Abstract
Objective
The study aim was to evaluate the histological relationship between osteoarthritis (OA) and articular cartilage in disuse atrophy induced by hindlimb unloading in a post-traumatic OA rat model.
Design
Forty male rats were divided into the 4 following experimental groups: control, hindlimb suspension (HS), OA induced by destabilization of the medial meniscus (OA), and OA induction after hindlimb suspension (HS-OA). Histological changes in the articular cartilage of the tibia were evaluated by the Osteoarthritis Research Society International (OARSI) scores and histomorphometrical analyses at 2, 4, and 8 weeks after OA induction.
Results
We confirmed that disuse atrophy of the articular cartilage was caused by thinning of the articular cartilage and the decrease in matrix staining for the nonloading period of 4 weeks. The OARSI scores and histomorphological analyses revealed that OA progressed significantly wider and deeper in the HS-OA group than in the OA group over time. In the sham group, disuse atrophy of the articular cartilage recovered at 2 weeks after reloading.
Conclusions
This study revealed that OA progressed faster in cartilage atrophy than in normal articular cartilage. Further studies are required for investigating the mechanisms of disuse atrophy of cartilage and its association with OA using the biochemical and immunohistochemical analysis.
Keywords: osteoarthritis, diagnosis, histology, scoring systems, research methods, joint unloading, articular cartilage, tissue, cartilage atrophy
Introduction
Mechanical stress, including joint loading, is an important factor in osteoarthritis (OA) onset and progression. Appropriate joint loading and exercise inhibit OA progression, 1 but excessive mechanical stress promotes OA progression.2,3 The guidelines published in 2014 and 2019 by the Osteoarthritis Research Society International (OARSI) on the nonsurgical treatment of OA include weight management (i.e., weight loss) as the core treatment for all individuals.4,5 In our previous study, we examined the histological effect of unloading condition on OA progression by combining hindlimb suspension and a monosodium iodoacetate–induced rat model of OA. 6 That study revealed new histological evidence showing that an unloading condition suppressed articular cartilage degeneration. 6 Those findings clearly showed both clinically and histologically that joint loading on the articular cartilage is an important factor for the development and progression of OA.
Additionally, mechanical stress by joint loading is essential for histological and functional maintenance of the articular cartilage, which has high mechanical reactivity and responds to its mechanical environment according to the type and amount of mechanical stress depending on the environment. 7 Under no joint loading, the articular cartilage undergoes disuse histological changes reflected by thinning of the cartilage and a decrease in matrix staining.7,8 Vincent et al. 7 defined this histological change as cartilage atrophy. In our previous study, we analyzed the histological effects of joint unloading on the articular cartilage by knee compartments using a hindlimb suspension rat model. 9 This result showed that the unloading condition caused articular cartilage thinning for 2 weeks in the patellofemoral compartment and for 4 weeks in the medial tibiofemoral compartment. 9
In recent years, there has been debate on whether or not cartilage atrophy is related to OA. 7 We speculated that the articular cartilage in disuse atrophy is vulnerable to mechanical loading and may be more likely to develop OA and that cartilage atrophy induced by unloading condition may promote development of OA. However, at present, the mechanism of the histological relationship between OA and the disuse cartilage atrophy has not been elucidated. The study aim was to investigate the histological relationship between OA and articular cartilage in disuse atrophy induced by hindlimb unloading in a post-traumatic OA rat model.
Methods
Experimental Animals and Animal Care
This study protocol was approved by the Animal Research Committee of the Graduate School of Medicine of Kanazawa University (Kanazawa, Japan; approval no. 204125) and was conducted in accordance with the ARRIVE guidelines 10 and the Guidelines for the Care and Use of Laboratory Animals of Kanazawa University.
Forty male Wistar rats (8 weeks old) were purchased from Japan SLC (Shizuoka, Japan) and housed under normal conditions for 1 week before starting the experiments to acclimatize the animals to their new environment. The rats were housed 1 per cage in a sanitary ventilated room under controlled temperature and humidity conditions, and a 12-hour light/dark cycle with ad libitum access to food and water.
