Graphical abstract
Keywords: COVID-19, SARS-CoV-2, coronavirus, innate immune response, interferon stimulated genes
Abstract
Recognition of viral infections by various pattern recognition receptors (PRRs) activates an inflammatory cytokine response that inhibits viral replication and orchestrates the activation of adaptive immune responses to control the viral infection. The broadly active innate immune response puts a strong selective pressure on viruses and drives the selection of variants with increased capabilities to subvert the induction and function of antiviral cytokines. This revolutionary process dynamically shapes the host ranges, cell tropism and pathogenesis of viruses. Recent studies on the innate immune responses to the infection of human coronaviruses (HCoV), particularly SARS-CoV-2, revealed that HCoV infections can be sensed by endosomal toll-like receptors and/or cytoplasmic RIG-I-like receptors in various cell types. However, the profiles of inflammatory cytokines and transcriptome response induced by a specific HCoV are usually cell type specific and determined by the virus-specific mechanisms of subverting the induction and function of interferons and inflammatory cytokines as well as the genetic trait of the host genes of innate immune pathways. We review herein the recent literatures on the innate immune responses and their roles in the pathogenesis of HCoV infections with emphasis on the pathobiological roles and therapeutic effects of type I interferons in HCoV infections and their antiviral mechanisms. The knowledge on the mechanism of innate immune control of HCoV infections and viral evasions should facilitate the development of therapeutics for induction of immune resolution of HCoV infections and vaccines for efficient control of COVID-19 pandemics and other HCoV infections.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has led to an unprecedented global public health crisis. SARS-CoV-2 belongs to the family of Coronaviridae, which is comprised of a diverse group of enveloped positive-sense single-stranded RNA viruses with broad host ranges in vertebrates1, 2 (Figure 1 ). While four human coronaviruses (HCoVs), including HCoV-229E, HCoV-OC43, HCoV-NL63 and HCoV-HKU1, cause mild upper respiratory tract infections, three coronaviruses (CoVs) have breached species barriers within the last 20 years to infect humans. These zoonotic CoVs have caused severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and COVID-19, with fatality rates of 10%, 36%, and 0.1 to 2%, respectively.3, 4 Despite the availability of several effective SARS-CoV-2 vaccines, SARS-CoV-2 has undergone rapid evolution with the selection of variants with increased infectivity and resistance to vaccine-induced immunity and thus, become the dominant lineages.5, 6, 7, 8, 9
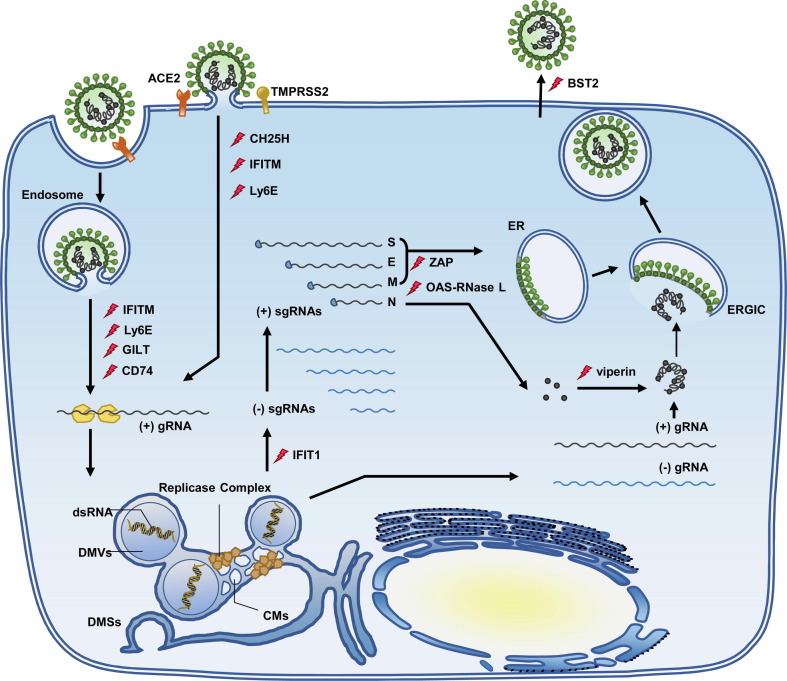
Figure 1.
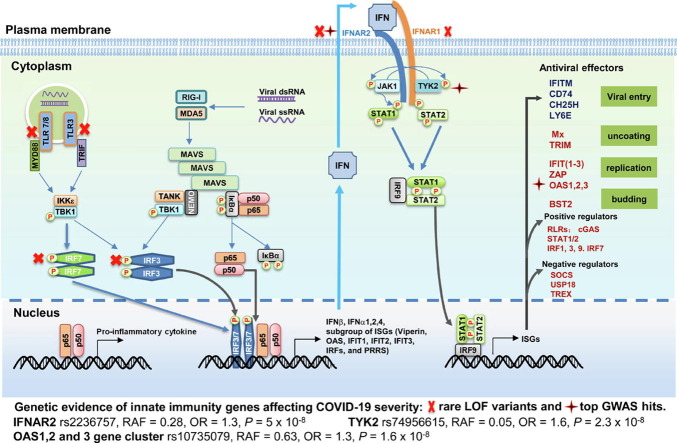
Mechanism of innate immune activation and control of viral infection. Recognition of viral RNA by cytoplasmic RIG-I-like receptors or endosomal TLRs activates signaling cascades leading to the production of IFNs, inflammatory cytokines and chemokines. IFNs bind to their specific receptors to activate JAK-STAT signaling pathway to induce the expression of hundreds of ISGs. While many of ISGs encode proteins that regulate innate immune response, some of them directly inhibit distinct steps of viral replication. Genetic evidence of innate immunity genes affecting COVID-19 severity was revealed in a GWAS report with hits in IFNAR2, TYK2 and OAS1 (RAF, risk allele frequency in the UK Biobank) as well as in studies that discovered at least 3.5% of patients with life-threatening COVID-19 carry rare loss-of-function (LOF) genetic variants in innate immunity genes (TLR3, TLR7, IRF7, IRF3, IFNAR1 and IFNAR2). These genes impair the type I and II IFN response to SARS-COVID-2 and are marked with a red cross. See text for further interpretation.
As illustrated in Figure 1, viral infections are promptly sensed by infected cells to mount an innate immune response leading to the transcriptional activation of genes for the production of intracellular antiviral proteins and secretion of cytokines, most prominently, the type I and type III interferons (IFN). While the antiviral proteins limit viral replication in infected cells, the IFNs induce hundreds of IFN-stimulated genes that establish an antiviral state to protect uninfected cells from oncoming infections.10 Clinical studies indicate that type I interferons (IFN-I) play critical roles in the control and pathogenesis of HCoVs, including SARS-CoV-2. This notion is supported by the findings that mutations rendering the gene products incapable of producing or responding to IFN-I are associated with life-threatening COVID-19 pneumonia.11, 12 In addition, autoantibodies capable of neutralizing IFN-I were found to dampen ISG induction in patients and are associated with severe COVID-19.13, 14 Here we review the recent progress on innate immune response in the control and pathogenesis of HCoV infections with emphasis on the pathobiological role and therapeutic efficacy of type I IFNs and their antiviral mechanisms against HCoVs. Better understanding of HCoV interaction with the IFN-mediated innate immunity should facilitate the discovery and development of novel therapeutic agents for the treatment of COVID-19 and vaccines to control the COVID-19 pandemic.
Role of IFN response in HCoV pathogenesis
HCoVs induce innate immune response via activation of multiple intracellular pattern recognition receptors
Viral infection of cells is sensed by pattern recognition receptors (PRRs) that bind unique structural motifs of viral components, i.e., pathogen associated molecular patterns (PAMPs).15 Similar to other RNA viruses, HCoV genomic RNA and its replication intermediates can be detected by the cytoplasmic RNA receptors, such as retinoic acid-inducible gene 1 (RIG-I) and melanoma differentiation-associated gene 5 (MDA5), or endosomal RNA sensors, including toll-like receptor (TLR) 3, 7 and 816, 17 (Figure 1). PRR recognition of viral RNA activates signaling cascades, leading to the activation of downstream transcription factors, including interferon regulator factor (IRFs) and nuclear factor κB (NF-κB). Transcriptional activation of IRF3/7 and NF-κB coordinately induces the expression of type I and type III IFNs to restrict viral replication as well as inflammatory cytokines and chemokines to recruit specific subsets of leukocytes and facilitate the activation of adaptive immune responses for the resolution of viral infections.16, 17, 18
RIG-I and MDA5 are ubiquitously expressed in various cell types and have been shown to be responsible for SARS-CoV-2 induction of IFN response in airway epithelial cells among other cell types.19, 20, 21 Two viral RNA fragments spanning nt24001–25000 and nt27001–28000 of SARS-CoV-2 genome significantly stimulated type I IFN production upon transfection into HEK293 cells, suggesting the viral RNA structural motifs or PAMPs recognized by RIG-I and/or MDA5 are likely located in these regions.22 SARS-CoV-2 RNAs in infected cells have the methylated adenosine bases at the nitrogen 6 position (m6A). Recent studies revealed that depletion of host cell m6A methyltransferase METTL3 decreased levels of m6A in SARS-CoV-2 RNA, but increased RIG-I binding and induction of IFN-I and other inflammatory cytokines.23 In addition, Yamanda et al. suggested RIG-I can also bind the 3′ non-translational region of SARS-CoV-2 RNA genome in human lung epithelial cells via its helicase domain to directly inhibit viral RNA replication independently of type I/III IFN production.24
Unlike RIG-I-like receptors, TLR7 and TLR8 are primarily expressed in dendritic cells (DCs), B lymphocytes and monocytes/macrophages. DCs play a critical role in viral immunity as they connect the innate immune response to the adaptive immune response when presented with viral antigens.25 While plasmacytoid DCs (pDCs) are the primary producers of IFN-I in response to viral infections,26 conventional DCs (cDCs) recognize various pathogens to produce inflammatory cytokines and activate T lymphocytes.27 In an in vitro human peripheral blood mononuclear cell (PBMC)-based experimental system, SARS-CoV-2 activates a robust TLR7/8-dependent response leading to the production of type I and III IFNs as well as inflammatory cytokines in the absence of productive viral replication. Type I IFNs are produced by pDCs in response to SARS-CoV-2 infection via a neuropilin-1-dependent, but ACE-2 independent mechanism.28 Based on the structural features of known TLR7/8 ligands,29, 30 a total of 491 GU-rich RNA fragments have been identified as putative immunostimulatory ligands within the SARS-CoV-2 genomic RNA and two of them were synthesized and validated as direct activators of endosomal TLR7/8 in a MyD88-depenent manner in DCs.31
HCoVs subvert the induction of innate immune response and signal transduction of IFNs via multiple mechanisms
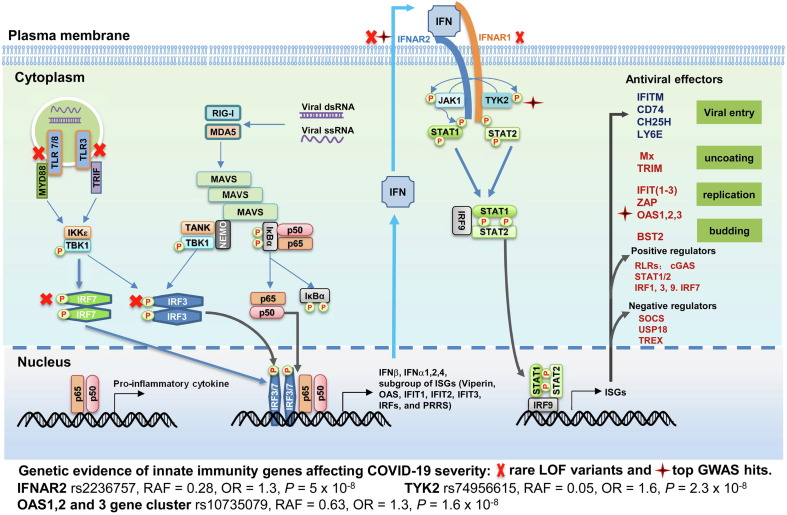
The broadly active antiviral response induced by PRR recognition of the viral pathogens puts strong selective pressure on viruses. This evolutionary process drives the selection of the variant viruses that have higher capabilities to evade or counteract the innate antiviral immune response. Similar to other viruses, HCoVs encode multiple proteins to subvert the innate immune response (Figure 2 ).32 Approximately 10 SARS-CoV and SARS-CoV-2 proteins, including membrane protein (M), nucleocapsid protein (N), and non-structural protein (Nsp) 10, 14, 15 and 16, were found to play a role in counteracting the production of IFNs and induction of ISGs (reviewed in.33 SARS-CoV-2 Nsp5, the main protease, targets RIG-I and MAVS proteins for degradation.34 ISG15 conjugation (ISGylation) of MDA5 at the CARD domain promotes its oligomerization and triggers the activation of IFN and inflammatory cytokine response to the infection of CoVs and other RNA viruses. This ISG15-dependent MDA5 activation can be antagonized by SARS-CoV-2 papain-like protease catalyzed de-ISGylation.35 SARS-CoV and MERS-CoV endonuclease (EndoU, Nsp15) efficiently prevents the early activation of double-stranded RNA (dsRNA) sensors, such as MDA5, 2′-5′-oligoadenylate synthase (OAS) and double‐stranded RNA‐dependent protein kinase (PKR). EndoU localizes at the site of viral RNA synthesis within the RNA replication complex, suggesting that CoVs have evolved a viral RNA decay pathway to evade early innate and intrinsic antiviral host cell responses.36 SARS-CoV-2 infection also disrupts the function of IFNAR1, JAK and Tyk2 proteins to block IFN signal transduction and induction of ISGs (reviewed in 17, 32). Through the unbiased screen of SARS-CoV-2 proteins for inhibition of IFN response, many were identified to inhibit IFN-I response through distinct mechanisms.37, 38 For example, Nsp6 inhibits IRF3 phosphorylation by binding to TBK1; NSP13 interacts with and impedes TBK1 phosphorylation; and ORF6 inhibits IRF3 nuclear translocation via sequestration of karyopherin α 2 (KPNA2). Interestingly, the Nsp1 and Nsp6 of SARS-CoV-2 interrupt IFN-I signaling more efficiently than those of SARS-CoV and MERS-CoV.37
Figure 2.
