Summary
Aim
Merkel cell carcinoma (MCC) is an aggressive cutaneous neuroendocrine carcinoma, with an increasing worldwide incidence. It presents as a painless red to purple nodule on sun-exposed skin. MCC is presumed to arise from resident cutaneous Merkel cells. The pathogenesis of MCC is likely multifactorial with immunosuppression, UV-induced skin damage, and Merkel cell polyomavirus contributing to the development. The diagnosis of MCC relies upon characteristic morphologic features and use of immunohistochemical stains. Histologically, the differential diagnosis of Merkel cell carcinoma includes the ‘small round cell’ tumor group, particularly metastatic small cell carcinoma and hematological malignancies. This study investigates the expression of NeuN antibody, which recognizes the protein NeuN, normally present in most neuronal cell types and neuronal tumors, in Merkel cell carcinomas.
Methods and results
Fifteen cases of Merkel cell carcinoma (7 men and 7 women; mean age 74 years) were retrieved from the institute database between the years 2011-2020. The immunohistochemical profile was investigated: CK20 (14/14), Neurofilament, (12/12), Synaptophysin (14/14); Chromogranin A (11/13), PAX5 (10/12), TDT (5/12), CK7 (1/14), TTF1 (0/14). Infection by Polyoma virus was detected in 11 of 14 patients. Most tumors showed middle/strong expression of NeuN. No cutaneous structures, or epidermal Merkel cells, showed expression of NeuN. The expression of NeuN was investigated in 17 primary small cell lung carcinomas: 2 cases were positive for Neu-N.
Conclusions
Awareness of the staining pattern of Neu-N could aid in diagnosis of Merkel cell carcinoma, avoiding misinterpretation and erroneous diagnosis with other tumors.
Key words: Merkel cell carcinoma, neuroendocrine carcinoma, NeuN, Merkel cell polyomavirus, small cell lung carcinoma
Introduction
In 1972, Toker 1 described rare primary cutaneous neuroendocrine carcinoma, with cytoplasmatic neurosecretory granules, resembling those in Merkel cell mechanoreceptor cells 2. Merkel cell carcinoma (MCC) is an aggressive tumor: the 10-year survival rates are 71%, 48%, and 20% for localized, regional, and distant disease, respectively 3. It has a predilection for sun-exposed skin of the head and neck (50%) and extremities (40%); primary tumors of the trunk are uncommon (10%) 4-6. The clinical presentation usually appears as an erythematous or purplish nodule 4. MCC accounts for less than 1% of all cutaneous malignancies, and is increasing over the last few decades 7. White people are affected 25-fold more than other ethnic groups 8. At diagnosis, the median age is 76 years 6. Patients with immunosuppression and solid organ transplant show an increased risk of MCC development 4,6,9. Large local surgical excision, with 1-2 cm margins and sentinel lymph node biopsy is indicated. MCC may arise from two independent mechanisms: Merkel cell polyomavirus (MCPyV) or ultraviolet-induced mutagenesis 10-13. MCC can therefore be distinguished into two molecular subclasses with diagnostic and prognostic significance 14: virus-negative MCC (VN-MCC) and virus-positive MCC (VP-MCC). MCC is composed of monotonous, blastic-appearing cells, with a salt-and-pepper chromatin and high nuclear to cytoplasmic ratio. It is centered on the dermis and the growth pattern can be nodular, infiltrative, or a mixture. By immunohistochemistry, MCC stains with a broad-spectrum cytokeratin (CAM5.2, AE1/AE3, and cytokeratin 903) with almost 100% sensitivity. The classic marker of MCC is cytokeratin 20 (CK20), especially with dot-like reactivity. CK20 is highly sensitive (about 95% of MCC) and relatively specific 15. Chromogranin A, synaptophysin, CD56 (neural cell adhesion molecule), and neurofilament are expressed in most tumors. Thyroid transcription factor 1 (TTF-1) is typically not expressed by MCC.
NeuN antibody (NEUronal Nuclei) specifically recognizes the DNA-binding neuron-specific protein NeuN, which is present in most central nervous system and peripheral nervous system neuronal cell types and tumors. NeuN protein distributions are apparently restricted to neuronal nuclei, perikaryal and some proximal neuronal processes in both fetal and adult brain.
In this study, we examined Neu-N immunoreactivity in 15 MCCs (14 patients) and compared NeuN staining with frequently used immunohistochemical markers utilized in the diagnosis of MCC. Nuclear staining intensity and extent were semi-quantitatively analyzed. Moreover, we explored Neu-N immunoreactivity in 17 primary neuroendocrine small cell lung carcinomas.
