Abstract
Objectives
There is an urgent need to develop therapeutic strategies to improve the treatment outcome of Alzheimer’s disease. The treatment strategy of gene therapy mediated by nanocarrier systems brings new hope for the treatment of Alzheimer’s disease. ROCK2 is involved in various pathological processes of Alzheimer’s disease and may be a potential target for the treatment of Alzheimer’s disease. Our previous study indicated that PEG-PEI/siROCK2 [polyethyleneglycol-polyethyleneimine deliver ROCK2-siRNA, (PPSR)] prevented Aβ42-induced neurotoxicity and showed a promising prospect for the treatment of Alzheimer’s disease. However, whether PPSR has an effect on the microglial inflammation in Alzheimer’s disease is still unclear.
Materials and methods
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay was used to detect the cytotoxicity of PEG-PEI and PPSR in primary microglial cells. Real-time PCR and western blotting were used to assess the expression of ROCK2 and nucleotide oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3)/caspase 1 pathway in primary microglial cells. ELISA assay was used to measure the effect of PPSR on attenuating the lipopolysaccharide (LPS) + Aβ-induced increase in IL-1β.
Results
PEG-PEI concentration less than 20 μg/ml and the N/P (molar ratio of PEG-PEI amino/siRNA phosphate) ratio of PPSR less than 50 showed no significant cytotoxicity in primary microglia cells. PPSR could effectively inhibit the expression of ROCK2 in primary microglial cells. A further study revealed that PPSR attenuates the LPS+Aβ-induced increase in IL-1β without affecting cell viability. In addition, we found that PPSR suppressed the Aβ-induced NLRP3/caspase 1 pathway in primary microglial cells.
Conclusion
PPSR inhibits Aβ42-induced microglial inflammation via NLRP3/caspase 1 pathway.
Keywords: alzheimer disease, microglia, NLRP3, polyethylene glycol-polyethyleneimine, Rho-associated kinase 2
Introduction
Alzheimer’s disease is the most frequent form of dementia in the elderly and is defined by the combined presence of amyloid β-protein (Aβ) and tau protein [1,2]. The number and proportion of those who are suffering from Alzheimer’s disease continue to increase worldwide. By 2050, Alzheimer’s disease is expected to affect 13.8 million adults [3]. However, at present, there is no successful therapy to stop or slow the progression of Alzheimer’s disease. Thus much research has focused on the mechanisms and treatment strategies underlying Alzheimer’s disease.
Inflammation plays a pivotal role in the pathogenesis of Alzheimer’s disease [4]. Excessive neuroimmune inflammation damages neurons, which release a variety of toxic contents such as Aβ42. A disturbed balance between the production and degradation of Aβ can drive inflammatory processes in astrocytes and microglial cells and initiate a vicious circle [5], eventually leading to neuronal death and brain tissue atrophy. Therefore, the inflammation of microglia may be a promising treatment target for Alzheimer’s disease.
Gene therapy has shown significant potential for the treatment of Alzheimer’s disease in preclinical trials [6]. However, the challenge of gene therapy is well-tolerated and effective delivery vectors. Nanocarriers are promising gene therapy carriers for central nervous system diseases due to their biosafety and ability to cross the blood-brain barrier [7]. Polyethylenimine (PEI) is one of the most successful polycationic carriers and when engrafted by hydrophilic PEG has enhanced biocompatibility and efficiency when used for nonviral gene delivery [8]. At present, polyethyleneglycol-polyethyleneimine (PEG-PEI) vectors have been applied to Alzheimer’s disease in our early experiments and achieved good results [9].
Rho-kinase, also known as ROCK, is widely recognized as a member of the serine/threonine-protein kinase family, which plays crucial roles in various cellular functions such as cell migration, proliferation and survival [10]. Aberrant activation of the Rho/ROCK pathway has already been noticed in multiple disorders of the central nervous system, including Alzheimer’s disease [11]. Our previous studies have found that ROCK2 was a vital target gene for the therapy of Alzheimer’s disease. In vitro, our studies showed that PEG-PEI/siROCK2 [polyethyleneglycol-polyethyleneimine deliver ROCK2-siRNA, (PPSR)] prevented Aβ42-induced neurotoxicity [9]. In vivo, we found that intracranial injection of PPSR improved the cognitive impairments of senescence-accelerated mouse (SAM) [12]. Therefore, ROCK2 may be a crucial target gene for the therapy of Alzheimer’s disease. But whether there is a relationship between ROCK2 and microglial inflammation in Alzheimer’s disease is still unclear.
With these backgrounds, we synthesized PPSR to inhibit the expression of ROCK2 in primary microglial cells. Our study aims to evaluate the biologic properties and molecular mechanism of PPSR on Aβ42-induced microglial inflammation. The results showed that PPSR inhibited Aβ42-induced microglial inflammation via the nucleotide oligomerization domain (NOD)-like receptor family pyrin domain containing 3 (NLRP3)/caspase 1 pathway, which provides evidence to support the therapeutic potential of PPSR in Alzheimer’s disease.