SARS-CoV-2 genome structure and functions of viral proteins in viral replication and evasion of innate immune response. SARS-CoV-2 has a single-stranded, positive sense RNA with a cap at its 5′ end and a poly (A) tail of 30–60 nt in length at the 3′ end. The genome encodes 16 non-structural proteins (Nsp1 to Nsp16) and 4 structural proteins (S, spike; E, envelope; M, membrane and N, nucleocapsid) as well as eight accessory proteins. Their functions in viral replication and evasion of innate immune response are listed.
The unique interactions between a specific virus and its host cells at various molecular levels lead to the virus- and cell type-specific transcriptome responses. This phenomenon is highlighted by the transcriptome analysis of SARS-CoV-2 infection. In comparison to other respiratory viruses, such as SARS-CoV, Influenza A virus (IAV), respiratory syncytial virus and parainfluenza virus 3, SARS-CoV-2 infection induces a low and delayed type I and type III IFN response, but increased levels of chemokines and inflammatory cytokines, such as IL-6 and IL-1RA.39 A single cell RNA sequencing analysis of SARS-CoV-2 tropism and antiviral response in primary human airway epithelium revealed that the virus predominantly infects ciliated cells and induction of IFN response within infected cells was low and heterogeneous, while heavily infected secretory cells expressed abundant IL-6.40 Furthermore, SARS-CoV-2 induction of IFN and inflammatory cytokines is tissue specific and associated with clinical conditions. For instance, high levels of IFN-III and modest levels of IFN-I were induced in the cells of the upper airways of patients with high viral burden and mild symptoms, whereas patients experiencing severe COVID-19 symptoms had elevated expression of IFN-I and IFN-III in the cells of the lower airway.41
Innate immune response plays critical roles in HCoV pathogenesis
The correlation between the severity of COVID-19 symptoms and the profiles of IFN and inflammatory cytokine responses indicates the important role of PRR-mediated innate immune response in the SARS-CoV-2 pathogenesis. Specifically, a clinical study with a cohort of COVID-19 patients with varying symptom severities revealed that patients with severe clinical conditions were associated with highly impaired type I interferon responses. This impairment was characterized by undetectable IFN-β levels, low IFN-α production and persistent viral load in their blood, and strong inflammatory responses with increased TNFα and IL-6 productions.42 Additionally, a longitudinal analysis of the natural killer (NK) cells from 205 COVID-19 patients exhibited early elevation of IFN-I in plasma with increased NK cell expression of ISGs and genes involved in IFN-I signaling. This trend was associated with early severe disease, whereas upregulation of TNF-α-induced genes in NK cells was indicative of moderate symptoms. These findings suggest the persistent dysregulation of innate immune responses in NK cells are associated with the severity of COVID-19 manifestation.43
Interestingly, genetic studies in mice argue the protective role of IFN response in CoV infection. For instance, genetic ablation of IFN-I receptor (IFNAR) protected mice from lethal infection by SARS-CoV.41 In addition, blockade of IFN signaling with an anti-IFNAR2 antibody attenuated the overactive inflammation in SARS-CoV-2 infected MISTRG6-hACE2 mice.44 However, accumulating evidence supports a protective role of IFN response in SARS-CoV-2 infection in humans. As illustrated in Figure 1, it was reported recently by several research groups that inborn genetic defects at the loci involved in the TLR3- and IRF7-dependent induction of IFN-I are enriched in patients with life-threatening COVID-19 pneumonia.11, 45, 46, 47 Additionally, a genome-wide association study revealed several gene variants encoding IFN-induced antiviral proteins (OAS1, OAS2 and OAS3) or components involved in IFN signal transduction, such as interferon receptor gene IFNAR2 and tyrosine kinase 2 (TYK2), are associated with the increased risk of suffering from severe COVID-19 symptoms.48 It was estimated that the rare loss-of-function variants in human genes involved in recognizing viral infections and stimulating interferon production or antiviral signaling may account for as many as 3.5% of severe life-threatening COVID-19.11 In support of these genetic findings, higher titers of neutralizing autoantibodies targeting IFN-I were found in 10% of patients with severe COVID-19 pneumonia, however, were not detected in asymptomatic patients or those with mild symptoms.13 The prevalence of these autoantibodies targeting IFN-I was significantly increased in patients over the age of 70 and involved in approximately one-fifth of COVID-19 fatalities.49 These findings thus imply the critical role of IFN response in the control and pathogenesis of SARS-CoV-2 infection. The discrepancy observed between human genetic studies and mice models implies the critical role of species-specific virus-host interaction in the pathogenesis of SARS-CoV-2 infection.
In comparison with the highly pathogenic CoVs, the innate immunity response and its role in the pathogenesis of the common cold HCoVs remains largely unknown. HCoV-229E was found to induce more potent IFN-I response than SARS-CoV does50 and double transgenic hAPN +/+ Stat1 −/− mice gain susceptibility to HCoV-229E infection,51 suggesting that IFN-I and STAT1 pathway play an essential role in the control of HCoV-229E infection. Apparently, the pathobiological role of IFN response in the infection of common cold HCoVs deserves further investigation.
Antiviral effects and therapeutic efficacy of IFNs to HCoV infections
Despite the IFN evasion and antagonistic strategy utilized by CoVs, most HCoVs are sensitive to IFN-I and IFN-III in cultured cells (Table 1 ). However, HCoV-OC43 is enhanced by all three types of IFNs through the induction of IFITM2/3 to facilitate its cellular entry.52 Although replication levels of SARS-CoV and SARS-CoV-2 are comparable in infected cells in culture, SARS-CoV-2 is more sensitive to IFN-I.53 IFN-α treatment was found to reduce viral load and ameliorate pathogenesis by SARS-CoV and MERS-CoV in cynomolgus macaques and rhesus macaques, respectively.54, 55 Moreover, a type III IFN, IFN-λ1a, effectively suppressed the replication of a mouse-adapted SARS-CoV-2 in mice.56 Hoagland et al. found when IFN-α was administered to hamsters intranasally prophylactically or at earlier times (one day) post infection, it reduced viral load and SARS-CoV-2 pathogenesis through the elevation of ISG expression.57 However, intranasal administration of IFN-α to hamsters after onset of symptoms did not improve clinical outcomes of SARS-CoV-2 infection, but was rather associated with signs of more severe disease.58
Table 1.
Susceptibility of human coronavirus to interferon treatment.
| HCoV | Type of Study | IFN | Efficacy | References |
|---|---|---|---|---|
| SARS-CoV-2 | In vitro | IFNβ, IFNλ1 | Inhibition in primary human bronchial epithelial cells. | 211, 212 |
| IFNγ | No effect in primary human bronchial epithelial cells. | 211 | ||
| IFNβ, IFNγ, IFNλ1 | Inhibition of viral replication in Calu-3 cells. | 211 | ||
| IFN-α, IFN-λ | Inhibition of viral replication in human Calu-3 and simian Vero E6 cells. | 213 | ||
| Animal model of hamster | IFN-I (IFN-α A/D) | Intranasal administration of IFN-I limits SARS-CoV-2 replication and inflammation in hamsters. | 57 | |
| Retrospective Observational Study | IFN-α2b | Early administration of IFN-α2b was associated with the reduction of in-hospital mortality in comparison to patients that did not receive IFN-α2b treatment. | 59 | |
| Clinical trial (phase 2) | IFN-β1b | Early treatment with triple combination of interferon IFNβ-1b, lopinavir-ritonavir, and ribavirin was associated with reduced duration of viral shedding compared to the lopinavir-ritonavir treatment group. | 214 | |
| Peg- IFN-λ1 | Acceleration of viral decline and clearance in COVID-19 outpatients. | 62 | ||
| Randomized Controlled Trial | Peg IFN-λ1a | Neither reduced duration of viral shedding nor improved symptoms. | 61 | |
| MERS-CoV | In vitro | IFN-β | Inhibition of viral replication in Calu-3 cells. | 215 |
| IFN-β | Inhibition of viral replication in Vero E6 cells. | 216 | ||
| Animal models | IFN-α2b | Treatment of MERS-CoV infected rhesus macaques with IFN-α2b and ribavirin reduces viral replication, moderates the host response, and improves clinical outcomes. | 55 | |
| IFN-β1b | IFN-β1b improved clinical, radiological, and pathological outcomes while reducing the viral load of the infected Common Marmoset. | 217 | ||
| Combination of lopinavir, ritonavir and IFN-β (LPV/RTV-IFNb) | Therapeutic LPV/RTV-IFN-β improves pulmonary function but does not reduce viral replication or severe lung pathology in human DPP4 mouse model. | 215 | ||
| Observational Study | Ribavirin and/or rIFN-α2a, rIFN-α2b, or rIFN-β1a | RBV/rIFN (RBV and/or rIFN-α2a, rIFN-α2b, or rIFN-β1a) therapy was commonly used in critically ill MERS patients but was not associated with reduction in 90-day mortality or faster MERS-CoV RNA clearance. | 218 | |
| Retrospective cohort study | Ribavirin and IFN-α2a | In patients with severe MERS-CoV infection, ribavirin and interferon alfa-2a therapy is associated with significantly improved survival at 14 days, but not at 28 days. | 219 | |
| Randomized Controlled Trial | IFN-β1b | Early treatment of IFN-β1b and lopinavir-ritonavir led to lower mortality in comparison to the placebo treatment. | 60 | |
| SARS-CoV | In vitro | IFN-α | IFN lead to a modest reduction in viral titer in Calu-3 cells. | 53 |
| IFN alfacon 1 | Inhibit viral yield in Calu-3 cell line. | 220 | ||
| IFN-α, IFN-β | IFN-α and IFN-β protected cells from CPE and reduced viral replication in Vero, Caco2 and Frhk-4 cells. | 221, 222, 223 | ||
| Animal model | IFN-α | IFN-α significantly reduces viral replication and excretion in cynomolgus macaques. | 54 | |
| Retrospective cohort study | IFN alfacon 1 plus corticosteroids | The combination of Interferon alfacon-1 plus corticosteroids was associated with reduced disease-associated impaired oxygen saturation, more rapid resolution of radiographic lung abnormalities, and lower levels of creatine kinase. | 224 | |
| HCoV-NL63 | Clinical trial (Single case) | IFN-α2b | Treatment of IFN-α2b eliminated NL63 infection in a leukemia patient. | 225 |
| HCoV-OC43 | In vitro | IFN-α, IFNγ, IFNλ | Enhanced viral infection in cells via the induction of IFITM2/3. | 52 |
Similar to the results obtained from animal models infected with SARS-CoV-2, Wang et al. found in a retrospective clinical study that IFN-α2b treatment of COVID-19 patients was only beneficial if the treatment started within 5 days of post-hospital admission, whereas IFN treatment administered later resulted in a slower recovery and extended hospital stay.59 Likewise, patients with MERS-CoV infection gained beneficial outcomes with earlier treatment regimens of IFN-β.60 However, the clinical efficacy of pegylated IFN-λ for COVID-19 remains controversial. Peg-IFN-λ-1a did not ameliorate symptoms or reduce SARS-CoV-2 viral shedding time in outpatients with mild to moderate COVID-19.61, 62 However, another study showed peg-IFN-λ accelerated viral clearance in COVID-19 outpatients with a high baseline of viral load.62 Collectively, these results indicate that administration of IFN-α may not always be beneficial for all the patients, but may be useful for subsets of patients who bear a high viral load and receive it in the early stage of infection.
Systematic identification of IFN-induced cellular proteins that restrict HCoV infection.
IFNs control viral infections by inducing the expression of IFN-stimulated genes (ISGs) that coordinately restrict viral replication.63 The ISGs that modulate viral replication can be identified by systematic gain-of-function or loss-of-function genetic screens.64, 65, 66 Currently, four independent gain-of-function genetic screens were performed to identify ISGs inhibiting the infection of SARS-CoV-2 and HCoV-229E.
Martin-Sancho et al. screened 399 ISGs with sequence validated full-length cDNA clones in HEK293 cells through the co-transfection of V5-epitope tagged individual ISG in the lentiviral expression vector, pLX304, with plasmids expressing human ACE2 and TMPRSS2.67 A total of 65 ISGs were identified to significantly inhibit SARS-CoV-2 infection while 38 ISGs were further validated in HEK293-ACE2-derived cell lines stably expressing a V5-tagged individual ISG. Eight ISGs, including MyD88, STAT1, STAT2, DDX60, ELF1, REC8, TRIM21 and CLEC4D, were identified as upstream signaling components and regulators of PRR/IFN pathways which execute their antiviral activity through the transcriptional activation of other cellular proteins. The remaining ISGs were comprised of well characterized IFN-induced direct-acting antiviral effector proteins (IFITM2, IFITM3, BST2, Viperin and IFIT) as well as unfamiliar ISGs that inhibit cellular entry (UBD and FAM46C) and viral RNA synthesis (SPATS2L, DNAJC6, RGSS2, LOC152225, ZBP1, B4GALT5). A highly enriched cluster of endoplasmic reticulum (ER)/Golgi-resident ISGs inhibits virion assembly/egress (ERLIN1, APOL2, HSPA8, etc). While IFITM2, IFITM3, ZBP1 and IFIT1 were demonstrated to restrict multiple RNA viral infections, eight ISGs, including ER/Golgi-resident proteins NAPA, APOL2, and ERLIN1, were found to inhibit the replication of both SARS-CoV and SARS-CoV-2, but did not have this effect on the other twenty RNA viruses tested. These results imply that regulation of the membrane composition at sites relevant for viral replication or trafficking is a key target for IFN-induced innate immune control of SARS-CoV-2 infection (Figure 2).