The experimental protocol is shown in Fig. 1 . The rats were divided into 4 groups: control (CON, n = 5), hindlimb suspension (HS, n = 5), post-traumatic OA (OA, n = 15; 5 rats each for 2, 4, and 8 weeks), and OA after HS (HS-OA, n = 15; 5 rats each for 2, 4, and 8 weeks). The rats in the CON group were kept in a physiological environment, and the rats in the HS group were subjected to tail suspension for 4 weeks. After this period, the rats in both groups were sacrificed and used to identify the disuse histological changes of cartilage due to the unloading environment and to confirm the histological condition before OA induction in the OA and HS-OA groups. OA was induced by surgical destabilization of the medial meniscus (DMM). In the OA group, the DMM was performed after the rats were housed under normal conditions for 4 weeks. In the HS-OA group, the DMM was performed after the rats were subjected to HS for 4 weeks. After OA induction, the rats in the HS-OA group were not subjected to tail suspension. Rats subjected to HS were allowed to walk freely using only their forelimbs. In the OA and HS-OA group, we set the experimental period to 2, 4, and 8 weeks after OA induction. After the start of the experiment, no further interventions, including range of motion exercise, were performed during the experimental period. No analgesics or anti-inflammatory drugs were administered.
Figure 1.
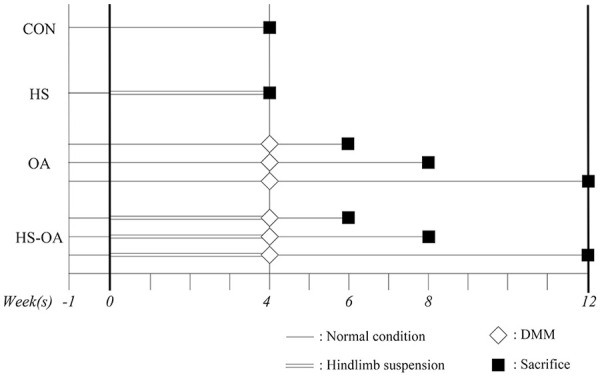
Schematic of the experimental protocol. The rats in the CON group (n = 5) and OA group (n = 15) were kept under normal conditions for 4 weeks, and the rats in the HS group (n = 5) and HS-OA group (n = 15) were subjected to tail suspension for 4 weeks. The rats in the OA and HS-OA groups underwent DMM surgery on their left knee and sham surgery on their right knee and were randomly assigned to 3 experimental periods (n = 5, respectively). After each experimental period, the rats were euthanized, and assessed by histological analyses. CON, control; OA, osteoarthritis; HS, hindlimb suspension; DMM, destabilization of the medial meniscus.
Hindlimb Suspension
In the HS and HS-OA groups, the rats were subjected to tail suspension for 4 weeks. HS was performed according to the tail suspension method described in our previous studies.6,9,11 For details see Supplementary Method 1.
Surgical Induction of OA
The same highly experienced operators (I.T. and K.T.) performed all DMM surgeries. In the OA and HS-OA groups, the DMM was induced by transecting the medial meniscotibial ligament (MMTL) in the left knee joint as described previously.12-14 In the OA and HS-OA groups, for internal controls, a sham operation was performed on the right knee joint by using the same approach without MMTL transection.15,16 For details, see Supplementary Method 1.
Histological Preparation
Decalcified paraffin sections were prepared for histology as described previously. 6 Both knees were excised frontally to evaluate the histological changes in the medial tibiofemoral joints. Three paraffin sections (3-µm thickness) spaced at 200-µm intervals were stained with hematoxylin–eosin and toluidine blue to evaluate the severity of cartilage lesions. 17 The stained sections were viewed under a light microscope and imaged by using a digital camera (BX-51 and DP-74; Olympus Corporation, Tokyo, Japan).
