Abstract
OBJECTIVE
COVID-19 is a rapidly changing and developing emergency that requires constant re-evaluation of available data. We report a systematic review and meta-analysis based on all published high-quality data up to and including June 3, 2021 on the maternal and neonatal outcomes in pregnant women infected with COVID-19.
DATA SOURCES
PubMed, SCOPUS, MEDLINE, ClinicalTrials.gov, and Web of Science databases were queried from inception up to June 3, 2021.
STUDY ELIGIBILITY CRITERIA
We included all clinical studies (prospective and retrospective cohort studies, case-control studies, case series, and rapid communications) that reported data on any maternal and neonatal outcomes of pregnant women with COVID-19.
METHODS
The data were analyzed as pooled proportions or odds ratios and 95% confidence intervals in meta-analysis models.
RESULTS
We included 111 studies enrolling 42,754 COVID-19-positive pregnant women. From COVID-19-positive pregnant women, the incidence rates were 53.2% (95% confidence interval, 48–58.4) for cesarean delivery, 41.5% (95% confidence interval, 36.3–46.8) for spontaneous vaginal delivery, and 6.4% (95% confidence interval, 4.5–9.2) for operative delivery. The rates of some adverse neonatal events, including premature delivery (16.7%; 95% confidence interval, 12.8–21.5) and low birthweight (16.7%; 95% confidence interval, 12.8–21.5) were relatively high in mothers infected with COVID-19. Vertical transmission (3.5%; 95% confidence interval, 2.7–4.7), neonatal death (3%; 95% confidence interval, 2–4), stillbirth (1.9%; 95% confidence interval, 1.5–2.4), and maternal mortality (0.012%; 95% confidence interval, 0.010–0.014) were rare adverse events. The mean birthweight was 3069.7 g (95% confidence interval, 3009.7–3129.8 g). In the comparative analysis, COVID-19 significantly increased the risk of premature delivery (odds ratio, 1. 48 [95% confidence interval, 1.22–1.8]), preeclampsia (odds ratio, 1.6 [95% confidence interval, 1.2–2.1]), stillbirth (odds ratio, 2.36 [95% confidence interval, 1.24–4.462]), neonatal mortality (odds ratio, 3.35 [95% confidence interval, 1.07–10.5]), and maternal mortality (odds ratio, 3.08 [95% confidence interval, 1.5–6.3]). The pooled analyses were homogenous, with mild heterogeneity in premature delivery and preeclampsia outcomes.
CONCLUSION
The data must be interpreted with caution as limited data are available, and no complete assessment of bias is possible at this time. Our data suggest that pregnant women who test positive for COVID-19 seem to be at a higher risk of lower birth weights and premature delivery. There is no evidence at this time of the sharply increased maternal mortality that was seen previously with both the 2003 SARS and 2012 MERS pandemics.
Keywords: coronavirus, COVID-19 in pregnancy, COVID-19 pregnancy outcomes, pregnancy outcomes, SARS-CoV-2
AJOG Global Reports at a Glance.
Why was this study conducted?
With the constant evolution of the COVID-19 pandemic, a periodic assessment of the available high-quality evidence is important in making informed decisions regarding the care of pregnant women infected with COVID-19.
Key findings
Like in previous systematic reviews, we found an increased risk of premature delivery and cesarean delivery rates in mothers infected with COVID-19. We did not find any evidence of the significant spike in maternal mortality that was seen with both the 2003 SARS and 2012 MERS coronavirus strains.
What does this add to what is known?
The large number of studies analyzed add strength to the notion that obstetricians may expect a higher incidence of preterm deliveries in mothers infected with COVID-19. It also adds strength to the consideration of respective changes in treatment plans such as antenatal steroid administration.
Introduction
The COVID-19 pandemic, which was caused by the 2019 novel coronavirus (2019-nCoV) (first isolated in China in December 2019), has grown to unprecedented proportions in modern times.1 Even now, the consequences of infection with COVID-19 in pregnant women are not fully understood. This is largely because of the shortage of sufficient evidence in this regard. Previous published articles, which scrutinized the effects of infection with earlier beta coronaviruses, showed that infected pregnant women were more susceptible to developing sepsis and acute respiratory distress syndrome. This warranted critical admission to the intensive care unit.2 Medical literature reveals that all-cause pneumonia has been linked to preterm labor, premature rupture of membranes, fetal growth restriction, and fetal death in addition to neonatal demise.3,4
The most recent large systematic review and meta-analysis on this topic, performed by Matar et al,5 concluded that the clinical manifestations of pregnant women who were infected with COVID-19 were similar to nonpregnant individuals who had this disease. Nonetheless, the authors of this study found that pregnant women who had confirmed COVID-19 had higher rates of cesarean deliveries and preterm births than the average reported statistics globally. One of the limitations of this review by Matar et al5 and another recent review by Kasraeian et al6 was the small sample size of the reported patients with 137 and 86 patients, respectively. A large cohort of studies regarding the impact of COVID-19 infection on pregnant women along with the effects of the virus on the fetus continues to be published. Consequently, we aimed to implement this comprehensive systematic review and meta-analysis to appraise the contemporary literature and dissect the effects of COVID-19 on pregnant women and their babies. We build on the previous literature and have included all the published quality data up to and including a publication date of June 3, 2021, with a total of 111 included studies totaling 42,754 infected pregnant patients.
Methods
We followed the MOOSE (Meta-analysis of Observational Studies in Epidemiology) statement guidelines during the preparation of this systematic review and meta-analysis.7 In addition, the reporting of this study was according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist.8
Search strategy and eligibility criteria
The relevant articles were retrieved from 5 major databases (PubMed, SCOPUS, MEDLINE, ClinicalTrials.gov, and Web of Science databases) from December 1, 2019 to June 3, 2021. A comprehensive search was done using the following search strategy: (“COVID-19” OR “SARS-CoV-2”) AND (“maternal outcomes” OR “neonatal outcomes” OR pregnan*). In addition, we performed a manual search of the references of the included articles. Two reviewers independently screened the titles and abstracts of the search results to define the initially eligible studies. Further full-text screening of the initially eligible studies was performed to determine the articles that would be finally included in this meta-analysis. Disagreements were settled by discussion, and the final decision was made by a third reviewer.
Inclusion and exclusion criteria
We included all prospective and retrospective cohort studies, case series, short communications, and case-control studies that reported data on the clinical characteristics and the maternal and neonatal outcomes of pregnant women with COVID-19. There were no restrictions on time or country of origin. Reviews, single case reports, non-English studies, expert opinions, letters to the editor, and studies without analyzable data were excluded from this study. The authors noted that some studies relative to our analyzed outcomes were excluded because they were published as a single case report or letter to the editor, when in fact, their subject matter could have qualified as a cohort study.
Data extraction
The extracted data included the first author, year of publication, study design, country, income, sample size, age of pregnant women, and COVID-19 infection confirmation method. Furthermore, we extracted the following outcomes of interest: (1) Maternal coexisting comorbidities including gestational diabetes and preeclampsia (2) Maternal delivery outcomes including either emergency or elective cesarean delivery, spontaneous vaginal delivery, preterm delivery (defined as before 37 weeks’ gestation,) and operative delivery, intensive care unit (ICU) admission, and the maternal mortality rate (3) Neonatal outcomes including low birthweight babies, premature delivery, neonatal birthweight, neonatal intensive care unit (NICU) admission, neonatal death, fetal death or stillbirth, and vertical transmission of SARS-CoV-2 infection. Two different investigators performed the data extraction in parallel to prevent errors. Discrepancies were then resolved by consensus. A third investigator was assigned to decide in the event that any discrepancies could not be resolved by the 2 extracting investigators.
Risk of bias assessment and strength of evidence
We assessed the quality of the included observational studies according to the quality assessment tools of the National Heart, Lung, and Blood Institute.9 We used both the tools of the observational cohort and case-control studies, which are composed of questions assessing the risk of bias and confounders. Each question was answered by “yes,” “no,” “not applicable,” “not reported,” or “cannot determine.” Then each study was given a score to guide the overall quality as either “poor,” “fair,” or “good.” In addition, the strength of evidence was evaluated by the Grading of Recommendations Assessment Development and Evaluation (GRADE) tool.10 A summary of the results of our risk of bias assessment can be found in supplemental Tables S1 and S2.