However, ectopic expression of more than 500 arrayed individual ISGs in ACE2-expressing and interferon regulatory factor 3 (IRF3)–deficient A549 cells (A549-Npro-ACE2) identified only six ISGs that inhibit SARS-CoV-2 infection/replication without inducing substantial toxicity or inducing interferon-stimulated response element (ISRE) expression68. While OAS1 efficiently inhibited SARS-CoV-2 infection of A549-ACE2 cells irrespective of serine protease transmembrane serine protease 2 (TMPRSS2) expression status, other five ISGs, including NCOA7, UNC93B1, CCARB2, ANKF1 and ZBTB42, only inhibited SARS-CoV-2 infection in the absence of TMPRSS2 expression, suggesting TMPRSS2-mediated SARS-CoV-2 entry evades the restriction of these antiviral factors.69, 70
By inducible CRISPR activation (CRISPRa) screen of pooled 414 ISGs for identification of individual ISGs that confer resistance to the cytopathic effects of SARS-CoV-2 in A549 lung adenocarcinoma cells, Oded Danziger et al. identified several previously known SARS-CoV-2 restriction factors, such as LY6E, CD74, IFITM1 and OAS1, as well as new candidate ISGs with putative antiviral activity to SARS-CoV-2, such as ERLIN1, ADPRHL2 and CTSS.71 In comparison to a cDNA over-expression screen, CRISPRa activates the transcription of ISG gene from its endogenous promoter enabling expression of multiple gene isoforms in physiologically relevant levels. This approach offers clear advantage for the identification of ISGs with only one of its isoforms restricting SARS-CoV-2 infection, such as CD74.72
Through the screening of more than 350 human ISG cDNAs in human hepatoma cells (Huh7), Pfaender at al. identified 33 ISGs that inhibited HCoV-229E infection by more than 20% at 24 h post infection.73 Some broad-spectrum antiviral effectors aside from the PRR/IFN signaling modulators, such as IFITM2, IFITM3, BST2, IFIT1, OAS2, OAS3, and LY6E, were identified to inhibit HCoV-229E infection. Several ISGs that inhibit SARS-CoV-2, including APOL2, RAB27A, FAM46A and FAM46C, also inhibit HCoV-229E. However, ISGs that potently inhibit HCoV-229E, such as SQLE, SLC1A1, and STARD5, were not identified to inhibit SARS-CoV-2. This finding suggests that there are overlapping but distinct antiviral networks induced by IFNs to restrict different HCoVs.
ISGs that inhibit distinct steps of HCoV replication
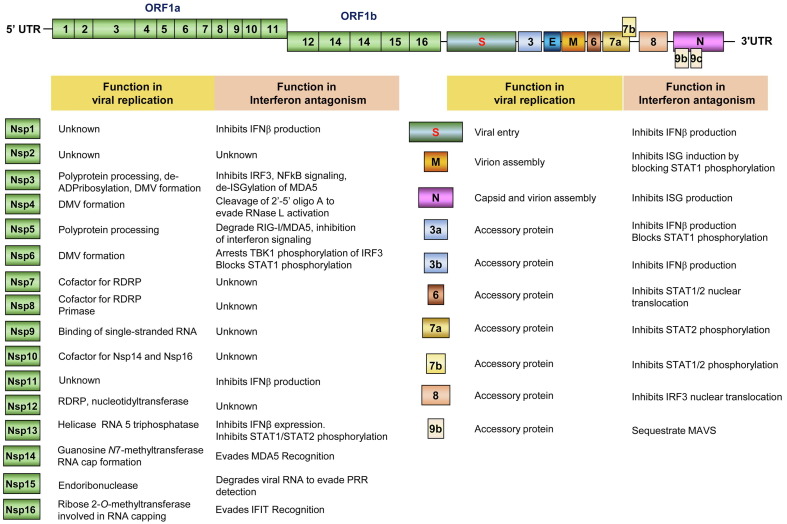
As summarized in Table 2 and illustrated in Figure 3 , many IFN-induced proteins have been identified to directly inhibit specific steps of HCoV replication. The antiviral properties and modes of action of well-studied ISGs as well as viral strategies to evade their actions will be further discussed.
Table 2.
ISGs directly-inhibiting coronavirus replication.
| ISG | Activity | Mechanism | References |
|---|---|---|---|
| ISGs inhibit the cellular entry of HCoVs | |||
| IFITM1, IFITM2 and IFITM3 | Inhibits cellular entry of all the known HCoVs except for HCoV-OC43, which uses human IFITM2 and IFITM3 as entry factors to facilitate its infection of target cells. | Inhibition or promotion of the fusion of viral envelopes with cellular membranes. | 52, 78, 86, 226, 227, 228 |
| LY6E | Inhibits cellular entry of all the known HCoVs. | Interrupts the fusion of the viral envelope and cellular membranes as well as impairs syncytia formation. | 73, 76 |
| CH25H | Inhibits cellular entry of SARS-CoV-1, SARS-CoV-2 and MERS-CoV. | Inhibits the fusion of viral envelopes and cellular plasma membranes through the reduction of cholesterol in the plasma membrane. | 132, 133 |
| GILT | Inhibits cellular entry of SARS-CoV-1. | Inhibition of SARS-CoV-1 entry in lysosomes via disruption of the stability and activity of cathepsin L. | 121 |
| CD74 | Inhibits the cellular entry of SARS-CoV-1 and SARS-CoV-2. | Inhibition of cathepsin L-mediated cleavage of viral spike proteins. | 72 |
| FAM46C, UBD, CLEC4D | Inhibit viral entry mediated by spike protein of SARS-CoV-2. | To be determined. | 67 |
| ISGs that bind viral RNA and inhibit viral replication | |||
| IFIT1, IFIT3 and IFIT5 | Inhibit SARS-CoV-2 RNA replication. | Prevention of active viral RNA replication by sequestering the single-stranded 5′-ppp or 2′-O-unmethylated RNA. | 67 |
| IFIT2 | Inhibits MHV RNA replication. | 167, 229 | |
| ZAP | Restricts SARS-CoV-2 replication. | Binds CpG dinucleotides in viral RNA to mark it for degradation | 142, 143, 144 |
| OAS1-RNase L | Restricts SARS-CoV-2 replication. | Synthesize 2′-5′oligo (A) that actives RNase L to degrade viral and cellular RNA. | 19 |
| SPATS2L, DNAJC6, RGSS2, LOC152225, ZBP1 B4GALT5 | To be determined. | 67 | |
| ER-Golgi-associated proteins that inhibit virion assembly and egress | |||
| Viperin | Inhibits PEDV replication. | Restricts viral replication and/or assembly via interactions with N proteins. | 230 |
| BST2 | Inhibits the release of HCoV-229E and SARS-CoV-2 virion particles. | Tethers virions on the cellular surfaces or intracellular membranes. | 67, 193, 202 |
| HSPA8, CNP, Rab27a, ERLIN1 | Inhibits late-stage SARS-CoV-2 replication. | To be determined. | 67 |
Figure 3.
IFN-induced antiviral proteins inhibit distinct steps of CoV replication. Systematic genetic screens identified more than 30 ISGs that directly inhibit SARS-CoV-2 and/or HCoV-229E replication. The replication steps inhibited by some of these ISGs are illustrated.
Multiple IFN-induced proteins inhibit HCoV entry into host cells
Interferon-induced transmembrane proteins (IFITMs)
The human IFITM family has five members, including IFITM1, IFITM2, IFITM3, IFITM5 and IFITM10. However, only IFITM1, IFITM2, and IFITM3 are IFN-inducible and inhibit the cellular entry of a broad spectrum of enveloped viruses.74, 75 IFITMs inhibit the entry of multiple HCoVs, including HCoV-NL63, 229E, MERS-CoV, SARS-CoV, SARS-CoV-2,76, 77, 78, 79 while IFITM2 and IFITM3 facilitate the entry of HCoV-OC43.52, 78 As potent viral restriction factors, IFITM genes evolve in vertebrates under the selective pressure of microorganism infection.80 Thus, a dozen of single-nucleotide polymorphisms (SNPs) have been identified in the human population, some of which associate with severity and prognosis of viral infection (reviewed in 81). SNP rs12252 and rs34481144, two well-studied IFITM3 SNPs associated with severe outcomes of IAV infection, have also be reported to associate with COVID-19 mortality or increased risk of hospitalization.82, 83, 84
IFITM proteins are widely expressed in human tissues and cell types. IFITM1 primarily localizes in the cellular plasma membrane and early endosomes,85 whereas IFITM2 and IFITM3 mainly co-localize with Rab7, CD63, and lysosome-associated membrane protein 1 (LAMP1) in late endosomes and lysosomes.86, 87 The cellular entry of HCoVs requires sequential protease processing of the viral spike glycoprotein to trigger membrane fusion. This processing takes place in either the plasma membrane or the endolysosomal membranes, depending on the structural property of the viral spike protein and the subcellular distribution of cellular protease that cleave viral spike protein. Accumulating evidence supports the notion that the presence of IFITM proteins at the site of viral membrane fusion is essential for their restriction of virus entry. Thus, the entry route of CoVs will largely determine their susceptibility to the different IFITMs. Although SARS-CoV-2 utilizes both early endosomes and lysosomes to fulfill infectious entry,88 its infection is mainlyrestricted by IFITM2 and the removal of the furin cleavage site in the spike protein renders SARS-CoV-2 entry predominant via late endosomes and makes the infection more sensitive to IFITM2 restriction.89 Moreover, the depletion of IFITM2 attenuates the antiviral effect of IFN-I to SARS-CoV-2 infection.89 Interestingly, SARS-CoV-2 utilizes S1/S2 furin cleavage sites and TMPRSS2 to escape the endosomal IFITM2 and IFITM3 restriction, which contribute to SARS-CoV-2 transmission in ferrets.70 However, a recent study reported that the endogenous expression but not artificial overexpression of IFITMs aids SARS-CoV-2 infection in human lung cells.90 The discrepancy of those studies is not clear and deserves further investigation. It is important to point out that IFITMs can be incorporated into the viral envelope and IFITM proteins on both viral and cellular membranes can restrict the infectious entry of various enveloped viruses. The restriction occurs through the blockade of viral fusion, but not the endocytosis of virions into the cells.91, 92, 93, 94
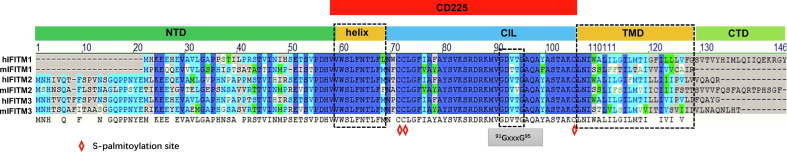
IFITMs belong to the CD225 transmembrane protein superfamily which share the CD225 domain and play important roles in many cellular events through the regulation of membrane fusion, such as neurotransmission, immunity and metabolism.95, 96 As illustrated in Figure 4 , the CD225 domain is defined by two hydrophobic regions separated by a cytoplasmic intracellular loop. One of the hydrophobic regions is a transmembrane helix and the other is an intramembrane helix. Recent studies revealed that the intramembrane helix of IFITMs is amphipathic and required for antiviral activity.97 It was reported recently that the amphipathic helix spanning amino acid residues 59–68 partitions into lipid-disordered domains at the site of IAV fusion to block the fusion pore formation by inducing negative membrane curvature and increasing the membrane lipid order and stiffness.98 Furthermore, a 91GxxxG95 motif in the CD225 domain of IFITM3 drives its oligomerization, which is essential for its antiviral functions.99 In addition to directly modulating membrane physical property at the site of membrane fusion, IFITMs may also inhibit viral entry through the regulation of endosomal trafficking. IFITM3 inhibition of the cellular entry of reovirus, a naked capsid virus, seems to support this notion.100 Moreover, site-specific fluorophore tagging and live-cell imaging studies revealed that IFITM3 on endocytic vesicles engages with and promotes the trafficking of incoming virus particles to lysosomes in a S-palmitoylation dependent manner.101 S-palmitoylation of IFITMs, a conserved post-translational modification for all the IFITM proteins, may assist IFITM conformation alteration, association with specific membrane domains or interaction with other proteins and is required for restriction of all the viruses examined.102, 103 It is thus likely that the S-palmitoylation of IFITMs functions in both inhibition of membrane fusion and direction of incoming virions to lysosomes for degradation.104
Figure 4.
Domain structure and critical motifs of interferon-induced transmembrane (IFITM) proteins. (A) Membrane topology of IFITM proteins. (B) Domain structure of human and mouse IFITM proteins. NTD, N-terminal domain; CTD, C-terminal domain, Helix, amphipathic alpha helix; CIL, conserved intracellular loop; TMD, Transmembrane domain. CD225 domain contains amphipathic alpha helix and CIL domains. The oligomerization motif and cysteines for palmitoylation are indicated.
LY6E
LY6E (Lymphocyte antigen 6 complex, locus E), also referred to as TSA-1 (Thymic Shared Antigen-1), SCA-2 (Stem Cell Antigen-2) or RIG-E (Retinoic Acid Inducible Gene E), is a member of Ly-6/uPAR protein family (Lymphocyte antigen-6/urokinase-type plasminogen activator receptor).105 LY6E was initially found to be expressed in immature CD4− CD8− thymocytes106 and functions in T cell development and activation.107 However, LY6E is also found to be an IFN-inducible protein108 and undergoes active transcription in various organs such as the liver, uterus, spleen, ovary, lung, and brain.105 As a GPI-anchored protein, LY6E is enriched in the lipid-raft microdomain of the plasma membrane and endosomes109 and functions in modulating the infectious entry of multiple enveloped viruses.79, 110 Specifically, LY6E differentially regulates HIV-1 entry in a CD4-dependant manner111, 112 and enhances the infectivity of multiple enveloped RNA viruses, including Yellow Fever Virus (YFV), Dengue Virus (DENV), West Nile Virus (WNV), Chikungunya virus (CHIKV), OYANG virus (ONV) and influenza A virus (IAV/PR8).109 LY6E also serves as the receptor for the endogenous retroviral envelope in mice, Syncytin-A (SynA). This interaction is essential for syncytiotrophoblast formation during the development of the murine placenta.113
LY6E potently restricts the cellular entry of multiple CoVs, including mouse hepatitis virus (MHV) and all the HCoVs examined,73, 76 through inhibition of viral spike protein-induced membrane fusion.73 Its critical role in the control of CoVs infection is demonstrated by the observation that mice lacking LY6E in their immune cells were highly susceptible to MHV infection.73 Interestingly, unlike IFITMs, amphotericin B treatment does not attenuate the restriction of HCoVs by LY6E, suggesting LY6E inhibits CoV spike protein induced membrane fusion via a distinct mechanism.76
CD74
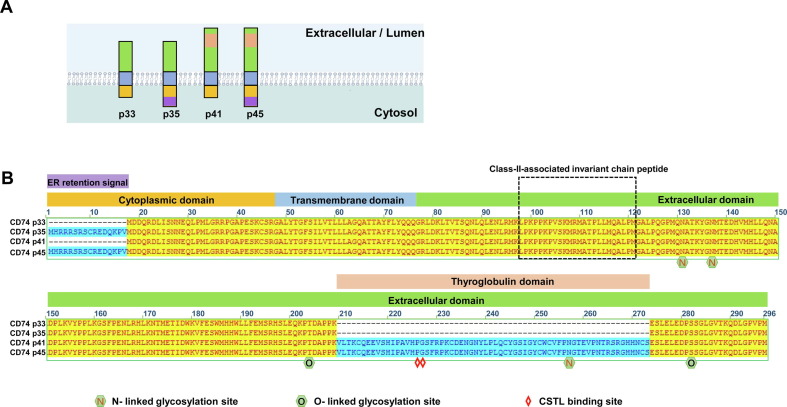
CD74 (also designated as the invariant chain protein, Ii) is primarily expressed in antigen-presenting cells as an MHC class II chaperone. In macrophages, CD74 serves as a receptor for migration inhibitory factors (MIF) and is expressed on macrophages that participate in inflammatory responses.114 CD74 is a type II transmembrane protein and its intracellular domain (ICD) is essential for the activation of NFκB and other signaling pathways upon MIF binding. In humans, four CD74 isoforms(p31, p35, p41 and p43) are expressed due to alternative splicing. As shown in Figure 5 , these isoforms differ in the presence or absence of N-terminal ER-retention signal and internal thyroglobulin domain.115
Figure 5.
