Abstract
During the coronavirus disease 2019 (COVID-19) pandemic, it were reported that COVID-19 patients could have cutaneous symptoms, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was observed on the skin of COVID-19 patients, which indicated that the skin is one target of SARS-CoV-2. Meanwhile, reports about SARS-CoV-2 transmission through food cold-chain overpacks emerged. With the fact that SARS-CoV-2 could survive on the skin for more than 9 h, the skin could be implicated in SARS CoV-2 transmission. Angiotensin-converting enzyme 2 (ACE2), a critical membrane protein for SARS-CoV-2 that enters a host cell, was recognized to be associated with the risk of SARS-CoV-2 infection. Therefore, tissues that express ACE2 might have the potential to be infected by and transmit SARS-CoV-2. The skin is one such tissue that expresses ACE2. However, unlike the lung that expresses ACE2 on the upper-most epithelial layer, the skin is composed of different layers of cells that function as a barrier, and cells under the top epidermal layer express ACE2. Since the skin barrier is the first line of protection, the typical position of ACE2-expressing cells in the skin implies that the skin barrier function could be the mediator of SARS-CoV-2. In our study, we found that ACE2 could be expressed in the skin, and its expression level is increased in psoriasis, an inflammatory disease of the skin with barrier dysfunction. Additionally, by applying the SARS-CoV-2 pseudovirus on mouse models with or without deteriorated skin barrier, we found that the SARS-CoV-2 pseudovirus could infect the skin and lungs of mouse models, and when the skin barrier was impaired, more SARS-CoV-2-infected cells could be found. Thus, we hypothesized that a deteriorated condition of the skin barrier might increase the risk of SARS-CoV-2 infection through the skin.
Abbreviations: ACE2, angiotensin-converting enzyme 2; AD, atopic dermatitis; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Keywords: Skin barrier, ACE2, Psoriasis, Atopic dermatitis, SARS-CoV-2
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first discovered at the end of 2019, and it spread worldwide [1]. During the coronavirus disease 2019 (COVID-19) pandemic, there were reports that SARS-CoV-2 was transmitted through food cold-chain overpacks [2], and that SARS-CoV-2 could survive on the skin for more than 9 h [3], which brought about the question whether SARS-CoV-2 could transmit through the skin.
Hypothesis
Considering the fact that the SARS-CoV-2 was present on the skin [11], we evaluated two hypotheses. Our first hypothesis was that the skin was implicated in SARS CoV-2. Another fact that evoked our further hypothesis was that a well-structured skin barrier is the first line of protection against external microbes. If the skin was implicated in the SARS CoV-2 transmission, the function of the skin barrier could be the mediator. Thus, our second hypothesis was that skin barrier could be the mediator of the impact of SARS-CoV-2 on the skin and the well-structured skin barrier is a potential weapon against transmission. In other words, SARS-CoV-2 might transmit through the skin while the skin barrier function could be the mediator.
Evaluation of the hypothesis
There were several indications to support our hypothesis regarding the implication of the skin in SARS-CoV-2 transmission. First, the skin is one of the organs that can express angiotensin-converting enzyme 2 (ACE2) [8], [9], [10]. ACE2 on the cell surface serves as the binding point of SARS-CoV-2, facilitating the entry of the virus and subsequent infection [4], [5]. Thus, ACE2 expression was considered to be associated with susceptibility to SARS-CoV-2 [6]. Secondly, there were reports of coronavirus disease 2019 (COVID-19) patients having dermatological symptoms [11], [12], [13], such as chilblains-like and pernio-like skin lesions. SARS-CoV-2 particles were found in the skin endothelial cells of the COVID-19 patients through electron microscopy [12], and a study reported that skin rashes could be an orphan symptom in some patients [14], for whom SARS-CoV-2 infection was first recognized through only skin-related symptoms, but without respiratory symptoms, which might provoke the thought that the skin was first infected in this patient. When SARS-CoV-2 is transmitted through the respiratory tract, numerous cells, known as alveolar epithelial type II cells, which express ACE2 on the surface of the tract [5], were infected. This led to severe lung injury, which could eventually result in death. The symptoms on the skin of COVID-19 patients might be caused by systematic response to the SARS-CoV-2 infection, which has not yet been verified. However, with the fact that SARS-CoV-2 virus was detected in the skin, it is more likely that these symptoms were caused by SARS-CoV-2 infecting the skin directly. Thirdly, the SARS-CoV-2 virus was found to be present on cold chain overpacks, and people who work in such cold chain environments could be infected by SARS-CoV-2 as patient zero in certain areas. The skin is the only organ that could directly be in contact with the SARS-CoV-2 contaminated surface in such environments, and SARS-CoV-2 could survive on the skin for over 9 h. This implied that the skin could be another target of the SARS-CoV-2, and a milieu for SARS-CoV-2 transmission [7]. All of the above support our first hypothesis that the skin is implicated in SARS-CoV-2 transmission.
