Abstract
Introduction:
Satiety, defined as the duration of the sensation of fullness, is usually measured by validated visual analog scales (VAS) for appetite. Gastric function plays a key role in food intake regulation. However, the association between gastric emptying (GE) and VAS-appetite is unknown.
Methods:
In this cross-sectional study, 134 participants [age=39±0.8 years, BMI=38±0.5 kg/m2, 67% females] completed simultaneous measurements of GE and VAS-appetite. After a 320kcal meal, GE was measured by scintigraphy and appetite by validated 100mm VAS for 240 min. Then, in the same day, satiation, defined as calories consumed to terminate meal, and measured by ad libitum meal. Percent of meal retention in the stomach, VAS area under curve (AUC0–240min), and overall appetite score (OAS) were calculated. Pearson correlation (ρ) determined the association of GE with VAS-appetite and satiation. Appetite components were also analyzed by quartiles based on GE120min.
Results:
GE120min was correlated with sensation of VAS-hungerAUC(0–240min) (ρ=0.24, p=0.004), fullnessAUC(0–240min) (ρ=0.16, p=0.05), and OASAUC(0–240min) (ρ=0.20, p=0.02). Patients with rapid GE120min had a mean increase in VAS-hungerAUC(0–240min) by 32 mm/min (15.62%, p=0.03) compared to normal/slow GE120min.
Conclusions:
GE is associated with the sensations of appetite, and rapid GE is associated with increased appetite, which may contribute to weight gain.
Keywords: Eating behavior, hunger, satiety, stomach, gastric physiology, calorie consumption
INTRODUCTION
The control of food intake is a regulated and complex process, which is highly influenced and often overridden by hedonic components(1, 2). The stages of food intake regulation are hunger, satiation, and satiety. Hunger represents the sensation that encourages the initiation of food consumption. Satiation is the sensation of fullness during a meal that aids in inducing the termination of a meal(2). Satiety is the period of time in which the fullness sensation persists(3). These different stages are regulated by homeostatic and hedonic cues that coalesce to control eating behavior.
The homeostatic regulation is driven by the brain-gut-adipose tissue axis. The hunger system in the hypothalamus is always turned “on”(4, 5). Satiety starts when an individual has reached satiation, terminated the meal, and the hunger system is in “off” mode. Peripheral signals, mainly from the distal gut, adipose tissue, and visceral afferent vagal fibers communicate with the brainstem and hypothalamus to signal this sensation of fullness, slow down the upper gut motor functions, enhance nutrient digestion, and maximize calorie absorption. This slowing of gastric emptying and reduced upper small bowel motility is mediated by the ileal brake(6–8). Gastric emptying is associated with caloric intake during the next meal(9, 10). However, it is a complex process that varies depending on the consistency, volume, macronutrient, and caloric content of the food consumed(11, 12).
The importance of satiety in food intake regulation and related disorders such as obesity has led researchers and clinicians to develop reliable and validated measurements of satiety, reviewed in detail elsewhere(13). The most commonly-used satiety tests are the appetite rating scales; however, other measurements are more complex, such as blood biomarkers (e.g. satiety gut hormones)(13), gastric functions testing (e.g. gastric emptying, volume or distention)(5), and functional brain magnetic resonance imaging(14). The appetite rating scales are commonly measured using 100-mm visual analog scales (VAS) for hunger, fullness, satisfaction, and desire to eat. The overall appetite score (OAS) is calculated as OAS = [satiety + fullness + (100 − hunger) + (100 − desire to eat)]/4, where 100 indicates more appetite and 0 indicates less appetite(15, 16). Although the appetite rating by VAS is widely adopted, the test has a moderate reproducibility (20–60%) and high inter-individual variability(17, 18). Furthermore, a recent meta-analysis of 462 scientific manuscripts showed that appetite scores do not reliably predict energy intake(19). Gibbons et al., explain these limitations suggesting that “subjective sensations do not provide the full picture of appetite control and other variables are contributing to satiety”(13).
In pursuit of more objective tools to measure satiety, gastric emptying has been used as an alternative test for postprandial satiety(9, 20). In particular, gastric emptying has been used to study subsequent food intake(9, 21), fasting and postprandial ghrelin(22, 23), and weight loss outcomes with satiety-reducing interventions, e.g. exenatide(24), liraglutide(21, 25), and intragastric balloons(26–28). The gold standard test for measuring gastric emptying is scintigraphy with a standard radiolabeled meal, which is reproducible (coefficient of variation 9–25%) over short-, intermediate-, and long-term in healthy volunteers(29). Furthermore, gastric emptying is accelerated in obesity when compared to healthy weight controls(20), and patients with faster gastric emptying gain more weight than those with normal gastric emptying when followed prospectively for 4 years(30).
However, the association between GE and appetite symptoms measured simultaneously after ingesting a standard meal has not been studied. Thus, it is key to understand whether GE correlates with appetite sensations in order to be used as markers for postprandial satiety. We hypothesize that percentage of solids emptied during the GE test is positively associated with the sensation of fullness in the assessment of appetite. Therefore, this cross-sectional study aimed to evaluate the correlation between gastric emptying of solids measured simultaneously with a visual analog scale for appetite, and satiety sensations in participants with obesity.
METHODS
Participants
A total of 134 subjects between 18 and 65 years old, with obesity (BMI >30 kg/m2) and no evidence of active psychiatric symptoms, eating disorders (specifically, bulimia), or alcohol use disorders were prospectively enrolled as part of ClinicalTrials.gov NCT03374956 trial. Here we report the baseline measurements prior to inclusion into the trial, which studied the effect of anti-obesity medication based on phenotypes. The study was approved by the Mayo Clinic Institutional Review Board (IRB # 17–003449) and all participants gave written informed consent following a thorough explanation of the study details. Women of childbearing potential had a negative pregnancy test within 48 hours before testing. All the studies were performed at the Mayo Clinic Clinical Research Trials Unit (CRTU) after an 8-hour fasting period. The exclusion criteria were history of bariatric procedures, use of medications that may alter gastrointestinal motility, appetite, or absorption (e.g. orlistat, neuromodulatory agents, etc.) within the last 6 months, and history of hypersensitivity to the components of the study material or meals.