Histological Analyses
To clarify the histological changes that occur in OA, we quantitatively evaluated the articular cartilage of the tibia in the medial tibiofemoral joint by using the OA cartilage histopathology assessment system. 18 The OARSI scoring system, consisting of 6 grades and 4 stages on a scale from 0 (normal) to 24 (severe cartilage lesion), was used for semiquantitative evaluation of cartilage lesion severity. 18 The maximum OARSI score was the highest score among 3 sections and provides a measure of the “severity” of the cartilage lesions. The summed OARSI score was the total of the 3 section scores and provides a measure of the “extent” of the cartilage lesions. 19
To evaluate quantitatively calcified cartilage and subchondral bone damage of the tibia in the medial tibiofemoral joint, we used the calcified cartilage and subchondral bone damage score. 20 This score was established by Gerwin et al. 20 and ranges from 0 to 5, with higher values indicating severe subchondral lesion. 20 For details, see Supplementary Method 2.
All histological scores using these scoring systems were determined by 2 blinded and trained independent observers (M.H., a pathologist, and I.T.). In our previous study, interclass correlation coefficients for the intra- and interrater reliabilities of the OARSI score with 95% confidence intervals were excellent: 0.94 (0.92-0.95) and 0.91 (0.89-0.93), respectively. 6
Histomorphometrical Analyses
Histomorphometrical analyses for articular cartilage were performed to evaluate the following four parameters: the cartilage thickness, intensity of matrix staining with toluidine blue, chondrocyte density, and osteophyte length.8,9,21 For details, see Supplementary Method 1.
Statistical Analysis
All statistical analyses were performed by using JMP 14 software (SAS Institute, Cary, NC, USA). All data were statistically analyzed as parametric data. The sample size was 5 for each group. Descriptive statistics were calculated as the median with interquartile range for the OARSI and subchondral bone scores and as the mean with standard deviation for body weight and histomorphometrical data. We considered P < 0.05 as indicative of statistical significance for all analyses; exact P values are shown in the figures. For all the data, we performed analysis of variance followed by the post hoc Tukey’s honest significant difference test.
A sample size calculation was performed by using the sample size and power tool in G Power 3.1 (free; available at https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower.html), 22 based on pilot experimental data of the main parameter and the maximum and summed OARSI scores at 4 weeks, including the first 5 rats, in the OA and HS-OA group. For these scores, a minimum of 3 and 5 were required in the OA and HS-OA group, respectively, with a power of 0.80 and a significance level of P < 0.05. Therefore, we set the sample size to 5 rats per group.
Results
General Condition
Within the first few minutes after discontinuation of the inhalation-induced anesthesia with isoflurane, all animals regained consciousness and mobility. None of the rats exhibited any signs of tail infection or died during the experimental period. Inflammation was macroscopically and microscopically well-controlled. The body weights of the rats throughout the experimental period are shown in Supplementary Result 1.
Cartilage Atrophy
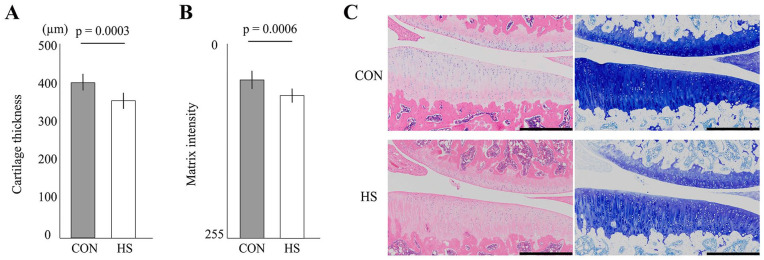
In Fig. 2 , the unloading condition for 4 weeks induced histological changes in the articular cartilage. This disuse change included thinning and a decrease in matrix staining, which are typical for cartilage atrophy. For details, see Supplementary Result 2.
Figure 2.