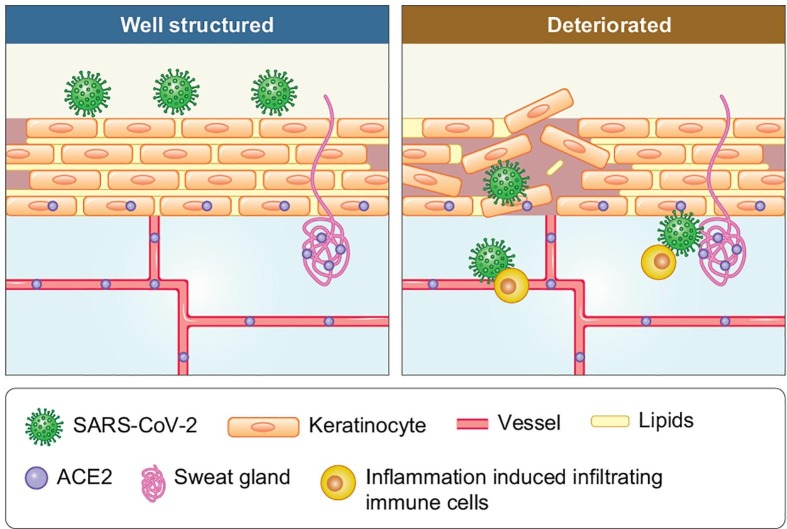
As the “great wall” of the human body, the skin barrier protects us from external microbes. Further, the well-structured skin barrier can also block SARS-CoV-2 from entering the skin. Then, in which circumstances can the SARS-CoV-2 pass the barrier? Unlike the lung, which contains only one layer of epithelial cells as a barrier to the external microbial environment, the skin is composed of tight junctions of keratinocytes and lipids that could be recognized as the “bricks and cement of a wall,” respectively. The expression of ACE2 in the normal skin was observed not on the surface, as in the lungs or gut, but on the stratum basale, sweat glands, and blood vessel epithelia, which are located at the bottom or under the skin barrier [7], [9], [10], [12], [16], [17]. Thus, the target ACE2-expressing cells of SARS-CoV-2 can be located in the innermost layer of the “bricks” and in the basement. If there are fractures between the external brick layers of the wall, infectious particles, such as SARS-CoV-2, present on the external layer, gain contact with the vulnerable bricks of the innermost bricklayer (Fig. 1 ). Thus, in certain pre-existing cutaneous conditions with high ACE2 expression and barrier dysfunction, SARS-CoV-2 may be transmitted through the skin, and the well-structured skin barrier can be a potential weapon against transmission while the skin barrier function acts as the mediator.
Fig. 1.
The hypothesis that deteriorated skin barrier might increase the risk of SARS-CoV-2 transmitting through skin.
Empirical data
Our hypothesis stands on the fact that the skin could express ACE2, and that skin barrier dysfunction was associated with the skin acting as a weapon against external microbes. We evaluated the skin ACE2 expression in psoriasis and atopic dermatitis (AD), typical skin diseases with barrier dysfunction [22], and normal skin through RNAseq. Results showed that the skin could express ACE2 in all the three conditions, and patients with psoriasis had the highest expression of ACE2 on the skin among the three conditions (Fig. 2 ). We used the SARS-CoV-2 pseudovirus-Luciferase (PSV001, Sino Biological, Beijing, China), which contained recombinant pseudo-type lentiviral particles of SARS-CoV-2 spike protein, to mimic SARS-CoV-2 cell infection [18]. Additionally, imiquimod- and calcipotriol-induced psoriatic and atopic dermatitis (AD) mouse models with humanized ACE2 (Shanghai Model Organisms Center, Inc., Shanghai, China) were used to verify whether the SARS-CoV-2 could transmit through the skin in conditions with a deteriorated barrier function. The mice(eight weeks)were divided into three groups with three mice each: psoriatic mice, which received application of imiquimod 10 mg (3 M Pharmaceuticals)per ear for 5 days; AD mice, which received application of calcipotriol (MedChemExpress, Cat. No.: HY-10001) 3 nmol per ear; and control mice, which received application of vaseline 10 mg per ear for 5 days (Fargon) (mice without a deteriorated barrier). All groups were reared in one cage (specific-pathogen-free conditions, P3 lab, The National Institute of Parasitic Diseases, Shanghai, China) and treated with the SARS-CoV-2 pseudovirus (2 × 103 copies/ml) at a dose of 30 μl per ear. The skin and lung tissues were obtained 48 h after the virus treatment. The mice were anesthetized for 48 h after the viral treatment if the virus was transmitted through the mouth or eyes [19], [20]. Besides, we additionally reared three mice in the same environment, and anesthetized them for 48 h without applying the pseudo virus topically. Thereafter, fluorescence-activated cell sorting was performed. The amount of green fluorescent protein cells detected in the skin and lung tissues of the pseudovirus-treated mice with barrier dysfunction was significantly higher than that in the control group (Fig. 3 ). No green fluorescent protein cells were observed in the lung of the three mice reared in the same environment without the topically applied pseudovirus, which could prove that the SARS-CoV-2 was not transmitted through the lung/respiratory tract.
