Abstract
The rapid spread of the highly contagious Omicron variant of SARS-CoV-2 along with its high number of mutations in the spike gene has raised alarm about the effectiveness of current medical countermeasures. To address this concern, we measured neutralizing antibodies against Omicron in three important settings: (1) post-vaccination sera after two and three immunizations with the Pfizer/BNT162b2 vaccine, (2) convalescent sera from unvaccinated individuals infected by different variants, and (3) clinical-stage therapeutic antibodies. Using a pseudovirus neutralization assay, we found that titers against Omicron were low or undetectable after two immunizations and in most convalescent sera. A booster vaccination significantly increased titers against Omicron to levels comparable to those seen against the ancestral (D614G) variant after two immunizations. Neither age nor sex were associated with differences in post-vaccination antibody responses. Only three of 24 therapeutic antibodies tested retained their full potency against Omicron and high-level resistance was seen against fifteen. These findings underscore the potential benefit of booster mRNA vaccines for protection against Omicron and the need for additional therapeutic antibodies that are more robust to highly mutated variants.
One Sentence Summary:
Third dose of Pfizer/BioNTech COVID-19 vaccine significantly boosts neutralizing antibodies to the Omicron variant compared to a second dose, while neutralization of Omicron by convalescent sera, two-dose vaccine-elicited sera, or therapeutic antibodies is variable and often low.
INTRODUCTION
In November 2021 a new SARS-CoV-2 variant, named Omicron (Pango lineage B.1.1.529), was identified as a variant of concern (VOC). Its rapid spread in Africa and unusually high number of mutations, especially in the spike gene, has triggered intense international efforts to track the variant’s spread and evaluate its effects on the potency of therapeutics and vaccines. The predominant strain of Omicron has mutations in the spike gene encoding 15 amino acid changes in the receptor binding domain (RBD) of the spike surface protein (G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, and Y505H). The RBD mediates virus attachment to the ACE2 receptor on target cells and is the principal target of neutralizing antibodies that contribute to protection against SARS-CoV-2. Many of these RBD changes have been previously reported to reduce the effectiveness of several therapeutic neutralizing antibodies (reviewed in Corti et al(1)). A recent study reports that the full complement of RBD substitutions in the Omicron spike compromises the potency of over 85% of 247 anti-RBD monoclonal antibodies (mAbs) tested(2). Preliminary reports indicate substantial immune evasion to two-dose vaccine-elicited sera(3–7), booster-elicited sera(8–16), genotype-varying convalescent sera(3, 5, 6), and several mAbs(2, 6). However, study populations and methods vary widely among the studies to date, and many lack critical details about host characteristics. Moreover, studies have not examined how host demography predicts these neutralizing humoral responses, and examination of how infection by a broader diversity of SARS-CoV-2 Delta and non-Delta genotypes is important for further insights into how genetic diversity may correlate with cross-neutralizing antibody responses.
Here we used a pseudovirus neutralization assay(16) to measure antibody neutralization of SARS-CoV-2 Omicron in three important contexts: (1) antibodies induced after two or three doses of the Pfizer-BioNTech Covid-19 (Pfizer/BNT162b2 mRNA) vaccine, (2) antibodies induced from infection by different SARS-CoV-2 variants and (3) therapeutic antibodies under emergency use authorization (EUA) or in later stages of clinical development. We compared the magnitude of neutralization escape by Omicron to D614G (referred to as wild type, WT) and Delta SARS-CoV-2 variants to help inform public health decisions and offer further data toward correlate of protection research.
RESULTS
Three immunizations of the Pfizer/BNT162b2 mRNA COVID-19 vaccine significantly boosts neutralizing antibodies to the Omicron variant compared to two-vaccinations.
The emergence of the Omicron variant coincided with recommendations for booster immunizations, particularly for at risk populations. We studied the neutralization titers of 39 generally healthy, adult healthcare workers participating in the Prospective Assessment of SARS-CoV-2 Seroconversion study (PASS study, Table 1)(17) who received the full primary series (1st and 2nd) and booster (3rd) immunizations with the Pfizer/BNT162b2 vaccine. We chose to study sera at peak responses after the full primary series vaccination rather than after 6 months because 6-months titers are often very low(10, 18).
Table 1.
Demographic data for participants receiving Pfizer/BNT162b2 initial vaccine series and booster
| N (%) | |
|---|---|
| Sex | |
| Female | 25 (64.1) |
| Male | 14 (35.9) |
| Race | |
| White | 26 (66.6%) |
| Asian | 8 (20.5%) |
| Black | 4 (10.3%) |
| Multiracial | 1 (2.6%) |
| Occupation | |
| Nurse | 11 (28.2%) |
| Physician | 11 (28.2%) |
| Physical/Occupational/Recreational Therapist | 9 (23.1%) |
| Medical Technician | 3 (7.7%) |
| Lab Personnel | 3 (7.7%) |
| Social Worker | 1 (2.6%) |
| Psychologist | 1 (2.6%) |
| Anti-N seroconversion after vaccination and before boost | |
| Positive | 17 (43.6%) |
| Negative | 22 (56.3%) |
| Age | |