Domain structure and splicing variants of CD74. (A) Membrane topology of CD74 proteins. (B) The topogenic feature and key structure domains or motifs are indicated. The structural features of the four CD74 variants are highlighted.
CD74 is an IFN-inducible protein. The p41 isoform specifically inhibited the endosomal entry of Ebola virus (EBOV) and HCoVs, including SARS-CoV-2.72 This isoform contains the thyroglobulin domain, lacks ER retention signal, and normally accumulates in endosomes. The CD74 thyroglobulin domain inhibits cathepsins and when its cathepsin L binding site is mutated it completely abolishes its ability to restrict viral entry.72, 116 Accordingly, apart from its roles in antigen presentation and inflammation, CD74 functions as a crucial restriction factor which inhibits endolysosomal entry of viruses through the inhibition of cathepsin cleavage of viral envelope proteins.
GILT
GILT (Gamma-interferon-inducible lysosomal thiol reductase, also referred to as IFI30) is a soluble thiol reductase responsible for the reduction of protein disulfide bonds in specific conditions. This enzyme is abundantly expressed in the lysosomes of professional antigen-presenting cells (APCs), such as macrophages, dendritic cells and B lymphocytes, but can be induced by IFN-γ in other cell types.117, 118 The role of GILT in adaptive immunity has been well characterized. Specifically, GILT promotes the MHC class II-restricted presentation of exogenous antigens containing disulfide bonds. GILT can also facilitate the MHC class I-restricted recognition of such antigens by CD8+ T cells via cross presentation.119, 120
GILT is synthesized as a 35-kDa precursor and is directed to the endocytic pathway via the mannose-6 phosphate receptor (M6PR). The enzyme is transported into the lysosomes which will provide the optimal acidic pH for its thiol reductase activity.118, 120 Mature GILT utilizes two cysteine residues in the 72CXXC75 reductase motif to reduce the disulfide bonds of endocytosed antigens, thus, facilitating antigen processing and presentation by MHC-II and MHC-I.119, 120
Recent studies revealed that GILT only inhibited the cellular entry of SARS-CoV, EBOV, and LASV, all of which enter host cells through the lysosome.121 Furthermore, GILT mutations that impair the thiol reductase activity or disrupt N-linked glycosylation, an essential post-translational modification for lysosomal localization, largely compromised its restriction of viral entry. Additionally, the induction of GILT expression reduced the level and activity of cathepsin L, the lysosomal endopeptidase enzyme required for the lysosomal entry of susceptible RNA viruses. These results suggest that GILT is a novel antiviral ISG that specifically inhibits the entry of selected enveloped RNA viruses in lysosomes through the inhibition of cathepsin L activity.
CH25H
Cholesterol-25-hydroxylase (CH25H), a conserved antiviral ISG in mammalian species, contributes to the regulation of cholesterol biosynthesis. CH25H encodes an ER-associated enzyme that converts cholesterol to a soluble antiviral factor, 25-hydroxycholesterol (25HC).122 The expression of CH25H and 25HC treatment is known to broadly inhibit the multiple enveloped viral infections.79, 123, 124, 125, 126 Studies have shown that CH25H-knockout mice exhibited increased susceptibility to murine gamma herpesvirus 68 (MHV68) infection.124 Through the oxidation of cholesterol, 25HC becomes a soluble oxysterol capable of inhibiting the entry of many enveloped viruses through the disruption of membrane fusion, due to the alteration of the membrane’s hydrophobicity or cholesterol composition.125 In support of its antiviral effects, the significant restriction of HIV replication and rescued T-cell depletion upon the administration of 25HC has been demonstrated in mice models.124 Administration of 25HC also reduced viremia and protected mice and rhesus macaques from ZIKV infection.125
In addition to directly interfering with membrane fusion, CH25H abrogates LASV G1 glycoprotein N-glycan maturation, thus suppressing the production of infectious virions.127 CH25H inhibited HCV infection at post entry stages by suppressing the maturation of SREBPs which are critical transcription factors for host lipid biosynthesis.128 Another study demonstrated that 25HC inhibited HCV infection through blocking the formation of the membranous web which serves as a viral RNA replication organelle.129 Interestingly, CH25H is involved in B cell migration through the generation of 7α,25-OHC, a chemokine that directs immune cell migration.130, 131
CH25H and 25HC have demonstrated the ability to inhibit cellular entry of many CoVs, including SARS-CoV,132, 133 SARS-CoV-2,132, 133 MERS-CoV,132 porcine epidemic diarrhea virus (PEDV),134 and Porcine deltacoronavirus (PDCoV).135 Of note, a potent antiviral effect of 25HC was observed in mouse model by using a mice-adapted SARS-CoV-2 strain MASCp6.136 Mechanistically, CH25H inhibits CoV cellular entry by interfering with S protein-mediated membrane fusion.132, 133 Particularly, internalized 25HC accumulates in the late endosomes and lysosomes to inhibit membrane fusion.133 Plasma membrane fusion is also restricted by 25HC through the activation of ER-resident acyl-CoA: cholesterol acyltransferase (ACAT) which depletes the accessible cholesterol from the plasma membrane.132
IFN-induced cellular proteins targeting HCoV RNA
Zinc finger antiviral protein (ZAP)
ZAP is an RNA binding protein containing an N-terminal ∼227-aa RNA-binding domain (RBD) with 4 CCCH zinc fingers (ZnF1 to ZnF4), a WWE domain, and a C-terminal poly(ADP-ribose) polymerase domain which is present in the ZAP-L isoform but not in the ZAP-S isoform.137 ZAP broadly inhibits the infection of various RNA viruses through the detection of specific viral RNAs that have a greater frequency of CG dinucleotides than cellular RNAs. These dinucleotides promote viral RNA degradation via its co-factor, KHNYN, a presumed endonuclease that is important for ZAP antiviral activity against retroviruses138 or the RNA exosome.139 Crystal structural analysis of the protein-RNA complex containing the N-terminal 4-zinc finger human ZAP RNA-binding domain (RBD) and the CG dinucleotide-containing RNA revealed the formation of a CG-binding pocket from the four zinc fingers on the ZAP RBD surface specifically accommodating the CG dinucleotide.140 However, ZAP induces the pregenomic RNA degradation of hepatitis B virus through the recognition of a stem-loop (ɛ) structure at the 5′ terminal region of the viral RNA in the 4-zinc finger-dependent manner.141
Recent studies indicated that ZAP restricts SARS-CoV-2 by targeting CpG dinucleotides in viral RNA sequences.142, 143, 144 Interestingly, SARS-CoV-2 and two closely-related animal CoVs isolated from horseshoe bats, RaTG13 and RmYN02, exhibited remarkable CpG suppression in comparison to other human and bat CoVs.142 These findings imply the CG depression observed in SARS-CoV-2 genome may be a result of adaptive selection to evade ZAP restriction, which highlights the critical role of ZAP in the control of CoVs infection, thus, shaping the evolution of viruses.145, 146
2′,5′-Oligoadenylate synthetases (OAS)/RNase L pathway
The 2′,5′-oligoadenylate (2-5A) synthetase (OAS)-RNase L system is a potent IFN-induced antiviral pathway that restricts the infection of a diverse group of viruses. Three human OAS species, OAS1, OAS2, and OAS3, can bind cytosolic dsRNA and be activated to synthesize 2′-5′ oligoadenylates (2-5A).147 Binding of 2-5A to RNase-L induces its dimerization and activation of nuclease activity. This results in the degradation of both the cellular and viral RNA as well as the consequential inhibition of viral replication and induction of infected cell death.10 Although OAS proteins are highly IFN-inducible, the basal levels of OAS expression can be detected in many cell types. Interestingly, although all three human OAS proteins synthesize 2-5A upon binding of dsRNA, it was demonstrated that only OAS3 is primarily responsible for the activation of RNase L in cells infected with a diverse group of viruses, including CoVs.148 The dominant role of OAS3 in viral activation of RNase L may be attributed to its higher binding affinity binding to dsRNA. However, it was reported recently that the induction or over-expression of OAS1, but not OAS2 or OAS3, by CRISPR activation or cDNA transfection significantly protects against SARS-CoV-2 induced cytopathic effects and reduces viral yield. Further mechanistic analysis demonstrated that OAS1 inhibition of SARS-CoV-2 is dependent on its oligoadenylate synthesis activity.71 A knockout of RNase L in A549ACE2 cells increased replication of SARS-CoV-2 and cytopathic effect.19 Moreover, OAS1 genetic variants were found to be associated with infection and morbidity of SARS-CoV while the single nucleotide polymorphisms (SNPs) in the OAS loci are linked to COVID-19 mortality48, 149, 150 (Figure 1). A Neanderthal isoform of OAS1 in individuals of European ancestry (rs4767027-T and rs10774671-G) was associated with reduced COVID-19 susceptibility and severity.151 OAS1 alternative splicing is regulated by the rs10774671-G allele, which increases the isoform p46. The p46 isoform has a higher enzymatic activity against viruses than the p42 isoform.152 Mechanistically, it was reported by two independent research groups that OAS1 p46 isoform is prenylated at the terminus, which targets the ER membrane to enhance its accessibility to viral RNA for more efficient activation thus increasing the antiviral activity to viruses that replicate their RNA genome in membranous organelles. These viruses include flaviviruses, piconaviruses and human coronaviruses, such as SARS-CoV-2.68, 153
As anticipated, CoVs have evolved multiple strategies to counteract OAS-RNase L pathway to facilitate their replication. For example, MHV employs Nsp2, an accessory protein that possesses 2′,5′-phosphodiesterase activity, to cleave 2′,5′-oligoadenylates and prevent RNase L activation.154, 155 MERS-CoV as well as closely-related bat CoVs also encode accessory proteins Nsp4A and Nsp4B with phosphodiesterase activity to inhibit the activation of OAS-RNase L pathway.156, 157 The critical role of OAS-RNase L system in defending CoV infection and pathogenesis is highlighted by the observation that Nsp2 antagonism of this intricate dsRNA sensor pathway is required for MHV replication in the liver and induction of hepatitis.154 However, the result that OAS1 restricts SARS-CoV-2 replication in A549 cells and protects SARS-CoV-2 infection and pathogenesis in humans suggests that SARS-CoV-2 may not evade this antiviral pathway. Indeed, a recent study on SARS-CoV-2 activation of innate immune response in the relevant respiratory tract derived cells shows that SARS-CoV-2 activates OAS–RNase L and PKR, while inducing minimal levels of interferon. These phenomena indicate viral SARS-CoV-2 evasion of RIG-I/MDA5 detection in contrast to MERS-CoV infection, which evades the activation of all the three dsRNA detection pathways.19 It will be interesting to uncover why OAS1, but not OAS2 or OAS3, restricts SARS-CoV-2 infection and whether different CoVs activate distinct OAS species.
IFIT1 inhibits viral protein translation
There are 4 members of the interferon-induced proteins with tetratricopeptide repeats (IFITs) family found humans which include: IFIT1 (ISG56), IFIT2 (ISG54), IFIT3 (ISG60) and IFIT5 (ISG58).158, 159 The expression of IFITs can be induced by the activation of a IFN regulatory factor (IRF) family of transcription factors in viral infection and by dsRNA recognition independently of IFN production. IFIT proteins contain multiple copies of tetratricopeptide repeats (TPR), a conserved motif composed of 34 amino acid residues, which adopt a helix–turn–helix structure.158, 159 The TPRs of IFITs coalesce into distinct super helical subdomains that form clamp-shaped structures.160, 161, 162
IFITs execute their antiviral function by specifically interacting with a range of cellular and viral RNAs and proteins. For instance, IFIT1 and IFIT2 bind to the multisubunit eukaryotic translation initiation factor 3 (eIF3) which inhibits viral and cellular mRNA translation initiation.163, 164 IFIT1 can bind to the 5′ ends of the mRNA lacking the ribose 2′-O methylation on the first cap-proximal nucleotide, a hallmark of some viral mRNAs but not cellular mRNAs. This binding selectively inhibits the translation of viral mRNA.165, 166, 167 In addition, IFIT1 also specifically recognizes and sequesters the viral RNA replication intermediates with uncapped 5′-ppp termini to inhibit viral RNA replication.160, 168 Therefore, IFIT1 is a unique pattern recognition receptor and antiviral effector molecule,169 whereas, other human IFIT members do not have a preferable binding affinity to those viral RNA molecular patterns.165, 170
IFIT1 has been shown to inhibit the replication of RNA viruses which produce RNA with 5′-ppp, such as VSV and Rift valley fever virus.170 IFIT1 also inhibits HCV171 and parainfluenza virus 3 and 5.172, 173 Four groups of viruses, including CoVs, flaviviruses, reoviruses, and poxviruses, encode enzymes to catalyze the methylation at the 2′-O-ribose of the first viral RNA nucleotide for evasion of recognition by IFIT1.174 SARS-CoV and SARS-CoV-2 Nsp10/Nsp16 heterodimer forms a 2′-O methyltransferase.175, 176 Mutant CoVs lacking 2′-O methyltransferase activity were observed to be more vulnerable to antiviral action of IFN and IFIT than their wild-type counterparts.177 Furthermore, attenuated vaccines with mutations at Nsp10 or/and Nsp16 have been developed due to the observation that targeting 2′-O-MTase activity will reduce viral proliferation and virulence.178, 179, 180
BST-2 inhibits the release of HCoV virions
Bone marrow stromal cell antigen 2 (BST-2), also referred to as CD317 or tetherin, is a type II transmembrane protein with a short intracellular N-terminal domain, an extracellular coil-coil domain and a glycosyl phosphatidyl inositol (GPI) membrane anchor at its C terminus. The GPI membrane anchor directs BST-2 association with cholesterol-enriched lipid rafts in the cellular membrane. BST2 traffics through the ER and Golgi and localizes in the plasma membrane and endosomes.181, 182 BST2 blocks the release of many enveloped viruses from infected cells, including members of the following families: Retroviridae (HIV-1),183, 184 Arenaviridae (Lassa virus),185 Paramyxoviridae (Nipah and Hendra virus),186, 187 Orthomyxoviidae (Influenza A virus),188, 189 Rhabdoviridae (vesicular stomatitis virus),94 Filoviridae (Ebola and Marburg viruses),183 and Hepadnaviridae (Hepatitis B virus).190 Accumulating evidence suggests BST-2 is incorporated into budding viral particles and through coil-coil domain mediated homo-oligomerization with other BST-2 molecules in the cellular membranes, viral particles become 'tethered' to the cell surface or intracellular membranes.191 Unlike HIV and many other viruses that bud from the plasma membrane, CoVs bud in the ER-Golgi apparatus intermediate compartment (ERGIC) and are transported to the plasma membrane inside vesicles.192 However, it has been shown that BST2 also restricts the replication of HCoV-229E and SARS-CoV-2 by tethering progeny virions on the cellular surface and intracellular membranes.67, 193 This observation suggests that BST-2 can also restrict viruses that bud in the ERGIC and are then released from the cell via vesicle fusion.