Fig. 2.
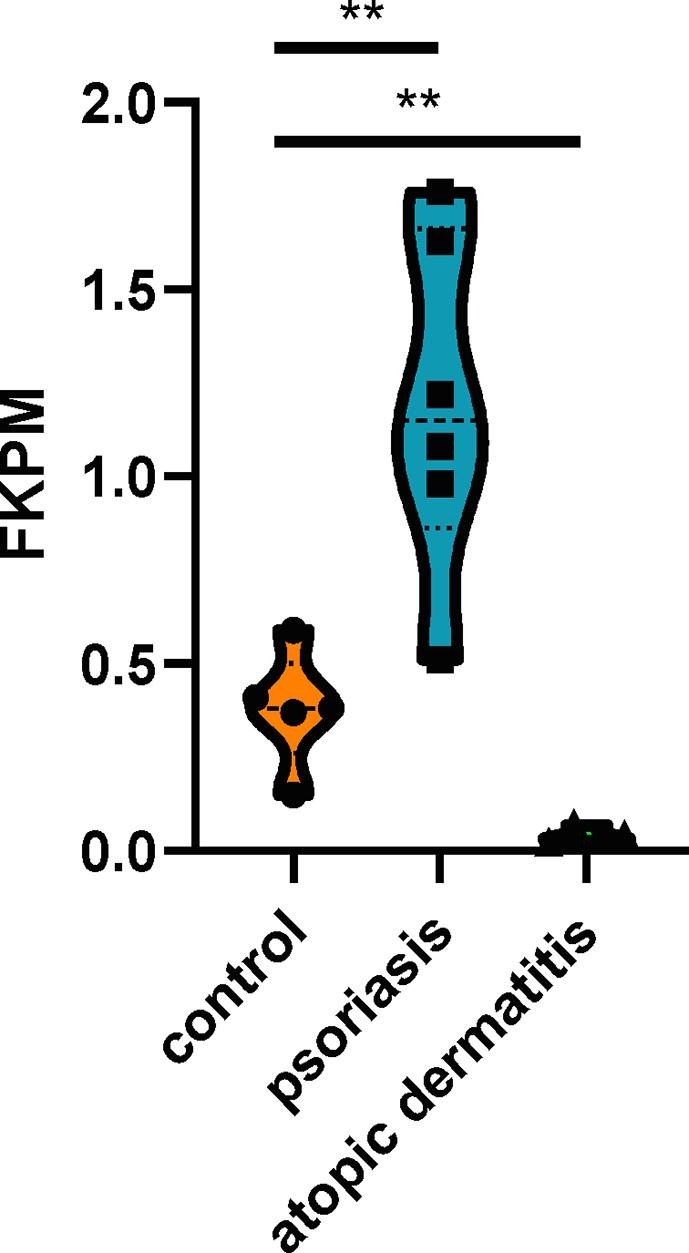
The expression of ACE2 in the lesion skin of patients with psoriasis and atopic dermatitis as well as the skin of normal control.
Fig. 3.