Study Visit Overview
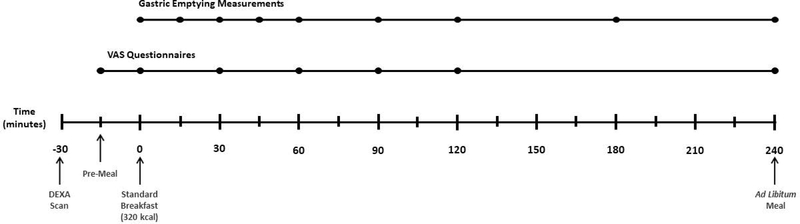
Participants reported to the Research unit between 6 am and 8 am. All participants confirmed overnight fasting status with at least 8 hours from the last meal and completed measurements of satiety and satiation on the same day. Participants underwent a body composition scan by dual energy X-ray absorptiometry (DEXA) at arrival to the research unit. After a standardized breakfast, satiety was measured concurrently with gastric emptying of solids by scintigraphy and appetite VAS. Satiation was assessed by quantification of total calories consumed after an ad libitum meal 240 minutes after the standardized breakfast (Figure 1). Both meals (i.e. standard breakfast and ad libitum meal) were provided in the Gastroenterology Lab of CRTU, participants were isolated from each other, and external visual food cues were minimized.
Figure 1. Study Timeline:
Participants presented to the Clinical Research Unit after overnight fasting. DEXA scan was performed upon patient arrival. VAS was recorded 15 minutes before a 320kcal breakfast (Pre-meal), after meal termination, and then every 30 minutes for the next 120 minutes and at 240 minutes. Gastric emptying was measured by scintigraphy with 99m Tc-radiolabeled eggs in the provided breakfast; images were acquired every 15 minutes for the first 60 minutes, and then at 90, 120, 180 and 240 minutes. An ad libitum meal was provided at 240 minutes.
Measurements of Satiety
A standard breakfast of 320kcal, 30% fat meal consisting of two 99mTc-radiolabeled eggs, toast, and 80 ml of skim milk was given to participants. The appetite rating scale was measured using a validated, standard, 100mm appetite VAS for hunger, and fullness(17, 31). Appetite VAS was assessed 15 minutes before the standard meal, after meal termination, then every 30 minutes for the first 120 minutes, and at 240 minutes for a total of 7 assessments. Following the last appetite VAS, participants were provided an ad libitum meal (see details below). Gastric emptying of solids was measured using the gold standard scintigraphy technique of two 99mTc-radiolabeled eggs that were included in the standardized breakfast. Images were acquired after breakfast termination, then every 15 minutes for the first 60 minutes, and at 60, 90, 120, 180, and 240 minutes (20, 29, 32).
Measurement of Satiation
The ad libitum meal was performed 240 minutes after standard breakfast and included all you can eat lasagna, vanilla pudding, and skim milk(20). Participants were invited to eat as much as they could until reaching maximal fullness. During the feeding paradigm there was no time limit to consume the meal. The ad libitum meal included: vegetable lasagna [Stouffers®, Nestle USA, Inc, Solon, OH; nutritional analysis of each 326g box: 420kcal, 17g protein (16% of energy), 38g carbohydrate (37% of energy), and 22g fat (47% of energy)]; vanilla pudding [Hunts®, Kraft Foods North America, Tarrytown, NY; nutritional analysis of each 99g carton: 130kcal, 1g protein (3% of energy), 21g carbohydrate (65% of energy), and 4.5g fat (32% of energy)]; and skim milk [nutritional analysis of each 236mL carton: 90kcal, 8g protein (36% of energy), 13g carbohydrate (64% of energy), and 0g fat]. The total amount (g and kcal) of food consumed and the kcal of each macronutrient at the ad libitum meal were analyzed by a registered dietitian, using validated software (ProNutra 3.0; Viocare Technologies Inc, Princeton, NJ).
Study Endpoints
Prior studies in healthy controls and obesity show about 50% of meal retained in the stomach at 90–120 minutes(20, 29, 32); therefore, this is the most relevant time period to assess the relationship between gastric emptying and VAS appetite.
Statistical Analysis
Data are shown as mean and one standard error of the mean (SEM) unless otherwise noted. Student T-test was used to compare differences in continuous variables by gender. The primary analysis was the Pearson correlation (ρ) of the percentage of meal retention at 120 minutes (GE120min) during the GE study, with concurrent VAS appetite (33, 34). Our secondary analysis was the Pearson correlation of GE and VAS appetite at each of the a priori specified time points; and the correlation of GE and VAS appetite with the number of calories consumed at the ad libitum meal. The analysis was adjusted for age, gender, BMI and percentage of body fat. An additional analysis was performed by quartiles using the GE120min; Q1 was defined as the rapid GE group and the remaining patients in Q2-Q4 were defined as having normal/slow GE. Area under the curve (AUC) was calculated using all VAS time points (AUC0–240min). Plots and statistical analyses were performed using JMP®, Version 14.1.0. SAS Institute Inc., Cary, NC, 1989–2019. Two-tailed P-values ≤0.05 were considered statistically significant.
RESULTS
Demographics and Participant Characteristics
A total of 134 participants with obesity completed the GE, satiety, and satiation tests. Participant characteristics were (mean ± SEM): age 39 ± 0.8 years, BMI 38 ± 0.5 kg/m2, and 67% female (Table 1).
Table 1.
Demographics and Clinical Characteristics. Data shown in mean ± SEM.
