Abstract
Background
When compared to the general population, persons with an intellectual disability have lower life expectancy, higher morbidity, and more difficulty finding and obtaining healthcare. Organisational interventions are used to reconfigure the structure or delivery of healthcare services. This is the first update of the original review.
Objectives
To assess the effects of organisational interventions of healthcare services for the mental and physical health problems of persons with an intellectual disability.
Search methods
For this update we searched CENTRAL, MEDLINE, EMBASE, CINAHL and other databases, from April 2006 to 4 September 2015. We checked reference lists of included studies and consulted experts in the field.
Selection criteria
Randomised controlled trials of organisational interventions of healthcare services aimed at improving care of mental and physical health problems of adult persons with an intellectual disability.
Data collection and analysis
We employed standard methodological procedures as outlined in the Cochrane Handbook of Systematic Reviews of Interventions, in addition to specific guidance from the Cochrane Effective Practice and Organisation of Care (EPOC) Group.
Main results
We identified one new trial from the updated searches.
Seven trials (347 participants) met the selection criteria. The interventions varied but had common components: interventions that increased the intensity and frequency of service delivery (4 trials, 200 participants), community‐based specialist behaviour therapy (1 trial, 63 participants), and outreach treatment (1 trial, 50 participants). Another trial compared two active arms (traditional counselling and integrated intervention for bereavement, 34 participants).
The included studies investigated interventions dealing with the mental health problems of persons with an intellectual disability; none focused on physical health problems. Four studies assessed the effect of organisational interventions on behavioural problems for persons with an intellectual disability, three assessed care giver burden, and three assessed the costs associated with the interventions. None of the included studies reported data on the effect of organisational interventions on adverse events. Most studies were assessed as having low risk of bias.
It is uncertain whether interventions that increase the frequency and intensity of delivery or outreach treatment decrease behavioural problems for persons with an intellectual disability (two and one trials respectively, very low certainty evidence). Behavioural problems were slightly decreased by community‐based specialist behavioural therapy (one trial, low certainty evidence). Increasing the frequency and intensity of service delivery probably makes little or no difference to care giver burden (MD 0.03, 95% CI ‐3.48 to 3.54, two trials, moderate certainty evidence). It is uncertain whether outreach treatment makes any difference for care giver burden (one trial, very low certainty evidence). There was very limited evidence regarding costs, with low to very low certainty evidence for the different interventions.
Authors' conclusions
There is very limited evidence on the organisation of healthcare services for persons with an intellectual disability. There are currently no well‐designed studies focusing on organising the health services of persons with an intellectual disability and concurrent physical problems. There are very few studies of organisational interventions targeting mental health needs and the results of those that were found need corroboration. There is an urgent need for high‐quality health services research to identify optimal health services for persons with an intellectual disability and concurrent physical problem.
Keywords: Humans, Persons with Mental Disabilities, Health Services for Persons with Disabilities, Health Services for Persons with Disabilities/organization & administration, Mental Disorders, Mental Disorders/therapy, Mental Health Services, Mental Health Services/organization & administration, Randomized Controlled Trials as Topic
Plain language summary
Healthcare services for adults with an intellectual disability
Background
Adults with an intellectual disability often have difficulty in receiving the healthcare they need. Compared to other adults who do not have an intellectual disability, they have poorer health and have more difficulty finding, getting to, and paying for healthcare. This happens for both physical and mental healthcare needs.
Review question
We conducted a review of the literature to assess the effects of different ways to organise services. This is the first update of a previously published review.
Study characteristics
We searched for all relevant studies until 4 September 2015. We found seven studies, six of which we identified previously and one retrieved for this update. All of the studies assessed the impact of the intervention on the mental health of persons with an intellectual disability; none considered the physical health. Those studies used different interventions, including giving persons with an intellectual disability more health services, psychological support, and treating them at home, instead of at the hospital. Studies mainly looked at how the interventions helped the behavioural problems of those with an intellectual disability, how much burden they caused the care givers, and how much they cost. No study assessed adverse events.
Key results
Community‐based behaviour therapy might decrease behavioural problems. We are uncertain whether other interventions make any difference in reducing behavioural problems. There was limited evidence about how those interventions helped care givers to deal with the burden of caring for their relatives with an intellectual disability, or how much they might cost compared with the usual care already provided.
Authors' conclusions
There is little information on different ways to organise services for people with intellectual disabilities. Most studies focused on people who had intellectual disabilities and mental health problems. There were no studies on people who had intellectual disabilities and physical problems.
Summary of findings
Summary of findings for the main comparison. Increasing the intensity and frequency of service delivery compared to standard treatment for persons with an intellectual disability.
| Increasing the intensity and frequency of service delivery compared to standard treatment for persons with an intellectual disability | |||
| Patient or population: persons with an intellectual disability Setting: United Kingdom and United States Intervention: increasing the intensity and frequency of service delivery Comparison: standard treatment | |||
| Outcomes | No. of participants (studies) | Certainty of the evidence (GRADE) | Impact |
| Behavioural problems | 66 (2 RCTs) | ⊕⊝⊝⊝ very low 1 2 3 4 | It is uncertain whether increasing the frequency and intensity of service delivery decreases behavioural problems. |
| Care giver burden | 50 (2 RCTs) | ⊕⊕⊕⊝ moderate 2 3 | Increasing the frequency and intensity of service delivery probably makes little or no difference to care giver burden. |
| Costs | 104 (1 RCT) | ⊕⊕⊝⊝ low 3 5 6 | Increasing the frequency and intensity of service delivery may make little to no difference to costs. |
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 Downgraded for indirectness as main outcomes were surrogates.
2 Downgraded for risk of bias as unclear allocation concealment and random sequence generation.
3 Downgraded for imprecision based on sample size and wide CIs.
4 Downgraded for inconsistency based on different direction of the effects.
5 Downgraded for risk of bias as blinding not done and subjective main outcome.
6 Downgraded for indirectness as sub‐analysis of a broader study.
Summary of findings 2. Community‐based specialist behaviour therapy compared to standard treatment for persons with an intellectual disability.
| Community‐based specialist behaviour therapy compared to standard treatment for persons with an intellectual disability | |||
| Patient or population: persons with an intellectual disability Setting: United Kingdom Intervention: community‐based specialist behaviour therapy Comparison: standard treatment | |||
| Outcomes | No. of participants (studies) | Certainty of the evidence (GRADE) | Impact |
| Behavioural problems | 63 (1 RCT) | ⊕⊕⊝⊝ low 1 2 3 | Community‐based specialist behaviour therapy may slightly decrease behavioural problems. |
| Costs | 63 (1 RCT) | ⊕⊕⊝⊝ low 1 2 | Community‐based specialist behaviour therapy may make little or no difference to costs. |
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 Downgraded for risk of bias as unclear protection against contamination and selective reporting; subjective main outcome.
2 Downgraded for imprecision based on sample size and wide confidence intervals.
3 Downgraded for indirectness as main outcome was surrogate.
Summary of findings 3. Outreach treatment compared to hospital treatment for persons with an intellectual disability.
| Outreach treatment compared to hospital treatment for persons with an intellectual disability | |||
| Patient or population: persons with an intellectual disability Setting: The Netherlands Intervention: outreach treatment Comparison: hospital treatment | |||
| Outcomes | No. of participants (studies) | Certainty of the evidence (GRADE) | Impact |
| Behavioural problems | 49 (1 RCT) | ⊕⊝⊝⊝ very low 1 2 3 | It is uncertain whether outreach treatment decreases behavioural problems. |
| Care giver burden | 49 (1 RCT) | ⊕⊝⊝⊝ very low | It is uncertain whether outreach treatment decreases care giver burden. |
| Costs | 49 (1 RCT) | ⊕⊝⊝⊝ very low | It is uncertain whether outreach treatment decreases costs. |
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 Downgraded for risk of bias as unclear allocation concealment and random sequence generation; surrogate main outcome; high risk of selective reporting.
2 Downgraded for indirectness as main outcome was surrogate; generalisability of intervention unclear.
3 Downgraded for imprecision based on sample size and wide confidence intervals.
Background
With an onset during the developmental period, intellectual disability is characterised by significant limitations in both intellectual and adaptive functioning. Adaptive functioning deficits are expressed in conceptual, social, and practical domains (DSM‐5). Approximately 1% of the world's population has an intellectual disability (Maulik 2011). It is more common in developing countries due to more frequent injuries at birth, childhood brain infections, and iodine deficiency. Other common causes include genetic factors (e.g. Down syndrome, Fragile X syndrome, Prader‐Willi syndrome), prenatal exposure to alcohol, and environmental hazards.
In developed countries, the disparity in life expectancy and morbidity between persons with an intellectual disability and the general population has decreased in recent years; however, a real difference still exists (Dieckmann 2015; Frid 1999; Lin 2013; Patja 2001; Van Schrojenstein 1997). Common causes of mortality for persons with an intellectual disability include neoplasms, and respiratory, cardiovascular and nervous system diseases (Patja 2001; Tyrer 2009). Results vary according to methodology, however recent studies have found that between 32% and 45% of adult persons with an intellectual disability also experience mental ill‐health of some type (Cooper 2007; Morgan 2008). The co‐occurrence of intellectual disability and mental illness is sometimes referred to as a 'dual diagnosis' (Morgan 2008). Individuals also often present with challenging behaviour. In this review we use specific categories to describe clinical problems in most instances; when we use the term 'dual diagnosis' it is consistent with the preceding definition.
The best way to organise healthcare services for persons with an intellectual disability has been debated since the de‐institutionalisation of services for this population started in developed countries (Alexander 2002; Aspray 1999; Hassiotis 2000; Lake 2014; O'Hara 2000). De‐institutionalisation has been credited with improving the lives of persons with an intellectual disability; however, in doing so it has shifted the responsibility of the many specialised healthcare needs to the community without sufficient preparation or financial support. Different countries have developed various models of care to deal with this shift in responsibility. In England, for example, Community Learning Disability Teams were created to provide a diverse range of clinical services to meet the comprehensive mental and physical health needs of persons with an intellectual disability (O'Hara 2000). This model of care has been criticised for frequently bypassing mainstream primary care services. This review uses the term 'mainstream' to describe healthcare that could potentially be used by any person in the general population including persons with an intellectual disability. Jurisdictions like the United States have relied more on the mainstream healthcare system; however, this model of care has been criticised for its insufficient capacity to effectively manage the specialised needs of this population (Bouras 2004; Lennox 2002). Well‐designed research on organisational interventions may provide insight to resolve such dilemmas and may prove useful to decrease the disparities in health outcomes that exist between persons with an intellectual disability and the general population.
There is general agreement among review articles that the mainstream health system has lagged in providing adequate healthcare to this population (Alborz 2005; Durvasula 2001; Fisher 2004; Havercamp 2006; Krahn 2006; Lennox 2015; Ouellette Kuntz 2005). Using unmet needs to measure health disparity, the National Health Interview Survey in the United States (US) showed that persons with an intellectual disability were 1.89 times more likely to report unmet healthcare needs than persons with no functional limitations (Anderson 2003). Larson 2005 conducted a review of access to healthcare among persons with an intellectual disability. It found that for persons with an intellectual disability, between 3.2% and 50% experience an unmet medical need and between 1.2% and 27% experience an unmet mental health need. In addition, people living on their own or with family members were less likely to get routine healthcare than those in community or institutional residential settings. Unmet healthcare need is a serious issue among persons with an intellectual disability and effective interventions and models of care need to be identified in order to decrease health disparities in this population.
The situation for persons with an intellectual disability in developed countries is summarised well in a report by the US Secretary of Health and Human Services: compared with other populations, adults with an intellectual disability experience poorer health and more difficulty in finding, getting to, and paying for appropriate healthcare (US PHS 2002). The lifetime direct and indirect economic costs for intellectual disability have been estimated at $1,014,000 per person in the US (CDCP 2004). These costs will surely increase over the years as more persons with an intellectual disability acquire age‐related health problems, and if their health needs continue to go unmet.
A number of options for organising healthcare services for persons with an intellectual disability have been proposed. Models of care range from enhancements to already existing mainstream healthcare to specialty care programs which specifically target the health needs of this population. The purpose of this systematic review was to evaluate recent, high‐quality research on health services interventions for persons with an intellectual disability, in order to provide the highest evidence on how to care effectively for this population. This is the first update of the original review.
Objectives
To assess the effects of organisational interventions of healthcare services for the mental and physical health problems of persons with an intellectual disability.
Methods
Criteria for considering studies for this review
Types of studies
In this update, we limited eligible study designs to only include randomised controlled trials (RCTs), as RCTs have become an accepted and more commonly‐used method to conduct health services research related to persons with an intellectual disability (Robotham 2011).
Previously, we had included other study designs and identified a single controlled before and after (CBA) study (Lowe 1996) and an interrupted time series (ITS) (Allen 1998) despite reviewing 16 years of research (Balogh 2008). These studies were not of the highest quality and the results from the studies were consistent with findings from the RCTs.
We did not restrict studies by publication status or language.
Types of participants
Persons with an intellectual disability and concurrent physical, mental, or behavioural problems (16 years and older). We excluded studies specifically targeting children and adolescents with intellectual disability, as healthcare services for that group are often different from services for young adults and adults (Barelds 2009).
Types of interventions
Researchers have identified components of healthcare services that can be targeted to improve outcomes in disease management (Gilbody 2003; Wagner 1996; Wagner 1998). These include:
Developing and implementing evidence‐based guidelines or protocols;
Supporting guidelines or protocols through health provider education and reminders and increased interaction between generalists and specialists;
Supporting self‐management for the patient; and
Reorganising the health service such that it meets the needs of the target population.
This last item is the focus of this review.
The Cochrane Effective Practice and Organisation of Care (EPOC) Review Group categorises interventions in further detail and, using an adapted version of its taxonomy, we included the following organisational interventions (EPOC 2015):
a) Revision of professional roles: Shifting roles among health professionals or expanding roles to include new tasks; also known as 'professional substitution', 'boundary encroachment'.
b) Clinical multidisciplinary teams: Creating a new team of health professionals of different disciplines or adding new members to the team who work together to care for patients; includes changing the caseload of the team or members of the team, or changing the frequency of episodes of care by the team or members of the team.
c) Formal integration of services: Bringing together services across sectors or teams, or organising services to bring all services together at one time; also called 'seamless care'.
d) Continuity of care: Arranging for follow‐up or case management; includes co‐ordination of assessment, treatment and arrangement for referrals.
e) Changes to the setting/site of service delivery: Includes home‐based, hospital‐based (inpatient and outpatient), and peripatetic interventions; excludes comparisons to institution‐based residential settings.
f) Changes in scope and nature of services: Includes adding elements of care that were not previously available (e.g., social care, psychological support).
In countries where de‐institutionalisation has taken place, researchers commonly examined the effect of change from institution‐based residential settings to community‐based residential settings. These were considered out of scope for this review and are considered elsewhere (Lynch 1997; Young 1998). Financial interventions were also excluded.
Types of outcome measures
Possible outcomes included, but were not restricted to, those listed below. Type of outcome measure was not used as an inclusion or exclusion criterion.
Primary outcomes
Behavioural problems (e.g., Aberrant Behavior Checklist, Aman 1985);
Physical health (e.g., Functional Independence Measure, Keith 1987);
Adverse events (e.g., worsening of pre‐existing symptoms);
Care giver burden (e.g., Uplift/Burden scale, Pruchno 1990);
Health system use, including costs.