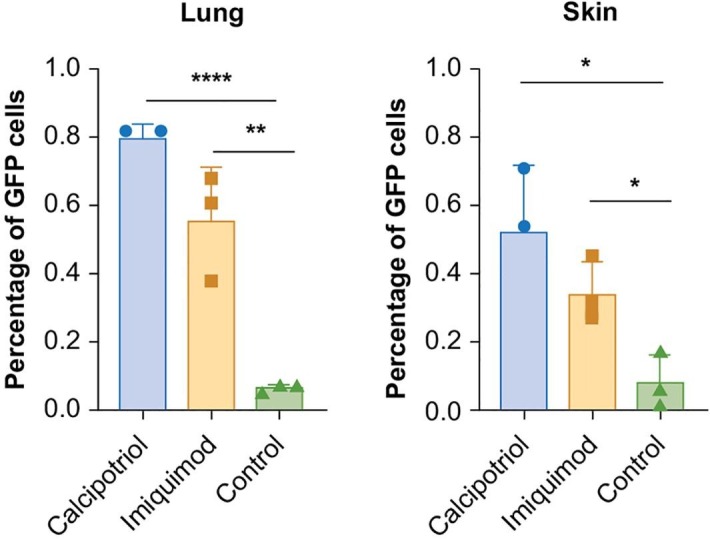
The percentage of GFP cells in mouse models with induced psoriasis, atopic dermatitis and group control.
Discussion
With the COVID-19 pandemic, establishing a method of preventing SARS-CoV-2 spread has become increasingly urgent. Thus, elucidating possible ways of SARS-CoV-2 transmission may encourage the development of new methods to reduce the spread of COVID-19. The expression of ACE2 in the skin indicates the possibility that the skin might be another entry point for the SARS-CoV-2. The situation of the wall fracture, herein referred to as barrier dysfunction of the skin, may vary. From our clinical work, we found that in psoriasis, a typical inflammatory skin disease with barrier dysfunction, treatment with interleukin (IL)-17 antibody resulted in a downregulation of ACE2 expression in the skin, while the skin barrier dysfunction and skin inflammatory status were restored [15]. This made us wonder whether the deteriorated skin barrier or skin inflammation could be associated with ACE2 expression. In our study, we could not conclude whether ACE2 was associated with skin barrier function; however, the results implied that the psoriatic form of the skin inflammation with barrier dysfunction might enable a higher risk for SARS-CoV-2 transmission in certain circumstances. However, another article demonstrated that the incidence of COVID-19 in psoriatic patients was not elevated [21]. Nevertheless, the conclusions were drawn in the context of a normal situation, and whether patients were in a circumstance where exposure to SARS-CoV-2 occurred only through the skin surface was not determined. Thus, the article could only conclude that psoriasis patients do not have an elevated risk of contracting COVID-19 in most conditions. When the environment contains surfaces contaminated by SARS-CoV-2, the risk of transmission though the deteriorated skin barrier cannot be ruled out. The protection of skin barriers could be more necessary for psoriasis patients.
The use of a SARS-CoV-2 pseudovirus showed that the skin could be a potential target of SARS-CoV-2, and that the cutaneous condition could affect the infection risk. Our previous work revealed that ACE2 is expressed not on the skin surface, but in the dermis and the cells between the dermis and epidermis. A deteriorated skin barrier provides various infectious agents, such as SARS-CoV-2, the opportunity to penetrate the epidermis. This study showed that based on the physical structure of the skin barrier, the SARS-CoV-2 S protein binding the ACE2-expressing cell in the stratum basale and dermis held a higher risk of being infected by SARS-CoV-2 when the skin barrier was deteriorated. Furthermore, this study showed that the deteriorated skin barrier enabled SARS-CoV-2 infection of cells in both the skin and lungs. Moreover, psoriasis might have increased the risk of SARS-CoV-2 transmission through the skin in certain circumstances. The inflammatory environment, along with the deteriorated skin barrier, could be an opportunity for SARS-CoV-2 transmission. An elevated ACE2 level in skin inflammatory disorders with a deteriorated barrier provides a suitable environment for such an opportunity.
Conclusions
With the indications above, we concluded that the skin is implicated in SARS CoV-2 transmission while the skin barrier might be a mediator that affects SARS-CoV-2 transmission through the skin. Since we use pseudo virus not real SARS-CoV-2 virus to evaluate our hypotheses, more work should be done to verify whether SARS-CoV-2 could transmit through skin. And clinical data should be gathered in order to clarify if patients with deteriorated skin barrier function gained higher risk of infected by SARS-CoV-2. Most importantly, our hypotheses implied the protection of the skin barrier be a solution of protecting against SARS-CoV-2 more adequately.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements:
Consent Statement/Ethical approval: Not required.