Statistical analysis
Comprehensive Meta-Analysis software version 3 was used for quantitative synthesis. Dichotomous events and no events were pooled as weighted proportions and odds ratios (OR) with 95% confidence intervals (CI), whereas the pooled rates of proportions were calculated through the Freeman–Tukey transformation meta-analysis of proportions using MedCalc (Version 15.0; MedCalc Software, Ostend, Belgium). For continuous outcomes, we used mean difference with 95% CIs and a random effects meta-analysis model. A P value <.05 was considered statistically significant. Heterogeneity among studies was assessed by visual inspection and using the I-square (I2) and chi-squared tests. Chi-square P values of <.1 or I2 >50% were considered as indicators of a significant heterogeneity. When heterogeneity was encountered, we changed from a fixed effect to a random effects model (when possible) to attempt to solve the heterogeneity. We also attempted to solve it by omitting 1 study from the analysis, also referred to as the “leave-on-out” method.
Results
Study selections
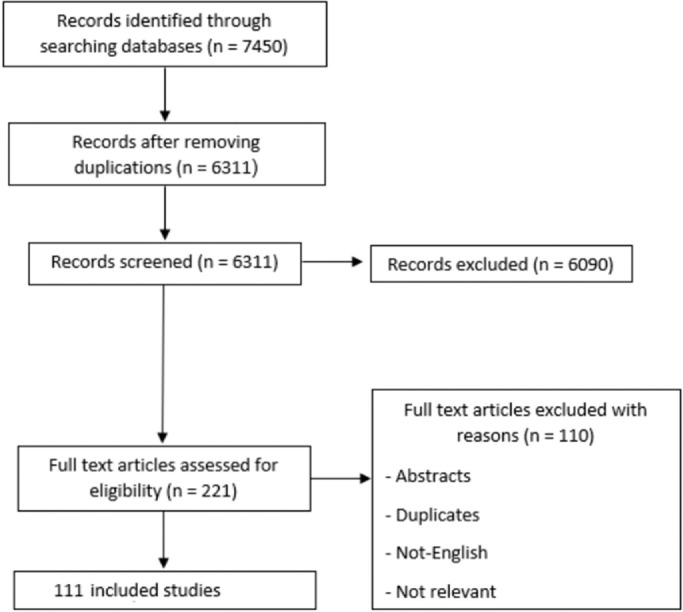
Database searching resulted in 7450 references. After duplicate removal by Endnote X8.0.1 (Build 1044) (Clarivate Analytics, London, United Kingdom), 6311 records were eligible for title and abstract screening. Thus, 221 reports were initially marked as eligible for inclusion. The full-text articles of these reports were examined, and 111 articles were included in the final systematic review and meta-analysis. A complete list of articles is included in (Appendix 1). The flow of data collection and screening process are shown in (Figure 1).
Figure 1.
PRISMA flow diagram
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Marchand. COVID-19 outcomes in pregnancy. Am J Obstet Gynecol Glob Rep 2022.
Baseline characteristics and strength of evidence
The summary and baseline characteristics of the included studies are shown in the (Table). Our systematic review included 111 studies that comprised a total of 42,754 infected pregnant women. The included studies varied in their design as prospective and retrospective cohort studies, case series, and case-control studies.
Table.
Summary and baseline characteristics of the included studies
| Study ID | Study design | Data source | Country | Setting | Income | Sample size | Mean age | COVID-19 confirmed by the following: | Main maternal and neonatal outcomes reported | Control group (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Abedzadeh-Kalahroudi 2021 | Cohort | Facility-based | Iran | The exposed group: Referral Hospital of Kashan University of Medical Sciences (Shahid Beheshti Hospital). The nonexposed group: Midwifery clinics to receive prenatal care. |
Middle-income | 150 (56 positive) | 31.6 (Exposed Group) | qRT-PCR, or based on clinical manifestations, laboratory findings, and positive findings on CT scan. |
C-delivery, preeclampsia, preterm labor, and fetal distress | Not applicable |
| Ahlberg 2020 | Registry-based | Sweden | Karolinska University Hospital, Stockholm | High-income | 759 (155 positive) | 32.1 (Positive)/ 32.0 (Negative) | RT-PCR | Preeclampsia, breastfeeding at discharge, gestational diabetes, preterm birth, induction of labor, epidural analgesia, mode of delivery, postpartum hemorrhage, 5-min Apgar score, large for gestational age, small for gestational age, major birth defect, and stillbirth | Not applicable | |
| Ajith 2021 | Retrospective | Registry-based | India | Tertiary care center (Referral center for 2 northern districts of Kerala) | Low-income | 350 COVID-19–positive pregnancies / 223 delivered | NA | Antigen test or RT-PCR | Stillbirth, mode of delivery, breastfeeding and rooming-in, and infected neonates | Not applicable |
| Anand 2020 | Cohort | Facility-based | India | Vardhman Mahavir Medical College & Safdarjung Hospital, New Delhi |
Low-income | 69 | 26.7 | RT-PCR OR (SARS-CoV 2 specific RdRp (RNA-dependent RNA polymerase) gene or Sarbeco subgenus ORF-1b-nsp14b gene) | Intrauterine death, neonatal infectivity, and viral load | Not applicable |
| Antoun 2020 | Prospective cohort | Facility-based | United Kingdom | University Hospitals of Birmingham | High-income | 23 | 29.3 | RT-PCR | Cesarean delivery, vaginal delivery, maternal mortality, preeclampsia, postpartum hemorrhage, preterm birth, ICU, birthweight, 5-min Apgar score <7 and vertical transmission. | Not applicable |
| Anuk 2021 | Prospective case-control | Population-based | Turkey | Ankara City Hospital | Middle-income | 70 | 30 (cases)/ 29 (controls) | RT-PCR | Maternal-fetal Doppler parameters | Not applicable |
| Bachani 2020 | Retrospective | Registry-based | India | Medical college affiliated tertiary care hospital | Low-income | 57 | 26.71 | qRT-PCR | Maternal mortality, neonatal infectivity, and disease's severity | Not applicable |
|
Badr 2020 |
Retrospective case-control |
Registry-based |
France and Belgium |
(1) Antoine Béclère, Clamart, Paris, France; (2) Bicêtre Hospital, Le Kremlin-Bicêtre, France; (3) Centre Hospitalier Sud Francilien, Corbeil-Essonnes, France; and (4) Brugmann University Hospital, Brussels, Belgium. |
High-income |
83 |
31.97 |
RT-PCR |
ICU |
Not applicable |
| Barbero 2020 | Retrospective Cohort | Registry-based | Spain | Tertiary care center, Hospital Universitario “12 de Octubre,” Madrid | High-income | 91 | 33.15 | NP swab or suggestive radiological findings | Pneumonia, hospitalization rate, ICU admission, COVID-19 severe forms, demographic characteristics, pregnancy-related conditions and presenting symptoms, rate of cesarean delivery, preterm birth, and mortality rates. | Not applicable |
| Blitz 2020 | Retrospective (Research Letters) | Registry-based | United States | large hospital system in New York State |
High-income | 82 | RT-PCR | ICU | Not applicable | |
| BRANDT 2020 | Case-control | Population-based | United States | Robert Wood Johnson University Hospital, a 139 regional perinatal center in New Brunswick, New Jersey |
High-income | 183 | 30.3 for the COVID-19 group, 30.9 for the control group | Quantitative PCR | Adverse maternal outcomes: Preeclampsia, venous thromboembolism, antepartum admission, maternal ICU admission, need for mechanical ventilation, supplemental oxygen, or maternal death. Adverse neonatal outcomes: respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, 5-min Apgar score <5, persistent category 2 fetal heart rate tracing despite intrauterine resuscitation, or neonatal death. |
Not applicable |
| Campbell 2020 | Retrospective | Registry-based | United States | 3 Yale New Haven Health hospitals in southern Connecticut |
High-income | 30 | RT-PCR | Cesarean delivery, preterm birth, vertical transmission, 5-min Apgar score <7, and birthweight | Not applicable | |
| Cheng 2020 | Retrospective | Registry-based | China | Renmin Hospital of Wuhan University | Middle-income | 31 | 29 | RT-PCR | Neonatal mortality, NICU, preterm birth, maternal mortality, ICU, vertical transmission, fetal Stillbirth, 5-min Apgar score <7, birthweight <2500 g, and birthweight | Not applicable |
| Cohen 2020 | Results of a French national survey | Population-based | France | internet platform | High-income | 88 | 31 | RT-PCR, Serology or lung CT-scanner | Cesarean delivery and Gestational diabetes. | Not applicable |
| Cojocaru 2020 | Quality improvement | Facility-based | United States | University of Maryland Medical System | High-income | 86 | 30.4 | PCR | Maternal bonding, ICU admission, transmission | Not applicable |
| Cribiù 2020 | Cohort | Facility-based | Spain | Fondazione IRCCS Ca’ Granda — Ospedale Maggiore Policlinico, Milan, and Department of Pathology, University of Basel |