Viruses have evolved many strategies to evade BST2 restriction by inducing its degradation or directing it away from its site of action on the cellular surface. For instance, primate lentiviruses employ at least three accessory proteins including Vpu, Nef, and Env, to counteract tetherin.194, 195 HIV-1′s Vpu interacts with BST2 via its transmembrane domains and directs BST2 to the lysosome and/or the proteasome for degradation.196, 197, 198 HIV-2 and some SIV strains utilize their Env and Nef proteins, respectively, to downregulate BST2 expression on the cellular surface through sequestrating it to endocytic compartments.199, 200, 201 SARS-CoV ORF7a selectively binds to unglycosylated BST2 to prevent its glycosylation, which is required for restriction of SARS-CoV release from infected cells. However, the interaction between SARS-CoV-2 ORF7a and BST2 does not depend on BST2 glycosylation status.67, 202 In addition, both SARS-CoV and SARS-CoV-2 spike proteins downregulate BST2 to facilitate viral spread.203, 204 However, BST-2 can be hijacked by human cytomegalovirus to enhance cellular entry. In other studies, murine BST2 was found to promote measles virus infection in brains of permissive mice and in primary neuron cultures.205, 206
Perspectives
The activation and evasion of the innate immune response are the key determinants of viral host range and cell tropism and thus, effect cross-species transmission and pathogenesis. In addition to antiviral drugs that directly target viral proteins or RNA for specific inhibition of viral replication, drugs that disrupt the viral evasion of host innate immune response should facilitate immune control and resolution of viral infections.207 Furthermore, mutant viruses that fail to counteract the innate immune restriction could be candidates for attenuated vaccines, which could induce more efficient and durable protection from HCoV infection and/or diseases.208, 209 Therefore, having a better understanding of the molecular mechanism of innate immune control and HCoV evasion should facilitate the discovery and development of novel antiviral drugs and vaccines for the treatment of CoV diseases and prevention of CoV infections. These developments will particularly be important for future zoonotic CoV epidemics in humans.210
While recent intensive investigation on the innate immune response to SARS-CoV-2 infection uncovered important features of the virus-host cell interactions and provided many mechanistic insights, some important questions on the role and mechanisms of innate immune response in SARS-CoV-2 pathogenesis have yet to be answered. First, the innate immune response of specific cell types, such as immune cells (NK cells and pDCs) and distinct cell types in the lung epithelium, to CoV pathogenesis should be further investigated. Second, it appears that CoVs can activate all the three families of known cytoplasmic dsRNA sensors, i.e., RIG-I-like receptors, PKR, and OAS. The mechanisms underlying the activation of these dsRNA receptors and their contributions to CoV pathogenesis and control should be dissected. Third, it is rather surprising that OAS3 is primarily responsible for RNase L activation in the infection of CoVs and several other RNA viruses.19 However, forward genetic screen and population genetics studies demonstrated that OAS1 restricts SARS-CoV-2 infection and plays a critical role in SARS-CoV-2 pathogenesis.71 Therefore, further investigation on the mechanisms of activation and biological function of the distinct members of the OAS family in the infection and pathogenesis of CoVs and other viruses is warranted. Finally, it is important to apply our understanding on the innate immune restriction and viral evasion of PRR- and IFN-mediated antiviral immune responses in the discovery and development of therapeutic and prophylactic medicines to control the infection of CoVs and other viruses. Particularly, the development of small molecular antiviral agents disrupting the key interaction between the viral and cellular proteins for the evasion of IFN restriction of viral replication hold great promise in the treatment of coronavirus diseases.
CRediT authorship contribution statement
Xuesen Zhao: Conceptualization, Writing – original draft, Writing – review & editing, Funding acquisition, Supervision. Danying Chen: Writing – original draft, Visualization. Xinglin Li: Writing – original draft, Visualization. Lauren Griffith: Writing – review & editing. Jinhong Chang: Writing – review & editing, Funding acquisition. Ping An: Writing – review & editing, Visualization. Ju-Tao Guo: Conceptualization, Writing – review & editing, Visualization, Funding acquisition, Supervision.
Acknowledgments
Acknowledgements
This work was partially supported by grants from the National Institute of Health, USA (AI134732) and the Commonwealth of Pennsylvania through the Hepatitis B Foundation. This work was also generously supported by grants from National Science Foundation of China (81772173 and 81971916) and National Science and Technology Mega-Project of China (2018ZX10301-408-002) to X.Z.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Edited by Alex Compton
Data availability
No data was used for the research described in the article.
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of V The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Beltran W.F., Lam E.C., St Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372e9–2383e9. doi: 10.1016/j.cell.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mlcochova P., Kemp S., Dhar M.S., Papa G., Meng B., Ferreira I., et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021 doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C., Ginn H.M., Dejnirattisai W., Supasa P., Wang B., Tuekprakhon A., et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220e13–4236e13. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J., Liu Y., Xia H., Zou J., Weaver S.C., Swanson K.A., et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature. 2021;596:273–275. doi: 10.1038/s41586-021-03693-y. [DOI] [PubMed] [Google Scholar]
- 9.Kozlov M. Waning COVID super-immunity raises questions about Omicron. Nature. 2021 doi: 10.1038/d41586-021-03674-1. [DOI] [PubMed] [Google Scholar]
- 10.Sadler A.J., Williams B.R. Interferon-inducible antiviral effectors. Nature Rev. Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stertz S., Hale B.G. Interferon system deficiencies exacerbating severe pandemic virus infections. Trends Microbiol. 2021;29:973–982. doi: 10.1016/j.tim.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Combes A.J., Courau T., Kuhn N.F., Hu K.H., Ray A., Chen W.S., et al. Global absence and targeting of protective immune states in severe COVID-19. Nature. 2021;591:124–130. doi: 10.1038/s41586-021-03234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Park A., Iwasaki A. Type I and Type III Interferons - Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowery S.A., Sariol A., Perlman S. Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19. Cell Host Microbe. 2021;29:1052–1062. doi: 10.1016/j.chom.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultze J.L., Aschenbrenner A.C. COVID-19 and the human innate immune system. Cell. 2021;184:1671–1692. doi: 10.1016/j.cell.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Renner D.M., Comar C.E., Whelan J.N., Reyes H.M., Cardenas-Diaz F.L., et al. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2022643118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin X., Riva L., Pu Y., Martin-Sancho L., Kanamune J., Yamamoto Y., et al. MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2020.108628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorne L.G., Reuschl A.K., Zuliani-Alvarez L., Whelan M.V.X., Turner J., Noursadeghi M., et al. SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J. 2021;40 doi: 10.15252/embj.2021107826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kouwaki T., Nishimura T., Wang G., Oshiumi H. RIG-I-Like Receptor-Mediated Recognition of Viral Genomic RNA of Severe Acute Respiratory Syndrome Coronavirus-2 and Viral Escape From the Host Innate Immune Responses. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.700926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N., Hui H., Bray B., Gonzalez G.M., Zeller M., Anderson K.G., et al. METTL3 regulates viral m6A RNA modification and host cell innate immune responses during SARS-CoV-2 infection. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada T., Sato S., Sotoyama Y., Orba Y., Sawa H., Yamauchi H., et al. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nature Immunol. 2021;22:820–828. doi: 10.1038/s41590-021-00942-0. [DOI] [PubMed] [Google Scholar]
- 25.Cabeza-Cabrerizo M., Cardoso A., Minutti C.M., Pereira da Costa M. Dendritic Cells Revisited. Annu. Rev. Immunol. 2021;39:131–166. doi: 10.1146/annurev-immunol-061020-053707. [DOI] [PubMed] [Google Scholar]
- 26.Greene T.T., Zuniga E.I. Type I Interferon Induction and Exhaustion during Viral Infection: Plasmacytoid Dendritic Cells and Emerging COVID-19 Findings. Viruses. 2021;13 doi: 10.3390/v13091839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swiecki M., Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nature Rev. Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Severa M., Diotti R.A., Etna M.P., Rizzo F., Fiore S., Ricci D., et al. Differential plasmacytoid dendritic cell phenotype and type I Interferon response in asymptomatic and severe COVID-19 infection. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu T. Structural insights into ligand recognition and regulation of nucleic acid-sensing Toll-like receptors. Curr. Opin. Struct. Biol. 2017;47:52–59. doi: 10.1016/j.sbi.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Diebold S.S., Massacrier C., Akira S., Paturel C., Morel Y., Reis e Sousa C. Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur. J. Immunol. 2006;36:3256–3267. doi: 10.1002/eji.200636617. [DOI] [PubMed] [Google Scholar]
- 31.Salvi V., Nguyen H.O., Sozio F., Schioppa T., Gaudenzi C., Laffranchi M., et al. SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight. 2021;6 doi: 10.1172/jci.insight.150542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beyer D.K., Forero A. Mechanisms of Antiviral Immune Evasion of SARS-CoV-2. J. Mol. Biol. 2021:167265. doi: 10.1016/j.jmb.2021.167265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sa Ribero M., Jouvenet N., Dreux M., Nisole S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Qin C., Rao Y., Ngo C., Feng J.J., Zhao J., et al. SARS-CoV-2 Nsp5 Demonstrates Two Distinct Mechanisms Targeting RIG-I and MAVS To Evade the Innate Immune Response. mBio. 2021:e0233521. doi: 10.1128/mBio.02335-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G., Lee J.H., Parker Z.M., Acharya D., Chiang J.J., van Gent M., et al. ISG15-dependent activation of the sensor MDA5 is antagonized by the SARS-CoV-2 papain-like protease to evade host innate immunity. Nature Microbiol. 2021;6:467–478. doi: 10.1038/s41564-021-00884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kindler E., Gil-Cruz C., Spanier J., Li Y., Wilhelm J., Rabouw H.H., et al. Early endonuclease-mediated evasion of RNA sensing ensures efficient coronavirus replication. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia H., Cao Z., Xie X., Zhang X., Chen J.Y., Wang H., et al. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nature Commun. 2020;11:3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181:1036e9–1045e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiege J.K., Thiede J.M., Nanda H.A., Matchett W.E., Moore P.J., Montanari N.R., et al. Single cell resolution of SARS-CoV-2 tropism, antiviral responses, and susceptibility to therapies in primary human airway epithelium. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., et al. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramer B., Knoll R., Bonaguro L., ToVinh M., Raabe J., Astaburuaga-Garcia R., et al. Early IFN-alpha signatures and persistent dysfunction are distinguishing features of NK cells in severe COVID-19. Immunity. 2021 doi: 10.1016/j.immuni.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sefik E., Qu R., Kaffe E., Zhao J., Junqueira C., Mirza H., et al. Viral replication in human macrophages enhances an inflammatory cascade and interferon driven chronic COVID-19 in humanized mice. bioRxiv. 2021 2021.09.27.461948. [Google Scholar]
