Abstract
Styrylchromones (SC) are a group of oxygen-containing heterocyclic compounds, which are characterized by the attachment of a styryl group to the chromone core. SC can be found in nature or can be chemically synthesized in the laboratory. As their presence in nature is scarce, the synthetic origin is the most common. Two types of SC are known: 2-styrylchromones and 3-styrylchromones. However, 2-styrylchromones are the most common, being more commonly found in nature and which chemical synthesis is more commonly described. A wide variety of SC has been described in the literature, with different substituents in different positions, the majority of which are distributed on the A- and/or B-rings. Over the years, several biological activities have been attributed to SC. This work presents a comprehensive review of the biological activities attributed to SC and their structure-activity relationship, based on a published literature search, since 1989. The following biological activities are thoroughly revised and discussed in this review: antioxidant, antiallergic, antiviral, antibacterial, antifungal, anti-inflammatory, and antitumoral, affinity and selectivity for A3 adenosine receptors, neuroprotective, and α-glucosidase inhibition. In general, SC are composed by a promising scaffold with great potential for the development of new drugs.
1. Introduction
Chromones are a group of oxygen-containing heterocyclic compounds with a benzoannelated γ-pyrone ring. The chromone ring system or chromone core (4H-chromen-4-ones or 4H-1-benzopyran-4-one, Figure 1) is found in several derivatives, and it is claimed as responsible for several biological activities, such as anti-inflammatory, antioxidant, anticancer, antiviral, and antimicrobial. Therefore, the chromone core is a privileged scaffold in drug discovery processes [1, 2].
Figure 1.
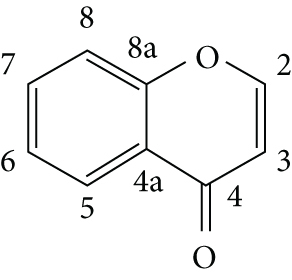
Chemical structure and numbering of the chromone core [1].
Among the compounds derived from chromones, there is a group characterized by having a styryl group attached to the chromone core, known as styrylchromones (SC) [3]. Over the years, several biological activities have been attributed to SC, such as antioxidant, anti-inflammatory, antimicrobial, antiviral, antitumor, neuroprotective, and antiallergic [3, 4]. This versatility has attracted the attention of several authors to the study of this group of chromones. Despite the various studies that already exist, there is still much more to discover and explore about the potential of SC. Thus, the present review gives a comprehensive insight into the biological activities of SC and their structure-activity relationship (SAR), based on the available information. This work provides an overview of the two types of SC that are known: 2-styrylchromones (2-SC) and 3-styrylchromones (3-SC), as well as their natural occurrence and synthetic approaches. Furthermore, their following biological activities are systematically described and discussed in terms of SAR: antioxidant, antiallergic, antiviral, antibacterial, antifungal, anti-inflammatory, and antitumoral. The neuroprotective activity, affinity and selectivity for A3 adenosine receptors, and α-glucosidase inhibition are also reported in this review.
2. Research Method
The bibliographic search for this review was carried out in the PubMed database using the keywords “styrylchromone” and “styryl-4H-chromen-4-one”. In the bibliographic search, 43 results were obtained, of which 4 were review articles (one of which was written in Japanese) and 39 were original articles. All the retrieved articles, written in English, from 1989 to the present (March 2021) have been analysed. From these articles, the cited references referring the biological activities of the SC were also included in this review. Furthermore, this review only includes studies with isolated compounds of synthetic and natural origin that are well characterized. Studies with SC's extracts or glycosylated derivatives were not included. Articles that do not present the methodologies used and with inconsistencies in the studied SC structures were also not included. The results presented throughout this review are presented according to the information available in the correspondent cited articles. Thus, the form of presentation of the results varies depending on the information available and may have the error associated (standard deviation or standard error of the mean) or not.
3. Styrylchromones
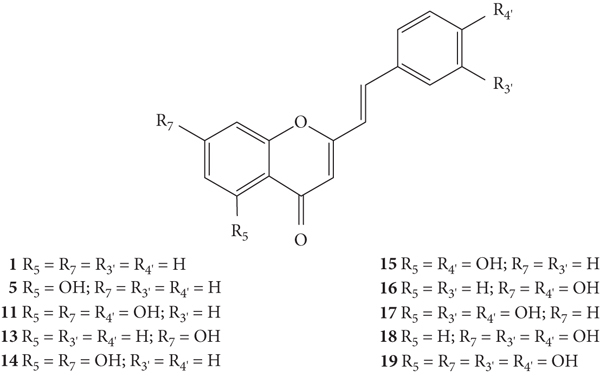
Styrylchromones belong to the group of compounds derived from chromones. These compounds have at least 17 carbons and are characterized by the attachment of a styryl group to the chromone core. Depending on the linking position of the styryl group, different SC are obtained. The position of this bond and the ring substituents determine the nomenclature of these compounds. The most common are 2-styrylchromones (2-SC) or 2-styryl-4H-chromen-4-one (1, Figure 2), where the bond occurs at C-2, and some 3-styrylchromones (3-SC) (2, Figure 2) are also known [3, 5].
Figure 2.
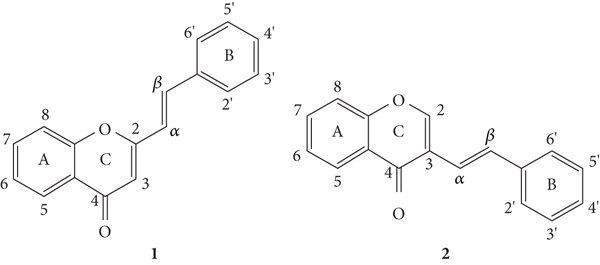
Chemical structures of 2-SC (1) and 3-SC (2) and numbering system to be adopted throughout this review [3].
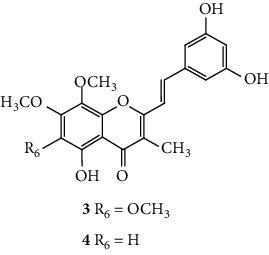
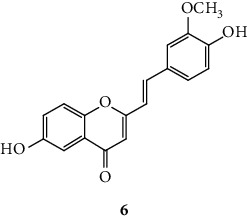
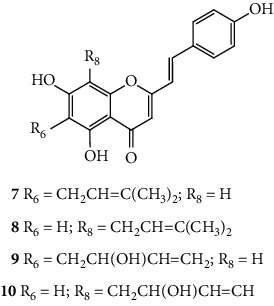
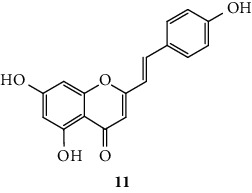
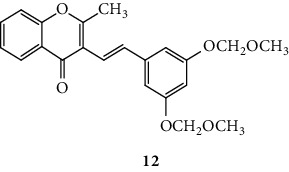
SC are mostly of synthetic origin since their presence in nature is scarce. Gerwick and co-workers [6], in 1986, in the north coast of Puerto Rico, collected a material that was identified as the marine cryptophyte Hormothamnion enteromorphoides [6]. From this cryptophyte, was isolated the first natural SC-hormothamnione (3, Table 1). Subsequently, Gerwick [7] revised the taxonomy of the material previously collected, concluding that it was erroneously identified. The marine cryptophyte was instead a Chrysophaeum taylori, from which they also isolated a new derivative – 6-desmethoxyhormothamnione (4, Table 1). Thus, hormothamnione (3) and its 6-desmethoxy analogue (4) are 2-SC that have been isolated from the marine cryptophyte Chrysophaeum taylori [7]. Several years after the isolation of the first natural SC, Yoon and co-workers [8] isolated a new 2-SC (5, Table 1) from the methanolic extract of the rhizomes of Imperata cylindrica. Years later, Yang and co-workers [9] isolated 2-SC 6 (Table 1) from the ethanolic extract of Chinese eaglewood. Chinese eaglewood is a resinous wood from the three of Aquilaria sinensis (Lour.) Gilg (Thymelaeaceae) [9]. Also, from an ethanolic extract, but from of the bark of Platanus × acerifolia (Aiton) Willd, five new 2-SC were isolated: platachromones A–D (7–10, Table 1) and 2-SC 11 (Table 1) [10]. Jung and co-workers [11] and Chaniad and co-workers [12] also isolated 2-SC 11 from the methanolic extract of the heartwood of Juniperus chinensis and from the ethanolic extract of the bulbils of Dioscorea bulbifera, respectively [11, 12]. All compounds isolated from natural sources mentioned above were 2-SC. However, to the best of our knowledge, only one natural 3-SC was yet isolated: 3-SC 12 (Table 1) was isolated from the aerial parts of Erucaria microcarpa [13]. In some cases, SC derivatives were first chemically synthesized and only later were isolated from natural sources; examples of this are 2-SC 5 and 11 [14, 15].
Table 1.
Chemical structures and natural occurring 2-SC and 3-SC.
| 2-SC | ||
| Chemical structure | Natural occurrence∗ | Ref. |
|
| ||
|
Chrysophaeum taylori | [6, 7] |
|
Imperata cylindrica | [8] |
|
Aquilaria sinensis (Lour.) Gilg (Thymelaeaceae) | [9] |
|
Platanus × acerifolia (Aiton) Willd | [10] |
|
Platanus × acerifolia (Aiton) Willd | [10] |
| Juniperus chinensis | [11] | |
| Dioscorea bulbifera | [12] | |
| 3-SC | ||
| Chemical structure | Natural occurrence∗ | Ref. |
|
| ||
|
Erucaria microcarpa | [13] |
∗According to the information provided in the original article.
Several approaches have been used and developed for the chemical synthesis of SC, leading to the obtention of a wide variety of compounds. The most common approach to 2-SC synthesis is the Baker-Venkataraman rearrangement, but there are others such as Allan-Robinson condensation, aldol condensation/oxidative cyclization, cyclization of an acetylenic ketone, condensation of 2-methylchromones with benzaldehydes, intramolecular Wittig reaction, and Knoevenagel condensation. In the synthesis of 3-SC, approaches such as oxidative rearrangement of 2′-hydroxychalcones to isoflavones using thallium (III) nitrate, Wittig reaction of 3-formylchromones with benzylic ylides, Knoevenagel condensation of chromone-3-carboxaldehyde with phenylacetic acids in the presence of potassium tert-butoxide under microwave irradiation, and Heck reaction of 3-bromochromone with styrene are used. These methodologies have been thoroughly reviewed and reported by several authors until now [3, 16–19].
4. Biological Activities
Several biological activities have been attributed to SC (Figure 3), namely, antioxidant, antiallergic, antiviral, antibacterial, antifungal, anti-inflammatory, antitumoral, neuroprotective, affinity and selectivity for A3 adenosine receptors, and α-glucosidase inhibitory activity. Despite the several biological activities described, the in vivo absorption, distribution, metabolization, and excretion of SC, to the best of our knowledge, have not yet been reported in the literature. Below, the biological activities described for SC will be systematically described and discussed in terms of SAR.
Figure 3.
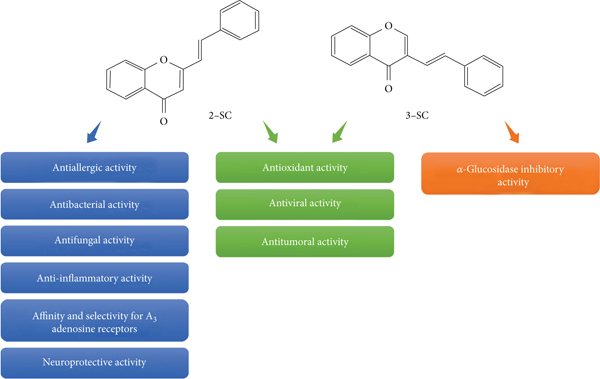
Biological activities described for 2-SC and 3-SC.
4.1. Antioxidant Activity
Antioxidants are substances that prevent or delay oxidative degradation of the substrate, protecting it against the action of reactive species. Reactive species are atoms or molecules, free and non-free radicals, which are extremely reactive [20, 21]. Several reactive species are generated during biological processes, such as reactive oxygen and nitrogen species (ROS and RNS, respectively), and this endogenous production has a physiological essential role in the organism [20, 21].
Oxidative stress can be understood as an imbalance between the production of reactive pro-oxidant species and the organism's capacity to counteract them by its antioxidant systems. Indeed, the human body has its own endogenous antioxidant system, able to fight against the oxidative attack and it is crucial for regular cellular functions [20]. The overproduction of these reactive pro-oxidant species produces severe damage in different biological functions and cell constituents, such as lipids, DNA, enzymes, or proteins, implicated in the development of various diseases as cancer, inflammatory diseases, or even ageing [20, 21]. Therefore, it became clear that, in some circumstances, the body's production of antioxidants is not enough to counteract this overproduction of reactive pro-oxidant species and their deleterious effects. Thus, the consumption of external antioxidants, as in the diet, is believed to be helpful, reinforcing the endogenous antioxidant defence system and bringing health benefits [20, 21]. Several 2-SC and 3-SC have shown potential as antioxidant agents and are below discussed, and all the observed antioxidant effects are summarized in Table 2, for 2-SC, and Table 3, for 3-SC.
Table 2.
2-SC studied for the antioxidant activity and summary of the observed antioxidant effects. The table display the antioxidant activity shown for the studied 2-SC according to the criterion: activity greater or equal to 30%.
| 2-SC | Observed effect(s) | Ref. |
|---|---|---|
| 3 | Scavenging of DPPH• | [32] |
| 5 | Inhibition of XO and high oxidation potential | [22, 27] |
| 11 | Inhibition of XO; protective activity against t-BHP-induced hepatotoxicity in rat hepatocytes; scavenging effect against ROS and RNS (O2•−; H2O2; HOCl; 1O2; ROO• and ONOO−); and moderate oxidation potential | [22, 24, 26, 27] |
| 13 | Inhibition of XO; scavenging effect against ROS (O2•− and HOCl); and high oxidation potential | [22, 26, 27] |
| 14 | Inhibition of XO; scavenging effect against ROS and RNS (HOCl; 1O2; ROO•; ONOO− and •NO); and high oxidation potential | |
| 15 | Inhibition of XO; protective activity against t-BHP-induced hepatotoxicity in rat hepatocytes; scavenging effect against ROS and RNS (H2O2 and ONOO−); and moderate oxidation potential | [22, 24, 26, 27] |
| 16 | Inhibition of XO; protective activity against t-BHP-induced hepatotoxicity in rat hepatocytes; scavenging effect against ROS and RNS (O2•−; HOCl; 1O2; ROO• and ONOO−); and moderate oxidation potential | |
| 17 - 19 | Inhibition of XO; protective activity against t-BHP-induced hepatotoxicity in rat hepatocytes; inhibition of Cu2+-induced oxidation of isolated human serum LDL; scavenging effect against ROS and RNS (O2•−; H2O2; HOCl; 1O2; ROO•; ONOO− and •NO); and low oxidation potential | [22, 24–27] |
| 20 | Inhibition of Cu2+-induced oxidation of isolated human serum LDL; scavenging effect against ROS and RNS (O2•−; H2O2; HOCl; 1O2; ROO•; ONOO− and •NO); and low oxidation potential | [25–27] |
| 21 | Scavenging effect against ROS and RNS (O2•−; HOCl; 1O2; ROO•; ONOO− and •NO); and moderate oxidation potential | [26, 27] |
| 22 - 24 | Scavenging effect against ROS and RNS (O2•−; H2O2; HOCl; 1O2; ROO•; ONOO− and •NO); metal chelating capacity; and reducing activity | [28] |
| 25 | Scavenging effect against ROS and RNS (O2•−; HOCl; 1O2; ROO•; ONOO−, without NaHCO3, and •NO) | [29] |
| 26, 27 | Scavenging effect against ROS and RNS (O2•−; HOCl; 1O2; ROO•; ONOO− and •NO) | |
| 28 - 32 | Scavenging of O2•− | [30] |
| 33 | Inhibition of reactive species-induced oxidation of DCFH-DA | [32] |
1O2: singlet oxygen; t-BHP: tert-butyl hydroperoxide; DCFH-DA: 2′,7′-dichlorodihydrofluorescein diacetate; DPPH•: 2,2-Diphenyl-1-picrylhydrazyl radical; H2O2: hydrogen peroxide; HOCl: hypochlorous acid; LDL: low-density lipoproteins; •NO: nitric oxide radical; ONOO−: peroxynitrite anion; RNS: reactive nitrogen species; ROS: reactive oxygen species; ROO•: peroxyl radical; O2•−: superoxide anion radical; XO: xanthine oxidase.
Table 3.
3-SC studied for the antioxidant activity and summary of the observed antioxidant effects.
1O2: singlet oxygen; DPPH•: 2,2-diphenyl-1-picrylhydrazyl radical.
4.1.1. 2-Styrylchromones
Fernandes and co-workers [22] evaluated the profile of some synthetic 2-SC derivatives (1, 5, 11, and 13–19, Figure 4) as inhibitors of xanthine oxidase (XO). XO is a highly versatile enzyme and exhibits a broad activity toward reducing substrates [22]. During these reactions with XO, ROS are generated, such as superoxide anion radical (O2•−) and hydrogen peroxide (H2O2) [22, 23]. All the tested 2-SC derivatives were found to be inhibitors of XO in a concentration-dependent manner, considering the studied concentration range (0.625–100 μM), except for 1 (an unsubstituted 2-SC) that was practically inactive [concentration of the tested compound that produce 50% inhibition (IC50) > 100 μM]. Some of the 2-SC were more potent than the tested positive control, allopurinol (IC50 = 5.43 ± 0.08 μM). 2-SC 19 was the most active, with an IC50 value of 0.55 ± 0.03 μM, followed by 18, 14, and 17 (IC50 values of 2.03 ± 0.19 μM, 2.52 ± 0.08 μM, and 4.36 ± 0.57 μM, respectively). The structure of the most active compounds indicates that the presence of the catechol group (C-3′ and C-4′) on the B-ring of the derivatives (17-19) potentiates XO inhibition. The presence of two −OH groups linked to the A-ring also contributes to the inhibition, especially at C-5 and C-7 (19) leading to an increase in the activity when compared with the presence of just one −OH on the A-ring (17 and 18). This effect is also observed in the result obtained for 2-SC 14, which, despite having only −OH groups at C-5 and C-7 on the A-ring, proved to be a potent XO inhibitor [22].
Figure 4.
Some of the previous 2-SC derivatives (11 and 15–19, Figure 4) were also evaluated as having a possible protective activity against the tert-butylhydroperoxide (t-BHP)-induced pro-oxidant hepatotoxicity in freshly isolated rat hepatocytes [24]. All the tested 2-SC exhibited in vitro hepatoprotective activity, which was reflected in the preservation of the integrity of the plasma membrane of rat hepatocytes. In this study, the results and the associated error were presented only in graphs, activity values not being mentioned. The tested compounds can be divided into two groups: the group that has an −OH at C-4′ on the B-ring (11, 15, and 16) and the group that has a catechol group (C-3′ and C-4′) on the B-ring (17–19). As in the previous study, the 2-SC derivatives that have the catechol group also proved to be much more active in this assay. For the group that has an −OH at C-4′ on the B-ring (11, 15, and 16), the concentration range tested was 25–200 μM, while for the group that has a catechol group on the B-ring (17–19), it was 3.125–50 μM. These two groups showed differences in what concerns the qualitative and quantitative preservation of biochemical homeostasis: 2-SC 17–19 exerted their hepatoprotective effect through the prevention of lipid peroxidation and the inhibition of reduced glutathione depletion and oxidized glutathione formation, these effects being comparable to those demonstrated by the used positive control, quercetin; whereas 2-SC 11, 15, and 16 only partially prevented lipid peroxidation and had no effect on glutathione levels. Thus, 2-SC 17–19 can be considered the most promising antioxidant 2-SC in this study [24].
Filipe and co-workers [25] synthesized some already known 2-SC (17–19, Figure 5) and a new 2-SC (20, Figure 5). The authors studied their inhibitory effect on Cu2+-induced oxidation of isolated human serum low-density lipoproteins (LDL), an in vitro model of lipid peroxidation, via radical chain reaction. The results and the associated error were presented only in graphs, activity values not being mentioned. In this study, the most active 2-SC were the least hydroxylated one (20), with −OH groups only at C-3′ and C-4′ on the B-ring, and the 2-SC 17, with an additional −OH at C-5 on the A-ring. The results observed in the assay of the inhibition of conjugated diene formation by 2-SC during Cu2+-induced LDL lipid peroxidation showed that 2-SC 17 and 20 (3 μM) and the used positive control (quercetin, 3 μM) totally inhibited the formation of conjugated dienes. 2-SC 18 and 19 (0.75 and 3 μM) were less active. These compounds have the catechol group on the B-ring, but 18 has only one −OH at C-7 on the A-ring and 19 has −OH at C-5 and C-7 on the A-ring [25]. Despite this rather inactivity in this system, 2-SC 19 was above considered the most effective XO inhibitor [22, 25]. Thus, for the systems tested in this study, the antioxidant potential of 2-SC is not necessarily determined by the number of −OH substituents, contrary to what was concluded in the studies mentioned above [22, 24], since the increase of the number of −OH groups led to a decrease in the antioxidant effect of these 2-SC [25]. The number of −OH groups present may have influenced the partitioning of this hydrophobic 2-SC into LDL leading to the variations observed in its antioxidant effectiveness. In what concerns the study of the electron donating properties by pulse radiolysis in micellar solutions, Filipe and co-workers [25] found that all the tested 2-SC were equally capable to react with O2•− and tryptophan radical in cationic micelles. When electron transfer reactions were carried out in neutral micelles, 2-SC 20 presented a higher effect when compared to the other tested compounds, since it was the one that exhibited a measurable reactivity [25].
Figure 5.
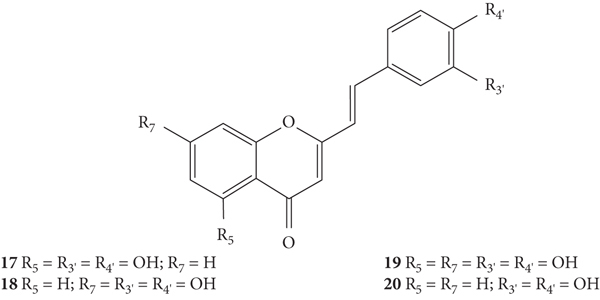
Chemical structures of 2-SC 17–20 [25].
The in vitro scavenging activities of ROS and RNS by several 2-SC derivatives (1, 5, 11, and 13–21, Figure 6) were also evaluated by Gomes and co-workers [26]. The scavenging activities of these 2-SC were tested against the following reactive species: O2•−, H2O2, hypochlorous acid (HOCl), singlet oxygen (1O2), peroxyl radical (ROO•), peroxynitrite anion (ONOO−), and nitric oxide radical (•NO), using various generating systems and probes, according to the reactive species to be detected [26]. Considering the −OH substituents present on the B-ring of the 2-SC, they can be divided into three groups: group 1, with a catechol group (C-3′ and C-4′) on the B-ring (17–20); group 2, with −OH at C-4′ on the B-ring (11, 15, 16, and 21); and group 3, without any substitution on the B-ring (1, 5, 13, and 14). The studied concentration range and the used positive controls varied according to the reactive species under study. Under the tested experimental conditions, 2-SC 19 and 18 (IC50 values of 48.9 ± 1.2 μM and 51.0 ± 1.4 μM, respectively) were the most active O2•− scavengers. The IC50 found for the tested positive control (propyl gallate) was 24.6 ± 3.2 μM. 2-SC 11, 16, 17, and 20 were also able to scavenge O2•−, while the 2-SC from group 3 presented very low (13 and 14) or no activity at all (1 and 5). The same did not apply to H2O2 scavenging activity, since only 2-SC from group 1 showed a significant scavenging effect. 2-SC 17 and 19 were the most active (IC50 values of 48.9 ± 5.5 μM and 50.3 ± 7.8 μM, respectively). They were even more active than the used positive control, ascorbic acid (IC50 = 625.5 ± 49.6 μM). The other compounds were less efficient (11 and 15) or completely ineffective (1, 5, 13, 14, 16, and 21) up to the highest tested concentration (250 μM). 2-SC 11, 14, and 19 were the most effective scavengers of HOCl, with IC50 values of 8.5 ± 0.3 μM, 5.7 ± 0.7 μM and 3.9 ± 0.2 μM, respectively. This results' pattern was similar for 1O2 scavenging activity; however, the most effective compound was 17 (IC50 = 4.9 ± 0.6 μM). The other compounds of group 1 also showed activity but were less effective than the used positive control, penicillamine (IC50 = 4.9 ± 0.2 μM). The results for ROO• scavenging activity showed that all the compounds from group 1 were able to delay the ROO•-dependent oxidation of fluorescein, even more than some other 2-SC from the other groups. In group 2, only 2-SC 15 was not effective, whereas from group 3, only 14 was active. The ONOO− scavenging was evaluated in the presence and absence of NaHCO3. The relative order of potencies of the 2-SC was the same for both conditions. From all the tested 2-SC, 20 was the most potent, with an IC50 value of 0.24 ± 0.02 μM, in the absence of NaHCO3, and 0.44 ± 0.02 μM in its presence. The 2-SC 20 was more potent than the positive control, ebselen (IC50 values of 0.91 ± 0.06 μM in the absence of NaHCO3 and 4.63 ± 0.44 μM in its presence). Compounds from group 1 were the most effective, and all the compounds from group 2 also showed scavenging effect, while in group 3, only 2-SC 14 was active. In what concerns •NO scavenging activity, 2-SC from group 1 were the most active [IC50 varying from 0.24 ± 0.04 (17) to 0.51 ± 0.13 μM (19)], being more potent than the used positive control, rutin (IC50 = 3.66 ± 0.43 μM). The 2-SC from the other groups were less efficient (11, 14, 16, and 21) or completely ineffective (1, 5, 13, and 15). In summary, 2-SC from group 1 showed scavenging activity against all the detected ROS and RNS, while 2-SC from group 2 had a variable effect depending on the number and position of the −OH substituents on the A-ring, and for some reactive species, they did not show any activity. The activity of 2-SC from group 3 was generally low or inexistent. Therefore, for most of the reactive species studied, the catechol on the B-ring appears to play an important role in the scavenging activity. However, for HOCl scavenging activity, the −OH substituents on the A-ring assumed high importance, despite the existence of −OH substituents on the B-ring. The authors also tested some flavonoids structurally related to the tested 2-SC, which allowed to conclude that the styryl moiety also contributes to their observed outstanding antioxidant activity. A possible explanation for this fact might be the contribution of the styryl pattern to the stabilization of the radicals that are formed during the scavenging reactions [26].
Figure 6.
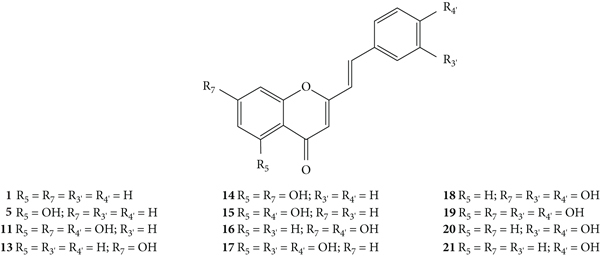
Considering these antioxidant properties, the electrochemical behaviour of these same 2-SC (1, 5, 11, and 13–21, Figure 6) was studied by cyclic voltammetry in order to understand the mechanism of ROS and RNS scavenging activity [27]. Cyclic voltammetry is an electroanalytic technique to determine the redox properties of molecules in solution. Higher scavenging effects corresponded to the lowest oxidation potentials, relating their electrochemical behaviour to the antioxidant capacity. The obtained results showed that 2-SC from group 1, with a catechol group on the B-ring, presented lower values of oxidation potentials corresponding to the oxidation of the 3′,4′-OH of the catechol group, while for the compounds from group 2, the values of the oxidation potentials correspond to the oxidation of −OH at C-4′ on the B-ring. While for the compounds 5, 13, and 14 from group 3, the values of oxidation potentials corresponded to the −OH groups present on the A-ring. For 2-SC 1, without −OH substituents, there were no detectable peaks. Correlations between the oxidation peak potentials and the scavenging activity against some ROS and RNS were also analysed. For H2O2, 1O2 and ONOO− were found to have significant correlations, indicating that the scavenging mechanism against them is based on redox reactions; whereas for O2•− and HOCl, no significant correlations were found. The authors further suggested that the oxidation reaction involves only one electron leading to the formation of a semiquinone, which in turn undergoes disproportionation, and forms an orthoquinone and regenerates the initial 2-SC structure [27].
