Abstract
Expression of the α6β4 integrin increases the invasive potential of carcinoma cells by a mechanism that involves activation of phosphoinositide 3-OH kinase (PI3K). In the present study, we investigated the signaling pathway by which the α6β4 integrin activates PI3K. Neither the α6 nor the β4 cytoplasmic domain contains the consensus binding motif for PI3K, pYMXM, indicating that additional proteins are likely to be involved in the activation of this lipid kinase by the α6β4 integrin. We identified insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the activation of PI3K by the α6β4 integrin. IRS-1 and IRS-2 are cytoplasmic adapter proteins that do not contain intrinsic kinase activity but rather function by recruiting proteins to surface receptors, where they organize signaling complexes. Ligation of the α6β4 receptor promotes tyrosine phosphorylation of IRS-1 and IRS-2 and increases their association with PI3K, as determined by coimmunoprecipitation. Moreover, we identified a tyrosine residue in the cytoplasmic domain of the β4 subunit, Y1494, that is required for α6β4-dependent phosphorylation of IRS-2 and activation of PI3K in response to receptor ligation. Most importantly, Y1494 is essential for the ability of the α6β4 integrin to promote carcinoma invasion. Taken together, these results imply a key role for the IRS proteins in the α6β4-dependent promotion of carcinoma invasion.
Cell adhesion molecules play an important role in normal epithelia, and changes in their expression and function contribute to the progression of epithelial cells to invasive, metastatic carcinoma. For example, cell-cell interactions in many tumors are altered through a quantitative decrease in cadherin expression, which reduces intercellular adhesion (5). This disruption of cell-cell adhesion is permissive for increased cell motility. Cell adhesion can also be modified through qualitative changes in receptor function that promote the dynamic adhesion that is required for motile, invasive cells (31). In recent years, a significant amount of evidence to suggest that the α6β4 integrin is a member of this category of adhesion receptors has accumulated. Specifically, the expression of this integrin receptor is maintained in carcinoma cells but it functions in a manner distinct from its role in normal epithelial cells (55). The involvement of the β4 integrin subunit in carcinoma cell biology was initially suggested by its identification as a tumor-related antigen expressed in metastatic cancer (22). Since then, many studies have reported a strong association of β4 expression with solid-tumor progression. For example, the β4 subunit is not expressed in the normal thyroid but its expression correlates with the progression to invasive thyroid carcinoma (62). The β4 subunit is also expressed in androgen receptor-negative invasive prostate carcinomas and at the leading edges of invading gastric carcinomas (6, 75). Moreover, expression of the β4 subunit correlates with a poor prognosis in patients with squamous cell, breast, and colon carcinomas (23, 71, 82). These correlative data have been supported more recently by functional studies that have provided mechanistic insight into how the α6β4 integrin contributes to tumor progression. In previous studies, we demonstrated that the α6β4 integrin can increase the invasive potential of breast carcinoma cells, a finding that has been confirmed for other cell types as well (12, 21, 64, 67). Furthermore, expression of the α6β4 receptor increases the survival of p53 mutant carcinoma cells (2, 74). Given that invasion and survival are two critical functions of metastatic cells, it is important to understand in more detail the mechanism of action of the α6β4 integrin in tumor cells.
In normal epithelia, the α6β4 integrin functions as a receptor for the laminin family of extracellular matrix proteins and mediates the stable attachment of epithelial cells to the underlying basement membrane (7, 41). Many studies, including those involving knockout of the β4 subunit, have substantiated the importance of the adhesive contributions of the α6β4 integrin to normal epithelial function (19, 81). In the absence of the β4 subunit, and more specifically the β4 cytoplasmic domain, a lethal blistering of the epithelium, which is known as epidermolysis bullosa, occurs (19, 81). In carcinoma cells, the α6β4 integrin also functions as a laminin receptor and the β4 subunit interacts with the actin cytoskeleton to promote the formation of actin-rich structures that are important for cell motility (54, 56). However, in addition to its mechanical involvement in mediating adhesive interactions, the α6β4 integrin activates intracellular signaling pathways that are essential for the ability of this receptor to promote tumor progression (51, 52, 64). For example, our analysis of the mechanism involved in the α6β4-dependent promotion of invasion revealed that phosphoinositide 3-OH kinase (PI3K) activation by the receptor is essential for this function (64). The ability of the α6β4 integrin to promote PI3K activation is greater than that observed for other β1 integrins, which supports the increased potential of this integrin to promote carcinoma invasion. Activation of PI3K by the α6β4 integrin is also required for the ability of this integrin to promote carcinoma cell survival through the activation of the Akt kinase (2, 3, 74).
PI3K is a lipid kinase that phosphorylates the D3 position of inositol lipids to form the products phosphatidylinositol (PtdIns)-3-P, PtdIns-3,4-P2, and PtdIns-3,4,5-P3 (76). These D3 phosphoinositides are expressed at very low levels in unstimulated cells, but their levels are increased in response to many different stimuli, supporting their role as second messengers. A major function of the D3 phosphoinositides is to bind and recruit signaling molecules to the plasma membrane, where they can interact with other regulatory and effector molecules (76). The involvement of PI3K in carcinoma cell biology has been proposed from both direct and indirect evidence. As mentioned above, PI3K activity promotes carcinoma invasion and survival, and it has also been implicated in promoting anchorage-independent growth (20, 34, 35, 64). An avian sarcoma virus that encodes the catalytic subunit of PI3K transforms chicken embryo fibroblasts, suggesting that PI3K can also play a role in the early stages of tumor initiation (11). Finally, PTEN, a lipid phosphatase that regulates the levels of the PI3K lipid products, is frequently mutated or deleted in tumors (9, 18). The identification of the PTEN gene as a tumor suppressor gene demonstrates the importance of tightly regulating the activity of PI3K. In light of these findings, the relevance of determining how the α6β4 integrin activates the PI3K signaling pathway is evident.
The α6β4 integrin is distinct from other integrin receptors because the β4 subunit contains a 1,000-amino-acid cytoplasmic domain (27, 70, 72). This large intracellular domain is important for many of the known α6β4-dependent functions. For example, the β4 cytoplasmic domain is essential for hemidesmosome formation in normal epithelial cells and it is required for promoting carcinoma cell invasion (44, 64). In the absence of the β4 cytoplasmic domain, the α6β4 receptor is not capable of activating PI3K or other signaling pathways that have been shown to be activated by this integrin, including the mitogen-activated protein kinase (MAPK) pathway (64). Although a number of proteins that interact with the β4 cytoplasmic domain in hemidesmosomes have been identified, very little is known about the structural requirements for signaling by this integrin or the specific interactions that occur with downstream effectors to initiate signals (48, 57). Recently, the binding site in the β4 cytoplasmic domain for the adapter protein Shc, which recruits Grb2 and Sos to promote Ras activation, has been identified (16, 43). However, the binding motif for the p85 regulatory subunit of PI3K, YMXM, is not present in the β4 cytoplasmic domain, which suggests that alternative mechanisms are required to recruit this lipid kinase (10).
In the present study, we have examined the mechanism by which the α6β4 integrin activates the PI3K signaling pathway. In light of the fact that the β4 subunit cytoplasmic domain does not contain a binding site to directly interact with PI3K, we sought to identify the intermediate proteins that are responsible for recruiting PI3K to the plasma membrane in response to α6β4 ligation. We identified two members of the insulin receptor substrate (IRS) family, IRS-1 and IRS-2, which are specific mediators of α6β4-dependent activation of PI3K (84). In addition, we investigated the structural requirements of the β4 subunit cytoplasmic domain for PI3K activation. Through this analysis we identified a specific tyrosine residue in the β4 subunit, Y1494, that is required for α6β4-dependent activation of PI3K and, importantly, for the ability of this integrin receptor to promote carcinoma invasion.
MATERIALS AND METHODS
Cells and antibodies.
MDA-MB-435 cells were grown in Dulbecco's modified Eagle's medium (DMEM; Biowhittaker) supplemented with 10% fetal calf serum (Sigma), 1% penicillin-streptomycin (Gibco), and 1% GlutaMax (Gibco). T47D cells were grown in DMEM supplemented with 10% fetal calf serum, 1% penicillin-streptomycin, 1% GlutaMax, and 5 μg of insulin (Gibco)/ml. The rat monoclonal antibody that recognizes the α6-integrin subunit (135-13C) was a gift from Rita Falcioni, and the mouse monoclonal antibody that recognizes the β4-integrin subunit (UM-A9) was purchased from Ancell. The IRS-1-specific polyclonal antibody was purchased from Santa Cruz Biotechnology. The IRS-2-specific polyclonal antibody and the 4G10 phosphotyrosine-specific monoclonal antibody were purchased from Upstate Biotechnology Inc. The RC-20 biotinylated-phosphotyrosine-specific monoclonal antibody was purchased from Transduction Labs. The p85-specific polyclonal antiserum was a gift from Alex Toker.
Integrin clustering.
Cells were removed from their dishes with trypsin and washed twice with RPMI medium containing 25 mM HEPES (RH) and 0.1% heat inactivated bovine serum albumin (BSA; RH-BSA). After being washed, the cells were resuspended in the same buffer at a concentration of 2 × 106 cells/ml and incubated for 30 min with integrin-specific antibodies or in buffer alone. The cells were washed once, resuspended in the same buffer, and added to plates that had been coated overnight with anti-mouse immunoglobulin G (IgG). After a 30-min incubation at 37°C, the cells were washed twice with cold phosphate-buffered saline (PBS) and solubilized at 4°C for 10 min in a 20 mM Tris buffer, pH 7.4, containing 0.14 M NaCl, 1% NP-40, 10% glycerol, 1 mM sodium orthovanadate, 2 mM phenylmethylsulfonyl fluoride, and 5 μg of aprotinin, pepstatin, and leupeptin/ml. Nuclei were removed by centrifugation at 12,000 × g for 10 min. For laminin attachment assays, cells were added to plates (100 mm in diameter) that had been coated overnight with 150 μg of laminin-1 and incubated for 45 min at 37°C.
Immunoprecipitation and immunoblotting.