- 45.van der Made C.I., Simons A., Schuurs-Hoeijmakers J., van den Heuvel G., Mantere T., Kersten S., et al. Presence of Genetic Variants Among Young Men With Severe COVID-19. JAMA. 2020;324:663–673. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asano T., Boisson B., Onodi F., Matuozzo D., Moncada-Velez M., Maglorius Renkilaraj M.R.L., et al. X-linked recessive TLR7 deficiency in ∼1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 2021;6 doi: 10.1126/sciimmunol.abl4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abolhassani H., Vosughimotlagh A., Asano T., Landegren N., Boisson B., Delavari S., et al. X-Linked TLR7 Deficiency Underlies Critical COVID-19 Pneumonia in a Male Patient with Ataxia-Telangiectasia. J. Clin. Immunol. 2021 doi: 10.1007/s10875-021-01151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pairo-Castineira E., Clohisey S., Klaric L., Bretherick A.D., Rawlik K., Pasko D., et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591:92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 49.Bastard P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Manry J., et al. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci. Immunol. 2021;6 doi: 10.1126/sciimmunol.abl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang B.S., Chan K.H., Cheng V.C., Woo P.C., Lau S.K., Lam C.C., et al. Comparative host gene transcription by microarray analysis early after infection of the Huh7 cell line by severe acute respiratory syndrome coronavirus and human coronavirus 229E. J. Virol. 2005;79:6180–6193. doi: 10.1128/JVI.79.10.6180-6193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lassnig C., Sanchez C.M., Egerbacher M., Walter I., Majer S., Kolbe T., et al. Development of a transgenic mouse model susceptible to human coronavirus 229E. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8275–8280. doi: 10.1073/pnas.0408589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao X., Guo F., Liu F., Cuconati A., Chang J., Block T.M., et al. Interferon induction of IFITM proteins promotes infection by human coronavirus OC43. Proc. Natl. Acad. Sci. U. S. A. 2014;111:6756–6761. doi: 10.1073/pnas.1320856111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lokugamage K.G., Hage A., de Vries M., Valero-Jimenez A.M., Schindewolf C., Dittmann M., et al. Type I Interferon Susceptibility Distinguishes SARS-CoV-2 from SARS-CoV. J. Virol. 2020;94 doi: 10.1128/JVI.01410-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F., van Amerongen G., et al. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nature Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falzarano D., de Wit E., Rasmussen A.L., Feldmann F., Okumura A., Scott D.P., et al. Treatment with interferon-alpha2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nature Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dinnon K.H., 3rd, Leist S.R., Schafer A., Edwards C.E., Martinez D.R., Montgomery S.A., et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 2020;586:560–566. doi: 10.1038/s41586-020-2708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoagland D.A., Moller R., Uhl S.A., Oishi K., Frere J., Golynker I., et al. Leveraging the antiviral type I interferon system as a first line of defense against SARS-CoV-2 pathogenicity. Immunity. 2021;54:557e5–570e5. doi: 10.1016/j.immuni.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bessiere P., Wasniewski M., Picard-Meyer E., Servat A., Figueroa T., Foret-Lucas C., et al. Intranasal type I interferon treatment is beneficial only when administered before clinical signs onset in the SARS-CoV-2 hamster model. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang N., Zhan Y., Zhu L., Hou Z., Liu F., Song P., et al. Retrospective Multicenter Cohort Study Shows Early Interferon Therapy Is Associated with Favorable Clinical Responses in COVID-19 Patients. Cell Host Microbe. 2020;28:455e2–464e2. doi: 10.1016/j.chom.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arabi Y.M., Asiri A.Y., Assiri A.M., Balkhy H.H., Al Bshabshe A., Al Jeraisy M., et al. Interferon Beta-1b and Lopinavir-Ritonavir for Middle East Respiratory Syndrome. N. Engl. J. Med. 2020;383:1645–1656. doi: 10.1056/NEJMoa2015294. [DOI] [PubMed] [Google Scholar]
- 61.Jagannathan P., Andrews J.R., Bonilla H., Hedlin H., Jacobson K.B., Balasubramanian V., et al. Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial. Nature Commun. 2021;12:1967. doi: 10.1038/s41467-021-22177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feld J.J., Kandel C., Biondi M.J., Kozak R.A., Zahoor M.A., Lemieux C., et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med. 2021;9:498–510. doi: 10.1016/S2213-2600(20)30566-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schneider W.M., Chevillotte M.D., Rice C.M. Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang D., Guo H., Xu C., Chang J., Gu B., Wang L., et al. Identification of three interferon-inducible cellular enzymes that inhibit the replication of hepatitis C virus. J. Virol. 2008;82:1665–1678. doi: 10.1128/JVI.02113-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schoggins J.W., MacDuff D.A., Imanaka N., Gainey M.D., Shrestha B., Eitson J.L., et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J., Ding S.C., Cho H., Chung B.C., Gale M., Jr., Chanda S.K., et al. A short hairpin RNA screen of interferon-stimulated genes identifies a novel negative regulator of the cellular antiviral response. mBio. 2013;4:e00385–e00413. doi: 10.1128/mBio.00385-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin-Sancho L., Lewinski M.K., Pache L., Stoneham C.A., Yin X., Becker M.E., et al. Functional landscape of SARS-CoV-2 cellular restriction. Mol. Cell. 2021;81:2656e8–2668e8. doi: 10.1016/j.molcel.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wickenhagen A., Sugrue E., Lytras S., Kuchi S., Noerenberg M., Turnbull M.L., et al. A prenylated dsRNA sensor protects against severe COVID-19. Science. 2021;374:eabj3624. doi: 10.1126/science.abj3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khan H., Winstone H., Jimenez-Guardeño J., Graham C., Doores K., Goujon C., et al. TMPRSS2 promotes SARS-CoV-2 evasion from NCOA7-mediated restriction. bioRxiv. 2021 doi: 10.1371/journal.ppat.1009820. 2021.07.23.453488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peacock T.P., Goldhill D.H., Zhou J., Baillon L., Frise R., Swann O.C., et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nature Microbiol. 2021;6:899–909. doi: 10.1038/s41564-021-00908-w. [DOI] [PubMed] [Google Scholar]
- 71.Danziger O., Patel R.S., DeGrace E.J., Rosen M.R., Rosenberg B.R. Inducible CRISPR activation screen for interferon-stimulated genes identifies OAS1 as a SARS-CoV-2 restriction factor. bioRxiv. 2021;2021 doi: 10.1371/journal.ppat.1010464. 2021.09.22.461286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruchez A., Sha K., Johnson J., Chen L., Stefani C., McConnell H., et al. MHC class II transactivator CIITA induces cell resistance to Ebola virus and SARS-like coronaviruses. Science. 2020;370:241–247. doi: 10.1126/science.abb3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfaender S., Mar K.B., Michailidis E., Kratzel A., Boys I.N., V'Kovski P., et al. LY6E impairs coronavirus fusion and confers immune control of viral disease. Nature Microbiol. 2020;5:1330–1339. doi: 10.1038/s41564-020-0769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siegrist F., Ebeling M., Certa U. The small interferon-induced transmembrane genes and proteins. J. Interferon Cytokine Res. 2011;31:183–197. doi: 10.1089/jir.2010.0112. [DOI] [PubMed] [Google Scholar]
- 75.Bailey C.C., Zhong G., Huang I.C., Farzan M. IFITM-Family Proteins: The Cell's First Line of Antiviral Defense. Annu. Rev. Virol. 2014;1:261–283. doi: 10.1146/annurev-virology-031413-085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao X., Zheng S., Chen D., Zheng M., Li X., Li G., et al. LY6E Restricts Entry of Human Coronaviruses, Including Currently Pandemic SARS-CoV-2. J. Virol. 2020;94 doi: 10.1128/JVI.00562-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng M., Zhao X., Zheng S., Chen D., Du P., Li X., et al. Bat SARS-Like WIV1 coronavirus uses the ACE2 of multiple animal species as receptor and evades IFITM3 restriction via TMPRSS2 activation of membrane fusion. Emerging Microbes Infect. 2020;9:1567–1579. doi: 10.1080/22221751.2020.1787797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao X., Sehgal M., Hou Z., Cheng J., Shu S., Wu S., et al. Identification of Residues Controlling Restriction versus Enhancing Activities of IFITM Proteins on Entry of Human Coronaviruses. J. Virol. 2018;92 doi: 10.1128/JVI.01535-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Majdoul S., Compton A.A. Lessons in self-defence: inhibition of virus entry by intrinsic immunity. Nature Rev. Immunol. 2021 doi: 10.1038/s41577-021-00626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Compton A.A., Roy N., Porrot F., Billet A., Casartelli N., Yount J.S., et al. Natural mutations in IFITM3 modulate post-translational regulation and toggle antiviral specificity. EMBO Rep. 2016;17:1657–1671. doi: 10.15252/embr.201642771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao X., Li J., Winkler C.A., An P., Guo J.T. IFITM Genes, Variants, and Their Roles in the Control and Pathogenesis of Viral Infections. Front. Microbiol. 2018;9:3228. doi: 10.3389/fmicb.2018.03228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alghamdi J., Alaamery M., Barhoumi T., Rashid M., Alajmi H., Aljasser N., et al. Interferon-induced transmembrane protein-3 genetic variant rs12252 is associated with COVID-19 mortality. Genomics. 2021;113:1733–1741. doi: 10.1016/j.ygeno.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gomez J., Albaiceta G.M., Cuesta-Llavona E., Garcia-Clemente M., Lopez-Larrea C., Amado-Rodriguez L., et al. The Interferon-induced transmembrane protein 3 gene (IFITM3) rs12252 C variant is associated with COVID-19. Cytokine. 2021;137 doi: 10.1016/j.cyto.2020.155354. [DOI] [PubMed] [Google Scholar]
- 84.Cuesta-Llavona E., Albaiceta G.M., Garcia-Clemente M., Duarte-Herrera I.D., Amado-Rodriguez L., Hermida-Valverde T., et al. Association between the interferon-induced transmembrane protein 3 gene (IFITM3) rs34481144 / rs12252 haplotypes and COVID-19. Curr. Res. Virol. Sci. 2021;2 doi: 10.1016/j.crviro.2021.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li K., Jia R., Li M., Zheng Y.M., Miao C., Yao Y., et al. A sorting signal suppresses IFITM1 restriction of viral entry. J. Biol. Chem. 2015;290:4248–4259. doi: 10.1074/jbc.M114.630780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang I.C., Bailey C.C., Weyer J.L., Radoshitzky S.R., Becker M.M., Chiang J.J., et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jia R., Xu F., Qian J., Yao Y., Miao C., Zheng Y.M., et al. Identification of an endocytic signal essential for the antiviral action of IFITM3. Cell. Microbiol. 2014;16:1080–1093. doi: 10.1111/cmi.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Winstone H., Lista M.J., Reid A.C., Bouton C., Pickering S., Galao R.P., et al. The Polybasic Cleavage Site in SARS-CoV-2 Spike Modulates Viral Sensitivity to Type I Interferon and IFITM2. J. Virol. 2021;95 doi: 10.1128/JVI.02422-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prelli Bozzo C., Nchioua R., Volcic M., Koepke L., Kruger J., Schutz D., et al. IFITM proteins promote SARS-CoV-2 infection and are targets for virus inhibition in vitro. Nature Commun. 2021;12:4584. doi: 10.1038/s41467-021-24817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li K., Markosyan R.M., Zheng Y.M., Golfetto O., Bungart B., Li M., et al. IFITM proteins restrict viral membrane hemifusion. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Compton A.A., Bruel T., Porrot F., Mallet A., Sachse M., Euvrard M., et al. IFITM proteins incorporated into HIV-1 virions impair viral fusion and spread. Cell Host Microbe. 2014;16:736–747. doi: 10.1016/j.chom.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Desai T.M., Marin M., Chin C.R., Savidis G., Brass A.L., Melikyan G.B. IFITM3 restricts influenza A virus entry by blocking the formation of fusion pores following virus-endosome hemifusion. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weidner J.M., Jiang D., Pan X.B., Chang J., Block T.M., Guo J.T. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J. Virol. 2010;84:12646–12657. doi: 10.1128/JVI.01328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coomer C.A., Rahman K., Compton A.A. CD225 Proteins: A Family Portrait of Fusion Regulators. Trends Genet. 2021;37:406–410. doi: 10.1016/j.tig.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 96.John S.P., Chin C.R., Perreira J.M., Feeley E.M., Aker A.M., Savidis G., et al. The CD225 domain of IFITM3 is required for both IFITM protein association and inhibition of influenza A virus and dengue virus replication. J. Virol. 2013;87:7837–7852. doi: 10.1128/JVI.00481-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chesarino N.M., Compton A.A., McMichael T.M., Kenney A.D., Zhang L., Soewarna V., et al. IFITM3 requires an amphipathic helix for antiviral activity. EMBO Rep. 2017;18:1740–1751. doi: 10.15252/embr.201744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo X., Steinkuhler J., Marin M., Li X., Lu W., Dimova R., et al. Interferon-Induced Transmembrane Protein 3 Blocks Fusion of Diverse Enveloped Viruses by Altering Mechanical Properties of Cell Membranes. ACS Nano. 2021;15:8155–8170. doi: 10.1021/acsnano.0c10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rahman K., Coomer C.A., Majdoul S., Ding S.Y., Padilla-Parra S., Compton A.A. Homology-guided identification of a conserved motif linking the antiviral functions of IFITM3 to its oligomeric state. eLife. 2020;9 doi: 10.7554/eLife.58537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anafu A.A., Bowen C.H., Chin C.R., Brass A.L., Holm G.H. Interferon-inducible transmembrane protein 3 (IFITM3) restricts reovirus cell entry. J. Biol. Chem. 2013;288:17261–17271. doi: 10.1074/jbc.M112.438515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spence J.S., He R., Hoffmann H.H., Das T., Thinon E., Rice C.M., et al. IFITM3 directly engages and shuttles incoming virus particles to lysosomes. Nature Chem. Biol. 2019;15:259–268. doi: 10.1038/s41589-018-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yount J.S., Moltedo B., Yang Y.Y., Charron G., Moran T.M., Lopez C.B., et al. Palmitoylome profiling reveals S-palmitoylation-dependent antiviral activity of IFITM3. Nature Chem. Biol. 2010;6:610–614. doi: 10.1038/nchembio.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blaskovic S., Blanc M., van der Goot F.G. What does S-palmitoylation do to membrane proteins? FEBS J. 2013;280:2766–2774. doi: 10.1111/febs.12263. [DOI] [PubMed] [Google Scholar]
- 104.Garst E.H., Lee H., Das T., Bhattacharya S., Percher A., Wiewiora R., et al. Site-Specific Lipidation Enhances IFITM3 Membrane Interactions and Antiviral Activity. ACS Chem. Biol. 2021;16:844–856. doi: 10.1021/acschembio.1c00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mao M., Yu M., Tong J.H., Ye J., Zhu J., Huang Q.H., et al. RIG-E, a human homolog of the murine Ly-6 family, is induced by retinoic acid during the differentiation of acute promyelocytic leukemia cell. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5910–5914. doi: 10.1073/pnas.93.12.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Godfrey D.I., Masciantonio M., Tucek C.L., Malin M.A., Boyd R.L., Hugo P. Thymic shared antigen-1. A novel thymocyte marker discriminating immature from mature thymocyte subsets. J. Immunol. 1992;148:2006–2011. [PubMed] [Google Scholar]