Gomes and co-workers [28] also published a work where a new group of 2-SC (22–24, Figure 7) was synthesized, with −OCH3 or −OH groups at C-7, C-8, C-3′, and C-4′ on the A- and B-rings, and studied their scavenging effects against the same ROS (O2•−, H2O2, HOCl, 1O2, and ROO•) and RNS (ONOO− and •NO). The metal chelating capacity and reducing activity of these compounds were also evaluated, as they are indicators of antioxidant activity [28]. The methodologies for the ROS and RNS scavenging assays used were similar to the previously mentioned studies of these authors [26, 28]. All the tested 2-SC showed O2•− scavenging activity, and the obtained IC50 values were much lower than the positive control, tiron (IC50 = 287.7 ± 32.0 μM). 2-SC 23 was the most active (IC50 = 17.8 ± 3.8 μM). This means that the −OH groups on the A- and B-rings favour this activity and their methylation decreases the scavenging effect [28]. Comparing with the before tested 2-SC [26], this new group of 2-SC demonstrated an improved O2•− scavenging activity, as they presented lower IC50 values [28]. H2O2 was scavenged by 2-SC 22 (IC50 = 701.9 ± 58.2 μM) that was the most active and by 2-SC 23 (IC50 = 799.3 ± 60.9 μM). The positive control, ascorbic acid, presented an IC50 value of 567.4 ± 33.0 μM. All the tested 2-SC were able to scavenge HOCl but were less effective than the used positive control, lipoic acid (IC50 = 1.95 ± 0.06 μM). 2-SC 22 was the most active (IC50 = 4.67 ± 0.64 μM). For this reactive specie and once more, the methylation of −OH on the B-ring decreased the activity. All the tested 2-SC were able to scavenge 1O2, being more effective than the positive control, ascorbic acid (IC50 = 10.2 ± 1.5 μM). The pattern of results of this assay was similar to the ones obtained for the O2•− scavenging assay. The results for the ROO• scavenging activity showed that all the 2-SC were able to delay the ROO•-dependent oxidation of fluorescein. Also, for the •NO scavenging activity, all the tested 2-SC were active and more potent than the positive control, rutin (IC50 = 1.17 ± 0.05 μM). In terms of ONOO– scavenging activity, in the absence of NaHCO3, 2-SC 24 was the most active (IC50 = 0.59 ± 0.08 μM), being more potent than the positive control, ebselen (IC50 = 1.00 ± 0.11 μM). In the presence of NaHCO3, 2-SC 22 was the most active (IC50 = 0.93 ± 0.21 μM), being more potent than the positive control, ebselen (IC50 = 5.27 ± 0.32 μM). Overall, the presented results demonstrate that the methylation of −OH on the B-ring decreases the scavenging of ROS and RNS. Depending on the reactive species, −OH at C-8 on the A-ring sometimes favoured the scavenging effects, namely, for O2•− and •NO. In what concerns the reducing activity, all the tested 2-SC were able to significantly reduce ferric ion. According to the figure presented by the authors, the order of potencies found was 22 > 24 > 23, which indicates that methylation of the −OH groups in the A- and B-rings improved the iron-reducing capacity. The determination of metal chelating capacity showed spectral changes of 2-SC with the addition of iron (II), indicating the formation of complexes [28].
Figure 7.
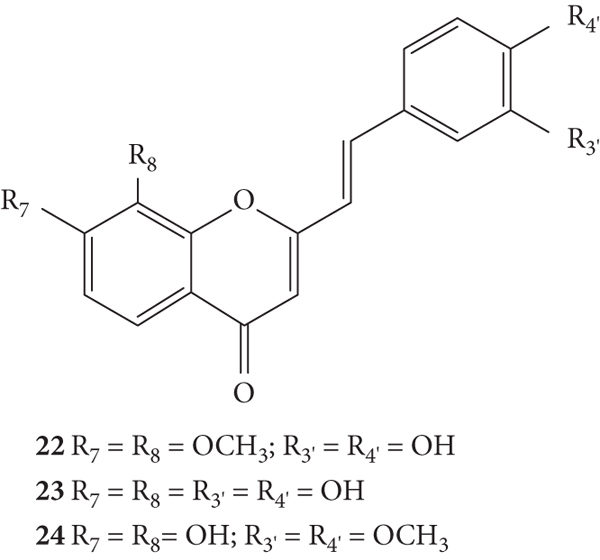
Chemical structures of 2-SC 22–24 [28].
Sousa and co-workers [29] synthesized 3-hydroxy-2-SC (25–27, Figure 8) and evaluated their scavenging activity against ROS and RNS (O2•−, H2O2, HOCl, 1O2, ROO•, ONOO−, and •NO) [29]. The methodologies used were similar to the previously mentioned ones [26, 28]. The 3-hydroxy-2-SC synthesized in this study are derived from the 2-SC studied by Gomes and co-workers [28] (22–24) by introducing an −OH at C-3 on the C-ring. O2•− was scavenged by 2-SC 25 and 27, for which the IC50 values were much lower than those found for the positive control used, quercetin (IC50 values of 35 ± 2 μM, 30 ± 1 μM, and 67 ± 7 μM, respectively), and compound 26 was less efficient than the previous ones. In this case, the methylation of −OH at C-7 and C-8 on the A-ring decreased the scavenging activity [29]. Comparing these 3-hydroxy-2-SC with 2-SC 22–24, tested by Gomes and co-workers [28], the presence of an −OH at C-3 on the C-ring does not seem to be relevant for the O2•− scavenging effect [29]. Also, none of the 3-hydroxy-2-SC showed any H2O2 scavenging activity, up to the highest tested concentration (1000 μM). These results indicate that an −OH at C-3 on the C-ring is not favourable for the scavenging activity of H2O2, when compared with the previously presented 2-SC (22–24), which showed activity [28]. All the tested 2-SC were able to scavenge HOCl. These compounds were even better scavengers than their structurally related previously presented 2-SC (22–24) [28], with much lower IC50 values. All the tested 2-SC were also able to scavenge 1O2, and the IC50 values obtained were low when compared with the previously tested 2-SC (22–24) [28], leading to the conclusion that the presence of an −OH at C-3 on the C-ring is also important for the 1O2 scavenging activity. The results of the ROO• scavenging activity showed that all the tested 2-SC were able to delay the ROO•-dependent oxidation of fluorescein. However, these compounds were less active than their analogous 2-SC (22–24) [28]. In the •NO scavenging assays, all the tested compounds were more potent than the positive control, quercetin (IC50 = 1.3 ± 0.1 μM). These results, when compared to the previously presented 2-SC (22–24) [28], suggest that an −OH at C-3 on the C-ring does not improve their •NO scavenging potential. The scavenging of ONOO− was evaluated in the absence of NaHCO3, and the IC50 values of the tested 2-SC varied from 0.29 ± 0.02 (27) to 0.57 ± 0.02 μM (25), being all more potent than the positive control, quercetin (IC50 = 0.76 ± 0.06 μM). In the presence of NaHCO3, only 2-SC 26 and 27 were active (IC50 values of 1.3 ± 0.3 μM and 0.98 ± 0.09 μM, respectively). Overall, the introduction of −OH at C-3 on the C-ring considerably improved the ONOO− scavenging potential, when compared to the previously tested 2-SC (22–24) [28, 29]. Generally, all the tested 2-SC showed to be potent scavengers of most of the evaluated reactive species, being more effective than the used positive control, quercetin. For the majority of the evaluated reactive species, the catechol on the B-ring appears to play an important role and the presence of −OH at C-3 on the C-ring improves the scavenging activity. The authors, in addition to 2-SC, also tested some flavonoids structurally identical to the 2-SC tested and concluded that the styryl moiety also contributes to the observed outstanding antioxidant activity, for some reactive species (HOCl, 1O2, and ONOO−), since the 2-SC showed lower IC50 values than the flavonoids [29].
Figure 8.
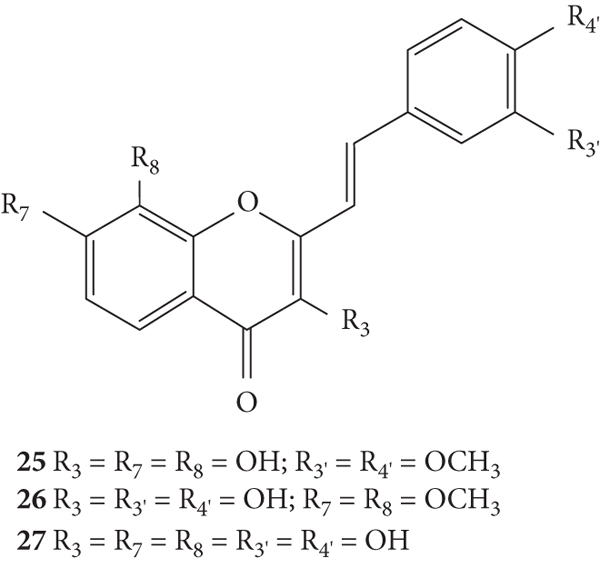
Chemical structures of 2-SC 25–27 [29].
The O2•− scavenging activity of 2-SC 28–32 (Figure 9) was determined by the nitroblue tetrazolium chloride (NBT) photo reduction method [30]. All the tested compounds exhibited a good antioxidant activity, being more potent than the tested positive controls [butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), vitamin C, and vitamin E]. In this study, the results were presented as IC50 values but the associated error was not indicated, even though the assays were performed in triplicate. 2-SC 31 and 32 were the most potent (IC50 values of 234 μM and 243 μM, respectively), followed by 28 and 30 (IC50 values of 268 μM and 275 μM, respectively) and finally 29 (IC50 = 287 μM). The authors concluded that the same number of −OH and −OCH3 groups led to variations in activity due to their position in the 2-SC structure and the number and position of −OH groups play a vital role in the antioxidant activity of 2-SC [30]. However, as the results were similar and were presented without indicating the associated error, it is difficult to draw solid conclusions about SAR as there is no guarantee about the magnitude of the difference between the results presented. In this study, the used methodology is significantly different from the one used in the studies mentioned above [26, 28, 29]. In the NBT photo reduction method, the O2•− generation was light induced by riboflavin, while in the other studies, O2•− was generated by the β-nicotinamide adenine dinucleotide (NADH)/phenazine methosulphate (PMS)/O2 system [26, 28–31]. Therefore, the results cannot be directly compared with those previously obtained [26, 28, 29].
Figure 9.
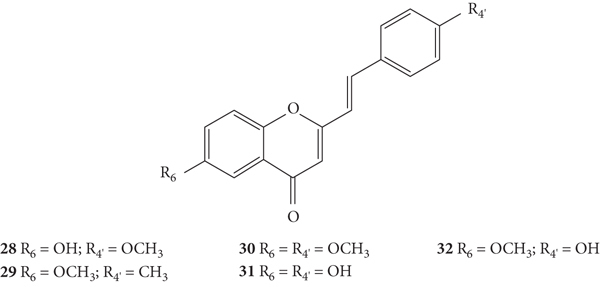
Chemical structures of 2-SC 28–32 [30].
The antioxidant activities of hormothamnione (3, Figure 10) and hormothamnione diacetate (33, Figure 10) were evaluated by Takamatsu and co-workers [32] by two antioxidant systems: 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) solution-based chemical assay and 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) cellular-based assay, in promyelocytic human leukaemia-60 (HL-60) cells. In this study, the results were presented as IC50 values but the associated error was not indicated. The hormothamnione (3) only showed moderate antioxidant activity in the DPPH assay but did not show significant activity in the DCFH-DA assay. On the contrary, hormothamnione diacetate (33) showed no activity in DPPH assay but showed good activity in the DCFH-DA assay (IC50 = 18.3 μM), which may indicate that it requires metabolic activation for antioxidant activity. The tested positive controls (vitamin E and vitamin C) showed strong activity in DPPH assay. In DCFH-DA assay, vitamin E and vitamin C exhibited IC50 values of 255 μM and 9.7 μM, respectively. The differences in the antioxidant activity of 2-SC 3 and 33 may be due to the di-OCOCH3 at C-3′ and C-5′ on the B-ring in 2-SC 33, while 2-SC 3 presents −OH in the same positions [32].
Figure 10.
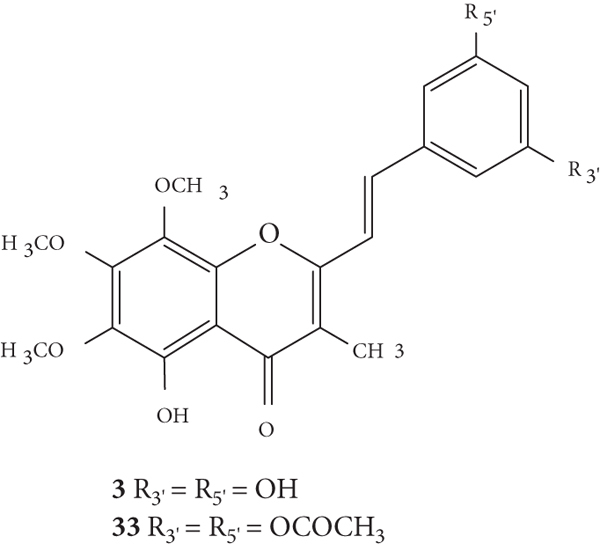
Chemical structures of 2-SC 3 and 33 [32].
The above-mentioned structural characteristics that demonstrated to favour the antioxidant activity of 2-SC are summarized in Figure 11.
Figure 11.
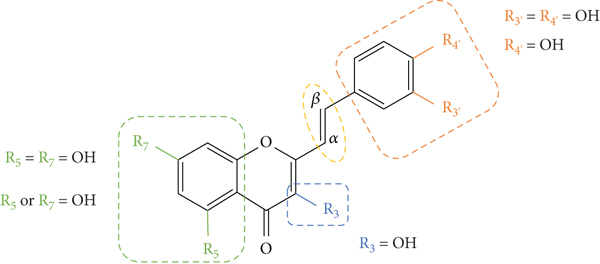
Structural characteristics that seem to favour the 2-SC antioxidant activity.
4.1.2. 3-Styrylchromones
Hashem [13] showed the ability of the 3-SC 12 (Figure 12) to scavenge 1O2. This 3-SC was isolated from the aerial parts of Erucaria microcarpa and was well characterized in this article. Its antioxidant activity was determined by electron spin resonance. 3-SC 12 showed a positive reaction when mixed with 1O2, which was converted to oxygen, producing a free radical compound. This radical compound was rapidly combined with another radical compound to give a stable compound. This study suggests that the −OCH3 groups and the conjugations of double bonds in the compound can possibly contribute to the 1O2 scavenging activity [13].
Figure 12.
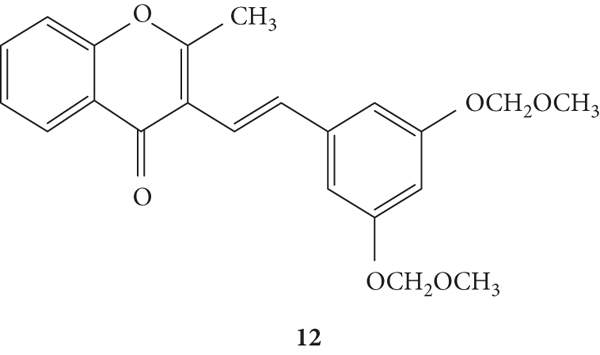
Chemical structure of 3-SC 12 [13].
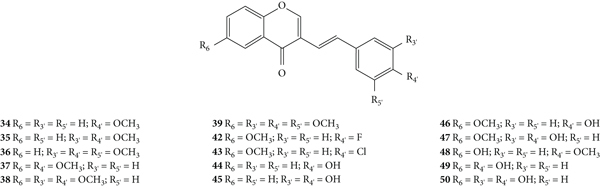
The antioxidant activity of a series of 3-SC derivatives (34–50, Figure 13) was evaluated by the DPPH free radical scavenging assay [33]. The synthesized compounds present several substituents such as halogen atoms (−F and −Cl), −OCH3 and −OH groups in both A- and B-rings. In this study, the results were presented as the concentration of compounds that produce 50% of the maximum effect (EC50) but the associated error and the number of assays done were not indicated. 3-SC 45, 47, and 50 were shown to be as or more potent than the tested positive control, ascorbic acid (EC50 = 23 μM). In contrast, 3-SC 34–43 and 48 did not show DPPH• scavenging activity. These results indicate that the presence of an −OCH3 group or a halo (−F or −Cl) substituent (34–43 and 48) on the B-ring did not favour the scavenging effect, while the existence of −OH groups is favourable. Indeed, 3-SC with −OH at C-3′ and C-4′ on the B-ring (45 and 47) were more potent than the 3-SC, with just an −OH at C-4′ on the B-ring (44 and 46). This behaviour is similar to that previously observed for 2-SC: 2-SC with −OH at C-3′ and C-4′ on the B-ring (17–20) were more active than 2-SC with only one −OH at C-4′ on the B-ring (11, 15, 16, and 21) [26]. The introduction of an −OH at C-6 on the A-ring did not increase the scavenging activity (34 versus 48). In conclusion, this study corroborates the central role of −OH groups on the B-ring [33].
Figure 13.
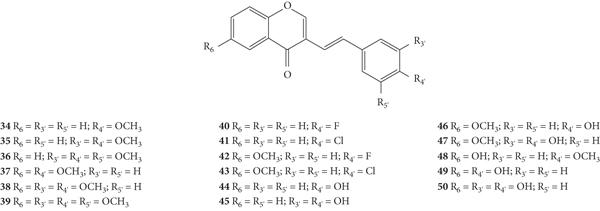
Chemical structures of 3-SC 34–50 [33].
The above-mentioned structural characteristics that demonstrated to favour the antioxidant activity of 3-SC are summarized in Figure 14.
Figure 14.
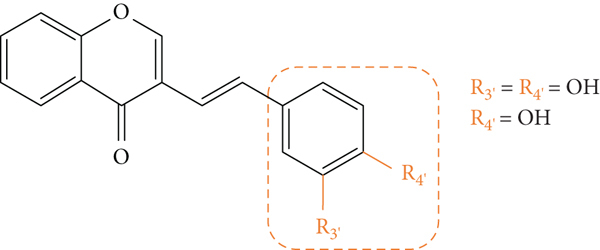
Structural characteristics that seem to favour the 3-SC antioxidant activity.
4.2. Antiallergic Activity
Allergy is one of the most common diseases and refers to abnormal adaptive immune response against non-infectious environmental substances (allergens). This disease is characterized by the development of signs and symptoms of hypersensitivity reactions upon exposure to certain substances. Consequently, the production of allergen-specific immunoglobulin E and allergen-specific T-cell production occurs. In some cases, the allergic reaction can be extremely severe or even fatal, as in the case of anaphylactic reaction. The prevalence of allergy is increasing worldwide, which leads to the search for more effective therapeutic responses to this pathology [34]. Some 2-SC have shown potential as antiallergic agents and are below discussed, and all the observed antiallergic effects are summarized in Table 4. There are no studies on the antiallergic activity of 3-SC, to the best of our knowledge.
Table 4.
2-SC studied for the antiallergic activity, their chemical structures, and summary of the observed antiallergic effects.
| Chemical structure | 2-SC | Observed effects | Ref. |
|---|---|---|---|
|
51-55 | Antiallergic activity in the passive cutaneous anaphylaxis test in rats; and inhibition of histamine release from passively sensitized rat peritoneal cells | [4] |
4.2.1. 2-Styrylchromones
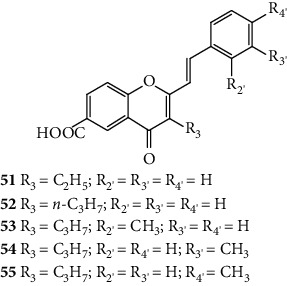
The antiallergic activity of 2-SC has already been summarized in two previous reviews [4, 16]. In 1979, the antiallergic potential of some 2-SC, all substituted with −COOH at C-6 on the A-ring, was evaluated (51–55, Table 4). The tested 2-SC displayed antiallergic activity, when orally administered to rats, in the passive cutaneous anaphylaxis test. This assay consisted in a sensitization test using rat serum rich in homocytotropic antibodies. These antibodies are responsible for triggering the release of pharmacological mediators of anaphylaxis. The antiallergic potential of 2-SC was also evaluated when the administration was parenteral, and also in this case, the studied 2-SC showed antiallergic effects in the passive cutaneous anaphylaxis test. These 2-SC were also able to inhibit histamine release from rat peritoneal cells passively sensitized with immunoglobulin E antibodies. The authors analysed the SAR, and some conclusions were drawn: the smaller alkyl groups, such as ethyl (51) and n-propyl (52), at C-3 on the C-ring, increased the oral activity. The introduction of a single −CH3 on the B-ring (53 and 55) increased the biological responses [4].
The above-mentioned structural characteristics that demonstrated to favour the antiallergic activity of 2-SC are summarized in Figure 15.
Figure 15.
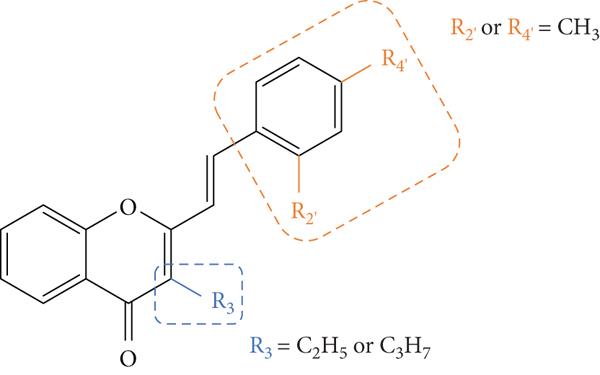
Structural characteristics that seem to favour the 2-SC antiallergic activity.
4.3. Antiviral Activity
Antivirals are a class of compounds that are used to treat viral infections and can be produced by living organism or obtained by chemical synthesis. An antiviral agent is capable to interfere in one or more steps of viral infection, arresting the viral replication cycle [35]. By attacking the virus's replication process, antivirals prevent the viral load from increasing to a point where it can cause pathogenesis, allowing the body's innate immune system to neutralize and eradicate the virus [35]. Viruses use the host's machinery to replicate and survive; thus, attacking the virus can also affect host cells causing deleterious effects, which can result in serious side effects to the host [35]. Since viral diseases are extremely widespread, the challenge is to find antivirals that interfere with viral replication without deleterious effects in the host cells. Other limitations of antivirals are related to the high mutation rate of virus, which favours the appearance of resistant viral strings. These questions make it essential to continually search and develop new antiviral compounds [35, 36]. Several 2-SC and 3-SC have shown potential as antiviral agents against human rhinovirus (HRV), murine norovirus (MNV), human immunodeficiency virus type 1 (HIV-1), and enterovirus (EV) and are below discussed. All the observed antiviral effects are summarized in Table 5, for 2-SC, and Table 6, for 3-SC.
Table 5.
2-SC studied for the antiviral activity and summary of the observed antiviral effects. The table displays the antiviral activity shown for the studied 2-SC according to the criterion: activity greater or equal to 30%.
| 2-SC | Observed effect(s) | Ref. |
|---|---|---|
| 1 | Anti-rhinovirus activity against HRV 1B in HeLa cells; and anti-norovirus activity against MNV in RAW cells | [37, 40] |
| 5 | Anti-norovirus activity against MNV in RAW cells | [40] |
| 11 | Anti-HIV-1 integrase activity | [12] |
| 21 | Anti-rhinovirus activity against HRVs 1B and 14 in HeLa cells | [37] |
| 56 | Anti-rhinovirus activity against HRV 14 in HeLa cells | |
| 57, 58 | Anti-rhinovirus activity against HRV 1B in HeLa cells; and anti-norovirus activity against MNV in RAW cells | [37, 40] |
| 59 | Anti-rhinovirus activity against HRVs 1B and 14 in HeLa cells | [37] |
| 60 | Anti-rhinovirus activity against HRV 1B in HeLa cells | |
| 61 | Anti-rhinovirus activity against HRVs 1B and 14 in HeLa cells | |
| 62, 63 | [38] | |
| 64 | Anti-rhinovirus activity against HRV 1B in HeLa cells | |
| 65, 66 | Anti-rhinovirus activity against HRVs 1B and 14 in HeLa cells | |
| 67, 68 | Anti-rhinovirus activity against HRV 1B in HeLa cells | |
| 69 | Anti-rhinovirus activity against HRVs 1B and 14 in HeLa cells | |
| 70 | Anti-rhinovirus activity against HRVs 1B and 14 in HeLa cells; and anti-norovirus activity against MNV in RAW cells | [38, 40] |
| 71 | Anti-rhinovirus activity against HRV 14 in HeLa cells | [38] |
| 72 | Anti-rhinovirus activity against HRV 1B in HeLa cells | |
| 73 | Anti-rhinovirus activity against HRVs 1B and 14 in HeLa cells | |
| 74 | Anti-rhinovirus activity against HRV 1B in HeLa cells | |
| 76 | ||
| 77 | Anti-rhinovirus activity against HRVs 1B and 14 in HeLa cells | |
| 78 | Anti-rhinovirus activity against HRV 1B in HeLa cells | [39] |
| 79, 80 | Anti-rhinovirus activity against HRV 14 in HeLa cells | |
| 81 - 83 | Anti-norovirus activity against MNV in RAW cells | [40] |
HeLa: cervical epithelioid carcinoma cells; HIV-1: human immunodeficiency virus type 1; HRV: human rhinovirus; MNV: murine norovirus; RAW cells: murine macrophage cell line RAW 264.7.
Table 6.
3-SC studied for the antiviral activity and summary of the observed antiviral effects. The table displays the antiviral activity shown for the studied 3-SC according to the criterion: activity greater or equal to 30%.
| 3-SC | Observed effect(s) | Ref. |
|---|---|---|
| 2 | Anti-rhinovirus activity against HRVs 1B and 14 in HeLa cells; and anti-enterovirus activity against EV 71 in HEp-2 cells | [44] |
| 41 | Anti-rhinovirus activity against HRVs 1B and 14 in HeLa cells | |
| 84 | Anti-rhinovirus activity against HRV 1B in HeLa cells; and anti-enterovirus activity against EV 71 in HEp-2 cells | |
| 85 | Anti-rhinovirus activity against HRVs 1B and 14 in HeLa cells; and anti-enterovirus activity against EV 71 in HEp-2 cells |
EV: enterovirus; HeLa: cervical epithelioid carcinoma cells; HEp-2: human epithelial type 2 cells; HRV: human rhinovirus.