Materials and methods
Cell culture and treatment
Primary microglial cells were cultured according to our previously published protocol [13]. Briefly, P1 C57BL/6J mice were stripped of meninges and minced in a hepes-balanced salt solution (Mediatech Inc., Herndon, Virginia, USA). All animals used for cell isolation were treated according to the legal and ethical requirements of the University of Sun Yat-sen University (Approval Number: IACUC-2021020801). Cells were dissociated and then cultured in F12/Dulbecco’s Modified Eagle Media (DMEM) medium (Gibco, Invitrogen, Carlsbad, California, USA) containing 10% heat-inactivated fetal bovine serum (Gibco, Invitrogen) at 104 cells/cm2. The cell culture medium was replaced with fresh complete media every 2 days. Microglial cells were harvested by tapping the flasks and collecting the floating cells on day 12. Microglia were pelleted by centrifugation and resuspended in F12/DMEM with 10% fetal bovine serum and maintained at 37°C under 5% CO2. The next day, microglia were incubated with PPSR, PEG-PEI, siROCK2 or PBS for 24 h. The microglia were then primed with lipopolysaccharide (LPS) (50 ng/mL) for 3 h and treated with 2.5 μM Aβ42 for 6 h in a serum-free DMEM containing 1% P/S.
Preparation of Aβ fibrils
Aβ42 oligomers were prepared as previously described [14]. Briefly, Aβ1-42 (Bachem, H-1368) dissolved in dimethyl sulfoxide (DMSO) with a final concentration of 500 μM and stored at −80 °C. To prepare Aβ fibrils, Aβ1-42 was diluted to a concentration of 50 μM by adding DMEM-F12 with 10% FBS for 24 h at 37 °C. After preparation of Aβ fibrils, they were diluted with cell culture medium to 2.5 μM. To confirm the fibrillization of Aβ, thioflavin S (Sigma, T1892) was added to the solution and then Aβ can be identified by an apple green color under a fluorescence microscope (Leica DM IRB, Wetzlar, Germany). To analyze the images, a spectrofluorometer VICTOR X4 (PerkinElmer, Massachusetts, USA) was used at an excitation of 405 nm and an emission of 535 nm.
Preparation of polyethyleneglycol-polyethyleneimine deliver ROCK2-siRNA complexes
PEI (MW = 25 kD), monomethoxy PEG (mPEG-OH, MW = 2 kD), N-hydroxysuccinimide (NHS) and dicyclohexylcarbodiimide (DCC) were purchased from Sigma-Aldrich (St Louis, Missouri, USA). PEG-PEI was synthesized internally using techniques previously reported in our laboratory [8]. The siROCK2 that targeted ROCK2 mRNA in mice was purchased from GenePharma (Shanghai, China). PPSR complexes were synthesized and characterized as described previously by our laboratory [8]. The amount of delivery agent (PEG-PEI) to complex the small interfering RNA (siROCK2) was based on various N/P (molar ratio of PEG-PEI amino/siRNA phosphate) ratios. The PPSR solution was vortexed for a short time and further incubated for 30 min at room temperature before use.
In-vitro transfection
For transfection experiments, the primary microglial cells were seeded in a 24-well plate at a density of 5 × 104 cells/well at 37 °C in a humidified atmosphere of 95% air and 5% CO2. The original medium in each well was replaced with optiMEM (Invitrogen). PPSR complexes with N/P ratios of 50 were added to the cells and incubated for 6 h at 37 °C. After that, the medium was replaced with the same volume of fresh complete medium, for another 18-h incubation.
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay
The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) cell metabolism assay was used to evaluate the cytotoxicity of PPSR complexes. Briefly, the primary microglial cells were seeded in a 96-well plate and treated according to the set conditions. After that, the cells were incubated with MTT reagent (0.5 mg/ml) for 4 h at 37 °C. Then, the MTT solution was removed and 100 μL of DMSO was added to dissolve the resulting formazan crystals. The optical density (OD) was measured using a Synergy plate reader (Bio-TEK instruments, Winooski, Vermont, USA) at 570 nm with a reference wavelength of 630 nm. The relative cell viability rates was calculated as follows: Cell viability (%) = (ODsample − ODblank)/(ODcontrol − ODblank) × 100%.
ELISA
NLRP3 inflammasome activation by Aβ was assessed by measuring IL-1β and tumor necrosis factor α (TNFα) secretion. The supernatants were centrifuged and harvested for ELISAs. Levels of IL-1β (R&D Systems, MLB00C) and TNFα (BMS2034 from Invitrogen) in cell-cultured supernatants samples were measured using ELISA kits according to the manufacturers’ instructions. The OD was analyzed at 450 nm photometrically with a microplate reader (Infinite M200; Tecan). The concentration of IL-1β and TNFα was quantified using the relevant standard curves.