High-income | 37 (21 positive) | 31.3 (cases)/ 35 (controls) | PCR | Mode of delivery, indications for labor induction, and neonatal outcomes | Not applicable |
| Cruz-Lemini 2021 | Prospective | Facility-based | Spain | by the Spanish Obstetric Emergency group in 42 hospitals | High-income | 604 (174 positive asymptomatic) | 32.6 (cases)/ 33.2 (controls) | PCR | Onset of labor, type of delivery, Preeclampsia, thrombotic risk, perinatal complications, neonatal data, and causes of NICU admission | Not applicable |
| Di Guardo 2021 | Retrospective cohort | Registry-based | Italy | Department of Gynecology and Obstetrics of 2 tertiary referral hospitals |
High-income | 145 | 31.5 | qRT- PCR |
Maternal death, neonatal death, vertical transmission, and preterm birth. | Not applicable |
| Di Mascio 2020 | Retrospective cohort | Registry-based | 22 different countries in Europe, United States, South America, Asia and Australia | 73 centers | 388 | 32.2 | RT-PCR | Maternal mortality and morbidity, including ICU admission, mechanical ventilation use, and death. | Not applicable | |
| Dıaz-Corvillon 2020 | Cross-sectional study | Population-based | Chile | Obstetrics & Gynecology Department of Clínica Dávila, Santiago | Middle-income | 37 | 29.9 | RT-PCR | Cesarean delivery, Instrumental delivery, neonatal mortality, preterm birth, NICU, fetal stillbirth, birthweight, birthweight <2500 g, 5-min Apgar score <7, and vertical transmission | 546 |
| Dumitriu 2020 | Retrospective cohort | Registry-based | United States | NewYork–Presbyterian Morgan Stanley Chil- dren's Hospital or NewYork–Presbyterian Allen Hospital |
High-income | 100 | 28.8 | Cobas or Xpert Xpress PCR (except for 1 –> symptomatic but negative) | Neonatal infectivity, maternal COVID-19 status, and neonatal characteristics and clinical courses | Not applicable |
| Facchetti 2020 | Retrospective | Registry-based | Italy | Brescia Spedali Civili Hospital | High-income | 15 | 35.1 | RT-PCR | Induction of labor, neonatal mortality, NICU, preterm birth, fetal Stillbirth, gestational diabetes, 5-min Apgar score <7, birthweight, birthweight <2500 g and vertical transmission. | Not applicable |
| Farghaly 2020 | Retrospective Cohort | Registry-based | United States | Brookdale Hospital Medical Center, New York |
High-income | 15 | 33.4 | RT-PCR | Cesarean delivery, vaginal delivery, NICU, preterm birth, birthweight and vertical transmission. | 64 |
| Flaherman 2020 | Prospective cohort | Registry-based | United States | Pregnancy Coronavirus Outcomes Registry (PRIORITY) |
High-income | 179 | 31.5 | RT-PCR | Vaginal delivery, NICU, ICU, preterm birth, birthweight and vertical transmission. | 84 |
| Gale 2020 | Prospective cohort | Registry-based | United Kingdom | British Paediatric Surveillance Unit | High-income | 66 infected neonates | - | NA | Gestational age at delivery, mode of transmission, and disease's severity | Not applicable |
| Gaspar 2021 | Retrospective | Registry-based | Portugal | Maternity of a Central Hospital in the Center Region |
High-income | 12 | 35.58 | RT-PCR | Condition's severity, maternal mortality, spontaneous abortions, preterm births, cesarean sections, and vertical transmission | Not applicable |
| Ghema 2021 | Descriptive | Facility-based | Morocco | neonatal ICU of Harouchi Mother and Child Hospital in Casablanca | Low-income | 30 neonates | - | PCR | Maternal symptoms, ICU admission, median gestational age at delivery, and neonatal infectivity | Not applicable |
| Goyal 2020 | Prospective observational | Facility-based | India | Department of Obstetrics and Gynecology at All India Institute of Medical Sciences, Jodhpur |
Low-income | 633 (COVID−19 period)/ 32 (Infected)/ 1116 (pre-COVID−19) | - | RT-PCR | Institutional deliveries, ICU admission, antenatal visits, and maternal and fetal outcomes in COVID positive. | Not applicable |
| Gulersen 2020 | Retrospective Cohort | Registry-based | United States | Long Island Jewish Medical Center, Northwell Health, Queens, New Year |
High-income | 50 | 29.3 | RT-PCR | Vaginal delivery, gestational diabetes, and birthweight | 50 |
| Handley 2020 | Cohort | Registry-based | United States | (GeoBirth) From 2 Penn Medicine hospitals, Philadelphia |
High-income | 8867 (Total)/ 2992 (Pandemic period)/ 86 (Infected) | — | — | Stillbirth, overall preterm birth, spontaneous preterm birth, iatrogenic preterm birth |
Not applicable |
| Hcinia 2021 | Prospective cohort | Facility-based | France | Department of Obstetrics and Gynecology of the Centre Hospitalier de L'Ouest Guyanais (referral center of western French Guiana) |
High-income | 507 (137 positive) | 25.7 (positive)/ 26.3 (negative) | PCR | Disease's severity, maternal death, ICU admission and oxygen support (noninvasive ventilation, endotracheal intubation), mode of delivery, preterm delivery, acute fetal distress, postpartum hemorrhage and transfusion, late miscarriages, stillbirth, neonatal death, NICU admission, respiratory distress, seizures, Apgar score 7 at 1 min, umbilical venous lactate ≥5 mmol/L at birth, and low birthweight |
Not applicable |
| He 2020 | Retrospective | Registry-based | China | Tongji Hospital affiliated to Huazhong University of Science & Technology, Wuhan |
Middle-income | 22 neonates | - | Based on the "New Coronavirus Pneumonia Prevention and Control Program 7th Edition." |
Neonatal clinical characteristics, routine blood test, liver and kidney functions, and SARS-COV2 antibodies | Not applicable |
| HuiYang 2020 | Retrospective | Registry-based | China | Patients in Wuhan, China | Middle-income | 27 | 29.91 | RT-PCR or clinically confirmed | Cesarean delivery, vaginal delivery, neonatal mortality, maternal mortality, gestational diabetes, preeclampsia, preterm birth, birthweight, birthweight <2500 g, neonatal asphyxia and vertical transmission. | Not applicable |
| Hui Yang 2020 | Observation | Registry-based | China | Patients in Wuhan, China | Middle-income | 13 | 30.2 | RT-PCR | Cesarean delivery, vaginal delivery, NICU and vertical transmission. | 42 |
| Jenabi 2020 | Case-control | Population-based | Iran | Hospitals of Hamadan Province | Middle-income | 90 | 29.47 (Symptomatic)/ 28.78 (Asymptomatic) | rRT-PCR | C-section, low birthweight, preterm labor, preeclampsia, hospitalization, and neonatal death | Not applicable |
| Knight 2020 | Prospective cohort | Population-based | United Kingdom | UK Obstetric Surveillance System | High-income | 472 | RT-PCR | Cesarean delivery, vaginal delivery, neonatal mortality, NICU, preterm birth, maternal mortality, ICU, vertical transmission, fetal Stillbirth and Iatrogenic preterm birth. | Not applicable | |
| Leon-Abarca 2020 | Retrospective analysis | Registry-based | Mexico | Patients across Mexico | Middle-income | 3434 | RT-PCR | ICU | Not applicable | |
| Liu 2020 | Retrospective case-control | Registry-based | China | Two centers in China | Middle-income | 21 | 31 | RT-PCR | ICU, 5-min Apgar score <7, preterm birth | Not applicable |
| Llorca 2021 | Cohort | Facility-based | Spain | University Hospital Marqués de Valdecilla (HUMV), Santander |
High-income | 1167 (14 were positive) | 34 | RT-PCR and ELISA | Mode of delivery, COVID-19 incidence, and pathology in pregnancy | Not applicable |
| Lokken 2021 | Retrospective cohort | Registry-based | United States | 22 large hospitals, and 13 clinic systems providing prenatal care in Washington State |
High-income | 240 | 28.7 | PCR | Disease severity, hospitalization because of COVID-19, ICU admission, maternal mortality, final pregnancy outcome, COVID-19 at final outcome, and recovery | Not applicable |
| Lopian 2020 | Cohort | Facility-based | Israel | Mayanei Hayeshua Medical Center (MHMC) in Bnei Brak |