Secondary outcomes
Psychological health (e.g., Psychiatric Assessment Schedule for Adults with a Developmental Disability Checklist, Moss 1997);
Quality of life (e.g., Quality of Life Questionnaire, Schalock 1993).
Search methods for identification of studies
Electronic searches
We searched the following databases from April 2006 to 4 September 2015: MEDLINE, CINAHL, EMBASE, PsycINFO, and Cochrane Library (CDSR, DARE, CENTRAL, HTA, NHS‐EED, Methods). EPOC's Trials Search Coordinator developed the search strategies in consultation with the authors (Appendix 1).
Searching other resources
For this update, we also searched the databases PDQ‐Evidence (PDQ‐Evidence 2015), US National Institutes of Health Trial Registry (ClinicalTrials.gov 2015) and WHO International Clinical Trials Registry Platform (ICTRP 2015). We checked the reference list of included studies.
Data collection and analysis
Selection of studies
For this update, one review author (DCGB) screened all titles and abstracts and excluded those that were not eligible, after independently piloting the inclusion criteria with another author (RB). Two review authors (RB and DCGB) applied the inclusion criteria for the full‐texts, following the same methodology as applied for the original review, with a third review author (CAM) solving discrepancies.
Data extraction and management
We used a modified data collection form from the Cochrane EPOC Review Group, that included sections on inclusion criteria, interventions, study participants, setting, methods, outcome measures, results, and quality criteria. Two review authors (of RB, CAM, and DCGB) independently extracted data and assessed the quality of the studies. We resolved disagreements with a third review author (CAM).
Assessment of risk of bias in included studies
We used the Cochrane Collaboration's tool for assessing risk of bias and additional criteria developed by EPOC (EPOC 2012; Higgins 2011b). Two review authors (RB and CAM) independently extracted 'Risk of bias' data from included studies. We employed the following criteria: 1) random sequence generation, 2) concealment of allocation, 3) no important concerns in relation to baseline measures, 4) completeness of outcome data, 5) blinding, 6) protection against contamination, 7) selective outcome reporting, and 8) other biases. The protection against bias rating was assigned in the following manner: 'unclear risk' if no relevant information was reported in the study; 'high risk' if the study authors specifically describe the item and it does not meet EPOC criteria; and 'low risk' if the study authors specifically describe the item and it meets EPOC criteria.
Measures of treatment effect
We used RevMan 5.1 for all statistical analyses (RevMan 2011) in the first iteration of the review, and RevMan 5.3 for analyses in the current review (RevMan 2014). In the absence of heterogeneity, we used a pooled effect estimate from a fixed‐effect meta‐analysis. For studies showing statistical heterogeneity we applied a random‐effects model. The mean difference (MD) was used to calculate a summary statistic of final values from studies reporting an outcome using the same measure (Higgins 2011a). When results came from different outcome measures, we used the standardised mean difference (SMD). This standardises the results of the trials to a uniform scale before being combined.
Data synthesis
In most instances, it was not possible to pool study results due to substantial heterogeneity. Instead we summarised results and characteristics of all included studies in tables. The complexity of health service interventions means that they may not fit precisely into single a priori defined categories. Campbell et al. have suggested a framework for evaluating interventions that are complex or made up of interconnected parts (Campbell 2000). Consistent with this framework, we attempted to specify the 'active ingredients' of the interventions of included studies.
We created 'Summary of findings' tables using the primary outcomes: behavioural problems, physical problems, care giver burden, health system use including costs, and adverse events. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and risk of bias) to assess the certainty of the evidence as it relates to the primary outcomes (Guyatt 2008). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook (Higgins 2011b). We justified all decisions to down‐ or up‐grade the certainty of evidence using footnotes.
Assessment of heterogeneity
Clinical heterogeneity was defined as between‐study variability in the participants, interventions, and outcomes and evaluated among the included studies (Higgins 2011a). We only considered studies with similar study populations, interventions and outcome measures as having low clinical heterogeneity that warranted meta‐analysis. For studies where pooling was appropriate we evaluated statistical heterogeneity. Statistical heterogeneity is variability in the treatment effects being evaluated among the different trials (Higgins 2011a). We evaluated it using a Chi2 test and I2, employing a P value of 0.10 rather than the usual 0.05 to determine statistical significance for the Chi2 test due to the small sample sizes and low number of included studies. This increases the power of the test to detect heterogeneity. A value of I2 greater than 50% was considered to represent significant heterogeneity. Due to the low number of studies in the current review, an in‐depth investigation of statistical heterogeneity was of limited value; when warranted, however, we applied strategies described in the Cochrane Handbook of Systematic Reviews of Interventions to address heterogeneity (Higgins 2011a).
Subgroup analysis and investigation of heterogeneity
We did not plan to conduct a subgroup analysis and did not conduct a subgroup analysis a posteriori.
Sensitivity analysis
We did not plan to conduct a sensitivity analysis and did not conduct a sensitivity analysis a posteriori.
Results
Description of studies
Results of the search
The searches for this update retrieved 1048 references, of which we short‐listed 37 for full‐text assessment. From those we identified one eligible trial (Figure 1).
1.
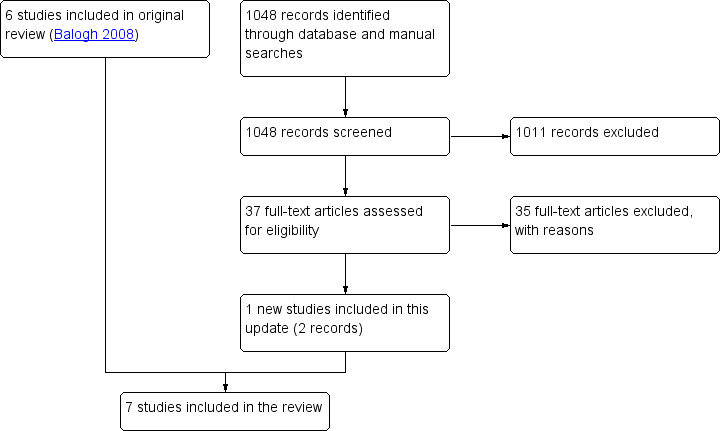
Flow diagram.
Included studies
We included seven RCTs in this update, six identified previously (Coelho 1993; Dowling 2006; Hassiotis 2001; Martin 2005; Oliver 2005; van Minnen 1997) and one new study (Hassiotis 2009), which are described in the Characteristics of included studies. One of the studies (Hassiotis 2001) was identified through the reference list of included studies and the remainder came from electronic databases.
Study participants were mainly in their thirties and early forties (Coelho 1993; Hassiotis 2009; Martin 2005; Oliver 2005; van Minnen 1997) and the majority were male (Coelho 1993; Dowling 2006; Hassiotis 2001; Hassiotis 2009; Martin 2005; Oliver 2005; van Minnen 1997). The sample size for included studies was small (range 20 to 63) (Coelho 1993; Dowling 2006; Hassiotis 2009; Martin 2005; Oliver 2005; van Minnen 1997), with the exception of the study by Hassiotis 2001 (104 participants). The studies used population samples from England (Hassiotis 2001; Hassiotis 2009; Martin 2005; Oliver 2005), the United States (Coelho 1993), the Netherlands (van Minnen 1997), and from across the United Kingdom (Dowling 2006). All studies except one (van Minnen 1997) specified the level of intellectual disability of the participants, which ranged from mild to severe/profound. Participants' level of intellectual disability varied across studies, from studies mainly including participants with mild disability (Martin 2005; Oliver 2005), those that included a mix of participants with mild and moderate disability (Coelho 1993; Hassiotis 2001), and studies that also included participants with severe disability (Dowling 2006; Hassiotis 2009). All the studies included persons with an intellectual disability who also had psychological or behavioural problems ranging in severity from bereavement (Dowling 2006) to severe psychotic illness (Hassiotis 2001). None of the studies identified physical health problems among participants.
Interventions
All of the studies evaluated complex interventions and several fit into more than one category of the modified EPOC taxonomy of interventions. Some studies stated which components of the intervention were thought to be most responsible for expected outcomes. None of the studies used an intervention focusing on formal integration of services. Characteristics of included studies provides more detail on the nature of the intervention and control groups.
Four studies manipulated the intensity and frequency of service delivery (Coelho 1993; Hassiotis 2001; Martin 2005; Oliver 2005). Coelho et al. described an intervention with "differences in the frequency and intensity of participant involvement" when compared to the control treatment and where the intervention emphasised contact with participants in their natural environment (Coelho 1993). The degree of specialisation in intellectual disability among the case managers was similar in both groups. Hassiotis 2001 used intensive case management as an intervention and specified smaller case‐load size to differentiate from the control treatment. The intervention and control were mainstream management strategies meant for the general population. This study's results were taken from a larger study which included persons without an intellectual disability (Burns 1999). Martin et al. identified frequency of contact as the main criterion for assertive community treatment (Martin 2005). In their study on assertive community treatment, Oliver et al. stated that the intervention was best measured in terms of frequency and types of contact (Oliver 2005). The control conditions for Martin 2005 and Oliver 2005 were standard care, which included services provided by Community Learning Disability Teams.
Dowling 2006 changed the role of care givers of persons with an intellectual disability to include bereavement work. Control conditions consisted of mainstream bereavement counselling provided by trained bereavement counsellors with no experience working with persons with an intellectual disability. The intervention in Hassiotis 2009 consisted of a community‐based specialist behaviour therapy team in addition to standard care, which in the United Kingdom includes services from Community Learning Disability Teams. The control group received standard care only as provided by the same Community Learning Disability Teams. van Minnen 1997 studied an outreach intervention provided by a multidisciplinary team which included a care coordinator: they identified the setting as the most important difference between the hospital‐treated controls versus the participants in the intervention group, who were seen in their home environment.
Outcomes assessed
Five studies measured aspects of behaviour (Coelho 1993; Dowling 2006; Hassiotis 2009; Martin 2005; van Minnen 1997) and two evaluated overall psychological and psychiatric function (Martin 2005; Oliver 2005). No study included measures of physical functioning or problems. Only three studies included measures of quality of life (Hassiotis 2001; Martin 2005; Oliver 2005) and care giver burden as an outcome (Martin 2005; Oliver 2005; van Minnen 1997), and two studies assessed the effect of organisational interventions on unmet needs (Hassiotis 2001; Martin 2005). Three studies reported the costs associated with the interventions (Hassiotis 2001; Hassiotis 2009; van Minnen 1997), with one study also reporting days in hospital and hospital readmissions (Hassiotis 2001). No study reported adverse events.
Excluded studies
For this update, we retrieved full‐text articles for 37 studies, of which 27 were clearly not eligible. Of the remaining 10, we excluded 8 (Bergström 2013; Chadwick 2009; Darrow 2011; Hassiotis 2011; Hassiotis 2014; Lennox 2008; Raghavan 2009; Willner 2013) after further screening and included 1 (Hassiotis 2009; 2 records) in this update (Characteristics of excluded studies).
Risk of bias in included studies
Allocation
Two studies clearly described adequate methods to conceal allocation to the control or intervention groups (Hassiotis 2001; Hassiotis 2009). It was unclear whether the remaining three adequately concealed allocation (Coelho 1993; Dowling 2006; Martin 2005; Oliver 2005; van Minnen 1997).
Blinding
Two studies had a low risk for detection bias (Hassiotis 2001; Oliver 2005) and three were unclear (Hassiotis 2009; Martin 2005; van Minnen 1997). The remaining studies had high risk of bias, as they had subjective outcomes and did not blind the outcome assessment (Coelho 1993; Dowling 2006). Due to the nature of the interventions, blinding of study participants and personnel was not possible.
Incomplete outcome data
Two studies were at high risk for attrition bias, due to missing outcome data (Dowling 2006; Hassiotis 2001). Four studies reported complete outcome data (Coelho 1993; Hassiotis 2009; Oliver 2005; van Minnen 1997). For one of the studies there was insufficient reporting on attrition and exclusions to permit a judgement (Martin 2005).
Selective reporting
For five of the studies it was unclear whether there were any reporting biases, as it was not possible to identify a protocol, although the studies reported all of the outcomes defined within the publication (Coelho 1993; Dowling 2006; Hassiotis 2009; Martin 2005; Martin 2005). One study had a high risk for selective reporting (van Minnen 1997), as it did not report on all the outcomes pre‐specified, and another study had a low risk (Hassiotis 2001), as it reported all outcomes defined for the protocol.
Other potential sources of bias
None of the included studies conducted a formal power calculation to identify the number of participants required to identify a clinically‐important difference. All studies ensured that a 'baseline measurement' of patient outcome was done prior to the introduction of the intervention (Figure 2; Figure 3).
2.
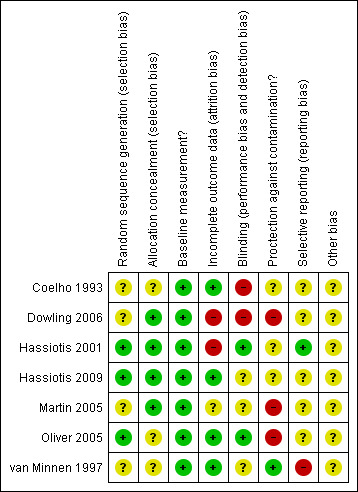
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
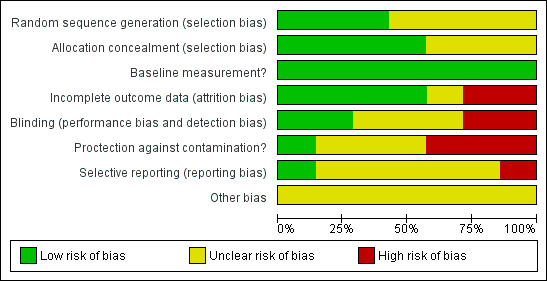
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See: Table 1; Table 2; Table 3
Effects of organisational interventions on behavioural problems and comorbid psychological and psychiatric function
We are uncertain whether increasing the frequency and intensity of service delivery decreases behavioural problems in persons with an intellectual disability (66 participants; 2 studies; very low certainty evidence). One study employed the American Association on Mental Deficiency Adaptive Behavior Scale and found fewer maladaptive behaviours for participants receiving the active treatment (mean difference (MD) ‐12.89, 95% confidence interval (CI) ‐24.9 to ‐0.88), and a similar number of adaptive behaviours (MD 10.6, 95% CI ‐37.5 to 24.9) (Coelho 1993; Analysis 1.1). Martin 2005 reported a similar mean difference between groups at 6‐month follow‐up, using the Aberrant Behavior Checklist (ABC) (MD 0.91, 95% CI ‐1.56 to 3.39). Community‐based specialist behaviour therapy may slightly decrease behavioural problems, at 3‐month (MD ‐0.89, 95% CI ‐1.74 to ‐0.04) and 12‐month follow‐up (MD ‐0.88, 95% CI ‐1.66 to ‐0.11), as measured by the ABC (63 participants; 1 study; low certainty evidence; Analysis 1.2). We are uncertain whether an outreach treatment group decreases behavioural problems (49 participants; 1 study; very low certainty evidence; Analysis 1.3). One study, comparing a counselling intervention with an integrated intervention using the ABC, found improvements in irritability (MD 7.1, 95% CI 3.4 to 10.7), lethargy (MD 7.4, 95% CI 3.1 to 11.7), stereotypy (MD 2.3, 95% CI 0.4 to 4.2) and hyperactivity (MD 6.5, 95% CI 2.1 to 10.8), but not inappropriate speech (MD ‐0.5, 95% CI ‐3.8 to 2.9), among participants who received bereavement counselling from bereavement counsellors with little experience working with persons with an intellectual disability (Dowling 2006; Analysis 1.4).
1.1. Analysis.
Comparison 1 Effect of organisational interventions on behavioural problems, Outcome 1 Interventions that increased the intensity and frequency of service delivery.
| Interventions that increased the intensity and frequency of service delivery | ||
|---|---|---|
| Study | Behavioural problems | Notes |
| Coelho 1993 |
AAMD Adaptive Behaviour At baseline: T: 199.4 (SD 28.5); C: 206.0 (SD 30.0) At post‐test: T: 211.9 (SD 30.9); C: 201.3 (SD 29.0) Mean difference at post‐test: 10.6, 95% CI ‐37.5 to 24.9 Treatment by time interaction: F(1,3) = 5.76, P < 0.001 AAMD Maladaptive Behaviour At baseline: T: 53.7 (SD 22.5); C: 53.4 (SD 27.5) At post‐test: T: 40.1 (SD 20.3); C: 53.0 (SD 29.0) Mean difference at post‐test: ‐12.9, 95% CI ‐24.9 to ‐0.88 Treatment by time interaction: F(1,3) = 6.05, P < 0.001 MMBS At baseline: T: 12.7 (SD 7.0); C: 12.0 (SD 7.6) At post‐test: T: 6.5 (SD 4.6); C: 11.5 (SD 6.4) Mean difference at post‐test: ‐5, 95% CI ‐7.7 to ‐3.7 Treatment by time interaction: F(1,3) = 5.57, P < 0.001 |
Mean difference between groups for post‐intervention scores and treatment by time interaction (active treatment model and the standard case management treatment). The American Association on Mental Deficiency Adaptive Behavior Scale has 110 items split in two sections (adaptive and maladaptive behaviour) and is completed by an informant. Higher scores represent more adaptive or less maladaptive behaviour. The Michigan Maladaptive Behaviour Scale covers 20 behaviour areas, from which the informant selects those relevant for the participant, then rating the frequency of occurrence. Higher scores represent more maladaptive behaviour. |
| Martin 2005 |
ABC – total score Adjusted mean difference: 0.91, 95% CI: ‐1.56 to 3.39 |
Adjusted difference between groups (standard treatment and assertive treatment) at 6m |
1.2. Analysis.
Comparison 1 Effect of organisational interventions on behavioural problems, Outcome 2 Community‐based specialist behaviour therapy.
| Community‐based specialist behaviour therapy | ||
|---|---|---|
| Study | Behavioural problems | Notes |
| Hassiotis 2009 |
ABC irritability Mean difference: ‐0.21, 95% CI ‐0.50 to 0.08 ABC lethargy Mean difference: ‐0.56, 95% CI ‐0.97 to 0.15 ABC stereotypy Mean difference: 0.06, 95% CI ‐0.33 to 0.45 ABC hyperactivity Mean difference: ‐0.56, 95% CI ‐0.97 to 0.15 ABC inappropriate speech Mean difference: ‐0.21, 95% CI ‐0.50 to 0.08 ABC – Total score 3 months Mean difference: ‐0.89, 95% CI ‐1.74 to ‐0.04 24 months Mean difference: ‐0.88, 95% CI ‐1.66 to ‐0.11 |
Mean difference between groups for pre‐ and post‐intervention scores (standard treatment and behaviour treatment plus standard treatment) Difference in mean transformed scores; transformed scores are the square root of raw scores. Scores are adjusted for each baseline ABC subscale score and retrospective time period (3‐, 6‐, and 24m). |
1.3. Analysis.
Comparison 1 Effect of organisational interventions on behavioural problems, Outcome 3 Outreach treatment group.
| Outreach treatment group | ||
|---|---|---|
| Study | Behavioural problems | Outcomes |
| van Minnen 1997 |
PIMRA‐I Mean difference: ‐1.2, 95% CI ‐4.9 to 2.6 PIMRA‐SR Mean difference: 0.1, 95% CI ‐3.7 to 3.9 RSMB Mean difference = 1.0, 95% CI ‐4.4 to 6.4 |
Mean difference between groups (hospital treatment and outreach treatment) at 6m The Psychopathology Inventory for Mentally Retarded Adults can be completed by an informant or self‐reported, with 56 items each. The Reiss Screen for Maladaptive Behavior has 38 items and is completed by an informant. Higher scores represent more behavioural problems. |
1.4. Analysis.
Comparison 1 Effect of organisational interventions on behavioural problems, Outcome 4 Counselling intervention.
| Counselling intervention | ||
|---|---|---|
| Study | Behavioural problems | Notes |
| Dowling 2006 |
ABC irritability Mean change after treatment CI: 6.1 (SD 4.41); II: ‐0.9 (SD 5.78) Mean difference in change: 7.1, 95% CI 3.4 to 10.7 ABC lethargy Mean change after treatment CI: 5.7 (SD 6.37); II: ‐1.8 (SD 4.52) Mean difference in change: 7.4, 95% CI 3.1 to 11.7 ABC stereotypy Mean change after treatment CI: 1.5 (SD 2.04); II: ‐0.8 (SD 3.41) Mean difference in change: 2.3, 95% CI 0.4 to 4.2 ABC hyperactivity Mean change after treatment CI: 6.2 (SD 6.53); II: ‐0.3 (SD 4.49) Mean difference in change: 6.5, 95% CI 2.1 to 10.8 ABC inappropriate speech Mean change after treatment CI: ‐0.1 (SD 5.46); II: 0.4 (SD 2.11) Mean difference in change: ‐0.5, 95% CI ‐3.8 to 2.9 HoNOS‐LD Mean change after treatment CI: 7.4 (SD 7.03); II: 0.4 (SD 6.69) Mean difference in change: 7.0, 95% CI 1.9 to 12.1 |
Mean difference between groups for pre‐ and post‐intervention scores (traditional counselling by volunteer bereavement counsellors, and an integrated intervention delivered by care givers which offered bereavement specific support) The Aberrant Behavior Checklist – Community has five scales and is completed by an informant. Higher scores represent more aberrant behaviour. The Health of the Nation Outcome Scales for People with Learning Disabilities has 18 items and is completed by an informant. Higher scores represent more behavioural problems. |
Two studies assessed the effects on psychological functioning of an intervention that increased the intensity and frequency of service delivery, using the Global Assessment of Function scale (Martin 2005; Oliver 2005). There was insufficient evidence that the interventions made a difference to persons with an intellectual disability, both for disability (MD 1.05, 95% CI ‐4.05 to 6.16, Analysis 2.1) and symptomatology (MD ‐0.76, 95% CI ‐6.07 to 4.55; Analysis 2.2).
2.1. Analysis.
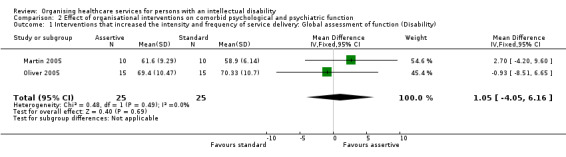
Comparison 2 Effect of organisational interventions on comorbid psychological and psychiatric function, Outcome 1 Interventions that increased the intensity and frequency of service delivery: Global assessment of function (Disability).
2.2. Analysis.
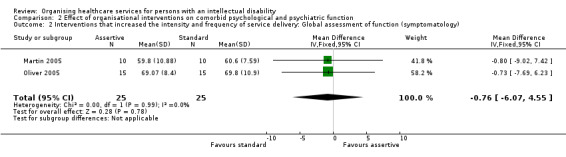
Comparison 2 Effect of organisational interventions on comorbid psychological and psychiatric function, Outcome 2 Interventions that increased the intensity and frequency of service delivery: Global assessment of function (symptomatology).
Effects of organisational interventions on adverse events
None of the included studies reported data on the effect of organisational interventions on adverse events.
Effects of organisational interventions on physical health
None of the included studies reported data on the effect of organisational interventions on physical health.
Effects of organisational interventions on quality of life and unmet needs
We are uncertain whether organisational interventions improve the quality of life of persons with an intellectual disability in the short term (Martin 2005; Oliver 2005) and long term (Hassiotis 2001). Three studies assessed the effects of interventions that increased the intensity and frequency of service delivery on quality of life, using different scales (see Analysis 3.1 and Analysis 3.2 for details). Pooled analysis of two trials indicated that quality of life was similar for both groups (SMD ‐0.20, 95% CI ‐0.75 to 0.36; 50 participants). One study reported a similar number of health and care needs at 6 months between participants who received increased service delivery and those who received standard community treatment (Martin 2005), whereas another study found that, at 24 months, those in the increased service delivery group reported a smaller number of needs (MD ‐1.65, 95% CI ‐2.98 to ‐0.32) and unmet needs (MD ‐1.29, 95% CI ‐2.52 to ‐0.06) (Hassiotis 2001).
3.1. Analysis.
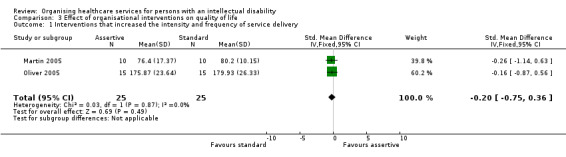
Comparison 3 Effect of organisational interventions on quality of life, Outcome 1 Interventions that increased the intensity and frequency of service delivery.
3.2. Analysis.
Comparison 3 Effect of organisational interventions on quality of life, Outcome 2 Interventions that increased the intensity and frequency of service delivery.
| Interventions that increased the intensity and frequency of service delivery | ||
|---|---|---|
| Study | Quality of life | Notes |
| Hassiotis 2001 |
LQLP No difference between groups at 24m (data not reported) |
The Lancashire Quality of Life Profile assesses nine domains, including work and education, family and social relations, and health. |
Effects of organisational interventions on care giver burden
Increasing the frequency and intensity of service delivery probably makes little or no difference to care giver burden as measured by the Uplift/Burden scale (MD 0.03, 95% CI ‐3.48 to 3.54; 50 participants; 2 studies; moderate certainty evidence; Analysis 5.1), both at 3 months (Oliver 2005) and 6 months (Martin 2005). One study comparing outreach treatment with hospital treatment reported that care giver burden as assessed with the Nijmegen Child‐Rearing Situation Questionnaire was similar at baseline and follow‐up, for care givers of persons with an intellectual disability receiving outreach treatment (MD 5.1, 95% CI ‐3.57 to 13.77) (49 participants; very low certainty evidence; Analysis 5.2). Results were not reported for the group receiving hospital treatment.
5.1. Analysis.
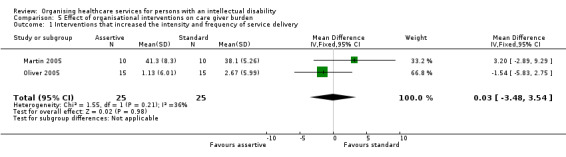
Comparison 5 Effect of organisational interventions on care giver burden, Outcome 1 Interventions that increased the intensity and frequency of service delivery.
5.2. Analysis.
Comparison 5 Effect of organisational interventions on care giver burden, Outcome 2 Outreach treatment group.
| Outreach treatment group | ||
|---|---|---|
| Study | Care giver burden | Notes |
| van Minnen 1997 |
NCSQ T: Baseline: 64.5 (SD 14.1), Endpoint: 59.4 (SD 15.7) Mean difference: 5.1, 95% CI: ‐3.57 to 13.77 |
The Nijmegen Child‐Rearing Situation Questionnaire has 46 items measuring the burden of caring for a person with an intellectual disability. Mean difference for treatment group from baseline to endpoint for 6m (control group not assessed at endpoint). |
Effects of organisational interventions on health systems use
Increasing the frequency of service delivery (104 participants; 1 study; low certainty evidence; Analysis 6.1) and community‐based behaviour therapy (63 participants; 1 study; low certainty evidence; Analysis 6.2) may make little to no difference to costs. We are uncertain whether outreach treatment decreases costs (Analysis 6.3). van Minnen 1997 reported that mean total costs for groups at 28 weeks favoured outreach treatment when compared with hospital treatment ($24,221 and $41,134, respectively), but as the authors did not report the standard deviation we were not able to calculate the mean difference between groups.
6.1. Analysis.
Comparison 6 Effect of organisational interventions on costs, Outcome 1 Interventions that increased the intensity and frequency of service delivery.
| Interventions that increased the intensity and frequency of service delivery | ||
|---|---|---|
| Study | Costs | Notes |
| Hassiotis 2001 |
Total costs T: 23,808 (SD 20,583); C: 28,983 (SD 30,719) Mean difference: ‐5,175, CI 95% ‐15,692 to 5,342 Health authority costs T: 11,175 (SD 14,808); C: 21,213 (SD 29,712) Mean difference: ‐10,038, CI 95% ‐19,542 to ‐534 Staffed accommodation costs T: 9,983 (SD 13,363); C: 5,068 (SD 10,412) Mean difference: 4,916, CI 95% 188 to 9,643 |
Mean difference between groups (intensive and standard case management) at 24m Values in British pound (£), 1999 |
6.2. Analysis.
Comparison 6 Effect of organisational interventions on costs, Outcome 2 Community‐based specialist behaviour therapy.
| Community‐based specialist behaviour therapy | ||
|---|---|---|
| Study | Costs | Notes |
| Hassiotis 2009 |
Total costs, including treatment T: 1,415 (SD 1,349); C: 3,615 (SD 8,239) Mean difference: ‐2,200, CI 95% ‐5,127 to 728 Total costs, excluding treatment T: 665 (SD 1,293); C: 3,219 (SD 8,229) Mean difference: ‐2,554, CI 95% ‐5,474 to 367 |
Mean difference between groups (behaviour therapy team and standard treatment) at 6m Values in British pound (£), 2009 |
6.3. Analysis.
Comparison 6 Effect of organisational interventions on costs, Outcome 3 Outreach treatment group.
| Outreach treatment group | ||
|---|---|---|
| Study | Costs | Notes |
| van Minnen 1997 |
Total costs T: 24,221 (SD not provided), C: 41,134 (SD not provided) |
Mean costs for groups (outreach and hospital treatment), for 28w Not possible to calculate mean difference Values in US dollar (US$), published in 1997 |
Hassiotis 2001 reported that persons with mild and borderline intellectual disability receiving care from intensive case management teams with small case‐loads spent fewer days in hospital (MD ‐57.5 days, CI 95% ‐110.9 to ‐4.2) and had fewer hospital readmissions for psychiatric reasons (MD ‐0.94, CI 95% ‐1.51 to ‐0.37) (Analysis 7.1).
