for the longitudinal association of dual sensory loss with all-cause mortality, with missing studies imputed via the trim-and-fill method.
eFigure 7. Leave-out-one influence analysis of the longitudinal association between dual sensory loss and all-cause mortality.
eFigure 8. Cumulative meta-analysis, by year published, of the longitudinal association between dual sensory loss and all-cause mortality.
eTable 1. MOOSE Checklist.
eTable 2. Evaluation of risk of bias using the Newcastle-Ottawa Scale (NOS) for cohort studies.
eTable 3. Evaluation of quality of pooled evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.
eTable 4. Random-effects meta-regression of log(HRs) against potential effect moderators (continuous and categorical study-level characteristics) for the longitudinal association of hearing loss with all-cause mortality.
eTable 5. Random-effects meta-regression of log(HRs) against potential effect moderators (continuous and categorical study-level characteristics) for the longitudinal association of dual sensory loss with all-cause mortality.
eTable 6. Meta-analyses in subgroups, stratified by categorical study-level characteristics for the longitudinal associations of hearing loss or dual sensory loss each with all-cause mortality.