References
- 1.Wang X., Ferro E.G., Zhou G., Hashimoto D., Bhatt D.L. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. JAMA. 2020;27:703–704. doi: 10.1001/jama.2020.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chi Y., Zheng S., Liu C., Wang Q. Transmission of SARS-CoV-2 on cold-chain food overpacks: A new challenge. J Global Health. 2021;11:03071. doi: 10.7189/jogh.11.03071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirose R., Ikegaya H., Naito Y., et al. Survival of SARS-CoV-2 and influenza virus on the human skin: Importance of hand hygiene in COVID-19. Clin Infect Dis: an official publication of the Infectious Diseases Society of America. 2020 doi: 10.1093/cid/ciaa1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan R., Zhang Y., Li Y., Xia L.u., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science (New York, N.Y.) 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181(5):1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith J.C., Sausville E.L., Girish V., Yuan M.L., Vasudevan A., John K.M., et al. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev Cell. 2020;53(5):514–529.e3. doi: 10.1016/j.devcel.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tembhre M.K., Parihar A.S., Sharma V.K., Imran S., Bhari N., Lakshmy R., et al. Enhanced expression of angiotensin-converting enzyme 2 in psoriatic skin and its upregulation in keratinocytes by interferon-γ: implication of inflammatory milieu in skin tropism of SARS-CoV-2. Br J Dermatol. 2021;184(3):577–579. doi: 10.1111/bjd.19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grzegrzolka J., Swiatko K., Pula B., Zamirska A., Olbromski M., Bieniek A., et al. ACE and ACE2 expression in normal and malignant skin lesions. Folia Histochem Cytobiol. 2013;51(3):232–238. doi: 10.5603/FHC.2013.0033. [DOI] [PubMed] [Google Scholar]
- 9.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue X., Mi Z., Wang Z., Pang Z., Liu H., Zhang F. High Expression of ACE2 on Keratinocytes Reveals Skin as a Potential Target for SARS-CoV-2. J Invest Dermatol. 2021;141(1):206–209.e1. doi: 10.1016/j.jid.2020.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouaziz J.D., Duong T.A., Jachiet M., Velter C., Lestang P., Cassius C., et al. Vascular skin symptoms in COVID-19: a french observational study. J Eur Acad Dermatol Venereol. 2020;34(9) doi: 10.1111/jdv.v34.910.1111/jdv.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colmenero I., Santonja C., Alonso‐Riaño M., Noguera‐Morel L., Hernández‐Martín A., Andina D., et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183(4):729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman E.E., McMahon D.E., Lipoff J.B., Rosenbach M., Kovarik C., Takeshita J., et al. Pernio-like skin lesions associated with COVID-19: A case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83(2):486–492. doi: 10.1016/j.jaad.2020.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatu A.L., Nadasdy T., Bujoreanu F.C. Familial Clustering of COVID-19 Skin Manifestations. Dermatol Ther. 2020 doi: 10.1111/dth.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Q., Chen L., Li X., Zheng J. If skin is a potential host of SARS-CoV-2, IL-17 antibody could reduce the risk of COVID-19. J Am Acad Dermatol. 2021;84(3):e173. doi: 10.1016/j.jaad.2020.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gambichler T., Reuther J., Stücker M., Stranzenbach R., Torres‐Reyes C., Schlottmann R., et al. SARS-CoV-2 spike protein is present in both endothelial and eccrine cells of a chilblain-like skin lesion. J Eur Acad Dermatol Venereol. 2021;35(3) doi: 10.1111/jdv.v35.310.1111/jdv.16970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santonja C., Heras F., Núñez L., Requena L. COVID-19 chilblain-like lesion: immunohistochemical demonstration of SARS-CoV-2 spike protein in blood vessel endothelium and sweat gland epithelium in a polymerase chain reaction-negative patient. Br J Dermatol. 2020;183(4):778–780. doi: 10.1111/bjd.19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie J., Li Q., Wu J., Zhao C., Hao H., Liu H., et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect. 2020;9(1):680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369(6499):50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z., Fu Y., Guo Z., Li J., Li J., Cheng H., et al. Transmission and prevention of SARS-CoV-2. Biochem Soc Trans. 2020;48(5):2307–2316. doi: 10.1042/BST20200693. [DOI] [PubMed] [Google Scholar]
- 21.Vispi M., Corradin T., Peccianti C., Feci L., Casini L., Pisani C., et al. Psoriasis, biological drugs and Coronavirus Disease 2019: Real life experience of two Italian provinces. Dermatol Reports. 2020;12(1) doi: 10.4081/dr.2020.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maglie R., Souza Monteiro de Araujo D., Antiga E., Geppetti P., Nassini R., De Logu F. The role of TRPA1 in skin physiology and pathology. Int J Mol Sci. 2021;22(6):3065. doi: 10.3390/ijms22063065. [DOI] [PMC free article] [PubMed] [Google Scholar]