7.1. Analysis.
Comparison 7 Effects of organisational interventions on health systems use, Outcome 1 Interventions that increased the intensity and frequency of service delivery.
| Interventions that increased the intensity and frequency of service delivery | ||
|---|---|---|
| Study | Health system use | Notes |
| Hassiotis 2001 | Mean number of days in hospital for psychiatric reasons T: 47.2 (SD 98.0); C: 104.8 (SD 159.5) Mean difference: ‐57.5 days, CI 95% ‐110.9 to ‐4.2 Number of hospital admissions for psychiatric reasons T: 0.55 (SD 0.97); C: 1.49 (SD 1.75) Mean difference: ‐0.94, CI 95% ‐1.51 to ‐0.37 |
Mean difference between groups (intensive and standard case management) at 24m |
Discussion
Summary of main results
This systematic review evaluated the effectiveness of the organisation of healthcare services for persons with an intellectual disability. Seven randomised controlled trials, of acceptable methodological quality, met the inclusion criteria. Although the interventions implemented by those trials varied, we were able to group them according to common components: interventions that increased the intensity and frequency of service delivery (Coelho 1993; Hassiotis 2001; Martin 2005; Oliver 2005), community‐based specialist behaviour therapy (Hassiotis 2009), and outreach treatment (van Minnen 1997). Another study compared two active arms: traditional counselling and an integrated intervention for bereavement (Dowling 2006).
For behavioural problems, it is uncertain whether increasing the frequency and intensity of service delivery and outreach treatment decreases those problems (two and one studies respectively, very low certainty evidence). Community‐based specialist behaviour therapy may slightly decrease behavioural problems for persons with an intellectual disability (one study, low certainty evidence). For care giver burden, increasing the frequency and intensity of service delivery probably makes little or no difference (two studies, moderate certainty evidence) and we are uncertain whether outreach treatment has any effect (one study, very low certainty evidence). Organising health services may make little or no difference to healthcare costs for persons with an intellectual disability. None of the included studies reported on the effect of organisational interventions on physical health or adverse events.
Overall completeness and applicability of evidence
The interpretation of the review depends on the jurisdiction in which it is being applied. Therefore the following discussion avoids making generalisations about local and national resources and values, with one caveat: the included studies were conducted in countries where the process of de‐institutionalisation has been ongoing for many years, and the results are applicable mostly to countries in the same situation. Even among countries where de‐institutionalisation has taken place, there are considerable differences in how health services for persons with an intellectual disability have been organised. In the UK, Community Learning Disability Teams are widespread, with as many as 350 identified in England alone. The vast majority of persons with moderate to profound intellectual disability use their services (Moss 2000; Slevin 2008). This is in contrast to the situation in countries like Australia and the United States, where specialist services for persons with an intellectual disability are less well developed (Chaplin 2004; Fletcher 1993; Lennox 1995). In these countries, persons with an intellectual disability have had to rely much more heavily on the same primary and specialist health services that are accessed by the general population. These examples stress the importance of interpreting study results within the context of jurisdictions with similar levels of standard care.
Care should be taken when generalising the results of this review due to differences in inclusion criteria and jurisdictions among the included studies. The English study by Hassiotis 2001 showed that intensive case management improved outcomes for persons with unrecognised mild or borderline intellectual disability and a concurrent psychotic illness. Martin 2005 and Oliver 2005, also in England, were not able to reproduce these findings in a population that had more significant cognitive impairments and a broader range of psychiatric and behavioural issues. The differences in study population may explain the different results and lack of generalisability of intervention effectiveness. Hassiotis 2001 identified a subset of persons not eligible for intellectual disability services at higher risk of experiencing adverse outcomes in the absence of intervention (Chaplin 2006; Higgins 2011a). The control and intervention groups from the Hassiotis 2001 study received only standard or intensive case management from mainstream health services: they did not benefit from the services provided by local Community Learning Disability Teams as did the study groups from Oliver 2002 and Martin 2005. This suggests a need for targeted and well‐coordinated services with clear descriptions of responsibilities so that the mental health and behaviour needs of persons with mild or borderline intellectual disability do not fall through service gaps (Hassiotis 2002; Moss 2000; Oliver 2002).
Another example of the lack of generalisability of effect is seen when comparing the Michigan state‐ (US) based study by Coelho et al. with the studies by Oliver et al. and Martin et al. (Coelho 1993; Martin 2005; Oliver 2005). In this case the study populations were similar but the jurisdictions were different. The different results may be because the investigations were carried out in jurisdictions with different health service structures. Michigan had no system of Community Learning Disability Teams like England's in place at the time of the study (Kelley 2006). As stated by Oliver 2005, the different results in England and Michigan depend substantially on the quality of the standard care. If Community Learning Disability Teams are providing services similar to the intervention being tested, then the effect shown in other jurisdictions may not be detected.
This review did not include studies examining organisational interventions addressing the health issues of children with intellectual disabilities, as medical and rehabilitative care for that age group might differ from that offered to adults, including specific transitional requirements that are absent from the care provided to adults (Barelds 2009). The next update to this review will consider expanding the age limit to include children.
The deliberations regarding the best way to organise healthcare services for persons with an intellectual disability are similar to dilemmas for patients in the general population with a chronic illness. In the review by Wagner et al. on care for patients with chronic illnesses, they found that health professionals "feel unprepared or are too rushed to meet the educational, behavioural, and psychosocial needs of chronically ill patients and their caregivers" (Wagner 1996, p. 512). There is evidence that specialised care is better suited to deal with the needs of persons from the general population with time‐consuming problems (Rich 1995). Condition‐specific specialised programs may prove effective for the general population and persons with an intellectual disability, but this may be at the detriment of coordinated care. Integrated care, where services are provided across sectors or teams, or organised to bring all services together at one time, provides one possible solution (EPOC 2015). This review, however, found no suitable studies that tested interventions of this nature, a result consistent with the findings of a systematic literature search on medical care for persons with an intellectual disability living in the community (Jansen 2006).
Persons with intellectual disabilities are a disadvantaged population who experience health inequities and are more likely to live in poorer neighbourhoods (Cooper 2011). This population is effectively and routinely excluded from research conducted in the general population due to the literacy skills often required to consent to participate (Lennox 2005). The studies included in this review successfully addressed this inequity, by assessing interventions specifically targeting this population. Moreover, some studies included participants with severe intellectual disabilities (Dowling 2006; Hassiotis 2009), who often are excluded from randomised trials (Martin 2005; Oliver 2005). The decision to exclude participants with severe intellectual disabilities is explained by barriers to conducting randomised studies with this specific sub‐population, beyond the control of researchers (Oliver 2002).
Certainty of the evidence
All studies included in this review were randomised controlled trials, most of which were assessed as having low risk of bias. We found no major inconsistency between trials, and overall the included participants were similar. However, we only identified 7 eligible studies, with samples ranging from 20 to 104 participants (average sample size of 50 participants), which contributed to increased imprecision and decreased certainty of the evidence. For each of the interventions analysed, we identified only one or two studies assessing each relevant outcome, which resulted in an overall rating of 'low to very low certainty' of the evidence.
Potential biases in the review process
Some challenges and limitations of the current review are summarised. We did not search for studies published before 1990. It is therefore possible that some appropriate studies were missed; this is unlikely, however, since concern over the quality of healthcare services provided in the community for persons with an intellectual disability is a relatively new development. For this update, only one author initially screened titles and abstracts, increasing the risk of missing potentially eligible studies. However, the application of the inclusion criteria to studies retrieved by the search was piloted independently by two authors prior to the screening of titles and abstracts, as to increase confidence in the single screener, and a sensitive approach was adopted.
The subtleties of complex interventions and lack of an agreed taxonomy made it hard to properly categorise the included studies. The taxonomy of interventions provided by the Cochrane EPOC Review Group was useful for identifying studies during the literature search but was not detailed enough to properly describe organisation of care interventions. This was especially true when trying to differentiate between 'assertive community treatment' and 'intensive case management'. For this purpose the framework developed by Campbell 2000 describing how complex interventions should be designed and evaluated was useful. The framework describes sequential phases for developing trials of complex interventions and recommends starting with a theoretical basis for an intervention and specifying its possible active ingredients.
Agreements and disagreements with other studies or reviews
A systematic review comparing specialist to mainstream psychiatric services for persons with an intellectual disability found 27 studies on the subject, 2 of which were randomised controlled trials (Chaplin 2004). The authors considered the evidence to be weak and insufficient to give clinicians and managers guidance as to the most suitable services to use or design. A recent systematic review on psychological therapies for people with intellectual disability identified 24 eligible trials, of which 8 were randomised controlled trials (Vereenooghe 2013). There was some evidence for cognitive‐behavioural therapy (CBT) for adults with intellectual disability and either anger or depression, but not for interpersonal functioning. There was insufficient evidence for other psychological therapies. A recently updated Cochrane systematic review on behavioural and cognitive‐behavioural interventions for outwardly‐directed aggressive behaviour in people with intellectual disabilities identified six studies, including five randomised controlled trials, all of which employed CBT (Ali 2015). Similar to our review, the authors concluded that methodologically high‐quality controlled trials are scarce for persons with an intellectual disability.
Authors' conclusions
Implications for practice.
Organisational interventions have the potential to improve health outcomes of persons with a dual diagnosis; however, this review found insufficient evidence to guide policy decisions about how to optimise services in different jurisdictions. The results of the meta‐analysis suggest that there is no evidence that assertive community treatment is superior to standard community treatment as practiced in England. This should not be taken as evidence that assertive community treatment is not effective; only that, to date, there is insufficient evidence to support it over standard treatment. A well‐conducted trial from England supported the use of community‐based specialist behaviour therapy teams to effectively address challenging behaviour (Hassiotis 2009). Implementing this approach more broadly in the United Kingdom could be considered, and research to corroborate the findings could be pursued elsewhere. Intensive case management could be considered when providing mental health services for persons with a dual diagnosis in the United States and for persons with a mild or borderline intellectual disability living in England.
Implications for research.
Most of the included studies of this review were assessed as having low risk of bias despite their small sample size. They require replication before firm conclusions can be drawn. Well‐designed appropriately‐powered studies focusing on organising the health services of persons with an intellectual disability and concurrent physical problems were conspicuously absent. Also missing were studies on integration of care interventions which may prove useful to resolve ongoing healthcare service debates. The debate over the effectiveness of mainstream versus specialised health services for persons with an intellectual disability remains unanswered and requires rigorous study. A study looking at the effects of Community Learning Disability Teams (or similar model) would be useful to guide countries that are considering adopting this approach as a standard of care. The objective of the current review was to identify effective methods of organising healthcare services for persons with an intellectual disability; decision makers, however, also need information on the efficient use of resources. Researchers should address economic issues more thoroughly.
Although researchers have identified challenges when conducting randomised controlled trials targeting persons with an intellectual disability (Lennox 2005; Oliver 2002), we found seven randomised trials, two of which included persons with a severe intellectual disability (Dowling 2006; Hassiotis 2009). Intervention trials using randomisation procedures are therefore feasible in this population and should be the first choice to test the effectiveness of organisational interventions. When a randomised trial is not possible, researchers could consider conducting a controlled clinical trial, controlled before‐and‐after study or an interrupted time series. To be rigorous, before‐and‐after studies require comparable control groups and interrupted time series require at least three observation points before and three observation points after the intervention. Future research should include sample size calculations to ensure adequate study power and measures of clinical, care giver burden, and quality of life outcomes. High‐quality health services research aimed at improving the lives of persons with an intellectual disability is possible, and long overdue.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2015 | New citation required but conclusions have not changed | Methods changed to align with current guidance. Study selection criteria revised to include RCTs only. Search strategies updated to reflect new terminology employed. This review includes seven studies. |
| 11 December 2015 | New search has been performed | New searches performed to 4 September 2015. One new study identified. |
History
Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 12 November 2008 | Amended | Minor changes |
| 9 November 2008 | Amended | Formatting changed slightly |
| 25 June 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank George Tomlinson for help with the statistical analyses and Sandra Langlands for guiding our initial literature search. Nicole Donaldson was an asset working with RevMan. Alain Mayhew was very supportive and provided comments and useful suggestions during each step of the study process. Patricia Oliver, Nick Bouras, and Elias Tsakanikos provided raw data to make a meta analysis possible. We thank Patrick Kelley, Kathy Lowe, David Allen, and Angela Hassiotis for the background information they provided. Jeremy Grimshaw, Jenn Torr, Mike Kerr, Sasha Shepperd, and Julia Worswick provided us with excellent and prompt editorial feedback. We thank Paul Miller for updating and conducting the searches for this update. National Institute for Health Research, via Cochrane Infrastructure funding to the Effective Practice and Organisation of Care Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Appendices
Appendix 1. Search strategies 2006‐2015
Medline (OVID)
1 exp intellectual disability/
2 exp learning disorders/
3 mentally disabled persons/
4 ((mental* or intellect* or development* or learning*) adj1 (deficien* or disabilit* or retard* or disorder* or impair*)).ti,ab.
5 (intellect* adj1 function*).ti,ab.
6 ((angelman* or bardet‐biedl* or brachmann‐de lange* or cat* cry* or cri‐du‐chat* or coffin‐lowry* or coffin* or crying cat* or de lange* or down* or fra* or fragile x or happy puppet* or labhart‐willi* or labhart‐willi‐prader‐fanconi* or laurence‐moon* or laurence‐moon‐bardet‐biedl* or laurence‐moon‐biedl* or x or martin‐bell* or prader‐labhart‐willi* or prader‐willi* or rett* or royer* or rubinstein* or rubinstein‐taybi* or willi‐prader* or william* or beuren*) adj2 syndrome*).ti,ab.
7 congenital hypothyroidism.ti,ab.
8 fetal alcohol spectrum disorders/
9 ((fetal or foetal) adj2 alcohol).ti,ab.
10 cretinism.ti,ab.
11 fetal iodine deficiency disorder.ti,ab.
12 foetal iodine deficiency disorder.ti,ab.
13 congenital myxedema.ti,ab.
14 congenital myxoedema.ti,ab.
15 rett* disorder.ti,ab.
16 danon disease.ti,ab.
17 antopol disease.ti,ab.
18 glycogen storage disease type 3.ti,ab.
19 glycogen storage disease 3.ti,ab.
20 glycogen storage disease type III.ti,ab.
21 glycogen storage disease III.ti,ab.
22 or/1‐21
23 case management/
24 patient care management/
25 home care services/
26 health services administration/
27 mental health services/
28 community mental health services/
29 patient care team/
30 health services accessibility/
31 delivery of health care/
32 case manag*.ti,ab.
33 ((home or domiciliary or community or outreach) adj2 (care or healthcare or program* or service* or treatment* or manag* or intervention*)).ti,ab.
34 ((multidisciplinary or interdisciplinary or multi‐disciplinary or inter‐disciplinary) adj team*).ti,ab.
35 outreach.ti,ab.
36 or/23‐35
37 22 and 36
38 exp randomized controlled trial/
39 controlled clinical trial.pt.
40 randomi#ed.ti,ab.
41 placebo.ab.
42 randomly.ti,ab.
43 Clinical Trials as topic.sh.
44 trial.ti.
45 or/38‐44
46 exp animals/ not humans/
47 45 not 46
48 37 and 47
49 limit 48 to yr="2006 ‐Current"
EMBASE (OVID)
1 exp *intellectual impairment/
2 exp *learning disorder/
3 *mental patient/
4 ((mental* or intellect* or development* or learning*) adj1 (deficien* or disabilit* or retard* or disorder* or impair*)).ti,ab.
5 (intellect* adj1 function*).ti,ab.
6 ((angelman* or bardet‐biedl* or brachmann‐de lange* or cat* cry* or cri‐du‐chat* or coffin‐lowry* or coffin* or crying cat* or de lange* or down* or fra* or fragile x or happy puppet* or labhart‐willi* or labhart‐willi‐prader‐fanconi* or laurence‐moon* or laurence‐moon‐bardet‐biedl* or laurence‐moon‐biedl* or x or martin‐bell* or prader‐labhart‐willi* or prader‐willi* or rett* or royer* or rubinstein* or rubinstein‐taybi* or willi‐prader* or william* or beuren*) adj2 syndrome*).ti,ab.
7 congenital hypothyroidism.ti,ab.
8 *fetal alcohol syndrome/
9 ((fetal or foetal) adj2 alcohol).ti,ab.
10 cretinism.ti,ab.
11 fetal iodine deficiency disorder.ti,ab.
12 foetal iodine deficiency disorder.ti,ab.
13 congenital myxedema.ti,ab.
14 congenital myxoedema.ti,ab.
15 rett* disorder.ti,ab.
16 danon disease.ti,ab.
17 antopol disease.ti,ab.
18 glycogen storage disease type 3.ti,ab.
19 glycogen storage disease 3.ti,ab.
20 glycogen storage disease type III.ti,ab.
21 glycogen storage disease III.ti,ab.
22 or/1‐21
23 case manag*.ti,ab.
24 ((home or domiciliary or community or outreach) adj2 (care or healthcare or program* or service* or treatment* or manag* or intervention*)).ti,ab.
25 ((multidisciplinary or interdisciplinary or multi‐disciplinary or inter‐disciplinary) adj team*).ti,ab.
26 outreach.ti,ab.
27 *case management/
28 *patient care/
29 *home care/
30 home mental health care/
31 *health service/
32 exp *mental health care/
33 *health care delivery/
34 or/23‐33
35 22 and 34
36 random*.ti,ab.
37 factorial*.ti,ab.
38 (crossover* or cross over*).ti,ab.
39 ((doubl* or singl*) adj blind*).ti,ab.
40 (assign* or allocat* or volunteer* or placebo*).ti,ab.
41 crossover procedure/
42 single blind procedure/
43 randomized controlled trial/
44 double blind procedure/
45 or/36‐44
46 exp animal/ not human/
47 45 not 46
48 35 and 47
49 limit 48 to yr="2006 ‐Current"
CINAHL (EBSCO)
S1 (MH "Intellectual Disability+")
S2 (MH "Learning Disorders+")
S3 (MH "Mentally Disabled Persons")
S4 (mental* or intellect* or development* or learning*) N1 (deficien* or disabilit* or retard* or disorder* or impair*)
S5 (intellect* N1 function*)
S6 ((angelman* or bardet‐biedl* or brachmann‐de lange* or cat* cry* or cri‐du‐chat* or coffin‐lowry* or coffin* or crying cat* or de lange* or down* or fra* or fragile x or happy puppet* or labhart‐willi* or labhart‐willi‐prader‐fanconi* or laurence‐moon* or laurence‐moon‐bardet‐biedl* or laurence‐moon‐biedl* or x or martin‐bell* or prader‐labhart‐willi* or prader‐willi* or rett* or royer* or rubinstein* or rubinstein‐taybi* or willi‐prader* or william* or beuren*) N2 syndrome*)
S7 congenital hypothyroidism
S8 ((fetal or foetal) N2 alcohol)
S9 cretinism
S10 fetal iodine deficiency disorder
S11 foetal iodine deficiency disorder
S12 congenital myxedema
S13 congenital myxoedema
S14 rett* disorder
S15 danon disease
S16 antopol disease
S17 glycogen storage disease type 3
S18 glycogen storage disease 3
S19 glycogen storage disease type III
S20 glycogen storage disease III
S21 (MH "Fetal Alcohol Syndrome")
S22 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21
S23 case N0 manag*
S24 (home or domiciliary or community or outreach) N2 (care or healthcare or program* or service* or treatment* or manag* or intervention*)
S25 (multidisciplinary or interdisciplinary or multi‐disciplinary or inter‐disciplinary) N0 team*
S26 outreach
S27 (MH "Case Management")
S28 (MH "Multidisciplinary Care Team")
S29 (MH "Home Health Care")
S30 (MH "Psychiatric Home Care")
S31 (MH "Health Services Administration")
S32 (MH "Community Mental Health Services")
S33 (MH "Mental Health Services")
S34 (MH "Health Services Accessibility")
S35 (MH "Health Care Delivery")
S36 S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35
S37 S22 AND S36
S38 PT randomized controlled trial
S39 PT clinical trial
S40 TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly)
S41 (MH "Clinical Trials+")
S42 (MH "Random Assignment")
S43 S38 OR S39 OR S40 OR S41 OR S42
S44 S37 AND S43
S45 S37 AND S43 Limiters ‐ Published Date: 20060101‐20151231; Exclude MEDLINE records
PsycINFO (OVID)
1 exp intellectual development disorder/
2 cognitive impairment/
3 fetal alcohol syndrome/
4 exp learning disorders/
5 ((mental* or intellect* or development* or learning*) adj1 (deficien* or disabilit* or retard* or disorder* or impair*)).ti,ab.
6 (intellect* adj1 function*).ti,ab.
7 ((angelman* or bardet‐biedl* or brachmann‐de lange* or cat* cry* or cri‐du‐chat* or coffin‐lowry* or coffin* or crying cat* or de lange* or down* or fra* or fragile x or happy puppet* or labhart‐willi* or labhart‐willi‐prader‐fanconi* or laurence‐moon* or laurence‐moon‐bardet‐biedl* or laurence‐moon‐biedl* or x or martin‐bell* or prader‐labhart‐willi* or prader‐willi* or rett* or royer* or rubinstein* or rubinstein‐taybi* or willi‐prader* or william* or beuren*) adj2 syndrome*).ti,ab.
8 congenital hypothyroidism.ti,ab.
9 ((fetal or foetal) adj2 alcohol).ti,ab.
10 cretinism.ti,ab.
11 fetal iodine deficiency disorder.ti,ab.
12 foetal iodine deficiency disorder.ti,ab.
13 congenital myxedema.ti,ab.
14 congenital myxoedema.ti,ab.
15 rett* disorder.ti,ab.
16 danon disease.ti,ab.
17 antopol disease.ti,ab.
18 glycogen storage disease type 3.ti,ab.
19 glycogen storage disease 3.ti,ab.
20 glycogen storage disease type III.ti,ab.
21 glycogen storage disease III.ti,ab.
22 or/1‐21
23 case management/
24 home care/
25 exp mental health services/
26 health care delivery/
27 exp mental health programs/
28 teams/
29 community services/
30 case manag*.ti,ab.
31 ((home or domiciliary or community or outreach) adj2 (care or healthcare or program* or service* or treatment* or manag* or intervention*)).ti,ab.
32 ((multidisciplinary or interdisciplinary or multi‐disciplinary or inter‐disciplinary) adj team*).ti,ab.
33 outreach.ti,ab.
34 or/23‐33
35 22 and 34
36 exp clinical trial/
37 random*.ti,ab.
38 ((clinical or control*) adj3 trial*).ti,ab.
39 ((singl* or doubl* or trebl* or tripl*) adj5 (blind* or mask*)).ti,ab.
40 (volunteer* or control group or controls).ti,ab.
41 placebo/ or placebo*.ti,ab.
42 or/36‐41
43 35 and 42
44 limit 43 to yr="2006 ‐Current"
Cochrane Library
#1 [mh "intellectual disability"]
#2 [mh "learning disorders"]
#3 [mh "mentally disabled persons"]
#4 ((mental* or intellect* or development* or learning*) near/1 (deficien* or disabilit* or retard* or disorder* or impair*)):ti,ab
#5 (intellect* near/1 function*):ti,ab
#6 ((angelman* or bardet‐biedl* or brachmann‐de lange* or cat* cry* or cri‐du‐chat* or coffin‐lowry* or coffin* or crying cat* or de lange* or down* or fra* or fragile x or happy puppet* or labhart‐willi* or labhart‐willi‐prader‐fanconi* or laurence‐moon* or laurence‐moon‐bardet‐biedl* or laurence‐moon‐biedl* or x or martin‐bell* or prader‐labhart‐willi* or prader‐willi* or rett* or royer* or rubinstein* or rubinstein‐taybi* or willi‐prader* or william* or beuren*) near/2 syndrome*):ti,ab
#7 congenital hypothyroidism:ti,ab
#8 [mh "fetal alcohol spectrum disorders"]
#9 ((fetal or foetal) near/2 alcohol):ti,ab
#10 cretinism:ti,ab
#11 fetal iodine deficiency disorder:ti,ab
#12 foetal iodine deficiency disorder:ti,ab
#13 congenital myxedema:ti,ab
#14 congenital myxoedema:ti,ab
#15 rett* disorder:ti,ab
#16 danon disease:ti,ab
#17 antopol disease:ti,ab
#18 glycogen storage disease type 3:ti,ab
#19 glycogen storage disease 3:ti,ab
#20 glycogen storage disease type III:ti,ab
#21 glycogen storage disease III:ti,ab
#22 {or #1‐#21}
#23 [mh "case management"]
#24 [mh ^"patient care management"]
#25 [mh ^"home care services"]
#26 [mh ^"health services administration"]
#27 [mh "mental health services"]
#28 [mh "community mental health services"]
#29 [mh ^"patient care team"]
#30 [mh "health services accessibility"]
#31 [mh ^"delivery of health care"]
#32 case manag*:ti,ab
#33 ((home or domiciliary or community or outreach) near/2 (care or healthcare or program* or service* or treatment* or manag* or intervention*)):ti,ab
#34 ((multidisciplinary or interdisciplinary or multi‐disciplinary or inter‐disciplinary) next team*):ti,ab
#35 outreach:ti,ab
#36 {or #23‐#35}
#37 #22 and #36 Publication Year from 2006 to 2015
Data and analyses
Comparison 1. Effect of organisational interventions on behavioural problems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Interventions that increased the intensity and frequency of service delivery | Other data | No numeric data | ||
| 2 Community‐based specialist behaviour therapy | Other data | No numeric data | ||
| 3 Outreach treatment group | Other data | No numeric data | ||
| 4 Counselling intervention | Other data | No numeric data |
Comparison 2. Effect of organisational interventions on comorbid psychological and psychiatric function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Interventions that increased the intensity and frequency of service delivery: Global assessment of function (Disability) | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.05 [‐4.05, 6.16] |
| 2 Interventions that increased the intensity and frequency of service delivery: Global assessment of function (symptomatology) | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐6.07, 4.55] |
Comparison 3. Effect of organisational interventions on quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Interventions that increased the intensity and frequency of service delivery | 2 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.75, 0.36] |
| 2 Interventions that increased the intensity and frequency of service delivery | Other data | No numeric data |
Comparison 4. Effect of organisational interventions on unmet needs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Interventions that increased the intensity and frequency of service delivery | Other data | No numeric data |
4.1. Analysis.
Comparison 4 Effect of organisational interventions on unmet needs, Outcome 1 Interventions that increased the intensity and frequency of service delivery.
| Interventions that increased the intensity and frequency of service delivery | ||
|---|---|---|
| Study | Unmet needs | Needs |
| Hassiotis 2001 |
CAN number of needs T: 6.20 (SD 2.38); C: 7.85 (SD 3.61) Mean difference: ‐1.65, CI 95% ‐2.98 to ‐0.32 CAN number of unmet needs T: 1.50 (SD 1.80); C: 2.79 (SD 3.54) Mean difference: ‐1.29, CI 95% ‐2.52 to ‐0.06 |
The Camberwell Assessment of Need covers 22 topics, including accommodation and relations. Results for participants with an IQ ≤85 only Mean difference between groups (intensive and standard case management) at 24m |
| Martin 2005 |
CANDID health and care needs Adjusted mean difference: 0.25, 95% CI: ‐0.98 to 1.47 |
The Camberwell Assessment of Need for Adults with Developmental and Intellectual Disabilities covers 25 topics, including health and care needs. Adjusted difference between groups (standard treatment and assertive treatment) at 6m |
Comparison 5. Effect of organisational interventions on care giver burden.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Interventions that increased the intensity and frequency of service delivery | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐3.48, 3.54] |
| 2 Outreach treatment group | Other data | No numeric data |
Comparison 6. Effect of organisational interventions on costs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Interventions that increased the intensity and frequency of service delivery | Other data | No numeric data | ||
| 2 Community‐based specialist behaviour therapy | Other data | No numeric data | ||
| 3 Outreach treatment group | Other data | No numeric data |
Comparison 7. Effects of organisational interventions on health systems use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Interventions that increased the intensity and frequency of service delivery | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Coelho 1993.
| Methods | Study design: parallel RCT Types of interventions: Multidisciplinary team + Continuity of care + Change to setting/site of service |
|
| Participants | Clinical problem: Persons with moderate to mild ID with a DSM‐III‐R diagnosis of mental illness or behavioural complications concerning mental illness; residing in community Setting: Michigan, USA Sample size (N): I: 23; C: 23 Gender (male): 61% (for entire sample) Age (M): I: 34 years; C: 33 years |
|
| Interventions | C: Standard case management specialising in ID. Community mental health agency administers range of multidisciplinary mental health services coordinated by case manager specialising in ID.
(Caseload average 35 participants per case manager. Approximately 0.5 episodes of direct care per week). I: Intensive case management specialising in ID. Intervention provides services described in C as well as greater direct contact services with participants in their natural environment. Team members specialised in ID. (Caseload between 7‐10 participants per professional. Approximately 2 episodes of direct care per week). |
|
| Outcomes | Behaviour:
Adaptive behaviour: Relevant section of American Association of Mental Deficiency Adaptive Behaviour Scale (AAMD‐ABS) Maladaptive behaviour: Relevant section of AAMD‐ABS Michigan Maladaptive Behaviour Scale (MMBS) Quality of life and burden measures NR |
|
| Notes | Stratified by level of maladaptive behaviour before randomised ensuring similarity between groups. Used repeated measures analysis of variance to identify significant interaction term. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "24 persons were randomly assigned to the active treatment and 23 persons to the traditional treatment model" (pg 38) |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment provided in paper. |
| Baseline measurement? | Low risk | Group differences at baseline were accounted for using "2x4 (grouped by time) analysis of variance with repeated measures." (page 40). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Almost complete follow‐up. "One person in the active treatment died, leaving a final sample of 46 participants for analysis" (pg 38) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Main outcome not objective; staff conducting assessment not blinded to group allocation. “Thus, the participant, the participant’s own case manager, with staff familiar with the participant (e.g., day program and residential staff) assisted in the completion of assessments and returned them to the agency’s Evaluation Office for analyses” (pg. 38) |
| Proctection against contamination? | Unclear risk | No description of protection against contamination provided in paper. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not found. No description of reporting biases provided in the paper; all relevant outcomes in the methods section are reported in the results section. |
| Other bias | Unclear risk | No other biases reported. |
Dowling 2006.
| Methods | Study design: parallel RCT Types of interventions: Revision of professional roles |
|
| Participants | Clinical problem: Adults with ID who experienced significant bereavement (death of sibling or parent); excluded persons with dementia or psychosis Setting: United Kingdom Sample size (N): I: 11; C: 23 Gender (male): 41% (for entire sample) Age (range): I: < 30 to > 60 years; C: 30 to 60 years |
|
| Interventions | C: Standard mainstream control group. Trained bereavement counsellors with no experience working with persons with ID deliver counselling in fixed setting of client’s choice.
(Initially 1 episode of direct care per week, then every other week: average of 15 episodes of care per client). I: Bereavement work provided by care givers (family or paid worker) who know client well but have no bereavement counselling experience. (Frequency of care not reported). |
|
| Outcomes | Behaviour: Aberrant Behaviour Checklist‐Community (ABC‐C) comprising scales for 1) irritability, 2) lethargy, 3) stereotypy, 4) hyperactivity, 5) inappropriate speech Health of the Nation Outcome Scales for People with Learning Disabilities (HoNOS‐LD) Quality of life and burden measures NR |
|
| Notes | t‐test used to compare magnitude of change between pre‐ and post‐intervention scores. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The allocation sequence was generated by the research team” (pg. 279) “Cluster randomization was used to allocate residential units to one or other of the two interventions” (pg. 279) |
| Allocation concealment (selection bias) | Low risk | “The allocation sequence was generated by the research team, with an independent observer present to verify that allocation was indeed concealed” (pg. 279) |
| Baseline measurement? | Low risk | Difference in groups greater than 10% for some outcome measures. Analysis took baseline into consideration by comparing change of scores between groups. “An independent samples t‐test was used to compare the magnitude of change between the pre‐ and postintervention scores for both standardized measures, between the counselling group and the integrated intervention group, in an 'intention to treat' analysis”. (pg. 279) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | They recruited and had consent for 56 total participants – of the 24 assigned to integrated intervention, only “9 attempted and 2 completed” for a total of 11, and of the 32 assigned to counselling intervention, “20 completed intervention”. (Fig 1, pg. 280) Therefore less than 80% were followed up. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Main outcome not objective; staff conducting assessment not blinded to group allocation. Participants and personnel not blinded to allocation. |
| Proctection against contamination? | High risk | Contamination of interventions may have occurred. "The success in this particular case may have been influenced by the fact that the two people, delivering the intervention were also, coincidentally, bereavement counsellors." (page 286). |
| Selective reporting (reporting bias) | Unclear risk | Protocol not found; all relevant outcomes in the methods section are reported in the results section. |
| Other bias | Unclear risk | No other biases addressed in the paper. |
Hassiotis 2001.
| Methods | Study design: parallel RCT Types of interventions: Multidisciplinary team + Continuity of care |
|
| Participants | Clinical problem: Patients with severe psychotic illness with mild intellectual disability (IQ range 51‐70) or borderline IQ (IQ range 71‐85) Setting: London and Manchester, England Sample size (N): I: 50; C: 54 Gender (male): 65% (for entire sample) Age (median): 36.5 years |
|
| Interventions | C: Standard mainstream case management: Case manager is trained mental health professional responsible for direct care and coordinating health and social inputs outside of hospital; member of multidisciplinary team (1 case manager: 30‐35 clients). I: Intensive mainstream case management. Same as C but smaller case load (1 case manager: 10‐15 clients). |
|
| Outcomes | Mean number of days in hospital for psychiatric reasons Quality of life: Lancashire quality of life profile Burden measures NR |
|
| Notes | Results are from larger study including persons without low IQ score. 95% confidence intervals show effect of intervention in persons with lower IQ. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done by an independent statistical centre. |
| Allocation concealment (selection bias) | Low risk | A centralised randomisation scheme was used. |
| Baseline measurement? | Low risk | Baseline measurements were collected and were similar in both groups. Analyses were performed adjusting for baseline levels. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up < 90% for objective outcomes; missing data between 2% and 32% for subjective outcomes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Main outcomes for this sub‐study were objective (costs and days in hospital). Participants and personnel not blinded to allocation. |
| Proctection against contamination? | Unclear risk | No description of protection again contamination provided in paper. |
| Selective reporting (reporting bias) | Low risk | Main outcomes prespecified by the protocol included in the final report (hospital admission and length of stay; met and unmet needs; patient satisfaction; contact with case managers, cost‐effectiveness; referral and service utilisation). Medication compliance and added cost‐effectiveness not reported. |
| Other bias | Unclear risk | No other biases were reported in the paper. |
Hassiotis 2009.
| Methods | Study design: parallel RCT Types of interventions: Multidisciplinary team + Change in scope and nature of services |
|
| Participants | Clinical problem: Adults with ID and challenging behaviour Setting: Greater London (West Essex), England Sample size (N): I: 32; C: 31 Gender (male): I: 59%; C: 58% Ethnicity (Caucasian): I: 93%; C: 96% Age (median): I: 39.6 years; C: 41.3 years Level of ID: Mild/Moderate: I: 62.5%; C: 71.0%. Severe/Profound: I: 37.5%; C: 29.0% |
|
| Interventions | C: Standard treatment: Consists of five community intellectual disabilities teams ‐ each have a mix of psychiatrists, community nurses, occupational therapists, speech and language therapists, physiotherapists, and psychologists. Teams offer a range of interventions, such as pharmacotherapy, nursing and enhancement of adaptive skills. I: Behaviour therapy team: Provides a multi‐dimensional model of treatment including applied behaviour analysis and positive behaviour support. Team is comprised of one team coordinator, five full‐time behaviour specialists, two part‐time behaviour associate practitioners, and one administrator. The behaviour associate practitioners work directly with individuals and the care givers to support the implementation of the behaviour treatment plan. Care givers are expected to employ behavioural strategies, and training is provided to enhance their skills. |
|
| Outcomes | Behaviour: Aberrant Behaviour Checklist (ABC) comprising scales for 1) irritability, 2) lethargy, 3) stereotypy, 4) hyperactivity, 5) inappropriate speech. Psychiatric Assessment Schedule for Adutls with a Developmental Disability Checklist (PAS‐ADD); measures the presence of comorbid affective disorders, organic mental disorders, and psychotic disorders. Quality of life and burden measures NR |
|
| Notes | Scores of the ABC and its sub‐scales were skewed and therefore transformed using their square root. Common intervention effects were estimated for two pairs of ABC sub‐scale outcomes (total irritability paired with inappropriate speech; lethargy paired with hyperactivity). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Participants were randomly assigned individually to either the intervention or the standard treatment arm. One of the authors (R.B.) developed a computer‐driven randomization list that was stratified by clinical catchment area and based on a block size of four.” (pg. 1280) |
| Allocation concealment (selection bias) | Low risk | "A set of sealed envelopes, each bearing only the name of the area and a number, were held by an independent administrator." (page 1280) |
| Baseline measurement? | Low risk | “Assessments were made at baseline (when the participant enrolled in the trial but before randomization), at 3 months, and at 6 months (end of trial participation).” (pg. 1280) “We adjusted for baseline total score and time period”(pg. 1281) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Almost complete follow‐up (0.05% attrition rate). At the 3‐month follow‐up 2 people did not continue to participate and at 6‐month follow‐up, 1 person did not continue to participate. (Figure 1 page 1279). |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Main outcomes were not objective. No description of blinding of outcome assessment in paper. Participants and personnel not blinded to allocation. |
| Proctection against contamination? | Unclear risk | No description of protection against contamination in paper. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not found; all relevant outcomes in the methods section are reported in the results section. |
| Other bias | Unclear risk | “Because scores on the Aberrant Behavior Checklist and its subscale were highly skewed, we transformed them by taking their square root. However, in approaching normality, such transformations change the relative distances between data points. This makes interpretation of the data complex even though all data points remain in the same rank order as before. Although results can still be understood in terms of increasing scores, care must be used in interpreting results based on transformed data” (pg. 1281) “The effect for time period was assumed to be the same across all outcomes by including a common coefficient for each outcome in the model” (pg. 1281) “Given that randomization in a small trial may not eliminate systematic bias, we also adjusted the model for gender, age, level of disability, and mobility status but found no significant association” (pg. 1282) |
Martin 2005.
| Methods | Study design: parallel RCT Types of interventions: Multidisciplinary team + continuity of care |
|
| Participants | Clinical problem: Persons with mild to moderate intellectual disability and psychiatric disorder Setting: South‐east London, England Sample size (N): I: 10; C: 10 Gender (male): 50% (for entire sample) Age (M): 45 years (for entire sample) |
|
| Interventions | C: Standard community treatment team specialising in ID. (One member of team has direct contact no more than once per week). I: Assertive community treatment team specialising in ID. Same as C but, with as many contacts as needed from two professionals, one of whom acts as case‐coordinator. Author note: Frequency of contact was main criterion for identifying assertive community treatment (frequency of contact not reported). |
|
| Outcomes | Function and problem behaviour: Global Assessment of Function (GAF) Symptomatolgy; Disability
Aberrant Behaviour Checklist (ABC) Quality of Life: Quality of Life Questionnaire (QOLQ) Care giver burden: Uplift/Burden scale |
|
| Notes | Differences in baseline measurement between groups adjusted using ANCOVA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “[patients] were then randomized within strata...” (pg. 518) |
| Allocation concealment (selection bias) | Low risk | "Allocation was by stratified randomization by an independent statistician. The prognostic factors used in the randomization were gender and psychiatric diagnosis (psychosis vs. affective). Service users were randomly allocated to either ACT‐ID or SCT‐ID." (page 518) No description of allocation concealment in the paper. |
| Baseline measurement? | Low risk | Baseline measurements were collected. "Differences between groups at follow‐up adjusted for baseline level." (page 520) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description of incomplete outcome data in the paper. All 20 participants were followed up (confirmation obtained through communication with author). |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | At baseline, both the clinician and the research assistant were blind to the other’s assessment. Unclear if at follow‐up the research assistant was still blind to group allocation. (page 518). Participants and personnel not blinded to allocation. |
| Proctection against contamination? | High risk | "This use of narrow, specific criteria to define ACT might also have resulted in a blurring of the distinction between the two treatment arms." (page 521). |
| Selective reporting (reporting bias) | Unclear risk | Protocol not found; all relevant outcomes in the methods section are reported in the results section. |
| Other bias | Unclear risk | There are no other biases addressed in this paper |
Oliver 2005.
| Methods | Study design: parallel RCT Types of interventions: Multidisciplinary team |
|
| Participants | Clinical problem: Persons with mild to moderate intellectual disability and a (1) serious mental health disorder or (2) challenging behaviour, or both (1) and (2) Setting: England Sample size (N): I: 15; C: 15 Gender (male): 43% (for entire sample) Age (M): 40.53 years |
|
| Interventions | C: Standard community treatment team specialising in ID. Author note: standard = no more than one visit per week from any one professional.
(Average 9.87 intervention events from a professional over 3 weeks). I: Assertive community treatment team specialising in ID. Same as C, but more frequent contact. Author note: assertive = more than one visit per week from one or more professionals. (Average 16.8 intervention events from a professional over 3 weeks). |
|
| Outcomes | Function: Global Assessment of Function (GAF) Symptomatology; Disability Quality of Life: World Health Organisation Quality of Life‐Bref (WHOQOL‐Bref) Care giver burden: Uplift/Burden scale |
|
| Notes | Authors used two way ANOVA. Differences in outcome were compared using time x intervention type interaction term in model | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The trial had an external randomization officer based at a different site in London from either of the recruitment centres; randomization was generated using a random number list” (pg. 508) |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment in the paper |
| Baseline measurement? | Low risk | Baseline measurements were collected. "Using two way analysis of variance in which differences in outcomes were measured by time/treatment model interaction there was no significant difference in gains for clinical symptomatology (p = 0.80) and social functioning (p = 0.79) between assertive and standard groups... The WHO‐QOL‐Brief sub‐scale (p = 0.05) and psychological sub‐scale (p = 0.06) were not major outcomes but showed differences between the groups that favoured standard care." (page 510) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30 patients were randomly allocated to intervention groups, 30 patients were analysed at the end of the intervention. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Assessments done by a research associate blinded to group allocation. “All non‐diagnostic assessments were repeated after 12 weeks by the research associate masked to knowledge of treatment.” (page 510) Participants and personnel not blinded to allocation. |
| Proctection against contamination? | High risk | No description of protection against contamination in the paper. “The overlap between the two complex interventions was substantial and the lack of difference in outcome could be explained as much by lack of fidelity to the assertive model as by a genuine equivalence of the treatments” (pg. 511) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not found; all relevant outcomes in the methods section are reported in the results section. |
| Other bias | Unclear risk | No other bias identified. |
van Minnen 1997.
| Methods | Study design: parallel RCT Types of interventions: Multidisciplinary team + Changes to the setting/site of service delivery + Continuity of care |
|
| Participants | Clinical problem: Persons with mild or borderline intellectual disability and serious mental illness (i.e. require in‐patient hospitalisation) Setting: Netherlands Sample size (N): I: 25; C: 25 Gender (male): 76% (for entire sample) Age (M, SD): I: 31.4 years (12.6); C: 31.0 (10.8) |
|
| Interventions | C: Standard hospital treatment: 48‐bed facility specialising in treatment of people with dual diagnosis. Interventions include: psychopharmacological medication, behavioural therapy, social skills training, education, structured daily activities.
(Patient contact 24 hours/day). I: Outreach treatment team: One member of team visits patient in home environment; works with care givers involved in daily life. Other interventions similar to C. (Interventions average 1 hour per week per patient). |
|
| Outcomes | Psychiatric symptoms: Psychopathology Inventory for Mentally Retarded Adults Subject Response (PIMRA‐SR); Psychopathology Inventory for Mentally Retarded Adults Informant response (PIMRA‐I); Reiss Screen for Maladaptive Behaviour Quality of life measure NR Care giver burden: Nijmegen Child‐Rearing Situation Questionnaire (NCSQ) |
|
| Notes | Differences in baseline measurement between groups adjusted using ANCOVA Authors also conducted equivalence testing |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of random sequence generation in the paper |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment in the paper. |
| Baseline measurement? | Low risk | Baseline measurements were collected and were similar in both groups (Table 1) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “60 patients were randomized (30 patients to hospital treatment and 30 patients to outreach treatment). Ten patients (five in each group) dropped out before entering the study” (pg. 516) The final group of 50 patients were retained throughout the study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Main outcomes were not objective. No description of blinding of outcome assessment described in paper. Participants and personnel not blinded to allocation. |
| Proctection against contamination? | Low risk | "The outreach treatment group received treatment in their home environment, i.e., at home with their parents (n = 8), in sheltered accomodation (n = 7), in group homes or institutions for the care of mentally retarded people (n = 8), or in some other form of accomodation (n = 2)." (page 517). Unlikely that control group received treatment. No description of protection against contamination in the paper |
| Selective reporting (reporting bias) | High risk | “The NCSQ was administered to both groups at baseline, and after 14 and 28 weeks only to the outreach treatment group. No 14‐ and 28‐week measurements were obtained in the hospital group because most of the patients had been relocated from an institution to the hospital and were no longer in contact with the carers from their original institution.” (pg. 518) |
| Other bias | Unclear risk | "Equivalence testing (26, 27) was used to compare outreach and hospital treatment” (pg. 518) |
Characteristics of findings tables:
C: Control group conditions;
I: Intervention group conditions.
ID: intellectual disability.
ITS: interrupted time series.
RCT: randomised controlled trial.
CBA: controlled before and after study.
NR: not reported.
NS: not significant.
SD: standard deviation.
CI 95% confidence interval. * primary outcome was either identified by original study author or identified by review authors as best reflecting intervention
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 1998 | Interrupted time series study. Excluded a posteriori during the first update (2015) |
| Aronow 2005 | Pilot for upcoming RCT study Outcome measure is being tested No data available comparing intervention to control group |
| Arya 2002 | Study participants are children |
| Bergström 2013 | Participants did not have dual diagnosis |
| Caldwell 2006 | Not an organisational intervention |
| Calkins 1994 | Persons with intellectual disability not identified in study Participants must speak and read English |
| Chadwick 2009 | Evaluation of training program for staff working with persons with intellectual disability |
| Cooper 2006 | No outcome measure at baseline |
| Darrow 2011 | Participants did not have dual diagnosis |
| Goldsmith 2000 | Impact of intervention not studied |
| Greenswag 1990 | Study of children with Prader‐Willi syndrome |
| Guo 2001 | Does not evaluate effect of intervention on persons with intellectual disability alone Evaluation of mobile services on hospitalisation outcomes |
| Hassiotis 2011 | Not an organisational intervention |
| Hassiotis 2014 | Not an organisational intervention (study protocol) |
| Holmes 2004 | Persons with intellectual disability not identified in study Comparisons of community mental health centres |
| Hylkema 2009 | Evaluated the impact of a behavioural intervention on sleep in people with an intellectual disability |
| Jones 1997 | Randomised controlled trial of opportunistic health screening tool Published study is missing relevant and interpretable data Study authors contacted to obtain unpublished analysis |
| Lennox 2008 | Not an organisational intervention No baseline measure |
| Lowe 1996 | Controlled before and after study. Excluded a posteriori during the first update (2015) |
| MacDonald 2006 | Study sample is primarily children and not necessarily persons with an intellectual disability |
| McCabe 2006 | Randomised controlled trial of cognitive behavioural programme Outside scope of review |
| McKee 1994 | Pharmacy intervention in institution‐based residential setting Controlled before and after with no control group and interrupted time series |
| Merrick 2000 | Study participants are children |
| Raghavan 2009 | Included participants younger than 16 years old |
| Rudolph 1998 | Follow‐up study with no control group |
| Webb 1999 | Follow‐up study with no control group |
| Wells 1997 | Follow‐up study using general population as control |
| Willner 2013 | Not an organisational intervention |
| Young 2006 | Evaluation of institution versus community living services |
Differences between protocol and review
For this update, we changed the eligibility criteria for study design and only included randomised controlled trials and cluster randomised controlled trials. The original version of the review also included controlled before‐after (CBA) and interrupted time series (ITS), and it identified one CBA and one ITS. Their results were consistent with the RCTs identified, which have become an accepted and more commonly‐used method to conduct health services research for people with an intellectual disability. For these reasons and because of the increased time and resources required to screen references, the authors decided to include only RCTs.
For this update, the search strategy was updated to reflect a transition in the terminology used to refer to persons with an intellectual disability.
For this update, other changes were introduced to reflect Cochrane and EPOC current recommendations and methodological standards: a new outcome (adverse events); 'Risk of bias' tables; and 'Summary of findings' tables. A new author was added to the team (DCGB).
Contributions of authors
R Balogh conceived and designed the review with the supervision of A Colantonio and H Ouellette‐Kuntz. R Balogh, H Ouellette‐Kuntz, and A Colantonio prepared the protocol. For the original review (Balogh 2008), the search strategy, search result screening, retrieval of papers, screening of retrieved papers against inclusion criteria, appraisal of quality and extraction of data from papers was conducted by R Balogh and L Bourne. A Colantonio acted as a third referee on decisions for inclusion of papers. Y Lunsky provided clinical and policy perspective and wrote to authors of papers for additional information. H Ouellette‐Kuntz edited the review with A Colantonio, Y Lunsky, and L Bourne.
For this first update, D Gonçalves‐Bradley and R Balogh screened the search results and retrieved and screened papers against inclusion criteria. C McMorris acted as third review author for any discrepancies. R Balogh and C McMorris extracted data and performed critical appraisal. D Gonçalves‐Bradley, R Balogh, and C McMorris analysed the certainty of evidence and built the 'Summary of findings' tables. All authors revised and approved the final version of the review. R Balogh acts as guarantor of the review.
Sources of support
Internal sources
University of Ontario Institute of Technology, Canada.
University of Toronto, Canada.
Queen's University, Canada.
Centre for Addiction and Mental Health, University of Toronto, Canada.
National Institute for Health Research, via Cochrane Infrastructure to the Effective Practice and Organisation of Care group, UK.
External sources
No sources of support supplied
Declarations of interest
RB: none known. CAM: none known. YL: none known. HO‐K: none known. LB: none known. AC: none known. DCGB: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Coelho 1993 {published data only}
- Coelho RJ, Kelley PS, DeatsmanKelley C. An experimental investigation of an innovative community treatment model for persons with a dual diagnosis (DD/MI). Journal of Rehabilitation 1993;59:37‐42. [Google Scholar]
Dowling 2006 {published data only}
- Dowling S, Hubert J, White S, Hollins S. Bereaved adults with intellectual disabilities: a combined randomized controlled trial and qualitative study of two community‐based interventions. Journal of Intellectual Disability Research 2006;50:277‐87. [DOI] [PubMed] [Google Scholar]
Hassiotis 2001 {published data only}
- Hassiotis A, Ukoumunne OC, Byford S, Tyrer P, Harvey K, Piachaud J, et al. Intellectual functioning and outcome of patients with severe psychotic illness randomised to intensive case management: report from the UK700 trial. British Journal of Psychiatry 2001;178:166‐71. [DOI] [PubMed] [Google Scholar]
Hassiotis 2009 {published data only}
- Hassiotis A, Canagasabey A, Robotham D, Marston L, Romeo R, King M. Applied behaviour analysis and standard treatment in intellectual disability: 2‐year outcomes. British Journal of Psychiatry 2011;198(6):490‐1. [DOI: 10.1192/bjp.bp.109.076646] [DOI] [PubMed] [Google Scholar]
- Hassiotis A, Robotham D, Canagasabey A, Romeo R, Langridge D, Blizard R, et al. Randomized, single‐blind, controlled trial of a specialist behavior therapy team for challenging behavior in adults with intellectual disabilities. American Journal of Psychiatry 2009;166(11):1278‐85. [DOI] [PubMed] [Google Scholar]
Martin 2005 {published data only}
- Martin G, Costello H, Leese M, Slade M, Bouras N, Higgins S, et al. An exploratory study of assertive community treatment for people with intellectual disability and psychiatric disorders: conceptual, clinical, and service issues. Journal of Intellectual Disability Research 2005;49:516‐24. [DOI] [PubMed] [Google Scholar]
Oliver 2005 {published data only}
- Oliver PC, Piachaud J, Tyrer P, Regan A, Dack M, Alexander R, et al. Randomized controlled trial of assertive community treatment in intellectual disability: the TACTILD study. Journal of Intellectual Disability Research 2005;49:507‐15. [DOI] [PubMed] [Google Scholar]
van Minnen 1997 {published data only}
- Minnen A, Hoogduin CA, Broekman TG. Hospital versus outreach treatment of patients with mental retardation and psychiatric disorders: a controlled study. Acta Psychiatrica Scandinavica 1997;95:515‐22. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 1998 {published data only}
- Allen D. Brief report: changes in admissions to a hospital for people with intellectual disabilities following the development of alternative community services. Journal of Applied Research in Intellectual Disabilities 1998;11:156‐65. [Google Scholar]
Aronow 2005 {published data only}
- Aronow HU, Hahn JE. Stay well and healthy! Pilot study findings from an in‐home preventive healthcare programme for persons ageing with intellectual and/or developmental disabilities. Journal of Applied Research in Intellectual Disabilities Special Issue: Health Inequalities and People with Intellectual Disabilities 2005;18(2):163‐73. [Google Scholar]
Arya 2002 {published data only}
- Arya S. Delivery of services through itinerant service model. Journal of Personality and Clinical Studies 2002;18(1‐2):67‐72. [Google Scholar]
Bergström 2013 {published data only}
- Bergström H, Hagströmer M, Hagberg J, Elinder LS. A multi‐component universal intervention to improve diet and physical activity among adults with intellectual disabilities in community residences: a cluster randomised controlled trial. Research in Developmental Disabilities 2013;34:3847‐57. [DOI: 10.1016/j.ridd.2013.07.019] [DOI] [PubMed] [Google Scholar]
Caldwell 2006 {published data only}
- Caldwell J. Consumer‐directed supports: economic, health, and social outcomes for families. Mental Retardation: A Journal of Practices, Policy and Perspectives 2006;44(6):405‐17. [DOI] [PubMed] [Google Scholar]
Calkins 1994 {published data only}
- Calkins DR, Rubenstein LV, Cleary PD, Davies AR, Jette AM, Fink A, et al. Functional disability screening of ambulatory patients: a randomized controlled trial in a hospital‐based group practice. Journal of General Internal Medicine 1994;9(10):590‐2. [DOI] [PubMed] [Google Scholar]
Chadwick 2009 {published data only}
- Chadwick DD, Jolliffe J. A pilot investigation into the efficacy of a signing training strategy for staff working with adults with intellectual disabilities. British Journal of Learning Disabilities 2009;37(1):34‐42. [Google Scholar]
Cooper 2006 {published data only}
- Cooper SA, Morrison J, Melville C, Finlayson J, Allan L, Martin G, et al. Improving the health of people with intellectual disabilities: outcomes of a health screening programme after 1 year. Journal of Intellectual Disability Research 2006;50(Pt 9):667‐77. [DOI] [PubMed] [Google Scholar]
Darrow 2011 {published data only}
- Darrow SM. Examining multiple outcomes of staff training. Dissertation Abstracts International: Section B: The Sciences andEngineering 2012;72:7679. [Google Scholar]
Goldsmith 2000 {published data only}
- Goldsmith S, Cooray S, Johnston F, Williams G. Good practice, general practice: Identifying the health needs of people with learning disabilities. Journal of Clinical Governance 2000;8(2):83‐8. [Google Scholar]
Greenswag 1990 {published data only}
- Greenswag LR. A community outreach program for individuals with Prader‐Willi syndrome. Journal of Pediatric Health Care 1990;4(1):32‐8. [DOI] [PubMed] [Google Scholar]
Guo 2001 {published data only}
- Guo S, Biegel DE, Johnsen JA, Dyches H. Assessing the impact of community based mobile crisis services on preventing hospitalization. Psychiatric Services 2001;52(2):223‐8. [DOI] [PubMed] [Google Scholar]
Hassiotis 2011 {published data only}
- Hassiotis A, Serfaty M, Azam K, Strydom A, Martin S, Parkes C, et al. Cognitive behaviour therapy (CBT) for anxiety and depression in adults with mild intellectual disabilities (ID): a pilot randomised controlled trial. Trials 2011;12:95. [DOI: 10.1186/1745-6215-12-95] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hassiotis 2014 {published data only}
- Hassiotis A, Strydom A, Crawford M, Hall I, Omar R, Vickerstaff V, et al. Clinical and cost effectiveness of staff training in Positive Behaviour Support (PBS) for treating challenging behaviour in adults with intellectual disability: a cluster randomised controlled trial. BMC Psychiatry 2014;14:219. [DOI: 10.1186/s12888-014-0219-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holmes 2004 {published data only}
- Holmes AM, Deb P. Performance assessment in community mental health care and at risk populations. Health Care Financing Review 2004;26(1):75‐84. [PMC free article] [PubMed] [Google Scholar]
Hylkema 2009 {published data only}
- Hylkema T, Vlaskamp C. Significant improvement in sleep in people with intellectual disabilities living in residential settings by non‐pharmaceutical interventions. Journal of Intellectual Disability Research 2009;53(8):695‐703. [DOI] [PubMed] [Google Scholar]
Jones 1997 {published data only}
- Jones RG, Kerr MP. A randomized control trial of an opportunistic health screening tool in primary care for people with intellectual disability. Journal of Intellectual Disability Research 1997;41(5):409‐15. [DOI] [PubMed] [Google Scholar]
Lennox 2008 {published data only}
- Lennox NG, Rey‐Conde TF, Faint SL. A pilot of interventions to improve health care in adolescents with intellectual disability. Journal of Applied Research in Intellectual Disabilities 2008;21(5):484‐9. [Google Scholar]
Lowe 1996 {published data only}
- Lowe K, Felce D, Blackman D. Challenging behaviour: the effectiveness of specialist support teams. Journal of Intellectual Disability Research 1996;40:336‐47. [PubMed] [Google Scholar]
MacDonald 2006 {published data only}
- MacDonald A, Manji N, Evans S, Davies P, Daly A, Hendriksz C, et al. Home delivery of dietary products in inherited metabolic disorders reduces prescription and dispensing errors. Journal of Human Nutrition & Dietetics 2006;19(5):375‐81. [DOI] [PubMed] [Google Scholar]
McCabe 2006 {published data only}
- McCabe MP, McGillivray JA, Newton DC. Effectiveness of treatment programmes for depression among adults with mild/moderate intellectual disability. Journal of Intellectual Disability Research 2006;50(4):239‐47. [DOI] [PubMed] [Google Scholar]
McKee 1994 {published data only}
- McKee JR. Clinical pharmacy services in an intermediate care facility for the mentally retarded. Hospital Pharmacy 1994;29(3):228‐30. [PubMed] [Google Scholar]
Merrick 2000 {published data only}
- Merrick J, Shapira J. Preventive dental health for persons with Down syndrome. International Journal of Adolescent Medicine and Health 2000;12(1):81‐4. [DOI] [PubMed] [Google Scholar]
Raghavan 2009 {published data only}
- Raghavan R, Newell R, Waseem F, Small N. A randomized controlled trial of a specialist liaison worker model for young people with intellectual disabilities with challenging behaviour and mental health needs. Journal of Applied Research in Intellectual Disabilities 2009;22(3):256‐63. [Google Scholar]
Rudolph 1998 {published data only}
- Rudolph C, Lakin KC, Oslund JM, Larson W. Evaluation of outcomes and cost effectiveness of a community behavioral support and crisis response demonstration project. Mental Retardation 1998;36(3):187‐97. [DOI] [PubMed] [Google Scholar]
Webb 1999 {published data only}
- Webb OJ, Rogers L. Health screening for people with intellectual disability: the New Zealand experience. Journal of Intellectual Disability Research 1999;43(6):497‐503. [DOI] [PubMed] [Google Scholar]
Wells 1997 {published data only}
- Wells MB, Turner S, Martin DM, Roy A. Health gain through screening ‐ coronary heart disease and stroke: developing primary health care services for people with intellectual disability. Journal of Intellectual and Developmental Disability 1997;22(4):251‐63. [Google Scholar]
Willner 2013 {published data only}
- Willner P, Rose J, Jahoda A, Stenfert Kroese B, Felce D, MacMahon P, et al. A cluster randomised controlled trial of a manualised cognitive–behavioural anger management intervention delivered by supervised lay therapists to people with intellectual disabilities. Health Technology Assessment 2013; Vol. 17, issue 21. [DOI: 10.3310/hta17210] [DOI] [PMC free article] [PubMed]
Young 2006 {published data only}
- Young L. Community and cluster centre residential services for adults with intellectual disability: long‐term results from an Australian‐matched sample. Journal of Intellectual Disability Research 2006;50(Pt 6):419‐31. [DOI] [PubMed] [Google Scholar]
Additional references
Alborz 2005
- Alborz A, McNally R, Glendinning C. Access to health care for people with learning disabilities in the UK: mapping the issues and reviewing the evidence. Journal of Health Services and Research Policy 2005;10:173‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alexander 2002
- Alexander R, Regan A, Gangadharan S, Bhaumik S. Psychiatry of learning disability: a future with mental health?. Psychiatric Bulletin 2002;26:299‐301. [Google Scholar]
Ali 2015
- Ali A, Hall A, Blickwedel J, Hassiotis A. Behavioural and cognitive‐behavioural interventions for outwardly‐directed aggressive behaviour in people with intellectual disabilities. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD003406.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aman 1985
- Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. American Association on Mental Deficiency 1985;89(5):485‐91. [PubMed] [Google Scholar]
Anderson 2003
- Anderson LL, Larson SA, Lakin KC, Kwak N. Health insurance coverage and health care experiences of persons with disabilities in the NHIS‐D [Report 5] [Data Brief]. University of Minnesota, Research and Training Center on Community Living 2003.
Aspray 1999
- Aspray TJ, Francis RM, Tyrer SP, Quilliam SJ. Patients with learning disability in the community. BMJ 1999;318:476‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barelds 2009
- Barelds A, Goor I, Bos M, Heck G, J Schols. Care and service trajectories for people with intellectual disabilities: Defining its course and quality determinants from the client's perspective. Journal of Policy and Practice in Intellectual Disabilities 2009;6(3):163‐72. [DOI: 10.1111/j.1741-1130.2009.00224.x] [DOI] [Google Scholar]
Bouras 2004
- Bouras N, Holt G. Mental health services for adults with learning disabilities. British Journal of Psychiatry 2004;184:291‐2. [DOI] [PubMed] [Google Scholar]
Burns 1999
- Burns T, Creed F, Fahy T, Thompson S, Tyrer P, White I. Intensive versus standard case management for severe psychotic illness: a randomised trial. The Lancet 1999;353:2185‐9. [DOI] [PubMed] [Google Scholar]
Campbell 2000
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDCP 2004
- Centers for Disease Control and Prevention. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment, United States, 2003. Morbidity and Mortality Weekly Report 2004;53:57‐9. [PubMed] [Google Scholar]
Chaplin 2004
- Chaplin R. General psychiatric services for adults with intellectual disability and mental illness. Journal of Intellectual Disability Research 2004;48:1‐10. [DOI] [PubMed] [Google Scholar]
Chaplin 2006
- Chaplin R. Assertive outreach for people with intellectual disability and mental disorders. Journal of Intellectual Disability Research 2006;50:615‐6. [DOI] [PubMed] [Google Scholar]
ClinicalTrials.gov 2015
- US National Institutes of Health Trial Registry. https://clinicaltrials.gov/ Accessed 4 September 2015.
Cooper 2007
- Cooper SA, Smiley E, Morrison J, Williamson A, Allan L. Mental ill‐health in adults with intellectual disabilities: prevalence and associated factors. British Journal of Psychiatry 2007;196:27‐35. [DOI] [PubMed] [Google Scholar]
Cooper 2011
- Cooper SA, McConnachie A, Allan LM, Melville C, Smiley E, Morrison J. Neighbourhood deprivation, health inequalities and service access by adults with intellectual disabilities: a cross‐sectional study. Journal of Intellectual Disability Research 2011;55(3):313‐23. [DOI: 10.1111/j.1365-2788.2010.01361.x] [DOI] [PubMed] [Google Scholar]
Dieckmann 2015
- Dieckmann F, Giovis C, Offergeld J. The life expectancy of people with intellectual disabilities in Germany. Journal of Applied Research in Intellectual Disabilities 2015;28(5):373‐82. [DOI: 10.1111/jar.12193] [DOI] [PubMed] [Google Scholar]
DSM‐5
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM‐5. Washington, D.C.: American Psychiatric Association, 2013. [Google Scholar]
Durvasula 2001
- Durvasula S, Beange H. Health inequalities in people with intellectual disability: strategies for improvement. Health Promotion Journal of Australia 2001;11:27‐31. [Google Scholar]
EPOC 2012
- Cochrane Effective Practice and Organisation of Care Review Group. Data Collection Checklist: Risk of Bias. http://epoc.cochrane.org/resources (accessed 6 April 2012).
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). EPOC Taxonomy. https://epoc.cochrane.org/epoc‐taxonomy 2015. Accessed 1 October 2015.
Fisher 2004
- Fisher K. Health disparities and mental retardation. Journal of Nursing Scholarship 2004;36:48‐53. [DOI] [PubMed] [Google Scholar]
Fletcher 1993
- Fletcher RJ. Mental illness‐mental retardation in the United States: policy and treatment changes. Journal of Intellectual Disability Research 1993;37 Suppl:25‐33. [DOI] [PubMed] [Google Scholar]
Frid 1999
- Frid C, Drott P, Lundell B, Rasmussen F, Anneren G. Mortality in Down's syndrome in relation to congenital malformations. Journal of Intellectual Disability Research 1999;43:234‐41. [DOI] [PubMed] [Google Scholar]
Gilbody 2003
- Gilbody S, Whitty P, Grimshaw J, Thomas R. Educational and organisational interventions to improve the management of depression in primary care: a systematic review. Journal of the American Medical Association 2003;289:3145‐51. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, Schünemann HJ, for the GRADE Working Group. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hassiotis 2000
- Hassiotis A, Barron P, O'Hara J. Mental health services for people with learning disabilities. BMJ 2000;321:583‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hassiotis 2002
- Hassiotis, A. Community mental health services for individuals with intellectual disabilities issues and approaches to optimizing outcomes. Disease Management and Health Outcomes 2002;10:409‐17. [Google Scholar]
Havercamp 2006
- Havercamp SM, Scandlin D, Roth M. Health disparities among adults with developmental disabilities, adults with other disabilities, and adults not reporting disability in North Carolina. Public Health Reports 2006;119:418‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, [updated March 2011]. [Google Scholar]
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook of Systematic Reviews of Interventions. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, [updated March 2011]. [Google Scholar]
ICTRP 2015
- WHO International Clinical Trials Registry Platform. http://www.who.int/ictrp/en/ Accessed 4 September 2015.
Jansen 2006
- Jansen DEMC, Krol B, Groothoff JW, Post D. Towards improving medical care for people with intellectual disability living in the community: possibilities of integrated care. Journal of Applied Research in Intellectual Disabilities 2006;19:214‐8. [Google Scholar]
Keith 1987
- Keith, RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Advances in Clinical Rehabilitation 1987;1:6‐18. [PubMed] [Google Scholar]
Kelley 2006
- Kelley P. Personal communication, 11 October 2006.
Krahn 2006
- Krahn GL, Hammond L, Turner A. A cascade of disparities: health and health care access for people with intellectual disabilities. Mental Retardation and Developmental Disabilities Research Reviews 2006;12:70‐82. [DOI] [PubMed] [Google Scholar]
Lake 2014
- Lake JK, Palucka AM, Desarkar P, Hassiotis A, Lunsky Y. Chapter 22: inpatient mental health services. In: Tsakanikos E, McCarthy J editor(s). Handbook of Psychopathology in Intellectual Disability Research, Practice, and Policy. New York: Springer Science+Business Media, 2014. [Google Scholar]
Larson 2005
- Larson SA, Anderson L, Dlojanac R. Health promotion for persons with intellectual and developmental disabilities. In: Nehring W editor(s). Access to Health Care. Washington: American Association on Mental Retardation, 2005:129‐84. [Google Scholar]
Lennox 1995
- Lennox N, Chaplin R. The psychiatric care of people with intellectual disabilities: the perceptions of trainee psychiatrists and medical officers. Australian and New Zealand Journal of Psychiatry 1995;29:632‐37. [DOI] [PubMed] [Google Scholar]
Lennox 2002
- Lennox N. Health promotion and disease prevention. In: Prasher VP, Janicki MP editor(s). Physical Health of Adults with Intellectual Disabilities. Oxford: Blackwell Publishing Ltd, 2002:230‐49. [Google Scholar]
Lennox 2005
- Lennox N, Taylor M, Rey‐Conde T, Bain C, Purdie DM, Boyle F. Beating the barriers: recruitment of people with intellectual disability to participate in research. Journal of Intellectual Disability Research 2005;49:296‐305. [DOI] [PubMed] [Google Scholar]
Lennox 2015
- Lennox N, Driel ML, Dooren K. Supporting primary healthcare professionals to care for people with intellectual disability: a research agenda. Journal of Applied Research in Intellectual Disabilities 2015;28(1):33‐42. [DOI: 10.1111/jar.12132] [DOI] [PubMed] [Google Scholar]
Lin 2013
- Lin E, Selick A, Balogh RS, Isaacs BJ, Ouellette‐Kuntz HMJ, Klein‐Geltink JE, et al. Chapter 2: prevalence, demographic and disease profiles. In: Lunsky Y, Klein‐Geltink JE, Yates EA editor(s). Atlas on the Primary Care of Adults with Developmental Disabilities in Ontario. Toronto, ON: Institute for Clinical Evaluative Sciences, 2013. [Google Scholar]
Lynch 1997
- Lynch PS, Kellow JT, Willson VL. The impact of deinstitutionalization on the adaptive behavior of adults with mental retardation: a meta‐analysis. Education and Training in Mental Retardation and Developmental Disabilities 1997;32:255‐61. [Google Scholar]
Maulik 2011
- Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta‐analysis of population‐based studies. Research in Developmental Disabilities 2011;32(2):419‐36. [DOI: 10.1016/j.ridd.2010.12.018] [DOI] [PubMed] [Google Scholar]
Morgan 2008
- Morgan VA, Leonard H, Bourke J, Jablensky A. Intellectual disability co‐occurring with schizophrenia and other psychiatric illness: population‐based study. British Journal of Psychiatry 2008;193(5):364‐72. [DOI: 10.1192/bjp.bp.107.044461] [DOI] [PubMed] [Google Scholar]
Moss 1997
- Moss S, Ibbotson B, Prosser H, Goldberg D, Patel P, Simpson N. Validity of the PAS‐ADD for detecting psychiatric symptoms in adults with learning disability (mental retardation). Social Psychiatry and Psychiatric Epidemiology 1997;32(6):344‐54. [DOI] [PubMed] [Google Scholar]
Moss 2000
- Moss S, Bouras N, Holt G. Mental health services for people with intellectual disability: a conceptual framework. Journal of Intellectual Disability Research 2000;44:97‐107. [DOI] [PubMed] [Google Scholar]
O'Hara 2000
- O'Hara J. Learning disabilities services: primary care or mental health trust?. Psychiatric Bulletin 2000;24:368‐9. [Google Scholar]
Oliver 2002
- Oliver PC, Piachaud J, Done J, Regan A, Cooray S, Tyrer P. Difficulties in conducting a randomized controlled trial of health service interventions in intellectual disability: implications for evidence‐based practice. Journal of Intellectual Disability Research 2002;46:340‐5. [DOI] [PubMed] [Google Scholar]
Ouellette Kuntz 2005
- Ouellette Kuntz H, Garcin N, Lewis MES, Minnes P, Martin C, Holden JJA. Addressing health disparities through promoting equity for individuals with intellectual disability. Canadian Journal of Public Health.Revue Canadienne de Sante Publique 2005;96:S8‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patja 2001
- Patja K, Molsa P, Iivanainen M. Cause‐specific mortality of people with intellectual disability in a population‐based, 35‐year follow‐up study. Journal of Intellectual Disability Research 2001;45:30‐40. [DOI] [PubMed] [Google Scholar]
PDQ‐Evidence 2015
- PDQ‐Evidence. PDQ‐Evidence for Informed Health Policymaking. http://www.pdq‐evidence.org/en/ accessed 23 September 2015.
Pruchno 1990
- Pruchno R. The effects of help patterns on the mental health of spouse caregivers. Research on Ageing 1990;12:57‐71. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rich 1995
- Rich MW, Beckham V, Wittenberg C. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. The New England Journal of Medicine 1995;333:1190‐5. [DOI] [PubMed] [Google Scholar]
Robotham 2011
- Robotham D, King M, Canagasabey A, Inchley‐Mort S, Hassiotis A. Social validity of randomised controlled trials in health services research and intellectual disabilities: a qualitative exploration of stakeholder views. Trials 2011;12:144. [DOI: 10.1186/1745-6215-12-144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schalock 1993
- Schalock RL, Keith KD. Quality of Life Questionnaire Manual. Worthington, OH: IDS Publishing Corporation, 1993. [Google Scholar]
Slevin 2008
- Slevin E, Truesdale‐Kennedy M, McConkey R, Barr O, Taggart L. Community learning disability teams: developments, composition and good practice. Journal of Intellectual Disabilities 2008;12:59‐79. [DOI] [PubMed] [Google Scholar]
Tyrer 2009
- Tyrer F, McGrother C. Cause‐specific mortality and death certificate reporting in adults with moderate to profound intellectual disability. Journal of Intellectual Disability Research 2009;53(11):898‐904. [DOI: 10.1111/j.1365-2788.2009.01201.x] [DOI] [PubMed] [Google Scholar]
US PHS 2002
- United States Public Health Service Office of the Surgeon General. Closing the gap: a national blueprint to improve the health of persons with mental retardation. Report of the Surgeon General's Conference on Health Disparities and Mental Retardation 2002. [PubMed]
Van Schrojenstein 1997
- Schrojenstein Lantman de Valk HMJ, Akker M, Maaskant MA, Haveman MJ, Urlings HFJ, Kessels AGH, et al. Prevalence and incidence of health problems in people with intellectual disability. Journal of Intellectual Disability Research 1997;41:42‐51. [DOI] [PubMed] [Google Scholar]
Vereenooghe 2013
- Vereenooghe L, Langdon PE. Psychological therapies for people with intellectual disabilities: A systematic review and meta‐analysis. Research in Developmental Disabilities 2013;34(11):4085‐102. [DOI: 10.1016/j.ridd.2013.08.030] [DOI] [PubMed] [Google Scholar]
Wagner 1996
- Wagner EH, Austin BT, Korff M. Organizing care for patients with chronic illness. Milbank Quarterly 1996;74:511‐44. [PubMed] [Google Scholar]
Wagner 1998
- Wagner EH. Chronic disease management: what will it take to improve care for chronic illness?. Effective Clinical Practice 1998;1:2‐4. [PubMed] [Google Scholar]
Young 1998
- Young L, Sigafoos J, Suttie J, Ashman A, Grevell P. Deinstitutionalisation of persons with intellectual disabilities: a review of Australian studies. Journal of Intellectual and Developmental Disability 1998;23:155‐70. [Google Scholar]
References to other published versions of this review
Balogh 2008
- Balogh R, Ouellette‐Kuntz H, Bourne L, Lunsky Y, Colantonio A. Organising health care services for persons with an intellectual disability. Cochrane Database of Systematic Reviews 2008, Issue 8. [DOI: 10.1002/14651858.CD007492] [DOI] [PubMed] [Google Scholar]