Aliquots of cell extracts containing equivalent amounts of protein were incubated for 3 h at 4°C with antibodies and protein A- or protein G-Sepharose (Pharmacia) with constant agitation. The beads were washed three times in the extraction buffer. Laemmli sample buffer was added to the samples, which were then incubated at 100°C for 4 min. Immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose filters. The filters were blocked for 1 h using a 50 mM Tris buffer, pH 7.5, containing 0.15 M NaCl and 0.05% Tween 20 (TBST) and 5% (wt/vol) Carnation dry milk. The filters were incubated for 1 h in the same buffer containing primary antibodies. After three 10-min washes in TBST, the filters were incubated for 1 h in blocking buffer containing horseradish peroxidase (HRP)-conjugated secondary antibodies. After three 10-min washes in TBST, proteins were detected by enhanced chemiluminescence (Pierce). For RC-20 phosphotyrosine immunoblots, the filters were blocked for 1 h using a 10 mM Tris buffer, pH 7.5, containing 0.5 M NaCl and 0.1% Tween 20 (RC-20 buffer) and 2% (wt/vol) Carnation dry milk. The filters were washed briefly in RC-20 buffer and then incubated overnight at 4°C in RC-20 buffer containing 3% (wt/vol) BSA and a 1:500 dilution of the RC-20 antibody. After being washed, the filters were incubated for 1 h in blocking buffer containing HRP-conjugated streptavidin, and the proteins were detected by enhanced chemiluminescence.
PI3K kinase assay.
To assay PI3K activity, aliquots of cell extracts that contained equivalent amounts of protein were incubated for 3 h at 4°C with antibodies and protein A-Sepharose (Pharmacia). The Sepharose beads were washed twice with solubilization buffer and twice with a 10 mM HEPES buffer, pH 7, containing 0.1 mM EGTA (kinase buffer). After removal of the last wash, the beads were resuspended in kinase buffer containing 10 μg of sonicated crude brain lipids (Sigma), 100 μM ATP, 25 mM MgCl2, and 10 μCi of [γ-32P]ATP and incubated for 10 min at room temperature. The reaction was stopped by the addition of 60 μl of 2 N HCl and 160 μl of a 1:1 mixture of chloroform and methanol. Lipids were resolved by using thin-layer chromatography plates coated with potassium oxalate.
Site-directed mutagenesis.
The cloning of the human wild-type β4 cDNA (β4D) and its transfection into the MDA-MB-435 cell line have been described previously (13, 64). Tyrosine residues 1257 and 1494 in the β4 subunit were mutated to phenylalanine residues using the Quickchange site-directed mutagenesis kit (Stratagene). Briefly, overlapping primers containing the desired mutations were used to amplify the β4 cDNA and vector by PCR and the resulting point mutations were confirmed by dideoxy sequencing. The vectors containing the mutant β4 cDNAs were transfected into the MDA-MB-435 cell line using Lipofectamine (Gibco) according to the manufacturer's instructions. Neomycin-resistant cells were isolated by selective growth in medium containing G418 (0.8 mg/ml; Gibco). The stable transfectants were pooled, and subclones of cells that expressed the mutant β4 subunits on the cell surface were isolated by fluorescence-activated cell sorting (FACS). The human β4 integrin-specific monoclonal antibody, UM-A9 (Ancell), was used for this sorting and for subsequent analysis of the transfectants.
Analysis of integrin surface expression.
The relative surface expression of the β4-integrin subunit on the transfected MDA-MB-435 subclones was assessed by flow cytometry. For this purpose, aliquots of cells (5 × 105) were incubated for 45 min at room temperature with RH-BSA and either the β4-specific antibody or nonspecific mouse IgG (Sigma). The cells were washed two times with RH-BSA and then incubated with goat anti-mouse IgG coupled to Cy2 (Jackson Immunoresearch) for 45 min at room temperature. After being washed two times with RH-BSA, the cells were resuspended in PBS and analyzed by flow cytometry.
Invasion assay.
Matrigel invasion assays were performed as described previously (63, 64) using 6.5-mm-diameter Transwell chambers (8-μm pore size; Costar). Matrigel purified from the Englebreth-Holm-Swarm tumor was diluted in cold distilled water, added to the Transwells (5 μg/well), and dried in a sterile hood. The Matrigel was then reconstituted with medium for 1 h at 37°C before the addition of cells. Cells (0.5 × 105) were resuspended in serum-free DMEM containing 0.1% BSA, and cells were added to each well. Conditioned NIH 3T3 medium was added to the bottom wells of the chambers. After 4 h, the cells that had not invaded were removed from the upper faces of the filters using cotton swabs and the cells that had invaded to the lower surfaces of the filters were fixed in methanol and then stained with a 0.2% solution of crystal violet in 2% ethanol. Invasion was quantitated by visual counting. The mean of five individual fields in the center of the filter, where invasion was the highest, was obtained for each well.
Adhesion assays.
Adhesion assays were performed as described previously (63). Briefly, multiwell tissue culture plates (11.3 mm in diameter) were coated overnight at 4°C with 0.2 ml of PBS containing either murine laminin-1 (20 μg/ml) or rat collagen I (20 μg/ml). The wells were then washed with PBS and blocked with RH-BSA. Cells (105) were resuspended in RH-BSA and added to the protein-coated wells. After a 60-min incubation at 37°C, the wells were washed three times with RH at 37°C, fixed for 15 min with methanol, and stained with a 0.2% solution of crystal violet in 2% ethanol. The crystal violet stain was solubilized with a 1% solution of SDS, and adhesion was quantitated by measuring the absorbance at 595 nm.
RESULTS
Identification of IRS-1 and IRS-2 as intermediates in the activation of PI3K by the α6β4 integrin.
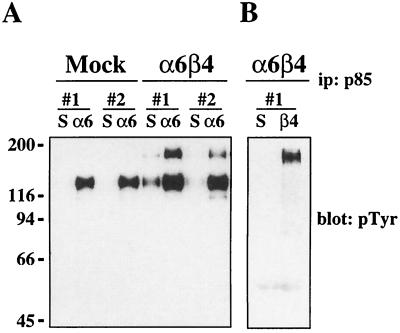
In previous work, we demonstrated that ligation of the α6β4 integrin promotes significantly more PI3K activity than ligation of the α6β1 integrin or other β1 integrins (64). PI3K is activated by recruitment of the p85 regulatory subunit to phosphotyrosine-containing binding motifs (pYMXM) (10). Neither the α6- nor the β4-subunit cytoplasmic domain contains the p85 consensus binding motif, which suggests that additional intermediate proteins are most likely involved in the α6β4-dependent activation of PI3K. To identify these intermediates, we analyzed the profile of phosphoproteins that associate with PI3K after α6β4 ligation. To do so, cell extracts from mock- (MDA-MB-435/mock) and β4-transfected (MDA-MB-435/β4) MDA-MB-435 cells that had been clustered with α6-specific antibody 135-13C were immunoprecipitated with a p85-specific antiserum and the associated proteins were detected by immunoblotting with phosphotyrosine-specific antibody RC-20. As shown in Fig. 1A, ligation of both α6β1 and α6β4 resulted in the interaction of PI3K with a 130-kDa phosphoprotein. However, an additional 180-kDa phosphoprotein coimmunoprecipitated with PI3K in both of the MDA-MB-435/β4 subclones after ligation with α6-specific antibodies. To confirm that this 180-kDa protein was specific for α6β4-dependent activation of PI3K, the MDA-MB-435/β4 transfectants were clustered with β4-specific antibodies, which will not ligate α6β1, and the p85-associated proteins were analyzed. When α6β4 was clustered in the absence of α6β1 ligation, only the 180-kDa protein was observed to coimmunoprecipitate with PI3K (Fig. 1B). The association of PI3K with the 180-kDa phosphoprotein only after ligation of the α6β4 integrin suggests that there is a unique mechanism for PI3K activation by the α6β4 receptor that is not utilized by the α6β1 receptor.
FIG. 1.
Analysis of PI3K-associated proteins. Two subclones each (#1 and #2) of the MDA-MB-435/mock and the MDA-MB-435/β4 transfectants were maintained in suspension or incubated with either α6- (A) or β4-specific (B) antibodies and allowed to adhere to either anti-rat IgG- or anti-mouse IgG-coated plates, respectively, for 30 min. Aliquots of cell extracts that contained equivalent amounts of protein were incubated with a polyclonal antiserum specific for the p85 subunit of PI3K and protein A-Sepharose for 3 h. The immune complexes were resolved by SDS–8% PAGE and then immunoblotted with phosphotyrosine-specific antibody RC-20. Mock, MDA-MB-435 cells transfected with the empty vector; α6β4, MDA-MB-435 cells transfected with the full-length β4 subunit; S, cells maintained in suspension; α6, cells clustered with an α6-specific antibody (135-13C); β4, cells clustered with a β4-specific antibody (UM-A9). ip, immunoprecipitation.
To understand further the mechanism of α6β4-dependent PI3K activation, we sought to identify the 180-kDa protein that was phosphorylated on tyrosine and associated with PI3K in response to α6β4 ligation. Given the molecular mass of this phosphoprotein, we first investigated if this protein was a growth factor receptor. This possibility was supported by the fact that the α6β4 integrin can associate with ErbB2 (21). However, the p85-associated 180-kDa protein was not expressed on the cell surface, as indicated by a test for surface biotinylation (data not shown).
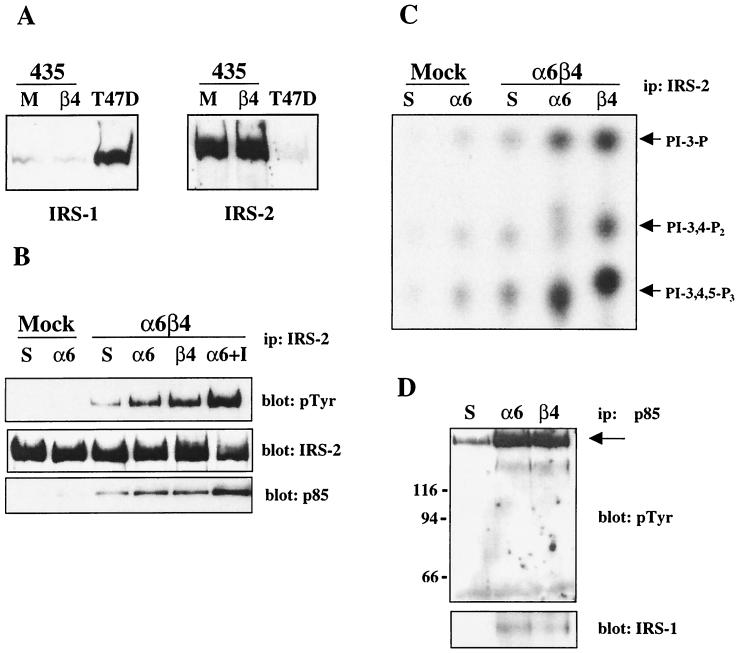
Next, we investigated the IRS adapter proteins. The IRS family members, which include IRS-1, IRS-2, IRS-3, and IRS-4, are 170- to 180-kDa proteins (except IRS-3, which is 60 kDa) that function as intermediate docking proteins downstream of the insulin and insulin-like growth factor 1 (IGF-1) receptors, as well as a number of cytokine receptors (84). In addition, the α5β1 integrin can promote IRS-1 phosphorylation in adipocytes (24). Importantly, the IRS proteins contain several PI3K binding sites, and these adapters are known to be involved in the activation of PI3K downstream of several of the receptors mentioned above (30, 33, 45, 69, 78, 86). To determine if the 180-kDa protein that coimmunoprecipitated with PI3K was an IRS family member, we first analyzed the expression of each IRS homolog in MDA-MB-435 cells. As shown in Fig. 2A, MDA-MB-435 cells express very low levels of IRS-1 but express high levels of IRS-2. IRS-3 and IRS-4 were not detected in these cells (data not shown). To investigate the potential involvement of IRS-2 in the α6β4-dependent activation of PI3K, cell extracts from MDA-MB-435/mock and MDA-MB-435/β4 cells that had been clustered with α6-specific antibodies were assayed for IRS-2 phosphorylation. As shown in Fig. 2B, IRS-2 was phosphorylated on tyrosine in response to clustering with α6-specific antibodies in the MDA-MB-435/β4 transfectants but not in the MDA-MB-435/mock transfectants. In addition, ligation with a β4-specific antibody also increased the tyrosine phosphorylation of IRS-2. Most importantly, the p85 subunit of PI3K associated with IRS-2 after ligation of the α6β4 receptor (Fig. 2B, bottom). As a positive control for IRS-2 phosphorylation, the MDA-MB-435/β4 cells were treated with IGF-1, which increased the phosphorylation of IRS-2 and its association with PI3K (Fig. 2B). The MDA-MB-435/mock and -β4 transfectants express equivalent levels of IRS-2, and therefore the lack of IRS-2 phosphorylation after ligation of α6β1 in the MDA-MB-435/mock transfectants is not due to a relative difference in protein expression levels (Fig. 2A).
FIG. 2.
Identification of IRS-1 and IRS-2 as α6β4-dependent PI3K-associated proteins. (A) Aliquots of cell extracts from the MDA-MB-435 transfectants and T47D breast carcinoma cells containing equivalent amounts of protein were resolved by SDS-PAGE and immunoblotted with antibodies specific for IRS-1 and IRS-2. 435, MDA-MB-435 cells; M, MDA-MB-435 cells transfected with the empty vector; β4, MDA-MB-435 cells transfected with the full-length β4 subunit. (B) MDA-MB-435 transfectants were maintained in suspension or incubated with either α6- or β4-specific antibodies and allowed to adhere to either anti-rat IgG- or anti-mouse IgG-coated plates, respectively, in the absence or presence of IGF-1 (100 ng/ml) for 30 min. Aliquots of cell extracts that contained equivalent amounts of protein were incubated with an IRS-2-specific polyclonal antibody and protein A-Sepharose for 3 h. The immune complexes were resolved by SDS–8% PAGE and then immunoblotted with RC-20 (top). The immunoblot was subsequently stripped and reprobed with IRS-2- (middle) and p85-specific (bottom) polyclonal antisera. ip, immunoprecipitation. (C) MDA-MB-435 transfectants were treated as described above, and aliquots of cell extracts that contained equivalent amounts of protein were incubated with an IRS-2-specific antibody and protein A-Sepharose for 3 h. After being washed, the beads were resuspended in kinase buffer and incubated for 10 min at room temperature. The phosphorylated lipids were resolved by thin-layer chromatography. Arrows, D3 phosphoinositides. (D) T47D cells were treated as described above, and aliquots of cell extracts that contained equivalent amounts of protein were incubated with a p85-specific antibody and protein A-Sepharose for 3 h. The immune complexes were resolved by SDS–8% PAGE and then immunoblotted with RC-20 (top). The immunoblot was subsequently stripped and reprobed with an IRS-1-specific polyclonal antibody (bottom). Mock, MDA-MB-435 cells transfected with the empty vector; α6β4, MDA-MB-435 cells transfected with the full-length β4 subunit; S, cells maintained in suspension; α6, cells clustered with an α6-specific antibody (135-13C); β4, cells clustered with a β4-specific antibody (UM-A9).
To confirm that PI3K is activated through IRS-2 in response to α6β4 ligation, in vitro kinase assays were performed on IRS-2 immune complexes. MDA-MB-435/mock and MDA-MB-435/β4 transfectants were clustered with α6- and β4-specific antibodies, and the cell extracts were immunoprecipitated with IRS-2 antibodies. The IRS-2 immunoprecipitates were assayed for their ability to phosphorylate crude brain phosphoinositides. As shown in Fig. 2C, ligation of the α6β4 integrin with both α6- and β4-specific antibodies resulted in a marked increase in PI3K activity associated with IRS-2, as demonstrated by the appearance of the PtdIns-3,4,5-P3 lipid product. In contrast, ligation of α6β1 in the MDA-MB-435/mock transfectants resulted in minimal IRS-2-associated PI3K activity.
IRS-1 and IRS-2 have considerable structural homology and both homologs contain multiple binding sites for PI3K (84). To determine if IRS-1 can also be involved in the activation of PI3K by the α6β4 integrin, we used T47D breast carcinoma cells, which express high levels of IRS-1 and low levels of IRS-2 (Fig. 2A). As shown in Fig. 2D (top), ligation of the α6β4 receptor using either α6- or β4-specific antibodies increased the association of PI3K with a 170-kDa phosphoprotein. An IRS-1-specific antibody recognized this phosphoprotein (bottom). Based on these results we conclude that both IRS-1 and IRS-2 can function as intermediate signaling proteins in the activation of PI3K by the α6β4 integrin. The 130-kDa phosphoprotein that associates with PI3K in response to α6β1 ligation has not been conclusively identified.
Adhesion to laminin-1 promotes α6β4-dependent IRS phosphorylation.
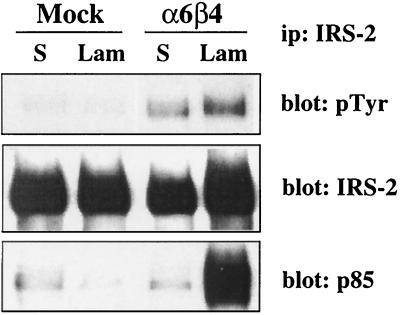
To confirm that the α6β4-dependent IRS signaling pathway that we identified by antibody clustering occurs in response to ligation of α6β4 by a natural extracellular matrix ligand, we assessed the phosphorylation of IRS-2 after adhesion of the MDA-MB-435/mock and MDA-MB-435/β4 transfectants to a laminin-1 substratum. As shown in Fig. 3, adhesion of the MDA-MD-435/mock transfectants to laminin-1 did not result in an increase in the tyrosine phosphorylation of IRS-2. Given that α6β1 is the major laminin receptor on the surfaces of these cells, these results confirm the data obtained using antibodies to cluster the α6β1 integrin (Fig. 2B). In contrast, adhesion of the MDA-MB-435/β4 transfectants to laminin-1 promoted the tyrosine phosphorylation of IRS-2 and the recruitment of PI3K to this adapter protein. Therefore, ligation of the α6β4 integrin by either laminin-1 or receptor-specific antibodies can activate the IRS-PI3K signaling pathway.
FIG. 3.
α6β4-dependent IRS-2 phosphorylation in response to laminin-1 adhesion. MDA-MB-435 transfectants were maintained in suspension or allowed to adhere to laminin-1-coated plates for 45 min. Aliquots of cell extracts that contained equivalent amounts of protein were incubated with an IRS-2-specific antibody and protein A-Sepharose for 3 h. The immune complexes were resolved by SDS–8% PAGE and then immunoblotted with RC-20 (top). The immunoblot was subsequently stripped and reprobed with IRS-2- (middle) and p85-specific (bottom) polyclonal antisera. Mock, MDA-MB-435 cells transfected with the empty vector; α6β4, MDA-MB-435 cells transfected with the full-length β4 subunit; S, cells maintained in suspension; Lam, cells adherent to a laminin substratum. ip, immunoprecipitation.
Analysis of tyrosine phosphorylation of the β4 subunit.
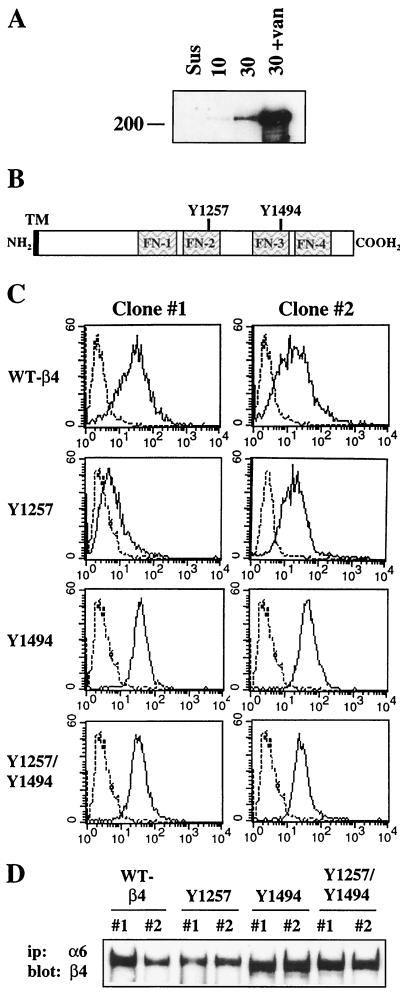
Having identified IRS-1 and IRS-2 as signaling intermediates in the pathway utilized by the α6β4 integrin to activate PI3K, we next investigated the mechanism by which this integrin receptor activates this pathway. It had been previously demonstrated that the β4 subunit is phosphorylated on tyrosine after ligation of the α6β4 receptor (43). We confirmed this finding in our transfected MDA-MB-435/β4 cells. As shown in Fig. 4A, a time-dependent increase in the tyrosine phosphorylation of the β4 subunit was observed after ligation of the α6β4 receptor with α6-specific antibodies. Addition of sodium orthovanadate markedly increased the level of tyrosine phosphorylation, indicating that tyrosine phosphorylation of the β4 subunit is regulated by tyrosine phosphatases in these cells (Fig. 4A) (43). To evaluate the role of β4-tyrosine phosphorylation in the activation of PI3K by the α6β4 integrin, we analyzed the β4 cytoplasmic domain for tyrosine residues that were located within known consensus binding or phosphorylation motifs. We identified two tyrosines that were of potential interest, Y1257 and Y1494 (Fig. 3B). Both of these tyrosines are located within immune T-cell inhibitory motifs (ITIM) that have the consensus sequence of L/VXXpYXL/V and that were initially identified in immune cell inhibitory coreceptors (79). ITIM sites are involved in regulating B- and T-cell receptor signaling, and they have been characterized as binding motifs for the SH2 domains of the SH2-containing tyrosine phosphatase 1 (SHP-1) and -2 protein tyrosine phosphatases and also for the SH2-containing inositol polyphosphate 5-phosphatase 1 (SHIP-1) and -2 lipid phosphatases (79).
FIG. 4.
Characterization of tyrosine mutants of the β4-integrin subunit. (A) MDA-MB-435/β4 transfectants were maintained in suspension or incubated with α6-specific antibodies and allowed to adhere to anti-rat IgG-coated plates in the absence or presence of 100 μM sodium orthovanadate (van) for 30 min. Aliquots of cell extracts that contained equivalent amounts of protein were incubated with a β4-specific antibody (UM-A9) and protein G-Sepharose for 3 h. The immune complexes were resolved by SDS–8% PAGE and then immunoblotted with RC-20. (B) Schematic of the β4-integrin subunit cytoplasmic domain, which indicates the tyrosine residues which were mutated to phenylalanine. TM, transmembrane domain. (C) Subclones of transfected MDA-MB-435 cells expressing the β4 subunit on the cell surface were isolated by FACS using a β4-specific antibody (UM-A9). MDA-MB-435 cells transfected with the wild-type (WT) and mutant human β4-integrin subunits were analyzed by flow cytometry using nonspecific mouse IgG (left peak) or UM-A9 (right peak). Shown are two representative subclones (clones 1 and 2) from each transfectant line. (D) Aliquots of cell extracts from the MDA-MB-435 transfectants were immunoprecipitated (ip) with an α6-specific antibody (135-13C). The immune complexes were resolved by SDS–6% PAGE and immunoblotted with a polyclonal antiserum that recognizes the C terminus of the β4 subunit.
To investigate the potential involvement of Y1257 and Y1494 in the α6β4-dependent activation of PI3K, Y1257 and Y1494 in the β4 subunit were individually mutated to phenylalanine residues. In addition, we also mutated both Y1257 and Y1494 to generate a double-ITIM-mutant β4 subunit (Y1257F/Y1494F). The mutant β4 subunits were stably expressed in the MDA-MB-435 cells, which lack endogenous β4 expression, and subclones expressing the β4 mutant proteins on the cell surface were isolated by FACS. As shown in the flow cytometry profiles for two individual subclones of each transfectant in Fig. 4C, all of the mutant β4 subunits were expressed on the cell surface. To confirm that the mutant β4 subunits associated with endogenous α6 subunits, cell extracts of the β4 mutant-expressing subclones were immunoprecipitated with an α6-specific antibody and then immunoblotted with an antiserum that recognizes the C terminus of the β4 subunit. As shown in Fig. 4D, all of the mutant β4 subunits formed heterodimers with the endogenous α6 subunits. Moreover, all of the mutants were recognized by the C-terminal antiserum, which indicates that they are all expressed as full-length proteins.
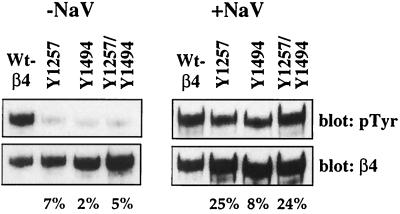
To determine if the β4 cytoplasmic domain is phosphorylated on either Y1257 or Y1494 in response to α6β4 ligation, we assayed the tyrosine phosphorylation of the mutant β4 subunits after clustering the receptors with β4-specific antibodies. Mutation of either Y1257 or Y1494 resulted in a significant decrease in the level of β4-tyrosine phosphorylation (2 to 7% of the wild-type level) after clustering with β4-specific antibodies (Fig. 5). Addition of sodium orthovanadate to the cells during the clustering markedly increased the tyrosine phosphorylation of the mutant β4 subunits. However, when the results were normalized for total β4 protein, a significant decrease in the tyrosine phosphorylation of the mutant proteins compared to the level of phosphorylation of the wild-type β4 subunit was still observed.
FIG. 5.
Analysis of tyrosine phosphorylation of the β4 subunit. MDA-MB-435/β4 transfectants were incubated with β4-specific antibodies and allowed to adhere to anti-mouse IgG-coated plates for 30 min. Aliquots of cell extracts that contained equivalent amounts of protein were incubated with protein A-Sepharose for 2 h. The immune complexes were resolved by SDS–6% PAGE and then immunoblotted with RC-20. The immunoblots were subsequently stripped and reprobed with a β4-specific polyclonal antiserum. Shown are representative subclones expressing the wild-type (WT) β4 subunit and each of the β4 mutant subunits. The percentages of phosphorylation of the mutant β4 subunits compared to that of the wild-type β4 subunit are indicated below. NaV, sodium orthovanadate.
Tyrosine 1494 in the β4 subunit is required for α6β4-dependent activation of PI3K.
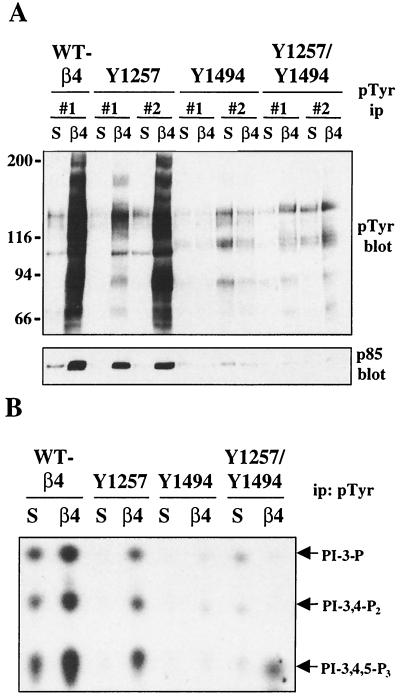
To evaluate the impact of mutating Y1257 and Y1494 in the β4-subunit cytoplasmic domain on the ability of the α6β4 receptor to activate downstream signaling pathways, we initially examined the ability of these α6β4 mutant receptors to promote increases in total cellular tyrosine phosphorylation after clustering with β4-specific antibodies. MDA-MB-435 cells that expressed the wild-type β4 and each of the mutant β4 subunits were clustered with β4-specific antibodies, and the cell extracts were incubated with phosphotyrosine-specific antibody 4G10. As shown in Fig. 6A (top), ligation of wild-type α6β4 resulted in a marked increase in total cellular tyrosine phosphorylation levels. A similar increase in tyrosine phosphorylation was observed in two individual subclones that expressed the Y1257F β4 subunit, indicating that Y1257 is not essential for α6β4-dependent promotion of tyrosine phosphorylation (Fig. 6A). Although one of the Y1257F subclones had a lower level of tyrosine phosphorylation than was observed for the wild-type β4 subclone, this level of phosphorylation correlated with the levels of surface expression of the β4 subunit in these cells (Fig. 4C). In contrast, none of the MDA-MB-435 subclones that expressed the Y1494F or the double-mutant Y1257F/Y1494F β4 subunits showed increases in cellular tyrosine phosphorylation levels in response to α6β4 clustering. These results suggest that Y1494 is essential for α6β4-dependent promotion of tyrosine phosphorylation.
FIG. 6.
Analysis of PI3K activity in the MDA-MB-435 mutant β4 transfectants. MDA-MB-435 transfectants were maintained in suspension or incubated with a β4-specific antibody (UM-A9) and allowed to adhere to anti-mouse IgG-coated plates for 30 min. Aliquots of cell extracts that contained equivalent amounts of protein were incubated with 4G10 and protein A-Sepharose for 3 h. (A) After being washed, the immune complexes were resolved by SDS–6% PAGE and immunoblotted with RC-20. Shown are one representative subclone expressing the wild-type (WT) β4 subunit and two representative subclones of each of the mutant β4 transfectants. (B) After being washed, the immune complexes were resuspended in a kinase reaction mixture and incubated for 10 min at room temperature. The phosphorylated lipids were resolved by thin-layer chromatography. Arrows, D3-phosphoinositides. S, cells maintained in suspension; β4, MDA-MB-435 cells clustered with the β4-specific antibody. ip, immunoprecipitation.
To determine if either Y1257 or Y1494 in the β4 cytoplasmic domain is required for PI3K activation by the α6β4 receptor, we assessed the association of PI3K with the phosphotyrosine immune complexes after ligation of α6β4 in the subclones that expressed these mutant β4 subunits. As shown in Fig. 6A (bottom), p85 association with the phosphotyrosine immune complexes increased after the clustering of the wild-type α6β4 and the Y1257F mutant α6β4 receptors. In contrast, an increase in p85 association with phosphotyrosine immune complexes in response to the clustering of the Y1494F and Y1257F/Y1494F mutant α6β4 receptors was not observed. The level of p85 subunit association with the phosphotyrosine immune complexes correlated well with the level of PI3K activity observed in in vitro kinase assays (Fig. 6B).
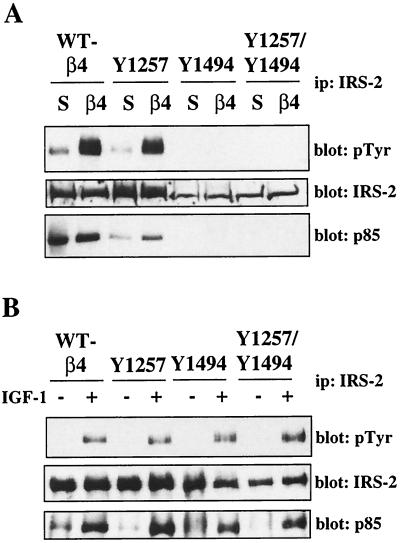
Our data suggest that IRS-2 is an important intermediate in the activation of PI3K by the α6β4 integrin. Therefore, we examined the ability of the mutant α6β4 receptors to promote IRS-2 tyrosine phosphorylation. As shown in Fig. 7A (top), mutation of Y1494 inhibited the ability of the α6β4 receptor to promote IRS-2 phosphorylation. Tyrosine phosphorylation of IRS-2 was also inhibited by the double Y1257F Y1494F β4 mutations. Moreover, the recruitment of p85 to IRS-2 in response to α6β4 ligation was prevented in the Y1494F-expressing and Y1257F Y1494F-expressing subclones (Fig. 6A, bottom). Although lower levels of IRS-2 expression were observed in the Y1494F-expressing and Y1257F- and Y1494F-expressing subclones, IRS-2 phosphorylation in response to α6β4 ligation was not detected even after prolonged exposure of the immunoblot. To confirm that the lack of IRS-2 phosphorylation in the subclones expressing the mutant β4 subunits was specific to α6β4-dependent signaling, the transfectants were treated with IGF-1, which promotes IRS-2 phosphorylation through IGF-1R. As shown in Fig. 7B, IRS-2 phosphorylation and PI3K recruitment in the wild-type and β4 mutant-expressing subclones after IGF-1 stimulation were equivalent. Taken together, our results indicate that Y1494 in the β4 subunit plays a pivotal role in the ability of the α6β4 integrin to activate PI3K.
FIG. 7.
Analysis of IRS-2 phosphorylation in the MDA-MB-435 mutant β4 transfectants. (A) MDA-MB-435 transfectants were maintained in suspension or incubated with β4-specific antibodies and allowed to adhere to anti-mouse IgG-coated plates for 30 min. WT, wild type; ip, immunoprecipitation. (B) MDA-MB-435 transfectants were incubated in the presence or absence of IGF-1 (100 ng/ml) for 5 min. Aliquots of cell extracts from equivalent numbers of cells were incubated with an IRS-2-specific antibody and protein A-Sepharose for 3 h. The immune complexes were resolved by SDS–6% PAGE and then immunoblotted with RC-20 (top). The immunoblots were subsequently stripped and reprobed with IRS-2-specified (middle) and p85-specific (bottom) polyclonal antisera.
Tyrosine 1494 in the β4 subunit is required for α6β4-dependent invasion.
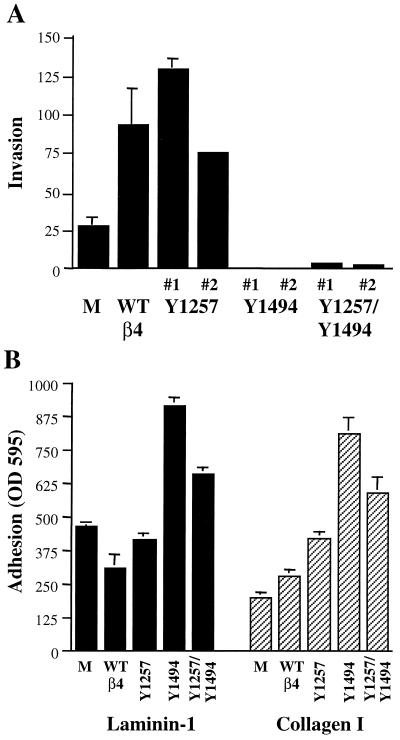
Expression of the β4 subunit increases the invasive potential of MDA-MB-435 cells, and we have hypothesized that this ability to promote invasion involves activation of PI3K by the α6β4 receptor (64). If this prediction is correct, expression of the Y1494F and Y1257F/Y1494F β4 mutant subunits in MDA-MB-435 cells should not increase their invasive potential. To address this question, subclones expressing the wild-type and mutant β4 subunits were assayed for their ability to invade Matrigel using a modified Boyden chamber assay. The MDA-MB-435 subclones expressing the Y1257F mutant subunit invaded to the same extent as the MDA-MB-435 subclone that expressed the wild-type β4 subunit (Fig. 8A). In contrast, the MDA-MB-435 subclones that expressed the Y1494F and Y1257F/Y1494F mutant β4 subunits did not invade. In fact, invasion was reduced below the level observed for the MDA-MB-435/mock transfectants, which suggests that the Y1494F and Y1257F/Y1494F mutant β4 subunits act in a dominant-negative manner for invasion.
FIG. 8.
Analysis of invasion and adhesion by the MDA-MB-435 mutant β4 transfectants. (A) MDA-MB-435 transfectants were assayed for their ability to invade Matrigel. Matrigel was diluted in cold distilled water, added to the upper well of Transwell chambers, and dried in a sterile hood. The Matrigel was reconstituted with medium, and the transfectants (5 × 104) were added to each well. Conditioned NIH 3T3 medium was added to the bottom wells of the chambers. After 4 h at 37°C, the cells that had not invaded were removed and the cells that had invaded to the lower surface of the filters were fixed, stained, and quantitated as described in Materials and Methods. The data shown are from one (mock and wild-type [WT] β4) or two (β4 mutants) individual subclones of each transfectant and are the mean values (± standard deviations [SD]) of a representative experiment done in triplicate. M, MDA-MB-435 cells transfected with vector alone; WT β4, MDA-MB-435 cells transfected with the wild-type β4 subunit. (B) MDA-MB-435 transfectants were assayed for their ability to adhere to laminin-1 and collagen I substrata. Forty-eight-well plates were coated overnight with 20 μg of laminin-1 or collagen I/ml (200 μl/well). The transfectants (105) were added to each well, and the plates were incubated for 1 h at 37°C. After being washed, the cells were fixed, stained, and quantitated as described in Materials and Methods. The data shown are the mean values (± SD) from a representative experiment done in triplicate.
The lack of invasion in the mutant β4 transfectants could result from a deficiency in adhesion. To examine if the observed decrease in invasion of the Y1494F and Y1257F/Y1494F transfectants was related to a decrease in cell adhesion, the ability of these cells to adhere to a laminin-1 or collagen I substrate was assessed. As shown in Fig. 8B, the Y1494F and Y1257F/Y1494F transfectants demonstrated a 1.5- to 2-fold higher level of adhesion to both substrates than the mock, wild-type β4, and Y1257 transfectants. The increased adhesion was not due to higher levels of receptor expression in the Y1494F and Y1257F/Y1494F transfectants (data not shown). Interestingly, the haptotactic migration of the Y1494F and Y1257F/Y1494F transfectants was significantly diminished on both laminin-1 and collagen I substrata (data not shown). The increased adhesive strength of the mutant β4 transfectants may inhibit their motility, which would impede the ability of these cells to invade (37). These results suggest that the Y1494 of the β4 subunit is important for regulating dynamic adhesion and that, in the absence of the IRS-2–PI3K signaling pathway activated through this tyrosine residue, other signals from the α6β4 receptor may promote stable adhesion.
DISCUSSION
Our results establish that the α6β4 integrin activates PI3K through the signaling adapters IRS-1 and IRS-2. This is a unique mechanism for α6β4 because the IRS proteins are not involved in PI3K activation downstream of the α6β1 receptor. We have also identified a specific tyrosine residue in the β4 cytoplasmic domain, Y1494, which is essential for the activation of PI3K and for the ability of the α6β4 integrin to promote carcinoma invasion. Taken together, our findings highlight a novel mechanism for α6β4-dependent signaling and for the ability of this integrin to promote carcinoma invasion.
Invasion is thought to be the essential first step for tumor cells in the metastatic cascade (14). The correlation between metastasis and patient mortality provides a strong impetus to elucidate the mechanisms involved in this transition. One approach to understanding how carcinoma cells acquire a motile, invasive phenotype is to dissect the cellular alterations that occur to drive this complex process. In this regard, previous work, including our own, has established that the α6β4 integrin can promote carcinoma invasion (12, 21, 64, 67). We determined that activation of PI3K was not only essential but also sufficient to increase the invasive potential of carcinoma cells, which emphasized the importance of investigating further the mechanism of α6β4 activation of this signaling pathway (64). We have now added to our understanding of this “invasion pathway” by establishing that IRS proteins IRS-1 and IRS-2 are upstream mediators in the activation of PI3K by the α6β4 integrin. This is the first report of an involvement of the IRS family in tumor invasion, and it establishes a new area of research for these proteins, which have been studied primarily for their role in metabolic regulation (84).
The role of the IRS proteins in α6β4-dependent promotion of tumor progression adds to other studies that have demonstrated an involvement of the IRS family in cancer. IRS-1 and IRS-2 are essential downstream effectors of IGF-1R, which is frequently overexpressed in tumors and which is a prognostic indicator of tumor recurrence and reduced patient survival (39, 77). Although only a limited number of studies have been performed to address directly the contribution of IRS function to cancer, the data to date support an important role for these proteins in tumor biology. For example, in breast cancer, an essential role for IRS-1 in IGF-1-dependent breast carcinoma cell survival and an involvement in IGF-1-dependent breast carcinoma cell growth have been observed (50). IRS-1 expression is regulated by estrogen, and the levels of IRS-1 are decreased in response to antiestrogens such as tamoxifen and ICI 182,780 (25, 60). This regulation of IRS-1 expression has been hypothesized to be a mechanism by which these antiestrogens inhibit breast carcinoma growth. Finally, high levels of IRS-1 expression in primary human breast cancers predict a greater incidence of recurrence and a decreased patient survival rate (58). Increased IRS-1 expression levels have also been observed in early stages of hepatocellular carcinoma, and a dominant-negative IRS-1 protein can reverse the malignant phenotype of transformed hepatocellular carcinoma cell lines (49, 73). Finally, IRS-1 and IRS-2 are both overexpressed in pancreatic cancer (36). It is intriguing to speculate that the correlations of IRS expression with tumor progression are related at least in part to the functions of the IRS proteins downstream of the α6β4 integrin.
The identification of IRS-1 and IRS-2 as signaling intermediates for the α6β4 integrin is significant because these proteins have the potential to regulate multiple signaling pathways downstream of this integrin receptor. IRS-1 and IRS-2 are cytoplasmic adapter proteins that do not contain intrinsic kinase activity but rather function by recruiting proteins to surface receptors, where they organize signaling complexes (84). These proteins belong to the IRS family, which includes IRS-1, IRS-2, IRS-3, and IRS-4. IRS-1 and IRS-2 are expressed ubiquitously, whereas the IRS-3 and IRS-4 homologs are more restricted in their localization (84). All of the IRS family members have homology and contain multiple binding motifs that are essential for their interaction with downstream effectors, which can include PI3K, Grb-2, SHP-2, Nck, Fyn, phospholipase C-γ, and Crk (4, 40, 46, 47, 66, 68, 69). With their ability to recruit such a variety of signaling molecules, the IRS proteins, not surprisingly, have been implicated in numerous cellular functions including mitogenesis, cell survival, gene transcription, and glucose transport (84). With regard to the involvement of the IRS proteins in α6β4-dependent signaling, we have identified PI3K as one downstream effector that is recruited to these adapter proteins in response to receptor ligation. The IRS-dependent activation of PI3K is important for the ability of α6β4 to promote invasion, an essential function of metastatic cells. Moreover, given the potential of the IRS proteins to interact with many other signaling effectors, other α6β4-dependent signals may also be regulated through these adapter proteins. For example, although we have observed MAPK activation in response to α6β4 ligation in the MDA-MB-435/β4 transfectants, we have not observed Shc phosphorylation, a proposed mechanism for MAPK activation (16, 43). An alternative mechanism for MAPK activation downstream of the α6β4 integrin could be IRS recruitment of Grb2. In support of this, we have not observed MAPK activation in the Y1494 mutant transfectants (data not shown).
Our demonstration that the α6β4 integrin is capable of stimulating the phosphorylation of both IRS-1 and IRS-2 raises the question of whether these homologs serve identical or distinct functions downstream of this integrin receptor. Although IRS-1 and IRS-2 share overall structural features and have some common effector binding sites, they also have unique phosphorylation sites (84). Furthermore, there are a number of reports that suggest that these homologs have different functions. For example, overexpression of IRS-1, but not IRS-2, in IRS-1 null fibroblasts restores IGF-1 stimulated cell cycle progression to the level observed in normal fibroblasts (8). In addition, IGF-1 stimulation of fetal brown adipocytes results in the association of Grb-2 with IRS-1 but not with IRS-2 (80). Differences in intracellular localization have also been demonstrated for IRS-1 and IRS-2, and this may explain some of the functional distinctions between these two homologs (28). The most striking evidence for functional differences in IRS-1 and IRS-2 comes from the phenotype of the IRS-1 and IRS-2 knockout mice. IRS-1 null mice are stunted in their growth, but they do not develop diabetes (1). In contrast, IRS-2 null mice develop insulin resistance in the liver and skeletal muscle and progressively lose their ability to regulate glucose homeostasis (85). The question of distinct IRS homolog function in carcinoma cells is relevant because differences in the expression and activity of IRS-1 and IRS-2 in tumor cells have been reported. For example, IRS-1 and IRS-2 are predominantly expressed in estrogen receptor positive (ER+) and ER− breast carcinoma cells, respectively (29). If IRS-1 and IRS-2 activate distinct downstream pathways, the function of the α6β4 receptor would depend on which IRS homolog was activated in response to receptor ligation. Therefore, although the α6β4 integrin can promote the phosphorylation of both IRS-1 and IRS-2, it is intriguing to speculate that α6β4-dependent signaling in carcinoma cells could be influenced by factors that differentially regulate the expression of the IRS proteins.
One issue that arises from our identification of IRS-1 and IRS-2 as downstream effectors of the α6β4 receptor is how the phosphorylation of these adapters is regulated by this integrin receptor. The IRS proteins were first discovered as signaling intermediates of the insulin receptor (IR), and they bind to a consensus sequence in the IR, where they are phosphorylated directly by the intrinsic receptor kinase domain (84). Although the IRS consensus binding site is also present in the IGF-1R and interleukin-4 (IL-4) receptor, not all receptors that promote IRS phosphorylation contain this binding motif, including the α6β4 integrin (15, 26, 32). In addition, none of these receptors possess intrinsic kinase domains. Therefore, alternative mechanisms for the recruitment and phosphorylation of the IRS proteins are necessary. Several models for α6β4-dependent activation of the IRS proteins could be proposed based on the mechanisms utilized by other members of this group of receptors that lack the IRS binding motif. One potential model involves the JAK family of tyrosine kinases, which includes JAK1, JAK2, JAK3, and Tyk2 (42). Several receptors, including those for alpha interferon, prolactin, growth hormone, and cytokines IL-2, -4, -7, and -15, associate with members of the JAK family, and these kinases recruit and phosphorylate the IRS proteins upon receptor stimulation (30, 53, 86). A second model involves the phosphorylation of the IRS proteins by members of the src-kinase family (17). However, it is not clear how the IRS proteins are recruited to receptor complexes for this src-dependent phosphorylation to occur. Focal adhesion kinase (FAK) has also been shown to associate with and promote phosphorylation of IRS-1 (38). However, the FAK-dependent phosphorylation may be indirect due to recruitment of src to the protein complexes. Finally, the possibility that the α6β4 receptor interacts with the IRS proteins through an association with other surface receptors is also valid. For example, the αvβ3 integrin can recruit IRS-1 indirectly to receptor complexes through an interaction with the insulin receptor (61, 83). The elucidation of the mechanism involved in the α6β4-dependent phosphorylation of the IRS proteins will provide additional targets for the disruption of tumor invasion.
We have identified a single amino acid in the β4 cytoplasmic domain, Y1494, which is involved in promoting the α6β4-IRS-PI3K invasion pathway. Mutation of this tyrosine residue inhibited not only α6β4-dependent activation of PI3K but also the ability of this receptor to promote carcinoma invasion. The fact that mutation of Y1494 had such a dramatic impact on α6β4-dependent signaling emphasizes the likelihood that this residue is an essential binding site for downstream effectors of the α6β4 receptor. We specifically selected Y1257 and Y1494 for mutation based on the location of these tyrosines within ITIM consensus binding motifs (79). The presence of both of these tyrosines within ITIM motifs might suggest that these residues have similar functions. However, it is clear that mutation of Y1257 in the β4 subunit does not disrupt the signaling functions of the α6β4 integrin, as was observed when Y1494 was mutated, even though both of these tyrosines appear to be phosphorylated in response to α6β4 ligation. These findings indicate that the two ITIM motifs in the β4 subunit do not function equally and that the specific sequences surrounding Y1257 and Y1494 must be important for regulating α6β4-dependent signals. ITIM motifs are known to be potential binding sites for the SH2 domains of tyrosine phosphatases SHP-1 and SHP-2 and lipid phosphatases SHIP-1 and SHIP-2 (79). SHP-1 and -2 phosphatases regulate tyrosine kinase signaling pathways, whereas the SHIP-1 and -2 phosphatases regulate the PI3K signaling pathway (reviewed in references 59 and 65). Disruption of either pathway could produce the phenotype we observed with the mutant receptors. For example, the phosphorylation of the β4 subunit is tightly regulated by tyrosine phosphatases, and this supports the potential involvement of SHP-2 (Fig. 5). The fact that IRS-2 is not phosphorylated in response to ligation of the Y1494 mutant receptor indicates that this tyrosine could be involved in the recruitment of IRS-2 to the receptor. However, it is also possible that Y1494 is essential for recruiting the kinase that regulates the phosphorylation of IRS-2 or other essential binding sites in the β4 subunit.
In summary, we have identified a novel mechanism for the activation of PI3K by the α6β4 integrin that involves IRS-1 and IRS-2 and requires Y1494 in the β4 cytoplasmic domain. Activation of this α6β4-IRS-PI3K signaling pathway promotes carcinoma invasion.
ACKNOWLEDGMENTS
I thank Yumiko Honzako for excellent technical assistance.
This work was supported by NIH grant CA81325 and U.S. Army grant DAMD17–97-1–7313.
REFERENCES
- 1.Araki E, Lipes M A, Patti M E, Bruning J C, Haag III B, Johnson R S, Kahn C R. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 2.Bachelder R E, Marchetti A, Falcioni R, Soddu S, Mercurio A M. Activation of p53 function in carcinoma cells by the alpha6beta4 integrin. J Biol Chem. 1999;274:20733–20737. doi: 10.1074/jbc.274.29.20733. [DOI] [PubMed] [Google Scholar]
- 3.Bachelder R E, Ribick M J, Marchetti A, Falcioni R, Soddu S, Davis K R, Mercurio A M. p53 inhibits alpha 6 beta 4 integrin survival signaling by promoting the caspase 3-dependent cleavage of AKT/PKB. J Cell Biol. 1999;147:1063–1072. doi: 10.1083/jcb.147.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beitner-Johnson D, Blakesley V A, Shen-Orr Z, Jimenez M, Stannard B, Wang L M, Pierce J, LeRoith D. The proto-oncogene product c-Crk associates with insulin receptor substrate-1 and 4PS. Modulation by insulin growth factor-I (IGF) and enhanced IGF-I signaling. J Biol Chem. 1996;271:9287–9290. doi: 10.1074/jbc.271.16.9287. [DOI] [PubMed] [Google Scholar]
- 5.Birchmeier W. E-cadherin as a tumor (invasion) suppressor gene. Bioessays. 1995;17:97–99. doi: 10.1002/bies.950170203. [DOI] [PubMed] [Google Scholar]
- 6.Bonaccorsi L, Carloni V, Muratori M, Salvadori A, Giannini A, Carini M, Serio M, Forti G, Baldi E. Androgen receptor expression in prostate carcinoma cells suppresses alpha6beta4 integrin-mediated invasive phenotype. Endocrinology. 2000;141:3172–3182. doi: 10.1210/endo.141.9.7640. [DOI] [PubMed] [Google Scholar]
- 7.Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Investig Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 8.Bruning J C, Winnay J, Cheatham B, Kahn C R. Differential signaling by insulin receptor substrate 1 (IRS-1) and IRS-2 in IRS-1-deficient cells. Mol Cell Biol. 1997;17:1513–1521. doi: 10.1128/mcb.17.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantley L C, Neel B G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantley L C, Songyang Z. Specificity in recognition of phosphopeptides by src-homology 2 domains. J Cell Sci Supplement. 1994;18:121–126. doi: 10.1242/jcs.1994.supplement_18.18. [DOI] [PubMed] [Google Scholar]
- 11.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 12.Chao C, Lotz M M, Clarke A C, Mercurio A M. A function for the integrin alpha6beta4 in the invasive properties of colorectal carcinoma cells. Cancer Res. 1996;56:4811–4819. [PubMed] [Google Scholar]
- 13.Clarke A S, Lotz M M, Mercurio A M. A novel structural variant of the human beta 4 integrin cDNA. Cell Adhesion Commun. 1994;2:1–6. doi: 10.3109/15419069409014197. [DOI] [PubMed] [Google Scholar]
- 14.Cotran R S, Kumar V, Collins T. Robbins pathologic basis of disease. 6th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1998. [Google Scholar]
- 15.Craparo A, O'Neill T J, Gustafson T A. Non-SH2 domains within insulin receptor substrate-1 and SHC mediate their phosphotyrosine-dependent interaction with the NPEY motif of the insulin-like growth factor I receptor. J Biol Chem. 1995;270:15639–15643. doi: 10.1074/jbc.270.26.15639. [DOI] [PubMed] [Google Scholar]
- 16.Dans M, Gagnoux-Palacios L, Blaikie P, Klein S, Mariotti A, Giancotti F G. Tyrosine phosphorylation of the beta 4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J Biol Chem. 2001;276:1494–1502. doi: 10.1074/jbc.M008663200. [DOI] [PubMed] [Google Scholar]
- 17.Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Src-family tyrosine kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J Biol Chem. 1999;274:20657–20663. doi: 10.1074/jbc.274.29.20657. [DOI] [PubMed] [Google Scholar]
- 18.Di Cristofano A, Pandolfi P P. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 19.Dowling J, Yu Q C, Fuchs E. Beta-4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 21.Falcioni R, Antonini A, Nistico P, Di Stefano S, Crescenzi M, Natali P G, Sacchi A. Alpha 6 beta 4 and alpha 6 beta 1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp Cell Res. 1997;236:76–85. doi: 10.1006/excr.1997.3695. [DOI] [PubMed] [Google Scholar]
- 22.Falcioni R, Sacchi A, Resau J, Kennel S J. Monoclonal antibody to human carcinoma-associated protein complex: quantitation in normal and tumor tissue. Cancer Res. 1988;48:816–821. [PubMed] [Google Scholar]
- 23.Falcioni R, Turchi V, Vittulo P, Navarra G, Ficari F, Cavaliere F, Sacchi A, Mariani-Costantini R. Integrin beta 4 expression in colorectal cancer. Int J Oncol. 1994;5:573–578. doi: 10.3892/ijo.5.3.573. [DOI] [PubMed] [Google Scholar]
- 24.Guilherme A, Czech M P. Stimulation of IRS-1-associated phosphatidylinositol 3-kinase and Akt/protein kinase B but not glucose transport by beta1-integrin signaling in rat adipocytes. J Biol Chem. 1998;273:33119–33122. doi: 10.1074/jbc.273.50.33119. [DOI] [PubMed] [Google Scholar]
- 25.Guvakova M A, Surmacz E. Tamoxifen interferes with the insulin-like growth factor I receptor (IGF-IR) signaling pathway in breast cancer cells. Cancer Res. 1997;57:2606–2610. [PubMed] [Google Scholar]
- 26.He W, Craparo A, Zhu Y, O'Neill T J, Wang L M, Pierce J H, Gustafson T A. Interaction of insulin receptor substrate-2 (IRS-2) with the insulin and insulin-like growth factor I receptors. Evidence for two distinct phosphotyrosine-dependent interaction domains within IRS-2. J Biol Chem. 1996;271:11641–11645. doi: 10.1074/jbc.271.20.11641. [DOI] [PubMed] [Google Scholar]
- 27.Hogervorst F, Kuikman I, von dem Borne A E, Sonnenberg A. Cloning and sequence analysis of beta-4 cDNA: an integrin subunit that contains a unique 118 kd cytoplasmic domain. EMBO J. 1990;9:765–770. doi: 10.1002/j.1460-2075.1990.tb08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue G, Cheatham B, Emkey R, Kahn C R. Dynamics of insulin signaling in 3T3–L1 adipocytes. Differential compartmentalization and trafficking of insulin receptor substrate (IRS)-1 and IRS-2. J Biol Chem. 1998;273:11548–11555. doi: 10.1074/jbc.273.19.11548. [DOI] [PubMed] [Google Scholar]
- 29.Jackson J G, White M F, Yee D. Insulin receptor substrate-1 is the predominant signaling molecule activated by insulin-like growth factor-I, insulin, and interleukin-4 in estrogen receptor-positive human breast cancer cells. J Biol Chem. 1998;273:9994–10003. doi: 10.1074/jbc.273.16.9994. [DOI] [PubMed] [Google Scholar]
- 30.Johnston J A, Wang L M, Hanson E P, Sun X J, White M F, Oakes S A, Pierce J H, O'Shea J J. Interleukins 2, 4, 7, and 15 stimulate tyrosine phosphorylation of insulin receptor substrates 1 and 2 in T cells. Potential role of JAK kinases. J Biol Chem. 1995;270:28527–28530. doi: 10.1074/jbc.270.48.28527. [DOI] [PubMed] [Google Scholar]
- 31.Juliano R L, Varner J A. Adhesion molecules in cancer: the role of integrins. Curr Opin Cell Biol. 1993;5:812–818. doi: 10.1016/0955-0674(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 32.Keegan A D, Nelms K, White M, Wang L M, Pierce J H, Paul W E. An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell. 1994;76:811–820. doi: 10.1016/0092-8674(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 33.Kellerer M, Koch M, Metzinger E, Mushack J, Capp E, Haring H U. Leptin activates PI-3 kinase in C2C12 myotubes via janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia. 1997;40:1358–1362. doi: 10.1007/s001250050832. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 35.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kornmann M, Maruyama H, Bergmann U, Tangvoranuntakul P, Beger H G, White M F, Korc M. Enhanced expression of the insulin receptor substrate-2 docking protein in human pancreatic cancer. Cancer Res. 1998;58:4250–4254. [PubMed] [Google Scholar]
- 37.Lauffenburger D A, Horwitz A F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 38.Lebrun P, Mothe-Satney I, Delahaye L, van Obberghen E, Baron V. Insulin receptor substrate-1 as a signaling molecule for focal adhesion kinase pp125(FAK) and pp60(src) J Biol Chem. 1998;273:32244–32253. doi: 10.1074/jbc.273.48.32244. [DOI] [PubMed] [Google Scholar]
- 39.Lee A V, Jackson J G, Gooch J L, Hilsenbeck S G, Coronado-Heinsohn E, Osborne C K, Yee D. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol. 1999;13:787–796. doi: 10.1210/mend.13.5.0274. [DOI] [PubMed] [Google Scholar]
- 40.Lee C H, Li W, Nishimura R, Zhou M, Batzer A G, Myers M G, Jr, White M F, Schlessinger J, Skolnik E Y. Nck associates with the SH2 domain-docking protein IRS-1 in insulin-stimulated cells. Proc Natl Acad Sci USA. 1993;90:11713–11717. doi: 10.1073/pnas.90.24.11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee E C, Lotz M M, Steele G D, Jr, Mercurio A M. The integrin alpha 6 beta 4 is a laminin receptor. J Cell Biol. 1992;117:671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu K D, Gaffen S L, Goldsmith M A. JAK/STAT signaling by cytokine receptors. Curr Opin Immunol. 1998;10:271–278. doi: 10.1016/s0952-7915(98)80165-9. [DOI] [PubMed] [Google Scholar]
- 43.Mainiero F, Pepe A, Wary K K, Spinardi L, Mohammadi M, Schlessinger J, Giancotti F G. Signal transduction by the alpha 6 beta 4 integrin: distinct beta 4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO J. 1995;14:4470–4481. doi: 10.1002/j.1460-2075.1995.tb00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murgia C, Blaikie P, Kim N, Dans M, Petrie H T, Giancotti F G. Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin beta4 cytoplasmic domain. EMBO J. 1998;17:3940–3951. doi: 10.1093/emboj/17.14.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers M G, Jr, Backer J M, Sun X J, Shoelson S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White M F. IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci USA. 1992;89:10350–10354. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myers M G, Jr, Mendez R, Shi P, Pierce J H, Rhoads R, White M F. The COOH-terminal tyrosine phosphorylation sites on IRS-1 bind SHP-2 and negatively regulate insulin signaling. J Biol Chem. 1998;273:26908–26914. doi: 10.1074/jbc.273.41.26908. [DOI] [PubMed] [Google Scholar]
- 47.Myers M G, Jr, Wang L M, Sun X J, Zhang Y, Yenush L, Schlessinger J, Pierce J H, White M F. Role of IRS-1–GRB-2 complexes in insulin signaling. Mol Cell Biol. 1994;14:3577–3587. doi: 10.1128/mcb.14.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niessen C M, Hulsman E H, Rots E S, Sanchez-Aparicio P, Sonnenberg A. Integrin alpha 6 beta 4 forms a complex with the cytoskeletal protein HD1 and induces its redistribution in transfected COS-7 cells. Mol Biol Cell. 1997;8:555–566. doi: 10.1091/mbc.8.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishiyama M, Wands J R. Cloning and increased expression of an insulin receptor substrate-1-like gene in human hepatocellular carcinoma. Biochem Biophys Res Commun. 1992;183:280–285. doi: 10.1016/0006-291x(92)91640-c. [DOI] [PubMed] [Google Scholar]
- 50.Nolan M K, Jankowska L, Prisco M, Xu S, Guvakova M A, Surmacz E. Differential roles of IRS-1 and SHC signaling pathways in breast cancer cells. Int J Cancer. 1997;72:828–834. doi: 10.1002/(sici)1097-0215(19970904)72:5<828::aid-ijc20>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 51.O'Connor K L, Nguyen B K, Mercurio A M. RhoA function in lamellae formation and migration is regulated by the alpha6beta4 integrin and cAMP metabolism. J Cell Biol. 2000;148:253–258. doi: 10.1083/jcb.148.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Connor K L, Shaw L M, Mercurio A M. Release of cAMP gating by the alpha6beta4 integrin stimulates lamellae formation and the chemotactic migration of invasive carcinoma cells. J Cell Biol. 1998;143:1749–1760. doi: 10.1083/jcb.143.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Platanias L C, Uddin S, Yetter A, Sun X J, White M F. The type I interferon receptor mediates tyrosine phosphorylation of insulin receptor substrate 2. J Biol Chem. 1996;271:278–282. doi: 10.1074/jbc.271.1.278. [DOI] [PubMed] [Google Scholar]
- 54.Rabinovitz I, Mercurio A M. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabinovitz I, Mercurio A M. The integrin alpha 6 beta 4 and the biology of carcinoma. Biochem Cell Biol. 1996;74:811–821. doi: 10.1139/o96-087. [DOI] [PubMed] [Google Scholar]
- 56.Rabinovitz I, Toker A, Mercurio A M. Protein kinase C-dependent mobilization of the alpha6beta4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol. 1999;146:1147–1160. doi: 10.1083/jcb.146.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rezniczek G A, de Pereda J M, Reipert S, Wiche G. Linking integrin alpha6beta4-based cell adhesion to the intermediate filament cytoskeleton: direct interaction between the beta4 subunit and plectin at multiple molecular sites. J Cell Biol. 1998;141:209–225. doi: 10.1083/jcb.141.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rocha R L, Hilsenbeck S G, Jackson J G, VanDenBerg C L, Weng C, Lee A V, Yee D. Insulin-like growth factor binding protein-3 and insulin receptor substrate-1 in breast cancer: correlation with clinical parameters and disease-free survival. Clin Cancer Res. 1997;3:103–109. [PubMed] [Google Scholar]
- 59.Rohrschneider L R, Fuller J F, Wolf I, Liu Y, Lucas D M. Structure, function, and biology of SHIP proteins. Genes Dev. 2000;14:505–520. [PubMed] [Google Scholar]
- 60.Salerno M, Sisci D, Mauro L, Guvakova M A, Ando S, Surmacz E. Insulin receptor substrate 1 is a target for the pure antiestrogen ICI 182,780 in breast cancer cells. Int J Cancer. 1999;81:299–304. doi: 10.1002/(SICI)1097-0215(19990412)81:2<299::AID-IJC21>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 61.Schneller M, Vuori K, Ruoslahti E. Alphabeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serini G, Trusolino L, Saggiorato E, Cremona O, De Rossi M, Angeli A, Orlandi F, Marchisio P C. Changes in integrin and E-cadherin expression in neoplastic versus normal thyroid tissue. J Natl Cancer Inst. 1996;88:442–449. doi: 10.1093/jnci/88.7.442. [DOI] [PubMed] [Google Scholar]
- 63.Shaw L M, Chao C, Wewer U M, Mercurio A M. Function of the integrin alpha 6 beta 1 in metastatic breast carcinoma cells assessed by expression of a dominant-negative receptor. Cancer Res. 1996;56:959–963. [PubMed] [Google Scholar]
- 64.Shaw L M, Rabinovitz I, Wang H H F, Toker A, Mercurio A M. Activation of phosphoinositide 3-OH kinase by the alpha-6-beta-4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 65.Siminovitch K A, Neel B G. Regulation of B cell signal transduction by SH2-containing protein-tyrosine phosphatases. Semin Immunol. 1998;10:329–347. doi: 10.1006/smim.1998.0125. [DOI] [PubMed] [Google Scholar]
- 66.Sozzani P, Hasan L, Seguelas M H, Caput D, Ferrara P, Pipy B, Cambon C. IL-13 induces tyrosine phosphorylation of phospholipase C gamma-1 following IRS-2 association in human monocytes: relationship with the inhibitory effect of IL-13 on ROI production. Biochem Biophys Res Commun. 1998;244:665–670. doi: 10.1006/bbrc.1998.8314. [DOI] [PubMed] [Google Scholar]
- 67.Sun H, Santoro S A, Zutter M M. Downstream events in mammary gland morphogenesis mediated by reexpression of the alpha2beta1 integrin: the role of the alpha6 and beta4 integrin subunits. Cancer Res. 1998;58:2224–2233. [PubMed] [Google Scholar]
- 68.Sun X J, Pons S, Asano T, Myers M G, Jr, Glasheen E, White M F. The Fyn tyrosine kinase binds Irs-1 and forms a distinct signaling complex during insulin stimulation. J Biol Chem. 1996;271:10583–10587. doi: 10.1074/jbc.271.18.10583. [DOI] [PubMed] [Google Scholar]
- 69.Sun X J, Rothenberg P, Kahn C R, Backer J M, Araki E, Wilden P A, Cahill D A, Goldstein B J, White M F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki S, Naitoh Y. Amino acid sequence of a novel integrin beta 4 subunit and primary expression of the mRNA in epithelial cells. EMBO J. 1990;9:757–763. doi: 10.1002/j.1460-2075.1990.tb08170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi M I, Menard S. Prognostic value of alpha 6 beta 4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res. 1998;4:407–410. [PubMed] [Google Scholar]
- 72.Tamura R N, Rozzo C, Starr L, Chambers J, Reichardt L F, Cooper H M, Quaranta V. Epithelial integrin alpha 6 beta 4: complete primary structure of alpha 6 and variant forms of beta 4. J Cell Biol. 1990;111:1593–1604. doi: 10.1083/jcb.111.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanaka S, Wands J R. A carboxy-terminal truncated insulin receptor substrate-1 dominant negative protein reverses the human hepatocellular carcinoma malignant phenotype. J Clin Investig. 1996;98:2100–2108. doi: 10.1172/JCI119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang K, Nie D, Cai Y, Honn K V. The beta4 integrin subunit rescues A431 cells from apoptosis through a PI3K/Akt kinase signaling pathway. Biochem Biophys Res Commun. 1999;264:127–132. doi: 10.1006/bbrc.1999.1496. [DOI] [PubMed] [Google Scholar]
- 75.Tani T, Karttunen T, Kiviluoto T, Kivilaakso E, Burgeson R E, Sipponen P, Virtanen I. Alpha 6 beta 4 integrin and newly deposited laminin-1 and laminin-5 form the adhesion mechanism of gastric carcinoma. Continuous expression of laminins but not that of collagen VII is preserved in invasive parts of the carcinomas: implications for acquisition of the invading phenotype. Am J Pathol. 1996;149:781–793. [PMC free article] [PubMed] [Google Scholar]
- 76.Toker A, Cantley L C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 77.Turner B C, Haffty B G, Narayanan L, Yuan J, Havre P A, Gumbs A A, Kaplan L, Burgaud J L, Carter D, Baserga R, Glazer P M. Insulin-like growth factor-I receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res. 1997;57:3079–3083. [PubMed] [Google Scholar]
- 78.Uddin S, Majchrzak B, Wang P C, Modi S, Khan M K, Fish E N, Platanias L C. Interferon-dependent activation of the serine kinase PI 3′-kinase requires engagement of the IRS pathway but not the Stat pathway. Biochem Biophys Res Commun. 2000;270:158–162. doi: 10.1006/bbrc.2000.2402. [DOI] [PubMed] [Google Scholar]
- 79.Unkeless J C, Jin J. Inhibitory receptors, ITIM sequences and phosphatases. Curr Opin Immunol. 1997;9:338–343. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 80.Valverde A M, Lorenzo M, Pons S, White M F, Benito M. Insulin receptor substrate (IRS) proteins IRS-1 and IRS-2 differential signaling in the insulin/insulin-like growth factor-I pathways in fetal brown adipocytes. Mol Endocrinol. 1998;12:688–697. doi: 10.1210/mend.12.5.0106. [DOI] [PubMed] [Google Scholar]
- 81.Vanderneut R, Krimpenfort P, Calafat J, Niessen C M, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta-4 null mice. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 82.van Waes C, Kozarsky K F, Warren A B, Kidd L, Paugh D, Liebert M, Carey T E. The A9 antigen associated with aggressive human squamous carcinoma is structurally and functionally similar to the newly defined integrin alpha 6 beta 4. Cancer Res. 1991;51:2395–2402. [PubMed] [Google Scholar]
- 83.Vuori K, Ruoslahti E. Association of insulin receptor substrate-1 with integrins. Science. 1994;266:1576–1578. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]
- 84.White M F. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl. 2):S2–S17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 85.Withers D J, Gutierrez J S, Towery H, Burks D J, Ren J M, Previs S, Zhang Y, Bernal D, Pons S, Shulman G I, Bonner-Weir S, White M F. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 86.Yamauchi T, Kaburagi Y, Ueki K, Tsuji Y, Stark G R, Kerr I M, Tsushima T, Akanuma Y, Komuro I, Tobe K, Yazaki Y, Kadowaki T. Growth hormone and prolactin stimulate tyrosine phosphorylation of insulin receptor substrate-1, -2, and -3, their association with p85 phosphatidylinositol 3-kinase (PI3-kinase), and concomitantly PI3-kinase activation via JAK2 kinase. J Biol Chem. 1998;273:15719–15726. doi: 10.1074/jbc.273.25.15719. [DOI] [PubMed] [Google Scholar]