| All (n=134) | Female (n=90) | Male (n=44) | Δ (95% CI) | p value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 39 ± 0.8 | 38.6 ± 0.9 | 39.8 ± 1.6 | 1.2 (−2.6–4.9) | 0.6 |
| Race, Caucasian (%) | 126 (96)a | 87 (97)b | 42 (95)c | NA | 0.54 |
| Weight, kg | 110.2 ± 1.9 | 103.9 ± 1.9 | 123.1 ± 3.7 | 19.2 (10.9 – 27.4) | <0.0001 |
| BMI, kg/m2 | 37.9 ± 0.5 | 37.8 ± 0.6 | 38.2 ± 0.9 | 0.4(−1.8 – 2.5) | 0.75 |
| DEXA Total body fat, % | 45 ± 0.8 | 45.3 ± 0.9 | 44.5 ± 1.2 | −0.7 (−3.9 – 2.4) | 0.63 |
| GE, %* | |||||
| 15 min | 92.8 ± 0.5 | 92.9 ± 0.5 | 92.8 ± 0.9 | −0.01 (−2.2 – 2) | 0.93 |
| 30 min | 88.3 ± 0.6 | 88.9 ± 0.6 | 87.2 ± 1.1 | −1.7 (−4.3 – 0.9) | 0.19 |
| 45 min | 83.9 ± 0.7 | 85.2 ± 0.7 | 81.3 ± 1.3 | −3.8 (−6.9 – −0.8) | 0.02 |
| 60 min | 78.5 ± 0.8 | 80.6 ± 0.8 | 74.3 ± 1.5 | −6.2 (−9.7 – −2.72) | 0.0007 |
| 90 min | 62.6 ± 1.2 | 66.4 ± 1.2 | 55 ± 2 | −11.3 (−16.1 – −6.5) | <0.0001 |
| 120 min | 46 ± 1.3 | 50.1 ± 1.4 | 37.6 ±2.3 | −12.6 (−17.9 – −7.17) | <0.0001 |
| 180 min | 19.9 ± 1.1 | 23.2 ± 1.3 | 13.3 ± 1.4 | −9.9 (−13.8 – −6) | <0.0001 |
| 240 min | 6.7 ± 0.6 | 8.4 ± 0.8 | 3.1 ± 0.5 | −5.4 (−7.3 – −3.4) | <0.0001 |
| T 1/2, min | 117.3 ± 2.3 | 124.2 ± 2.7 | 103.3 ± 3.5 | −20.9 (−29.8 – −12) | <.0001 |
| VAS Scores, mm | Δ (95% CI) | p value | |||
| Hunger | |||||
| Pre-meal | 56 ± 1.6 | 54.4 ± 1.9 | 59.5 ± 2.4 | 5.1 (−1.3 – 11.4) | 0.11 |
| Breakfast | 27 ± 1.8 | 25.5 ± 2.2 | 30.3 ± 2.8 | 4.9 (−2.4 – 20) | 0.19 |
| 30 min | 31.4 ± 1.8 | 28.6 ± 2.2 | 37.3 ± 2.9 | 8.7 (1.3 – 16.2) | 0.02 |
| 60 min | 38.5 ± 2.2 | 36.5 ± 2.8 | 42.6 ± 3.3 | 4.4 (−2.7 – 14.8) | 0.17 |
| 90 min | 43.1 ± 2.1 | 39.5 ± 2.6 | 50.4 ± 3 | 10.9 (2.9 – 18.9) | 0.008 |
| 120 min | 51.7 ± 2 | 49.8 ± 2.6 | 55.8 ± 3.4 | 6 (−2.5 – 14.6) | 0.16 |
| 240 min | 70.9 ± 1.4 | 70.8 ± 1.6 | 71.3 ± 2.7 | 0.5 (−5.9 – 6.9) | 0.88 |
| AUC(0–120 min), mm/min | 191.4 ± 8.3 | 179 ± 10.4 | 216.8 ± 12.8 | 37.9 (4.9– 70.7) | 0.03 |
| AUC(0–240 min), mm/min | 209.5 ± 6.4 | 202.8 ± 7.7 | 223.2 ± 11.1 | 20.5 (−6.6 – 47.5) | 0.14 |
| Fullness | |||||
| Pre-meal | 12.7 ± 1.3 | 11.9 ± 1.5 | 14.3 ± 2.5 | 2.4 (−3.5 – 8.5) | 0.42 |
| Breakfast | 60.6 ± 1.9 | 62.8 ± 2.3 | 56.1 ± 3.2 | −6.6 (−14.9 – 1.7) | 0.12 |
| 30 min | 54.5 ± 2 | 56.5 ± 2.5 | 50.4 ± 3.2 | −6.1 (−14.9 – 2.0) | 0.14 |
| 60 min | 51.7 ± 2 | 53.9 ± 2.5 | 47.5 ± 3.1 | −6.3 (−14.4 – 1.7) | 0.12 |
| 90 min | 45.7 ± 2.1 | 48.8 ± 2.6 | 39.4 ± 3.2 | −9.3 (−17.6 – −1.07) | 0.03 |
| 120 min | 38.5 ± 2 | 40 ± 2.5 | 35.5 ± 3.1 | −4.5 (−12.5 – 3.6) | 0.27 |
| 240 min | 20 ± 1.2 | 20 ± 1.4 | 20.31 ± 2.2 | 0.3 (−5 – 5.7) | 0.90 |
| AUC(0–120 min), mm/min | 251.8 ± 8.6 | 262.6 ± 10.6 | 229.8 ± 14.3 | −32.8 (−68.3 – 2.8) | 0.07 |
| AUC(0–240 min), mm/min | 168.3 ± 6.2 | 171 ± 7.4 | 163 ± 11.1 | −7.9 (−34.6 – 18.7) | 0.55 |
| OAS | |||||
| 120 min | 42.4 ± 1.8 | 44.7 ± 2.2 | 37.5 ± 2.5 | −7.2 (−14.1 – −0.35) | 0.04 |
| 240 min | 25.2 ± 1.1 | 22.5 ± 1.3 | 24.4 ± 2 | −1.2 (−6.1 – 3.8) | 0.64 |
| AUC(0–120 min), mm/min | 270.4 ± 7.5 | 282.7 ± 9.3 | 245.1 ± 11.7 | −37.5 (−67.3 – −7.8) | 0.01 |
| AUC(0–240 min), mm/min | 164.4 ± 5.3 | 171.5 ± 6.6 | 149.72 ± 8.6 | −21.8 (−43.4 – −0.21) | 0.05 |
| Satiation test | |||||
| Ad libitum meal, kcal | 905.1 ± 26.5 | 806.9 ± 23.5 | 1106 ± 53.3 | 299 (182 – 415.8) | <0.0001 |
| Protein, % | 22.7 ± 0.2 | 22.8 ± 0.2 | 22.5 ± 0.3 | −0.4 (−1.2 – 0.5) | 0.40 |
| Fat, % | 20.6 ± 0.2 | 20.4 ± 0.2 | 20.9 ± 0.3 | 0.4 (−0.3 – 1.2) | 0.27 |
| Carbohydrates, % | 56.7 ± 0.2 | 56.7 ± 0.2 | 56.6 ± 0.4 | −0.1 (−1.2 – 1.02) | 0.86 |
Abbreviations: Δ, mean difference between gender groups; CI, confidence interval; BMI, body mass index; GE, gastric emptying; VAS, visual analog scale; AUC, area under the curve; OAS, overall appetite score.