High-income | 21 | 30 | RT-PCR | ICU admission, mortality, mode of delivery, Apgar score, and vertical transmission | Not applicable |
| Lu Zhang 2020 | Retrospective observational study | Registry-based | China | Renmin Hospital of Wuhan University | Middle-income | 18 | 29.11 | RT-PCR or clinically confirmed | Cesarean delivery, vaginal delivery, gestational diabetes, preeclampsia, birthweight, preterm birth, and vertical transmission. | Not applicable |
| Luming Xu 2020 | Retrospective observational study | Registry-based | China | Wuhan Union Hospital | Middle-income | 5 | 28.8 | RT-PCR | Cesarean delivery, vaginal delivery, neonatal mortality, preterm birth, birthweight, birthweight <2500 g, Neonatal Mortality, 5-min Apgar score <7 and vertical transmission. | Not applicable |
| Mahajan 2021 | Retrospective | Registry-based | India | dedicated Covid-19 Hospital in Mumbai | Low-income | 879 | 26.97 | PCR | Twinning rate, term deliveries, spontaneous abortions, and hypertensive disorders of pregnancy | Not applicable |
| Martinez-Perez 2021 | Prospective cohort | Facility-based | Spain | Spanish Obstetric Emergency group in 45 hospitals |
High-income | 246 | 32.6 | RT-PCR | Cesarean delivery, vaginal delivery, Instrumental delivery, neonatal mortality, NICU, preterm birth, gestational diabetes, Postpartum hemorrhage, maternal mortality, ICU, vertical transmission, fetal Stillbirth and 5-min Apgar score <7. | 763 |
| Martinez-Portilla | Prospective cohort | Facility-based | Mexico | 475 monitoring hospitals dedicated to COVID-19 and located in all 32 states of Mexico |
Middle-income | 5183 pregnant and 175,998 nonpregnant | 28.5 (pregnant) | RT-PCR | Death, pneumonia, intubation, and ICU admission |
Not applicable |
| Maru 2020 | Retrospective cross-sectional study | Registry-based | United States | L&D Unit at Elmhurst 106 Hospital |
High-income | 46 | 30.2 | Cepheid rapid PCR | Cesarean delivery, vaginal delivery, preterm birth, and gestational diabetes | 78 |
| Mattar 2020 | Prospective cohort | Facility-based | Singapore | The National University Hospital, KK Women's and Children's Hospital (KKH), Singapore General Hospital |
High-income | 16 | 29.75 | RT-PCR | Severe disease, pregnancy loss, and vertical and horizontal transmission | Not applicable |
| Mattern 2021 | Prospective | Facility-based | France | The Antoine Be ´clère Hospital maternity ward (Paris area, France) |
High-income | 249 (20 Immunoglobulin G-positive) | 32.83 (IgG-positive) | Serology test | Gestational age at delivery, birthweight, and infected neonate | Not applicable |
| Molina 2020 | Retrospective | Registry-based | Spain | Mancha-Centro Hospital in Castile-La Mancha, Spain | High-income | 20 | 34.9 | Qualitative serologic positive antibody test and/or RT-PCR | Clinical characteristics, management, treatment, and obstetrical and neonatal outcomes | Not applicable |
| Moreno 2020 | Retrospective | Registry-based | United States | Flushing Hospital Medical Centre or Jamaica Hospital Medical Centre (JHMC) | High-income | 19 | 31.7 | rRT-PCR | Vertical transmission of COVID-19 | Not applicable |
| Nambair 2020 | Retrospective Cohort | Registry-based | India | Tertiary Referral Center in South India | Low-income | 350 | NA | RT-PCR | Mode of delivery, postpartum hemorrhage, NICU, infected neonates, and breastfeeding | Not applicable |
| Nayak 2020 | Retrospective | Registry-based | India | Department of Obstetrics and Gynaecology at Ter- tiary Care Hospital attached to a Medical College (Central Mumbai) |
Low-income | 977 (141 positive) | NA | PCR | Mode of delivery, Apgar score, and vertical transmission | Not applicable |
| Ochiai 2020 | Retrospective | Registry-based | Japan | Tertiary center, Keio University Hospital (in central Tokyo) |
High-income | 3 | 32 | RT-PCR or clinically confirmed | Cesarean delivery, vaginal delivery, NICU, preterm birth, birthweight, 5-min Apgar score <7, birthweight <2500 g, vertical transmission, and gestational diabetes | Not applicable |
| Oncel 2020 | Cohort | Facility-based | Turkey | 34 NICUs in Turkey | Middle-income | 125 | RT-PCR | Not applicable | ||
| Onwuzurike 2020 | Retrospective | Registry-based | United States | Department of Obstetrics & Gynecology, Brigham and Women's Hospital, Boston, MA. | High-income | 44 | 29.6 | PCR | Disease's severity, hospitalization, indication for delivery, pregnancy and neonatal outcomes, and postpartum care | Not applicable |
| Ozsurmeli 2021 | Retrospective cohort | Registry-based | Turkey | Istanbul Medeniyet University Göztepe Training and Research Hospital and University of Health Sciences Derince Training and Research Hospital | Middle-income | 24 | 26.9 | qRT-PCR | Clinical symptoms, mode of delivery, laboratory results, and disease's severity. | Not applicable |
| Pachtman 2020 | Retrospective | Registry-based | United States | Seven hospitals within Northwell Health, New York state | High-income | 20 | PCR | Pregnancy complications, clinical symptoms, and cardiac enzymes | Not applicable | |
| Patberg 2020 | Retrospective cohort | Registry-based | United States | NYU Winthrop Hospital | High-income | 133 (77 positive) | 29.9 (positive)/ 32.3 (negative) | PCR | Fetal vascular malperfusion abnormalities, mode of delivery, pregnancy complications, and neonatal infection. | Not applicable |
| Pecks 2020 | Retrospective | Registry-based | Germany | 121 German hospitals and from Kepler University Hospital Linz, Austria |
High-income | 247 | NA | NA | Outcomes in pregnant women regarding COVID-19, obstetrical pregnancy outcome, mode of delivery, gestational age, and neonatal outcomes | Not applicable |
| Peng 2020 | Retrospective | Registry-based | China | Hubei Province | Middle-income | 24 | 29.8 | RT-PCR | Cesarean delivery, neonatal mortality, preterm birth, gestational diabetes, birthweight, and vertical transmission | 21 |
| Pereira 2020 | Retrospective | Facility-based | Spain | Puerta de Hierro University Hospital Madrid, Spain |
High-income | 60 | 34 | RT-PCR | Clinical symptoms, disease's severity, mode of delivery, treatment, and lab results | Not applicable |
| Pierce-Williams 2020 | Cohort | Facility-based | United States | 12 US institutions | High-income | 64 | 33.2 | Laboratory testing meeting criteria for diagnosis of severe or critical COVID-19 as defined by the "Chinese Center for Disease Control and Prevention." |
Median duration from hospital admission to discharge, need for supplemental oxygen, intubation, cardiomyopathy, cardiac arrest, death, and timing of delivery. | Not applicable |
| Pineles 2020 | Retrospective Cohort | Registry-based | United States | A community hospital in Houston, Texas | High-income | 77 | RT-PCR | Cesarean delivery, neonatal mortality, preterm birth, NICU, fetal stillbirth, birthweight, and vertical transmission. | 858 | |
| Pirjani 2020 | Prospective cohort | Facility-based | Iran | Arash Hospital in Tehran, Iran | Middle-income | 199 (66 positive) | 30.97 (positive)/ 28.79 (negative) | RT-PCR and CT | Not applicable | |
| Prabhu et al,16 2020 | Prospective cohort | Facility-based | United States | NewYork Presbyterian-Weill Cornell Medical Center, New York Presbyterian-Lower Manhattan Hospital and New York Presbyterian-Queens, New York |
High-income | 70 | 31.24 | RT-PCR | Cesarean delivery, vaginal delivery, preterm birth, live birth, ICU admission, gestational diabetes, preeclampsia, vertical transmission, and fetal stillbirth, NICU, birthweight, and severe neonatal asphyxia. | 605 |
| Pu Yang 2020 | Retrospective | Registry-based | China | Zhongnan Hospital of Wuhan University | Middle-income | 7 | — | RT-PCR | Cesarean delivery, vaginal delivery, NICU, preterm birth, birthweight, neonatal asphyxia, and vertical transmission. | Not applicable |