- 107.Upadhyay G. Emerging Role of Lymphocyte Antigen-6 Family of Genes in Cancer and Immune Cells. Front. Immunol. 2019;10:819. doi: 10.3389/fimmu.2019.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schoggins J.W., Wilson S.J., Panis M., Murphy M.Y., Jones C.T., Bieniasz P., et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mar K.B., Rinkenberger N.R., Boys I.N., Eitson J.L., McDougal M.B., Richardson R.B., et al. LY6E mediates an evolutionarily conserved enhancement of virus infection by targeting a late entry step. Nature Commun. 2018;9:3603. doi: 10.1038/s41467-018-06000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu J., Liu S.L. Emerging Role of LY6E in Virus-Host Interactions. Viruses. 2019;11 doi: 10.3390/v11111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu J., Liang C., Liu S.L. CD4-Dependent Modulation of HIV-1 Entry by LY6E. J. Virol. 2019;93 doi: 10.1128/JVI.01866-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu J., Liang C., Liu S.L. Interferon-inducible LY6E Protein Promotes HIV-1 Infection. J. Biol. Chem. 2017;292:4674–4685. doi: 10.1074/jbc.M116.755819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bacquin A., Bireau C., Tanguy M., Romanet C., Vernochet C., Dupressoir A., et al. A Cell Fusion-Based Screening Method Identifies Glycosylphosphatidylinositol-Anchored Protein Ly6e as the Receptor for Mouse Endogenous Retroviral Envelope Syncytin-A. J. Virol. 2017;91 doi: 10.1128/JVI.00832-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leng L., Metz C.N., Fang Y., Xu J., Donnelly S., Baugh J., et al. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Strubin M., Berte C., Mach B. Alternative splicing and alternative initiation of translation explain the four forms of the Ia antigen-associated invariant chain. EMBO J. 1986;5:3483–3488. doi: 10.1002/j.1460-2075.1986.tb04673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mihelic M., Dobersek A., Guncar G., Turk D. Inhibitory fragment from the p41 form of invariant chain can regulate activity of cysteine cathepsins in antigen presentation. J. Biol. Chem. 2008;283:14453–14460. doi: 10.1074/jbc.M801283200. [DOI] [PubMed] [Google Scholar]
- 117.Luster A.D., Weinshank R.L., Feinman R., Ravetch J.V. Molecular and biochemical characterization of a novel gamma-interferon-inducible protein. J. Biol. Chem. 1988;263:12036–12043. [PubMed] [Google Scholar]
- 118.Arunachalam B., Phan U.T., Geuze H.J., Cresswell P. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT) Proc. Natl. Acad. Sci. U. S. A. 2000;97:745–750. doi: 10.1073/pnas.97.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hastings K.T., Lackman R.L., Cresswell P. Functional requirements for the lysosomal thiol reductase GILT in MHC class II-restricted antigen processing. J. Immunol. 2006;177:8569–8577. doi: 10.4049/jimmunol.177.12.8569. [DOI] [PubMed] [Google Scholar]
- 120.Singh R., Cresswell P. Defective cross-presentation of viral antigens in GILT-free mice. Science. 2010;328:1394–1398. doi: 10.1126/science.1189176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen D., Hou Z., Jiang D., Zheng M., Li G., Zhang Y., et al. GILT restricts the cellular entry mediated by the envelope glycoproteins of SARS-CoV, Ebola virus and Lassa fever virus. Emerg. Microbes Infect. 2019;8:1511–1523. doi: 10.1080/22221751.2019.1677446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao J., Chen J., Li M., Chen M., Sun C. Multifaceted Functions of CH25H and 25HC to Modulate the Lipid Metabolism, Immune Responses, and Broadly Antiviral Activities. Viruses. 2020;12 doi: 10.3390/v12070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Blanc M., Hsieh W.Y., Robertson K.A., Kropp K.A., Forster T., Shui G., et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity. 2013;38:106–118. doi: 10.1016/j.immuni.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu S.Y., Aliyari R., Chikere K., Li G., Marsden M.D., Smith J.K., et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. 2013;38:92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li C., Deng Y.Q., Wang S., Ma F., Aliyari R., Huang X.Y., et al. 25-Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity. 2017;46:446–456. doi: 10.1016/j.immuni.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saulle I., Ibba S.V., Vittori C., Fenizia C., Mercurio V., Vichi F., et al. Sterol metabolism modulates susceptibility to HIV-1 Infection. AIDS. 2020;34:1593–1602. doi: 10.1097/QAD.0000000000002591. [DOI] [PubMed] [Google Scholar]
- 127.Shrivastava-Ranjan P., Bergeron E., Chakrabarti A.K., Albarino C.G., Flint M., Nichol S.T., et al. 25-Hydroxycholesterol Inhibition of Lassa Virus Infection through Aberrant GP1 Glycosylation. mBio. 2016;7 doi: 10.1128/mBio.01808-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiang Y., Tang J.J., Tao W., Cao X., Song B.L., Zhong J. Identification of Cholesterol 25-Hydroxylase as a Novel Host Restriction Factor and a Part of the Primary Innate Immune Responses against Hepatitis C Virus Infection. J. Virol. 2015;89:6805–6816. doi: 10.1128/JVI.00587-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Anggakusuma R.-B., Berger C., Colpitts C.C., Boldanova T., Engelmann M., et al. Interferon-inducible cholesterol-25-hydroxylase restricts hepatitis C virus replication through blockage of membranous web formation. Hepatology. 2015;62:702–714. doi: 10.1002/hep.27913. [DOI] [PubMed] [Google Scholar]
- 130.Liu C., Yang X.V., Wu J., Kuei C., Mani N.S., Zhang L., et al. Oxysterols direct B-cell migration through EBI2. Nature. 2011;475:519–523. doi: 10.1038/nature10226. [DOI] [PubMed] [Google Scholar]
- 131.Hannedouche S., Zhang J., Yi T., Shen W., Nguyen D., Pereira J.P., et al. Oxysterols direct immune cell migration via EBI2. Nature. 2011;475:524–527. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang S., Li W., Hui H., Tiwari S.K., Zhang Q., Croker B.A., et al. Cholesterol 25-Hydroxylase inhibits SARS-CoV-2 and other coronaviruses by depleting membrane cholesterol. EMBO J. 2020;39 doi: 10.15252/embj.2020106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zang R., Case J.B., Yutuc E., Ma X., Shen S., Gomez Castro M.F., et al. Cholesterol 25-hydroxylase suppresses SARS-CoV-2 replication by blocking membrane fusion. Proc. Natl. Acad. Sci. U. S. A. 2020;117:32105–32113. doi: 10.1073/pnas.2012197117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang Y., Song Z., Wang M., Lan M., Zhang K., Jiang P., et al. Cholesterol 25-hydroxylase negatively regulates porcine intestinal coronavirus replication by the production of 25-hydroxycholesterol. Vet. Microbiol. 2019;231:129–138. doi: 10.1016/j.vetmic.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ke W., Wu X., Fang P., Zhou Y., Fang L., Xiao S. Cholesterol 25-hydroxylase suppresses porcine deltacoronavirus infection by inhibiting viral entry. Virus Res. 2021;295 doi: 10.1016/j.virusres.2021.198306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zu S., Deng Y.Q., Zhou C., Li J., Li L., Chen Q., et al. 25-Hydroxycholesterol is a potent SARS-CoV-2 inhibitor. Cell Res. 2020;30:1043–1045. doi: 10.1038/s41422-020-00398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li M.M.H., Aguilar E.G., Michailidis E., Pabon J., Park P., Wu X., et al. Characterization of Novel Splice Variants of Zinc Finger Antiviral Protein (ZAP) J. Virol. 2019;93 doi: 10.1128/JVI.00715-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ficarelli M., Wilson H., Pedro Galao R., Mazzon M., Antzin-Anduetza I., Marsh M., et al. KHNYN is essential for the zinc finger antiviral protein (ZAP) to restrict HIV-1 containing clustered CpG dinucleotides. Elife. 2019;8 doi: 10.7554/eLife.46767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhu Y., Chen G., Lv F., Wang X., Ji X., Xu Y., et al. Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:15834–15839. doi: 10.1073/pnas.1101676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Meagher J.L., Takata M., Goncalves-Carneiro D., Keane S.C., Rebendenne A., Ong H., et al. Structure of the zinc-finger antiviral protein in complex with RNA reveals a mechanism for selective targeting of CG-rich viral sequences. Proc. Natl. Acad. Sci. U. S. A. 2019;116:24303–24309. doi: 10.1073/pnas.1913232116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mao R., Nie H., Cai D., Zhang J., Liu H., Yan R., et al. Inhibition of hepatitis B virus replication by the host zinc finger antiviral protein. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nchioua R., Kmiec D., Muller J.A., Conzelmann C., Gross R., Swanson C.M., et al. SARS-CoV-2 Is Restricted by Zinc Finger Antiviral Protein despite Preadaptation to the Low-CpG Environment in Humans. mBio. 2020;11 doi: 10.1128/mBio.01930-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang Y., Song W., Chen S., Yuan Z., Yi Z. A bacterial artificial chromosome (BAC)-vectored noninfectious replicon of SARS-CoV-2. Antiviral Res. 2021;185 doi: 10.1016/j.antiviral.2020.104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kmiec D., Lista M.J., Ficarelli M., Swanson C.M., Neil S.J. S-farnesylation is essential for antiviral activity of the long ZAP isoform against RNA viruses with diverse replication strategies. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xia X. Extreme Genomic CpG Deficiency in SARS-CoV-2 and Evasion of Host Antiviral Defense. Mol. Biol. Evol. 2020;37:2699–2705. doi: 10.1093/molbev/msaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang Y., Mao J.M., Wang G.D., Luo Z.P., Yang L., Yao Q., et al. Human SARS-CoV-2 has evolved to reduce CG dinucleotide in its open reading frames. Sci. Rep. 2020;10:12331. doi: 10.1038/s41598-020-69342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hornung V., Hartmann R., Ablasser A., Hopfner K.P. OAS proteins and cGAS: unifying concepts in sensing and responding to cytosolic nucleic acids. Nature Rev. Immunol. 2014;14:521–528. doi: 10.1038/nri3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li Y., Banerjee S., Wang Y., Goldstein S.A., Dong B., Gaughan C., et al. Activation of RNase L is dependent on OAS3 expression during infection with diverse human viruses. Proc. Natl. Acad. Sci. U. S. A. 2016;113:2241–2246. doi: 10.1073/pnas.1519657113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.He J., Feng D., de Vlas S.J., Wang H., Fontanet A., Zhang P., et al. Association of SARS susceptibility with single nucleic acid polymorphisms of OAS1 and MxA genes: a case-control study. BMC Infect. Dis. 2006;6:106. doi: 10.1186/1471-2334-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Anisul M., Shilts J., Schwartzentruber J., Hayhurst J., Buniello A., Shaikho Elhaj Mohammed E., et al. A proteome-wide genetic investigation identifies several SARS-CoV-2-exploited host targets of clinical relevance. eLife. 2021;10 doi: 10.7554/eLife.69719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou S., Butler-Laporte G., Nakanishi T., Morrison D.R., Afilalo J., Afilalo M., et al. A Neanderthal OAS1 isoform protects individuals of European ancestry against COVID-19 susceptibility and severity. Nature Med. 2021;27:659–667. doi: 10.1038/s41591-021-01281-1. [DOI] [PubMed] [Google Scholar]
- 152.Bonnevie-Nielsen V., Field L.L., Lu S., Zheng D.J., Li M., Martensen P.M., et al. Variation in antiviral 2′,5′-oligoadenylate synthetase (2'5'AS) enzyme activity is controlled by a single-nucleotide polymorphism at a splice-acceptor site in the OAS1 gene. Am. J. Hum. Genet. 2005;76:623–633. doi: 10.1086/429391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Soveg F.W., Schwerk J., Gokhale N.S., Cerosaletti K., Smith J.R., Pairo-Castineira E., et al. Endomembrane targeting of human OAS1 p46 augments antiviral activity. eLife. 2021;10 doi: 10.7554/eLife.71047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao L., Jha B.K., Wu A., Elliott R., Ziebuhr J., Gorbalenya A.E., et al. Antagonism of the interferon-induced OAS-RNase L pathway by murine coronavirus ns2 protein is required for virus replication and liver pathology. Cell Host Microbe. 2012;11:607–616. doi: 10.1016/j.chom.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhao L., Birdwell L.D., Wu A., Elliott R., Rose K.M., Phillips J.M., et al. Cell-type-specific activation of the oligoadenylate synthetase-RNase L pathway by a murine coronavirus. J. Virol. 2013;87:8408–8418. doi: 10.1128/JVI.00769-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thornbrough J.M., Jha B.K., Yount B., Goldstein S.A., Li Y., Elliott R., et al. Middle East Respiratory Syndrome Coronavirus NS4b Protein Inhibits Host RNase L Activation. mBio. 2016;7:e00258. doi: 10.1128/mBio.00258-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Comar C.E., Goldstein S.A., Li Y., Yount B., Baric R.S., Weiss S.R. Antagonism of dsRNA-Induced Innate Immune Pathways by NS4a and NS4b Accessory Proteins during MERS Coronavirus Infection. mBio. 2019;10 doi: 10.1128/mBio.00319-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Diamond M.S., Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nature Rev. Immunol. 2013;13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fensterl V., Sen G.C. Interferon-induced Ifit proteins: their role in viral pathogenesis. J. Virol. 2015;89:2462–2468. doi: 10.1128/JVI.02744-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abbas Y.M., Pichlmair A., Gorna M.W., Superti-Furga G., Nagar B. Structural basis for viral 5'-PPP-RNA recognition by human IFIT proteins. Nature. 2013;494:60–64. doi: 10.1038/nature11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Feng F., Yuan L., Wang Y.E., Crowley C., Lv Z., Li J., et al. Crystal structure and nucleotide selectivity of human IFIT5/ISG58. Cell Res. 2013;23:1055–1058. doi: 10.1038/cr.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang Z., Liang H., Zhou Q., Li Y., Chen H., Ye W., et al. Crystal structure of ISG54 reveals a novel RNA binding structure and potential functional mechanisms. Cell Res. 2012;22:1328–1338. doi: 10.1038/cr.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hui D.J., Bhasker C.R., Merrick W.C., Sen G.C. Viral stress-inducible protein p56 inhibits translation by blocking the interaction of eIF3 with the ternary complex eIF2.GTP.Met-tRNAi. J. Biol. Chem. 2003;278:39477–39482. doi: 10.1074/jbc.M305038200. [DOI] [PubMed] [Google Scholar]
- 164.Guo J., Hui D.J., Merrick W.C., Sen G.C. A new pathway of translational regulation mediated by eukaryotic initiation factor 3. EMBO J. 2000;19:6891–6899. doi: 10.1093/emboj/19.24.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Habjan M., Hubel P., Lacerda L., Benda C., Holze C., Eberl C.H., et al. Sequestration by IFIT1 impairs translation of 2'O-unmethylated capped RNA. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kimura T., Katoh H., Kayama H., Saiga H., Okuyama M., Okamoto T., et al. Ifit1 inhibits Japanese encephalitis virus replication through binding to 5' capped 2'-O unmethylated RNA. J. Virol. 2013;87:9997–10003. doi: 10.1128/JVI.00883-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Abbas Y.M., Laudenbach B.T., Martinez-Montero S., Cencic R., Habjan M., Pichlmair A., et al. Structure of human IFIT1 with capped RNA reveals adaptable mRNA binding and mechanisms for sensing N1 and N2 ribose 2'-O methylations. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E2106–E2115. doi: 10.1073/pnas.1612444114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pichlmair A., Lassnig C., Eberle C.A., Gorna M.W., Baumann C.L., Burkard T.R., et al. IFIT1 is an antiviral protein that recognizes 5'-triphosphate RNA. Nature Immunol. 2011;12:624–630. doi: 10.1038/ni.2048. [DOI] [PubMed] [Google Scholar]