Histological effect of the unloading condition on the tibial articular cartilage. (A) Change in articular cartilage thickness. The tibial cartilage thickness was significantly thinned after 4 weeks of hindlimb suspension. (B) Change in the intensity of matrix staining by toluidine blue. The staining intensity was significantly decreased after 4 weeks of hindlimb suspension (HS). (C) Representative histological features of the tibial articular cartilage. In both groups, the articular cartilage surface was smooth and intact, but in the HS group, thinning of cartilage, and decreased staining intensity were observed. Scale bar = 500 μm.
Histological Scores
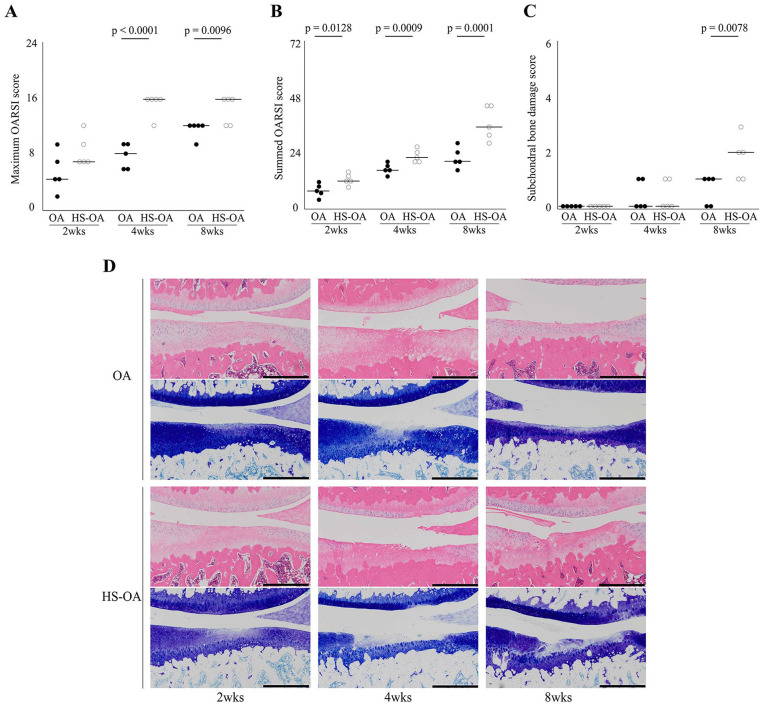
In Fig. 3 , OA progression was faster in the HS-OA group than in the OA group. The maximum OARSI score was higher in the HS-OA group than in the OA group at 4 and 8 weeks after OA induction. Additionally, the summed OARSI score was higher in the HS-OA group than in the OA group during all experimental periods. There was no significant difference in the subchondral bone damage scores between the 2 groups at 2 and 4 weeks; however, the score was higher in the HS-OA group than in the OA group at 8 weeks ( Figs. 3 and 4 ). For details, see Supplementary Method 2 and Supplementary Result 3.
Figure 3.
Histological effect of disuse atrophy of articular cartilage on OA progression. Time-dependent changes in the maximum OARSI score (A), summed OARSI score (B), and subchondral bone damage score (C). All scores significantly increased with time. (D) Representative histological osteoarthritic features of the tibial articular cartilage. At 2 and 4 weeks, the toluidine blue–stained area was decreased in the HS-OA group relative to that in the OA group. At 4 weeks, fibrillation and fissure were observed in the OA group and HS-OA group, and erosion was also observed in the latter. At 8 weeks, cartilage thinning, fibrillation, and fissure were observed in the OA group, and erosion and deformation of the articular surface were observed in the HS-OA group. Scale bar = 500 μm. OA, osteoarthritis; HS, hindlimb suspension; OARSI, Osteoarthritis Research Society International.