4.3.1. 2-Styrylchromones
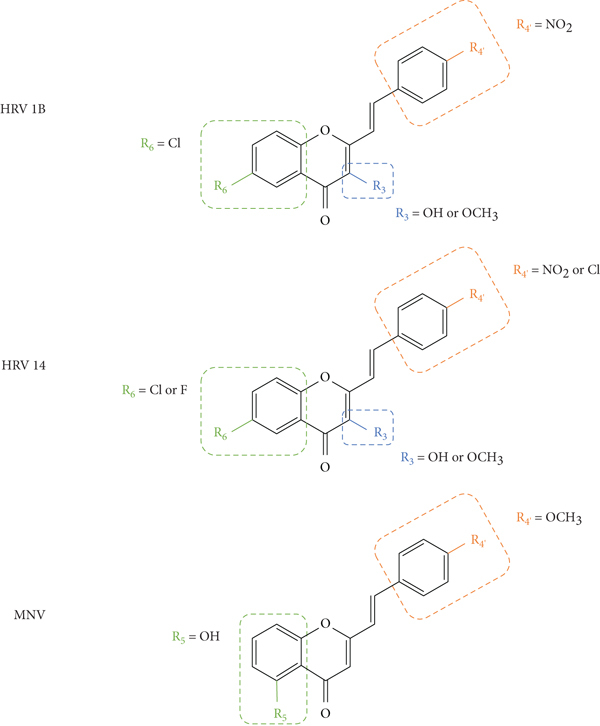
Desideri and co-workers [37] were the first to evaluate the antiviral potential of 2-SC, to the best of our knowledge. These authors tested the anti-rhinovirus activity of 2-SC 1, 21, and 56–61 (Figure 16) against two selected serotypes of HRV, 1B and 14. The HRV belongs to the picornavirus family, and is the most frequent cause of the common cold and has several serotypes, which makes it difficult to develop a vaccine, leading to continuous research in this field. In this work [37], serotypes 1B and 14 were selected as representative for viral groups B and A of HRVs, respectively. Antiviral activity was evaluated by a plaque reduction assay in cervical epithelioid carcinoma (HeLa) cell cultures infected with HRVs 1B and 14. First, the cytotoxicity of the compounds under study was evaluated, in vitro, by 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) colorimetric assay, to determine the maximum non-toxic concentration (MNTC). In this study, the error associated with the results was not indicated, even though the assays were performed in triplicate. 2-SC 58 was the most toxic, since its MNTC was the lowest (3.12 μM). The evaluation of the antiviral activity was done from the maximum concentration that did not affect cell viability and growth and was expressed as IC50 (concentration of compound that reduces the plaque number by 50%). All the tested 2-SC showed antiviral effect against HRV 1B, but the same did not happen against HRV 14, since 2-SC 58 and 60 were inactive. Despite this difference, some 2-SC had better activity against HRV 14 than against serotype 1B (21, 56, 59, and 61). 2-SC 59 was the most potent against both serotypes (IC50 values of 3.89 μM and 1.33 μM for HRVs 1B and 14, respectively). The known in vitro potent inhibitor of group B HRV replication, 4′,6-dichloroflavan, was used as positive control (IC50 = 0.025 μM). No known inhibitor was used as a control for HRV 14. For both serotypes, some compounds (21, 56, and 59–61), in addition to an effect on the plaque number, they also produced a reduction (about 50%) in viral plaque size. This fact suggested that these 2-SC caused a slowdown in the kinetics of viral replication. Analysing the structures of the tested compounds, the most active 2-SC (59) that displayed antiviral activity against both serotypes presents a single substituent, a -NO2 at C-4′ on the B-ring. Compared to 2-SC 59 (IC50 values of 3.89 μM and 1.33 μM against HRVs 1B and 14, respectively), the presence of an −OH at C-4′ on the B-ring (21, IC50 values of 9.29 μM and 6.41 μM against HRVs 1B and 14, respectively) or a −NH2 at C-4′ on the B-ring (61, IC50 values of 15.06 μM and 13.46 μM against HRVs 1B and 14, respectively) decreased the cytotoxicity, but also the antiviral effect against both serotypes; in the latter case, the decrease of the antiviral activity is more accentuated. The existence of −Cl at C-4' on the B-ring (56) leads to antiviral activity against serotype 14 but not against 1B, while the addition of a second −Cl at C-6 on the A-ring (60) increased the antiviral effect against HRV 1B but not against HRV 14, 2-SC 60 was inactive. The presence of a −CH3 or an −OCH3 at C-4′ on the B-ring (57 and 58, respectively) resulted in a modest or no effect against HRVs 1B and 14 [37].
Figure 16.
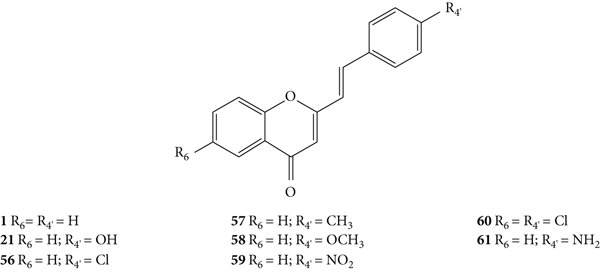
Chemical structures of 2-SC 1, 21, and 56–61 [37].
After this first approach, Desideri and co-workers [38] synthesized a new group of structurally related 2-SC (62–77, Figure 17) with −OH or −OCH3 at C-3 on the C-ring and studied their anti-rhinovirus activity against HRVs 1B and 14. The used methodology was the same with that of the previous study [37]. Also, in this study, the error associated with the results was not indicated, even though the assays were performed in triplicate. In this study [38], 2-SC 64, 69, 75, and 77 were the most toxic, since their MNTC was the lowest (3.12 μM). These new 2-SC were generally more toxic than those previously synthesized [37], since the majority presented MNTC values of 3.12 μM and 6.25 μM. All the tested 2-SC showed antiviral effect against HRV 1B, but the same did not happen against HRV 14, since two 2-SC were inactive (68 and 74). Despite this difference, some 2-SC had better activity against HRV 14 than against serotype 1B (63, 65, 69, 71, and 77). 2-SC 69 was the most potent against both serotypes (IC50 values of 0.94 μM and 0.73 μM against HRVs 1B and 14, respectively). 2-SC 70 also demonstrated high potency against both serotypes (IC50 values of 1.10 μM and 1.60 μM against HRVs 1B and 14, respectively). In this study, 4′,6-dichloroflavan was used as a positive control (IC50 = 0.025 μM), and again, no known inhibitor was used as a control for HRV 14. For both serotypes, some compounds (62, 64, 66, 69, 71, and 74, for HRV 1B, and 77 for HRV 14) in addition to an effect on plaque number also produced a reduction (about 50%) in viral plaque size. 2-SC 69 and 77 were the most potent 3-hydroxy- and 3-methoxy-2-SC, respectively (69, IC50 values of 0.94 μM and 0.73 μM for HRVs 1B and 14, respectively, and 77, IC50 values of 3.00 μM and 0.96 μM for HRVs 1B and 14, respectively). Analysing the structure of the tested 2-SC for antiviral activity against HRV 1B, 2-SC 69 was the most active and presents an −OH at C-3 on the C-ring, a −Cl at C-6 on the A-ring, and a −NO2 at C-4′ on the B-ring. 2-SC 70, which also showed high antiviral activity, has only one −OCH3 at C-3 on the C-ring. For the antiviral activity against HRV 14, 2-SC 69 and 77 were the most active and both have −Cl at C-6 on the A-ring and −NO2 at C-4′ on the B-ring, the difference being at C-3 on the C-ring, since 69 has an −OH and 77 has an −OCH3. For 2-SC 62 and 70, the opposite happens, since 70, with −OCH3 at C-3 on the C-ring, was more active than 62, with −OH at C-3 on the C-ring. Thus, the influence of −OH or −OCH3 at C-3 on the C-ring in the anti-rhinovirus activity varies according to the substituents present in the structure and its location [38]. The comparison between these results and those previously obtained by the same authors [37] allows a more in-dept analysis of the SAR. 2-SC 59 was considered the most active 2-SC in the previous study [37], however the IC50 values found were higher than the ones found for 2-SC 69, the most active in the present study. When comparing the structure of 2-SC 59 with the one of 2-SC 69, the first lacks the −Cl at C-6 on the A-ring and the −OH at C-3 on the C-ring. This may mean that these substituents at these positions may be essential for a higher anti-rhinovirus activity. 2-SC with −NO2 at C-4′ on the B-ring, without substituents on the A-ring, but with −OH or −OCH3 at C-3 on the C-ring (65 and 73, respectively) were less potent than 2-SC 59. Only these 2-SC showed a decrease in the antiviral effect with the introduction of −OH or −OCH3 at C-3 on the C-ring, since in general, the introduction of these groups enhanced the antiviral activity against both serotypes [37, 38].
Figure 17.
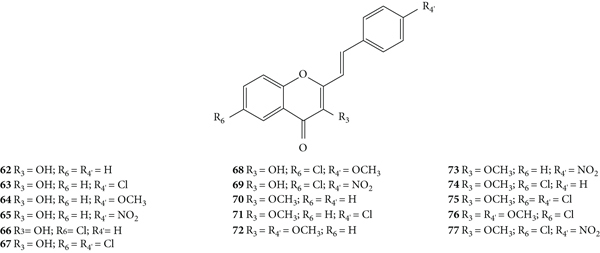
Chemical structures of 2-SC 62–77 [38].
These authors also evaluated the anti-rhinovirus activity against HRVs 1B and 14, resulting from the introduction of a −F at C-6 on the A-ring in hydroxylated or methoxylated 2-SC (78–80, Figure 18) [39]. The methodology used to assess the antiviral activity was the same used in the previous studies by these authors [37, 38]. Also, in this study, only a positive control for HRV 1B (4′6-dichloroflavan) was tested and the error associated with the results was not indicated [39]. 2-SC 78–80 were less toxic than those tested in the previous studies [37–39]. 2-SC 78 has only one −F at C-6 on the A-ring as a substituent and showed a weak potency against both serotypes [47% and 13% of inhibition against HRV 1B and 14, respectively, up to the highest tested concentration (12.5 μM)] [39]. 2-SC 79 and 80 interfered with HRV 14 replication (IC50 values of 9.09 μM and 12.03 μM, respectively), causing a reduction in viral plaque size (50 to 70%), and were inactive against serotype 1B. This effect suggests that these 2-SC may have a neutralizing action on the progeny virus and/or lead to a slowing down in the kinetics of viral replication. The results indicate that the introduction of −OH (79) or −OCH3 (80) at C-3 on the C-ring, when a −F atom is present at C-6 on the A-ring, enhanced the activity against serotype 14 and led to the loss of efficacy against serotype 1B [39]. Analysing the previous study [37] and comparing the obtained results with the present ones, it may be concluded that the introduction of only a −F at C-6 on the A-ring (78) decreased the activity against HRV 1B, when compared with the unsubstituted 2-SC 1 that was able to interfere with HRV 1B replication [39]. In conclusion, these new 2-SC (78–80) were not highly potent anti-rhinovirus agents and showed a preferential effect against HRV 14, contrary to the effects showed for 2-SC previously reported [37, 38].
Figure 18.
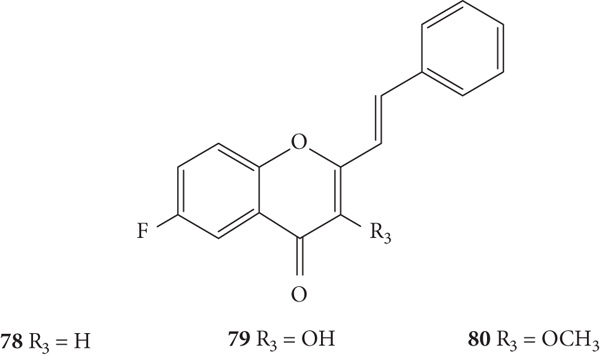
Chemical structures of 2-SC 78–80 [39].
Rocha-Pereira and co-workers [40] evaluated the potential anti-norovirus activity of 2-SC (1, 5, 21, 56–58, 62, 70, and 81–83, Figure 19) using MNV in murine macrophage cell line RAW 264.7 (RAW cells), by a plaque reduction assay. Human noroviruses (NoV) are the most frequent cause of outbreaks and sporadic cases of acute gastroenteritis and affect people of all ages [40, 41]. MNV was used in this study since it is genetically related to non-cultivable NoV and is able to replicate in RAW cells, being considered the best surrogate model for NoV [40]. First, the cytotoxicity of the compounds under study was evaluated, in vitro, by a colorimetric assay, to determine the concentration of compounds that cause 50% cytotoxicity (CC50) and MNTC. Most of the tested 2-SC showed little toxicity, presenting a relatively large margin of security, at the highest concentration tested (150 μM). However, 2-SC 62, 70, and 83 showed a different toxicity profile in RAW cells, with 62 and 83 being the most toxic (CC50 values of 52.1 ± 10.6 μM and 46.7 ± 8.7 μM, respectively). Antiviral activity was evaluated by a plaque reduction assay in RAW cells. The results were presented as IC50 values and the selectivity index (SI) was also presented. 2-SC 5 and 58 were the most active (IC50 values of 7.0 ± 0.7 μM and 7.4 ± 1.3 μM, respectively) and presented high SI values (>21.6 and >13.5, respectively). 2-SC 1, 57, 70, and 82 were less active but also demonstrated antiviral effects, and 21, 56, and 62 showed a weak anti-norovirus activity. In this study, the possibility of 2-SC 5 and 58 interfering in an early or late stage of MNV replication cycle was also evaluated. The antiviral effects of compounds decreased when they were added during the first hour of viral infection and remained when they were added after viral infection and for a continuous period of 48 h. Therefore, it was concluded that 2-SC 5 and 58 interfere more in the late stages of the virus life cycle that follow the enhancement of virus in cells. The obtained results showed that the type of substituent present at C-4′ on the B-ring influenced the potency of the anti-norovirus activity. As an example, this can be observed comparing the 4′-unsubstituted 2-SC 1 with 2-SC 21 (4′−OH substituted), 56 (4′−Cl substituted), 57 (4′−CH3 substituted), and 58 (4′−OCH3 substituted). The presence of −Cl or −OH at C-4′ on the B-ring led to a loss of the antiviral effect of 2-SC 56 or 21, respectively. 2-SC 57, with −CH3 at C-4′ on the B-ring, showed some antiviral activity but was approximately three times less potent than 2-SC 58, with −OCH3 at C-4′ on the B-ring. Thus, −OCH3 at C-4′ on the B-ring showed to be the best substituent, when there is no other substituent present in the 2-SC structure. In what concerns the C-5 substitutions on the A-ring, the presence of −OBn in 2-SC 81 led to a loss of antiviral effect, while the presence of −OH in 2-SC 5 led to one of the most active compounds. The existence of the −CH3 at C-α of the double bond of the styryl moiety led 2-SC 82 to show antiviral effect. The presence of the −OCH3 at C-3 on the C-ring appears to confer some antinorovirus activity to 2-SC 70 [40]. Some of the tested 2-SC in this study (1, 21, 56–58, 62, and 70) were also previously evaluated for their anti-rhinovirus activity [37, 38, 40]. 2-SC 1, 21, 56–58, 62, and 70 showed anti-norovirus and anti-rhinovirus activities (against both serotypes, 1B and 14), while 2-SC 58 was only effective against HRV 1B [37, 38, 40]. 2-SC 70 presented only a modest anti-norovirus effect but was considered as one of the most potent against both serotypes of rhinovirus [38, 40]. 2-SC 58, one the most active compounds for anti-norovirus activity, was inactive for HRV 14 and had little effect against HRV 1B [37, 40]. These results demonstrate a different interaction of the 2-SC with norovirus and rhinovirus and some selectivity, since the antiviral effects shown by the same 2-SC are very different for the two viruses, HRV and MNV [37, 38, 40].
Figure 19.
Chemical structures of 2-SC 1, 5, 21, 56–58, 62, 70, and 81–83 [40].
Chaniad and co-workers [12] isolated the 2-SC 11 (Figure 20) from the ethanolic extract of the bulbils of Dioscorea bulbifera and evaluated its inhibitory activity against the HIV-1 integrase through the multiplate integration assay [12]. HIV-1 is a human retrovirus of the lentivirus family that infects humans and results in the acquired immune deficiency syndrome (AIDS) [42]. HIV-1 integrase is one of the essential enzymes used for the replication of HIV, since it is responsible for the integration of viral DNA into the host cells [12, 42]. So, this enzyme is an attractive target for the development of novel anti-HIV drugs [12]. The anti-HIV-1 integrase activity of 2-SC 11 was evaluated and compared with the positive control, suramin (IC50 = 2.3 μM). 2-SC 11 was not much effective against the HIV-1 integrase, reaching only 47.89 ± 1.54% inhibition, at the highest tested concentration (100 μM) [12].
Figure 20.
Chemical structure of 2-SC 11 [12].
The above-mentioned structural characteristics that demonstrated to favour the antiviral activity of 2-SC against HRV and MNV are summarized in Figure 21.
Figure 21.
Structural characteristics that seem to favour the 2-SC antiviral activity against serotypes 1B and 14 of human rhinovirus (HRV) and murine norovirus (MNV).
4.3.2. 3-Styrylchromones
Shimada and co-workers [43] synthetized fifteen 3-SC (34–39 and 42–50, Figure 22) and studied their cytotoxicity and anti-HIV activity. For the anti-HIV assay, a human T-cell lymphotropic virus-I (HTLV-I) carrying human T-cell line MT4 that was infected with HIV-1IIIB was used [43]. HTLV-I is a human retrovirus of the oncornaviruses family [42]. First, the cytotoxicity of the 3-SC under study was evaluated by the MTT assay and the CC50 was determined. In this study, the error associated with the results was not indicated, even though the assays were performed in triplicate. According to their cytotoxicity, 3-SC can be divided into two groups: those with high to moderate toxicity (37, 39, 44–47, 49, and 50) and those with low toxicity (34–36, 38, 42, 43, and 48). For the anti-HIV assay, the results were expressed as SI. The SI was calculated by dividing the CC50 by the 50% cytoprotective concentration from HIV infection (EC50). All the tested 3-SC showed SI values of <1, meaning that none of the tested 2-SC protected the cells from the HIV infection. Four positive controls were used: dextran sulphate, curdlan sulphate, azidothymidine, and 2′,3′-dideoxycytidine, with SI values between 2445 and 20421 [43].
Figure 22.
Chemical structures of 3-SC 34–39 and 42–50 [43].
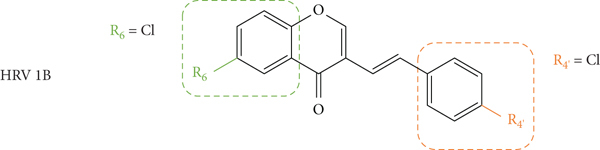
Conti and Desideri [44] also evaluated the potential antiviral activity of four 3-SC (2, 41, 84, and 85, Figure 23) against HRVs 1B and 14 and EV 71. The EV belongs to the family of picornavirus and its infection can constitute a serious health threat in children under young children [35]. Antiviral activity was evaluated by a plaque reduction assay in HeLa cell culture infected with HRV types 1B and 14 and in human epithelial type 2 (HEp-2) cell cultures infected with EV 71. First, the cytotoxicity of the 3-SC under study was evaluated by XTT assay, to determine the MNTC. The error associated with the results was not indicated, even though the assays were performed in triplicate. 4′,6-Dichloroflavan was included as a positive control for serotype 1B (IC50 = 0.026 μM), but no positive control was included for HRV 14 and EV 71. The tested compounds presented low cytotoxicity against HeLa and HEp-2 cells, and all the tested 3-SC were able to interfere with the replication of both HRV serotypes and EV 71, but the sensibility of each virus was considerably different. For HRV, the tested 3-SC exhibited a higher potency against the serotype 1B than against 14. 3-SC 84 was the most potent against HRV 1B (IC50 = 2.19 μM), while 3-SC 2 and 41 were the most potent against HRV 14 (IC50 values of 4.36 μM and 5.52 μM, respectively). The results seem to indicate that the presence of a −Cl at C-6 on the A-ring (85) or −Cl at C-4′ on the B-ring (41) slightly decreases the antiviral activity of these 3-SC against both HRV serotypes, when compared with unsubstituted 3-SC (2). When comparing the activity of 3-SC 84, with −Cl at C-6 on the A-ring and C-4′ on the B-ring, with 3-SC 2, it was observed that the presence of these substituents enhanced the antiviral activity of 3-SC against HRV 1B but decreased the antiviral effect against serotype 14. For the antiviral activity against EV 71, the tested 3-SC showed low activities and only reached 21.6–37.0% of inhibition, at the highest tested concentration (25 μM for 41, 84, and 85, and 50 μM for 2) [44].
Figure 23.
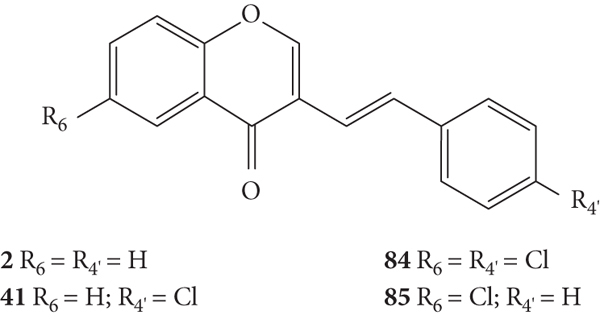
Chemical structures of 3-SC 2, 41, 84, and 85 [44].
The above-mentioned structural characteristics that demonstrated to favour the antiviral activity of 3-SC against HRV 1B are summarized in Figure 24.
Figure 24.
Structural characteristics that seem to favour the 3-SC antiviral activity against serotype 1B of human rhinovirus (HRV).
4.4. Antibacterial Activity
Antibacteria are a class of compounds that are used to treat bacterial infections. The antibacterial activity is associated with the compounds that kill bacteria or slow down their rate of growth, without being extensively toxic to nearby tissues. The wide use as well as the abuse of antibacterial agents has led to the development of multidrug-resistant bacteria. The emergence of drug-resistant bacteria has become the major cause of failure in the treatment of infectious diseases, since most bacteria are resistant to a minimum of one of the antibiotics that are generally used to eliminate the infection. The inefficacy of currently available antibiotics urges the searching for new types of antibacterial agents against the drug-resistant bacteria [45]. Several 2-SC have shown potential as antibacterial agents against Enterococcus faecium, Bacillus subtilis, Staphylococcus aureus, S. sciuri, S. xylosus, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Xanthomonas campestris, and Agrobacterium tumefaciens and are below discussed. All the observed antibacterial effects are summarized in Table 7. There are no studies on the antibacterial activity of 3-SC, to the best of our knowledge.
Table 7.
2-SC studied for the antibacterial activity and summary of the observed antibacterial effects.
| 2-SC | Observed effect(s) | Ref. |
|---|---|---|
| 1 | Antibacterial activity against X. campestis and A. tumafeciens | [48] |
| 13 | ||
| 28–32 | [30] | |
| 56 | [48] | |
| 58 | Antibacterial activity against B. subtilis (ATCC 6633), X. campestis and A. tumafeciens | [46, 48] |
| 78 | Antibacterial activity against B. subtilis (ATCC 6633), E. faecium (ATCC 51299), S. aureus (ATCC 29212 and ATCC 43300), S. sciuri (ATCC 29062) and S. xylosus (ATCC 35033) | [46] |
| 86–88 | Antibacterial activity against B. subtilis (ATCC 6633), S. aureus (ATCC 29212 and ATCC 43300) and E. coli (ATCC 25922) | |
| 89 | Antibacterial activity against B. subtilis (ATCC 6633), S. aureus (ATCC 29212 and ATCC 43300) and E. coli (ATCC 25922 and ATCC 35218) | |
| 90 | Antibacterial activity against B. subtilis (ATCC 6633), S. sciuri (ATCC 29062) and E. coli (ATCC 25922 and ATCC 35218) | |
| 91 | Antibacterial activity against B. subtilis (ATCC 6633) | |
| 92 | Antibacterial activity against B. subtilis (ATCC 6633) and S. aureus (ATCC 29212) | |
| 94–101 | Antibacterial activity against B. subtilis (NCIM-2063), S. aureus (NCIM-2901), E. coli (NCIM-2256) and P. aeruginosa (NCIM-2036) | [47] |
| 102 | Antibacterial activity against X. campestis and A. tumafeciens | [48] |
4.4.1. 2-Styrylchromones
Momin and co-workers [46] synthetized ten 2-SC (58, 78, and 86–93, Figure 25) and evaluated their antibacterial activity by the disc diffusion method against the Gram-positive bacteria E. faecium (ATCC 51299), B. subtilis (ATCC 6633), S. aureus (ATCC 29212 and ATCC 43300), S. sciuri (ATCC 29062), and S. xylosus (ATCC 35033) and the Gram-negative bacteria E. coli (ATCC 25922 and ATCC 35218), P. aeruginosa (ATCC 35032), and K. pneumoniae (ATCC 700603). In this study, we used two standard antibiotics as positive controls: tetracycline and ampicillin. In general, the 2-SC were more effective against the Gram-positive bacteria and less potent than the standard antibiotics used. The exception was 2-SC 93 which showed no antibacterial activity against any of the studied bacterial organisms. The other 2-SC showed to be effective against B. subtilis. Only 2-SC 78 showed antibacterial effect against E. faecium [diameter of inhibition zone (DIZ) = 10 ± 0.06 mm]. In what concerns S. aureus, two strains were studied: ATCC 29212 and ATCC 43300. 2-SC 78 and 86–89 were effective against both strains for ATCC 43300 strain; however, 2-SC 92 was only effective against the ATCC 29212 strain (DIZ = 9 ± 0.12 mm). 2-SC 78 were effective against S. sciuri and S. xylosus (DIZ values of 8 ± 0.06 mm and 10 ± 0.06 mm, respectively), while 2-SC 90 was only effective against S. sciuri (DIZ = 12 ± 0.06 mm). In what concerns the Gram-negative bacteria, the studied 2-SC did not show any antibacterial activity against P. aeruginosa and K. pneumoniae. 2-SC 86–90 showed activity against E. coli strain ATCC 25922, and additionally, 2-SC 89 and 90 also showed activity against E. coli strain ATCC 35218. Analysing the structures of the tested 2-SC, in general, the 2-SC with −F on the A- and/or B-rings (78 and 86–91) were effective against a large number of studied bacterial organisms, which seems to indicate that −F plays an important role for the antibacterial activity. 2-SC 78, with only one −F at C-6 on the A-ring, showed an inhibitory effect against all the studied Gram-positive bacteria but did not show any activity against any of the Gram-negative bacteria. 2-SC 90, with −F at C-7 on the A-ring and C-4′ on the B-ring, showed antibacterial activity only against two Gram-positive bacteria (B. subtilis and S. sciuri) but was more effective than 2-SC 78 against these specific strains. Additionally, 90 also showed an inhibitory effect against both strains of E. coli (Gram-negative bacteria). 2-SC 89, with −F at C-3′ and C-5′ on the B-ring, also exhibited inhibitory effect against both strains of E. Coli and against B. subtilis and both strains of S. aureus. The presence of only one −F at C-2′ (86) or at C-3′ (87) or at C-4′ (88) on the B-ring led these 2-SC to be effective against B. subtilis and both strains of S. aureus and only one strain of E. coli (ATCC 25922). The obtained results in this study seem to indicate that the position and number of −F substituents in the A- or B-rings influence the antibacterial activity of 2-SC. 2-SC 58, 92, and 93 have no −F substituents in their structure. 2-SC 58, with −OCH3 at C-4′ on the B-ring, was only active against B. subtilis. The presence of −OCH3 at C-3′ and C-4′ on the B-ring (92) led to an inhibitory effect against B. subtilis and one strain of S. aureus (ATCC 29212). The 2-SC 93 is a methylenedioxy derivative and displayed no antibacterial activity, which apparently indicates that this group does not favour this activity [46].
Figure 25.
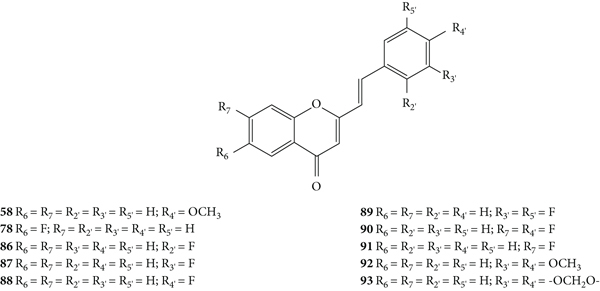
Chemical structures of 2-SC 58, 78, and 86–93 [46].
Nikam and co-workers [47] synthetized a novel series of 2-SC with 1,2,4-triazole ring at C-4′ on the B-ring (94–101, Figure 26) and evaluated their antibacterial activity by the agar dilution method against the Gram-positive bacteria B. subtilis (NCIM-2063) and S. aureus (NCIM-2901) and the Gram-negative bacteria E. coli (NCIM-2256) and P. aeruginosa (NCIM-2036). In this study, the results were presented as the minimum inhibitory concentration (MIC) but the associated error was not indicated, even though the assays were performed in triplicate. Taking this in consideration, 2-SC 96 was the most potent (MIC = 25 μg/mL) against S. aureus, being more potent than the positive control, ciprofloxacin (MIC = 50 μg/mL). 2-SC 94, 96, and 99 were the most potent against B. subtilis (MIC = 50 μg/mL), being equipotent with ciprofloxacin. In what concerns Gram-negative bacteria, 2-SC 94 was the most potent against E. coli (MIC = 25 μg/mL), being as powerful as ciprofloxacin, and against P. aeruginosa (MIC = 50 μg/mL). The positive control, ciprofloxacin, showed a MIC value of 25 μg/mL. Analysing the substitution pattern of the studied 2-SC, 94, only with 1,2,4-triazole ring at C-4′ on the B-ring as a substituent, showed an excellent antibacterial activity against all the bacterial panel tested, being the most potent for the majority of them, with exception of S. aureus. The introduction of a −CH3 at C-6 on the A-ring of 2-SC 94 resulted in 95 and led to a significant loss of antibacterial activity, which was recovered with the addition of a second −CH3 at C-8 on the A-ring (96). The presence of a single −Cl at C-6 on the A-ring in 2-SC 99 made it equipotent with a ciprofloxacin against B. subtilis but decreased its antibacterial potential against the other tested bacterial strains, when compared to 2-SC 94. The addition of a second −Cl at C-8 on the A-ring to 2-SC 99, giving 100, improved the antibacterial effect against S. aureus and E. coli but decreased the potential against B. subtilis and P. aeruginosa. The introduction of a −F or a −Br at C-6 on the A-ring (98 and 101, respectively) led to a weaker antibacterial activity, when compared to 2-SC 94. The tested 2-SC were also evaluated for their absorption, distribution, metabolism, excretion, and toxicity (ADMET). All synthesized 2-SC showed to obey the Lipinski's rule of five and Veber rule, which indicates good drug-like properties. It has also been suggested that 2-SC have a good oral bioavailability, due the values obtained for the total polar surface area (TPSA) (<100), logarithm of partition coefficient of compound between n-octanol and water (log P < 5), and molecular weight (<500). The number of rotatable (<25) and rigid (<10) bonds, the number of hydrogen bond acceptors (<10) and donors (<5) indicated that these 2-SC have good intestinal bioavailability [47].
Figure 26.
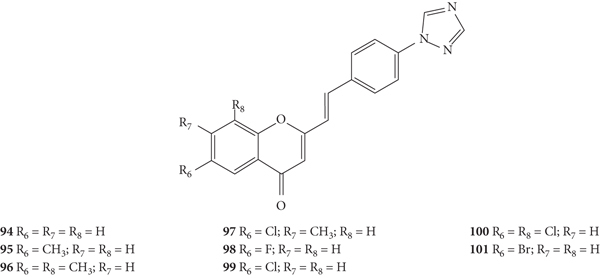
Chemical structures of 2-SC 94–101 [47].
Ujwala and co-workers [48] evaluated the antibacterial activity of five 2-SC (1, 13, 56, 58, and 102, Figure 27) by the agar cup method against Xanthomonas campestris and Agrobacterium tumefaciens. In this study, the results were expressed as DIZ values but the authors did not indicate the associated error. The results of DIZ were presented for each of the volumes of the compounds used, 50 μL and 100 μL, with no concentration of solutions being indicated. Thus, two values of DIZ will always be indicated, which correspond in the same order to 50 μL and 100 μL. Streptomycin was used as a positive control, and only a volume of the compound (10 μL) was tested, thus only one DIZ value was reported. All the tested 2-SC exhibited some antibacterial effect against the tested bacteria. The order of potencies that can be established, based on the reported values, against X. campestris is 56 > 58 > 102 > 13 > 1 (DIZ values of 4.2 and 11.2 mm; 4.0 and 10.8 mm; 4.1 and 9.8 mm; 3.6 and 9.5 mm; and 1.2 and 4.2 mm, respectively). Streptomycin showed a DIZ value of 15 mm. The order of potencies that may be suggested against A. tumefaciens is the same: 56 > 58 > 102 > 13 > 1, with DIZ values of 3.9 and 9.6 mm; 3.8 and 8.9 mm; 3.7 and 8.6 mm; 3.4 and 7.8 mm; and 2.2 and 5.3 mm, respectively. For streptomycin, the DIZ value was 12 mm against A. tumefaciens. In what concerns the structure of the tested 2-SC, the presence of a −Cl at C-4′ on the B-ring in 2-SC 56 appears to increase the antibacterial potential against both tested bacteria, compared with unsubstituted 2-SC 1. The 2-SC 58, with −OCH3 at C-4′ on the B-ring, and 102, with −OCH3 at C-4′ and −OH at C-7 on the B- and A-rings, also had a greater antibacterial activity than the unsubstituted 2-SC 1 and the 2-SC 13, with only one −OH at C-7 on the A-ring. In conclusion, the 2-SC with −Cl or −OCH3 at C-4′ on the B-ring resulted in a better antibacterial activity against X. campestris and A. tumefaciens [48].
Figure 27.
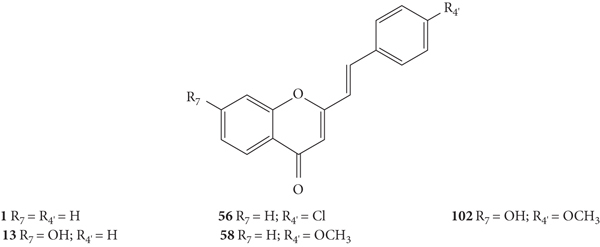
Chemical structures of 2-SC 1, 13, 56, 58, and 102 [48].
The same authors also evaluated the antibacterial activity of a series of 2-SC derivatives with −OH, −CH3, and −OCH3 at C-6 on the A-ring and/or C-4′ on the B-ring (28–32, Figure 28) by the same previous method against X. campestris and A. tumefaciens [30]. In this study, the results were expressed as DIZ values but the authors did not indicate the associated error. The results of DIZ were presented for each of the volumes of the compounds used, 50 μL and 100 μL, with no concentration of solutions being indicated. Thus, two values of DIZ will always be indicated, which correspond in the same order to 50 μL and 100 μL. Streptomycin was used as a positive control and only a volume of the compound (10 μL) was tested, thus only one DIZ value was reported. All the tested 2-SC exhibited some antibacterial effect against the tested bacteria, and 2-SC 31 was the most active one (DIZ values of 5.7 and 11.3 mm against X. campestris and 5.2 and 11.0 mm against A. tumefaciens). Streptomycin showed a DIZ value of 15 and 12 mm against X. campestris and A. tumefaciens, respectively. In what concerns the structure of the tested 2-SC, the presence of −OH at C-6 on the A-ring and C-4′ on the B-ring (31) appears to favour the antibacterial potential against both tested bacteria, while the presence of −OCH3 at C-6 on the A-ring and C-4′ on the B-ring appears to decrease the activity (30) [30].
Figure 28.
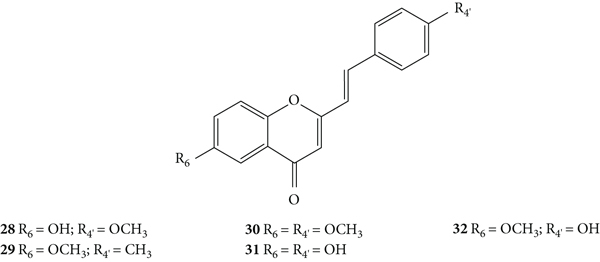
Chemical structures of 2-SC 28–32 [30].
The existing works on the antibacterial activity of 2-SC include studies with different strains of Gram-positive and Gram-negative bacteria. The antibacterial activity demonstrated by 2-SC showed some variability, and it is not possible to define a clear SAR for each strain, resulting in the establishment of a generic SAR for the antibacterial activity. Thus, the above-mentioned structural characteristics that demonstrated to favour the antibacterial activity of 2-SC are summarized in Figure 29.
Figure 29.
Structural characteristics that seem to favour the 2-SC antibacterial activity.
4.5. Antifungal Activity
Antifungal agents are used to treat fungal infections, in which the most common types are cutaneous, and ringworm infections of the skin and nails [49, 50]. Another type of common fungal infections includes mucosal infections of the oral and genital tracts. Fungal infections can be superficial or invasive, the latter having a much lower incidence but are of great concern [49]. Invasive fungal infections have a higher incidence in immunocompromised individuals, as their immune system is weakened. The body's immune system plays an essential role and has effective mechanisms for the prevention fungal infections [49, 50]. Fungi are omnipresent and a generally benign part of our environment, for healthy individuals. Antifungal agents work by arresting or killing pathogenic fungal cells in preference to normal cells [50]. The development of successful antifungal agents becomes urgent, especially with the emergence of new fungal pathogens and knowing that fungal infections substantially contribute to human mortality [49, 50]. Several 2-SC have shown potential as antifungal agents against Candida albicans, Aspergillus niger, Aspergillus flavus, and Penicillium chrysogenum and are below discussed. All the observed antifungal effects are summarized in Table 8. There are no studies on the antifungal activity of 3-SC, to the best of our knowledge.
Table 8.
2-SC studied for the antifungal activity and summary of the observed antifungal effects.
4.5.1. 2-Styrylchromones
Nikam and co-workers [47] evaluated the antifungal potential of 2-SC 94–101 (Figure 30) against C. albicans (NCIM-3417), A. niger (NCIM-1196), and A. flavus (NCIM-539) by the agar dilution method. In this study, the results were presented as MIC values but the associated error was not indicated, even though the assays were performed in triplicate. 2-SC 94 and 96 were the most potent (MIC = 25 μg/mL) against C. albicans, being two times more potent that positive control, fluconazole (MIC = 50 μg/mL). For the A. niger, 2-SC 94 and 96–98 were the most potent (MIC = 25 μg/mL) and were equipotent with fluconazole. Against A. flavus, 2-SC 94, 96, and 98 were the most potent (MIC = 25 μg/mL), being equipotent with fluconazole. Analysing the substitution pattern, all the tested 2-SC have a 1,2,4-triazole ring at C-4′ on the B-ring. Interestingly, 2-SC 94, with only a 1,2,4-triazole ring at C-4′ on the B-ring as a substituent, showed an excellent antifungal activity against all the tested fungal strains. The presence of only one −CH3 at C-6 on the A-ring (95) led to a decrease in antifungal activity, when compared to 2-SC 94 and 96. The presence of a −Cl or −Br at C-6 on the A-ring (99 and 101, respectively) also led a decrease in antifungal potential compared to 2-SC 94. The existence of a −Cl at C-6 and C-8 on the A-ring (100) also did not favour antifungal activity. The introduction of −F at C-6 on the A-ring (98) maintained the antifungal activity against A. niger and A. flavus but decreased the potential against C. albicans, when compared to 2-SC 94. The studied 2-SC were also evaluated for their ADMET, as already mentioned in Section 4.4.1. In order to understand the possible mechanism of antifungal activity, docking studies were performed to rationalize the 2-SC binding interaction at the active site of fungal enzyme cytochrome P450 lanosterol 14 α-demethylase. The 1,2,4-triazole ring at C-4′ on the B-ring was mainly responsible for the interaction in the active site. Overall, a good binding interaction was shown between 2-SC 94–101 and the active site of fungal enzyme cytochrome P450 lanosterol 14 α-demethylase [47].
Figure 30.
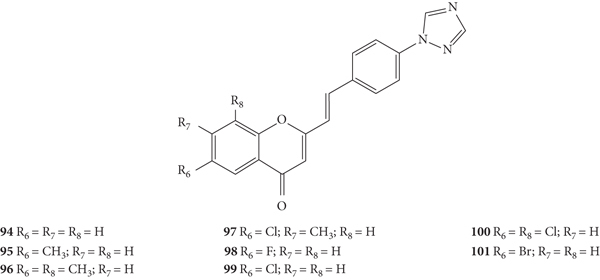
Chemical structures of 2-SC 94–101 [47].
Ujwala and co-workers [48] evaluated the antifungal activity of 2-SC 1, 13, 56, 58, and 102 (Figure 31) by the disc diffusion method against A. niger and P. chrysogenum. The results were expressed as DIZ values, but the authors did not indicate the associated error. The results of DIZ were presented for each of the volumes of the compounds used, 50 μL and 100 μL, with no concentration of solutions being indicated. Thus, two values of DIZ will always be indicated, which correspond in the same order to 50 μL and 100 μL. Nystatin was used as a positive control and only a volume of the compound (10 μL) was tested, thus only one DIZ value was reported. All tested 2-SC exhibited antifungal activity. 2-SC 56 was the most active against both tested fungal strains (DIZ values of 7.5 and 13.4 mm against A. niger and 6.9 and 12.2 mm against P. chrysogenum); nystatin presented DIZ values of 19 and 13 mm against A. niger and P. chrysogenum, respectively. 2-SC 56 has a −Cl at C-4′ on the B-ring, suggesting that this feature contributes to the increase of antifungal activity, when compared to the unsubstituted 2-SC 1.The 2-SC 58, with −OCH3 at C-4′ on the B-ring, and 2-SC 102, with −OCH3 at C-4′ on the B-ring and −OH at C-7 on the A-ring, also had a greater antifungal activity. 2-SC 13, with only one −OH at C-7 on the A-ring, showed antifungal activity lower than those mentioned above (56, 58, and 102) but higher than 2-SC 1. Thus, 2-SC with −Cl or −OCH3 at C-4′ on the B-ring had better antifungal activity, suggesting that these groups may be responsible for the greater effects [48].
Figure 31.
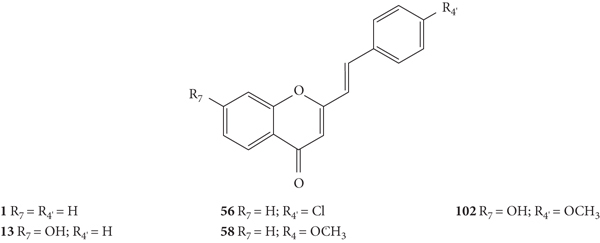
Chemical structures of 2-SC 1, 13, 56, 58, and 102 [48].
The same authors evaluated the antifungal activity of 2-SC 28–32 (Figure 32), with −OH, −CH3, and −OCH3 at C-6 and/or C-4′ on the A- and B-rings, against A. niger and P. chrysogenum, by the disc diffusion method [30]. The results were expressed as DIZ values, but the authors did not indicate the associated error. The results of DIZ were presented for each of the volumes of the compounds used, 50 μL and 100 μL, with no concentration of solutions being indicated. Thus, two values of DIZ will always be indicated, which correspond in the same order to 50 μL and 100 μL. Nystatin was used as a positive control and only a volume of the compound (10 μL) was tested, thus only one DIZ value was reported. 2-SC 30 was the most active against both tested fungal strains (DIZ values of 6.7 and 11.9 mm against A. niger and 6.3 and 11.7 mm against P. chrysogenum); nystatin presented DIZ values of 19 and 13 mm against A. niger and P. chrysogenum, respectively. These results indicate that the presence of −OCH3 simultaneously at C-6 on the A-ring and C-4′ on the B-ring appears to favour the antifungal potential. 2-SC 28, with −OH at C-6 on the A-ring and an −OCH3 at C-4′ on the B-ring, also showed antifungal potential, as well as 2-SC 32, with −OCH3 at C-6 on the A-ring and an −OH at C-4′ on the B-ring. 2-SC 31 has −OH simultaneously at C-6 on the A-ring and C-4′ on the B-ring and showed a decrease in the antifungal effects when compared to 2-SC 30 and 28. 2-SC 29 has an −OCH3 at C-6 on the A-ring, while in C-4′ on the B-ring, it has an −CH3 group. This structure showed a greater decrease in antifungal effects compared to 2-SC 30, which may indicate that the −CH3 group may not favour the antifungal activity. Thus, the −OCH3 group can be identified as contributing for the antifungal activity of the 2-SC but its effects vary according to their position and the additional substituents present in the 2-SC scaffold [30].
Figure 32.
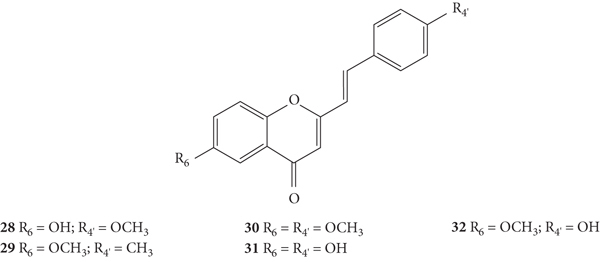
Chemical structures of 2-SC 28–32 [30].
The studies on the antifungal activity of 2-SC included different substitution patterns of 2-SC and diverse methodologies used in each study, which difficults the establishment of a specific SAR for each fungus. Thus, it was only possible to establish a generic SAR for antifungal activity. The above-mentioned structural characteristics that demonstrated to favour the antifungal activity of 2-SC are summarized in Figure 33.
Figure 33.
Structural characteristics that seem to favour the 2-SC antifungal activity.
4.6. Anti-Inflammatory Activity
Inflammation is the body's first response to processes such as infection, tissue injury, cell death, and degeneration. The inflammatory process involves the innate and the adaptive immune responses. The adaptive immune system involves the activity of more specialized cells such as B and T cells, while the innate immune system involves the activity of various cells. During inflammatory responses, numerous inflammatory mediators are synthetized and secreted and they are usually divided into pro- and anti-inflammatory mediators. The search for compounds that are able to regulate these mediators and interfere with the inflammation mechanisms is still ongoing [51]. Several 2-SC have shown potential as anti-inflammatory agents through several mechanisms that are discussed below, such as the inhibition of lymphocyte function-associated molecule-1/intercellular cell adhesion molecule-1 (LFA-1/ICAM-1)-mediated cell adhesion, interference with the arachidonic acid metabolic pathways, inhibition of nuclear factor kappa B (NF-κB) activation, and modulation of cytokines/chemokine production. All the observed anti-inflammatory effects are summarized in Table 9. The anti-inflammatory activity of SC has already been reviewed [3, 4]; the most recent review article was published in 2017. To the best of our knowledge, since this year, no article has been published describing the anti-inflammatory activity of SC. Thus, in this work, this activity will be presented and discussed in more detail than in the two previously referred reviews [3, 4]. There are no studies on the anti-inflammatory activity of 3-SC, to the best of our knowledge.
Table 9.
2-SC studied for the anti-inflammatory activity and summary of the observed anti-inflammatory effects.
| 2-SC | Observed effect(s) | Ref. |
|---|---|---|
| 1 | Inhibition of LTB4 production in human neutrophils | [54] |
| 5 | ||
| 11 | Inhibition of LTB4 production in human neutrophils and COX-1 activity | |
| 15 | Inhibition of LTB4 production in human neutrophils, NF-kB activation and production of TNF-α and IL-6; and induction of the production of IL-1β and IL-8 | [54, 59] |
| 16 | Inhibition of LTB4 production in human neutrophils; and reducing activity | [54] |
| 17 | Inhibition of LTB4 production in human neutrophils; metal chelating capacity; reducing activity; inhibition of NF-kB activation and production of TNF-α, IL-1β, IL-6 and IL-8 | [54, 59] |
| 18, 19 | Inhibition of LTB4 production in human neutrophils and COX-1 activity; metal chelating capacity; and reducing activity | [54] |
| 20 | Inhibition of LTB4 production in human neutrophils; metal chelating capacity; and reducing activity | |
| 21 | Inhibition of LTB4 production in human neutrophils, NF-kB activation and production of TNF-α, IL-1β and IL-6 | [54, 59] |
| 33 | Inhibition of cell-cell adhesion between HL-60 cells and CHO-ICAM-1 cells | [52] |
CHO: Chinese hamster ovary; COX-1: cyclooxygenase-1; HL-60: promyelocytic human leukaemia-60; ICAM-1: intercellular cell adhesion molecule-1; IL: interleukin; LT: leukotriene; NF-κB: nuclear factor kappa B; TNF-α: tumor necrosis factor α.
4.6.1. 2-Styrylchromones
Takamatsu and co-workers [52] evaluated the profile of hormothamnione (3, Figure 34) and hormothamnione diacetate (33, Figure 34) as inhibitors of the adhesion of HL-60 cells to Chinese hamster ovary (CHO)-ICAM-1 cells through LFA-1/ICAM-1 interaction. LFA-1 is a leukocyte integrin adhesion molecule which promotes intercellular adhesion of most leukocytes to each other and to other cells, playing a crucial role in immunological and inflammatory reactions [52, 53]. ICAM-1 is a glycosylated integral membrane protein, that is expressed by most tissues at low levels. Its expression readily increases by the action of inflammatory cytokines. ICAM-1 plays an important role in inflammation and in T-cell-mediated host defence system. LFA-1 typically binds to ICAM-1, and this interaction mediates a cell adhesion pathway that is important in the progression of inflammatory response [52, 53]. In this study, the expression of LFA-1 occurred in HL-60 cells, while CHO-ICAM-1 cells express high levels of ICAM-1. The authors only indicated the associated error for some results. Thus, the results presented here are in accordance with the information provided in the original article. First, a primary screening was carried out to the selection of candidates of cell adhesion inhibitors, among the compounds selected for the study, using cell aggregation assay and XTT method. After this screening, the cytotoxicity of the selected compounds to HL-60 cells was evaluated by XTT assay, whereas to CHO-ICAM-1 cells, the MTT assay was used. 2-SC 3 and 33 did not affect the cell proliferation of HL-60 cells but did affect the proliferation of CHO-ICAM-1 cells. 2-SC 3 and 33 inhibited cell aggregation of HL-60 cells (MIC values of 3.1 and 2.6 μM, respectively). 2-SC 3 did not significantly inhibit the cell adhesion of HL-60 to CHO-ICAM-1, while 2-SC 33 potently inhibited this adhesion (IC50 = 1.5 ± 0.2 μM). Cytochalasin B was used as positive control and showed to inhibit cell adhesion of HL-60 to CHO-ICAM-1 cells with an IC50 value of 1.2 ± 0.2 μM. The difference between the results obtained for 2-SC 3 and 33, regarding the inhibition of cell-cell adhesion, may be attributed to the −OCOCH3 present at C-3′ and C-5′ on the B-ring of 2-SC 33, whereas 2-SC 3 has −OH at the same positions. Thus, as 2-SC 33 inhibited cell-cell adhesion between HL-60 cells and CHO-ICAM-1 cell, it may prove to be beneficial in the treatment of inflammation [52].
Figure 34.
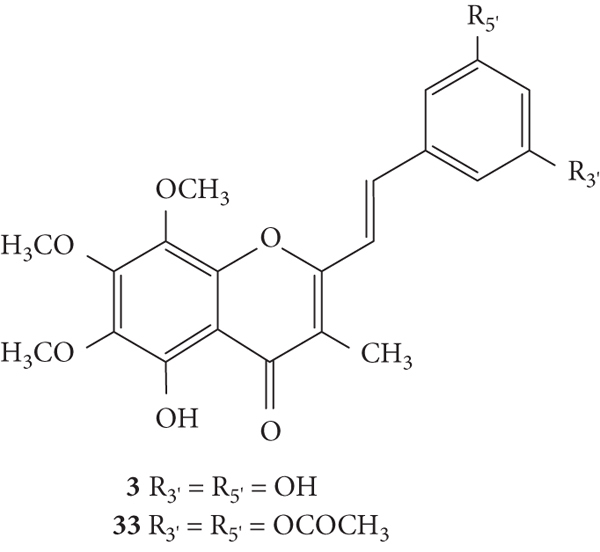
Chemical structures of 2-SC 3 and 33 [52].