RNA extraction and quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) and cDNA was synthesized according to the manufacturer’s instructions using the PrimeScript RT Reagent Kit (Takara). The quantitative real-time PCR (qRT-PCR) reaction was performed by using the all in-One q-PCR Mix Kit (GeneCopoeia, AOPR-0200) in the LightCycler 480II PCR System (LightCycler 480II, Roche, USA). Differences between samples and controls in gene expression were quantified using the 2−ΔΔCt method. Primers were provided as follows: ROCK2 forward, GCGATGCTGAGCCTGATGA and reverse, GCACAGGCAATGACAA -CCAT. β-actin forward, GTGACGTTGACATCCGTAAAGA and reverse GCCGGACTCATCGTACTCC.
Western blotting
The total amount of protein was extracted using cytosolic/nuclear protein lysis buffer (Beyotime, P0027). Protein concentrations were measured using the bicinchoninic acid assay kit (ThermoFisher Pierce) according to the manufacturer’s instructions. For western blotting, protein lysates were separated by SDS-PAGE and transferred to polyvinyl difluoride membrane. The membranes were incubated with primary antibodies against ROCK2 (Cell Signaling Technology, 1:1000,), NLRP3 (Cell Signaling Technology, 1:500), caspase-1 (Santa-Cruz Biotechnology, 1:50), IL-1β (Santa-Cruz Biotechnology, 1:50), β-actin (Cell Signaling Technology, 1:1000) and glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling Technology, 1:1000) at 4 °C overnight, followed by incubation with anti-rabbit IgG (MultiSciences, 1:5000) or anti-mouse IgG (MultiSciences, 1:5000) at room temperature for 1 h. Protein bands were determined using an enhanced chemiluminescence western blot detection kit (KeyGEN BioTECH, KGP1126).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 20.0 (IBM, Armonk, New York, USA). All experiments were performed in triplicate and each assay was repeated at least three times independently. Data are presented as mean ± SD and P values <0.05 are considered statistically significant. Comparisons between two groups were analyzed with Student’s t-test. Multiple comparisons of more than two groups were conducted with one-way analysis of variance followed by Bonferroni’s post hoc test.
Results
Neurotoxicity of Polyethyleneglycol-polyethyleneimine and Polyethyleneglycol-polyethyleneimine deliver ROCK2-siRNA-induced in primary microglial cells
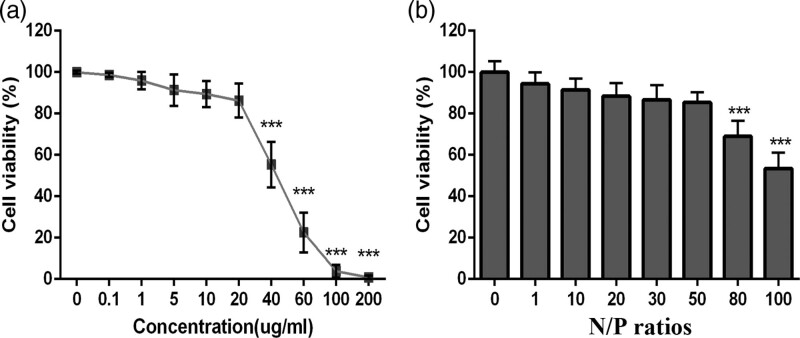
To verify the biosafety of PEG-PEI and PPSR, we first tested their cytotoxicity in primary microglial cells. As shown in Fig. 1a, the cytotoxicity of PEG-PEI increased with the increase of concentration. When PEG-PEI concentration exceeded 20 μg/mL, the cell viability decreased significantly(P < 0.001, Figure. 1a). The cytotoxicity of PPSR complexes correspondingly increased with the increase of the N/P ratio. When the N/P ratio reached 50, the cell viability was 85.3% (Fig. 1b). Cell viability was remarkably decreased when the N/P ratio exceeded 50 (P < 0.001, Fig. 1b). These results indicated that a PEG-PEI concentration less than 20 μg/mL and N/P ratio of PPSR less than 50 showed good biosafety in primary microglia cells.
Fig. 1.
In vitro cytotoxicity of PEG-PEI and PPSR in primary microglial cells determined by an MTT assay. (a) Primary microglial cells treated with PEG-PEI at various concentrations from 0 to 200 μg/ml. (b) Primary microglial cells treated with PPSR at various N/P ratios from 0 to 100. The dose of PPSR was 100 nM in each well and incubation time was 24 h. Values are mean ± SD (n = 3). ***P < 0.001 vs. control. PPSR, polyethyleneglycol-polyethyleneimine deliver ROCK2-siRNA;.