| Qiancheng 2020 | Retrospective | Registry-based | China | The Central Hospital of Wuhan |
Middle-income | 28 | 30 | RT-PCR | Cesarean delivery, vaginal delivery, preterm birth, ICU admission, gestational diabetes, vertical transmission, fetal stillbirth, NICU, birthweight, birthweight <2500 g, severe neonatal asphyxia and Neonatal Mortality. | Not applicable |
| Qing-Lei Zeng 2020 | Retrospective | Registry-based | China | 12 centers in Henan and Shaanxi Provinces, China |
Middle-income | 2 | — | RT-PCR | Cesarean delivery, vaginal delivery, neonatal mortality, preterm birth, maternal mortality, vertical transmission | Not applicable |
| Reale 2020 | Prospective cohort | Facility-based | United States | Four large hospitals: 2 academic medi- cal centers and 2 community hospitals |
High-income | 93 | 29.6 | RT-PCR | Cesarean delivery and gestational diabetes | 2852 |
| Ríos-Silva 2020 | Retrospective Cohort | Registry-based | Mexico | The open national database of COVID-19 [12] from the Ministry of Health of Mexico. |
Middle-income | 29 | 448 | RT-PCR | ICU admission and maternal mortality | Not applicable |
| Rizzo 2021 | Prospective case-control | Population-based | Italy | The Division of Maternal Fetal Medicine, Università di Roma Tor Vergata, Italy | High-income | 49 | 30.4 | RT-PCR | Birthweight | 98 |
| Rong Yang 2020 | Retrospective Cohort | Registry-based | China | The Maternal and Child Health Information Management System of Wuhan (MCHIMS) |
Middle-income | 65 | _ | RT-PCR | Cesarean delivery, vaginal delivery, gestational diabetes, preeclampsia, preterm birth, birthweight, neonatal asphyxia, and vertical transmission | Not applicable |
| Sahin 2020 | Prospective cohort | Facility-based | Turkey | Turkish Ministry of Health Ankara City Hospital |
Middle-income | 29 | 26.38 | RT-PCR | Cesarean delivery, vaginal delivery, preeclampsia, preterm delivery, ICU admission, NICU, vertical transmission, and birthweight | 8 |
| Sahin 2020 "update" | Prospective cohort | Facility-based | Turkey | Turkish Ministry of Health Ankara City Hospital |
Middle-income | 533 | 28.04 | RT-PCR | Cesarean delivery, vaginal delivery, preterm delivery, ICU admission, gestational diabetes, preeclampsia, maternal mortality, NICU, vertical transmission, and birthweight | Not applicable |
| Sakowicz 2020 | Retrospective cohort | Registry-based | United States | Northwestern Memorial Hospital or affiliated outpatient clinics |
High-income | 101 | 30 | PCR | Gestational diabetes. | 1317 |
| Salvatore 2020 | Observation cohort | Facility-based | United States | New York Presbyterian—Komansky Children's Hospital, Weill Cornell Medicine, New York Presbyterian—Lower Manhattan Hospital, and New York Presbyterian—Queens |
High-income | 78 | _ | RT-PCR | Cesarean delivery, vaginal delivery, NICU, preterm delivery, birthweight, birthweight <2500 g, and vertical transmission | Not applicable |
| Samadi 2021 | Cross-sectional study | Population-based | Iran | Forghani Hospital in Qom, a tertiary referral hospital | Middle-income | 258 | 29.5 | RT-PCR or lung CT scan or both | Cesarean delivery, vaginal delivery, ICU, maternal mortality, gestational diabetes, and preeclampsia | Not applicable |
| San-juan 2020 | Retrospective cohort | Registry-based | Spain | Department of Obstetrics of the University Hospital “12 de Octubre” (Madrid, Spain) |
High-income | 32 | 32 | RT-PCR | Cesarean delivery, vaginal delivery, ICU, gestational diabetes, preterm delivery, birthweight, 5-min Apgar score <7, birthweight, and vertical transmission. | Not applicable |
| Santana 2021 | Retrospective cohort | Registry-based | Spain | University Hospital La Paz, Madrid, Spain | High-income | 29 | 31.9 | RT-PCR | Cesarean delivery, vaginal delivery, preterm delivery, ICU admission, maternal mortality, gestational diabetes, vertical transmission, 5-min Apgar score <7, and birthweight | Not applicable |
| Santhosh 2021 | Retrospective | Registry-based | Oman | A tertiary care center in Muscat, Oman | High-income | 60 | 32 | RT-PCR | Cesarean delivery, vaginal delivery, instrumental delivery, ICU, gestational diabetes, preeclampsia, preterm delivery, birthweight, birthweight <2500 g, fetal stillbirth, vertical transmission, and postpartum hemorrhage | Not applicable |
| Savasi 2020 | Prospective cohort | Prospective cohort | Italy | 12 maternity hospitals in Northern Italy including L. Sacco (Milan), Mangi- agalli (Milan), S. Gerardo MBBM Foundation (Mon- za), Papa Giovanni XXIII (Bergamo), and San Matteo (Pavia) as hub maternity hospitals, and Hospitals of Padua, Florence, Lecco, Trento, Modena, Seriate and Piacenza. |
High-income | 77 | 32 | RT-PCR | Cesarean delivery, vaginal delivery, ICU, NICU, preterm delivery, birthweight, vertical transmission | Not applicable |
| Savirón-Cornudella 2020 | Retrospective cohort | Registry-based | Spain | The Hospital Universitario General de Villalba, located in the North of Madrid |
High-income | 6 | 27.83 | RT-PCR | Cesarean delivery, vaginal delivery, NICU, birthweight, and vertical transmission. | Not applicable |
| Savirón-Cornudella 2020 | Retrospective cohort | Registry-based | Spain | The Villalba General University Hospital, Madrid and the Miguel Servet University Hospital, Zaragoza, Spain. | High-income | 22 | 29.2 | RT-PCR | Cesarean delivery, vaginal delivery, instrumental delivery, NICU, gestational diabetes, postpartum hemorrhage, preterm delivery, birthweight, 5-min Apgar score <7, and vertical transmission. | 1189 |
| Schwartz,13 2020 | Retrospective cohort | Registry-based | Iran | Ten hospitals in different cities throughout Iran |
Middle-income | 22 neonates | Cesarean delivery, preterm delivery, birthweight, birthweight <2500 g, and vertical transmission. | Not applicable | ||
| Sherer 2020 | Retrospective cohort | Registry-based | United States | Johns Hopkins Hospital | High-income | 22 | 27 | RT-PCR | Cesarean delivery, vaginal delivery, NICU | 11 |
| Shmakov 2020 | Prospective observational study | Registry-based | Russia | The National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russia Federation |
Middle-income | 66 | 30.3 | RT-PCR | Cesarean delivery, vaginal delivery, instrumental delivery, ICU, preterm delivery, birthweight, vertical transmission, and maternal mortality | Not applicable |
| Singh 2021 | Observational study | Registry-based | India | Tata Main Hospital, Jamshedpur, a tertiary care hospital in Eastern India |
Low-income | 132 | 27.5 | RT-PCR | Cesarean delivery, vaginal delivery, instrumental delivery, postpartum hemorrhage, NICU, preterm delivery, birthweight, fetal stillbirth, neonatal mortality, and vertical transmission. | Not applicable |
| Smithgall 2020 | Retrospective | Registry-based | United States | Academic hospital, Columbia University Irving Medical Center in New York City | High-income | 51 | 32.3 | RT-PCR | Cesarean delivery, vaginal delivery, neonatal mortality, preterm birth, 5-min Apgar score <7, and vertical transmission. | 25 |
| Soffer 2021 | Retrospective cohort | Registry-based | United States | Large academic medical center serving patients from multiple communities |
High-income | 67 | 31 | RT-PCR | ICU and gestational diabetes | Not applicable |
| Soto-Torres 2020 | Retrospective case-control | Registry-based | United States | The Maternal-Fetal Medicine Division of the University of Texas McGovern Medical School Department of Obstetrics and Gynecology |
High-income | 106 | 28 | RT-PCR | Cesarean delivery, vaginal delivery, preeclampsia, NICU, preterm delivery, fetal stillbirth, and birthweight | 103 |
| Suyuthi 2020 | Descriptive study | Registry-based | Indonesia | Dr Wahidin Sudirohusodo Hospital | Middle-income | 26 | _ | RT-PCR | Cesarean delivery, vaginal delivery, maternal mortality, gestational diabetes, preeclampsia, neonatal mortality, neonatal asphyxia, vertical transmission | Not applicable |
| Tug 2020 | Retrospective study | Registry-based | Turkey | Four tertiary centers (Şehit Prof Dr İlhan Varank Training and Research Hospital, İstanbul; Kartal Dr Lütfi Kırdar Training and Research Hospital, İstanbul; Darıca Farabi Training and Research Hospital, Kocaeli; Medeniyet University Hospital, İstanbul) |
Middle-income | 188 | 31 | RT-PCR (8 confirmed with imaging studies only) | Cesarean delivery, vaginal delivery, ICU, preterm delivery, gestational diabetes, preeclampsia, and vertical transmission. | Not applicable |
| Villalaın 2020 | Retrospective cohort | Registry-based | Spain | Hospital Universitario 12 de Octubre, a large teaching hospital in the south of Madrid |
High-income | 673 | 32 | RT-PCR | Cesarean delivery, vaginal delivery, NICU, fetal stillbirth, gestational diabetes, preterm delivery, birthweight, 5-min Apgar score <7, and vertical transmission | Not applicable |
| Vintzileos 2020 | Retrospective cohort | Registry-based | United States | The NYU Winthrop Hospital of the NYU Langone Health System; |
High-income | 32 | 31 | RT-PCR | Vertical transmission | Not applicable |
| Vivanti 2020 | Retrospective | Registry-based | France | Four tertiary referral obstetrical units in the Paris metropolitan area included in the study were Antoine Béclère, Clamart; Bicêtre Hospital, Le Kremlin Bicêtre; Louis-Mourier, Colombes; and Centre Hospitalier SudFrancilien, Evry |
High-income | 100 | 33.1 | RT-PCR (1 confirmed with imaging studies only) | Cesarean delivery, vaginal delivery, induction of labor, premature delivery, neonatal mortality, maternal mortality, preeclampsia, ICU, fetal death/stillbirth, NICU, and vertical transmission. | Not applicable |
| Vizheh 2021 | Retrospective cohort | Registry-based | Iran | Three hospitals—Arash, Imam Khomeini, and Shariati |
Middle-income | 110 | 32.02 | RT-PCR | Cesarean delivery, vaginal delivery, preterm delivery, neonatal mortality, maternal mortality, ICU, gestational diabetes, preeclampsia, NICU, birthweight, and vertical transmission. | Not applicable |
| Wang 2020 | Retrospective | Registry-based | China | The Central Hospital of Wuhan, China | High-income | 30 | 29.9 | RT-PCR or imaging studies | Cesarean delivery and vaginal delivery. | Not applicable |
| Wang 2020 | Retrospective cohort | Registry-based | United States | Boston Medical Center |
High-income | 53 | 29.8 | RT-PCR | Cesarean delivery, vaginal delivery, preeclampsia, induction of labor, and preterm birth | 760 |
| Wei 2020 | Retrospective | China | Tongji Hospital, Wuhan, China | Middle-income | 17 | 33.3 | RT-PCR | ICU and maternal mortality. | Not applicable | |
| Wei Liu 2020 | Retrospective | Registry-based | China | Tongji Hospital and HuangShi Maternal and Child Healthcare Hospital |
Middle-income | 15 | 32 | RT-PCR | Cesarean delivery, vaginal delivery, gestational diabetes, postpartum hemorrhage, NICU, preterm birth, birthweight, and vertical transmission. | 16 |
| Wu 2020 | Retrospective | Registry-based | China | Renmin Hospital, Wuhan Uni- versity, and Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology |
Middle-income | 29 | 29.59 | RT-PCR or chest CT scan | Cesarean delivery, vaginal delivery, preterm birth, NICU, gestational diabetes, and postpartum hemorrhage | Not applicable |
| Xu 2020 | Retrospective observational study | Registry-based | China | The west campus of Union hospital | Middle-income | 34 | 30 | RT-PCR | Cesarean delivery, vaginal delivery, gestational diabetes, preeclampsia, neonatal mortality, fetal stillbirth, NICU, severe neonatal asphyxia, and vertical transmission. | Not applicable |
| Yan 2020 | Retrospective | Registry-based | China | 25 hospitals in China | Middle-income | 116 | 30.8 | RT-PCR or clinically confirmed | Cesarean delivery, vaginal delivery, gestational diabetes, preeclampsia, neonatal mortality, fetal stillbirth, preterm birth, birthweight, neonatal mortality, maternal mortality, NICU, ICU, neonatal asphyxia, and vertical transmission. | Not applicable |
| Yao 2021 | Retrospective cohort | Registry-based | United States | Department of Obstetrics and Gynecology, Loma Linda University |
High-income | 50 | 28.86 | RT-PCR | Preterm birth | Not applicable |
| Yazihan 2020 | Prospective case-control | Population-based | Turkey | Ankara City Hospital | Middle-income | 95 | 29 | RT-PCR | Preterm birth, gestational diabetes and preeclampsia. | 92 |
| Yin 2020 | Retrospective cohort | Registry-based | China | Wuhan Union and Tongji hospitals of Huazhong University of Science and Technology. |
Middle-income | 31 | 31 | RT-PCR | Cesarean delivery, vaginal delivery, neonatal mortality, preterm birth, vertical transmission, fetal stillbirth, 5-min Apgar score <7, birthweight <2500 g, and birthweight | Not applicable |
| Yingchun Zeng 2020 | Retrospective cohort | Registry-based | China | Wuhan Union Hospital | Middle-income | 14 | 31 | RT-PCR | Cesarean delivery, vaginal delivery, preterm birth, maternal mortality, vertical transmission | Not applicable |
| Yu 2020 | Retrospective descriptive | Registry-based | China | Tongji hospital | Middle-income | 7 | 31.75 | RT-PCR | Cesarean delivery, vaginal delivery, neonatal mortality, fetal stillbirth, preterm birth, birthweight, maternal mortality, vertical transmission | Not applicable |
| Zambrano 2020 | Report | Population-based | United States | Women across the United States | High-income | 409,462 (23,434 infected pregnant & 386,028 nonpregnant) | NA | Laboratory-confirmed | Signs and symptoms of COVID-19, ICU admission, and death | Not applicable |
| Zou 2020 | Retrospective analysis | Registry-based | China | Tongji Hospital in Wuhan |
Middle-income | 6 | 31 | RT-PCR | Cesarean delivery, 5-min Apgar score <7, neonatal mortality, maternal mortality, vertical transmission | Not applicable |
CT, computerized tomography; ICU, intensive care unit; IgG, immunoglobulin G; NICU, neonatal intensive care unit; NYU, New York University; OR, odds ratio; qRT-PCR, real time quantitative reverse transcription-polymerase chain reaction; RT-PCR, reverse transcription-polymerase chain reaction.
Marchand. COVID-19 outcomes in pregnancy. Am J Obstet Gynecol Glob Rep 2022.
Regarding the cohort and population-based studies, 43 studies were of poor quality, 41 were of fair quality, and the other 19 were of good quality, according to the National Institutes of Health (NIH) quality assessment tool for observational cohort studies. However, according to the NIH quality assessment tool for observational case-control studies, only 1 study was of poor quality, 3 were of fair quality, and the other 4 were of good quality. As for publication bias, most of our constructed funnel plots were asymmetrical, and the further Egger tests were significant (Appendix 2). However, the outcomes of spontaneous vaginal delivery and cesarean delivery were symmetrical with no small-study effects. Supplemental Table S1 shows the detailed risk of bias assessment for cohort studies, whereas Supplemental Table S2 shows detailed risk of bias for case-control studies.
Maternal outcomes
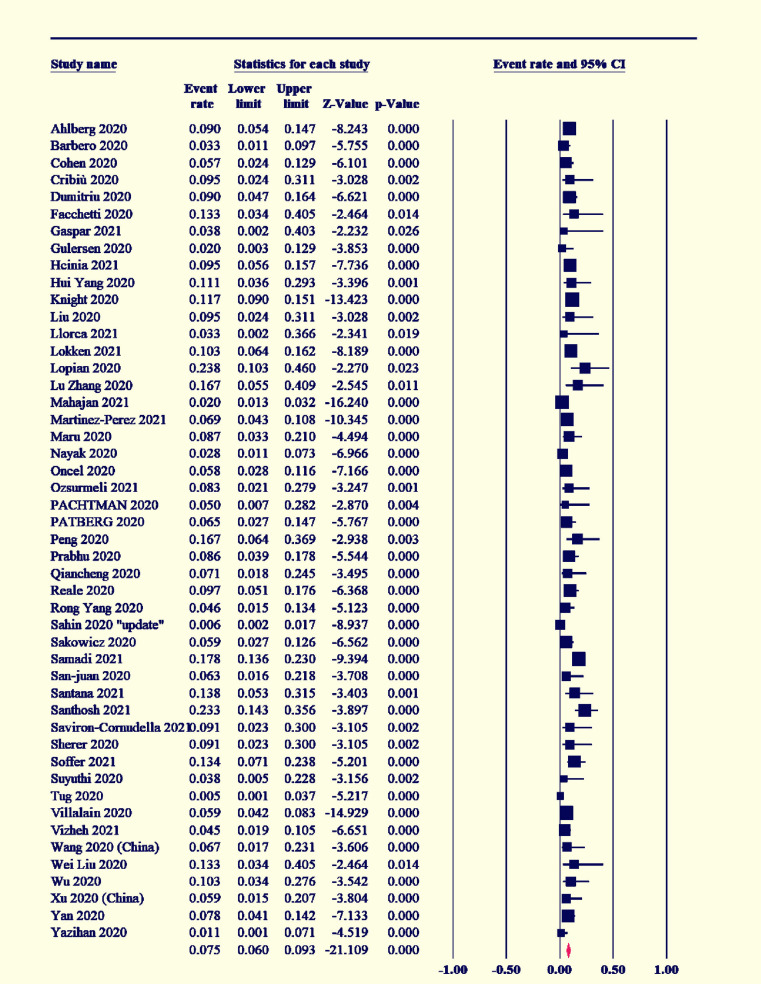
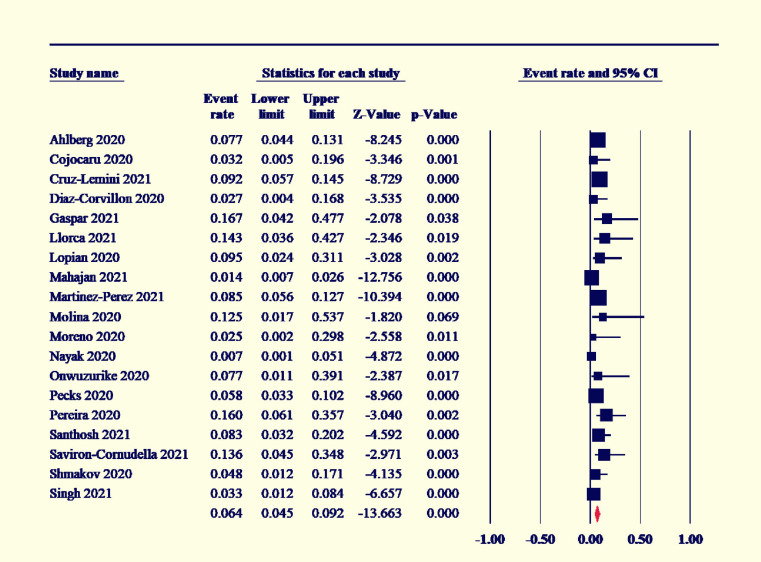
Among the COVID-19-positive women, 7.5% had gestational diabetes (95% CI [6–9.3]; I2˃50%) (Figure 2), and preeclampsia existed in 7% (95% CI [5.5–8.9]; I2˃50%) (Figure 3). The maternal mortality rate was 1.2% (95% CI [1–1.4]; I2<50%) (Figure 4), and 4.6% of COVID-19-positive women were admitted to the ICU (95% CI [3.4–6.2]; I2<50%) (Figure 5).