: % of meal retention at each time point
3% Asian, 1% African American
2% Asian, 1% African American
5% Asian
Measurements of Gastric Emptying, Appetite, and Ad libitum meal
The mean GE T1/2 was 117.3 ± 2.3 minutes and the GE120min was 46 ± 1.3%. The VAS AUC from 0–240 minutes for hunger (hungerAUC(0–240min)) was 209.5 ± 6.4 mm/min, was 168.3 ± 6.2 mm/min for fullnessAUC(0–240min), and 164.4 ± 5.3 mm/min for VAS OASAUC(0–240min). The mean VAS at 120 min (VAS120min) was 51.7 ± 2 mm for hunger, 38.5 ± 2 mm for fullness, and 42.4 mm ± 1.8 for OAS. The mean ad libitum meal intake was 905.1 ± 26.5 kcal. The mean macronutrient intake during the ad libitum meal was protein 22.7 ± 0.2 %, fat 20.6 ± 0.2 %, and carbohydrate 56.7 ± 0.2 % (Table 1). The coefficient of variation was 33% for GE120min and 49% for VAS OAS120min.
Effect of Gender on measurements of Gastric Emptying, VAS Appetite, and Satiation tests.
There were statistically significant differences when analyzed by gender (Table 1). The GE was significantly slower in females with a difference of −11.3% (95% confidence interval [CI], −16.1 to −6.5; p<0.0001), −12.6% (95% CI, −17.9 to −7.17; p<0.0001), −9.9% (95% CI, −13.8 to −6; p<0.0001), and −5.4% (95% CI, −7.3 to −3.4; p<0.0001) at 90, 120, 180, and 240 min, respectively. VAS scores were significantly lower in females at 90 minutes, with a difference of 10.9 mm (95% CI, 2.9 to 18.9; p=0.008) for hunger, and higher for fullness with a difference of −9.3 mm (95% CI, −17.6 to −1.07; p=0.03). VAS OAS was significantly higher in females at 120 minutes, with a −7.2 mm (95% CI, −14.1 to −0.35; p=0.04). We did not observe significant differences in VASAUC(0–240 min) for hunger, desire to eat, fullness, or satisfaction when analyzed by gender. Total kcal consumed during the ad libitum meal were significantly higher in males with a difference of 299 kcal (95% CI, 182 to 415.8; p<0.0001). There was no difference in the percentage of macronutrient intake between females and males.
Relationship between Gastric emptying and Appetite sensations
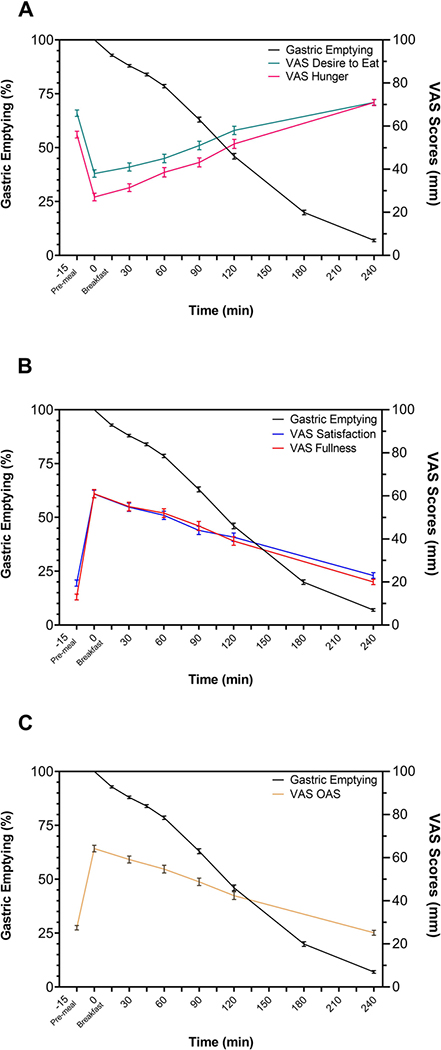
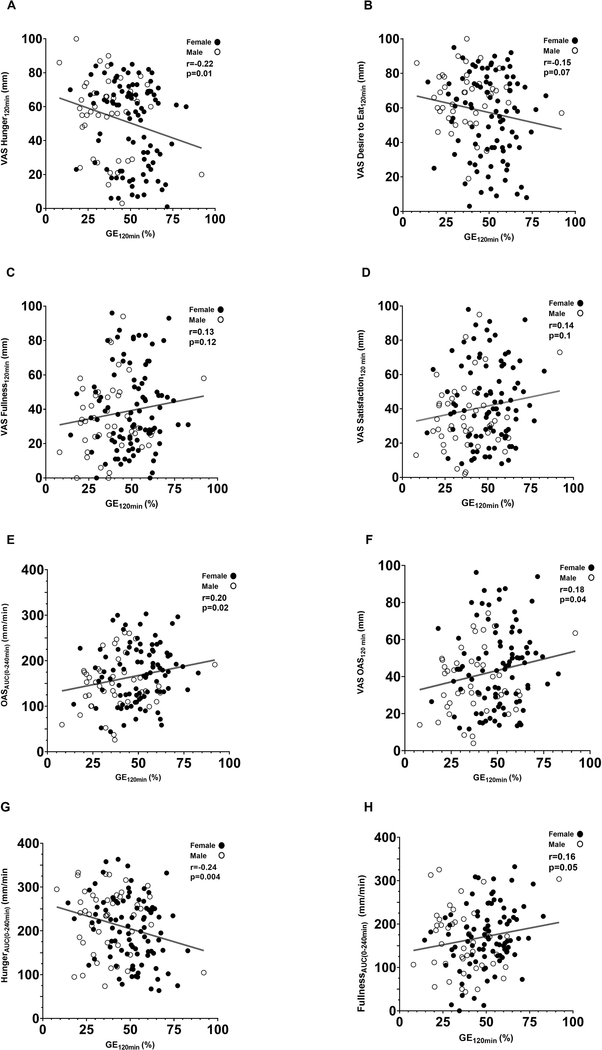
GE, hunger, and fullness curves are provided in Figure 2. Figure 2 demonstrates the interaction of the gastric emptying percentage with the decrease in fullness and the increase in hunger after a standard breakfast. GE120min positively correlated with VAS FullnessAUC(0–240 min) (ρ=0.16, p=0.05), VAS OASAUC(0–240 min) (ρ=0.20, p=0.02) and VAS OAS120min (ρ=0.18, p=0.04). A negative correlation was observed with GE120min and VAS HungerAUC(0–240 min) (ρ=−0.24, p=0.004), and VAS Hunger120min (ρ=−0.22, p=0.01) (Figure 3). The correlation between GE120min and sensation of appetite remained significant when adjusted for age, BMI and percentage of body fat.
Figure 2. GE and VAS Appetite measured simultaneously.
A) VAS for hunger and desire to eat (mean ± SEM) plotted against GE. After overnight fasting, hunger and desire to eat are higher, with a rapid decrease in hunger and desire to eat after the standard 320 kcal breakfast. Within the next 240 min, both sensations return to the fasting levels – increase hunger and desire to eat. B) VAS for fullness and satisfaction (mean ± SEM) which are low during fasting, with a rapid increase after breakfast and with a gradual reduction in time. C) VAS OAS (mean ± SEM) plotted against GE display an inverse curve where lower scores indicate higher appetite.
Figure 3. Correlation between GE120min and Appetite VAS 120 min.
Percentage of meal retention at 120 minutes and its correlation with A) VAS Hunger120 min, B) VAS Desire to eat120 min, C) VAS Fullness120 min, D) VAS Satisfaction120 min, E) VAS OAS120 min, F) VAS OASAUC(0–240 min), G) VAS HungerAUC(0–240 min), and H) VAS FullnessAUC(0–240 min). Female = dark dots; male = white dots.
Relationship between Rapid Gastric Emptying and Appetite sensations
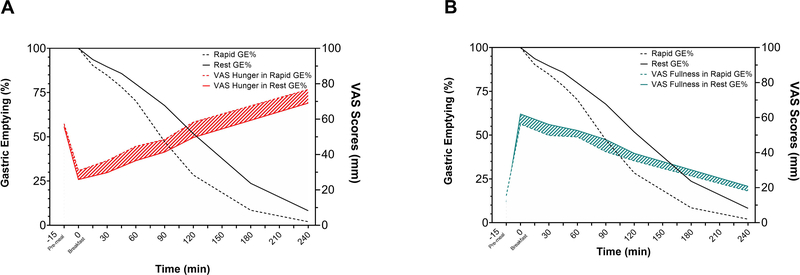
Rapid gastric emptying was defined as the fast GE120min quartile per gender (Table 2). When compared to normal/slow GE, patients with rapid GE had a statistically significant higher sensation of VAS hunger: AUC0–240min mean increase of 32 mm/min (15.6%, p=0.03), VAS hunger at 120 minutes, mean increase of 8.7 mm (p=0.07), and at 240 minutes, mean increase of 7.7 mm (p=0.008) (Figure 4A). Conversely, we did not observe a significant difference between rapid and normal/slow GE in sensation of VAS fullness by AUC0–240min mean decrease of 18 mm/min (p=0.21), VAS fullness at 120 minutes mean decrease of 4.3 mm (p=0.34), or at 240 minutes mean decrease of 2.7 mm (p=0.31) (Figure 4B). Full details about demographics and VAS scores in rapid and rest GE groups are provided in Supplementary Table 1.
Table 2.
Gastric Emptying and VAS appetite test in rapid GE quartile group compared to rest (other 3 quartiles) group. Data shown in mean ± SEM.
| Rapid (n=33) | Rest (n=101) | p value | ||
|---|---|---|---|---|
| GE | ||||
| GE T 1/2, min | 88.6 ± 2.3 | 126.7 ± 2.3 | <.0001 | |
| VAS Scores, mm | ||||
| Hunger | Δ (95% CI) | p value | ||
| 120 min | 58.3 ± 3.8 | 49.6 ± 2.4 | −8.7 (−17.8 – 0.5) | 0.07 |
| 240 min | 76.7 ± 2.1 | 69 ± 1.7 | −7.7 (−13.2 – −2.1) | 0.008 |
| AUC(0–240 min), mm/min | 233.2 ± 11.9 | 201.7 ± 7.4 | −32 (−59.7 – −3.2) | 0.03 |
| Fullness | ||||
| 120 min | 35.3 ± 3.8 | 39.6 ± 2.3 | 4.3 (−4.7 – 13.3) | 0.34 |
| 240 min | 18.1 ± 2.1 | 20.8 ± 1.4 | 2.7 (−2.6 – 7.9) | 0.31 |
| AUC(0–240 min), mm/min | 154.9 ± 13.2 | 172.8 ± 6.9 | 18 (−12.1 – 47.9) | 0.21 |
| OAS | ||||
| 120 min | 39 ± 3.5 | 43.5 ± 2 | 16.9 (−3.7 – 12.7) | 0.28 |
| 240 min | 22.6 ± 2.1 | 26 ± 1.3 | 3.4 (−1.6 – 8.5) | 0.18 |
| AUC(0–240 min), mm/min | 151.7 ± 10.7 | 168.5 ± 6.1 | 4.5 (−7.8 – 41.6) | 0.17 |
Abbreviations: Δ, mean difference between rapid and rest groups; CI, confidence interval; GE, gastric emptying; VAS, visual analog scale; AUC, area under the curve; OAS, overall appetite score.
Figure 4. GE rate and VAS Appetite plotted by quartile performance.
GE rate (mean ± SEM) classified by quartile emptying rate performance (Rapid vs Rest) associated with A) VAS hunger and B) VAS fullness. Rapid GE is associated with higher hunger scores and lower sensation of fullness.
Correlations between Gastric Emptying and VAS Appetite with Satiation measurements
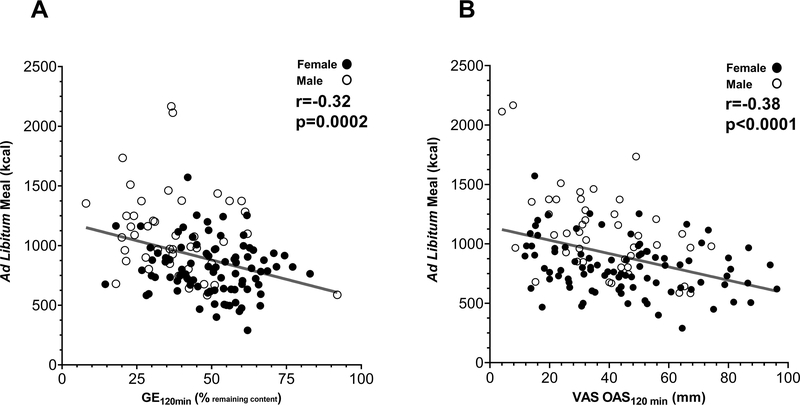
Both VAS appetite sensations (i.e. hunger, fullness, desire to eat, satisfaction) and GE120min were associated with the subsequent calories consumed at ad libitum meal. GE120min and VAS OAS120min were negatively correlated with calories consumed in the ad libitum meal (ρ= −0.32 p=0.002; and (ρ =−0.38, p<0.0001, respectively) (Figure 5A and 5B).
Figure 5. GE120min and VAS Appetite correlation with ad libitum meal.
A) GE120 min, percentage of meal retention at 120 min during the GE study, and B) VAS OAS120min, correlated negatively with total calories consumed in the ad libitum meal 240 minutes after breakfast. Female = dark dots; male = white dots.
DISCUSSION
Our studies, based on real-time, simultaneous measurements of gastric emptying and visual analog scale measurements of appetite, show that gastric emptying is associated with the sensations of appetite, and subsequent calorie intake at an ad libitum meal. These observations support the role of gastric emptying in homeostatic food intake regulation.
As expected, when the stomach is full after a meal, the sensation of hunger is lower and the sensation of fullness is higher; on the contrary, when the stomach is empty, the sensation of hunger is higher and fullness lower. This observation is very consistent among participants immediately after the standard breakfast and prior to the next meal. However, at 120 min postprandially, there is high variability in both the appetite sensation (49%) and the gastric emptying (33%). This variability represents the heterogeneity in satiety, which is defined as the duration of the sensation of fullness or return to hunger. Nonetheless, the high heterogeneity in the current tests should not hold back from proposing to use the 120 minutes postprandial time point as a key determinant and measurement of satiety. The biology of satiety can be evaluated either as the duration of fullness (VAS fullness), return to hunger (VAS hunger), or percent of meal retention at 120 minutes during the GE scintigraphy study. GE T1/2 has been previously associated with individual gastrointestinal symptoms such as early satiation, nausea, bloating, or the symptom complex of dyspepsia(9, 35–38); as well as being a biomarker of diseases such as gastroparesis(39).
In the pursuit of a gold-standard measurement of satiety, we must consider both the homeostatic and hedonic components in the regulation of food intake, and attempt to test homeostatic satiety mechanisms, while separating homeostatic from the hedonic mechanisms. The reported VAS measurements of the sensations of appetite are commonly regarded as subjective and are highly influenced by the hedonic component of feeding behavior. Thus, it is important to identify and validate more objective measurements of satiety, which will not be affected by the emotional status of the individual. In our current study, we demonstrate that gastric emptying can be considered an objective measurement of satiety since it correlates in real-time with the sensation of appetite, and its subcomponents (hunger, desire to eat, fullness, satisfaction, and overall appetite) after a standard meal. As an example of the subjective measurement of the appetite score, a higher hunger sensation or lower fullness sensation does not correlate with acceleration or delay in GE. On the contrary, participants with rapid gastric emptying experienced a 15.6% increase in the sensation of hunger compared to participants with normal GE. This example illustrates how a homeostatic constituent, like GE, can serve as a more objective proxy of appetite sensation, non-influenced by the hedonic component of food intake regulation. These observations are important since the objective physiological assessment of GE may constitute a useful indirect tool of the homeostatic measurement of satiety – or return to hunger. Additionally, establishing an objective measurement of satiety such as GE at 120 minutes after a standardized meal may be valuable in physiologic and interventional studies where reproducibility between individuals and consistent measurements at different laboratories is crucial.
Gastric emptying is a critical factor in the gut-brain axis regulating food intake. GE correlates with hunger hormones like ghrelin (40) and satiety hormones like GLP-1 and PYY, all of which influence GE (41). Furthermore, rapid gastric emptying is associated with more weight gain in 4 years compared to normal gastric emptying in young adults(30), and physical activity accelerates gastric emptying and increases ghrelin(42). Moreover, the identification of rapid GE can help to individualize treatment strategies in patients with obesity to achieve better weight loss outcomes(43). Thus, in two pilot studies, we have shown that patients with obesity and rapid GE respond better to GLP-1 analogs, exenatide(24), and liraglutide(21). More recently, abnormal postprandial satiety – defined as rapid GE – was identified as a key phenotype in a novel obesity classification based on pathophysiological and behavioral traits(43). Furthermore, the classification was used to guide antiobesity pharmacotherapy in a 312 patient’s pragmatic trial and showed that patients treated with liraglutide and abnormal postprandial satiety had 2x more weight loss than patients with normal postprandial satiety(43). This suggests the clinical relevance of GE as an objective measurement of postprandial satiety.
The calorie intake of the next meal – or expected satiation – is influenced by the nutritional properties of the previous meal, homeostatic food intake signals, and learned behavior about expected satiation potential. The high impact of the psychological facet of food intake increases the difficulty of predicting calorie intake or appetite. A meta-analysis examining VAS appetite revealed the absence of a significant correlation between the VAS appetite scores and energy intake(19). In the current study of 134 patients, we have shown that both GE and VAS correlated with calorie intake in the subsequent meal.
Strengths and weaknesses:
The reported study strengths are the large sample size, the tests performed simultaneously on the same day, in participants with obesity who had minimal or no comorbidities that may influence the GE or VAS, such as advanced diabetes, or binge eating disorders. Furthermore, the measured tests were conducted with the gold-standard, in particular the GE test, with its well-characterized performance(29). The conducted study also has limitations. This type of study needs to be replicated in healthy weight participants and more diverse patients with obesity and obesity-related comorbidities. Furthermore, other variables such as gastric accommodation, enteroendocrine hormones, and hedonic characteristics should be considered in the measurement of satiety and satiation.
In conclusion, we report that, in real-time, simultaneous measurements, gastric emptying, and visual analog scale for appetite are correlated and both are well-validated surrogates of postprandial satiety. However, both have high coefficient of variance among our cohort of patients with obesity. Rapid GE is associated with increased appetite, which may contribute to weight gain.
Supplementary Material
RESEARCH IN CONTEXT.
What is already known about this subject?
Satiety, defined as the duration of the sensation of fullness, is usually measured by validated visual analog scales (VAS) for appetite.
Gastric function plays a key role in food intake regulation.
The association between gastric emptying (GE) and VAS-appetite is unknown.
What are the new findings in your manuscript?
Gastric emptying is associated with the sensations of appetite. However, both have a high variability.
Both gastric emptying and VAS-appetite are associated with subsequent ad libitum meal intake
Rapid gastric emptying is associated with increased appetite.
How might your results change the direction of research or the focus of clinical practice?
Both gastric emptying and VAS for appetite are well validated test for postprandial satiety.
Gastric emptying may be an objective measurement for postprandial satiety.
Acknowledgements:
We thank participants in the studies, the nurses and staff of the Mayo Clinic Clinical Research Trials Unit (supported by Mayo Clinic Center for Clinical and Translational Science [CCaTS] grant UL1-TR000135), and Michael Ryks and Deborah Rhoten for excellent technical support.
Funding: Dr. Acosta is supported by NIH (NIH K23-DK114460, C-Sig P30DK84567), Mayo Clinic Center for Individualized Medicine – Gerstner Career Development Award. Dr. Camilleri receives funding related to obesity from the National Institutes of Health (NIH RO1-DK67071).
Disclosure: Dr. Acosta is a stockholder in Gila Therapeutics, Phenomix Sciences; he serves as a consultant for Rhythm Pharmaceuticals, General Mills. Dr. Camilleri is a stockholder in Phenomix Sciences and Enterin and serves as a consultant to Takeda, Allergan, Rhythm, Kallyope, and Arena with compensation to his employer, Mayo Clinic. Dr. Abu Dayyeh has served as a consultant for Boston Scientific, Metamodix, BFKW, DyaMx, and USGI Medical, has received research support for Boston Scientific, Apollo Endosurgery, USGI, Spatz Medical, GI Dynamics, Caim Diagnostics, Aspire Bariatrics, and Medtronic, and has been a speaker for Johnson & Johnson, Endogastric Solutions, and Olympus.
Funding Sources: The funding source had no involvement in the study design, in collection, analysis, and interpretation of the data, in writing the report, or in the decision to submit the paper for publication.
The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis, and the decision to submit for publication.
Data Sharing: Data collected for the study, including individual de-identified participant data, as well as study protocol, and informed consent will be available to interested parties with publication, after signing of a data access agreement. Data may be requested by contacting Dr. Andres Acosta M.D, Ph.D., at Acosta.andres@mayo.edu.
Footnotes
Clinical trial registration: The studies reported here constitute baseline measurements prior to inclusion in the therapeutic trial NCT03374956.
REFERENCES
- 1.Berthoud H-R, Münzberg H, Morrison CD. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology. 2017;152(7):1728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blundell JE, Halford JC. Regulation of nutrient supply: the brain and appetite control. Proc Nutr Soc. 1994;53(2):407–18. Epub 1994/07/01. doi: 10.1079/pns19940046. [DOI] [PubMed] [Google Scholar]
- 3.Strubbe JH, Woods SC. The timing of meals. Psychol Rev. 2004;111(1):128–41. Epub 2004/02/06. doi: 10.1037/0033-295X.111.1.128. [DOI] [PubMed] [Google Scholar]
- 4.Acosta A, Abu Dayyeh BK, Port JD, Camilleri M. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut. 2014;63(4):687–95. Epub 2014/01/10. doi: 10.1136/gutjnl-2013-306235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri M Peripheral mechanisms in appetite regulation. Gastroenterology. 2015;148(6):1219–33. Epub 2014/09/23. doi: 10.1053/j.gastro.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, et al. The ileal brake--inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25(4):365–74. Epub 1984/04/01. doi: 10.1136/gut.25.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiller RC, Trotman IF, Adrian TE, Bloom SR, Misiewicz JJ, Silk DB. Further characterisation of the ‘ileal brake’ reflex in man--effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut. 1988;29(8):1042–51. Epub 1988/08/01. doi: 10.1136/gut.29.8.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Read NW, McFarlane A, Kinsman RI, Bates TE, Blackhall NW, Farrar GB, et al. Effect of infusion of nutrient solutions into the ileum on gastrointestinal transit and plasma levels of neurotensin and enteroglucagon. Gastroenterology. 1984;86(2):274–80. [PubMed] [Google Scholar]
- 9.Halawi H, Camilleri M, Acosta A, Vazquez-Roque M, Oduyebo I, Burton D, et al. Relationship of gastric emptying or accommodation with satiation, satiety, and postprandial symptoms in health. Am J Physiol Gastrointest Liver Physiol. 2017;313(5):G442–G7. Epub 2017/08/05. doi: 10.1152/ajpgi.00190.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Lorenzo C, Williams CM, Hajnal F, Valenzuela JE. Pectin delays gastric emptying and increases satiety in obese subjects. Gastroenterology. 1988;95(5):1211–5. Epub 1988/11/01. doi: 10.1016/0016-5085(88)90352-6. [DOI] [PubMed] [Google Scholar]
- 11.Goyal RK, Guo Y, Mashimo H. Advances in the physiology of gastric emptying. Neurogastroenterol Motil. 2019;31(4):e13546. Epub 2019/02/12. doi: 10.1111/nmo.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt JN, Smith JL, Jiang CL. Effect of meal volume and energy density on the gastric emptying of carbohydrates. Gastroenterology. 1985;89(6):1326–30. Epub 1985/12/01. doi: 10.1016/0016-5085(85)90650-x. [DOI] [PubMed] [Google Scholar]
- 13.Gibbons C, Hopkins M, Beaulieu K, Oustric P, Blundell JE. Issues in Measuring and Interpreting Human Appetite (Satiety/Satiation) and Its Contribution to Obesity. Curr Obes Rep. 2019;8(2):77–87. Epub 2019/05/01. doi: 10.1007/s13679-019-00340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis ST, Eldeghaidy S. Imaging methodologies and applications for nutrition research: what can functional MRI offer? P Nutr Soc. 2015;74(2):89–98. doi: 10.1017/S0029665114001530. [DOI] [PubMed] [Google Scholar]
- 15.Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, et al. Appetite control: methodological aspects of the evaluation of foods. Obes Rev. 2010;11(3):251–70. Epub 2010/02/04. doi: 10.1111/j.1467-789X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flint A, Kapitza C, Zdravkovic M. The once-daily human GLP-1 analogue liraglutide impacts appetite and energy intake in patients with type 2 diabetes after short-term treatment. Diabetes, Obesity and Metabolism. 2013;15(10):958–62. doi: 10.1111/dom.12108. [DOI] [PubMed] [Google Scholar]
- 17.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. Epub 2000/03/07. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 18.Raben A, Tagliabue A, Astrup A. The reproducibility of subjective appetite scores. Br J Nutr. 1995;73(4):517–30. Epub 1995/04/01. doi: 10.1079/bjn19950056. [DOI] [PubMed] [Google Scholar]
- 19.Holt GM, Owen LJ, Till S, Cheng YY, Grant VA, Harden CJ, et al. Systematic literature review shows that appetite rating does not predict energy intake. Crit Rev Food Sci. 2017;57(16):3577–82. doi: 10.1080/10408398.2016.1246414. [DOI] [PubMed] [Google Scholar]
- 20.Acosta A, Camilleri M, Shin A, Vazquez-Roque MI, Iturrino J, Burton D, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology. 2015;148(3):537–46 e4. Epub 2014/12/09. doi: 10.1053/j.gastro.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halawi H, Khemani D, Eckert D, O’Neill J, Kadouh H, Grothe K, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol. 2017;2(12):890–9. Epub 2017/09/30. doi: 10.1016/S2468-1253(17)30285-6. [DOI] [PubMed] [Google Scholar]
- 22.Blom WA, Lluch A, Vinoy S, Stafleu A, van den Berg R, Holst JJ, et al. Effects of gastric emptying on the postprandial ghrelin response. Am J Physiol Endocrinol Metab. 2006;290(2):E389–95. Epub 2005/09/29. doi: 10.1152/ajpendo.00238.2005. [DOI] [PubMed] [Google Scholar]
- 23.Nelson AD, Camilleri M, Acosta A, Busciglio I, Linker Nord S, Boldingh A, et al. Effects of ghrelin receptor agonist, relamorelin, on gastric motor functions and satiation in healthy volunteers. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2016. Epub 2016/06/11. doi: 10.1111/nmo.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acosta A, Camilleri M, Burton D, O’Neill J, Eckert D, Carlson P, et al. Exenatide in obesity with accelerated gastric emptying: a randomized, pharmacodynamics study. Physiol Rep. 2015;3(11). Epub 2015/11/07. doi: 10.14814/phy2.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. International journal of obesity. 2014;38(6):784–93. Epub 2013/09/04. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrichello S, Badurdeen D, Hedjoudje A, Neto MG, Yance R, Veinert A, et al. The Effect of the Intra-gastric Balloon on Gastric Emptying and the DeMeester Score. Obes Surg. 2020;30(1):38–45. Epub 2019/06/30. doi: 10.1007/s11695-019-04039-4. [DOI] [PubMed] [Google Scholar]
- 27.Vargas EJ, Bazerbachi F, Calderon G, Prokop LJ, Gomez V, Murad MH, et al. Changes in Time of Gastric Emptying After Surgical and Endoscopic Bariatrics and Weight Loss: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2019. Epub 2019/04/08. doi: 10.1016/j.cgh.2019.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Nava G, Jaruvongvanich V, Storm AC, Maselli DB, Bautista-Castano I, Vargas EJ, et al. Personalization of Endoscopic Bariatric and Metabolic Therapies Based on Physiology: a Prospective Feasibility Study with a Single Fluid-Filled Intragastric Balloon. Obes Surg. 2020. Epub 2020/04/15. doi: 10.1007/s11695-020-04581-6. [DOI] [PubMed] [Google Scholar]
- 29.Camilleri M, Iturrino J, Bharucha A, Burton D, Shin A, Jeong ID, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24(12):1076. doi: 10.1111/j.1365-2982.2012.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pajot G, Camilleri M, Calderon G, Davis J, Eckert D, Burton D, et al. Association between gastrointestinal phenotypes and weight gain in younger adults: a prospective 4-year cohort study. Int J Obes (Lond). 2020. Epub 2020/05/18. doi: 10.1038/s41366-020-0593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calderon G, McRae A, Rievaj J, Davis J, Zandvakili I, Linker-Nord S, et al. Ileo-colonic delivery of conjugated bile acids improves glucose homeostasis via colonic GLP-1-producing enteroendocrine cells in human obesity and diabetes. EBioMedicine. 2020;55:102759. Epub 2020/04/29. doi: 10.1016/j.ebiom.2020.102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vazquez Roque MI, Camilleri M, Stephens DA, Jensen MD, Burton DD, Baxter KL, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology. 2006;131(6):1717–24. Epub 2006/11/08. doi: 10.1053/j.gastro.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Hill AJ, Blundell JE. Comparison of the Action of Macronutrients on the Expression of Appetite in Lean and Obese Human-Subjects. Ann Ny Acad Sci. 1989;575:529–31. [Google Scholar]
- 34.Rolls BJ, Bell EA, Castellanos VH, Chow M, Pelkman CL, Thorwart ML. Energy density but not fat content of foods affected energy intake in lean and obese women. Am J Clin Nutr. 1999;69(5):863–71. Epub 1999/05/08. doi: 10.1093/ajcn/69.5.863. [DOI] [PubMed] [Google Scholar]
- 35.Cassilly DW, Wang YR, Friedenberg FK, Nelson DB, Maurer AH, Parkman HP. Symptoms of gastroparesis: use of the gastroparesis cardinal symptom index in symptomatic patients referred for gastric emptying scintigraphy. Digestion. 2008;78(2–3):144–51. Epub 2008/11/27. doi: 10.1159/000175836. [DOI] [PubMed] [Google Scholar]
- 36.Stanghellini V, Tosetti C, Paternico A, Barbara G, Morselli-Labate AM, Monetti N, et al. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110(4):1036–42. Epub 1996/04/01. doi: 10.1053/gast.1996.v110.pm8612991. [DOI] [PubMed] [Google Scholar]
- 37.Sarnelli G, Caenepeel P, Geypens B, Janssens J, Tack J. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98(4):783–8. Epub 2003/05/10. doi: 10.1111/j.1572-0241.2003.07389.x. [DOI] [PubMed] [Google Scholar]
- 38.Talley NJ, Locke GR 3rd, Lahr BD, Zinsmeister AR, Tougas G, Ligozio G, et al. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut. 2006;55(7):933–9. Epub 2005/12/03. doi: 10.1136/gut.2005.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camilleri M Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356(8):820–9. Epub 2007/02/23. doi: 10.1056/NEJMcp062614. [DOI] [PubMed] [Google Scholar]
- 40.Levin F, Edholm T, Schmidt PT, Gryback P, Jacobsson H, Degerblad M, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 2006;91(9):3296–302. Epub 2006/06/15. doi: 10.1210/jc.2005-2638. [DOI] [PubMed] [Google Scholar]
- 41.Hellstrom PM, Gryback P, Jacobsson H. The physiology of gastric emptying. Best Pract Res Clin Anaesthesiol. 2006;20(3):397–407. Epub 2006/11/04. doi: 10.1016/j.bpa.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Davis J, Camilleri M, Eckert D, Burton D, Joyner M, Acosta A. Physical activity is associated with accelerated gastric emptying and increased ghrelin in obesity. Neurogastroenterol Motil. 2020:e13879. Epub 2020/05/12. doi: 10.1111/nmo.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acosta A, Camilleri M, Dayyeh BA, Calderon G, Gonzalez D, McRae A, et al. Selection of Anti-obesity Medications based on Phenotypes Enhances Weight Loss: A Pragmatic Trial in an Obesity Clinic. Obesity Journal. 2021;Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.