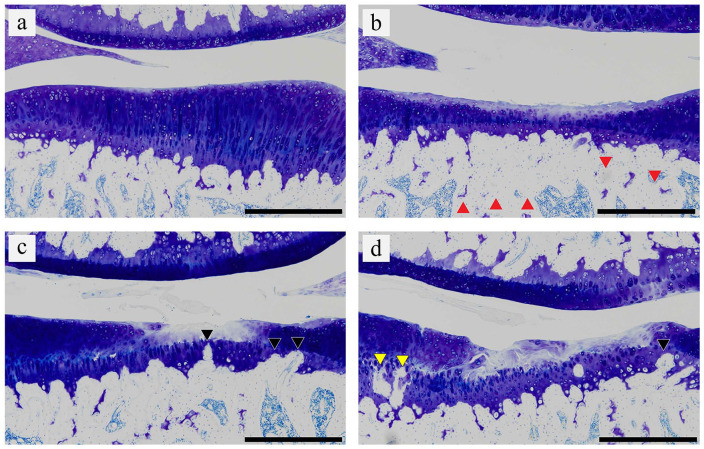
Figure 4.
Representative histological changes in calcified cartilage and subchondral bone. According to the scoring system, (a) the histological image in the sham limb at 8 weeks was grade 0. (b, c, and d) The histological images in the operated limb at 8 weeks in the HS-OA group were grades 1, 2, and 3, respectively. Grade 0 indicates no remarkable histological change; grade 1 indicates increased basophilia and minimal focal marrow change (red triangle); grade 2 indicates mild focal fragmentation of calcified cartilage of the tidemark and thickening of subchondral bone (black triangle); and grade 3 indicates mild to marked fragmentation of calcified cartilage/subchondral bone loss (yellow triangle). For grade definition, see Supplementary Method 2. Scale bar = 500 μm. OA, osteoarthritis; HS, hindlimb suspension.
Histomorphometrical Analyses
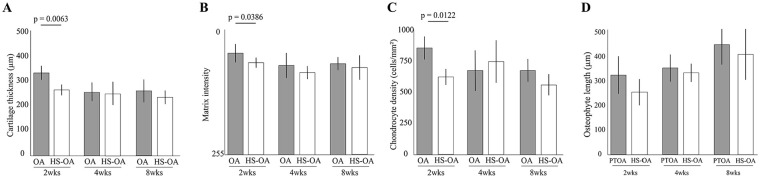
Significant differences in the cartilage thickness, matrix intensity, and chondrocyte density between both groups were detected at 2 weeks but not at 4 and 8 weeks ( Fig. 5 ). There was no significant difference in osteophyte length between the 2 groups during the experimental period. For details, see Supplementary Result 4. In the sham limb, the significant decrease in cartilage thickness and matrix staining caused by the unloading condition disappeared at 2 weeks after the surgery (Supplementary Results 2 and 4).
Figure 5.
Histomorphological results of disuse atrophy of articular cartilage on osteoarthritis (OA) progression. Time-dependent changes in cartilage thickness (A), matrix intensity (B), chondrocyte density (C), and osteophyte length (D). Significant differences were observed at 2 weeks in all parameters except osteophyte length. However, at 4 and 8 weeks, there were no significant differences in any of the parameters.
Discussion
The purpose of this study was to investigate the histological influence of disuse atrophy of the articular cartilage on progression of OA induced by DMM in an HS rat model. Our results showed that the histological change in both the articular cartilage and subchondral bone in the HS-OA group was significantly more severe and faster than that in the OA group, and this histological finding suggested that disuse atrophy of the articular cartilage accelerated OA progression. Specifically, the histological change in the HS-OA group progressed more widely at 2 weeks after surgery, and the histological changes in the HS-OA group progressed more widely and deeply at 4 and 8 weeks. Additionally, the subchondral bone lesion was more severe in the HS-OA group at 8 weeks, and the marked fragmentation of the calcified cartilage/subchondral bone was observed only in the HS-OA group. In the histomorphometric analysis results, decreased chondrocyte density, and matrix staining and thinning of articular cartilage were observed at 2 weeks in the HS-OA group. The histological findings of this study are new histological evidence of the onset and progression of OA and will be very useful for basic and clinical studies.