Gene-silencing effect of polyethyleneglycol-polyethyleneimine deliver ROCK2-siRNA in Primary microglial cells
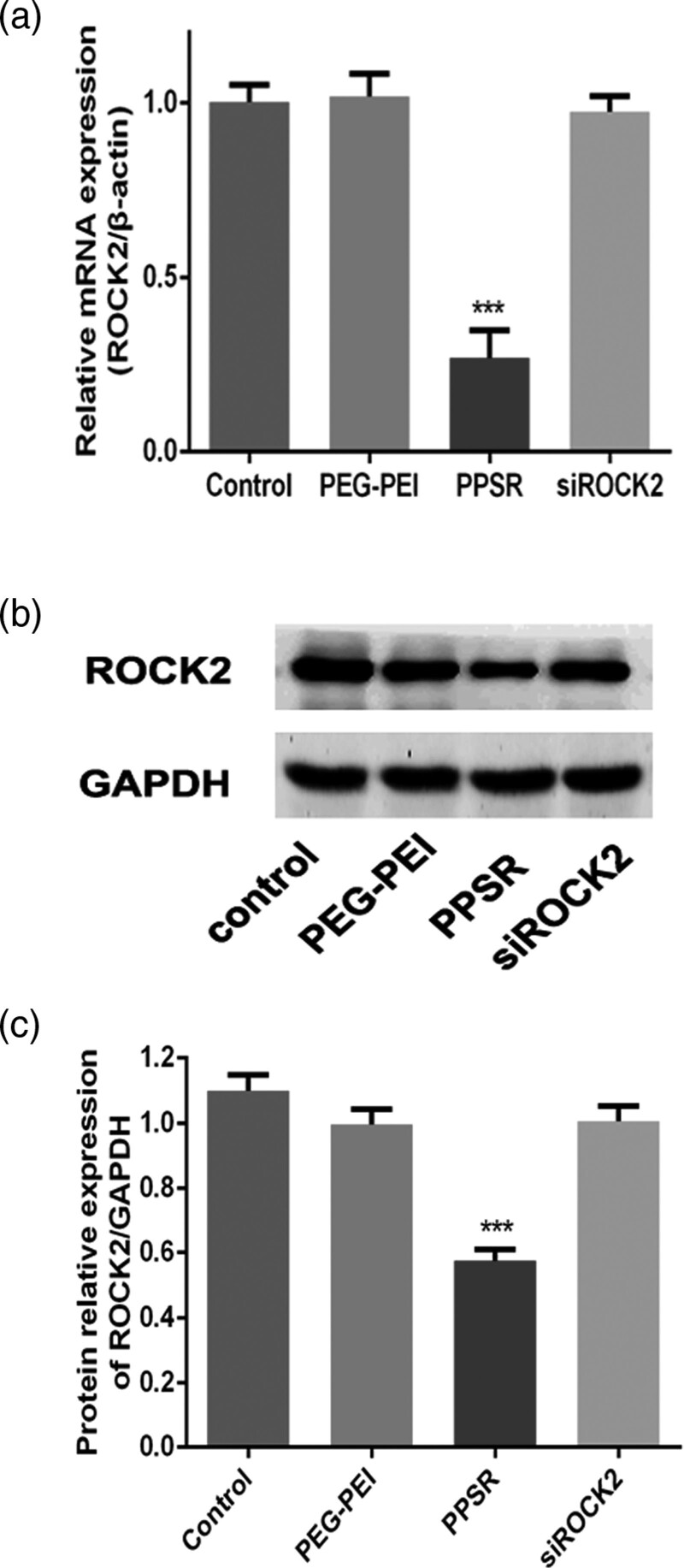
The Gene-silencing effect of PPSR in primary microglial cells was evaluated at both the mRNA and protein levels of ROCK2. As shown in Fig. 2a, PPSR complexes significantly inhibited ROCK2 mRNA expression compared with the control group (P < 0.001, Fig. 2a). The protein expression of ROCK2 level was significantly decreased in the PPSR group compared with the control group (P < 0.001, Fig. 2b and c). These results indicated that PPSR effectively inhibits the expression of ROCK2 in primary microglial cells.
Fig. 2.
Gene-silencing effect of PPSR in primary microglial cells. (a) The gene-silencing effect of PPSR (100 nM) in primary microglial cells was detected by RT-PCR. (b) The gene-silencing effect of PPSR (100 nM) in primary microglial cells was detected by western blotting. (c) The relative protein expression levels of ROCK2 in each group. The control was treated with PBS. Values are mean ± SD, ***P < 0.001, compared with the control. PPSR, polyethyleneglycol-polyethyleneimine deliver ROCK2-siRNA; RT-PCR, reverse transcription-PCR
Polyethyleneglycol-polyethyleneimine deliver ROCK2-siRNA attenuates the lipopolysaccharide+Aβ-induced increase of IL-1β in primary microglial cells without affecting cell viability
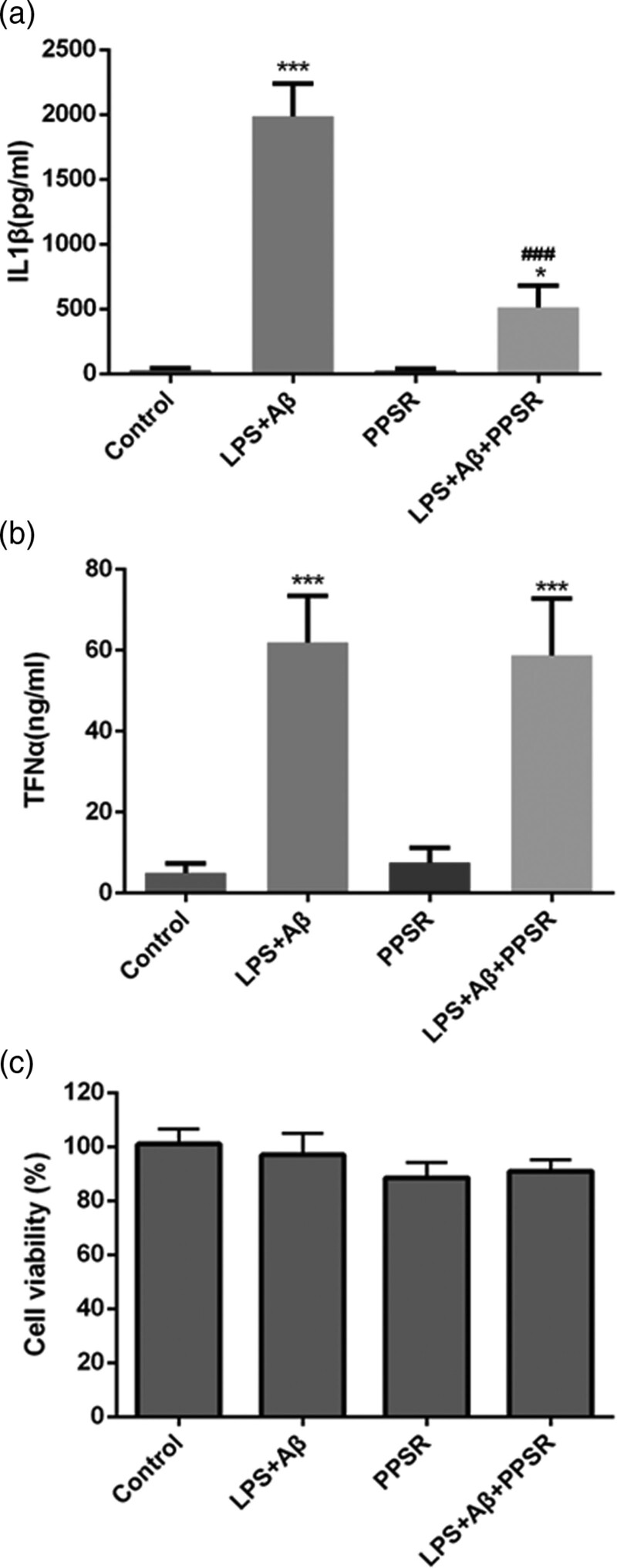
To investigate the effect of PPSR on Aβ-induced secretion of inflammatory cytokines in primary microglial cells, the levels of IL-1β and TNFα were measured in the supernatant fractions of the microglia using ELISA. The results revealed that LPS+Aβ (50 ng/ml LPS plus 2.5 μM Aβ42) significantly increased the expression of IL-1β and TNFα (P < 0.001, Fig. 3a and b). The level of IL-1β was significantly reduced in the LPS+Aβ+PPSR group compared with the LPS+Aβ group (P < 0.001, Fig. 3a and b). However, the level of TNFα showed no significant difference between the LPS+Aβ+PPSR group and the LPS+Aβ group (P > 0.05, Fig. 3a and b). These results indicated that PPSR attenuates the LPS+Aβ-induced increase of IL-1β in primary microglial cells. To test whether the treatment of the four groups affected the viability of primary microglial cells, the MTT assay was performed. There was no significant difference in cell viability among the groups (P > 0.05, Fig. 3c). Together, these results indicated that PPSR attenuates the LPS+Aβ-induced increase of IL-1β in primary microglial cells without affecting cell viability.
Fig. 3.
PPSR attenuates the LPS + Aβ-induced increase in IL-1β without affecting cell viability. (a) The IL-1β level was measured in the supernatants of the microglia 24 h later using ELISA. (b) The levels of TNFα were measured in the supernatant fractions of the microglia 24 h later using ELISA. Primary microglial cells were transfected with the indicated PPSR and preactivated with LPS (50 ng/mL) for 3 h before the Aβ fibrils (2.5 μM) were added. (c) In-vitro cytotoxicity of four groups in primary microglial cells was determined by an MTT assay. The control was treated with PBS. Values are mean ± SD, ***P < 0.001, compared with the control. PPSR, polyethyleneglycol-polyethyleneimine deliver ROCK2-siRNA; IL, interleukin; LPS, lipopolysaccharide; TNFα, tumor necrosis factor α
Polyethyleneglycol-polyethyleneimine deliver ROCK2-siRNA suppressed the Aβ-induced NLRP3/caspase 1 pathway in primary microglial cells
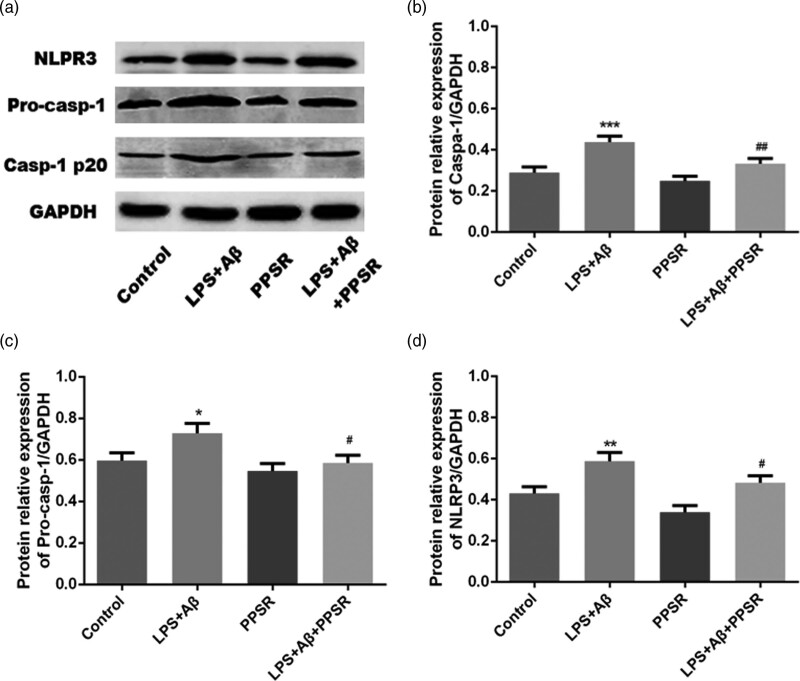
To explore the effect of PPSR on IL-1β pathway activation, the expression of NLRP3, pro-caspase-1 and caspase-1 in each group of primary microglial cells was measured by western blotting. The protein expression levels of NLRP3, pro-caspase-1 and caspase-1 were significantly increased in the LPS + Aβ group compared with the control group (P < 0.01, Fig. 4a–d). These results indicated that PPSR suppresses the Aβ-induced NLRP3/caspase 1 pathway in primary microglial cells.
Fig. 4.
PPSR suppressed Aβ-induced NLRP3/caspase 1 pathway response Primary microglial cells. (a) Western blot analysis of inflammasome complex (NLRP3, pro-CASP1 and CASP1 p20). (b–d) The relative protein expression levels of NLRP3, pro-CASP1 and CASP1 p20 in each group. The control was treated with PBS. Values are mean ± SD, ***P < 0.001, compared with the control. PPSR, polyethyleneglycol-polyethyleneimine deliver ROCK2-siRNA; LPS, lipopolysaccharide; NLRP3, NOD-like receptor family pyrin domain containing 3; NOD, nucleotide oligomerization domain.
Discussion and conclusion
Since its discovery in 1996, ROCK has been shown to contribute to several physiological processes, especially in neurite growth and sprouting [15]. Inhibition of the Rho/ROCK pathway has proven efficacious in a transgenic mouse model of Alzheimer’s disease [16]. We have also demonstrated that the inactivation of the Rho/ROCK signaling pathway can alleviate the impairment of Alzheimer’s disease [12]. To study the relationship between ROCK2 and microglial inflammation in Alzheimer’s disease, we successfully synthesized the PPSR compound and confirmed that PPSR alleviated Aβ42-induced microglial inflammation by targeting the NLRP3/caspase 1 pathway.
PEG-PEI copolymer has potential medical applications in drug and gene delivery [17,18]. Compared with viral vectors, polymer materials have advantages of nonimmunogenicity and high gene transfer efficiency. Based on previous experiments, we successfully synthesized PEG-PEI and PPSR. But there are still some concerns about the biosafety of the PEG-PEI copolymer, which are related to its concentration and N/P ratios. Our study showed that the toxicity increased with the concentration of PEG-PEI. These findings are consistent with other experimental studies showing that as the polymer concentration increased, the cytotoxicity gradually increased [19]. Our study also confirmed an increase in toxicity of PPSR with increasing N/P ratios, which is in accordance with other nanomaterials [20]. Increased N/P results in better transfection efficiency but with more potent cytotoxicity. Thus, to obtain lower cytotoxicity and higher transfection efficiency, PEG-PEI concentration less than 20 μg/mL and the N/P ratio of PPSR less than 50 were selected to treat primary microglial cells in vitro. Our experiment finally confirmed that the PPSR at N/P of 50 effectively inhibited the expression of ROCK2, which was consistent with our previous study [8].