Figure 2.
Forest plot of event rate with 95% CI for gestational diabetes mellitus
CI, confidence interval.
Marchand. COVID-19 outcomes in pregnancy. Am J Obstet Gynecol Glob Rep 2022.
Figure 3.
Forest plot of event rate with 95% CI for preeclampsia
CI, confidence interval.
Marchand. COVID-19 outcomes in pregnancy. Am J Obstet Gynecol Glob Rep 2022.
Figure 4.
Forest plot of event rate with 95% CI for the maternal mortality rate
CI, confidence interval.
Marchand. COVID-19 outcomes in pregnancy. Am J Obstet Gynecol Glob Rep 2022.
Figure 5.
Forest plot of event rate with 95% CI for the maternal ICU admission rate
CI, confidence interval; ICU, intensive care unit.
Marchand. COVID-19 outcomes in pregnancy. Am J Obstet Gynecol Glob Rep 2022.
Delivery outcomes
Among the COVID-19-positive pregnant women, 53.2% had an emergency, indicated, or elective cesarean delivery (95% CI [48–58.4]; I2<50%) (Supplement Figure S1); 41.5% had spontaneous vaginal delivery (95% CI [36.3–46.8]; I2<50%) (Supplement Figure S2); and 6.4% of them had an operative vaginal delivery (95% CI [4.5–9.2]; I2<50%) (Supplement Figure S3).
Neonatal outcomes
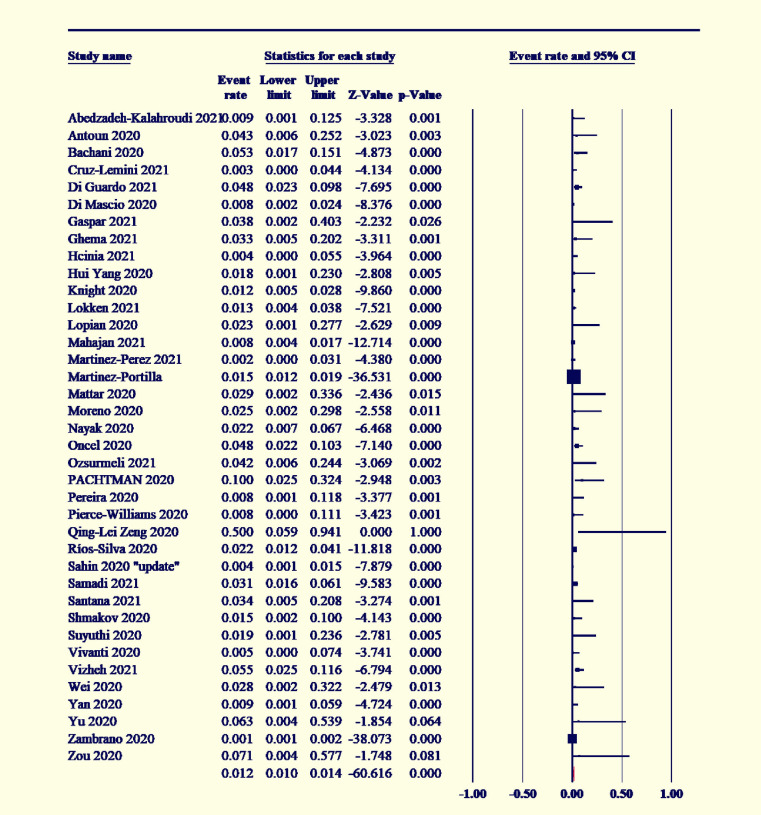
The overall pooled proportion for low birthweight in the babies of COVID-19-positive women was 16.7% (95% CI [12.8–21.5]; I2˃50 %) (Supplement Figure S4). The premature delivery rate was estimated to be 20% (95% CI [17.1–23.3]; I2˃50%) (Supplement Figure S5). The pooled mean difference of the neonatal birthweight was 3069.7 g (95% CI [3009.7–3129.8 g]; I2<50%) (Supplement Figure S6). Almost 32.9% of the delivered neonates needed NICU admission (95% CI [17.6–31.6]; I2˃50%) (Supplement Figure S7). The neonatal death rate was 3.0% (95% CI [2–4]; I2˃50%) (Supplement Figure S8), and 1.9% experienced fetal death or stillbirth (95% CI [1.5–2.4]; I2˃50%) (Supplement Figure S9). The overall vertical transmission rate of SARS-CoV-2 infection was 3.5% (95% CI [2.7–4.7]; I2˃50%) (Supplement Figure S10).
Comparative analysis with associated odds ratios
We implemented further comparative analysis between positive and negative COVID-19 patients to assess the possible associated risks. Pooled ORs were not statistically significant in most reported outcomes (Appendix 3). However, COVID-19 significantly increased the risk of premature delivery (OR, 1.48 [95% CI, 1.22–1.8]; I2=23.99%), preeclampsia (OR, 1.6; 95% CI, 1.2–2.1); I2=30.98), stillbirth (OR, 2.36 [95% CI, 1.24–4.462]; I2=5.54%), neonatal mortality (OR, 3.35; 95% CI, 1.07–10.5]; I2=0%), and maternal mortality (OR, 3.08 [95% CI, 1.5–6.3]; I2=0%). The pooled analyses were homogenous, with mild heterogeneity in the premature delivery and preeclampsia outcomes.
Sensitivity and subgroups analyses
We performed further sensitivity and consequent subgroups analyses according to the quality of included studies to confirm the robustness of our analysis (Appendix 4). These analyses revealed no significant difference in most of our reported outcomes when considering all the quality variations. However, the premature delivery rate was relatively higher in poor quality articles (20% [95% CI, 14.9–26.8]), whereas the frequency of stillbirth events was notably higher in good quality articles (3.6% [95% CI, 1.1–11.8]). Furthermore, the reported vertical transmission rates were lower in good quality articles (26% [95% CI, 0.015–0.046]).
Discussion
In this systematic review and meta-analysis, we pooled all available data from the literature to provide high-quality evidence regarding the clinical characteristics and the outcomes of pregnancy and delivery in COVID-19-positive pregnant women. Our analysis showed that 7.5% of pregnant women with COVID-19 had gestational diabetes, 7% had preeclampsia, and 4.6% needed ICU admission. A high rate of cesarean delivery (53.2%) was observed among pregnant women with COVID-19, with another 6.4% requiring an operative vaginal delivery. Li et al11 hypothesized that this rise in cesarean delivery deliveries is because of regulatory modifications to cope up with the pandemic. Furthermore, several reports showed that many pregnant cases with COVID-19 were indicated for emergency cesarean delivery because of maternal causes such as premature rupture of membrane and worsening respiratory status in patients with severe disease.12,13 Maternal mortality was low (1.2%), according to the pooled data from the included studies. This is in stark contrast to the mortality rates reported in previous coronavirus infections such as the Middle East respiratory syndrome and severe acute respiratory syndrome.14 Pooled data demonstrated that premature delivery accounted for approximately 20% of the total deliveries in pregnant women with COVID-19. Fetal death or stillbirth was relatively low (1.9%), and of the delivered neonates, 32.9% needed NICU admission. The authors recognize with great seriousness that these numbers represent significant increases of serious morbid complications (stillbirth, maternal death, neonatal death) over the general population but stand by the positive aspect that these numbers are relatively low in consideration of the subset of third trimester pregnancies with serious respiratory disease. In addition, the authors plan future analyses with subgroup calculation to differentiate studies by socioeconomic status of the country of origin. Our initial delving in this analysis has not shown any of these incidences to be significantly related to the originating country's socioeconomic factors.
Vertical transmission is a major concern in the setting of a global pandemic. Most of the included studies screened newborns for SARS-CoV-2 infection using nasopharyngeal swabs and reverse transcription-polymerase chain reaction (RT-PCR), and most studies performed testing at 24 hours of life. Vertical transmission was reported in 3.5%. Few studies currently include data as to whether these RT-PCR results correlate with clinical symptoms of disease in the newborn later, so this cannot not be explored as an outcome at this time. Therefore, the authors feel that the question of vertical transmission is still largely unanswered, though the low rate of SARS-CoV-2 detection by RT-PCR in newborns is reassuring.
Khoury et al15 studied the differences in the maternal and neonatal outcomes between initially symptomatic and asymptomatic pregnant women. Interestingly, the symptomatic patients had a higher risk of cesarean delivery and preterm birth than asymptomatic COVID-19-positive patients. This may give clinicians cause to alter some treatment plans, and in some circumstances, it may decrease the threshold for the administration of antenatal steroids secondary to the higher rates of preterm delivery in COVID-19 infected women. Prabhu et al16 showed that initially symptomatic women developed more postpartum fever than asymptomatic COVID-19-positive patients. Previous reports attributed this increased postpartum fever to a cytokine storm in response to the SARS-CoV-2 viral infection.17,18
Our results were similar to recently published systematic reviews and meta-analyses. Islam et al19 in 2020 reported that 66.38% of pregnant women had a cesarean delivery and 33.62% had a vaginal delivery. Di Toro et al20 in 2021 found that the rate of maternal ICU admission was 8%, that of preeclampsia was 7%, and that of preterm birth was 23%. However, the rate of cesarean delivery was slightly different, as they found that 85% of women underwent cesarean delivery.
Our study has several strengths. We executed a comprehensive systematic review and meta-analysis to investigate and describe the pregnancy outcomes among pregnant individuals infected with COVID-19. We reported as many outcomes as possible pertaining to the maternal clinical features and the fetal or neonatal outcomes among COVID-19-positive pregnant women. Methodologically, the MOOSE and PRISMA guidelines were followed throughout the steps of this study to ensure high-quality reporting. Nonetheless, few caveats warrant attention while interpreting the results of this meta-analysis. The observational nature of the included studies (most retrospective) is an important limitation. Most of the included studies were of moderate quality, and in most cases, the heterogeneity could not be resolved. Another concern is that most of the included pregnant women were in the third trimester, so the results of this meta-analysis cannot be generalized to pregnant women in the first and second trimesters. Lastly, there is an international hurry to publish COVID-19 studies, some of which may unfortunately affect the quality and reliability of the data. Unfortunately, the inclusion of such studies affects the quality and scientific evidence synthesized during the conduct of systematic review and meta-analysis reports. We hope that our comprehensive approach in this report provides a robust summary for practicing obstetricians making evidence-based clinical decisions when caring for pregnant women with COVID-19.
Conclusion
Pregnant women with COVID-19 are at a significantly higher risk of cesarean delivery and premature delivery than uninfected pregnant women. Given the fact that these results are based on observational studies, further well-designed investigations are warranted to guide an evidence-based clinical practice. Being more vulnerable to unfavorable maternal and neonatal complications, clinicians may consider altering treatment plans to prepare for possible morbidities, most notably the consideration of steroids for the increased possibility of preterm delivery in COVID-19 infected women. Fortunately, despite these findings, there is still no evidence at this time of the sharply increased maternal mortality that was seen previously with both the 2003 SARS and 2012 MERS pandemics.
Acknowledgments
The Marchand Institute for Minimally Invasive Surgery would like to acknowledge the efforts of all of the students, researchers, residents, and fellows at the institute who put their time and effort into these projects without compensation, only for the betterment of women's health. We firmly assure them that the future of medicine belongs to them.
Footnotes
This manuscript has been reviewed by the institutional review board at the Marchand Institute and was deemed exempt (June 2021).
The data used were exempt from consent to participate or publish secondary to the nature of the study being a systematic review retrospectively looking at previously published data.
The Marchand Institute remains committed to diversity and tolerance in its research and actively maintains a workplace free of racism and sexism. More than half of the authors for this study are female, and many represent diverse backgrounds and underrepresented ethnic groups.
The authors declare no conflict of interest.
No authors received any payment for this work, and all the work was carried out by them voluntarily.
Patient consent was not obtained because it is not applicable to systematic reviews.
All the supporting data are included or referenced in this manuscript. No additional data were used in this study by the authors.
This study was registered with the International Prospective Register of Systematic Reviews under registration number CRD42021239772.
Cite this article as: Marchand G, Patil AS, Masoud AT, et al. Systematic review and meta-analysis of COVID-19 maternal and neonatal clinical features and pregnancy outcomes to June 3, 2021. Am J Obstet Gynecol Glob Rep 2022;2:100049.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.xagr.2021.100049.
Appendix. Supplementary materials
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation. Obstet Gynecol. 2020;135:999–1002. doi: 10.1097/AOG.0000000000003873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Getahun D, Ananth CV, Peltier MR, Smulian JC, Vintzileos AM. Acute and chronic respiratory diseases in pregnancy: associations with placental abruption. Am J Obstet Gynecol. 2006;195:1180–1184. doi: 10.1016/j.ajog.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Chen YH, Keller J, Wang IT, Lin CC, Lin HC. Pneumonia and pregnancy outcomes: a nationwide population-based study. Am J Obstet Gynecol. 2012;207:288. doi: 10.1016/j.ajog.2012.08.023. e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matar R, Alrahmani L, Monzer N, et al. Clinical presentation and outcomes of pregnant women with coronavirus disease 2019: a systematic review and meta-analysis. Clin Infect Dis. 2021;72:521–533. doi: 10.1093/cid/ciaa828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasraeian M, Zare M, Vafaei H, et al. COVID-19 pneumonia and pregnancy; a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020 doi: 10.1080/14767058.2020.1763952. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG, Group PRISMA. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 9.National Heart, Lung and Blood Institute. Study quality assessment tools. 2021. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed July 5, 2021.
- 10.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N, Han L, Peng M, et al. Maternal and neonatal outcomes of pregnant women with coronavirus disease 2019 (COVID-19) pneumonia: a case-control study. Clin Infect Dis. 2020;71:2035–2041. doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H, Hu B, Zhan S, Yang LY, Xiong G. Effects of severe acute respiratory syndrome coronavirus 2 infection on pregnant women and their infants. Arch Pathol Lab Med. 2020;144:1217–1222. doi: 10.5858/arpa.2020-0232-SA. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020;144:799–805. doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222:415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoury R, Bernstein PS, Debolt C, et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at five New York City Medical Centers. Obstet Gynecol. 2020;136:273–282. doi: 10.1097/AOG.0000000000004025. [DOI] [PubMed] [Google Scholar]
- 16.Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548–1556. doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smulian JC, Bhandari V, Vintzileos AM, et al. Intrapartum fever at term: serum and histologic markers of inflammation. Am J Obstet Gynecol. 2003;188:269–274. doi: 10.1067/mob.2003.11. [DOI] [PubMed] [Google Scholar]
- 18.Riley LE, Celi AC, Onderdonk AB, et al. Association of epidural-related fever and noninfectious inflammation in term labor. Obstet Gynecol. 2011;117:588–595. doi: 10.1097/AOG.0b013e31820b0503. [DOI] [PubMed] [Google Scholar]
- 19.Islam MM, Poly TN, Walther BA, et al. Clinical characteristics and neonatal outcomes of pregnant patients with COVID-19: a systematic review. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.573468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di TF, Gjoka M, Di LG, et al. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clinical Microbiology and Infection. 2021;27:36–46. doi: 10.1016/j.cmi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.