Materials and methods
Formalin-fixed paraffin-embedded tissue samples of 15 MCC were retrospectively selected from the cases diagnosed in Departments of Pathology, between the years 2011-2020. Clinical data including the tumor site and size were collected from the medical documents. Immunohistochemistry was performed on whole-section and semi-quantitatively graded as extent: 0 (< 5%), 1 + (5-25%), 2 + (25-50%), and 3 + (> 50). Fixation tissue is carried out in 10% neutral buffered formalin for 24 hours. Once tissue is embedded in paraffin, a 3-4 micron tissue section is cut onto charged glass slides. The detection system for immunostaining is BOND Polymer Refine Detection on staining platform LEICA BOND III for these antibodies: Cytokeratin 7 (clone OV-TL 12/30; Menarini; 1:500), 10 min at 37°C with Bond Epitope Retrieval Enzyme for antibodies; synaptophysin (clone 27G12; Leila; 1:200); chromogranin A (clone DAK-A3; Dako; 1:100); Cytokeratin 20 (clone KS20.8; Menarini; 1:500); PAX5 (polyclonal; Biocare; 1:50); TDT (E9266; Leica; 1:100); TTF1 (8G7G3/1; Menarini; 1:100); neurofilament (2F11; Menarini; 1:500); NeuN (A60; Millipore; 1:500); Merkel Cell Polyomavirus (CM2B4; Santa Cruz; 1:100) (test performed in Laboratory of ASST Brescia).
Results
At review, the diagnosis of MMC was confirmed for all cases. Clinical and histopathological data of the cases are summarized in Table I. There were 7 men and 7 women with age range from 52-95 years; mean age was 74 years. In our series, 71% of the tumors were present in the extremities, 21% in the head and neck region and 8% in the trunk. A female of 72 years old had MCC in two different sites, right knee (case 13a) and left elbow (case 13b). Largest diameter of the tumor was ≤ 2 cm in 5 cases (pT1 in AJCC 8th Ed.), and > 2 cm but ≤ 5 cm in 5 cases (pT2 in AJCC 8th Ed.); in 5 cases it was not available. Ten patients had lymph node removal, with lymph node metastasis in 7 patients. Case 6 had metastasis to both the breasts.
Table I.
Clinicopathological characteristics.
| Case No. | Sex/Age | Location | pT AJCC 8th Ed. | Lymph node removal | Lymph node location | Lymph node metastases | Number of metastatic lymph nodes | Number of isolated lymph nodes |
|---|---|---|---|---|---|---|---|---|
| Case 1 | F/73 | left knee | pT2 | Yes | left groin | Yes | 3 | 3 |
| Case 2 | F/70 | right buttock | pT2 | Yes | right groin | Yes | 1 | 1 |
| Case 3 | F/88 | right ear | NA* | No | - | - | - | - |
| Case 4 | F/61 | left forearm | pT1 | Yes | left axilla | Yes | 1 | 1 |
| Case 5 | M/74 | right arm | NA* | Yes | right axilla | Yes | 1 | 4 |
| Case 6 | F/95 | face | pT2 | Yes | left axilla | Yes | 1 | 1 |
| Case 7 | F/83 | right forearm | pT1 | Yes | right axilla | No | 0 | 1 |
| Case 8 | M/78 | right arm | NA* | Yes | right axilla | No | 0 | 2 |
| Case 9 | M/81 | left forearm | pT2 | Yes | left axilla | No | 0 | 4 |
| Case 10 | M/69 | left shoulder | NA* | Yes | left axilla | Yes | 6 | 9 |
| Case 11 | M/80 | face | pT1 | No | - | - | - | - |
| Case 12 | M/52 | chest | NA* | Yes | right axilla | Yes | 4 | 14 |
| Case 13a | F/72 | right knee | pT2 | No | - | - | - | - |
| Case 13b | F/72 | left elbow | pT1 | No | - | - | - | - |
| Case 14 | M/66 | right calf | pT1 | No | - | - | - | - |
| 7M 7F |
5 pT1 5 pT2 |
10 Yes 4 No |
8 Axilla 2 Groin |
7 Yes 3 No |
*NA - Not avable.
Detailed characteristic of the immunophenotype of our cases of MCC (Fig. 1) is given in Table II. All cases were positive for Neu-N (middle or strong nuclear expression; cytoplasmic or membranous staining were not observed), CK20 (cytoplasmic paranuclear dot staining), Neurofilament (cytoplasmic paranuclear dot staining), and Synaptophysin, and negative for thyroid transcription factor-1 (TTF1). Most cases (78.5%) showed > 25% of tumor cells positive for NeuN. NeuN showed no staining of cutaneous structures, neither normal epidermal Merkel cells. Infection by Polyoma virus was detected in 12 out of 15 MCCs (80%).
Figure 1.
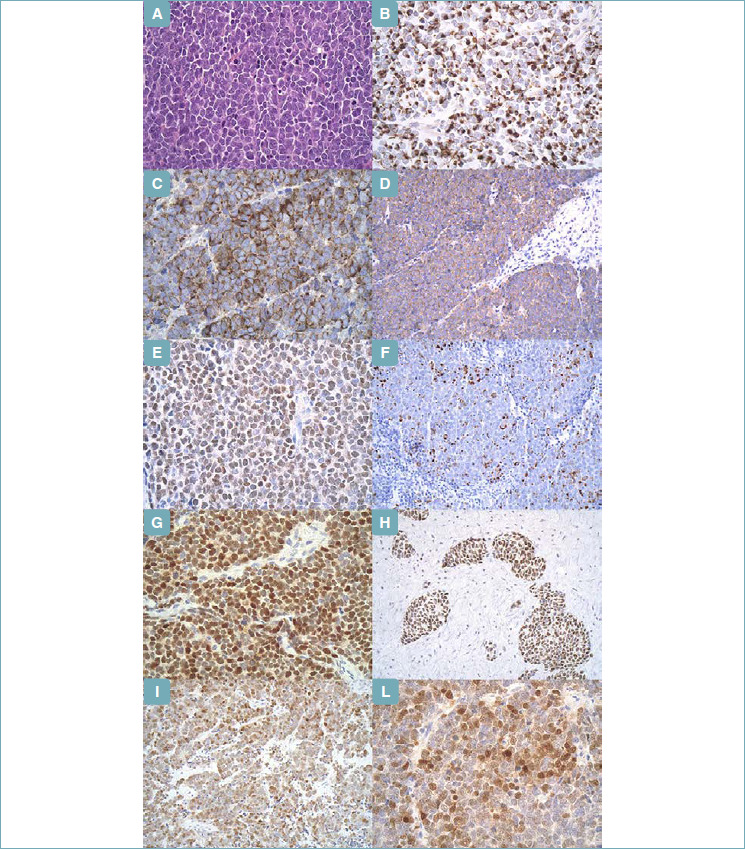
Merkel Cell Carcinoma. (A) (Hematoxylin & Eosin; x40) - Classic cytomorphology displaying small round blue cells with neuroendocrine chromatin, scant cytoplasm, and frequent mitoses. (B) (Immunohistochemistry; x40) - CK20, perinuclear dot pattern. (C) (Immunohistochemistry; x40) - Chromogranin A, granular cytoplasmic pattern. (D) (Immunohistochemistry; x20) - Synaptophysin, granular cytoplasmic pattern. (E) (Immunohistochemistry; x40) - PAX5, nuclear expression. (F) (Immunohistochemistry; x40) - Neurofilament, perinuclear dot pattern. (G) (Immunohistochemistry; x20) - MC Polyoma virus, nuclear expression. (H) (Immunohistochemistry; x20) - TDT, nuclear expression. (I) (Immunohistochemistry; x20) - NeuN, nuclear expression. (L) (Immunohistochemistry; x40) - NeuN, nuclear expression.
Table II.
Detailed characteristics of the immunophenotype. Staining extent (0: < 5%, 1+: 5%-25%, 2+: 25%-50%, and 3+: > 50%) was semi-quantitatively analyzed.
| Case No. | CK7 | CK20 | Chromog. | Synapt. | TTF 1 | Polyoma virus | PAX5 | NF | TDT | Neu-N |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 0 | 3+ | 3+ | 3+ | 0 | 3+ | 3+ | 1+ | 0 | 1+ |
| Case 2 | 0 | 3+ | 3+ | 3+ | 0 | 3+ | 3+ | 2+ | 0 | 3+ |
| Case 3 | 0 | 3+ | 3+ | 3+ | 0 | 3+ | 3+ | 1+ | 0 | 1+ |
| Case 4 | 0 | 2+ | 2+ | 3+ | 0 | 3+ | 3+ | 3+ | 0 | 3+ |
| Case 5 | 0 | 3+ | 3+ | 3+ | 0 | 3+ | 3+ | 2+ | 0 | 1+ |
| Case 6 | 0 | 3+ | 1+ | 3+ | 0 | 3+ | 3+ | 1+ | 1+ | 3+ |
| Case 7 | 0 | 3+ | 0 | 1+ | 0 | 3+ | 3+ | 1+ | 1+ | 1+ |
| Case 8 | 0 | 3+ | 0 | 3+ | 0 | 3+ | 3+ | 2+ | 3+ | 3+ |
| Case 9 | 0 | 3+ | 3+ | 3+ | 0 | 3+ | 3+ | 1+ | 1+ | 3+ |
| Case 10 | 0 | 2+ | 3+ | 3+ | 0 | 0 | 0 | 1+ | 0 | 2+ |
| Case 11 | 2+ | 2+ | 2+ | 3+ | 0 | 0 | 3+ | 1+ | 1+ | 3+ |
| Case 12 | 0 | 3+ | 2+ | 3+ | 0 | 0 | 0 | 3+ | 0 | 3+ |
| Case 13a | 0 | 3+ | - | 3+ | 0 | 3+ | - | - | - | 2+ |
| Case 13b | 0 | 3+ | 2+ | 3+ | 0 | 3+ | - | - | - | 2+ |
| Case 14 | 0 | 3+ | 3+ | 1+ | 0 | 3+ | - | 3+ | - | 3+ |
| 1/14 7,1% |
14/14 100% |
11/13 84,6% |
14/14 100% |
0/14 0% |
11/14 78,6% |
10/12 83,3% |
13/13 100% |
5/12 41,6% |
14/14 100% |
Two cases (11.7%) of 17 primary small cell lung carcinomas were positive for Neu-N in 25% to 50% of tumor cells (score 2 +), with low nuclear intensity (Fig. 2).
Figure 2.
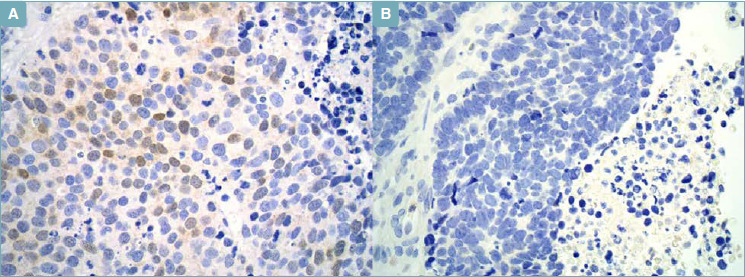
Small Cell Lung Carcinoma. (A) (Immunohistochemistry; x40) - NeuN, nuclear expression in > 25%, but < 50% cells. (B) (Immunohistochemistry; x40) - NeuN, negative.
Discussion
MCC is an aggressive tumor arising on sun-damaged skin of the elderly, associated with immunosuppression and senescence. The course of the disease is characterized by lymph node metastases and local recurrences. MCPyV and UV-induced mutagenesis play fundamental roles in the pathogenesis of MCPyV-positive and MCPyV-negative, respectively. MCPyV-positive MCCs constitute approximately 80% of all cases 12. MCC is recognized by its morphologic features and by its classic immunophenotypic properties. The differential diagnosis of MCC includes cutaneous carcinomas, especially basal cell carcinoma, melanoma, lymphoma, and Ewing sarcoma. The characteristic immunohistochemical profile of MCC includes expression of cytokeratins and neuroendocrine markers (chromogranin A, synaptophysin). Metastatic neuroendocrine carcinomas can be indistinguishable from MCC. Pathologists use CK20 to confirm the diagnosis of MCC, as 80-90% of tumors express CK20 positivity, especially in paranuclear dot-like pattern. However, CK20 is focally expressed in 14% of small cell carcinoma of the lung, and it is frequently positive in neuroendocrine carcinomas arising in the salivary glands and genitourinary tract 15. Moreover, CK20 may be focal or absent in a minority of MCC, especially MCPyV-negative 17. In 2010, McCalmont et al. 17 showed that expression of neurofilament, especially in paranuclear dots, could be demonstrated in 95% of MCCs, and that this antibody can be included in the panel for evaluation of MCCs. Neurofilament has lower sensitivity in MCPyV-negative (50-75%) than MCPyV-positive (75-100%) 12. Neurofilament is often expressed even in CK20-negative cases 18. MCCs are typically described as negative for CK7. However, up to 30% in various studies express CK7. In our study, we detected one out of fifteen cases (7.1%). CK7 and TTF-1 help to distinguish between MCC and a cutaneous metastasis from a small cell carcinoma of the lung or other site. However, no marker is completely sensitive and specific to distinguish MCC from small cell lung carcinoma. Indeed, Pasternak et al. 14 reported that about a third of MCCPy-N express TTF-1, although only weakly and focally. Moreover, CK20 can occasionally be positive in small cell carcinoma of the lung 16.
Paired box gene 5 (PAX5) a B-cell transcription factor expressed early on cell B-cell development, was found to be reactive against various non-hematopoietic tissues, including neuroendocrine carcinomas and MCC 19. Sur et al. 20 showed that 53% (8/15) of MCC expressed terminal deoxynucleotidyl transferase (TdT), a DNA polymerase that has greatest activity in early B cells and T cells. We detected expression of TdT in 5/12 (41.6%) MCCs. Merkel cell carcinoma expressing TdT and/or PAX5 can be a potential diagnostic pitfall, as it may result in misdiagnosis as B-lymphoblastic lymphoma, particularly since the latter is often negative for CD45.
The aim of our study was to evaluate the expression of a neuron-associated protein in primary cutaneous neuroendocrine carcinoma to determine whether it has a role in diagnostic surgical pathology. In this study, we detected NeuN as nuclear staining in all 15 cases of MCC, mostly in > 25% cells (78.5%), enabling easy and rapid decision of positivity and negativity, and only in 2 (11.7%) of 17 primary small cell lung carcinomas. In 2005, Shuangshoti et al. 21 demonstrated NeuN immunoreactivity in 56% of epithelial neuroendocrine tumors (19/34): 4 of 7 (57%) carcinoid; 4 of 5 (90%) atypical carcinoid; 11 of 22 (50%) small and large cell neuroendocrine carcinoma. The authors investigated cases of neuroendocrine epithelial tumor with pulmonary, gastric, ovarian, hepatic, colic, pancreatic, cervical, and vaginal primitiveness, but only one case of skin neuroendocrine carcinoma that was negative. The two lung carcinoids studied by the authors were negative, while the only lung neuroendocrine carcinoma was positive, although the cytoplasmic or nuclear site of expression was not specified. Shuangshoti et al. 21 used a different antibody (mouse-derived monoclonal antibody A60, Chemicon International) and although NeuN is known to express primarily in the neuronal nuclei, cytoplasmic granular staining reminiscent of that observed with chromogranin was detected in 11 of NeuN positive cases. The immunoreactivity was localized in the nucleus in 8 cases. None showed nuclear/cytoplasmic co-expression.
In our study, with the exception of neurofilament and synaptophysin compared to NeuN, other immunohistochemical markers, i.e. chromogranin A, showed expression in a lesser number of patients. CK20 was expressed in all MCC, but as noted previously, there are tumors, both MCC and pulmonary neuroendocrine showing aberrant immunophenotype. Therefore, clinical correlation, radiologic studies, and novel antibodies may be required to achieve the correct diagnosis.
The expression of NeuN expands the spectrum of immunoreactivity of this rare tumor and may prove to be a useful addition to a diagnostic panel to prevent diagnostic pitfalls. However, the expression by primary small cell lung carcinomas must be considered, so it is necessary to evaluate NeuN expression in neuroendocrine carcinomas of other sites and other simulating tumors as well.
Figures and tables
References
- 1.Toker C. Trabecular carcinoma of the skin. Arch Dermatol 1972;105:107-110. [PubMed] [Google Scholar]
- 2.Tang CK, Toker C. Trabecular carcinoma of the skin: an ultrastructural study. Cancer 1978;42:2311-2321. https://doi.org/10.1002/1097-0142(197811)42:5<2311::aid-cncr2820420531>3.0.co;2-l [DOI] [PubMed] [Google Scholar]
- 3.Cook DL, Frieling GW. Merkel cell carcinoma: a review and update on current concepts. Diagnostic Histopathology 2016;22:4:127-133. [Google Scholar]
- 4.Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol 2018;78:433-42. https://doi.org/10.1016/j.jaad.2017.12.004 10.1016/j.jaad.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 5.Harms KL, Healy MA, Nghiem P, et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol 2016;23:3564-3571. https://doi.org/10.1245/s10434-016-5266-4 10.1245/s10434-016-5266-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harms PW. Update on Merkel cell carcinoma. Clin Lab Med. 2017;37:485-501. https://doi.org/10.1016/j.cll.2017.05.004 10.1016/j.cll.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 7.Agelli M, Clegg LX, Becker JC, et al. The etiology and epidemiology of Merkel cell carcinoma. Curr Probl Cancer 2010;34:14-37. https://doi.org/10.1016/j.currproblcancer.2010.01.001 10.1016/j.currproblcancer.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 8.Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol 2018;78:457e63. e452. https://doi.org/10.1016/j.jaad.2017.10.028 10.1016/j.jaad.2017.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet 2002;359:497-498. https://doi.org/10.1016/S0140-6736(02)07668-7 10.1016/S0140-6736(02)07668-7 [DOI] [PubMed] [Google Scholar]
- 10.Harms PW, Vats P, Verhaegen ME, et al. The distinctive mutational spectra of polyomavirus- negative Merkel cell carcinoma. Cancer Res 2015;75:3720-3727. https://doi.org/10.1158/0008-5472.CAN-15-0702 10.1158/0008-5472.CAN-15-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong SQ, Waldeck K, Vergara IA, et al. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res 2015;75:5228-5234. https://doi.org/10.1158/0008-5472.CAN-15-1877 10.1158/0008-5472.CAN-15-1877 [DOI] [PubMed] [Google Scholar]
- 12.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008;319:1096-100. https://doi.org/10.1126/science.1152586 10.1126/science.1152586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moshiri AS, Doumani R, Yelistratova L, et al. Polyoma virus negative Merkel cell carcinoma: a more aggressive subtype based on analysis of 282 cases using multimodal tumor virus detection. J Invest Dermatol 2017;137:819-27. https://doi.org/10.1016/j.jid.2016.10.028 10.1016/j.jid.2016.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasternak S, Carter MD, Ly TY, Doucette S, Walsh NM. Immunohistochemical profiles of different subsets of Merkel cell carcinoma. Hum Pathol 2018;82:232-238. https://doi.org/10.1016/j.humpath.2018.07.022 10.1016/j.humpath.2018.07.022 [DOI] [PubMed] [Google Scholar]
- 15.Chan JK, Suster S, Wenig BM, Tsang WY, Chan JB, Lau AL. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol 1997;21:226-234. https://doi.org/10.1097/00000478-199702000-00014 10.1097/00000478-199702000-00014 [DOI] [PubMed] [Google Scholar]
- 16.Miner AG, Patel RM, Wilson DA, et al. Cytokeratin 20-negative Merkel cell carcinoma is infrequently associated with the Merkel cell polyomavirus. Mod Pathol 2015;28:498-504. https://doi.org/10.1038/modpathol.2014.148 10.1038/modpathol.2014.148 [DOI] [PubMed] [Google Scholar]
- 17.McCalmont TH. Paranuclear dots of neurofilament reliably identify Merkel cell carcinoma. J Cutan Pathol 2010;37:822-823. https://doi.org/10.1111/j.1600-0560.2010.01567_1.x 10.1111/j.1600-0560.2010.01567_1.x [DOI] [PubMed] [Google Scholar]
- 18.Stanoszek LM, Chan MP, Palanisamy N, et al. Neurofilament is superior to cytokeratin 20 in supporting cutaneous origin for neuroendocrine carcinoma. Histopathology 2019;74:504-513. https://doi.org/10.1111/his.13758 10.1111/his.13758 [DOI] [PubMed] [Google Scholar]
- 19.Dong HY, Liu W, Cohen P, et al. B-cell specific activation protein encoded by the PAX-5 gene is commonly expressed in merkel cell carcinoma and small cell carcinomas. Am J Surg Pathol 2005;29:687-692. https://doi.org/10.1097/01.pas.0000155162.33044.4f 10.1097/01.pas.0000155162.33044.4f [DOI] [PubMed] [Google Scholar]
- 20.Sur M, Al Ardati H, Ross C, Alowami S. TdT expression in Merkel cell carcinoma: potential diagnostic pitfall with blastic hematological malignancies and expanded immunohistochemical analysis. Mod Pathol 2007;20:1113-1120. https://doi.org/10.1038/modpathol.3800936 10.1038/modpathol.3800936 [DOI] [PubMed] [Google Scholar]
- 21.Shuangshoti S, Mujananon S, Chaipipat M, et al. Expression of neuronal nuclear antigen (NeuN) in epithelial neuroendocrine carcinoma. Appl Immunohistochem Mol Morphol 2005. ;13:265-267. https://doi.org/10.1097/01.pai.0000137360.34201.21 10.1097/01.pai.0000137360.34201.21 [DOI] [PubMed] [Google Scholar]