Articular cartilage is an avascular tissue composed of a specialized matrix of collagens, proteoglycans, and non-collagen proteins in which chondrocytes constitute the unique cellular component. 23 Articular cartilage provides a low-friction gliding surface that acts as a shock absorber to minimize peak pressure on the subchondral bone. 24 Although the details of the mechanism of cartilage atrophy are unknown, it is thought that the main factors are reduced synthesis of proteoglycan and a mechanoadaptive response to reduced load. 7 Many researchers have reported basic evidence of cartilage atrophy in both humans25,26 and animals.8,27 To summarize, the histological hallmark of disuse cartilage atrophy is thinning of the cartilage and reduced matrix staining. 7 These 2 characteristics are common to OA, but their mechanisms are different. Disuse cartilage atrophy results in thinning of cartilage and a decrease in matrix content due to a decrease in chondrocyte matrix synthesis, and the surface is smooth. 7 On the other hand, in OA, chondrocytes secrete proteases, such as metalloprotease and aggrecanase, resulting in a decrease in the matrix content and a rough surface.28,29 Therefore, disuse cartilage atrophy and OA are clearly different pathological conditions.
However, our study results revealed that these 2 pathologies are related and that cartilage atrophy is a condition in which OA is prone to progress. In this regard, Vincent et al. 7 speculated as follows:
Chondrocytes have mechanostats and set a certain threshold. This threshold changes as the cartilage mechanoadapts and will be different for each individual according to genetics, the mechanical integrity of the tissue, and the amount of load usually experienced by the joint. When the mechanical load is high and when the load is moderate but the joint has lost its mechanoprotective mechanisms by cartilage atrophy, chondrocytes determine that the stimulus has crossed a threshold, the stimulus is recognized as detrimental, and the chondrocytes activate OA-related pathways.
However, regarding disuse atrophy of articular cartilage, its onset mechanism and the changes in chondrocyte metabolism and mechanical strength have not been clarified. To clarify the pathophysiology of OA in more detail, further research is needed from multiple fields on disuse atrophy of articular cartilage.
Furthermore, the results of this study provided interesting and potential data on disuse atrophy of articular cartilage. In the sham limb of the OA and HS-OA groups, the histological changes in disuse atrophy, which occurred for 4 weeks before OA induction, generally recovered 2 weeks after the OA induction (Supplementary Results 2 and 4). It is known that cartilage thickness changes depending on the load, and there are some reports that it increased with repeated exercise.30,31 In addition, the metabolism of proteoglycan reportedly is relatively fast (half-life: 8 days), 32 and synthesis of proteoglycan increased after injury. 33 These findings suggest that disuse atrophy of articular cartilage may be a reversible histological change that is expected to be restored by reloading.
This study has one major limitation. In this study, detailed histology was conducted as an analysis method. However, a single analytical method is not sufficient to provide a detailed picture of the disuse atrophy of articular cartilage and its association with OA. Therefore, further multifaceted and more comprehensive studies such as immunohistochemical staining and in situ hybridization, analysis to investigate proteins such as Western blotting and enzyme-linked immunosorbent assay, analysis to examine gene expression such as polymerase chain reaction, analysis to investigate the mechanical strength of articular cartilage, and analysis of synovial membrane and synovial fluid would be essential.
In conclusion, the histological change of both the articular cartilage and subchondral bone was severe in the HS-OA group, and OA progress faster in cartilage atrophy than in normal articular cartilage. Clinically, health care professionals, such as orthopedic surgeons and physiotherapists, should consider the possibility that when patients restart exercise after long-term bed rest, such as walking or standing up, exercise may overload the articular cartilage, which may lead to onset and progression of OA. In addition to the histological results of this study, further studies are required for investigating the mechanisms of disuse atrophy of cartilage and its association with OA using the biochemical analysis for protein and gene expression and for synovial membrane and synovial fluid.