| Mean age ± SD (range) | 45 ± 11 (26 – 69) |
| Time between second vaccine and sample collection | |
| Mean days ± SD (range) | 30 ± 11 (28 – 34) |
| Time between second vaccine and booster dose | |
| Mean days ± SD (range) | 267 ± 14 (218–310) |
| Time between booster dose and sample collection | |
| Mean days +/− SD (range) | 43 ± 17 (7 – 93) |
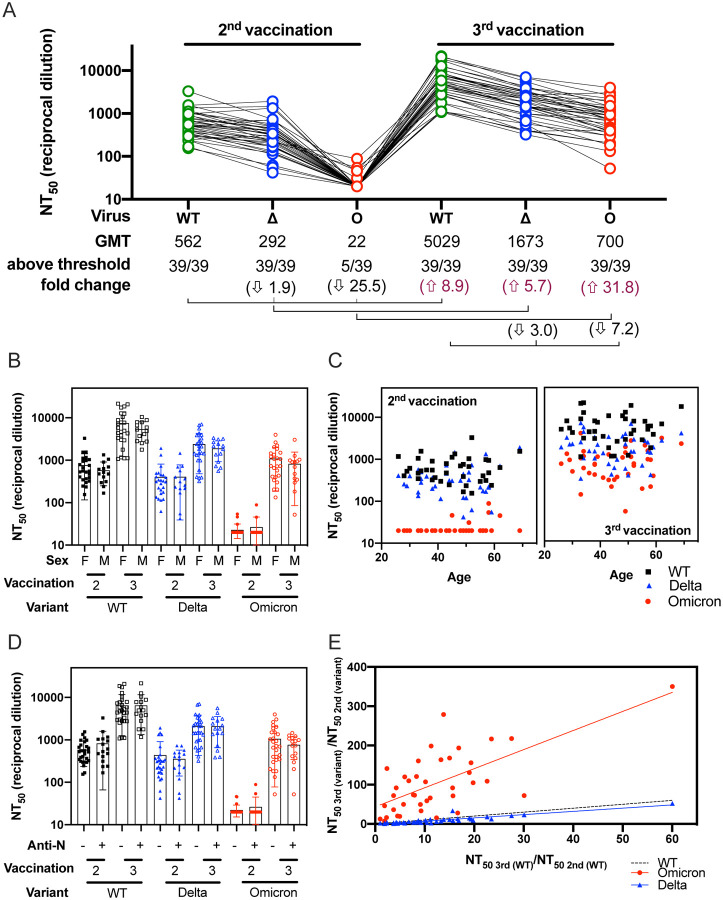
Neutralization activity against Omicron above the threshold of our assay (1:40 dilution) was only observed in 12.8% (5/39) of serum samples obtained at a mean of 30 ± 11 days after the 2nd Pfizer/BNT162b2 vaccination (Figure 1A). After the 2nd vaccination neutralization titers against Omicron (geometric mean titer, GMT 22) were 25.5-fold lower than neutralization titers against WT (GMT 562). By contrast, neuralization titers against Delta (GMT 292) were 1.9-fold lower than WT. Neutralization titers from the same individuals collected 43 ± 17 days after the 3rd Pfizer/BNT162b2 vaccination were 8.9-fold greater against WT (GMT 5029) compared to titers after the 2nd vaccination. The titers against Omicron after the 3rd vaccination (GMT 700) showed 31.8-fold increase compared to titers after the 2nd vaccination, whereas titers against Delta after the 3rd vaccination (GMT 1673) were only 5.7-fold higher than the titers after the 2nd vaccination. Importantly, all individuals had measurable neutralizing titers against Omicron after the 3rd vaccination, highlighting the potential for increased protection by a booster vaccine.
Fig. 1. Sensitivity of the Omicron variant to neutralization by Pfizer/BNT162b2 vaccinee sera.
(A) Neutralization assays were performed using lentiviral pseudoviruses bearing SARS-CoV-2 WT (B.1 lineage, D614G), Delta (Δ, B.1.617.2 lineage, T19R, G142D, E156-, F157-, R158G, L452R, T478K, D614G, P681R and D950N) and Omicron (O, B.1.1.529 lineage, A67V, del69–70, T95I, del142–144, Y145D, del211, L212I, ins214EPE, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K and L981F) with sera from 39 healthcare workers after immunization with two and three doses of Pfizer/BNT162b2 vaccine. Sera was obtained at a mean of 30 ± 11 days after immunization with two doses and at a mean of 43 ± 17 days after booster vaccination. Each serum was run in duplicate in two independent experiments against each pseudovirus to determine the 50% neutralization titer (NT50). The geometric means titers (GMT), the number of NT50 above threshold (1:40) and the fold change are indicated. Titers below 1:40 were set at 20 to calculate GMTs. Arrows indicate increase or decrease relative to WT. Connecting lines indicate serum from the same individual. The demographic information for this sera cohort is provided in Table 1. (B) NT50 by sex after 2nd or 3rd vaccination. (C) NT50 by age after 2nd and 3rd vaccination. (D) 2nd or 3rd vaccination NT50 according to anti-N (nucleocapsid protein) seroconversion between 2nd and 3rd vaccination. (E) The ratios between the neutralization titers after the 3rd and the 2nd immunization for Omicron and Delta were plotted against the corresponding ratios for WT. For panels B-E, black squares correspond to WT, blue triangles correspond to Delta and red circles correspond to Omicron.
We also evaluated how sex or age of the individual might affect the titers in sera after vaccination. We did not observe a trend according to sex or age after the 2nd or 3rd vaccination (Figure 1B and 1C, respectively). A total of 17/39 individuals had a positive anti-N (SARS-CoV-2 nucleoprotein) seroconversion during regular scheduled blood sampling between the 2nd and the 3rd immunization(17). No symptoms of COVID-19 were reported by the subjects, possibly indicative of silent infection or exposure to SARS-CoV-2 or other coronaviruses. We did not find any trends when comparing differences in neutralizing antibody titers between individuals who had anti-N seroconversion and those who did not (Figure 1D). To assess the breadth of neutralization responses against Omicron induced by boosting, we compared the change in titers against Omicron or Delta relative to WT after the 2nd and 3rd vaccination. To account for variability in the antibody titers from one individual to another, we calculated the ratio between the neutralization titers after the 3rd and the 2nd vaccination for each variant and plotted this ratio for Omicron and Delta against WT (Figure 1E). We observed that the ratios of NT50 titer from the 3rd to the 2nd vaccination for Omicron relative to the corresponding ratios for WT were higher than the ratios for Delta relative to WT, suggesting that the 3rd vaccination broadened responses to the distantly-related variant.
Neutralization of Omicron by convalescent sera from individuals infected by different variants shows lower neutralization titers compared to the infecting variant.
To investigate the potency of infection-induced neutralizing antibodies against Omicron, we compared neutralization titers against WT, Delta, and Omicron in convalescent sera obtained at a mean of 30.2 ± 9.3 days post-symptom onset from unvaccinated individuals infected with WT, Alpha, Beta, or Delta variants who were enrolled in the Epidemiology, Immunology, and Clinical Characteristics of Emerging Infectious Diseases with Pandemic Potential (EPICC) study(19). Genotypes of the infecting variants were sequenced for all cases (Table 2 and Supplementary Table). These convalescent sera were complemented by a Beta-convalescent serum from another protocol.
Table 2:
Characteristics of unvaccinated infections providing convalescent sera
| N = 39 | |
|---|---|
| Gender | |
| Female | 14 (35.9%) |
| Male | 25 (64.1%) |
| Race | |
| White | 29 (74.4%) |
| Asian | 1 (2.6%) |
| Black | 6 (15.4%) |
| Multiracial | 3 (7.7%) |
| Age | |
| Mean age ± SD (range) | 41.1 ± 20 (1.4 – 73.2) |
| Charlson comorbidity index | |
| 0 | 20 (51.3%) |
| 1–2 | 10 (25.6%) |
| 3–4 | 5 (12.8%) |
| >5 | 4 (10.3%) |
| Time between infection symptom onset and sample collection | |
| Mean days ± SD (range) | 30.2 ± 9.3 (14.0 – 51.0) |
| Severity of initial infection | |
| Outpatient | 23 (59.0%) |
| Hospitalized | 16 (41.0%) |
| Infecting genotype * | |
| AY.119 | 1 (2.6%) |
| AY.14 | 2 (5.1%) |
| AY.25 | 3 (7.7%) |
| AY.44 | 1 (2.6%) |
| AY.47 | 1 (2.6%) |
| AY.62 | 1 (2.6%) |
| AY.74 | 1 (2.6%) |
| B.1 | 10 (25.6%) |
| B.1.1.7 | 5 (12.8%) |
| B.1.2 | 6 (15.4%) |
| B.1.351 | 1 (2.6%) |
| B.1.617.2 | 7 (17.9%) |
Genotypes assigned based on Pango 3.1.17 (2021-12-06). The genotype of the infecting variant was determined in all cases except for one, a traveler who had moderate-severe Covid-19 (outpatient) in the Republic of South Africa during the peak of the Beta (B.1.351) wave in January 2021 (FDA IRB Study # 2021-CBER-045).
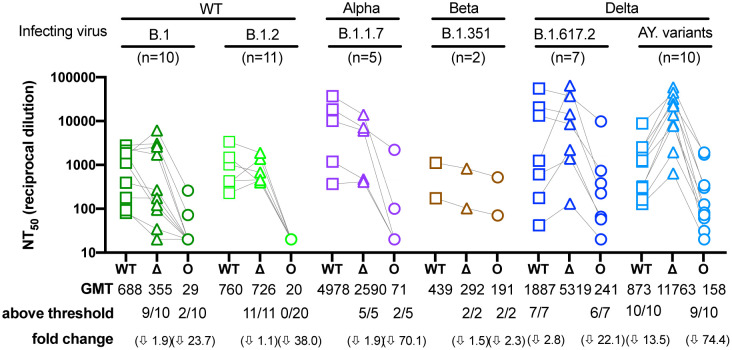
Figure 2 shows that the Delta variant was modestly more resistant to neutralization than WT by sera from most individuals infected with WT, Alpha, or Beta viruses (1.1 to 1.9-fold), while neutralization titers were much more reduced against Omicron (2.3 to 70.1- fold). A total of 2/10 and 0/20 individuals infected with WT (B.1 and B.1.2) variants, respectively, had a response above the threshold against Omicron, whereas 2/5 and 2/2 individuals infected with Alpha (B.1.1.7) and Beta (B.1.351) variants, respectively, were above threshold. Convalescent sera from individuals infected with Delta (B.1.617 and AY.14/.25/.44/.47/.62/.74/.119) variants generally had higher neutralization titers against Delta (2.8 to 13.5-fold) compared to WT, indicating more focused antibody responses to epitopes in the Delta spike. Convalescent sera from these Delta-infected individuals showed 22.1 to 74.4-fold lower neutralization titers against Omicron compared to Delta. However, a total of 6/7 individuals infected with the B.1.617.2 variant and 9/10 individuals infected with an AY variant had titers above threshold against Omicron.
Fig. 2. Sensitivity of the Omicron variant to neutralization by convalescent sera.
Neutralization assays were performed using convalescent sera from persons infected with genotyped variants from B.1, B.1.2, B.1.1.7, B.1.351, B.1.617.2, AY.14, AY.25, AY.44, AY.47, AY.62, AY.74 or AY.119 lineages (Table 2 and Supplementary Table). Both B.1 and B.1.2 have no mutations in the receptor binding domain and were therefore considered WT, whereas some of the AY mutants have additional mutations in the RBD relative to B.1.617.2. Each serum was run in duplicate against WT, Delta, and Omicron to determine the NT50. The geometric means (GMT), the number of NT50 above threshold (1:40) and the fold change are indicated. Titers that did not inhibit at the lowest dilution tested (1:40) were assigned a titer of 20 for GMT calculations. Arrows indicate decrease relative to the infecting variant. Connecting lines indicate serum from the same individual. Data shown represent two independent experiments each with an intra-assay duplicate. Squares correspond to WT, triangles correspond to Delta, and circles correspond to Omicron.
Boosting reduces apparent antigenic differences between WT and Omicron variants.
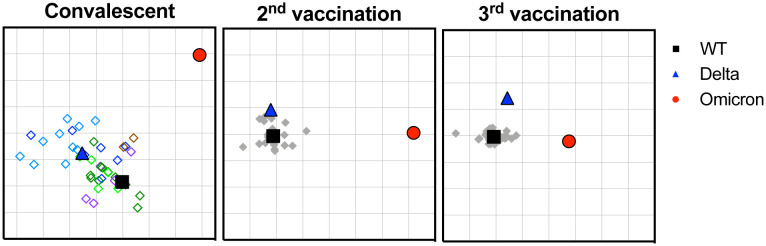
We applied antigenic cartography to explore how the convalescent and post-vaccination sera distinguish the different spike antigens. Antigenic maps were made separately using neutralizing antibody titers from individuals infected with the different variants or from individuals after the 2nd or 3rd vaccination (Figure 3). Convalescent sera were more heterogeneous compared to the post-vaccination, with each convalescent serum clustering closer to the infecting variant, as expected. The 3rd post-vaccination sera were more tightly clustered around WT compared to the 2nd post-vaccination sera. The antigenic distances between Omicron and WT were large for all three sera sets, but the antigenic distance between Omicron and WT were smaller after the 3rd vaccination (7.2-fold drop) compared to either convalescent or the 2nd vaccination sera (49.4-fold drop and 39.4-fold drop, respectively), in agreement with the titer changes in Figure 1A. Small distance changes between WT and Delta were observed for all three sera sets (2.0 to 3.6-fold difference).
Fig. 3. Antigenic cartography of convalescent and vaccinee sera against WT, Delta and Omicron.
Antigenic maps were separately generated from convalescent (left panel), 2nd vaccination (middle panel) or 3rd vaccination (right panel) sera. Convalescent sera are shown in diamonds as follows: B.1 (dark green), B.1.2 (light green), B.1.1.7 (purple), B.1.351 (brown), AY variants (light blue), and B.1.617.2 (dark blue). Gray diamonds correspond to post-vaccination sera. Each grid square corresponds to 2-fold dilution in the neutralization assay. Black squares correspond to WT variant. Blue triangles correspond to Delta variant. Red circle corresponds to Omicron variant.
The potency of most therapeutic antibody products is compromised against Omicron.
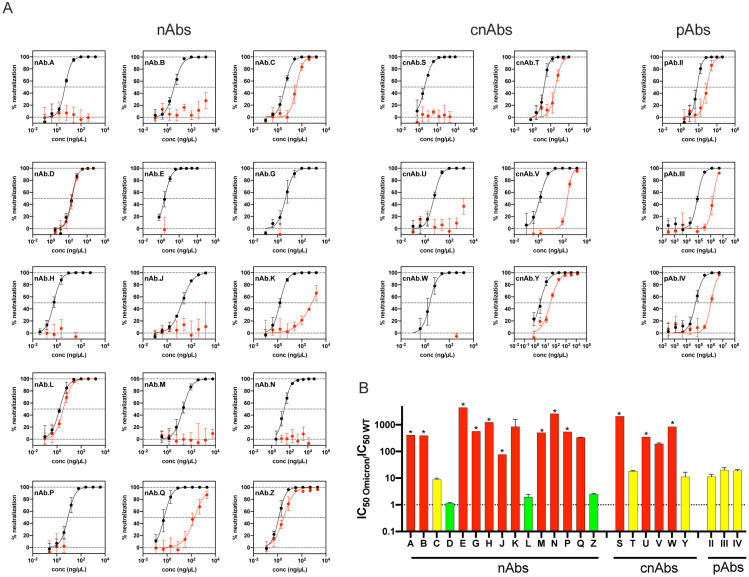
As part of the US government COVID-19 response effort to speed development of therapeutics for COVID-19, we assessed the neutralization of Omicron by 24 therapeutic antibody products currently under EUA or in late stages of clinical development. This panel includes 15 single therapeutic neutralizing antibody products (nAbs), six combination nAbs (cnAbs) and three polyclonal antibody preparations (pAbs). The manufacturers provided these clinical products for side-by-side comparisons of potency against variants but required blind coding of these antibody products for publications. Previously, we reported that several single substitutions in the spike protein of other variants conferred resistance to some of these products(20), but a similar assessment has not been performed on the Omicron spike.
Figure 4A shows the neutralization curves for each product against WT and Omicron. To quantify the relative drop in nAb potency we calculated ratios between the 50% inhibitory concentration of Omicron to WT (Figure 4B). These ratios do not account for the absolute potencies of the nAbs but do allow for a uniform comparison of the changes in potency against Omicron relative to WT for all the antibody products. We note that the majority of successful clinical trials were performed when predominant strains had a ratio of near 1 compared to WT. However, absolute potency using IC50 ng/ml as a measure has not been established as a correlate of protection. We defined ratios between of 5 to 50 as a benchmark for partial or moderate resistance and ratios of > 50 as a benchmark for more complete resistance relative to WT. While the clinical relevance of the IC50 changes has not been determined for any in vitro assay, these cutoffs were chosen because the therapeutic levels of antibody therapeutics may be high enough to overcome low levels of resistance. By the fold-change measure, only three of 15 nAbs retained near full potency against Omicron compared to WT, and only one retained partial potency. Two cnAbs retained partial potency, while the remaining four cnAbs showed complete loss of neutralization potency. All three pAbs showed reduced neutralization potency (ratios 13–17) against Omicron, in agreement with the data from convalescent and vaccinated individuals. These findings raise concerns that many of the available therapeutic antibody products may not be effective against Omicron.
Fig. 4. Neutralization of Omicron by therapeutic antibodies.
(A) Neutralization curves for each one of the 24 therapeutic antibody products against WT (black) and Omicron (red). (B) Bar graph showing the ratio between the IC50 of Omicron and WT for all the antibody products. The sensitivity of the Omicron variant against 15 monoclonal antibodies (nAbs), 6 combination nAbs products (cnAbs), and 3 polyclonal antibodies (pAbs). Red indicates IC50 resistance ratios >50, yellow indicates moderate resistance with IC50 ratios between 5–50, and green indicates sensitivity comparable to WT with IC50 ratios <5. Antibodies for which complete neutralization was not achieved at the highest concentration tested are denoted by *. Data shown represent two independent experiments each with an intra-assay duplicate.
DISCUSSION
Neutralizing antibodies are widely accepted as an important component of protection against SARS-CoV-2 infection and disease (COVID-19), but efforts to assess antibody titers that correlate with protection are complicated by many factors. These include potential redundancy and synergism of different components of the humoral, cellular, and innate immune system, and differences in variant fitness and host genetics, age, and prior immunity. The risk of infection can also be confounded by human behavior and local public health measures, while measurements of antibody titers can vary with different laboratory methods. Therefore, differences in study populations and laboratory methods are important considerations for assessing the impact of immune evasion by Omicron on medical countermeasures.
Here, using the same lentiviral pseudovirus neutralization platform we measured the change in potency of 24 clinical-stage therapeutic antibodies against Omicron compared to WT (D614G) and compared neutralizing antibodies in sera from two well-characterized cohorts of subjects in prospective clinical studies. Our findings show that most vaccinated individuals have low or undetectable titers against Omicron after the second Pfizer/BNT162b2 vaccination, similar to findings reported by others(3–7). However, the third vaccination significantly increased neutralizing titers to levels significantly higher than those elicited by the second vaccination, in agreement with other preliminary studies(8–14). It is notable that the post-2nd vaccination and post-3rd vaccination sampling times were similar, indicating that this boost does not simply reflect time since last vaccine. We found no association between sex or age with these neutralizing immune responses, although it is important to note that the study samples were from generally healthy adults, and that the post-vaccine-dose sampling time was overall short (43 ± 17 days). It has been shown that infection followed by vaccination results in neutralizing antibody titers comparable or higher to titers after two vaccinations(3, 10, 14, 21). The PASS study included anti-N antibody testing on all blood samples for detection of silent infections. The neutralizing antibody titers among the 17 asymptomatic individuals who seroconverted for N antibodies between the 2nd and 3rd immunization were not higher than those who did not seroconvert. Other studies have shown high antibody titers in convalescent individuals after the 1st or 2nd vaccination(10, 11). We did not see an increase after the 3rd vaccination in individuals who seroconverted for N-antibodies. This may be due to reduced antigen load from incident asymptomatic infection or having reached a maximum response after vaccination.
The antigenic cartography analysis suggests that the Omicron variant appears to be antigenically distant from WT and Delta, but this distance seems to decrease after the 3rd vaccination. The apparent broadening of responses to Omicron may be due to boosting of titers to cross-reactive epitopes or improved antibody affinity to shared epitopes, or both. For the convalescent sera, high titers generally correlated with the highest cross-neutralization to Omicron. Continued studies of the breadth against multiple variants and duration of responses after booster vaccinations are urgently needed. In both vaccination and convalescent individuals, our studies suggest that booster vaccinations, even with the original ancestral vaccine antigen, could be beneficial in protecting against Omicron, in agreement with the rapidly accumulating data from many sources(3, 5, 6).
Lastly, nine of the fifteen clinical-grade nAbs under EUA or in late stages of clinical development had no measurable IC50 against Omicron compared to WT. Also, while most nAbs and cnAbs lost measurable potency, all polyclonal antibodies retained measurable, though reduced with IC50. Careful selection of therapeutic antibodies is needed according to variant prevalence. As Omicron continues to acquire additional mutations the products that remain effective could be jeopardized, underscoring the risk associated with this variant and its derivatives.
In summary, our findings indicate that booster doses of mRNA COVID-19 vaccines may afford an increase in protection against Omicron by inducing higher levels of neutralizing antibodies compared to two vaccine doses or the levels of neutralizing antibodies induced by SARS-CoV-2 infection from different variants. The strengths of this study include representation of a broad diversity of genotypes, including within-Delta diversity, comparable sampling times between convalescent and vaccinated subjects, the use of cartography methods, and the availability of subject demographics to interpret how host characteristics may influence Omicron humoral immunity. The limitations of our study include the relatively small numbers of study subjects, restricted timing of sera collection, and the use of a pseudovirus platform as a surrogate to authentic SARS-CoV-2 viruses. Ultimately neutralization titers need to be tied to clinical outcomes. The rapid and unpredictable evolution of SARS-CoV-2 requires continued development and assessments of medical countermeasures.
MATERIALS AND METHODS
Vaccinated participants
Details of the PASS study protocol, including the inclusion/exclusion criteria, have been published(17). Full details are in the Supplemental Material. Briefly, generally healthy healthcare workers without a history of SARS-CoV-2 infection at screening were enrolled. The study began in August 2020 and involved monthly research clinic visits to obtain serum for longitudinal SARS-CoV-2 antibody testing. The subset of participants included in this study received three doses of Pfizer/BNT162b2 vaccine; none had a PCR-confirmed SARS-CoV-2 infection during follow-up. Participants’ serially-collected serum samples were screened for immunoglobulin G (IgG) reactivity with SARS-CoV-2 spike protein and nucleocapsid protein (N) using a multiplex microsphere-based immunoassay, as described(22).
Unvaccinated infections - study population and general study design
The EPICC study is a cohort study of U.S. Military Health System (MHS) beneficiaries that includes those with a history of SARS-CoV-2 infection, as described previously(19). Full details are in Supplemental Material. Enrollment occurred at six Military Treatment Facilities (MTFs). Demographic, comorbidity, COVID illness characteristics, and vaccination status were obtained from the clinical case report form and/or the MHS Data Repository. Biospecimen collection included serial serum samples and upper respiratory specimen swabs.
Diagnosis of SARS-CoV-2 infection and genotyping of infections used for convalescent sera
SARS-CoV-2 infection was determined by positive clinical laboratory PCR test performed at the enrolling clinical site, or a follow-up upper respiratory swab collected as part of the EPICC study. The specific clinical PCR assay employed at the MTF varied. The follow-up PCR assay (for EPICC specimens) was the SARS-CoV-2 (2019-nCoV) CDC qPCR Probe Assay research use only kits (IDT, Coralville, IA)(23); details are in the Supplemental Material. Whole viral genome sequencing was performed on extracted SARS-CoV-2 RNA from PCR-positive specimens; details are in the Supplemental Material. The Pango classification tool (https://cov-lineages.org/) was used for genotype classification (version 3.1.17).
Ethics
The PASS (IDCRP-126) and EPICC (IDCRP-085) studies were approved by the Uniformed Services University of the Health Sciences Institutional Review Board (IRB) in compliance with all applicable Federal regulations governing the protection of human participants. All PASS and EPICC study participants provided informed consent. The convalescent Beta sera, obtained from a traveler who had moderate-severe Covid-19 in the Republic of South Africa during the peak of the Beta (B.1.351) wave in January 2021, was obtained with informed consent and covered under the US Food and Drug Administration IRB approved expedited protocol # 2021-CBER-045.
SARS-Cov-2 pseudovirus production and neutralization assays
Lentiviral pseudoviruses were generated and used in neutralization assays, as previously described(24). The Omicron spike expression plasmid was generously provided by Nicole Doria-Rose (Vaccine Research Center, National Institutes of Health). Neutralization assays were performed on 293T-ACE2/TMPRSS2 cells stably expressing ACE2 and transmembrane serine protease 2 (BEI # NR-55293). Twenty-four clinical-stage therapeutic antibody products were provided by pharmaceutical companies to support the US government COVID-19 response efforts. The antibody identities are blinded for publications per an agreement with the companies. Neutralization curves were normalized to virus only controls and fitted using nonlinear regression curve (GraphPad Prism, La Jolla, CA). The antibody concentration or sera dilution corresponding to 50% neutralization was defined as NT50 for sera or IC50 for antibody products. Data reported are averages from at least two independent experiments, each with intra-assay duplicates.
Antigenic cartography
ACMACS antigenic cartography software (https://acmacs-web.antigenic-441cartography.org/) was used to create a geometric interpretation of neutralization titers against the WT, Delta, and Omicron variants. Each square in the map indicates one antigenic unit, corresponding to two-fold dilution of the antibody in the neutralization assay. Antigenic distance is measured in any direction of the map.
Supplementary Material
Table S1: SARS-CoV-2 genotypes for the infecting variants
Supplementary materials and methods
Acknowledgments:
The authors wish to also acknowledge all who have contributed to the EPICC (IDCRP-085) COVID-19 study.
Brooke Army Medical Center, Fort Sam Houston, TX: Col J. Cowden; LTC M. Darling; S. DeLeon; Maj D. Lindholm; LTC A. Markelz; K. Mende; S. Merritt; T. Merritt; LTC N. Turner; CPT T. Wellington
Carl R. Darnall Army Medical Center, Fort Hood, TX: LTC S. Bazan; P.K Love
Fort Belvoir Community Hospital, Fort Belvoir, VA: N. Dimascio-Johnson; MAJ E. Ewers; LCDR K. Gallagher; LCDR D. Larson; A. Rutt
Henry M. Jackson Foundation, Inc., Bethesda, MD: P. Blair; J. Chenoweth; D. Clark
Madigan Army Medical Center, Joint Base Lewis McChord, WA: S. Chambers; LTC C. Colombo; R. Colombo; CAPT C. Conlon; CAPT K. Everson; COL P. Faestel; COL T. Ferguson; MAJ L. Gordon; LTC S. Grogan; CAPT S. Lis; COL C. Mount; LTC D. Musfeldt; CPT D. Odineal; LTC M. Perreault; W. Robb-McGrath; MAJ R. Sainato; C. Schofield; COL C. Skinner; M. Stein; MAJ M. Switzer; MAJ M. Timlin; MAJ S. Wood
Naval Medical Center Portsmouth, Portsmouth, VA: S. Banks; R. Carpenter; L. Kim; CAPT K. Kronmann; T. Lalani; LCDR T. Lee; LCDR A. Smith; R. Smith; R. Tant; T. Warkentien
Naval Medical Center San Diego, San Diego, CA: CDR C. Berjohn; S. Cammarata; N. Kirkland; D. Libraty; CAPT (Ret) R. Maves; CAPT (Ret) G. Utz
Tripler Army Medical Center, Honolulu, HI: S. Chi; LTC R. Flanagan; MAJ M. Jones; C. Lucas; LTC (Ret) C. Madar; K. Miyasato; C. Uyehara
Uniformed Services University of the Health Sciences, Bethesda, MD: B. Agan; L. Andronescu; A. Austin; C. Broder; CAPT T. Burgess; C. Byrne; COL K Chung; J. Davies; C. English; N. Epsi; C. Fox; M. Fritschlanski; M. Grother; A. Hadley; COL P. Hickey; E. Laing; LTC C. Lanteri; LTC J. Livezey; A. Malloy; R. Mohammed; C. Morales; P. Nwachukwu; C. Olsen; E. Parmelee; S. Pollett; S. Richard; J. Rozman; J. Rusiecki; E. Samuels; M. Sanchez; A. Scher; CDR M. Simons; A. Snow; K. Telu; D. Tribble; L. Ulomi
United States Air Force School of Medicine, Dayton, OH: SSgt T. Chao; R. Chapleau; M. Christian; A. Fries; C. Harrington; S. Huntsberger; K. Lanter; E. Macias; J. Meyer; S. Purves; K. Reynolds; J. Rodriguez; C. Starr
Womack Army Medical Center, Fort Bragg, NC: B. Barton; LTC D. Hostler; LTC J. Hostler; MAJ K. Lago; C. Maldonado; J. Mehrer
United States Army Medical Research Institute of Infectious Diseases, Frederick, MD: MAJ J. Kugelman
William Beaumont Army Medical Center, El Paso, TX: MAJ T. Hunter; J. Mejia; R. Mody; R. Resendez; P. Sandoval; M. Wayman
Walter Reed National Military Medical Center, Bethesda, MD: I. Barahona; A. Baya; A. Ganesan; MAJ N. Huprikar; B. Johnson
Walter Reed Army Institute of Research, Silver Spring, MD: S. Peel
We also thank Douglas Pratt and Hector Izurieta (US Food and Drug Administration) for critical review of the manuscript.
Funding Statement:
This work was supported by awards from the Defense Health Program (HU00012020067, HU00012120094, HU00012120104) and the National Institute of Allergy and Infectious Disease (HU00011920111). The PASS and EPICC protocols were executed by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed by the Uniformed Services University of the Health Sciences (USUHS) through a cooperative agreement by the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). This project has also been funded in part by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIAID/NIH), under an interagency agreement with USUHS (Y1-AI-5072), and by the Intramural Research Program of NIAID. Additional support was provided by institutional research funds from the US Food and Drug Administration (FDA) and NIAID/NIH through an interagency agreement (AAI21013-001-00000) with Center for Biologics Evaluation and Research, FDA, as part of the US Government COVID-19 response efforts.
Potential conflicts of interest.
S. D. P., T. H. B, and D.R.T. report that the Uniformed Services University (USU) Infectious Diseases Clinical Research Program (IDCRP), a US Department of Defense institution, and the Henry M. Jackson Foundation (HJF) were funded under a Cooperative Research and Development Agreement to conduct an unrelated phase III COVID-19 monoclonal antibody immunoprophylaxis trial sponsored by AstraZeneca. The HJF, in support of the USU IDCRP, was funded by the Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense to augment the conduct of an unrelated phase III vaccine trial sponsored by AstraZeneca. Both of these trials were part of the US Government COVID-19 response.
Footnotes
Publisher's Disclaimer: Disclaimer: E. Laing, T. Burgess, D. Tribble, A Fries, C. Broder, E. Mitre, M. Hollis-Perry, S. Lusvarghi, S. Pollett, SN. Neerukonda, W. Wang, R. Wang, R. Vassell, LC. Katzelnick, L Bentley, AE. Eakin, KJ. Erlandson and CD. Weiss are service members or employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Publisher's Disclaimer: The contents of this publication are the sole responsibility of the author (s) and do not necessarily reflect the views, opinions, or policies of Uniformed Services University of the Health Sciences (USUHS); the Department of Defense (DoD); the Departments of the Army, Navy, or Air Force; the Defense Health Agency, Brooke Army Medical Center; Walter Reed National Military Medical Center; Naval Medical Center San Diego; Madigan Army Medical Center; United States Air Force School of Aerospace Medicine; Fort Belvoir Community Hospital; the Henry M. Jackson Foundation for the Advancement of Military Medicine Inc; the National Institutes of Health; the Office of the Assistance Secretary for Preparedness and Response, US Department of Health and Human Services (HHS); the Biomedical Advanced Research and Development Authority, HHS; or the US Food and Drug Administration. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government. The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46.
References and Notes
- 1.Corti D., Purcell L. A., Snell G., Veesler D., Tackling COVID-19 with neutralizing monoclonal antibodies. Cell 184, 3086–3108 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao Y., Wang J., Jian F., Xiao T., Song W., Yisimayi A., Huang W., Li Q., Wang P., An R., Wang J., Wang Y., Niu X., Yang S., Liang H., Sun H., Li T., Yu Y., Cui Q., Liu S., Yang X., Du S., Zhang Z., Hao X., Shao F., Jin R., Wang X., Xiao J., Wang Y., Xie X. S., B.1.1.529 escapes the majority of SARS-CoV-2 neutralizing antibodies of diverse epitopes. bioRxiv, 2021.2012.2007.470392 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rössler A., Riepler L., Bante D., Laer D. v., Kimpel J., SARS-CoV-2 B.1.1.529 variant (Omicron) evades neutralization by sera from vaccinated and convalescent individuals. medRxiv, 2021.2012.2008.21267491 (2021). [Google Scholar]
- 4.Lu L., Mok B. W.-Y., Chen L., Chan J. M.-C., Tsang O. T.-Y., Lam B. H.-S., Chuang V. W.-M., Chu A. W.-H., Chan W.-M., Ip J. D., Chan B. P.-C., Zhang R., Yip C. C.-Y., Cheng V. C.-C., Chan K.-H., Hung I. F.-N., Yuen K.-Y., Chen H., To K. K.-W., Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. medRxiv, 2021.2012.2013.21267668 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikemura N., Hoshino A., Higuchi Y., Taminishi S., Inaba T., Matoba S., SARS-CoV-2 Omicron variant escapes neutralization by vaccinated and convalescent sera and therapeutic monoclonal antibodies. medRxiv, 2021.2012.2013.21267761 (2021). [Google Scholar]
- 6.Cameroni E., Saliba C., Bowen J. E., Rosen L. E., Culap K., Pinto D., De Marco A., Zepeda S. K., di Iulio J., Zatta F., Kaiser H., Noack J., Farhat N., Czudnochowski N., Havenar-Daughton C., Sprouse K. R., Dillen J. R., Powell A. E., Chen A., Maher C., Yin L., Sun D., Soriaga L., Gustafsson C., Chu H., Franko N. M., Logue J., Iqbal N. T., Mazzitelli I., Geffner J., Grifantini R., Gori A., Riva A., Giannini O., Ceschi A., Ferrari P., Franzetti-Pellanda A., Garzoni C., Hebner C., Purcell L., Piccoli L., Pizzuto M. S., Walls A. C., Telenti A., Virgin H. W., Lanzavecchia A., Veesler D., Snell G., Corti D., Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. bioRxiv, 2021.2012.2012.472269 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cele S., Jackson L., Khan K., Khoury D., Moyo-Gwete T., Tegally H., Scheepers C., Amoako D., Karim F., Bernstein M., Lustig G., Archary D., Smith M., Ganga Y., Jule Z., Reedoy K., San J. E., Hwa S.-H., Giandhari J., Blackburn J. M., Gosnell B. I., Karim S. A., Hanekom W., NGS-SA, C.-K. Team, von Gottberg A., Bhiman J., Lessells R. J., Moosa M.-Y. S., Davenport M., de Oliveira T., Moore P. L., Sigal A., SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv, 2021.2012.2008.21267417 (2021). [Google Scholar]
- 8.Kaku C. I., Champney E. R., Normark J., Johnson C. E., Ahlm C., Sakharkar M., Ackerman M. E., Forsell M. N. E., Walker L. M., Broad anti-SARS-CoV-2 antibody immunity induced by heterologous ChAdOx1/mRNA-1273 prime-boost vaccination. medRxiv, 2021.2012.2013.21267598 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Beltran W. F., St Denis K. J., Hoelzemer A., Lam E. C., Nitido A. D., Sheehan M. L., Berrios C., Ofoman O., Chang C. C., Hauser B. M., Feldman J., Gregory D. J., Poznansky M. C., Schmidt A. G., Iafrate A. J., Naranbhai V., Balazs A. B., mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. medRxiv, 2021.2012.2014.21267755 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruell H., Vanshylla K., Tober-Lau P., Hillus D., Schommers P., Lehmann C., Kurth F., Sander L. E., Klein F., mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. medRxiv, 2021.2012.2014.21267769 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt F., Muecksch F., Weisblum Y., Silva J. D., Bednarski E., Cho A., Wang Z., Gaebler C., Caskey M., Nussenzweig M. C., Hatziioannou T., Bieniasz P. D., Plasma neutralization properties of the SARS-CoV-2 Omicron variant. medRxiv, 2021.2012.2012.21267646 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basile K., Rockett R. J., McPhie K., Fennell M., Johnson-Mackinnon J., Agius J. E., Fong W., Rahman H., Ko D., Donavan L., Hueston L., Lam C., Arnott A., Chen S. C.-A., Maddocks S., O’Sullivan M. V., Dwyer D. E., Sintchenko V., Kok J., Improved neutralization of the SARS-CoV-2 Omicron variant after Pfizer-BioNTech BNT162b2 COVID-19 vaccine boosting. bioRxiv, 2021.2012.2012.472252 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemet I., Kliker L., Lustig Y., Zuckerman N. S., Erster O., Cohen C., Kreiss Y., Alroy-Preis S., Regev-Yochay G., Mendelson E., Mandelboim M., Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron infection. medRxiv, 2021.2012.2013.21267670 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilhelm A., Widera M., Grikscheit K., Toptan T., Schenk B., Pallas C., Metzler M., Kohmer N., Hoehl S., Helfritz F. A., Wolf T., Goetsch U., Ciesek S., Reduced Neutralization of SARS-CoV-2 Omicron Variant by Vaccine Sera and monoclonal antibodies. medRxiv, 2021.2012.2007.21267432 (2021). [Google Scholar]
- 15.Doria-Rose N., Shen X., Schmidt S. D., O’Dell S., McDanal C., Feng W., Tong J., Eaton A., Maglinao M., Tang H., Atmar R. L., Lyke K. E., Wang L., Zhang y., Gaudinski M. R., Black W. P., Gordon I., Guech M., Ledgerwood J. E., Misasi J. N., Widge A., Roberts P. C., Beigel J., Korber B., Pajon R., Mascola J. R., Montefiori D. C., Booster of mRNA-1273 Vaccine Reduces SARS-CoV-2 Omicron Escape from Neutralizing Antibodies. medRxiv, 2021.2012.2015.21267805 (2021). [Google Scholar]
- 16.Carreño J. M., Alshammary H., Tcheou J., Singh G., Raskin A., Kawabata H., Sominsky L., Clark J., Adelsberg D. C., Bielak D., Gonzalez-Reiche A. S., Group P. P. S., Srivastava K., Sordillo E. M., Bajic G., van Bakel H., Simon V., Krammer F., Activity of convalescent and vaccine serum against a B.1.1.529 variant SARS-CoV-2 isolate. medRxiv, 2021.2012.2020.21268134 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Jackson-Thompson B. M., Goguet E., Laing E. D., Olsen C. H., Pollett S., Hollis-Perry K. M., Maiolatesi S. E., Illinik L., Ramsey K. F., Reyes A. E., Alcorta Y., Wong M. A., Davies J., Ortega O., Parmelee E., Lindrose A. R., Moser M., Graydon E., Letizia A. G., Duplessis C. A., Ganesan A., Pratt K. P., Malloy A. M., Scott D. W., Anderson S. K., Snow A. L., Dalgard C. L., Powers J. H. 3rd, Tribble D., Burgess T. H., Broder C. C., Mitre E., Prospective Assessment of SARS-CoV-2 Seroconversion (PASS) study: an observational cohort study of SARS-CoV-2 infection and vaccination in healthcare workers. BMC Infect Dis 21, 544 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laing E. D., Weiss C. D., Samuels E. C., Coggins S. A. A., Wang W., Wang R., Vassell R., Sterling S. L., Tso M. A., Conner T., Goguet E., Moser M., Jackson-Thompson B. M., Illinik L., Davies J., Ortega O., Parmelee E., Hollis-Perry M., Maiolatesi S. E., Wang G., Ramsey K. F., Reyes A. E., Alcorta Y., Wong M. A., Lindrose A. R., Duplessis C. A., Tribble D. R., Malloy A. M. W., Burgess T. H., Pollett S. D., Olsen C. H., Broder C. C., Mitre E., Durability of antibody responses and frequency of clinical and subclinical SARS-CoV-2 infection six months after BNT162b2 COVID-19 vaccination in healthcare workers. medRxiv, 2021.2010.2016.21265087 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richard S. A., Pollett S. D., Lanteri C. A., Millar E. V., Fries A. C., Maves R. C., Utz G. C., Lalani T., Smith A., Mody R. M., Ganesan A., Colombo R. E., Colombo C. J., Lindholm D. A., Madar C., Chi S., Huprikar N., Larson D. T., Bazan S. E., English C., Parmelee E., Mende K., Laing E. D., Broder C. C., Blair P. W., Chenoweth J. G., Simons M. P., Tribble D. R., Agan B. K., Burgess T. H., E. C.-C. S. Group, COVID-19 Outcomes Among US Military Health System Beneficiaries Include Complications Across Multiple Organ Systems and Substantial Functional Impairment. Open Forum Infect Dis 8, ofab556 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lusvarghi S., Wang W., Herrup R., Neerukonda S. N., Vassell R., Bentley L., Eakin A. E., Erlandson K. J., Weiss C. D., Key substitutions in the spike protein of SARS-CoV-2 variants can predict resistance to monoclonal antibodies, but other substitutions can modify the effects. J Virol, JVI0111021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schubert M., Bertoglio F., Steinke S., Heine P. A., Ynga-Durand M. A., Zuo F., Du L., Korn J., Milošević M., Wenzel E. V., Maass H., Krstanović F., Polten S., Pribanić-Matešić M., Brizić I., Piralla A., Baldanti F., Hammarström L., Dübel S., Šustić A., Marcotte H., Strengert M., Protić A., Pan Hammarström Q., Čičin-Šain L., Hust M., Human serum from SARS-CoV-2 vaccinated and COVID-19 patients shows reduced binding to the RBD of SARS-CoV-2 Omicron variant in comparison to the original Wuhan strain and the Beta and Delta variants. medRxiv, 2021.2012.2010.21267523 (2021). [Google Scholar]
- 22.Laing E. D., Sterling S. L., Richard S. A., Epsi N. J., Coggins S., Samuels E. C., Phogat S., Yan L., Moreno N., Coles C. L., Drew M., Mehalko J., Merritt S., Mende K., Munster V., de Wit E., Chung K. K., Millar E. V., Tribble D. R., Simons M. P., Pollett S. D., Esposito D., Lanteri C., Clifton G. T., Mitre E., Burgess T. H., Broder C. C., Antigen-based multiplex strategies to discriminate SARS-CoV-2 natural and vaccine induced immunity from seasonal human coronavirus humoral responses. medRxiv, (2021). [Google Scholar]
- 23.Jangra S., Ye C., Rathnasinghe R., Stadlbauer D., g. Personalized Virology Initiative study, Krammer F., Simon V., Martinez-Sobrido L., Garcia-Sastre A., Schotsaert M., SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neerukonda S. N., Vassell R., Lusvarghi S., Wang R., Echegaray F., Bentley L., Eakin A. E., Erlandson K. J., Katzelnick L. C., Weiss C. D., Wang W., SARS-COV-2 Delta variant displays moderate resistance to neutralizing antibodies and spike protein properties of higher soluble ACE2 sensitivity, enhanced cleavage and fusogenic activity. bioRxiv, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: SARS-CoV-2 genotypes for the infecting variants
Supplementary materials and methods