- 169.Diamond M.S. IFIT1: A dual sensor and effector molecule that detects non-2'-O methylated viral RNA and inhibits its translation. Cytokine Growth Factor Rev. 2014;25:543–550. doi: 10.1016/j.cytogfr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kumar P., Sweeney T.R., Skabkin M.A., Skabkina O.V., Hellen C.U., Pestova T.V. Inhibition of translation by IFIT family members is determined by their ability to interact selectively with the 5'-terminal regions of cap0-, cap1- and 5'ppp- mRNAs. Nucleic Acids Res. 2014;42:3228–3245. doi: 10.1093/nar/gkt1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Raychoudhuri A., Shrivastava S., Steele R., Kim H., Ray R., Ray R.B. ISG56 and IFITM1 proteins inhibit hepatitis C virus replication. J. Virol. 2011;85:12881–12889. doi: 10.1128/JVI.05633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Andrejeva J., Norsted H., Habjan M., Thiel V., Goodbourn S., Randall R.E. ISG56/IFIT1 is primarily responsible for interferon-induced changes to patterns of parainfluenza virus type 5 transcription and protein synthesis. J. Gen. Virol. 2013;94:59–68. doi: 10.1099/vir.0.046797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rabbani M.A., Ribaudo M., Guo J.T., Barik S. Identification of Interferon-Stimulated Gene Proteins That Inhibit Human Parainfluenza Virus Type 3. J. Virol. 2016;90:11145–11156. doi: 10.1128/JVI.01551-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hyde J.L., Diamond M.S. Innate immune restriction and antagonism of viral RNA lacking 2-O methylation. Virology. 2015;479–480:66–74. doi: 10.1016/j.virol.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Viswanathan T., Arya S., Chan S.H., Qi S., Dai N., Misra A., et al. Structural basis of RNA cap modification by SARS-CoV-2. Nature Commun. 2020;11:3718. doi: 10.1038/s41467-020-17496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen Y., Su C., Ke M., Jin X., Xu L., Zhang Z., et al. Biochemical and structural insights into the mechanisms of SARS coronavirus RNA ribose 2'-O-methylation by nsp16/nsp10 protein complex. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zust R., Cervantes-Barragan L., Habjan M., Maier R., Neuman B.W., Ziebuhr J., et al. Ribose 2'-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nature Immunol. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Menachery V.D., Yount B.L., Jr., Josset L., Gralinski L.E., Scobey T., Agnihothram S., et al. Attenuation and restoration of severe acute respiratory syndrome coronavirus mutant lacking 2'-o-methyltransferase activity. J. Virol. 2014;88:4251–4264. doi: 10.1128/JVI.03571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Menachery V.D., Gralinski L.E., Mitchell H.D., Dinnon K.H., 3rd, Leist S.R., Yount B.L., Jr, et al. Middle East Respiratory Syndrome Coronavirus Nonstructural Protein 16 Is Necessary for Interferon Resistance and Viral Pathogenesis. mSphere. 2017;2 doi: 10.1128/mSphere.00346-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chang L.J., Chen T.H. NSP16 2'-O-MTase in Coronavirus Pathogenesis: Possible Prevention and Treatments Strategies. Viruses. 2021;13 doi: 10.3390/v13040538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hinz A., Miguet N., Natrajan G., Usami Y., Yamanaka H., Renesto P., et al. Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain. Cell Host Microbe. 2010;7:314–323. doi: 10.1016/j.chom.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schubert H.L., Zhai Q., Sandrin V., Eckert D.M., Garcia-Maya M., Saul L., et al. Structural and functional studies on the extracellular domain of BST2/tetherin in reduced and oxidized conformations. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17951–17956. doi: 10.1073/pnas.1008206107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jouvenet N., Neil S.J., Zhadina M., Zang T., Kratovac Z., Lee Y., et al. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 2009;83:1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Neil S.J., Zang T., Bieniasz P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 185.Sakuma T., Noda T., Urata S., Kawaoka Y., Yasuda J. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 2009;83:2382–2385. doi: 10.1128/JVI.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hoffmann M., Nehlmeier I., Brinkmann C., Krahling V., Behner L., Moldenhauer A.S., et al. Tetherin Inhibits Nipah Virus but Not Ebola Virus Replication in Fruit Bat Cells. J. Virol. 2019;93 doi: 10.1128/JVI.01821-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kong W.S., Irie T., Yoshida A., Kawabata R., Kadoi T., Sakaguchi T. Inhibition of virus-like particle release of Sendai virus and Nipah virus, but not that of mumps virus, by tetherin/CD317/BST-2. Hiroshima J. Med. Sci. 2012;61:59–67. [PubMed] [Google Scholar]
- 188.Hu S., Yin L., Mei S., Li J., Xu F., Sun H., et al. BST-2 restricts IAV release and is countered by the viral M2 protein. Biochem. J. 2017;474:715–730. doi: 10.1042/BCJ20160861. [DOI] [PubMed] [Google Scholar]
- 189.Gnirss K., Zmora P., Blazejewska P., Winkler M., Lins A., Nehlmeier I., et al. Tetherin Sensitivity of Influenza A Viruses Is Strain Specific: Role of Hemagglutinin and Neuraminidase. J. Virol. 2015;89:9178–9188. doi: 10.1128/JVI.00615-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yan R., Zhao X., Cai D., Liu Y., Block T.M., Guo J.T., et al. The Interferon-Inducible Protein Tetherin Inhibits Hepatitis B Virus Virion Secretion. J. Virol. 2015;89:9200–9212. doi: 10.1128/JVI.00933-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gustin J.K., Douglas J.L. BST-2/tetherin: viral tether, viral sensor or both? Future Virol. 2013;8 doi: 10.2217/fvl.13.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Stertz S., Reichelt M., Spiegel M., Kuri T., Martinez-Sobrido L., Garcia-Sastre A., et al. The intracellular sites of early replication and budding of SARS-coronavirus. Virology. 2007;361:304–315. doi: 10.1016/j.virol.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang S.M., Huang K.J., Wang C.T. BST2/CD317 counteracts human coronavirus 229E productive infection by tethering virions at the cell surface. Virology. 2014;449:287–296. doi: 10.1016/j.virol.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Neil S.J. The antiviral activities of tetherin. Curr. Top. Microbiol. Immunol. 2013;371:67–104. doi: 10.1007/978-3-642-37765-5_3. [DOI] [PubMed] [Google Scholar]
- 195.Le Tortorec A., Willey S., Neil S.J. Antiviral inhibition of enveloped virus release by tetherin/BST-2: action and counteraction. Viruses. 2011;3:520–540. doi: 10.3390/v3050520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Douglas J.L., Viswanathan K., McCarroll M.N., Gustin J.K., Fruh K., Moses A.V. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism. J. Virol. 2009;83:7931–7947. doi: 10.1128/JVI.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mitchell R.S., Katsura C., Skasko M.A., Fitzpatrick K., Lau D., Ruiz A., et al. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Goffinet C., Allespach I., Homann S., Tervo H.M., Habermann A., Rupp D., et al. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe. 2009;5:285–297. doi: 10.1016/j.chom.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 199.Sauter D., Schindler M., Specht A., Landford W.N., Munch J., Kim K.A., et al. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe. 2009;6:409–421. doi: 10.1016/j.chom.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Le Tortorec A., Neil S.J. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 2009;83:11966–11978. doi: 10.1128/JVI.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hauser H., Lopez L.A., Yang S.J., Oldenburg J.E., Exline C.M., Guatelli J.C., et al. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology. 2010;7:51. doi: 10.1186/1742-4690-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Taylor J.K., Coleman C.M., Postel S., Sisk J.M., Bernbaum J.G., Venkataraman T., et al. Severe Acute Respiratory Syndrome Coronavirus ORF7a Inhibits Bone Marrow Stromal Antigen 2 Virion Tethering through a Novel Mechanism of Glycosylation Interference. J. Virol. 2015;89:11820–11833. doi: 10.1128/JVI.02274-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stewart H., Johansen K., McGovern N., Palmulli R., Carnell G., Heeney J., et al. SARS-CoV-2 spike downregulates tetherin to enhance viral spread. bioRxiv. 2021 2021.01.06.425396. [Google Scholar]
- 204.Wang S.M., Huang K.J., Wang C.T. Severe acute respiratory syndrome coronavirus spike protein counteracts BST2-mediated restriction of virus-like particle release. J. Med. Virol. 2019;91:1743–1750. doi: 10.1002/jmv.25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Viswanathan K., Smith M.S., Malouli D., Mansouri M., Nelson J.A., Fruh K. BST2/Tetherin enhances entry of human cytomegalovirus. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Miller K.D., Matullo C., Williams R., Jones C.B., Rall G.F. Murine BST2/tetherin promotes measles virus infection of neurons. Virology. 2021;563:38–43. doi: 10.1016/j.virol.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kim H.J., Jeong M.S., Jang S.B. Structure and Activities of the NS1 Influenza Protein and Progress in the Development of Small-Molecule Drugs. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22084242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Deng X., Chen Y., Mielech A.M., Hackbart M., Kesely K.R., Mettelman R.C., et al. Structure-Guided Mutagenesis Alters Deubiquitinating Activity and Attenuates Pathogenesis of a Murine Coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.01734-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Deng X., Buckley A.C., Pillatzki A., Lager K.M., Faaberg K.S., Baker S.C. Inactivating Three Interferon Antagonists Attenuates Pathogenesis of an Enteric Coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00565-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Szemiel A.M., Merits A., Orton R.J., MacLean O.A., Pinto R.M., Wickenhagen A., et al. In vitro selection of Remdesivir resistance suggests evolutionary predictability of SARS-CoV-2. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Busnadiego I., Fernbach S., Pohl M.O., Karakus U., Huber M., Trkola A., et al. Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2. mBio. 2020;11 doi: 10.1128/mBio.01928-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vanderheiden A., Ralfs P., Chirkova T., Upadhyay A.A., Zimmerman M.G., Bedoya S., et al. Type I and Type III Interferons Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. J. Virol. 2020;94 doi: 10.1128/JVI.00985-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Felgenhauer U., Schoen A., Gad H.H., Hartmann R., Schaubmar A.R., Failing K., et al. Inhibition of SARS-CoV-2 by type I and type III interferons. J. Biol. Chem. 2020;295:13958–13964. doi: 10.1074/jbc.AC120.013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y., et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sheahan T.P., Sims A.C., Leist S.R., Schafer A., Won J., Brown A.J., et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nature Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hart B.J., Dyall J., Postnikova E., Zhou H., Kindrachuk J., Johnson R.F., et al. Interferon-beta and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J. Gen. Virol. 2014;95:571–577. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chan J.F., Yao Y., Yeung M.L., Deng W., Bao L., Jia L., et al. Treatment With Lopinavir/Ritonavir or Interferon-beta1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset. J. Infect. Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Al Qasim E., et al. Ribavirin and Interferon Therapy for Critically Ill Patients With Middle East Respiratory Syndrome: A Multicenter Observational Study. Clin. Infect. Dis. 2020;70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y., et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect. Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kumaki Y., Day C.W., Wandersee M.K., Schow B.P., Madsen J.S., Grant D., et al. Interferon alfacon 1 inhibits SARS-CoV infection in human bronchial epithelial Calu-3 cells. Biochem. Biophys. Res. Commun. 2008;371:110–113. doi: 10.1016/j.bbrc.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zheng B., He M.L., Wong K.L., Lum C.T., Poon L.L., Peng Y., et al. Potent inhibition of SARS-associated coronavirus (SCOV) infection and replication by type I interferons (IFN-alpha/beta) but not by type II interferon (IFN-gamma) J. Interferon Cytokine Res. 2004;24:388–390. doi: 10.1089/1079990041535610. [DOI] [PubMed] [Google Scholar]
- 222.Tan E.L., Ooi E.E., Lin C.Y., Tan H.C., Ling A.E., Lim B., et al. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg. Infect. Dis. 2004;10:581–586. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B., Lho H., et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 225.Mayer K., Nellessen C., Hahn-Ast C., Schumacher M., Pietzonka S., Eis-Hubinger A.M., et al. Fatal outcome of human coronavirus NL63 infection despite successful viral elimination by IFN-alpha in a patient with newly diagnosed ALL. Eur. J. Haematol. 2016;97:208–210. doi: 10.1111/ejh.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Shi G., Kenney A.D., Kudryashova E., Zani A., Zhang L., Lai K.K., et al. Opposing activities of IFITM proteins in SARS-CoV-2 infection. EMBO J. 2021;40 doi: 10.15252/embj.2020106501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Buchrieser J., Dufloo J., Hubert M., Monel B., Planas D., Rajah M.M., et al. Syncytia formation by SARS-CoV-2-infected cells. EMBO J. 2020;39 doi: 10.15252/embj.2020106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shi G., Chiramel A.I., Majdoul S., Lai K.K., Das S., Beare P.A., et al. Rapalogs downmodulate intrinsic immunity and promote cell entry of SARS-CoV-2. bioRxiv. 2021 doi: 10.1172/JCI160766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Daffis S., Szretter K.J., Schriewer J., Li J., Youn S., Errett J., et al. 2'-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wu J., Chi H., Fu Y., Cao A., Shi J., Zhu M., et al. The antiviral protein viperin interacts with the viral N protein to inhibit proliferation of porcine epidemic diarrhea virus. Arch. Virol. 2020;165:2279–2289. doi: 10.1007/s00705-020-04747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.