Supplemental Material
Supplemental material, sj-pdf-1-car-10.1177_1947603520982350 for Disuse Atrophy of Articular Cartilage Induced by Unloading Condition Accelerates Histological Progression of Osteoarthritis in a Post-traumatic Rat Model by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE
Supplemental material, sj-pdf-2-car-10.1177_1947603520982350 for Disuse Atrophy of Articular Cartilage Induced by Unloading Condition Accelerates Histological Progression of Osteoarthritis in a Post-traumatic Rat Model by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE
Supplemental material, sj-pdf-3-car-10.1177_1947603520982350 for Disuse Atrophy of Articular Cartilage Induced by Unloading Condition Accelerates Histological Progression of Osteoarthritis in a Post-traumatic Rat Model by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/car.
Acknowledgments and Funding: The author thanks the members of the Department of Human Pathology at the Kanazawa University Graduate School of Medicine for offering advice regarding the histopathological techniques. The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a JSPS KAKENHI grant-in-aid for Young Scientists B (number: 17K13051) and for Early Career Scientists (number: 20K19444).
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study protocol was approved by the Animal Research Committee of the Graduate School of Medicine of Kanazawa University (Kanazawa, Japan; approval no. 204125).
Animal Welfare: This study was conducted in accordance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines and the Guidelines for the Care and Use of Laboratory Animals of Kanazawa University.
ORCID iD: Ikufumi Takahashi  https://orcid.org/0000-0003-1924-3101
https://orcid.org/0000-0003-1924-3101
References
- 1. Iijima H, Ito A, Nagai M, Tajino J, Yamaguchi S, Kiyan W, et al. Physiological exercise loading suppresses post-traumatic osteoarthritis progression via an increase in bone morphogenetic proteins expression in an experimental rat knee model. Osteoarthritis Cartilage. 2017;25(6):964-75. [DOI] [PubMed] [Google Scholar]
- 2. Tsezou A. Osteoarthritis year in review 2014: genetics and genomics. Osteoarthritis Cartilage. 2014;22(12):2017-24. [DOI] [PubMed] [Google Scholar]
- 3. Moyer RF, Ratneswaran A, Beier F, Birmingham TB. Osteoarthritis year in review 2014: mechanics–basic and clinical studies in osteoarthritis. Osteoarthritis Cartilage. 2014;22(12):1989-2002. [DOI] [PubMed] [Google Scholar]
- 4. McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-88. [DOI] [PubMed] [Google Scholar]
- 5. Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578-89. [DOI] [PubMed] [Google Scholar]
- 6. Takahashi I, Matsuzaki T, Kuroki H, Hoso M. Joint unloading inhibits articular cartilage degeneration in knee joints of a monosodium iodoacetate-induced rat model of osteoarthritis. Osteoarthritis Cartilage. 2019;27(7):1084-93. [DOI] [PubMed] [Google Scholar]
- 7. Vincent TL, Wann AKT. Mechanoadaptation: articular cartilage through thick and thin. J Physiol. 2019;597(5):1271-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nomura M, Sakitani N, Iwasawa H, Kohara Y, Takano S, Wakimoto Y, et al. Thinning of articular cartilage after joint unloading or immobilization. An experimental investigation of the pathogenesis in mice. Osteoarthritis Cartilage. 2017;25(5):727-36. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi I, Matsuzaki T, Kuroki H, Hoso M. Disuse histological changes of an unloading environment on joint components in rat knee joints. Osteoarthr Cartil Open. 2019;1(1-2):e100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage. 2012;20(4):256-60. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi I, Matsuzaki T, Yoshida S, Kitade I, Hoso M. Differences in cartilage repair between loading and unloading environments in the rat knee. J Jpn Phys Ther Assoc. 2014;17(1):22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061-9. [DOI] [PubMed] [Google Scholar]
- 13. Ulici V, Kelley KL, Azcarate-Peril MA, Cleveland RJ, Sartor RB, Schwartz TA, et al. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthritis Cartilage. 2018;26(8):1098-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iijima H, Aoyama T, Ito A, Tajino J, Nagai M, Zhang X, et al. Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthritis Cartilage. 2014;22(7):1036-43. [DOI] [PubMed] [Google Scholar]