Gomes and co-workers [54] evaluated the anti-inflammatory potential of 2-SC 1, 5, 11, and 13–21 (Figure 35) by studying their interference with the arachidonic acid metabolic pathways. The authors assessed the ability of these 2-SC to inhibit cyclooxygenase (COX) -1 and -2 (ovine and human recombinant origin, respectively) and leukotriene (LT) B4 production (in human neutrophils). The metal chelating activity and reducing power of these compounds were also evaluated [54]. Arachidonic acid is a polyunsaturated fatty acid and an integral constituent of biological cell membranes, conferring their fluidity and flexibility [55]. Arachidonic acid is metabolized by COXs and 5-lipoxygenase, resulting in the synthesis of prostanoids and leukotrienes, respectively. COX-1 and COX-2 are isoforms of the COXs. COX-1 is a constitutive enzyme expressed in all tissues that induces an acute inflammation in response to an inflammatory stimuli, while COX-2 is an inducible isoform, usually absent in most tissues and that is upregulated by inflammatory stimuli as cytokines, hormones, and growth factors. COX-1 is involved in several homeostatic processes, such as the modulation of platelet aggregation and the preservation of gastrointestinal mucosal integrity, so its inhibition affects not only the inflammatory response but also the normal function of the body. COX-2 is expressed primarily in cells involved in inflammation, e.g., macrophages and fibroblasts, so its selective inhibition is preferable for an anti-inflammatory activity [55–57]. As mentioned above, COXs are involved in the metabolization of arachidonic acid to prostanoids, which include prostaglandins, thromboxanes, and prostacyclins. The prostanoids are lipid mediators and are involved in physiologic and pathologic processes, namely, in inflammation. Prostaglandins are pro-inflammatory mediators and their biosynthesis is increased during the inflammatory response. These mediators increase vascular permeability and are involved in common inflammation signalling processes, such as pain, oedema, and redness [55, 56, 58]. LTB4 is a LT that induces inflammation via its chemotactic and degranulating actions on polymorphonuclear lymphocytes [55]. In the study of Gomes and co-workers, the results were expressed as percentages of inhibition and the values are indicated by the authors in the “Results” Section. Thus, the results presented here are in accordance with the information provided in the original article, where only a few activity values were mentioned. In what concerns the inhibition of COX-1 and COX-2 by 2-SC, this was determined in a cell-free system by quantifying the levels of prostaglandin F2α, produced by catalysis of arachidonic acid. The results were expressed as the percent inhibition of COX-1 or COX-2 activity. 2-SC 17–20 were able to inhibit COX-1. The 2-SC 19 was the most potent, significantly inhibiting the enzyme's activity at the concentrations of 100 and 250 μM (46.0 ± 5.6% and 74.4 ± 12.8%, respectively). From 2-SC with −OH at C-4′ on the B-ring (11, 15, 16, and 21), only 2-SC 11 and 16 inhibited COX-1 at a concentration of 250 μM, with 2-SC 11 being significantly more potent (66.5 ± 11.4% inhibitory effect). None of the other 2-SC (1, 5, 13, 14, 15, and 21) was able to inhibit COX-1 and none of the studied 2-SC inhibited COX-2 at the tested concentrations (100 and 250 μM). Indomethacin (1 μM), a non-selective COX inhibitor, and a selective COX-2 inhibitor, celecoxib (10 μM), were tested as positive controls. Indomethacin showed 26.6 ± 6.6% inhibitory effect of COX-1 and 92.6 ± 2.1% inhibitory effect of COX-2. Celecoxib inhibited 68.2 ± 3.0% of COX-2 activity. In general, 2-SC with −OH groups at C-3′ and C-4′ on the B-ring appear to be better COX-1 inhibitors than 2-SC with −OH at C-4′ on the B-ring or unsubstituted B-ring. All the tested 2-SC demonstrated inhibitory effects of LTB4 production by human neutrophils. A lipoxygenase inhibitor, nordihydroguaiaretic acid (NDGA), at a concentration of 1 μM, and two concentrations of 2-SC (10 and 25 μM) were tested, but only the results for the concentration 25 μM of SC were shown. In general, 2-SC 17–20, with −OH at C-3′ and C-4′ on the B-ring, were more effective inhibitors of LTB4 production than the other 2-SC (1, 5, 11, 13–16, and 21). 2-SC 17, 19, and 20 were the most effective compounds, reaching ≈90% inhibitory effect. Among the 2-SC that have in common an −OH at C-4′ on the B-ring (11, 15, 16, and 21), 2-SC 15 was the most effective with 78.0 ± 9.0% inhibitory effect. In general, 2-SC without any substitution in the B-ring (1, 5, 13, and 14) were less effective. 2-SC 1 was the most effective of these group of compounds (48.9 ± 10.3% inhibitory effect). NDGA (1 μM) reached 76.1 ± 11.0% inhibitory effect. In what concerns the reducing power, 2-SC 16–20 were able to reduce ferric ion, at the final concentration of 25 μM, being 2-SC 17–20 more efficient reducers than the positive control, ascorbic acid. 2-SC 17–20 also showed metal chelating capacity. In this study, the authors established correlations with the results obtained in a previous work [26] with these 2-SC. Thus, a significant correlation was established between inhibition of LTB4 production and the reducing activity and between inhibition of LTB4 production and the scavenging activity against 1O2 and ONOO— (with and without NaHCO3) of the tested 2-SC [54]. Overall, 2-SC with substitutions on the B-ring (−OH at C-3′ and C-4′ or −OH at C-4′) appear to have a more significant influence on the inhibition of LTB4 production in human neutrophils than 2-SC with substitutions on the A-ring (−OH at C-5 and/or C-7 or unsubstituted). In this study, it was also proposed that inhibition of LTB4 production by 2-SC probably involves the inhibition of 5-lipoxygenase. The majority of 5-lipoxygenase inhibitors act in the catalytic domain of the enzyme, reducing or chelating iron from the active site or by eliminating radical intermediates in the iron redox cycle. Thus, the 2-SC that were most effective in inhibiting the production of LTB4 (17–20) were also strong reducers, supporting the idea that the inhibition of 5-lipoxygenase is related to the mechanism that leads to the inhibition of LTB4 production by 2-SC. Significant correlations were found between the inhibition of LTB4 production and the scavenging activity against 1O2 and ONOO— also indicating that the elimination of radical intermediates may contribute to the LTB4 production inhibitory effect. For the inhibition of COX-1 activity, it has been proposed that the mechanism by which 2-SC inhibit COX-1 activity probably consists in the scavenging of the radical intermediates involved in COX enzyme catalysis. This can be corroborated by the fact that only 2-SC that previously showed high ROS and RNS scavenging activity [26] have been effective [54]. The fact that 2-SC only inhibits COX-1 activity seems to indicate some selectivity for this isoform.
Figure 35.
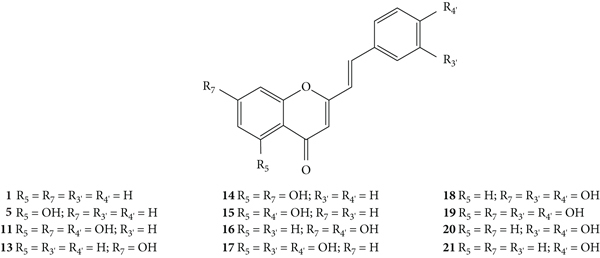
Gomes and co-workers [59] also evaluated the effects of several 2-SC derivatives (1, 5, 11, and 13–21, Figure 35) on lipopolysaccharide (LPS)-induced NF-κB activation and consequent production of pro-inflammatory cytokines/chemokine, using a human monocytic leukaemia cell line (THP-1). NF-κB is one of the most important transcription factors and a regulator of immune and inflammatory responses. When not stimulated, the NF-κB is located in the cytoplasm, but when activated, it translocates to the nucleus and induces the expression of many mediators of the inflammation, including cytokines, enzymes, and chemokines. The activation of NF-κB can occur due to the action of several pro-inflammatory stimuli, such as cytokines, e.g., tumor necrosis factor (TNF)-α or interleukin (IL)-1, and infectious agents. The cytokines and chemokine evaluated in this study, e.g., TNF-α, IL-1β, IL-6, and IL-8, are inflammatory mediators, involved in the regulation of the inflammatory response. Cytokines act as signalling molecules, participating in the inflammatory response, mainly regulating their intensity, propagation, and duration. There are several types of cytokines and their specific function depends on the cell type that produce them and their location. Chemokines are generally produced by leukocytes and endothelial cells and their main function is to induce cell migration. Thus, chemokines chemoattract leukocytes to inflammation sites and induce integrin expression [60, 61]. The cytotoxicity of the tested 2-SC to THP-1 cells was evaluated for the concentration of 50 μM and none showed cytotoxicity. For NF-κB activation, 2-SC 17 was the most active (65.7 ± 11.5% inhibitory effect, 50 μM) and exhibited a concentration-dependent effect. The other 2-SC (1, 5, 11, 13, 14, and 16–20) displayed a lower inhibition of NF-κB activation. The ability of 2-SC 15, 17, and 21 to reduce pro-inflammatory cytokines/chemokine production was tested for the concentration of 50 μM. In the original article, the obtained results and the associated errors were presented only in graphs and the exact activity values were not mentioned. The three tested 2-SC were able to almost completely inhibit the production of IL-6. 2-SC 17 was capable to significantly inhibit the production of TNF-α, IL-1β, IL-6, and IL-8, being the most potent. 2-SC 15 also inhibited production of TNF-α and IL-6 but appears to induce the production of IL-1β and IL-8. 2-SC 21 inhibited the production of TNF-α, IL-1β, and IL-6, while it appears to induce the production of IL-8, in a lower extent. Thus, 2-SC 15, 17, and 21 showed to be capable to significantly inhibit the NF-κB activation and to reduce the production of the pro-inflammatory cytokines/chemokine. However, 2-SC 17 was the most active for the two assays. The three 2-SC with better activity (15, 17, and 21) have in common the presence of an −OH at C-4′ on the B-ring, which seems to indicate that this substitution favours the inhibition of NF-κB activation and the reduction of cytokines/chemokine production. In addition to the −OH at C-4′ on the B-ring, the simultaneous presence of an −OH at C-3′ on the B-ring (catechol group) in 2-SC 17 seemed to potentiate the anti-inflammatory activity [59].
The above-mentioned structural characteristics that demonstrated to favour the anti-inflammatory activity of 2-SC are summarized in Figure 36.
Figure 36.
Structural characteristics that seem to favour the 2-SC anti-inflammatory activity.
4.7. Antitumoral Activity
A tumor can be defined as an abnormal mass of tissue, resulting from the abnormal growth of cells in the body. Tumors, also known as neoplasms, may be benign or malignant. Benign tumors are slow-growing masses; they remain localized, neither spreading nor invading the surrounding tissues. These types of tumors are less harmful, are easily removed by surgery, and generally do not grow back after removal. On the other hand, malignant tumors, commonly known as cancer, are invasive and tend to spread through metastases and to reappear after their removal. The growth of these tumors is characterized by a disordered and uncontrolled multiplication of cells, causing cancer cells to gain a degree of self-sufficiency [62, 63]. Cancer can be considered as a genetic disease and include different types of cancer diseases. This is one of the main causes of death in the world, strongly affecting the world population, since its incidence is increasing [62]. Thus, these cancer diseases have become a worldwide health problem, which leads to the search for effective responses to these pathologies. Several 2-SC and 3-SC have shown potential as antitumoral agents and are below discussed. The observed antitumor effects, in the published works that studied tumor and normal cell lines, are summarized in Table 10, for 2-SC, and Table 11, for 3-SC.
Table 10.
Summary of the 2-SC in which the antitumoral activity was studied in works using tumor and normal cell lines and description of the observed effects in each type of cells used. The table displays the antitumoral activity shown for the studied 2-SC according to the criterion: selectivity index greater or equal to 2.
| 2-SC | Studied cell lines | Observed effect(s) | Ref. | |
|---|---|---|---|---|
| Tumoral | Non-tumoral | |||
| 1 | Ca9-22, HSC-2, HSC-3, HSC-4, HSG and HL-60 cells | HGF, HPC and HPLF cells | Decreased cell viability of Ca9-22, HSC-2, HSC-3, HSC-4, HSG and HL-60 cells | [64, 66] |
| 30 | Ca9-22, HSC-2, HSC-3 and HSC-4 cells | Decreased cell viability of Ca9-22, HSC-2, HSC-3 and HSC-4 cells; and apoptosis induction of HSC-2 cells | [66] | |
| 56 | Decreased cell viability of Ca9-22, HSC-2, HSC-3 and HSC-4 cells | |||
| 57 | HSC-2, HSC-3, HSG and HL-60 cells | Decreased cell viability of HSC-2, HSC-3, HSG and HL-60 cells | [64] | |
| 58 | MCF-7, NCI-H460, Ca9-22, HSC-2, HSC-3, HSC-4, HSG and HL-60 cells | MRC-5 cells, human lymphocytes, HGF, HPLF and HPC cells | Growth inhibition of MCF-7 and NCI-H460 cells; cell cycle arrest at the G2/M phase of MCF-7 and NCI-H460 cells; microtubule-stabilizing antimitotic effect in MCF-7 and NCI-H460 cells; weak inhibition of peripheral human lymphocytes proliferation; decreased cell viability of Ca9-22, HSC-2, HSC-3, HSC-4, HSG and HL-60 cells; apoptosis induction of HSC-2 cells; DNA fragmentation induction in HL-60 and HSC-2 cells; and activation of caspase 3, 8 and 9 in HL-60 cells | [64–66] |
| 88 | Ca9-22, HSC-2, HSC-3 and HSC-4 cells | HGF, HPLF and HPC cells | Decreased cell viability of Ca9-22, HSC-2, HSC-3 and HSC-4 cells | [66] |
| 92 | Ca9-22, HSC-2, HSC-3, HSC-4, HSG and HL-60 cells | Decreased cell viability of Ca9-22, HSC-2, HSC-3, HSC-4, HSG and HL-60 cells | [64, 66] | |
| 103 | HSC-2, HSC-3, HSG and HL-60 cells | Decreased cell viability of HSC-2, HSC-3, HSG and HL-60 cells; DNA fragmentation induction in HL-60 and HSC-2 cells; and activation of caspase 3, 8 and 9 in HL-60 cells | [64] | |
| 104 - 108 | Ca9-22, HSC-2, HSC-3 and HSC-4 cells | Decreased cell viability of Ca9-22, HSC-2, HSC-3 and HSC-4 cells | [66] | |
| 110, 111 | ||||
| 114, 115 | ||||
| 152 | AREc32 and K562 cells | Peripheral blood mononuclear cells | Nrf2-inducing activity in AREc32 cells; decreased cell viability and proliferation of K562 cells | [70] |
AREc32: human mammary epithelial adenocarcinoma cell line; Ca9-22: human oral squamous cell carcinoma Ca9-22; HGF: human gingival fibroblast; HL-60: promyelocytic human leukaemia-60 cell line; HPC: human pulp cell; HPLF: human periodontal ligament fibroblast; HSC-2: human squamous cell carcinoma-2; HSC-3: human squamous cell carcinoma-3; HSC-4: human squamous cell carcinoma-4; HSG: human submandibular gland carcinoma; K562: human chronic myeloid leukaemia; MCF-7: breast adenocarcinoma cell line; MRC-5: diploid embryonic lung fibroblast cell line; NCI-H460: non-small cell lung cancer; Nrf2: nuclear factor erythroid 2-related factor 2.
Table 11.
Summary of the 3-SC which antitumoral activity was studied in works using tumor and normal cell lines and description of the observed effects in each type of cells used. The table displays the antitumoral activity shown for the studied 3-SC according to the criterion: selectivity index greater or equal to 2.
| 3-SC | Studied cell lines | Observed effect(s) | Ref. | |
|---|---|---|---|---|
| Tumoral | Non-tumoral | |||
| 2 | Ca9-22, HSC-2, HSC-3 and HSC-4 cells | HGF, HPLF and HPC cells | Decreased cell viability of Ca9-22, HSC-2, HSC-3 and HSC-4 cells | [78] |
| 34 | [43] | |||
| 36 - 39 | ||||
| 41 | High cytotoxicity against HGF, HPLF and HPC cells | [78] | ||
| 42 - 47 | Decreased cell viability of Ca9-22, HSC-2, HSC-3 and HSC-4 cells | [43] | ||
| 49, 50 | ||||
| 165 | [78] | |||
| 167 | High cytotoxicity against HGF, HPLF and HPC cells | |||
| 168 | Decreased cell viability of Ca9-22, HSC-2, HSC-3 and HSC-4 cells; induction of apoptosis and mitotic arrest in HSC-2 cells | |||
| 169 | Decreased cell viability of Ca9-22, HSC-2, HSC-3 and HSC-4 cells; and high cytotoxicity against HGF, HPLF and HPC cells | |||
| 170 - 174 | Decreased cell viability of Ca9-22, HSC-2, HSC-3 and HSC-4 cells | |||
| 175 | Decreased cell viability of Ca9-22, HSC-2, HSC-3 and HSC-4 cells; induction of apoptosis and mitotic arrest in HSC-2 cells | |||
Ca9-22: human oral squamous cell carcinoma Ca9-22; HGEP: primary gingival epithelial cell; HOK: human oral keratinocyte; HPC: human pulp cell; HGF: human gingival fibroblast; HPLF: human periodontal ligament fibroblast; HSC-2: human squamous cell carcinoma-2; HSC-3: human squamous cell carcinoma-3; HSC-4: human squamous cell carcinoma-4.
4.7.1. 2-Styrylchromones
Gerwick and co-workers [6] were the first to describe the antitumor potential of 2-SC, to the best of our knowledge. These authors evaluated the cytotoxic potential of the natural derivative hormothamnione (3, Figure 37). In this study, the associated error, the number of assays done, and the positive control used were not mentioned. The hormothamnione (3) was defined as a potent cytotoxic agent, by the inhibition of the cell growth of P388 lymphocytic leukaemia and HL-60 cell lines, with IC50 values of 4.6 ng/mL and 0.1 ng/mL, respectively. The possible mechanism of action of hormothamnione (3) was studied by the evaluation of macromolecule biosynthesis (DNA, RNA, and protein), through radioactive precursor incorporation studies in HL-60 and KB cells (human epidermoid carcinoma). The 2-SC 3 demonstrated to be effective in the inhibition of RNA synthesis, apparently being a selective inhibitor (IC50 values of 1.0 ng/mL and 140 ng/mL for HL-60 and KB cells, respectively), but was not effective in the inhibition of the synthesis of DNA and proteins [6].
Figure 37.
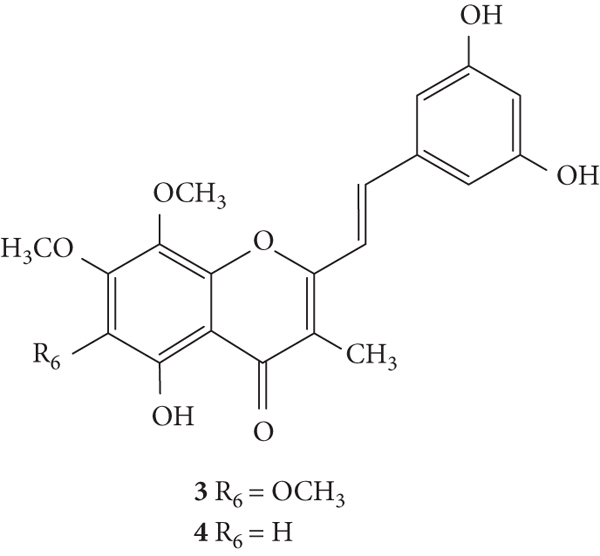
Gerwick [7] also evaluated the antitumor potential of 6-desmethoxyhormothamnione (4, Figure 37). Again, the associated error, the number of assays done, and positive control were not indicated. 2-SC 4 showed good cytotoxicity in 9 KB cells (median lethal dose~1 μg/mL) [7].
Momoi and co-workers [64] evaluated the cytotoxic activity of 2-SC 1, 57, 58, 92, 93, and 103 (Figure 38) against three normal oral human cells [human gingival fibroblast (HGF), human pulp cell (HPC), and human periodontal ligament fibroblast (HPLF)] and four human tumor cell lines [squamous cell carcinoma (HSC-2 and HSC-3), human submandibular gland carcinoma (HSG), and HL-60]. Cytotoxic activity was assessed by the MTT assay, except for HL-60 cells for which the viable cell number was determined by the trypan blue exclusion assay. The CC50 was calculated and tumor specificity of the studied 2-SC was evaluated by SI, presented by the authors as an interval of values. The associated error and the number of assays done were not indicated and a positive control was also not mentioned. All the tested 2-SC showed higher cytotoxicity against tumor cell lines than against normal cells. 2-SC 58 and 103 (SI values of 10.0–13.8 and 7.5–27.3, respectively) were the most cytotoxic against tumor cell lines, followed by 2-SC 92 (SI values of 2.1–8.2). These results indicate that 2-SC with −OCH3 on the B-ring (58, 92, and 103) showed greater tumor specificity. The log P was also theoretically calculated, and a relationship was established between SI and log P, in which 2-SC 58, 92, and 103 showed low log P values (2–2.8), despite the greater SI. Low log P indicates greater hydrophilicity, which makes it more difficult to compounds to cross the cell phospholipid bilayer and produce the desired effects. In this case, despite the low values of log P, the 2-SC were able to affect the cell viability, as demonstrated by the SI values. The effect of NADH on the cytotoxic activities of 2-SC 58 and 103 in HSC-2 cells was also evaluated, which allowed to assess whether cell viability was affected by enzymatic reactions of 2-SC with NADH. Lower concentrations of NADH (0.05–0.5 mM) slightly reduced the cytotoxic activity of the two 2-SC, whereas a higher concentration of NADH (5 mM) improved the cytotoxic activity of the 2-SC 58. In this study, it was also evaluated whether the tested 2-SC could induce apoptosis-associated characteristics, such as internucleosomal DNA fragmentation and caspase activation, in the tumor cell lines, and whether radical-mediated reactions were involved in the cytotoxicity induction. In HL-60 cells, 2-SC 58 and 103 induced internucleosomal DNA fragmentation, with this fragmentation being more accentuated at 24 h. The authors also indicated that apoptotic bodies were produced. In HSC-2 cells, 2-SC 58 and 103 induced the production of large DNA fragments without induction of internucleosomal DNA fragmentation. 2-SC 58 and 103 were able to activate caspases 3, 8, and 9 in HL-60 cells, but the activation of caspases by 2-SC 58 was superior to the positive control, UV irradiation. The authors suggested that 2-SC 58 and 103 activated the non-mitochondrial extrinsic and the mitochondrial intrinsic apoptotic pathways, involved with caspases 8 and 9, respectively, and that they induce caspase 3 activation by both these pathways, leading to DNase activation inducing internucleosomal DNA fragmentation, which may depend on the type of cell line. It was also assessed whether radical-mediated reactions were involved in the induction of cytotoxicity. In these assays, all the tested 2-SC did not produce a detectable amount of radicals, under alkaline condition (pH 10 and 12.5), and did not scavenge the O2•— generated by the hypoxanthine-xanthine oxidase reaction. As mentioned, the results indicate that 2-SC 58, 92, and 103 showed greater tumor specificity. These 2-SC have in common the presence of −OCH3 on the B-ring: 58, at C-4′; 92, at C-3′ and C-4′; 103, at C-3′, C-4′, and C-5′. The other 2-SC (1, 57, and 93) have less tumor specificity than those above mentioned. 2-SC 1 is unsubstituted; 2-SC 57 has only one −CH3 at C-4′ on the B-ring, instead of an −OCH3 (58). The decrease in the tumor specificity was even more accentuated with the presence of an −OCH2O−, forming a ring with C-3′ and C-4′ on the B-ring (93), when compared to 92, with −OCH3 at C-3′ and C-4′ on the B-ring. Based on the results of the assay with different concentrations of NADH, it was proposed that the addition of NADH stimulated the formation of orthoquinone in 2-SC 92 and 103 and quinone in 2-SC 58 by enzymatic demethylation of the −OCH3 groups. This hypothesis is supported by the fact that 2-SC 58 and 103 showed tumor specificity and apoptosis-inducing activity, even though these 2-SC have not been oxidized under alkaline conditions, since they did not produce a detectable amount of radicals. On the other hand, 2-SC 93 with −OCH2O− at C-3′ and C-4′ on the B-ring did not show any tumor specificity, which may indicate that this group is not dehydrolized due to ether bonding, which means that the cytotoxic orthoquinone does not form. In conclusion, 2-SC 58 and 103 appear to be very promising compounds, relating −OCH3 groups to the cytotoxic activity against human tumor cell lines [64].
Figure 38.
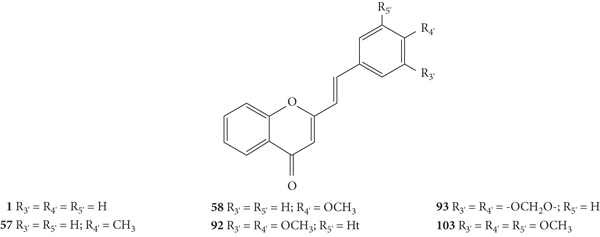
Chemical structures of 2-SC 1, 57, 58, 92, 93, and 103 [64].
Marinho and co-workers [65] also studied 2-SC 58 (Figure 38). The authors evaluated whether 2-SC 58, identified as a potent growth inhibitor of human tumor cell lines, may be equally potent for non-tumor cell lines. In this study, the following human tumor cell lines were used: breast adenocarcinoma cells (MCF-7) and non-small cell lung cancer (NCI-H460) and human non-tumor cell line: diploid embryonic lung fibroblast cell (MRC-5). 2-SC 58 (2.2–180 μM) was able to inhibit the growth of MCF-7 and NCI-H460 cells in a concentration-dependent manner [concentration of compound that produce 50% of cell growth inhibition (GI50) of 4.4 ± 0.8 μM and 5.2 ± 0.3 μM, respectively]. Interestingly, 2-SC 58 was five to six times less potent at inhibiting the growth of MRC-5 cells (GI50 = 26.9 ± 3.2 μM). Regarding proliferation of peripheral human lymphocytes, 2-SC 58 (9.4–600 μM) showed to be a weak inhibitor (IC50 = 74.7 ± 4.8 μM). The cell cycle of non-tumor MRC-5 cells does not appear to be affected by 2-SC 58, since the cells presented a profile of DNA content similar to the untreated control. On the other hand, 2-SC 58 led to the hold in the G2/M phase of the cell cycle of MCF-7 cells in a concentration- and time-dependent manner. Also, for NCI-H460 cells, the effects of 2-SC 58 on the cell cycle were concentration and time dependent and led to an arrest at the G2/M phase of the cell cycle. These results led the authors to analyse the effect of 2-SC 58 (2.2–180 μM) on confluent (non-proliferative) and non-confluent (exponential growth) cells with the assumption that 2-SC 58 specifically affects mitosis. The results of both cell lines showed a high cellular protein content for the confluent cells, indicating that they were less sensitive to the 2-SC 58 treatment than non-confluent cells. The evaluation of cell growth by DNA synthesis for the NCI-H460 cell line indicates an inhibition of cell growth in non-confluent cells, in a concentration-dependent manner. The evaluation of the effects of 2-SC 58 (100 μM) on cytoskeleton components (actin filaments and microtubules) of MCF-7 and NCI-H460 cells during the interphase showed that the microtubule cytoskeleton appeared rich and intact in both cell lines, similar to the untreated cells of the control. Mitotic cells have undergone serious alterations, such as monopolar microtubule arrays, and rarely, normal mitotic spindles were found. For actin filaments, no significant changes were detected in both cell lines after 2-SC 58 treatment. These results suggest that 2-SC 58 specifically affects microtubules during mitosis. In the in vitro tubulin polymerization assay, 2-SC 58 (100–500 μM) led to a slight increase in tubulin polymerization, in a concentration-dependent manner, and the highest concentration tested (500 μM) resulted in the maximum polymerization plateau. In conclusion, 2-SC 58 appears to be a potential microtubule-stabilizing agent and a selective proliferation inhibitor of human tumor cell lines over normal cells, acting preferentially on proliferating cells [65].
Uesawa and co-workers [66] investigated the cytotoxic activity of 2-SC 1, 30, 56, 58, 88, 92, and 104–115 (Figure 39) against four human oral squamous cell carcinoma (Ca9-22, derived from gingival tissue; HSC-2, HSC-3, and HSC-4, derived from tongue) and three human oral mesenchymal cells (HGF, HPLF, and HPC). Cytotoxic activity was assessed by the MTT assay, and the results were presented as CC50, for each cell line (without the associated error) and the mean values of the four tumor cell lines and the three normal cells (with the associated error), SI, and potency-selectivity expression (PSE). Doxorubicin is an anthracycline anticancer drug and was used as a positive control. 2-SC 58 was the most cytotoxic against tumor cell lines (CC50 values between 1.2 and 2.6 μM and mean CC50 = 1.9 ± 0.6 μM), followed by 2-SC 30 (CC50 values between 2.8 and 4.9 μM and mean CC50 = 3.8 ± 1.1 μM). Doxorubicin showed a mean CC50 value of 0.101 ± 0.046 μM against tumor cell lines and 6.8 ± 5.5 μM against normal oral cells. For normal oral cell lines, 2-SC 113 was the most cytotoxic (mean CC50 = 27.3 ± 16.8 μM). The SI calculation showed that the 2-SC 30 has a high tumor specificity (SI = 89.1), followed by 2-SC 58 (SI = 84.1). The PSE values showed that 2-SC 58 has a high potency-selectivity expression (PSE = 4443.4), followed by 2-SC 30 (PSE = 2364.8). Therefore, the tested tumor oral cell lines were more sensitive to 2-SC 30 and 58 than normal oral cell lines. Thus, the effect of 2-SC 30 (8–32 μM) and 58 (4–16 μM) on the cell cycle of HSC-2 cells was also analysed for 24 h. 2-SC 30 and 58 led to an accumulation of the cell population in the subG1 and G2/M phase and induced mitotic arrest. The subG1 population is a marker of apoptosis. Thus, these two 2-SC induced apoptosis of HSC-2 cell more potently than the positive control, actinomycin D (1 μM) [66]. The results obtained for 2-SC 58 were in line with those obtained in the previous study [65], where this 2-SC demonstrated an antiproliferative and microtubule-stabilizing effect [65]. Quantitative structure-activity relationship (QSAR) analysis of all the tested 2-SC, in this study, indicated that tumor specificity was correlated with the following descriptors: topological and 3D shape, size, electric state, and ionization potential [66]. The study of these authors also reinforces the idea, previously mentioned [64], that the −OCH3 on the B-ring are related to the cytotoxic activity, more specifically at C-4′ on the B-ring [66]. Looking at the structure of the most cytotoxic derivatives, 2-SC 58 has an −OCH3 at C-4′ on the B-ring, while 2-SC 30 has an −OCH3 at C-6 on the A-ring and C-4′ on the B-ring [66].
Figure 39.
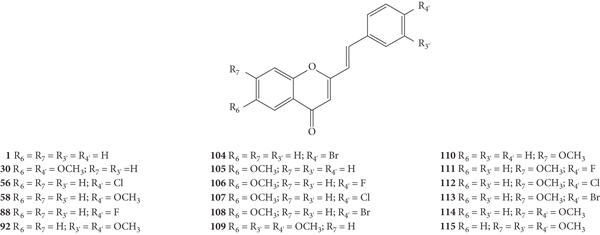
Chemical structures of 2-SC 1, 30, 56, 58, 88, 92, and 104–115 [66].
Shaw and co-workers [67] synthesized a novel series of 2-SC with −OCH3 at various positions on the A-ring and diverse substituents and modifications on the B-ring (116–131, Figure 40) and evaluated the antiproliferative activity against five human carcinoma cell lines [prostate carcinoma cell (PC-3), non-small cell lung adenocarcinoma cell (A549), mammary gland adenocarcinoma cell (BT483), HeLa, and hepatocellular carcinoma cell (SKHep)]. Cell proliferation studies were performed by the MTT assay and no standard compound was mentioned. Only 2-SC 125 exhibited toxicity against PC-3 cells (IC50 = 28.9 ± 0.7 μM). 2-SC 116, 117, 121, 123–125, and 131 exhibited cytotoxicity against the A549 cell line, with IC50 values from 15.6 ± 2.6 (121) to 24.2 ± 1.5 μM (124). Results of cytotoxicity against BT483 cells showed that the majority of 2-SC had moderate antiproliferative activity [IC50 values from 18.2 ± 2.3 (126) to 30.9 ± 1.1 μM (129)], except for 2-SC 116, 119, 122, and 127 that were not active. Finally, 2-SC 121, 125, and 131 demonstrated good antiproliferative activity against BT483 cells, with IC50 values of 16.6 ± 2.4 μM, 15.5 ± 1.9 μM, and 16.6 ± 1.3 μM, respectively. The HeLa cell line was the most sensitive to the tested 2-SC, since most the tested 2-SC had better antiproliferative effects against this cell line when compared to the other tested cell lines, only 2-SC 119 and 124 were not active. 2-SC 129 was the most cytotoxic against the HeLa cell line (IC50 = of 4.9 ± 1.0 μM). The results obtained for the SKHep cell line showed that 2-SC 119, 120, 125, 127, and 128 were not active and 2-SC 124 and 129 were the most potent (IC50 values of 12.5 ± 2.9 μM and 12.4 ± 1.5 μM, respectively). The results of 2-SC 129 obtained for the HeLa cell line led the authors to make a mechanistic study of its antiproliferative effects. Thus, HeLa cells were treated with several concentrations of 2-SC 129 (1–10 μM), and the results showed that suppression of cell proliferation was concentration dependent. The effects of 2-SC 129 (5–20 μM) on HeLa cell morphology were also studied, and significant morphological changes have occurred, such as cell rounding and detachment from the substratum. The effects of 2-SC 116, 122, and 129 on apoptosis and cell cycle profile of HeLa cells were also evaluated to establish a relationship between the antiproliferative effect and cell cycle arrest. 2-SC 116 and 122 (20 μM) led to some cell cycle arrest in the subG1 phase, while 2-SC 129 (5–20 μM) induced cell cycle arrest in the subG1 phase in a concentration-dependent manner, which may indicate that the antiproliferative effect of 2-SC 129 observed in HeLa cells may be due to DNA fragmentation, resulting in cell death. Therefore, the effect of 2-SC 129 (5 and 10 μM) on DNA fragmentation in HeLa cells was also studied, by 4′,6-diamidino-2-phenylindole (DAPI) staining, and this fragmentation clearly occurred after treatment with 10 μM of 2-SC 129. The possibility of 2-SC 129 (10 μM) to have a microtubule-stabilizing effect was also evaluated by in vitro microtubule polymerization assay, and no effect was observed, suggesting that the antiproliferative effect on HeLa cells occurs by the other mechanisms mentioned above. In this study, the 3D-QSAR model was established by comparative molecular field analysis (CoMFA) on the BT483 cell line, to investigate how the 2-SC substituents influence the activities of the BT483 cell line. For this analysis, it was assumed that each 2-SC would adopt a conformation in its lowest energy, since the binding site is unknown. The results of the predictive model were in line with those obtained in cellular model. In what concerns the SAR, in this study, it is difficult to establish a relationship between the antiproliferative effects against the studied cell lines and the structures of the studied 2-SC, since the observed cytotoxic effects varied considerably among cell lines and regardless of the common structural characteristics among the studied 2-SC [67].
Figure 40.
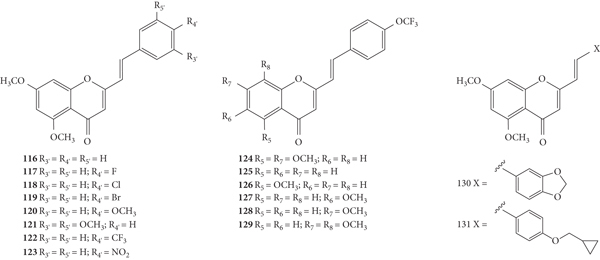
Chemical structures of 2-SC 116–131 [67].
Lin and co-workers [68] synthesized 2-SC 1, 56, 58, 59, 92, 93, 103, 104, 125, and 132–147 (Figure 41), with several substituents on the A- and/or B-rings, and evaluated their growth inhibition activity against a panel of human carcinoma cell lines, by MTT assay, with concentrations varying between 0.1 and 1 mM, and the results were presented as GI50.
Figure 41.
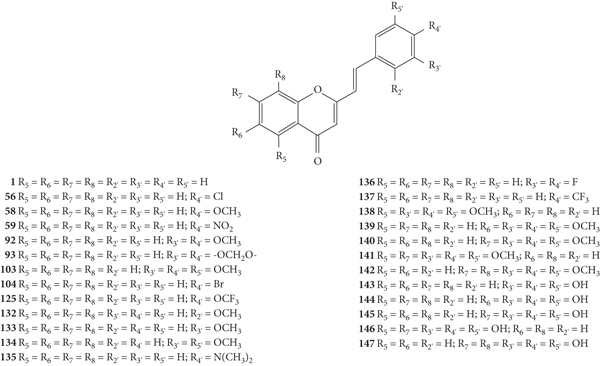
Chemical structures of 2-SC 1, 56, 58, 59, 92, 93, 103, 104, 125, and 132–147 [68].
For 2-SC 1, 56, 58, 59, 92, 93, 104, 125, and 132–137, growth inhibition against gastric carcinoma (AGS), HeLa, ovarian carcinoma (OVCA), SKHep, and large lung carcinoma (H460) was assessed. In addition, for 2-SC 103, growth inhibition against BT483 and colorectal adenocarcinoma (SW620) cell lines was also studied. For 2-SC 138–147, growth inhibition against AGS, PC-3, H460, and SW620 cell lines was studied. 2-SC 138 was the most active growth inhibitor (GI50 = 1.3 ± 0.07 μM) of AGS cells, followed by 2-SC 103 (GI50 = 2 ± 0.2 μM). 2-SC 1, 56, 92, 93, 104, 125, 132–134, and 143–147 did not inhibit AGS cell growth. Results of growth inhibition of 2-SC against HeLa cells showed that 2-SC 58, 59, 103, and 136 were the most potent, with GI50 varying from 6 ± 1 (103) to 9.9 ± 1 μM (136). 2-SC 56, 92, 132, and 134 did not inhibit HeLa cell growth. Against OVCA cells, 2-SC 56, 58, 103, and 135 were the most active, with GI50 values between 6 ± 1 (58) and 11 ± 1 μM (56), and 59, 93, 125, 132, 134, and 137 did not inhibit OVCA cell growth. 2-SC 58 was the most potent growth inhibitor (GI50 = 5 ± 1 μM) against the SKHep cells, and 1, 92, 93, 125, 132, and 135 did not inhibit SKHep cell growth. Results of growth inhibition of 2-SC against H460 cells showed that 2-SC 58, 103, 135, 139, and 141 presented the lowest GI50 values, varying from 6 ± 1 (103) to 10 ± 2 μM (139 and 141), and 1, 92, 93, 132, 138, 140, and 142–147 did not inhibit their growth. Only 2-SC 138 and 139 were able to inhibit the growth of PC-3 cells (GI50 values of 20 ± 4 μM and 29 ± 5 μM, respectively). For SW620 cells, 2-SC 138, 139, and 141 were able to inhibit their growth, while 2-SC 140 and 142–147 did not. Only 2-SC 103 was tested against BT483 cells and a GI50 value of 10 ± 2 μM was found. Doxorubicin was tested as a positive control against all carcinoma cell lines and showed GI50 values between 0.02 ± 0.01 and 1.76 ± 0.71 μM. The results obtained in this study reinforces the idea, previously mentioned [64, 66], that the presence of −OCH3 on the B-ring is related to the antitumoral activity. The results obtained for 2-SC 58 show that an −OCH3 at C-4′ on the B-ring is favourable for the inhibition of growth against the studied carcinoma cell lines, with GI50 values being relatively low. 2-SC 58 was one of the 2-SC with the best activity. 2-SC 103 that has −OCH3 at C-3′, C-4′ and C-5′ on the B-ring also presented good activity. Thus, the position that seems to most favour the activity is −OCH3 at C-4′ on the B-ring (58) or simultaneously at C-3′, C-4′, and C-5′ on the B-ring (103). In addition to −OCH3, the tested 2-SC also displayed other groups as substituents on the B-ring. Interestingly, different halogen substituents resulted in different activities: 2-SC 104, with −Br at C-4′ on the B-ring, showed growth inhibition activity against HeLa, OVCA, SKHep, and H460 cells; 2-SC 136, with −F at C-3′ and C-4′ on the B-ring, showed activity against all the studied carcinoma cells; 2-SC 56, with −Cl at C-4′ on the B-ring, demonstrated activity against OVCA, SKHep, and H460 cells. The introduction of −CF3 into C-4′ on the B-ring led 2-SC 137 to display activity against AGS, HeLa, SKHep, and H460 cells. In turn, the introduction of an −OCF3 into C-4′ on the B-ring led 2-SC 125 to only display activity against HeLa and H460 cells. 2-SC 59 has a −NO2 at C-4′ on the B-ring and was only inactive against OVCA cells and showed better activity than 2-SC 125 and 137. Since the 2-SC 103 demonstrated good activity, analogues were synthesized with −OCH3 at C-3′, C-4′ and C-5′ on the B-ring but with modifications on the A-ring (138–142). In general, the analogues (138–142) were less active than 2-SC 103, except for 2-SC 138 against AGS cells, which showed great potency. Another series of 2-SC (143–147) was also synthesized, but now with −OH at C-3′, C-4′, and C-5′ on the B-ring and modifications in several positions on the A-ring. However, none of these 2-SC showed growth inhibitory activity against the studied carcinoma cell lines, which seems to indicate that the simultaneous presence of −OH groups on the A- and B-rings does not favour this activity. The importance of double bond of the styryl moiety and the rigidity of the 2-SC structure for growth inhibitory activity was also evaluated. For this, a structure analogous to 2-SC 103 was tested, in which the double bond of the styryl moiety was hydrogenated to a single bond. This analogue and 2-SC 103 were tested against seven carcinoma cell lines (AGS, BT483, HeLa, OVCA, SKHep, H460, and SW620 cells). The new analogue showed a decreased activity when compared to 2-SC 103. Thus, the double bond of the styryl moiety seems to favour the growth inhibitory activity [68].
Yang and co-workers [10] isolated 2-SC 7–11 (Figure 42) and reported their cytotoxic activity against human hepatocyte carcinoma (HepG2), human breast adenocarcinoma (MDA-MB-231), human hepatocarcinoma (SMMC-7721), and KB cell lines. 2-SC 7–10 are also known as platachromones A–D. The cytotoxicity of 2-SC (0.5–100 μg/mL) was assayed by the MTT assay, and taxol was used as positive control. In general, 2-SC 7–11 showed to be more effective against HepG2 and KB cells, being more cytotoxic for these two cell lines than for MDA-MB-231 and SMMC-7721. 2-SC 7 was the most cytotoxic against HepG2 cells (IC50 = 6.8 ± 0.3 μM), followed by 2-SC 8 (IC50 = 7.4 ± 0.1 μM). 2-SC 8 showed to be the most potent against MDA-MD-231 cells (IC50 = 8.7 ± 0.3 μM), followed by 2-SC 7 (IC50 = 11.9 ± 0.6 μM). 2-SC 9 was the most cytotoxic against KB cells (IC50 = 3.0 ± 0.2 μM) and SMMC-7721 (IC50 = 14.6 ± 0.3), and 11 was considered inactive in the last ones. Taxol was tested against the four cancer cell lines and showed IC50 values that ranged from 0.04 ± 0.002 to 1.1 ± 0.2 μM. These 2-SC have in common an −OH at C-5 and C-7 on the A-ring and at C-4′ on the B-ring, and their structure differ in the substituents present at C-6 and C-8 on the A-ring. The −CH2CH=C(CH3)2 group seems to contribute to the greater cytotoxic effect observed against HepG2 and MDA-MB-231 cells (7 and 8), while the −CH2CH(OH)CH=CH2 group seems to favour cytotoxicity against SMMC-7721 and KB cells (9 and 10). In conclusion, the presence of substituents at C-6 and C-8 on the A-ring (7–10) seems to favour the cytotoxic activity over the unsubstituted structure (11) at these positions [10].
Figure 42.
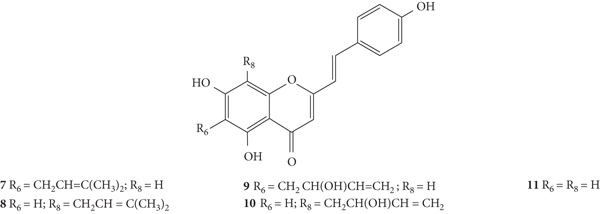
Chemical structures of 2-SC 7–11 [10].
Lee and co-workers [69] synthesized a series of 2-SC based on the lavendustin A structure (148–151, Figure 43) and reported their cytotoxic activity against A549, human colon cancer (HCT-15), KB, and malignant melanoma (SK-MEL-2) cell lines. The cytotoxicity of 2-SC was assayed by the sulforhodamine B (SRB) assay, and cisplatin was used as positive control. 2-SC 148 was the most cytotoxic against A549 cells (IC50 = 17.9 ± 2.9 μg/mL). 2-SC 151 showed potent cytotoxicity against HCT-15 (IC50 = 9.22 ± 1.8 μg/mL). Only 2-SC 148 showed cytotoxicity against KB cells (IC50 = 34.98 ± 6.6 μg/mL). None of the 2-SC 148–151 showed cytotoxic activity against SK-MEL-2 cells, up to a concentration of 100 μg/mL. The positive control, cisplatin, was tested against four cancer cell lines and showed IC50 values that ranged from 0.56 ± 0.12 to 1.65 ± 0.12 μg/mL. 2-SC 148–151 have in common an −OH at C-7 on the A-ring and a substituent structurally derived from lavendustin A at C-8 on the A-ring, and differ in the substituents on the B-ring. 2-SC 148 that has no substituent on the B-ring exhibited cytotoxicity against A549, HCT-15, and KB cells. In turn, 2-SC 149 that has an −OCH3 at C-4′ on the B-ring showed a decrease in the cytotoxic effect when compared to 2-SC 148, as it only showed effect against A549 and HCT-15 cells and with higher IC50 values. 2-SC 150, with −OCH3 at C-3′ and C-4′ on the B-ring, also revealed a decrease in the cytotoxic effect when compared with the activity of 2-SC 148. The presence of −Br at C-3′ on the B-ring (151) seems to favour the cytotoxic effect against HCT-15 cells, but to decrease the activity against A549 cells, when compared to 2-SC 148. In general, in the presence of a substituent structurally derived from lavendustin A at C-8 on the A-ring, the modifications at C-3′ and/or C-4′ on the B-ring did not favour the cytotoxic activity [69].
Figure 43.
Chemical structures of 2-SC 148–151 [69].
Talhi and co-workers [70] evaluated the anticarcinogenic potential of 2-SC 152–154 (Figure 44). These authors measured the induction of the cytoprotective Kelch ECH-associating protein 1-nuclear factor erythroid 2-related factor 2 (Keap1-Nrf2) signalling pathway through the luciferase reporter assay and also evaluated the cytotoxic activity through the SRB assay, in human mammary epithelial adenocarcinoma MCF-7 (AREc32) cells. The antileukemic activity of 2-SC 152 and 153 was also evaluated against human chronic myeloid leukaemia cells (K562) [70]. The Nrf2 is a transcription factor that regulates cytoprotective genes and mediates the cellular response to inducers (e.g., electrophiles and oxidants) in the regulatory regions of target cytoprotective genes. Keap1 is a cysteine-rich repressor protein that provides Nrf2 degradation when bound to it. Thus, the Keap1-Nrf2 pathway is the major regulator of cytoprotective responses and plays a role in cancer prevention, being considered a chemopreventive pathway [71, 72]. The ability of 2-SC 152–154 (1.56–100 μM) to activate the Keap1-Nrf2 pathway was evaluated and compared to xanthohumol and sulforaphane, which were tested as positive controls, in AREc32 cells. The results were presented as the concentration required to increase luciferase activity by fivefold in relation to solvent control (designated as C5 value), but the authors did not mention the associated error. 2-SC 152–154 presented a concentration-dependent activity and showed C5 values of 2.9 μM, 18 μM, and 10.2 μM, respectively. The xanthohumol and sulforaphane presented C5 values of 7.8 μM and 4.8 μM, respectively. Thus, 2-SC 152 had better Nrf2-inducing potential than the two positive controls. 2-SC 152–154 did not show cytotoxic effects against AREc32 cells. 2-SC 152 was able to reduce the viability and proliferation (IC50 = 4.5 ± 1.9 μM, after 72 h of treatment), while 153 did not affect cell proliferation or induce the death of K562 cells. When the effect of 2-SC 152 on the viability of peripheral blood mononuclear cells from healthy donors was evaluated, this compound did not affect the viability of these cells, which may indicate selective toxicity for cancer cells. The obtained results revealed that the introduction of −OCH3 at C-3′ and C-4′ (153) or only at C-4′ (154) on the B-ring reduced the Nrf2-inducing activity, when compared with 2-SC 152. The same conclusion can be drawn for the antiproliferative activity against the K562 cell line, since 2-SC 153 did not have an antiproliferative effect and 2-SC 152 did. In this study, the antioxidant activity of 2-SC 152–154 was also evaluated, using the ferric-reducing antioxidant power and DPPH methods. However, these 2-SC demonstrated very low or non-existent activity, therefore this study was not mentioned in the Section 4.1 [70].
Figure 44.
Chemical structures of 2-SC 152–154 [70].
Ragab and co-workers [73] synthetized 2-SC 155–163 (Figure 45), with a furan ring attached at C-6 and C-7 on the A-ring. The compounds were screened for a first single dose of 10 μM against a panel of sixty cell lines, where cells were continuously exposed to the compounds for 48 h and cell viability or growth was determined by the SRB assay. The panel of sixty cancer lines represented nine types of cancer: leukaemia, lung, colon, central nervous system (CNS), melanoma, ovarian, renal, prostate, and breast. In this study, the error associated with the results was not indicated. In the first screening, 2-SC 160 showed growth inhibitory activity against four leukaemia cell lines (% GI values between 60 and 93%), two non-small cell lung cancer cell lines (% GI values of 95% and 88%), one CNS cancer cell line (% GI = 67%), two ovarian cancer cell lines (% GI values of 60% and 68%), three renal cancer cell lines (% GI values between 61 and 66%), one prostate cancer cell line (% GI = 72%), and one breast cancer cell line (% GI = 86%). 2-SC 163 inhibited the growth of five leukaemia cell lines (% GI values between 64 and 95%), two non-small cell lung cancer cell lines (% GI values of 80% and 84%), four colon cancer cell lines (% GI values between 67 and 82%), two CNS cancer cell lines (% GI values of 69% and 99%), three melanoma cell lines (% GI values between 63 and 76%), four ovarian cancer cell lines (% GI values between 78 and 88%), three renal cancer cell lines (% GI values between 63 and 95%), two prostate cancer cell lines (% GI values of 87% and 93%), and one breast cancer cell line (% GI = 70%). After this primary screening, 2-SC 160 and 163 (0.01–100 μM) were selected by the authors for an assay against cell lines of the nine types of cancer mentioned above. In this assay, GI50, the compound concentration resulting in total growth inhibition (TGI), and LC50 (compound concentration affecting 50% net loss in initial cells, at the end of the 48 h incubation period) were calculated for each cell line. Summarizing and in general, 2-SC 163 (median GI50 = 1.9 μM) was more active than 160 (median GI50 = 2.45 μM) against the tested cell lines. The structures of these two 2-SC differ in the substituent present at C-4′ on the B-ring: 160 has a −Cl, while 163 has −N(CH3)2. These results indicate that the −N(CH3)2 at C-4′ on the B-ring favours the growth inhibitory activity in relation to the −Cl in the same position. After the results are obtained in the screening, the authors submitted the 2-SC 160 and 163 to the kinase inhibition assay to determine whether the activity of these 2-SC was related to their enzyme inhibition ability of protein kinase targets. Thus, the profile of 2-SC 160 and 163 against cyclin-dependent kinase (CDK) 2/cyclin E1, CDK4/cyclin D1, and glycogen synthase kinase 3-beta (GSK-3β) was performed [73]. CDKs play an important role in the regulation of the cell cycle, controlling gene transcription and other processes, while GSK-3β is an enzyme that regulates numerous cellular signalling pathways and is involved in a wide range of cellular processes, such as cell cycle regulation and proliferation [74, 75]. Profiling results for the CDK2/cyclin E1 protein kinase showed that 2-SC 160 and 163 did not inhibit the enzyme activity. 2-SC 160 and 163 showed weak inhibition against the CDK4/cyclin D protein kinase target but caused stimulation of GSK-3β activity (5% and 13%, respectively) instead of inhibiting it. Given that 2-SC 163 was very potent in cell growth inhibition assays, it was not expected to stimulate of GSK-3β activity, but to inhibit it. Thus, this result led the authors to progress to in vivo studies with 2-SC 163 against breast cancer induced in female BALB/mice (Ehrlich solid tumor), in an attempt to correlate the high in vitro antiproliferative activity with the stimulation of GSK-3β activity. The tumor was induced and grew for two weeks, after which the mice received intraperitoneal injections of 2-SC 163 (3 mg/kg and 6 mg/kg) for five days, daily. The treatment with 2-SC 163 inhibited the growth of the tumor in a dose-dependent manner. The treatment with 2-SC 163 at low and high dose (3 mg/kg and 6 mg/kg, respectively) significantly decreased the level of cyclin D1 in the tumor, whereas it increased the level of GSK-3β, when compared to the untreated group. Thus, the 2-SC 163 antitumor effect was accompanied by a significant decrease in cyclin D1 levels and a significant increase in GSK-3β levels. These results seem to indicate that tumor suppression is partially mediated by an increase in GSK-3β and a decrease in cyclin D1 levels, which suggests that cyclin D1 levels can be reduced by the activation of GSK-3β that phosphorylates cyclin D1, leading to its proteolysis [73].
Figure 45.
Chemical structures of 2-SC 155–163 [73].
Peixoto and co-workers [76] studied the effects of 2-SC 164 (Figure 46) on rat liver mitochondrial bioenergetics related with a potential cancer chemopreventive activity. Mitochondrial assays can be used as models to study the cellular toxicity, since mitochondria is involved in the regulation of apoptosis [76, 77]. The influence of 2-SC 164 on the membrane potential was evaluated by the detection of changes induced in mitochondrial membrane energization and respiration. 2-SC 164 (200 and 500 nmol/mg mitochondrial protein) decreased the mitochondrial membrane potential, which was more pronounced at the highest concentration. 2-SC 164 showed an inhibitory effect, in a concentration-dependent manner, on respiratory rates. Thus, the decrease in transmembrane potential induced by 164 can result from the inhibition in any respiratory complex (II, III, or IV), since it must interact with the phosphorylation system and the mitochondrial redox chain. The authors, in an attempt to elucidate the exact action of 2-SC 164 in the respiratory redox chain and in the phosphorylation system (directly on the respiratory complexes or by an uncoupler effect), evaluated the effect of 2-SC 164 on the respiratory complexes (II, III, and IV) and in the mitochondria swelling. 2-SC 164 decreased the ATP synthase and ATPase activities (80% and 50%, respectively) and inhibited succinate dehydrogenase activity (17%). 2-SC 164 partially inhibited cytochrome c oxidase (27%), however it inhibited succinate cytochrome c reductase (≈62%). These results may indicate that 2-SC 164 essentially interacts with electron transfer at the level of complex III. Experiments in the osmotic potassium acetate medium showed that in the presence of 2-SC 164, only a small mitochondrial swelling was observed, in a concentration-dependent manner, when compared to carbonyl cyanide-p-trifluoromethoxyphenylhydrazone, used as a positive control. In conclusion, the results of this study indicate that the possible toxicological effect of 2-SC 164 may be related to the inhibition of the phosphorylation system and the electron transfer via the respiratory chain of rat liver mitochondria, especially in complex III (succinate cytochrome c reductase). The authors also suggested that 2-SC 164 may induce apoptosis through a decrease in the cellular energetic charge and promotion of the release of cytochrome c in the cytosol. Thus, the induction of mitochondrial cytochrome c release, shown in these biochemical studies, can subsequently trigger an apoptotic mechanism, an indicator of the potential cancer chemopreventive activity of 2-SC 164 [76].
Figure 46.
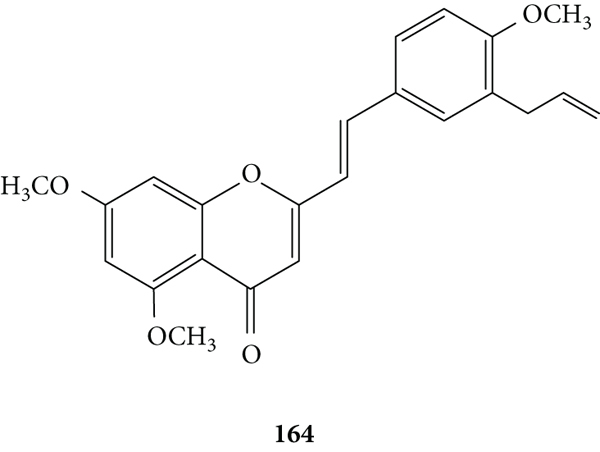
Chemical structure of 2-SC 164 [76].
The great number of studies found in the literature about 2-SC antitumoral activity covers a wide variety of tumor cell lines, some also include normal cell lines, but not all the studies. Moreover, they comprise a wide range of 2-SC with numerous substituents in different locations, making it difficult to indicate which type of cancer is most studied and to establish a SAR. However, the above-mentioned structural characteristics that demonstrated to favour the antitumoral activity of 2-SC are summarized in Figure 47.
Figure 47.
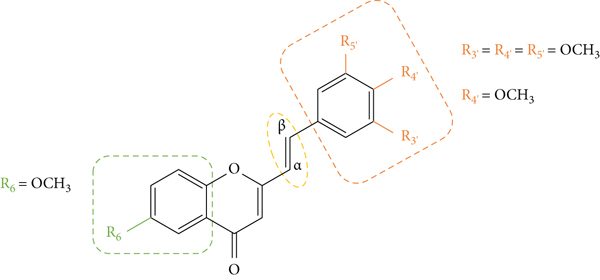
Structural characteristics that seem to favour the 2-SC antitumoral activity.
4.7.2. 3-Styrylchromones
Shimada and co-workers [43] synthetized fifteen 3-SC (34–39, and 42–50, Figure 48) and investigated their cytotoxic activity against four human oral squamous cell carcinoma (Ca9-22, HSC-2, HSC-3, and HSC-4) and three human normal oral cells (HGF, HPLF, and HPC) and performed the QSAR analysis. Cytotoxic activity was assessed by the MTT assay, and CC50 and SI were calculated and presented. The authors mentioned the obtained CC50 values for each cell line, without the associated error, and the mean of the CC50 values obtained for the four tumor cell lines and the three normal cells, with the associated error. 3-SC 46 was the most cytotoxic against the four tumor cell lines (mean CC50 = 2.0 ± 1.2 μM), followed by 37 (mean CC50 = 6.4 ± 2.4 μM). Against normal oral cells, 3-SC 46 and 37 showed lower cytotoxicity (mean CC50 values of 138 ± 116 μM and 258 ± 126 μM, respectively). 3-SC 39 was also quite cytotoxic against tumor cell lines (mean CC50 = 13 ± 6.1 μM) and showed lower cytotoxicity against normal cell lines (mean CC50 = 339 ± 183 μM). Thus, these 3-SC showed high tumor specificity (SI values of 69, 40.3, and 26.1) and their results can be compared to those obtained for the positive controls, doxorubicin and 5-fluorouracil (SI values of >26.0 and >55.6, respectively). Only 3-SC 48 did not show cytotoxicity against tumor cell lines nor against normal oral cell lines. In what concerns QSAR analysis, several chemical descriptors were used. The effects of functional groups on cytotoxicity of the studied 3-SC against tumor cell lines were evaluated, and the substituents −OCH3 at C-6 on the A-ring and −OH at C-4′ on the B-ring were defined to be crucial to increase the cytotoxicity against tumor cell lines. Accordingly, 3-SC 37 that has −OCH3 at C-6 on the A-ring and at C-4′ on the B-ring showed a greater cytotoxicity and tumor specificity than 3-SC 39, with −OCH3 at C-6 on the A-ring and at C-3′, C-4′ and C-5′ on the B-ring. 3-SC 38 and 39 have −OCH3 at C-6 on the A-ring and at C-3′ and C-4′ on the B-ring, but 39 also have an −OCH3 at C-5′ on the B-ring and showed better tumor specificity than 38, which indicates that this additional −OCH3 may contribute to the cytotoxic activity when in the presence of multiple −OCH3 groups on the B-ring. 3-SC 34 (with −OCH3 at C-4′ on the B-ring), 35 (with −OCH3 at C-3′ and C-4′ on the B-ring), and 36 (with −OCH3 at C-3′, C-4′ and C-5′ on the B-ring) showed low tumor specificity, which seems to indicate that the presence of −OCH3 groups only on the B-ring (without substituents at C-6 on the A-ring) decreases the cytotoxic activity against tumor cell lines. When an −OCH3 at C-6 on the A-ring is present, −Cl at C-4′ on the B-ring (43) seems to favour the cytotoxic activity against tumor cell lines over the presence of −F in the same position (42). 3-SC 44 (with −OH at C-4′ on the B-ring) and 45 (with −OH at C-3′ and C-4′ on the B-ring) have no substituents in the A-ring and showed better tumor specificity than 3-SC also without substituents in the A-ring but with −OCH3 on the B-ring, in the same positions (34–36), which may indicate that −OH on the B-ring is important for the cytotoxic activity against tumor cell lines. In 3-SC 46, −OCH3 is at C-6 on the A-ring and −OH is at C-4′ on the B-ring; while in 3-SC 48, −OCH3 is at C-4′ on the B-ring and −OH is at C-6 on the A-ring; this inversion of the substituents' positions led to the loss of 3-SC 48 cytotoxic activity. As mentioned, 3-SC 46 has an −OCH3 at C-6 on the A-ring and −OH at C-4′ on the B-ring and it showed better tumor specificity than 47, which has an additional −OH at C-3′ on the B-ring. Moreover, 3-SC 49 and 50 both have −OH at C-6 on the A-ring and C-4′ on the B-ring but 50 has an additional −OH at C-3′ on the B-ring that led to a better tumor specificity than 49. This result indicates that when C-6 on the A-ring is −OH instead of −OCH3, the addition of −OH to the B-ring can improve tumor specificity. In conclusion, 3-SC 46, with −OCH3 at C-6 on the A-ring and −OH at C-4′ on the B-ring, was the most cytotoxic and showed high tumor specificity, which indicates that these two groups, at these positions, favour the cytotoxic activity against the studied tumor cell lines [43].
Figure 48.
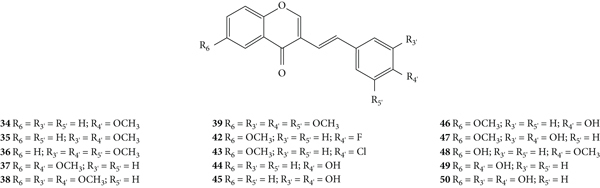
As described, 3-SC 37, 39, and 46 (Figure 48) showed to have much higher cytotoxicity against four epithelial human oral squamous cell carcinoma lines (Ca9-22, HSC-2, HSC-3, and HSC-4) than against human normal mesenchymal cells (HGF, HPLF, and HPC) [43]. These results led Sakagami and co-workers [5] to continue the study of the tumor specificity of 3-SC 37, 39, and 46 against both epithelial malignant and non-malignant cells through the MTT assay. As in the previous study [43], the cytotoxicity activity against epithelial malignant cell lines had already been tested; in the present study [5], the authors only tested the cytotoxicity activity against epithelial non-malignant cell lines [human oral keratinocyte (HOK) and primary gingival epithelial cell (HGEP)]. The obtained results show that 3-SC 37, 39, and 46 were less cytotoxic against epithelial non-malignant cell lines (CC50 values between 19 and 800 μM) than against epithelial malignant cell lines (mean CC50 values between 2.0 ± 1.2 and 13 ± 6.1 μM) [5, 43]. Doxorubicin and 5-fluorouracil were tested as positive controls and were highly cytotoxic against epithelial non-malignant cell lines, showing weak tumor specificity (SI values between 0.2 and 8.7). So, 3-SC 37, 39, and 46 demonstrated greater tumor specificity than the positive controls [5]. The PSE was also calculated to identify 3-SC that have both good potency and selective toxicity, and 3-SC 46 showed high potency-selectivity to malignant tumors (PSE values between 475 and 20000). To determine the minimum treatment time required for cytotoxicity induction, pulse-chase experiments were performed, where HSC-2 cells were incubated until 47 h with increasing concentrations of 37, 39, and 46 (0–1000 μM), and the relative viable cell number was determined by the MTT assay. 3-SC 46 was the most cytotoxic (CC50 values varied between 18 and <0.39, for 1 h and 47 h of exposure, respectively), followed by 37 (CC50 values varied between 61 and 2.4 μM, for 1 h and 47 h of exposure, respectively) and 39 (CC50 values varied between 65 and 2.2 μM, for 1 h and 47 h of exposure, respectively). In this assay, it was also observed that high concentrations (100–1000 μM) of 3-SC 37 and 46 led to a cell death greater than 90%, while 3-SC 39, for the same range of concentrations, was less cytotoxic, showing a 30% cell viability. The obtained results led the authors to conclude that 3-SC 46 had the highest cytotoxicity and tumor specificity among the three 3-SC (37, 39, and 46), with cytotoxicity being triggered at early 3 h after treatment. Therefore, the effects of 3-SC 46 on fine cell structures and the type of cell death were evaluated, as well as the metabolic profile induced by this 3-SC in HSC-2 cells. 3-SC 46 (3 and 10 μM) induced concentration-dependent mitochondrial vacuolization, which suggests induction of mitochondrial injury. 3-SC 46 (10 μM) suppressed autophagy and induced apoptotic cell death. In what concerns metabolomic analysis, 3-SC 46 (1, 3, and 10 μM) did not significantly affect major cellular metabolic pathways, however the authors reported changes in metabolites involved in amino acid and glycerophospholipid metabolism [5].
Takao and co-workers [78] synthetized fourteen 3-SC (2, 40, 41, and 165–175, Figure 49) and investigated their tumor specificity. The authors evaluated the cytotoxic activity of these 3-SC against four human oral squamous cell carcinoma cell lines (Ca9-22, HSC-2, HSC-3, and HSC-4) and three human normal oral mesenchymal cells (HGF, HPLF, and HPC). In addition, QSAR analyses were performed. First, the cytotoxic activity of 3-SC (0.3–400 μM) was assessed by the MTT assay, and CC50, SI, and PSE were calculated and presented. In this study, the associated error to CC50 values was not indicated, even though the determination of the relative viable cell number was performed in triplicate. The CC50 values were presented for each cell line and the mean of the CC50 values of the four tumor cell lines, and the three normal cells were also presented, with the associated error. Doxorubicin (0.078–10 μM) and 5-fluorouracil (7.8–1000 μM) were used as positive controls. 3-SC 168 and 175 were the most cytotoxic against the tumor cell lines (mean CC50 values of 0.6 ± 0.27 μM and 0.65 ± 0.34 μM, respectively), followed by 169 (mean CC50 = 1.1 ± 0.31 μM). Against normal oral cells, 3-SC 168 and 175 showed low cytotoxicity (mean CC50 values of 181.9 ± 195.0 μM and 118.8 ± 142.6 μM, respectively), while 3-SC 169 showed high toxicity (mean CC50 values of 4.1 ± 1.6 μM). Thus, 3-SC 168 and 175 showed high tumor specificity (SI values of 301.1 and 182.0, respectively), being better than doxorubicin and 5-fluorouracil (SI values of 54.9 and 16.2, respectively); and 169 showed low tumor specificity (SI = 3.7). 3-SC 41 and 167 were also quite cytotoxic against tumor cell lines (mean CC50 values of 5.3 ± 0.8 μM and 6.5 ± 2.6 μM, respectively), but nevertheless also showed high cytotoxicity against normal oral cell lines (mean CC50 values of 3.7 ± 1.1 μM and 6.1 ± 0.8 μM, respectively). 3-SC 168 showed a high potency-selectivity expression (PSE = 49842.4), followed by 175 (PSE = 27898.1), being these values higher than those obtained for the positive controls (PSE = 24953.8 for doxorubicin and PSE = 26.2 for 5-fluorouracil). These results indicate that all tumor oral cell lines tested were more sensitive to 3-SC 168 and 175 than normal oral cells. Thus, the effects of 3-SC 168 (1–4 μM) and 175 (2–8 μM) on the apoptosis induction and growth arrest were further monitored by cell cycle analysis of HSC-2 cells. 3-SC 168 and 175 and the positive control, actinomycin D (1 μM), led to an accumulation of cell population in the subG1; however, only 3-SC 168 and 175 induced the accumulation at G2/M phase cells and induced mitotic arrest. Thus, the results demonstrated that these two 3-SC induced the apoptosis and mitotic arrest in HSC-2 cell more potently than actinomycin D. The QSAR analysis of all the tested 3-SC indicated that tumor specificity was correlated with the following descriptors: topological and 3D shape, size, electric state, aromaticity index, energy of the lowest unoccupied molecular orbital (LUMO), and partial charges. The authors also indicated that the presence of −OCH3 at C-6 on the A-ring reduced the tumor specificity and the presence of −OCH3 at C-7 on the A-ring led to an increase in tumor specificity. In fact, the 3-SC with the highest tumor specificity (168) has only −OCH3 at C-7 on the A-ring. Furthermore, the fact that 3-SC 166, with only an −OCH3 at C-6 on the A-ring, has a very low tumor specificity is an example that demonstrates that in addition to the type of substituents, their position also influences the tumor specificity. In general, the introduction of halogen atoms (−F and −Cl) at C-4′ on the B-ring (3-SC 40 and 41) reduced the tumor specificity, even in the presence of additional −OCH3 at C-7 on the A-ring (169 and 170). Unsubstituted 3-SC 2 also showed low tumor specificity. For 3-SC 165, 167, and 171, a low tumor specificity was found. These three 3-SC have in common the −N(CH3)2 group at C-4′ on the B-ring, which does not seem to favour tumor specificity. In conclusion, this study suggests that the −OCH3 group at C-7 on the A-ring favours the cytotoxic activity against the studied tumor cell lines and improves the tumor specificity of 3-SC [78].
Figure 49.
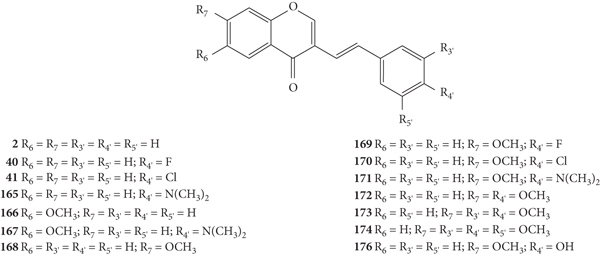
Chemical structures of 3-SC 2, 40, 41, and 165–175 [78].
As for 2-SC, studies found in the literature on the antitumor activity of 3-SC include different tumor cell lines and a wide range of 3-SC with various substituents in different locations, making it difficult to establish a clear SAR. However, the above-mentioned structural characteristics that demonstrated to favour the antitumoral activity of 3-SC are summarized in Figure 50.
Figure 50.
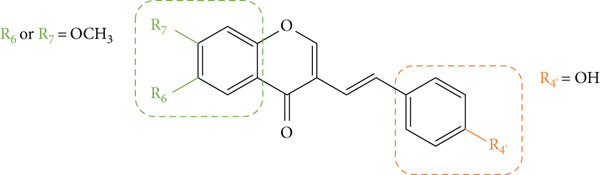
Structural characteristics that seem to favour the 3-SC antitumoral activity.
4.8. Other Activities
In addition to the biological activities already described in the previous topics of this review, several SC have shown other less explored biological properties, such as affinity and selectivity for A3 adenosine receptors, neuroprotective activity, and α-glucosidase inhibitory activity and are below discussed.
Affinity and selectivity for A3 adenosine receptors have only been reported in one study, to the best of our knowledge, in which three 2-SC were studied. Adenosine receptors are important targets for drug development as they have been associated with several biological functions, such as immune regulation and vascular function, but also with pathological processes, such as inflammatory and neurodegenerative diseases. Karton and co-workers [79] evaluated the potential of 2-SC 176–178 (Table 12) as A3 adenosine antagonists, which the authors described to serve as anti-inflammatory, cerebroprotective, or antiasthmatic molecules. For this purpose, the affinity and selectivity of these 2-SC for A1, A2A, and A3 adenosine receptors were evaluated and the observed effects are described in Table 12 [79].
Table 12.
2-SC studied for A3 adenosine antagonists, their chemical structures, and summary of the observed effects.
| Chemical structure | 2-SC | Observed effect(s) | Ref. |
|---|---|---|---|
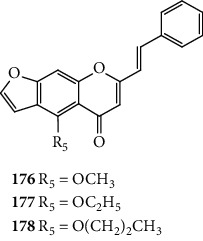
|
176 | Binding affinity for A1, A2A and A3 adenosine receptors | [79] |
| 177 | Binding affinity for A1, A2A and A3 adenosine receptors; and selectivity for A3 adenosine receptor | ||
| 178 | Binding affinity for A1, A2A and A3 adenosine receptors |
The potential of 2-SC as neuroprotective agents has only been studied in four studies, to the best of our knowledge. Yoon and co-workers [8] evaluated the neuroprotective activity of the natural 2-SC 5 (Table 13) against glutamate-induced neurotoxicity in primary cultures of Sprague-Dawley rat cortical cells. Yang and co-workers [9] isolated the 2-SC 6 (Table 13) from the ethanolic extract of Chinese eaglewood and studied its neuroprotective activities against glutamate-induced and corticosterone-induced neurotoxicity in P12 pheochromocytoma and human U251 glioma cells, respectively. In another study, Jung and co-workers [11] isolated a 2-SC (11, Table 13) and evaluated its inhibitory potential against β-site amyloid precursor protein-cleaving enzyme 1 (BACE1), acetylcholinesterase (AChE), and butyrylcholinesterase (BChE). Years later, Takao and co-workers [80] synthetized a series of 2-SC (1, 30, 56, 58, 88, 92, and 104–115, Table 13) and evaluated their inhibitory activity against recombinant human monoamine oxidase (MAO)-A and MAO-B. The observed effects are described in Table 13.
Table 13.
2-SC studied for the neuroprotective activity, their chemical structures, and summary of the observed neuroprotective effects.
| Chemical structure | 2-SC | Observed effect(s) | Ref. |
|---|---|---|---|
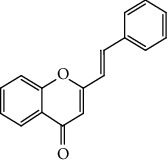
|
1 | Inhibition of MAO-A and MAO-B activities, displaying MAO-B selectivity | [80] |
|
| |||
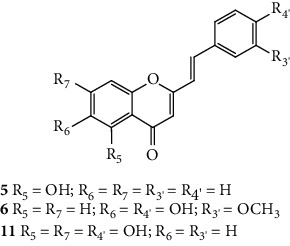
|
5 | NA | [8] |
| 6 | Neuroprotective activity against glutamate-induced neurotoxicity in P12 pheochromocytoma cells | [9] | |
| 11 | Inhibition of AChE, BChE and BACE1 | [11] | |
|
| |||
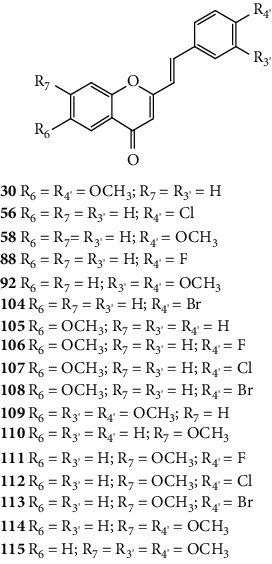
|
30 | Inhibition of MAO-A and MAO-B activities | [80] |
| 56 | Inhibition of MAO-A and MAO-B activities, displaying MAO-B selectivity | ||
| 58 | |||
| 88 | |||
| 92 | |||
| 104 - 111 | |||
| 112, 113 | Inhibition of MAO-B activity, displaying MAO-B selectivity | ||
| 114 | Inhibition of MAO-A and MAO-B activities, displaying MAO-B selectivity | ||
| 115 | Inhibition of MAO-B activity, displaying MAO-B selectivity | ||
NA: no activity was found under tested experimental conditions. AChE: acetylcholinesterase; BACE1: β-site amyloid precursor protein cleaving enzyme 1; BChE: butyrylcholinesterase; MAO: monoamine oxidase.
Remarkably, a new series of 2-SC has been synthetized and evaluated for their possible application in the diagnostic as imaging probes for cerebral amyloid-β plaques and for abnormal prion protein aggregates. Even though it is not a biological activity, it demonstrates the great potential of 2-SC in the medical diagnostic field. For more detailed information, the works by Ono and co-workers [81], Fuchigami and co-workers [82], and Fuchigami and co-workers [83] elegantly describe these properties of 2-SC.
The potential of 3-SC as inhibitors of α-glucosidase has only been studied once, to the best of our knowledge. α-Glucosidase is a carbohydrase enzyme that catalyses the final step in the digestive process of carbohydrates. The inhibition of this enzyme activity is one of the therapeutic approaches for the treatment of diabetes mellitus [84]. Takao and co-workers [33] evaluated the α-glucosidase inhibitory activity of a series of 3-SC (34–50, Table 14), and the observed effects are described in Table 14.
Table 14.
3-SC studied for α-glucosidase inhibitory activity, their chemical structures, and summary of the observed effects.
| Chemical structure | 3-SC | Observed effect(s) | Ref. |
|---|---|---|---|
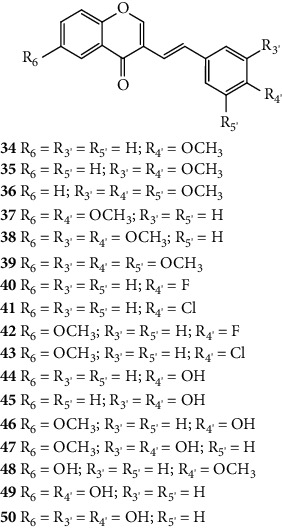
|
34 - 39 | NA | [33] |
| 40, 41 | Inhibition of α-glucosidase | ||
| 42 | NA | ||
| 43 - 45 | Inhibition of α-glucosidase | ||
| 46 | NA | ||
| 47 - 50 | Inhibition of α-glucosidase |
NA: no activity was found under tested experimental conditions.
5. Discussion
Several biological activities have been attributed to SC. Currently, two types of SC have been studied: 2-SC and 3-SC. The presence of SC in nature is scarce, however 2-SC are more common and are also the most chemically synthesized. Thus, they are most often reported in the literature for their biological properties. 2-SC have been reported to display antioxidant, antiallergic, antiviral, antibacterial, antifungal, anti-inflammatory, antitumor, neuroprotective properties and affinity and selectivity for A3 adenosine receptors. 3-SC have only been attributed to have the following activities: antioxidant, antiviral, antitumor properties, and α-glucosidase inhibitory activities. This work presents a comprehensive review of the biological activities attributed to SC and their SAR. However, the number of existing reports as well as the variety of structural changes between the studied SC and the experimental differences between studies make it difficult to establish a generic SAR. Nevertheless, for each biological activity, some important structural aspects were identified.
The antioxidant activity of SC is one of the most studied biological activities, with many associated reports in the literature. These studies refer to different antioxidant effects, which leads to significant differences regarding the type of important substituents and their location in the structure. Nevertheless, in general, the presence of −OH groups on the B-ring seems to play an important role for this activity, particularly the presence of a catechol group. The presence of only one −OH at C-4′ on the B-ring also revealed some importance, however it was overcome by the presence of the catechol group in the same ring. In addition, the presence of −OH groups on the A-ring also seems to contribute to the antioxidant effects, especially if they are at C-5 and C-7 on the A-ring. Studies for the scavenging effects of ROS and RNS have shown that, depending on the reactive species, the styryl moiety also influences the antioxidant activity of 2-SC and can improve it. The importance of the role of −OH groups (C-3′ and/or C-4′) on the B-ring was also confirmed, because when they were methylated, the scavenging activity of 2-SC decreased. In contrast, the presence of −OCH3 at C-3′ and C-4′ on the B-ring improved the iron reducing activity of 2-SC. Finally, the presence of −OH at C-3 on the C-ring also seems to favour the scavenging activity against ROS and RNS. The studies of the antioxidant activity of 3-SC also corroborate the importance of the −OH groups on the B-ring for their antioxidant effects.
For the antiallergic activity, the presence of only one −CH3 on the B-ring (C-2′ or C-4′) seems to increase it. The presence of small alkyl groups at C-3 on the C-ring also seems to contribute to the antiallergic activity of the studied 2-SC.
The antiviral potential of SC has been studied for different viruses, and the same SC have demonstrated different interactions with the different studied viruses and viruses' strains. These differences between the antiviral effects shown by SC indicate some selectivity, being an advantage for their use as antiviral agents. The analysis of the antiviral activity of 2-SC showed that it is significantly influenced by the substituents present in the structure and its location, however it is difficult to indicate in a generic way which substituents play the most important role. The presence of −Cl at C-6 on the A-ring, −OH or −OCH3 at C-3 on the C-ring, and −NO2 at C-4′ on the B-ring seems to favour 2-SC antiviral activity against serotype 1B of HRV. These substituents also seem to be important for the antiviral activity of 2-SC against serotype 14 of HRV; however, the presence of −F at C-6 on the A-ring and −Cl at C-4′ on the B-ring also seems to contribute to this activity. 2-SC antiviral activity against MNV was also studied and demonstrated the importance of the presence of the −OH group at C-5 on the A-ring and the −OCH3 group at C-4′ on the B-ring for the antiviral activity against MNV. The inhibitory activity of 2-SC against the HIV-1 integrase was another of the studied antiviral effects, but only one 2-SC was evaluated, which was not very effective against the HIV-1 integrase. In what concerns 3-SC antiviral activity, there are few studies related to their antiviral properties, however in the reports found in the literature, 3-SC have shown few antiviral effects. 3-SC were studied against HIV, EV, and the serotypes 1B and 14 of HRV. The studied 3-SC did not show anti-HIV activity. In addition, it was only possible to conclude that the presence of −Cl at C-6 on the A-ring and C-4′ on the B-ring seems to play an important role for the antiviral effect against HRV 1B. The studied 3-SC showed low efficacy against EV and HRV 14, so it was not possible to establish a SAR.
As previously mentioned, for the antibacterial and antifungal activities of SC, only 2-SC were studied and there are no reports of 3-SC with antibacterial and antifungal potential, to the best of our knowledge. The antibacterial activity of 2-SC was studied for different strains of Gram-positive and Gram-negative bacteria, with the tested 2-SC showing some variability in what concerns the results observed for each strain and between Gram-positive and negative bacteria, which makes it difficult to establish a clear SAR. Additionally, the studied 2-SC included several substituents which did not present coherent activities patterns, which also makes it difficult to establish of a specific SAR. Thus, only a generic SAR has been established for the antibacterial activity. The antibacterial activity of 2-SC seems to be influenced by the position and the number of −F substituents present on the A- or B-rings, as well as by the existence of −Cl or −OH at C-4′ on the B-ring. The C-6 on the A-ring and C-4′ on the B-ring also seem to be important substituents' positions for the antibacterial effects of 2-SC. For most 2-SC, the authors who reported their antibacterial properties, also assessed their antifungal potential. As reported for the antibacterial activity, the 2-SC studied included different substitution patterns. This fact, combined with differences in the used methodology between studies, only allowed the establishment of a generic SAR for antifungal activity. Similar to what was reported for the bacterial activity, the presence of the 1,2,4-triazole ring at C-4′ on the B-ring appears to contribute to the antifungal activity, as well as the existence of −Cl at C-4′ on the B-ring. The −OCH3 group at C-4′ on the B-ring also seems important for this activity. Summarizing, in part, the substituents that favour antibacterial activity also favour antifungal activity, which seems to indicate a weak selectivity by 2-SC.
Several anti-inflammatory mechanisms have been described for 2-SC, namely, inhibition of LFA-1/ICAM-1-mediated cell adhesion, interference with the arachidonic acid metabolic pathways, inhibition of NF-κB activation, and modulation of cytokines/chemokine production. Despite the reported studies being quite different and evaluating several mediators, the presence of −OH at C-3′ and C-4′ on the B-ring seems to generically favour the anti-inflammatory activity, similarly to what has been demonstrated for the antioxidant activity.
The antitumor potential is one of the most explored biological activities in the literature for SC. The antitumor properties of 2-SC are widely described, however there are few studies describing the antitumoral effects of 3-SC. Some of the existing studies, in addition to the evaluation of the effects of SC on tumor cell lines, also evaluate their effects on normal cells, which allows to understand whether the effects of SC are tumor selective. In addition to selectivity, it is important to know whether SC are also cytotoxic to normal cells. This fact is determinant for their use as possible antitumor agents, since the major objective is to demonstrate cytotoxicity against tumor cells, affecting as little as possible normal cells. Unfortunately, many of the studies reported did not include normal cell lines in their evaluation, making it difficult to understand if and in what extension SC are selective for tumor cell lines. In the cases where both cell types were studied, in general, SC were cytotoxic to tumor cells and did not show cytotoxicity to normal cells. These studies cover a wide variety of tumor cell lines, so it is difficult to indicate which type of cancer is most studied and to establish SAR. Nevertheless, in general, the presence of −OCH3 groups on the B-ring seems to play an important role in the antitumor activity performed by 2-SC, especially when the −OCH3 is at C-4′ on the B-ring. Furthermore, the simultaneous presence of −OCH3 groups at C-3′, C-4′, and C-5′ on the B-ring also seems to potentiate the antitumor effects of 2-SC. The styryl moiety seems to favour the growth inhibitory activity. The existence of −OCH3 at C-6 on the A-ring also appear to favour cytotoxic activity against tumor cell lines. All in all, the studies of the antitumoral activity of 3-SC seem to indicate that the presence of −OCH3 at C-6 or C-7 on the A-ring and a−OH at C-4′ on the B-ring favour cytotoxic activity against tumor cell lines.
The neuroprotective activity of SC is one of the biological activities less explored in the literature, since only the possible neuroprotective effects of three 2-SC were reported. Among these three 2-SC studied, only two showed neuroprotective properties: neuroprotective activity against glutamate-induced neurotoxicity in P12 pheochromocytoma cells and inhibition of AChE, BChE, and BACE1. Since the neuroprotective activity of 2-SC has been less studied and the effects evaluated are very different, it is difficult to identify the substituents that favour their effect, because in each study, a different 2-SC was evaluated.
As mentioned in the beginning of this section, in addition to the biological activities mentioned above, other activities have also been reported for SC, namely, affinity and selectivity for the A3 adenosine receptor, inhibitory activity of MAO-A and MAO-B, and inhibitory activity of α-glucosidase. In what concerns the affinity and selectivity of 2-SC for adenosine receptors, the presence of −OC2H5 at C-5 on the A-ring appears to favour the affinity and selectivity for the A3 adenosine receptor. For the MAO inhibitory activity, the presence of −OCH3 at C-6 on the A-ring and halogenated groups (−F, −Cl, or −Br) at C-4′ on the B-ring favoured the MAO-A inhibitory activity; while the presence of −OCH3 at C-6 on the A-ring and −Cl or −Br at C-4′ on the B-ring favoured the MAO-B inhibitory activity and selectivity. The α-glucosidase inhibitory activity of 3-SC was influenced by the presence of the −OH group at C-6 on the A-ring and −OH at C-3′ and C-4′ or at C-4′ on the B-ring; however, the 3-SC with −OH at C-3′ and C-4′ on the B-ring was more potent than the 3-SC with just an −OH at C-4′ on the B-ring.
6. Conclusions
This work demonstrates that SC exhibit a large variety of biological properties. Throughout this work, it can be observed that a wide variety of SC structures has already been studied, most of which with substituent variations on the A- and/or B-rings. The type of substituents in the SC structure and their location assume an essential role in each one of the activities shown. This variety, added to the experimental differences between studies, makes it difficult to establish a generic SAR for each activity. The antioxidant and antitumor activities of SC are undoubtedly the most explored ones in the literature. In conclusion, SC present a molecular scaffold that demonstrates an enormous potential for the development of new drugs envisioning the treatment of several oxidative process-related diseases, namely, inflammatory, carcinogenic, and neurodegenerative.
Acknowledgments
This work received financial support from PT national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through the project UIDB/50006/2020 and from the European Union (FEDER funds through the Operational Competitiveness Program (COMPETE2020) POCI-01-0145-FEDER-029253—Project PTDC/MED-QUI/29253/2017). Mariana Lucas acknowledges FCT and ESF (European Social Fund) through Programa Operacional Regional do Norte (NORTE 2020) for her PhD grant (2021.06746.BD). Marisa Freitas acknowledges her contract under the Scientific Employment Stimulus - Individual Call (CEEC Individual) 2020.04126.CEECIND/CP1596/CT0006.
Abbreviations
- 2-SC:
2-Styrylchromones
- 3-SC:
3-Styrylchromones
- CC50:
Concentration that causes 50% of cytotoxicity
- CDK:
Cyclin-dependent kinase
- CHO:
Chinese hamster ovary
- CNS:
Central nervous system
- COX:
Cyclooxygenase
- DCFH-DA:
2′,7′-Dichlorodihydrofluorescein diacetate
- DIZ:
Diameter of inhibition zone
- DPPH•:
2,2-Diphenyl-1-picrylhydrazyl radical
- EC50:
Concentration that produces 50% of the maximum effect
- EV:
Enterovirus
- GI:
Growth inhibition
- GI50:
Concentration that produces 50% of cell growth inhibition
- GSK-3β:
Glycogen synthase kinase 3-beta
- HIV-1:
Human immunodeficiency virus type 1
- HRV:
Human rhinovirus
- IC50:
Concentration that produces 50% of inhibition
- ICAM-1:
Intercellular cell adhesion molecule-1
- IL:
Interleukin
- Keap1:
Kelch ECH-associating protein 1
- LDL:
Low-density lipoproteins
- LFA-1:
Lymphocyte function-associated molecule-1
- log P:
Logarithm of partition coefficient of compound between n-octanol and water
- LT:
Leukotriene
- MAO:
Monoamine oxidase
- MIC:
Minimum inhibitory concentration
- MNTC:
Maximum non-toxic concentration
- MNV:
Murine norovirus
- MTT:
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NA:
No activity
- NADH:
β-Nicotinamide adenine dinucleotide
- NF-κB:
Nuclear factor kappa B
- NoV:
Human noroviruses
- Nrf2:
Nuclear factor erythroid 2-related factor 2
- PSE:
Potency-selectivity expression
- QSAR:
Quantitative structure-activity relationship
- Ref.:
Reference
- RNS:
Reactive nitrogen species
- ROS:
Reactive oxygen species
- SAR:
Structure-activity relationship
- SC:
Styrylchromones
- SI:
Selectivity index
- SRB:
Sulforhodamine B
- t-BHP:
tert-Butyl hydroperoxide
- TNF-α:
Tumor necrosis factor-α
- XO:
Xanthine oxidase
- XTT:
2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide.
Contributor Information
Eduarda Fernandes, Email: egracas@ff.up.pt.
Daniela Ribeiro, Email: dsribeiro@ff.up.pt.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gaspar A., Matos M. J., Garrido J., Uriarte E., Borges F. Chromone: a valid scaffold in medicinal chemistry. Chemical Reviews . 2014;114(9):4960–4992. doi: 10.1021/cr400265z. [DOI] [PubMed] [Google Scholar]
- 2.Keri R. S., Budagumpi S., Pai R. K., Balakrishna R. G. Chromones as a privileged scaffold in drug discovery: a review. European Journal of Medicinal Chemistry . 2014;78:340–374. doi: 10.1016/j.ejmech.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 3.Santos C. M. M., Silva A. M. S. An overview of 2-styrylchromones: natural occurrence, synthesis, reactivity and biological properties. European Journal of Organic Chemistry . 2017;2017(22):3115–3133. doi: 10.1002/ejoc.201700003. [DOI] [Google Scholar]
- 4.Gomes A., Freitas M., Fernandes E., Lima J. L. F. C. Biological activities of 2-styrylchromones. Mini-Reviews in Medicinal Chemistry . 2010;10(1):1–7. doi: 10.2174/138955710791112550. [DOI] [PubMed] [Google Scholar]
- 5.Sakagami H., Shimada C., Kanda Y., et al. Effects of 3-styrylchromones on metabolic profiles and cell death in oral squamous cell carcinoma cells. Toxicology Reports . 2015;2:1281–1290. doi: 10.1016/j.toxrep.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerwick W. H., Lopez A., van Duyne G. D., Clardy J., Ortiz W., Baez A. Hormothamnione, a novel cytotoxic styrylchromone from the marine cyanophyte Hormothamnion enteromorphoides grunow. Tetrahedron Letters . 1986;27(18):1979–1982. doi: 10.1016/S0040-4039(00)84426-3. [DOI] [Google Scholar]
- 7.Gerwick W. H. 6-Desmethoxyhormothamnione, a new cytotoxic styrylchromone from the marine cryptophyte Chrysophaeum taylori. Journal of Natural Products . 1989;52(2):252–256. doi: 10.1021/np50062a005. [DOI] [PubMed] [Google Scholar]
- 8.Yoon J. S., Lee M. K., Sung S. H., Kim Y. C. Neuroprotective 2-(2-phenylethyl)chromones of Imperata cylindrica. Journal of Natural Products . 2006;69(2):290–291. doi: 10.1021/np0503808. [DOI] [PubMed] [Google Scholar]
- 9.Yang L., Qiao L., Xie D., et al. 2-(2-Phenylethyl)chromones from Chinese eaglewood. Phytochemistry . 2012;76:92–97. doi: 10.1016/j.phytochem.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Yang C.-H., Yang Y., Liu J. H. Platachromone A-D: Cytotoxic 2-styrylchromones from the bark of Platanus × acerifolia (Aiton) Willd. Phytochemistry Letters . 2013;6(3):387–391. doi: 10.1016/j.phytol.2013.05.003. [DOI] [Google Scholar]
- 11.Jung H. J., Jung H. A., Min B.-S., Choi J. S. Anticholinesterase and β-site amyloid precursor protein cleaving enzyme 1 inhibitory compounds from the heartwood of Juniperus chinensis. Chemical and Pharmaceutical Bulletin . 2015;63(11):955–960. doi: 10.1248/cpb.c15-00504. [DOI] [PubMed] [Google Scholar]
- 12.Chaniad P., Wattanapiromsakul C., Pianwanit S., Tewtrakul S. Anti-HIV-1 integrase compounds from Dioscorea bulbifera and molecular docking study. Pharmaceutical Biology . 2016;54(6):1077–1085. doi: 10.3109/13880209.2015.1103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashem F. A. Phenolic compounds ofErucaria microcarpaBoiss. And their effect as scavengers for singlet oxygen. Journal of Herbs, Spices & Medicinal Plants . 2007;12(3):27–41. doi: 10.1300/J044v12n03_03. [DOI] [Google Scholar]
- 14.Pinto D. C. G. A., Silva A. M. S., Cavaleiro J. A. S. A convenient synthesis of new (E)-5-hydroxy-2-styrylchromones by modifications of the Baker–Venkataraman method. New Journal of Chemistry . 2000;24(2):85–92. doi: 10.1039/a908539d. [DOI] [Google Scholar]
- 15.Santos C. M. M., Silva A. M. S., Cavaleiro J. A. S. Synthesis of new hydroxy-2-styrylchromones. European Journal of Organic Chemistry . 2003;2003(23):4575–4585. doi: 10.1002/ejoc.200300468. [DOI] [Google Scholar]
- 16.Cavaleiro J. A. S., Price W. A., Silva A. M. S. 2-Styrlchromones: biological action, synthesis and reactivity. Heterocycles . 1993;36(11):2601–2612. doi: 10.3987/REV-93-457. [DOI] [Google Scholar]
- 17.Silva A. M. S., Pinto D. C. G. A., Cavaleiro J. A. S., Levai A., Patonay T. Synthesis and reactivity of styrylchromones. ARKIVOC . 2004;2004(7):106–123. doi: 10.3998/ark.5550190.0005.709. [DOI] [Google Scholar]
- 18.Silva V. L. M., Silva A. M. S., Pinto D. C. G. A., Cavaleiro J. A. S., Vasas A., Patonay T. Syntheses of (E)- and (Z)-3-styrylchromones. Monatshefte für Chemie - Chemical Monthly . 2008;139(11):1307–1315. doi: 10.1007/s00706-008-0926-0. [DOI] [Google Scholar]
- 19.Rocha D. H. A., Pinto D., Silva A. One-pot synthesis of 3-Methylflavones and their transformation into (E)-3-Styrylflavones via Wittig reactions. Synlett . 2013;24(20):2683–2686. doi: 10.1055/s-0033-1340013. [DOI] [Google Scholar]
- 20.Pisoschi A. M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. European Journal of Medicinal Chemistry . 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 21.Gomes A., Fernandes E., Lima J. L. F. C. Fluorescence probes used for detection of reactive oxygen species. Journal of Biochemical and Biophysical Methods . 2005;65(2-3):45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes E., Carvalho F., Silva A. M. S., et al. 2-Styrylchromones as novel inhibitors of xanthine oxidase. A structure-activity study. Journal of Enzyme Inhibition and Medicinal Chemistry . 2002;17(1):45–48. doi: 10.1080/14756360290019944. [DOI] [PubMed] [Google Scholar]
- 23.Battelli M. G., Polito L., Bortolotti M., Bolognesi A. Xanthine oxidoreductase-derived reactive species: physiological and pathological effects. Oxidative Medicine and Cellular Longevity . 2016;2016:8. doi: 10.1155/2016/3527579.3527579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes E., Carvalho M., Carvalho F., et al. Hepatoprotective activity of polyhydroxylated 2-styrylchromones against tert-butylhydroperoxide induced toxicity in freshly isolated rat hepatocytes. Archives of Toxicology . 2003;77(9):500–505. doi: 10.1007/s00204-003-0480-9. [DOI] [PubMed] [Google Scholar]
- 25.Filipe P., Silva A. M. S., Morlière P., et al. Polyhydroxylated 2-styrylchromones as potent antioxidants. Biochemical Pharmacology . 2004;67(12):2207–2218. doi: 10.1016/j.bcp.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 26.Gomes A., Fernandes E., Silva A. M. S., et al. 2-Styrylchromones: novel strong scavengers of reactive oxygen and nitrogen species. Bioorganic & Medicinal Chemistry . 2007;15(18):6027–6036. doi: 10.1016/j.bmc.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 27.Gomes A., Fernandes E., Garcia M. B. Q., et al. Cyclic voltammetric analysis of 2-styrylchromones: relationship with the antioxidant activity. Bioorganic & Medicinal Chemistry . 2008;16(17):7939–7943. doi: 10.1016/j.bmc.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 28.Gomes A., Neuwirth O., Freitas M., et al. Synthesis and antioxidant properties of new chromone derivatives. Bioorganic & Medicinal Chemistry . 2009;17(20):7218–7226. doi: 10.1016/j.bmc.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 29.Sousa J. L. C., Proença C., Freitas M., Fernandes E., Silva A. M. S. New polyhydroxylated flavon-3-ols and 3-hydroxy-2-styrylchromones: synthesis and ROS/RNS scavenging activities. European Journal of Medicinal Chemistry . 2016;119:250–259. doi: 10.1016/j.ejmech.2016.04.057. [DOI] [PubMed] [Google Scholar]
- 30.Rao V. M., Ujwala B., Priyadarsini P., Krishna Murthy P. Synthesis, antioxidant and antimicrobial activity of three new 2-styrylchromones and their analogues. Der Pharma Chemica . 2016;8(7):1–6. [Google Scholar]
- 31.Anto R. J., Kuttan G., Kuttan R., Sathyanarayana K., Rao M. N. A. Tumor-reducing and antioxidant activities of sydnone-substituted chalcones. Journal of Clinical Biochemistry and Nutrition . 1994;17(2):73–80. doi: 10.3164/jcbn.17.73. [DOI] [Google Scholar]
- 32.Takamatsu S., Hodges T. W., Rajbhandari I., Gerwick W. H., Hamann M. T., Nagle D. G. Marine natural products as novel antioxidant prototypes. Journal of Natural Products . 2003;66(5):605–608. doi: 10.1021/np0204038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takao K., Ishikawa R., Sugita Y. Synthesis and biological evaluation of 3-styrylchromone derivatives as free radical scavengers and α-glucosidase inhibitors. Chemical and Pharmaceutical Bulletin . 2014;62(8):810–815. doi: 10.1248/cpb.c14-00351. [DOI] [PubMed] [Google Scholar]
- 34.Galli S. J., Tsai M., Piliponsky A. M. The development of allergic inflammation. Nature . 2008;454(7203):445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villa T. G., Feijoo-Siota L., Rama J. L. R., Ageitos J. M. Antivirals against animal viruses. Biochemical Pharmacology . 2017;133:97–116. doi: 10.1016/j.bcp.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M.-Z., Chen Q., Yang G.-F. A review on recent developments of indole-containing antiviral agents. European Journal of Medicinal Chemistry . 2015;89:421–441. doi: 10.1016/j.ejmech.2014.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desideri N., Conti C., Mastromarino P., Mastropaolo F. Synthesis and anti-rhinovirus activity of 2-styrylchromones. Antiviral Chemistry and Chemotherapy . 2000;11(6):373–381. doi: 10.1177/095632020001100604. [DOI] [PubMed] [Google Scholar]
- 38.Desideri N., Mastromarino P., Conti C. Synthesis and evaluation of antirhinovirus activity of 3-hydroxy and 3-methoxy 2-styrylchromones. Antiviral Chemistry and Chemotherapy . 2003;14(4):195–203. doi: 10.1177/095632020301400404. [DOI] [PubMed] [Google Scholar]
- 39.Conti C., Mastromarino P., Goldoni P., Portalone G., Desideri N. Synthesis and anti-rhinovirus properties of fluoro-substituted flavonoids. Antiviral Chemistry and Chemotherapy . 2005;16(4):267–276. doi: 10.1177/095632020501600406. [DOI] [PubMed] [Google Scholar]
- 40.Rocha-Pereira J., Cunha R., Pinto D. C. G. A., Silva A. M. S., Nascimento M. S. J. (E)-2-Styrylchromones as potential anti-norovirus agents. Bioorganic & Medicinal Chemistry . 2010;18(12):4195–4201. doi: 10.1016/j.bmc.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Patel M. M., Hall A. J., Vinjé J., Parashar U. D. Noroviruses: a comprehensive review. Journal of Clinical Virology . 2009;44(1):1–8. doi: 10.1016/j.jcv.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Sleasman J. W., Goodenow M. M. In: Principles of Molecular Medicine . Jameson J. L., editor. Totowa, NJ: Humana Press; 1998. [Google Scholar]
- 43.Shimada C., Uesawa Y., Ishii-Nozawa R., et al. Quantitative structure-cytotoxicity relationship of 3-styrylchromones. Anticancer Research . 2014;34(10):5405–5411. [PubMed] [Google Scholar]
- 44.Conti C., Desideri N. New 4H-chromen-4-one and 2H-chromene derivatives as anti-picornavirus capsid-binders. Bioorganic & Medicinal Chemistry . 2010;18(17):6480–6488. doi: 10.1016/j.bmc.2010.06.103. [DOI] [PubMed] [Google Scholar]
- 45.Singh K. In: Applications of Targeted Nano Drugs and Delivery Systems . Singh K., Mishra A., Sharma D., editors; Mohapatra S. S., Ranjan S., Dasgupta N., Mishra R. K., Thomas S., editors. Elsevier; 2019. [Google Scholar]
- 46.Momin M., Ramjugernath D., Chenia H., Koorbanally N. A. Synthesis and evaluation of novel fluorinated 2-styrylchromones as antibacterial agents. Journal of Chemistry . 2013;2013:13. doi: 10.1155/2013/436758.436758 [DOI] [Google Scholar]
- 47.Nikam M. D., Mahajan P., Damale M., et al. Novel amalgamation of 2-styrylchromones and 1,2,4-triazole: synthesis, antimicrobial evaluation and docking study. Letters in Drug Design & Discovery . 2015;12(8):650–660. doi: 10.2174/157018081208150730160521. [DOI] [Google Scholar]
- 48.Ujwala B., Priyadarsini P., Rao V. M. Synthesis and bio-activity evaluation of 2-styrylchromones. International Journal of Pharma and Bio Sciences . 2013;4(1):199–206. [Google Scholar]
- 49.Brown G. D., Denning D. W., Gow N. A. R., Levitz S. M., Netea M. G., White T. C. Hidden killers: human fungal infections. Science Translational Medicine . 2012;4(165):p. 165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 50.Howard K. C., Dennis E. K., Watt D. S., Garneau-Tsodikova S. A comprehensive overview of the medicinal chemistry of antifungal drugs: perspectives and promise. Chemical Society Reviews . 2020;49(8):2426–2480. doi: 10.1039/C9CS00556K. [DOI] [PubMed] [Google Scholar]
- 51.Azab A., Nassar A., Azab A. Anti-inflammatory activity of natural products. Molecules . 2016;21(10):p. 1321. doi: 10.3390/molecules21101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takamatsu S., Nagle D. G., Gerwick W. H. Secondary metabolites from marine cyanobacteria and algae inhibit LFA-1/ICAM-1 mediated cell adhesion. Planta Medica . 2004;70(2):127–131. doi: 10.1055/s-2004-815488. [DOI] [PubMed] [Google Scholar]
- 53.Martz E. In: Encyclopedia of Immunology . Second Edition. Delves P. J., editor. Oxford: Elsevier; 1998. [DOI] [Google Scholar]
- 54.Gomes A., Fernandes E., Silva A. M. S., et al. Anti-inflammatory potential of 2-styrylchromones regarding their interference with arachidonic acid metabolic pathways. Biochemical Pharmacology . 2009;78(2):171–177. doi: 10.1016/j.bcp.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 55.Hanna V. S., Hafez E. A. A. Synopsis of arachidonic acid metabolism: a review. Journal of Advanced Research . 2018;11:23–32. doi: 10.1016/j.jare.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cipollone F., Cicolini G., Bucci M. Cyclooxygenase and prostaglandin synthases in atherosclerosis: recent insights and future perspectives. Pharmacology & Therapeutics . 2008;118(2):161–180. doi: 10.1016/j.pharmthera.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Ribeiro D., Freitas M., Lima J. L. F. C., Fernandes E. Proinflammatory pathways: the modulation by flavonoids. Medicinal Research Reviews . 2015;35(5):877–936. doi: 10.1002/med.21347. [DOI] [PubMed] [Google Scholar]
- 58.Ricciotti E., FitzGerald G. A. Prostaglandins and inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology . 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomes A., Capela J., Ribeiro D., et al. Inhibition of NF-kB activation and cytokines production in THP-1 monocytes by 2-styrylchromones. Medicinal Chemistry . 2015;11(6):560–566. doi: 10.2174/1573406411666150209114702. [DOI] [PubMed] [Google Scholar]
- 60.Ramesh G., MacLean A. G., Philipp M. T. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators of Inflammation . 2013;2013:20. doi: 10.1155/2013/480739.480739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turner M. D., Nedjai B., Hurst T., Pennington D. J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta . 2014;1843(11):2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 62.Korgaonkar N., Yadav K. S. Understanding the biology and advent of physics of cancer with perspicacity in current treatment therapy. Life Sciences . 2019;239, article 117060 doi: 10.1016/j.lfs.2019.117060. [DOI] [PubMed] [Google Scholar]
- 63.U. S. National Institutes of Health: National Cancer Institute SEER Training Modules: Cancer Terms. 2020. https://training.seer.cancer.gov/disease/cancer/terms.html .
- 64.Momoi K., Sugita Y., Ishihara M., et al. Cytotoxic activity of styrylchromones against human tumor cell lines. In Vivo . 2005;19(1):157–163. [PubMed] [Google Scholar]
- 65.Marinho J., Pedro M., Pinto D. C. G. A., et al. 4 ′-Methoxy-2-styrylchromone a novel microtubule-stabilizing antimitotic agent. Biochemical Pharmacology . 2008;75(4):826–835. doi: 10.1016/j.bcp.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 66.Uesawa Y., Nagai J., Shi H., et al. Quantitative structure–cytotoxicity relationship of 2-styrylchromones. Anticancer Research . 2019;39(12):6489–6498. doi: 10.21873/anticanres.13863. [DOI] [PubMed] [Google Scholar]
- 67.Shaw A. Y., Chang C.-Y., Liau H.-H., et al. Synthesis of 2-styrylchromones as a novel class of antiproliferative agents targeting carcinoma cells. European Journal of Medicinal Chemistry . 2009;44(6):2552–2562. doi: 10.1016/j.ejmech.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 68.Lin C., Lu P.-J., Yang C.-N., Hulme C., Shaw A. Y. Structure-activity relationship study of growth inhibitory 2-styrylchromones against carcinoma cells. Medicinal Chemistry Research . 2013;22(5):2385–2394. doi: 10.1007/s00044-012-0232-6. [DOI] [Google Scholar]
- 69.Lee K. Y., Nam D. H., Moon C. S., Seo S. H., Lee J. Y., Lee Y. S. Synthesis and anticancer activity of lavendustin a derivatives containing arylethenylchromone substituents. European Journal of Medicinal Chemistry . 2006;41(8):991–996. doi: 10.1016/j.ejmech.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Talhi O., Brodziak-Jarosz L., Panning J., et al. One-pot synthesis of benzopyran-4-ones with cancer preventive and therapeutic potential. European Journal of Organic Chemistry . 2016;2016(5):965–975. doi: 10.1002/ejoc.201501278. [DOI] [Google Scholar]
- 71.Baird L., Dinkova-Kostova A. T. The cytoprotective role of the Keap1–Nrf2 pathway. Archives of Toxicology . 2011;85(4):241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 72.Kansanen E., Kuosmanen S. M., Leinonen H., Levonen A.-L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biology . 2013;1(1):45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ragab F. A., Yahya T. A. A., El-Naa M. M., Arafa R. K. Design, synthesis and structure-activity relationship of novel semi-synthetic flavonoids as antiproliferative agents. European Journal of Medicinal Chemistry . 2014;82:506–520. doi: 10.1016/j.ejmech.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 74.Morgan D. O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annual Review of Cell and Developmental Biology . 1997;13(1):261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 75.Grimes C. A., Jope R. S. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Progress in Neurobiology . 2001;65(4):391–426. doi: 10.1016/S0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 76.Peixoto F., Barros A. I. R. N. A., Silva A. M. S. Interactions of a new 2-styrylchromone with mitochondrial oxidative phosphorylation. Journal of Biochemical and Molecular Toxicology . 2002;16(5):220–226. doi: 10.1002/jbt.10042. [DOI] [PubMed] [Google Scholar]
- 77.Knobeloch L. M., Blondin G. A., Read H. W., Harkin J. M. Assessment of chemical toxicity using mammalian mitochondrial electron transport particles. Archives of Environmental Contamination and Toxicology . 1990;19(6):828–835. doi: 10.1007/BF01055047. [DOI] [PubMed] [Google Scholar]
- 78.Takao K., Hoshi K., Sakagami H., et al. Further quantitative structure-cytotoxicity relationship analysis of 3-styrylchromones. Anticancer Research . 2020;40(1):87–95. doi: 10.21873/anticanres.13929. [DOI] [PubMed] [Google Scholar]
- 79.Karton Y., Jiang J. L., Ji X. D., et al. Synthesis and biological activities of flavonoid derivatives as A3 adenosine receptor antagonists. Journal of Medicinal Chemistry . 1996;39(12):2293–2301. doi: 10.1021/jm950923i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takao K., Endo S., Nagai J., et al. 2-Styrylchromone derivatives as potent and selective monoamine oxidase B inhibitors. Bioorganic Chemistry . 2019;92, article 103285 doi: 10.1016/j.bioorg.2019.103285. [DOI] [PubMed] [Google Scholar]
- 81.Ono M., Maya Y., Haratake M., Nakayama M. Synthesis and characterization of styrylchromone derivatives as β-amyloid imaging agents. Bioorganic & Medicinal Chemistry . 2007;15(1):444–450. doi: 10.1016/j.bmc.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 82.Fuchigami T., Ogawa A., Yamashita Y., et al. Development of alkoxy styrylchromone derivatives for imaging of cerebral amyloid-β plaques with SPECT. Bioorganic & Medicinal Chemistry Letters . 2015;25(16):3363–3367. doi: 10.1016/j.bmcl.2015.05.048. [DOI] [PubMed] [Google Scholar]
- 83.Fuchigami T., Yamashita Y., Kawasaki M., et al. Characterisation of radioiodinated flavonoid derivatives for SPECT imaging of cerebral prion deposits. Scientific Reports . 2015;5(1):p. 18440. doi: 10.1038/srep18440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumar S., Prakash O., Kumar S., Narwal S. α-Glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacognosy Reviews . 2011;5(9):19–29. doi: 10.4103/0973-7847.79096. [DOI] [PMC free article] [PubMed] [Google Scholar]