Previous studies have confirmed that the excessive activation of microglia resulted in significant changes in its structure and function, releasing massive neuroimmune inflammatory factors such as TNF-α and IL-1β [21]. Since Aβ42 oligomers cannot directly lead to the production of TNF-α and IL-1β in microglia, while LPS combined with Aβ42 oligomers can significantly increase the secretion of TNF-α and IL-1β [22], we used LPS+Aβ oligomers to simulate microglia cells as the cell model of Alzheimer’s disease. Our results demonstrated that LPS+Aβ pretreatment could aggravate the inflammatory response in primary microglial cells, causing increased release of inflammatory factors such as IL-1β and TNFα, which were consistent with previous studies [23]. Our results also revealed that PPSR could reduce the LPS+Aβ-induced increase of IL-1β in primary microglial cells, but did not affect the increased production of TNFα. These findings suggested that ROCK2 does not completely ameliorate the microglial cell inflammation induced by LPS+Aβ, but specifically inhibits the IL-1β inflammatory pathway. However, the precise mechanism underlying the role of ROCK2 and IL-1β in primary microglial cells remains unclear.
The inflammasome, a multiprotein complex, specifically cleaves proinflammatory cytokine precursors into activated proinflammatory cytokines that mature and are secreted, leading to widespread inflammation in the brain [24]. Recently, attention has turned to the NLRP3 inflammasome. The core of the NLRP3 inflammasome is the NOD receptor family of NLRP3 proteins. Therapies targeted specifically to the NLRP3 inflammasome could relieve the dysfunctions following Alzheimer’s disease [25]. Aβ42 is reported to activate the NLRP3 inflammasome in microglial cells, leading to the production of proinflammatory cytokines and inflammation [26], which is consistent with our findings. Researchers proved that inhibiting the NLRP3 inflammasome promotes clearance of amyloid-β and cognitive function in APP/PS1 mice [27]. Therefore, we wanted to explore whether PPSR affects IL-1β through the NLRP3 pathway.
At present, there are few studies on the relationship between ROCK and NRLP3. Previous studies revealed that the inhibition of ROCK2 significantly reduced the expression of NLRP3 and therefore suppressed the inflammatory response induced by NLRP3 [28]. Our results showed that inhibiting ROCK2 significantly reduced the expression of NLRP3, which was consistent with the previous reports. The increase and activation of NLRP3 mediate the activation of the NLRP3/caspase1 pathway and the release of IL-18/IL-1β [29]. Aβ deposition leads to an increase in IL-1β, which is produced in a biologically inactive form and requires caspase-1 activation and secretion [30]. Therefore, according to the above studies, we speculated that PPSR could inhibit IL-1β by suppressing the expression of NLRP3.
In summary, our study demonstrated that PPSR inhibits Aβ42-induced microglial inflammation via NLRP3/caspase1 pathway. But, how PPSR regulates the NLRP3/caspase1 pathway and its specific mechanism are still unclear, which need further study. Overall, this study revealed a new mechanism of Alzheimer’s disease, indicating that PPSR is a potent therapeutic strategy for Alzheimer’s disease.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [81801046 to YYL], the Natural Science Foundation of Guangdong, China [2018A030313085 to YYL, 2020A1515010111 to ZL, 2018A030313427 to LMW], and the Science and Technology Planning Project of Guangzhou City (202002030393 to LMW).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Dr. Yunyun Liu, Dr. Han Zhang and Dr. Anping Peng contributed equally to the writing of this article.
Limin Wang is the co-corresponding author.
Reference
- 1.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer’s disease. Lancet 2016; 388:505–517. [DOI] [PubMed] [Google Scholar]
- 2.Barnett R. Alzheimer’s disease. Lancet 2019; 393:1589. [DOI] [PubMed] [Google Scholar]
- 3.Taylor CA, Greenlund SF, McGuire LC, Lu H, Croft JB. Deaths from Alzheimer’s Disease - United States, 1999-2014. MMWR Morb Mortal Wkly Rep 2017; 66:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira ST, Clarke JR, Bomfim TR, De Felice FG. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement 2014; 10:S76–S83. [DOI] [PubMed] [Google Scholar]
- 5.Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: the role of microglia and astrocytes. Aging Cell 2004; 3:169–176. [DOI] [PubMed] [Google Scholar]
- 6.Tedeschi DV, da Cunha AF, Cominetti MR, Pedroso RV. Efficacy of gene therapy to restore cognition in Alzheimer’s disease: a systematic review. Curr Gene Ther 2021; 21:246–257. [DOI] [PubMed] [Google Scholar]
- 7.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet 2014; 15:541–555. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Liu Z, Wang Y, Liang YR, Wen X, Hu J, et al. Investigation of the performance of PEG-PEI/ROCK-II-siRNA complexes for Alzheimer’s disease in vitro. Brain Res 2013; 1490:43–51. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Yang X, Lei Q, Li Z, Hu J, Wen X, et al. PEG-PEI/siROCK2 protects against Aβ42-induced neurotoxicity in primary neuron cells for Alzheimer disease. Cell Mol Neurobiol 2015; 35:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov 2005; 4:387–398. [DOI] [PubMed] [Google Scholar]
- 11.Burton A. NSAIDS and Alzheimer’s disease: it’s only Rock and Rho. Lancet Neurol 2004; 3:6. [DOI] [PubMed] [Google Scholar]
- 12.Wen X, Wang L, Liu Z, Liu Y, Hu J. Intracranial injection of PEG-PEI/ROCK II-siRNA improves cognitive impairment in a mouse model of Alzheimer’s disease. Int J Neurosci 2014; 124:697–703. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Zhang Y, Peng J, Wang H, Li X, Li X, et al. Autophagy alleviates ethanol-induced memory impairment in association with anti-apoptotic and anti-inflammatory pathways. Brain Behav Immun 2019; 82:63–75. [DOI] [PubMed] [Google Scholar]
- 14.Cho MH, Cho K, Kang HJ, Jeon EY, Kim HS, Kwon HJ, et al. Autophagy in microglia degrades extracellular β-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy 2014; 10:1761–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salminen A, Suuronen T, Kaarniranta K. ROCK, PAK, and Toll of synapses in Alzheimer’s disease. Biochem Biophys Res Commun 2008; 371:587–590. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, et al. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science 2003; 302:1215–1217. [DOI] [PubMed] [Google Scholar]
- 17.Tian HY, Deng C, Lin H, Sun J, Deng M, Chen X, Jing X. Biodegradable cationic PEG-PEI-PBLG hyperbranched block copolymer: synthesis and micelle characterization. Biomaterials 2005; 26:4209–4217. [DOI] [PubMed] [Google Scholar]
- 18.Yang S, Wang Z, Ping Y, Miao Y, Xiao Y, Qu L, et al. PEG/PEI-functionalized single-walled carbon nanotubes as delivery carriers for doxorubicin: synthesis, characterization, and in vitro evaluation. Beilstein J Nanotechnol 2020; 11:1728–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo X, Feng M, Pan S, Wen Y, Zhang W, Wu C. Charge shielding effects on gene delivery of polyethylenimine/DNA complexes: PEGylation and phospholipid coating. J Mater Sci Mater Med 2012; 23:1685–1695. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Shu S, Cheung GS, Wei X. Effect of miR-146a/bFGF/PEG-PEI nanoparticles on inflammation response and tissue regeneration of human dental pulp cells. Biomed Res Int 2016; 2016:3892685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spangenberg EE, Green KN. Inflammation in Alzheimer’s disease: lessons learned from microglia-depletion models. Brain Behav Immun 2017; 61:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luciunaite A, McManus RM, Jankunec M, Racz I, Dansokho C, Dalgediene I, et al. Soluble abeta oligomers and protofibrils induce NLRP3 inflammasome activation in microglia. J Neurochem 2020; 155:650–661. [DOI] [PubMed] [Google Scholar]
- 23.Fu W, Vukojevic V, Patel A, Soudy R, MacTavish D, Westaway D, et al. Role of microglial amylin receptors in mediating beta amyloid (Aβ)-induced inflammation. J Neuroinflammation 2017; 14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nizami S, Hall-Roberts H, Warrier S, Cowley SA, Di Daniel E. Microglial inflammation and phagocytosis in Alzheimer’s disease: potential therapeutic targets. Br J Pharmacol 2019; 176:3515–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng YS, Tan ZX, Wu LY, Dong F, Zhang F. The involvement of NLRP3 inflammasome in the treatment of Alzheimer’s disease. Ageing Res Rev 2020; 64:101192. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi A, Kaneko N, Takeda H, Sawasaki T, Morikawa S, Zhou W, et al. Amyloid β directly interacts with NLRP3 to initiate inflammasome activation: identification of an intrinsic NLRP3 ligand in a cell-free system. Inflamm Regen 2018; 38:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav Immun 2017; 61:306–316. [DOI] [PubMed] [Google Scholar]
- 28.Gao Z, Li Q, Zhang Y, Gao X, Li H, Yuan Z. Ripasudil alleviated the inflammation of RPE cells by targeting the miR-136-5p/ROCK/NLRP3 pathway. BMC Ophthalmol 2020; 20:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015; 21:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013; 493:674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]