- 15. Ratneswaran A, Beier F. An approach towards accountability: suggestions for increased reproducibility in surgical destabilization of medial meniscus (DMM) models. Osteoarthritis Cartilage. 2017;25(11):1747-50. [DOI] [PubMed] [Google Scholar]
- 16. Loeser RF, Olex AL, McNulty MA, Carlson CS, Callahan MF, Ferguson CM, et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 2012;64(3):705-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmitz N, Laverty S, Kraus VB, Aigner T. Basic methods in histopathology of joint tissues. Osteoarthritis Cartilage. 2010;18(Suppl 3):S113-S116. [DOI] [PubMed] [Google Scholar]
- 18. Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13-29. [DOI] [PubMed] [Google Scholar]
- 19. Iijima H, Aoyama T, Ito A, Tajino J, Yamaguchi S, Nagai M, et al. Exercise intervention increases expression of bone morphogenetic proteins and prevents the progression of cartilage-subchondral bone lesions in a post-traumatic rat knee model. Osteoarthritis Cartilage. 2016;24(6):1092-102. [DOI] [PubMed] [Google Scholar]
- 20. Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18(Suppl 3):S24-34. [DOI] [PubMed] [Google Scholar]
- 21. Iijima H, Aoyama T, Ito A, Tajino J, Nagai M, Zhang X, et al. Immature articular cartilage and subchondral bone covered by menisci are potentially susceptive to mechanical load. BMC Musculoskelet Disord. 2014;15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2_suppl):175-91. [DOI] [PubMed] [Google Scholar]
- 23. Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11(3):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77-95. [DOI] [PubMed] [Google Scholar]
- 25. Benichou C, Wirotius JM. Articular cartilage atrophy in lower limb amputees. Arthritis Rheum. 1982;25(1):80-2. [DOI] [PubMed] [Google Scholar]
- 26. Vanwanseele B, Eckstein F, Knecht H, Stüssi E, Spaepen A. Knee cartilage of spinal cord-injured patients displays progressive thinning in the absence of normal joint loading and movement. Arthritis Rheum. 2002;46(8):2073-8. [DOI] [PubMed] [Google Scholar]
- 27. Moriyama H, Yoshimura O, Kawamata S, Takayanagi K, Kurose T, Kubota A, et al. Alteration in articular cartilage of rat knee joints after spinal cord injury. Osteoarthritis Cartilage. 2008;16(3):392-8. [DOI] [PubMed] [Google Scholar]
- 28. Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteo-arthritis Cartilage. 2004;12:31-3. [DOI] [PubMed] [Google Scholar]
- 29. Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5(2_suppl):94-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52(11):3507-14. [DOI] [PubMed] [Google Scholar]
- 31. Hamann N, Zaucke F, Heilig J, Oberländer KD, Brüggemann GP, Niehoff A. Effect of different running modes on the morphological, biochemical, and mechanical properties of articular cartilage. Scand J Med Sci Sports. 2014;24(1):179-88. [DOI] [PubMed] [Google Scholar]
- 32. Mankin HJ, Lippiello L. The turnover of adult rabbit articular cartilage. J Bone Joint Surg Am. 1969;51(8):1591-600. [PubMed] [Google Scholar]
- 33. Meachim G. The effect of scarification on articular cartilage in the rabbit. J Bone Joint Surg Br. 1963;45-B(1):150-61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-car-10.1177_1947603520982350 for Disuse Atrophy of Articular Cartilage Induced by Unloading Condition Accelerates Histological Progression of Osteoarthritis in a Post-traumatic Rat Model by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE
Supplemental material, sj-pdf-2-car-10.1177_1947603520982350 for Disuse Atrophy of Articular Cartilage Induced by Unloading Condition Accelerates Histological Progression of Osteoarthritis in a Post-traumatic Rat Model by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE
Supplemental material, sj-pdf-3-car-10.1177_1947603520982350 for Disuse Atrophy of Articular Cartilage Induced by Unloading Condition Accelerates Histological Progression of Osteoarthritis in a Post-traumatic Rat Model by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE